Abstract
This review contains the results of Polish (Central Europe) ethnomedical studies that describe the treatment of urinary tract diseases with wild and cultivated plants. The study includes only the plants that are used to treat the urinary tract, excluding prostate diseases. A review of the literature was carried out to verify the pharmacological use of the plants mentioned in the interviews. Based on this, the study reviews the pharmacological activities of all the recorded species and indicates their most important chemical compounds. Fifty-three species (belonging to 30 families) were selected for the study. The Compositae (eight species), Rosaceae (six species), and Apiaceae (six species) are the most common families used in the treatment of urinary diseases in Polish folk medicine. Both in vitro and in vivo studies have confirmed that many of these plant species have beneficial properties, such as diuretic, antihyperuricemic, antimicrobial, and anti-inflammatory activity, or the prevention of urinary stone formation. These effects are exerted through different mechanisms, for example, through the activation of bradykinin B2 receptors, inhibition of xanthine oxidase, or inhibition of Na+-K+ pump. Many plants used in folk medicine are rich in phytochemicals with proven effectiveness against urinary tract diseases, such as rutin, arbutin, or triterpene saponins.
1. Introduction
Urological diseases are most commonly associated with the filtration and excretion of urine from the body. In males, health problems can manifest in the urinary tract and/or the reproductive organs, while in females these issues usually affect only the urinary tract. There are many urologic disorders and diseases. They include infections of the urinary tract, urinary lithiasis, urinary incontinence and disorders of micturition, hereditary diseases, and other common urological conditions [1,2,3].
Urinary tract infections (UTIs) occur in the entire population, but young children, pregnant women, postmenopausal women, and men with prostatic hyperplasia are especially susceptible to them [1,2,3].
Urolithiasis is among the most commonly diagnosed diseases of the urinary tract. The frequency of its occurrence is about 9% of the general population [4,5]. Urolithiasis occurs in about 10% of men and 6% of women [6,7]. Over a period of five years, the risk of disease recurrence and the chance of second stone formation is 30% to 50% [8,9].
There are four categories of plants utilized in the treatment of urological diseases—botanical diuretics, urinary antiseptic and anti-adhesion agents, antinephrotoxic botanicals, and herbs used in the treatment of benign prostatic hyperplasia. Numerous plants are used as diuretics in traditional medicine. In many cases, their activity has been confirmed by preliminary clinical trials, both in healthy people and patients with urologic diseases, though the mechanism of their action often remains unclear [10]. Although these traditional herbal drugs are called diuretics, it would be more accurate to describe them as aquaretics. They usually contain flavonoids, volatile oils, saponins, or tannins, which promote blood flow in the kidneys, leading to a higher glomerular filtration rate and increased urine volume. However, they do not decrease the resorption of Na+ and Cl− in the renal tubules; these electrolytes are retained in the body instead of being excreted with water [11]. Increased urine flow can help with the prevention of kidney stone formation [8]. Diuretic herbs can also prove useful for minor infections, which can be alleviated due to increased urine volume. Some herbs exhibit antibacterial properties, which, in combination with increased urinary output, are useful in combating infection. There are two major mechanisms of this process—targeted killing of microbes and interference in their adhesion to epithelial cells [10].
It is believed that rural, traditional folk medicine is more prevalent in underdeveloped communities, for example, in Eastern Europe (Poland, Ukraine, Lithuania, and Belarus). Traditional folk medicine and self-treatment are strongly associated with Polish culture and tradition. Between the 1940s and 1980s, it was associated with ignorance. Folk treatment methods were vigorously condemned, while folk healers were forced to abandon their practice by the “socialist health service”. However, folk treatment methods did not disappear in rural regions; ethnological, sociological, and medical studies suggest that they seem to be enjoying social acceptance [12,13,14].
The present paper collects information about plants used to treat urological diseases in Polish folk medicine and reviews their pharmacological activities, most important phytochemicals, and mechanisms of action. We described 53 species belonging to 30 plant families. The greatest number of plant species used to treat urinary diseases were found to come from the Compositae (eight species), Rosaceae (six species), and Apiaceae (six species). The plants were identified based on ethnopharmacological studies, Polish herbalist literature, and interviews with Polish traditional healers. We used PubMed, Scopus, Google Scholar, Web of Science, Science Direct, and other databases to search for the ethnomedicinal uses of these plants, as well as phytochemistry, pharmacology, toxicity, and clinical studies. The search terms included the name of plant or phytochemical and the words “diuretic”, “urinary”, “UTI”, or “urolithiasis”. Plant names have been checked and updated with the online website The Plant List version 1.1 (www.theplantlist.org (accessed between 1 April 2023 and 30 July 2023)) of the Royal Botanic Gardens, Kew, and Missouri Botanical Garden and the online website Atlas of Vascular Plants of Poland (http://www.atlas-roslin.pl (accessed between 1 April 2023 and 30 July 2023)). The common names of the plants were identified by referring to research articles.
Table 1 presents the plant nomenclature, main Polish name, English common name, the part used, utilization, and administration. Most often, dried material was used. Moreover, in Table 1, plants are listed alphabetically by plant family, and then by species.

Table 1.
Ethnopharmacological characteristics of plants reported for the treatment of urinary diseases in Poland.
2. Plants Whose Therapeutic Effect on the Urinary Tract Has Been Confirmed by Scientific Findings (in Alphabetical Order)
Based on the literature data, we found that the described plants show mainly diuretic and natriuretic effects. Table 2 presents the results of the researched studies, including the experimental model, extract used, dose, control and reference substance, duration, number of animals or participants, and the effect on UV and UNa. The remaining test results are described in the text for individual plants or are included in the table in the ‘other effects found’ column: in this way, the text complements the information in the table rather than duplicates it.

Table 2.
Summary of research reporting effects on urinary volume and sodium excretion. The plants are listed alphabetically.
2.1. Achillea millefolium L.
The Compositae is one of the best-known families, incorporating numerous flowering plants classified into approximately 23,000 species. One such species is Achillea, whose members have numerous pharmaceutical properties. They are recommended as effective tonics, sedatives, and diuretics [26]. In addition, it is common to see the consumption of herbal teas from different species of Achillea in folk medicine. Many of the varied therapeutic usages have been confirmed by new in vitro and in vivo experimental and clinical studies. Various authors note that Achillea millefolium has important traditional and ethnomedicinal uses when drunk as a tea, including treating urinary disorders; it has been used in various countries, particularly in Europe [26,83,84]. The varied effects of these plants may be due to the presence of numerous secondary metabolites, i.e., flavonoids, phenolic acids, terpenoids, and sterols. Souza et al. [62] demonstrated that oral administration of A. millefolium extract in rats effectively increases diuresis. For example, hydroethanolic extract at the dose of 300 mg/kg increased diuresis by approximately 30–60% between 4 and 8 h after administration. This effect is dependent on the activation of bradykinin B2 receptors and the activity of cyclooxygenase.
2.2. Acorus calamus L.
For centuries, Acorus calamus has been used in Polish folk medicine to treat urinary diseases [85]. It is also used in modern medicine. Ghelani et al. [15] report that an ethanolic extract of A. calamus rhizome demonstrates diuretic and antiurolithiatic activity in an experimental animal model (male Wistar albino rats). Their results indicate that the ethanolic extract of A. calamus significantly increases diuresis and reduces the urinary excretion of phosphate, oxalate, calcium, and uric acid (compared to urolithiatic control rats). At the dose of 750 mg/kg, the diuretic effect was comparable to that of furosemide. The urine volume increased from 1.88 ± 0.18 mL (control) to 3.78 ± 0.11 mL (A. calamus extract at the dose of 750 mg/kg) and 4.02 ± 0.17 mL (furosemide at the dose of 15 mg/kg).
2.3. Aegopodium podagraria L.
Aegopodium podagraria is a perennial herb of the carrot family (Apiaceae). It is native to Europe, western and eastern Siberia, the Caucasus, and Central Asian mountainous regions. It has been naturalized in North America. Koyro et al. showed that the essential oil from the flowers has diuretic and uricosuric activities [63]. Another study examined the influence of the extract and tincture of the aerial parts of A. podagraria in rats that received excess fructose with hydrochlorothiazide [64]. The authors observed that the tested extract significantly enhances kaliuresis.
2.4. Amaranthus retroflexus L.
Amaranthus retroflexus is an upright annual herb that was probably introduced into Europe from North America. Currently, it is common throughout Poland [86]. The plant is known to have a number of toxic effects; for example, Osweiler et al. [87] demonstrated that A. retroflexus induced the production of perirenal edema in pigs. Microscopical lesions include renal tubular degeneration and necrosis. [88] observed that acute renal failure is associated with Amaranthus species, including A. retroflexus ingestion by lambs.
