Repurposing EGFR Inhibitors for Oral Cancer Pain and Opioid Tolerance
Abstract
1. Introduction
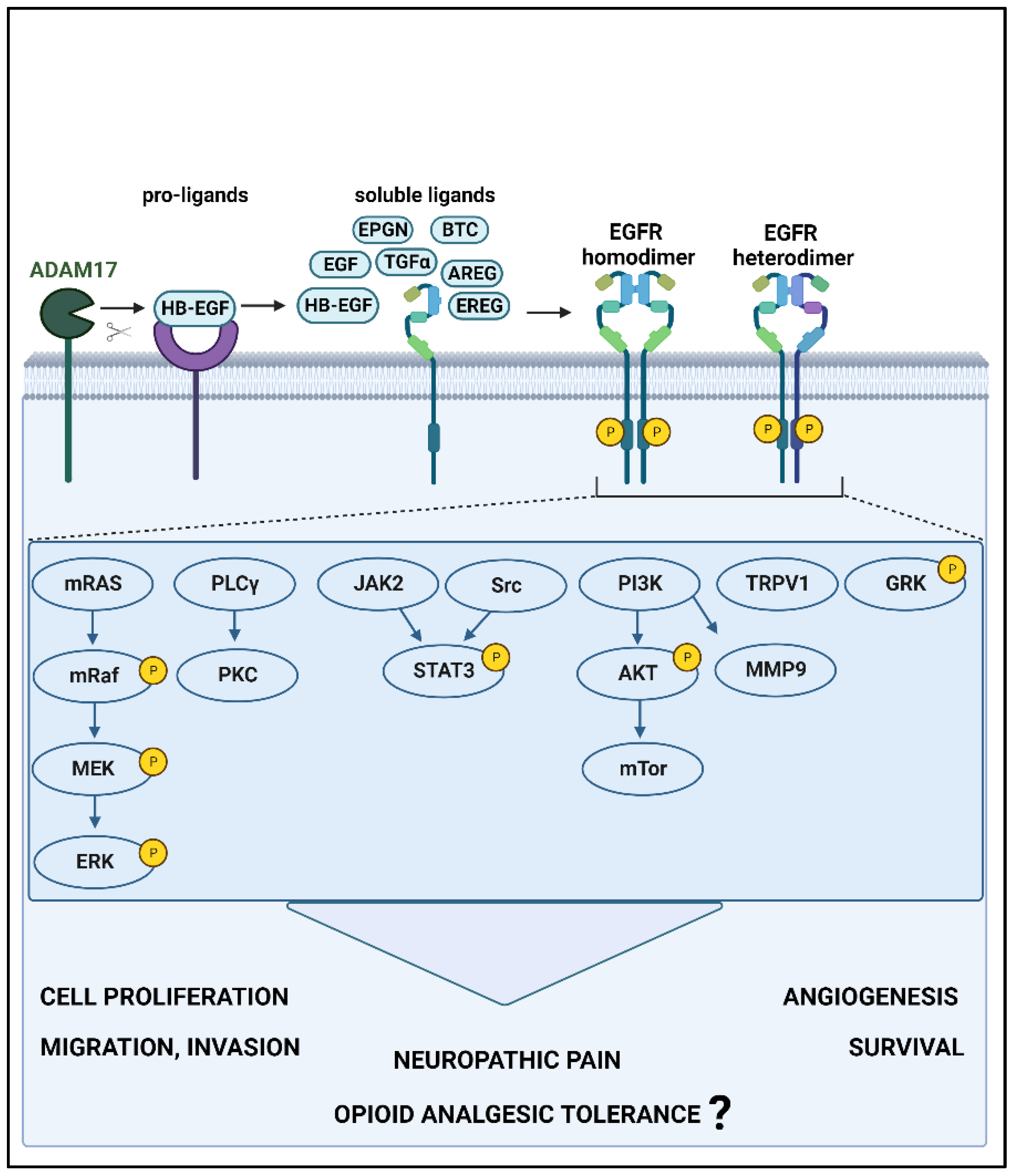
2. EGFR and Its Known Signaling Pathways
3. EGFR in Oral Cancer Treatment in Clinical Studies
4. EGFR in Pain and Morphine Tolerance
4.1. Clinical Studies and Genetic Associations
4.2. EGFR Involvement in Pain in Animal Models
| Category | Procedure or Animal Models | Species | EGFR Activation | EGFR Inhibition | Ref. | ||
|---|---|---|---|---|---|---|---|
| Induced by | Behavioral Outcomes | Induced by | Behavioral Outcomes | ||||
| Nociceptive | None | Mouse | HB-EGF injected into the paw | Mechanical allodynia | [44] | ||
| Mouse | HB-EGF mimicking toxin (intraplanar) | Mechanical allodynia, thermal hypersensitivity | [80] | ||||
| Rat | EGF (i.t.) | Mechanical allodynia | [25] | ||||
| Mouse | Epiregulin (i.t.) | Mechanical allodynia, thermal hypersensitivity | [43] | ||||
| ADAM17 hypomorphic mutant | Mouse | Reduced release of EGFR ligands | Reduced mechanical allodynia, heat hypersensitivity, and cold hypersensitivity | [81] | |||
| Inflammatory | Intraplantar Injection of formalin | Mouse | Epiregulin (i.t.) | Analgesic during the early phase; Aggravated late-phase nociceptive behavior (lick/bite) | [43] | ||
| Mouse | EGF, amphiregulin betacellulin TGFα (i.t.) | No effect | |||||
| Mouse | AG 1478, gefitinib, lapatinib (i.p.) | Reversal of late-phase nociceptive behavior (licking/biting) | |||||
| Injection of Complete Freund’s adjuvant | Mouse | AG 1478, gefitinib, lapatinib (i.p.) | Reversal of mechanical allodynia | [43] | |||
| Injection of carrageenan | Mouse | AG 1478, gefitinib, lapatinib (i.p.) | Reversal of thermal hypersensitivity | [43] | |||
| Anterior cruciate ligament, transection, and partial medial meniscectomy | Rat | AG1478 (infusion) | Reduced osteoarthritis at 4 and 7, not 10 wk postsurgery in males | [75,82] | |||
| DMM-induced osteoarthritis | Mouse | Downregulation of TGFα in DRGs by miR-183 | Reduced mechanical allodynia at 8 wk postsurgery in males | [73] | |||
| DMM-induced osteoarthritis | Mouse | HB-EGF overexpression, or TGFα (intra-articular) | Reversal of mechanical allodynia after 1 week postsurgery | Gefitinib (oral) | No reversal of mechanical allodynia after 1 wk postsurgery | [78] | |
| DMM-induced osteoarthritis in EGFR knockout mice | Mouse | Reduced intra-articular EGFR expression | Development of mechanical allodynia 1 month postsurgery | [83] | |||
| Tibial loading of 6 Newton | Mouse | Intra-articular HB-EGF overexpression | Reversal of mechanical allodynia | [77] | |||
| Intra-colonic infusion of acetic acid | Rat | Lower EGF levels in plasma and colon | Development of visceral hypersensitivity | [84] | |||
| Neuropathic | Spared nerve injury | Mouse | EGFR inhibitor III (i.p.) | Reversal of mechanical allodynia | [85] | ||
| Mouse | AG 1478, gefitinib, lapatinib (i.p.) | Reversal of mechanical allodynia | [43] | ||||
| Chronic constriction injury | Mouse | AG 1478, gefitinib, lapatinib (i.p.) | Reversal of mechanical allodynia | [43] | |||
| Rat | Erlotinib (i.p.) | Reversal of mechanical allodynia, thermal hypersensitivity, cold hypersensitivity | [86] | ||||
| Gefitinib, AG 1478, falnidamol, EGFRi 324674 (i.p.) | Reversal of mechanical allodynia | ||||||
| Lapatinib, afatinib (i.p.) | Reversal of mechanical allodynia | ||||||
| Geniposide (i.p.) | Reversal of mechanical allodynia, thermal hypersensitivity | [87] | |||||
