Abstract
Ketamine is a potential rapid-onset antidepressant characterized by sympathomimetic effects. However, the question of ketamine’s use in treating adolescents’ major depressive disorder (MDD) is still discussed. Thus, we aimed to study the acute effect of ketamine infusion treatment on sympathetic regulation using electrodermal activity (EDA) in addition to an assessment of depressive symptomatology in MDD adolescents. Twenty hospitalized adolescent girls with MDD (average age: 15.0 ± 1.46 yrs.) were examined before and two hours after a single intravenous infusion of ketamine. EDA was continuously recorded for 6 min, and depressive symptoms were assessed before and two hours after ketamine administration. The evaluated parameters included skin conductance level (SCL), nonspecific electrodermal responses (NS-SCRs), MADRS (questions no. 1–10, total score), and CDI (items A–E, total score). EDA parameters showed no significant changes after the ketamine treatment, and depressive symptoms were significantly reduced after the ketamine infusion. The analysis revealed a significant negative correlation between index SCL and CDI-A, CDI-E, and the total CDI score and between index NS-SCRs and MADRS no. 4 before the ketamine treatment. In conclusion, ketamine improved depressive symptomatology without a significant effect on EDA, indicating its potential safety and efficiency as an acute antidepressant intervention in adolescent MDD.
1. Introduction
Depression is a serious mental disorder in adolescence characterized by a 19% prevalence [1]. Notably, depression is associated with a high risk of suicide, which represents one of the leading causes of death in people aged 15 to 24 [2]. Moreover, the quality of life associated with depressive disorder, particularly during adolescence, is significantly altered, including a reduced ability to manage normal daily activities and decreased performance in the educational and social fields [3]. Thus, the safe and effective treatment of depressive disorder in adolescence is very important.
The first-choice antidepressant treatment for adolescents is therapy via selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine following the current NICE treatment recommendations for an adequate SSRI treatment trial lasting at least 8 weeks at or above the minimally recommended dose [4]. Similarly, Dwyer et al. defined an adequate trial of SSRI treatment for adolescent depression as at least 6–8 weeks of treatment on the minimally recommended FDA dose with appropriate titration up to the maximally recommended tolerated dose [5]. Moreover, an adequate MDD treatment response to first-line interventions is defined by at least a 50% reduction in symptoms [6,7]. However, it has been reported that at least 40% of depressive adolescents do not adequately respond to SSRI treatment [5,6]. Moreover, adolescents not responding to first-line MDD treatments have higher suicide rates, greater impairments to their academic and social skills, and more conflicts with family and peers [8]. Importantly, Dwyer et al. (2020) defined adolescent treatment-resistant depression (TRD) as adolescent MDD in which significant depressive symptoms are exhibited despite the patient receiving an adequate trial of a first-line antidepressant agent (i.e., an SSRI such as fluoxetine, escitalopram, or sertraline) and psychotherapy [5]. However, pharmacotherapy interventions for adolescent TRD are still being extensively discussed. In this context, the discovery of the rapid antidepressant effects of intravenous ketamine [9] brought about an effective treatment for adolescent MDD, particularly in TRD patients. Ketamine is a chiral molecule with two enantiomers, R-ketamine and S-ketamine. The mixture of both enantiomers in equal parts is called racemic ketamine, and this represents the most commonly used form of ketamine in clinical settings. The isolation of enantiomeric S-ketamine (esketamine), which has a four-fold higher affinity to N-methyl-D-aspartate (NMDA) receptors, and its availability as an intranasal spray approved by the FDA and EMA for use in adult TRD, could present a more practical option compared to intravenous racemic ketamine [10,11,12]. However, despite its potential benefit, the side effects of esketamine, such as dissociative symptoms, are of comparable severity to intravenous racemic ketamine [13]. Moreover, a recent systematic review and meta-analysis concluded that intravenous ketamine had better efficiency than intranasal esketamine for MDD treatment [14]. While numerous studies have confirmed the fast-acting antidepressant, anti-suicidal, and anti-hedonic effects of ketamine in adults [15,16,17,18], the safety and efficacy of ketamine administration for adolescent depression are still unclear and understudied [19].
From a pharmacokinetic point of view, ketamine metabolism is primarily linked to enzymatic processes in the liver. Specifically, the enzyme responsible for the formation of the main metabolite, norketamine (a pharmacologically active metabolite with analgesic effects), is cytochrome CYP3A4 [20,21]. In the following metabolic processes, ketamine and norketamine undergo hydroxylation, while their hydroxylated derivatives are conjugated with glucuronic acid in the liver microsomal glucuronosyltransferase system. Glucuronidation increases their solubility, facilitating their excretion in bile and urine [22,23,24]. With regard to adolescence, differences in ketamine pharmacokinetics are assumed to occur primarily at the enzymatic level of the metabolism, which is clinically manifested as the need for a higher dose of ketamine per kilogram to produce an anesthetic effect compared to adults. This necessity of a higher dose of ketamine in adolescence is also caused by developmental differences in cerebral circulation and metabolism, the insufficient myelination of neurons, and age-linked changes in the cardiovascular system, such as a higher cardiac output [25,26]. The elimination half-life of ketamine is relatively short (approximately 3 h), but several studies have pointed to slower elimination times after repeated ketamine administration [27,28]. In adolescence, ketamine elimination is faster compared to in adulthood [29]. Moreover, ketamine clearance is around 79 l/h/70 kg in adults [30], while in the pediatric population, it can reach values of up to 90 l/h/70 kg [31].
In addition, the pharmacodynamics of ketamine are complex, including action on various receptors—NMDA, serotonergic, opioid, dopaminergic, sigma, γ-aminobutyric acid type A (GABA-A), nicotinic, and muscarinic acetylcholine receptors—as well as on sodium, potassium, hyperpolarization-activated cyclic nucleotide (HCN) channels, L-type calcium channels, and monoamine transporters [32]. However, the antidepressant effect of ketamine is currently primarily attributed to its action on the postsynaptic glutamate NMDA receptors of GABA inhibitory interneurons in the form of non-competitive antagonism [33]. The subsequent disinhibition of glutamatergic neurons is manifested by a sudden release of glutamate from presynaptic neurons into the synaptic cleft in the medial prefrontal cortex and hippocampus. Next, the released glutamate molecules bind to α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)’s postsynaptic receptors, with their activation leading to the disinhibition of the translation of synaptic proteins and brain-derived neurotrophic factor (BDNF). In general, this cascade of reactions leads to an increase in the number of synaptic signaling proteins and novel functional dendritic synapses, allowing massive synaptogenesis to occur in the prefrontal cortex [34]. Therefore, it is assumed that ketamine can restore the physiological connectivity between the prefrontal cortex and the limbic system, which plays a key role in the emotional regulation/dysregulation associated with depressive disorder [35].