2.5. Apium graveolens L.
In traditional medicine, some medicinal plants, i.e., corn silk, barley, and celery were used to relieve renal pain. Apium graveolens is a popular vegetable that can be added to many dishes, such as salads. It has been used in Chinese medicine to decrease blood pressure and in Arabian medicine to relieve renal pain [10,65,89,90]. [65] observed that celery (at the dose of 8 g/kg/day) increased urinary Ca2+ excretions (from 2.05 ± 0.07 to 5.01 ± 0.05 mmol/L) in an experimental model of nephrocalcinosis, and caused a significant reduction in serum creatinine (from 97.3 ± 0.5 to 87.2 ± 0.63 mmol/L) and blood urea nitrogen (from 7.3 ± 0.2 to 5.7 ± 0.5 mmol/L). Renal functions were analyzed on the first, fifth and 10th day.
2.6. Arctostaphylos uva-ursi (L.) Spreng.
Arctostaphylos uva-ursi folium has been used for therapeutic purposes in Europe and America, particularly in the treatment of lower urinary tract infections. The key constituents of the dried leaves are arbutin (a phenolic glycoside) and its derivate hydroquinone, which both show antiseptic activity in inflammation of the urinary tract [36,91]. Although arbutin is the major pharmacological active constituent of the A. uva-ursi leaves extract, experimental studies indicate that their global pharmacological action requires the use of the whole extract. Haslam [92] described that the dried leaves of Arctostaphylos uva-ursi have a soothing, astringent effect and that they can be used as a diuretic in kidney disorders and aliments of the bladder and urinary tract. The principal phenolic metabolites in the leaves are arbutin, gallotannins, and galloyl esters of arbutin.
2.7. Betula pendula Roth and Betula pubescens Ehrh.
Although over a hundred Betula species are distributed all over the globe, we only know of seven species used in traditional medicine, B. pendula among them. Crude extracts, fractions, and phytochemical constituents isolated from B. pendula have demonstrated a wide spectrum of in vivo and in vitro biological properties [93]. Extracts of B. pendula leaves have been used in arthritic diseases and to relieve rheumatic pain. In addition, different experiments suggest that these extracts have mild diuretic activity and anti-inflammatory properties. B. pendula extract reduces the growth and proliferation of activated T lymphocytes in a dose-dependent manner; this has been attributed to the action of secondary metabolites (i.e., phenolic acids and flavonoids) within the leaves [94,95]. Also, an ethanol–water (1:1; v/v) extract from the Betula spp. leaves has been found to have an antiadhesive effect against the binding of uropathogenic Escherichia coli to the bladder cell surface (cell line T24). Decreased bacterial adhesion (IC50 415 mg/mL ± 7.19) was observed, and this action is linked with urinary tract infection prevention [66].
2.8. Bidens tripartita L.
Another member of the Composite is Bidens tripartita. It is also known in oriental medicine, where it is used as a diuretic and diaphoretic in nephrolithiasis. It was also valued for its antiseptic and anti-inflammatory properties [27,28,96].
2.9. Carum carvi L.
The dried fruits of Carum carvi are used in traditional medicine as a carminative and have been found to be effective against gastrointestinal complications [19]. In Moroccan traditional medicine, the ripe fruits of C. carvi are used as diuretics; however, this activity has not been investigated in controlled studies. On the other hand, Lahlou et al. [68] report that the aqueous extract of C. carvi demonstrated diuretic properties in healthy rats.
2.10. Daucus carota L.
Daucus carota commonly known as carrot, has been used traditionally in folk medicine to treat nephrosis and other urinary disorders. Sodimbaku et al. [69] have observed that carrot has dose-dependent nephroprotective activity against gentamicin-induced nephrotoxicity in rats. Both used doses (200 and 400 mg/kg) had significant effects. The dose of 400 mg/kg decreased the levels of serum urea (from 79.10 ± 2.21 to 45.96 ± 2.37 mg/dL), blood urea nitrogen (from 37.23 ± 1.36 to 20.21 ± 3.38 mg/dL), uric acid (from 4.87 ± 0.29 to 2.83 ± 0.35 mg/dL), and creatinine (from 2.03 ± 0.08 to 1.04 ± 0.08 mg/dL). Moreover, concurrent treatment with D. carota extract significantly inhibited gentamicin-induced weight loss.
2.11. Elsholtzia ciliata (Thunb.) Hyl.
Elsholtzia ciliata is widely distributed throughout Korea, China, and Europe. In Poland, it grows mainly in the eastern regions [97,98]. An in vivo study found that ethanol extract from this plant inhibits renal interstitial fibrosis induced by unilateral ureteral obstruction. This effect may be mediated by inhibiting the expression of KIM1 (kidney injury molecule 1), TNF-α (tumor necrosis factor α), TGF-β (transforming growth factor β), Smad3, and MMP 9 (matrix metalloproteinase 9) proteins, which are markers of inflammation and renal histopathological alterations. An in vitro study based on MTT (3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay showed that E. ciliata ethanolic extract has no cytotoxic effect, even at a concentration of 200 µg/mL. The study was conducted for 24 h on mouse macrophage cells (RAW 264.7) and human renal mesangial cells [70].
2.12. Elymus repens (L.) Gould
Elymus repens originates from the temperate regions of Europe and Central Asia. Nowadays, it can be found in Africa as well. Traditionally, it is used as a soothing diuretic and to alleviate pain and spasming of the urinary tract. It is often used in urinary system disorders in children (e.g., urinary incontinence and enuresis), to combat the symptoms of urinary disease, urinary calculi, and urinary infections (like prostatitis, urethritis, or cystitis) [99]. In a clinical study, the effects of administrating A. repens with potassium citrate were assessed in patients with nephrolithiasis. After five months the treated group exhibited a significant reduction in the diameter (−3.6 ± 0.9 mm vs. 0.0 ± 0.8 mm) and total number of stones (−1.0 ± 0.2 vs. 0.0 ± 0.2 stones). A reduction in uric acid urinary excretion was noted as well [71]. It was also shown, that under in vitro conditions, E. repens had antiadhesive activity towards uropathogenic Escherichia coli. It inhibited the binding of E. coli to the surface of bladder cells (cell line T24). Decreased bacterial adhesion (IC25 630 mg/mL) was observed, and this effect has been linked to the prevention of urinary tract infections [66]. In a clinical trial in patients with micturition disorders, an ethanolic extract of Elymus repens caused a reduction in urinary incontinence, nycturia, dysuria, and tenesmus due to adenoma of prostate, cystitis, and prostatitis [72].
2.13. Equisetum arvense L.
The genus Equisetum encompasses 30 species of rush-like, long-standing herbs. An extract from Equisetum arvense is used to relieve pain and increase diuresis. It is highly effective in removing water from the body. This effect is due to the action of various components, i.e., equisetonin, potassium, magnesium, calcium, ascorbic acid, and caffeic acid [100,101,102]. Recently, Carneiro et al. [35] noted that a standardized, dried E. arvense extract showed a diuretic effect stronger than hydrochlorothiazide in healthy male volunteers. In addition, E. arvense did not have a significant effect on the urinary excretion of catabolites and electrolytes. It was also deemed safe for oral consumption.
2.14. Foeniculum vulgare Mill.
Foeniculum vulgare is a biennial medicinal and aromatic plant. Its bulbs and fronds have a culinary application [103]. Bardai et al. [21] showed that administration of F. vulgare fruit extract significantly increased urinary volume, as well as potassium and sodium excretion. The dosage was chosen according to the dose used in Moroccan traditional medicine.
2.15. Fraxinus excelsior L.
The Fraxinus genus has been used in traditional medicine in many regions of the globe. It is valued for its diuretic properties and has been used in the treatment of rheumatic pain, arthritis, cystitis, constipation, dropsy, and itching scalp. In Polish folk medicine, the bark and leaves of Fraxinus excelsior, which is native to Europe, are used to treat various conditions, including wounds, diarrhea, and dysentery [42]. F. excelsior leaf extracts have been used to promote renal excretion. Aqueous and ethanolic extracts can be used to make spray-dried powders, which increase the excretion of chloride and sodium ions, potassium, and urea. The presence of flavonoids is likely the reason for their diuretic activity [43]. Moreover, the daily oral administration of aqueous extract from Fraxinus excelsior increased urination and promoted urinary excretion of sodium, potassium, and chlorides in hypertensive rats [44].