| Lumbar spinal nerve ligation | Rat | Imatinib, gefitinib, EGF-scavenging molecule (i.t.) | No reversal of mechanical allodynia | [25] | |||
| Injection of oxaliplatin | Mouse | Erlotinib, gefitinib, AG 1478 (i.p.) | Reversal of mechanical allodynia | [86] | |||
| Mixed | Injecting cancer cell supernatant into the tongue | Mouse | Cetuximab (i.p.) | Reversal of orofacial nociceptive behavior | [19] | ||
| Chronic DRG compression | Rat | Gefitinib, EGFR siRNA (i.t.) | Reversal of mechanical allodynia, thermal hypersensitivity, cold hypersensitivity | [88] | |||
4.3. Downstream Signaling Cascade of EGFR Signaling in Pain
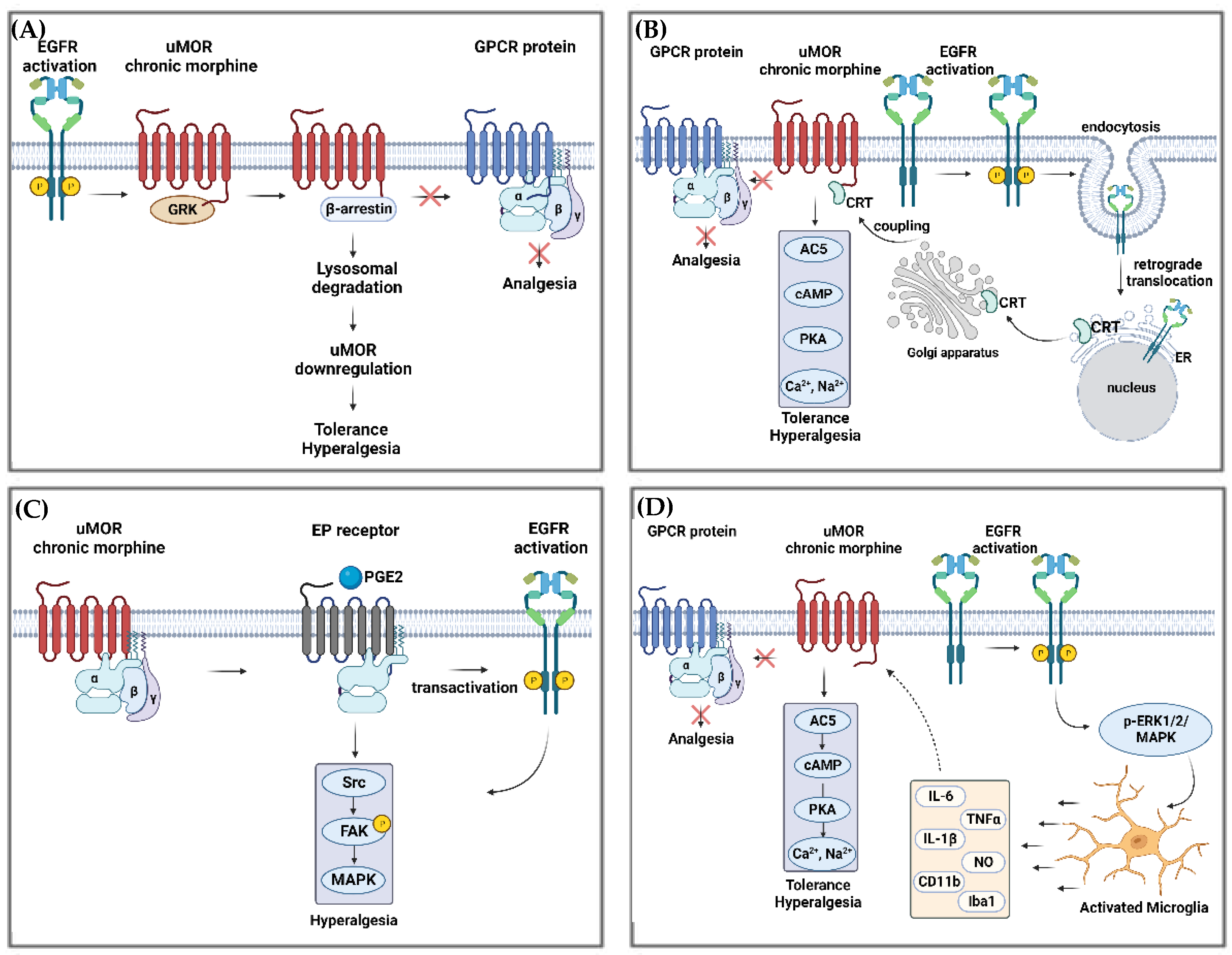
4.4. EGFR Involvement in Morphine Tolerance in Animal Models
4.5. Possible Downstream Signaling Cascades of EGFR in Opioid Tolerance
4.5.1. EGFR and MOR Interactions
- (1)
- Receptor tolerance: loss of surface MOR receptors, phosphorylation of MOR, internalization/endocytosis, sequestration/recycling, and downregulation/desensitization [99];
- (2)
- Cellular tolerance and withdrawal: upregulation of cAMP, increase in adenylyl cyclase (AC) activity and sensitization [45];
- (3)
4.5.2. EGFR and NMDA Receptor Interactions
5. Challenges
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connelly, S.T.; Schmidt, B.L. Evaluation of pain in patients with oral squamous cell carcinoma. J. Pain 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Epstein, J.B.; Elad, S.; Eliav, E.; Jurevic, R.; Benoliel, R. Orofacial pain in cancer: Part II—Clinical perspectives and management. J. Dent. Res. 2007, 86, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Schmidt, B.L. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Ye, Y.; Aouizerat, B.E.; Patel, Y.K.; Viet, D.T.; Chan, K.C.; Ono, K.; Doan, C.; Figueroa, J.D.; Yu, G.; et al. Targeting the endothelin axis as a therapeutic strategy for oral cancer metastasis and pain. Sci. Rep. 2020, 10, 20832. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Miaskowski, C. Oral Pain in the Cancer Patient. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz003. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell. Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef]
- Ye, Y.; Jensen, D.D.; Viet, C.T.; Pan, H.L.; Campana, W.M.; Amit, M.; Boada, M.D. Advances in Head and Neck Cancer Pain. J. Dent. Res. 2022, 101, 1025–1033. [Google Scholar] [CrossRef]
- Borges, J.P.; Mekhail, K.; Fairn, G.D.; Antonescu, C.N.; Steinberg, B.E. Modulation of Pathological Pain by Epidermal Growth Factor Receptor. Front. Pharmacol. 2021, 12, 642820. [Google Scholar] [CrossRef]
- Kersten, C.; Cameron, M.G.; Laird, B.; Mjaland, S. Epidermal growth factor receptor-inhibition (EGFR-I) in the treatment of neuropathic pain. Br. J. Anaesth. 2015, 115, 761–767. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ahmed, E. Cancer Pain Syndromes. Hematol. Oncol. Clin. N. Am. 2018, 32, 371–386. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Salvemini, D. Cancer and orofacial pain. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e665–e671. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Hamamoto, D.T.; Simone, D.A.; Wilcox, G.L. Mechanism of cancer pain. Mol. Interv. 2010, 10, 164–178. [Google Scholar] [CrossRef]
