Michelia compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage
Abstract
1. Introduction
2. Results
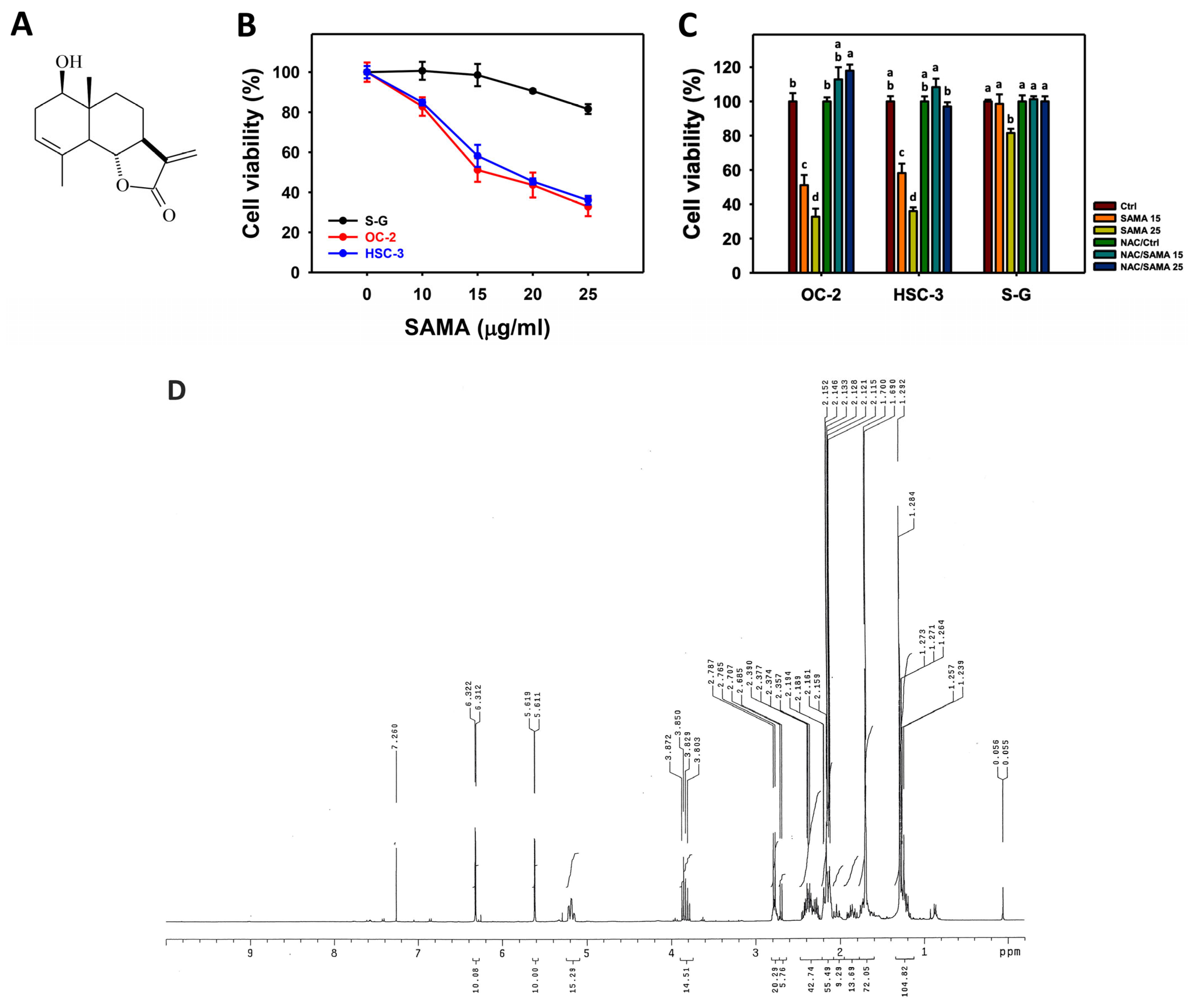
2.1. Proliferation-Regulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
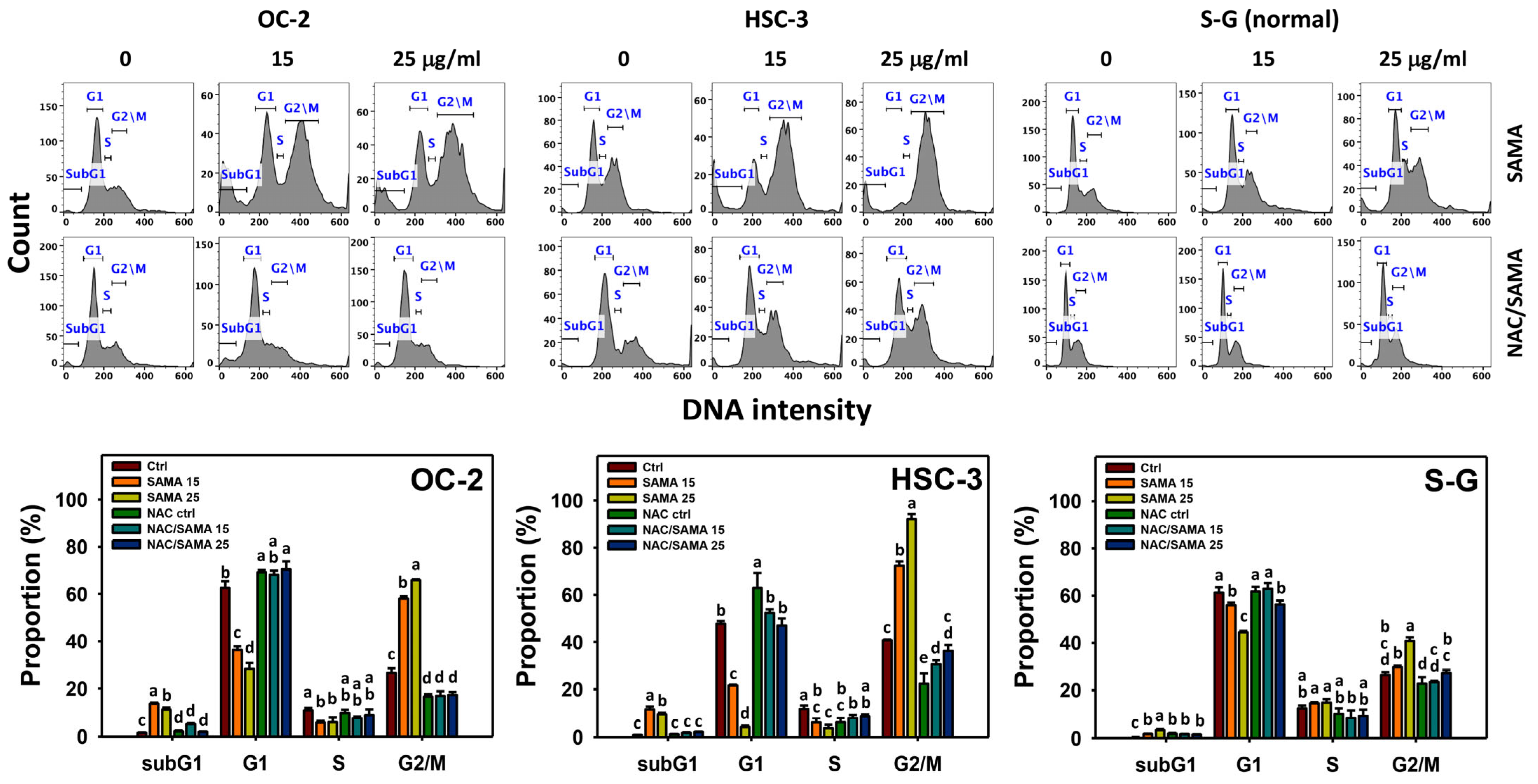
2.2. Cell Cycle-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
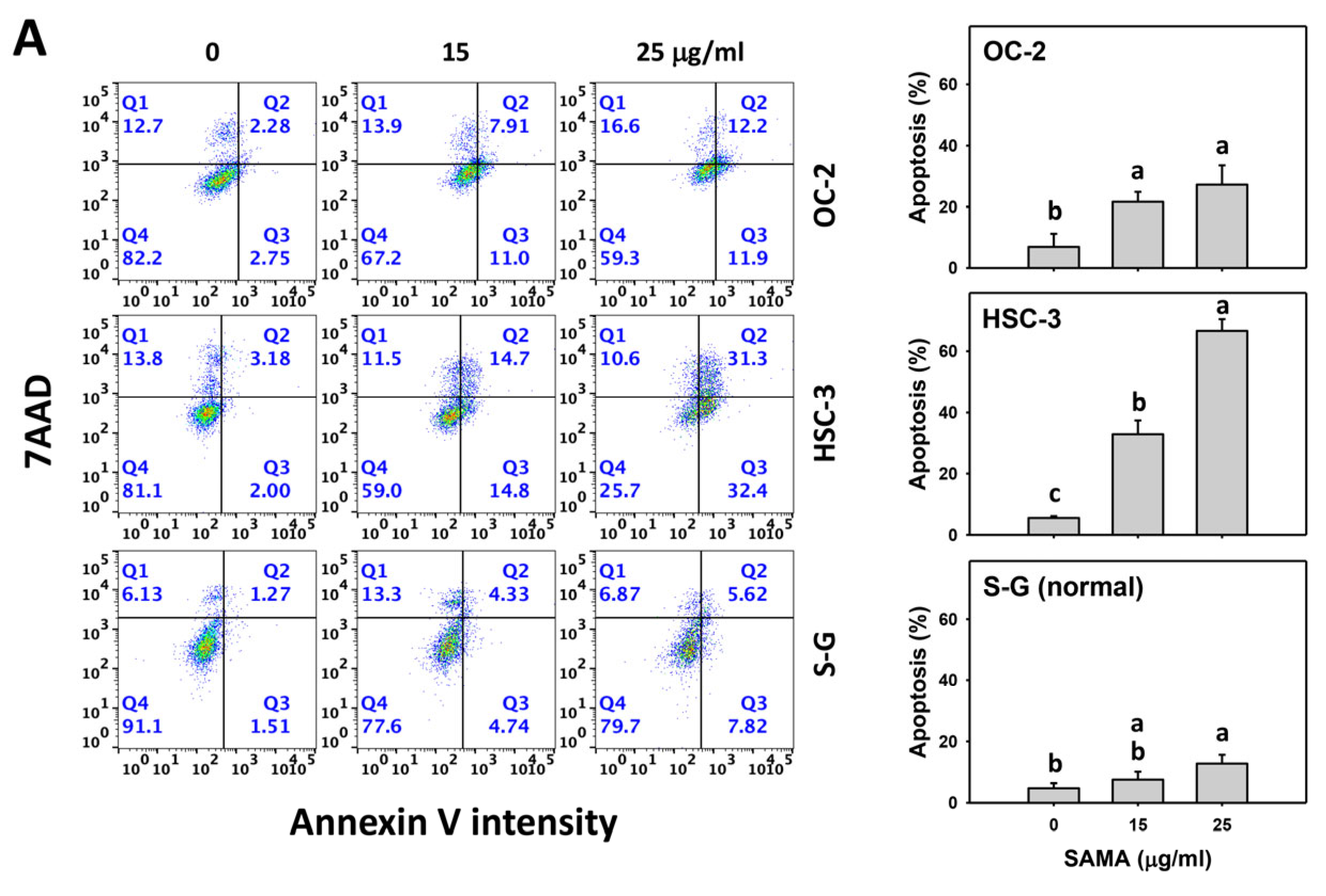
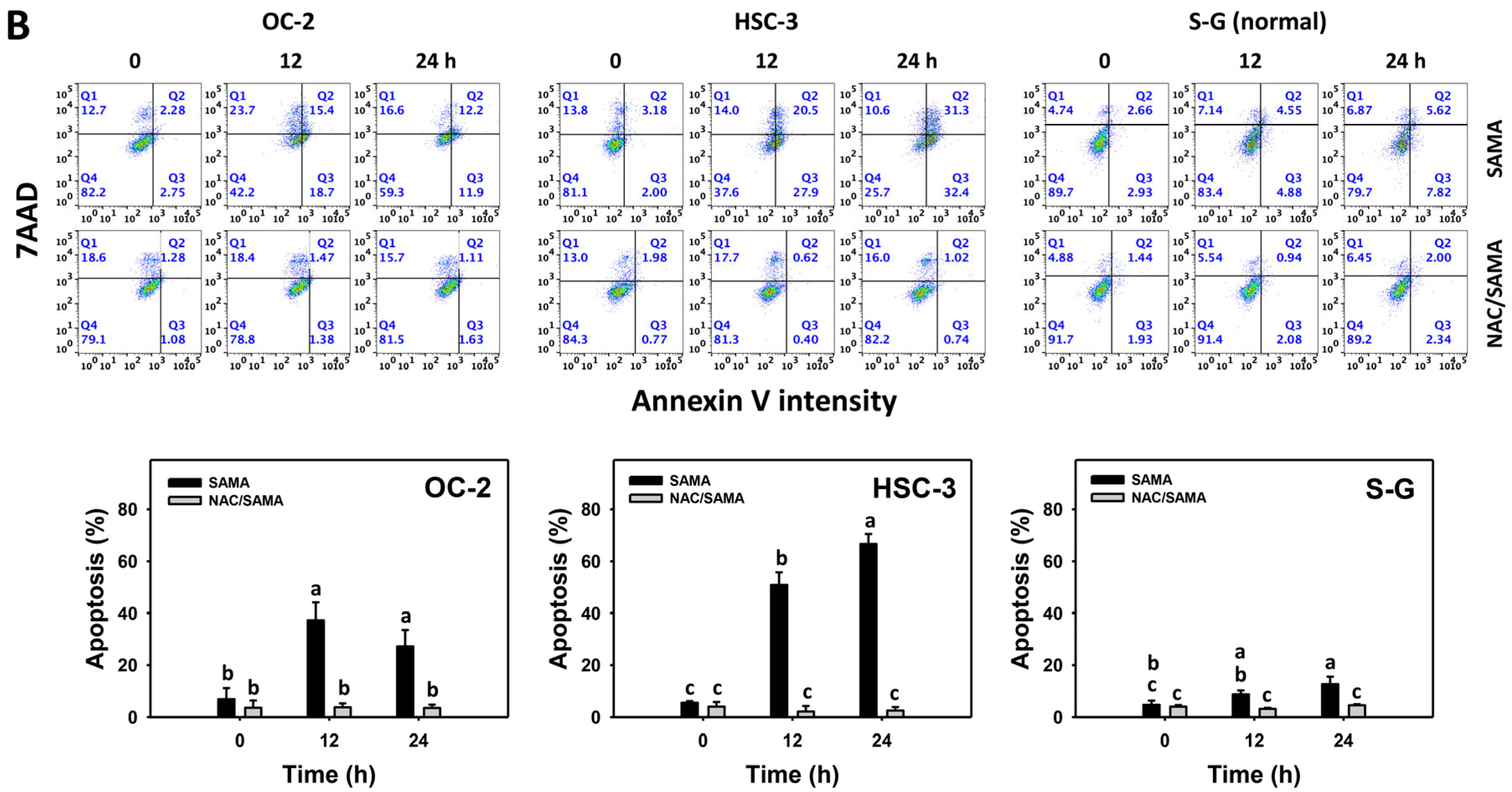
2.3. Apoptosis-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
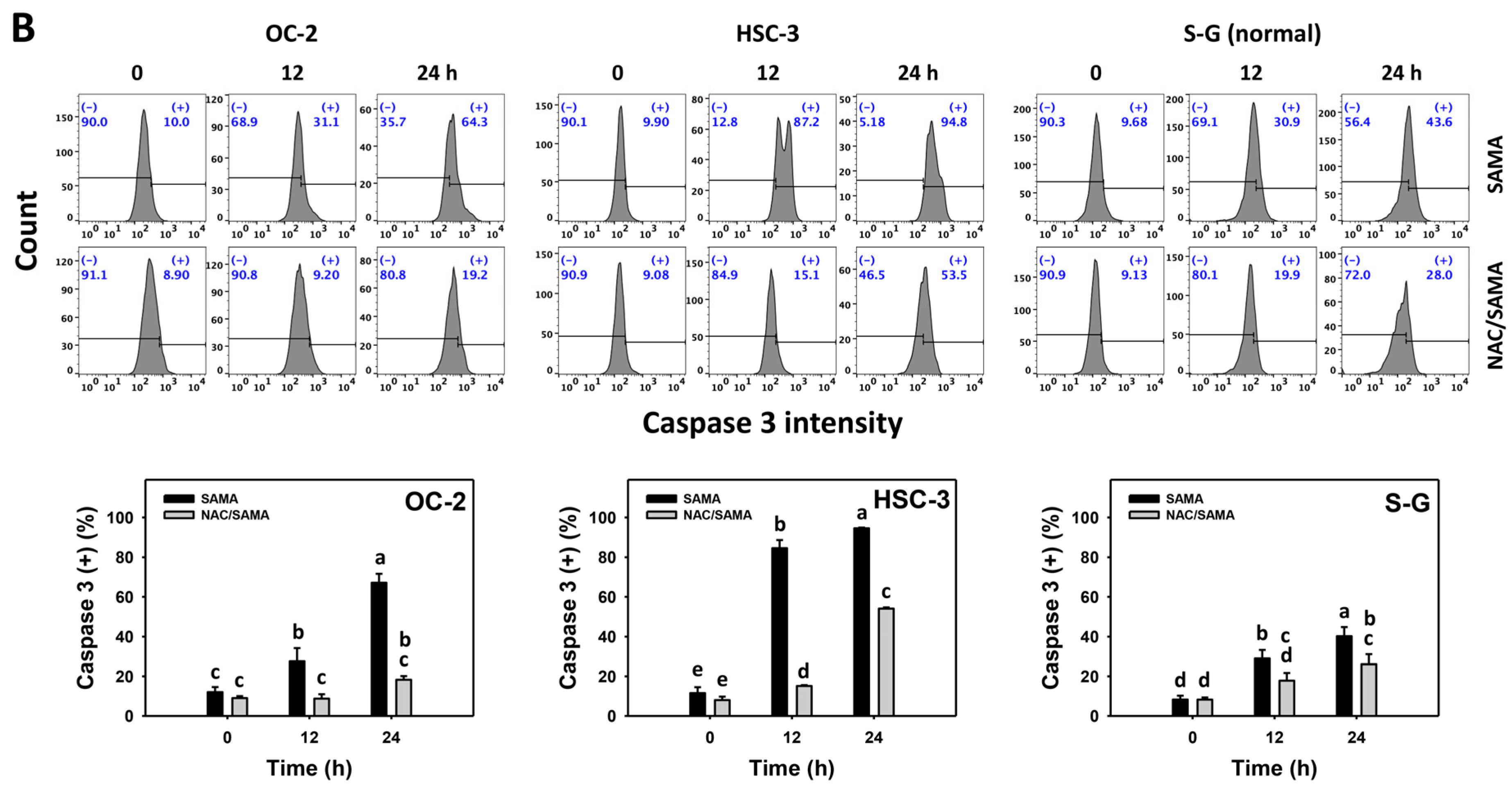
2.4. Caspase 3-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
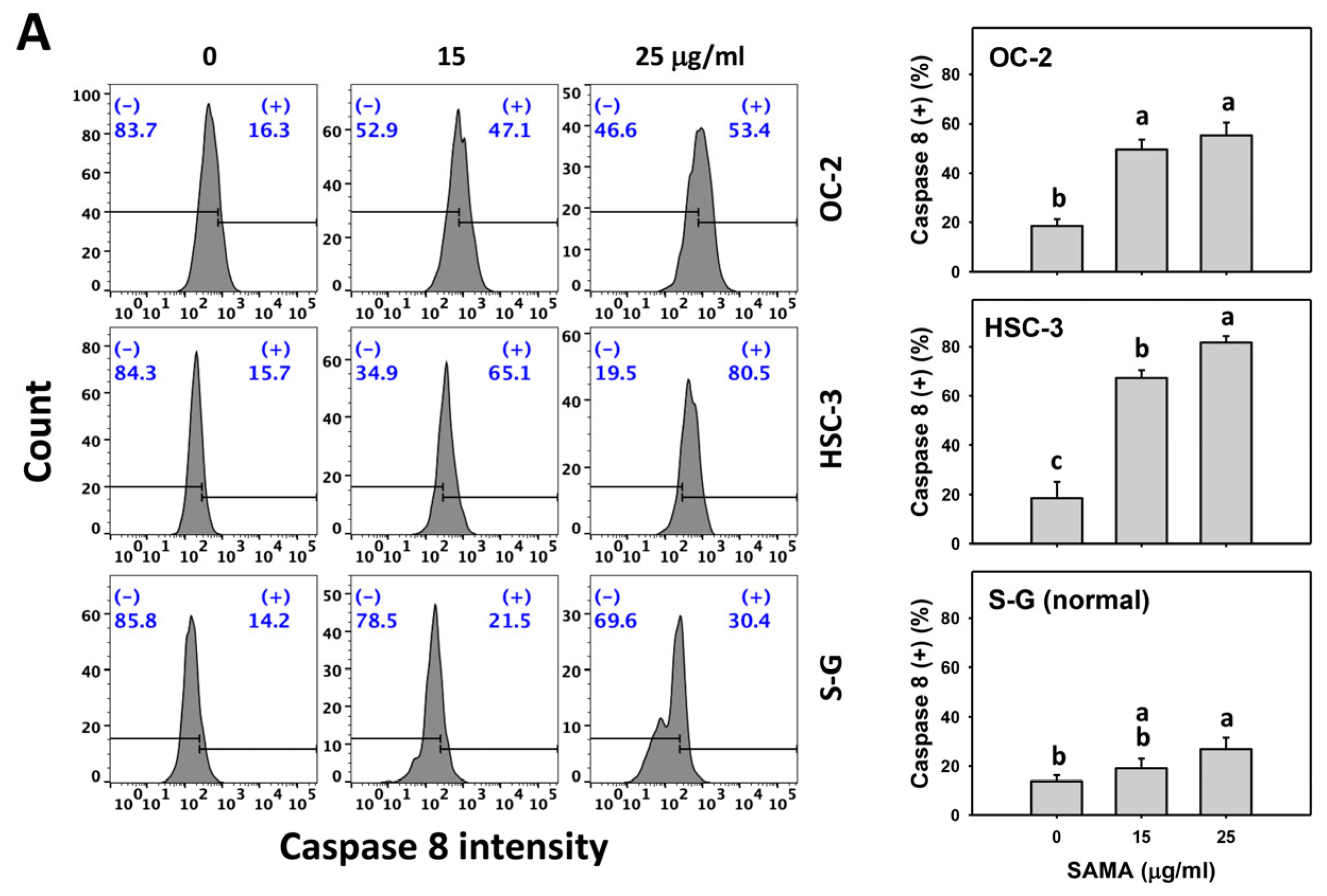
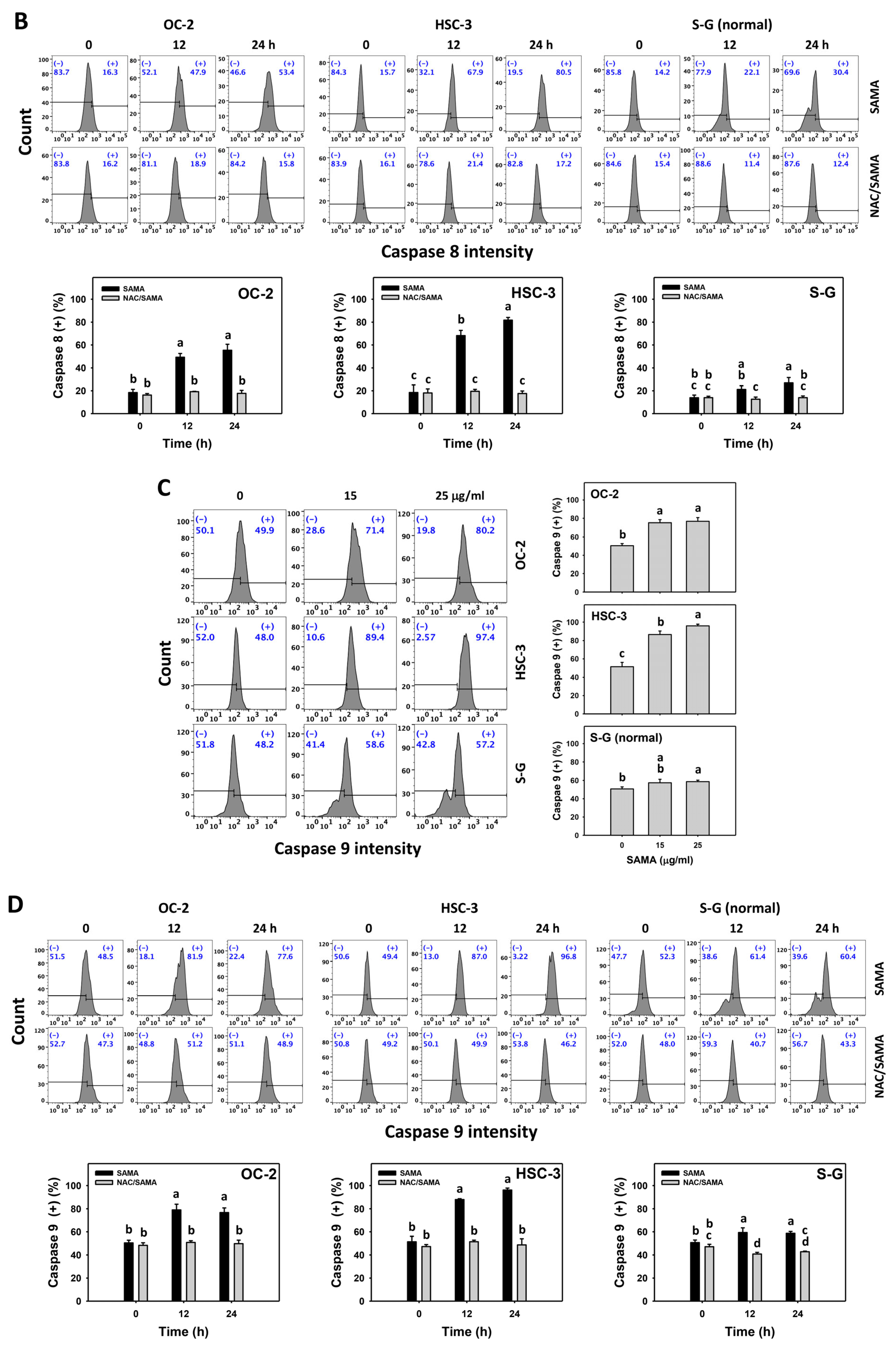
2.5. Caspase 8- and 9-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
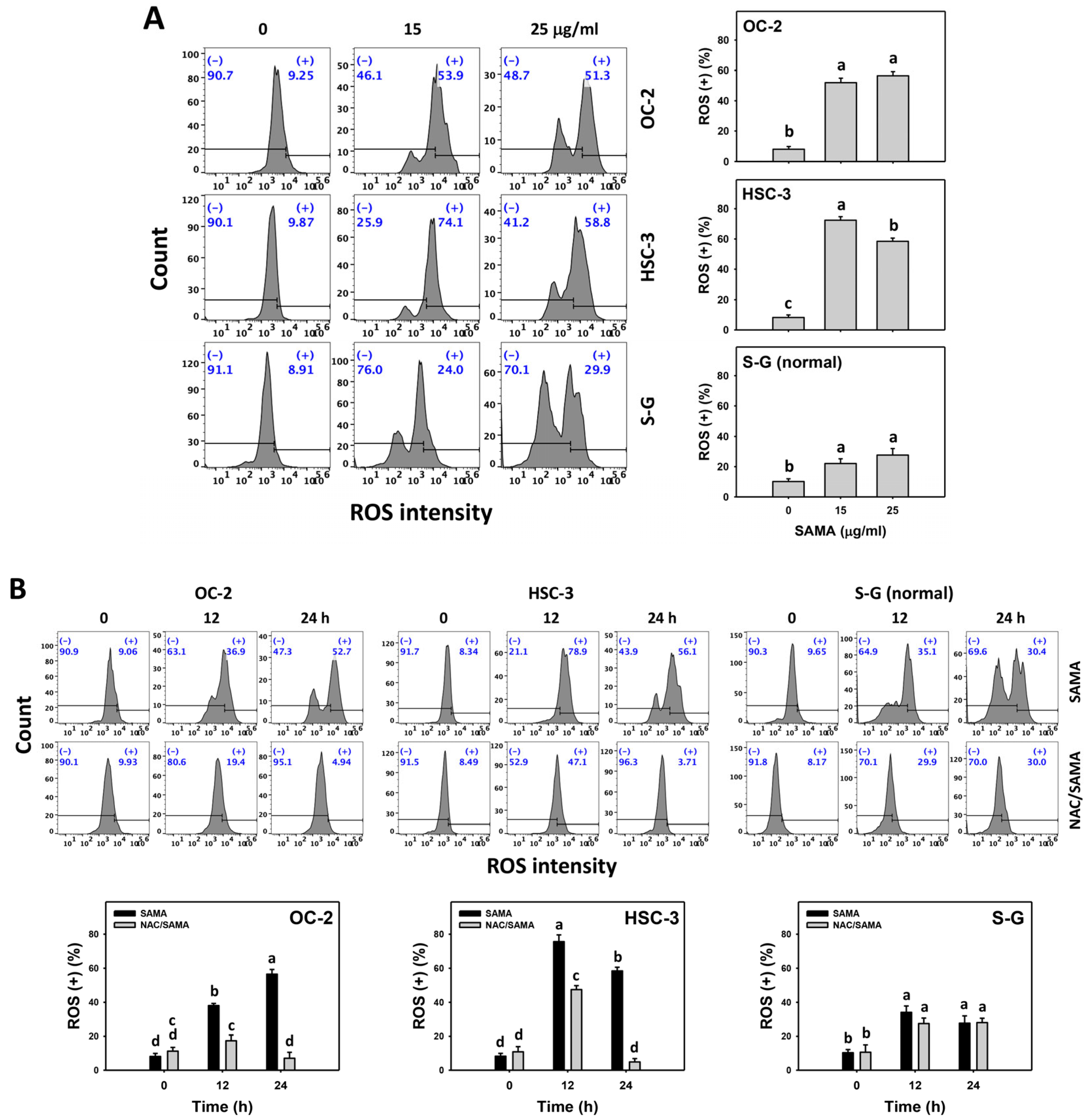
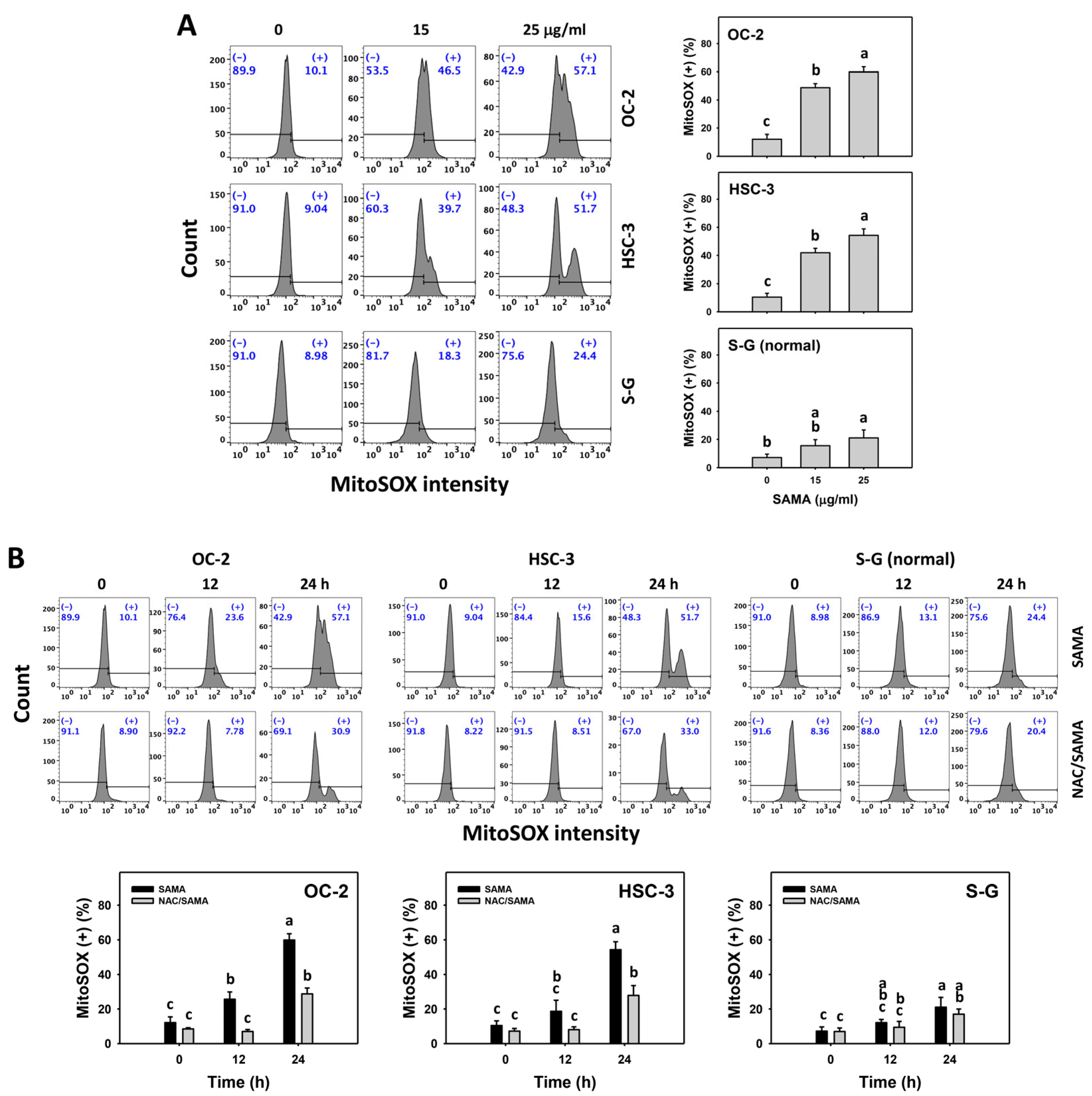
2.6. Oxidative Stress (Reactive Oxygen Species (ROS) and Mitochondrial Superoxide (MitoSOX))-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
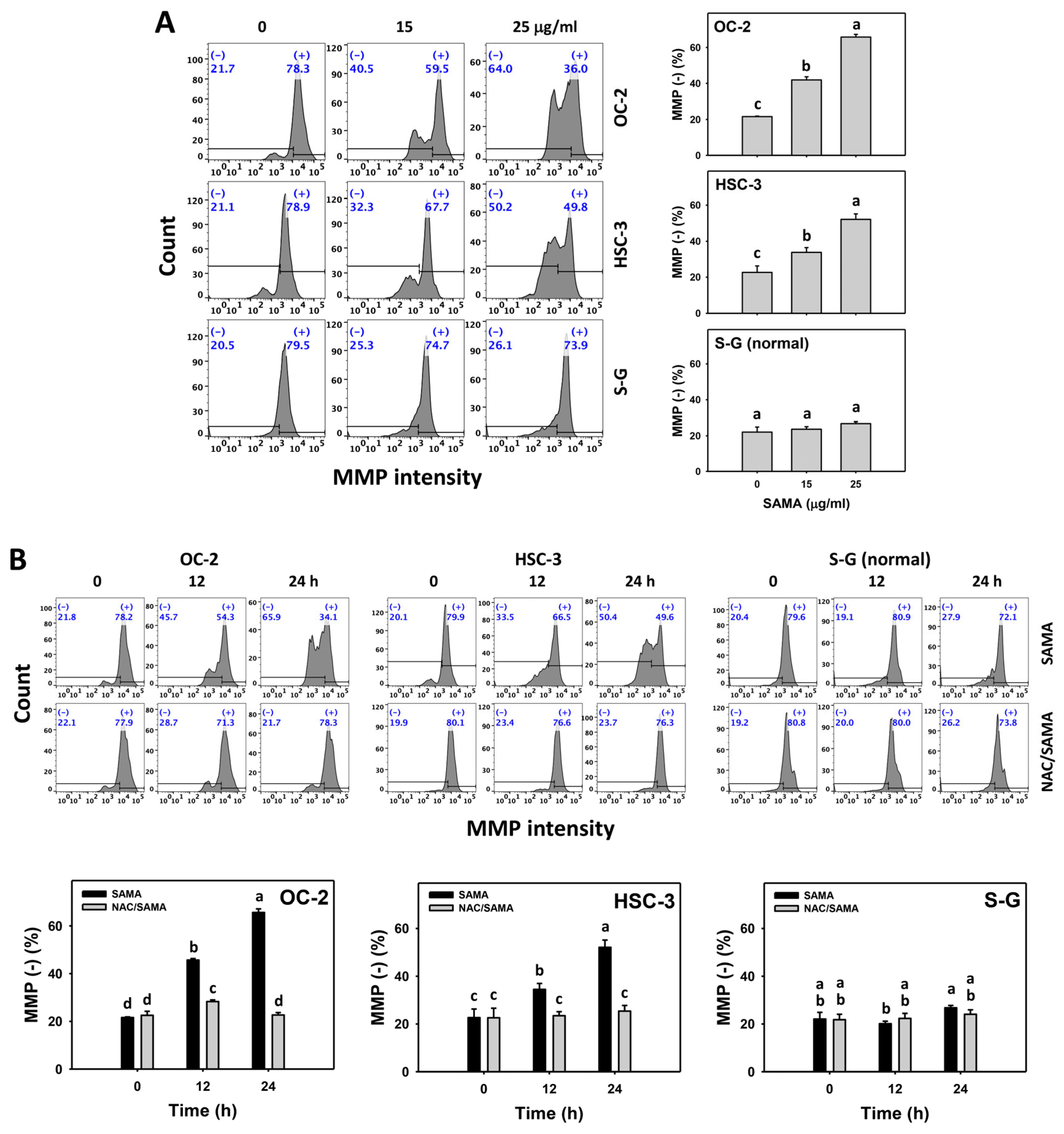
2.7. Mitochondrial Membrane Potential (MMP)-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
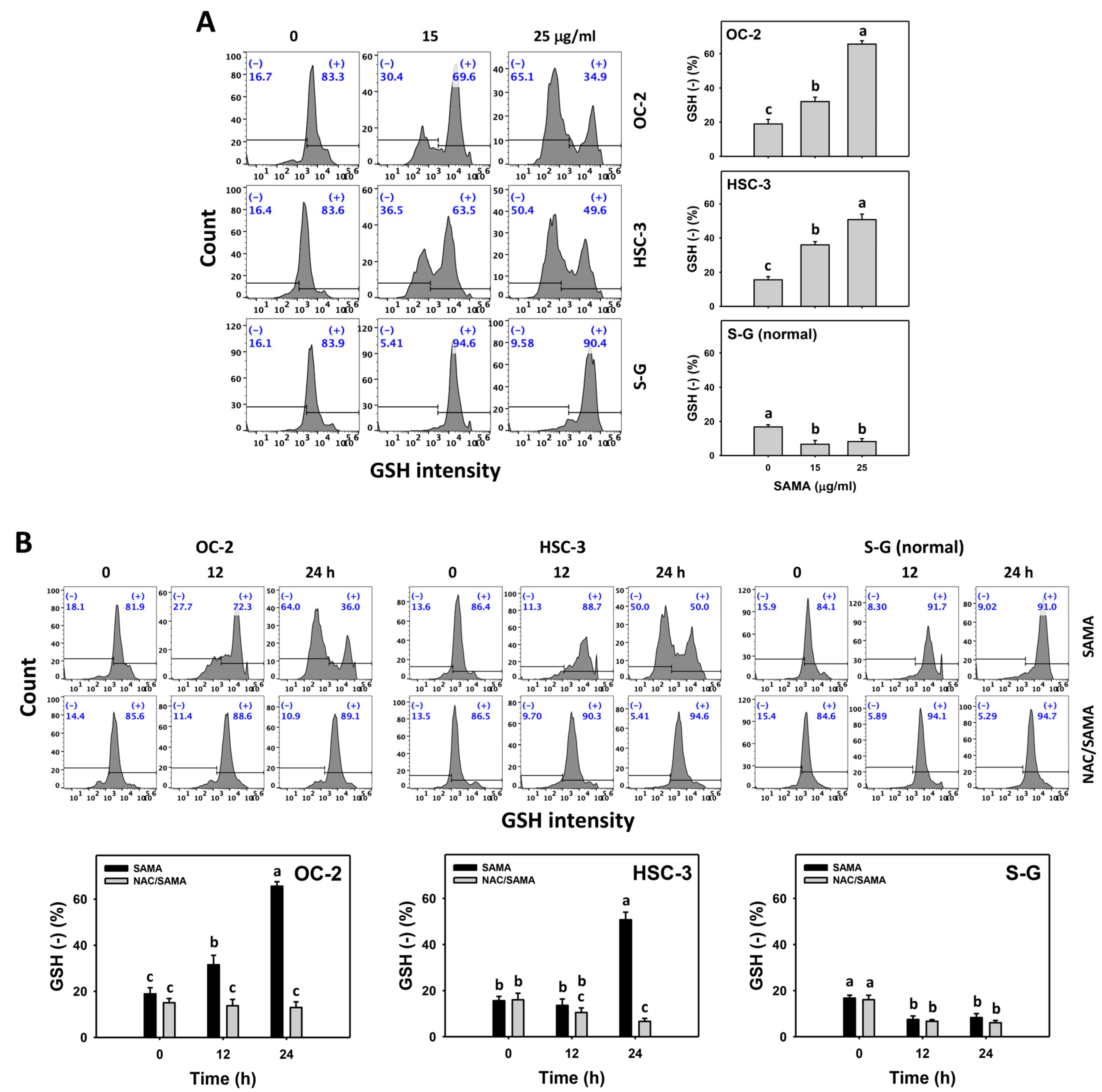
2.8. Glutathione (GSH)-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
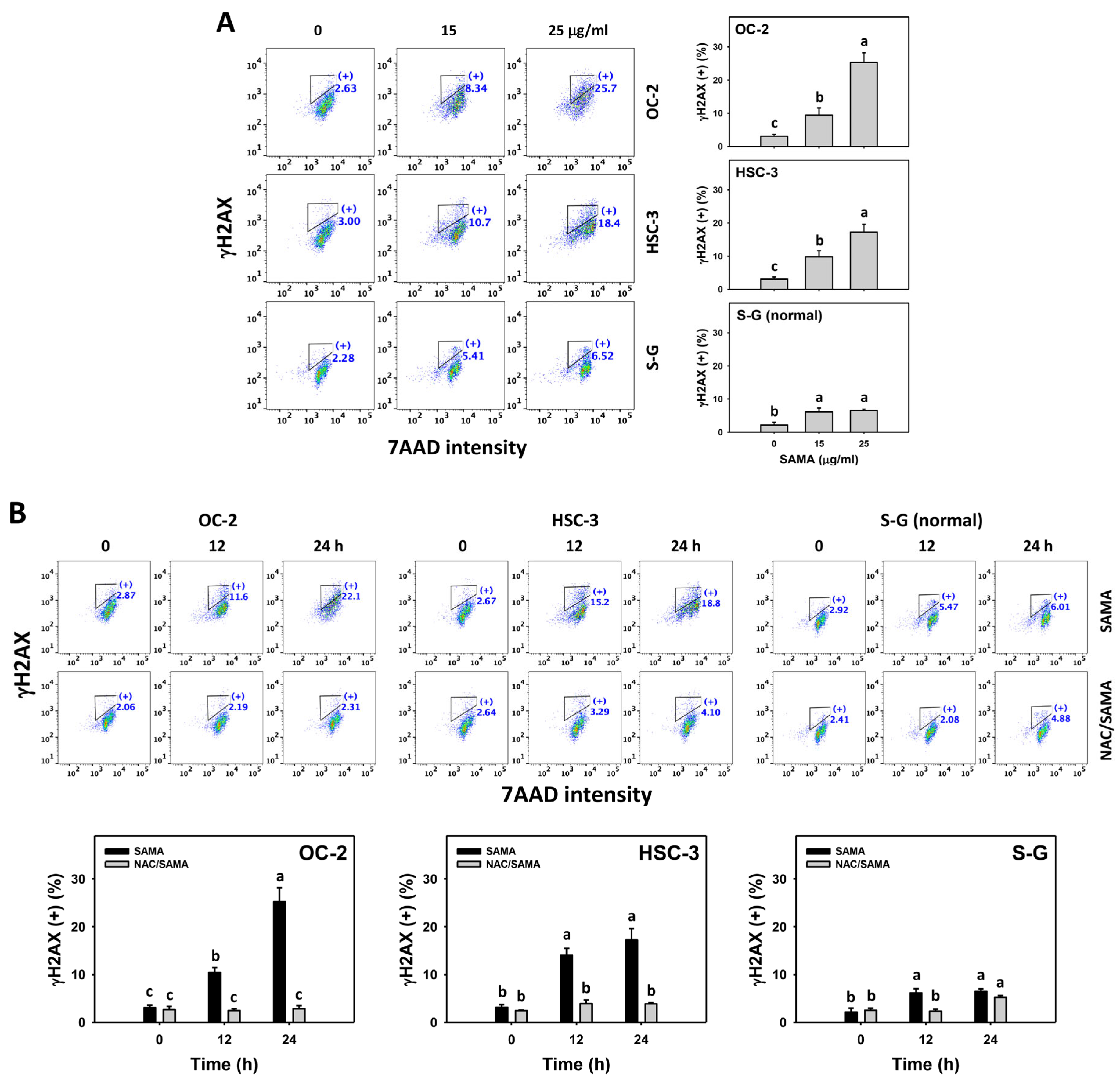
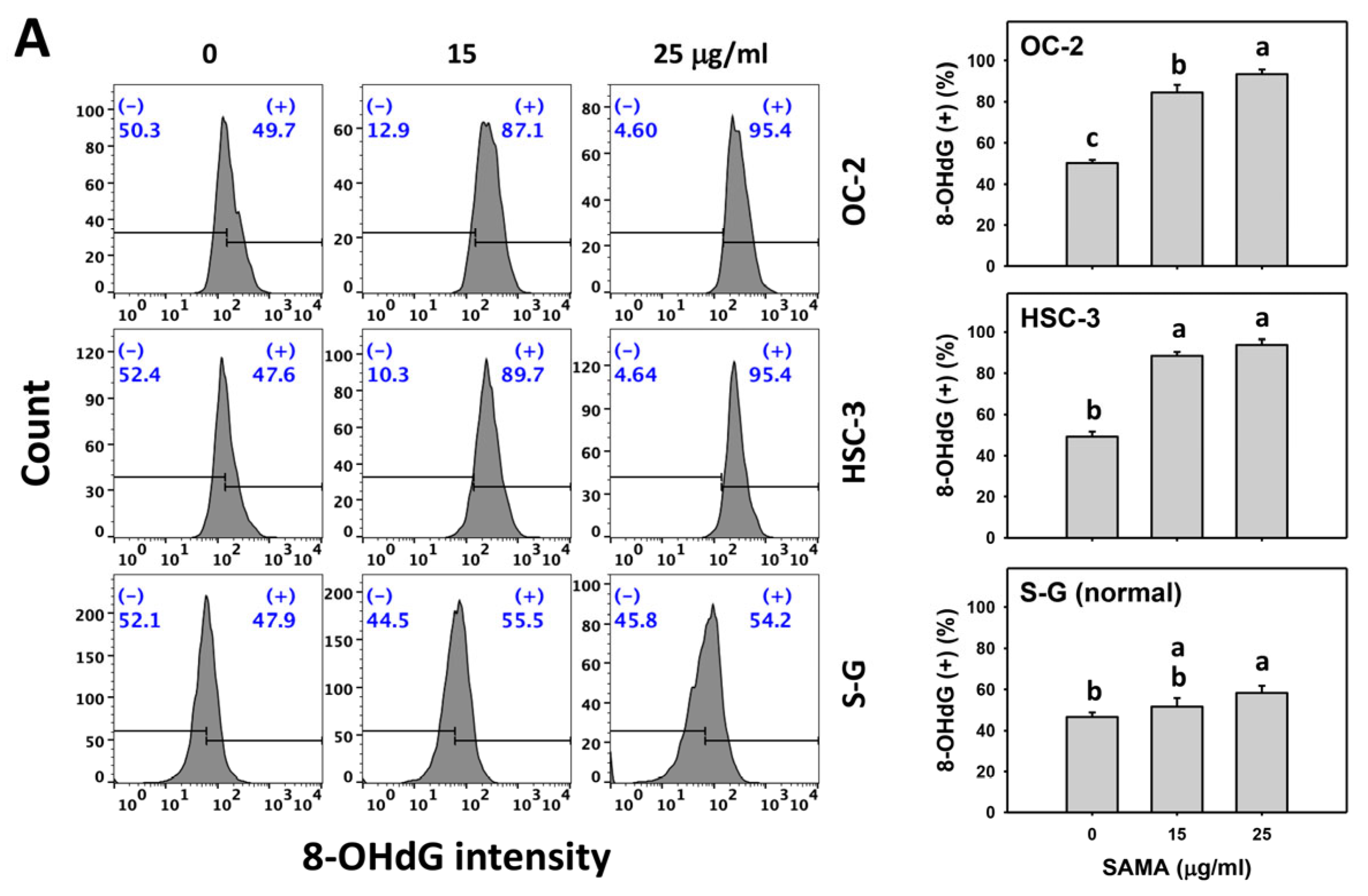
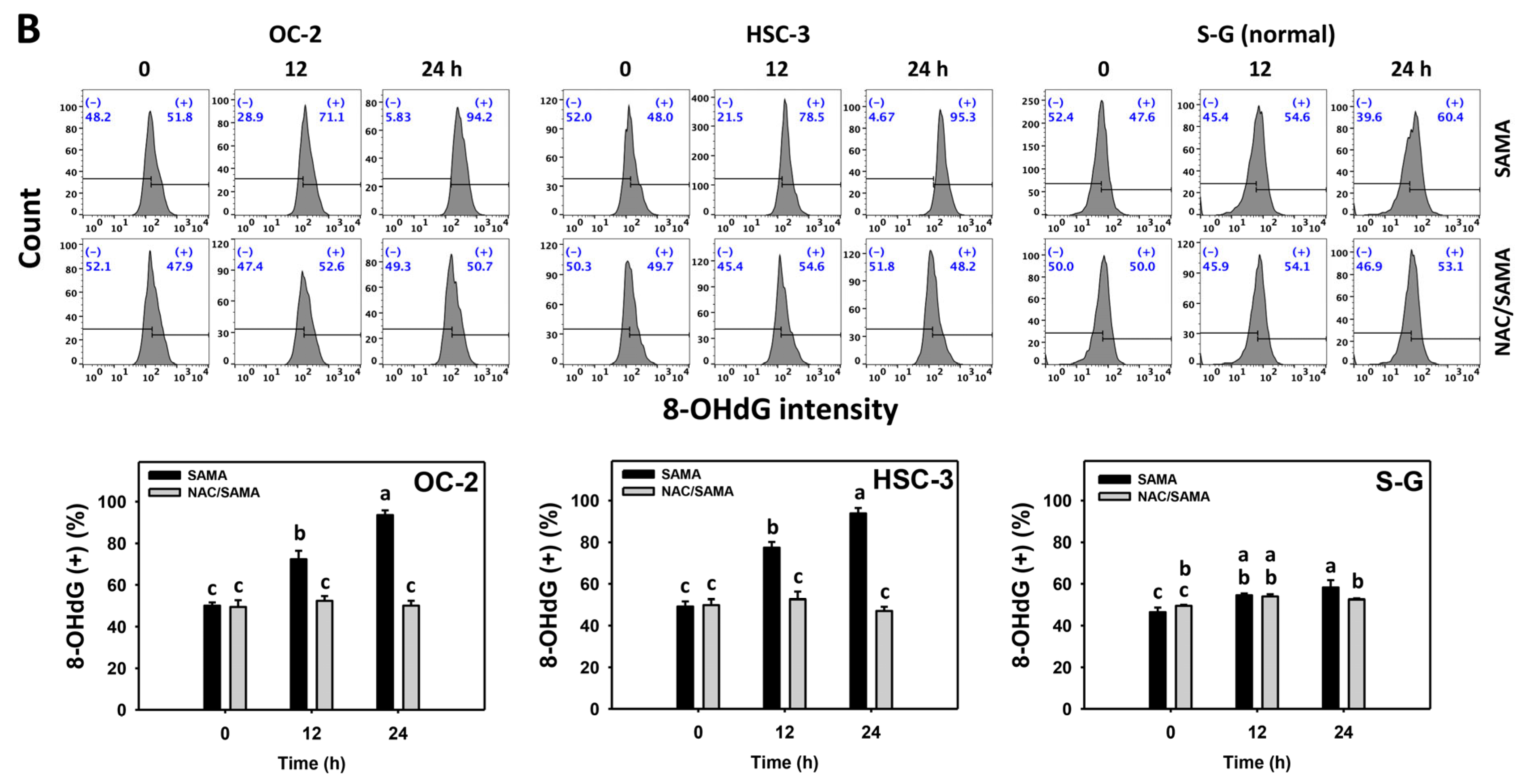
2.9. DNA Damage-Modulating Effects of SAMA: Oral Cancer Cells vs. Normal Cells
3. Discussion
3.1. Antiproliferative Effects of SAMA in Different Types of Cancer Cells
3.2. SAMA Causes Oxidative Stress of Oral Cancer Cells
3.3. SAMA Causes G2/M Arrest, Apoptosis, and DNA Damage
3.4. Selective Antiproliferation Mechanism of SAMA
3.5. Potential Application of SAMA in Combined Treatment
3.6. Future Research of SAMA in Oral Cancer Treatment
4. Materials and Methods
