Magnetic Hyperthermia Therapy for High-Grade Glioma: A State-of-the-Art Review
Abstract
1. Introduction
2. MHT Workflow
2.1. Nanoparticles
2.2. MPI
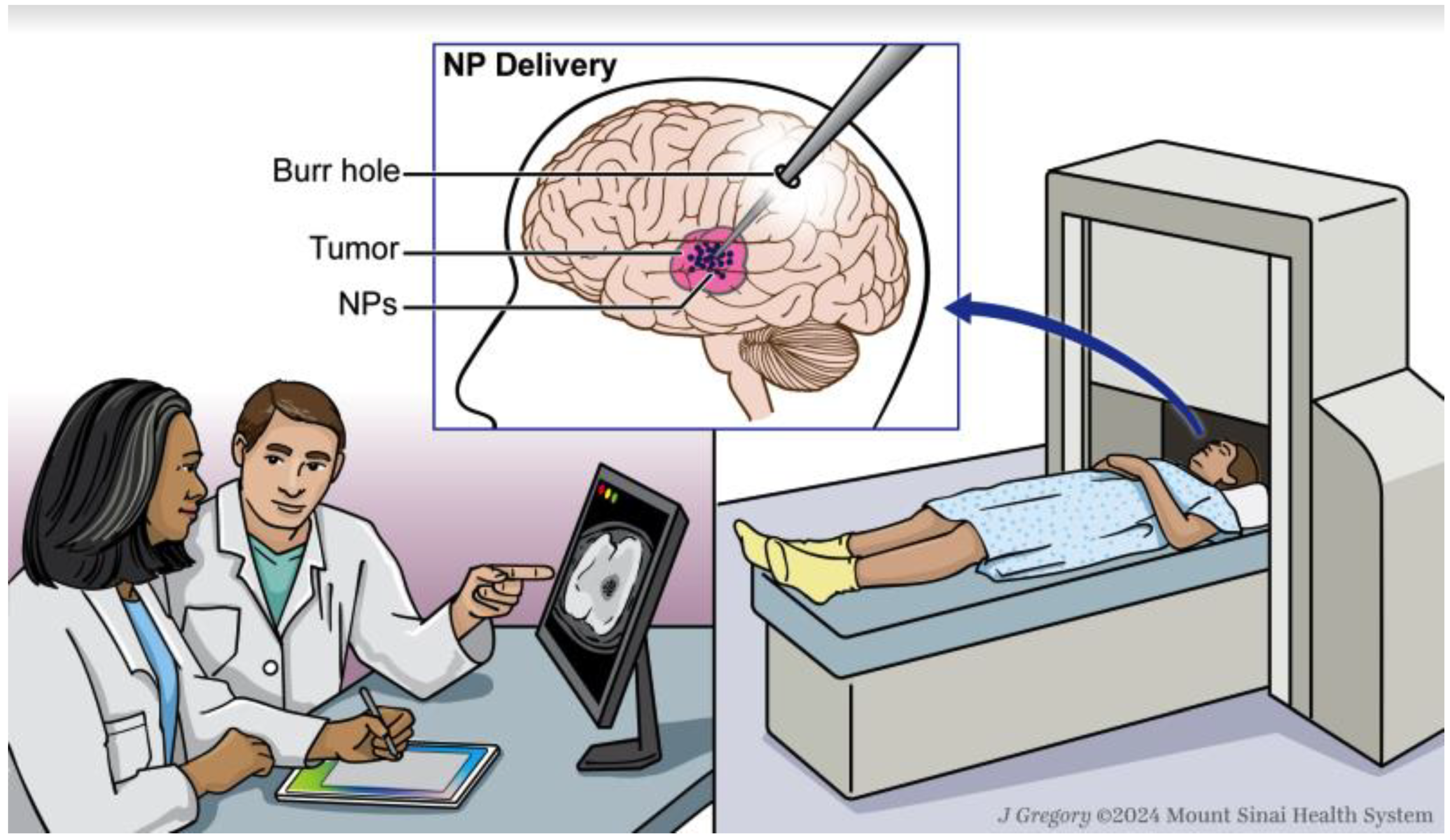
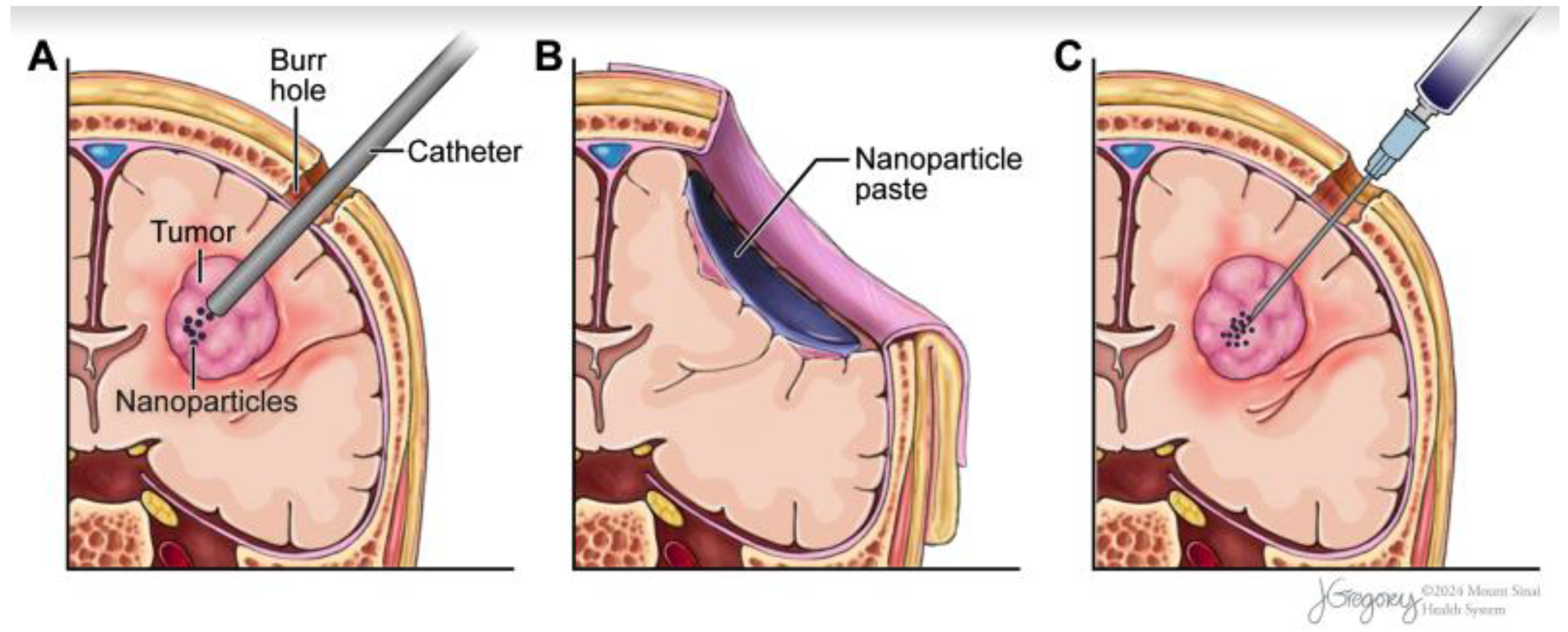
2.3. Nanoparticle Delivery
2.4. AMF
2.5. MHT-Mediated Enhancement of Chemotherapy and Radiation
2.6. MHT for Glioma Clinical Impact
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Francis, S.S.; Ostrom, Q.T.; Cote, D.J.; Smith, T.R.; Claus, E.; Barnholtz-Sloan, J.S. The Epidemiology of Central Nervous System Tumors. Hematol. Oncol. Clin. N. Am. 2022, 36, 23–42. [Google Scholar] [CrossRef]
- Gisina, A.; Kholodenko, I.; Kim, Y.; Abakumov, M.; Lupatov, A.; Yarygin, K. Glioma Stem Cells: Novel Data Obtained by Single-Cell Sequencing. Int. J. Mol. Sci. 2022, 23, 14224. [Google Scholar] [CrossRef]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular Signature and Crossroads with Tumor Microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Schupper, A.J.; Hadjipanayis, C.G. Novel Approaches to Targeting Gliomas at the Leading/Cutting Edge. J. Neurosurg. 2023, 139, 760–768. [Google Scholar] [CrossRef]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Jesu Raj, J.G.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia Treatment Advances for Brain Tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Chanenchuk, T.; Racanelli, A.; Price, G.; Hadjipanayis, C.G. Laser Hyperthermia: Past, Present, and Future. Neuro. Oncol. 2022, 24, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Schupper, A.J.; Bouras, A.; Anastasiadou, M.; Kleinberg, L.; Kraitchman, D.L.; Attaluri, A.; Ivkov, R.; Hadjipanayis, C.G. Neurosurgical Applications of Magnetic Hyperthermia Therapy. Neurosurg. Clin. N. Am. 2023, 34, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic Hyperthermia Therapy for the Treatment of Glioblastoma: A Review of the Therapy’s History, Efficacy and Application in Humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of Magnetic Hyperthermia for Glioblastoma Multiforme Therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Sun, J.; Ma, Y. Hyperthermia Targeting the Tumor Microenvironment Facilitates Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 595207. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical Magnetic Hyperthermia Requires Integrated Magnetic Particle Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef]
- Attaluri, A.; Kandala, S.K.; Zhou, H.; Wabler, M.; DeWeese, T.L.; Ivkov, R. Magnetic Nanoparticle Hyperthermia for Treating Locally Advanced Unresectable and Borderline Resectable Pancreatic Cancers: The Role of Tumor Size and Eddy-Current Heating. Int. J. Hyperth. 2020, 37, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-Controlled Magnetic Nanoparticles Hyperthermia Inhibits Primary Tumor Growth and Metastases Dissemination. Nanomedicine 2020, 25, 102171. [Google Scholar] [CrossRef] [PubMed]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an Adjuvant Cancer Therapy in Combination with Radiotherapy versus Radiotherapy Alone for Recurrent/Progressive Glioblastoma: A Systematic Review. J. Neurooncol. 2021, 152, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Savliwala, S.; Chiu-Lam, A.; Unni, M.; Rivera-Rodriguez, A.; Fuller, E.; Sen, K.; Threadcraft, M.; Rinaldi, C. Chapter 13—Magnetic Nanoparticles. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–221. ISBN 9780128166628. [Google Scholar]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. Int. J. Hyperth. 2019, 36, 687–701. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Pankhurst, Q.A. Suitability of Commercial Colloids for Magnetic Hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shah, N.; Huffman, G.P. Size Dependence of Magnetic Parameters and Surface Disorder in Magnetite Nanoparticles. J. Appl. Phys. 2009, 105, 07B501. