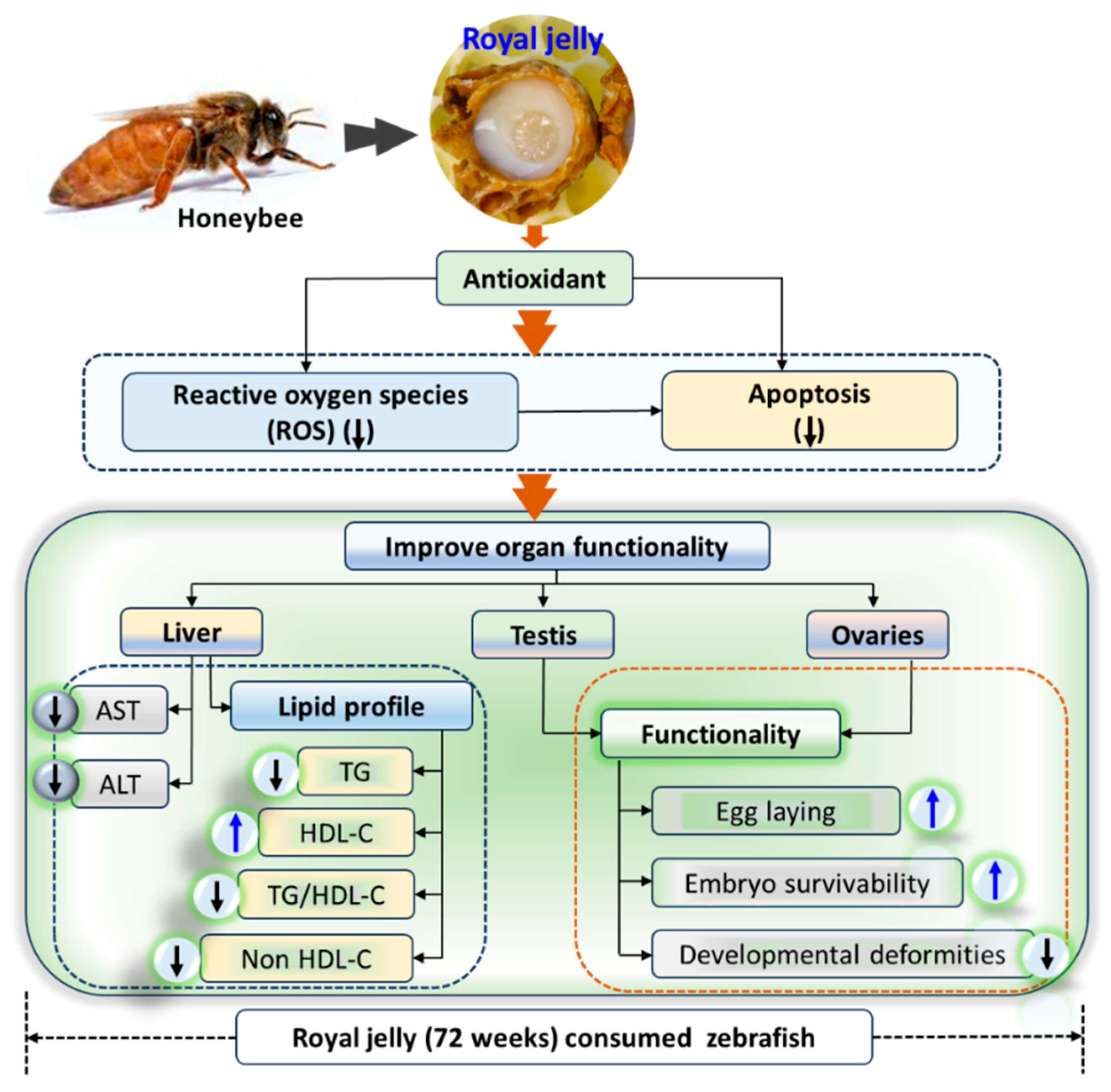
Long-Term Supplementation of Royal Jelly (Raydel®) Improves Zebrafish Growth, Embryo Production and Survivability, Blood Lipid Profile and Functionality of Vital Organs: A 72-Weeks’ Consumption Study
Abstract
1. Introduction
2. Results
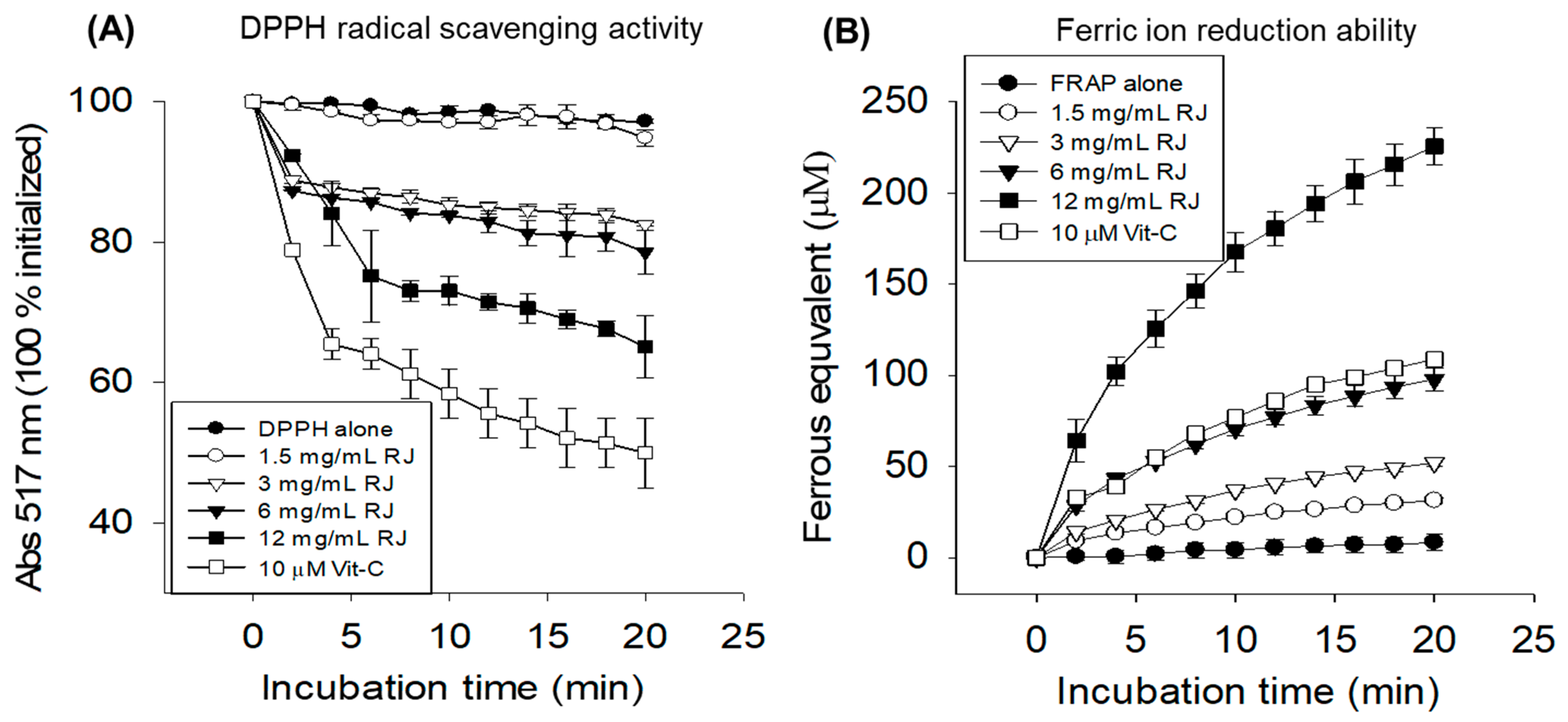
2.1. In Vitro Antioxidant Activity of Royal Jelly
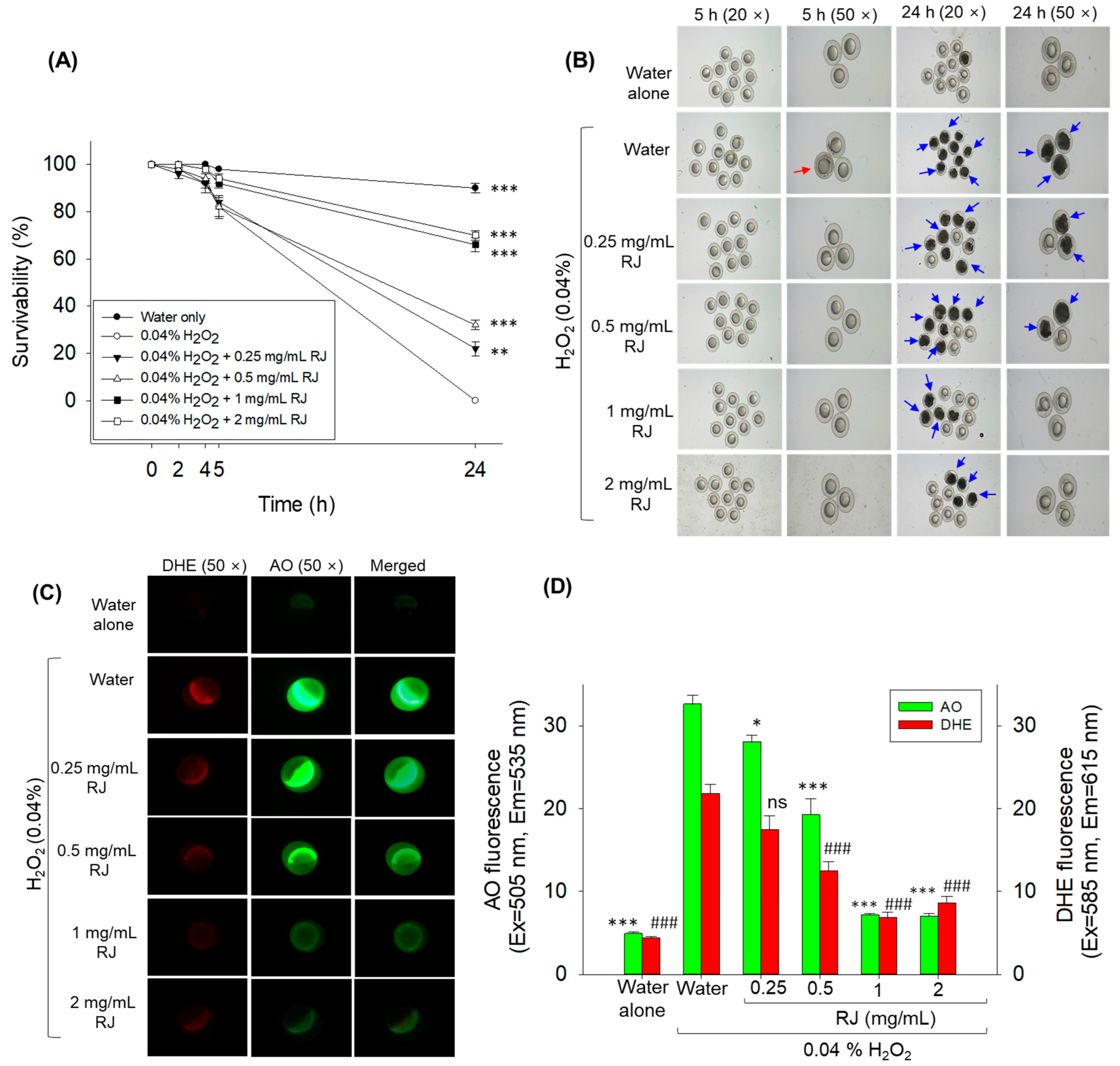
2.2. Protective Effect of Royal Jelly against H2O2 Exposure in Zebrafish Embryos
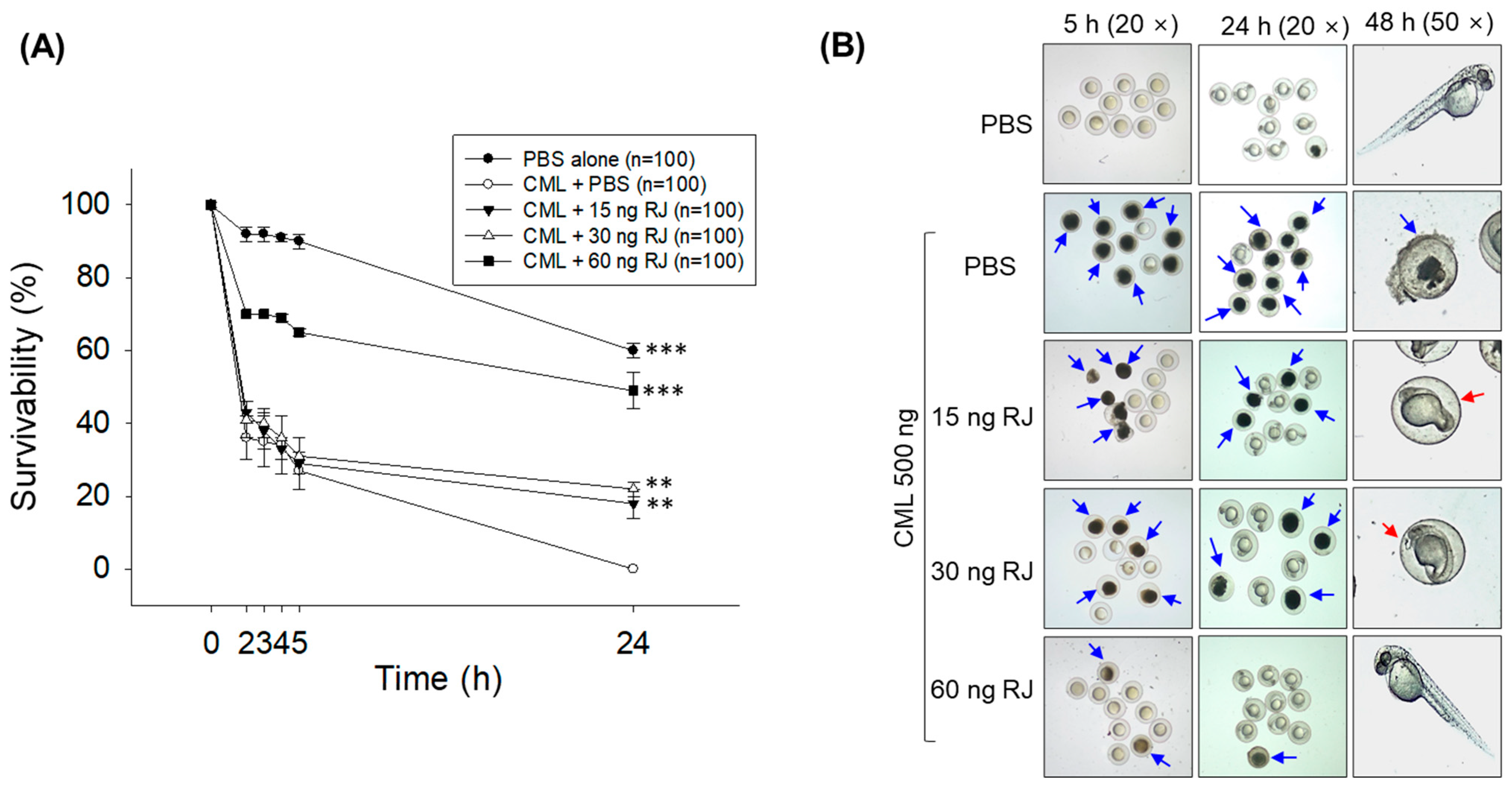
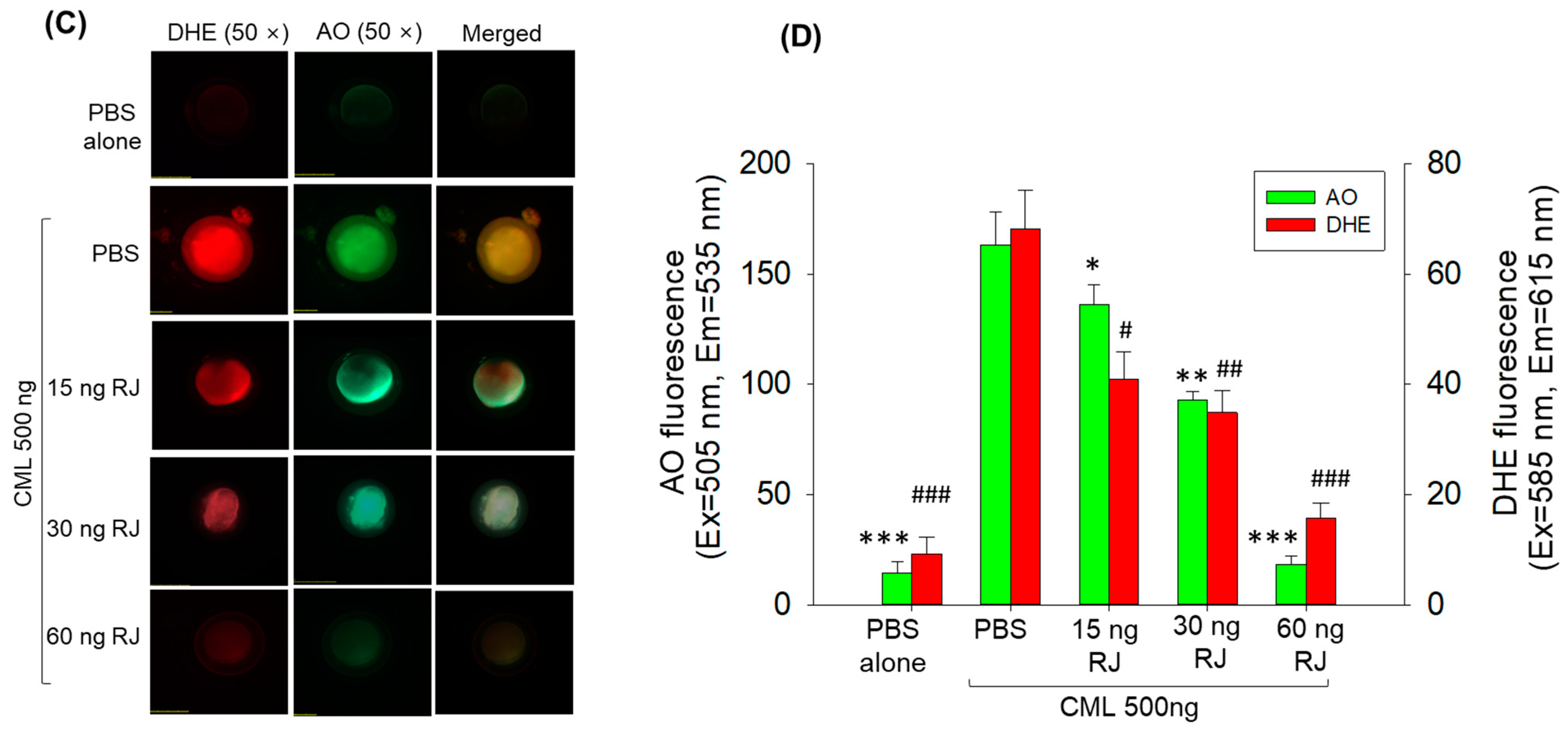
2.3. Royal Jelly Prevents Carboxymethyllysine (CML)-Induced Acute Embryo Death
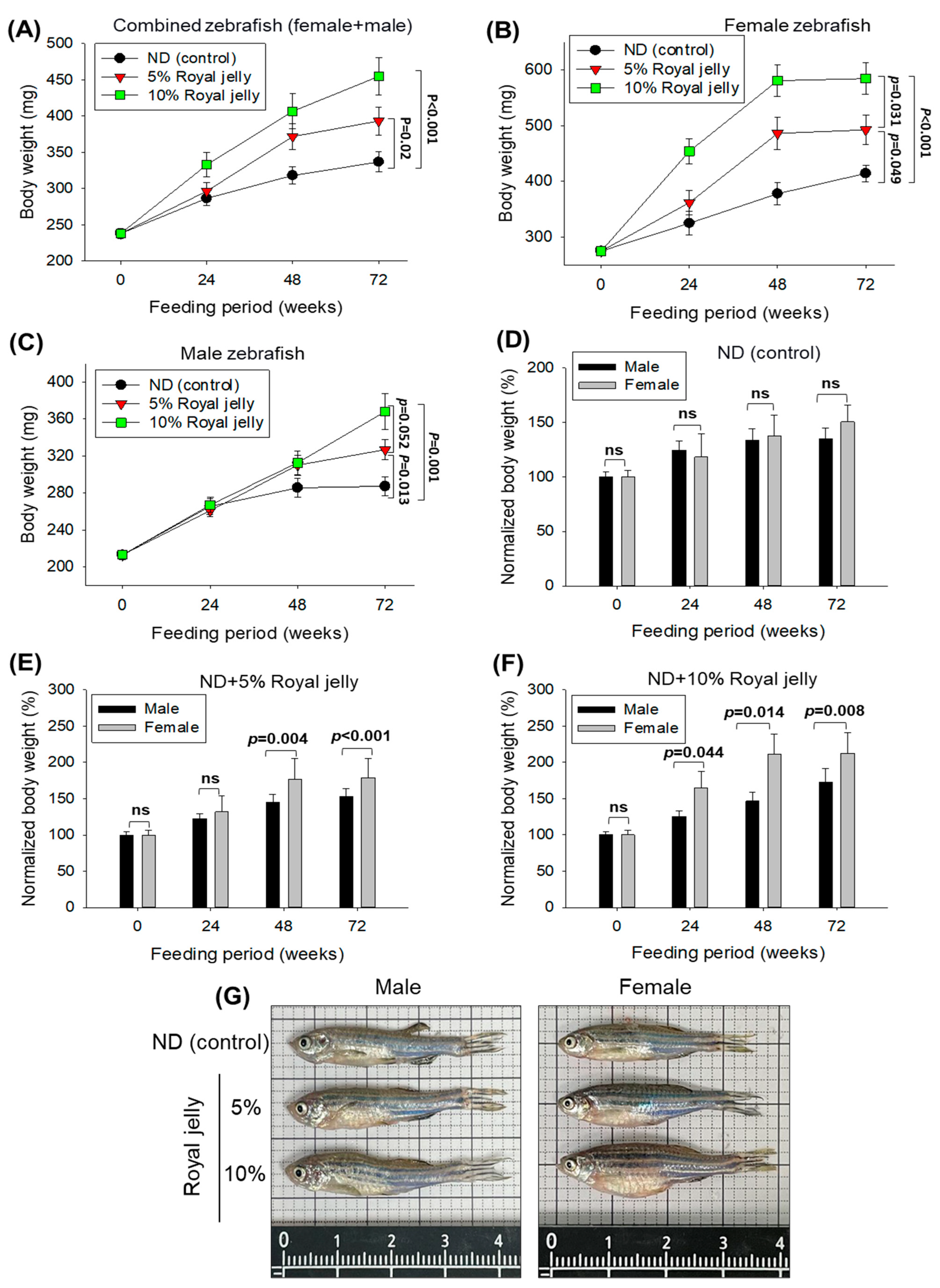
2.4. Body Weight and Survivability of Zebrafish
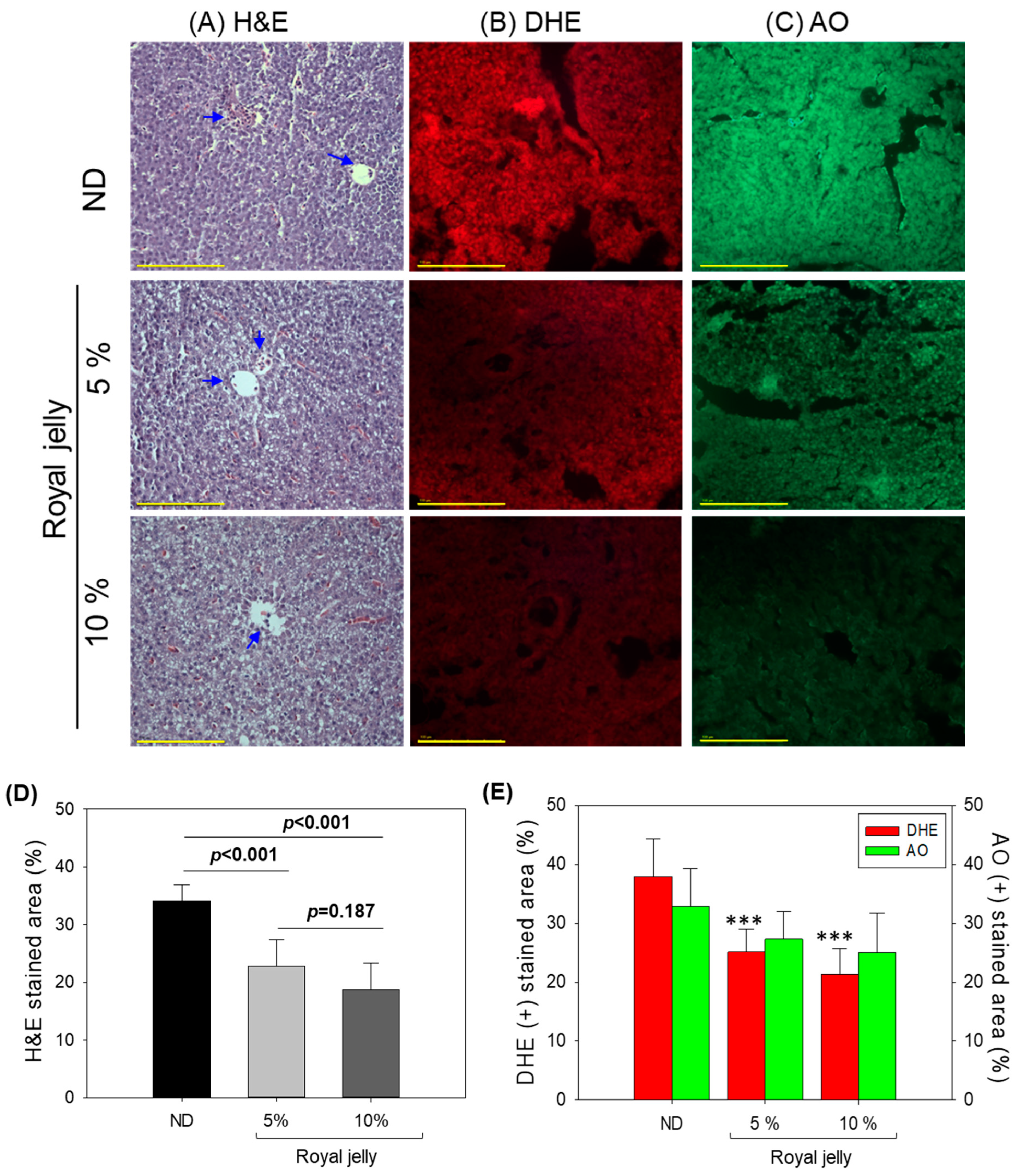
2.5. Evaluation of the Liver
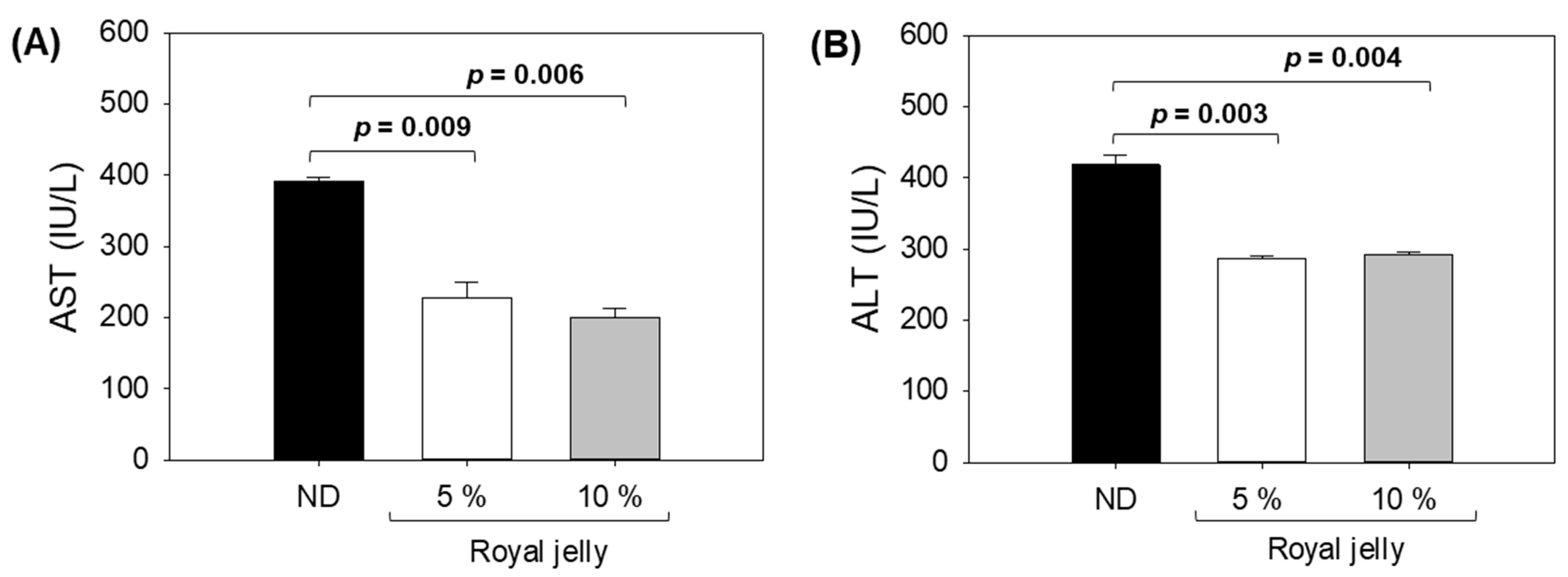
2.6. Hepatic Function Biomarkers
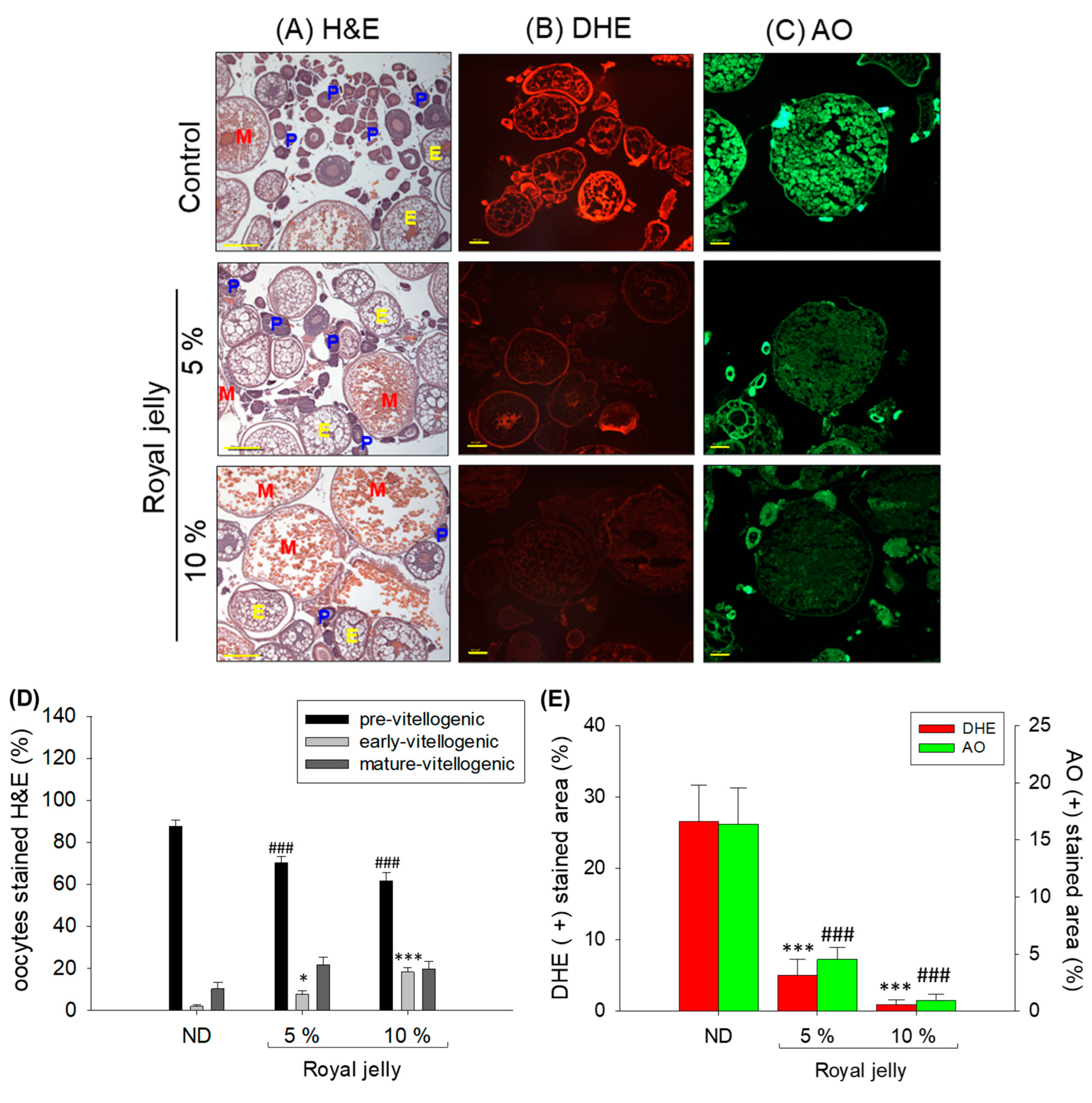
2.7. Effect of Royal Jelly Consumption on Ovaries
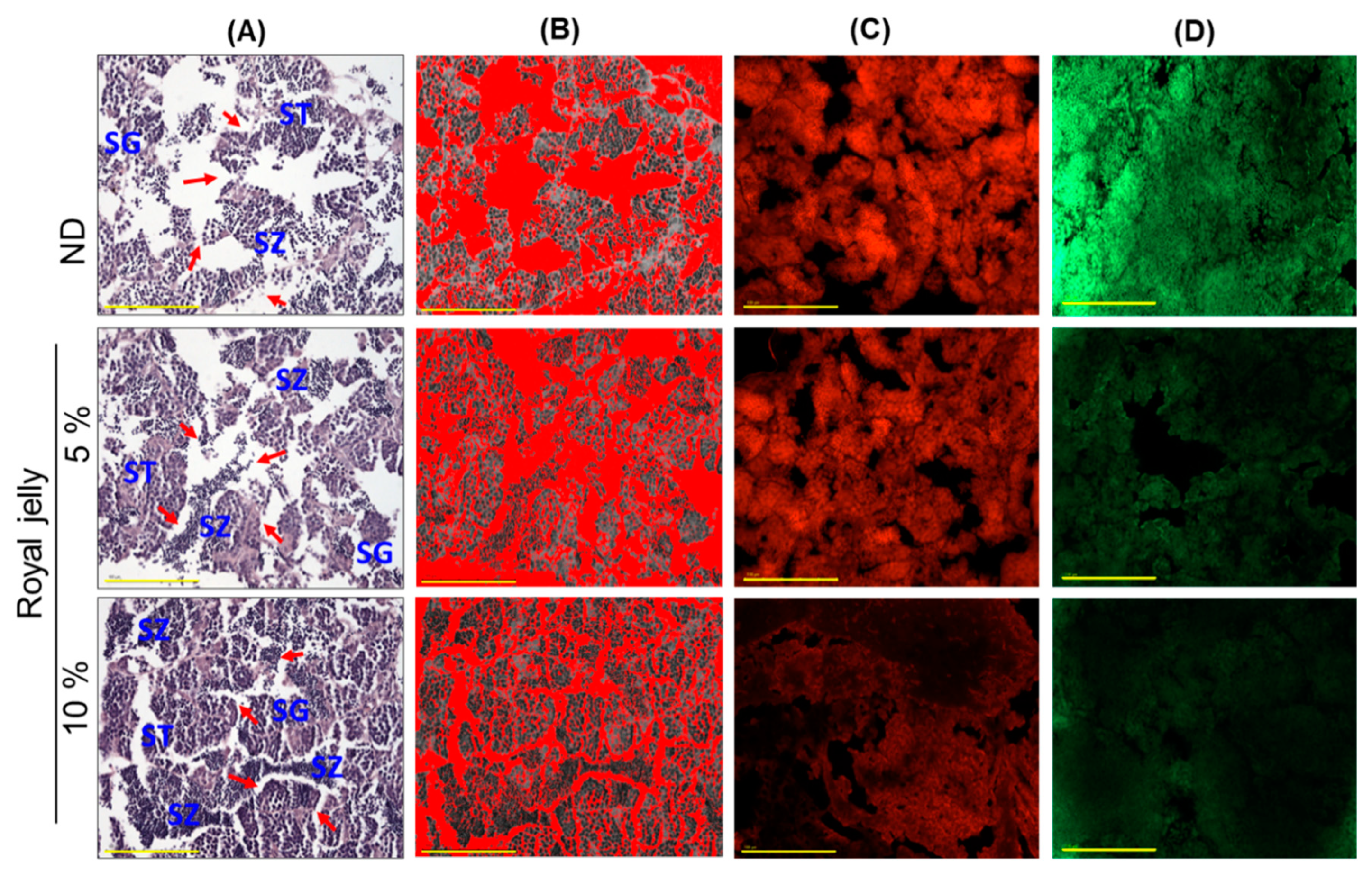
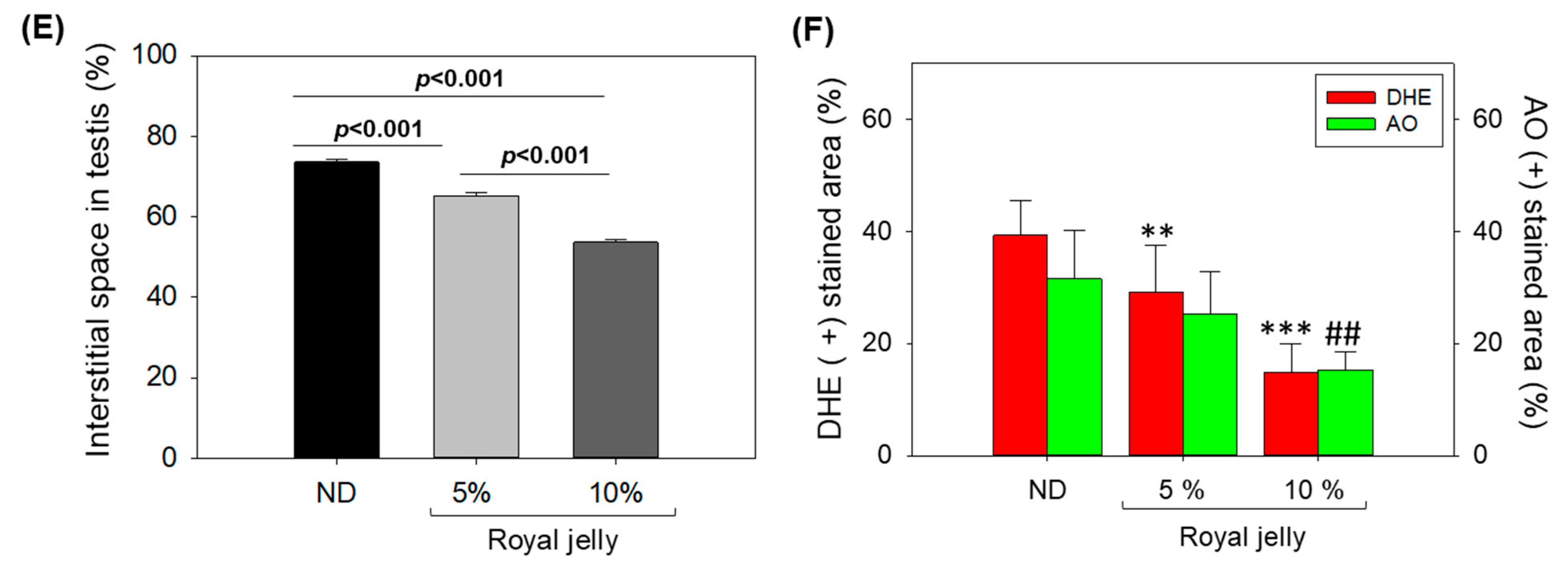
2.8. Effect of Royal Jelly Consumption on Testis
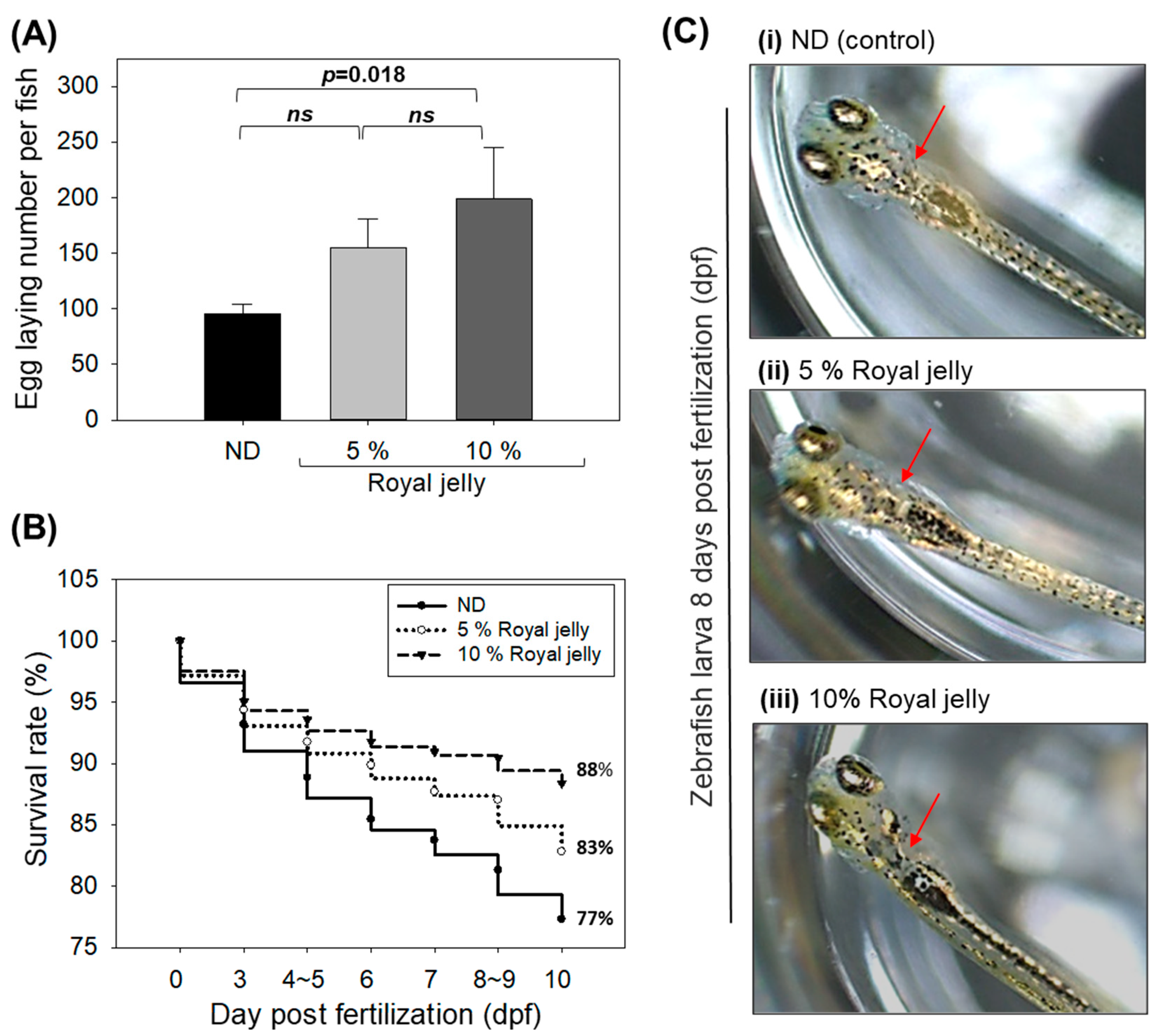
2.9. Effect of Royal Jelly Consumption on Egg-Laying Ability
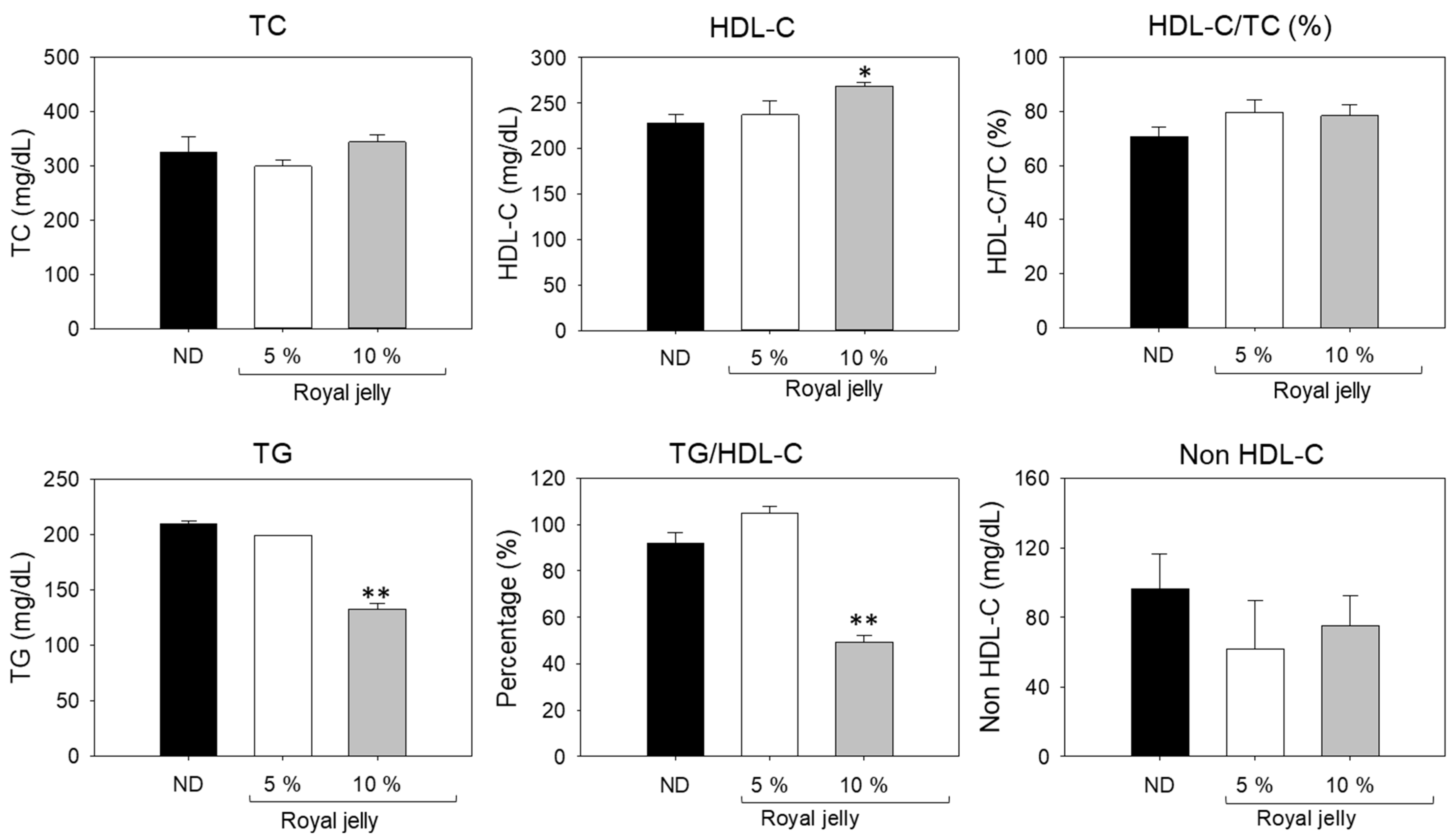
2.10. Blood Lipid Profile
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Antioxidant Activity
4.3. Zebrafish Husbandry