4.1. Preparation of SAMA
4.2. Oxidative Stress Remover
4.3. Cell Culture and Viability Assay
4.4. Cell Cycle
4.5. Apoptosis
4.6. Caspase 3, 8, and 9 Activation
4.7. ROS and MitoSOX Content
4.8. MMP Content
4.9. GSH Content
4.10. γH2AX DNA Damage
4.11. 8-OHdG DNA Damage
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Chung, C.H.; Yang, Y.H.; Wang, T.Y.; Shieh, T.Y.; Warnakulasuriya, S. Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J. Oral Pathol. Med. 2005, 34, 460–466. [Google Scholar] [CrossRef]
- Almangush, A.; Makitie, A.A.; Triantafyllou, A.; de Bree, R.; Strojan, P.; Rinaldo, A.; Hernandez-Prera, J.C.; Suarez, C.; Kowalski, L.P.; Ferlito, A.; et al. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol. 2020, 107, 104799. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chou, W.Y.; Chang, C.W.; Lin, M.C.; Wang, C.P.; Lou, P.J.; Chen, T.C. Chemoprevention of oral cancer: A review and future perspectives. Head Neck 2023, 45, 1045–1059. [Google Scholar] [CrossRef]
- Riva, G.; Cravero, E.; Pizzo, C.; Briguglio, M.; Iorio, G.C.; Cavallin, C.; Ostellino, O.; Airoldi, M.; Ricardi, U.; Pecorari, G. Sinonasal side effects of chemotherapy and/or radiation therapy for head and neck cancer: A literature review. Cancers 2022, 14, 2324. [Google Scholar] [CrossRef]
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, T.H.; Kao, C.L.; Yeh, H.C.; Li, H.T.; Chen, C.Y. Secondary metabolites of fruits of Michelia compressa var. compressa. Chem. Nat. Compd. 2023, 59, 1002–1004. [Google Scholar] [CrossRef]
- Cheng, K.K.; Nadri, M.H.; Othman, N.Z.; Rashid, S.; Lim, Y.C.; Leong, H.Y. Phytochemistry, bioactivities and traditional uses of Michelia x alba. Molecules 2022, 27, 3450. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, V.H.; Jayanthi, M.K.; Rashmi, H.R.; Shivamurthy, V.K.N.; Patil, S.M.; Shirahatti, P.S.; Ramu, R. New insights on the phytochemical intervention for the treatment of neuropsychiatric disorders using the leaves of Michelia champaca: An in vivo and in silico approach. Pharm. Biol. 2022, 60, 1656–1668. [Google Scholar]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef]
- Neganova, M.E.; Afanas’eva, S.V.; Klochkov, S.G.; Shevtsova, E.F. Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull. Exp. Biol. Med. 2012, 152, 720–722. [Google Scholar] [CrossRef]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects-involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Jalal, S.; Ahmad, B.; Zhang, T.; Guo, L.; Huang, L. SANTAMARINE: Mechanistic studies on multiple diseases. Chem. Biol. Drug Des. 2020, 95, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.K.; Patra, A.; Talapatra, B. Parthenolide and a new germacranolide, 11, 13-dehydrolanuginolide, from Michelia lanuginosa. Phytochemistry 1973, 12, 1827. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Z.Y.; Zhu, M.C.; Dong, M.; Wang, S.M.; Shi, Q.W.; Zhang, M.L.; Wang, Y.F.; Huo, C.H.; Kiyota, H.; et al. Antitumour activities of sesquiterpene lactones from Inula helenium and Inula japonica. Z. Für Naturforschung C 2012, 67, 375–380. [Google Scholar]
- Oh, J.H.; Kim, J.; Karadeniz, F.; Kim, H.R.; Park, S.Y.; Seo, Y.; Kong, C.S. Santamarine shows anti-photoaging properties via inhibition of MAPK/AP-1 and stimulation of TGF-β/Smad signaling in UVA-irradiated HDFs. Molecules 2021, 26, 3585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Yang, H.Y.; Yang, M.; Fang, J.; Gao, K. Inhibition of thioredoxin reductase by santamarine conferring anticancer effect in HeLa cells. Front. Mol. Biosci. 2021, 8, 710676. [Google Scholar] [CrossRef]