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic Particle Hyperthermia: Nanoparticle Magnetism and Materials Development for Cancer Therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Gomes, J.A.; Sousa, M.H.; Bakuzis, A.F. Magnetic Hyperthermia Investigation of Cobalt Ferrite Nanoparticles: Comparison between Experiment, Linear Response Theory, and Dynamic Hysteresis Simulations. J. Appl. Phys. 2012, 111, 123902. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.-M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo-Fesenko, O.; Marciello, M.; Morales, M.d.P.; Baldomir, D.; Martinez-Boubeta, C. Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Gabelova, A.; Svitkova, B.; Ursinyova, M.; Kubovcikova, M.; Antal, I.; Khmara, I.; Jurikova, A.; Molcan, M.; et al. Effect of Magnetic Nanoparticles Coating on Cell Proliferation and Uptake. J. Magn. Magn. Mater. 2019, 472, 66–73. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Cytotoxicity Suppression and Cellular Uptake Enhancement of Surface Modified Magnetic Nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Biehl, P.; Von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef]
- Strable, E.; Bulte, J.W.M.; Moskowitz, B.; Vivekanandan, K.; Allen, M.; Douglas, T. Synthesis and Characterization of Soluble Iron Oxide−Dendrimer Composites. Chem. Mater. 2001, 13, 2201–2209. [Google Scholar] [CrossRef]
- Quaglia, F.; Ostacolo, L.; Nese, G.; Canciello, M.; De Rosa, G.; Ungaro, F.; Palumbo, R.; La Rotonda, M.I.; Maglio, G. Micelles Based on Amphiphilic PCL-PEO Triblock and Star-Shaped Diblock Copolymers: Potential in Drug Delivery Applications. J. Biomed. Mater. Res. A 2008, 87, 563–574. [Google Scholar] [CrossRef]
- Lacava, L.M.; Lacava, Z.G.; Da Silva, M.F.; Silva, O.; Chaves, S.B.; Azevedo, R.B.; Pelegrini, F.; Gansau, C.; Buske, N.; Sabolovic, D.; et al. Magnetic Resonance of a Dextran-Coated Magnetic Fluid Intravenously Administered in Mice. Biophys. J. 2001, 80, 2483–2486. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S.G. Lactoferrin and Ceruloplasmin Derivatized Superparamagnetic Iron Oxide Nanoparticles for Targeting Cell Surface Receptors. Biomaterials 2004, 25, 3029–3040. [Google Scholar] [CrossRef]
- Albert, E.L.; Che Abdullah, C.A.; Shiroshaki, Y. Synthesis and Characterization of Graphene Oxide Functionalized with Magnetic Nanoparticle via Simple Emulsion Method. Results Phys. 2018, 11, 944–950. [Google Scholar] [CrossRef]
- Venkatesha, N.; Poojar, P.; Ashwini, R.; Qurishi, Y.; Geethanath, S.; Srivastava, C. Ultrafine Graphene Oxide–CoFe2O4 Nanoparticle Composite as T1 and T2 Contrast Agent for Magnetic Resonance Imaging. RSC Adv. 2016, 6, 17423–17429. [Google Scholar] [CrossRef]
- Crespo, P.; de la Presa, P.; Marín, P.; Multigner, M.; Alonso, J.M.; Rivero, G.; Yndurain, F.; González-Calbet, J.M.; Hernando, A. Magnetism in Nanoparticles: Tuning Properties with Coatings. J. Phys. Condens. Matter 2013, 25, 484006. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Duce, S.L.; Brown, S.; Lee, S.; Melzer, A.; Cuschieri, A.; André, P. Engineered Biocompatible Nanoparticles for in Vivo Imaging Applications. J. Am. Chem. Soc. 2010, 132, 15022–15029. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; de la Presa, P.; Alonso, J.M.; Rueda, T.; Martínez, A.; Crespo, P.; Morales, M.P.; Gonzalez-Fernandez, M.A.; Valdés, J.; Rivero, G. Hyperthermia HeLa Cell Treatment with Silica-Coated Manganese Oxide Nanoparticles. J. Phys. Chem. C 2010, 114, 1976–1981. [Google Scholar] [CrossRef]
- Fernandes, T.; Nogueira, H.I.S.; Amorim, C.O.; Amaral, J.S.; Daniel-da-Silva, A.L.; Trindade, T. Chemical Strategies for Dendritic Magneto-Plasmonic Nanostructures Applied to Surface-Enhanced Raman Spectroscopy. Chemistry 2022, 28, e202202382. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P.; Trubetskoy, V.S. Which Polymers Can Make Nanoparticulate Drug Carriers Long-Circulating? Adv. Drug Deliv. Rev. 1995, 16, 141–155. [Google Scholar] [CrossRef]
