Abstract
Isoflavones are a group of (poly)phenols, also defined as phytoestrogens, with chemical structures comparable with estrogen, that exert weak estrogenic effects. These phytochemical compounds have been targeted for their proven antioxidant and protective effects. Recognizing the increasing prevalence of cardiovascular diseases (CVD), there is a growing interest in understanding the potential cardiovascular benefits associated with these phytochemical compounds. Gut microbiota may play a key role in mediating the effects of isoflavones on vascular and endothelial functions, as it is directly implicated in isoflavones metabolism. The findings from randomized clinical trials indicate that isoflavone supplementation may exert putative effects on vascular biomarkers among healthy individuals, but not among patients affected by cardiometabolic disorders. These results might be explained by the enzymatic transformation to which isoflavones are subjected by the gut microbiota, suggesting that a diverse composition of the microbiota may determine the diverse bioavailability of these compounds. Specifically, the conversion of isoflavones in equol—a microbiota-derived metabolite—seems to differ between individuals. Further studies are needed to clarify the intricate molecular mechanisms behind these contrasting results.
1. Introduction
Oxidative stress and inflammation are pivotal players involved in the disruption of endothelial equilibrium, leading to its dysfunction and affecting the overall cardiometabolic system [1,2]. This imbalance is the main cause of the development of cardiovascular disease (CVD) and related vascular and metabolic disorders, such as obesity, diabetes, hypertension, dyslipidemia, and atherosclerotic vascular diseases [2]. For these reasons, a better understanding of the mechanisms and risk factors involved in these processes, as well as effective strategies for prevention and management are necessary. Genetic, environmental, and lifestyle factors, such as diet, play a critical role in the development of CVD [3,4]. Diets rich in plant-based foods have been reported to substantially reduce the risk of CVD [5,6,7]. One of the main mechanisms, among others, is hypothesized to depend on their content in (poly)phenol, natural compounds with a variety of properties related to their chemical composition, which can also exert effects on human health. A recent summary of evidence suggests that a higher intake of certain (poly)phenol classes is associated with a lower risk of hypertension [8], CVD [9], and CVD-related mortality [10].
A peculiar group of (poly)phenols are phytoestrogens—molecules with a chemical structure similar to estradiol—which can exert some effects on the human system through the interaction with estrogen receptors (ERs) [11]. Isoflavones, mainly represented by daidzein and genistein, are phytoestrogens that raised great interest for their potentially hormone-related relevant effects on human health [11]. Many studies have demonstrated that isoflavone intake was associated with reduced menopausal symptoms [12], decreased incidence of different types of cancers (breast and prostate cancers) [13], and osteoporosis [14,15]. Isoflavones per se have a low bioavailability, while they are metabolized by enteric cells and the gut microbiota to produce more available and active metabolites [16,17]. In particular, equol is a gut microbiota-derived metabolite showing a greater estrogenic effect than isoflavones [18,19]. However, while all animals are able to convert isoflavones in equol, human studies demonstrated that individuals may have substantially different responses to isoflavone intake (such as being equol-producers and equol-non-producers) depending on genetic factors (given the considerable inconsistencies observed by geographical area [20]. In this regard, investigation of the gut microbiota composition and the differences between equol-producers and equol-non-producers is important to understand other potential variables related to the ability to metabolize isoflavones. Improving our knowledge on this matter would allow the development of possible interventions to modify gut microbiota in order to increase equol production and the benefit derived. In this review, we will discuss the clinical evidence known to date on isoflavone interventions and vascular outcomes. Furthermore, we will sum up the implication of gut microbiota in isoflavone metabolism and the main molecular mechanisms of action of major gut-derived metabolites.
2. Gut Microbiota and Its Implication in Cardiovascular Disease
The microbiota is defined as the whole microorganisms that cohabit inside/outside of a host [21]. It is composed of bacteria, archaea, viruses, and eukaryotes, although most of the studies are focused on the analysis of bacterial composition. In the last few years, researchers have moved their attention to the microbiota that inhabit the gut for its implication in health and disease [22]. The gut microbiota is composed mainly of the phyla Firmicutes and Bacteroidetes, followed by Proteobacteria and Actinobacteria [23]. Generally, a low Firmicutes/Bacteroidetes ratio is considered a health marker while on the contrary a high ratio is attributed to an unhealthy status [24,25]. More deeply, alterations in the microbiota composition have been correlated to different pathologies, such as neuropsychiatric disorders, obesity, irritable bowel disease, and CVD [21]. Oxidative stress and inflammation are risk factors involved in the pathogenesis of CVD [26,27] and gut microbiota play a key role in their regulation [28]. Indeed, a healthy microbiota is associated with reduced harmful metabolite production and tight junction integrity, which is linked to lower inflammation and oxidative stress, whereas an unhealthy microbiota is correlated with the production of harmful metabolites, such as trimethylamine (TMA) and p-cresol, increased gut permeability (leaky gut), and the onset of a low-grade inflammatory state [29,30,31] (Figure 1).
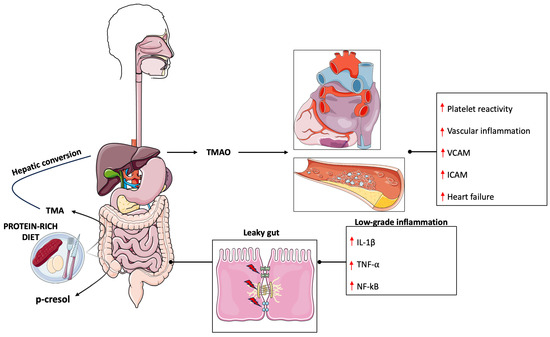
Figure 1.
Gut-microbiota derived metabolites involved in cardiovascular disease. High protein intake leads to an increased production of TMA and p-cresol that negatively impacts gut permeability leading to systemic low-grade inflammation. IL-1beta (Interleukin-1 beta), ICAM (Intercellular Adhesion Molecule 1), NF-kB (Nuclear factor kappa B), TMA (Trimethylamine), TMAO (Trimethylamine N-Oxide), TNF-alpha (Tumor necrosis factor alpha), VCAM (Vascular cell adhesion protein). ↑ denotes increase.