2.16. Humulus lupulus L.
Humulus lupulus is well-known across the world for its use in the brewing industry. The major compounds isolated from mature hop cones include chalcones, bitter acids, and terpenes [104]. Moreover, hops have a long history of use in folk medicine, particularly to treat sleep disturbances. In addition, some in vitro and in vivo experiments support the value of hops as a traditional antibacterial and antifungal remedy, while others show them to have diuretic activity.
2.17. Juniperus communis L.
In both folk and official medicine, juniper berries (Juniperus communis) are believed to have diuretic, antiseptic, stomachic, and carminative activities [11]. Juniper berries contain essential oil (between 0.5 and 2%), which is a source of phenolic compounds, carbohydrates, fatty acids, and sterols. However, Stanić et at. [73] found that the diuretic activity of juniper berries is the result of the action of essential oil and hydrophilic constituents.
2.18. Lycopodium clavatum L.
Lycopodium clavatum is a pteridophyte that grows abundantly in subtropical and tropical regions and in numerous European countries. This plant is used to help with digestion, relieve gastric inflammation, and for the treatment of chronic kidney disorders [105]. It has been shown that L. clavatum can play a role in alternative treatment of gout by inhibiting xanthine oxidase. Alcoholic and aqueous extracts (50–100 μg/mL) of the whole plant inhibited oxidase activity by 13–58% [106].
2.19. Nigella sativa L.
The seeds of Nigella sativa have been used in the Indian subcontinent, Europe, and Arabian countries for culinary purposes and as a remedy for several illnesses and conditions such as hypertension, asthma, inflammation, diabetes, bronchitis, cough, headache, fever, influenza, dizziness, and eczema. The seeds and seed oil are used as a carminative and in food as a spice [51], although they are seldom cultivated as a spice in Poland (atlas-roslin.pl). Zaoui et al. [50] showed, that dichloromethane extract from N. sativa seeds has a strong diuretic effect in rats with spontaneous hypertension. The diuretic activity of the extract was approximately 16% stronger than that of frusemide, which increased diuresis by 30%. Apart from increased diuresis, the excretion of sodium, chloride, potassium, and urea was higher as well.
2.20. Petroselinum crispum (Mill.) Fuss
Petroselinum crispum, commonly known as parsley, is used as carminative, gastro tonic, diuretic, and antiseptic of the urinary tract. In addition, in Bulgarian phytotherapy, various parts of P. hortense (leaves, seeds, and roots) are thought to have diuretic activity [107].
De Ribeiro et al. [74] investigated the action of aqueous-ethanol extract from the seeds of P. sativum which was administered to rats. Increased urinary volume and sodium excretion were observed, and these effects were similar to those of furosemide. Yarnell et al. [10] noted that P. crispum has strong potency which was compared to properties of other herbal diuretics, including Taraxacum officinale and Ononis compestris, whose potential was described as medium. The main active compounds of P. sativum are phenolics, which include flavonoids such as appinin and apigenin. These compounds possess a wide range of biological effects, diuretic activity among them [107]. Moreover, Kreydiyyeh and Usta [75] observed that aqueous parsley seed extract increased diuresis. Its mechanism of action appears to be mediated by the inhibition of Na+-K+ pump that leads to a reduction in Na+ and K+ reabsorption, which in turn, causes osmotic water flow into the lumen and diuresis. In another study, Alyami and Rabah [76] reported that parsley leaf tea induced no significant differences in urine volume, pH, sodium, chloride, magnesium, potassium, creatinine, urea, citric acid, or uric acid. It did not induce a significant reduction in urinary tract stone formation as well. Moreover, Saeidi et al. [77] showed that aqueous extracts of P. sativum had a therapeutic effect on calcium oxalate stones in rats and reduced the number of calcium oxalate deposits. It has been observed that P. sativum extract significantly increases the calcium level and decreases the magnesium level in serum.
2.21. Plantago major L.
Plantago major, a perennial herb, is found wild throughout the whole of Europe and temperate regions of Asia. Every part of the plant has been used in traditional medicines that are used to treat cough, diarrhea, dysentery, infections, pain, or urinary tract calculus [47]. An ethanolic extract of P. major significantly inhibited the growth of calcium oxalate crystals (dihydrate variety) in vitro. It has been shown, that P. major extract was better than allopurinol and potassium citrate in the reduction in the risk of unfavorable renal outcomes [78].
2.22. Rosa canina L.
Rosa canina, a plant native to Poland can also be found in other European countries [108]. Its fruit is extensively used worldwide in foods such as jelly and jam, in various beverages such as tea, and in traditional medicine to treat urate metabolism disorders. Urate is the end product of purine metabolism. It is produced from hypoxanthine after double enzyme catalysis by xanthine oxidase (XO), which is carried out in the liver. An imbalance in serum urate production and excretion induces hyperuricemia, which can also develop into gout and kidney stones, and accelerate the progression of renal diseases. In vitro studies have shown that R. canina extracts inhibited XO activity and significantly decreased the levels of serum urate eight hours after administration. It is suggested that R. canina hot water extract can serve as a functional food which could be beneficial for patients with a high level of urate. It could also be used in the treatment of hyperuricemic patients [109]. It has also been shown, that aqueous extract lowered the levels of renal and urinary calcium, decreased the number and size of kidney CaOx calculi, and promoted citrate excretion without affecting the urinary concentrations of oxalate, or urine volume and pH [79].
2.23. Sambucus ebulus L. and Sambucus nigra L.
Sambucus ebulus is a herbaceous plant well-known in traditional European medicine for its healing effects in many disorders; however, its toxicity limits its value as food [110]. Dimkov [16] indicates that a decoction from S. ebulus has diuretic and diaphoretic properties. Also, Beaux et al. [80] report increased excretion of sodium in rats after the administration of S. nigra flower extract. In addition, Walz and Chrubasik [81] indicate that S. nigra concentrate can be administered without the risk of adverse effects, even in patients afflicted with idiopathic nephrolithiasis. They observed that S. nigra concentrate did not change the urinary pH or hydrogen ion levels. The solubility of stone-inducing ions remained unaffected as well.
2.24. Taraxacum campylodes G.E.Haglund
Taraxacum campylodes is a widespread perennial of the Asteraceae family. It is commonly seen as a weed but contains a large variety of chemical compounds with healing potential. Most of the active substances found in T. campylodes are phenols and terpenes; however, carbohydrates, proteins, fatty acids, vitamins, and minerals are also present. This range of compounds has resulted in the plant being used as a natural drug in the treatment of gout and diarrhea, as well as problems associated with the bladder, spleen, and liver [111]. Leaf ethanol extract (1 g/mL) demonstrated a diuretic effect in a group of women treated with the extract every five hours for four days, with no side effects observed [112,113,114].
2.25. Urtica dioica L. and Urtica urens L.
Urtica dioica is a perennial plant widely distributed throughout the temperate and tropical regions of the globe, common throughout Poland [86]. Traditionally, U. dioica leaves and roots are known for a wide range of ethnomedicinal uses. Urtica urens has similar pharmacological properties [115]. The aqueous extract of the aerial part of U. dioica at a low dose (4 mg/kg/h) increased diuresis by 11%, and by 84% at a high dose (24 mg/kg/h). Moreover, low and high doses induced natriuresis by 28% and 143%, respectively [59]. Various preparations from U. dioica have been investigated experimentally, but only stinging nettle juice, tea, stew, encapsulated fresh freeze-dried leaf powder, and proprietary extracts have been used in human studies [115,116]. In vitro studies have shown that, like Betula spp. and Elymus repens, Urtica spp. shows an antiadhesive effect against the binding of uropathogenic E. coli to the surface of bladder cells (IC25 630 mg/mL) [66]. However, while U. dioica ethanolic extract at a dose of 1g/kg (p.o) had no effect on diuresis, its administration at a dose of 500 mg/kg (i.p) resulted in a significant increase of the urine output [115].
2.26. Vaccinium myrtillus L.
Vaccinium myrtillus is a small deciduous shrub that is very popular in Poland and other European countries. The leaves are used in folk medicine as decoctions and infusions for treating conditions associated with the urinary tract thanks to their astringent and antiseptic properties. Vaccinium vitis idaea is another evergreen small shrub growing in Europe; its berries are known to have the same properties as V. myrtillus fruits, while the leaves have diuretic and urinary antiseptic activities, which have been attributed to their high concentration of tannins, arbutin, and arbutin derivatives [117].
2.27. Viola tricolor L.
Viola tricolor has a long history in phytomedicine. Its aerial parts have been described and used in Europe for centuries; they were utilized in the therapy of skin disorders and upper-respiratory problems and were used as a diuretic [60]. V. tricolor inhabits the lowland and lower mountain areas of Poland [118]. Its anti-inflammatory and diuretic properties have been attributed to the presence of saponins (5.98%) and flavonoids (1.81–1.99%). In a study conducted on rats, Viola tricolor tincture was found to have a moderate diuretic effect (diuretic index was 1.103, saluretic index of Na+ was 1.181, and saluretic index of K+ was 1.365) [61].
