Use of Medicinal Plants in the Process of Wound Healing: A Literature Review
Abstract
1. Introduction
2. Methods
2.1. Information Sources, Searching, and Selection of Studies
2.2. Eligibility Criteria
2.3. Results
3. Classification of Wounds
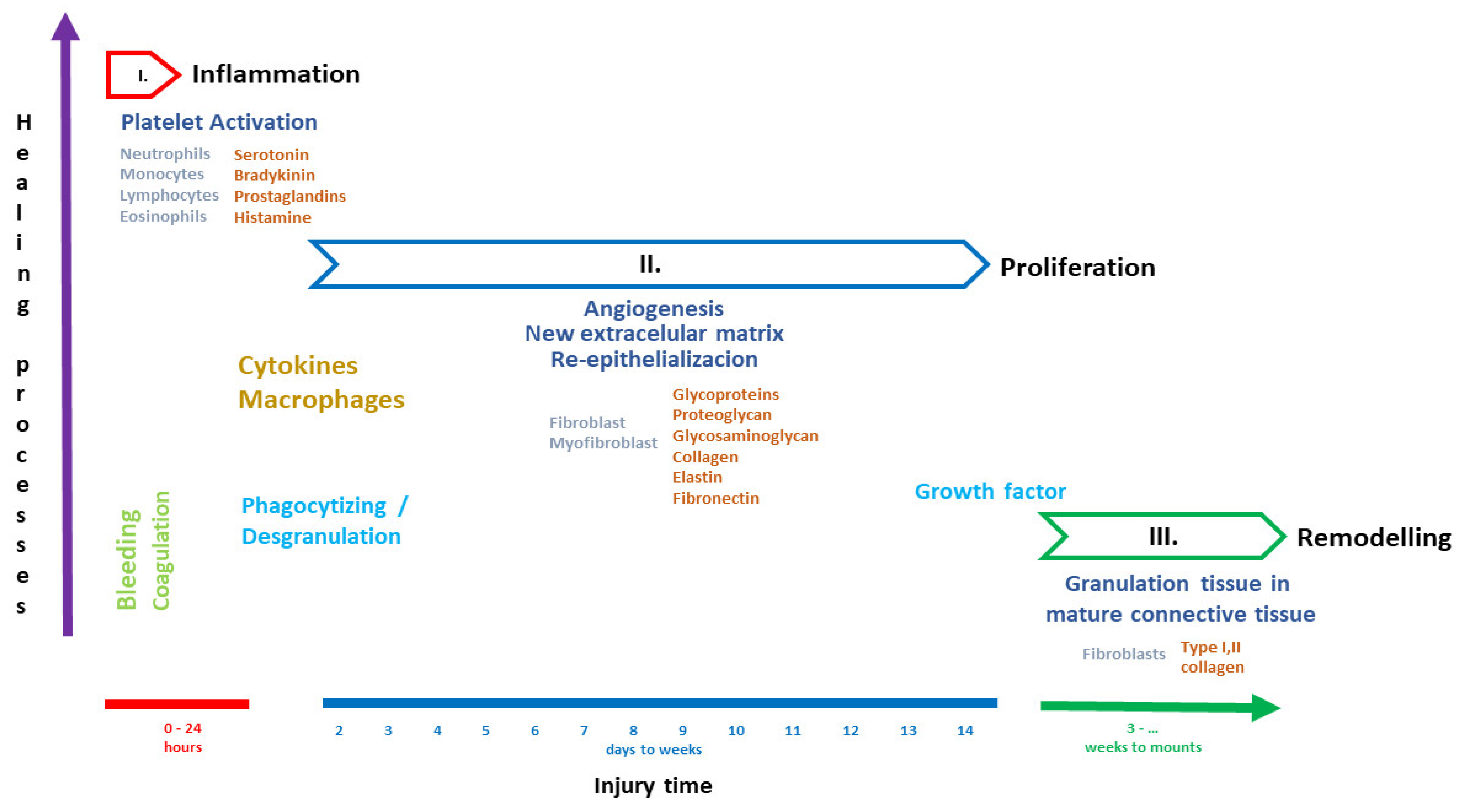
3.1. Healing Process
3.2. Inflammation
3.3. Proliferation
3.4. Remodeling
4. Medicinal Plants Used for Wound Healing
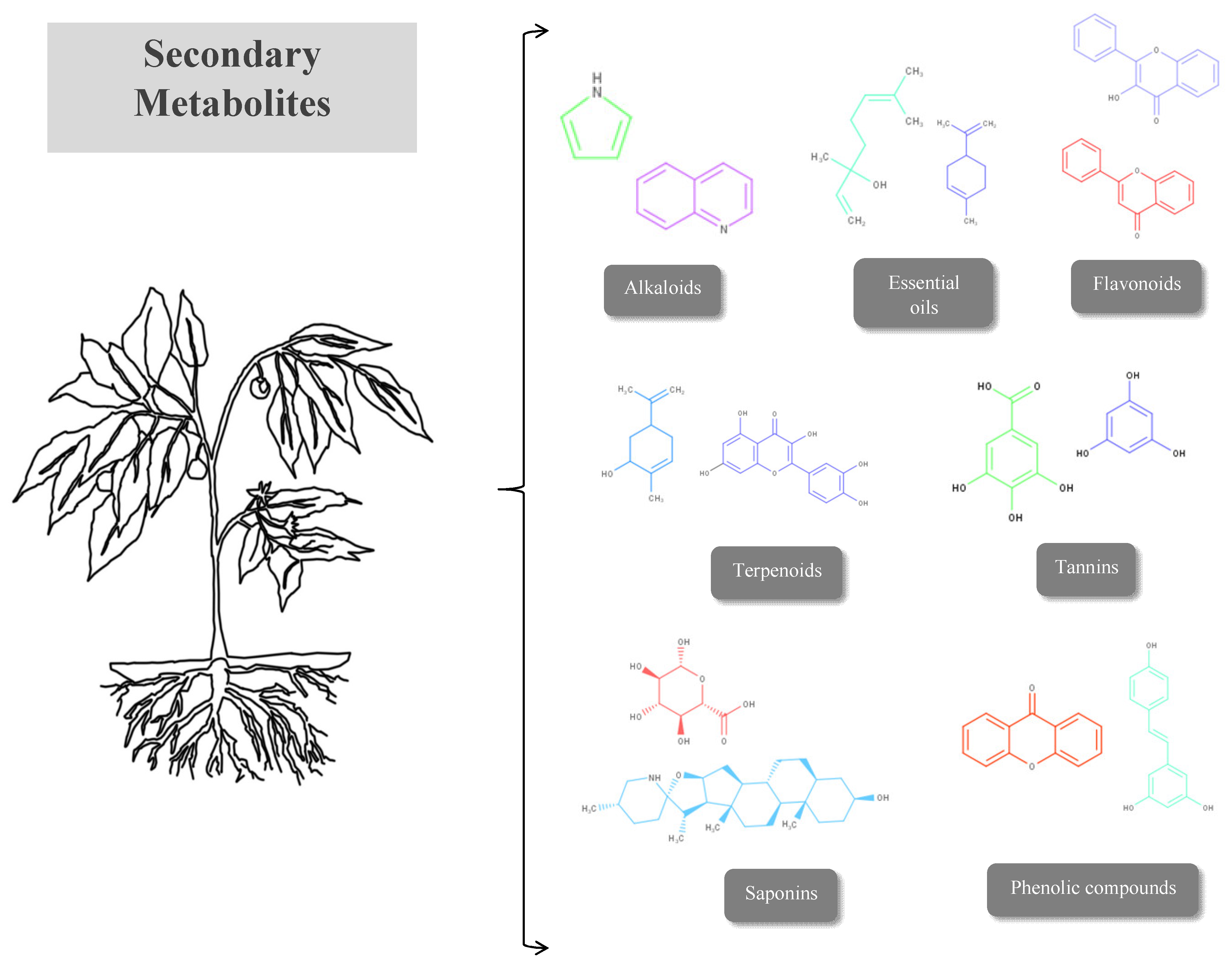
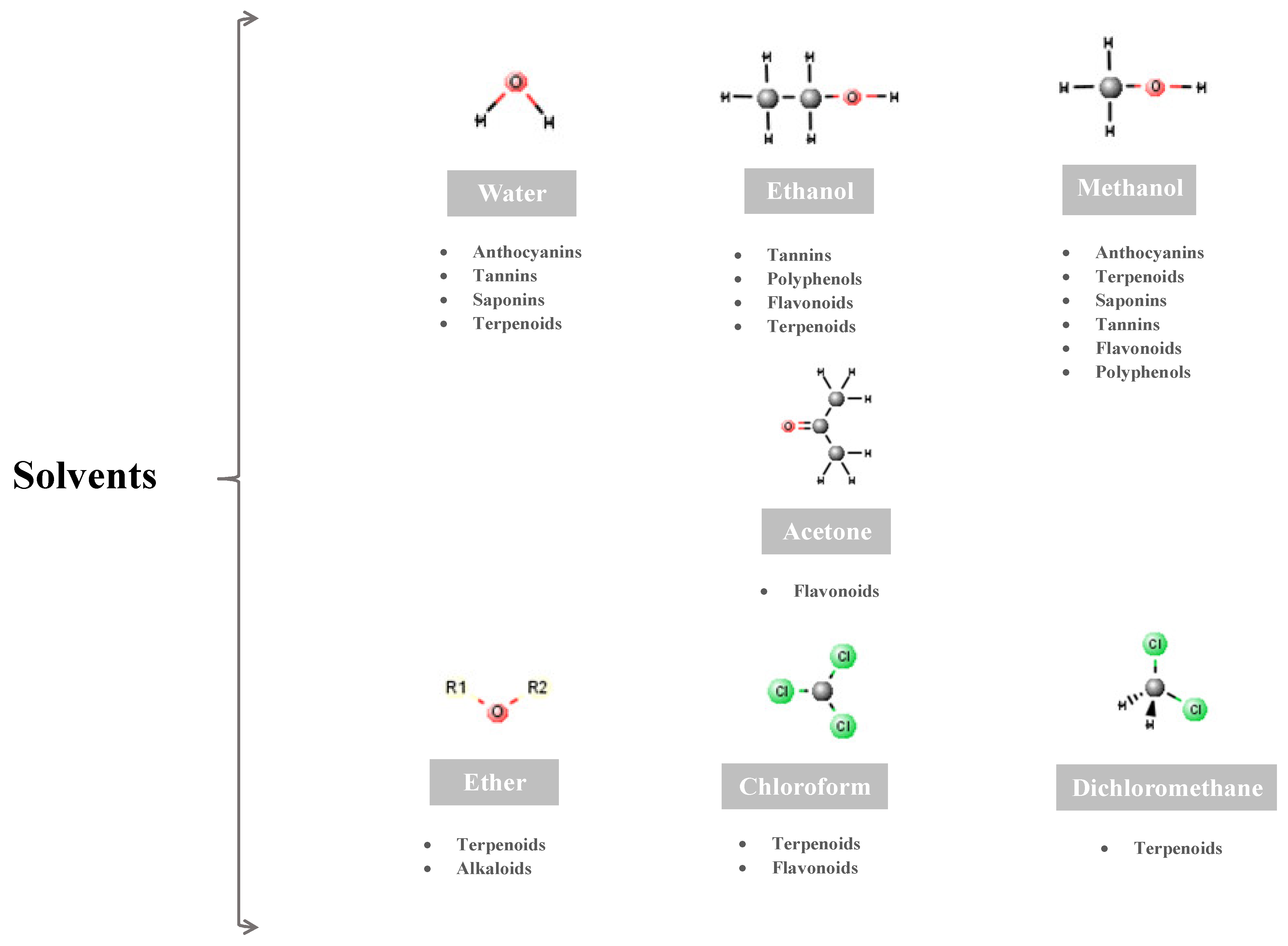
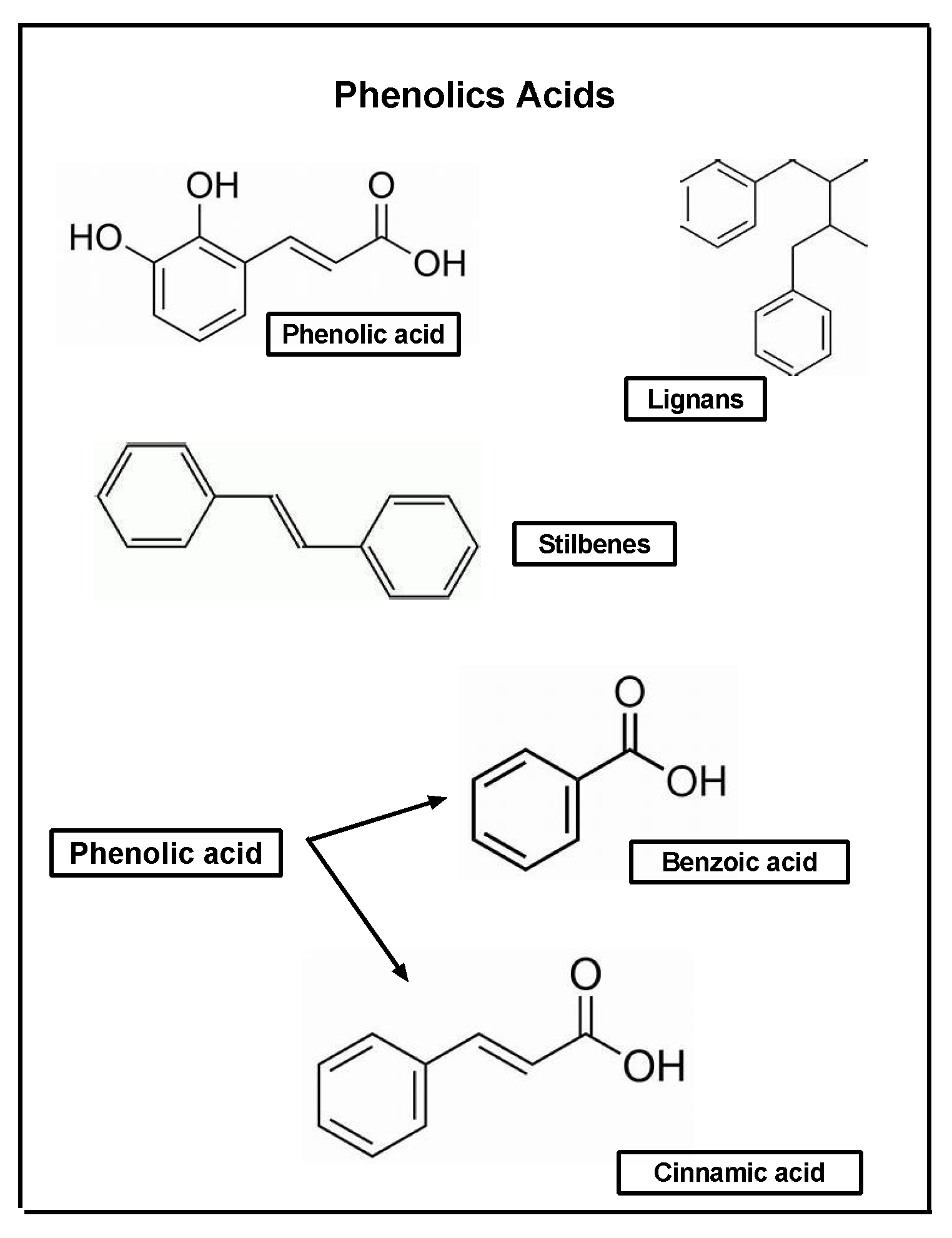
5. Bioactive Phytocompounds with Wound-Healing Properties
6. Activity of Bioactive Phytochemicals in Wound Healing
6.1. Essential Oils
6.2. Polyphenols
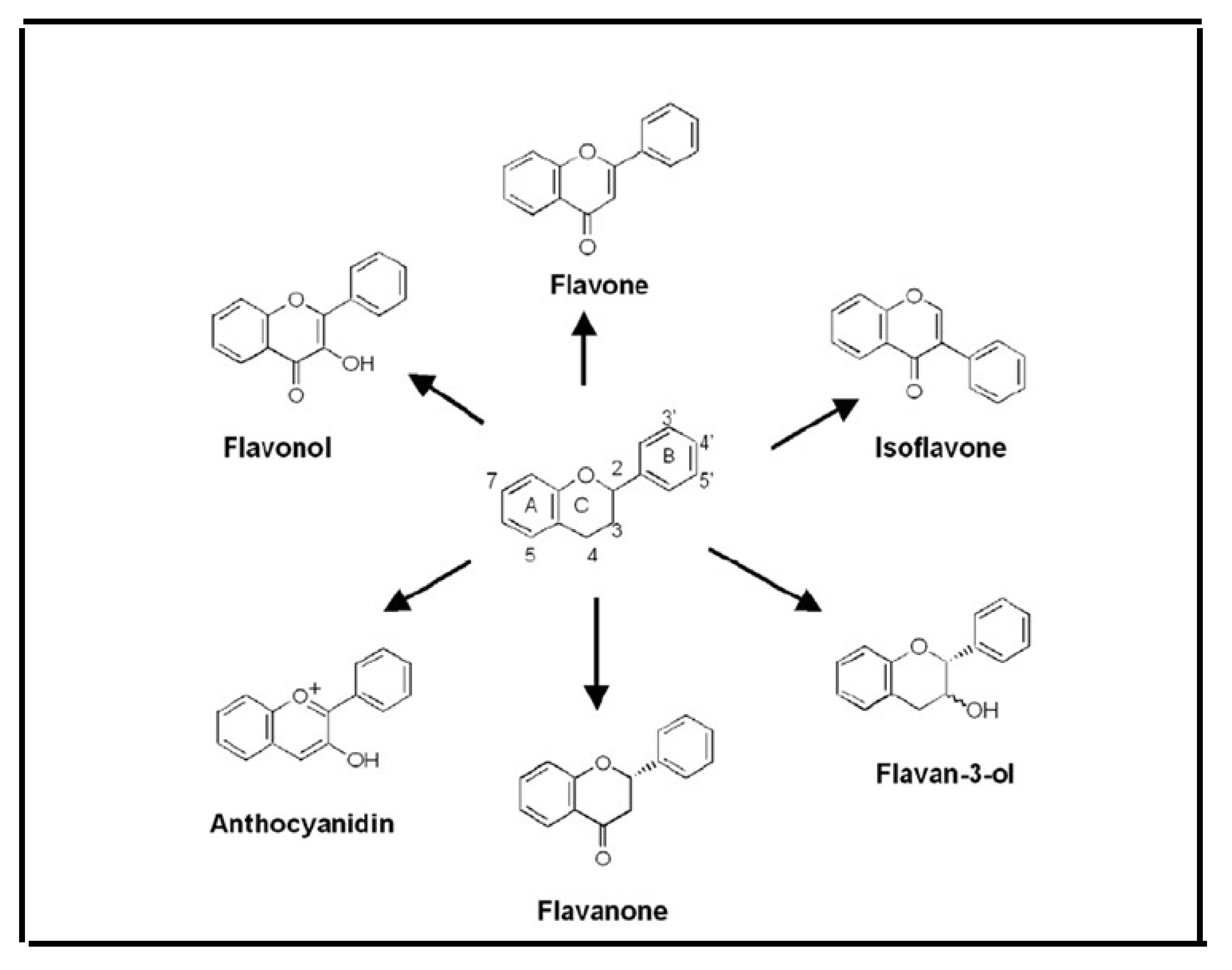
6.3. Flavonoids
7. Mechanisms of Effects of Phytochemicals on Wound-Healing Agents
7.1. Antioxidant Activities of Wound-Healing Agents
7.2. Anti-Inflammatory Properties of Wound-Healing Agents
7.3. Antimicrobial Effects of Wound-Healing Agents
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strodtbeck, F. Physiology of wound healing. Newborn Infant. Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Singer, A.J.; Clarck, R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), S12–S34. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.G.L. Alterações do processo de cicatrização de queimaduras em indivíduos diabéticos. Rev. Bras. Queimaduras 2013, 12, 42–48. [Google Scholar]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C. Processo de cura das feridas: Cicatrização fisiológica. Rev. Med. 2010, 89, 125–131. [Google Scholar] [CrossRef]
- Mandelbaum, S.H.; Di Santis, E.P.; Mandelbaum, M.H.S.A. Cicatrization: Current concepts and auxiliary resources—Parte I. An. Bras. Dermatol. 2003, 72, 393–410. [Google Scholar] [CrossRef]
- Mallefet, P.; Dweck, A.C. Mechanism of Wound Healing Examined. Personal. Care 2008, 9, 75–83. [Google Scholar]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Patologia Básica, 9th ed.; Elsevier: Rio de Janeiro, Brazil, 2013; p. 928. [Google Scholar]
- Lamano, T.L.C. Patologia Geral. Inflamação; Universidade de São Paulo: Ribeirão Preto, Brazil, 2008. [Google Scholar]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar]
