New Pyrazolyl Thioureas Active against the Staphylococcus Genus
Abstract
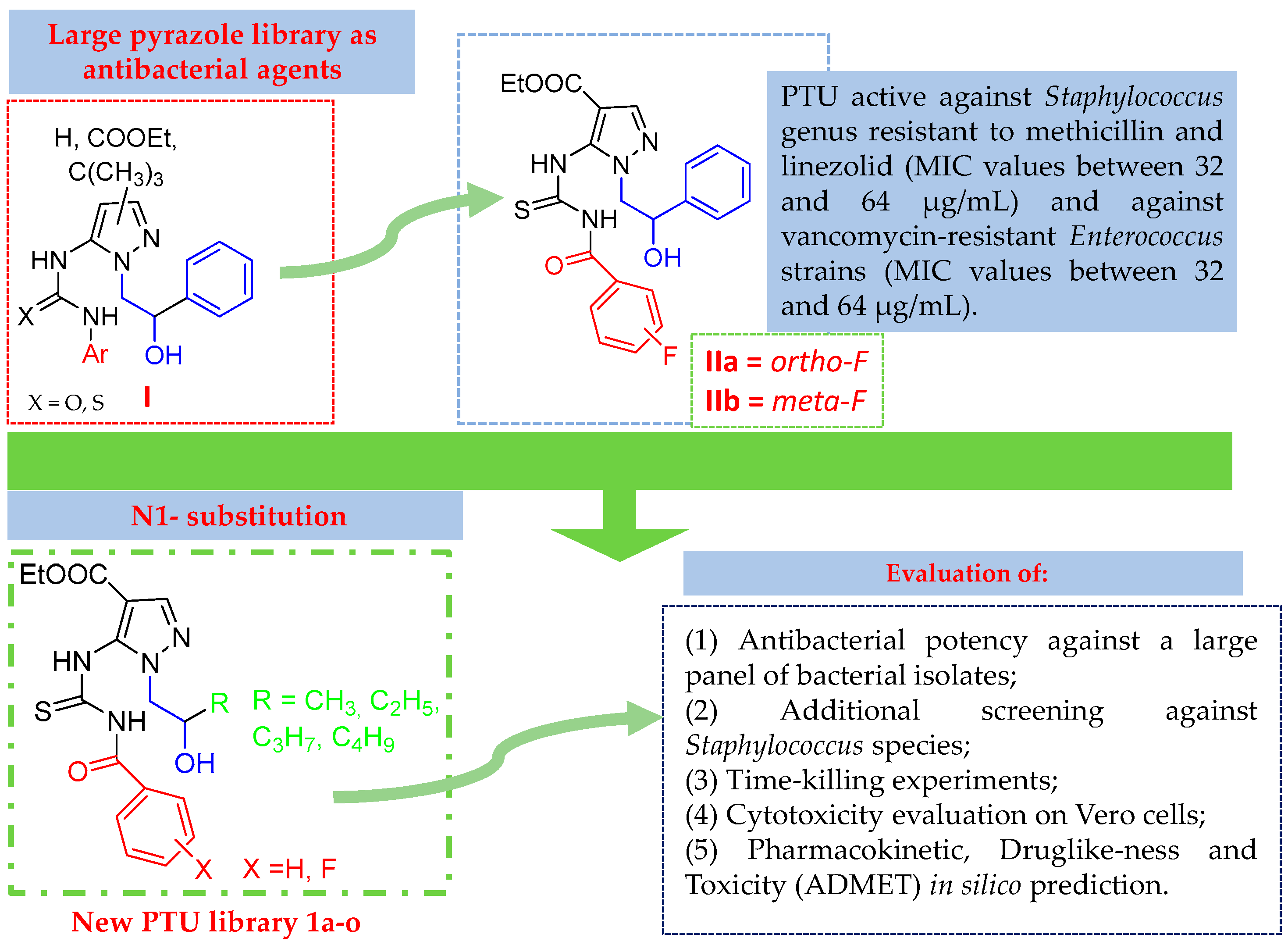
1. Introduction
2. Results and Discussion
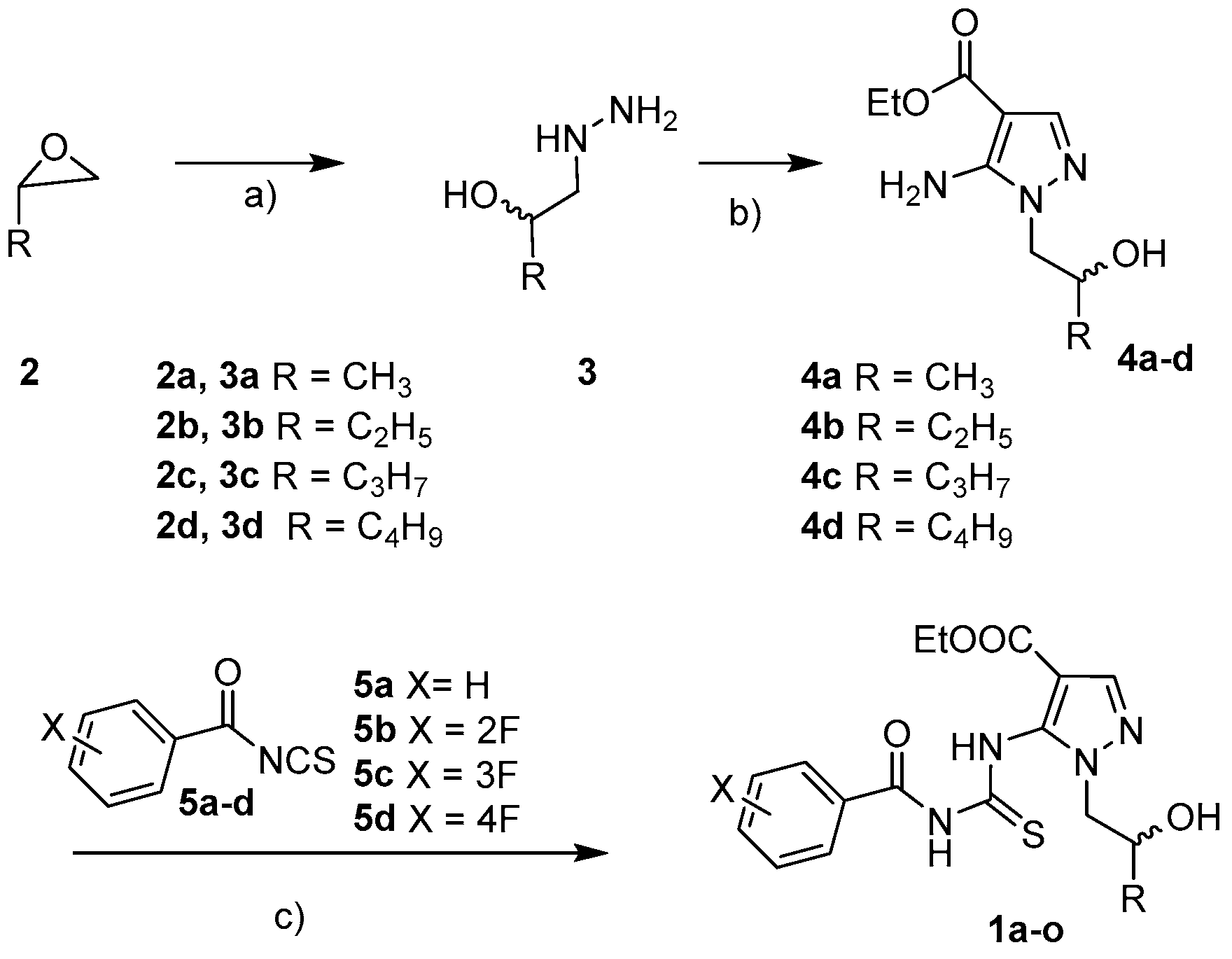
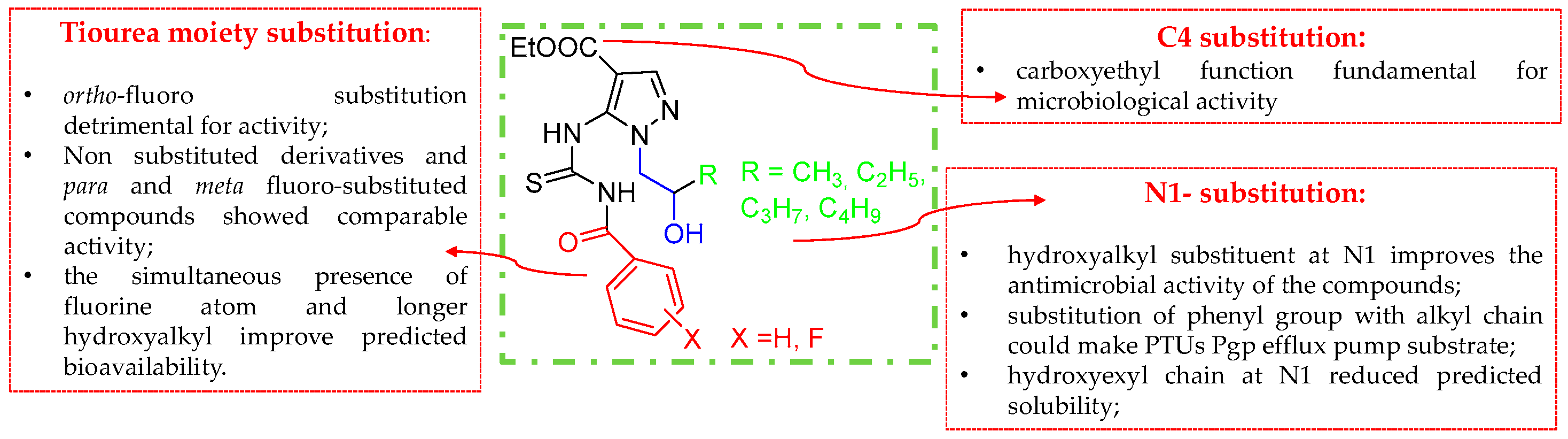
2.1. Chemistry
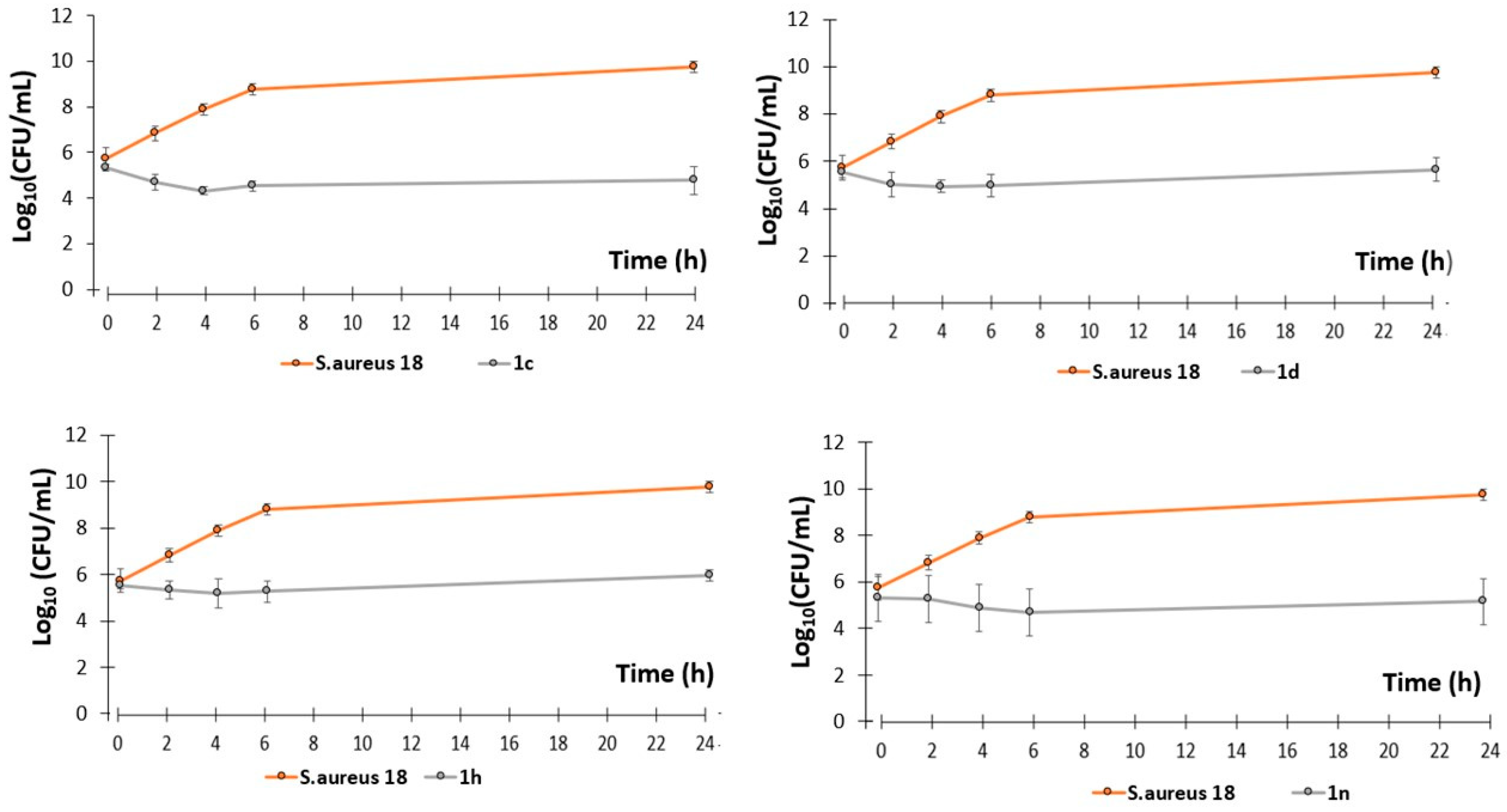
2.2. Antibacterial Activity
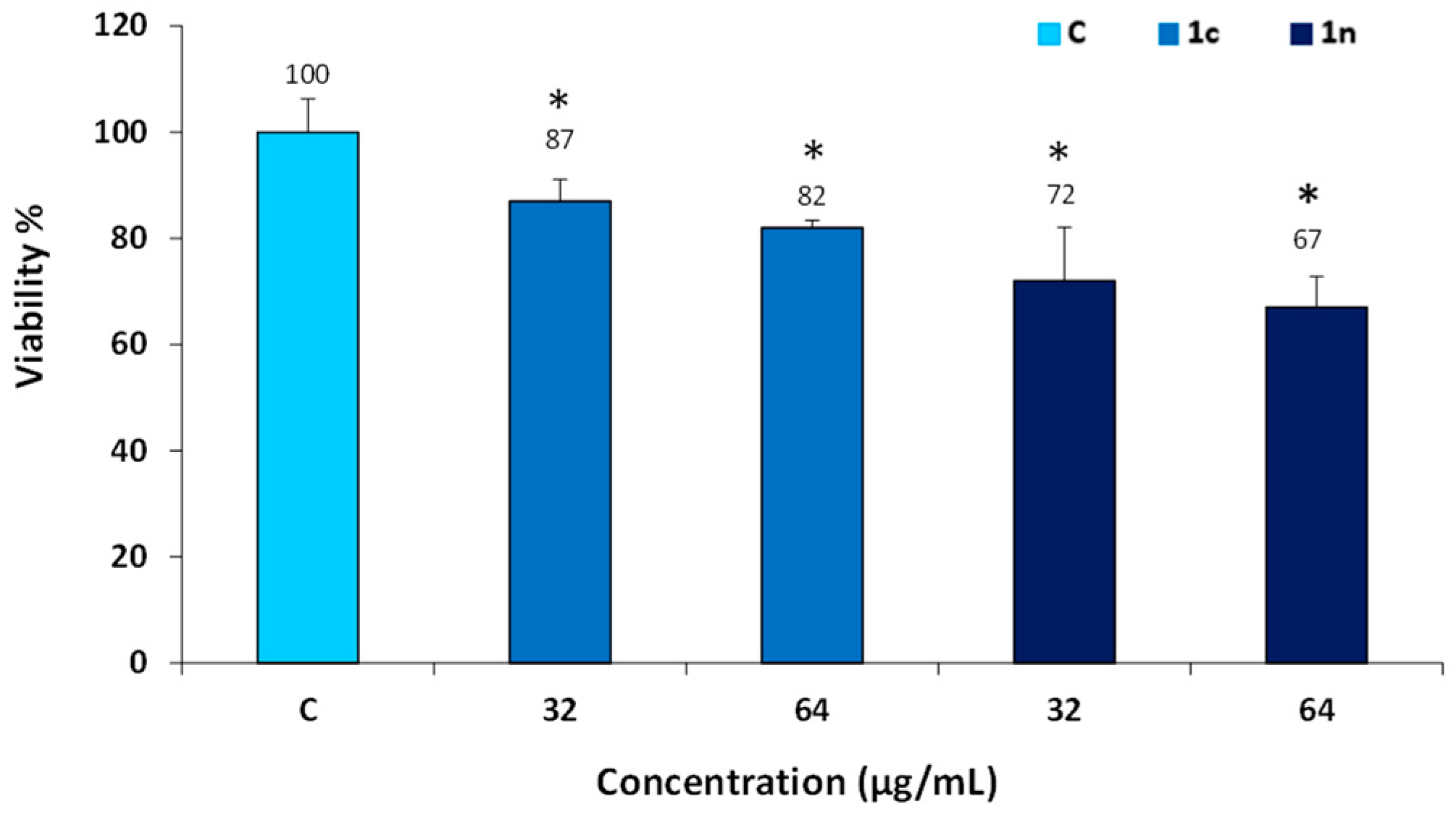
2.3. Cytotoxicity Evaluation
2.4. Pharmacokinetic Properties, Drug-Likeness and Toxicity (ADMET) Prediction
| 1a | 1b–d | 1e | 1f–h | 1i | 1j–l | 1m | 1n–o | IIa,b | |
|---|---|---|---|---|---|---|---|---|---|
| Physicochemical Property | |||||||||
| MW (g/mol) | 376.43 | 394.42 | 390.46 | 408.45 | 404.48 | 422.47 | 418.51 | 436.50 | 456.49 |
| Fraction Csp3 | 0.29 | 0.29 | 0.33 | 0.33 | 0.37 | 0.37 | 0.40 | 0.40 | 0.18 |
| Rotatable bonds | 10 | 10 | 11 | 11 | 12 | 12 | 13 | 13 | 11 |
| H-bond acceptors | 5 | 6 | 5 | 6 | 5 | 6 | 5 | 6 | 6 |
| H-bond donors | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TPSA a (Å2) | 137.57 | 137.57 | 137.57 | 137.57 | 137.57 | 137.57 | 137.57 | 137.57 | 137.57 |
| Lipophilicity | |||||||||
| LogP b | 2.06 | 2.16 | 2.59 | 2.69 | 2.95 | 3.05 | 3.49 | 3.59 | 3.26 |
| Water solubility | |||||||||
| Solubility (mg/mL) c | 0.282 | 0.203 | 0.133 | 0.095 | 0.080 | 0.057 | 0.037 | 0.026 | 0.018 |