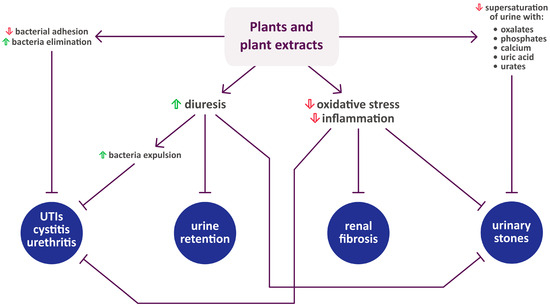
Figure 1 summarizes the activity of each plant listed in Section 2, while Figure 2 shows their mechanisms of action in urinary diseases and disorders.
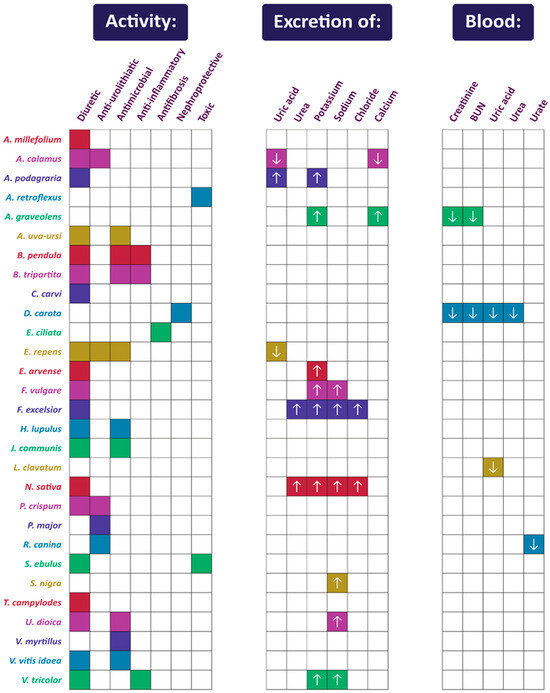
Figure 1.
Summary of activities of plants used in Polish folk medicine to treat urinary disorders (confirmed by scientific studies). The arrows indicate an increase (upward-facing arrow) or decrease (downward-facing arrow) in the excretion or blood concentration of compounds and markers. Compilation of data from Section 2.
3. Phytochemicals Important in the Treatment of Urinary Diseases
Apium graveolens L., Arctostaphylos uva-ursi (L.) Spreng, Betula spp., Elymus repens (L.) Gould, Equisetum arvense L., Juniperus communis L., Levisticum officinale W.D.J. Koch, Ononis spinosa L., Petroselinum crispum (Mill.) Fuss, Solidago virgaurea L., Taraxacum campylodes G.E.Haglund, Urtica spp., Vaccinium myrtillus L., Vaccinium vitis-idaea L., and Viola tricolor L. are all known to have diuretic and urinary tract disinfectant activity [18]. These herbs usually contain monosaccharides, flavonoids, volatile oils, saponins, terpenes, or tannins, which increase urine volume by promoting kidney blood flow and raising the glomerular filtration rate. Nevertheless, unlike synthetic diuretics, they do not reduce the resorption of Na+ and Cl− in the renal tubules [11]. Lien et al. [122] identified the most common chemical ingredients in plants used in the treatment of kidney disease and/or kidney protection, and their possible mechanisms of action in traditional Chinese medicine. Antioxidant polyphenols can prevent nephropathy by interacting with free radicals or reactive oxygen species. Many antioxidants contain a component part bound to oxidizable functional groups like ferulic acid and isoferulic acid, tannins, flavonoids, and isoflavonoids [123].
Some essential fatty acids can exhibit both anti-inflammatory and pro-inflammatory properties, modulating the immune response [122]. Arbutin is a phenolic glycoside that shows antimicrobial and anti-inflammatory activity. It can decrease the production of inflammatory cytokines, and reduce the expression of inducible NO synthase (iNOS). About 65% of arbutin undergoes hydrolyzation to hydroquinone, which takes place primarily in the intestines. The main mechanism of the antimicrobial activity of hydroquinone is tied to the destruction of the bacterial cell wall [124]. The antimicrobial effect of quinones is partially attributed to their ability to form irreversible complexes with proteins, specifically with nucleophilic amino acids. They are thought to affect adhesins exposed on the surface, enzymes bound to membranes, and cell wall polypeptides [125,126].
Flavonoids are produced by plants partly in response to microbial infection. The mechanism of action of both flavonoids and tannins is thought to be similar to quinones. They inactivate microbial adhesions, cell envelope transport proteins, and enzymes, and possibly inactivate the microorganisms directly. It has been observed that tannins elicit antimicrobial activity against filamentous fungi, yeasts, and bacteria [125,126].
Rutin is a flavonoid that can be found in several plant species used in the treatment of urinary diseases, including Fraxinus excelsior, Matricaria chamomilla, or Viola tricolor [42,60,125]. It can decrease the levels of oxalate and calcium in the kidneys and urine. Oxalate and calcium are the main components of urinary stones. This effect is thought to be due to the inhibition of oxalate synthesis and the increase in calcium sequestration by nitric oxide [127,128]. Moreover, Kappel et al. [129] have shown, that rutin increases the uptake of calcium into skeletal muscles, which is mediated through mitogen-activated kinase (MEK) and protein kinase A (PKA) signaling pathways.
There are many different mechanisms of action of diuretic or aquaretic drugs. Based on their site of action, diuretics can be classified into thiazide (inhibition of the Na+/Cl− transporter), loop (inhibition of the Na+/K+/2Cl−), potassium-sparing diuretics, and carbonic anhydrase inhibitors. Some aquaretics block the V2 vasopressin receptor reducing aquaporin 2 (AQP2) water channel and sodium/glucose transporter 2 (SGLT2) inhibitors. New mechanisms of action of diuretic compounds (for example, inhibition of adenosine A1 receptors or urea transporters) are also being discovered [130].
Luteolin (at a dose of 3 mg/kg) showed diuretic activity in normotensive and spontaneously hypertensive rats. Moreover, in normotensive rats, it did not change the calcium levels in urine, which is an important aspect of urinary stone formation prevention. Importantly, luteolin did not increase potassium excretion, unlike thiazide and loop diuretics. The authors suggest that its mechanism of action might be linked to muscarinic acetylcholine receptors [131]. Similar to luteolin, gallic acid also increased diuresis without increasing the potassium excretion in rats at a dose of 3 mg/kg. The effect was similar to that of hydrochlorothiazide (a clinical reference thiazide-type diuretic). However, here, the effect was not dependent on the activation of muscarinic acetylcholine receptors. Instead, the mechanism of action of gallic acid might be linked to endogenous prostanoid generation [132].
Triterpene saponins can stimulate microcirculation due to their surfactant properties. The diuretic action of these compounds is believed to be associated with local irritation of kidney epithelia. The diuresis caused by plants such as Ononis spp., Betula spp., and Solidago species is relatively mild, and the effect might originate from the accompanying flavonoids and essential oils. An alternative theory is that the potassium content of these plants is, in fact, the diuretic agent [133]. Unfortunately, the mechanisms of action of many plant-derived diuretic compounds remain unknown.
Table 3 summarizes the most commonly used plants in Polish folk medicine for the treatment of urinary diseases, and their main phytochemicals. Flavonoids were found in every plant species listed in the table. Phenolic acids, coumarins, tannins, and terpenes were also common.

Table 3.
The most commonly used plants in Polish folk medicine for the treatment of urinary diseases, and their main phytochemicals.
The chemical composition of many plants used in folk medicine to treat urinary diseases remains unclear, as does the composition of plants used in contemporary medicine. Experimental data on the pharmacological effects of these plants are insufficient. Some of the plants from the traditional folk pharmacopeia are still in use, but a large group has been discarded, and the body of scientific evidence on the effectiveness and safety of their use can be sparse. A precise chemical analysis of the composition of these plants, based on in vivo and in vitro studies, may allow for a rediscovery of valuable therapeutics for the treatment of urinary diseases. The following plants have a long history of use in folk medicine; however, the literature indicates they are rarely used in contemporary Polish phytomedicine and merit further analysis: Allium ursinum L., Angelica sylvestris L., Bryonia alba L., Elsholtzia ciliata (Thunb.) Hyl., Galium aparine L., Onopordum acanthium L., Quercus robur L., Raphanus raphanistrum subsp. sativus (L.) Domin, Sanguisorba officinalis L., Silene vulgaris (Moench) Garcke, Stellaria media (L.) Vill., Trifolium arvense L., and Trigonella caerulea (L.) Ser. These plants have been used in folk medicine, but according to literature sources, are not used in contemporary Polish phytomedicine [134].