- Xie, J.; Xu, C.; Kohler, N.; Hou, Y.; Sun, S. Controlled PEGylation of Monodisperse Fe3O4 Nanoparticles for Reduced Non-specific Uptake by Macrophage Cells. Adv. Mater. 2007, 19, 3163–3166. [Google Scholar] [CrossRef]
- Bender, P.; Bogart, L.K.; Posth, O.; Szczerba, W.; Rogers, S.E.; Castro, A.; Nilsson, L.; Zeng, L.J.; Sugunan, A.; Sommertune, J.; et al. Structural and Magnetic Properties of Multi-Core Nanoparticles Analysed Using a Generalised Numerical Inversion Method. Sci. Rep. 2017, 7, 45990. [Google Scholar] [CrossRef]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic Nanocrystal Assemblies as Versatile Magnetic Particle Hyperthermia Mediators. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Coral, D.F.; Zélis, P.M.; Marciello, M.; Morales, M.d.P.; Craievich, A.; Sánchez, F.H.; van Raap, M.B.F. Effect of Nanoclustering and Dipolar Interactions in Heat Generation for Magnetic Hyperthermia. Langmuir 2016, 32, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T.K. High Performance Multi-Core Iron Oxide Nanoparticles for Magnetic Hyperthermia: Microwave Synthesis, and the Role of Core-to-Core Interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Costo, R.; Grüttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis Methods to Prepare Single- and Multi-Core Iron Oxide Nanoparticles for Biomedical Applications. Dalton Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Paez-Muñoz, J.M.; Gámez, F.; Fernández-Afonso, Y.; Gallardo, R.; Pernia Leal, M.; Gutiérrez, L.; de la Fuente, J.M.; Caro, C.; García-Martín, M.L. Optimization of Iron Oxide Nanoparticles for MRI-Guided Magnetic Hyperthermia Tumor Therapy: Reassessing the Role of Shape in Their Magnetocaloric Effect. J. Mater. Chem. B Mater. Biol. Med. 2023, 11, 11110–11120. [Google Scholar] [CrossRef]
- Fink, C.; Gevaert, J.J.; Barrett, J.W.; Dikeakos, J.D.; Foster, P.J.; Dekaban, G.A. In Vivo Tracking of Adenoviral-Transduced Iron Oxide-Labeled Bone Marrow-Derived Dendritic Cells Using Magnetic Particle Imaging. Eur. Radiol. Exp. 2023, 7, 42. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, Y.; Xing, X.; Xiao, J.; Chen, H.; Zhang, H.; Wang, D.; Zhang, Y.; Zhang, G.; Wu, Z.; et al. Manganese-Deposited Iron Oxide Promotes Tumor-Responsive Ferroptosis That Synergizes the Apoptosis of Cisplatin. Theranostics 2021, 11, 5418–5429. [Google Scholar] [CrossRef]
- Hanini, A.; Lartigue, L.; Gavard, J.; Kacem, K.; Wilhelm, C.; Gazeau, F.; Chau, F.; Ammar, S. Zinc Substituted Ferrite Nanoparticles with Zn0.9Fe2.1O4 Formula Used as Heating Agents for in Vitro Hyperthermia Assay on Glioma Cells. J. Magn. Magn. Mater. 2016, 416, 315–320. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, N.; Huang, Y.; He, R.; Xu, S.; Yuan, W. Coordination of Injectable Self-Healing Hydrogel with Mn-Zn Ferrite@mesoporous Silica Nanospheres for Tumor MR Imaging and Efficient Synergistic Magnetothermal-Chemo-Chemodynamic Therapy. Chem. Eng. J. 2020, 401, 126100. [Google Scholar] [CrossRef]
- Razumov, I.A.; Zav’yalov, E.L.; Troitskii, S.Y.; Romashchenko, A.V.; Petrovskii, D.V.; Kuper, K.E.; Moshkin, M.P. Selective Cytotoxicity of Manganese Nanoparticles against Human Glioblastoma Cells. Bull. Exp. Biol. Med. 2017, 163, 561–565. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron Oxide Nanorods as High-Performance Magnetic Resonance Imaging Contrast Agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic Imaging Using the Nonlinear Response of Magnetic Particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Gleich, B.; Weizenecker, J.; Borgert, J. Experimental Results on Fast 2D-Encoded Magnetic Particle Imaging. Phys. Med. Biol. 2008, 53, N81. [Google Scholar] [CrossRef]
