Cardioprotective Effect of Hydroalcohol Extract of Andaliman (Zanthoxylum acanthopodium DC.) Fruits on Doxorubicin-Induced Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemicals Constituent
2.2. Chemical Constituents of Hydroalcohol Extract
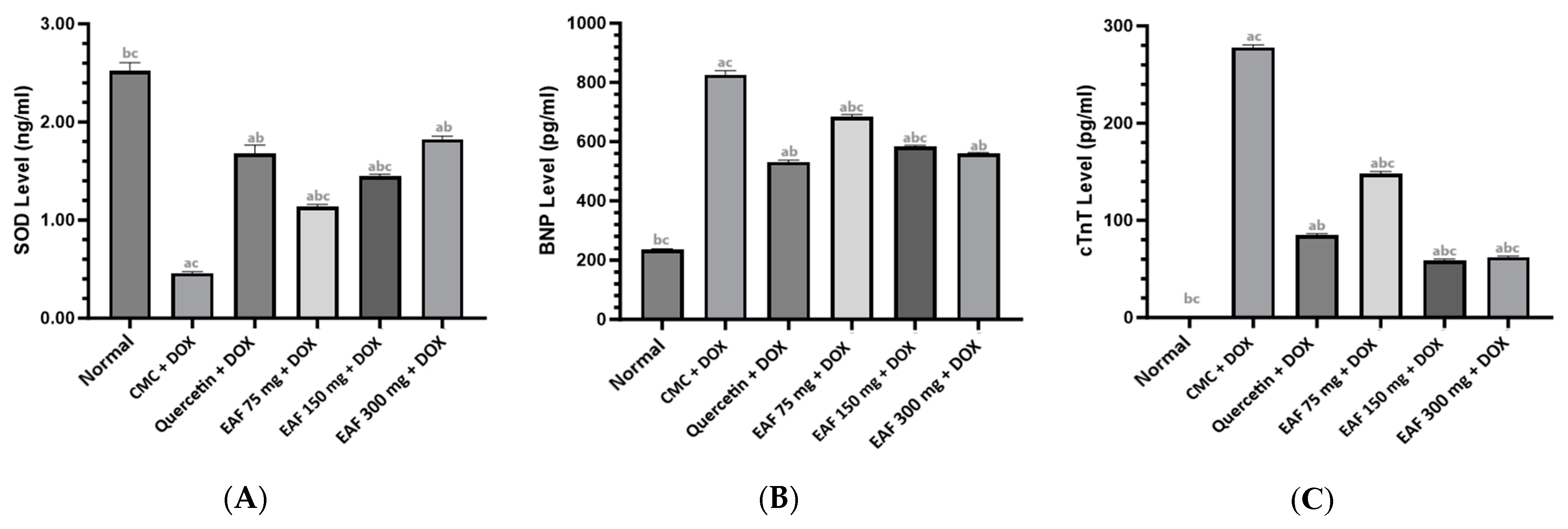
2.3. Results of SOD, BNP, and cTnT Measurements
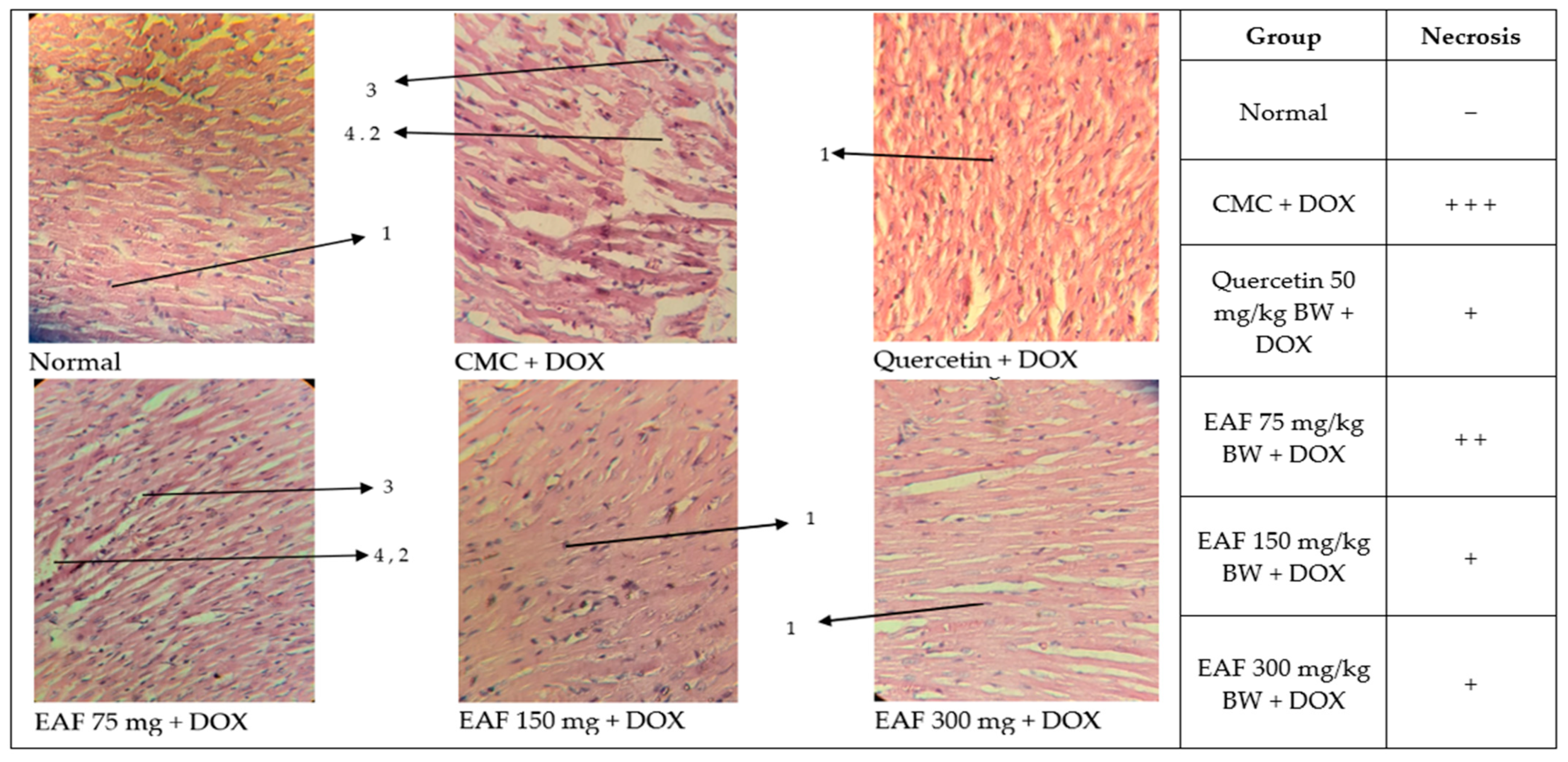