The main chemical compounds of selected plants used in Polish folk medicine to treat urinary diseases and their potential molecular targets of action are presented in Figure 3.
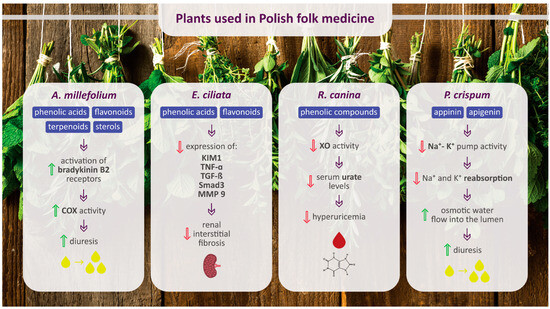
Figure 3.
Main chemical compounds of selected plants used in Polish folk medicine to treat urinary diseases and their potential molecular targets of action. Upward-facing green arrows indicate an increase, while downward-facing red arrows indicate a decrease. COX, cyclooxygenase; KIM1, kidney injury molecule 1; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor β; MMP 9, matrix metalloproteinase 9; XO, xanthine oxidase.
4. Conclusions
There are many plants used to treat urological diseases in Polish folk medicine. They are prescribed for various conditions, including UTIs, urolithiasis, ischuria, edema, or cystitis. Currently, both in vitro and in vivo studies have confirmed that many of these plant species have beneficial properties, such as diuretic, antihyperuricemic, antimicrobial, and anti-inflammatory activity, or prevention of urinary stone formation. These effects are exerted through different mechanisms, for example, through the activation of bradykinin B2 receptors, the inhibition of xanthine oxidase, or the inhibition of Na+-K+ pumps. Many plants used in folk medicine are rich in phytochemicals with proven effectiveness against urinary tract diseases, such as rutin, arbutin, or triterpene saponins. The present study constitutes a good basis for future comparison of Polish folk and contemporary medicine, with the aim of restoring the use of phytochemicals with proven activities. Many of the plants presented above (e.g., Petroselinum crispum (parsley), Ribes nigrum (blackcurrant), and Apium graveolens (celery root)) are common components of the everyday diet, which makes them easily accessible for people who are ill or at risk of urinary tract diseases. Furthermore, plant-based medicines which have been used for generations can serve as a basis for creating new, inexpensive, and safe drugs. Conducting well-controlled and high-quality human clinical experiments in this area is encouraged.
Author Contributions
Conceptualization, M.B., B.O. and W.R.; writing—original draft preparation, N.S. and K.U.; writing—review and editing, M.B. and B.O.; visualization, M.B. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ADH, antidiuretic hormone; AP, aerial parts; AQP2, V2 vasopressin receptor reducing aquaporin 2; Ba, bark; b.w., body weight; d, day; Fl, flower; FS, flowering shoots; Fr, fruit; h, hour; HCTZ, hydrochlorothiazide; In, inflorescence; iNOS, inducible NO synthase; i.p., intraperitoneal; JB, juniper berry; KIM1, kidney injury molecule 1; MEK, mitogen-activated kinase; MMP, matrix metalloproteinase; LS, leaf stalk; Lf, leaf; m, month; PKA, protein kinase A; Rz, rhizome; p.o., oral administration; Ro, root; Se, seed; SGLT2, sodium/glucose transporter 2; SHR, spontaneously hypertensive rats; Sp, spores; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; UPEC, uropathogenic Escherichia coli; UNa, urinary sodium; UTI, urinary tract infection; UV, urine volume; w, week; WP, whole plant; XO, xanthine oxidase.
References
- Lee, J.B.L.; Neild, G.H. Urinary Tract Infection. Medicine 2007, 35, 423–428. [Google Scholar] [CrossRef]
- Weissman, S.J.; Warren, J.W.; Mobley, H.I.T.; Donnenberg, M.S. Host–Pathogen Interactions and Host Defense Mechanisms. In Diseases of the Kidney and Urinary Tract; Schrier, R.W., Ed.; Wolters Kluwer Health, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 816–831. [Google Scholar]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney Stones: A Global Picture of Prevalence, Incidence, and Associated Risk Factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar] [PubMed]
- Gambaro, G.; Croppi, E.; Bushinsky, D.; Jaeger, P.; Cupisti, A.; Ticinesi, A.; Mazzaferro, S.; D’Addessi, A.; Ferraro, P.M. The Risk of Chronic Kidney Disease Associated with Urolithiasis and Its Urological Treatments: A Review. J. Urol. 2017, 198, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wilson, D.M.; O’Fallon, W.M.; Malek, R.S.; Kurland, L.T. Renal Stone Epidemiology: A 25-Year Study in Rochester, Minnesota. Kidney Int. 1979, 16, 624–631. [Google Scholar] [CrossRef]
- Abbagani, S.; Gundimeda, S.D.; Varre, S.; Ponnala, D.; Mundluru, H.P. Kidney Stone Disease: Etiology and Evaluation. Int. J. Appl. Biol. Pharm. 2010, 1, 175–182. [Google Scholar]
- Hesse, A.; Tiselius, H.-G.; Siener, R.; Hoppe, B. Urinary Stones: Diagnosis, Treatment, and Prevention of Recurrence; S. Karger AG: Basel, Switzerland, 2009; ISBN 978-3-8055-9149-2. [Google Scholar]
- Keshavarzi, B.; Yavar Ashayeri, N.; Moore, F.; Irani, D.; Asadi, S.; Zarasvandi, A.; Salari, M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals 2016, 6, 131. [Google Scholar] [CrossRef]
- Yarnell, E. Botanical Medicines for the Urinary Tract. World J. Urol. 2002, 20, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V.C. Chapter Four: Kidney, Urinary Tract, and Prostate Problems. In Tyler’s Herbs of Choice: The Therapeutic Use of Phytomedicinals; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 59–76. [Google Scholar]
- Tylkowa, D. Medycyna Ludowa w Kulturze Wsi Karpat Polskich; Ossolineum: Wrocław, Poland, 1989. [Google Scholar]
- Penkala-Gawęcka, D. Medycyna Komplementarna w Polsce i Jej Badanie. Lud 1991, 74, 43–54. [Google Scholar]
- Piątkowski, W.; Majchrowska, A. Health, Illness and Dying in Polish Folk Medicine. Health Sci. 2015, 5, 214–224. [Google Scholar]
- Ghelani, H.; Chapala, M.; Jadav, P. Diuretic and Antiurolithiatic Activities of an Ethanolic Extract of Acorus calamus L. Rhizome in Experimental Animal Models. J. Tradit. Complement. Med. 2016, 6, 431–436. [Google Scholar] [CrossRef]
- Dimkov, P. Bulgarian Folk Medicine. Naturopathy and Natural Life; Publishing House of Bulgarian Academy of Science: Sofia, Bulgaria, 1977. [Google Scholar]
- Dévora Gutiérrez, S.; Hernández-Luis, F.; Martín-Herrera, D.; Morales Marrero, C.C.; Abdala, S. Diuretic Activity of Sambucus nigra L. ssp. Palmensis (Link) R. Bolli, an Endemic Canary Islands Species. Bol. Latinoam. Caribe Plantas Med. Aromat. 2023, 22, 500–507. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Kowal-Gierczak, B.; Niedworok, J. Fitoterapia i Leki Roślinne; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2007. [Google Scholar]
- Johri, R.K. Cuminum cyminum and Carum carvi: An Update. Pharmacogn. Rev. 2011, 5, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ismail, J.; Shebaby, W.N.; Daher, J.; Boulos, J.C.; Taleb, R.; Daher, C.F.; Mroueh, M. The Wild Carrot (Daucus carota): A Phytochemical and Pharmacological Review. Plants 2023, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- El Bardai, S.; Lyoussi, B.; Wibo, M.; Morel, N. Pharmacological Evidence of Hypotensive Activity of Marrubium vulgare and Foeniculum vulgare in Spontaneously Hypertensive Rat. Clin. Exp. Hypertens. 2001, 23, 329–343. [Google Scholar] [CrossRef]
- Shokry, A.A.; El-Shiekh, R.A.; Kamel, G.; Bakr, A.F.; Ramadan, A. Bioactive Phenolics Fraction of Hedera helix L. (Common Ivy Leaf) Standardized Extract Ameliorates LPS-Induced Acute Lung Injury in the Mouse Model through the Inhibition of Proinflammatory Cytokines and Oxidative Stress. Heliyon 2022, 8, e09477. [Google Scholar] [CrossRef] [PubMed]
