Antipsychotics and Risks of Cardiovascular and Cerebrovascular Diseases and Mortality in Dwelling Community Older Adults
Abstract
1. Introduction
2. Results
2.1. Antipsychotics Exposure
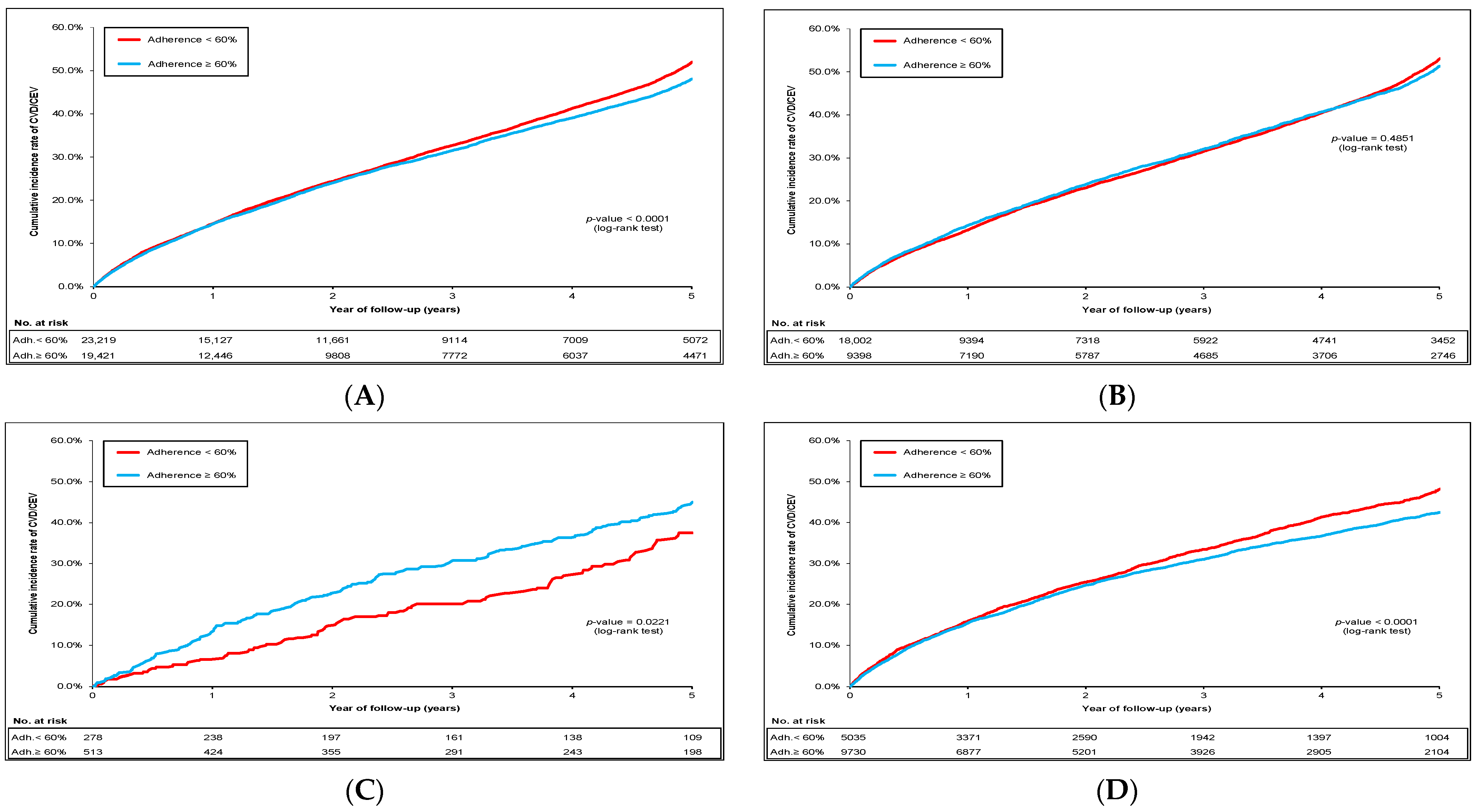
2.2. Cumulative Incidence of CVD/CEV and Mortality
2.3. Hazard Ratios for CVD/CEV Risks
2.4. Hazard Ratios for Mortality Risks
2.5. Sensitivity Analyses
3. Discussion
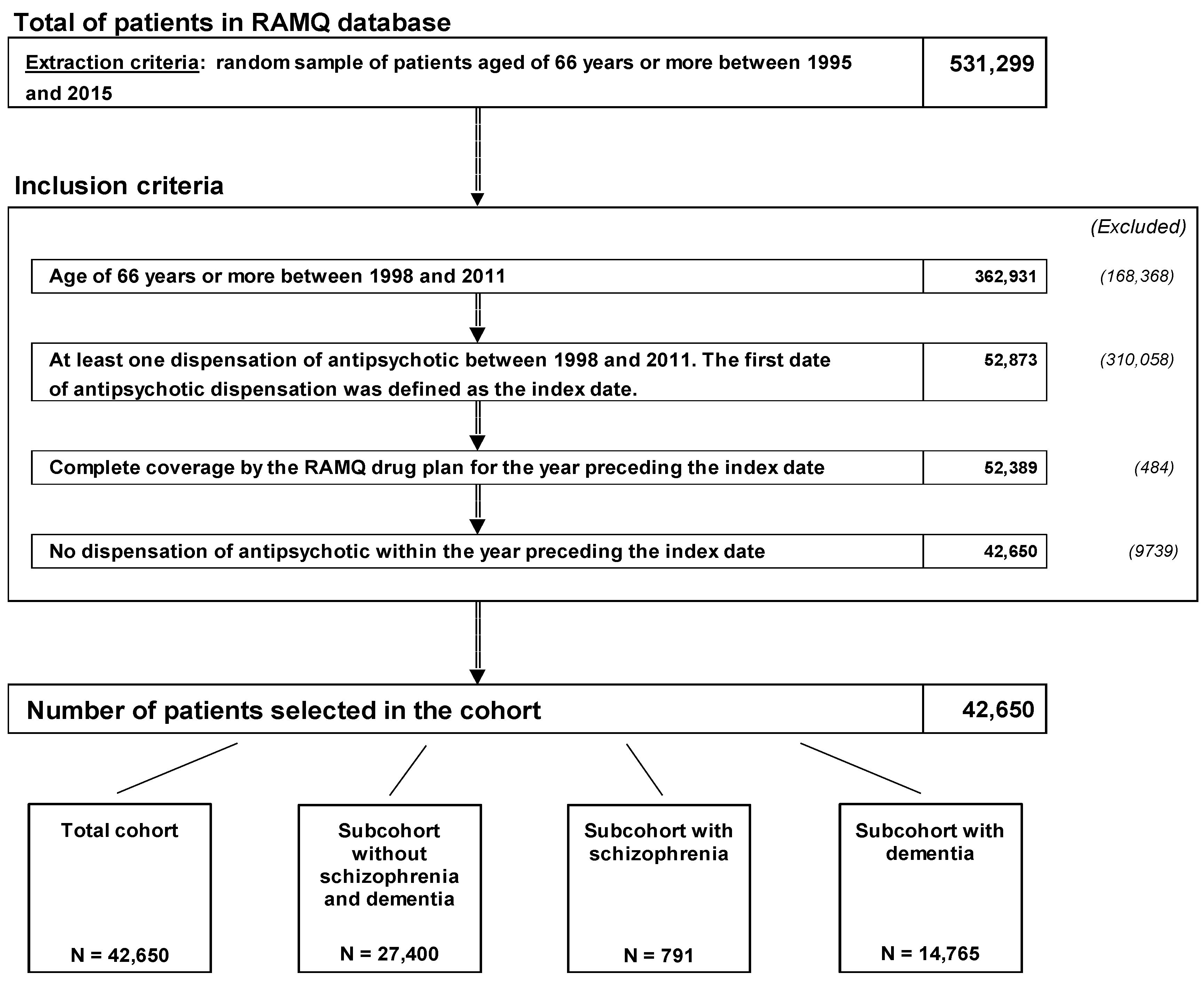
4. Methods
4.1. Data Source and Ethics Declarations
4.2. Cohort Definition
4.3. Adherence Level of Antipsychotics
4.4. Outcomes
4.5. Covariates
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakouni, H.; Berbiche, D.; Vasiliadis, H.M. Off-label use of antipsychotics and associated factors in community living older adults. Aging Ment. Health 2019, 23, 158–165. [Google Scholar] [CrossRef]
- Masand, P.S. Side effects of antipsychotics in the elderly. J. Clin. Psychiatry 2000, 61 (Suppl. 8), 43–49; discussion 50–41. [Google Scholar]
- Lee, S.H.; Hsu, W.T.; Lai, C.C.; Esmaily-Fard, A.; Tsai, Y.W.; Chiu, C.C.; Wang, J.; Chang, S.S.; Lee, C.C. Use of antipsychotics increases the risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 1167–1178. [Google Scholar] [CrossRef]
- Whitaker, R. How the FDA approved an antipsychotic that failed to show a meaningful benefit but raised the risk of death. BMJ 2023, 382, 1801. [Google Scholar] [CrossRef]
- Tampi, R.R.; Tampi, D.J.; Balachandran, S.; Srinivasan, S. Antipsychotic use in dementia: A systematic review of benefits and risks from meta-analyses. Ther. Adv. Chronic Dis. 2016, 7, 229–245. [Google Scholar] [CrossRef]
- Rogowska, M.; Thornton, M.; Creese, B.; Velayudhan, L.; Aarsland, D.; Ballard, C.; Tsamakis, K.; Stewart, R.; Mueller, C. Implications of Adverse Outcomes Associated with Antipsychotics in Older Patients with Dementia: A 2011–2022 Update. Drugs Aging 2023, 40, 21–32. [Google Scholar] [CrossRef]
- Mueller, C.; John, C.; Perera, G.; Aarsland, D.; Ballard, C.; Stewart, R. Antipsychotic use in dementia: The relationship between neuropsychiatric symptom profiles and adverse outcomes. Eur. J. Epidemiol. 2021, 36, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.J.; Espinet, A.J. Increased All-Cause Mortality by Antipsychotic Drugs: Updated Review and Meta-Analysis in Dementia and General Mental Health Care. J. Alzheimers Dis. Rep. 2018, 2, 1–26. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Lolk, A.; Valentin, J.B.; Andersen, K. Cumulative dosages of antipsychotic drugs are associated with increased mortality rate in patients with Alzheimer’s dementia. Acta Psychiatr. Scand. 2016, 134, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, A.; Jensen-Dahm, C.; Wimberley, T.; Svendsen, J.H.; Ishtiak-Ahmed, K.; Laursen, T.M.; Waldemar, G.; Gasse, C. Effect of antipsychotics on mortality risk in patients with dementia with and without comorbidities. J. Am. Geriatr. Soc. 2022, 70, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Yunusa, I.; Alsumali, A.; Garba, A.E.; Regestein, Q.R.; Eguale, T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw. Open 2019, 2, e190828. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Mortality Database. 25th May Update. 2020. Available online: https://platform.who.int/mortality (accessed on 15 May 2023).
- European Commission. Eurostat Database. 2016. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 15 May 2023).
- Zhu, J.; Hou, W.; Xu, Y.; Ji, F.; Wang, G.; Chen, C.; Lin, C.; Lin, X.; Li, J.; Zhuo, C.; et al. Antipsychotic drugs and sudden cardiac death: A literature review of the challenges in the prediction, management, and future steps. Psychiatry Res. 2019, 281, 112598. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, S.; Koh, C.H.; Kaza, N.; Jackson, C.A. Antipsychotic drug use and risk of stroke and myocardial infarction: A systematic review and meta-analysis. BMC Psychiatry 2019, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, T.; Huybrechts, K.; Olfson, M.; Schneeweiss, S.; Bobo, W.V.; Doraiswamy, P.M.; Devanand, D.P.; Lucas, J.A.; Huang, C.; Malka, E.S.; et al. Comparative mortality risks of antipsychotic medications in community-dwelling older adults. Br. J. Psychiatry 2014, 205, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Ashraf, A.; Baker, D.; Al-Omary, M.S.; Savage, L.; Ekmejian, A.; Singh, R.S.H.; Brienesse, S.; Majeed, T.; Gordon, T.; et al. Clozapine and incidence of myocarditis and sudden death—Long term Australian experience. Int. J. Cardiol. 2017, 238, 136–139. [Google Scholar] [CrossRef]
- Davies, E.A.; O’Mahony, M.S. Adverse drug reactions in special populations—The elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef]
