Terpenes from Cecropia Species and Their Pharmacological Potential
Abstract
1. Introduction
2. Ethnopharmacological Aspects of the Genus Cecropia
3. Terpenes Identified from the Genus Cecropia
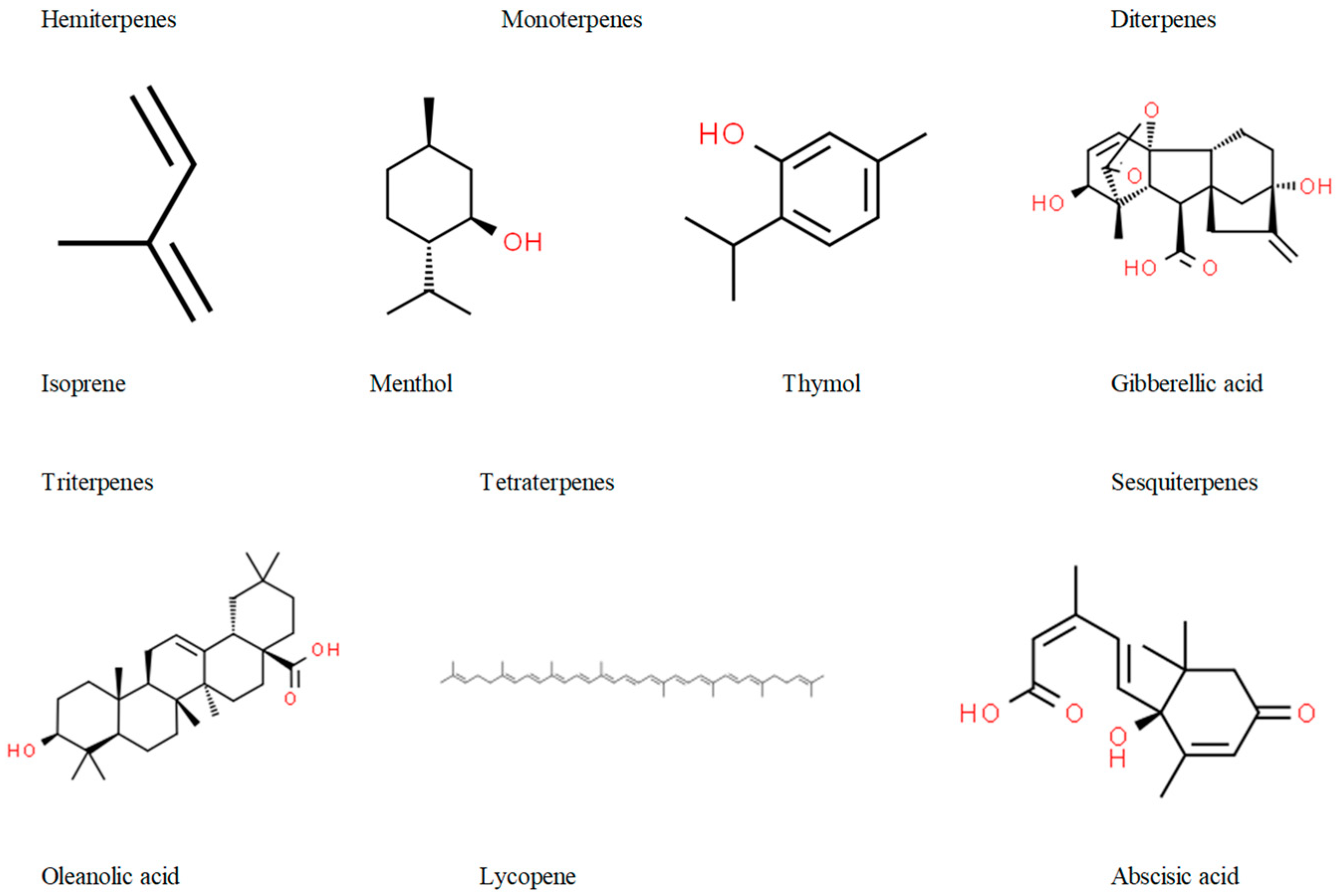
3.1. Importance of Terpenes
3.2. Methods for Terpenes Isolation from Cecropia Species
3.3. Cecropia Terpenes’ Pharmacology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berg, C.C.; Rosselli, P.F.; Davidson, D.W. Cecropia. In Flora Neotropica; New York Botanical Garden Press: New York, NY, USA, 2005; Volume 94, pp. 1–230. [Google Scholar]
- Cecropia Loefl.|Plants of the World Online|Kew Science. Plants of the World Online. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:331474-2 (accessed on 20 November 2023).
