Discovery and Development of Caffeic Acid Analogs as Versatile Therapeutic Agents
Abstract
1. Introduction
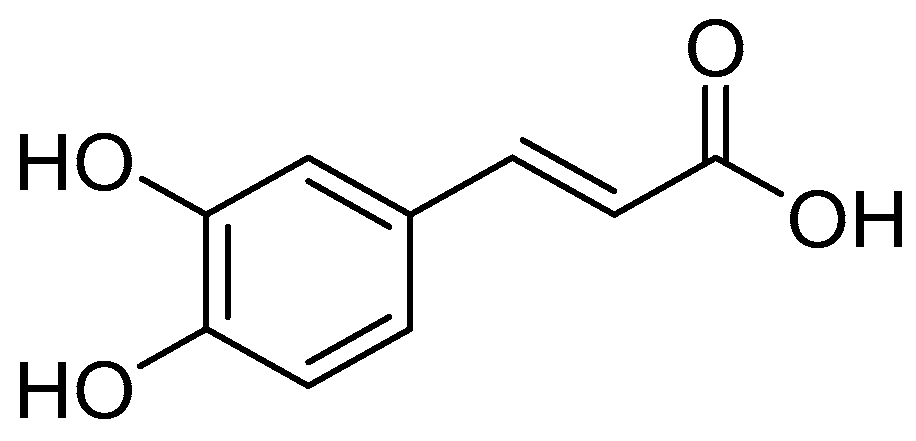
2. Caffeic Acid Derivatives
2.1. Caffeic Acid Ester Derivatives
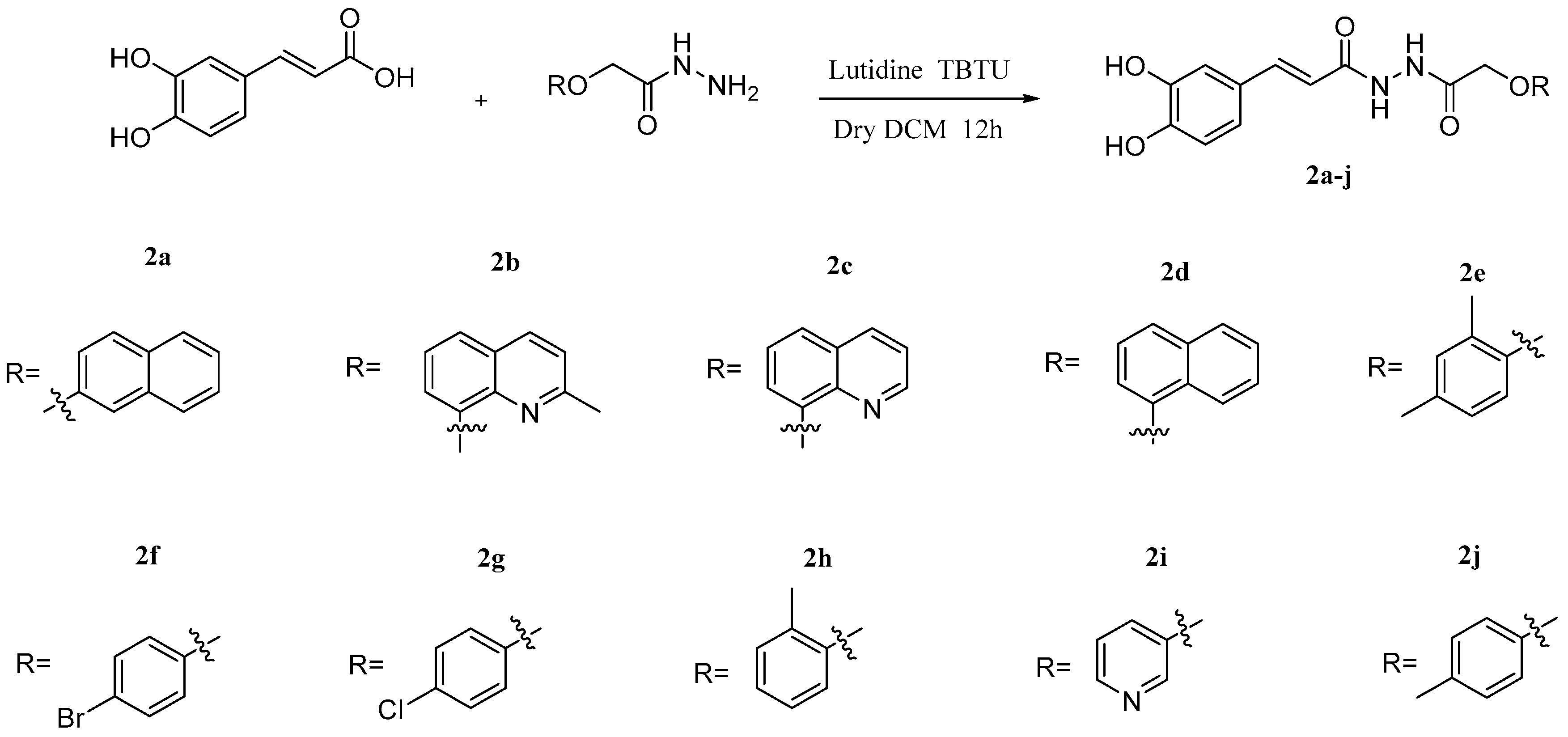
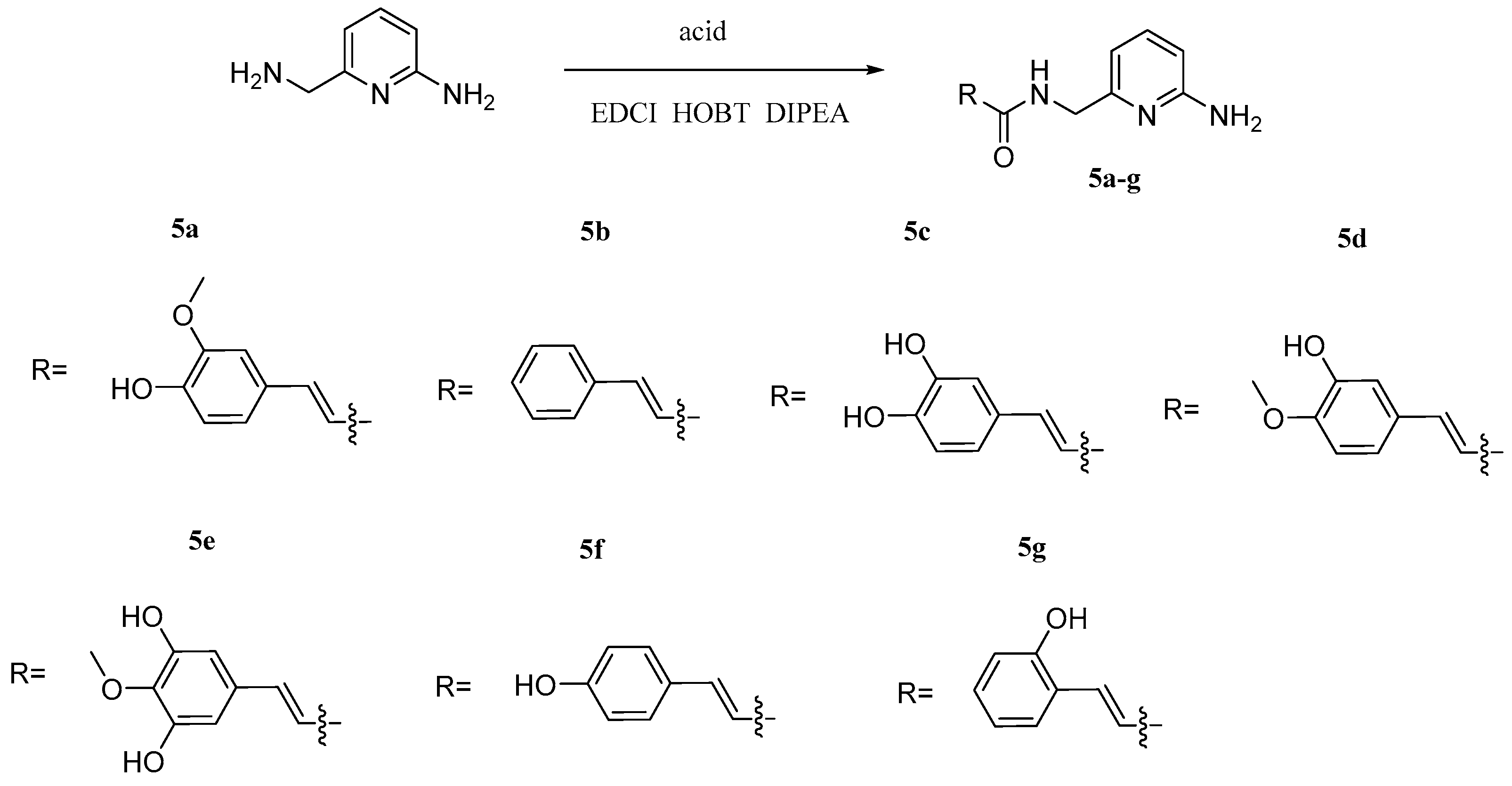
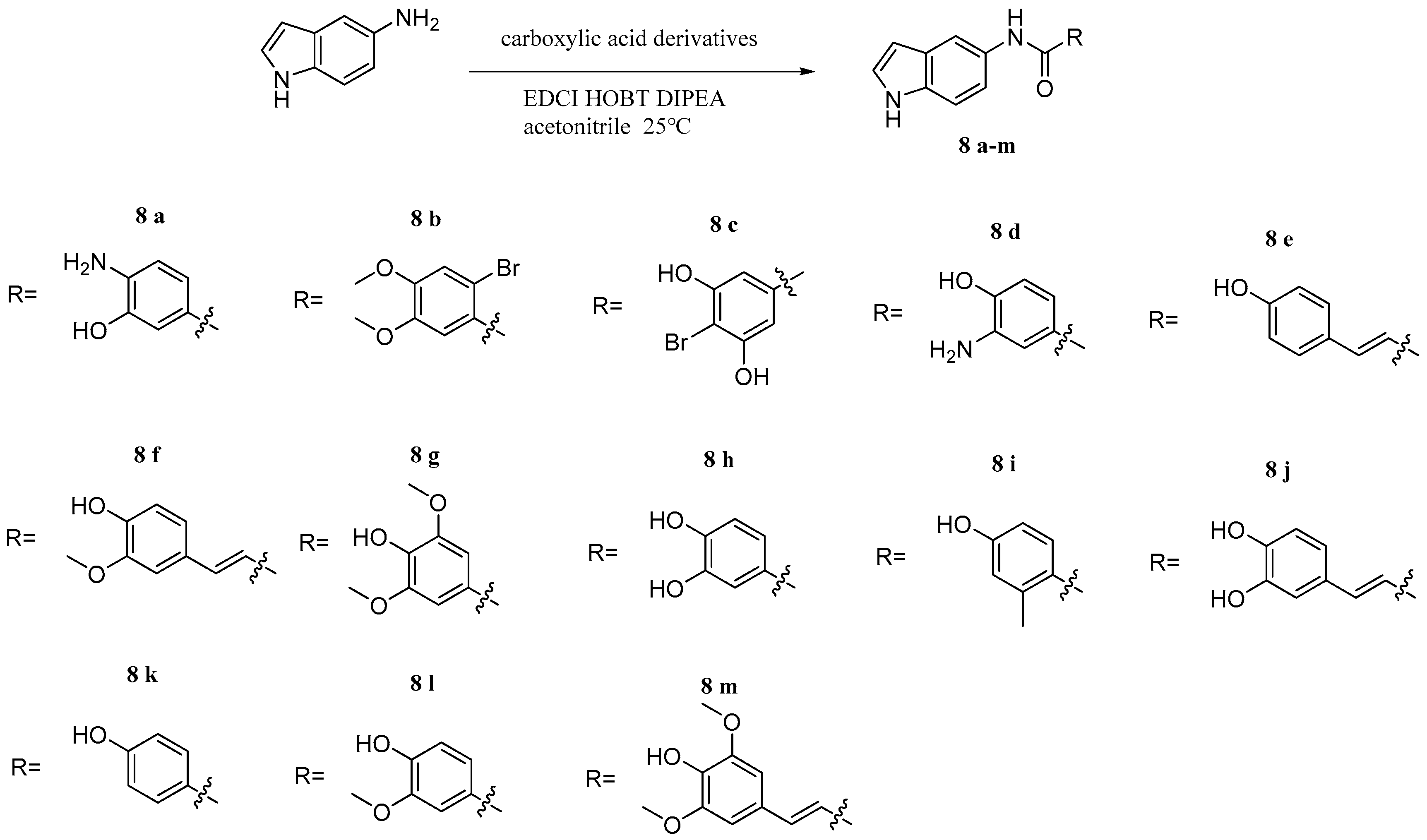
2.2. Caffeic Acid Amides Derivatives
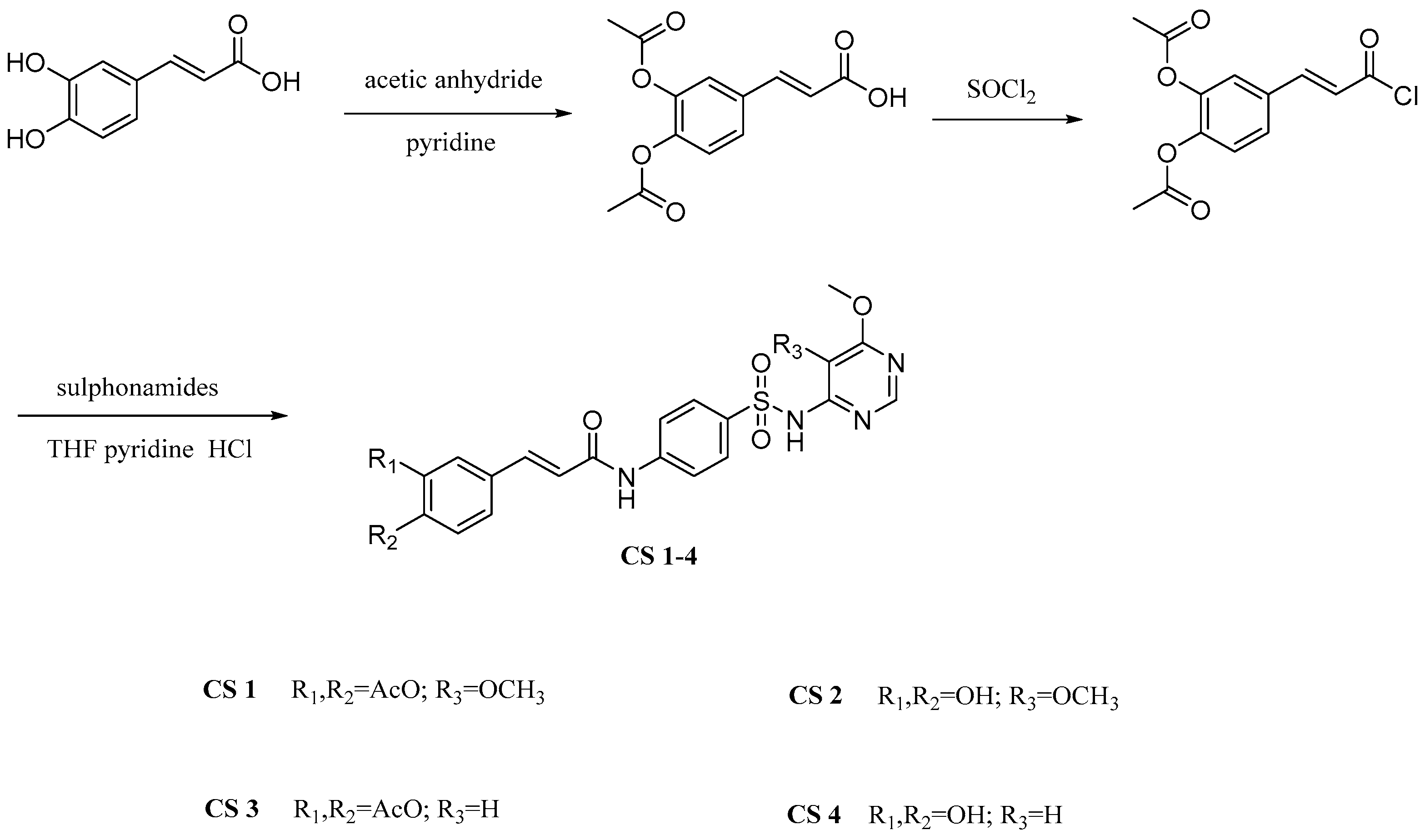
2.3. Caffeic Acid Hybrids
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavlikova, N. Caffeic Acid and Diseases-Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Lin, I.C.; Huang, K.C.; Chen, H.Y.; Ali, M.; Huang, Y.J.; Hsia, S.M. Caffeic acid’s role in mitigating polycystic ovary syndrome by countering apoptosis and ER stress triggered by oxidative stress. Biomed. Pharmacother. 2023, 166, 115327. [Google Scholar] [CrossRef]
- Sakae, K.; Nonaka, D.; Kishida, M.; Hirata, Y.; Fujiwara, R.; Kondo, A.; Noda, S.; Tanaka, T. Caffeic acid production from glucose using metabolically engineered Escherichia coli. Enzym. Microb. Technol. 2023, 164, 110193. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.W.; Liu, H.; Guo, J.; Long, S.H. Hybrid molecules based on caffeic acid as potential therapeutics: A focused review. Eur. J. Med. Chem. 2022, 243, 114745. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Hon, P.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Pechlivanova, D.; Rangelov, M.; Todorova, N.; Stoyanova, T.; Assenov, B.; Todorov, P. A novel cinnamic and caffeic acid-conjugated peptide analogs with anticonvulsant and analgesic potency: Comparative analyses of trans/cis isomers. Drug Dev. Res. 2024, 85, e22236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Q.; Hong, H.; Qian, Z.; Wan, B.; Xia, M. Caffeic acid phenethyl ester inhibits neuro-inflammation and oxidative stress following spinal cord injury by mitigating mitochondrial dysfunction via the SIRT1/PGC1α/DRP1 signaling pathway. J. Transl. Med. 2024, 22, 304. [Google Scholar] [CrossRef]
- Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; Menezes, G.D.; da Silva, R.A.; Nebo, L.; da Silva, G.C.D.; Milhim, B.H.G.D.; Teixeira, I.D.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilheus Virus In Vitro. Viruses 2023, 15, 494. [Google Scholar] [CrossRef]
