Efficiency of NZ2114 on Superficial Pyoderma Infected with Staphylococcus pseudintermedius
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentrations (MICs) of NZ2114 and Antibiotics against S. pseudintermedius
2.2. Drug Sensitivity of S. pseudintermedius
2.3. Effect of Formulations on Activity of NZ2114
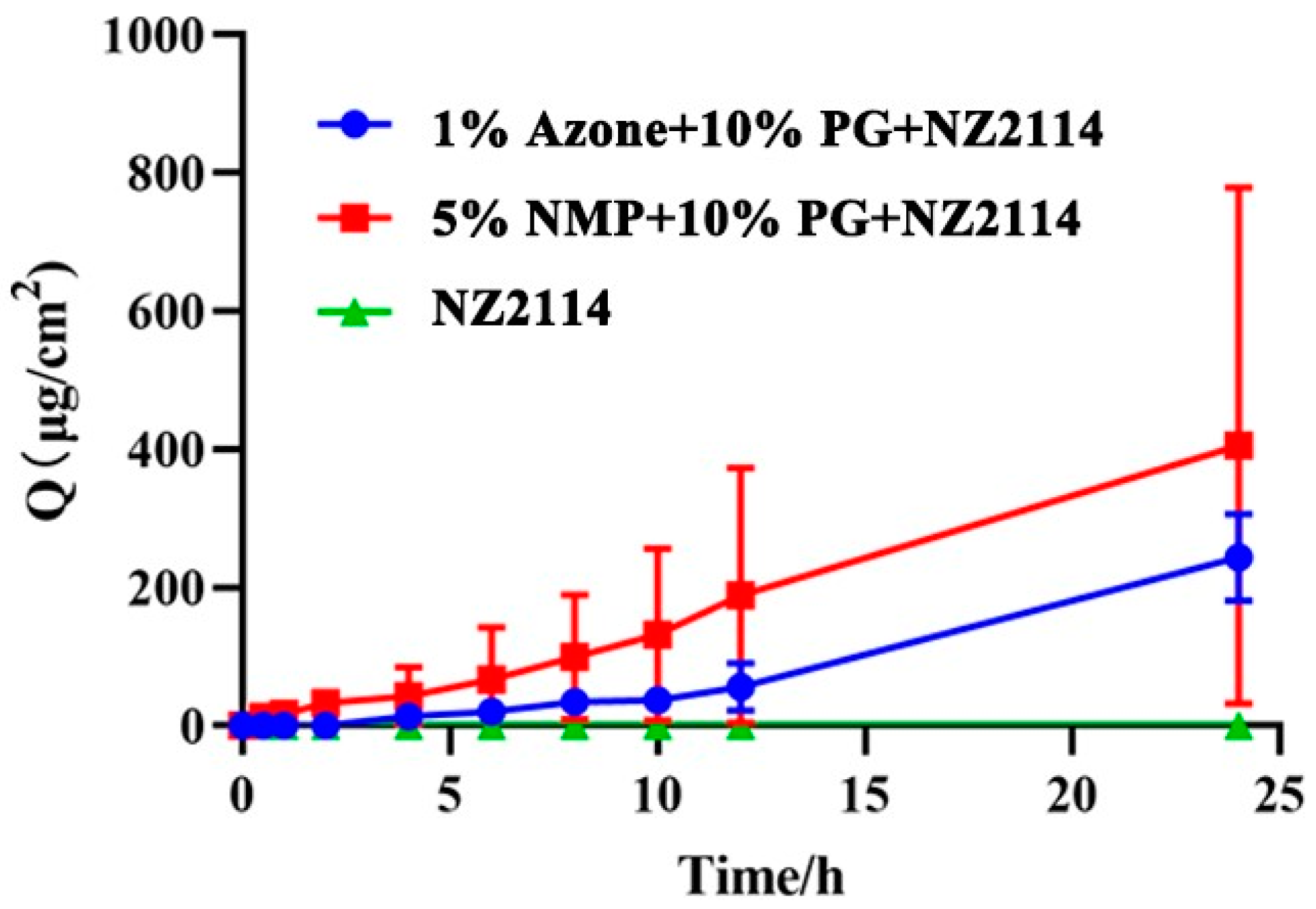
2.4. Penetrating Effect of NZ2114 Spray on Mouse Skin
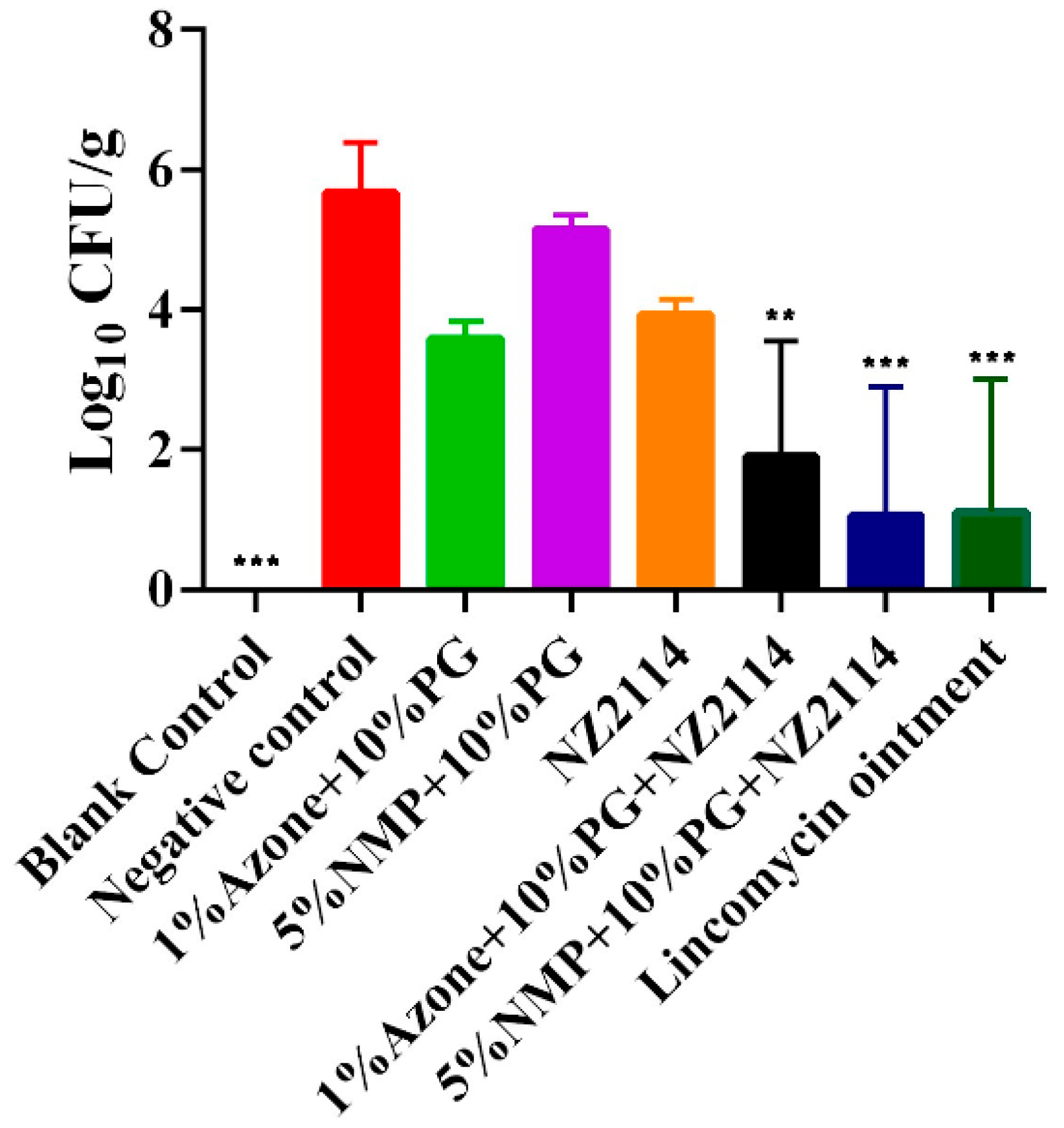
2.5. Antimicrobial Effect of NZ2114 Spray on Superficial Pyoderma in Mice
2.6. Effects of NZ2114 Sprays on Skin Irritation in Mice
3. Discussion
4. Materials and Methods
4.1. Strains, Reagents and Mice
4.2. MICs of NZ2114 against S. pseudointermedia
4.3. S. pseudintermedius Susceptibility Assay
4.4. Formulation Screening for NZ2114 Spray
4.5. In Vitro Skin Penetration Experiments
4.6. Superficial Pyoderma Assay
4.7. Skin Irritation Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stegmann, R.; Burnens, A.; Maranta, C.A.; Perreten, V. Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J. Antimicrob. Chemother. 2010, 65, 2047–2048. [Google Scholar] [CrossRef]
- Bäumer, W.; Jacobs, M.; Tamamoto-Mochizuki, C. Efficacy study of a topical treatment with a plant extract with antibiofilm activities using an in vivo model of canine superficial pyoderma. Vet. Dermatol. 2020, 31, 86–89. [Google Scholar] [CrossRef]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections (1): Diagnosis based on clinical presentation, cytology and culture. Vet. Rec. 2013, 172, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, W.; Bizikova, P.; Jacob, M.; Linder, K.E. Establishing a canine superficial pyoderma model. J. Appl. Microbiol. 2017, 122, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Moodley, A.; Ghibaudo, G.; Guardabassi, L. Carriage of methicillin-resistant Staphylococcus pseudintermedius in small animal veterinarians: Indirect evidence of zoonotic transmission. Zoonoses Public Heal. 2011, 58, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Larsuprom, L.; Rungroj, N.; Lekcharoensuk, C.; Pruksakorn, C.; Kongkiatpaiboon, S.; Chen, C.; Sukatta, U. In vitro antibacterial activity of mangosteen (Garcinia mangostana Linn.) crude extract against Staphylococcus pseudintermedius isolates from canine pyoderma. Vet. Dermatol. 2019, 30, 487-e145. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.T.C.d.; Toledo Borges, A.B.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial peptides as an alternative for the eradication of bacterial biofilms of multi-drug resistant bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, Q.; Mao, R.; Hao, Y.; Ma, X.; Teng, D.; Fan, H.; Wang, J.H. Effect of NZ2114 against Streptococcus dysgalactiae biofilms and its application in murine mastitis model. Front. Microbiol. 2022, 13, 1010148. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.H.; de la Fuente-Nunez, C.; Franco, O.L. Editorial: Antimicrobial peptides: Molecular design, structure-function relationship, and biosynthesis optimization. Front. Microbiol. 2022, 13, 888540. [Google Scholar] [CrossRef]