- Keys_Search. Key to the Flowering Plant Families of the Neotropics. Key Search. Available online: https://keys.lucidcentral.org/search/key-to-the-flowering-plant-families-of-the-neotropics/ (accessed on 20 December 2023).
- Francis, A.; Tan, K.; Chong, K.Y.; Nghiem, T.; Tan, H. The Distribution and Ecology of Cecropia Species (Urticaceae) In Singapore. Nat. Singap. 2010, 3, 199–209. [Google Scholar]
- Luengas-Caicedo, P.E.; Braga, F.C.; Brandão, G.C.; Oliveira, A.B.D. Seasonal and Intraspecific Variation of Flavonoids and Proanthocyanidins in Cecropia glaziovi Sneth. Leaves from Native and Cultivated Specimens. Z. Für Naturforschung C 2007, 62, 701–709. [Google Scholar] [CrossRef]
- Costa, G.M.; Schenkel, E.P.; Reginatto, F.H. Chemical and Pharmacological Aspects of the Genus Cecropia. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Chávez, J.O.; Velanda, J.R.; Pabón, M.G. Perfil neurofarmacológico de la fracción butanólica de las hojas de Cecropia peltata L. Rev. Colomb. Cienc. Químico-Farm. 2013, 42, 244–259. [Google Scholar]
- Montoya Peláez, G.L.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic Triterpenes from Cecropia telenitida with Immunomodulatory Activity on Dendritic Cells. Rev. Bras. Farmacogn. 2013, 23, 754–761. [Google Scholar] [CrossRef]
- Miller, J.S. Medicinal Plants of Brazil By Walter B. Mors, Carlos Toledo Rizzini, and Nuno Alvares Pereira (Federal University of Rio de Janeiro [Mors, Pereira], Rio de Janeiro Botanical Garden [Rizzini]). Reference Publications, Inc., Algonac, MI. 2000. 15 × 22.5 Cm. $60.00. ISBN 0-917256-42-5. J. Nat. Prod. 2001, 64, 1258–1259. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, J.; Chomicki, G.; Renner, S.S. Recurrent Breakdowns of Mutualisms with Ants in the Neotropical Ant-Plant Genus Cecropia (Urticaceae). Mol. Phylogenetics Evol. 2017, 111, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Treiber, E.L.; Gaglioti, A.L.; Romaniuc-Neto, S.; Madriñán, S.; Weiblen, G.D. Phylogeny of the Cecropieae (Urticaceae) and the Evolution of an Ant-Plant Mutualism. Syst. Bot. 2016, 41, 56–66. [Google Scholar] [CrossRef]
- Fáveri, S.B.; Vasconcelos, H.L. The Azteca-Cecropia Association: Are Ants Always Necessary for Their Host Plants? Biotropica 2004, 36, 641–646. [Google Scholar] [CrossRef]
- Davidson, D.W. Cecropia and Its Biotic Defenses; New York Botanical Garden Press: New York, NY, USA, 2005; Available online: https://collections.lib.utah.edu/details?id=702798 (accessed on 20 December 2023).
- Gianoli, E.; Sendoya, S.; Vargas, F.; Mejía, P.; Jaffé, R.; Rodríguez, M.; Gutiérrez, A. Patterns of Azteca Ants’ Defence of Cecropia Trees in a Tropical Rainforest: Support for Optimal Defence Theory. Ecol. Res. 2008, 23, 905–908. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Farmacopeia Brasileira 1ª Edição/1926—Revogada. Fitoterapia Brasil. Available online: https://fitoterapiabrasil.com.br/biblioteca-virtual/farmacopeia-brasileira-1a-edicao1926-revogada (accessed on 20 December 2023).
