Inhibitory Effect of Luteolin on Spike S1 Glycoprotein-Induced Inflammation in THP-1 Cells via the ER Stress-Inducing Calcium/CHOP/MAPK Pathway
Abstract
1. Introduction
2. Results
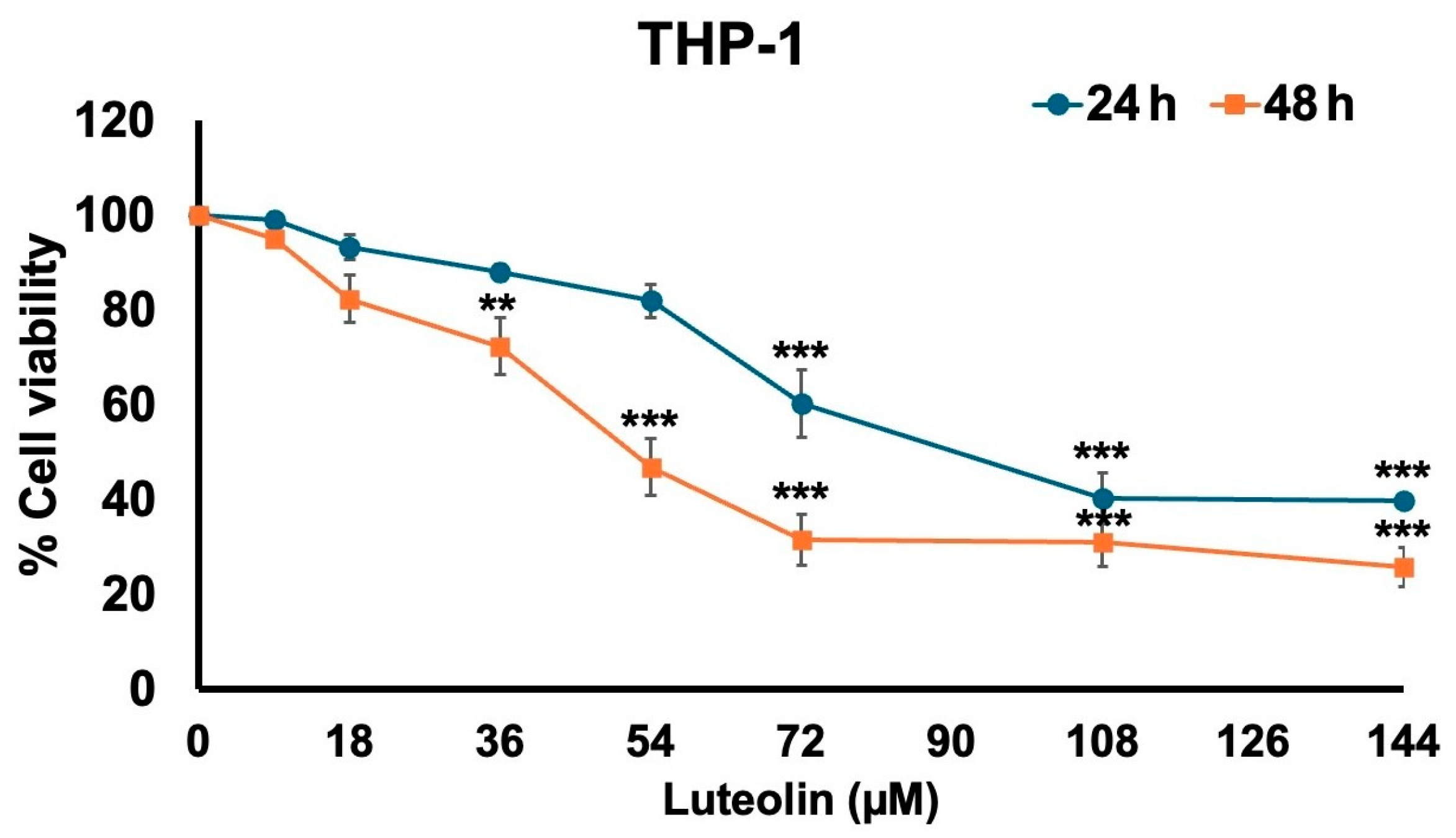
2.1. Effect of Luteolin on THP-1 Cell Viability
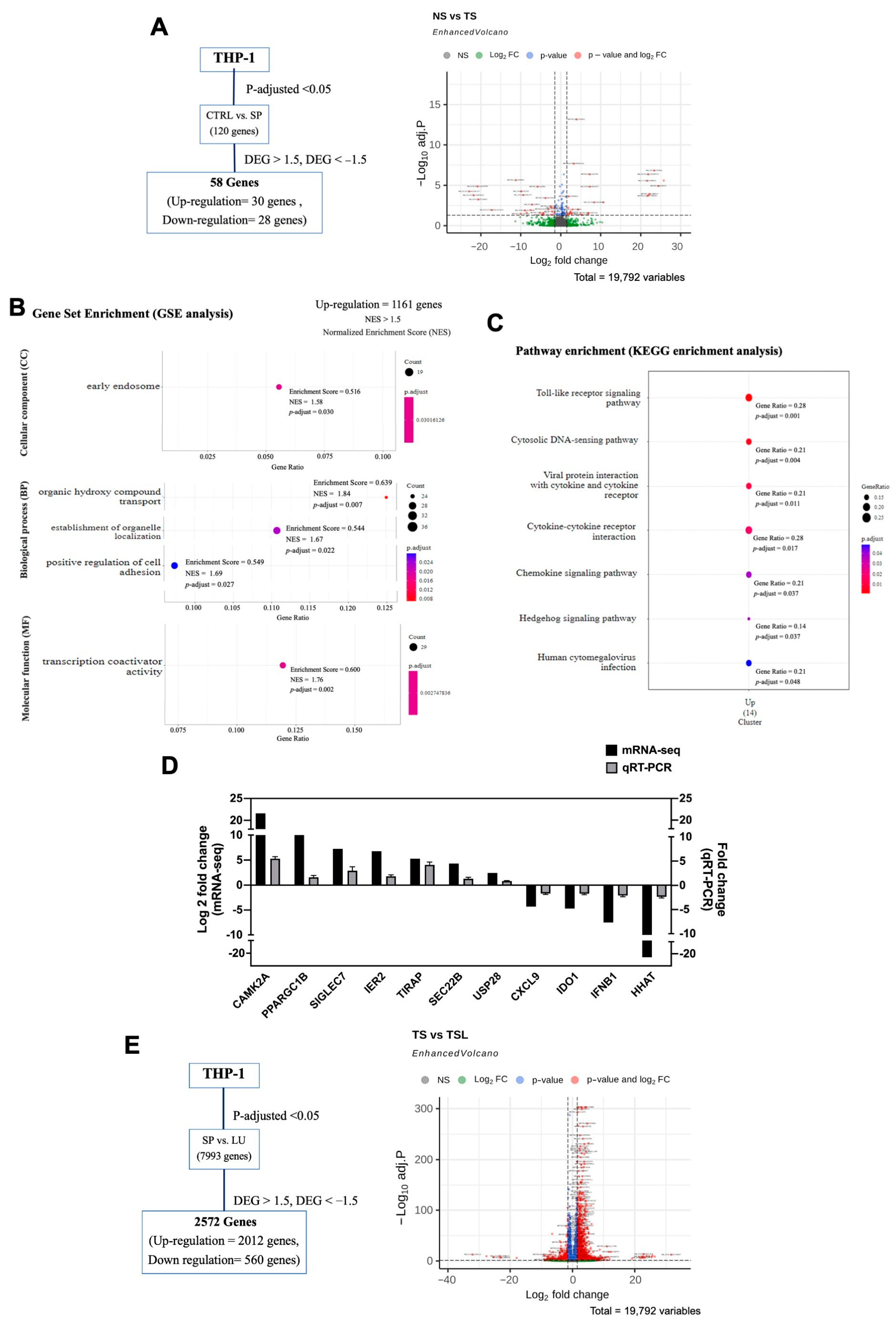
2.2. Differential Expression Analysis of Untreated THP-1 Cells and SARS-CoV-2 Spike S1-Induced THP-1
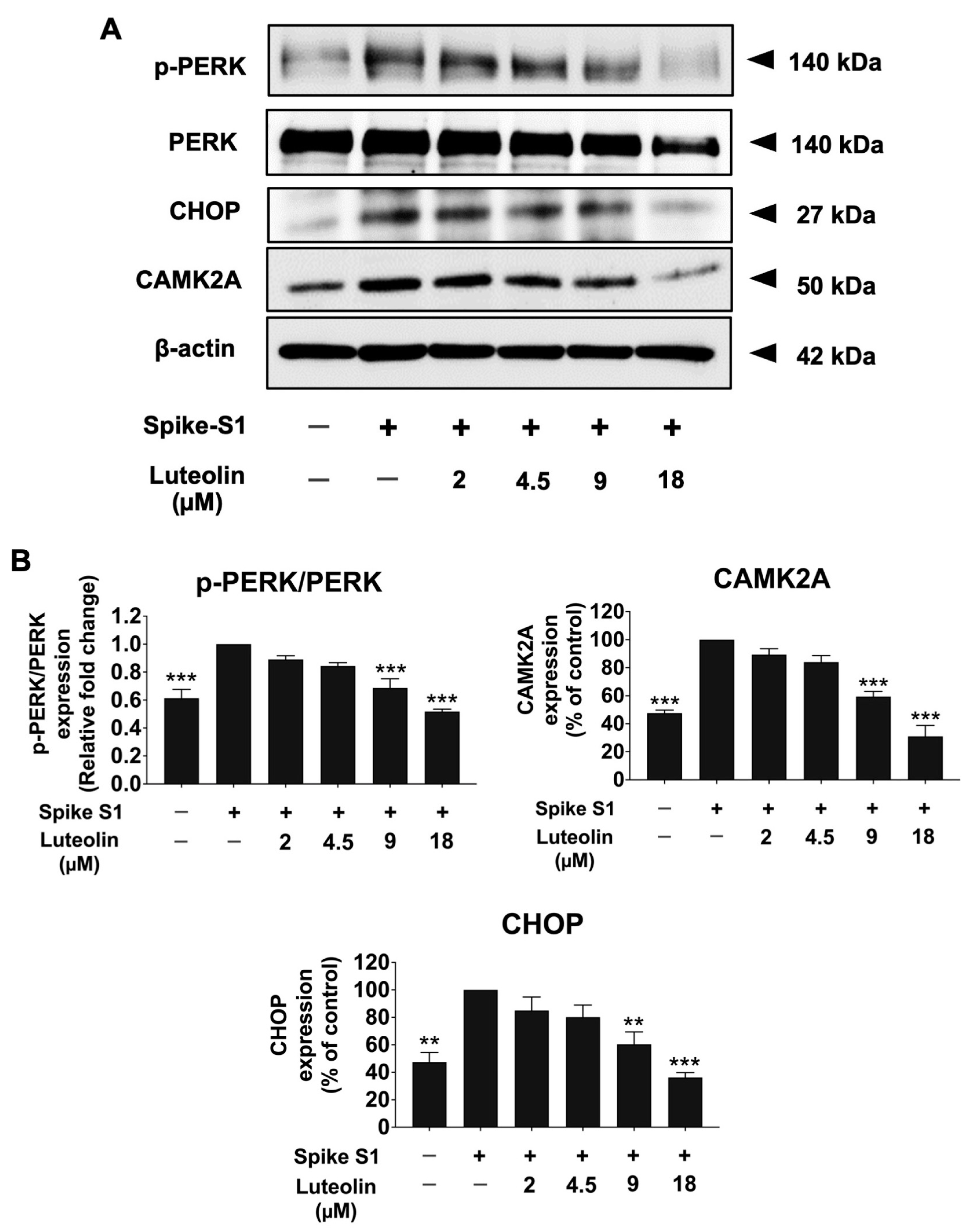
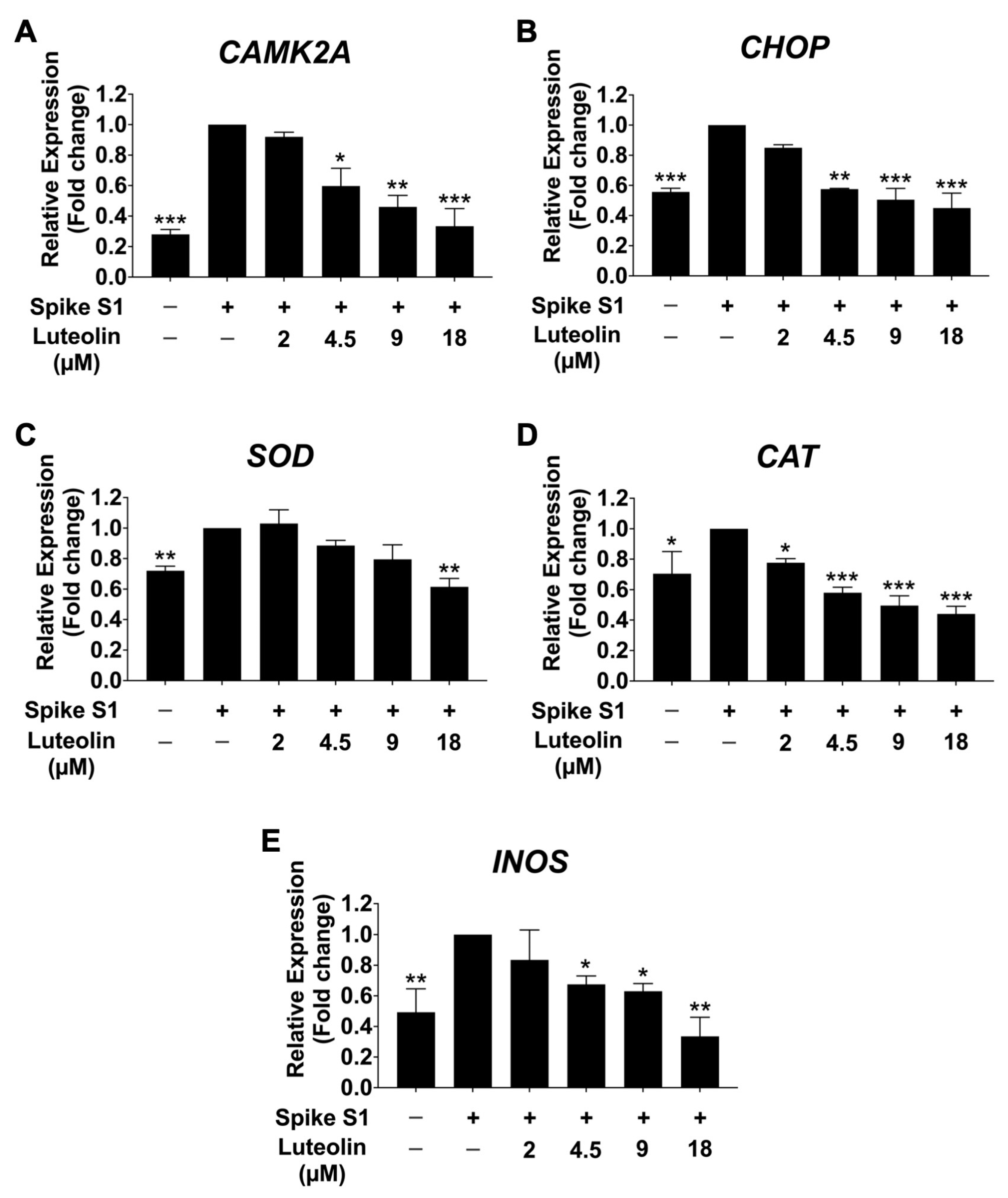
2.3. The Effect of Luteolin on the Expression of ER Stress Markers (CAMK2A/CHOP) in SARS-CoV-2 Spike S1-Induced THP-1 Cells
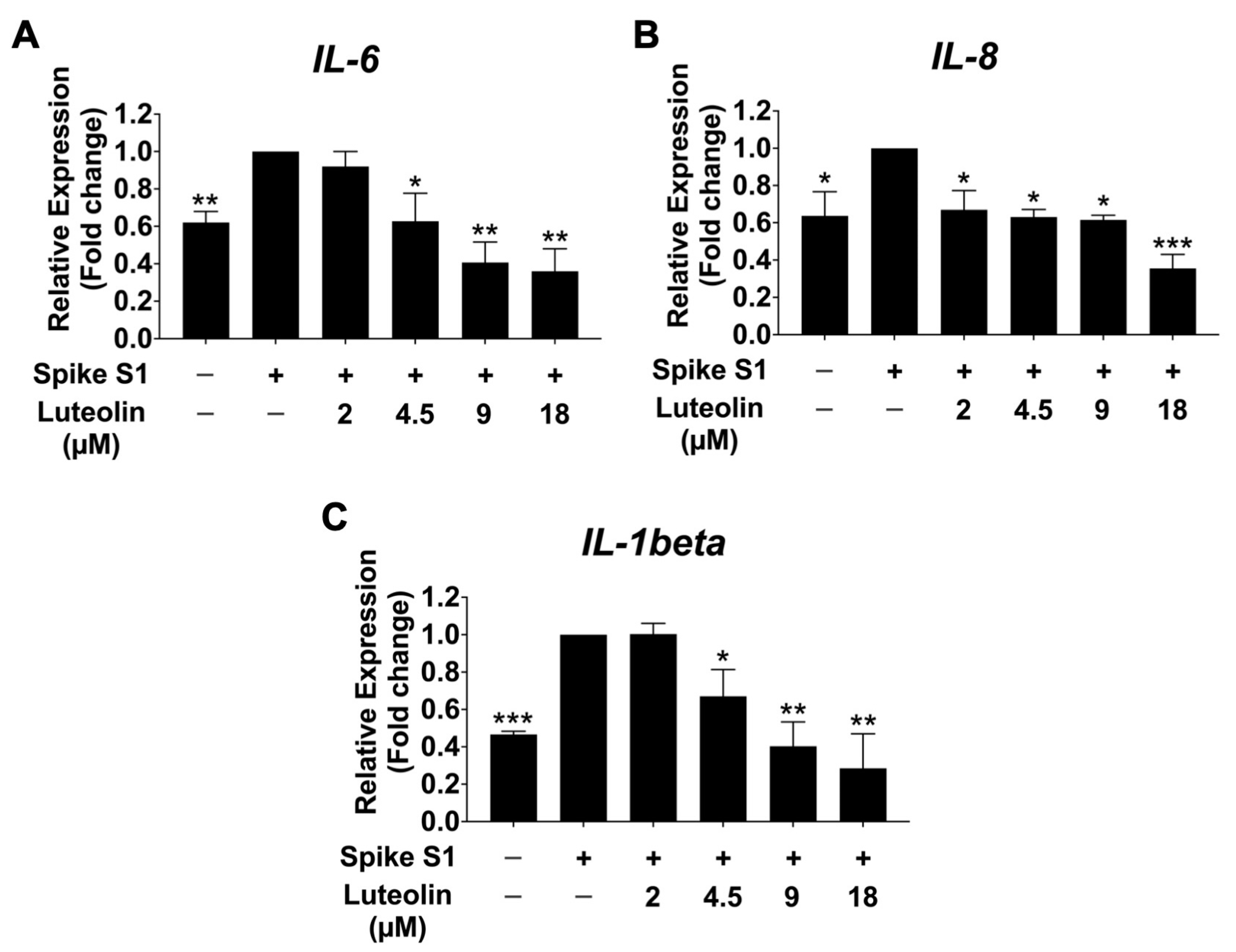
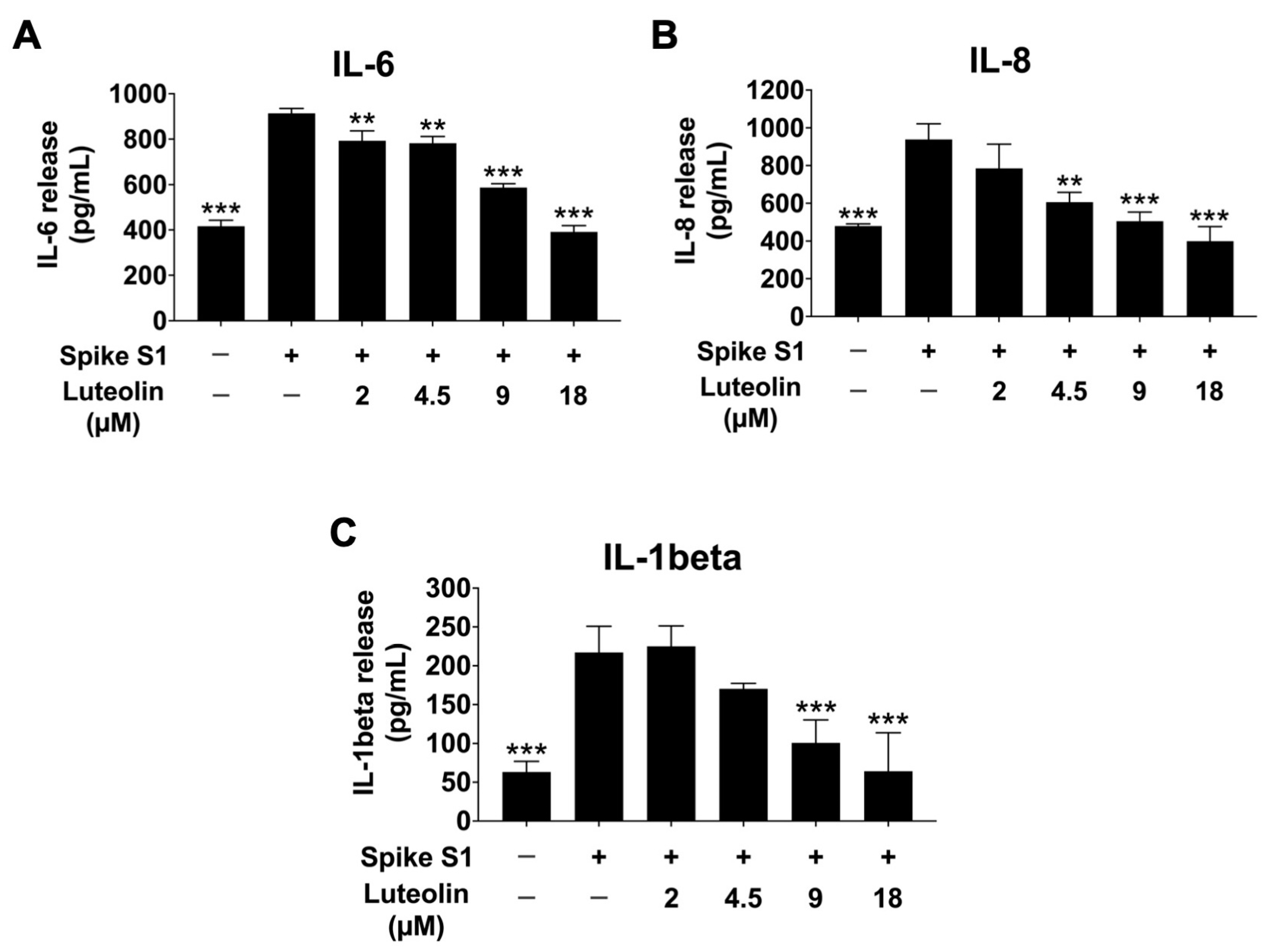
2.4. The Effect of Luteolin on Inflammatory Cytokine Release in SARS-CoV-2 Spike S1-Induced THP-1 Cells
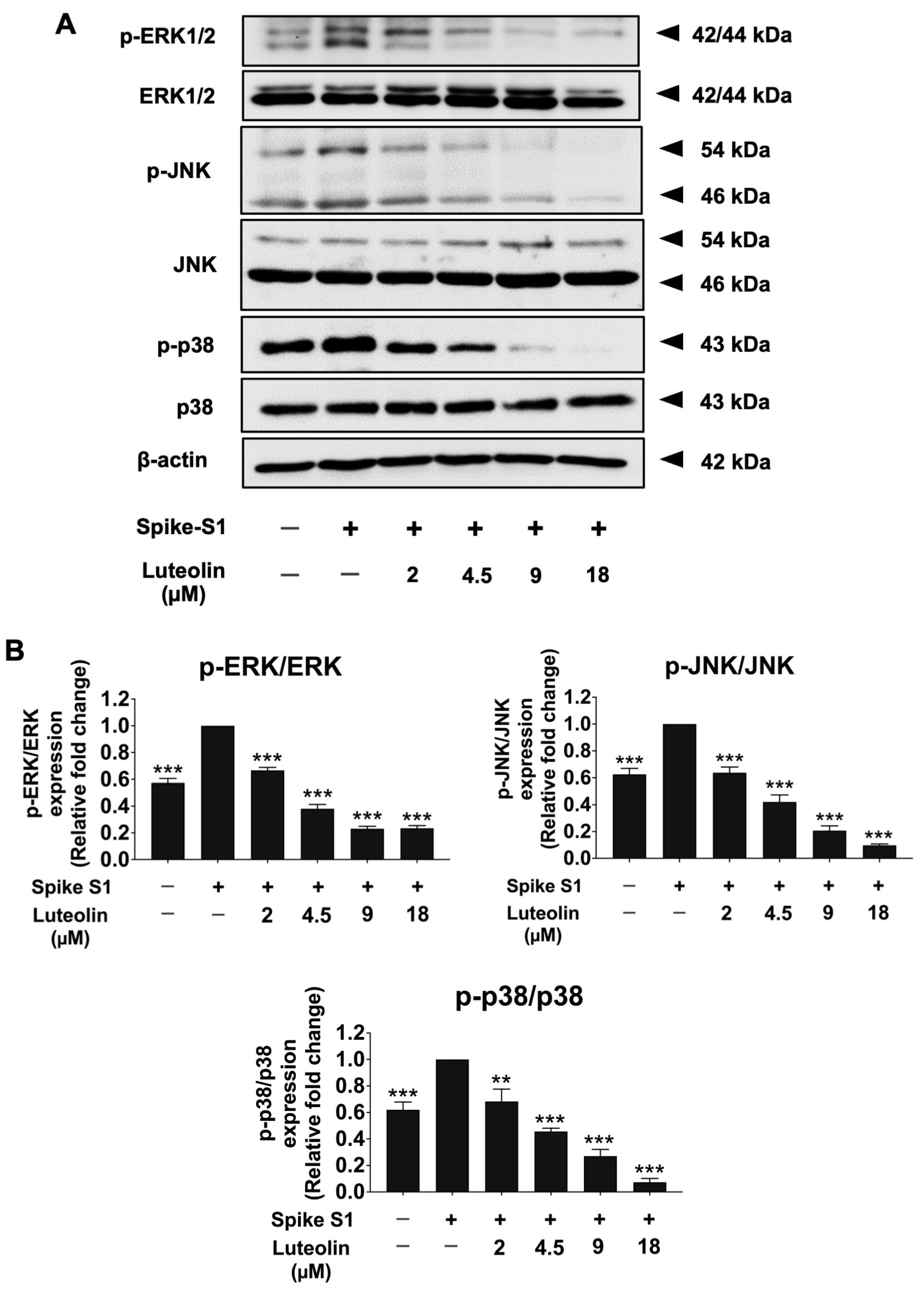
2.5. The Effect of Luteolin on MAPK Signaling Pathway in SARS-CoV-2 Spike S1-Induced THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. Total RNA Isolation and Expression Analysis
4.5. mRNA Sequencing Data Analysis
4.6. Determination of Gene Expressions by RT-qPCR Analysis
4.7. Determination of Cytokine Release
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Darif, D.; Hammi, I.; Kihel, A.; Saik, I.E.I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462. [Google Scholar] [CrossRef]
- Qudus, M.S.; Tian, M.; Sirajuddin, S.; Liu, S.; Afaq, U.; Wali, M.; Liu, J.; Pan, P.; Luo, Z.; Zhang, Q. The roles of critical pro-inflammatory cytokines in the drive of cytokine storm during SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28751. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Holt, M.R.; Heads, R.J. SARS-CoV-2 Spike S1 glycoprotein is a TLR4 agonist, upregulates ACE2 expression and induces pro-inflammatory M1 macrophage polarisation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Lepiarz-Raba, I.; Al-Hindawi, A.A. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation 2021, 44, 1865–1877. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 spike protein promotes MAPK and NF-kB activation in human lung cells and inflammatory cytokine production in human lung and intestinal epithelial cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar] [PubMed]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation 2020, 43, 95–108. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.-S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Peng, W.-H.; Tsai, K.-D.; Hsu, S.-L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of flavonoids in management of inflammatory disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–322. [Google Scholar]
- Zhu, J.; Yan, H.; Shi, M.; Zhang, M.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L. Luteolin inhibits spike protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) binding to angiotensin-converting enzyme 2. Phytother. Res. 2023, 37, 3508–3521. [Google Scholar] [CrossRef]
- Munafò, F.; Donati, E.; Brindani, N.; Ottonello, G.; Armirotti, A.; De Vivo, M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci. Rep. 2022, 12, 10571. [Google Scholar] [CrossRef]
- Bracken, C.; Beauverger, P.; Duclos, O.; Russo, R.J.; Rogers, K.A.; Husson, H.; Natoli, T.A.; Ledbetter, S.R.; Janiak, P.; Ibraghimov-Beskrovnaya, O. CaMKII as a pathological mediator of ER stress, oxidative stress, and mitochondrial dysfunction in a murine model of nephronophthisis. Am. J. Physiol. Ren. Physiol. 2016, 310, F1414–F1422. [Google Scholar] [CrossRef]
- Cai, B.; Kasikara, C.; Doran, A.C.; Ramakrishnan, R.; Birge, R.B.; Tabas, I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal. 2018, 11, eaar3721. [Google Scholar] [CrossRef]