- Li, Z.P.; You, Y.S.; Wang, J.D.; He, L.P. Underlying disease may increase mortality risk in users of atypical antipsychotics. World J. Psychiatry 2022, 12, 1112–1114. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qiu, L.K.; Li, Z.P.; He, L.P.; Zhou, L.L. Underlying reasons for the decline in physical activity during COVID-19. World J. Psychiatry 2022, 12, 999–1001. [Google Scholar] [CrossRef]
- Naylor, C.; Parsonage, M.; McDaid, D.; Knapp, M.; Fossey, M.; Galea, A. Long-Term Conditions and Mental Health: The Cost of Co-Morbidities; King’s Fund and Centre for Mental Health: London, UK, 2012. [Google Scholar]
- Grenard, J.L.; Munjas, B.A.; Adams, J.L.; Suttorp, M.; Maglione, M.; McGlynn, E.A.; Gellad, W.F. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. J. Gen. Intern. Med. 2011, 26, 1175–1182. [Google Scholar] [CrossRef]
- St-Pierre, M.; Sinclair, I.; Elgbeili, G.; Bernard, P.; Dancause, K.N. Relationships between psychological distress and health behaviors among Canadian adults: Differences based on gender, income, education, immigrant status, and ethnicity. SSM Popul. Health 2019, 7, 100385. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, S.Y.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Yoon, J.S.; Kim, J.M. Comorbidity of depression with physical disorders: Research and clinical implications. Chonnam Med. J. 2015, 51, 8–18. [Google Scholar] [CrossRef]
- Prior, A.; Fenger-Gron, M.; Larsen, K.K.; Larsen, F.B.; Robinson, K.M.; Nielsen, M.G.; Christensen, K.S.; Mercer, S.W.; Vestergaard, M. The Association Between Perceived Stress and Mortality Among People With Multimorbidity: A Prospective Population-Based Cohort Study. Am. J. Epidemiol. 2016, 184, 199–210. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef]
- Celano, C.M.; Millstein, R.A.; Bedoya, C.A.; Healy, B.C.; Roest, A.M.; Huffman, J.C. Association between anxiety and mortality in patients with coronary artery disease: A meta-analysis. Am. Heart J. 2015, 170, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Balaram, K.; Balachandran, S. Psychopharmacology in the Elderly: Why Does Age Matter? Psychiatr. Clin. N. Am. 2022, 45, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Riordan, H.J.; Antonini, P.; Murphy, M.F. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia: Risk factors, monitoring, and healthcare implications. Am. Health Drug Benefits 2011, 4, 292–302. [Google Scholar] [PubMed]