- Benoliel, R.; Epstein, J.; Eliav, E.; Jurevic, R.; Elad, S. Orofacial pain in cancer: Part I—Mechanisms. J. Dent. Res. 2007, 86, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.; Aouizerat, B.E.; Ye, Y.; Dang, D.; Asam, K.; Bhattacharya, A.; Howard, T.; Patel, Y.K.; Viet, D.T.; Figueroa, J.D.; et al. Neurotrophin Pathway Receptors NGFR and TrkA Control Perineural Invasion, Metastasis, and Pain in Oral Cancer. Adv. Biol. (Weinh) 2022, 6, e2200190. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Campana, W.M.; Scheff, N.N.; Nguyen, T.H.; Jeong, S.H.; Wall, I.; Wu, A.K.; Zhang, S.; Kim, H.; Bhattacharya, A.; et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 2020, 161, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral Mucositis Induced By Anticancer Therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef]
- Paice, J.A. Cancer pain management and the opioid crisis in America: How to preserve hard-earned gains in improving the quality of cancer pain management. Cancer 2018, 124, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A. Navigating Cancer Pain Management in the Midst of the Opioid Epidemic. Oncology 2018, 32, 403. [Google Scholar]
- Scheff, N.N.; Ye, Y.; Conley, Z.R.; Quan, J.W.; Lam, Y.V.R.; Klares, R., 3rd; Singh, K.; Schmidt, B.L.; Aouizerat, B.E. A disintegrin and metalloproteinase domain 17-epidermal growth factor receptor signaling contributes to oral cancer pain. Pain 2020, 161, 2330–2343. [Google Scholar] [CrossRef]
- Ye, Y.; Salvo, E.; Romero-Reyes, M.; Akerman, S.; Shimizu, E.; Kobayashi, Y.; Michot, B.; Gibbs, J. Glia and Orofacial Pain: Progress and Future Directions. Int. J. Mol. Sci. 2021, 22, 5345. [Google Scholar] [CrossRef]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFalpha promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Tu, N.H.; Jensen, D.D.; Anderson, B.M.; Chen, E.; Jimenez-Vargas, N.N.; Scheff, N.N.; Inoue, K.; Tran, H.D.; Dolan, J.C.; Meek, T.A.; et al. Legumain Induces Oral Cancer Pain by Biased Agonism of Protease-Activated Receptor-2. J. Neurosci. 2021, 41, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cuevas, F.G.; Reyna-Jeldes, M.; Velazquez-Miranda, E.; Coddou, C. Transactivation of receptor tyrosine kinases by purinergic P2Y and adenosine receptors. Purinergic Signal. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wu, L.; Zhang, Y.; Sun, Y.; Chen, J.W.; Subudhi, S.; Ho, W.; Lee, G.Y.; Wang, A.; Gao, X.; et al. Co-Targeting IL-6 and EGFR signaling for the treatment of schwannomatosis and associated pain. bioRxiv 2023, Preprint. [Google Scholar]
- Puig, S.; Donica, C.L.; Gutstein, H.B. EGFR Signaling Causes Morphine Tolerance and Mechanical Sensitization in Rats. eNeuro 2020, 7, ENEURO.0460-18.2020. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Hu, R.; Yan, J.; Wang, Z.; Li, W.; Jiang, H. Morphine promotes microglial activation by upregulating the EGFR/ERK signaling pathway. PLoS ONE 2021, 16, e0256870. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, V.J.; Chawla, P.A. Epidermal growth factor receptor inhibitors as potential anticancer agents: An update of recent progress. Bioorg. Chem. 2021, 116, 105393. [Google Scholar] [CrossRef]
- Donier, E.; Gomez-Sanchez, J.A.; Grijota-Martinez, C.; Lakoma, J.; Baars, S.; Garcia-Alonso, L.; Cabedo, H. L1CAM binds ErbB receptors through Ig-like domains coupling cell adhesion and neuregulin signalling. PLoS ONE 2012, 7, e40674. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- The Human Protein Atlas: EGFR Expression. 2023. Volume 2023, v23.0. Available online: proteinatlas.org (accessed on 22 September 2023).