- Mehmood, T.; Maryam, A.; Tian, X.; Khan, M.; Ma, T. Santamarine inhibits NF-kB and STAT3 activation and induces apoptosis in HepG2 liver cancer cells via oxidative stress. J. Cancer 2017, 8, 3707–3717. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Yan, J.; Khan, M.; Yu, X. Santamarine inhibits NF-kappaB activation and induces mitochondrial apoptosis in A549 lung adenocarcinoma cells via oxidative stress. Biomed. Res. Int. 2017, 2017, 4734127. [Google Scholar] [CrossRef]
- Koc, E.; Celik-Uzuner, S.; Uzuner, U.; Cakmak, R. The detailed comparison of cell death detected by annexin V-PI counterstain using fluorescence microscope, flow cytometry and automated cell counter in mammalian and microalgae cells. J. Fluoresc. 2018, 28, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shih, Y.L.; Lee, M.H.; Au, M.K.; Chen, Y.L.; Lu, H.F.; Chung, J.G. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase- and mitochondria-dependent signaling pathways. Molecules 2017, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar] [PubMed]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Teppo, H.R.; Soini, Y.; Karihtala, P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxidative Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef]
- Wang, J.; Sun, D.; Huang, L.; Wang, S.; Jin, Y. Targeting reactive oxygen species capacity of tumor cells with repurposed drug as an anticancer therapy. Oxidative Med. Cell. Longev. 2021, 2021, 8532940. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Tang, J.Y.; Chen, S.R.; Hou, M.F.; Jeng, J.H.; Cheng, Y.B.; Chang, H.W. Antiproliferation effects of marine-sponge-derived methanol extract of Theonella swinhoei in oral cancer cells in vitro. Antioxidants 2022, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, C.; Malfa, G.A.; Tomasello, B.; Bianchi, S.; Acquaviva, R. Natural compounds and glutathione: Beyond mere antioxidants. Antioxidants 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Chu, K.B.; Moon, E.K.; Kim, S.S.; Quan, F.S. Sensitization to oxidative stress and G2/M cell cycle arrest by histone deacetylase inhibition in hepatocellular carcinoma cells. Free Radic. Biol. Med. 2020, 147, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Lin, S.C.; Su, J.H.; Chen, Y.K.; Lin, C.C.; Chan, H.L. Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Spector, S.; Hardy, L.; Zhao, S.; Saluk, A.; Alemane, L.; Spector, N.L. Tumor selective G2/M cell cycle arrest and apoptosis of epithelial and hematological malignancies by BBL22, a benzazepine. Proc. Natl. Acad. Sci. USA 2000, 97, 7494–7499. [Google Scholar] [CrossRef]
- Lee, E.J.; Min, H.Y.; Joo Park, H.; Chung, H.J.; Kim, S.; Nam Han, Y.; Lee, S.K. G2/M cell cycle arrest and induction of apoptosis by a stilbenoid, 3,4,5-trimethoxy-4′-bromo-cis-stilbene, in human lung cancer cells. Life Sci. 2004, 75, 2829–2839. [Google Scholar] [CrossRef]
- Zhang, R.; Loganathan, S.; Humphreys, I.; Srivastava, S.K. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J. Nutr. 2006, 136, 2728–2734. [Google Scholar] [CrossRef]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1“ peaks on DNA content histograms. Cytom. Part A 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Shiau, J.P.; Lee, M.Y.; Tang, J.Y.; Huang, H.; Lin, Z.Y.; Su, J.H.; Hou, M.F.; Cheng, Y.B.; Chang, H.W. Marine sponge Aaptos suberitoid extract improves antiproliferation and apoptosis of breast cancer cells without cytotoxicity to normal cells in vitroes. Pharmaceuticals 2022, 15, 1575. [Google Scholar] [CrossRef]
- Chen, B.H.; Chang, H.W.; Huang, H.M.; Chong, I.W.; Chen, J.S.; Chen, C.Y.; Wang, H.M. (-)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma h1299 cells. J. Agric. Food Chem. 2011, 59, 2284–2290. [Google Scholar] [CrossRef]