- Hagg, S.; Jonsson, A.K.; Spigset, O. Risk of venous thromboembolism due to antipsychotic drug therapy. Expert Opin. Drug Saf. 2009, 8, 537–547. [Google Scholar] [CrossRef]
- Burghardt, K.J.; Seyoum, B.; Mallisho, A.; Burghardt, P.R.; Kowluru, R.A.; Yi, Z. Atypical antipsychotics, insulin resistance and weight; a meta-analysis of healthy volunteer studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 83, 55–63. [Google Scholar] [CrossRef]
- Go, C.; Kim, S.; Kim, Y.; Sunwoo, Y.; Eom, S.H.; Yun, J.; Shin, S.; Choi, Y.J. A Real-World Data Driven Pharmacovigilance Investigation on Drug-Induced Arrhythmia Using KAERS DB, a Korean Nationwide Adverse Drug Reporting System. Pharmaceuticals 2023, 16, 1612. [Google Scholar] [CrossRef]
- Pratt, N.L.; Roughead, E.E.; Ramsay, E.; Salter, A.; Ryan, P. Risk of hospitalization for stroke associated with antipsychotic use in the elderly: A self-controlled case series. Drugs Aging 2010, 27, 885–893. [Google Scholar] [CrossRef]
- Sacchetti, E.; Turrina, C.; Cesana, B.; Mazzaglia, G. Timing of stroke in elderly people exposed to typical and atypical antipsychotics: A replication cohort study after the paper of Kleijer, et al. J. Psychopharmacol. 2010, 24, 1131–1132. [Google Scholar] [CrossRef]
- Fife, D.; Blacketer, C.; Knight, R.K.; Weaver, J. Stroke Risk Among Elderly Users of Haloperidol and Typical Antipsychotics Versus Atypical Antipsychotics: A Real-World Study From a US Health Insurance Claims Database. Am. J. Geriatr. Psychiatry 2021, 29, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Giorgianni, F.; Rea, F.; Lucenteforte, E.; Lombardi, N.; Mugelli, A.; Vannacci, A.; Liperoti, R.; Kirchmayer, U.; Vitale, C.; et al. All-cause mortality and antipsychotic use among elderly persons with high baseline cardiovascular and cerebrovascular risk: A multi-center retrospective cohort study in Italy. Expert Opin. Drug Metab. Toxicol. 2019, 15, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.P. Association of clinical characteristics with sudden cardiac arrest in people with type 2 diabetes with and without CVD: A longitudinal case-control study on primary care data Diabetologia 2023; Oral presentation in Session: Cardiovascular complications in type 1 and type 2 diabetics; Abstract 172.