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Cai, W.Q.; Zeng, L.S.; Wang, L.F.; Wang, Y.Y.; Cheng, J.T.; Zhang, Y.; Han, Z.W.; Zhou, Y.; Huang, S.L.; Wang, X.W.; et al. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front. Oncol. 2020, 10, 1249. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Liu, Z.C.; Xie, B.-F.; Li, Z.-M.; Feng, G.-K.; Yang, D.; Zenga, Y.-X. EGFR tyrosine kinase inhibitor AG1478 inhibits cell proliferation and arrests cell cycle in nasopharyngeal carcinoma cells. Cancer Lett. 2001, 169, 27–32. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Xu, L.; Zhu, X.; Liu, W.; Tian, L.; Chen, Y.; Wang, Y.; Nagendra, B.V.P.; Jia, S.; et al. The upregulation of EGFR in the dorsal root ganglion contributes to chronic compression of dorsal root ganglions-induced neuropathic pain in rats. Mol. Pain. 2019, 15, 1744806919857297. [Google Scholar] [CrossRef]
- Huerta, J.J.; Diaz-Trelles, R.; Naves, F.J.; Llamosas, M.M.; Del Valle, M.E.; Vega, J.A. Epidermal growth factor receptor in adult human dorsal root ganglia. Anat. Embryol. (Berl.) 1996, 194, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, X.; Fan, Y.; Jiang, P.; Zhao, Y.; Yang, Y.; Han, S.; Xu, B.; Chen, B.; Han, J.; et al. Single-cell analysis reveals dynamic changes of neural cells in developing human spinal cord. EMBO Rep. 2021, 22, e52728. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Xu, L.; Zhu, Y.; Zhao, L.L.; Li, X.D.; Yang, W.W.; Chen, J.; Gu, M.; Gu, X.S.; et al. Evolution of the ErbB gene family and analysis of regulators of Egfr expression during development of the rat spinal cord. Neural Regen. Res. 2022, 17, 2484–2490. [Google Scholar] [PubMed]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: Recent advances. F1000Research 2016, 5, 2270. [Google Scholar] [CrossRef]
- Byeon, H.K.; Ku, M.; Yang, J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Inoue, H.; Furukawa, M.; Kakudo, K.; Nozaki, M. Heparin-binding epidermal growth factor-like growth factor is a potent regulator of invasion activity in oral squamous cell carcinoma. Oncol. Rep. 2012, 27, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Simabuco, F.M.; Kawahara, R.; Yokoo, S.; Granato, D.C.; Miguel, L.; Agostini, M.; Aragao, A.Z.; Domingues, R.R.; Flores, I.L.; Macedo, C.C.; et al. ADAM17 mediates OSCC development in an orthotopic murine model. Mol. Cancer 2014, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.C.; Chew, K.Y.; Tan, E.L.; Khoo, S.P. The effect of epiregulin on epidermal growth factor receptor expression and proliferation of oral squamous cell carcinoma cell lines. Cancer Cell Int. 2014, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Smith, S.B.; Khoutorsky, A.; Magnussen, C.A.; Samoshkin, A.; Sorge, R.E.; Cho, C.; Yosefpour, N.; Sivaselvachandran, S.; Tohyama, S.; et al. Epiregulin and EGFR interactions are involved in pain processing. J. Clin. Investig. 2017, 127, 3353–3366. [Google Scholar] [CrossRef] [PubMed]
- Wangzhou, A.; Paige, C.; Neerukonda, S.V.; Naik, D.K.; Kume, M.; David, E.T.; Dussor, G.; Ray, P.R.; Price, T.J. A ligand-receptor interactome platform for discovery of pain mechanisms and therapeutic targets. Sci. Signal. 2021, 14, eabe1648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, G.; Cao, X. EGFR dependent subcellular communication was responsible for morphine mediated AC superactivation. Cell Signal. 2013, 25, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef]
- Kumar, S.; Noronha, V.; Patil, V.; Joshi, A.; Menon, N.; Prabhash, K. Advances in pharmacotherapy for head and neck cancer. Expert. Opin. Pharmacother. 2021, 22, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.; Formento, J.L.; Francoual, M.; Milano, G.; Schneider, M.; Dassonville, O.; Demard, F. Characterization, quantification, and potential clinical value of the epidermal growth factor receptor in head and neck squamous cell carcinomas. Head. Neck. 1991, 13, 132–139. [Google Scholar] [CrossRef]
- Rubin Grandis, J.; Melhem, M.F.; Barnes, E.L.; Tweardy, D.J. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996, 78, 1284–1292. [Google Scholar] [CrossRef]
- Psyrri, A.; Seiwert, T.Y.; Jimeno, A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am. Soc. Clin. Oncol. Educ. Book. 2013, 33, 246–255. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zouhair, A.; Azria, D.; Ozsahin, M. The epidermal growth factor receptor (EGFR) in head and neck cancer: Its role and treatment implications. Radiat. Oncol. 2006, 1, 11. [Google Scholar] [CrossRef]
- Grandis, J.R.; Tweardy, D.J. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993, 53, 3579–3584. [Google Scholar] [PubMed]