2.4. Cardiac Histopathology Results
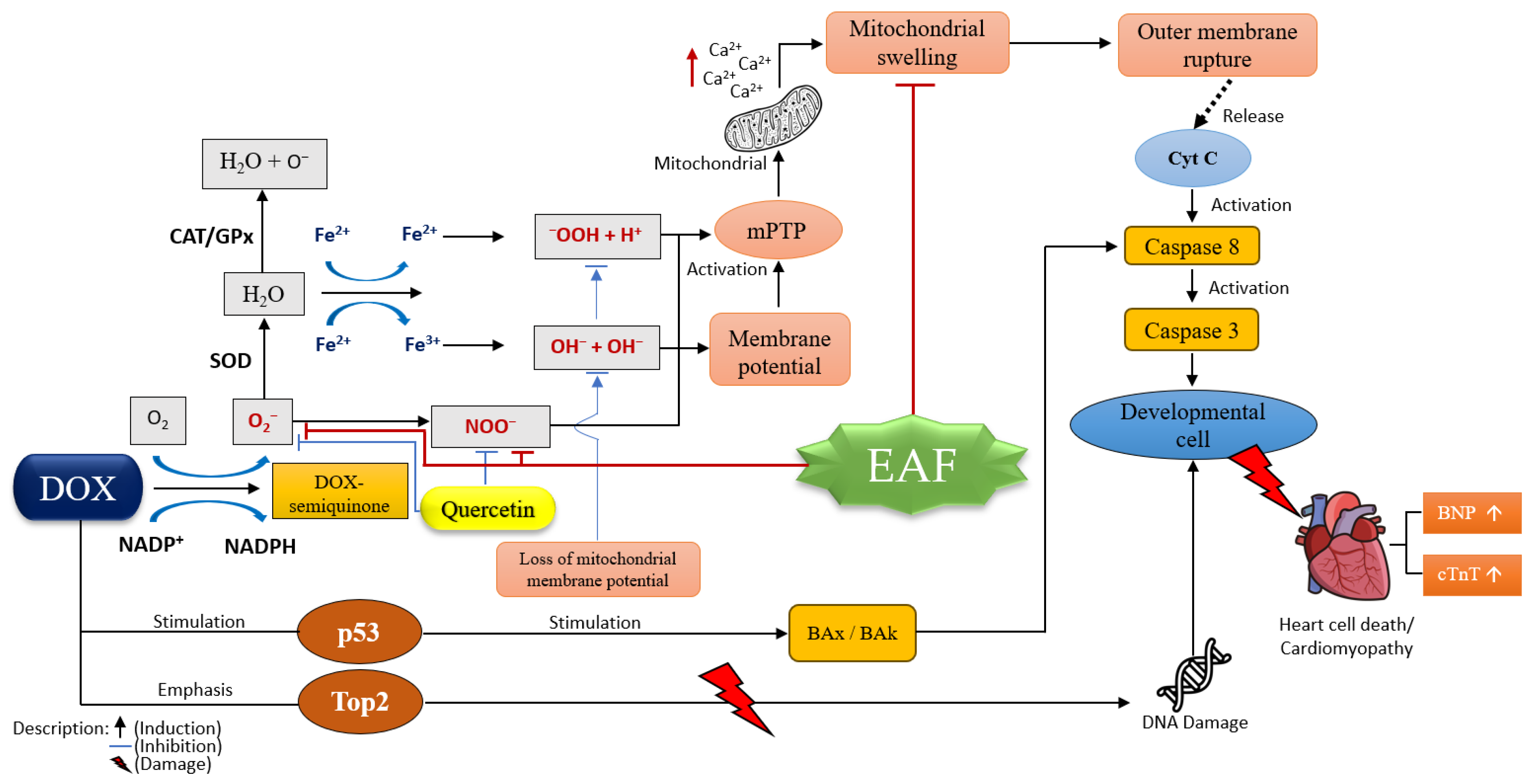
3. Discussion
4. Materials and Methods
4.1. Plants, Tools and Chemicals
4.2. Preparation of Extract of Andaliman Fruit
4.3. Phytochemicals Constituent Analysis with LC-HRMS
4.4. Analysis of Chemical Constituents by GC-MS
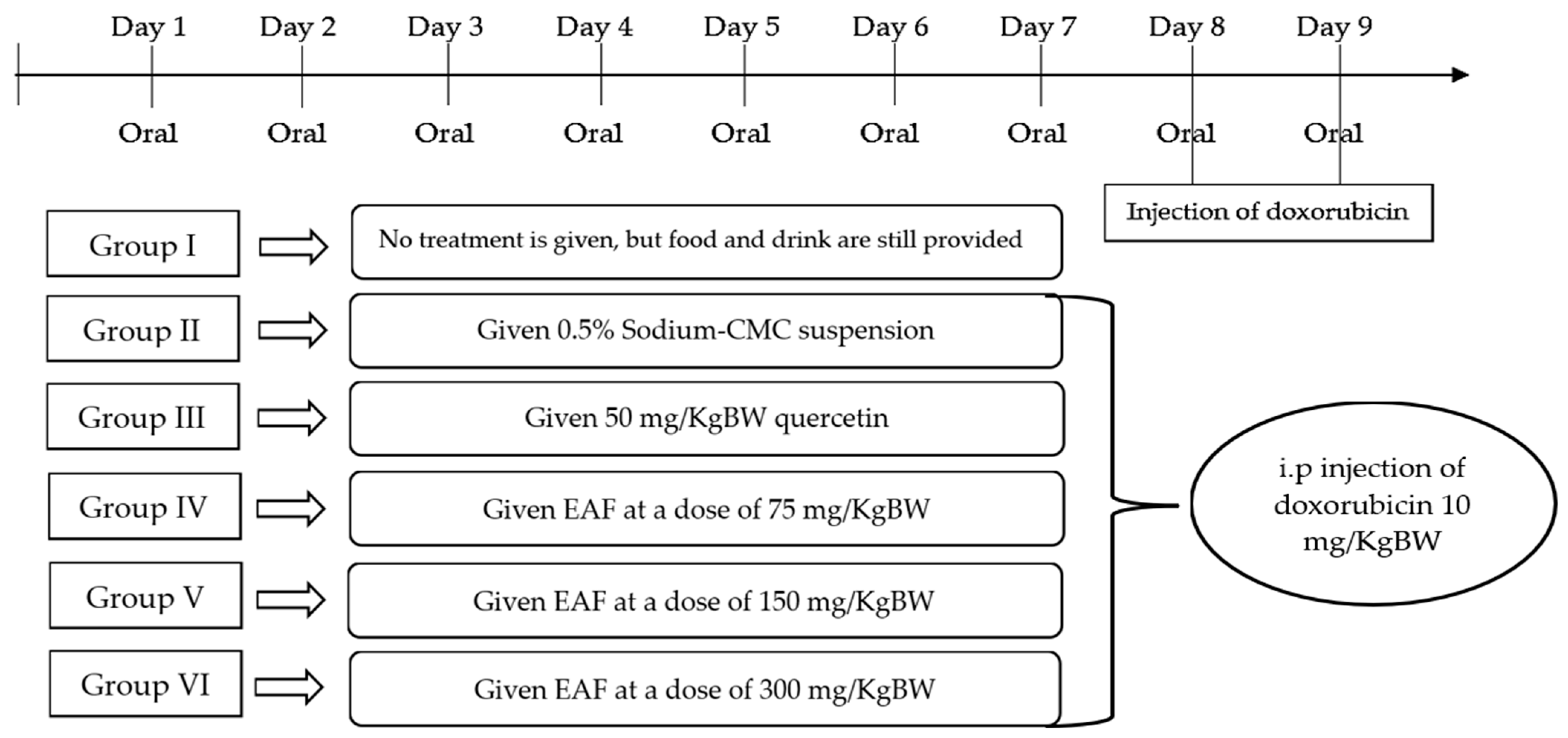
4.5. Animals and Study Design
- Group I: Normal control test animals were not given any treatment, but food and drink were still provided.
- Group II: Negative control test animals were given 0.5% sodium-CMC suspension.
- Group III: Positive control test animals were given 50 mg/kg BW quercetin.
- Group IV: test animals were given EAF at 75 mg/kg BW.
- Group V: test animals were given EAF at 150 mg/kg BW.
- Group VI: test animals were given EAF at 300 mg/kg BW.