- Sun, R.; Wu, T.; Xing, S.; Wei, S.; Bielicki, J.K.; Pan, X.F.; Zhou, M.Y.; Chen, J.B. Caffeic acid protects against atherosclerotic lesions and cognitive decline in ApoE−/− mice. J. Pharmacol. Sci. 2023, 151, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, H.; Araya-Valdes, G.; Cortes, G.; Jara, J.A.; Catalan, M. Pharmacological Effects of Caffeic Acid and Its Derivatives in Cancer: New Targeted Compounds for the Mitochondria. Adv. Exp. Med. Biol. 2022, 1401, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Kostic, S.; Vilotic, A.; Pirkovic, A.; Dekanski, D.; Borozan, S.; Nacka-Aleksic, M.; Vrzic-Petronijevic, S.; Jovanovic Krivokuca, M. Caffeic acid protects human trophoblast HTR-8/SVneo cells from H2O2-induced oxidative stress and genotoxicity. Food Chem. Toxicol. 2022, 163, 112993. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Ma, S.; Kang, H.; Zhou, C.; Jin, L.; Zhang, X.; Zhang, Y.; Yuan, Y.; Shu, P. A Review of the Phytochemistry and Biological Activities of Echinopsis Radix. Molecules 2024, 29, 2267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sun, C.J.; Fan, R.Y.; Xu, Z.; Su, S.L.; Shang, E.X.; Zhang, W.; Duan, J.A. Comparative pharmacokinetic study on phenolic acids and flavonoids in normal and microcirculation dysfunction rats plasma by UPLC-TQ/MS/MS after oral administration of Salvia miltiorrhiza stem-leaf extracts. Heliyon 2024, 10, e30910. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Yuniarti, N.; Prasasty, V.D.; Mungkasi, S.; Waskitha, S.S.W.; Yanuar, M.R.S.; Riswanto, F.D.O. Caffeic Acid in Spent Coffee Grounds as a Dual Inhibitor for MMP-9 and DPP-4 Enzymes. Molecules 2023, 28, 7182. [Google Scholar] [CrossRef]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Novel Caffeic Acid–Zinc Acetate Complex: Studies on Promising Antidiabetic and Antioxidative Synergism Through Complexation. Med. Chem. 2023, 19, 147–162. [Google Scholar]
- Bassil, D.; Makris, D.P.; Kefalas, P. Oxidation of caffeic acid in the presence of L-cysteine: Isolation of 2-cysteinylcaffeic acid and evaluation of its antioxidant properties. Food Res. Int. 2005, 38, 395–402. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Mauricio, A.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Ou, T.T.; Wu, C.H.; Wang, C.J. Prevention of Diet-Induced Hyperlipidemia and Obesity by Caffeic Acid in C57BL/6 Mice through Regulation of Hepatic Lipogenesis Gene Expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef] [PubMed]