- Chen, D.; Ejlskov, L.; Laustsen, L.M.; Weye, N.; Sorensen, C.L.B.; Momen, N.C.; Dreier, J.W.; Zheng, Y.; Damgaard, A.J.; McGrath, J.J.; et al. The Role of Socioeconomic Position in the Association Between Mental Disorders and Mortality: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol. Drug Saf. 2017, 26, 1033–1039. [Google Scholar] [CrossRef]
- Wang, S.V.; Schneeweiss, S.; Berger, M.L.; Brown, J.; de Vries, F.; Douglas, I.; Gagne, J.J.; Gini, R.; Klungel, O.; Mullins, C.D.; et al. Reporting to Improve Reproducibility and Facilitate Validity Assessment for Healthcare Database Studies V1.0. Pharmacoepidemiol. Drug Saf. 2017, 26, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Schmidt, S.A.J.; Wing, K.; Ehrenstein, V.; Nicholls, S.G.; Filion, K.B.; Klungel, O.; Petersen, I.; Sorensen, H.T.; Dixon, W.G.; et al. La declaration RECORD-PE (Reporting of Studies Conducted Using Observational Routinely Collected Health Data Statement for Pharmacoepdemiology): Directives pour la communication des etudes realisees a partir de donnees de sante observationelles collectees en routine en pharmacoepidemiologie. CMAJ 2019, 191, E689–E708. [Google Scholar] [CrossRef]
- Gatto, N.M.; Reynolds, R.F.; Campbell, U.B. A Structured Preapproval and Postapproval Comparative Study Design Framework to Generate Valid and Transparent Real-World Evidence for Regulatory Decisions. Clin. Pharmacol. Ther. 2019, 106, 103–115. [Google Scholar] [CrossRef]
- Wang, S.V.; Pinheiro, S.; Hua, W.; Arlett, P.; Uyama, Y.; Berlin, J.A.; Bartels, D.B.; Kahler, K.H.; Bessette, L.G.; Schneeweiss, S. STaRT-RWE: Structured template for planning and reporting on the implementation of real world evidence studies. BMJ 2021, 372, m4856. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Rassen, J.A.; Brown, J.S.; Rothman, K.J.; Happe, L.; Arlett, P.; Dal Pan, G.; Goettsch, W.; Murk, W.; Wang, S.V. Graphical Depiction of Longitudinal Study Designs in Health Care Databases. Ann. Intern. Med. 2019, 170, 398–406. [Google Scholar] [CrossRef]
- Rapport Annuel de Gestion 2005–2006. Régie de l’Assurance Maladie du Québec, Québec, Bibliothèque et Archives nationales du Québec, 2006. ISBN-13: 978-2-550-47511-8.
- Blais, C.L.L.; Hamel, D.; Brown, K.; Rinfret, S.; Cartier, R.; Giguère, M.; Carroll, C.; Beauchamp, C.; Bogaty, P. Évaluation des Soins et Surveillance des Maladies Cardiovasculaires de Santé Publique du Québec et de L’institut National d’Excellence en Santé et Services Sociaux; Gouvernement du Québec, Institut National de Santé Publique, Institut National d’Excellence en Santé et des Services Sociaux: Quebec City, QC, Canada, 2012; pp. 1–9.
- Perreault, S.; Shahabi, P.; Cote, R.; Dumas, S.; Rouleau-Mailloux, E.; Feroz Zada, Y.; Provost, S.; Mongrain, I.; Dorais, M.; Huynh, T.; et al. Rationale, design, and preliminary results of the Quebec Warfarin Cohort Study. Clin. Cardiol. 2018, 41, 576–585. [Google Scholar] [CrossRef]
- Roy, L.; Zappitelli, M.; White-Guay, B.; Lafrance, J.P.; Dorais, M.; Perreault, S. Agreement Between Administrative Database and Medical Chart Review for the Prediction of Chronic Kidney Disease G category. Can. J. Kidney Health Dis. 2020, 7, 2054358120959908. [Google Scholar] [CrossRef]