Many studies have investigated the role of the microbiota in CVD onset and pathological raise in blood pressure (BP). When comparing the microbiota composition of healthy with hypertensive and pre-hypertensive participants it was observed a significant increase in the abundance of pro-inflammatory taxa such as Prevotella and Klebsiella genera and a significant reduction of Faecalibacterium, Oscillibacter, Roseburia, Bifidobacterium, Coprococcus, and Butyrivibrio. These alterations were also associated with changes in circulating metabolites, such as increased levels of stearic acid in hypertensive patients, and possibly related to the onset of a low-grade inflammatory state [32]. Similar results have demonstrated decreased levels of Faecalibacterium prausnitzii and Lachnospiraceae family and increased levels of Ruminococcus, Prevotella, Hungatella, and Succinclasticum genera [33]. Furthermore, recent studies described a case of bacteria translocation from the gut to the heart [34] and the detection of gut microbial DNA in the plaques [35]. Gut dysbiosis is strictly correlated to an alteration in gut permeability that leads to the translocation of bacteria products into host circulation resulting in a proinflammatory state [30]. Indeed, when the gut barrier is impaired, lipopolysaccharides (LPS) can cross the intestinal lumen, reaching the circulation and binding the Toll-like receptor (TLR) on the surface of immune cells [36] triggering an inflammatory process that leads to the production of proinflammatory cytokines [37]. In a recent study, it was observed that increased LPS concentrations were predictive of major adverse cardiac events in subjects with atrial fibrillation [38]. However, it should be noted that other factors contribute to the development of CVD. In this regard, it is also important to consider that change in the microbiota composition led to an alteration in microbial-derived metabolites which could affect the physiological function of different organs. One of the most famous and discussed metabolites produced by gut bacteria in literature is TMA [39]. TMA is produced starting from different precursors, such as choline [40], phosphatidylcholine [41] and carnosine [42]. TMA reaches systemic circulation and is further metabolized in the liver by the enzyme flavin monooxygenases to produce trimethylamine N-oxide (TMAO) [43]. Circulating levels of TMAO have been associated with CVD outcome [44,45] and it was observed to promote platelet reactivity [46,47], vascular inflammation [48,49], and heart failure [50,51]. However, TMAO is not the only metabolite associated with CVD. Indeed, another candidate showed a strong association with a major adverse cardiac event identified as phenylacetylglutamine (PAG), a metabolite produced during gut phenylalanine metabolism [52]. A study conducted on 4000 participants showed an increase in the incidence of major adverse cardiac events like heart attack, stroke, and death associated with PAG [52]. PAG was shown to interact with adrenergic receptors (ARs) which are involved in heart disease and platelet functions [53,54,55]; and its detrimental effects could be attenuated by β-blocker carvedilol [52]. However, more studies are necessary to further characterize the function of PAG in host physiology.
3. Isoflavones Metabolism and Impact on Gut Microbiota Composition
The phytoestrogen isoflavones, of which genistein and daidzein are the main studied, are flavonoid compounds that, due to their molecular structure and size, resemble vertebrate steroid estrogen and interact with their receptors [15]. The orally ingested phytoestrogens are mainly in the form of glycosides (genistin and daidzin) that are rapidly cleaved during the passage in the intestinal lumen by the glycosidase enzyme produced by the intestinal epithelium [16] or by the bacteria in the small intestine [17] to release the aglycone. The latter is rapidly absorbed and modified in the liver to be released in the colon and further metabolized by colon bacteria [56]. Daidzein and genistein, the aglycone of daidzin and genistin, are converted by gut bacteria to produce equol or 5-OH-equol, respectively [18,19]. However, while all animals tested in in vivo experiments demonstrated to produce equol, in humans only 25–50% of subjects (named equol-producers) possess the capability to produce it while the great part of humans does not synthesize equol but convert daidzein and genistein into O-desmethylangolensin (O-DMA) and 6′-OH-O-DMA, respectively [19,20]. The inter-individual difference in equol production could be attributed to the microbiota composition. Many studies showed that bacteria with the capacity to convert daidzein into equol are largely of the Coriobacteriaceae family. Among these, different bacteria included in the Eggerthella genus [57], such as Eggerthella julong 732 strain [58], and the species Adlercreutzia equolifaciens have been observed to produce equol [59]. Other strains that have been reported to be equol-producers without being included in the Coriobacteriaceae family are Lactococcus garvieae 20–92 [60] and Pediococcus pentosaceus [61]. Genomic analysis identified three genes in the daidzein-equol conversion which are daidzein reductase (DZNR), dihydrodaidzein reductase (DHDR) and tetrahydrodaidzein reductase (THDR) [20]. Analysis of the GC content in bacteria outside the Coriobacteriaceae family revealed higher GC content in these genes compared to the overall genomic GC content, suggesting that these genes were acquired through horizontal transfer [62,63]. Different studies have observed that isoflavone and isoflavone-derived metabolite supplementation can influence microbiota composition. Dietary supplementation with soy bars containing isoflavones led to an increase in Bifidobacterium genus with a greater increase of Bifidobacterium and Eubacterium observed in equol-producers versus non-equol-producers [64]. In another study, equol supplementation was positively correlated with an increase in Bifidobacterium and negatively correlated with Clostridium cluster IV [65]. Similar results have been observed in an in vivo study, which demonstrated that isoflavone supplementation was associated with a significant change in the microbiota composition with an increase in SCFA production and in Bifidobacterium spp. [66]. Similarly, in another in vivo study isoflavones administration was associated with the augmented composition of bacteria belonging to the genus Bifidobacterium, Akkermansia, Bacteroides and Firmicutes compared to the control [67]. Another study aimed to evaluate the effects of genistein supplementation on glucose metabolism and adipose tissue browning, investigated the genistein-mediated microbiota changes showing a significant increase in Ruminiclostridium_5, Ruminiclostridium_9, and Blautia which were significantly correlated with browning markers and glucose tolerance [68].
4. Clinical Evidence on the Effect of Isoflavones on Vascular Outcomes
Isoflavones, present mainly in soy and soy-based foods, represent a subclass of flavonoids that comprises a group of molecules with pleiotropic effects, including weak estrogenic activity. Various studies have demonstrated the beneficial effects of phytoestrogens on vascular and endothelial parameters (Table 1).