- Aminov, R. Editorial: Insights in antimicrobials, resistance, and chemotherapy: 2021. Front. Microbiol. 2022, 13, 1037326. [Google Scholar] [CrossRef]
- Wang, C.; Hong, T.; Cui, P.; Wang, J.; Xia, J. Antimicrobial peptides towards clinical application: Delivery and formulation. Adv. Drug Deliv. Rev. 2021, 175, 113818. [Google Scholar] [CrossRef]
- Li, X.; Hao, Y.; Yang, N.; Mao, R.; Teng, D.; Wang, J. Plectasin: From evolution to truncation, expression, and better druggability. Front. Microbiol. 2023, 14, 1304825. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Ma, X.; Wei, L.; Wang, J.H. Antibacterial peptide NZ2114-loaded hydrogel accelerates Staphylococcus aureus-infected wound healing. Appl. Microbiol. Biotechnol. 2022, 106, 3639–3656. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.F.; Wu, T.T.; Wang, W.S.; Li, B.L.; Wang, M.; Chen, L.L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Garbacz, K.; Neubauer, D.; Kamysz, W. In vitro efficiency of antimicrobial peptides against Staphylococcal pathogens associated with canine pyoderma. Animals 2020, 10, 470. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, D.; Mao, R.; Wang, X.; Xi, D.; Hu, X.; Wang, J. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 681–694. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Structure and function of the stratum corneum extracellular matrix. J. Investig. Dermatol. 2012, 132, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Y.; Gu, Z. Punching and electroporation for enhanced transdermal drug delivery. Theranostics 2018, 8, 3688–3690. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Sapra, B.; Jain, S. Innovations in transdermal drug delivery: Formulations and techniques. Recent. Pat. Drug Deliv. Formul. 2007, 1, 23–36. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Lee, P.J.; Langer, R.; Shastri, V.P. Role of N-methyl pyrrolidone in the enhancement of aqueous phase transdermal transport. J. Pharm. Sci. 2005, 94, 912–917. [Google Scholar] [CrossRef]
- Cilurzo, F.; Vistoli, G.; Selmin, F.; Gennari, C.G.; Musazzi, U.M.; Franzé, S.; Lo Monte, M.; Minghetti, P. An insight into the skin penetration enhancement mechanism of N-methylpyrrolidone. Mol. Pharm. 2014, 11, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.; Peck, J.; Williams, D.G.; Pugh, W.J.; Allan, G. Mechanisms of action of skin penetration enhancers/retarders: Azone and analogues. Int. J. Pharm. 1996, 141, 17–25. [Google Scholar] [CrossRef]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2020, 312, 337–352. [Google Scholar] [CrossRef]
