Effect of Arthrospira (Spirulina) maxima on Cadmium-Chloride-Induced Alterations in Sexual Behavior and Fertility in Male Wistar Rats
Abstract
1. Introduction
2. Results
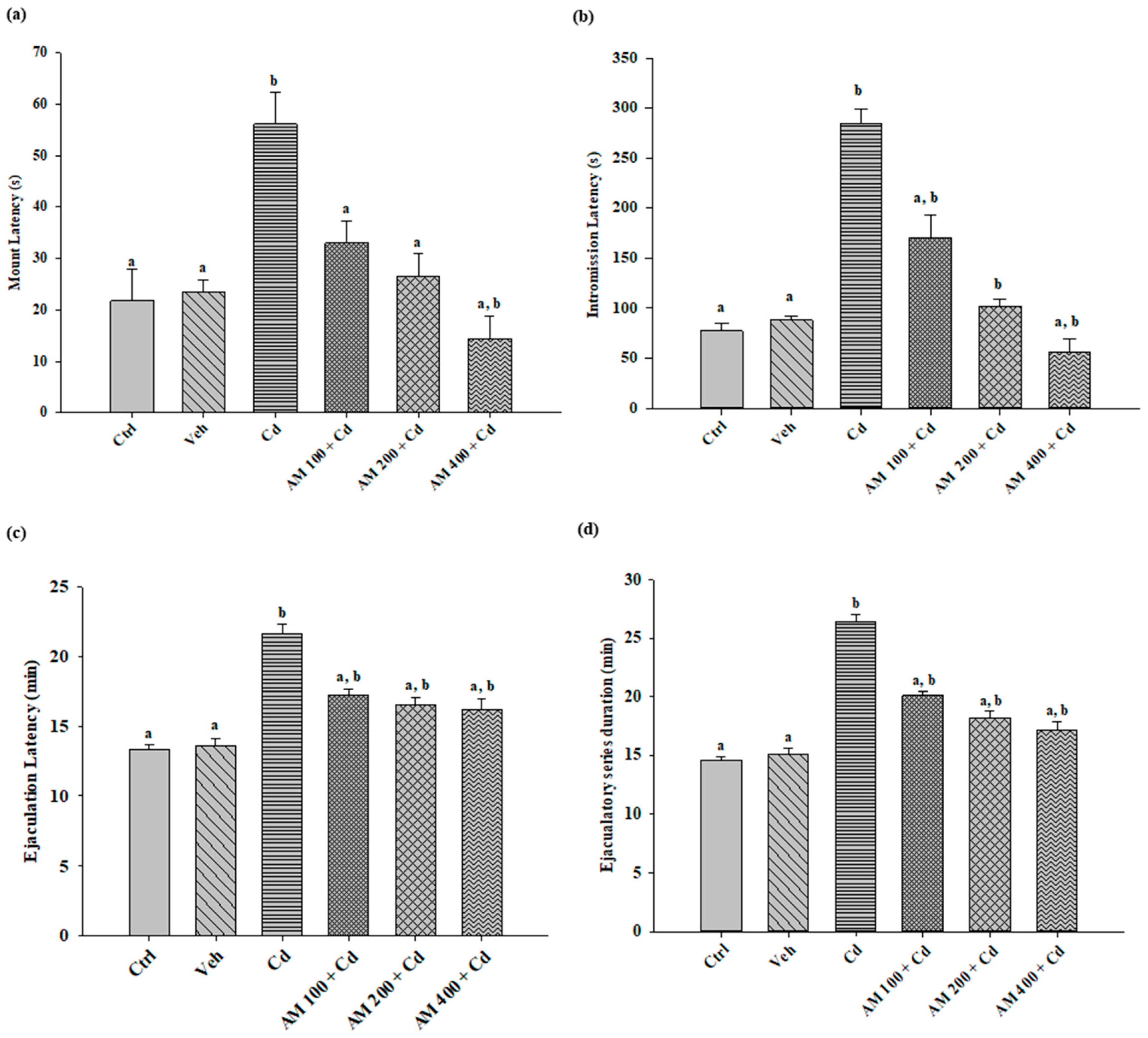
2.1. Sexual Behavior
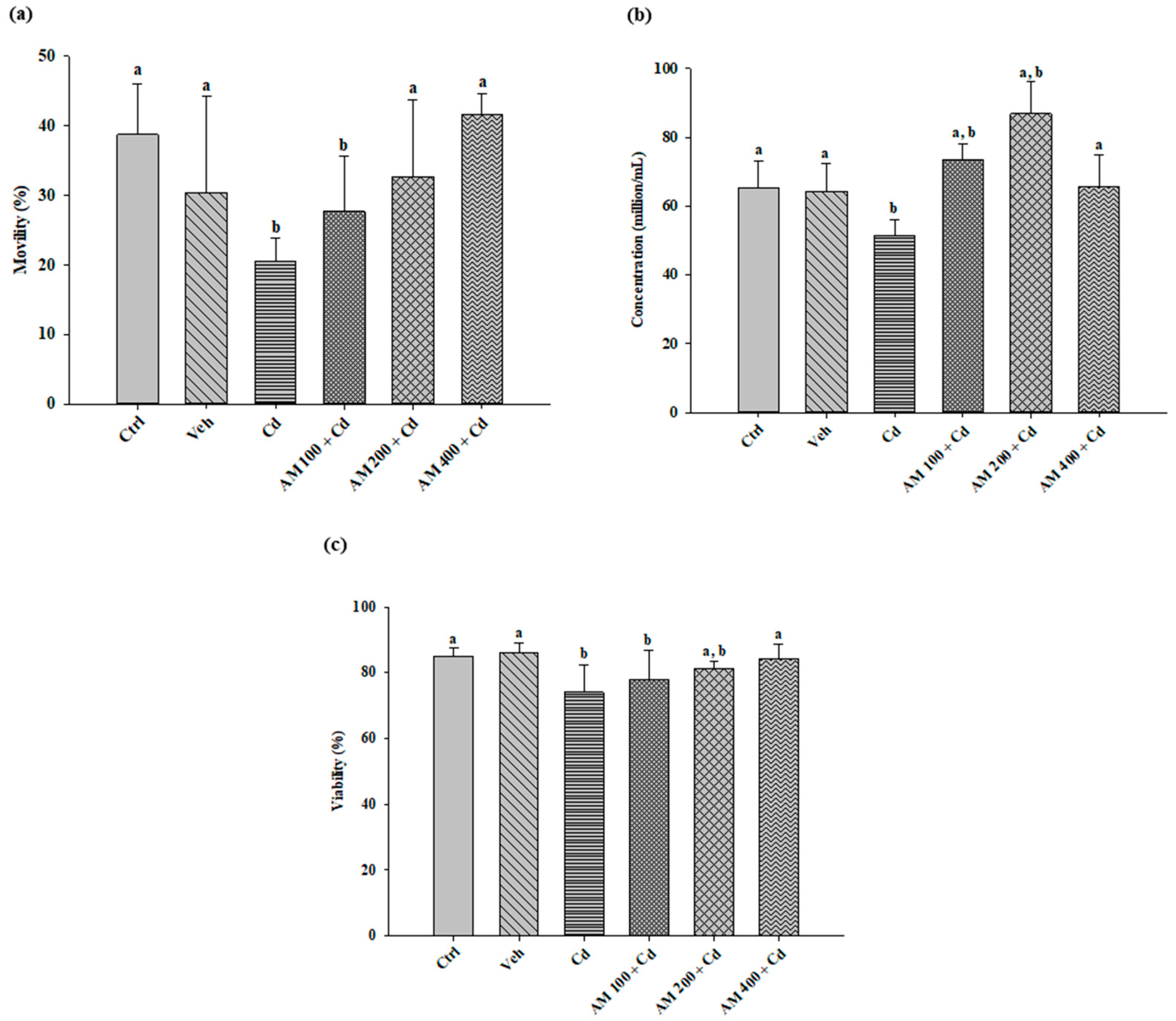
2.2. Semen Quality
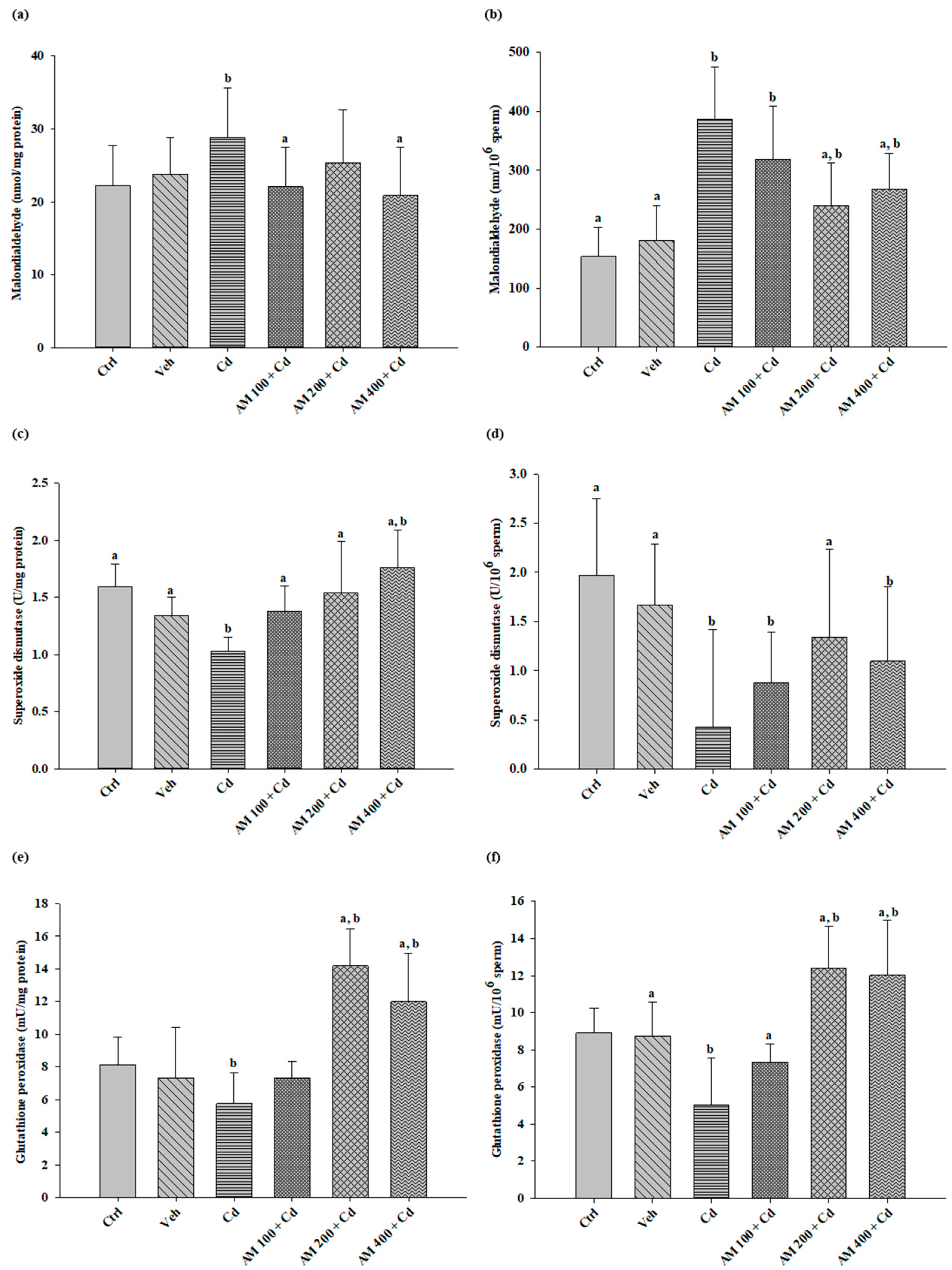
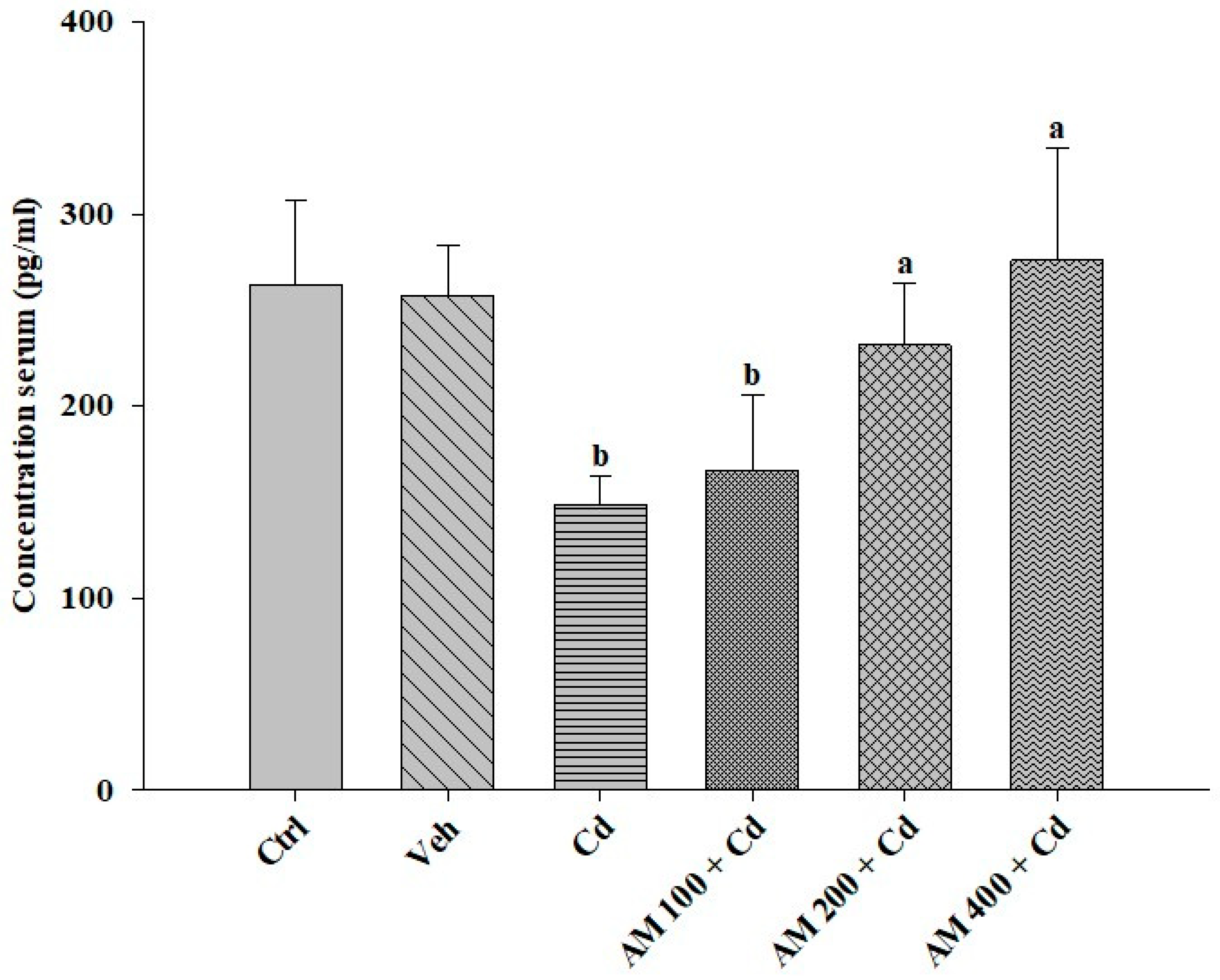
2.3. Biochemical Assays
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Preparation of Females for Mating, Selection of Animals, and Preparation for Experiments
4.3. Treatment and Experimental Groups
4.4. Sexual Performance Evaluation
4.5. Sample Collection
4.6. Sperm Quality Analysis
4.6.1. Evaluation of Sperm Motility and Sperm Count
4.6.2. Evaluation of Sperm Viability
4.7. Biochemical Analyses
4.7.1. Lipid Peroxidation
4.7.2. Superoxide Dismutase
4.7.3. Glutathione Peroxidase
4.8. Determination of Testosterone Concentration
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Yassien, E.E.; Hassan, H.M. Mitigation of Lead Neurotoxicity by the Ethanolic Extract of Laurus Leaf in Rats. Ecotoxicol. Environ. Saf. 2020, 192, 110297. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Williams, P.L.; Chiu, Y.H.; Gaskins, A.J.; Nassan, F.L.; Dadd, R.; Petrozza, J.; Hauser, R.; Chavarro, J.E. Secular Trends in Semen Parameters among Men Attending a Fertility Center between 2000 and 2017: Identifying Potential Predictors. Environ. Int. 2018, 121 Pt 2, 1297–1303. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert. Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, X.; Ge, R.S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Li, S. Relationship between Cadmium Content in Semen and Male Infertility: A Meta-Analysis. Environ. Sci. Pollut. Res. 2018, 26, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Machado-Neves, M. Effect of Heavy Metals on Epididymal Morphology and Function: An Integrative Review. Chemosphere 2022, 291 Pt 2, 133020. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. Ecotoxicol. Environ. Saf. 2019, 182, 109398. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ma, Y.; Zhu, J.; Zou, H.; Liu, Z. Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility. Cells 2022, 11, 3601. [Google Scholar] [CrossRef]
- Iqbal, T.; Cao, M.; Zhao, Z.; Zhao, Y.; Chen, L.; Chen, T.; Li, C.; Zhou, X. Damage to the Testicular Structure of Rats by Acute Oral Exposure of Cadmium. Int. J. Environ. Res. Public Health 2021, 18, 6038. [Google Scholar] [CrossRef]
- Mannino, F.; Pallio, G.; Imbesi, C.; Scarfone, A.; Puzzolo, D.; Micali, A.; Freni, J.; Squadrito, F.; Bitto, A.; Minutoli, L. Beta-Caryophyllene, a Plant-Derived CB2 Receptor Agonist, Protects SH-SY5Y Cells from Cadmium-Induced Toxicity. Int. J. Mol. Sci. 2023, 24, 15487. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Benko, F.; Ďuračka, M.; Baňas, Š.; Lukáč, N.; Tvrdá, E. Biological Relevance of Free Radicals in the Process of Physiological Capacitation and Cryocapacitation. Oxygen 2022, 2, 164–176. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Maria Varoni, E.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R. Oxidative Stress: Relevance, Evaluation, and Management. In Male Infertility in Reproductive Medicine; CRC Press: Boca Raton, FL, USA, 2019; pp. 119–128. [Google Scholar] [CrossRef]
- Paulo Cardoso, J.; Cocuzza, M.; Elterman, D. Optimizing Male Fertility: Oxidative Stress and the Use of Antioxidants. World J. Urol. 2019, 37, 1029–1034. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Soni, Y.; Patel, P.; Parekh, N.; Ramasamy, R. A Systematic Review and Evidence-Based Analysis of Ingredients in Popular Male Fertility Supplements. Urology 2020, 136, 133–141. [Google Scholar] [CrossRef]
- Ahmadip, S.; Bashirip, R.; Ghadiri-Anarip, A.; Nadjarzadehp, A. Antioxidant Supplements and Semen Parameters: An Evidence Based Review. Int. J. Reprod. Biomed. 2016, 14, 729. [Google Scholar] [CrossRef]