Furthermore, a recent meta-analysis provided evidence for an association between autonomic nervous system dysfunction and mental disorders characterized by emotional dysregulation in youths [36]. Specifically, sympathetic neural mechanisms play a key role in human cardiovascular health and disease, including hypertension and heart failure [37]. In this respect, electrodermal activity (EDA) represents a promising, noninvasive tool for measuring sympathetic regulation, which is closely related to emotional and cognitive states [38]. In other words, EDA reflects changes in the electrical properties of the skin related to the sudomotor activity of the eccrine sweat glands that are regulated via the cholinergic sympathetic nervous system [39,40,41]. The basic principle of EDA evaluation is the measurement of water and electrolyte secretion between the two electrodes placed on the surface of the skin on the phalanges, palms, or feet. As the glands on the palms or soles are also activated in response to emotional stress, EDA can reflect an individual´s level of emotional arousal [42]. With respect to adolescent depression, recent studies have revealed sympathetic hypoactivity, indexed by a reduced EDA, which is associated with a higher risk of potential cardiovascular complications [43,44]. However, studies concerning sympathetic regulation/dysregulation and the acute effect of ketamine treatment are rare. Although previous studies have shown that ketamine administration may be associated with increases in blood pressure and heart rate, indicating a sympathomimetic effect [45,46], there are no studies on the acute effect of ketamine on sympathetic regulation indexed by EDA in adolescent depression.
This study is focused on two goals. The first aim is to study the acute effect of ketamine treatment on sympathetic regulation using EDA in major depressive disorder in adolescence. The second goal is to assess the acute effect of ketamine treatment on depressive symptomatology in severe episodes of adolescent MDD. To the best of our knowledge, this is the first study to analyze the acute effect of ketamine on sympathetic neural activity and depressive symptomatology in major depression and severe episodes in adolescents.
2. Results
2.1. Characteristics of the Studied Group
The basic descriptive characteristics of the evaluated MDD group are presented in Table 1.

Table 1.
Characteristics of the studied group.
2.2. The EDA and Hemodynamic Parameters
No significant changes were found in the SCL and NS_SCRs parameters before and after treatment (p = 0.820 and p = 0.823, respectively). Similarly, the mean values of the hemodynamic parameters—HR, SBP, and DBP—did not show significant changes before and after treatment (p = 0.106, p = 0.544, and p = 0.776, respectively). All results are summarized in Table 2.

Table 2.
Summary descriptive statistics for all evaluated parameters.
2.3. Depressive Symptomatology
The MADRS no. 1, MADRS no. 2, MADRS no. 3, MADRS no. 6, MADRS no. 7, MADRS no. 8, MADRS no. 9, MADRS no. 10, and the MADRS total score indices were significantly higher before treatment compared to after the treatment period (p < 0.001, effect size = 1.532; p < 0.001, effect size = 1.394; p < 0.001, effect size = 1.268; p = 0.003, effect size = 0.954; p < 0.001, effect size = 1.448; p = 0.006, effect size = 0.794; p = 0.001, effect size = 0.818; p < 0.001, effect size = 1.701; and p < 0.001, effect size = 1.574, respectively). No significant changes were found in the remaining parameters. The CDI A, CDI E, and CDI total score indices were significantly higher before treatment compared to after the treatment period (p = 0.012, effect size = 0.389; p = 0.002, effect size = 0.572; and p = 0.018, effect size = 0.361, respectively). No significant changes were observed in the remaining parameters. All results are summarized in Table 2.
2.4. Correlation Analysis between EDA and Depressive Symptoms
Before ketamine treatment, correlation analysis revealed significant negative correlations between index SCL and CDI total score, CDI A, and CDI E (r = −0.546, p = 0.013; r = −0.527, p = 0.017; and r= −0.632, p = 0.003, respectively) and a significant negative correlation between index NS_SCRs and MADRS no. 4 (r = −0.588, p = 0.006) (Figure 1). No significant correlations were found between the EDA parameters and the remaining depression parameters.
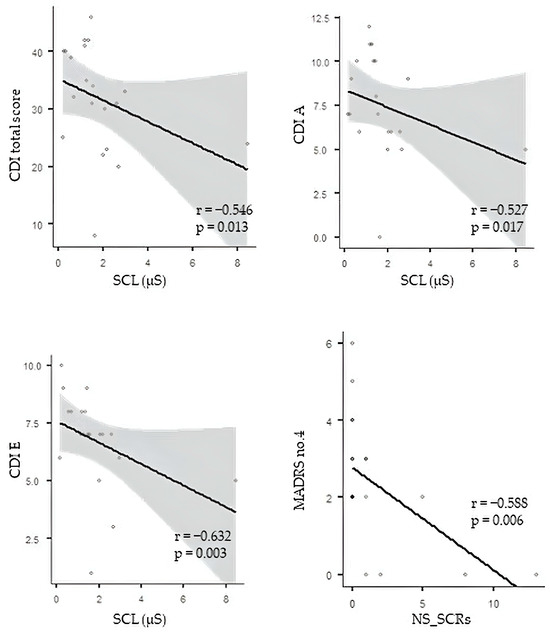
Figure 1.
Correlation analysis between EDA and depression measures. CDI—Children´s Depression Inventory; SCL—skin conductance level; MADRS—Montgomery–Asberg Depression Rating Scale; NS_SCRs—nonspecific electrodermal responses. The circles represent individual MDD patients.
In contrast, after the ketamine treatment, the correlation analysis revealed no significant correlations between the evaluated EDA indices (SCL and NS_SCR) or the CDI and MADRS total scores and individual items.
2.5. Correlation Analysis between Hemodynamic Measures and Depressive Symptoms
Correlation analysis revealed no significant correlations between the evaluated hemodynamic measures (HR, SBP, and DBP) or the CDI and MADRS total score and individual items.
3. Discussion
This study explored, for the first time, the efficacy and safety of acute intravenous ketamine administration by assessing depressive symptomatology in concert with an evaluation of ketamine’s acute effect on sympathetic regulation using EDA in the little-studied field of adolescent depression. Our results revealed significantly decreased depressive symptoms as evaluated using CDI and MADRS, indicating the rapid efficacy of ketamine administration; this decrease in symptoms was associated with non-significant changes in EDA parameters after ketamine treatment. Moreover, significant negative correlations between EDA parameters and several depressive symptoms before treatment indicate that the severity of depression is negatively associated with sympathetic hypoactivity before the ketamine treatment but not two hours after of the ketamine administration. Several mechanisms explaining this finding are suggested.