4.4. Zebrafish Embryo Collection and Evaluation of Embryo-Protective Effect of Royal Jelly
4.5. Microinjection of Zebrafish Embryos
4.6. Fluorescent Staining for ROS and Apoptosis
4.7. Zebrafish Fed with Royal Jelly
4.8. Effect of Royal Jelly Supplementation on Egg-Laying and Embryo Survivability of Zebrafish
4.9. Histological Analysis
4.10. Fluorescent Imaging for Reactive Oxygen Species (ROS) and Apoptosis
4.11. Blood Lipid Profile and Hepatic Function Biomarker Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on royal jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2, 7074209. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R. Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Argüelles, S. Bee Products: Royal Jelly and Propolis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128124918. [Google Scholar]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Mureşan, C.I.; Dezmirean, D.S.; Marc, B.D.; Suharoschi, R.; Pop, O.L.; Buttstedt, A. Biological properties and activities of major royal jelly proteins and their derived peptides. J. Funct. Foods 2022, 98, 105286. [Google Scholar] [CrossRef]
- Yu, X.; Tu, X.; Tao, L.; Daddam, J.; Li, S.; Hu, F. Royal Jelly fatty acids: Chemical composition, extraction, biological activity, and prospect. J. Funct. Foods 2023, 111, 105868. [Google Scholar] [CrossRef]