- Cheon, C.; Ko, S.G. Synergistic effects of natural products in combination with anticancer agents in prostate cancer: A scoping review. Front. Pharmacol. 2022, 13, 963317. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert. Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the use of natural compounds in combination with chemotherapy drugs for tumor treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Covenas, R.; Munoz, M. Combination therapy of chemotherapy or radiotherapy and the neurokinin-1 receptor antagonist aprepitant: A new antitumor strategy? Curr. Med. Chem. 2023, 30, 1798–1812. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Ramos-Garcia, P.; Esteban, F. Significance of the overexpression of substance P and its receptor NK-1R in head and neck carcinogenesis: A systematic review and meta-analysis. Cancers 2021, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Tsao, M.; Chiu, L.; Popovic, M.; Milakovic, M.; Lam, H.; DeAngelis, C. Efficacy of the combination neurokinin-1 receptor antagonist, palonosetron, and dexamethasone compared to others for the prophylaxis of chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2018, 7, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aranda, M.; Tellez, T.; McKenna, L.; Redondo, M. Neurokinin-1 receptor (NK-1R) antagonists as a new strategy to overcome cancer resistance. Cancers 2022, 14, 2255. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.J.; Meng, M.; Liu, Y.; Su, T.; Kwan, H.Y. Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol. Lett. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, J.; Sun, J.; Chen, Z.; Gong, Y.; Bi, L.; Lu, Y.; Yao, J.; Zhu, W.; Hou, A.; et al. Chinese herbal medicine combined with EGFR-TKI in EGFR mutation-positive advanced pulmonary adenocarcinoma (CATLA): A multicenter, randomized, double-blind, placebo-controlled trial. Front. Pharmacol. 