- Racioppi, L.; Noeldner, P.K.; Lin, F.; Arvai, S.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J. Biol. Chem. 2012, 287, 11579–11591. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Gallenga, C.; Ross, R.; Kritas, S.; Frydas, I.; Younes, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar] [PubMed]
- Kosyreva, A.; Dzhalilova, D.; Lokhonina, A.; Vishnyakova, P.; Fatkhudinov, T. The role of macrophages in the pathogenesis of SARS-CoV-2-associated acute respiratory distress syndrome. Front. Immunol. 2021, 12, 682871. [Google Scholar] [CrossRef]
- Chiok, K.; Hutchison, K.; Miller, L.G.; Bose, S.; Miura, T.A. Proinflammatory Responses in SARS-CoV-2 and Soluble Spike Glycoprotein S1 Subunit Activated Human Macrophages. Viruses 2023, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Albarakati, A.J.A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Abdel Moneim, A.E. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 2020, 47, 2591–2603. [Google Scholar] [CrossRef]
- Kim, H.-J.; Khan, I.; Shahidullah, A.; Halimi, S.M.A.; Rauf, A.; Lee, J.-Y.; Kim, Y.-J.; Kim, B.-Y.; Park, W. Diospyrin modulates inflammation in poly I: C-induced macrophages via ER stress-induced calcium-CHOP pathway. Processes 2020, 8, 1050. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, J.-Y.; Kim, Y.-J.; Kim, H.-J.; Park, W. Rubi Fructus water extract alleviates lps-stimulated macrophage activation via an er stress-induced calcium/CHOP signaling pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef]
- Kou, J.-j.; Shi, J.-z.; He, Y.-y.; Hao, J.-j.; Zhang, H.-y.; Luo, D.-m.; Song, J.-k.; Yan, Y.; Xie, X.-m.; Du, G.-h. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Lo, C.W.; Lii, C.K.; Lin, K.S.; Li, C.C.; Liu, K.L.; Yang, Y.C.; Chen, H.W. Luteolin, apigenin, and chrysin inhibit lipotoxicity–induced NLRP3 inflammasome activation and autophagy damage in macrophages by suppressing endoplasmic reticulum stress. Environ. Toxicol. 2024, 39, 4120–4133. [Google Scholar] [CrossRef]