- Allan, V.; Ramagopalan, S.V.; Mardekian, J.; Jenkins, A.; Li, X.; Pan, X.; Luo, X. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J. Comp. Eff. Res. 2020, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bjerre, L.M.; Farrell, B.; Hogel, M.; Graham, L.; Lemay, G.; McCarthy, L.; Raman-Wilms, L.; Rojas-Fernandez, C.; Sinha, S.; Thompson, W.; et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: Evidence-based clinical practice guideline. Can. Fam. Physician 2018, 64, 17–27. [Google Scholar] [PubMed]
- Leucht, S.; Crippa, A.; Siafis, S.; Patel, M.X.; Orsini, N.; Davis, J.M. Dose-Response Meta-Analysis of Antipsychotic Drugs for Acute Schizophrenia. Am. J. Psychiatry 2020, 177, 342–353. [Google Scholar] [CrossRef] [PubMed]


| Initial Cohort | Cohort after IPTW | |||||
|---|---|---|---|---|---|---|
| Adherence Level | Adherence Level | |||||
| <60% (n = 23,219) | ≥60% (n = 19,431) | Absolute Standardized Difference | <60% (n = 23,219) | ≥60% (n = 19,431) | Absolute Standardized Difference | |
| Age at group entry, mean years (SD) * | 78.7 (6.9) | 81.7 (6.8) | 0.43 | 80.4 (7.3) | 80.4 (6.8) | 0.01 |
| Male, % | 42.6 | 36.7 | 0.12 | 39.8 | 39.9 | <0.01 |
| Prevalence within 3-year prior cohort entry, % | ||||||
| Hypertension | 69.8 | 71.1 | 0.02 | 70.5 | 70.9 | 0.01 |
| Diabetes mellitus | 26.8 | 26.6 | <0.01 | 26.8 | 27.2 | 0.01 |
| Dyslipidemia | 32.1 | 27.8 | 0.09 | 29.9 | 30.3 | 0.01 |
| Stroke | 12.9 | 16.0 | 0.09 | 14.9 | 15.1 | 0.01 |
| Coronary artery disease excluding MI | 44.4 | 45.0 | 0.01 | 45.1 | 45.8 | 0.01 |
| Myocardial infarction | 8.3 | 8.6 | 0.01 | 8.8 | 8.9 | 0.01 |
| Heart failure | 17.7 | 20.2 | 0.06 | 19.4 | 20.0 | 0.01 |
| Atrial fibrillation | 17.0 | 18.7 | 0.05 | 18.4 | 18.9 | 0.01 |
| Major bleeding | 12.9 | 12.0 | 0.03 | 12.5 | 12.8 | 0.01 |
| Systemic embolism | 1.7 | 1.3 | 0.03 | 1.6 | 1.7 | 0.01 |
| CKD with eGRF < 30 mL/min † | 3.0 | 2.8 | 0.01 | 3.1 | 3.9 | 0.04 |
| Acute renal failure | 9.4 | 10.2 | 0.03 | 10.2 | 10.7 | 0.02 |
| Liver disease | 2.9 | 2.5 | 0.02 | 2.7 | 2.7 | <0.01 |
| COPD | 38.6 | 36.9 | 0.04 | 38.1 | 38.5 | 0.01 |
| Neurologic disease | 20.9 | 26.6 | 0.14 | 24.6 | 24.9 | 0.01 |
| Hypothyroidism | 19.1 | 21.0 | 0.05 | 20.2 | 20.4 | 0.01 |
| Malign cancer | 52.9 | 28.8 | 0.51 | 40.6 | 39.6 | 0.02 |
| Dementia | 21.7 | 50.1 | 0.62 | 36.3 | 36.2 | <0.01 |
| Medical procedures in 3-year prior cohort entry, % | ||||||
| Percutaneous coronary intervention/CABG | 3.4 | 2.2 | 0.07 | 2.8 | 2.9 | 0.01 |
| Medical procedures for a defibrillator | 1.8 | 2.2 | 0.02 | 2.0 | 2.0 | <0.01 |
| Medication in 1-year prior to cohort entry | ||||||
| Diuretics | 35.5 | 37.4 | 0.04 | 36.9 | 37.4 | 0.01 |
| Inhibitors of renin-angiotensin system | 40.4 | 41.9 | 0.03 | 41.2 | 41.5 | 0.01 |
| Beta-blockers | 32.1 | 32.7 | 0.01 | 32.3 | 32.8 | 0.01 |
| Spironolactone or eplerenone | 3.0 | 2.9 | 0.01 | 3.0 | 3.1 | <0.01 |
| Digoxin | 5.5 | 7.0 | 0.06 | 6.4 | 6.5 | <0.01 |
| Hydralazine | 0.5 | 0.5 | <0.01 | 0.5 | 0.5 | <0.01 |
| Nitrates | 17.8 | 18.9 | 0.03 | 18.7 | 19.0 | 0.01 |