- Almajano, M.P.; Carbo, R.; Delgado, M.E.; Gordon, M.H. Effect of pH on the antimicrobial activity and oxidative stability of oil-in-water emulsions containing caffeic acid. J. Food Sci. 2007, 72, C258–C263. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chen, R.F.; Chen, J.Y.; Chu, Y.C.; Wang, H.M.; Chou, H.L.; Chang, W.C.; Fong, Y.; Chang, W.T.; Wu, C.Y.; et al. Protective effect of caffeic acid on paclitaxel induced anti-proliferation and apoptosis of lung cancer cells involves NF-κB pathway. Int. J. Mol. Sci. 2012, 13, 6236–6245. [Google Scholar] [CrossRef]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Fernandes, S.; Catarino, C.; Santos-Silva, A.; Paiva-Martins, F. Caffeic acid phenolipids in the protection of cell membranes from oxidative injuries. Interaction with the membrane phospholipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183727. [Google Scholar] [CrossRef]
- Vissers, A.; Kiskini, A.; Hilgers, R.; Marinea, M.; Wierenga, P.A.; Gruppen, H.; Vincken, J.P. Enzymatic Browning in Sugar Beet Leaves (Beta vulgaris L.): Influence of Caffeic Acid Derivatives, Oxidative Coupling, and Coupled Oxidation. J. Agric. Food Chem. 2017, 65, 4911–4920. [Google Scholar] [CrossRef]
- Becker, C.; Urlic, B.; Jukic Spika, M.; Klaring, H.P.; Krumbein, A.; Baldermann, S.; Goreta Ban, S.; Perica, S.; Schwarz, D. Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives, and Sucrose while Losing Chlorophylls, Beta-Carotene and Xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef]
- Medeiros, J.; Xu, S.; Pickering, G.J.; Kemp, B.S. Influence of Caffeic and Caftaric Acid, Fructose, and Storage Temperature on Furan Derivatives in Base Wine. Molecules 2022, 27, 7891. [Google Scholar] [CrossRef]
- Jöhrer, K.; Pérez, M.G.; Kircher, B.; Çiçek, S.S. Flavones, Flavonols, Lignans, and Caffeic Acid Derivatives from Dracocephalum moldavica and Their In Vitro Effects on Multiple Myeloma and Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 14219. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, A.; Thomas, R.; van Belkum, A.; Neela, V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed Res. Int. 2013, 2013, 392058. [Google Scholar] [CrossRef] [PubMed]
- Lukac, M.; Slobodnikova, L.; Mrva, M.; Dusekova, A.; Garajova, M.; Kello, M.; Sebova, D.; Pisarcik, M.; Kojnok, M.; Vrtak, A.; et al. Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. Int. J. Mol. Sci. 2024, 25, 1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Xu, J.R.; Ji, D.D. Synthesis of N-hydroxycinnamoyl amide derivatives and evaluation of their anti-oxidative and anti-tyrosinase activities. Bioorg. Med. Chem. 2019, 27, 114918. [Google Scholar] [CrossRef]
- Zhu, M.; Shan, Q.; Ma, L.; Wen, J.J.; Dong, B.A.; Zhang, G.N.; Wang, M.H.; Wang, J.X.; Zhou, J.M.; Cen, S.; et al. Design and biological evaluation of cinnamic and phenylpropionic amide derivatives as novel dual inhibitors of HIV-1 protease and reverse transcriptase. Eur. J. Med. Chem. 2021, 220, 113498. [Google Scholar] [CrossRef]