- Rowat, A.C.; Kitson, N.; Thewalt, J.L. Interactions of oleic acid and model stratum corneum membranes as seen by 2H NMR. Int. J. Pharm. 2006, 307, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Fang, L.; Li, T.; Wang, M.; Zhao, L.; He, Z. Effect of permeation enhancers and organic acids on the skin permeation of indapamide. Int. J. Pharm. 2008, 350, 43–47. [Google Scholar] [CrossRef]
- Loeffler, A.; Cobb, M.A.; Bond, R. Comparison of a chlorhexidine and a benzoyl peroxide shampoo as sole treatment in canine superficial pyoderma. Vet. Rec. 2011, 169, U249–U291. [Google Scholar] [CrossRef]
- Uri, M.; Buckley, L.M.; Marriage, L.; McEwan, N.; Schmidt, V.M. A pilot study comparing in vitro efficacy of topical preparations against veterinary pathogens. Vet. Dermatol. 2016, 27, e39. [Google Scholar] [CrossRef] [PubMed]
- Valentine, B.K.; Dew, W.; Yu, A.; Weese, J.S. In vitro evaluation of topical biocide and antimicrobial susceptibility of Staphylococcus pseudintermedius from dogs. Vet. Dermatol. 2012, 23, 493-e95. [Google Scholar] [CrossRef] [PubMed]
- Rana, E.A.; Islam, M.Z.; Das, T.; Dutta, A.; Ahad, A.; Biswas, P.K.; Barua, H. Prevalence of coagulase-positive methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in dogs in Bangladesh. Vet. Med. Sci. 2022, 8, 498–508. [Google Scholar] [CrossRef]
- Zaffaroni, A.A. An enterprise in biomedical innovation. Technovation 1981, 1, 135–146. [Google Scholar] [CrossRef]
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Clares, B. Enhancing topical analgesic administration: Review and prospect for transdermal and transbuccal drug delivery systems. Curr. Pharm. Des. 2015, 21, 2867–2882. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Jain, A.; Jain, P.; Kurmi, J.; Jain, D.; Jain, R.; Chandel, S.; Sahu, A.; Mody, N.; Upadhaya, S.; Jain, A. Novel strategies for effective transdermal drug delivery: A review. Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 219–272. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J. Transdermal drug delivery by passive diffusion and iontophoresis: A review. Med. Res. Rev. 1993, 13, 569–621. [Google Scholar] [CrossRef]
- Nino, M.; Calabro, G.; Santoianni, P. Topical delivery of active principles: The field of dermatological research. Dermatol. Online J. 2010, 16, 4. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Wang, J.; Baba, K.; Oki, Y.; Hiruta, Y.; Ito, M.; Ito, S.; Kanazawa, H. Transcutaneous drug delivery by liposomes using fractional laser technology. Lasers Surg. Med. 2017, 49, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, L.X.; Peng, X.T.; Gong, H.N.; Wang, J.Q.; Lu, J.R.; Xu, H. Effects of conventional surfactants on the activity of designed antimicrobial peptide. Langmuir 2020, 36, 3531–3539. [Google Scholar] [CrossRef]