Several RCTs explored the effect of soy and soy-derived products, including soy legumes, soy flour, and soy protein, in both healthy and disease-affected individuals, reporting contradictory results. In a double-blind, RCT 179 healthy participants (96 men and 83 postmenopausal women) with a mean age of 62 years were supplemented with 56 g/day of powdered soy protein (providing 118 mg of total isoflavones, 75.6 mg genistein, 36.96 mg daidzein, 5.04 mg glycitein) or placebo: results showed that soy supplementation reduced mean BP (from 93 ± 1 to 87 ± 1 mmHg; p < 0.01), SBP (from 130 ± 2 to 123 ± 2 mmHg; p < 0.05) and DBP (from 76 ± 1 to 72 ± 1 mmHg; p < 0.01) compared to the placebo group; furthermore, it was observed a significant improvement of the femoro-dorsal PWV (from 11.1 ± 0.2 to 10.3 ± 0.2; p = 0.02) and a significant reduction in brachial artery FMD only in men (p < 0.05) compared to the placebo group [69]. In a crossover RCT, 23 healthy volunteers aged between 60 and 70 years were recruited to analyze the effects of 64 g/day of soy nuts containing 174 mg of isoflavones: after the intervention, a significant increase in FMD (p = 0.040) was reported in the soy nuts group compared to the control group [70]. Similar findings were reported in studies on individuals at CVD risk. A double-blind, parallel-group dietary intervention RCT evaluated the effects of 20 g/day of soy powder supplementation (providing 80 mg of isoflavones) on BP in 50 men with relatively higher BP and/or total cholesterol levels with a mean age of 52 years. Results showed that soy supplementation was able to significantly reduce SBP (from 142.0 ± 3.0 to 131.2 ± 3.1 mmHg, p = 0.001) and DBP (from 87.1 ± 1.8 to 82.0 ± 1.8 mmHg, p = 0.002) compared to baseline values, while no differences were observed for the placebo group [71]. In a double-blind, RCT it was investigated the effects of 40 g/day of soybean consumption (providing 79.4 mg total isoflavone, 44.9 mg genistein, 26.5 mg daidzein, 4.9 mg of glycitein) on BP in 302 participants (mean age of 49 years) with untreated BP for 12 weeks: results showed a significant decrease in SBP (−7.88, 95% CI: −4.66 to −11.1) and DBP (−5.27, 95% CI: −3.05 to −7.49) [72]. However, other studies conducted on healthy and unhealthy individuals using soy-derived products reached null results. A double-blind RCT, which investigated the effects of isoflavone intake among 89 hypercholesterolaemic participants with a mean age of 60 years who were randomly divided to receive 30 g/daily of soy protein (providing 100 mg of isoflavones) or 30 g/day of placebo (casein) for 24 weeks, reported no differences in FMD after treatment [73]. Furthermore, a double-blind, RCT conducted on 180 postmenopausal women (mean age 59 years) evaluated the effects of 100 mg/day of isoflavone supplementation (providing 35 mg daidzin, 59 mg genistin, 4 mg glycitin) combining soy or milk protein, or placebo on BP showed that after 6 months of treatment no differences between groups were observed [74]. In another double-blind RCT, 61 postmenopausal women (mean age 50 years) were supplemented with 33 g/day of soy-derived products, providing 54 mg of isoflavones, or placebo for 8 weeks leading to no differences were observed for SBP and DBP [75]. Similar results have been obtained from a parallel-group, double-blind, RCT conducted on 253 postmenopausal women (mean age 56 years) and supplemented with 40 g/day of soy flour (providing 49.3 mg isoflavones) or 40 g low-fat milk powder plus 63 mg daidzein or placebo for 6 months on BP and endothelial function leading to no differences independently of the type of treatment [76].
Other RCTs focused on the investigation of the isoflavone-containing capsules on vascular outcomes, reporting contradictory findings. A double-blind, RCT evaluated the effects of genistein tablets administration (providing 54 mg/day of genistein) or placebo on 60 healthy postmenopausal women, with a mean age of 56 years, for 6 months. At the end of the treatment genistein group showed a significant increase in the brachial artery diameter (from 3.9 ± 0.8 to 4.4 ± 0.7 mm; p < 0.05 vs. placebo and p < 0.01 vs. before genistein) accompanied by an increase in the brachial arterial blood flow (from 25 ± 5 to 75 ± 7 mm; p < 0.05 vs. placebo and p < 0.01 vs. before genistein) during reactive hyperemia. No effects were observed concerning other parameters [77]. In another double-blind, RCT involving 85 postmenopausal women supplemented with 70 mg/day of isoflavone capsule (providing 38 mg glycitin, 20 mg daidzin, and 12.4 mg genistin) or placebo for 12 weeks demonstrated that isoflavone supplementation was able to decrease SBP (from 116.1 ± 14.3 to 110.8 ± 11 mmHg, p < 0.05) and DBP (from 74.6 ± 10 to 71.6 ± 7.7 mmHg, p < 0.05) in the treated group compared to baseline [78]. Other works, conducted on unhealthy participants, showed similar results. In a double-blind RCT conducted on 102 participants with prior ischaemic stroke (mean age 66 years) and supplemented for 12 weeks with 80 mg/day of isoflavone capsules (providing 80 mg purified isoflavones) or placebo, demonstrated that isoflavone consumption was correlated with a significant improvement of brachial FMD (OR 0.32, 95% CI: 0.13 to 0.80, p = 0.014) reversing their endothelial dysfunction status [79]. Another double-blind RCT investigated the effects of genistein tablets administration (providing 54 mg of genistein) for 6 months on 20 postmenopausal women (mean age of 58 years) with metabolic syndrome. At the end of the study, genistein-supplemented participants showed a significant increase of FMD (from 3.2 ± 4.9 to 8.9 ± 3.1) compared to the baseline (p < 0.001) and placebo group (p = 0.04) after 6 months [80]. A double-blind, RCT, including 108 postmenopausal women with metabolic syndrome, investigated the effects of 54 mg/day of genistein for 12 months: results showed a significant reduction of SBP (from 135.7 to 123.7 mmHg, p < 0.001 vs. baseline and p < 0.0002 vs. placebo group) and DBP (from 78.7 to 74.5 mmHg, p = 0.047 vs. baseline and p = 0.0541 vs. placebo group) [81]. However, other works showed opposite results. A double-blind, parallel, RCT conducted on 24 postmenopausal women with a mean age of 50 years demonstrated that 81.02 mg/day of isoflavone tablets supplementation (providing 44.02 mg daidzein, 27.08 mg glycitein and 9.92 mg genistein) for 6 weeks had no effects on BP and other cardiovascular parameters compared to the placebo group [82]. Similarly, a 2-year prospective, double-blind, RCT conducted on 431 postmenopausal women with a mean age of 57 years, and supplemented with isoflavone tablets (providing 300 mg of isoflavones), showed a slight reduction in SBP and DBP in both groups not associated with the treatment [83]. In another double-blind, RCT conducted on 50 obese postmenopausal women (mean age 60 years) supplemented with 4 isoflavone capsules daily (providing 70 mg/day of isoflavone, 44 mg daidzein, 16 mg glycitein, 10 mg genistein) or placebo for 6 months: results did not show any significant change in the treated group [84]. A double-blind, case–control study aimed to investigate the effects on cardiovascular functions of one-year genistein intervention in 22 postmenopausal women with metabolic syndrome (mean age 55 years). After the daily consumption of two tablets containing 54 mg of genistein, no significant results were detected in SBP and DBP compared to the placebo [85]. Similar results have been observed in a double-blind, RCT including 82 patients with non-alcoholic fatty liver disease (mean age of 43 years) that received 250 mg/day of genistein capsules or placebo for 8 weeks. After intervention, no differences were observed in SBP and DBP [86]. Also in another double-blind, RCT which included 38 peritoneal dialysis patients who randomly received 100 mg of soy isoflavone tablets (providing 63.72 mg genistin, 2.98 mg genistein, 26.42 mg daidzin, 3.5 mg daidzein, 2.28 mg glycitin, 1.1 mg glycitein) or a placebo for 8 weeks did not show any significant change related to treatment [87].