- Martínez-Galero, E.; Pérez-Pastén, R.; Perez-Juarez, A.; Fabila-Castillo, L.; Gutiérrez-Salmeán, G.; Chamorro, G. Preclinical Antitoxic Properties of Spirulina (Arthrospira). Pharm. Biol. 2015, 54, 1345–1353. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G.; Politécnico Nacional Avenida Wilfrido Massieu, I.; Adolfo López Mateos Del Gustavo Madero, U.A. Aspectos Nutricionales y Toxicológicos de Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [CrossRef]
- Bobescu, E.; Bălan, A.; Moga, M.A.; Teodorescu, A.; Mitrică, M.; Dima, L. Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women? Mar. Drugs 2020, 18, 651. [Google Scholar] [CrossRef] [PubMed]
- Anvar, A.A.; Nowruzi, B. Bioactive Properties of Spirulina: A Review. Microb. Bioact. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baroty, G.S. Spirulina Maxima L-Asparaginase: Immobilization, Antiviral and Antiproliferation Activities. Recent. Pat. Biotechnol. 2020, 14, 154–163. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Thu Thao, N.T.; Wijerathna, H.M.S.M.; Lee, J.; Edussuriya, M.; Choi, D.; Saravana Kumar, R. In Vitro and in Vivo Anticandidal Efficacy of Green Synthesized Gold Nanoparticles Using Spirulina Maxima Polysaccharide. Process Biochem. 2020, 92, 138–148. [Google Scholar] [CrossRef]
- Ovando, C.A.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional Properties and Health Benefits of Bioactive Peptides Derived from Spirulina: A Review. Food Rev. Int. 2016, 34, 34–51. [Google Scholar] [CrossRef]
- Ferreira-Hermosillo, A.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Hepatoprotective Effects of Spirulina Maxima in Patients with Non-Alcoholic Fatty Liver Disease: A Case Series. J. Med. Case Rep. 2010, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Castro-García, S.Z.; Chamorro-Cevallos, G.; Quevedo-Corona, L.; McCarty, M.F.; Bobadilla-Lugo, R.A. Beneficial Effects of Phycobiliproteins from Spirulina Maxima in a Preeclampsia Model. Life Sci. 2018, 211, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sámano, J.; Torres-Montes de Oca, A.; Luqueño-Bocardo, O.I.