- Choi, E.Y.; Jin, J.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Effects of luteolin on the release of nitric oxide and interleukin-6 by macrophages stimulated with lipopolysaccharide from Prevotella intermedia. J. Periodontol. 2011, 82, 1509–1517. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kim, M.-Y.; Cho, J.Y. Immunopharmacological activities of luteolin in chronic diseases. Int. J. Mol. Sci. 2023, 24, 2136. [Google Scholar] [CrossRef]
- Xagorari, A.; Roussos, C.; Papapetropoulos, A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002, 136, 1058–1064. [Google Scholar] [CrossRef]
- Kuo, M.-Y.; Liao, M.-F.; Chen, F.-L.; Li, Y.-C.; Yang, M.-L.; Lin, R.-H.; Kuan, Y.-H. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFκB pathways in mice with endotoxin-induced acute lung injury. Food Chem. Toxicol. 2011, 49, 2660–2666. [Google Scholar] [CrossRef]
- Ansari, W.A.; Ahamad, T.; Khan, M.A.; Khan, Z.A.; Khan, M.F. Luteolin: A dietary molecule as potential anti-COVID-19 agent. Res. Sq. 2020, submitted. [CrossRef]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306. [Google Scholar] [CrossRef]
- Baxter, E.; Graham, A.; Re, N.; Carr, I.; Robinson, J.; Mackie, S.; Morgan, A. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M (IFNγ+ LPS), M (IL-4) and M (IL-10) phenotypes. J. Immunol. Methods 2020, 478, 112721. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-P.; Wang, H.-Z. A TETRAZOLIUM-BASED (MTT) ASSAY FOR MEASURING. Kaohsiung J. Med. Sci. 1993, 9, 610–615. [Google Scholar]
- Chumphukam, O.; Pintha, K.; Khanaree, C.; Chewonarin, T.; Chaiwangyen, W.; Tantipaiboonwong, P.; Suttajit, M.; Khantamat, O. Potential anti-mutagenicity, antioxidant, and anti-inflammatory capacities of the extract from perilla seed meal. J. Food Biochem. 2018, 42, e12556. [Google Scholar] [CrossRef]
- Lu, M.; Berglund, E.; Larsson, C.; Höög, A.; Farnebo, L.-O.; Bränström, R. Calmodulin and calmodulin-dependent protein kinase II inhibit hormone secretion in human parathyroid adenoma. J. Endocrinol. 2011, 208, 31. [Google Scholar] [CrossRef] [PubMed]