4.6. Preparation of Blood Serum and Cardiac Organs
4.7. Measurement of Superoxide Dismutase, Brain Natriuretic Peptide, and Troponin T
4.7.1. SOD Measurement Procedure
4.7.2. BNP Measurement Procedure
4.7.3. cTnT Measurement Procedure
4.8. Histopathological Observation
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.; Roth, G.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.; Reddy, K.S.; Paccaud, F.; Horton, S.; Chaturvedi, V. Cardiovascular disease. In Disease Control Priorities in Developing Countries, 2nd ed.; Oxford University Press: New York, NY, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11767/ (accessed on 1 December 2023).
- WHO. 2021. Cardiovascular Diseases. WHO. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 1 December 2023).
- Feingold, K.R. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; South MDText.com, Inc.: Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570127/ (accessed on 16 April 2021).
- Shin, H.R.; Song, S.; Cho, J.A.; Ly, S.Y. Atherogenic Index of Plasma and Its Association with Risk Factors of Coronary Artery Disease and Nutrient Intake in Korean Adult Men: The 2013–2014 KNHANES. Nutrients 2022, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R. High Cholesterol Disorders, Myocardial Infarction and Its Therapeutics. World J. Cardiovasc. Dis. 2023, 13, 433–469. [Google Scholar] [CrossRef]
- Zoltani, C.K. Cardiovascular toxicity biomarkers. In Biomarkers in Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Gaber, S.A.; Mokhemer, S.A.; Kandeel, M.; Sedik, W.F.; Nair, A.B.; Venugopala, K.N.; Khalil, H.E.; Al-Dhubiab, B.E.; Mohamed, M.Z. Pregnenolone Inhibits Doxorubicin-Induced Cardiac Oxidative Stress, Inflammation, and Apoptosis—Role of Matrix Metalloproteinase 2 and NADPH Oxidase 1. Pharmaceuticals 2023, 16, 665. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S. Trabectedin and lurbinectedin: Mechanisms of action, clinical impact, and future perspectives in uterine and soft tissue sarcoma, ovarian carcinoma, and endometrial carcinoma. Front. Oncol. 2022, 12, 914342. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, Y.; Chen, S.; Wu, J.; Zhu, W.; Liu, H.; Chen, M.; Xu, B. Curative Effect and Survival Assessment Comparing Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin and Cisplatin as Neoadjuvant Therapy for Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 678896. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Chubarev, V.N.; Smolyarchuk, E.A.; Drozdov, V.N.; Krasnyuk, I.I.; Liu, J.; Fan, R.; Tse, E.; Shikh, E.V.; Sukocheva, O.A. Pharmacogenetics of Drug Metabolism: The Role of Gene Polymorphism in the Regulation of Doxorubicin Safety and Efficacy. Cancers 2022, 14, 5436. [Google Scholar] [CrossRef]
- Kübler, W.; Haass, M. Cardioprotection: Definition, classification, and fundamental principles. Heart 1996, 75, 330–333. [Google Scholar] [CrossRef]
- Cherney, D.; Odutayo, A.; Aronson, R.; Ezekowitz, J.; Parker, J.D. Sodium Glucose Cotransporter-2 Inhibition and Cardiorenal Protection: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2511–2524. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free. Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Lan, H.R.; Li, X.M.; Jin, K.T. A systematic review of the potential chemoprotective effects of resveratrol on doxorubicin-induced cardiotoxicity: Focus on the antioxidant, antiapoptotic, and anti-inflammatory activities. Oxidative Med. Cell. Longev. 2021, 2021, 2951697. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Asirvatham, R.; Tomy, D.V. Cardioprotective Effect of Marsdenia tenacissima and Sansevieria roxburghiana in Doxorubicin-induced Cardiotoxicity in Rats in vivo: The Role of Dresgenin and Lupeol. Turk. J. Pharm. Sci. 2021, 18, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Satria, D.; Dalimunthe, A.; Pertiwi, D.; Muhammad, M. Phytochemicals, proximate composition, minerals and volatile oil analysis of Zanthoxylum acanthopodium DC. fruits. F1000Research 2023, 12, 227. [Google Scholar] [CrossRef]
- Syari, D.M.; Rosidah, R.; Hasibuan, P.A.Z.; Haro, G.; Satria, D. Evaluation of Cytotoxic Activity Alkaloid Fractions of Zanthoxylum acanthopodium DC. Fruits. Open Access Maced. J. Med. Sci. 2019, 7, 3745–3747. [Google Scholar] [CrossRef] [PubMed]
- Adrian, A.; Syahputra, R.A.; Juwita, N.A.; Astyka, R.; Lubis, M.F. Andaliman (Zanthoxylum acanthopodium DC.) a herbal medicine from North Sumatera, Indonesia: Phytochemical and pharmacological review. Heliyon 2023, 9, e16159. [Google Scholar] [CrossRef] [PubMed]
- Megawati, E.R.; Bangun, H.; Putra, I.B.; Rusda, M.; Syahrizal, D.; Jusuf, N.K.; Eyanoer, P.C.; Lubis, R.R.; Amin, M.M. Phytochemical Analysis by FTIR of Zanthoxylum acanthopodium, DC Fruit Ethanol Extract, N-hexan, Ethyl Acetate and Water Fraction. Med. Arch. 2023, 77, 183–188. [Google Scholar] [CrossRef]
- Sihotang, Y.; Silalahi, J.; Anjelisa, P. Cardioprotective effect of ethylacetate extract of Zanthoxylum acanthopodium DC. against doxorubicin-induced cardiotoxicity in rats. Int. J. PharmTech Res. 2016, 9, 249–253. Available online: www.sphinxsai.com (accessed on 1 December 2023).