| Solubility class d | S | S | S | S | S | S | MS | MS | MS |
| Pharmacokinetics | |||||||||
| GI absorption | high | high | high | high | high | low | high | low | low |
| BBB permeant | no | no | no | no | no | no | no | no | no |
| P-gp substrate | yes | yes | yes | yes | yes | yes | yes | yes | no |
| CYP1A2 inhibitor | no | no | no | no | no | no | no | no | no |
| CYP2C19 inhibitor | no | no | yes | yes | yes | yes | yes | yes | yes |
| CYP2C9 inhibitor | no | no | yes | yes | yes | yes | yes | yes | yes |
| CYP2D6 inhibitor | no | no | no | no | no | no | no | no | yes |
| CYP3A4 inhibitor | no | no | yes | yes | yes | yes | yes | yes | yes |
| Drug-likeness | |||||||||
| Lipinski violations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medicinal chemistry | |||||||||
| PAINS alerts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brenk alerts | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis of Ethyl 5-amino-1-(2-hydroxyhexyl)-1H-pyrazole-4-carboxylate 4d
3.1.3. General Synthesis of 5-Thioureido Pyrazoles 1a–o
3.2. Microbiological Evaluation
3.2.1. Bacterial Species Considered in This Study
3.2.2. Determination of the Minimal Inhibitory Concentrations (MICs)
3.2.3. Killing Curves
3.3. Maintenance of Cell Cultures
3.3.1. Cell Viability Index
MTT Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. YJBM 2017, 90, 269–281. [Google Scholar]