- Billings, C.; Langley, M.; Warrington, G.; Mashali, F.; Johnson, J.A. Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures. Int. J. Mol. Sci. 2021, 22, 7651. [Google Scholar] [CrossRef]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. AJNR Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining Magnetic Particle Imaging and Magnetic Fluid Hyperthermia in a Theranostic Platform. Phys. Med. Biol. 2017, 62, 3483–3500. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Münkel, C.; Grüttner, C.; Levan, P.; von Elverfeldt, D.; Graeser, M.; et al. In Situ Theranostic Platform Combining Highly Localized Magnetic Fluid Hyperthermia, Magnetic Particle Imaging, and Thermometry in 3D. Theranostics 2024, 14, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, P.W.; Saritas, E.U.; Croft, L.R.; Kim, T.N.; Krishnan, K.M.; Schaffer, D.V.; Conolly, S.M. X-Space MPI: Magnetic Nanoparticles for Safe Medical Imaging. Adv. Mater. 2012, 24, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.D.; Jung, S.; Choi, S.H.; Kim, D.-H. Local Drug Delivery Strategies for Glioblastoma Treatment. Brain Tumor Res. Treat. 2022, 10, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.C.; Platt, S.R.; Holmes, S.; Kent, M.; Robinson, K.; Howerth, E.; Eagleson, J.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Convection-Enhanced Delivery of Cetuximab Conjugated Iron-Oxide Nanoparticles for Treatment of Spontaneous Canine Intracranial Gliomas. J. Neurooncol. 2018, 137, 653–663. [Google Scholar] [CrossRef]
- Pablico-Lansigan, M.H.; Situ, S.F.; Samia, A.C.S. Magnetic Particle Imaging: Advancements and Perspectives for Real-Time in Vivo Monitoring and Image-Guided Therapy. Nanoscale 2013, 5, 4040–4055. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Gill, T.; Barua, N.U.; Woolley, M.; Bienemann, A.S.; Johnson, D.E.; O’Sullivan, S.; Murray, G.; Fennelly, C.; Lewis, O.; Irving, C.; et al. In Vitro and in Vivo Testing of a Novel Recessed-Step Catheter for Reflux-Free Convection-Enhanced Drug Delivery to the Brain. J. Neurosci. Methods 2013, 219, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kida, Y.; Tanaka, T.; Hattori, K.; Matsui, M.; Amemiya, Y. Interstitial Hyperthermia of Malignant Brain Tumors by Implant Heating System: Clinical Experience. J. Neurooncol. 1991, 10, 153–163. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined Intracavitary Thermotherapy with Iron Oxide Nanoparticles and Radiotherapy as Local Treatment Modality in Recurrent Glioblastoma Patients. J. Neurooncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.-H. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar]
- Hemery, G.; Genevois, C.; Couillaud, F.; Lacomme, S.; Gontier, E.; Ibarboure, E.; Lecommandoux, S.; Garanger, E.; Sandre, O. Monocore vs. Multicore Magnetic Iron Oxide Nanoparticles: Uptake by Glioblastoma Cells and Efficiency for Magnetic Hyperthermia. Mol. Syst. Des. Eng. 2017, 2, 629–639. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy; Codon Publications: Singapore, 2017. [Google Scholar]
- Zhao, Y.-H.; Wang, Z.-F.; Pan, Z.-Y.; Péus, D.; Delgado-Fernandez, J.; Pallud, J.; Li, Z.-Q. A Meta-Analysis of Survival Outcomes Following Reoperation in Recurrent Glioblastoma: Time to Consider the Timing of Reoperation. Front. Neurol. 2019, 10, 286. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-Enhanced Delivery to the Central Nervous System. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Desjardins, A. Convection-Enhanced Delivery for High-Grade Glioma. Neurooncol. Pract. 2022, 9, 24–34. [Google Scholar] [CrossRef]
- Sharma, A.; Jangam, A.; Shen, J.L.Y.; Ahmad, A.; Arepally, N.; Rodriguez, B.; Borrello, J.; Bouras, A.; Kleinberg, L.; Ding, K.; et al. Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device. Cancers 2023, 15, 327. [Google Scholar] [CrossRef]