Table 1.
The main characteristic of the selected randomized clinical trials concerning isoflavones and cardiovascular risk factors.
Table 1.
The main characteristic of the selected randomized clinical trials concerning isoflavones and cardiovascular risk factors.
| Author, Year, Country | Study Design | Participants (Mean Age) | Duration | Treatment | Isoflavones Constituent (Daily Intake) | Comparison | Main Findings |
|---|---|---|---|---|---|---|---|
| Teede, 2001, Australia [69] | Double-blind, placebo-controlled | 179 healthy participants (96 men and 83 postmenopausal women) (62 y) | 3 mo | 56 g/d powdered soy protein isolate | 118 mg isoflavones, 75.6 mg genistein, 36.96 mg daidzein, 5.04 mg glycitein | Placebo (casein) | Significant reduction in BP (p < 0.01) and PWV(FD) improvement (p = 0.02). Brachial artery FMD was significantly reduced only in men (p < 0.05). |
| Squadrito, 2002, Italy [77] | Double-blind, placebo-controlled | 60 healthy postmenopausal women (56 y) | 6 mo | Genistein tablets | 54 mg genistein | Placebo tablets | Increase in brachial artery diameter and brachial artery blood flow (p < 0.01). |
| Sagara, 2004, UK [71] | Double-blind, placebo-controlled | 50 men with relatively higher BP and/or total cholesterol (52 y) | 5 wk | Diet containing at least 20 g/d of soy powder | At least 80 mg of isoflavones | Placebo diet | Decrease in SBP (p = 0.001) and DBP (p = 0.002) compared to baseline. |
| He, 2005, China [72] | Double-blind, controlled | 302 participants untreated BP (49 y) | 12 wk | 40 g/d of isolated soybean protein supplement | 79.4 mg total isoflavone, 44.9 mg genistein, 26.5 mg daidzein, 4.9 mg/d of glycitein | 40 g/d of complex carbohydrate | Reduction in SBP (p = 0.01) and DBP (p = 0.007). |
| Hermansen, 2005, Denmark [73] | Double-blind, placebo-controlled | 89 hypercholesterolaemic subjects (60 y) | 24 wk | Soy supplement with 30 g/d soy protein 9 g/d and cotyledon fibre | 100 mg isoflavones | Placebo (30 g/d casein) | No differences in FMD. |
| Aubertin-Leheudre, 2008, Canada [84] | Double-blind, placebo-controlled | 50 obese postmenopausal women (60 y) | 6 mo | Isoflavone capsules | 70 mg total isoflavones, 44 mg daidzein, 16 mg glycitein, 10 mg genistein | Placebo capsules | No differences were observed. |
| Chan, 2008, China [79] | Double-blind, placebo-controlled | 102 participants with prior ischaemic stroke (66 y) | 12 wk | Isoflavone capsules | 80 mg purified isoflavones | Placebo (powdered cellulose) | Improvement of brachial FMD (p = 0.014). |
| Wong, 2012, USA [82] | Double-blind, placebo-controlled | 24 postmenopausal women (50 y) | 6 wk | Isoflavone tables | 81.02 mg total isoflavone (44.02 mg daidzein, 27.08 mg glycitein and 9.92 mg genistein) | Placebo tablets (<1.0 mg aglycone) | No effects were observed comparing treated and placebo groups. |
| Irace, 2013, Italy [80] | Double-blind, placebo-controlled | 20 postmenopausal women with metabolic syndrome (58 y) | 6 mo | Genistein tablets | 54 mg genistein | Placebo tablets | Significant increase of FMD compared with placebo group (p < 0.001). |
| Kim, 2013, South Korea [78] | Double-blind, placebo-controlled | 85 postmenopausal women (53 y) | 12 wk | Isoflavone capsules | 70 mg total isoflavone (38 mg glycitin, 20 mg daidzein, and 12.4 mg genistein) | Placebo capsules | Significant reduction of SBP and DBP compared to baseline (p < 0.05). |
| Liu, 2013, China [74] | Double-blind, placebo-controlled | 180 postmenopausal women with pre or early diabetes (59 y) | 6 mo | (i) 15 g soy + 100 mg isoflavones; (ii) 15 g milk protein + 100 mg isoflavone | 100 mg total isoflavones (35 mg daidzein, 59 mg genistein, 4 mg glycitin | Placebo (15 g milk protein) | Subgroup analysis among pre and hypertensive women showed a significant reduction in SBP (p < 0.05) and sICAM1 compared to placebo group (p = 0.02). |
| Squadrito, 2013, Italy [81] | Double-blind, placebo-controlled | 108 postmenopausal women with MetS (58 y) | 12 mo | Genistein tablets | 54 mg genistein | Placebo tablets | Significant reduction of SBP (p < 0.0002) and DBP (p = 0.0541). |
| Cheng, 2015, China [83] | Double-blind, placebo-controlled | 431 postmenopausal women (57 y) | 2 y | Isoflavone tablets | 300 mg isoflavone aglycone | Placebo tablets | No differences were observed between treatment groups. |
| Husain, 2015, Iran [75] | Double-blind, placebo-controlled | 61 postmenopausal women (50 y) | 8 wk | 33 g of soy in the form of biscuits | 54 mg isoflavones | Placebo biscuits | No differences were observed after treatment. |
| Liu, 2015, China [76] | Double-blind, placebo-controlled | 253 postmenopausal women (56 y) | 6 mo | (i) 40 g soy flour; (ii) 40 g low-fat milk powder + 63 mg daidzein | (i) 49.3 mg isoflavones, (ii) 63 mg daidzein | Placebo (40 g low-fat milk powder) | No differences were observed after treatment. |
| De Gregorio, 2017, Italy [85] | Double-blind, placebo-controlled | 22 postmenopausal women with MetS (55 y) | 12 mo | Genistein tablets | 54 mg genistein | Placebo tablets | No significant findings were found in SBP and DBP in the genistein group. |
| Amanat, 2018, Iran [86] | Double-blind, placebo-controlled | 82 patients with NAFLD (43 y) | 8 wk | Genistein capsule | 250 mg genistein | Placebo capsule (cornstarch) | No differences in SBP and DBP were observed. |
| Movahedian, 2021, Iran [87] | Double-blind, placebo-controlled | 38 peritoneal dialysis patients (soy group: 54 y; placebo group: 51 y) | 8 wk | Soy isoflavone tablets | 63.72 mg genistein, 2.98 mg genistein, 26.42 mg daidzein, 3.5 mg daidzein, 2.28 mg glycitin, 1.1 mg glycitein | Placebo tablets (starch) | SBP and DBP did not significantly change at the end of the treatment. |
| Tischmann, 2022, The Netherlands [70] | Single-blind, controlled, crossover | 23 healthy volunteers (64 y) | 2 × 8 wk (8 wk washout) | 64 g/d soy nuts | 174 mg isoflavones | No treatment | A significant increase in FMD (p = 0.040) was detected following the soy nut intervention compared to the placebo. |
Abbreviations: BP (blood pressure); d (day); DBP (diastolic blood pressure); FD (femoro-dorsal); FMD (flow-mediated dilation); mo (month); PWV (pulse wave velocity); RCT (randomized clinical trial); SBP (systolic blood pressure); sICAM1 (soluble intercellular cell adhesion molecule 1); wk (week); y (year).