2019, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Tang, J.Y.; Yang, K.H.; Chang, F.R.; Hou, M.F.; Yen, C.Y.; Chang, H.W. The impact of oxidative stress and AKT pathway on cancer cell functions and its application to natural products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chang, Y.L.; Liu, S.T.; Chen, G.S.; Lee, S.P.; Huang, S.M. Differential cytotoxicity mechanisms of copper complexed with disulfiram in oral cancer cells. Int. J. Mol. Sci. 2021, 22, 3711. [Google Scholar] [CrossRef]
- Huang, W.Z.; Liu, T.M.; Liu, S.T.; Chen, S.Y.; Huang, S.M.; Chen, G.S. Oxidative status determines the cytotoxicity of ascorbic acid in human oral normal and cancer cells. Int. J. Mol. Sci. 2023, 24, 4851. [Google Scholar] [CrossRef]
- Wong, D.Y.; Chang, K.W.; Chen, C.F.; Chang, R.C. Characterization of two new cell lines derived from oral cavity human squamous cell carcinomas—OC1 and OC2. J. Oral Maxillofac. Surg. 1990, 48, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Chan, Y.H.; Shiau, J.P.; Farooqi, A.A.; Tang, J.Y.; Chen, K.L.; Yen, C.Y.; Chang, H.W. The neddylation inhibitor MLN4924 inhibits proliferation and triggers apoptosis of oral cancer cells but not for normal cells. Environ. Toxicol. 2024, 39, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Huang, C.C. Strontium peroxide-loaded composite scaffolds capable of generating oxygen and modulating behaviors of osteoblasts and osteoclasts. Int. J. Mol. Sci. 2022, 23, 6322. [Google Scholar] [CrossRef] [PubMed]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Hsieh, Y.C.; Li, L.H.; Chang, C.C.; Janouskova, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.T.; Chang, C.C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/beta-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Peng, B.R.; Hsu, K.C.; El-Shazly, M.; Shih, S.P.; Lin, T.E.; Kuo, F.W.; Chou, Y.C.; Lin, H.Y.; Lu, M.C. 