- Young, J.S.; Bernal, G.; Polster, S.P.; Nunez, L.; Larsen, G.F.; Mansour, N.; Podell, M.; Yamini, B. Convection-Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg. 2018, 117, e698–e704. [Google Scholar] [CrossRef]
- Stea, B.; Rossman, K.; Kittelson, J.; Shetter, A.; Hamilton, A.; Cassady, J.R. Interstitial Irradiation versus Interstitial Thermoradiotherapy for Supratentorial Malignant Gliomas: A Comparative Survival Analysis. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 591–600. [Google Scholar] [CrossRef]
- Stea, B.; Kittelson, J.; Cassady, J.R.; Hamilton, A.; Guthkelch, N.; Lulu, B.; Obbens, E.; Rossman, K.; Shapiro, W.; Shetter, A. Treatment of Malignant Gliomas with Interstitial Irradiation and Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 657–667. [Google Scholar] [CrossRef]
- Belova, N.A.; Acosta-Avalos, D. The Effect of Extremely Low Frequency Alternating Magnetic Field on the Behavior of Animals in the Presence of the Geomagnetic Field. J. Biophys. 2015, 2015, 423838. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Kötitz, R.; Weitschies, W.; Trahms, L.; Semmler, W. Investigation of Brownian and Néel Relaxation in Magnetic Fluids. J. Magn. Magn. Mater. 1999, 201, 102–104. [Google Scholar] [CrossRef]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic Fluid Hyperthermia: Advances, Challenges, and Opportunity. Int. J. Hyperth. 2013, 29, 706–714. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Stigliano, R.V.; Shubitidze, F.; Petryk, J.D.; Shoshiashvili, L.; Petryk, A.A.; Hoopes, P.J. Mitigation of Eddy Current Heating during Magnetic Nanoparticle Hyperthermia Therapy. Int. J. Hyperth. 2016, 32, 735–748. [Google Scholar] [CrossRef]
- Wust, P.; Gneveckow, U.; Johannsen, M.; Böhmer, D.; Henkel, T.; Kahmann, F.; Sehouli, J.; Felix, R.; Ricke, J.; Jordan, A. Magnetic Nanoparticles for Interstitial Thermotherapy--Feasibility, Tolerance and Achieved Temperatures. Int. J. Hyperth. 2006, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.Y.; Munir, N.; Kumaria, A.; Akhtar, Q.; Bullock, C.J.; Narayanan, A.; Fu, R.Z. Medical Device Advances in the Treatment of Glioblastoma. Cancers 2022, 14, 5341. [Google Scholar] [CrossRef] [PubMed]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015. [Google Scholar] [CrossRef]
- Hilschenz, I.; Körber, R.; Scheer, H.-J.; Fedele, T.; Albrecht, H.-H.; Mario Cassará, A.; Hartwig, S.; Trahms, L.; Haase, J.; Burghoff, M. Magnetic Resonance Imaging at Frequencies below 1 KHz. Magn. Reson. Imaging 2013, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Brezovich, I.A.; Meredith, R.F. Practical Aspects of Ferromagnetic Thermoseed Hyperthermia. Radiol. Clin. N. Am. 1989, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, 31, 70–75. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 84. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia—Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef]
- Tansi, F.L.; Maduabuchi, W.O.; Hirsch, M.; Southern, P.; Hattersley, S.; Quaas, R.; Teichgräber, U.; Pankhurst, Q.A.; Hilger, I. Deep-Tissue Localization of Magnetic Field Hyperthermia Using Pulse Sequencing. Int. J. Hyperth. 2021, 38, 743–754. [Google Scholar] [CrossRef]
- Corry, P.M.; Robinson, S.; Getz, S. Hyperthermic Effects on DNA Repair Mechanisms. Radiology 1977, 123, 475–482. [Google Scholar] [CrossRef]
- Ihara, M.; Takeshita, S.; Okaichi, K.; Okumura, Y.; Ohnishi, T. Heat Exposure Enhances Radiosensitivity by Depressing DNA-PK Kinase Activity during Double Strand Break Repair. Int. J. Hyperth. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Khurana, N.; Laskar, S.; Bhattacharyya, M.K.; Bhattacharyya, S. Hsp90 Induces Increased Genomic Instability toward DNA-Damaging Agents by Tuning down RAD53 Transcription. Mol. Biol. Cell 2016, 27, 2463–2478. [Google Scholar] [CrossRef]