5. Mediating Effect of Equol-Production Status on Clinically Relevant Vascular Outcomes
The evidence from RCTs investigating the effects of soy and isoflavone supplementation on vascular outcomes indicates contrasting results. The discrepancies in the findings may be attributed, at least partially, to several factors influencing the real exposure to isoflavone metabolites. Among them, interindividual variations in the physiological response to isoflavone intake, related to the differences in the microbiota composition, and the possible interactions, including accumulating, synergistic and antagonistic effects, with other compounds from diet seems to play a major role [88].
A limited number of intervention studies explored how equolproduction status may influence the above-mentioned relation, generally reporting more pronounced effects among equol-producers compared to equol-non-producers. A double-blind RCT conducted among 190 postmenopausal women stratified into equol-producers and equol-non-producers tested the effects of 100 mg/day soy isoflavone supplementation on vascular markers. After 6 months of intervention the malondialdehyde (MDA) concentrations, an oxidative stress marker, were significantly lower in the soy-isoflavone equol-producers compared with equol-non-producers (p = 0.021). Although not statistically significant, similar results were also found for VCAM-1 and NO concentrations (p = 0.413 and p = 0.724, respectively) [89]. In line, another double-blind RCT comprising 202 postmenopausal women showed that 12-month supplementation with soy protein containing 99 mg isoflavones/d decreased systolic and diastolic blood pressure and improved endothelial function in the equol-producers, while when considering equol-non-producers systolic and diastolic blood pressure increased and endothelial function deteriorated during the trial [90]. A crossover RCT conducted on 60 postmenopausal women stratified based on the metabolic syndrome status and following a soy nut enriched diet for 8 weeks or control diet, reported significant reductions in diastolic BP (p = 0.02), TG (p = 0.02), C-reactive protein (CRP) (p = 0.01) and sICAM (p = 0.03) among women with MetS following soy-enriched diet, However, changes were observed only among equol-producers compared to control diet, but not among equol-non-producers. Likewise, when considering women without metabolic syndrome, only equol-producers had significant reductions in diastolic BP (p = 0.02) and CRP (p = 0.04) [91]. Among 270 equol-producing postmenopausal women a 6-month supplementation with whole soy, but not purified daidzein, decreased serum LDL-C and hs-CRP levels, when compared to the control group [92].
On the contrary, some studies exploring the effect of isoflavone supplementation on vascular inflammation markers reported no significant differences in the clinical outcomes between equol-producing and equol-non-producing individuals. In particular, a double-blind, crossover RCT comprising 117 postmenopausal women consuming either isoflavone-enriched or control cereal bars for 8 weeks did not observe any significant differences in the vascular and inflammatory markers in response to isoflavones or placebo between equol-producers and non-equol-producers [93]. Similarly, a crossover RCT conducted among 117 healthy postmenopausal women and exploring the effect of 50 mg/d isoflavone supplementation on different markers of CVD, reported no differences in response to isoflavones according to equol-production status [94]. Finally, an acute RCT conducted among male equol and non-equol-producers showed that after soy intake carotid-femoral PWV significantly improved in equol-producers at 24 h, which was significantly associated with plasma equol concentrations, while no vascular effects were observed in non-equol-producers at any time point [95].
Although the findings from the RCT suggest the differences in response to soy or isoflavone supplementation between equol-producers and nonproducers, with more pronounced clinical effects among the former, the majority has been conducted among postmenopausal women. Therefore, further research exploring the potential effects of clinically significant isoflavone doses among different populations, considering both males and females, is warranted to better elucidate the mediating effect of equol production status as well as food and dietary matrix, in the relation between isoflavone intake and vascular health.
6. Potential Mechanisms Mediating the Effect of Isoflavones and Gut-Derived Metabolite Equol on Endothelium
Isoflavones are also known as phytoestrogen for their similarity with estradiol and the ability to bind estrogen receptors (ERs) distinguishable into ER-alpha and ER-beta and distributed across different systems including the cardiovascular system [96]. Equol, the microbial-derived isoflavones metabolite, has aroused great interest for its greater positive effects on health compared to isoflavones per se (Figure 2).
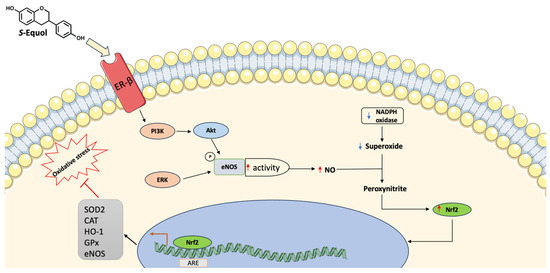
Figure 2.
Main equol mechanisms of action in equol-producers. Equol can increase eNOS activity resulting in higher levels of NO. This can react with superoxide producing peroxynitrite and increasing Nrf2 levels leading to upregulation of antioxidant genes. ARE (antioxidant response elements), CAT (Catalase), eNOS (Endothelial nitric oxide synthase), EC (Endothelial cell), ERK (Extracellular signal-regulated kinases), GPx (Glutathione peroxidase), HO-1 (Heme oxygenase 1), NO (Nitric oxide), PI3K (Phosphatidylinositol 3-kinase), NADPH (Nicotinamide adenine dinucleotide phosphate), SOD (Superoxide dismutase). ↑ denotes increase, ↓ denotes decrease.