- Barros, E.M.; Martin, M.J.; Selleck, E.M.; Lebreton, F.; Sampaio, J.L.M.; Gilmore, M.S. Daptomycin Resistance and Tolerance Due to Loss of Function in Staphylococcus aureus dsp1 and asp23. Antimicrob. Agents Chemother. 2018, 63, e01542-18. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.A.; Shore, A.C.; O’Connell, B.; Brennan, G.I.; Coleman, D.C. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: High prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J. Antimicrob. Chemother. 2020, 75, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrook, E.C.; Checkley, W.; Merlo, C.A.; Konstan, M.W.; Lechtzin, N.; Boyle, M.P. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010, 303, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.L.; Morgan, W.J.; Konstan, M.W.; Schechter, M.S.; Wagener, J.S.; Fisher, K.A.; Regelmann, W.E. Presence of methicillin resistant Staphylococcus Aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr. Pulmonol. 2007, 42, 513–518. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Zhang, G.; Tadi, D.A. Prevalence of antibiotic resistance of Staphylococcus aureus in cystic fibrosis infection: A systematic review and meta-analysis. JGAR 2013, in press. [Google Scholar] [CrossRef]
- Akil, N.; Muhlebach, M.S. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr. Pulmonol. 2018, 53, S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Del Campo, R.; Máiz, L.; Morosini, M.I.; Lamas, A.; Baquero, F.; Cantón, R. High prevalence in cystic fibrosis patients of multiresistant hospital-acquired methicillin-resistant Staphylococcus aureus ST228-SCCmecI capable of biofilm formation. J. Antimicrob. Chemother. 2008, 62, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-Pyrazoles in Medicinal Chemistry: A Review. Int. J. Mol. Sci. 2023, 24, 7834. [Google Scholar] [CrossRef]
- Verma, R.; Verma, S.K.; Rakesh, K.P.; Girish, Y.R.; Ashrafizadeh, M.; Sharath Kumar, K.S.; Rangappa, K.S. Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation. Eur. J. Med. Chem. 2021, 212, 113134. [Google Scholar] [CrossRef]
- Abu-Hashem, A.; El-Shazly, M. Synthesis of quinoxaline, pyrimidine, and pyrazole furochromone derivatives as cytotoxic agents. Monatsh. Chem. 2017, 148, 1853–1863. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Aly, A.S. Synthesis of New Pyrazole, Triazole, Thiazolidine, -Pyrimido[4,5-b] quinoline derivatives with Potential Antitumor Activity. Arch. Pharm. Res. 2012, 35, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.; Gouda, M.A. Synthesis and Antimicrobial Activity of Some Novel Quinoline, Chromene, Pyrazole Derivatives Bearing Triazolopyrimidine Moiety. J. Heter. Chem. 2017, 54, 850–858. [Google Scholar] [CrossRef]