- Maseehullah, M.; Zakir, M.; Anas, M.; Kazmi, M.H. Ethno-Pharmacology of Asaroon (Asarum europaeum L.) with Special Reference to Unani System of Medicine. J. Complement. Integr. Med. 2022, 19, 181–192. [Google Scholar] [CrossRef]
- Mohammed, G.J.; Hameed, I.H. Pharmacological Activities: Hepatoprotective, Cardio Protective, Anti-Cancer and Anti-Microbial Activity of (Raphanus raphanistrum subsp. sativus): A Review. Indian J. Public Health Res. Dev. 2018, 9, 212. [Google Scholar] [CrossRef]
- Mayer, J.G. The History of Valerian and Hops. Z. Phytother. 2003, 24, 70–81. [Google Scholar]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Sezik, E.; Yesilada, E.; Shadidoyatov, H.; Kulivey, Z.; Nigmatullaev, A.M.; Aripov, H.N.; Takaishi, Y.; Takeda, Y.; Honda, G. Folk Medicine in Uzbekistan. J. Ethnopharmacol. 2004, 92, 197–207. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Kosman, V.M.; Faustova, N.M.; Tesakova, S.V.; Makarov, V.G.; Galambosi, B. Anti-Inflammatory Activity of a HPLC-Fingerprinted Aqueous Infusion of Aerial Part of Bidens tripartita L. Phytomedicine 2010, 17, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Mołdoch, J.; Paduch, R.; Strzemski, M.; Szkutnik, J.; Tyszczuk-Rotko, K.; Dresler, S.; Szczepanek, D.; Wójciak, M. Polyphenolic Composition of Carlina acaulis L. Extract and Cytotoxic Potential against Colorectal Adenocarcinoma and Cervical Cancer Cells. Molecules 2023, 28, 6148. [Google Scholar] [CrossRef]
- Satmbekova, D.; Srivedavyasasri, R.; Orazbekov, Y.; Omarova, R.; Datkhayev, U.; Ross, S.A. Chemical and biological studies on Cichorium intybus L. Nat. Prod. Res. 2018, 32, 1343–1347. [Google Scholar] [CrossRef]
- Saeedi, M.; Khanavi, M.; Shahsavari, K.; Manayi, A. Matricaria chamomilla: An Updated Review on Biological Activities of the Plant and Constituents. Res. J. Pharmacogn. 2024, 11, 109–136. [Google Scholar] [CrossRef]
- Robertovna, G.E.; Alexeevich, K.D.; Alexeevich, S.A.; Petrovna, G.M.; Kenzhebaevna, O.K. A Traditional Medicine Plant, Onopordum acanthium L. (Asteraceae): Chemical Composition and Pharmacological Research. Plants 2019, 8, 40. [Google Scholar] [CrossRef]
- Sharef, A.Y.; Hamdi, B.A.; Alrawi, R.A.; Ahmad, H.O. Onopordum acanthium L. Extract Attenuates Pancreatic β-Cells and Cardiac Inflammation in Streptozocin-Induced Diabetic Rats. PLoS ONE 2023, 18, e0280464. [Google Scholar] [CrossRef]
- Ilhan, M.; Dereli, F.T.G.; Tümen, I.; Akkol, E.K. Anti-Inflammatory and Antinociceptive Features of Bryonia alba L.: As a Possible Alternative in Treating Rheumatism. Open Chem. 2019, 17, 23–30. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Freire, R.C.; Honório, T.C.; Zoghaib, I.; Cardoso, F.F.; Tresvenzol, L.M.; de Paula, J.R.; Sousa, A.L.; Jardim, P.C.; Cunha, L.C. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evid.-Based Complement. Altern. Med. 2014, 2014, 760683. [Google Scholar] [CrossRef]
- Kozlowski, J. Arctostaphyllos Uva-Ursi Spreng. [Common Bearberry]—An Indispensable Drug Plant [Ecology; Conservation]. Wiadomości Zielar. 1984, 9, 15–16. (In Polish) [Google Scholar]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Afzaal, M.; Saeed, F.; Ahmad, A.; Din, A.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Rafi, A.; et al. Biological Activities, Therapeutic Potential, and Pharmacological Aspects of Blackcurrants (Ribes nigrum L.): A Comprehensive Review. Food Sci. Nutr. 2023, 11, 5799–5817. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Khaksarian, M.; Rafieian-Kopaei, M.; Abbasi, N. Overview of the Therapeutic Effects of Origanum Vulgare and Hypericum Perforatum Based on Iran’s Ethnopharmacological Documents. J. Clin. Diagn. Res. 2018, 12, FE1–FE4. [Google Scholar] [CrossRef]
- Pourmirzaee Sheikhali Kelayeh, T.; Abedinzade, M.; Ghorbani, A. A Review on Biological Effects of Lamium album (White Dead Nettle) and Its Components. J. Herbmed Pharmacol. 2019, 8, 185–193. [Google Scholar] [CrossRef]
- Bharatan, V. Homeopathy and Systematics: A Systematic Analysis of the Therapeutic Effects of the Plant Species Used in Homeopathy. Homeopathy 2008, 97, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Iossifova, T. Chemical Components of Fraxinus Species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Casadebaig, J.; Jacob, M.; Cassanas, G.; Gaudy, D.; Baylac, G.; Puech, A. Physicochemical and Pharmacological Properties of Spray-Dried Powders from Fraxinus excelsior Leaf Extracts. J. Ethnopharmacol. 1989, 26, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Maghrani, M.; Zeggwagh, N.-A.; Haloui, M.; Michel, J.-B. Fraxinus excelsior L. Evokes a Hypotensive Action in Normal and Spontaneously Hypertensive Rats. J. Ethnopharmacol. 2005, 99, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Mocan, A.; Vlase, L.; Gheldiu, A.-M.; Crișan, G.; Ielciu, I.; Voștinaru, O.; Crișan, O. Evaluation of Polyphenolic Content, Antioxidant and Diuretic Activities of Six Fumaria Species. Molecules 2017, 22, 639. [Google Scholar] [CrossRef]
- Abate, L.; Bachheti, R.K.; Tadesse, M.G.; Bachheti, A. Ethnobotanical Uses, Chemical Constituents, and Application of Plantago lanceolata L. J. Chem. 2022, 2022, 1532031. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Duwiejua, M.; Zeitlin, I.; Gray, A.; Waterman, P. The Anti-Inflammatory Compounds of Polygonum bistorta: Isolation and Characterisation. Planta Med. 1999, 65, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Dudek, M.K.; Ciach, M.; Włodarczyk, M. Isolation and Structural Determination of Flavan-3-Ol Derivatives from the Polypodium vulgare L. Rhizomes Water Extract. Nat. Prod. Res. 2021, 35, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, A.; Cherrah, Y.; Lacaille-Dubois, M.A.; Settaf, A.; Amarouch, H.; Hassar, M. Diuretic and Hypotensive Effects of Nigella sativa in the Spontaneously Hypertensive Rat. Therapie 2000, 55, 379–382. [Google Scholar] [PubMed]
- Ali, B.H.; Blunden, G. Pharmacological and Toxicological Properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, A.; Peeters, L.; Foubert, K.; Piazza, S.; Vanden Berghe, W.; Hermans, N.; Pieters, L. In Vitro Biotransformation and Anti-Inflammatory Activity of Constituents and Metabolites of Filipendula ulmaria. Pharmaceutics 2023, 15, 1291. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS Based Metabolomics and Antioxidant Activity for Evaluation of Bioactive Compounds in Fragaria vesca Leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, X.; Huang, X.; Liu, F.; Zhang, Z.; Cao, L. The Identification of SQS/SQE/OSC Gene Families in Regulating the Biosynthesis of Triterpenes in Potentilla Anserina. Molecules 2023, 28, 2782. [Google Scholar] [CrossRef] [PubMed]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Valorization of Sweet Cherry (Prunus Avium) Wastes as a Source of Advanced Bioactive Compounds. In Mediterranean Fruits Bio-Wastes; Springer International Publishing: Cham, Switzerland, 2022; pp. 559–579. [Google Scholar]
- Sulimanec, A.; Kragić;, K.; Sekovanić;, A.; Jurasović;, J.; Panjkota Krbavčić;, I.; Vahčić;, N.; Vidaković, A.; Poljak, I.; Rumora Samarin, I. Chemical Characterization and Antioxidant Potential of the Rowan (Sorbus aucuparia L.) Fruits from Alpine-Dinaric Region of Croatia. Food Technol. Biotechnol. 2023, 61, 465–474. [Google Scholar] [CrossRef]