- Li, H.M.; Yu, S.P.; Fan, T.Y.; Zhong, Y.; Gu, T.; Wu, W.Y.; Zhao, C.; Chen, Z.; Chen, M.; Li, N.G.; et al. Design, synthesis, and biological activity evaluation of BACE1 inhibitors with antioxidant activity. Drug Dev. Res. 2020, 81, 206–214. [Google Scholar] [CrossRef]
- Peng, X.Y.; Zhao, A.N.; Huang, K.L.; Hu, T.J.; Liu, B.N.; Huang, Y.; Chen, H.L.; Chai, L.; Lin, C.W. Synthesis of Caffeic Acid Sulphonamide Derivatives and Preliminary Exploration of Their Biological Applications. Chem. Res. Chin. Univ. 2020, 36, 795–803. [Google Scholar] [CrossRef]
- Gabriele, E.; Brambilla, D.; Ricci, C.; Regazzoni, L.; Taguchi, K.; Ferri, N.; Asai, A.; Sparatore, A. New sulfurated derivatives of cinnamic acids and rosmaricine as inhibitors of STAT3 and NF-κB transcription factors. J. Enzyme Inhib. Med. Chem. 2017, 32, 1012–1028. [Google Scholar] [CrossRef]
- Nemadziva, B.; Le Roes-Hill, M.; Koorbanally, N.; Kudanga, T. Small laccase-catalyzed synthesis of a caffeic acid dimer with high antioxidant capacity. Process Biochem. 2018, 69, 99–105. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Oh, N.K.; Gouda, N.A.; Abdellattif, M.H.; Alshammari, S.O.; Abourehab, M.A.S.; Alshammari, Q.A.; Belal, A.; Kim, M.; Al-Karmalawy, A.A.; et al. Novel Hybrid Indole-Based Caffeic Acid Amide Derivatives as Potent Free Radical Scavenging Agents: Rational Design, Synthesis, Spectroscopic Characterization, In Silico and In Vitro Investigations. Metabolites 2023, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.; Lowen, N.; Blake, D.J. Caffeic Acid Esters Are Effective Bactericidal Compounds Against Paenibacilluslarvae by Altering Intracellular Oxidant and Antioxidant Levels. Biomolecules 2019, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Schultheis, M.; Hazra, S.; Hazra, B.; Moll, H.; Schurigt, U.; Holzgrabe, U. Antileishmanial lead structures from nature: Analysis of structure-activity relationships of a compound library derived from caffeic Acid bornyl ester. Molecules 2014, 19, 1394–1410. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Xie, J.C.; Sun, M.; Xiong, S.H.; Xu, C.F.; Zhang, Z.M.; Li, M.J.; Li, C.; Lin, L.M. Plant-Derived Caffeic Acid and Its Derivatives: An Overview of Their NMR Data and Biosynthetic Pathways. Molecules 2024, 29, 1625. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Ferreira, P.L.; Groppo, M.; Da Costa, F.B. Caffeic acid ester derivatives and flavonoids of genus Arnaldoa (Asteraceae, Barnadesioideae). Biochem. Syst. Ecol. 2019, 86, 103911. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Sousa, C.; Marques, J.T.; Vila-Vicosa, D.; de Granada-Flor, A.; Viana, A.S.; Santos, M.; Machuqueiro, M.; de Almeida, R.F.M. Differential targeting of membrane lipid domains by caffeic acid and its ester derivatives. Free Radic. Biol. Med. 2018, 115, 232–245. [Google Scholar] [CrossRef]
- Sardi, J.d.C.O.; Gullo, F.P.; Freires, I.A.; Pitangui, N.d.S.; Segalla, M.P.; Fusco-Almeida, A.M.; Rosalen, P.L.; Regasini, L.O.; Mendes-Giannini, M.J.S. Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn. Microbiol. Infect. Dis. 2016, 86, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Guo, R.; Fu, Z.M.; Chen, D.F. Effects of different ester chains on the antioxidant activity of caffeic acid. Bioorg. Chem. 2020, 105, 104341. [Google Scholar] [CrossRef]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In-vitro and in-vivo antimalarial activity of caffeic acid and some of its derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef]
- Choi, H.G.; Tran, P.T.; Lee, J.H.; Min, B.S.; Kim, J.A. Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza Bunge. Arch. Pharm. Res. 2018, 41, 64–70. [Google Scholar] [CrossRef]
- Balaha, M.; De Filippis, B.; Cataldi, A.; Di Giacomo, V. CAPE and Neuroprotection: A Review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla, B.; Robles, J. Antiradical properties of curcumin, caffeic acid phenethyl ester, and chicoric acid: A DFT study. J. Mol. Model. 2022, 28, 68. [Google Scholar] [CrossRef] [PubMed]
- Olgierd, B.; Kamila, Z.; Anna, B.; Emilia, M. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, M.; Wang, Y.; Wa, W.; Zheng, J. Interaction between caffeic acid/caffeic acid phenethyl ester and micellar casein. Food Chem. 2021, 349, 129154. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Pascual, C.; Gonzalez, R.; Torricella, R.G. Scavenging action of propolis extract against oxygen radicals. J. Ethnopharmacol. 1994, 41, 9–13. [Google Scholar] [CrossRef]
- Sud’ina, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V.; Varfolomeev, S.D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar] [CrossRef]
- Krol, W.; Scheller, S.; Czuba, Z.; Matsuno, T.; Zydowicz, G.; Shani, J.; Mos, M. Inhibition of neutrophils’ chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. J. Ethnopharmacol. 1996, 55, 19–25. [Google Scholar] [CrossRef]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar]