Indeed, it was observed that equol has higher antioxidant activity compared to isoflavones [97,98,99] and higher bioavailability because of its reduced propensity to bind serum proteins [100]. Furthermore, equol shows an increased affinity for ER-beta compared to daidzein [101] and it is more lipophilic than isoflavones, resulting in a greater bioavailability [102]. Different studies have investigated the beneficial effects of equol on health, even if results from human studies are more inconsistent because of the great variability that distinguishes individuals in equol-producers and equol-non producers [103,104,105]. Oxidative stress and inflammation are the pivotal causes of an increased risk of developing CVD [26,27]. Increased accumulation of oxidized lipids leads to atherosclerosis through the activation of proinflammatory pathways and the increase of proinflammatory cytokines such as interleukin- (IL) 1beta, tumor necrosis factor-alpha (TNF-alpha), and nuclear factor-kB (NF-kB) [26]. Proinflammatory cytokines induce the expression of vascular cell adhesion molecule-1 (VCAM-1) by endothelial cells (ECs) increasing the adhesion of leukocytes, monocytes, and T lymphocytes to the arterial wall [106,107]. Once adhered to the endothelium, monocytes enter the vessel wall and initiate the production of monocyte chemoattractant protein-1 (MCP-1) [106] and become the mature form macrophages and begin to accumulate cholesterol into the cytoplasm transforming into foam cells [108]. Several in vivo and in vitro studies reported the anti-inflammatory effects of equol which showed the ability to reduce the expression of inflammatory biomarkers, such as prostaglandin E2 [109] and MCP-1 [110] as well as the reduction of IL-6 release and its receptor [111]. Myeloperoxidase (MPO) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase are enzymatic sources of reactive oxygen species (ROS) that lead to the production of oxidized LDL (oxLDL) [112,113]. Equol protects from oxidative stress by reducing the lipid peroxidation product malondialdehyde (MDA), enhancing the antioxidant glutathione, or increasing the activities of the enzyme superoxide dismutase (SOD) [114,115] and increasing the expression of nuclear factor erythroid 2 (Nrf2) [116]. Equol also showed the ability to reduce the NADPH-induced superoxide production [97] and to increase the expression of phosphorylated-p38 mitogen-activated protein kinase and Bcl-2 [117]. Oxidative stress and inflammation cause damage to the endothelium and alter its functions. It was observed that equol can stimulate the phosphorylation of phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and enhance the activity of endothelial NO synthase (eNOS) through the binding to the ER-beta receptor [118,119]. Equol can activate eNOS also via ER-independent pathways through the activation of extracellular signal-regulated kinase (ERK) 1/2 and Akt [119]. Furthermore, equol can directly upregulate eNOS transcription [120,121] while eNOS gene contains an estrogen-response element, it is reasonable that the binding of equol to ER-beta can enhance eNOS expression [122]. The NO can react with the superoxide anion producing peroxynitrite and enhancing the nuclear accumulation of Nrf2, which in turn binds the antioxidant response elements (ARE) and increases the expression of antioxidant enzymes such as superoxide dismutase, catalase, glutathione-S-transferase, glutathione peroxidase, and heme oxygenase-1 [123].
7. Conclusions
In vivo and in vitro studies demonstrated the beneficial effects of isoflavones and, in particular, of the microbial-derived metabolite equol [96]. The interaction with ERs seems to be the main mechanism of action that recapitulates the cardioprotective effects exerted by equol [96]. However, according to up-to-date knowledge, analysis of gut microbiota revealed that isoflavone metabolism is restricted to bacteria belonging to the Coriobacteriaceae family and some species that seem to have received the genes involved in these metabolic pathways through horizontal transmission [19]. Furthermore, data reported in the literature did not result in univocal findings showing that interventions with dietary food sources of isoflavone seem to have more significant results when in the context of healthier diets; similarly, supplementation of isoflavones through tablets would provide more consistent benefits among healthier individuals. These discordant results are probably due to the effect of several factors that influence the real exposure to isoflavone metabolites. Among which are the interindividual variations in the physiological response to isoflavone intake, linked with the differences in the microbiota composition, providing more beneficial effects on equol-producers compared to nonproducers. Also, the possible interactions, including accumulating, synergistic and antagonistic effects, with other compounds from diet cannot be ruled out [88]. In this regard, it is necessary to further explore the relationship between microbiota, isoflavones, and equol production to understand which other factors are involved in these differences and how it could be possible to intervene in order to spread equol beneficial effects even to equol-non producers. In this context, a more complex approach of dietary interventions with synbiotics that promote the bioconversion of isoflavones to equol is warranted.
Author Contributions
Conceptualization, J.G. and G.G.; investigation, S.L., J.G., F.M.D.D. and G.G.; writing—original draft preparation, S.L., J.G., G.L.R., L.G., F.M.D.D. and G.G.; writing—review and editing, S.L., J.G., G.L.R., L.G., F.M.D.D., I.D.A., R.M.D., F.G. (Francesca Giampieri), J.L.Q., M.B., F.D., F.G. (Fabio Galvano) and G.G.; table visualization, S.L., J.G. and F.M.D.D.; figure visualization, S.L.; supervision, M.B., F.D., F.G. (Fabio Galvano) and G.G.; project administration, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
J.G. was supported by the co-financing of the European Union—FSE-REACT-EU, PON Research and Innovation 2014–2020 DM1062/2021; CUP: E65F21002560001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
The figures have been generated by using Servier Medical Art available at smart.servier.com (12 November 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ARE (antioxidant response elements); ARs (adrenergic receptors); BP (blood pressure); CAT (catalase); CVD (cardiovascular disease); DBP (diastolic blood pressure); DHDR (dihydrodaidzein reductase); DZNR (daidzein reductase); FMD (flow-mediated dilation); ECs (endothelial cells); eNOS (endothelial nitric oxide synthase); ERK (extracellular signal-regulated kinase); ERs (estrogen receptors); GC (guanine-cytosine); GPx (glutathione peroxidase); HO-1 (heme oxygenase 1); ICAM (intercellular adhesion molecule 1); IL (interleukin); LPS (lipopolysaccharides); MCP-1 (monocyte chemoattractant protein-1); MDA (malondialdehyde); MPO (myeloperoxidase); NADPH (nicotinamide adenine dinucleotide phosphate); NF-kB (nuclear factor-kB); Nrf2 (nuclear factor erythroid 2); O-DMA (O-desmethylangolensin); oxLDL (oxidized low-density lipoprotein); PAG (phenylacetylglutamine); PI3K (phosphatidylinositol 3-kinase); PI3K/Akt (phosphatidylinositol 3-kinase/protein kinase B); PWV (pulse wave velocity); RCT (randomized clinical trial); ROS (reactive oxygen species); SBP (systolic blood pressure); SCFAs (short chain fatty acids); SOD (superoxide dismutase); THDR (tetrahydrodaidzein reductase); TLR (Toll-like receptor); TMA (trimethylamine); TMAO (trimethylamine N-Oxide); TNF-alpha (tumor necrosis factor-alpha); VCAM-1 (vascular cell adhesion molecule-1).