- Botezan, S.; Baci, G.-M.; Bagameri, L.; Pașca, C.; Dezmirean, D.S. Current status of the bioactive properties of royal jelly: A comprehensive review with a focus on Its anticancer, anti-Inflammatory, and antioxidant effects. Molecules 2023, 28, 1510. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Oshvandi, K.; Aghamohammadi, M.; Kazemi, F.; Masoumi, S.Z.; Mazdeh, M.; Molavi, V.M. Effect of royal jelly capsule on quality of life of patients with multiple sclerosis: A double-blind randomized controlled clinical trial. Iran. Red Crescent Med. J. 2020, 22, e74. [Google Scholar]
- Shafiee, Z.; Hanifi, N.; Rashtchi, V. The effect of royal jelly on the level of consciousness in patients with traumatic brain injury: A double-blind randomized clinical trial. Nurs. Midwifery Stud. 2022, 11, 96–102. [Google Scholar]
- Peivandi, S.; Khalili, S.S.; Abbasi, Z.; Zamaniyan, M.; Gordani, N.; Moradi, S. Effect of Royal Jelly on Sperm Parameters and Testosterone Levels in Infertile Men. J. Maz. Univ. Med. Sci. 2022, 31, 43–52. [Google Scholar]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to: Anthocyanidins and proanthocyanidins (ID 1787, 1788, 1789, 1790, 1791); sodium alginate and ulva (ID 1873); vitamins, minerals, trace elements and standardised ginseng G115 extract (ID 8, 1673, 1674); vitamins, minerals, lysine and/or arginine and/or taurine (ID 6, 1676, 1677); plant-based preparation for use in beverages (ID 4210, 4211); Carica papaya L. (ID 2007); “fish