- Kordbacheh, F.; Farah, C.S. Current and Emerging Molecular Therapies for Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5471. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.; Vega-Villegas, M.E.; Lopez-Brea, M.F.; Marquez, R. Current situation of Panitumumab, Matuzumab, Nimotuzumab and Zalutumumab. Acta Oncol. 2008, 47, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Foncillas, J.; Sunakawa, Y.; Aderka, D.; Wainberg, Z.; Ronga, P.; Witzler, P.; Stintzing, S. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front. Oncol. 2019, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Gleber-Netto, F.O.; Rubin, M.L.; Qing, Y.; Du, R.; Kies, M.; Blumenschein, G., Jr.; Lu, C.; Johnson, F.M.; Bell, D.; et al. Induction chemotherapy with or without erlotinib in patients with head and neck squamous cell carcinoma amenable for surgical resection. Clin. Cancer Res. 2022, 28, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iida, M.; Dunn, E.F.; Ghia, A.J.; Wheeler, D.L. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene 2009, 28, 3801–3813. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Copland, J.A. Targeting lipid metabolism for the treatment of anaplastic thyroid carcinoma. Expert. Opin. Ther. Targets 2016, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Lu, H.; Luo, J.; Hong, Y.; Fan, Z. AMPK-mediated energy homeostasis and associated metabolic effects on cancer cell response and resistance to cetuximab. Oncotarget 2015, 6, 11507–11518. [Google Scholar] [CrossRef]
- Bezjak, A.; Tu, D.; Seymour, L.; Clark, G.; Trajkovic, A.; Zukin, M.; Ayoub, J.; Lago, S.; de Albuquerque Ribeiro, R.; Gerogianni, A.; et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J. Clin. Oncol. 2006, 24, 3831–3837. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Szczesna, A.; Juhasz, E.; Esteban, E.; Molinier, O.; Brugger, W.; Melezinek, I.; et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010, 11, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V.; Cadranel, J.; Cong, X.J.; Fairclough, D.; Finnern, H.W.; Lorence, R.M.; Miller, V.A.; Palmer, M.; Yang, J.C. Symptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib: Results of a randomized phase IIb/III trial (LUX-Lung 1). J. Thorac. Oncol. 2013, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.G.; Kersten, C. Differential effects of epidermal growth factor receptor inhibitors in a single patient with neuropathic pain. BMJ Case Rep. 2021, 14, e239385. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Hirsh, V.; Popat, S.; Cobo, M.; Fulop, A.; Dayen, C.; Trigo, J.M.; Gregg, R.; Waller, C.F.; Soria, J.C.; et al. Symptom and Quality of Life Improvement in LUX-Lung 8, an Open-Label Phase III Study of Second-Line Afatinib Versus Erlotinib in Patients with Advanced Squamous Cell Carcinoma of the Lung After First-Line Platinum-Based Chemotherapy. Clin. Lung Cancer 2018, 19, 74–83.e11. [Google Scholar] [CrossRef]
- Kersten, C.; Cameron, M.G. Cetuximab alleviates neuropathic pain despite tumour progression. BMJ Case Rep. 2012, 2012, bcr1220115374. [Google Scholar] [CrossRef]
- Kersten, C.; Cameron, M.G.; Mjaland, S. Epithelial growth factor receptor (EGFR)-inhibition for relief of neuropathic pain-A case series. Scand. J. Pain 2013, 4, 3–7. [Google Scholar] [CrossRef]
- Kersten, C.; Cameron, M.G.; Bailey, A.G.; Fallon, M.T.; Laird, B.J.; Paterson, V.; Mitchell, R.; Fleetwood-Walker, S.M.; Daly, F.; Mjaland, S. Relief of Neuropathic Pain Through Epidermal Growth Factor Receptor Inhibition: A Randomized Proof-of-Concept Trial. Pain. Med. 2019, 20, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.G.; Kersten, C. Prospective case series of neuropathic cancer pain in patients treated with an EGFR-inhibitor. Palliat. Med. 2022, 36, 1154–1162. [Google Scholar] [CrossRef]
- Verma, V.; Khoury, S.; Parisien, M.; Cho, C.; Maixner, W.; Martin, L.J.; Diatchenko, L. The dichotomous role of epiregulin in pain. Pain 2020, 161, 1052–1064. [Google Scholar] [CrossRef]
- Sit, D.; Bale, M.; Lapointe, V.; Olson, R.; Hsu, F. Association Between EGFR and ALK Mutation Status on Patient-Reported Symptoms After Palliative Radiation for Bone Pain in NSCLC. JTO Clin. Res. Rep. 2022, 3, 100371. [Google Scholar] [CrossRef]
- Li, S.; Poelmans, G.; van Boekel, R.L.M.; Coenen, M.J.H. Genome-wide association study on pharmacological outcomes of musculoskeletal pain in UK Biobank. Pharmacogenomics J. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhou, Y.; Zeng, B.; Yang, X.; Su, M. MicroRNA-183 attenuates osteoarthritic pain by inhibiting the TGFalpha-mediated CCL2/CCR2 signalling axis. Bone Jt. Res. 2021, 10, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, M.; Emrich, D.; Wolf, E.; Schneider, M.R. Increased activation of the epidermal growth factor receptor in transgenic mice overexpressing epigen causes peripheral neuropathy. Biochim. Biophys. Acta 2013, 1832, 2068–2076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Appleton, C.T.; Usmani, S.E.; Pest, M.A.; Pitelka, V.; Mort, J.S.; Beier, F. Reduction in disease progression by inhibition of transforming growth factor alpha-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis Rheumatol. 2015, 67, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Pozzi, A.; Boyd, S.K.; Clark, A.L. Integrin alpha1beta1 protects against signs of post-traumatic osteoarthritis in the female murine knee partially via regulation of epidermal growth factor receptor signalling. Osteoarthr. Cartil. 2016, 24, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ma, X.; Tong, W.; Doyran, B.; Sun, Z.; Wang, L.; Zhang, X.; Zhou, Y.; Badar, F.; Chandra, A.; et al. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc. Natl. Acad. Sci. USA 2016, 113, 14360–14365. [Google Scholar] [CrossRef]