References
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, C.K.; Yi, M.; Lui, K.O.; Huang, Y. Targeting endothelial dysfunction and inflammation. J. Mol. Cell. Cardiol. 2022, 168, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and cardiovascular disease: Effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef]
- Ding, Q.-Y.; Tian, J.-X.; Li, M.; Lian, F.-M.; Zhao, L.-H.; Wei, X.-X.; Han, L.; Zheng, Y.-J.; Gao, Z.-Z.; Yang, H.-Y.; et al. Interactions between therapeutics for metabolic disease, cardiovascular risk factors, and gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 530160. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef]
- Martini, D.; Godos, J.; Marventano, S.; Tieri, M.; Ghelfi, F.; Titta, L.; Lafranconi, A.; Trigueiro, H.; Gambera, A.; Alonzo, E.; et al. Nut and legume consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2021, 72, 871–878. [Google Scholar] [CrossRef]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hameed, A.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef]
- Soukup, S.T.; Stoll, D.A.; Danylec, N.; Schoepf, A.; Kulling, S.E.; Huch, M. Metabolism of daidzein and genistein by gut bacteria of the class coriobacteriia. Foods 2021, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- García, N.; Zazueta, C.; Aguilera-Aguirre, L. Oxidative stress and inflammation in cardiovascular disease. Oxid. Med. Cell. Longev. 2017, 2017, 5853238. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Yap, Z.H.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Carnevale, R.; Nocella, C.; Novo, M.; Santulli, M.; Cammisotto, V.; Menichelli, D.; Pignatelli, P.; Violi, F. Gut-Derived Serum Lipopolysaccharide is Associated with Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Heart Assoc. 2017, 6, e005784. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Wang, Y.-J.; Zhang, A.; Ding, Y.-J.; Zhang, X.-N.; Jia, Q.-J.; Zhu, Y.-P.; Li, Y.-Y.; Lv, S.-C.; Zhang, J.-P. TMA/TMAO in hypertension: Novel horizons and potential therapies. J. Cardiovasc. Transl. Res. 2021, 14, 1117–1124. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Buffa, J.A.; Wang, Z.; Warrier, M.; Schugar, R.; Shih, D.M.; Gupta, N.; Gregory, J.C.; Org, E.; Fu, X.; et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J. Thromb. Haemost. 2018, 16, 1857–1872. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Shimizu, I.; Shimada, A.; Nakahara, K.; Yanagisawa, S.; Kubo, M.; Fukuda, S.; Ishii, C.; Yamamoto, H.; Ishikawa, T.; et al. Brown adipose tissue dysfunction promotes heart failure via a trimethylamine N-oxide-dependent mechanism. Sci. Rep. 2022, 12, 14883. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22. [Google Scholar] [CrossRef]
- Huynh, K. Novel gut microbiota-derived metabolite promotes platelet thrombosis via adrenergic receptor signalling. Nat. Rev. Cardiol. 2020, 17, 265. [Google Scholar] [CrossRef]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef]
- Yokoyama, S.; Suzuki, T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Dufault-Thompson, K.; Hall, B.; Jiang, X. Taxonomic distribution and evolutionary analysis of the equol biosynthesis gene cluster. BMC Genomics 2022, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lim, J.; Kim, I.; Kim, D.; Kang, S.C. Isolation and identification of new bacterial stains producing equol from Pueraria lobata extract fermentation. PLoS ONE 2018, 13, e0192490. [Google Scholar] [CrossRef] [PubMed]
- Kawada, Y.; Yokoyama, S.; Yanase, E.; Niwa, T.; Suzuki, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota Food Health 2016, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Matthies, A.; Engst, W.; Blaut, M.; Braune, A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 2013, 79, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Chiba, Y. Evaluation of a natural S-equol supplement in treating premenstrual symptoms and the effect of the gut microbiota: An open-label pilot study. Neuropsychopharmacol. Rep. 2022, 42, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tousen, Y.; Matsumoto, Y.; Matsumoto, C.; Nishide, Y.; Nagahata, Y.; Kobayashi, I.; Ishimi, Y. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice. Br. J. Nutr. 2016, 116, 247–257. [Google Scholar] [CrossRef]
- Ghimire, S.; Cady, N.M.; Lehman, P.; Peterson, S.R.; Shahi, S.K.; Rashid, F.; Giri, S.; Mangalam, A.K. Dietary isoflavones alter gut microbiota and lipopolysaccharide biosynthesis to reduce inflammation. Gut Microbes 2022, 14, 2127446. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Zhang, Q.; Yu, M.; Xiao, X. Genistein improves glucose metabolism and promotes adipose tissue browning through modulating gut microbiota in mice. Food Funct. 2022, 13, 11715–11732. [Google Scholar] [CrossRef]
- Teede, H.J.; Dalais, F.S.; Kotsopoulos, D.; Liang, Y.L.; Davis, S.; McGrath, B.P. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 3053–3060. [Google Scholar] [CrossRef]
- Tischmann, L.; Adam, T.C.; Mensink, R.P.; Joris, P.J. Longer-term soy nut consumption improves vascular function and cardiometabolic risk markers in older adults: Results of a randomized, controlled cross-over trial. Clin. Nutr. 2022, 41, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Sagara, M.; Kanda, T.; NJelekera, M.; Teramoto, T.; Armitage, L.; Birt, N.; Birt, C.; Yamori, Y. Effects of dietary intake of soy protein and isoflavones on cardiovascular disease risk factors in high risk, middle-aged men in Scotland. J. Am. Coll. Nutr. 2004, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gu, D.; Wu, X.; Chen, J.; Duan, X.; Chen, J.; Whelton, P.K. Effect of soybean protein on blood pressure: A randomized, controlled trial. Ann. Intern. Med. 2005, 143, 1–9. [Google Scholar] [CrossRef]
- Hermansen, K.; Hansen, B.; Jacobsen, R.; Clausen, P.; Dalgaard, M.; Dinesen, B.; Holst, J.J.; Pedersen, E.; Astrup, A. Effects of soy supplementation on blood lipids and arterial function in hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2005, 59, 843–850. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: A 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Husain, D.; Khanna, K.; Puri, S.; Haghighizadeh, M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J. Am. Coll. Nutr. 2015, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Tomlinson, B.; Ho, S.; To, K.; Woo, J. Effect of whole soy and purified daidzein on ambulatory blood pressure and endothelial function—A 6-month double-blind, randomized controlled trial among Chinese postmenopausal women with prehypertension. Eur. J. Clin. Nutr. 2015, 69, 1161–1168. [Google Scholar] [CrossRef]
- Squadrito, F.; Altavilla, D.; Morabito, N.; Crisafulli, A.; D’Anna, R.; Corrado, F.; Ruggeri, P.; Campo, G.M.; Calapai, G.; Caputi, A.P.; et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis 2002, 163, 339–347. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Lee, O.; Lee, K.-H.; Lee, Y.-B.; Young, K.D.; Jeong, Y.H.; Choue, R. Isoflavone supplementation influenced levels of triglyceride and luteunizing hormone in Korean postmenopausal women. Arch. Pharm. Res. 2013, 36, 306–313. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Lau, K.-K.; Yiu, K.-H.; Li, S.-W.; Chan, H.-T.; Fong, D.Y.-T.; Tam, S.; Lau, C.-P.; Tse, H.-F. Reduction of C-reactive protein with isoflavone supplement reverses endothelial dysfunction in patients with ischaemic stroke. Eur. Heart J. 2008, 29, 2800–2807. [Google Scholar] [CrossRef]