- Ross, R.; Odland, G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J. Cell Biol. 1968, 39, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Liu, W.; Borjesson, D.L.; Curry, F.-R.E.; Miller, L.S.; Cheung, A.L.; Liu, F.-T.; Isseroff, R.R.; Simon, S.I. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Investig. Dermatol. 2008, 128, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Deppermann, C.; Cherpokova, D.; Nurden, P.; Schulz, J.-N.; Thielmann, I.; Kraft, P.; Vögtle, T.; Kleinschnitz, C.; Dütting, S.; Krohne, G.; et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J. Clin. Investig. 2013, 123, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.J.; Coutinho-Neto, J. Aspectos celulares da cicatrização. An. Bras. Dermatol. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Waldrop, J.; Doughty, D. Wound-healing Physiology. In Acute and Chronic Wounds: Nursing Management; Mosby Inc.: London, UK, 2000; pp. 17–39. [Google Scholar]
- Enoch, S.; Leaper, D. Basic science of wound healing. Surgery 2007, 26, 31–37. [Google Scholar]
- Eckes, B.; Nischt, R.; Krieg, T. Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair. 2010, 3, 4. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Waldrop, M.A.; Kolb, S.J. Current treatment options in neurology-SMA therapeutics. Curr. Treat Options Neurol. 2019, 21, 25. [Google Scholar] [CrossRef]
- Eming, S.A.; Brachvogel, B.; Odorisio, B.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef]
- Beldon, P. Basic science of wound healing. Surgery 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Michaelis, J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Rep. Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Rep. Regen. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Eo, H.; Lee, H.J.; Lim, Y. Ameliorative Effect of Dietary Genistein on Diabetes Induced Hyper-Inflammation and Oxidative Stress During Early Stage of Wound Healing in Alloxan Induced Diabetic Mice. Biochem. Biophys. Rev. Commun. 2016, 478, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.; Hong, H.S.; Son, Y. Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Rep. Reg. 2016, 24, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Romana-Souza, A.P.; Nascimento, A.P.; Monte-Alto-Costa, A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 611, 77–84. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- WHO Implementation of WHO Traditional Medicine Strategy 2014–2023; Bolletin; WHO: Geneva, Switzerland, 2013; ISBN 9789241506090.