- Quarta, S.; Mitric, M.; Kalpachidou, T.; Mair, N.; Schiefermeier-Mach, N.; Andratsch, M.; Qi, Y.; Langeslag, M.; Malsch, P.; Rose-John, S.; et al. Impaired mechanical, heat, and cold nociception in a murine model of genetic TACE/ADAM17 knockdown. FASEB J. 2019, 33, 4418–4431. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Pan, Z.; Yu, D.; Chen, P.; Zhang, X.; Wu, H.; Zhang, X.; An, C.; Chen, Y.; et al. Gefitinib for Epidermal Growth Factor Receptor Activated Osteoarthritis Subpopulation Treatment. EBioMedicine 2018, 32, 223–233. [Google Scholar] [CrossRef]
- Eagles, D.A.; Saez, N.J.; Krishnarjuna, B.; Bradford, J.J.; Chin, Y.K.; Starobova, H.; Mueller, A.; Reichelt, M.E.; Undheim, E.A.B.; Norton, R.S.; et al. A peptide toxin in ant venom mimics vertebrate EGF-like hormones to cause long-lasting hypersensitivity in mammals. Proc. Natl. Acad. Sci. USA 2022, 119, e2112630119. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Tran, P.B.; Das, R.; Ghoreishi-Haack, N.; Ren, D.; Miller, R.J.; Malfait, A.M. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 20602–20607. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Wei, Y.; Luo, L.; Li, J.; Zhong, L.; Yao, L.; Beier, F.; Nelson, C.L.; Tsourkas, A.; Liu, X.S.; et al. Activating EGFR Signaling Attenuates Osteoarthritis Development Following Loading Injury in Mice. J. Bone Miner. Res. 2022, 37, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.F.; Zhou, W.M.; Yang, Y.; Zhou, J.; Li, X.L.; Lin, L.; Zhang, H.J. Epidermal growth factor upregulates serotonin transporter and its association with visceral hypersensitivity in irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 13521–13529. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.J.F.; Rao, S.; Tejada, M.A.; Turnes, B.L.; Licht-Mayer, S.; Omura, T.; Brenneis, C.; Jacobs, E.; Barrett, L.; Latremoliere, A.; et al. Phenotypic drug screen uncovers the metabolic GCH1/BH4 pathway as key regulator of EGFR/KRAS-mediated neuropathic pain and lung cancer. Sci. Transl. Med. 2022, 14, eabj1531. [Google Scholar] [CrossRef]
- Mitchell, R.; Mikolajczak, M.; Kersten, C.; Fleetwood-Walker, S. ErbB1-dependent signalling and vesicular trafficking in primary afferent nociceptors associated with hypersensitivity in neuropathic pain. Neurobiol. Dis. 2020, 142, 104961. [Google Scholar] [CrossRef]
- Zhang, D.D.; Chen, Q.Q.; Yao, L. Geniposide Alleviates Neuropathic Pain in CCI Rats by Inhibiting the EGFR/PI3K/AKT Pathway And Ca(2+) Channels. Neurotox. Res. 2022, 40, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; He, Q.; Matsuda, M.; Han, Q.; Wang, K.; Bang, S.; Ding, H.; Ko, M.C.; Ji, R.R. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci. Transl. Med. 2020, 12, aaw6471. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Wang, X.; Lo, E.H. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol. Sci. 2009, 30, 336–340. [Google Scholar] [CrossRef]
- Latremoliere, A.; Costigan, M. GCH1, BH4 and pain. Curr. Pharm. Biotechnol. 2011, 12, 1728–1741. [Google Scholar] [CrossRef]
- Chen, Y.; Long, H.; Wu, Z.; Jiang, X.; Ma, L. EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol. Biol. Cell 2008, 19, 2973–2983. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.K.; Yu, L.; Li, Z.J.; Sung, J.J.; Zhang, S.T.; Cho, C.H. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of beta-adrenoceptors. J. Pharmacol. Exp. Ther. 2008, 326, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Capo-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Hu, R.; Sun, Y.; Xiang, L.; Yan, J.; Jiang, H. Activation of the spinal EGFR signaling pathway in a rat model of cancer-induced bone pain with morphine tolerance. Neuropharmacology 2021, 196, 108703. [Google Scholar] [CrossRef] [PubMed]
- Lueptow, L.M.; Fakira, A.K.; Bobeck, E.N. The Contribution of the Descending Pain Modulatory Pathway in Opioid Tolerance. Front. Neurosci. 2018, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Pan, H.L. High voltage-activated Ca(2+) channel currents in isolectin B(4)-positive and -negative small dorsal root ganglion neurons of rats. Neurosci. Lett. 2004, 368, 96–101. [Google Scholar] [CrossRef]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.J. Cellular neuroadaptations to chronic opioids: Tolerance, withdrawal and addiction. Br. J. Pharmacol. 2008, 154, 384–396. [Google Scholar] [CrossRef]
- Chen, S.R.; Prunean, A.; Pan, H.M.; Welker, K.L.; Pan, H.L. Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience 2007, 145, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Chen, S.R.; Chen, H.; Pan, H.L. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 2010, 30, 4460–4466. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, H.; Jin, D.; Pan, H.L. Brief Opioid Exposure Paradoxically Augments Primary Afferent Input to Spinal Excitatory Neurons via alpha2delta-1-Dependent Presynaptic NMDA Receptors. J. Neurosci. 2022, 42, 9315–9329. [Google Scholar] [CrossRef] [PubMed]