- Zaima, N.; Sugawara, T.; Goto, D.; Hirata, T. Trans geometric isomers of EPA decrease LXRα-induced cellular triacylglycerol via suppression of SREBP-1c and PGC-1β. J. Lipid Res. 2006, 47, 2712–2717. [Google Scholar] [CrossRef]
- Dharmadhikari, G.; Stolz, K.; Hauke, M.; Morgan, N.G.; Varki, A.; De Koning, E.; Kelm, S.; Maedler, K. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci. Rep. 2017, 7, 45319. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, L.; Wu, W.; Liao, Y.; Zhang, W.; Deng, Z.; Shen, J.; Yuan, Q.; Zheng, L.; Zhang, Y. Immediate early response protein 2 regulates hepatocellular carcinoma cell adhesion and motility via integrin β1-mediated signaling pathway. Oncol. Rep. 2017, 37, 259–272. [Google Scholar] [CrossRef]
- Dissanayeke, S.R.; Levin, S.; Pienaar, S.; Wood, K.; Eley, B.; Beatty, D.; Henderson, H.; Anderson, S.; Levin, M. Polymorphic variation in TIRAP is not associated with susceptibility to childhood TB but may determine susceptibility to TBM in some ethnic groups. PLoS ONE 2009, 4, e6698. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gu, B.; Zhang, Q.; Fu, G.; Wu, J.; Han, Z.; Cao, W.; Zou, J.; Mao, M.; Liu, J. Application of radiation hybrid in gene mapping. Sci. China Ser. C Life Sci. 1998, 41, 644–649. [Google Scholar] [CrossRef]
- Diefenbacher, M.E.; Popov, N.; Blake, S.M.; Schülein-Völk, C.; Nye, E.; Spencer-Dene, B.; Jaenicke, L.A.; Eilers, M.; Behrens, A. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J. Clin. Investig. 2014, 124, 3407–3418. [Google Scholar] [CrossRef]