Our study revealed an improvement in depressive symptomatology through a significant reduction in the MADRS and CDI total scores two hours after the intravenous administration of ketamine. These findings are in accordance with the few current studies dealing with the antidepressant effect of ketamine on adolescent depression [2,46,47,48]. The complex effect of ketamine on the central nervous system (CNS) is determined via a relatively large number of affected receptors [17,49,50,51,52,53,54]. More specifically, ketamine acts as a noncompetitive antagonist on postsynaptic glutamate NMDA receptors located on GABA inhibitory interneurons, leading to the disinhibition of glutamatergic neurons. Subsequently, a sudden release of glutamate from presynaptic neurons into the synaptic cleft in the medial prefrontal cortex and several subcortical structures related to emotional regulation is manifested [33]. The release of glutamate via these receptors and intracellular signaling pathways ultimately leads to an increase in synaptic signaling proteins and dendritic synapses [34]. In other words, the effect of ketamine may lead to synaptogenesis in the prefrontal cortex and to the restoration of the physiological connection between the prefrontal cortex and the limbic structures of the brain, which are key structures in emotional regulation [34,35]. In contrast, a recent study revealed that ketamine administration led to a decrease in the global brain connectivity of the prefrontal region [55]. However, several studies have also pointed to ketamine’s other antidepressant effects on the serotonergic [56,57], opioid [58], nicotinic [59,60], and sigma receptors [61,62,63], as well as HCN [64,65,66] and potassium channels [67]. Thus, ketamine’s antidepressant effect appears to be the result of complex mechanisms that have previously been extensively discussed [17]. Moreover, a significant improvement was achieved in the items focused on negative mood (CDI A) and negative self-esteem (CDI E) in this study. In the same way, the MADRS evaluation pointed to an improvement in apparent and reported sadness (no. 1 and no. 2), inner tension (no. 3), concentration difficulties (no. 6), lassitude (no. 7), the inability to feel (no. 8), pessimistic thoughts (no. 9), and suicidal thoughts (no. 10). It seems that ketamine administration is characterized by prompt effectiveness predominantly for affective and anhedonic symptoms (sadness, a loss of gratification, and a loss of feelings and affection toward others), and cognitive symptoms (a diminished ability to think and concentrate) without a significant influence on somatic symptoms (related to sleep, fatigue, and appetite). It is particularly important to note that ketamine administration led to a significant reduction in suicidal thoughts, thus significantly reducing the risk of suicide in depressive adolescents. For this reason, ketamine may be a great choice for acute antidepressant treatment for severe depressive disorder at adolescent age with a high suicide risk due to its rapid anti-suicidal effect. In addition, suicidality is correlated with the presence of anhedonia, defined as the inability to experience pleasure [68]. While several studies have reported the anti-anhedonic effect of ketamine in adults, there has been no evidence of its effect on anhedonia in adolescents [69]. Our study revealed a significant reduction in affective and anhedonic symptoms after an intravenous ketamine treatment in adolescent depressive patients. It is believed that this anti-anhedonic effect is due to the downstream regulation of dopaminergic activity via the glutamatergic system [70].
Furthermore, it is well known that depression linked to impaired sympathetic neural regulation represents a risk factor for cardiovascular complications [37,71,72]. Previous studies revealed that ketamine administration is predominantly associated with a sympathomimetic effect on the cardiovascular system, resulting in an increased heart rate or blood pressure [73,74,75,76,77]. Although it has been suggested that ketamine activates the sympathetic nervous system through direct action on the CNS [78,79], the mechanism behind this has not yet been fully elucidated. It is assumed that ketamine inhibits the formation of centrally synthesized nitric oxide through the reduction in NMDA receptor activity [75]. Moreover, other mechanisms of ketamine’s sympathomimetic action include the systemic release of catecholamines, the release of noradrenaline from sympathetic ganglion neurons, the inhibitory action of ketamine on the vagus nerve and centrally located muscarinic receptors, and the inhibition of the reuptake of noradrenaline in peripheral nerve endings, as well as in the myocardium [73,80,81]. Previously, increases in systolic and diastolic blood pressure, as well as heart rate, were observed following the administration of sub-anesthetic doses of ketamine [73,74,75,76,77]. However, recent studies demonstrated minor transient hemodynamic/neural changes (i.e., in blood pressure and heart rate) after ketamine treatment in adolescent depression [2,46]. Our study revealed a discrete, nonsignificant increase in heart rate without significant changes in blood pressure or EDA parameters. Thus, we assume that our findings can point to ketamine’s safety in terms of acute complications (e.g., cardiovascular) associated with sympathetic dysregulation in adolescent MDD. It is noteworthy that, as a measure of neural-mediated influences on the activity of sweat glands reflecting pure sympathetic cholinergic regulation, EDA cannot indicate the overall functioning of the sympathetic regulatory network. Therefore, the analysis of other sympathetically mediated effectors’ parameters, such as heart rate or blood pressure variabilities, may provide independent and distinct information on sympathetic regulation in response to ketamine treatment.
A remarkable result is the significant negative correlation between the SCL index and the CDI total score, CDI A (negative mood), and CDI E (negative self-esteem), associated with a negative correlation between index NS-SCRs and MADRS no. 4 (reduced sleep) before ketamine treatment. These findings point to a negative relationship between the severity of depressive symptoms and EDA parameters before acute ketamine treatment. Our results are in accordance with other studies revealing lower SCL values in subjects with more severe depressive symptoms [82,83,84,85,86,87]. In other words, MDD patients with more pronounced, subjectively interpreted depressive symptoms had lower tonic EDA activity before ketamine treatment that appeared to improve (no significant correlations between EDA and depressive symptoms) after the acute ketamine intervention.
Limitations of the Study
The limitations of this study include the relatively small number of depressed patients who participated, all of whom were the same gender (female). Therefore, further research is needed to validate our findings with a larger set of depressed patients including male adolescent patients. Our study was further limited by its open-label design. Therefore, a blind study with a placebo is needed to confirm our findings. Notably, the analysis of other parameters reflecting effectors’ sympathetically mediated regulatory mechanisms, such as blood pressure variability, could contribute to a more precise clarification of the relationship between the acute effect of ketamine and sympathetic regulation/dysregulation. Further research in this field is, therefore, needed.
4. Materials and Methods
4.1. Ethics Statement
This study was approved by the Ethics Committee of the Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, the Slovak Republic (56/2021), and by the Ethics Committee of University Hospital Martin, the Slovak Republic (143/2021). All procedures in our study were performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients’ legal representatives were properly informed about the study protocol and provided written informed consent.
4.2. Subjects
Initially, thirty adolescent female patients, aged 12–18 years, who were suffering from MDD and hospitalized at the Clinic of Psychiatry were examined. Severe MDD episodes were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [88], and confirmed by two independent specialists—child and adolescent psychiatrists.
The inclusion criteria were the following: adolescent age, the diagnosis of a severe episode of MDD according to the DSM-5 [88] confirmed by two independent specialists—child and adolescent psychiatrists, and an inadequate response to SSRI treatment after 8 weeks of treatment on a sufficient recommended dose [8].
The exclusion criteria were the following: a personal history of psychotic illnesses or a manic episode (assessed by two independent psychiatrists), the abuse of psychoactive substances, weight abnormalities (obesity, underweight, or overweight), or endocrinological, cardiovascular, neurological, and other diseases potentially affecting the activity of the autonomic nervous system. Moreover, all subjects were instructed not to use substances influencing autonomic nervous activity (caffeine and nicotine) for at least 12 h before the ketamine infusion therapy.