- Daga, M.A.; Ayala, T.S.; Menolli, R.A. A review of the anti-inflammatory and antimicrobial activities of the components of the cecropia genus. Asian J. Pharm. Clin. Res. 2020, 13, 13–20. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Ortíz, O.O.; Gupta, M.P.; Caballero-George, C. Pharmacognostic Evaluation of Ten Species of Medicinal Importance of Cecropia: Current Knowledge and Therapeutic Perspectives. Planta Med. 2021, 87, 764–779. [Google Scholar] [CrossRef]
- Pérez-Guerrero, C.; Herrera, M.D.; Ortiz, R.; Alvarez De Sotomayor, M.; Fernández, M.A. A Pharmacological Study of Cecropia obtusifolia Bertol Aqueous Extract. J. Ethnopharmacol. 2001, 76, 279–284. [Google Scholar] [CrossRef]
- Caballero-George, C.; Vanderheyden, P.M.L.; Solis, P.N.; Pieters, L.; Shahat, A.A.; Gupta, M.P.; Vauquelin, G.; Vlietinck, A.J. Biological Screening of Selected Medicinal Panamanian Plants by Radioligand-Binding Techniques. Phytomedicine 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Farmacopea Herbolaria de los Estados Unidos Mexicanos; Secretaría de Salud, Cecropia obtusifolia Bertol. 1840 1.ed.; Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos: Mexico City, Mexico, 2001.
- de Agra, M.F.; de Freitas, P.F.; Barbosa-Filho, J.M. Synopsis of the Plants Known as Medicinal and Poisonous in Northeast of Brazil. Rev. Bras. Farmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef]
- Arend, D.P.; Santos, T.C.D.; Cazarolli, L.H.; Hort, M.A.; Sonaglio, D.; Dos Santos, A.L.G.; Ribeiro-do-Valle, R.M.; Silva, F.R.M.B.; De Campos, A.M. In Vivo Potential Hypoglycemic and in Vitro Vasorelaxant Effects of Cecropia glaziovii Standardized Extracts. Rev. Bras. Farmacogn. 2015, 25, 473–484. [Google Scholar] [CrossRef]
- Sequeda, L.G. Phytochemical and Therapeutic Use of Cecropia mutisiana Mildbr. (Urticaceae) an Endemic Plant from Colombia. Pharmacologyonline 2015, 3, 62–64. [Google Scholar]
- Alonso-Castro, A.J.; Miranda-Torres, A.C.; González-Chávez, M.M.; Salazar-Olivo, L.A. Cecropia Obtusifolia Bertol and Its Active Compound, Chlorogenic Acid, Stimulate 2-NBDglucose Uptake in Both Insulin-Sensitive and Insulin-Resistant 3T3 Adipocytes. J. Ethnopharmacol. 2008, 120, 458–464. [Google Scholar] [CrossRef]
- Revilla-Monsalve, M.C.; Andrade-Cetto, A.; Palomino-Garibay, M.A.; Wiedenfeld, H.; Islas-Andrade, S. Hypoglycemic Effect of Cecropia obtusifolia Bertol Aqueous Extracts on Type 2 Diabetic Patients. J. Ethnopharmacol. 2007, 111, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Aarland, R.C.; Peralta-Gómez, S.; Sanchéz, C.M.; Parra-Bustamante, F.; Villa-Hernández, J.M.; León-Sánchez, F.D.d.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. A Pharmacological and Phytochemical Study of Medicinal Plants Used in Mexican Folk Medicine. Indian J. Tradit. Knowl. 2015, 14, 550–557. [Google Scholar]
- Lorenzi, H.; de Matos, F.J.A. Plantas Medicinais No Brasil: Nativas E Exóticas; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brasil, 2002. [Google Scholar]
- Aragão, D.M.O.; Guarize, L.; Lanini, J.; Da Costa, J.C.; Garcia, R.M.G.; Scio, E. Hypoglycemic Effects of Cecropia Pachystachya in Normal and Alloxan-Induced Diabetic Rats. J. Ethnopharmacol. 2010, 128, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Sticher, O. Triterpene einschließlich Steroide. In Pharmakognosie—Phytopharmazie; Hänsel, R., Sticher, O., Eds.; Springer-Lehrbuch; Springer: Berlin/Heidelberg, Germany, 2010; pp. 833–938. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The Role of Triterpenes in the Management of Diabetes Mellitus and Its Complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Biochemistry; Perveen, S., Mohammad Al-Taweel, A., Eds.; IntechOpen: Madison, WI, USA, 2021; Volume 21. [Google Scholar] [CrossRef]
- De Santana Souza, M.T.; Almeida, J.R.G.D.S.; De Souza Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; Dos Santos, M.R.V.; Quintans-Júnior, L.J. Structure–Activity Relationship of Terpenes with Anti-Inflammatory Profile—A Systematic Review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef]
- Myers, C.E.; Trepel, J.; Sausville, E.; Samid, D.; Miller, A.; Curt, G. Monoterpenes, Sesquiterpenes and Diterpenes as Cancer Therapy. Patent US5602184A, 11 February 1997. Available online: https://patents.google.com/patent/US5602184A/en (accessed on 20 December 2023).