- Alvim, N.A.T.; Ferreira, M.D.A.; Cabral, I.E.; Almeida Filho, A.J.D. O uso de plantas medicinais como recurso terapêutico: Das influências da formação profissional às implicações éticas e legais de sua aplicabilidade como extensão da prática de cuidar realizada pela enfermeira. Rev. Lat.-Am. Enferm. 2006, 14, 316–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carvalho, A.C.B.; Silveira, D. Drogas vegetais: Uma antiga nova forma de utilização de plantas medicinais. Brasília Méd. 2010, 48, 219–237. [Google Scholar]
- Silva, D.M.; Mocelin, K.R. O cuidado de enfermagem ao cliente portador de feridas sob a ótica do cuidado transcultural. Nursing 2007, 9, 8188. [Google Scholar]
- Aquino, D.; Silva, R.B.L.D.; Gomes, V.F.; Araújo, E.C.D. Nível de conhecimento sobre riscos e benefícios do uso de plantas medicinais e fitoterápicos de uma comunidade do Recife—PE. Rev. Enferm. UFPE Online 2007, 1, 107–110. [Google Scholar] [CrossRef]
- Sund, B. New Developments in Wound Care; Clínica Report; PJB Publications: New York, NY, USA, 2000; pp. 1–255. [Google Scholar]
- Wanda, A.; Dorsette, M. Rat models of skin wound healing: A review. Wound Rep. Regen. 2004, 12, 591–599. [Google Scholar]
- Jurjus, A.; Atiyeh, B.; Abdallah, I.; Jurjus, R.; Hayek, S.; Jaoude, M.; Gerges, A.; Tohme, R. Pharmacological modulation of wound healing in experimental burns. Burns 2007, 3, 892–907. [Google Scholar] [CrossRef]
- Agyare, C.; Bempah, S.B.; Boakye, Y.D.; Ayande, P.G.; Adarkwa-Yiadom, M.; Mensah, K.B. Evaluation of antimicrobial and wound healing potential of Justicia flava and Lannea welwitschii. Evid. Based Complement. Altern. Med. 2013, 2013, 632927. [Google Scholar] [CrossRef]
- Al-Bayaty, F.H.; Abdulla, M.A.; Hassan, M.I.A.; Ali, H.M. Effect of Andrographis paniculata leaf extract on wound healing in rats. Nat. Prod. Res. 2012, 26, 423–429. [Google Scholar] [CrossRef]
- Mengie, T.; Mequanente, S.; Nigussie, D.; Legesse, B.; Makonnen, E. Investigation of wound healing and anti-inflammatory activities of 80% methanol leaf extract of Achyranthes aspera L. (Amaranthaceae) in rats. J. Inflamm. Res. 2021, 14, 1775–1787. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Sean Tan, W.; Suresh Kumar, S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.; Arulselvan, P. In Vitro Wound Healing Potential of Stem Extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef]
- Kallappa, M.H.; Ganjihal, S.S.; Chavadi, D.V. Alternanthera triandra seed oil: A moderate source of ricinoleic acid and its possible industrial utilization. Ind. Crops Prod. 2004, 2, 133–136. [Google Scholar]
- Ofusori, A.E.; Raharjo, Y.; Ofusori, D.A.; Adekunle, V.O. A comparative study of dichloromethane and ethyl acetate root extracts of Celoisa trigyna: Phytochemical and wound healing effects analyses. J. Wound Manag. Res. 2023, 19, 87–95. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sarkar, R.; Sharma, A.; Yadav, K.K.; Kumar, A.; Roy, P.; Sen, T. Pharmacological studies on Buchanania lanzan Spreng.-A focus on wound healing with particular reference to anti-biofilm properties. Asian Pac. J. Trop. Biomed. 2013, 3, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Hung, C.-Y.; Tsai, T.-H.; Chak, K.-F. A Study of the wound healing mechanism of a traditional Chinese medicine, Angelica sinensis, using a proteomic approach. Evid.-Based Complement. Altern. Med. 2012, 2012, 467531. [Google Scholar] [CrossRef] [PubMed]
- Tanga, B.M.; Bang, S.; Fang, X.; Seo, C.; de Zoysa, M.; Saadeldin, M.I.M.; Lee, S.; Park, S.; Chung, S.-O.; Lee, G.-J.; et al. Centella asiatica extract in carboxymethyl cellulose at its optimal concentration improved wound healing in mice model. Heliyon 2022, 8, e12031. [Google Scholar] [CrossRef] [PubMed]
- Gohari, A.R.; Saeidnia, S.A. Review on Phytochemistry of Cuminum cyminum seeds and its standards from field to market. Pharmacogn. J. 2011, 3, 1–5. [Google Scholar] [CrossRef]
- Wu, J.-G.; Wei, Y.-J.; Ran, X.; Zhang, H.; Nian, H.; Qin, L.-P. Inhibitory effects of essential oil from rhizomes of Ligusticum chuanxiong on hypertrophic scarring in the rabbit ear model. Pharm. Biol. 2011, 49, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Kabesh, K.; Senthulkumar, P.; Ragunathan, R.; Kumar, R. Phytochemical análisis of Catharantus roseus plan extract and its anti-microbial acitivity. Int. J. Pure App. Biosci. 2015, 3, 162–172. [Google Scholar]
- Agyare, C.; Dwobeng, A.S.; Agyepong, N.; Boakye, Y.D.; Mensah, K.B.; Ayande, P.G.; Adarkwa-Yiradon, M. Antimicrobial, antioxidant, and wound healing properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Adv. Pharmacol. Sci. 2013, 2013, 692613. [Google Scholar] [CrossRef] [PubMed]
- Meenu, N.C.; Manokari, S.L.; Yogeswari, G.; Duraisami, R. Analysis of phytochemical constituents and antibacterial activity of Wrightia tinctoria: Traditional medicinal plant of India for application on wound dressing materials. Indian J. Trad. Knowl. 2022, 21, 48–54. [Google Scholar]
- Omale, J.; Victoria, I.A. Excision and incision wound healing potential of Saba florida (Benth) leaf extract in Rattus novergicus. Int. J. Pharm. Biomed. Res. 2010, 1, 101–107. [Google Scholar]
- Namgoong, S.; Lee, H.; Han, S.-K.; Lee, H.-W.; Jeong, S.-H.; Dhong, E.-S. Effect of Panax ginseng extract on the activity of diabetic fibroblasts in vitro. Int. Wound J. 2019, 16, 737–745. [Google Scholar] [CrossRef]
- Lu, T.; Gao, Y.; Duan, Y.; Cui, C.; Zhang, L.; Mingning, S. Panax notoginseng saponins improves healing of high glucose-induced wound through the GSK-3β/β-catenin pathway. Environ. Toxicol. 2022, 37, 1867–1877. [Google Scholar]
- Ali-Seyed, M.; Ayesha, S. Calotropis A multi-potential plant to human kind: Special focus on its wound healing efficacy. Biocatal. Agric. Biotechnol. 2020, 28, 101725. [Google Scholar] [CrossRef]
- Mali, R.P.; Raq, P.S.; Vikle, D.N. Wound healing activity of Calotropis procera root bark on diabetic rats. J. Drug Deliv. Ther. 2020, 10, 86–89. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2016, 523, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The Estimation of the Traditionally Used Yarrow (Achillea millefolium L. Asteraceae) Oil Extracts with Anti-Inflamatory Potential in Topical Application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.A.C.; Gardinassi, L.G.; Silveira, I.M.G.; Galucci, M.G.; Tomre, M.A.; Oliveira, J.F.D.; Moreira, M.R.A.; Meirelles, A.F.G.; Faccioli, L.H.; Tefé-Silva, C.; et al. Arctium lappa extracts suppresses inflammation and inhibits melanoma progression. Medines 2019, 6, 81. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, D.; Hu, X.; Wang, H.; Fu, W.; Fan, Z.; Chen, X.; Yu, F. Effect of volatile oil from Blumea balsamifera (L.) DC. leaves on wound healing in mice. J. Tradit. Chin. Med. 2014, 34, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The water fraction of Calendula officinalis hydroethanol extract stimulates in vitro and in vivo proliferation of dermal fibroblasts in wound healing. Phytother. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.M.; Lino Júnior, R.d.S.; Faustino Tresvenzol, L.M.; Vinaud, M.C.; de Paula, J.R.; Paulo, N.M. Wound healing and anti-inflammatory effect in animal models of Calendula officinalis L. growing in Brazil. Evid.-Based Complement. Altern. Med. 2012, 2012, 375671. [Google Scholar] [CrossRef]
- Gao, S.-Q.; Chang, C.; Niu, X.-Q.; Li, L.-J.; Zhang, Y.; Gao, J.-Q. Topical application of Hydroxysafflor yellow A accelerates the wound healing in streptozotocin induced T1DM rats. Eur. J. Pharmacol. 2018, 823, 72–78. [Google Scholar] [CrossRef]
- Balekar, N.; Nakpheng, T.; Katkam, N.G.; Srichana, T. Wedelia trilobata L. Phytochemical and Pharmacological Reviews. Chiang Mai J. Sci. 2014, 41, 590–605. [Google Scholar]
- Agyare, G.; Koffuor, A.; Boamah, V.E.; Adu, F.; Mensah, K.B.; Adu-Amoah, L. Antimicrobial and anti-inflammatory activities of Pterygota macrocarpa and Cola gigantea (Sterculiaceae). Evid.-Based Complement. Altern. Med. 2012, 2012, 902394. [Google Scholar] [CrossRef]
- Kuklkarni, M.; Singhai, R.G.; Bhise, K.; Tambe, R. Phytochemical screening, HPTLC studies screening of antioxidants activity of extracts of leaves of Spanthodea campanulate. J. Pharmacogn. Phytochem. 2014, 3, 8–13. [Google Scholar]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.-R.H. Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea. Molecules 2020, 25, 4870. [Google Scholar] [CrossRef]
- Nakuntwalai, W.; Thungmungmee, S.; Khobjai, W. Factor promoting wound healing: Radical scavenging and anti-inflammatory activity and growth factor promotion of Heliotropium indicum. Int. J. Appl. Pharm. 2019, 11, 44–48. [Google Scholar]
- Kojoma, M. Cultivation study of Lithospermum erythrorhizon to obtain “Shikon” as a purple dye and traditional medicine–root growth and shikonin derivatives content. In Proceedings of the ISHS Acta Horticulturae 1361: XXXI International Horticultural Congress (IHC2022): International Symposium on Natural Colorants from Plants, Angers, France, 14–20 August 2022; Räisänen, R., de la Sayette, A., Eds.; 2022. [Google Scholar]
- Kavousi, A.; Nikkhah, E.; Tayarani-Najaran, Z.; Javadi, B. Wound Healing Effects and Related Mechanims of Action of Methanol Extracts of Boswellia sacra and Commiphora myrrha Oleo-Gum Resins on Adult Human Dermal Fibroblasts (HDFa). Available online: https://ssrn.com/id3969804 (accessed on 9 January 2024).