- Gu, S.Y.; Gao, J.; Hou, X.M.; Ding, B.Y.; Zhang, W.; Gao, S.; Ding, X.Y. Effects of penetration enhancers on Shuangwu traumatic formula: In vitro percutaneous absorption and in vivo pharmacodynamic evaluation of an herb medicine. Eur. J. Pharm. Biopharm. 2009, 73, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hu, J.H. Influence of different penetration enhancer on in vitro transdermal effect of curcumin gel. Pharm. Care Res. 2015, 15, 357–360. [Google Scholar] [CrossRef]
- Priborsky, J.; Takayama, K.; Nagai, T.; Waitzova, D.; Elis, J. Evaluation of in Vitro and in Situ Transdermal Absorption of Drugs in Pig and Rat Skin. Chem. Pharm. Bull. 1987, 35, 4915–4920. [Google Scholar] [CrossRef]
- Choksi, N.Y.; Truax, J.; Layton, A.; Matheson, J.; Mattie, D.; Varney, T.; Tao, J.; Yozzo, K.; McDougal, A.J.; Merrill, J.; et al. United States regulatory requirements for skin and eye irritation testing. Cutan. Ocul. Toxicol. 2019, 38, 141–155. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protocols 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Koeth, L.M.; Apfalter, P.; Becker, K.; Gesu, G.; Martínez-Martínez, L.; Lahiri, S.D.; Alm, R.A.; Ambler, J.; Iaconis, J. Multi-center and multi-method evaluation of in vitro activities of ceftaroline against S. aureus. Diagn. Microbiol. Infect. Dis. 2016, 85, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hachem, C.Y.; Clarridge, J.E.; Reddy, R.; Flamm, R.; Evans, D.G.; Tanaka, S.K.; Graham, D.Y. Antimicrobial susceptibility testing of Helicobacter pylori comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn. Microbiol. Infect. Dis. 1996, 24, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Chuang, H.C.; Chang, B.Y.; Kuo, W.C.; Wang, C.H.; Gao, H.W.; Chiang, C.P. Combining microbubble contrast agent with pulsed-laser irradiation for transdermal drug delivery. Pharmaceutics 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.L.; Onunkwo, C.C.; Watts, C.J.; Sohnle, P.G. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb. Pathog. 2009, 47, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Shimo, T.; Yamazaki, M.; Noguchi, Y.; Mukawa, A.; Kato, H.; Ito, Y. Irritation test of dexamethasone valerate (dv-17) and other steroid ointments in rabbits: Skin and eye primary irritation tests, and skin cumulative irritation test. J. Toxicol. Sci. 1982, 7 (Suppl. S1), 1–13. [Google Scholar] [CrossRef]

| Strains | MIC | |||||||