- van den Tempel, N.; Zelensky, A.N.; Odijk, H.; Laffeber, C.; Schmidt, C.K.; Brandsma, I.; Demmers, J.; Krawczyk, P.M.; Kanaar, R. On the Mechanism of Hyperthermia-Induced BRCA2 Protein Degradation. Cancers 2019, 11, 97. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild Hyperthermia Inhibits Homologous Recombination, Induces BRCA2 Degradation, and Sensitizes Cancer Cells to Poly (ADP-Ribose) Polymerase-1 Inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef]
- Ko, S.H.; Ueno, T.; Yoshimoto, Y.; Yoo, J.S.; Abdel-Wahab, O.I.; Abdel-Wahab, Z.; Chu, E.; Pruitt, S.K.; Friedman, H.S.; Dewhirst, M.W.; et al. Optimizing a Novel Regional Chemotherapeutic Agent against Melanoma: Hyperthermia-Induced Enhancement of Temozolomide Cytotoxicity. Clin. Cancer Res. 2006, 12, 289–297. [Google Scholar] [CrossRef]
- Marino, A.; Camponovo, A.; Degl’Innocenti, A.; Bartolucci, M.; Tapeinos, C.; Martinelli, C.; De Pasquale, D.; Santoro, F.; Mollo, V.; Arai, S.; et al. Multifunctional Temozolomide-Loaded Lipid Superparamagnetic Nanovectors: Dual Targeting and Disintegration of Glioblastoma Spheroids by Synergic Chemotherapy and Hyperthermia Treatment. Nanoscale 2019, 11, 21227–21248. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.-S.; Martel, S. Remote Control of the Permeability of the Blood-Brain Barrier by Magnetic Heating of Nanoparticles: A Proof of Concept for Brain Drug Delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef]
- Salehi, A.; Paturu, M.R.; Patel, B.; Cain, M.D.; Mahlokozera, T.; Yang, A.B.; Lin, T.-H.; Leuthardt, E.C.; Yano, H.; Song, S.-K.; et al. Therapeutic Enhancement of Blood-Brain and Blood-Tumor Barriers Permeability by Laser Interstitial Thermal Therapy. Neurooncol. Adv. 2020, 2, vdaa071. [Google Scholar] [CrossRef]
- Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Radiosensitivity Enhancement of Radioresistant Glioblastoma by Epidermal Growth Factor Receptor Antibody-Conjugated Iron-Oxide Nanoparticles. J. Neurooncol. 2015, 124, 13–22. [Google Scholar] [CrossRef]
- Minaei, S.E.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Sensitization of Glioblastoma Cancer Cells to Radiotherapy and Magnetic Hyperthermia by Targeted Temozolomide-Loaded Magnetite Tri-Block Copolymer Nanoparticles as a Nanotheranostic Agent. Life Sci. 2022, 306, 120729. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, H.; Peng, B.; Zhang, S.; Ma, J.; Deng, G.; Zou, P.; Liu, J.; Chen, A.T.; Li, D.; et al. Vessel-Targeting Nanoclovers Enable Noninvasive Delivery of Magnetic Hyperthermia-Chemotherapy Combination for Brain Cancer Treatment. Nano Lett. 2021, 21, 8111–8118. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; Román, J.S.; Goya, G.F.; Hernández, R.; Mijangos, C. Chitosan Nanoparticles for Combined Drug Delivery and Magnetic Hyperthermia: From Preparation to in Vitro Studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, B.; Wang, Y.; Yao, Z.; Wang, X.; Feng, S.-S.; Tang, J. Thermochemotherapy Mediated by Novel Solar-Planet Structured Magnetic Nanocomposites for Glioma Treatment. J. Nanosci. Nanotechnol. 2012, 12, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, M.A.; Enriquez, D.M.; Salinas, A.D.; Garcia, R., Jr.; Trevino De Leo, C.; Lopez, S.A.; Martirosyan, K.S.; Chew, S.A. Application of Iron Oxide Nanoparticles to Control the Release of Minocycline for the Treatment of Glioblastoma. Future Med. Chem. 2021, 13, 1833–1843. [Google Scholar] [CrossRef]
- Koyama, Y.S.T. Standarlized Evaluation of Direct Effect of Chemotherapy for Solid Tumor. J. Jpn. Soc. Cancer Ther. Ed. 1989, 929. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted Therapy of Glioblastoma Stem-like Cells and Tumor Non-Stem Cells Using Cetuximab-Conjugated Iron-Oxide Nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef]