- Mocan, A.; Crişan, G.; Vlase, L.; Ivanescu, B.; Bădărău, A.S.; Arsene, A.L. Phytochemical Investigations on Four Galium Species (Rubiaceae) from Romania. Farmacia 2016, 64, 95–99. [Google Scholar]
- Jayakody, J.R.A.C.; Ratnasoori, W.D.; Fernando, W.A.N.A.; Weeraseker, K.R. Diuretic Activity of Leaves Extract of Hot Water Infusion of Ruta graveolens L. in Rats. J. Pharmacol. Toxicol. 2011, 6, 525–532. [Google Scholar] [CrossRef]
- Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute Diuretic, Natriuretic and Hypotensive Effects of a Continuous Perfusion of Aqueous Extract of Urtica dioica in the Rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rimkiene, S.; Ragazinskiene, O.; Savickiene, N. The Cumulation of Wild Pansy (Viola tricolor L.) Accessions: The Possibility of Species Preservation and Usage in Medicine. Medicina 2003, 39, 411–416. [Google Scholar] [PubMed]
- Toiu, A.; Muntean, E.; Oniga, I.; Voştinaru, O.; Tămaş, M. Pharmacognostic Research on Viola tricolor L. (Violaceae). Rev. Med. Chir. Soc. Med. Nat. Iasi 2009, 113, 264–267. [Google Scholar] [PubMed]
- De Souza, P.; Crestani, S.; da Silva, R.D.; Gasparotto, F.; Kassuya, C.A.; da Silva-Santos, J.E.; Gasparotto, A., Jr. Involvement of Bradykinin and Prostaglandins in the Diuretic Effects of Achillea millefolium L. (Asteraceae). J. Ethnopharmacol. 2013, 149, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Koyro, O.O.; Tovchiga, O.V.; Stepanova, S.I.; Shtrygol, Y. Study of the Composition of the Goutweed Flowers Essential Oil, Its Renal Effects and Influence on Uric Acid Exchange. Pharmacogn. Commun. 2012, 2, 46–49. [Google Scholar] [CrossRef]
- Tovchiga, O.V. Renal Effects of Goutweed (Aegopodium podagraria L.) Preparations in Rats with the Metabolic Disorders Induced by Fructose and Hydrochlorothiazide. Ukr. Biopharm. J. 2014, 4, 60–66. [Google Scholar]
- Al Jawad, F.H.; Al Razzuqi, R.A.M.; Al Jeboori, A.A. Apium Graveolens Accentuates Urinary Ca+2 Excretions in Experimental Model of Nephrocalcinosis. Int. J. Green Pharm. 2011, 5, 100–102. [Google Scholar] [CrossRef]
- Rafsanjany, N.; Lechtenberg, M.; Petereit, F.; Hensel, A. Antiadhesion as a Functional Concept for Protection against Uropathogenic Escherichia coli: In Vitro Studies with Traditionally Used Plants with Antiadhesive Activity against Uropathognic Escherichia coli. J. Ethnopharmacol. 2013, 145, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gaybullaev, A.; Kariev, S. Phytotherapy of Calcium urolithiasis with Extracts of Medicinal Plants: Changes of Diuresis, Urine PH and Crystalluria. ATI-Appl. Technol. Innov. 2012, 7, 59–66. [Google Scholar] [CrossRef]
- Lahlou, S.; Tahraoui, A.; Israili, Z.; Lyoussi, B. Diuretic Activity of the Aqueous Extracts of Carum carvi and Tanacetum vulgare in Normal Rats. J. Ethnopharmacol. 2007, 110, 458–463. [Google Scholar] [CrossRef]
- Sodimbaku, V.; Pujari, L.; Mullangi, R.; Marri, S. Carrot (Daucus carota L.): Nephroprotective against Gentamicin-Induced Nephrotoxicity in Rats. Indian J. Pharmacol. 2016, 48, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Kim, Y.-J.; Seo, C.-S.; Kim, H.-T.; Park, S.-R.; Lee, M.-Y.; Jung, J.-Y. Elsholtzia ciliata (Thunb.) Hylander Attenuates Renal Inflammation and Interstitial Fibrosis via Regulation of TGF-ß and Smad3 Expression on Unilateral Ureteral Obstruction Rat Model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef]
- Brardi, S.; Imperiali, P.; Cevenini, G.; Verdacchi, T.; Ponchietti, R. Effects of the Association of Potassium citrate and Agropyrum repens in Renal Stone Treatment: Results of a Prospective Randomized Comparison with Potassium citrate. Arch. Ital. Urol. Androl. 2012, 84, 61–67. [Google Scholar]
- Al-Snafi, A.E. Chemical Constituents and Pharmacological Importance of Agropyron repens—A Review. Res. J. Pharmacol. Toxicol. 2015, 1, 37–41. [Google Scholar]
- Stanić, G.; Samaržija, I.; Blažević, N. Time-Dependent Diuretic Response in Rats Treated with Juniper Berry Preparations. Phytother. Res. 1998, 12, 494–497. [Google Scholar] [CrossRef]
- Ribeiro, R.D.; de Barros, F.; de Melo, M.M.; Muniz, C.; Chieia, S.; das Graças Wanderley, M.; Gomes, C.; Trolin, G. Acute Diuretic Effects in Conscious Rats Produced by Some Medicinal Plants Used in the State of São Paulo, Brasil. J. Ethnopharmacol. 1988, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Usta, J. Diuretic Effect and Mechanism of Action of Parsley. J. Ethnopharmacol. 2002, 79, 353–357. [Google Scholar] [CrossRef]
- Alyami, F.A.; Rabah, D.M. Effect of Drinking Parsley Leaf Tea on Urinary Composition and Urinary Stones’ Risk Factors. Saudi J. Kidney Dis. Transpl. 2011, 22, 511–514. [Google Scholar]
- Saeidi, J.; Bozorgi, H.; Zendehdel, A.; Mehrzad, J. Therapeutic Effects of Aqueous Extracts of Petroselinum sativum on Ethylene Glycol-Induced Kidney Calculi in Rats. Urol. J. 2012, 9, 361–366. [Google Scholar]
- Abdul Aziz, S.; Lee See, T.; Yew Khuay, L.; Osman, K.; Azman Abu Bakar, M.; Kebangsaan Malaysia, U.; Raja Muda Abdul Aziz, J.; Lumpur Malaysia, K. In Vitro Effects of Plantago Major Extract on Urolithiasis. Malays. J. Med. Sci. 2005, 12, 22–26. [Google Scholar]
- Tayefi-Nasrabadi, H.; Sadigh-Eteghad, S.; Aghdam, Z. The Effects of the Hydroalcohol Extract of Rosa canina L. Fruit on Experimentally Nephrolithiasic Wistar Rats. Phytother. Res. 2012, 26, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Beaux, D.; Fleurentin, J.; Mortier, F. Effect of Extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) spreng. in Rats. Phytother. Res. 1999, 13, 222–225. [Google Scholar] [CrossRef]
- Walz, B.; Chrubasik, S. Impact of a Proprietary Concentrate of Sambucus nigra L. on Urinary PH. Phytother. Res. 2008, 22, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Rácz–Kotilla, E.; Racz, G.; Solomon, A. The Action of Taraxacum Officinale Extracts on the Body Weight and Diuresis of Laboratory Animals. Planta Med. 1974, 26, 212–217. [Google Scholar] [CrossRef]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. Revisited: Recent Findings Confirm the Traditional Use. Wien. Med. Wochenschr. 2007, 157, 312–314. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A Review on Phytochemistry and Medicinal Properties of the Genus Achillea. Daru 2011, 19, 173–186. [Google Scholar]
- Kuźniewski, E.; Augustyn-Puziewicz, J. Przewodnik Ziołolecznictwa Ludowego; PWN Wydawnictwo Naukowe: Warszawa, Poland, 1984. [Google Scholar]
- Rutkowski, L. Klucz Do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Osweiler, G.D.; Buck, W.B.; Bicknell, E.J. Production of Perirenal Edema in Swine with Amaranthus retroflexus. Am. J. Vet. Res. 1969, 30, 557–566. [Google Scholar]
- Kessell, A.; Boulton, J.; Krebs, G.; Quinn, J. Acute Renal Failure Associated with Amaranthus Species Ingestion by Lambs. Aust. Vet. J. 2015, 93, 208–213. [Google Scholar] [CrossRef]
- Fazal, S.S.; K Singla, R. Review on the Pharmacognostical & Pharmacological Characterization of Apium graveolens Linn. Indo Glob. J. Pharm. Sci. 2012, 2, 36–42. [Google Scholar] [CrossRef]