- Abu-Hashem, A. Synthesis, and biological activity of pyrimidines, quinolines, thiazines and pyrazoles bearing a common thieno moiety. Acta Pol. Pharm.-Drug Res. 2018, 75, 59–70. [Google Scholar]
- Abu-Hashem, A. Synthesis of new pyrazoles, oxadiazoles, triazoles, pyrrolotriazines and pyrrolotriazepines as potential cytotoxic agents. J. Heter. Chem. 2021, 58, 805–821. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; El Rhayam, Y.; Karrouchi, K.; Cherrah, Y.; Tarib, A.; Ansar, M.; Faouzi, M.E.A. Identification of the new progress on Pyrazole Derivatives Molecules as Antimicrobial and Antifungal Agents. West Afr. J. Med. 2022, 39, 1217–1244. [Google Scholar]
- Alam, M.A. Pyrazole: An emerging privileged scaffold in drug discovery. Future Med. Chem. 2023, 15, 2011–2023. [Google Scholar] [CrossRef]
- Ambade, S.S.; Gupta, V.K.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. A Review on Five and Six-Membered Heterocyclic Compounds Targeting the Penicillin-Binding Protein 2 (PBP2A) of Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 7008. [Google Scholar] [CrossRef]
- Brullo, C.; Caviglia, D.; Spallarossa, A.; Alfei, S.; Franzblau, S.G.; Tasso, B.; Schito, A.M. Microbiological Screening of 5-Functionalized Pyrazoles for the Future Development of Optimized Pyrazole-Based Delivery Systems. Pharmaceutics 2022, 14, 1770. [Google Scholar] [CrossRef]