protein” (ID 651); acidic water-based, non-alcoholic flavoured beverages containing calcium in the range of 0.3 to 0.8 mol per mol of acid with a pH not lower than 3.7 (ID 1170); royal jelly (ID 1225, 1226, 1227, 1228, 1230, 1231, 1326, 1328, 1329, 1982, 4696, 4697); foods low in cholesterol (ID 624); and foods low in trans-fatty acids (ID 672, 4333) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2083. [Google Scholar]
- Gupta, H.R.; Patil, Y.; Singh, D.; Thakur, M. Embryonic zebrafish model-A well-established method for rapidly assessing the toxicity of homeopathic drugs: Toxicity evaluation of homeopathic drugs using zebrafish embryo model. J. Pharmacopunct. 2016, 19, 319–328. [Google Scholar] [CrossRef]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A versatile animal model for fertility research. BioMed Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef]
- Ka, J.; Jin, S.-W. Zebrafish as an emerging model for dyslipidemia and associated diseases. J. Lipid Atheroscler. 2021, 10, 42–56. [Google Scholar] [CrossRef]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. In Current Protocols in Toxicology; Unit 1.7; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Trede, N.S.; Zapata, A.; Zon, L.I. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Nam, H.-S.; Kim, J.-E.; Na, H.-J.; del Carmen Dominguez-Horta, M.; Martinez-Donato, G. CIGB-258 exerts potent anti-inflammatory activity against carboxymethyllysine-induced acute inflammation in hyperlipidemic zebrafish via the protection of apolipoprotein AI. Int. J. Mol. Sci. 2023, 24, 7044. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem. Toxicol. 2013, 62, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Nabas, Z.; Haddadin, M.S.; Haddadin, J.; Nazer, I.K. Chemical composition of royal jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Pol. J. Food Nutr. Sci. 2014, 64, 171–180. [Google Scholar] [CrossRef]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Watanabe, S.; Suemaru, K.; Takechi, K.; Kaji, H.; Imai, K.; Araki, H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil-induced oral mucositis. J. Pharmacol. Sci. 2013, 121, 110–118. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Deng, Y.; Park, H.G.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antioxidant capacity of major royal jelly proteins of honeybee (Apis mellifera) royal jelly. J. Asia Pac. Entomol. 2020, 23, 445–448. [Google Scholar] [CrossRef]
- Xin, X.X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.-Q.; Shen, L.-R. Supplementation with major Royal-Jelly proteins increases lifespan, feeding, and fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef]