- Yuan, X.; Han, L.; Fu, P.; Zeng, H.; Lv, C.; Chang, W.; Runyon, R.S.; Ishii, M.; Han, L.; Liu, K.; et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab. Investig. 2018, 98, 783–798. [Google Scholar] [CrossRef]
- Koshak, A.; Algandaby, M.N.; Mujallid, M.I.; Abdel-Naim, A.B.; Alhakamy, N.A.; Fahmy, U.A.; Alfarsi, A.; Badr-Eldin, S.M.; Neamatallah, T.; Nasrullah, M.Z.; et al. Wound healing activity of Opuntia ficus-indica fixed oil formulated in a self-nanoemulsifying formulation. Int. J. Nanomed. 2021, 16, 3889–3905. [Google Scholar] [CrossRef] [PubMed]
- Fanani Hakim, R.; Dinni, F. Effect of Carica papaya extract toward incised wound healing process in mice (Mus musculus) clinically and histologically. Evid.-Bases Complemment. Altern. Med. 2019, 2019, 8306519. [Google Scholar] [CrossRef]
- Quillay Davila, M.A.; Arana Arias, Y.A.; Jaramillo Jaramillo, C.G.; Buelle, S.C.; Rojas de Astudillo, L.L.; Jaramillo Alcívar, V. Contenido de saponinas y actividad cicatrizante de Cecropia peltata y Parthenium hysterophorus. Rev. Cuba. Farm. 2018, 51. Available online: http://www.revfarmacia.sld.cu/index.php/far/article/view/250/147 (accessed on 9 January 2024).
- Agyare, C.; Ansah, A.O.; Ossei, P.P.S.; Apenteng, J.A.; Boakye, Y.D. Wound healing and anti-infective properties of Myrianthus arboreus and Alchornea cordifolia. Med. Chem. 2014, 4, 533–539. [Google Scholar] [CrossRef]
- Chen, W.C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complement. Altern. Med. 2012, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Kisseih, E.; Lechtenber, M.; Petereit, F.; Sendker, J.; Brandt, S.; Agyare, C.; Hensel, A. Phytochemical characterization and in vitro wound healing activity of leaf extracts from Combretum mucronatum Schum. & Thonn.: Oligomeric procyanidins as strong inductors of cellular differentiation. J. Ethnopharmacol. 2015, 174, 628–636. [Google Scholar]
- Jokar, A.; Masoomi, F.; Sadeghpour, O.; Nassiri-Toosi, M.; Hamedi, S. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J. Trd. Chin. Med. 2016, 36, 250–254. [Google Scholar]
- Khan, M.U.; Khalilullah, H.; Akhtar, J.; Elhasan, G.O. Terminalia chebula: An ephemeral glance. Int. J. Pharm. Phram. 2015, 7, 40–43. [Google Scholar]
- Rodrigues Dantas Araujo, E.; Costa da Silva, V.; Figueiredo de Lima, J.B.; Schlamb, J.; Freitas Fernandes-Pedrosa, M.; Silva Junior, A.A.; Rathinasabapathy, T.; Moncada, M.; Esposito, D.; Coelho Bernardo Guerra, G.; et al. Gel formulated with Bryophyllum pinnatum leaf extract promotes skin wound healing in vivo by increasing VEGF expresión: A novel potential active ingredient for pharmaceuticals. Front. Pharmacol. 2023, 13, 1104705. [Google Scholar] [CrossRef] [PubMed]
- Ullah Arshad, M.; Hassan, A. Medical treatment of various diseases through Nagarmotha (Cyperus rotundus) plant. Eur. J. Biol. Med. Sci. Res. 2022, 10, 26–43. [Google Scholar] [CrossRef]
- Bigonya, P.; Agrawal, S.; Verma, N.K. Potential wound healing activity of Euphoria hirta Linn total flavonoids fractions. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 149–156. [Google Scholar]
- Nwala, C.O.; Akaninwor, J.O.; Monanu, M.O. Phytochemical screening and wound healing activities of extracts of Jatropha curcas leaf formulated in a simple ointment base. Int. J. Eng. Sci. Invent. 2013, 2, 72–75. [Google Scholar]
- Kabran, F.A.; Okpekon, T.A.; Roblot, F.; Seon-Meniel, T.; Leblanc, K.; Bories, C.; Champy, P.; Yolou, S.F.; Loiseau, P.M.; Djakoure, L.A.; et al. Bioactive phloroglucinols from Mallotus oppositifolius. Fitoterapia 2015, 107, 100–104. [Google Scholar] [CrossRef]
- Ajirni, N.A.; Nazaruddin, A.; Sutriana, D.; Masyitha Isa, M. The effect of ethanol of Malacca leaves (Phyllanthus emblica) on the number of fibroblast cells in White rats (Tattus norvegicus) burns wound. Int. J. Trop. Vet. Biomed. Res. 2020, 5, 7–12. [Google Scholar] [CrossRef]
- Boakye, Y.D.; Agyare, C.; Ayande, P.G.; Asiamah, E.A.; Titloye, N.A. Wound healing activity of geraniin and aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Excel (Euphorbiaceae). Planta Med. 2014, 80, WS20. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Moon, M.Y.; Ryu, E.K.; Lee, J.S.; Chan, J. Identification of the phytochemical compounds and their type I procollagen induction in Astragalus membranaceus Sprouts grown under different light conditions. J. Appl. Pharmacetical. Sci. 2018, 8, 1–7. [Google Scholar]
- Tewtrakul, S.; Tungcharoen, P.; Sudsai, T.; Karalai, C.; Ponglimanont, C.; Yodsaoue, O. Antiinflammatory and wound healing effects of Caesalpinia sappan L. Phytother. Res. 2015, 29, 850–856. [Google Scholar] [CrossRef]
- Su, X.; Liu, X.; Wang, S.; Li, B.; Pan, T.; Liu, D.; Wang, F.; Diao, Y.; Li, K. Wound-healing promoting effect of total tannins from Entada phaseoloides (L.) Merr. In rats. Burns 2017, 43, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, M.; Sandoughdaran, S.; Shandiz, F.H.; Honary, S. The efficacy of Licorice root (Glycyrrhiza glabra) and yarrow (Achillea millefolium) in preventing radiation dermatitis in patients with breast cancer, a randomized, double-blinded, placebo-controlled clinical trial. Asian Pac. J. Cancer Care 2016, 1, 9. [Google Scholar] [CrossRef][Green Version]
- Saini, S.; Dhiman, A.; Nanda, S. Traditional Indian Medicinal plants with potential wound healing activity: A review. Int. J. Pharm. Sci. Res. 2009, 7, 1809–1891. [Google Scholar]
- Kannan, S.; Vijay Jesuraj, S.A.; Jeeva Kumar, E.S.; Saminathan, K.; Suthakaran, R.; Saminathan, K.; Suthakaran, R.; Ravi Kumar, M.; Parimala Devi, B. Wound healing activity of Mimosa pudica Linn formulation. Int. J. PharmTech. Res. 2009, 1, 1554–1558. [Google Scholar]
- Saini, P.; Kumar Verma, P. Evaluation of the wound healing properties of Mimosa pudica Linn. In streptozocin-induced diabetes mellitus in rats. Int. J. Pharm. Sci. Res. 2019, 10, 661–665. [Google Scholar]
- Xu, X.; Li, X.; Zhang, L.; Liu, Z.; Pan, Y.; Chen, D.; Bin, D.; Deng, Q.; Sun, Y.; Hoffman, R.M.; et al. Enhancement of wound healing by the Chinese medicine herbal mixture Sophora flavescens in a rat model of perianal ulceration. Vivo 2017, 31, 543–549. [Google Scholar]
- Lodhi, S.; Jain, A.; Jain, A.P.; Pawar, R.S.; Singhai, A.K. Effects of flavonoids from Martynia annua and Tephora purpurea on cutaneous wound healing. Avicenna J. Phytomed. 2016, 6, 578–591. [Google Scholar]
- Chokpaisam, J.; Chusri, S.; Ammuaikit, T.; Udomuksorn, W.; Voravuthikunchai, S.P. Potential wound healing activity of Cuercus infectoria formulation in diabetic rats. PeerJ 2017, 5, e3608. [Google Scholar] [CrossRef]
- Cheng, P.G.; Phan, C.-W.; Sabaratnam, V.; Abdullah, N.; Abdulla, M.A.; Kuppusamy, U.R. Polysaccharides-rich extract of Ganoderma lucidum (M.A. Curtis:Fr.) P. Karst accelerates wound healing in streptozotocin-induced diabetic rats. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Ngwoke, K.G.; Akwaqbulam, A.G.; Erhirhie, E.O.; Ajaghaku, D.L.; Chiedu Okoye, F.B.; Okechukwu Esimone, C. Antioxidant, Anti-inflammatory, Analgesic Properties, and Phytochemical Characterization of Stem Bark Extract and Fractions of Anthocleista nobilis. Pharmacogn. Res. 2018, 10, 81–87. [Google Scholar] [CrossRef]
- Bardaa, S.; Makni, K.; Boudaouara, O.; Bardaa, T.; Ktari, N.; Hachira, S.; Salah, R.B.; Kallel, R.; Sahnound, Z.; Boufi, S. Development and evaluation of the wound healing effect of a novel topical cream formula based on Ginkgo biloba extract on wounds in diabetic rats. BioMed Res. Int. 2021, 2021, 6474706. [Google Scholar] [CrossRef] [PubMed]
- Hariharapura, R.; Srinivasan, R.; Ashok, G.; Dongra, S.H.; Jagani, H.V.; Vijayan, P. Investigation of the antioxidant and hepatoprotective potential Hypericum mysorense. Antioxidants 2014, 3, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, M.R.; Cortese, M.; Vargas Peregrina, D.V.; Villa, C.; Lupidi, G.; Pruccoli, L.; Angeloni, C.; Tarozzi, A. Development of new extracts of Crocus sativus L. By products from two different Italian regions as new potential active ingredient in cosmetic formulations. Cosmetics 2021, 8, 51. [Google Scholar] [CrossRef]
- Jayapal, V.; Subha, V.; Pradeep, J.; Janardan Salwe, K.; Manimekalai, R.; Tahinamala, R.; Kumar, B.; Rayvathy, B.; Ravi Kumar, S. Evaluation of wound healing potential of the essential oil of Ocimum sanctum L. (Thulasi/basil) containing ointment in female Wistar albino rats. J. Pharmacogn. Phytochem. 2023, 12, 189–193. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, S.; Farahpour, M.R. Topical application of Salvia officinalis hydroethanolic leaf extract improves wound healing process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar]
- Guevara-Vazquez, A.M.; Marin-Tello, C.L. Wound healing activity of Allium cepa L. bulbs in a second-degree burn wound model in Holtzman rats. Vitae 2021, 28, 345737. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hyck, K.; Lim, D.-W.; Kim, J.-E.; Park, W.-H.; Park, S.-D. Ethanol Extract of Lycopodium serratum Thunb. Attenuates Lipopolysaccharide-Induced C6 Glioma Cells Migration via Matrix Metalloproteinase-9 Expression. Chin. J. Integr. Med. 2018, 24, 860–866. [Google Scholar] [CrossRef]
- Dutta, S.; Pattnak, A.K.; Bersa, S.E. Wound healing potential of methanolic extract and its fraction of Lawsonia alba Lam leaves formulated a topical gel. World J. Pharm. Res. 2016, 5, 1091–1109. [Google Scholar]
- El Massoudi, S.; Zinedine, A.; Rocha, J.M.; Benidir, M.; Najjari, I.; El Ghadraoui, L.; Benjelloun, M.; Errachidi, F. Phenolic composition and wound healing potential assessment of Moroccan Henna (Lawsonia inermis) Aqueous extract. Cosmetics 2023, 10, 92. [Google Scholar] [CrossRef]