- Platt, S.; Nduom, E.; Kent, M.; Freeman, C.; Machaidze, R.; Kaluzova, M.; Wang, L.; Mao, H.; Hadjipanayis, C.G. Canine Model of Convection-Enhanced Delivery of Cetuximab-Conjugated Iron-Oxide Nanoparticles Monitored with Magnetic Resonance Imaging. Clin. Neurosurg. 2012, 59, 107–113. [Google Scholar] [CrossRef]
- Jordan, A.; Wust, P.; Fähling, H.; John, W.; Hinz, A.; Felix, R. Inductive Heating of Ferrimagnetic Particles and Magnetic Fluids: Physical Evaluation of Their Potential for Hyperthermia. Int. J. Hyperth. 1993, 9, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Yim, R.L.H.; Leung, K.M.M.; Poon, C.C.M.; Irwin, M.G. Peri-Operative Management of Patients with Parkinson’s Disease. Anaesthesia 2022, 77 (Suppl 1), 123–133. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.-J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Nuttin, B.; Wu, H.; Mayberg, H.; Hariz, M.; Gabriëls, L.; Galert, T.; Merkel, R.; Kubu, C.; Vilela-Filho, O.; Matthews, K.; et al. Consensus on Guidelines for Stereotactic Neurosurgery for Psychiatric Disorders. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Racine, E. Ethics Guidance for Neurological and Psychiatric Deep Brain Stimulation. Handb. Clin. Neurol. 2013, 116, 313–325. [Google Scholar]
- Kim, S.; Palta, J. The Physics of Stereotactic Radiosurgery. In Principles and Practice of Stereotactic Radiosurgery; Chin, L.S., Regine, W.F., Eds.; Springer: New York, NY, USA, 2015; pp. 35–56. ISBN 9781461483632. [Google Scholar]
- Verhey, L.J.; Smith, V. The Physics of Radiosurgery. Semin. Radiat. Oncol. 1995, 5, 175–191. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Kavanagh, B.D.; Asher, A.; Harbaugh, R.E. Inception of a National Multidisciplinary Registry for Stereotactic Radiosurgery. J. Neurosurg. 2016, 124, 155–162. [Google Scholar] [CrossRef]
- Mueller, S. CED with Irinotecan Liposome Injection Using Real Time Imaging in Children with Diffuse Intrinsic Pontine Glioma (DIPG) (PNOC 009). Available online: https://clinicaltrials.gov/study/NCT03086616?cond=dipg&intr=CED&rank=1 (accessed on 20 December 2023).
- Gov, C. MTX110 by Convection-Enhanced Delivery in Treating Participants with Newly-Diagnosed Diffuse Intrinsic Pontine Glioma (PNOC015). Available online: https://clinicaltrials.gov/study/NCT03566199?cond=dipg&intr=CED&rank=2 (accessed on 20 December 2023).
- Therapeutics, Y.-M. 131I-Omburtamab Delivered by Convection-Enhanced Delivery in Patients with Diffuse Intrinsic Pontine Glioma. Available online: https://clinicaltrials.gov/study/NCT05063357?cond=dipg&intr=CED&rank=3 (accessed on 20 December 2023).
- Therapeutics, Y.-M. Convection-Enhanced Delivery of 124I-Omburtamab for Patients with Non-Progressive Diffuse Pontine Gliomas Previously Treated With External Beam Radiation Therapy. Available online: https://clinicaltrials.gov/study/NCT01502917?cond=dipg&intr=CED&rank=4 (accessed on 20 December 2023).
- Lemke, A.-J.; Senfft von Pilsach, M.-I.; Lübbe, A.; Bergemann, C.; Riess, H.; Felix, R. MRI after Magnetic Drug Targeting in Patients with Advanced Solid Malignant Tumors. Eur. Radiol. 2004, 14, 1949–1955. [Google Scholar] [CrossRef]
- de Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The Worldwide Incidence and Prevalence of Primary Brain Tumors: A Systematic Review and Meta-Analysis. Neuro Oncol. 2015, 17, 776–783. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Review of the National Nanotechnology Initiative. Small Wonders, Endless Frontiers: A Review of the National Nanotechnology Initiative; National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Morigi, V.; Tocchio, A.; Bellavite Pellegrini, C.; Sakamoto, J.H.; Arnone, M.; Tasciotti, E. Nanotechnology in Medicine: From Inception to Market Domination. J. Drug Deliv. 2012, 2012, 389485. [Google Scholar] [CrossRef]
- Nanomedicine Market Size, Growth, Trends, Report 2023–2032. Available online: https://www.precedenceresearch.com/nanomedicine-market (accessed on 20 December 2023).