| Statins | 41.2 | 38.4 | 0.06 | 39.4 | 39.8 | 0.01 |
| Antiarrhythmic (amiodarone or propafenone) | 2.4 | 2.3 | 0.01 | 2.4 | 2.4 | <0.01 |
| Warfarin | 12.0 | 12.4 | 0.01 | 12.4 | 12.8 | 0.01 |
| DOAC | 0.2 | 0.1 | 0.01 | 0.1 | 0.1 | <0.01 |
| Antiplatelets (without ASA) | 7.2 | 8.1 | 0.04 | 8.0 | 8.0 | <0.01 |
| Low-dose ASA | 44.0 | 47.1 | 0.06 | 45.7 | 46.3 | 0.01 |
| Antidiabetics | ||||||
| Metformin | 13.1 | 12.9 | 0.01 | 12.9 | 13.0 | <0.01 |
| Sulfonylurea | 8.9 | 8.9 | <0.01 | 8.9 | 9.1 | 0.01 |
| Thiazolidinediones | 1.7 | 1.7 | 0.01 | 1.7 | 1.7 | <0.01 |
| DPP-4 inhibitors | 0.2 | 0.2 | <0.01 | 0.2 | 0.2 | <0.01 |
| SGLT2 inhibitors | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Insulins | 3.7 | 3.7 | <0.01 | 3.8 | 4.1 | 0.01 |
| Other medications | ||||||
| Proton pump inhibitors | 42.4 | 37.9 | 0.09 | 40.6 | 41.0 | 0.01 |
| Antidepressant agents | 29.1 | 38.7 | 0.21 | 34.5 | 35.0 | 0.01 |
| Anticholinergics agents | 4.5 | 3.7 | 0.04 | 4.2 | 4.3 | 0.01 |
| Benzodiazepine | 49.6 | 52.6 | 0.06 | 51.4 | 52.2 | 0.02 |
| Polypharmacy (≥10 medications) | 56.5 | 54.0 | 0.05 | 55.9 | 56.8 | 0.02 |
| Health care services in 1-year prior cohort entry | ||||||
| Number of visit medicals, mean (SD) * | 11.5 (11.2) | 8.4 (9.7) | 0.30 | 10.0 (10.0) | 11.0 (17.6) | 0.07 |
| Emergency visit, mean (SD) * | 2.0 (2.7) | 2.1 (3.0) | 0.02 | 2.1 (2.9) | 2.2 (2.9) | 0.01 |
| Hospitalization (%) | 60.8 | 53.7 | 0.14 | 57.6 | 57.4 | <0.01 |
| CVD/CEV Outcomes | Mortality | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Total cohort | Adherence ≥ 60% (ref.: <60%) of total antipsychotics | 0.92 (0.89–0.96) | <0.0001 | 0.96 (0.94–0.98) | 0.0005 |
| Adherence ≥ 60% (ref.: <60%) of atypical antipsychotics | 0.93 (0.90–0.96) | <0.0001 | 0.74 (0.72–0.75) | <0.0001 | |
| Adherence ≥ 60% (ref.: <60%) of typical antipsychotics | 0.96 (0.87–1.06) | 0.3680 | 3.35 (3.22–3.50) | <0.0001 | |
| Sub-cohort without schizophrenia/dementia | Adherence ≥ 60% (ref.: <60%) of total antipsychotics | 0.94 (0.90–0.99) | 0.0086 | 0.98 (0.94–1.01) | 0.1272 |
| Adherence ≥ 60% (ref.: <60%) of atypical antipsychotics | 0.97 (0.92–1.01) | 0.1239 | 0.67 (0.65–0.70) | <0.0001 | |
| Adherence ≥ 60% (ref.: <60%) of typical antipsychotics | 0.79 (0.69–0.91) | 0.0007 | 3.58 (3.40–3.76) | <0.0001 | |
| Sub-cohort of patients with schizophrenia | Adherence ≥ 60% (ref.: <60%) of total antipsychotics | 1.37 (1.06–1.77) | 0.0165 | 0.92 (0.74–1.15) | 0.4698 |
| Adherence ≥ 60% (ref.: <60%) of atypical antipsychotics | 1.33 (1.04–1.70) | 0.0233 | 0.88 (0.71–1.09) | 0.2320 | |
| Adherence ≥ 60% (ref.: <60%) of typical antipsychotics | 0.89 (0.49–1.62) | 0.7053 | 1.25 (0.77–2.02) | 0.3695 | |
| Sub-cohort of patients with dementia | Adherence ≥ 60% (ref.: <60%) of total antipsychotics | 0.89 (0.83–0.94) | <0.0001 | 0.98 (0.94–1.02) | 0.3141 |
| Adherence ≥ 60% (ref.: <60%) of atypical antipsychotics | 0.87 (0.84–0.91) | <0.0001 | 0.88 (0.84–0.91) | <0.0001 | |
| Adherence ≥ 60% (ref.: <60%) of typical antipsychotics | 1.25 (1.04–1.51) | 0.0153 | 2.45 (2.23–2.69) | <0.0001 | |
| HR (95% CI) | p-Value | ||
|---|---|---|---|