- Bruno, O.; Brullo, C.; Bondavalli, F.; Schenone, S.; Spisani, S.; Falzarano, M.S.; Varani, K.; Barocelli, E.; Ballabeni, V.; Giorgio, C.; et al. 1-Methyl and 1-(2-hydroxyalkyl)-5-(3-alkyl/cycloalkyl/phenyl/naphthylureido)-1H-pyrazole-4-carboxylic acid ethyl esters as potent human neutrophil chemotaxis inhibitors. Bioorg. Med. Chem. 2009, 17, 3379–3387. [Google Scholar] [CrossRef]
- Lewis, R.T.; Bode, C.M.; Choquette, D.M.; Potashman, M.; Romero, K.; Stellwagen, J.C.; Teffera, Y.; Moore, E.; Whittington, D.A.; Chen, H.; et al. The discovery and optimization of a novel class of potent, selective, and orally bioavailable anaplastic lymphoma kinase (ALK) inhibitors with potential utility for the treatment of cancer. J. Med. Chem. 2012, 55, 6523–6540. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Model. 2004, 44, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.; Wehrle-Haller, B.; Sidibe, A.; Ponassi, M.; Iervasi, E.; Rosano, C.; Brullo, C.; Spallarossa, A. Novel 5-aminopyrazoles endowed with anti-angiogenetic properties: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2023, 260, 115727. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria (accessed on 23 June 2023).