- Matevossian, A.; Resh, M.D. Hedgehog Acyltransferase as a target in estrogen receptor positive, HER2 amplified, and tamoxifen resistant breast cancer cells. Mol. Cancer 2015, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Liu, L.-J.; Zhong, B.; Liu, T.-T.; Li, Y.; Yang, Y.; Ran, Y.; Li, S.; Tien, P.; Shu, H.-B. WDR5 is essential for assembly of the VISA-associated signaling complex and virus-triggered IRF3 and NF-κB activation. Proc. Natl. Acad. Sci. USA 2010, 107, 815–820. [Google Scholar] [CrossRef]
- Napolioni, V.; Pariano, M.; Borghi, M.; Oikonomou, V.; Galosi, C.; De Luca, A.; Stincardini, C.; Vacca, C.; Renga, G.; Lucidi, V. Genetic polymorphisms affecting IDO1 or IDO2 activity differently associate with aspergillosis in humans. Front. Immunol. 2019, 10, 890. [Google Scholar] [CrossRef]
- Liang, Y.-j.; Luo, J.; Lu, Q.; Zhou, Y.; Wu, H.-w.; Zheng, D.; Ren, Y.-y.; Sun, K.-y.; Wang, Y.; Zhang, Z.-s. Gene profile of chemokines on hepatic stellate cells of schistosome-infected mice and antifibrotic roles of CXCL9/10 on liver non-parenchymal cells. PLoS ONE 2012, 7, e42490. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lee, K.-T.; Lim, M.C.; Choi, J.-H. TRPV1 antagonist DWP05195 induces ER stress-dependent apoptosis through the ROS-p38-CHOP pathway in human ovarian cancer cells. Cancers 2020, 12, 1702. [Google Scholar] [CrossRef]
- Hannon, D.B.; Thompson, J.T.; Khoo, C.; Juturu, V.; Vanden Heuvel, J.P. Effects of cranberry extracts on gene expression in THP-1 cells. Food Sci. Nutr. 2017, 5, 148–159. [Google Scholar] [CrossRef]
- Wang, H.-w.; Wu, T.; Qi, J.-y.; Wang, Y.-q.; Luo, X.-p.; Ning, Q. Salidroside attenuates LPS-stimulated activation of THP-1 cell-derived macrophages through down-regulation of MAPK/NF-kB signaling pathways. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2013, 33, 463–469. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef]
| Gene Name | Log2 Fold Change | p-adj | ||
|---|---|---|---|---|
| NS vs. TS Gene | TS vs. TSL Gene | NS vs. TS Gene | TS vs. TSL Gene | |
| NM_171825|CAMK2A | 21.61 | −23.36 | 2.7 × 10−6 | 4.9 × 10−10 |
| NM_001172698|PPARGC1B | 10.69 | −8.11 | 1.3 × 10−3 | 1.3 × 10−3 |