- Kooti, W.; Mansouri, E.; Ghasemiboroon, M.; Harizi, M.; Ashtary-Larky, D.; Afrisham, R. The Effects of Hydroalcoholic Extract of Apium graveolens Leaf on the Number of Sexual Cells and Testicular Structure in Rat. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e17532. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Natural Therapeutics for Urinary Tract Infections—A Review. Futur. J. Pharm. Sci. 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Gruber, C.W.; Hertrampf, A.; Zehl, M.; Kopp, B.; Huber, R. An Aqueous Birch Leaf Extract of Betula Pendula Inhibits the Growth and Cell Division of Inflammatory Lymphocytes. J. Ethnopharmacol. 2011, 136, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Raudonis, R.; Janulis, V.; Viškelis, P. Quality Evaluation of Different Preparations of Dry Extracts of Birch (Betula pendula Roth) Leaves. Nat. Prod. Res. 2014, 28, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In Vivo Anti-Inflammatory and Anti-Allergic Activities of Cynaroside Evaluated by Using Hydrogel Formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef] [PubMed]
- Hojden, B. Marzymięta Grzebieniasta—Zapomniany Użyteczny Chwast. Wiadomości Zielar. 1995, 37, 7–8. [Google Scholar]
- Wang, F.; Liu, X.; Chen, Y.; An, Y.; Zhao, W.; Wang, L.; Tian, J.; Kong, D.; Xu, Y.; Ba, Y.; et al. Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology. Molecules 2022, 27, 6411. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jagtap, S.D.; Thapa, D.; Khyade, M.S.; Russell, W.R. Herbal Remedies for Urinary Stones Used in India and China: A Review. J. Ethnopharmacol. 2017, 203, 55–68. [Google Scholar] [CrossRef]
- Mamedova, K.T.; Gysejnova, I.D. Effect of Equisetum arvense L. on Diuresis. Dokl. Akad. Nauk. Azerbaidzhana 1996, 51, 175–179. [Google Scholar]
- Asgarpanah, J.; Roohi, E. Jinous Asgarpanah Phytochemistry and Pharmacological Properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6, 3689–3693. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Equisetum arvense: Pharmacology and Phytochemistry—A Review. AJPCR 2010, 3, 146–150. [Google Scholar]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum Vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Biswas, S.; Ranjan Madhu, N.; Roy Karmakar, S.; Jyoti Biswas, S.; Jyoti Biswas, S. A Better Understanding of Pharmacological Activities and Uses of Phytochemicals of Lycopodium clavatum: A Review. J. Pharmacogn. Phytochem. 2014, 3, 207–210. [Google Scholar]
- Kong, L.D.; Cai, Y.; Huang, W.W.; Cheng, C.H.K.; Tan, R.X. Inhibition of Xanthine Oxidase by Some Chinese Medicinal Plants Used to Treat Gout. J. Ethnopharmacol. 2000, 73, 199–207. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Sõukand, R.; Quave, C.L.; Pieroni, A.; Pardo-de-Santayana, M.; Tardío, J.; Kalle, R.; Łuczaj, Ł.; Svanberg, I.; Kolosova, V.; Aceituno-Mata, L.; et al. Plants Used for Making Recreational Tea in Europe: A Review Based on Specific Research Sites. J. Ethnobiol. Ethnomed. 2013, 9, 58. [Google Scholar] [CrossRef]
- Edwards, N.L. The Role of Hyperuricemia in Vascular Disorders. Curr. Opin. Rheumatol. 2009, 21, 132–137. [Google Scholar] [CrossRef]
- Jiménez, P.; Tejero, J.; Cordoba-Diaz, D.; Quinto, E.J.; Garrosa, M.; Gayoso, M.J.; Girbés, T. Ebulin from Dwarf Elder (Sambucus ebulus L.): A Mini-Review. Toxins 2015, 7, 648–658. [Google Scholar] [CrossRef]
- Lis, B.; Grabek-Lejko, D. Dandelion (Taraxacum officinale)—Potential Health Benefits. Nauka Przyr. Technol. 2016, 10, 37. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A Review on Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse Biological Activities of Dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- González-Castejón, M.; García-Carrasco, B.; Fernández-Dacosta, R.; Dávalos, A.; Rodriguez-Casado, A. Reduction of Adipogenesis and Lipid Accumulation by Taraxacum officinale (Dandelion) Extracts in 3T3L1 Adipocytes: An In Vitro Study. Phytother. Res. 2014, 28, 745–752. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical Review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar]
- Upton, R. Stinging Nettles Leaf (Urtica dioica L.): Extraordinary Vegetable Medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic Distribution in Liquid Preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Zając, M. Atlas Rozmieszczenia Roślin Naczyniowych w Polsce; Pracownia Choronologii Komputerowej Instytutu Botaniki Uniwersytetu Jagiellońskiego: Kraków, Poland, 2001. [Google Scholar]
- Shen, P.; Deng, X.; Li, T.; Chen, X.; Wu, X. Demethylzeylasteral Protects against Renal Interstitial Fibrosis by Attenuating Mitochondrial Complex I-Mediated Oxidative Stress. J. Ethnopharmacol. 2024, 327, 117986. [Google Scholar] [CrossRef]
- Mohammad, A.; Laboulaye, M.A.; Shenhar, C.; Dobberfuhl, A.D. Mechanisms of Oxidative Stress in Interstitial Cystitis/Bladder Pain Syndrome. Nat. Rev. Urol. 2024. [Google Scholar] [CrossRef]
- Hong, S.Y.; Qin, B.L. The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients 2023, 15, 3753. [Google Scholar] [CrossRef]
- Lien, E.J.; Lien, L.L.; Wang, R.; Wang, J. Phytochemical Analysis of Medicinal Plants with Kidney Protective Activities. Chin. J. Integr. Med. 2012, 18, 790–800. [Google Scholar] [CrossRef]
- Mehta, A. Pharmacology of Medicinal Plants with Antioxidant Activity. In Plants as a Source of Natural Antioxidants; Dubey, N.K., Ed.; CAB International: Oxfordshire, UK, 2015; pp. 225–244. [Google Scholar]
- Cela-López, J.M.; Camacho Roldán, C.J.; Gómez-Lizarraga, G.; Martínez, V. Effects of Itxasol© Components on Gene Expression in Bacteria Related to Infections of the Urinary Tract and to the Inflammation Process. Int. J. Mol. Sci. 2021, 22, 12655. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- Talapatra, S.K.; Talapatra, B. Chemistry of Plant Natural Products; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-642-45409-7. [Google Scholar]
- Zeng, X.; Xi, Y.; Jiang, W. Protective Roles of Flavonoids and Flavonoid-Rich Plant Extracts against Urolithiasis: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2125–2135. [Google Scholar] [CrossRef]
- Pawar, A.; Deshmukh, C.; Bhanudas, B.; Ghodasara, J. Inhibitory Effect of Rutin and Curcumin on Experimentally-Induced Calcium Oxalate Urolithiasis in Rats. Pharmacogn. Res. 2010, 2, 388. [Google Scholar] [CrossRef] [PubMed]
- Kappel, V.D.; Zanatta, L.; Postal, B.G.; Silva, F.R.M.B. Rutin Potentiates Calcium Uptake via Voltage-Dependent Calcium Channel Associated with Stimulation of Glucose Uptake in Skeletal Muscle. Arch. Biochem. Biophys. 2013, 532, 55–60. [Google Scholar] [CrossRef]
- Titko, T.; Perekhoda, L.; Drapak, I.; Tsapko, Y. Modern Trends in Diuretics Development. Eur. J. Med. Chem. 2020, 208, 112855. [Google Scholar] [CrossRef]
- Boeing, T.; da Silva, L.M.; Mariott, M.; de Andrade, S.F.; de Souza, P. Diuretic and Natriuretic Effect of Luteolin in Normotensive and Hypertensive Rats: Role of Muscarinic Acetylcholine Receptors. Pharmacol. Rep. 2017, 69, 1121–1124. [Google Scholar] [CrossRef]
- Schlickmann, F.; Boeing, T.; Mariano, L.N.; da Silva, R.D.; da Silva, L.M.; de Andrade, S.F.; de Souza, P.; Cechinel-Filho, V. Gallic Acid, a Phenolic Compound Isolated from Mimosa bimucronata (DC.) Kuntze Leaves, Induces Diuresis and Saluresis in Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 649–655. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995; ISBN 9780521329705. [Google Scholar]
- Ożarowski, A. Ziołolecznictwo. Poradnik Dla Lekarzy; Państwowy Zakład Wydawnictw Lekarskich: Warszawa, Poland, 1982. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).