According to the strict inclusion and exclusion criteria, the final homogeneous sample consisted of 20 adolescent females (average age: 15.0 years ± 1.46). We included only females in the study to achieve precise homogeneity of the studied group because of the different prevalence of depression (generally twice as common in females compared to males) [89] and sex-related differences in autonomic nervous system development [90], which might have affected the results of the electrodermal activity as an index of sympathetic neural control. The included MDD patients were previously treated with fluoxetine at a dose of 20 mg, and 7 patients had been treated with sertraline at a dose of 50 mg. Over the following four weeks, the dose of fluoxetine was increased to 40 mg and the dose of sertraline to 100 mg. A flow diagram for the study subjects is presented in Figure 2.
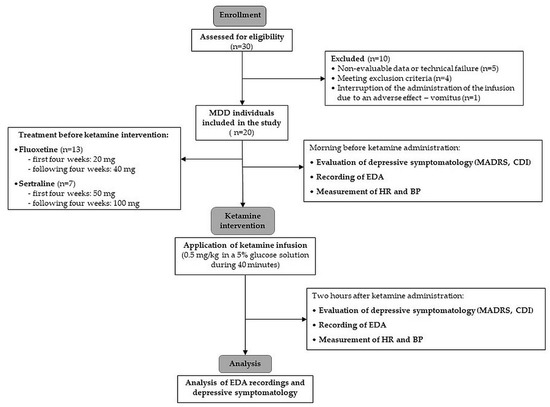
Figure 2.
Study flow diagram. MDD—major depressive disorder; MADRS—Montgomery–Asberg Depression Rating Scale; CDI—Children’s Depression Inventory; EDA—electrodermal activity; HR—heart rate; BP—blood pressure.
4.3. Continual Recording of Electrodermal Activity
EDA measurement was performed the morning before the ketamine infusion and two hours after the end of the ketamine infusion in order to meet the strict standard conditions necessary to avoid disturbing factors during EDA recording (e.g., silence and only one examiner); avoiding these factors could not be ensured during the ketamine infusion since the patients’ active cooperation was essential (due to, e.g., necessary communication, acute psychotherapy during infusion therapy, the presence of other necessary medical personnel, etc.). Additionally, according to some studies, ketamine’s immediate adverse effects (such as dissociative symptoms or a quick sympathomimetic effect) disappear within two hours after ketamine administration [91,92]. This was another reason for measuring EDA two hours after the ketamine infusion. At first, the participants were instructed to sit comfortably and rest in a special armchair for 10 min to avoid the potential effects of stress (from the laboratory environment and the presence of the examiner). Consequently, EDA was continuously recorded with a sampling frequency of 256 Hz (required via hardware) (FlexComp Infinity Biofeedback, Thought Technology, Montreal, QC, Canada) [93] and monitored using two dry Ag–AgCl bipolar electrodes placed on the middle phalanges of two fingers on the left (non-dominant) hand [93,94,95]. Before each examination, the EDA electrodes were carefully cleaned with an alcohol wipe. The baseline phase of the study lasted for 6 min.
EDA Evaluated Parameters
Raw EDA recordings were carefully checked, and rare artifacts were manually removed for data analysis. Next, the tonic EDA component was extracted using the 10th-order low-pass finite impulse response filter [96]. Furthermore, the index of the tonic level of the skin’s electrical conductivity—the skin conductance level (SCL, microSiemens (μS)) [97]—was evaluated as the average amplitude of the tonic EDA from 5 min of artifact-free recordings. The SCL evaluates quantitative alterations in the cholinergic sympathetic nervous system. The typical physiological values of the SCL depend on the size of the sensors used. For the 10 mm sensors used in this study, the range is from 0 to 30 μS [40]. Furthermore, the nonspecific electrodermal responses (NS-SCRs) indicating momentary arousal were evaluated as the frequency of spontaneous skin conductance responses occurring without external stimuli [38,41]. The threshold for NS-SCRs evaluation was 0.05 μS [41].
4.4. Assessment of Depressive Symptomatology
The clinical assessment of the participants, with a focus on depressive symptoms, was carried out using internationally accepted and standardized assessment scales meeting the criteria of validity and reliability. The following scales were used: the Montgomery–Asberg Depression Rating Scale (MADRS) and the self-reported Children’s Depression Inventory (CDI) scale. The participants were scored before receiving the ketamine infusion and two hours after the ketamine infusion [91,92]. The scales were provided and assessed by specialists from the fields of child and adolescent psychiatry.
4.4.1. The Montgomery–Asberg Depression Rating Scale
MADRS is one of the most commonly used depression rating scales with high inter-rater reliability, validity, and sensitivity to change. It consists of 10 items targeting the core symptoms of depressive disorder: apparent sadness (no. 1), reported sadness (no. 2), inner tension (no. 3), reduced sleep (no. 4), reduced appetite (no. 5), difficulties concentrating (no. 6), lassitude (no. 7), the inability to feel (no. 8), pessimistic thoughts (no. 9), and suicidal thoughts (no. 10). Individual items are rated on a scale from 0 to 6, while a rating can lie on the defined scale levels (0, 2, 4, 6) or between them (1, 3, 5) [98]. The total score ranges from 0 to 60, with a cut-off score for severe depression of 35 points [99].
4.4.2. The Children’s Depression Inventory
The CDI is an internationally accepted self-reported scale for depression in the child and adolescent population that meets the criteria of validity and reliability [100]. It consists of 27 multiple-choice items that are quantified using values from 0 to 2 according to severity. Based on the individual items, five basic groups of depression symptoms are evaluated: CDI A (negative mood), CDI B (interpersonal problems), CDI C (ineffectiveness), CDI D (anhedonia), and CDI E (negative self-esteem). The total score ranges from 0 to 54 and, according to the Kovacs, the recommended cut-off score in clinical settings was set at 13 [101].
4.5. Study Protocol
All MDD patients underwent basic laboratory examinations, pregnancy testing, and ECG examination to screen for somatic diseases, the presence of which met the exclusion criteria. All patients were instructed not to consume food for at least 6 h or liquids for at least 2 h prior to the infusion administration. As the MDD adolescents did not respond to previous SSRI antidepressants, SSRI treatment was discontinued, and the intravenous ketamine administration started on the 2nd or 3rd day of the patient’s hospitalization. Ketamine at a dose of 0.5 mg/kg was administered intravenously in the form of an infusion for 40 min under the supervision of specialists in the field of anesthesiology and intensive care medicine. A psychotherapeutic (supportive) intervention was conducted with the patient during the administration of the ketamine infusion. Moreover, the patient’s heart rate and blood pressure were measured using an automated oscillometric device (OMRON M6 Comfort, Kyoto, Japan) before and after ketamine administration. During infusion, vital functions (blood pressure and heart rate) and oxygen saturation were checked every 15 min and then every hour for 2 h after the infusion.