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jin, L.; Deng, H.; Wu, D.; Shen, Q.; Quan, Z.; Zhang, C.; Guo, H.-Y. Research and Development of Natural Product Tanshinone I: Pharmacology, Total Synthesis, and Structure Modifications. Front. Pharmacol. 2022, 13, 920411. [Google Scholar] [CrossRef] [PubMed]
- González-Cardenete, M.A.; González-Zapata, N.; Boyd, L.; Rivas, F. Discovery of Novel Bioactive Tanshinones and Carnosol Analogues against Breast Cancer. Cancers 2023, 15, 1318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rajendram, R.; Zhang, L. Effects of Oleanolic Acid and Maslinic Acid on Glucose and Lipid Metabolism. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1423–1429. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Xiang, L.; Chi, T.; Tang, Q.; Yang, X.; Ou, M.; Chen, X.; Yu, X.; Chen, J.; Ho, R.J.Y.; Shao, J.; et al. A Pentacyclic Triterpene Natural Product, Ursolic Acid and Its Prodrug US597 Inhibit Targets within Cell Adhesion Pathway and Prevent Cancer Metastasis. Oncotarget 2015, 6, 9295–9312. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Yadav, D.K. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 2021, 22, 12162. [Google Scholar] [CrossRef]
- Schmidt, M.E.P.; Pires, F.B.; Bressan, L.P.; Silva, F.V.D.; Lameira, O.; Rosa, M.B.D. Some Triterpenic Compounds in Extracts of Cecropia and Bauhinia Species for Different Sampling Years. Rev. Bras. Farmacogn. 2018, 28, 21–26. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Bijttebier, S.; Tuenter, E.; Custers, D.; Ortíz, O.O.; Pieters, L.; Caballero-George, C.; Apers, S.; Foubert, K. Phytochemical Characterization and Comparative Studies of Four Cecropia Species Collected in Panama Using Multivariate Data Analysis. Sci. Rep. 2019, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.D.G.; Simões, M.; Lúcio, K.A.; Oliveira, R.R.; Coelho Kaplan, M.A.; Gattass, C.R. Natural Triterpenoids from Cecropia Lyratiloba Are Cytotoxic to Both Sensitive and Multidrug Resistant Leukemia Cell Lines. Bioorganic Med. Chem. 2007, 15, 7355–7360. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Leitão, G.G.; Moraes, M.C.C.; Kaplan, M.A.C.; Lopes, D.; Carauta, J.P.P. Gradient Elution for Triterpene Separation from Cecropia Lyratiloba Miquel by HSCCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1985–1992. [Google Scholar] [CrossRef]
- Mosquera, C.; Panay, A.J.; Montoya, G. Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1. Molecules 2018, 23, 1444. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.; Valencia, L.M.; Giraldo-Dávila, D.; Combariza, M.Y.; Galeano, E.; Balcazar, N.; Panay, A.J.; Jerez, A.M.; Montoya, G. Pentacyclic Triterpene Profile and Its Biosynthetic Pathway in Cecropia Telenitida as a Prospective Dietary Supplement. Molecules 2021, 26, 1064. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.; Aquila, S.; Dade, M.; Giner, R.; Recio, M.d.C.; Spegazzini, E.; Buschiazzo, P.d.; Tournier, H.; Ríos, J.L. Anti-Inflammatory and Apoptotic Activities of Pomolic Acid Isolated from Cecropia pachystachya. Planta Med. 2008, 74, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Machado∥, E.C.; Yunes, R.A.; Malheiros, A.; Gomez, E.C.; Delle Monache, F. Two New 11 α, 12α-Epoxy-Ursan-28,13 β-Olides and Other Triterpenes from Cecropia catharinensis. Nat. Prod. Res. 2008, 22, 1310–1316. [Google Scholar] [CrossRef]
- Rosa, H.H.; Carvalho, P.; Ortmann, C.F.; Schneider, N.F.Z.; Reginatto, F.H.; Simões, C.M.O.; Silva, I.T. Cytotoxic Effects of a Triterpene-enriched Fraction of Cecropia Pachystachya on the Human Hormone-Refractory Prostate Cancer PC3 Cell Line. Biomed. Pharmacother. 2020, 130, 110551. [Google Scholar] [CrossRef]
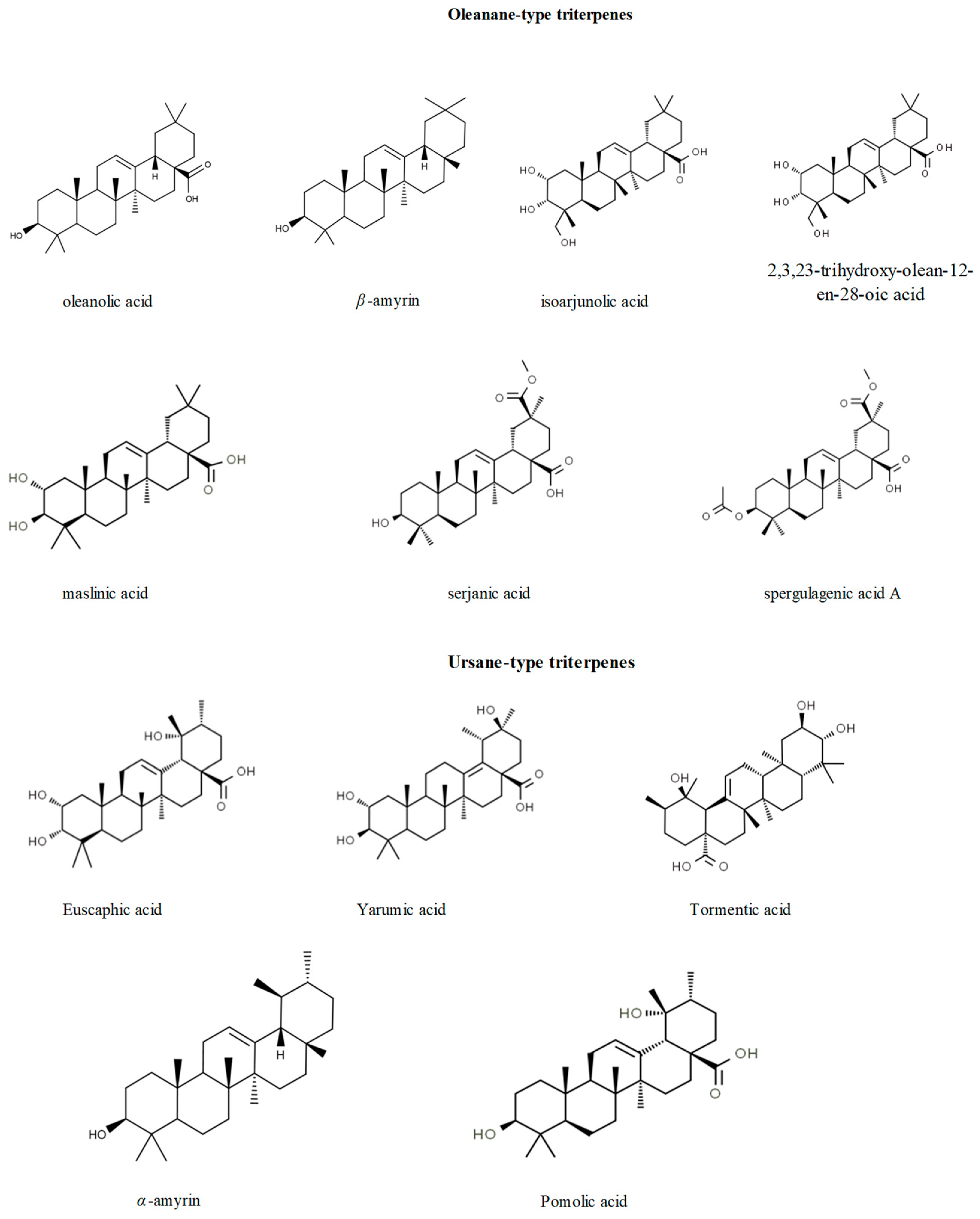
| Compounds | Cecropia Species | Ref. |
|---|---|---|
| Tentatively identified terpenes | ||
| serjanic acid, spergulagenic acid A, goreishic acid I, 20-hydroxy-ursolic acid, yarumic acid | C. telenitida | [8] |
| oleanolic acid, maslinic acid, α-amirin, β-amirin, lupeol | C. obtusa, C.palmata | [51] |