|---|---|---|---|---|---|---|---|---|
| NZ2114 a | Mupirocin | Ofloxacin | Lincomycin | |||||
| μg/mL | μM | μg/mL | μM | μg/mL | μM | μg/mL | μM | |
| S. pseudintermedius CGMCC 1.90001 | 2 | 0.45 | 0.25 | 0.50 | 0.5 | 1.38 | 1 | 2.26 |
| S. pseudintermedius CGMCC 1.90002 | 1 | 0.23 | 0.25 | 0.50 | 32 | 88.55 | >128 | >288.95 |
| S. pseudintermedius CGMCC 1.90003 | 2 | 0.45 | 0.25 | 0.50 | 0.5 | 1.38 | 1 | 2.26 |
| S. pseudintermedius CGMCC 1.90004 | 2 | 0.45 | 0.25 | 0.50 | 1 | 2.77 | >128 | >288.95 |
| S. pseudintermedius CGMCC 1.90005 | 2 | 0.45 | 0.25 | 0.50 | 1 | 2.77 | 4 | 9.03 |
| S. pseudintermedius CGMCC 1.90001 | S. pseudintermedius CGMCC 1.90002 | S. pseudintermedius CGMCC 1.90003 | S. pseudintermedius CGMCC 1.90004 | S. pseudintermedius CGMCC 1.90005 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D a (mm) | Susceptibility | D a (mm) | Susceptibility | D a (mm) | Susceptibility | D a (mm) | Susceptibility | D a (mm) | Susceptibility | |
| Penicillin (PEN) | 11.5 | R | 8.5 | R | 39.5 | S | 37.0 | S | 7.5 | R |
| Ampicillin (AMP) | 14.5 | I | 10.5 | R | 35.5 | S | 38.0 | S | 12.0 | R |
| Cefatriaxone (CRO) | 28.5 | S | 14.5 | I | 32.0 | S | 30.5 | S | 28.5 | S |
| Cefazolin (CZO) | 32.0 | S | 25.5 | S | 42.5 | S | 42.0 | S | 28.5 | S |
| Cefotaxime (CTX) | 36.5 | S | 23.5 | S | 42.5 | S | 37.5 | S | 33.5 | S |
| Imipenem (IPM) | 42.5 | S | 45.5 | S | 45.5 | S | 45.5 | S | 42.0 | S |
| Cefoxitin (FOX) | 35.5 | S | 32.0 | S | 30.5 | S | 34.5 | S | 33.5 | S |
| Oxacillin (OXA) | 27.0 | S | 25.5 | S | 29.5 | S | 36.0 | S | 22.5 | S |
| Vancomycin (VAN) | 15.5 | S | 17.3 | S | 14.5 | S | 17.0 | S | 17.5 | S |
| Kanamycin (KAN) | 23.5 | S | 9.5 | R | 23.0 | S | 7.0 | R | 12.0 | R |
| Azithromycin (AZM) | 22.0 | S | 6.0 | R | 23.5 | S | 6.0 | R | 6.0 | R |
| Erythromycin (ERY) | 25.5 | S | 6.0 | R | 22.0 | I | 6.0 | R | 7.0 | R |
| Tetracycline (TCY) | 20.5 | S | 9.3 | R | 22.5 | S | 21.0 | S | 8.0 | R |
| Ciprofloxacin (CIP) | 27.0 | S | 6.5 | R | 28.0 | S | 24.0 | S | 23.0 | S |
| Ofloxacin (OFX) | 24.8 | S | 6.0 | R | 24.5 | S | 22.0 | S | 20.5 | S |
| Nitrofurantoin (NIT) | 22.5 | S | 21.0 | S | 22.0 | S | 20.0 | S | 15.5 | I |
| Clindamycin (CLI) | 5 | R | 6.0 | R | 21.0 | I | 6.0 | R | 12.0 | R |
| Lincomycin (LM) | 5 | R | 6.0 | R | 19.0 | I | 6.0 | R | 10.0 | R |
| Trimethoprim (SXT) | 6 | R | 6.0 | R | 6.0 | R | 6.0 | R | 6.0 | R |
| Chloramphenicol (CHL) | 21 | S | 24.0 | S | 22.5 | S | 20.0 | S | 11.5 | R |
| Rifampicin (RIF) | 27.5 | S | 24.0 | S | 26.0 | S | 27.0 | S | 29.0 | S |
| Linezolid (LNZ) | 25 | S | 28.5 | S | 21.5 | S | 21.0 | S | 28.5 | S |
| Inhibition Zone Diameter/mm | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azone (%) | PG (%) | OA (%) | NMP (%) | LA (%) | ||||||||||