- Park, E.H.; Kahng, J.H. Suppressive effects of propolis in rat adjuvant arthritis. Arch. Pharm. Res. 1999, 22, 554–558. [Google Scholar] [CrossRef]
- Chen, Y.J.; Shiao, M.S.; Wang, S.Y. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs 2001, 12, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Liao, P.H.; Chen, W.K.; Yang, C.Y. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000, 153, 51–56. [Google Scholar] [CrossRef]
- Son, S.; Lobkowsky, E.B.; Lewis, B.A. Caffeic acid phenethyl ester (CAPE): Synthesis and X-ray crystallographic analysis. Chem. Pharm. Bull. (Tokyo) 2001, 49, 236–238. [Google Scholar] [CrossRef]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 1988, 44, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Shao, Y.; Huang, M.-T.; Chin, C.-K.; Ho, C.-T. Inhibitory effect of caffeic acid phenethyl ester on human leukemia HL-60 cells. Cancer Lett. 1996, 108, 211–214. [Google Scholar] [CrossRef]
- Chen, W.K.; Liao, P.H.; Tsai, C.F.; Lee, Y.-J. Synthesis of caffeic acid esters as antioxidants by esterification via acyl chlorides. Chin. Pharm. J 1999, 51, 271–278. [Google Scholar]
- Bankova, V.S. Synthesis of Natural Esters of Substituted Cinnamic-Acids. J. Nat. Prod. 1990, 53, 821–824. [Google Scholar] [CrossRef]
- Boutagy, J.; Thomas, R. Olefin synthesis with organic phosphonate carbanions. Chem. Rev. 1974, 74, 87–99. [Google Scholar] [CrossRef]
- Xia, C.; Hu, W. Synthesis of Caffeic Acid Esters. J. Chem. Res. 2005, 5, 332–334. [Google Scholar] [CrossRef]
- Ryu, Y.; Scott, A.I. Self-condensation of activated malonic acid half esters: A model for the decarboxylative Claisen condensation in polyketide biosynthesis. Tetrahedron Lett. 2003, 44, 7499–7502. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, Y.; Li, N.G.; Zhu, Y.; Duan, J.A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef] [PubMed]
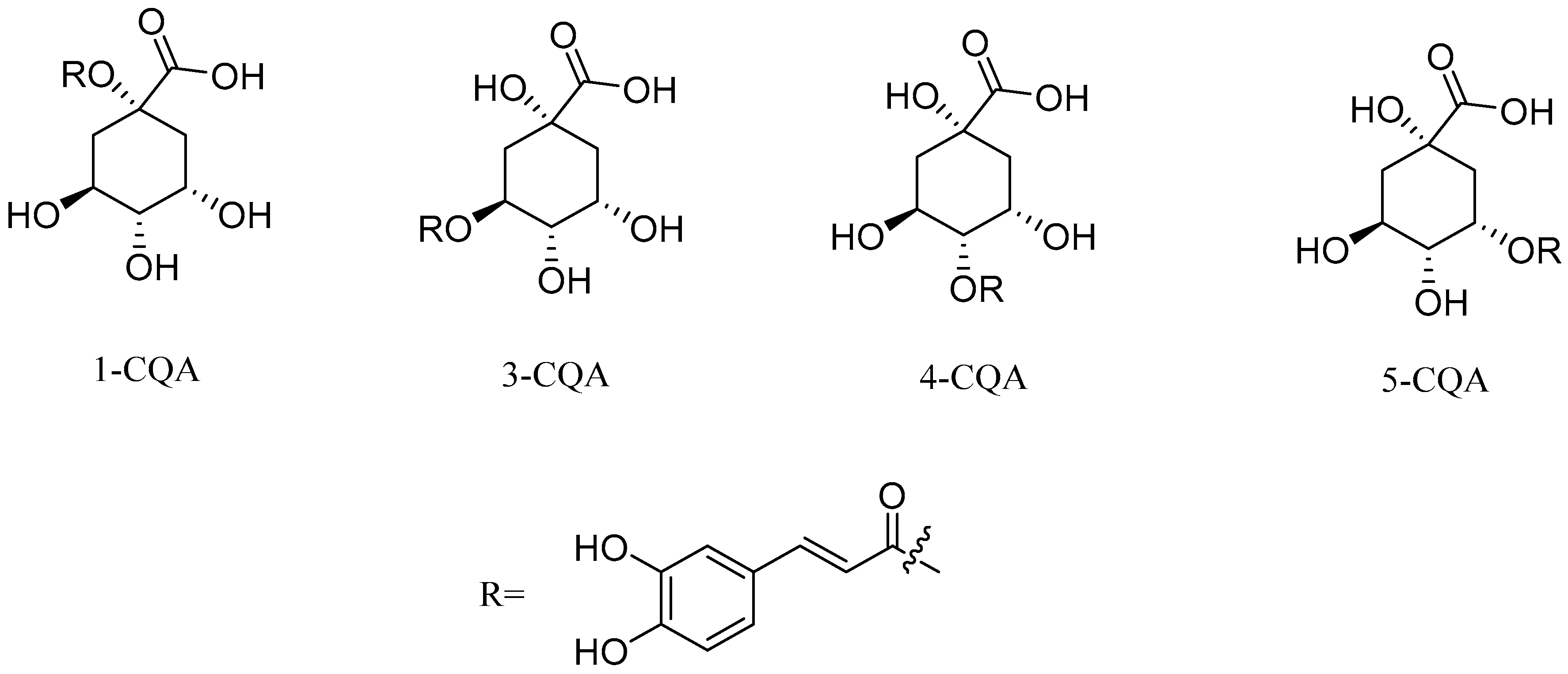
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.-y.; Shang, H.; Jia, Y.; Xu, X.-D.; Tian, Y.; Guo, P. Amino acid ester-coupled caffeoylquinic acid derivatives as potential hypolipidemic agents: Synthesis and biological evaluation. RSC Adv. 2021, 11, 1654–1661. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, J.; Kim, B.K.; Whang, W.K.; Min, H. Effects of caffeoylquinic acid analogs derived from aerial parts of Artemisia iwayomogi on adipogenesis. Food Sci. Biotechnol. 2023, 32, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tao, M.; Wu, T.; Zhuo, Z.; Xu, T.; Pan, S.; Xu, X. A promising strategy for investigating the anti-aging effect of natural compounds: A case study of caffeoylquinic acids. Food Funct. 2021, 12, 8583–8593. [Google Scholar] [CrossRef]