4.6. Statistical Analysis
The data were explored and analyzed in jamovi version 1.6.9 (Sydney, Australia). The Shapiro–Wilk normality test was used to evaluate data distributions (Gaussian/non-Gaussian). None of the analyzed data were normally distributed. Consequently, the Wilcoxon rank test was used for comparisons before and after treatment with Bonferroni correction to minimize the experimental and family error rate in multiple comparisons [102]. The associations between the MADRS questionnaire indices and SCL and NS-SCRs and between the CDI questionnaire indices and SCL and NS-SCRs were analyzed using Spearman’s rank-order correlation test with Bonferroni correction applied. The effect size, r, was calculated using G*Power 3.1.9.7 (Dusseldorf University, Dusseldorf, Germany) post hoc: the computation achieved power for the Wilcoxon signed-rank test (matched pairs) from the obtained parameters (means, SDs, and correlations). The parameters were expressed as means ± SDs. A value of p < 0.05 (two-tailed) was considered statistically significant.
5. Conclusions
Our study revealed a significant improvement in depressive symptomatology predominantly in affective, anhedonic, and cognitive symptoms after the administration of one ketamine infusion in patients with adolescent MDD. Further, ketamine significantly reduced suicidal thoughts, which represents an important finding from the perspective of the suicidality decrease in adolescent MDD. Additionally, the EDA parameters showed no significant changes after the ketamine treatment, indicating ketamine’s relative safety in terms of acute sympathetically mediated complications of adolescent major depression.
Author Contributions
Conceptualization, I.T. and I.O.; methodology, I.O., I.T. and S.N.; formal Analysis, Z.V. and I.F.; investigation, V.K., A.M., Z.M. and T.K.; resources, V.K. and A.M.; data curation, Z.V. and N.F.; writing—original draft preparation, V.K., A.M. and Z.V.; writing—editing, I.T. and N.F.; supervision, I.O., I.T. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Slovak Scientific Grant Agency under a VEGA grant (No. 1/0048/24).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Jessenius Faculty of Medicine in Martin, Comenius University, in Bratislava, the Slovak Republic (56/2021), and by the Ethics Committee of University Hospital Martin, the Slovak Republic (143/2021).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study and their legal representatives.
Data Availability Statement
Complete data are available upon reasonable request from the corresponding author.
Acknowledgments
The collective authors appreciate all the patients who participated in this study and their guardians.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global Prevalence of Depression and Elevated Depressive Symptoms among Adolescents: A Systematic Review and Meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Landeros-Weisenberger, A.; Johnson, J.A.; Londono Tobon, A.; Flores, J.M.; Nasir, M.; Couloures, K.; Sanacora, G.; Bloch, M.H. Efficacy of Intravenous Ketamine in Adolescent Treatment-Resistant Depression: A Randomized Midazolam-Controlled Trial. Am. J. Psychiatry 2021, 178, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.M.; Woodward, L.J. Mental Health, Educational, and Social Role Outcomes of Adolescents with Depression. Arch. Gen. Psychiatry 2002, 59, 225–231. [Google Scholar] [CrossRef] [PubMed]
- NICE. NICE Recommendations. Depression in Children and Young People: Identification and Management. Guidance. Available online: https://www.nice.org.uk/guidance/ng134/chapter/Recommendations (accessed on 10 February 2024).
- Dwyer, J.B.; Stringaris, A.; Brent, D.A.; Bloch, M.H. Annual Research Review: Defining and Treating Pediatric Treatment-resistant Depression. J. Child Psychol. Psychiatry 2020, 61, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.; Emslie, G.; Clarke, G.; Wagner, K.D.; Asarnow, J.R.; Keller, M.; Vitiello, B.; Ritz, L.; Iyengar, S.; Abebe, K.; et al. Switching to Another SSRI or to Venlafaxine with or without Cognitive Behavioral Therapy for Adolescents With SSRI-Resistant Depression. JAMA J. Am. Med. Assoc. 2008, 299, 901–913. [Google Scholar] [CrossRef]
- March, J.; Silva, S.; Petrycki, S.; Curry, J.; Wells, K.; Fairbank, J.; Burns, B.; Domino, M.; McNulty, S.; Vitiello, B.; et al. Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents with Depression: Treatment for Adolescents with Depression Study (TADS) Randomized Controlled Trial. JAMA 2004, 292, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, F.T.; Atwi, M.; Brent, D.A. Treatment-Resistant Depression in Adolescents: Review and Updates on Clinical Management. Depress. Anxiety 2011, 28, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide Consensus on the Clinical Management of Treatment-Resistant Depression in Italy: A Delphi Panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef]
- Correia-Melo, F.S.; Leal, G.C.; Carvalho, M.S.; Jesus-Nunes, A.P.; Ferreira, C.B.N.; Vieira, F.; Magnavita, G.; Vale, L.A.S.; Mello, R.P.; Nakahira, C.; et al. Comparative Study of Esketamine and Racemic Ketamine in Treatment-Resistant Depression. Medicine 2018, 97, e12414. [Google Scholar] [CrossRef]
- Schatzberg, A.F. A Word to the Wise About Intranasal Esketamine. Am. J. Psychiatry 2019, 176, 422–424. [Google Scholar] [CrossRef]
- Vlerick, L.; Devreese, M.; Peremans, K.; Dockx, R.; Croubels, S.; Duchateau, L.; Polis, I. Pharmacokinetics, Absolute Bioavailability and Tolerability of Ketamine after Intranasal Administration to Dexmedetomidine Sedated Dogs. PLoS ONE 2020, 15, e0227762. [Google Scholar] [CrossRef]