- Kanbur, M.; Eraslan, G.; Silici, S.; Karabacak, M. Effects of sodium fluoride exposure on some biochemical parameters in mice: Evaluation of the ameliorative effect of royal jelly applications on these parameters. Food Chem. Toxicol. 2009, 47, 1184–1189. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine. J. Bee Prod. Sci. 2011, 3, 8–19. [Google Scholar]
- Deb, S.; Puthanveetil, P.; Sakharkar, P. A population-based cross-sectional study of the association between liver enzymes and lipid levels. Int. J. Hepatol. 2018, 2018, 1286170. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Ali, D.; Moneim, A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Aparisi, Á.; Martín-Fernández, M.; Ybarra-Falcón, C.; Gil, J.F.; Carrasco-Moraleja, M.; Martínez-Paz, P.; Cusácovich, I.; Gonzalo-Benito, H.; Fuertes, R.; Marcos-Mangas, M.; et al. Dyslipidemia and inflammation as hallmarks of oxidative stress in COVID-19: A follow-up study. Int. J. Mol. Sci. 2022, 23, 15350. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Njeru, L.K.; Lattorff, H.M.G. African honeybee royal jelly: Phytochemical contents, free radical scavenging activity, and physicochemical properties. Food Biosci. 2020, 37, 100733. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kang, D.-J.; Nam, H.-S.; Kim, J.-H.; Kim, S.-Y.; Lee, J.-O.; Kim, B.