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Spirulina maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rebolledo, G.A.; Galar-Martínez, M.; García-Rodríguez, R.V.; Chamorro-Cevallos, G.A.; Hernández-Reyes, A.G.; Martínez-Galero, E. Antioxidant Effect of Spirulina (Arthrospira) Maxima on Chronic Inflammation Induced by Freund’s Complete Adjuvant in Rats. J. Med. Food 2015, 18, 865. [Google Scholar] [CrossRef] [PubMed]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Quevedo-Corona, L.; Pérez-Pastén-Borja, R.; Rivero-Ramírez, N.L.; Ríos-Castro, E.; Pérez-Gutiérrez, S.; Pérez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of Ethanol-Induced Gastric Ulcers in Rats Pretreated with Phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Montaño-González, R.I.; Gutiérrez-Salmeán, G.; Mojica-Villegas, M.A.; Cristóbal-Luna, J.M.; Briseño-Bugarín, J.; Chamorro-Cevallos, G. Phycobiliproteins extract from Spirulina protects against single-dose cadmium-induced reproductive toxicity in male mice. Environ. Sci. Pollut. Res. 2022, 29, 17441–17455. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef]
- Singh, K.B.; Kaushalendra-Rajan, J.P. Therapeutical and Nutraceutical Roles of Cyanobacterial Tetrapyrrole Chromophore: Recent Advances and Future Implications. Front. Microbiol. 2022, 13, 932459. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106 Pt A, 430–445. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Ru, Y.-F.; Liu, M.; Tang, J.-N.; Zheng, J.-F.; Wu, B.; Gu, Y.-H.; Shi, H.-J. Reproductive Effects of Cadmium on Sperm Function and Early Embryonic Development in Vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef]