- Manurung, R.D.; Ilyas, S.; Hutahaean, S.; Rosidah, R.; Simanullang, R.H. Effectivity of Nano herbal Andaliman (Zanthoxylum acanthopodium) to the Vascular Endothelial Growth Factor (VEGF) expression in burn wound in diabetic rats. In Proceedings of the 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), Medan, Indonesia, 14–16 July 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Suryanto, E.; Sastrohamidjojo, H.; Raharjo, S. Antiradical Activity of Andaliman (Zanthoxylumachanthopodium DC) Fruit Extract. Indones. Food Nutr. Prog. 2004, 11, 15–19. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Prananda, A.T.; Dalimunthe, A.; Harahap, U.; Syahputra, R.A.; Nugraha, S.E.; Situmorang, P.C.; Fah, Y.T.; Adrian Siahaan, J.M.; Velaro, A.J.; Bilakaya, B.; et al. Phytochemical profiling and cardioprotective activity of Vernonia amygdalina ethanol extract (VAEE) against ISO-induced cardiotoxicity in rats. Pharmacia 2023, 70, 758–796. [Google Scholar] [CrossRef]
- Hussein, M.A.; Islam, M.S.; Ali, A.A.; Mansour, M.S.; Bondok, M.; Salem, M.A.; Amein, A.S.; El-gizawy, H.A. Malva parviflora seed oil; Isolation, gas chromatographic profiling and its cardioprotective activity against myocardial infraction in animal model. J. King Saud Univ. Sci. 2023, 36, 103060. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.C.; Dass, C.R. Doxorubicin-induced cardiotoxicity: Causative factors and possible interventions. J. Pharm. Pharmacol. 2022, 74, 1677–1688. [Google Scholar] [CrossRef]
- Fan, R.; Wang, Y.; Zhang, J.; An, X.; Liu, S.; Bai, J.; Li, J.; Lin, Q.; Xie, Y.; Liao, J.; et al. Hyperhomocysteinaemia Promotes Doxorubicin-Induced Cardiotoxicity in Mice. Pharmaceuticals 2023, 16, 1212. [Google Scholar] [CrossRef] [PubMed]
- Brandão, S.R.; Reis-Mendes, A.; Neuparth, M.J.; Carvalho, F.; Ferreira, R.; Costa, V.M. The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases. Pharmaceuticals 2023, 16, 1613. [Google Scholar] [CrossRef] [PubMed]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular Biomarkers: Lessons of the Past and Prospects for the Future. Int. J. Mol. Sci. 2022, 23, 5680. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Nishikimi, T.; Nakagawa, Y. Potential pitfalls when interpreting plasma BNP levels in heart failure practice. J. Cardiol. 2021, 78, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Ponomaryova, T.D.; Drozd, D.D.; Kokorina, A.A.; Rusanova, T.Y.; Mishra, P.K.; Goryacheva, I.Y. Heart failure biomarkers BNP and NT-proBNP detection using optical labels. TrAC Trends Anal. Chem. 2022, 146, 116477. [Google Scholar] [CrossRef]