- Lukiswanto, B.S.; Miranti, A.; Sudjarwo, S.A.; Primarizky, H.; Yuniati, W.M. Evaluation of wound healing potential of pomegranate (Punica granatum) whole fruit extract on skin burn wound in rats (Rattus norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Balla, R.; Kaur, R.; Kaur, B.; Kaur, P. Hibiscus rosa sinensis Linn. A phytochemical and pharmacological review. Int. J. Health Sci. 2022, 6, 5165–5193. [Google Scholar]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Shams Ardekani, M.R.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mat. Sci. Eng. 2020, 114, 111039. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.H.; Mulla, S.M.; Ashariff, A.; Sreeharsha, N.; Meravanige, G.; Shiroorkar, P.N.; Basheeruddin Asdaq, S.M.; Khalid Answer, M.D.; Roopashree, T.S.; Karnatti, R.K. Exploring the topical gel of Thespesia populnea leaf extract for in vivo wound healing efficacy. Pharmacogn. Mag. 2022, 18, 519–523. [Google Scholar]
- Maan, P.; Singh Yadav, K.; Yadav, N.P. Wound healing activity of Azadirachta indica A. Juss stem bark in mice. Pharmacogn. Mag. 2017, 13 (Suppl. S2), S316–S320. [Google Scholar]
- Silva, D.F.; Lima, K.T.; Bastos, G.T.N.; Oliveira, J.A.R.; do Nascimento, L.A.S.; Costa, C.E.F.; Filho, N.R.; Concha, V.O.; Passos, M.F. PCL/Andiroba oil (Carapa guianensis Aubl.) hybrid film for wound healing applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Vrma, A. Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, J. Ointment of methanolic extract Ficus religiosa: Traditional approach in wound healing in rats. Int. J. Pharm. Sci. Res. 2016, 7, 5006–5011. [Google Scholar]
- Susanto, A.; Muhaimina, R.K.; Amalliya, A.; Sutjiatmo, A.B. The effectiveness of ethanolic of Moringa oleífera Lam. Gel on the wound healing process of the rat’s palate. J. Int. Dent. Med. Res. 2019, 12, 504–509. [Google Scholar]
- Kumar, M.; Kumar Gautan, M.; Singh, A.; Kumar Goel, R. Healing effect of Musa sapientum var. Paradisiaca in diabetic rats with co-occurring gastric ulcer: Cytokines and growth factor by PCR amplification. BMC Complement. Altern. Med. 2013, 13, 305. [Google Scholar] [CrossRef]
- Shrimali, H.; Kumar Mandal, U.; Nivsarkar, M.; Shrivastawa, N. Fabrication and evaluation of a medicated hydrogel film with embelin from Embelia ribes for wound healing activity. Future J. Pharm. Sci. 2019, 5, 12. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Sousa Brites, G.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Costa Branco, P.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Khuanekkaphan, M.; Noysang, C.; Khobjai, W. Anti-aging potential and phytochemicals of Centella asiática, nelumbo nucifera and Hibiscus sabdariffa extracts. J. Adv. Pharm. Technol. Res. 2020, 11, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Satish, S.S.; Anima, P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna J. Phytomed. 2015, 6, 295–304. [Google Scholar]
- Chaturvedi, A.P.; Kumar, M.; Tripathi, Y.B. Efficacy of Jasminum grandiflorum L. leaf extract on dermal wound healing in rats. Int. Wound J. 2013, 10, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Chen, Z.; Shi, J.; Qu, Y.; Zhang, J. Effect of Polysaccharides from Bletilla striata on the Healing of Dermal Wounds in Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 9212314. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Szopa, A. Paeonia suffruticosa (Mountan Peony)—A review of the chemical composition, traditional and profesional use in medicine, position in cosmetics industries and biotechnological studies. Plants 2022, 11, 3379. [Google Scholar] [CrossRef]
- Ayele, T.M.; Chekol Abebe, E.; Tilahum Muche, Z.; Mekonnen Agidew, M.; Shumet Yimer, Y.; Testaw Addis, G.; Dagnaw Baye, N.; Bogale Kassie, A.; Adela Alemu, M.; Gobezie Yblet, T.; et al. Evaluation of in vivo wound-healing and anti-inflammatory activities of solvent fractions of fruits of Argemone mexicana L. (Papaveraceae). Evid.-Based Complement. Altern. Med. 2022, 2022, 6154560. [Google Scholar] [CrossRef]
- Heba, E.; El Aty, A.; Zaazaa, A.M.; Mohamed, S.H.; El Dayem, S.A.; Foda, F. Promising Therapeutic Efficacy of Trigonella-foenum graecum and Bone Marrow-Derived Mesenchymal Stem Cells on Skeletal Muscle Atrophy in Experimental Rat Model. Biointerrface Res. Appl. Chem. 2023, 13, 133. [Google Scholar] [CrossRef]
- Somwanshi Sachim, B.; Hiremath Shivanand, N. In vivo evaluation of the wound healing activity of the Sesamum indicum L. seed extract in novel ethosomal vesicular system. J. Drug Del. Therap. 2018, 8, 411–420. [Google Scholar] [CrossRef]
- Ghanadian, M.; Soltani, R.; Homayouni, A.; Khorvash, F.; Jouabadi, S.M.; Abdollahzadeh, M. The Effect of Plantago Major Hydroalcoholic Extract on the Healing of Diabetic Foot and Pressure Ulcers: A Randomized Open-Label Controlled Clinical Trial. Int. J. Low Extrem. Wounds 2022. [Google Scholar] [CrossRef]
- Yang, W.-T.; Ke, C.-Y.; Wu, W.-T.; Harn, H.-J.; Teng, Y.-H.; Lee, R.-P. Effects of Angelica dahurica and Rheum officinale extracts on excisional wound healing in rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 1583031. [Google Scholar] [CrossRef] [PubMed]
- Budiawan, A.; Purwanto, A.; Puradewa, L.; Dwi Cahyani, E.; Endang Purwaningsh, C. Wound healing activity and flavonoid contentes of pursale (Portulaca grandiflora) of various varieties. RSC Adv. 2023, 13, 9871. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Jugreet, S.; Sinan, K.I.; Zengin, G.; Gunes, A.K.; Ramazan, C.; Josef, J.; Zoltan, C.; Angelin, P.; Angeles Flores, G.; et al. Pharmacological potential and chemical characrization of Bridelia ferruginea Benth.—A native tropical African Medicinal Plants. Antibiotics 2021, 10, 223. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Cen, Y. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int. J. Biol. Macromol. 2018, 112, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.T.; Pham Nguyen, M.T.; Oanh Nguyen, T.K.; Quynh Bui, T.P.; Ke, X.; Le, V.M. Phytochemical analysis and wound healing activity of Noni (Morinda citrifolia) leaf extract. J. Herbs. Spices Med. Plants 2020, 26, 379–393. [Google Scholar] [CrossRef]
- Wen, M.; Chen, Q.; Chen, W.; Yang, J.; Zhou, X.; Zhang, C.; Wu, A.; Lai, J.; Chen, J.; Mei, Q.; et al. A comprehensive review of Rubia cordifolia L.: Traditional uses, phytochemistry, pharmacological activities, and clinical applications. Front. Pharmacol. 2022, 13, 965390. [Google Scholar] [CrossRef]
- Azmil, L.; Shukla, I.; Goutam, A.; Allauddin Rao, C.H.V.; Jawaid, T.; Kamal, M.; Awaad, A.S.; Alqasoumi, S.I. In vitro wound healing activity of 1-hydroxy-5,7-dimethoxy-2-naphthalene-carboxaldehyde (HDNC) and other isolates of Aegle marmelos L.: Enhances keratinocytes motility via Wnt/β-catenin and RAS-ERK pathways. Saudi Pharm. J. 2019, 27, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutacea): A systematic Review of traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.N.; da Silveira, M.R.; Danielski, L.G.; Florentino, D.; Petronilho, F.; Piovezan, A.P. Anti-Inflammatory and Antioxidant Properties of Hydroalcoholic Crude Extract from Casearia sylvestris Sw. (Salicaceae). J. Ethnopharmacol. 2013, 147, 612–617. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Zeng, L.; Jin, J.-O. Rehmannia glutinosa polysaccharide induced an anti-cancer effect by activating natural killer cells. Int. J. Biol. Macromol. 2017, 105, 680–685. [Google Scholar] [CrossRef]
- Kil, Y.-S.; Park, J.; Han, A.-R.; Woo, H.; Seo, E.-K. A new 9,10-dihydrophenanthrene and cell proliferative 3,4-δ-dehydrotocopherols from Stemona tuberosa. Molecules 2015, 20, 5965–5974. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Abdulla, M.A.; Sanusi, J. The effect of Camellia sinensis on wound healing potential in an animal model. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Tang, Y.; Wu, L.; Tao, W.; Qian, Y.; Duan, J.-A. Pharmacokinetic profile and metabolite identification of yuanhuapine, a bioactive component in Daphne genka by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 112, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Wang, I.C.; Chen, J.J. Bioactive components from Ampelopsis japonica with antioxidant, glucosidase, and antiacetylcholinesterase activities. Antioxidants 2022, 11, 1228. [Google Scholar] [CrossRef]
- Irham, W.H.; Hardiyanti, R. Wound healing bioactivity of Curcuma longa Linn. Rasayan J. Chem. 2021, 14, 2386–2391. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Singh, B.B. Effect of foliage supplementation to Heteropogon contortus based diets on nutrients digestibility, gas and metabolites production in sheep and goat inoculums. Anim. Nutr. Feed Technol. 2016, 16, 439–450. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.-C.; Jung, W.-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Macromolecules 2015, 77, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, J.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Optimization of extraction parameters of total phenolics from Annona crassiflora Mart. (araticum) fruits using response surface methodology. Food Anal. Methods 2017, 10, 100–110. [Google Scholar] [CrossRef]
- George, E.; Kasipandi, M.; Vekataramana, M.; Kumar, K.N.; Allen, J.A.; Parimelazhagan, T.; Gopalan, N. In vitro anti-oxidant and cytotoxic analysis of Pogostemon mollis Benth. Bangladesh J. Pharmacol. 2016, 11, 148–158. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.-Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Silva, E.K.; Pereira, E.K.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of high-intensity ultrasound process parameters on the phenolic compound recovery from araticum peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Brazilian Cerrado fruit araticum (Annona crassiflora Mart.) as a potential source of natural antioxidant compounds. Int. Food Res. J. 2018, 25, 2005–2012. Available online: http://www.ifrj.upm.edu.my/25(05)2018/(33).pdf (accessed on 9 January 2024).