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
| MNP Type | MNP Shape Illustration | MNP Types Used in Glioma MHT Studies | Characteristic MNP Size | Study Type | Composition and Administration | Experimental Results |
|---|---|---|---|---|---|---|
| Iron Oxide |     | Magnetite (Fe3O4) [50] | ~12 nm (MagForce, used in Maier-Hoff) | Human (phase II clinical trial) | Coated with aminosilane AMF—100 kHz | Demonstrated an overall survival following a diagnosis of 23.2 months in human patients |
| Iron Oxide | Maghemite (γ-Fe2O3) [51] | 50 nm [52] (Synomag-D) | In vitro and in vivo (mouse model) | AMF—192 kHz IV injection | Delayed tumor growth | |
| Iron Oxide | Hematite, Ferric Oxide (α-Fe2O3) [53] | 3–100 nm | In vivo (mouse model) | PEGylated IV injection | Improved survival | |
| Ferrite |  | Manganese ferrite [54] | ~90 nm | In vivo (mouse model) | IV injection | Treatment effect and low systemic toxicity |
| Ferrite | Zinc ferrite [55] | ~11 nm | In vitro (U-87MG) | AMF—700 kHz | Sustained heating at 41.5 °C to trigger tumor cell death | |
| Ferrite | Mn-Zn ferrite [56] | 55 nm | In vitro and in vivo (mouse model) | Rhodamin B isothiocyanate (RBITC)-labeled/mesoporous silica-coated AMF—160 kHz | Inhibited tumor growth | |
| Other | Manganese oxide (MnO) [57] | 120–160 nm | In vitro (U-87MG and U-251 GBM cell lines) and in vivo | IV injection | Demonstrated highly selective cytotoxicity in U-87MG cell lines |
| Study Authors | Title | N (#) | MNP Delivery Modality | MNPs Used | Study Outcomes |
|---|---|---|---|---|---|
| Kobayashi et al., 1991 [74] | Interstitial hyperthermia of malignant brain tumors by implant heating system: clinical experience | 25 | Direct Implantation + CED | Fe-Pt Alloy | Successful treatment completion in 23 of 25 patients with a 34.8% overall response rate to treatment |
| Stea et al., 1992 [87] | Treatment of malignant gliomas with interstitial irradiation and hyperthermia | 28 | CED | Ni-S Alloy | Demonstrated feasibility of the interstitial MHT of brain tumors with ferromagnetic implants, with a median patient survival of 20.6 months from diagnosis |
| Stea et al., 1994 [86] | Interstitial irradiation versus interstitial thermoradiotherapy for supratentorial malignant gliomas: a comparative survival analysis | 62 | CED | Ni-S Alloy | The hazard of dying when treated with hyperthermia plus brachytherapy was 0.53 times that of the control group treated with brachytherapy alone |
| Maier-Hauff et al., 2007 [80] | Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme | 14 | Stereotactic Injection | Aminosilane-coated Fe3O4 | Treatment with a median maximum intratumoral temperature of 44.6 degrees C was tolerated in all 14 patients |
| Maier-Hauff et al., 2011 [50] | Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme | 66 | Stereotactic Injection | Aminosilane coated Fe3O4 | An overall survival after a primary tumor diagnosis of 23.4 months and an overall survival following a diagnosis of first tumor recurrence of 13.4 months |
| Grauer et al., 2019 [75] | Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients | 6 | Direct Implantation | Aminosilane-coated Fe3O4 | Demonstrated inflammatory reaction surrounding the resection cavity following intracavitary MHT in combination with radiation therapy, potentially triggering a potent antitumor immune response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, B.; Rivera, D.; Zhang, J.Y.; Brown, C.; Young, T.; Williams, T.; Huq, S.; Mattioli, M.; Bouras, A.; Hadjpanayis, C.G. Magnetic Hyperthermia Therapy for High-Grade Glioma: A State-of-the-Art Review. Pharmaceuticals 2024, 17, 300. https://doi.org/10.3390/ph17030300
Rodriguez B, Rivera D, Zhang JY, Brown C, Young T, Williams T, Huq S, Mattioli M, Bouras A, Hadjpanayis CG. Magnetic Hyperthermia Therapy for High-Grade Glioma: A State-of-the-Art Review. Pharmaceuticals. 2024; 17(3):300. https://doi.org/10.3390/ph17030300
Chicago/Turabian StyleRodriguez, Benjamin, Daniel Rivera, Jack Y. Zhang, Cole Brown, Tirone Young, Tyree Williams, Sakibul Huq, Milena Mattioli, Alexandros Bouras, and Constantinos G. Hadjpanayis. 2024. "Magnetic Hyperthermia Therapy for High-Grade Glioma: A State-of-the-Art Review" Pharmaceuticals 17, no. 3: 300. https://doi.org/10.3390/ph17030300
APA StyleRodriguez, B., Rivera, D., Zhang, J. Y., Brown, C., Young, T., Williams, T., Huq, S., Mattioli, M., Bouras, A., & Hadjpanayis, C. G. (2024). Magnetic Hyperthermia Therapy for High-Grade Glioma: A State-of-the-Art Review. Pharmaceuticals, 17(3), 300. https://doi.org/10.3390/ph17030300