| erythrodiol | C. palmata | [51] |
| euscaphic acid 28-O-glucoside (kaji-ichigoside F1), triterpenoid saponin-O-hexoside niga-ichigoside F2 and buergericic acid, 28-O-glucoside inseparable mixture. tormentic acid 28-O-glucoside (tormentoside) | C. peltata, C. insignis, C. hispidissima, C. obtusifolia | [52] |
| Isolated terpenes | ||
| euscaphic acid, tormentic acid, 2α-acetyl tormentic acid, 3β-acetyl tormentic acid | C. lyratiloba | [53] |
| tormentic acid, euscaphic acid, isoarjunolic acid, 3-acetyl tormentic acid | C. lyratiloba | [54] |
| isoyarumic acid | C. telenitida | [55] |
| arjunolic acid, isoyarumic acid, tormentic acid, hederagenic acid | C. telenitida | [56] |
| pomolic acid | C. pachystachia | [57] |
| maslinic acid, oleanolic acid, ursolic acid, pomolic acid, tormentic acid, 2-O-acetyl-tormentic acid, 2α,3β,19α,-trihidroxy-11α,12α,-epoxy-ursane-28,13β-olide, euscaphic acid, 2-O-acetyl-euscaphic acid | C. catharinensis | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enchev, P.; Zarev, Y.; Dakovska, A.; Rivera-Mondragón, A.; Kozuharova, E.; Ionkova, I. Terpenes from Cecropia Species and Their Pharmacological Potential. Pharmaceuticals 2024, 17, 399. https://doi.org/10.3390/ph17030399
Enchev P, Zarev Y, Dakovska A, Rivera-Mondragón A, Kozuharova E, Ionkova I. Terpenes from Cecropia Species and Their Pharmacological Potential. Pharmaceuticals. 2024; 17(3):399. https://doi.org/10.3390/ph17030399
Chicago/Turabian StyleEnchev, Preslav, Yancho Zarev, Anzhelica Dakovska, Andrés Rivera-Mondragón, Ekaterina Kozuharova, and Iliana Ionkova. 2024. "Terpenes from Cecropia Species and Their Pharmacological Potential" Pharmaceuticals 17, no. 3: 399. https://doi.org/10.3390/ph17030399
APA StyleEnchev, P., Zarev, Y., Dakovska, A., Rivera-Mondragón, A., Kozuharova, E., & Ionkova, I. (2024). Terpenes from Cecropia Species and Their Pharmacological Potential. Pharmaceuticals, 17(3), 399. https://doi.org/10.3390/ph17030399