- Singh, B.; Kung, S.; Pazdernik, V.; Schak, K.M.; Geske, J.; Schulte, P.J.; Frye, M.A.; Vande Voort, J.L. Comparative Effectiveness of Intravenous Ketamine and Intranasal Esketamine in Clinical Practice Among Patients with Treatment-Refractory Depression: An Observational Study. J. Clin. Psychiatry 2023, 84, 22m14548. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Ballard, E.D.; Bloch, M.H.; Mathew, S.J.; Murrough, J.W.; Feder, A.; Sos, P.; Wang, G.; Zarate, C.A.; Sanacora, G. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 2018, 175, 150–158. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.-H.; Keilp, J.G.; Moitra, V.K.; Parris, M.S.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; et al. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am. J. Psychiatry 2018, 175, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; d’Andrea, G.; Di Carlo, F.; De Risio, L.; Zoratto, F.; Miuli, A.; Benatti, B.; Vismara, M.; Pompili, E.; Nicolò, G.; et al. Comparing Fast-Acting Interventions for Treatment-Resistant Depression: An Explorative Study of Accelerated HF-rTMS versus Intranasal Esketamine. Brain Stimulat. 2023, 16, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Meshkat, S.; Rosenblat, J.D.; Ho, R.C.; Rhee, T.G.; Cao, B.; Ceban, F.; Danayan, K.; Chisamore, N.; Vincenzo, J.D.D.; McIntyre, R.S. Ketamine Use in Pediatric Depression: A Systematic Review. Psychiatry Res. 2022, 317, 114911. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Y.; Boulieu, R. Contribution of CYP3A4, CYP2B6, and CYP2C9 Isoforms to N-Demethylation of Ketamine in Human Liver Microsomes. Drug Metab. Dispos. 2002, 30, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fanta, S.; Kinnunen, M.; Backman, J.T.; Kalso, E. Population Pharmacokinetics of S-Ketamine and Norketamine in Healthy Volunteers after Intravenous and Oral Dosing. Eur. J. Clin. Pharmacol. 2015, 71, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Theurillat, R.; Lassahn, P.; Mevissen, M.; Thormann, W. CE Provides Evidence of the Stereoselective Hydroxylation of Norketamine in Equines. Electrophoresis 2009, 30, 2912–2921. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism and Metabolomics of Ketamine: A Toxicological Approach. Forensic Sci. Res. 2017, 2, 2–10. [Google Scholar] [CrossRef]
- Mion, G.; Villevieille, T. Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Edginton, A.N.; Schmitt, W.; Voith, B.; Willmann, S. A Mechanistic Approach for the Scaling of Clearance in Children. Clin. Pharmacokinet. 2006, 45, 683–704. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, C.H.; Nelson, W.L. The Relationship of Ketamine Requirement to Age in Pediatric Patients. Anesthesiology 1974, 40, 507–508. [Google Scholar] [CrossRef]
- White, P.F.; Schüttler, J.; Shafer, A.; Stanski, D.R.; Horai, Y.; Trevor, A.J. Comparative Pharmacology of the Ketamine Isomers. Br. J. Anaesth. 1985, 57, 197–203. [Google Scholar] [CrossRef]
- Adamowicz, P.; Kala, M. Urinary Excretion Rates of Ketamine and Norketamine Following Therapeutic Ketamine Administration: Method and Detection Window Considerations. J. Anal. Toxicol. 2005, 29, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.R.; Stiller, R.; Dayton, P. Pharmacokinetics of Ketamine (K) in Infants and Small Children. Anesthesiology 1982, 57, A428. [Google Scholar] [CrossRef]
- Kamp, J.; Olofsen, E.; Henthorn, T.K.; Van Velzen, M.; Niesters, M.; Dahan, A.; for the Ketamine Pharmacokinetic Study Group. Ketamine Pharmacokinetics: A Systematic Review of the Literature, Meta-analysis, and Population Analysis. Anesthesiology 2020, 133, 1192–1213. [Google Scholar] [CrossRef]
- Herd, D.; Anderson, B.J. Ketamine Disposition in Children Presenting for Procedural Sedation and Analgesia in a Children’s Emergency Department. Pediatr. Anesth. 2007, 17, 622–629. [Google Scholar] [CrossRef]
- Sleigh, J.; Harvey, M.; Voss, L.; Denny, B. Ketamine—More Mechanisms of Action than Just NMDA Blockade. Trends Anaesth. Crit. Care 2014, 4, 76–81. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Adams, T.G.; Kelmendi, B.; Esterlis, I.; Sanacora, G.; Krystal, J.H. Ketamine’s mechanism of action: A path to rapid-acting antidepressants. Depress. Anxiety 2016, 33, 689–697. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Anand, A.; Li, Y.; Wang, Y.; Wu, J.; Gao, S.; Bukhari, L.; Mathews, V.P.; Kalnin, A.; Lowe, M.J. Antidepressant Effect on Connectivity of the Mood-Regulating Circuit: An fMRI Study. Neuropsychopharmacology 2005, 30, 1334–1344. [Google Scholar] [CrossRef]
- Bellato, A.; Sesso, G.; Milone, A.; Masi, G.; Cortese, S. Systematic Review and Meta-Analysis: Altered Autonomic Functioning in Youths With Emotional Dysregulation. J. Am. Acad. Child Adolesc. Psychiatry 2023, 63, 216–230. [Google Scholar] [CrossRef]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic Neural Mechanisms in Human Cardiovascular Health and Disease. Mayo Clin. Proc. 2009, 84, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, J.; Watson, D.; Jones, R.; Rowe, M.A. Guide for Analysing Electrodermal Activity & Skin Conductance Responses for Psychological Experiments; CTIT Technical Report Series; Behavioural Brain Sciences Centre, University of Birmingham: Birmingham, UK, 2013. [Google Scholar]
- Andreassi, J.L. Psychophysiology: Human Behavior and Physiological Response, 4th ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2000; pp. xxvi, 458. ISBN 978-0-8058-2832-0. [Google Scholar]
- Dawson, M.E.; Schell, A.M.; Filion, D.L.; Berntson, G.G. The Electrodermal System; Cacioppo, J.T., Tassinary, L.G., Berntson, G., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 157–181. [Google Scholar]
- Boucsein, W. Electrodermal Activity; Springer: Boston, MA, USA, 2012; ISBN 978-1-4614-1125-3. [Google Scholar]
- Caruelle, D.; Gustafsson, A.; Shams, P.; Lervik-Olsen, L. The Use of Electrodermal Activity (EDA) Measurement to Understand Consumer Emotions—A Literature Review and a Call for Action. J. Bus. Res. 2019, 104, 146–160. [Google Scholar] [CrossRef]
- Mestanikova, A.; Ondrejka, I.; Mestanik, M.; Hrtanek, I.; Snircova, E.; Tonhajzerova, I. Electrodermal Activity in Adolescent Depression. In Pulmonary Infection and Inflammation; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 935, pp. 83–88. ISBN 978-3-319-44484-0. [Google Scholar]