- Arruda, H.S.; Fernandes, R.V.B.; Botrel, D.A.; Almeida, M.E.F. Frutos do Cerrado: Conhecimento e aceitação de Annona crassiflora Mart. (araticum) e Eugenia dysenterica Mart. (Caigata) por crianças utilizando o paladar e a visão. J. Health Biol. Sci. 2015, 3, 224–230. [Google Scholar] [CrossRef][Green Version]
- Chen, M.-X.; Huo, J.-M.; Hu, J.; Xu, Z.-P.; Zhang, X. Amaryllidaceae alkaloids from Crinum latifolium with cytotoxic, antimicrobial, antioxidant, and anti-inflammatory activities. Fitoterapia 2018, 130, 48–53. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Vieira, M.C.; Volobuff, C.R.F.; Silva, M.S.; Matos, A.I.; Cardoso, C.A.L.; Carvalho, J.E. In vitro biological screening of the anticholinesterase and antiproliferative activities of medicinal plants belonging to Annonaceae. Braz. J. Med. Biol. Res. 2015, 48, 308–315. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao-Hong, G.; Tang, J.; Liu, K. Antioxidant Activities of Hops (Humulus Lupulus) and Their Products. J. Am. Soc. Brew. Chem. 2018, 65, 116–121. [Google Scholar] [CrossRef]
- Zhang, I.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlet, J.; Shanmugam, K.; Munch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Fattahi, M.; Rahimi, R. Optimization of extraction parameters of phenolic antioxidants from leaves of Capparis spinosa using response surface methodology. Food Anal. Method 2016, 9, 2321–2334. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Masetto, T.E.; da Baldivia, D.S.; Vieira, M.C.; Zarate, N.A.H.; Pereira, Z.V. Potencial alelopático de cinco espécies da família Annonaceae. Braz. J. Bioscien. 2010, 8, 349–354. [Google Scholar]
- Scalise, A.; Bianchi, A.; Tartaglione, C.; Bolletta, E.; Pierangeli, M.; Torresetti, M.; Marazzi, M.; Di Benedetto, G. Microenvironment and Microbiology of Skin Wounds: The Role of Bacterial Biofilms and Related Factors. Semin. Vasc. Surg. 2015, 28, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural Wound Healing and Bioactive Natural Products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Bittner Fialová, S.; Rendeková, K.; Mucaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef] [PubMed]
- Amparo, T.R.; Seibert, J.B.; de Abreu Vieira, P.M.; Teixeira, F.F.M.; dos Santos, O.D.F.; de Souza, G.H.B. Herbal Medicines to the Treatment of Skin and Soft Tissue Infections: Advantages of the Multi-Targets Action. Phyther. Res. 2020, 34, 94–103. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, J.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple Biological Activities of Curcumin: A Short Review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.S.; Mamdouh, W.; Nasr, M.; ElShaer, A.; Polycarpou, E.; Abdel-Aziz, R.T.A.; Sammour, O.A. Quercetin Loaded CosmNutraceutical Electrospun Composite Nanofibers for Acne Alleviation: Preparation, Characterization and Experimental Clinical Appraisal. Int. J. Pharm. 2022, 612, 121309. [Google Scholar] [CrossRef] [PubMed]
- Dyja, R.; Jankowski, A. The Effect of Additives on Release and in Vitro Skin Retention of Flavonoids from Emulsion and Gel Semisolid Formulations. Int. J. Cosmet. Sci. 2017, 39, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Parveen, S.; Ghosh, P.; Ghatak, K.; Dasgupta, S. Flavonoid Loaded Nanoparticles as an Effective Measure to Combat Oxidative Stress in Ribonuclease A. Biochimie 2019, 162, 185–197. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial Activities of Green Synthesized Gums-Stabilized Nanoparticles Loaded with Flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Mala, L.; Lalouckova, K.; Skrivanova, E. Bacterial Skin Infections in Livestock and Plant-Based Alternativ es to Their Antibiotic Treatment. Animals 2021, 11, 2473. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. AsianAustralas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shah, A.M.A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine Mastitis: An Appraisal of Its Alternative Herbal Cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Tohma, H.; Gulcin, I.; Bursal, E.; Goren, A.; Alwasel, S.H.; Koksal, I. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. Food Meas. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Biproducts Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Ambika, P.P.S.; Chauhan, S.S.M. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Former. Nat. Prod. Lett. 2014, 28, 760–763. [Google Scholar] [CrossRef]
- Odeh, D.; Orsolic, N.; Berendika, M.; Dikic, D.; Drozdek, S.D.; Balbino, S.; Repajic, M.; Dragovic-Uzelac, V.; Jurcevic, I.L. Antioxidant and anti-atherogenic activities of essential oils from Myrtus communis L. and Laurus nobilis L. in rat. Nutrients 2022, 14, 1465. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Ferreira, S.; da Silva Medeiros, F.; Fujiwara, R.; de Souza Filho, J.; Pimenta, L. Nematicidal activity of Annona crassiflora leaf extract on Caenorhabditis elegans. Parasites Vectors 2015, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharm. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation Enhances the Biological Activity of Dietary Flavonoids by Altering Their Bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef]
- Hošek, J.; Závalová, V.; Šmejkal, K.; Bartoš, M. Effect of Diplacone on Lps-Induced Inflammatory Gene Expression in Macrophages. Folia Biol. 2010, 56, 124–130. [Google Scholar]
- Shin, H.J.; Shon, D.H.; Youn, H.S. Isobavachalcone Suppresses Expression of Inducible Nitric Oxide Synthase Induced by Toll-like Receptor Agonists. Int. Immunopharmacol. 2013, 15, 38–41. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Nkuete, A.H.L.; Ngameni, B.; Eloff, J.N. Anti-Inflammatory and Anticholinesterase Activity of Six Flavonoids Isolated from Polygonum and Dorstenia Species. Arch. Pharm. Res. 2017, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J. Licochalcone Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting NF-KB Activation. Inflammation 2016, 39, 569–574. [Google Scholar] [CrossRef]
- Jia, T.; Qiao, J.; Guan, D.; Chen, T. Anti-Inflammatory Effects of Licochalcone A on IL-1β-Stimulated Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- Cha, S.M.; Cha, J.D.; Jang, E.J.; Kim, G.U.; Lee, K.Y. Sophoraflavanone G Prevents Streptococcus mutans Surface Antigen I/IIInduced Production of NO and PGE2 by Inhibiting MAPK-Mediated Pathways in RAW 264.7 Macrophages. Arch. Oral Biol. 2016, 68, 97–104. [Google Scholar] [CrossRef]
- Wun, Z.Y.; Lin, C.F.; Huang, W.C.; Huang, Y.L.; Xu, P.Y.; Chang, W.T.; Wu, S.J.; Liou, C.J. Anti-Inflammatory Effect of Sophoraflavanone G Isolated from Sophora flavescens in Lipopolysaccharide-Stimulated Mouse Macrophages. Food Chem. Toxicol. 2013, 62, 253–361. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm Activity of Bioactive Hop Compounds Humulone, Lupulone and Xanthohumol toward Susceptible and Resistant Staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef]
- Hartkorn, A.; Hoffmann, F.; Ajamieh, H.; Vogel, S.; Heilmann, J.; Gerbes, A.L.; Vollmar, A.M.; Zahler, S. Antioxidant Effects of Xanthohumol and Functional Impact on Hepatic Ischemia-Reperfusion Injury. J. Nat. Prod. 2009, 72, 1741–1747. [Google Scholar] [CrossRef]
- Cho, Y.C.; Kim, H.J.; Kim, Y.J.; Lee, K.Y.; Choi, H.J.; Lee, I.S.; Kang, B.Y. Differential Anti-Inflammatory Pathway by Xanthohumol in IFN-γ and LPS-Activated Macrophages. Int. Immunopharmacol. 2008, 8, 567–573. [Google Scholar] [CrossRef]
- Lupinacci, E.; Meijerink, J.; Vincken, J.P.; Gabriele, B.; Gruppen, H.; Witkamp, R.F. Xanthohumol from Hop (Humulus lupulus L.) Is an Efficient Inhibitor of Monocyte Chemoattractant Protein-1 and Tumor Necrosis Factor-α Release in LPS-Stimulated RAW 264.7 Mouse Macrophages and U937 Human Monocytes. J. Agric. Food Chem. 2009, 57, 7274–7281. [Google Scholar] [CrossRef]