| 0.5 | 1 | 2 | 5 | 10 | 20 | 1 | 5 | 10 | 1 | 5 | 1 | 3 | 5 | |
| Positive Control | 14.70 ± 0.67 | |||||||||||||
| Blank Control | 16.67 ± 0.58 | 17.17 ± 0.29 | 18.00 ± 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NZ2114 sprays | 17.17 ± 0.29 | 18.17 ± 0.76 | 18.23 ± 0.58 | 15.83 ± 1.04 | 16.33 ± 1.19 | 16.37 ± 0.58 | 0 | 0 | 0 | 15.17 ± 0.29 | 14.67 ± 0.58 | 0 | 0 | 0 |
| Administration | Groups | Test Substance | Total Score | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | ||||||||||
| 1 | 24 | 48 | 72 | 1 | 24 | 48 | 72 | |||
| Single dose | Intact skin | NZ2114 sprays | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blank Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Negative Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Positive Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Damaged skin | NZ2114 sprays | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blank Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Negative Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Positive Control | 8 | 7 | 7 | 6 | 2.7 | 2.3 | 2.3 | 2 | ||
| Multiple doses | Intact skin | NZ2114 sprays | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blank Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Negative Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Positive Control | 9 | 9 | 9 | 9 | 3 | 3 | 3 | 3 | ||
| Damaged skin | NZ2114 sprays | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blank Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Negative Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Positive Control | 12 | 9 | 9 | 9 | 4 | 3 | 3 | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Huang, Y.; Li, Y.; Teng, D.; Mao, R.; Hao, Y.; Wei, L.; Wang, J. Efficiency of NZ2114 on Superficial Pyoderma Infected with Staphylococcus pseudintermedius. Pharmaceuticals 2024, 17, 277. https://doi.org/10.3390/ph17030277
Yang N, Huang Y, Li Y, Teng D, Mao R, Hao Y, Wei L, Wang J. Efficiency of NZ2114 on Superficial Pyoderma Infected with Staphylococcus pseudintermedius. Pharmaceuticals. 2024; 17(3):277. https://doi.org/10.3390/ph17030277
Chicago/Turabian StyleYang, Na, Yan Huang, Yuanyuan Li, Da Teng, Ruoyu Mao, Ya Hao, Lingyun Wei, and Jianhua Wang. 2024. "Efficiency of NZ2114 on Superficial Pyoderma Infected with Staphylococcus pseudintermedius" Pharmaceuticals 17, no. 3: 277. https://doi.org/10.3390/ph17030277
APA StyleYang, N., Huang, Y., Li, Y., Teng, D., Mao, R., Hao, Y., Wei, L., & Wang, J. (2024). Efficiency of NZ2114 on Superficial Pyoderma Infected with Staphylococcus pseudintermedius. Pharmaceuticals, 17(3), 277. https://doi.org/10.3390/ph17030277