- Erath, S.A.; Su, S.; Tu, K.M. Electrodermal Reactivity Moderates the Prospective Association Between Peer Victimization and Depressive Symptoms in Early Adolescence. J. Clin. Child Adolesc. Psychol. 2018, 47, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, O.; Di Vincenzo, J.D.; Rodrigues, N.B.; Cha, D.S.; Lee, Y.; Greenberg, D.; Teopiz, K.M.; Ho, R.C.; Cao, B.; Lin, K.; et al. Safety, Tolerability, and Real-World Effectiveness of Intravenous Ketamine in Older Adults with Treatment-Resistant Depression: A Case Series. Am. J. Geriatr. Psychiatry 2021, 29, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Amatya, P.; Roback, M.G.; Albott, C.S.; Westlund Schreiner, M.; Ren, Y.; Eberly, L.E.; Carstedt, P.; Samikoglu, A.; Gunlicks-Stoessel, M.; et al. Intravenous Ketamine for Adolescents with Treatment-Resistant Depression: An Open-Label Study. J. Child Adolesc. Psychopharmacol. 2018, 28, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.J.; Hasassri, E. Ketamine for Adolescent Depression: An Overview and Considerations for Future Directions. Am. J. Psychiatry Resid. J. 2022, 17, 2–4. [Google Scholar] [CrossRef]
- Weber, G.; Yao, J.; Binns, S.; Namkoong, S. Case Report of Subanesthetic Intravenous Ketamine Infusion for the Treatment of Neuropathic Pain and Depression with Suicidal Features in a Pediatric Patient. Case Rep. Anesthesiol. 2018, 2018, 9375910. [Google Scholar] [CrossRef]
- Kohrs, R.; Durieux, M.E. Ketamine: Teaching an Old Drug New Tricks. Anesth. Analg. 1998, 87, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, S.; Bayliss, D.A. HCN1 Channel Subunits Are a Molecular Substrate for Hypnotic Actions of Ketamine. J. Neurosci. 2009, 29, 600–609. [Google Scholar] [CrossRef]
- Durieux, M.E. Inhibition by Ketamine of Muscarinic Acetylcholine Receptor Function. Anesth. Analg. 1995, 81, 57–62. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. NMDA Receptor Antagonists Ketamine and PCP Have Direct Effects on the Dopamine D2 and Serotonin 5-HT2 Receptors—Implications for Models of Schizophrenia. Mol. Psychiatry 2002, 7, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Ko, F.; Tallerico, T. Dopamine Receptor Contribution to the Action of PCP, LSD and Ketamine Psychotomimetics. Mol. Psychiatry 2005, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Lisek, M.; Boczek, T.; Ferenc, B.; Zylinska, L. Regional Brain Dysregulation of Ca2+-Handling Systems in Ketamine-Induced Rat Model of Experimental Psychosis. Cell Tissue Res. 2016, 363, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Dubois, J.; Field, R.M.; Fishburn, F.; Gundran, A.; Ho, W.C.; Jawhar, S.; Kates-Harbeck, J.; Aghajan, Z.M.; Miller, N.; et al. Measuring Acute Effects of Subanesthetic Ketamine on Cerebrovascular Hemodynamics in Humans Using TD-fNIRS. Sci. Rep. 2023, 13, 11665. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Mendez-David, I.; Defaix, C.; Guiard, B.P.; Tritschler, L.; David, D.J.; Gardier, A.M. Ketamine Treatment Involves Medial Prefrontal Cortex Serotonin to Induce a Rapid Antidepressant-like Activity in BALB/cJ Mice. Neuropharmacology 2017, 112, 198–209. [Google Scholar] [CrossRef]
- Qing-Ping, W.; Nakai, Y. The Dorsal Raphe: An Important Nucleus in Pain Modulation. Brain Res. Bull. 1994, 34, 575–585. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Blasey, C.; Sudheimer, K.; Pannu, J.; Pankow, H.; Hawkins, J.; Birnbaum, J.; Lyons, D.M.; Rodriguez, C.I.; et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 2018, 175, 1205–1215. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Picciotto, M.R. Nicotine Receptors and Depression: Revisiting and Revising the Cholinergic Hypothesis. Trends Pharmacol. Sci. 2010, 31, 580–586. [Google Scholar] [CrossRef]
- Moaddel, R.; Abdrakhmanova, G.; Kozak, J.; Jozwiak, K.; Toll, L.; Jimenez, L.; Rosenberg, A.; Tran, T.; Xiao, Y.; Zarate, C.A.; et al. Sub-Anesthetic Concentrations of (R,S)-Ketamine Metabolites Inhibit Acetylcholine-Evoked Currents in A7 Nicotinic Acetylcholine Receptors. Eur. J. Pharmacol. 2013, 698, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Lambert, D.G. Anaesthesia-Related Drugs and SARS-CoV-2 Infection. Br. J. Anaesth. 2021, 127, e32–e34. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.J.; Elliott, M.; Seminerio, M.J.; Matsumoto, R.R. Evaluation of Sigma (σ) Receptors in the Antidepressant-like Effects of Ketamine in Vitro and in Vivo. Eur. Neuropsychopharmacol. 2012, 22, 308–317. [Google Scholar] [CrossRef]
- Wang, J.; Mack, A.L.; Coop, A.; Matsumoto, R.R. Novel Sigma (σ) Receptor Agonists Produce Antidepressant-like Effects in Mice. Eur. Neuropsychopharmacol. 2007, 17, 708–716. [Google Scholar] [CrossRef]
- Kim, C.S.; Chang, P.Y.; Johnston, D. Enhancement of Dorsal Hippocampal Activity by Knockdown of HCN1 Channels Leads to Anxiolytic- and Antidepressant-like Behaviors. Neuron 2012, 75, 503–516. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, T.; Yuan, Z.; Wei, Z.; Yamaki, V.N.; Huang, M.; Huganir, R.L.; Cai, X. Essential Roles of AMPA Receptor GluA1 Phosphorylation and Presynaptic HCN Channels in Fast-Acting Antidepressant Responses of Ketamine. Sci. Signal. 2016, 9, ra123. [Google Scholar] [CrossRef]
- Han, Y.; Heuermann, R.J.; Lyman, K.A.; Fisher, D.; Ismail, Q.-A.; Chetkovich, D.M. HCN-Channel Dendritic Targeting Requires Bipartite Interaction with TRIP8b and Regulates Antidepressant-like Behavioral Effects. Mol. Psychiatry 2017, 22, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.P.; Lücken, M.D.; Brivio, E.; Karamihalev, S.; Kos, A.; De Donno, C.; Benjamin, A.; Yang, H.; Dick, A.L.W.; Stoffel, R.; et al. Ketamine Exerts Its Sustained Antidepressant Effects via Cell-Type-Specific Regulation of Kcnq2. Neuron 2022, 110, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Demyttenaere, K.; Janka, Z.; Aarre, T.; Bourin, M.; Canonico, P.L.; Carrasco, J.L.; Stahl, S. The Other Face of Depression, Reduced Positive Affect: The Role of Catecholamines in Causation and Cure. J. Psychopharmacol. Oxf. Engl. 2007, 21, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Wiglusz, M.S.; Gałuszko-Wegielnik, M.; Włodarczyk, A.; Cubała, W.J. Antianhedonic Effect of Repeated Ketamine Infusions in Patients With Treatment Resistant Depression. Front. Psychiatry 2021, 12, 704330. [Google Scholar] [CrossRef] [PubMed]