- Araldi, D.; Ferrari, L.F.; Levine, J.D. Mu-opioid Receptor (MOR) Biased Agonists Induce Biphasic Dose-dependent Hyperalgesia and Analgesia, and Hyperalgesic Priming in the Rat. Neuroscience 2018, 394, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Araldi, D.; Ferrari, L.F.; Levine, J.D. Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J. Neurosci. 2015, 35, 12502–12517. [Google Scholar] [CrossRef] [PubMed]
- Clayton, C.C.; Bruchas, M.R.; Lee, M.L.; Chavkin, C. Phosphorylation of the mu-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol. Pharmacol. 2010, 77, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Allouche, S.; Noble, F.; Marie, N. Opioid receptor desensitization: Mechanisms and its link to tolerance. Front. Pharmacol. 2014, 5, 280. [Google Scholar] [CrossRef]
- Tang, Y.; Ye, M.; Du, Y.; Qiu, X.; Lv, X.; Yang, W.; Luo, J. EGFR signaling upregulates surface expression of the GluN2B-containing NMDA receptor and contributes to long-term potentiation in the hippocampus. Neuroscience 2015, 304, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, I.E.; Helton, T.D.; Petrou, V.I.; Mirshahi, T.; Ehlers, M.D.; Logothetis, D.E. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J. Neurosci. 2007, 27, 5523–5532. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Jin, D.; Chen, S.R.; Pan, H.L. NMDA Receptors at Primary Afferent-Excitatory Neuron Synapses Differentially Sustain Chemotherapy- and Nerve Trauma-Induced Chronic Pain. J. Neurosci. 2023, 43, 3933–3948. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.R.; Chen, H.; Zhang, J.; Pan, H.L. Increased alpha2delta-1-NMDA receptor coupling potentiates glutamatergic input to spinal dorsal horn neurons in chemotherapy-induced neuropathic pain. J. Neurochem. 2019, 148, 252–274. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Chen, S.R.; Chen, H.; Xie, J.D.; Sirrieh, R.E.; MacLean, D.M.; Zhang, Y.; Zhou, M.H.; Jayaraman, V.; et al. The alpha2delta-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions. Cell Rep. 2018, 22, 2307–2321. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, S.R.; Pan, H.L. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev. Clin. Pharmacol. 2011, 4, 379–388. [Google Scholar] [CrossRef]
- Deng, M.; Chen, S.R.; Chen, H.; Luo, Y.; Dong, Y.; Pan, H.L. Mitogen-activated protein kinase signaling mediates opioid-induced presynaptic NMDA receptor activation and analgesic tolerance. J. Neurochem. 2019, 148, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, M.J.; Inoue, K.; Akiduki, S.; Osugi, T.; Imamura, T.; Ishida, N.; Ohtomi, M. Identification of addicsin/GTRAP3-18 as a chronic morphine-augmented gene in amygdala. Neuroreport 2002, 13, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, M.; Zhou, Q.; Xue, Y.; Wang, L.; Bil De Arce, V.J.; Zhang, X.; Jiang, W. Antisense oligonucleotide knockdown of mGlu(5) receptor attenuates the antinociceptive tolerance and up-regulated expression of spinal protein kinase C associated with chronic morphine treatment. Eur. J. Pharmacol. 2012, 683, 78–85. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Xu, H.; Ma, X.; Jiang, W.; Xu, T. Inhibitory effect of spinal mGlu(5) receptor antisense oligonucleotide on the up-regulated expression of spinal G protein associated with chronic morphine treatment. Eur. J. Pharmacol. 2014, 723, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, L.J.; Babey, A.M. Synthesis of the Mechanisms of Opioid Tolerance: Do We Still Say NO? Cell. Mol. Neurobiol. 2021, 41, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Garzon, J.; Rodriguez-Munoz, M.; Sanchez-Blazquez, P. Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: Molecular implications for opioid tolerance. Curr. Drug Abuse Rev. 2012, 5, 199–226. [Google Scholar] [CrossRef]
- Sanchez-Blazquez, P.; Rodriguez-Munoz, M.; Berrocoso, E.; Garzon, J. The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain. Eur. J. Pharmacol. 2013, 716, 94–105. [Google Scholar] [CrossRef]
- Suina, K.; Tsuchihashi, K.; Yamasaki, J.; Kamenori, S.; Shintani, S.; Hirata, Y.; Okazaki, S.; Sampetrean, O.; Baba, E.; Akashi, K.; et al. Epidermal growth factor receptor promotes glioma progression by regulating xCT and GluN2B-containing N-methyl-d-aspartate-sensitive glutamate receptor signaling. Cancer Sci. 2018, 109, 3874–3882. [Google Scholar] [CrossRef]
- Oyagi, A.; Oida, Y.; Kakefuda, K.; Shimazawa, M.; Shioda, N.; Moriguchi, S.; Kitaichi, K.; Nanba, D.; Yamaguchi, K.; Furuta, Y.; et al. Generation and characterization of conditional heparin-binding EGF-like growth factor knockout mice. PLoS ONE 2009, 4, e7461. [Google Scholar] [CrossRef]
- Garcia Campelo, M.R.; Zhou, C.; Ramalingam, S.S.; Lin, H.M.; Kim, T.M.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Goodman, E.; Mehta, M.; et al. Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort. J. Clin. Med. 2022, 12, 112. [Google Scholar] [CrossRef]