13-Acetoxysarcocrassolide exhibits cytotoxic activity against oral cancer cells through the interruption of the Keap1/Nrf2/p62/SQSTM1 pathway: The need to move beyond classical concepts. Mar. Drugs 2020, 18, 382. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, G.; Gorini, G.; Liuzzi, F.; Solaini, G.; Baracca, A. Hypoxia and IF(1) expression promote ROS decrease in cancer cells. Cells 2018, 7, 64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.-I.; Chen, K.-L.; Yen, C.-Y.; Chen, C.-Y.; Chien, T.-M.; Shu, C.-W.; Chen, Y.-H.; Jeng, J.-H.; Chen, B.-H.; Chang, H.-W. Michelia compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage. Pharmaceuticals 2024, 17, 230. https://doi.org/10.3390/ph17020230
Lu H-I, Chen K-L, Yen C-Y, Chen C-Y, Chien T-M, Shu C-W, Chen Y-H, Jeng J-H, Chen B-H, Chang H-W. Michelia compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage. Pharmaceuticals. 2024; 17(2):230. https://doi.org/10.3390/ph17020230
Chicago/Turabian StyleLu, Hsin-I, Kuan-Liang Chen, Ching-Yu Yen, Chung-Yi Chen, Tsu-Ming Chien, Chih-Wen Shu, Yu-Hsuan Chen, Jiiang-Huei Jeng, Bing-Hung Chen, and Hsueh-Wei Chang. 2024. "Michelia compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage" Pharmaceuticals 17, no. 2: 230. https://doi.org/10.3390/ph17020230
APA StyleLu, H.-I., Chen, K.-L., Yen, C.-Y., Chen, C.-Y., Chien, T.-M., Shu, C.-W., Chen, Y.-H., Jeng, J.-H., Chen, B.-H., & Chang, H.-W. (2024). Michelia compressa-Derived Santamarine Inhibits Oral Cancer Cell Proliferation via Oxidative Stress-Mediated Apoptosis and DNA Damage. Pharmaceuticals, 17(2), 230. https://doi.org/10.3390/ph17020230