-J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- (NRC) National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2010. [Google Scholar]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-Term Supplementation on of ozonated sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic zebrafish: Suppression of oxidative stress and inflammation against carboxymethyllysine toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. In Basic Methods in Microscopy; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2006; Chapter 4. [Google Scholar]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Ozonated sunflower oil exerted potent anti-inflammatory activities with enhanced wound healing and tissue regeneration abilities against acute toxicity of carboxymethyllysine in zebrafish with improved blood lipid profile. Antioxidants 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Baek, S.H. Cuban policosanol (Raydel®) potently protects the liver, ovary, and testis with an improvement in dyslipidemia in hyperlipidemic zebrafish: A comparative study with three Chinese policosanols. Molecules 2023, 28, 6609. [Google Scholar] [CrossRef]
- Umali, J.; Hawkey-Noble, A.; French, C.R. Loss of foxc1 in zebrafish reduces optic nerve size and cell number in the retinal ganglion cell layer. Vision Res. 2019, 156, 66–72. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Baek, S.-H.; Bahuguna, A. Consumption of policosanol (Raydel®) improves hepatic, renal, and reproductive functions in zebrafish: In Vivo comparison study among Cuban, Chinese, and American policosanol. Pharmaceuticals 2024, 17, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Nam, H.-S.; Bahuguna, A.; Kim, J.-E. Long-Term Supplementation of Royal Jelly (Raydel®) Improves Zebrafish Growth, Embryo Production and Survivability, Blood Lipid Profile and Functionality of Vital Organs: A 72-Weeks’ Consumption Study. Pharmaceuticals 2024, 17, 324. https://doi.org/10.3390/ph17030324
Cho K-H, Nam H-S, Bahuguna A, Kim J-E. Long-Term Supplementation of Royal Jelly (Raydel®) Improves Zebrafish Growth, Embryo Production and Survivability, Blood Lipid Profile and Functionality of Vital Organs: A 72-Weeks’ Consumption Study. Pharmaceuticals. 2024; 17(3):324. https://doi.org/10.3390/ph17030324
Chicago/Turabian StyleCho, Kyung-Hyun, Hyo-Seon Nam, Ashutosh Bahuguna, and Ji-Eun Kim. 2024. "Long-Term Supplementation of Royal Jelly (Raydel®) Improves Zebrafish Growth, Embryo Production and Survivability, Blood Lipid Profile and Functionality of Vital Organs: A 72-Weeks’ Consumption Study" Pharmaceuticals 17, no. 3: 324. https://doi.org/10.3390/ph17030324
APA StyleCho, K.-H., Nam, H.-S., Bahuguna, A., & Kim, J.-E. (2024). Long-Term Supplementation of Royal Jelly (Raydel®) Improves Zebrafish Growth, Embryo Production and Survivability, Blood Lipid Profile and Functionality of Vital Organs: A 72-Weeks’ Consumption Study. Pharmaceuticals, 17(3), 324. https://doi.org/10.3390/ph17030324