- Rekowska, A.; Rola, P.; Wojcik-Superczynska, M.; Chmielewska, I.; Krawczyk, P.; Milanowski, J. Efficacy of Osimertinib in Lung Squamous Cell Carcinoma Patients with EGFR Gene Mutation-Case Report and a Literature Review. Curr. Oncol. 2022, 29, 3531–3539. [Google Scholar] [CrossRef]
- Yue, L.; Wentao, L.; Xin, Z.; Jingjing, H.; Xiaoyan, Z.; Na, F.; Tonghui, M.; Dalin, L. Human epidermal growth factor receptor 2-positive metastatic breast cancer with novel epidermal growth factor receptor -ZNF880 fusion and epidermal growth factor receptor E114K mutations effectively treated with pyrotinib: A case report. Medicine 2020, 99, e23406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Wei, J.G.; Chen, Y.Y.; Wang, J.F. Efficacy and safety of pembrolizumab monotherapy in EGFR-mutant squamous cell lung cancer with PD-L1 over-expression: A case report. Medicine 2022, 101, e30099. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs. Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival among Patients with RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef]
- Liam, C.K.; Ahmad, A.R.; Hsia, T.C.; Zhou, J.; Kim, D.W.; Soo, R.A.; Cheng, Y.; Lu, S.; Shin, S.W.; Yang, J.C.; et al. Randomized Trial of Tepotinib Plus Gefitinib versus Chemotherapy in EGFR-Mutant NSCLC with EGFR Inhibitor Resistance Due to MET Amplification: INSIGHT Final Analysis. Clin. Cancer Res. 2023, 29, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, P.; Zhao, D.; Shi, X.; Guo, R.; Gao, W.; Shu, Y. Safety, efficacy, and pharmacokinetics of SH-1028 in EGFR T790M-positive advanced non-small cell lung cancer patients: A dose-escalation phase 1 study. Cancer 2023, 129, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Xi, D.; Bai, Y.; Liu, L.; Ma, Y.; Yin, Z.; Chen, H. Case Report: Chemotherapy-free treatment with camrelizumab and anlotinib for elderly patients with KRAS and TP53 mutated advanced lung cancer. Front. Pharmacol. 2023, 14, 1026135. [Google Scholar] [CrossRef] [PubMed]
- Dal Lago, L.; Uwimana, A.L.; Coens, C.; Vuylsteke, P.; Curigliano, G.; Brouwers, B.; Jagiello-Gruszfeld, A.; Altintas, S.; Tryfonidis, K.; Poncet, C.; et al. Health-related quality of life in older patients with HER2+ metastatic breast cancer: Comparing pertuzumab plus trastuzumab with or without metronomic chemotherapy in a randomised open-label phase II clinical trial. J. Geriatr. Oncol. 2022, 13, 582–593. [Google Scholar] [CrossRef]
- Chen, H.; Jia, B.; Zhang, Q.; Zhang, Y. Meclofenamic Acid Restores Gefinitib Sensitivity by Downregulating Breast Cancer Resistance Protein and Multidrug Resistance Protein 7 via FTO/m6A-Demethylation/c-Myc in Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 870636. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.J.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2022, 28, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, S.W.; Zhang, S.; Li, P.; Zhao, C.; Zhao, X.B.; Wang, C.H.; Zhang, J.; Wang, B.; Liu, P.N. Phase II trial of icotinib in adult patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J. Neurosurg. 2022, 1, jns22699. [Google Scholar] [CrossRef] [PubMed]

| Animal Models | EGFR Activation | EGFR Inhibition | Ref. | |||
|---|---|---|---|---|---|---|
| Induced by | Behavioral Outcomes | Induced by | Behavioral Outcomes | |||
| i.t. morphine | Rat | EGF (4 days) | Production of pre-tolerance and thermal hypersensitivity | [25] | ||
| Lumbar spinal nerve ligation Morphine (i.t. or subcutaneous) | Rat | Gefitinib (i.t. or subcutaneous) | Reversal of tolerance mechanical allodynia and thermal hypersensitivity | [25] | ||
| EGF-scavenging molecule (i.t.) | Reversal of tolerance and mechanical allodynia | |||||
| Injecting Walker 256 mammary gland carcinoma cells into tibias + morphine (i.t.) | Rat | AG 1478 (i.t.) | Reversal of tolerance and mechanical allodynia | [94] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, M.D.; Zhang, M.; Liu, N.; Viet, C.T.; Xie, T.; Jensen, D.D.; Amit, M.; Pan, H.; Ye, Y. Repurposing EGFR Inhibitors for Oral Cancer Pain and Opioid Tolerance. Pharmaceuticals 2023, 16, 1558. https://doi.org/10.3390/ph16111558
Santi MD, Zhang M, Liu N, Viet CT, Xie T, Jensen DD, Amit M, Pan H, Ye Y. Repurposing EGFR Inhibitors for Oral Cancer Pain and Opioid Tolerance. Pharmaceuticals. 2023; 16(11):1558. https://doi.org/10.3390/ph16111558
Chicago/Turabian StyleSanti, Maria Daniela, Morgan Zhang, Naijiang Liu, Chi T. Viet, Tongxin Xie, Dane D. Jensen, Moran Amit, Huilin Pan, and Yi Ye. 2023. "Repurposing EGFR Inhibitors for Oral Cancer Pain and Opioid Tolerance" Pharmaceuticals 16, no. 11: 1558. https://doi.org/10.3390/ph16111558
APA StyleSanti, M. D., Zhang, M., Liu, N., Viet, C. T., Xie, T., Jensen, D. D., Amit, M., Pan, H., & Ye, Y. (2023). Repurposing EGFR Inhibitors for Oral Cancer Pain and Opioid Tolerance. Pharmaceuticals, 16(11), 1558. https://doi.org/10.3390/ph16111558