- Lally, N.; Nugent, A.C.; Luckenbaugh, D.A.; Niciu, M.J.; Roiser, J.P.; Zarate, C.A. Neural Correlates of Change in Major Depressive Disorder Anhedonia Following Open-Label Ketamine. J. Psychopharmacol. 2015, 29, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Citi, L.; Saul, J.P.; Barbieri, R. Measures of Sympathetic and Parasympathetic Autonomic Outflow from Heartbeat Dynamics. J. Appl. Physiol. 2018, 125, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G. Depression as a Cardiovascular Disorder: Central-Autonomic Network, Brain-Heart Axis, and Vagal Perspectives of Low Mood. Front. Netw. Physiol. 2023, 3, 1125495. [Google Scholar] [CrossRef] [PubMed]
- Liebe, T.; Li, S.; Lord, A.; Colic, L.; Krause, A.L.; Batra, A.; Kretzschmar, M.A.; Sweeney-Reed, C.M.; Behnisch, G.; Schott, B.H.; et al. Factors Influencing the Cardiovascular Response to Subanesthetic Ketamine: A Randomized, Placebo-Controlled Trial. Int. J. Neuropsychopharmacol. 2017, 20, 909–918. [Google Scholar] [CrossRef]
- Dowdy, E.G.; Kaya, K. Studies of the Mechanism of Cardiovascular Responses to CI-581. Anesthesiology 1968, 29, 931–942. [Google Scholar] [CrossRef]
- Okamoto, H.; Hoka, S.; Kawasaki, T.; Okuyama, T.; Takahashi, S. L-Arginine Attenuates Ketamine-Induced Increase in Renal Sympathetic Nerve Activity. Anesthesiology 1994, 81, 137–146. [Google Scholar] [CrossRef]
- Irnaten, M.; Wang, J.; Chang, K.S.K.; Andresen, M.C.; Mendelowitz, D. Ketamine Inhibits Sodium Currents in Identified Cardiac Parasympathetic Neurons in Nucleus Ambiguus. Anesthesiology 2002, 96, 659–666. [Google Scholar] [CrossRef]
- Mendelowitz, D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. Physiology 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Kienbaum, P.; Heuter, T.; Pavlakovic, G.; Michel, M.C.; Peters, J. S (+)-Ketamine Increases Muscle Sympathetic Activity and Maintains the Neural Response to Hypotensive Challenges in Humans. Anesthesiology 2001, 94, 252–258. [Google Scholar] [CrossRef]
- Couch, G.A.; White, M.P.; De Gray, L.E. Central Nervous System Stimulants: Basic Pharmacology and Relevance to Anaesthesia and Critical Care. Anaesth. Intensive Care Med. 2020, 21, 503–511. [Google Scholar] [CrossRef]
- Lalonde, G. Miller’s Anesthesia, Eighth Edition: Ronald D. Miller, Neal H. Cohen, Lars I. Eriksson, Lee A. Fleisher, Jeanine P. Wiener-Kronish, William L. Young. Elsevier, Philadelphia, 2015, 449$, 3576 Pages. IBSN 978-0-7020-5283-5. Can. J. Anesth. Can. Anesth. 2015, 62, 558–559. [Google Scholar] [CrossRef]
- Salt, P.J.; Barnes, P.K.; Beswick, F.J. Inhibition of Neuronal and Extraneuronal Uptake of Noradrenaline by Ketamine in the Isolated Perfused Rat Heart. Br. J. Anaesth. 1979, 51, 835–838. [Google Scholar] [CrossRef]
- Bonnet, A.; Naveteur, J. Electrodermal Activity in Low Back Pain Patients with and without Co-Morbid Depression. Int. J. Psychophysiol. 2004, 53, 37–44. [Google Scholar] [CrossRef]
- Carney, R.M.; Hong, B.A.; Kulkarni, S.; Kapila, A. A Comparison of EMG and SCL in Normal and Depressed Subjects. Pavlov. J. Biol. Sci. 1981, 16, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Miquel, M.; Fuentes, I.; Garcia-Merita, M.; Rojo, L. Habituation and Sensitization Processes in Depressive Disorders. Psychopathology 1999, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, J.; Gross, J.J.; Wilhelm, F.H.; Najmi, S.; Gotlib, I.H. Crying Threshold and Intensity in Major Depressive Disorder. J. Abnorm. Psychol. 2002, 111, 302–312. [Google Scholar] [CrossRef]
- Tsai, J.L.; Pole, N.; Levenson, R.W.; Muñoz, R.F. The Effects of Depression on the Emotional Responses of Spanish-Speaking Latinas. Cultur. Divers. Ethnic Minor. Psychol. 2003, 9, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Jeon, H.J.; Yu, H.Y.; Byun, S. Automatic Detection of Major Depressive Disorder Using Electrodermal Activity. Sci. Rep. 2018, 8, 17030. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Platt, J.M.; Bates, L.; Jager, J.; McLaughlin, K.A.; Keyes, K.M. Is the US Gender Gap in Depression Changing Over Time? A Meta-Regression. Am. J. Epidemiol. 2021, 190, 1190–1206. [Google Scholar] [CrossRef]
- Harteveld, L.M.; Nederend, I.; Ten Harkel, A.D.J.; Schutte, N.M.; De Rooij, S.R.; Vrijkotte, T.G.M.; Oldenhof, H.; Popma, A.; Jansen, L.M.C.; Suurland, J.; et al. Maturation of the Cardiac Autonomic Nervous System Activity in Children and Adolescents. J. Am. Heart Assoc. 2021, 10, e017405. [Google Scholar] [CrossRef]
- Srivastava, S.; Gangwar, R.; Kumar, A. Safety and Efficacy of Ketamine Infusion in Late Onset Depression, and Conversion to Treatment Response. Indian J. Psychiatry 2015, 57, 328. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Beyer, C.; Wilkinson, S.T.; Ostroff, R.B.; Qayyum, Z.; Bloch, M.H. Ketamine as a Treatment for Adolescent Depression: A Case Report. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Thought Technology Ltd. FlexComp Infiniti Hardware Manual 2003. Available online: https://thoughttechnology.com/content/docs/manual/SA7560%20rev.%204%20FlexComp%20Infiniti%20User%20Manual%20(2).pdf (accessed on 12 December 2023).
- Blain, S.; Power, S.D.; Sejdic, E.; Mihailidis, A.; Chau, T. A Cardiorespiratory Classifier of Voluntary and Involuntary Electrodermal Activity. Biomed. Eng. OnLine 2010, 9, 11. [Google Scholar] [CrossRef]
- Poh, M.; Swenson, N.C.; Picard, R.W. A Wearable Sensor for Unobtrusive, Long-Term Assessment of Electrodermal Activity. IEEE Trans. Biomed. Eng. 2010, 57, 1243–1252. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power Spectral Density Analysis of Electrodermal Activity for Sympathetic Function Assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef]
- Turpin, G.; Grandfield, T. Electrodermal Activity. In Encyclopedia of Stress; Elsevier: Amsterdam, The Netherlands, 2007; pp. 899–902. ISBN 978-0-12-373947-6. [Google Scholar]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Müller, M.J.; Szegedi, A.; Wetzel, H.; Benkert, O. Moderate and Severe Depression. J. Affect. Disord. 2000, 60, 137–140. [Google Scholar] [CrossRef]
- Smucker, M.R.; Craighead, W.E.; Craighead, L.W.; Green, B.J. Normative and Reliability Data for the Children’s Depression Inventory. J. Abnorm. Child Psychol. 1986, 14, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar] [PubMed]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).