| NM_016543|SIGLEC7 | 7.25 | −7.76 | 1.5 × 10−5 | 8.4 × 10−9 |
| MSTRG.2975|USP28 | 6.76 | −5.39 | 3.1 × 10−2 | 8.2 × 10−3 |
| MSTRG.7674|IER2 | 5.29 | −5.82 | 4.5 × 10−2 | 3.2 × 10−4 |
| MSTRG.3069|TIRAP | 4.30 | −3.34 | 1.0 × 10−2 | 4.6 × 10−3 |
| MSTRG.904|SEC22B | 2.44 | −1.77 | 1.6 × 10−2 | 1.2 × 10−2 |
| NM_001170587|HHAT | −20.65 | 31.70 | 0.01 | 7.4 × 10−13 |
| MSTRG.15555|IFNB1 | −7.50 | 3.06 | 0.01 | 1.4 × 10−1 |
| MSTRG.15072|IDO1 | −4.32 | 5.66 | 0.03 | 5.7 × 10−6 |
| MSTRG.11698|CXCL9 | −4.72 | 7.08 | 0.04 | 5.6 × 10−7 |
| Gene Product | Primer Sequences | References |
|---|---|---|
| CAMK2A | Forward: 5′-ACC AGC TCT TCG AGG AAT TG-3′ Reverse: 5′-GTG ACC AGG TCG AAG ATC AG-3′ | [42] |
| PPARGC1B | Forward: 5′-ATG ACT CCG AGC TCT TCC AG-3′ Reverse: 5′-CGA AGC TGA GGT GCA TGA TA-3′ | [43] |
| SIGLEC7 | Forward: 5′-AAG AAG CCA CCA ACA ATG AG-3′ Reverse: 5′-CAG TTA GAC AAG AGG AAT AAG TTC-3′ | [44] |
| IER2 | Forward: 5′-CCA AAG TCA GCC GCA AAC GA-3′ Reverse: 5′-TTT CTT CCA GAC GGG CTT TCT TGC-3′ | [45] |
| TIRAP | Forward: 5′-CTC TGA GAA TAA GAT GTT TCC-3′ Reverse: 5′-ACG CAG ACG TCA TAG TCT TT-3′ | [46] |
| SEC22B | Forward: 5′-GGC CAA TAG ACG AGA TCT GT-3′ Reverse: 5′-CTT AGT CAA CCT GTG CCA GC-3′ | [47] |
| USP28 | Forward: 5′-CCG AAC AGT TCT GCG TGC T-3′ Reverse: 5′-CAC CGG CTG TGA AGC TGA-3′ | [48] |
| HHAT | Forward: 5′-GGG TGC TTG TTT CTG AGA TTT G-3′ Reverse: 5′-GGG TAC ACT ATC CTG TGG TTT C-3′ | [49] |
| IFNB1 | Forward: 5′-CAG CAA TTT TCA GTG TCA GCA AGC T-3′ Reverse: 5′-TCA TCC TGT CCT TGA GGC AGT AT-3′ | [50] |
| IDO1 | Forward: 5′-TCA CAG ACC ACA AGT CAC AG-3′ Reverse: 5′-GCA AGA CCT TAC GGA CAT CT-3′ | [51] |
| CXCL9 | Forward: 5′-CCA GTA GTG AGA AAG GGT CGC-3′ Reverse: 5′-TGG GGC AAA TTG TTT AAG GTC TT-3′ | [52] |
| CHOP | Forward: 5′-TTG CCT TTC TCC TTC GGG AC-3′ Reverse: 5′-CAG TCA GCC AAG CCA GAG AA-3′ | [53] |