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Bactericidal Activity of Non-Cytotoxic Cationic Nanoparticles against Clinically and Environmentally Relevant Pseudomonas spp. Isolates. Pharmaceutics 2021, 13, 1411. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.P.; Brownlee, K.G.; Denton, M.; Peckham, D.G. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. Am. J. Respir. Med. 2003, 2, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Biswas, L.; Götz, F. Molecular Mechanisms of Staphylococcus and Pseudomonas Interactions in Cystic Fibrosis. Front. Cell. Infect. Microbiol. 2022, 11, 824042. [Google Scholar] [CrossRef]
- Parkins, M.D.; Elborn, J.S. Newer antibacterial agents and their potential role in cystic fibrosis pulmonary exacerbation management. J. Antimicrob. Chemother. 2010, 65, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; D’Ursi, P.; Pedemonte, N.; Urbinati, C.; Ford, R.C.; Cichero, E.; Uggeri, M.; Orro, A.; Fossa, P. Recent Strategic Advances in CFTR Drug Discovery: An Overview. Int. J. Mol. Sci. 2020, 21, 2407. [Google Scholar] [CrossRef] [PubMed]
- Raslan, M.A.; Raslan, S.A.; Shehata, E.M.; Mahmoud, A.S.; Sabri, N.A. Advances in the Applications of Bioinformatics and Chemoinformatics. Pharmaceuticals 2023, 16, 1050. [Google Scholar] [CrossRef] [PubMed]
- Balbi, A.; Anzaldi, M.; Macciò, C.; Aiello, C.; Mazzei, M.; Gangemi, R.; Castagnola, P.; Miele, M.; Rosano, C.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem. 2011, 46, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Delpe-Acharige, A.; Zhang, M.; Eschliman, K.; Dalecki, A.; Covarrubias-Zambrano, O.; Minjarez-Almeida, A.; Shrestha, T.; Lewis, T.; Al-Ibrahim, F.; Leonard, S.; et al. Pyrazolyl Thioureas and Carbothioamides with an NNSN Motif against MSSA and MRSA. ACS Omega 2021, 6, 6088–6099. [Google Scholar] [CrossRef] [PubMed]
- Ommi, O.; Naiyaz Ahmad, M.; Gajula, S.N.R.; Wanjari, P.; Sau, S.; Agnivesh, P.K.; Sahoo, S.K.; Kalia, N.P.; Sonti, R.; Nanduri, S.; et al. Synthesis and pharmacological evaluation of 1,3-diaryl substituted pyrazole based (thio)urea derivatives as potent antimicrobial agents against multi-drug resistant Staphylococcus aureus and Mycobacterium tuberculosis. RSC Med. Chem. 2023, 14, 1296–1308. [Google Scholar] [CrossRef]
- Allen, L.; Allen, L.; Carr, S.B.; Davies, G.; Downey, D.; Egan, M.; Forton, J.T.; Gray, R.; Haworth, C.; Horsley, A.; et al. Future therapies for cystic fibrosis. Nat. Commun. 2023, 14, 693. [Google Scholar] [CrossRef]
- Esposito, C.; Kamper, M.; Trentacoste, J.; Galvin, S.; Pfister, H.; Wang, J. Advances in the Cystic Fibrosis Drug Development Pipeline. Life 2023, 13, 1835. [Google Scholar] [CrossRef]
- Singh, J.; Yeoh, E.; Fitzgerald, D.A.; Selvadurai, H. A systematic review on the use of bacteriophage in treating Staphylococcus aureus and Pseudomonas aeruginosa infections in cystic fibrosis. Paediatr. Respir. Rev. 2023, 48, 3–9. [Google Scholar] [CrossRef]
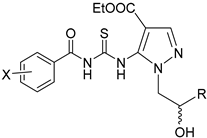 | ||||
|---|---|---|---|---|
| Compound | R | X | Melting Point (°C) | Yield (%) |
| 1a | CH3 | H | 106–108 °C | 64% |
| 1b | CH3 | o-F | Yellow oil | 45% |
| 1c | CH3 | m-F | Yellow oil | 35% |
| 1d | CH3 | p-F | 131–133 °C | 39% |
| 1e | C2H5 | H | Yellow oil | 55% |
| 1f | C2H5 | o-F | Yellow oil | 41% |
| 1g | C2H5 | m-F | Yellow oil | 40% |
| 1h | C2H5 | p-F | 120–122 °C | 50% |
| 1i | C3H7 | H | 126–128 °C | 55% |
| 1j | C3H7 | o-F | Yellow oil | 36% |
| 1k | C3H7 | m-F | Yellow oil | 51% |
| 1l | C3H7 | p-F | 134–136 °C | 61% |
| 1m | C4H9 | H | 108–110 °C | 43% |
| 1n | C4H9 | m-F | Yellow oil | 47% |
| 1o | C4H9 | p-F | 114–116 °C | 94% |
| MIC (µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | 1a | 1b | 1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | 1k | 1l | 1m | 1n | 1o | oxa |