- Pecoraro, M.; Marzocco, S.; Belvedere, R.; Petrella, A.; Franceschelli, S.; Popolo, A. Simvastatin Reduces Doxorubicin-Induced Cardiotoxicity: Effects beyond Its Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 7573. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhang, C.; Liu, N.; Cao, L.; Wang, C.; Feng, Q.; Yao, D.; Long, M.; Jiang, P. Involvement of neurotrophic signaling in doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2020, 19, 1129–1135. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Rienoviar, R.; Setyaningsih, D. Studi Senyawa Aroma Ekstrak Andaliman (Zanthoxylum acanthopodium) dari Beberapa Pelarut Menggunakan Gas Chromatography—Mass Spectra (GC-MS). War. Ind. Has. Pertan. 2018, 35, 85. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A.; et al. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-κB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Jabbari, A.; Babaeipour, V. Bacterial cellulose as a potential biopolymer for wound care. A review. Int. J. Polym. Mater. Polym. Biomater. 2023, 73, 455–477. [Google Scholar] [CrossRef]
- Farokhcheh, M.; Hejazian, L.; Akbarnejad, Z.; Pourabdolhossein, F.; Hosseini, S.M.; Mehraei, T.M.; Soltanpour, N. Geraniol improved memory impairment and neurotoxicity induced by zinc oxide nanoparticles in male wistar rats through its antioxidant effect. Life Sci. 2021, 282, 119823. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Properties of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. Int. Sch. Res. Not. 2013, 2013, 459530. [Google Scholar] [CrossRef]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. LC-MS/MS analysis of flavonoid compounds from Zanthoxylum zanthoxyloides extracts and their antioxidant activities. Nat. Prod. Commun. 2017, 12, 1865–1868. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. Pathol. Basis Vet. Dis. Expert Consult. 2017, 1, 2–43.e19. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxidat. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of reactive oxygen species in aging and age-related diseases: A review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Tiwari, V.; Wilson, D.M. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Satria, D.; Silalahi, J.; Haro, G.; Ilyas, S.; Hasibuan, P.A.Z. Cell Cycle Inhibition of Ethylacetate Fraction of Zanthoxylum acanthopodium DC. Fruit against T47D Cells. Open Access Maced. J. Med. Sci. 2019, 7, 726–729. [Google Scholar] [CrossRef]