| SOD | Forward: 5′-GGT GTG GCC GAT GTG TCT AT-3′ Reverse:: 5′-CCT TTG CCC AAG TCA TCT GC-3′ | [54] |
| CAT | Forward: 5′-TGT TGC TGG AGA ATC GGG TTC-3′ Reverse: 5′-TCC CAG TTA CCA TCT TCT GTG TA-3′ | [54] |
| INOS | Forward: 5′-TGA ACT ACG TCC TGT CCC CT-3′ Reverse: 5′-CTC TTC TCT TGG GTC TCC GC-3′ | [55] |
| GAPDH | Forward: 5′-TCA ACA GCG ACA CCC AC-3′ Reverse: 5′-GGG TCT CTC TCT TCC TCT TGT G-3′ | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umsumarng, S.; Dissook, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Sangphukieo, A.; Thongkumkoon, P.; Dejkriengkraikul, P. Inhibitory Effect of Luteolin on Spike S1 Glycoprotein-Induced Inflammation in THP-1 Cells via the ER Stress-Inducing Calcium/CHOP/MAPK Pathway. Pharmaceuticals 2024, 17, 1402. https://doi.org/10.3390/ph17101402
Umsumarng S, Dissook S, Arjsri P, Srisawad K, Thippraphan P, Sangphukieo A, Thongkumkoon P, Dejkriengkraikul P. Inhibitory Effect of Luteolin on Spike S1 Glycoprotein-Induced Inflammation in THP-1 Cells via the ER Stress-Inducing Calcium/CHOP/MAPK Pathway. Pharmaceuticals. 2024; 17(10):1402. https://doi.org/10.3390/ph17101402
Chicago/Turabian StyleUmsumarng, Sonthaya, Sivamoke Dissook, Punnida Arjsri, Kamonwan Srisawad, Pilaiporn Thippraphan, Apiwat Sangphukieo, Patcharawadee Thongkumkoon, and Pornngarm Dejkriengkraikul. 2024. "Inhibitory Effect of Luteolin on Spike S1 Glycoprotein-Induced Inflammation in THP-1 Cells via the ER Stress-Inducing Calcium/CHOP/MAPK Pathway" Pharmaceuticals 17, no. 10: 1402. https://doi.org/10.3390/ph17101402
APA StyleUmsumarng, S., Dissook, S., Arjsri, P., Srisawad, K., Thippraphan, P., Sangphukieo, A., Thongkumkoon, P., & Dejkriengkraikul, P. (2024). Inhibitory Effect of Luteolin on Spike S1 Glycoprotein-Induced Inflammation in THP-1 Cells via the ER Stress-Inducing Calcium/CHOP/MAPK Pathway. Pharmaceuticals, 17(10), 1402. https://doi.org/10.3390/ph17101402