- Cho, Y.C.; You, S.K.; Kim, H.J.; Cho, C.W.; Lee, I.S.; Kang, B.Y. Xanthohumol Inhibits IL-12 Production and Reduces Chronic Allergic Contact Dermatitis. Int. Immunopharmacol. 2010, 10, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimarães, J.T.; Guardão, L.; Azevedo, I.; Soares, R. XanthohumolSupplemented Beer Modulates Angiogenesis and Inflammation in a Skin Wound Healing Model. Involvement of Local Adipocytes. J. Cell. Biochem. 2012, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic Flavonoids with Antimicrobial Activity: A Review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-Alanine Ligase as a New Target for the Flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular Dynamics Study on the Biophysical Interactions of Seven Green Tea Catechins with Lipid Bilayers of Cell Membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Kusuda, M.; Inada, K.; Ogawa, T.O.; Yoshida, T.; Shiota, S.; Tsuchiya, T.; Hatano, T. Polyphenolic Constituent Structures of Zanthoxylum piperitum Fruit and the Antibacterial Effects of Its Polymeric Procyanidin on Methicillin-Resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2006, 70, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Chan, H.T. Chemistry and Pharmacology of Artocarpin: An Isoprenyl Flavone from Artocarpus Species. Syst. Rev. Pharm. 2018, 9, 58–63. [Google Scholar] [CrossRef]
- Dej-Adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and Antimicrobial Activities from Thai Medicinal Plants. Arch. Pharm. Res. 2014, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Septama, A.W.; Panichayupakaranant, P. Synergistic Effect of Artocarpin on Antibacterial Activity of Some Antibiotics against Methicillin-Resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli. Pharm. Biol. 2016, 54, 686–691. [Google Scholar] [CrossRef]

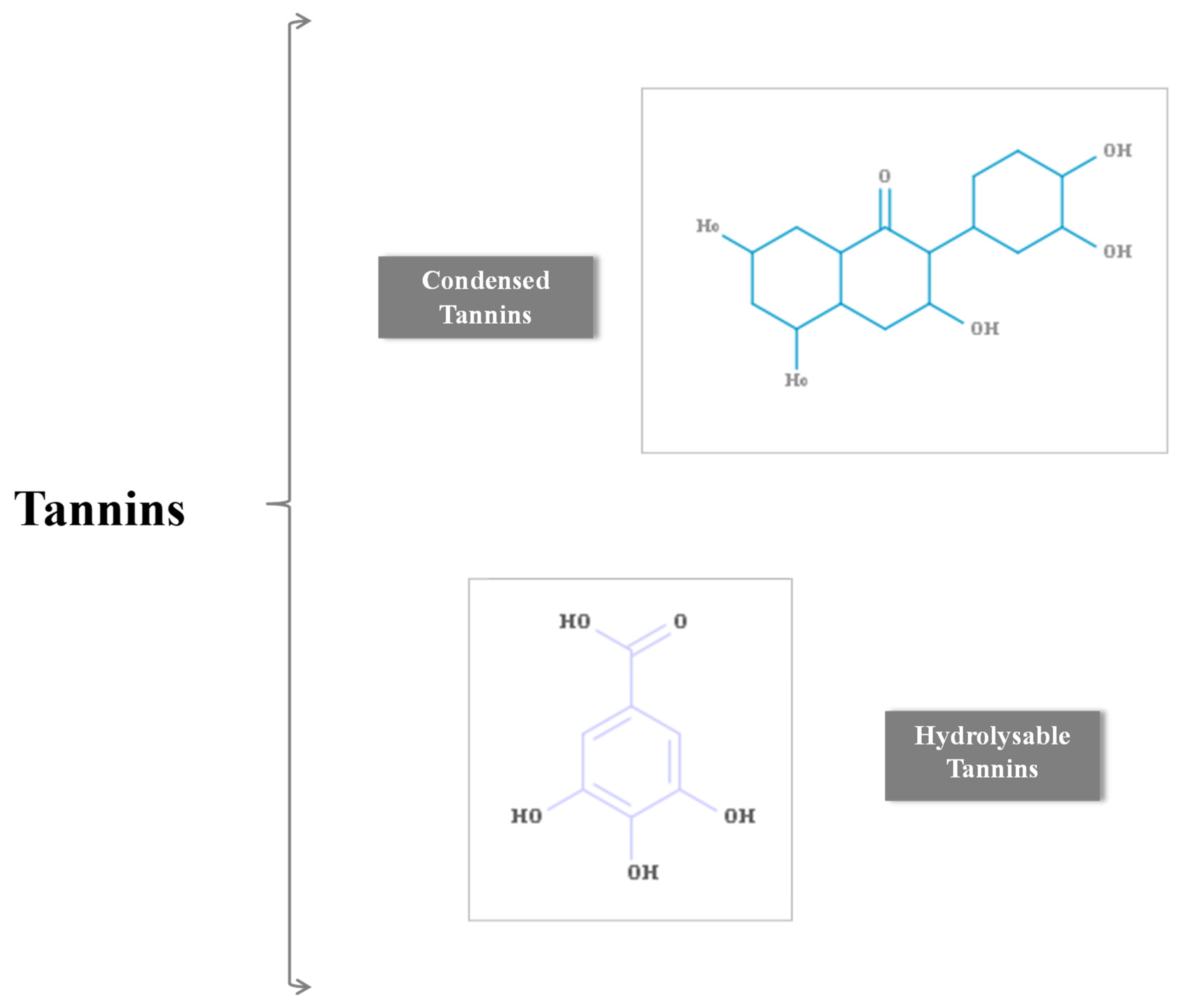
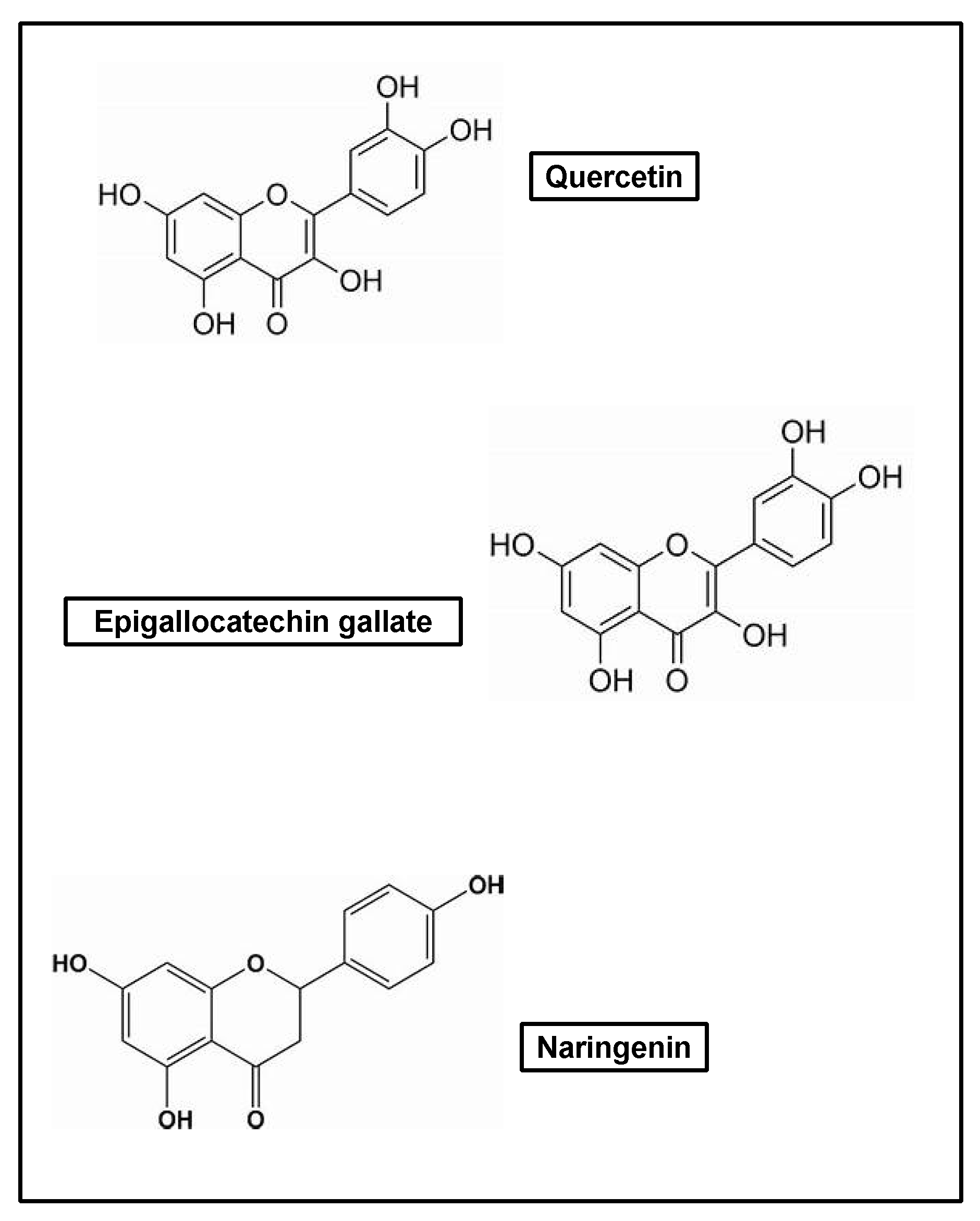
| Classification | Type |
|---|---|
| Cause | Pathological: resulting from a pathology (pressure ulcer, neoplasia). Surgical or traumatic: resulting from surgery or trauma. Iatrogenic: resulting from procedures or treatment with radiotherapy. |
| Evolution | Acute: wounds of easy resolution, rupture of vascularization, and mediate triggering of homeostasis (cuts, scoring, burns). Chronic: long-lasting wounds (deviation from the physiological cicatricial process). |
| Presence of infection | Clean: free of microorganisms. Clean-contaminated: lesions less than 6 h between trauma and initial care. Infected: presence of local infectious agent. Contaminated: wounds serviced more than 6 h after trauma. |
| Regarding tissue impairment | Stage I: skin integrates with signs of hyperemia, discoloration, or hardening. Stage II: the epidermis and dermis are ruptured, with subcutaneous tissue showing hyperemia, blisters, and a shallow crater. Stage III: total loss of cutaneous tissue, necrosis of the subcutaneous tissue to the muscular fascia. Stage IV: great tissue destruction with necrosis reaching muscles, tendons, and bones. |
| Degree of openness | Open: wounds in which the edges of the skin do not touch. Closed: wounds where the edges of the skin are juxtaposed. |
| Family | Genus | Part Used/Type Extraction | Compounds | Ref. |
|---|---|---|---|---|
| Acanthaceae | Justicia flava | Leaf/Methanol | Alkaloids, Flavonoids, Glycosides, Tannins | [41] |
| A. paniculata | Leaf/10% aqueous extract | Diterpenoids | [42] | |
| Amaranthaceae | Achyranthes aspera | Leaf/Ethanol | Flavonoids, Saponins, Tepernoids | [43] |
| A. sessilis | Stem and Leaf/Methanol | 2,4-dihydroxy-2,5-dimethyl-3(2H)-furan-3-one, hexadecanoic acid, 2-1,2,4-trioxolane,3-phenyl-, palmitate-ethyl-, L-glutamic acid. | [44] | |
| A.triandra | Air seed/Petroleum ether | Oil ricinoleic acid | [45] | |
| Celosia argentea | Root/Dichloromethane and ethyl acetate | Terpenoids | [46] | |
| Anacardiaceae | Buchanania lanzan S. | Root/Petroleum ether | Alkaloids, Flavonoids, Polyphenols, Steroids | [47] |
| Lannea welwitschii Hiern | Leaf/Methanol | Alkaloids, Flavonoids, Glycosides, Steroids, Tannins | [41] | |
| Apiaceae | Angelica sinensis | Leaf/Ethanol | n-buthylidenephthalide and proteins | [48] |
| Centella asiatica | Leaf/Methanol | Asiaticoside, Madecassic acid, Madecassoside asiatic acid, Triterpenes, | [49] | |
| Cuminum cyminum | Seeds | Essential oils | [50] | |
| L. striatum | Rhizoma | Essential oils | [51] | |
| Apocyanaceae | Catharanthus roseus | Leaf/Aqueous and Methanol | Alkaloids, Phenols, Proteins, Saponins, Tannins | [52] |
| S. hispidus | Leaf and Root | Alkaloids, Flavonoids, Saponins, Tannins, | [53] | |
| Wrightia tinctoria | Leaf/Aqueous | Alkaloids, Flavonoids, Phenolics, Saponins, Tannins | [54] | |
| Saba florida | Leaf/Methanol 99.9% | Total extract | [55] | |
| Araliaceae | Panax ginseng | Panax ginseng saponins (PGS) | Ginsenoside Rb1 (G-Rb1) | [56] |
| Panax notoginseng | Panax notoginseng saponins (PNS) | High-glucose (HG-30Mn) | [57] | |
| Asclepiadaceae | Calotropis giganthea | Root Bark | Taraxasteroryl isovalerate, Gigantin, Giganteol, Isogiganteol, α-amyrin-3-amyrin, Taraxasterol | [58] |
| Calotropis procera | Root bark/Ethanol | Alkaloids, Flavonoids, Steroids, Tannins | [59] | |
| Asphodelaceae | Aloe vera | Leaf/Acetonic extract | Polymers | [60] |
| Asteraceae | Achillea millefolium | Aerial parts | Yarrow Oil | [61] |
| Arctium lappa | Ground bark/Ethanol | Alkaloids, Flavonoids, Lignans, Phenolic acid, Tannins, Terpenoids | [62] | |
| Blumea balsamifera | Leaf/Methanol 95% | Flavonoids, Nonvalatile constituents | [63] | |
| Calendula officinalis | Flowers/Hydroethanol | Rutin, Quercetin-3-O-glucoside | [64,65] | |
| Carthamus tinctorius | Saflowers | Hydroxysaflow yellow A (HSYA) | [66] | |
| Wedelia trilobata | Leaves/Ethylacetate, Chloroform:Methanol | Kaura-9(11),16-dien-19-oic acid | [67] | |
| Bignoniaceae | Kigelia africana | Leaves/Roots/Methanol | Flavonoids, Carbohydrates, Sapogenetic glycosides, Saponins, Steroids | [68] |
| S. campanulata | Leaf/Methnol | Flavonoids, Phenols, Saponins, Steroids | [69] | |
| Tecoma capensis | Shoots/Hydroalcoholic | Myrecetin | [70] | |
| Boraginaceae | H. indicum | Leaf/Ethanol | Crude extract | [71] |
| L. erythrorhizon | Root | Purification of Shikonin | [72] | |
| Burseracea | Boswelia sacra | Leaf/Methanol | Oil | [73] |
| C. myrrha | Leaf/Methanol | Oleo-gum-resins | [73,74] | |
| Cactaceae | O. ficus-indica | Seed/Oil extraction | OFI-SNEDDSs | [75] |
| Caricaceae | Carica papaya | Papaya fruit extraction | Crude extract | [76] |
| Cecropiaceae | Cecropia peltata | Leaf | Saponins | [77] |
| Myrianthus arboreus | Leaves/Ethanol | Alkaloids, Flavonoids, Glycosides, Sterols, Tannins, Terpenoids | [78] | |
| Caprifoliacea | Locinera japonica | Flowers/Ethanol | Chlorogenic acid | [79] |
| Combretaceae | C. mucronatum | Leaf/Ethanol | Procyanidin B2 | [80] |
| Terminalia chebula | Fruit extraction | Anthraquinone, Flavonoids, Sapogenins, Saponins, Steroids, Tannins | [81] | |
| Terminalia arjuna | Fruit extraction/Methanol | Anthraquinones, Carbohydrates, Flavonol, Glucose sorbitol, Hydrolyzable Tannins | [82] | |
| Crassulaceae | Bryophylum pinnatum Lam | Leaf/Aqueous | Patulitin-O-deoxy-hexoside-O-hexoside, Quercetin-O-hexoside, Quercetin-O-deoxy-hexoside-O-pentoside | [83] |
| Cyperacea | Cyperus rotundus L | Aerial part/Methanol | Alkaloids, Phenols | [84] |
| Euphorbiacea | Alchornea cordifolia (Schum & Thonn) | Leaf/Ethanol | Quercetin, Hyperin, Guaijaverin | [78] |