- Ujah, G.A.; Nna, V.U.; Agah, M.I.; Omue, L.O.; Leku, C.B.; Osim, E.E. Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male Wistar rats. Andrologia 2018, 50, e12866. [Google Scholar] [CrossRef]
- Hassan, W.A.; El-kashlan, A.M.; Mohamed, N.A. Egyptian date palm pollen ameliorates testicular dysfunction induced by cadmium chloride in adult male rats. J. Am. Sci. 2012, 8, 659–669. [Google Scholar]
- Cheitlin, M.D. Sexual Activity and Cardiac Risk. Am. J. Cardiol. 2005, 96 (Suppl. 2), 24–28. [Google Scholar] [CrossRef]
- Dye, C.A.; Engelstein, E.; Swearingen, S.; Murphy, J.; Larsen, T.; Volgman, A.S. Sex, Rhythm & Death: The Effect of Sexual Activity on Cardiac Arrhythmias and Sudden Cardiac Death. Front. Cardiovasc. Med. 2022, 9, 987247. [Google Scholar] [CrossRef]
- Bu, T.; Mi, Y.; Zeng, W.; Zhang, C. Protective Effect of Quercetin on Cadmium-Induced Oxidative Toxicity on Germ Cells in Male Mice. Anat. Rec. 2011, 294, 520–526. [Google Scholar] [CrossRef]
- Strumylaite, L.; Bogusevicius, A.; Abdrachmanovas, O.; Baranauskiene, D.; Kregzdyte, R.; Pranys, D.; Poskiene, L. Cadmium Concentration in Biological Media of Breast Cancer Patients. Breast Cancer Res. Treat. 2011, 125, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm Motility and Morphology Changes in Rats Exposed to Cadmium and Diazinon. Reprod. Biol. Endocrinol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Ourique, G.M.; Saccol, E.M.H.; Pês, T.S.; Glanzner, W.G.; Schiefelbein, S.H.; Woehl, V.M.; Baldisserotto, B.; Pavanato, M.A.; Gonçalves, P.B.D.; Barreto, K.P. Protective Effect of Vitamin E on Sperm Motility and Oxidative Stress in Valproic Acid Treated Rats. Food Chem. Toxicol. 2016, 95, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, Z.; Zhu, Y.; Li, S.; Zhang, L.; Luo, Y. Effects of Paternal Cadmium Exposure on the Sperm Quality of Male Rats and the Neurobehavioral System of Their Offspring. Exp. Ther. Med. 2015, 10, 2356–2360. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.M.; Yusuf, K. Improvement of Mercuric Chloride-Induced Testis Injuries and Sperm Quality Deteriorations by Spirulina Platensis in Rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; El Awdan, S.A.; Ebaid, H.; Alhazza, I.M. Antioxidant Potential of Spirulina Platensis Mitigates Oxidative Stress and Reprotoxicity Induced by Sodium Arsenite in Male Rats. Oxid. Med. Cell. Longev. 2016, 2016, 7174351. [Google Scholar] [CrossRef]
- Adi, P.J.; Burra, S.P.; Vataparti, A.R.; Matcha, B. Calcium, Zinc and Vitamin E Ameliorate Cadmium-Induced Renal Oxidative Damage in Albino Wistar Rats. Toxicol. Rep. 2016, 3, 591–597. [Google Scholar] [CrossRef]
- Obembe, O.O.; Raji, Y. Effects of Aqueous Extract of Moringa Oleifera Seed on Cadmium-Induced Reproductive Toxicity in Male Wistar Rats. Afr. Health Sci. 2018, 18, 653–663. [Google Scholar] [CrossRef]
- McCarty, M.F.; Iloki Assanga, S.B.; Lewis Luján, L.; O’Keefe, J.H.; DiNicolantonio, J.J. Nutraceutical Strategies for Suppressing NLRP3 Inflammasome Activation: Pertinence to the Management of COVID-19 and Beyond. Nutrients 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, B.I.; Marković, S.D.; Dordević, N.Z.; Trbojević, I.S.; Štajn, A.Š.; Saičić, Z.S. Cadmium-Induced Lipid Peroxidation and Changes in Antioxidant Defense System in the Rat Testes: Protective Role of Coenzyme Q10 and Vitamin E. Reprod. Toxicol. 2010, 29, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 3, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Djuric, A.; Begic, A.; Gobeljic, B.; Ninkovic, M.; Stanojevic, I.; Vojvodic, D.; Pantelic, A.; Zebic, G.; Prokic, V.; Dejanovic, B.; et al. Oxidative Stress, Bioelements and Androgen Status in Testes of Rats Subacutely Exposed to Cadmium. Food Chem. Toxicol. 2015, 86, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M. Antioxidant Effect of Vitamin E Treatment on Some Heavy Metals-Induced Renal and Testicular Injuries in Male Mice. Saudi J. Biol. Sci. 2011, 18, 63–72. [Google Scholar] [CrossRef]