- Nazliniwaty; Hanafiah, O.A.; Pertiwi, D.; Satria, D.; Muhammad, M. Antioxidant activity, total phenolic and total flavonoid content of hydroalcoholic extract of Artocarpus lacucha Buch-Ham. Leaves. AIP Conf. Proc. 2021, 2342, 080010. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Harahap, U.; Sitorus, P.; Satria, D. The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon 2020, 6, e04449. [Google Scholar] [CrossRef]
- Dalimunthe, A.; Sitorus, P.; Satria, D. Phytochemicals Constituent Analysis and Cell Cycle Inhibition Effect of Ethanol Extract of Litsea cubeba Lour. Heartwood Towards MCF-7/HER-2 Cell Line. Rasayan J. Chem. 2021, 14, 1447–1450. [Google Scholar] [CrossRef]
- Satria, D.; Silalahi, J.; Haro, G.; Ilyas, S.; Hasibuan, P.A.Z. Chemical analysis and cytotoxic activity of N-hexane fraction of Zanthoxylum acanthopodium DC. fruits. Rasayan J. Chem. 2019, 12, 803–808. [Google Scholar] [CrossRef]
- Angela, I.F.D.; Dalimunthe, A.; Harahap, U.; Satria, D. Effect of andaliman (Zanthoxylum acanthopodium DC.) ethanol extract on doxorubicin-induced toxicity on hematology in male rats. J. Drug Deliv. Ther. 2023, 13, 27–29. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View from the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.D.; Patel, K.R. Enzyme Immunoassay and Enzyme-Linked Immunosorbent Assay. J. Investig. Dermatol. 2013, 133, e12. [Google Scholar] [CrossRef] [PubMed]
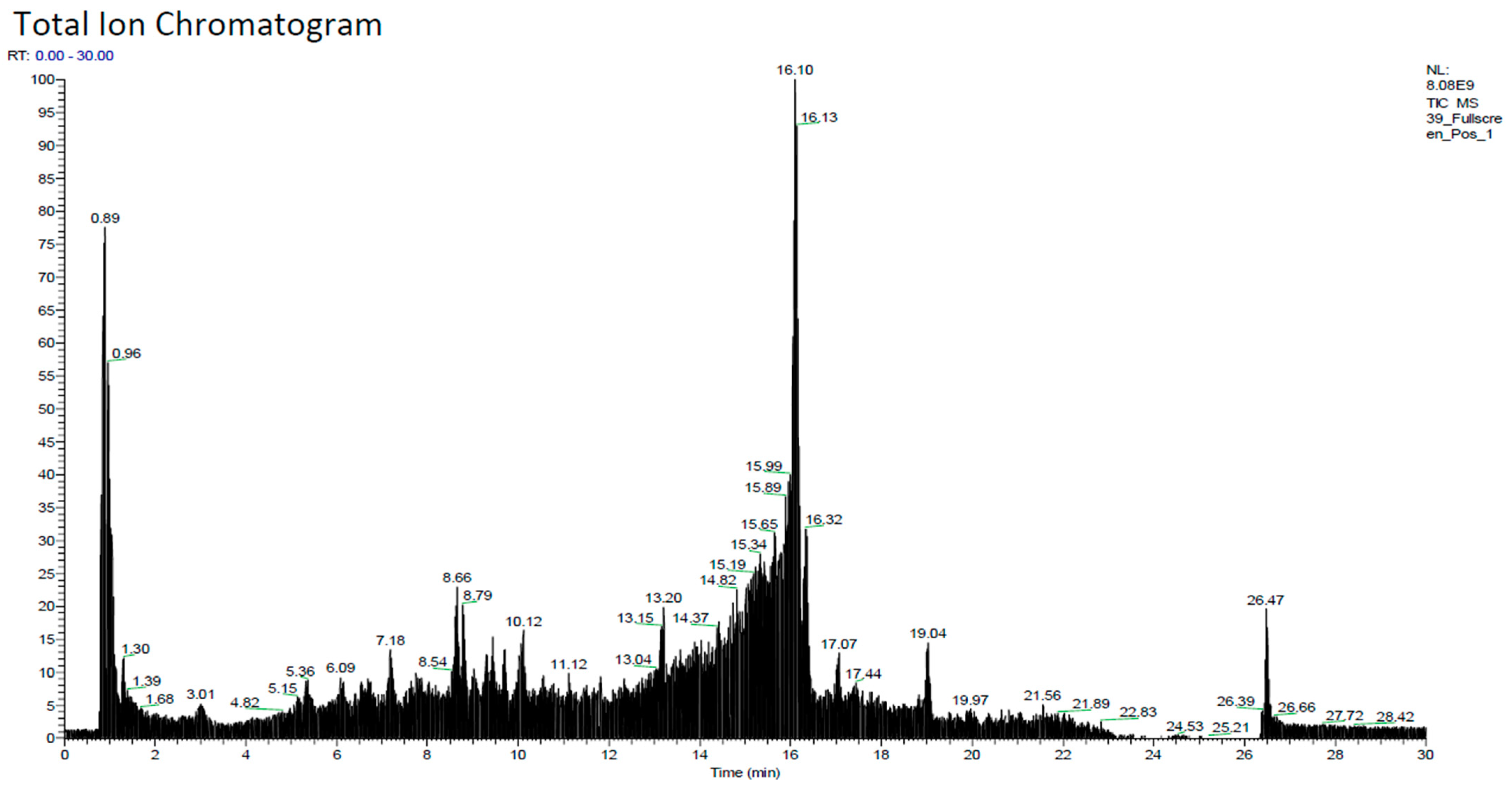
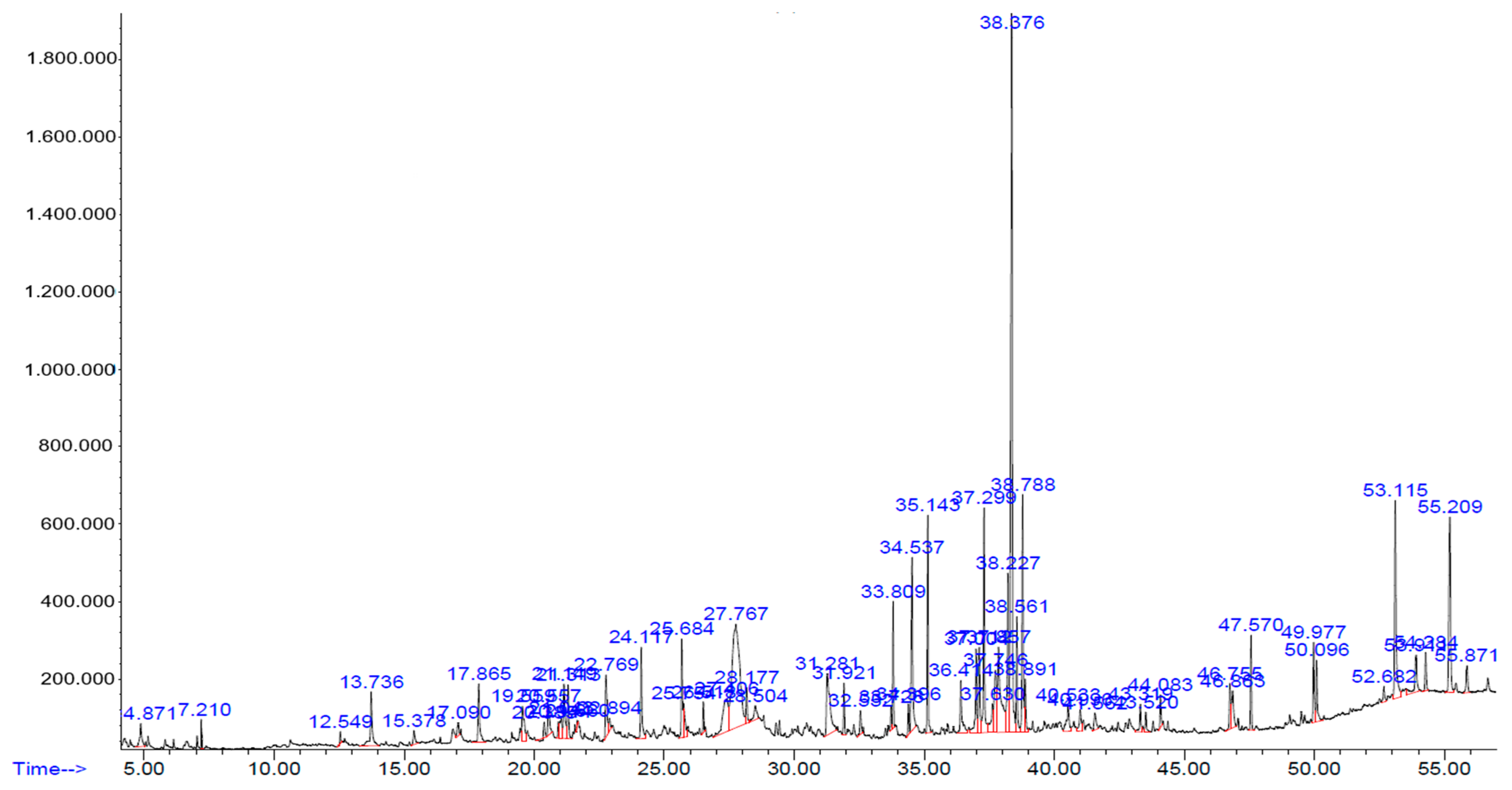
| N | Name | Formula | Molecular Weight | Retention Time (min) |
|---|---|---|---|---|
| 1 | 3-[(2-phenyl-1H-imidazol-4-yl)methylene]-1,3-dihydro-2H-indol-2-one | C18H13N3O | 287.10427 | 14.391 |
| 2 | 4,7,8-trimethoxyfuro[2,3-b]quinoline | C14H13NO4 | 259.0833 | 10.768 |
| 3 | N,N-Dimethyltryptamine | C12H16N2 | 188.13068 | 5.157 |
| 4 | Luotonin A | C18H11N3O | 285.08876 | 15.777 |
| 5 | Citral | C10H16O | 152.11952 | 5.187 |
| 6 | 4-Coumaric acid | C9H8O3 | 164.04676 | 6.542 |
| 7 | Quercetin | C15H10O7 | 302.04158 | 9.615 |
| 8 | Nootkatone | C15H22O | 218.16611 | 17.545 |
| 9 | 7,7-dimethyl-3-spiro(4,4,-dimethyl-2,6-dioxocyclohexyl)-1,2,3,4,5,6,7,8-octahydro-5-quinolinone | C18H25NO3 | 303.1797 | 8.645 |
| 10 | 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6,8-bis[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-one | C27H30O15 | 594.15666 | 5.707 |
| 11 | Isorhamnetin | C16H12O7 | 316.05708 | 11.129 |
| 12 | Berberine | C20H17NO4 | 335.11437 | 9.951 |
| 13 | Ferulic acid | C10H10O4 | 194.05724 | 18.404 |