- Wu, W.; Qian, W.; Hao, H.; Kang, Y.; Wang, Y.; Deng, Y.; Ni, C.; Huang, J.; Weng, W. Determination of caffeoylquinic acid derivatives in Azolla imbricata by chitosan-based matrix solid-phase dispersion coupled with HPLC-PDA. J. Pharm. Biomed. Anal. 2019, 163, 197–203. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, P.; Ma, Y.; Wang, K.; Chang, X.; Bai, Y.; Zhang, D.; Yang, L. Simultaneous Quantitative Determination of Six Caffeoylquinic Acids in Matricaria chamomilla L. with High-Performance Liquid Chromatography. J. Chem. 2019, 2019, 4352832. [Google Scholar] [CrossRef]
- Wu, Q.Z. Advances in research of caffeoylquinic acid compounds. Chin. Wild Plant Resour. 2020, 39, 48–60. [Google Scholar]
- Skała, E.; Makowczyńska, J.; Wieczfinska, J.; Kowalczyk, T.; Sitarek, P. Caffeoylquinic Acids with Potential Biological Activity from Plant In vitro Cultures as Alternative Sources of Valuable Natural Products. Curr. Pharm. Des. 2020, 26, 2817–2842. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, 202000051. [Google Scholar] [CrossRef] [PubMed]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity on digestive enzyme, antioxidant, anti-proliferative and antibacterial activities of the extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Han, J.; Lv, Q.-Y.; Jin, S.-Y.; Zhang, T.-T.; Jin, S.-X.; Li, X.-Y.; Yuan, H.-L. Comparison of anti-bacterial activity of three types of di-O-caffeoylquinic acids in Lonicera japonica flowers based on microcalorimetry. Chin. J. Nat. Med. 2014, 12, 108–113. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
- Wu, M.Y.; Liu, C.C.; Lee, S.C.; Kuo, Y.H.; Hsieh, T.J. N-Octyl Caffeamide, a Caffeic Acid Amide Derivative, Prevents Progression of Diabetes and Hepatic Steatosis in High-Fat Diet Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 8948. [Google Scholar] [CrossRef]
- Lee, Y.L.; Hsu, L.H.; Kuo, Y.H.; Lee, C.C. Caffeic amide derivatives inhibit allergen-induced bone marrow-derived dendritic cell maturation. Pharmacol. Rep. 2019, 71, 194–200. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Zabiulla; Grisha, S.; Mohammed, Y.H.E.; Vivek, H.K.; Ara Khanum, S. Molecular docking and synthesis of caffeic acid analogous and its anti-inflammatory, analgesic and ulcerogenic studies. Bioorg. Med. Chem. Lett. 2021, 33, 127743. [Google Scholar] [CrossRef]