- Castillo-Castañeda, P.C.; Gaxiola-Robles, R.; Labrada-Martagón, V.; Vargas, B.A.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Oxidative Damage to Proteins Related to Metals and Antioxidant Defenses in Breastmilk. Nutr. Hosp. 2017, 34, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mouro, V.G.S.; Martins, A.L.P.; Silva, J.; Menezes, T.P.; Gomes, M.L.M.; Oliveira, J.A.; Melo, F.C.S.A.; Matta, S.L.P. Subacute Testicular Toxicity to Cadmium Exposure Intraperitoneally and Orally. Oxid. Med. Cell. Longev. 2019, 2019, 3429635. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Wang, H.; Zhou, S.; Li, L.; Chen, X.; Wang, G.; Liu, J.; Ge, H.S.; Ge, R.S. A Brief Exposure to Cadmium Impairs Leydig Cell Regeneration in the Adult Rat Testis. Sci. Rep. 2017, 7, 6337. [Google Scholar] [CrossRef]
- Babaknejad, N.; Bahrami, S.; Moshtaghie, A.A.; Nayeri, H.; Rajabi, P.; Golshan Iranpour, F. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol. Trace Element Res. 2017, 185, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Požgajová, M.; Navrátilová, A.; Kovár, M. Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12380. [Google Scholar] [CrossRef]
- de Aluja, A.S. Animales de laboratorio y la Norma Oficial Mexicana (NOM-062-ZOO-1999) [Laboratory animals and official Mexican norms (NOM-062-ZOO-1999)]. Gac. Med. Mex. 2002, 138, 295–298. [Google Scholar] [PubMed]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Description of a new method of ovariectomy in female rats. Rev. Bras. Reumatol. 2012, 52, 462–470. (In Portuguese) [Google Scholar]
- Ikbal, M.O.; Belgin, B.; Ebru, B.; Burak, C.; Sevgi, G.; Semra, E. Determination of acute and chronic effects of cadmium on the cardiovascular system of rats. Toxicol. Mech. Methods 2009, 19, 308–317. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A. Effect of Aqueous Extract of Massularia Acuminata Stem on Sexual Behaviour of Male Wistar Rats. Evid. Based Complement. Altern. Med. 2011, 2011, 738103. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Saitoh, H.; Sano, M.; Tomita, I.; Ohkawa, M.; Ikekawa, T. Studies on Antioxidant Effects of Hypsizigus marmoreus. II. Effects of Hypsizigus marmoreus for Antioxidant Activities of Tumor-Bearing Mice. Yakugaku Zasshi 1998, 118, 476–481. [Google Scholar] [CrossRef]
- Andersen, H.R.; Nielsen, J.B.; Nielsen, F.; Grandjean, P. Antioxidative enzyme activities in human erythrocytes. Clin. Chem. 1997, 43, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candelaria, G.-C.; Rosa Virginia, G.-R.; María Angélica, M.-V.; Yuliana, G.-M.; José Melesio, C.-L.; Germán, C.-C. Effect of Arthrospira (Spirulina) maxima on Cadmium-Chloride-Induced Alterations in Sexual Behavior and Fertility in Male Wistar Rats. Pharmaceuticals 2024, 17, 332. https://doi.org/10.3390/ph17030332
Candelaria G-C, Rosa Virginia G-R, María Angélica M-V, Yuliana G-M, José Melesio C-L, Germán C-C. Effect of Arthrospira (Spirulina) maxima on Cadmium-Chloride-Induced Alterations in Sexual Behavior and Fertility in Male Wistar Rats. Pharmaceuticals. 2024; 17(3):332. https://doi.org/10.3390/ph17030332
Chicago/Turabian StyleCandelaria, Galván-Colorado, García-Rodríguez Rosa Virginia, Mojica-Villegas María Angélica, García-Martínez Yuliana, Cristóbal-Luna José Melesio, and Chamorro-Cevallos Germán. 2024. "Effect of Arthrospira (Spirulina) maxima on Cadmium-Chloride-Induced Alterations in Sexual Behavior and Fertility in Male Wistar Rats" Pharmaceuticals 17, no. 3: 332. https://doi.org/10.3390/ph17030332
APA StyleCandelaria, G.-C., Rosa Virginia, G.-R., María Angélica, M.-V., Yuliana, G.-M., José Melesio, C.-L., & Germán, C.-C. (2024). Effect of Arthrospira (Spirulina) maxima on Cadmium-Chloride-Induced Alterations in Sexual Behavior and Fertility in Male Wistar Rats. Pharmaceuticals, 17(3), 332. https://doi.org/10.3390/ph17030332