| 14 | (-)-Caryophyllene oxide | C15H24O | 220.18181 | 11.753 |
| 15 | 8,8-dimethyl-2H,8H-pyrano[3,2-g]chromen-2-one | C14H12O3 | 228.07764 | 13.956 |
| 16 | Jasmonic acid | C12H18O3 | 210.12242 | 5.209 |
| 17 | Kaempferol | C15H10O6 | 286.04656 | 10.929 |
| 18 | Luotonin F | C18H11N3O2 | 301.0837 | 13.011 |
| N | Compounds | Retention Time (min) | Area (%) |
|---|---|---|---|
| 1 | Geraniol | 17.862 | 1.24 |
| 2 | 2-methoxy-4-vinylphenol | 19.564 | 1.10 |
| 3 | Geranic acid | 20.559 | 0.76 |
| 4 | Geranyl acetate | 21.315 | 0.87 |
| 5 | 6-hydroxy-3,7-dimethyl-2,7-octadienyl acetate | 25.689 | 1.47 |
| 6 | 2,2,4-trimethyl-1,3-pentanediol di isobutyrate | 26.520 | 0.35 |
| 7 | Alpha cadinol | 28.171 | 0.59 |
| 8 | N,N-dimethyltryptamine | 31.284 | 2.92 |
| 9 | 2,6,10-dodecatrien-1-ol,3,7,11-trimethyl acetate | 31.927 | 0.70 |
| 10 | Hexadecanoid acid | 33.805 | 1.48 |
| 11 | n-hexadecanoid acid | 34.536 | 3.50 |
| 12 | 9,12,15-octadecatrienoic acid | 37.107 | 1.60 |
| 13 | Phytol | 37.296 | 3.57 |
| 14 | 9,12-octadecadienoic acid | 37.750 | 1.82 |
| 15 | Lineloic acid ethyl ester | 38.229 | 2.91 |
| 16 | Furo[2,3-b]quinoline,4,7,8-trimethoxy | 41.569 | 0.45 |
| 17 | (+)-Sesamin | 53.113 | 4.25 |
| 18 | Stigmasterol | 54.285 | 0.63 |
| 19 | Gamma sitosterol | 55.205 | 3.52 |
| 20 | Beta amyrin | 55.873 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalimunthe, A.; Satria, D.; Sitorus, P.; Harahap, U.; Angela, I.F.D.; Waruwu, S.B. Cardioprotective Effect of Hydroalcohol Extract of Andaliman (Zanthoxylum acanthopodium DC.) Fruits on Doxorubicin-Induced Rats. Pharmaceuticals 2024, 17, 359. https://doi.org/10.3390/ph17030359
Dalimunthe A, Satria D, Sitorus P, Harahap U, Angela IFD, Waruwu SB. Cardioprotective Effect of Hydroalcohol Extract of Andaliman (Zanthoxylum acanthopodium DC.) Fruits on Doxorubicin-Induced Rats. Pharmaceuticals. 2024; 17(3):359. https://doi.org/10.3390/ph17030359
Chicago/Turabian StyleDalimunthe, Aminah, Denny Satria, Panal Sitorus, Urip Harahap, Intan Farah Diba Angela, and Syukur Berkat Waruwu. 2024. "Cardioprotective Effect of Hydroalcohol Extract of Andaliman (Zanthoxylum acanthopodium DC.) Fruits on Doxorubicin-Induced Rats" Pharmaceuticals 17, no. 3: 359. https://doi.org/10.3390/ph17030359
APA StyleDalimunthe, A., Satria, D., Sitorus, P., Harahap, U., Angela, I. F. D., & Waruwu, S. B. (2024). Cardioprotective Effect of Hydroalcohol Extract of Andaliman (Zanthoxylum acanthopodium DC.) Fruits on Doxorubicin-Induced Rats. Pharmaceuticals, 17(3), 359. https://doi.org/10.3390/ph17030359