| Euphoria hirta | Whole plant/Methanol | Alkaloids, Flavonoids, Glycosides, Proteins, Saponins, Tannins | [85] | |
| Jatropha curcas L. | Flowers/Methanol | Alkaloids, Flavonoids, Glycoside, Saponins, Tannins | [86] | |
| Mallotus oppositifolius (Geiseler) | Leaf/Ethanol | Aspinidiol B, methylene bis-aspidinol, α-tocoferol | [87] | |
| P. emblica L. | Leaves/Ethanol | Flavonoids, Saponins, Tannins | [88] | |
| P. muellerianus (Kuntze) | Leaf/Aqueous | Geranin | [89] | |
| Fabaceae | Astragalus membranaceus Sprants | Seeds/Ethanol | Tryptophan, Linoleic acid, Adenine | [90] |
| Caesalpinia sappan L. | Wood | Sappachalcone | [91] | |
| Entada phaseoloides | Total Tannins | [92] | ||
| Glycyrrhiza glabra L. | Root/Ethanol | Glycyrrhiza cream | [93] | |
| Indigofera enneaphylla L. | Whole plant/Petroleum ether, Ethyl Acetate, Ethanol | Flavonoids, Saponins, Tannins | [94] | |
| Mimosa pudica L. | Seeds/Ethyl Acetate Root/Petroleum ether | Alkaloids, Glycosides, Phytosterol | [95,96] | |
| Sophora flavescens | Compound, Sophora flavescen lotion | [97] | ||
| Tephrosia purpurea | Aerial plants/Ethanol | Flavonoids, TPF-A 7 peaks | [98] | |
| Fagaceae | Quercus infectoria Oliver | Nutgails/Ethanol | Pharmaceutical formulations | [99] |
| Ganodermataceae | Ganoderma lucidum | Fruting bodies/Hot water | Polysaccharides 25.1% Ganodermic acid A | [100] |
| Gentianaceae | Anthocleista nobilis G. Don | Stem bark/Ethanol Ethyl Acetate Buthanol n-Hexane | Isovitexin and Isovitexin-2”-O-xyl Isovitexin Apigenin monoglycoside p-Hydroxybenzoic acid, Sarasinside | [101] |
| Ginkgoaceae | Ginkgo biloba L. | Leaf/Aqueous | Myricerin, Quercetin, Kaempferol, Isorhamnitin, Terpenes lactones, Ginkgolic acid | [102] |
| Hypericaceae | Hypericu mysorense | Parts plant/Methanol | Flavonoids, Saponins, Tannins | [103] |
| Iridaceae | Crocus sativus L. | Stigmas/Glycerin/water/Ethanol | Flavonoids, Anthocyanins | [104] |
| Lamiaceae | Occimum sanctum L. | Leaf/Water | Essential Oil | [105] |
| Rosmarinus officinalis | Aerial parts/Hydrodistillation | Essential Oil | [106] | |
| Salvia miltiorrhiza | Leaf/Hydroethanolic | Flavonoids, Total Phenols | [107] | |
| Lauraceae | Cinnamomum cassia | Cinnamon Oils | [74] | |
| Liliaceae | Allium cepa L. | Onion/Ethanol 95% | Alkaloids, Flavonoids, Phenols, Tannins | [108] |
| Lycopodiaceae | Lycopodium serratum | Aerial parts/Ethanol | Crude etanol extract | [109] |
| Lythraceae | Lawsonia alba | Leaf/Methanol | Coumarin, Flavonoid, Steroid, Tannin, Terpenoid | [110] |
| Lawsonia inermis L. | Leaf/Aqueous | Total Phenols, Total Flavonoids, Total Tannins, Saponins | [111] | |
| Punica granatum L. | Fruit whole | Pomegranate are Tannins, Flavonoids, Punicic acid, Phytoestrogen | [112] | |
| Malvaceae | Hibiscus rosa sinensis L. | Flowers/Methanol | Phenolic compounds, Flavonoids, Essential Oils, Anthocyanins | [113] |
| Malva sylvestris | Flowers/Ethanol:Water (80:20) | Total phenolic, Flavonoids, Anthocyanin | [114] | |
| Thespesia populnea L. | Fruit/Aqueous | Glycosides, Flavonoids, Alkaloids, Phytosterol, Quercetin, Rutin, Lupeal | [115] | |
| Martyniaccae | Martynia annua | Leaf/Ethanol | Glycosides, Phenols, Flavonoids, Tannins, Anthocyanins MAF-C 7 peaks | [98] |
| Meliaceae | A. indica A. Juss | Steam bark/Water:Ethanol | Crude | [116] |
| Carapa guianensis Aubl | Andiroba seed oil | Lauric axid, Myristic, Palmitic acid, Stearic acid, Oleic acid, Linoleica cid, Lignoceric acid, Palmitoleic acid, Heptadecanoic acid, Arachidic acid, Behenic acid | [117] | |
| Mimosaceae | Prosopis cineraria | Leaves/Petroleum ether | Protocatechuic acid, Caffeic acid, Chlorogenic acid, Ferrulic acid | [118] |
| Moraceae | Ficus religiosa L. | Leaves/Methanol | Glycosides, Alkaloids, Tannins, Terpenoids | [119] |
| Moringaceae | Moringa oleífera Lam. | Leaves/Ethanol | Flavonoids, Phenolic acids | [120] |
| Musaceae | Musa sapientum L. | Fruits/Ethanol | Saponins, Flavonoids, Glycosides, Steroids, Alkaloids | [121] |
| Myrsinaceae | Embelia ribes Burn. | Fruits/Petroleum ether | Embelin | [122] |
| Myrtaceae | Eucalyptus globulus | Leaves/Hydrodistillation | 1,8-cineole content 72.3%, α-pinone 9.4% | [123] |
| Nymphaeaceae | Nelumbo nucifera | Aerial part/Ethanol | 30 peaks Ethanol,2-(-Octadecinyloxy, γ-sitosterol, Hexadecanoic acid | [124] |
| Oleaceae | Jasminum auriculatum Vahl. | Leaves/Petroleum ether | Alkaloids, Carbohydrates, Flavonoids, Phenolic compounds, Saponins, Steroids, Tannins, Tepernoids | [125] |
| Jasminum grandiflorum L. | Leaves/Methanol | Crude | [126] | |
| Orchidaceae | Bletilla striata | Root/Boiled water | Polysaccharide content (65.3%) | [127] |
| Paeoniaceae | Paeonia suffruticosa | Bark root/Alcohol | Flavonoids, Phenolic acid, Polysaccharide, Saponins | [128] |
| Papaveraceae | Argemone mexicana L. | Fruits/Methanol | Alkaloids, Flavonoids, Glycosides, Saponins, Steroids, Tannins, Terpenoids | [129] |
| Papilionaceae | Trigonella foenum-graecum | Aerial part/Methanol | Flavonoids | [130] |
| Pedaliaceae | Sesamum indicum | Seed/Ethanol | Sesame Oil | [131] |
| Plantaginaceae | Plantago | Leaves/Distilled water | Polyphenolic compounds | [132] |
| Polygonaceae | Rheum officinale | Powders/Ethanol | TMC extracts | [133] |
| Potulacaceae | Portulaca grandiflora | Total plant/Ethanol | Alkaloids, Flavonoids, Saponins, Terpenoids | [134] |
| Phyllanthaceae | Bridelia ferruginea Benth. | Leaves/Methanol Stem barks/Ethyl Acetate | High phenolic content High flavonoids content | [135] |
| Rosaceae | Sanguisorba officinalis | Polysaccharide | [136] | |
| Rubiaceae | Morinda citrifolia L. | Leaf | Alkaloids, Coumarins, Flavonoids, Saponins, Tannins, Triterpenes | [137] |
| Rubia cordifolia L. | 100 Compounds bicyclic peptides, terpenes, polysaccharides, Flavonoids, Quinones | [138] | ||
| Rutaceae | Aegle marmelos L. | Flower/Ethanol 60% | Aegelin, Cineol, Cuminaldheyde, Luvangetin, Eugenol | [139] |
| Zanthoxylum bungeanum Maxim | 140 constituents of this plant | Alkaloids, Fatty acids, Flavonoids, Tepernoids, Flavonoids | [140] | |
| Salicaceae | Casearia sylvestris | Leaves/Hydroalcoholic | Crude extract | [141] |
| Scrophulariaceae | Rehmannia glutinosa | Polysaccharides | [142] | |
| Stemonaceae | Stemona tuberosa | 9,10-dihydro-5-methoxy-8-methyl-2,7-phenanthrenediol | [143] | |
| Theaceae | Camellia sinensis | Tea leaves/Methanol | [144] | |
| Thymelaeaceae | Daphne genkwa Sie. | Diterpenoids/yuanhuapine | [145] | |
| Vitaceae | Ampelopsis japonica | Root/Methanol | Catechin, Gallic acid, Kaempferol, Euscaphic acid, Resveratrol, Epicatechin | [146] |
| Zingiberaceae | Curcuma longa Linn | Extracts | Alkaloids, Flavonoids, Phenolic, Saponins, Terpenoids, Steroids | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. https://doi.org/10.3390/ph17030303
Cedillo-Cortezano M, Martinez-Cuevas LR, López JAM, Barrera López IL, Escutia-Perez S, Petricevich VL. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals. 2024; 17(3):303. https://doi.org/10.3390/ph17030303
Chicago/Turabian StyleCedillo-Cortezano, Mayra, Luis Ruben Martinez-Cuevas, Jesús A. Márquez López, Ingrid L. Barrera López, Samantha Escutia-Perez, and Vera L. Petricevich. 2024. "Use of Medicinal Plants in the Process of Wound Healing: A Literature Review" Pharmaceuticals 17, no. 3: 303. https://doi.org/10.3390/ph17030303
APA StyleCedillo-Cortezano, M., Martinez-Cuevas, L. R., López, J. A. M., Barrera López, I. L., Escutia-Perez, S., & Petricevich, V. L. (2024). Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals, 17(3), 303. https://doi.org/10.3390/ph17030303