- Irace, C.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; Arcoraci, V.; Minutoli, L.; Di Benedetto, A.; Di Vieste, G.; et al. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur. J. Clin. Invest. 2013, 43, 1025–1031. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef]
- Wong, W.W.; Taylor, A.A.; Smith, E.O.; Barnes, S.; Hachey, D.L. Effect of soy isoflavone supplementation on nitric oxide metabolism and blood pressure in menopausal women. Am. J. Clin. Nutr. 2012, 95, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-C.; Lo, S.-C.; Tsai, K.-S.; Tu, S.-T.; Wu, J.-S.; Chang, C.-I.; Chen, C.-L.; Shaw, N.-S.; Peng, H.-Y.; Wang, S.-Y.; et al. Effects of high-dose phytoestrogens on circulating cellular microparticles and coagulation function in postmenopausal women. J. Formos. Med. Assoc. 2015, 114, 710–716. [Google Scholar] [CrossRef][Green Version]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Isoflavones and clinical cardiovascular risk factors in obese postmenopausal women: A randomized double-blind placebo-controlled trial. J Womens Health (Larchmt) 2008, 17, 1363–1369. [Google Scholar] [CrossRef]
- De Gregorio, C.; Marini, H.; Alibrandi, A.; Di Benedetto, A.; Bitto, A.; Adamo, E.B.; Altavilla, D.; Irace, C.; Di Vieste, G.; Pancaldo, D.; et al. Genistein Supplementation and Cardiac Function in Postmenopausal Women with Metabolic Syndrome: Results from a Pilot Strain-Echo Study. Nutrients 2017, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef]
- Movahedian, M.; Tabibi, H.; Atabak, S.; Hedayati, M.; Rahmani, L.; Yari, Z. Effects of Soy Isoflavones on Glycemic Parameters and Blood Pressure in Peritoneal Dialysis Patients: A Randomized, Double Blind, Placebo-Controlled Trial. Iran. J. Kidney Dis. 2021, 1, 134–142. [Google Scholar] [PubMed]
- Mena, P.; Del Rio, D. Gold standards for realistic (poly)phenol research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef] [PubMed]
- Pusparini; Yenny; Hidayat, A. Effect of soy isoflavone supplementation on endothelial dysfunction and oxidative stress in equol-producing postmenopausal women. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 71–79. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Bots, M.L.; Grobbee, D.E.; Lampe, J.W.; van der Schouw, Y.T. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am. J. Clin. Nutr. 2005, 81, 189–195. [Google Scholar] [CrossRef][Green Version]
- Acharjee, S.; Zhou, J.-R.; Elajami, T.K.; Welty, F.K. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metab. Clin. Exp. 2015, 64, 236–243. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Ho, S.; To, K.; Tomlinson, B.; Woo, J. Whole soy, but not purified daidzein, had a favorable effect on improvement of cardiovascular risks: A 6-month randomized, double-blind, and placebo-controlled trial in equol-producing postmenopausal women. Mol. Nutr. Food Res. 2014, 58, 709–717. [Google Scholar] [CrossRef]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bügel, S.; Koebnick, C.; Reimann, M.; Ferrari, M.; Branca, F.; Talbot, D.; Dadd, T.; et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2005, 82, 1260–1268, quiz 1365. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Hall, W.L.; Williams, C.M. Does genotype and equol-production status affect response to isoflavones? Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc. Nutr. Soc. 2006, 65, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.-M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases-From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef] [PubMed]
- Jackman, K.A.; Woodman, O.L.; Chrissobolis, S.; Sobey, C.G. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007, 1141, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Wang, J.; Morazzoni, P.; Hodis, H.N.; Sevanian, A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: An antioxidant mechanism for cell-mediated LDL modification. Free Radic. Biol. Med. 2003, 34, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Izquierdo Pulido, M.; Sánchez-González, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, Soy Isoflavones, and Protein Intake in Relation to Mortality from All Causes, Cancers, and Cardiovascular Diseases: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500.e17. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. American Heart Association Nutrition Committee Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein E-deficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.-C.; Yamashita, S.; Murata, M.; Kumazoe, M.; Tachibana, H. Equol suppresses inflammatory response and bone erosion due to rheumatoid arthritis in mice. J. Nutr. Biochem. 2016, 32, 101–106. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Clinical application of NOX activity and other oxidative biomarkers in cardiovascular disease: A critical review. Antioxid. Redox Signal. 2015, 23, 514–532. [Google Scholar] [CrossRef]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 1994, 94, 437–444. [Google Scholar] [CrossRef]
- Choi, E.J. Evaluation of equol function on anti- or prooxidant status in vivo. J. Food Sci. 2009, 74, H65-71. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Jiang, S.; Zheng, C.; Tian, Z.; Lin, X. Equol Inhibits LPS-Induced Oxidative Stress and Enhances the Immune Response in Chicken HD11 Macrophages. Cell. Physiol. Biochem. 2015, 36, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jiang, S.; Jiang, Z.; Zheng, C.; Gou, Z. Effects of equol on H2O2-induced oxidative stress in primary chicken intestinal epithelial cells. Poult. Sci. 2016, 95, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-E.; Kim, S.Y.; Jo, H.H.; Hwang, S.J.; Chae, B.; Kwon, D.J.; Lew, Y.O.; Lim, Y.-T.; Kim, J.H.; Kim, E.J.; et al. Antioxidant effects of equol on bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 2008, 375, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.; Shi, L.; Wang, L.; Chen, J.; Kang, C.; Zhu, J.; Mi, M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef]
- Joy, S.; Siow, R.C.M.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006, 281, 27335–27345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, X.; Weakley, S.M.; Kougias, P.; Lin, P.H.; Yao, Q.; Chen, C. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Nutr. 2010, 140, 12–17. [Google Scholar] [CrossRef]
- Ohkura, Y.; Obayashi, S.; Yamada, K.; Yamada, M.; Kubota, T. S-equol Partially Restored Endothelial Nitric Oxide Production in Isoflavone-deficient Ovariectomized Rats. J. Cardiovasc. Pharmacol. 2015, 65, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Johnson, H.; Jing, X.; Michalkiewicz, T.; Huang, Y.-W.; Lane, R.H.; Konduri, G.G. Persistent pulmonary hypertension alters the epigenetic characteristics of endothelial nitric oxide synthase gene in pulmonary artery endothelial cells in a fetal lamb model. Physiol. Genom. 2018, 50, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Priestley, J.R.C.; Kautenburg, K.E.; Casati, M.C.; Endres, B.T.; Geurts, A.M.; Lombard, J.H. The NRF2 knockout rat: A new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H478–H487. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).