| Total cohort | Users of high dose vs. low dose of typical antipsychotic agents (≥10 mg vs. <10 mg Eq-Olan) * | 1.67 (1.59–1.74) | <0.0001 |
| Sub-cohort without schizophrenia/dementia | Users of high dose vs. low dose of typical antipsychotic agents (≥10 mg vs. <10 mg Eq-Olan) | 1.64 (1.56–1.73) | <0.0001 |
| Sub-cohort of patients with schizophrenia | Users of high dose vs. low dose of typical antipsychotic agents (≥10 mg vs. <10 mg Eq-Olan) | 1.24 (0.74–2.09) | 0.4142 |
| Sub-cohort of patients with dementia | Users of high dose vs. low dose of typical antipsychotic agents (≥10 mg vs. <10 mg Eq-Olan) | 1.69 (1.53–1.88) | <0.0001 |
| Incident Rate of ≥60% 100 PY (95% CI) | Incident Rate of <60 100 PY (95% CI) | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Glaucoma | 0.4 (0.3–0.6) | 0.3 (0.2–0.5) | 1.25 (0.71–2.22) | 0.4410 |
| Hyperthyroidism | 0.08 (0.01–0.16) | 0.11 (0.03–0.20) | 0.72 (0.23–2.27) | 0.5754 |
| HR (95% CI) | E-Value Corresponding to the CI Bound Closest to 1 | E-Value for HR Point Estimate | |||
|---|---|---|---|---|---|
| Total cohort | CVD/CEV risk | Atypical antipsychotics | 0.93 (0.90–0.96) | 1.25 | 1.36 |
| Mortality risk | Typical antipsychotics | 3.55 (3.39–3.71) | 6.24 | 6.56 | |
| Atypical antipsychotics | 0.67 (0.65–0.69) | 2.26 | 2.35 | ||
| Sub-cohort without schizophrenia/dementia | CVD/CEV risk | Typical antipsychotics | 0.79 (0.69–0.91) | 1.43 | 1.85 |
| Mortality risk | Typical antipsychotics | 3.58 (3.40–3.76) | 6.26 | 6.62 | |
| Atypical antipsychotics | 0.67 (0.65–0.70) | 2.21 | 2.35 | ||
| Sub-cohort with schizophrenia | CVD/CEV risk | Atypical antipsychotics | 1.33 (1.04–1.70) | 1.24 | 1.99 |
| Sub-cohort with dementia | CVD/CEV risk | Typical antipsychotics | 1.25 (1.04–1.51) | 1.24 | 1.81 |
| Atypical antipsychotics | 0.87 (0.82–0.92) | 1.39 | 1.56 | ||
| Mortality risk | Typical antipsychotics | 2.45 (2.23–2.69) | 3.89 | 4.33 | |
| Atypical antipsychotics | 0.88 (0.84–0.71) | 1.67 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perreault, S.; Boivin Proulx, L.-A.; Brouillette, J.; Jarry, S.; Dorais, M. Antipsychotics and Risks of Cardiovascular and Cerebrovascular Diseases and Mortality in Dwelling Community Older Adults. Pharmaceuticals 2024, 17, 178. https://doi.org/10.3390/ph17020178
Perreault S, Boivin Proulx L-A, Brouillette J, Jarry S, Dorais M. Antipsychotics and Risks of Cardiovascular and Cerebrovascular Diseases and Mortality in Dwelling Community Older Adults. Pharmaceuticals. 2024; 17(2):178. https://doi.org/10.3390/ph17020178
Chicago/Turabian StylePerreault, Sylvie, Laurie-Anne Boivin Proulx, Judith Brouillette, Stéphanie Jarry, and Marc Dorais. 2024. "Antipsychotics and Risks of Cardiovascular and Cerebrovascular Diseases and Mortality in Dwelling Community Older Adults" Pharmaceuticals 17, no. 2: 178. https://doi.org/10.3390/ph17020178
APA StylePerreault, S., Boivin Proulx, L.-A., Brouillette, J., Jarry, S., & Dorais, M. (2024). Antipsychotics and Risks of Cardiovascular and Cerebrovascular Diseases and Mortality in Dwelling Community Older Adults. Pharmaceuticals, 17(2), 178. https://doi.org/10.3390/ph17020178