| Gram-positive | ||||||||||||||||
| S. aureus 17 * | 128 | >128 | 64 | 128 | 128 | >128 | 64 | 128 | 64 | >128 | 64 | >128 | 64 | 64 | >128 | 256 |
| S. aureus 18 * | 128 | >128 | 64 | 64 | 64 | >128 | 128 | 128 | 64 | >128 | 64 | >128 | 64 | 64 | >128 | 512 |
| S. aureus 187 * | 64 | >128 | 32 | 32 | 64 | >128 | 64 | 64 | 64 | >128 | 32 | >128 | 32 | 32 | >128 | 512 |
| S. aureus 195 * | 64 | >128 | 64 | 64 | 64 | >128 | 64 | 64 | 64 | >128 | 64 | >128 | 32 | 64 | >128 | 256 |
| S. epidermidis 22 * | 32 | >128 | 64 | 32 | 32 | >128 | >128 | 64 | 32 | >128 | 64 | >128 | 32 | 64 | >128 | 256 |
| S. epidermidis 171 * | 64 | >128 | 128 | 32 | 64 | >128 | 128 | 32 | 64 | >128 | 64 | >128 | 64 | 64 | >128 | 128 |
| S. epidermidis 181 ** | 64 | >128 | 64 | 32 | 64 | >128 | 128 | 32 | 64 | >128 | 128 | >128 | 64 | 128 | >128 | 256 |
| E. faecalis 365 # | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| E. faecalis 28 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| E. faecium 152 # | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| E. faecium 158 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| Gram-negative | ||||||||||||||||
| E. coli 462 (NDM, CR) | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| E. coli 475 (CR) | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| P. aeruginosa 1V MDR | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| P. aeruginosa 6G MDR | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | - |
| MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Strains | 1a | 1d | 1e | 1h | 1i | 1m | oxa |
| S. saprophyticus 41 | >128 | >128 | >128 | >128 | >128 | >128 | 0.5 |
| S. capitis 71 * | 128 | 64 | 128 | 128 | 128 | 128 | 64 |
| S. capitis 121 | >128 | 128 | >128 | 128 | >128 | >128 | 0.25 |
| S. warneri 74 * | >128 | >128 | >128 | >128 | >128 | >128 | 64 |
| S. simulans 94 * | >128 | >128 | >128 | >128 | >128 | >128 | 16 |
| S. simulans 163 * | >128 | >128 | >128 | >128 | >128 | >128 | 16 |
| S. lugdunensis 96 | 32 | 32 | 32 | 32 | 32 | 32 | 1 |
| S. lugdunensis 137 * | 32 | 32 | 16 | 32 | 16 | 32 | 16 |
| S. haemoliticus 115 * | 128 | 64 | 64 | 64 | 64 | 128 | 64 |
| S. haemoliticus 193 * | >128 | 128 | 128 | 128 | >128 | >128 | 16 |
| S. haemoliticus 174 | >128 | >128 | 128 | >128 | >128 | >128 | 0.25 |
| S. hominis 124 * | 128 | 128 | 128 | 64 | 128 | >128 | 16 |
| S. hominis 125 * | 128 | 128 | 128 | 128 | 128 | 128 | 24 |
| S. auricularis 136 * | 32 | 32 | 16 | 32 | 16 | 16 | 16 |
| Cpd | LD50 (mg/kg) | Reliability (R.I.) |
|---|---|---|
| 1a | 2600 | 0.45 |
| 1b–d | 1600 | 0.39 |
| 1e | 2800 | 0.45 |
| 1f–h | 1700 | 0.39 |
| 1i | 3000 | 0.45 |
| 1j–l | 1900 | 0.40 |
| 1m | 3300 | 0.45 |
| 1n–o | 2000 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schito, A.M.; Caviglia, D.; Penco, S.; Spallarossa, A.; Cichero, E.; Tasso, B.; Brullo, C. New Pyrazolyl Thioureas Active against the Staphylococcus Genus. Pharmaceuticals 2024, 17, 376. https://doi.org/10.3390/ph17030376
Schito AM, Caviglia D, Penco S, Spallarossa A, Cichero E, Tasso B, Brullo C. New Pyrazolyl Thioureas Active against the Staphylococcus Genus. Pharmaceuticals. 2024; 17(3):376. https://doi.org/10.3390/ph17030376
Chicago/Turabian StyleSchito, Anna Maria, Debora Caviglia, Susanna Penco, Andrea Spallarossa, Elena Cichero, Bruno Tasso, and Chiara Brullo. 2024. "New Pyrazolyl Thioureas Active against the Staphylococcus Genus" Pharmaceuticals 17, no. 3: 376. https://doi.org/10.3390/ph17030376
APA StyleSchito, A. M., Caviglia, D., Penco, S., Spallarossa, A., Cichero, E., Tasso, B., & Brullo, C. (2024). New Pyrazolyl Thioureas Active against the Staphylococcus Genus. Pharmaceuticals, 17(3), 376. https://doi.org/10.3390/ph17030376