- Wen, S.; Cao, C.; Ge, J.; Yang, W.; Wang, Y.; Mou, Y. Research Progress of H2S Donors Conjugate Drugs Based on ADTOH. Molecules 2022, 28, 331. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Yang, X.H.; Ou, R.Y.; Ouyang, Y.; Wang, S.N.; Chen, Z.W.; Wen, S.J.; Pi, R.B. Synthesis and evaluation of multifunctional ferulic and caffeic acid dimers for Alzheimer’s disease. Nat. Prod. Res. 2017, 31, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, R.; Zhou, Z.; Liu, R.; Wen, J. Efficacy and safety of caffeic acid tablets in the treatment of thrombocytopenia: A systematic review and meta-analysis. Medicine 2023, 102, e35353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bi, Z.; Li, Y.; Zheng, H.; Li, L.; Ouyang, J.; Wang, B.; Bi, Y. Sodium Ferulate Modified Gene Expression Profile of Oxidized Low-Density Lipoprotein-Stimulated Human Umbilical Vein Endothelial Cells. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 302–313. [Google Scholar] [CrossRef]







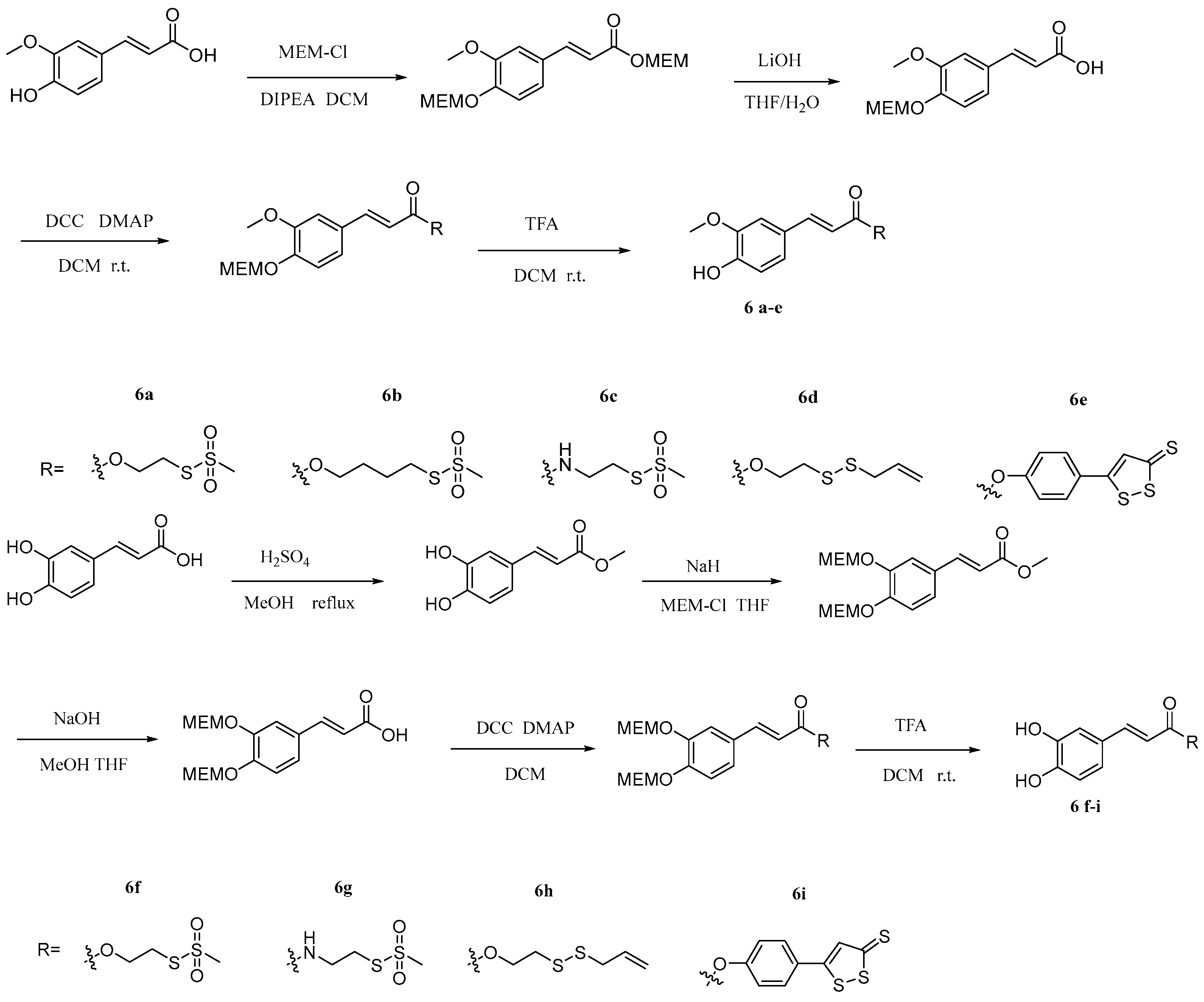


| Types of Derivatives | Chemical Structures | Biological Activities | References |
|---|---|---|---|
| CA-ester derivatives | CA-phenethyl ester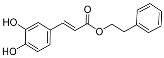 | Showed antibacterial activity MIC50 5.2 ± 0.8 µg/mL for S. mutans | [31] |
Compound 1g | Showed antibacterial activity MIC50 32 µg/mL for Candida albicans | [32] | |
5-CQA | Showed antibacterial activity MIC50 8–16 µg/mL for Stenotrophomonas maltophilia | [33] | |
Compound CAP10 | Showed antibacterial activity MIC50 13 μM for Candida albicans | [34] | |
| CA-amides derivatives | Compound 3k | Showed antioxidant activity IC50 18.6 μM | [35] |
Compound 4′d | Showed antiviral activity IC50 0.081 nM for HIV-1 protease | [36] | |
Compound 5a | Showed antioxidant activity IC50 67.85 μM | [37] | |
| CA-hybrids | Compound CS2 | Showed antioxidant activity IC50 40.29 μM | [38] |
Compound 6i | Showed antitumor activity IC50 46.7 μM for HCT-116 cells | [39] | |
Compound Phellinsin A | Showed antioxidant activity IC50 0.29 mM | [40] | |
Compound 8j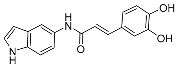 | Showed antioxidant activity IC50 4774.37 μM | [41] |
| Synthetic Methods | Reagents | Solvent | Reaction Temperature | References |
|---|---|---|---|---|
| Direct Catalysis Method | Caffeic acid, phenethyl alcohol, toluene-p-sulfonic acid | Benzene | Reflux | [64] |
| Direct Catalysis Method | Caffeic acid, phenethyl alcohol, dicyclohexylcarbodiimide and dimethylaminopyridine | THF/CH2Cl2 (1:1) | Room temperature | [65] |
| Halogen-Substituted Hydrocarbon Method | Caffeic acid, β-phenyl ethyl bromide, sodium hydroxide | Hexamethylphosphoramide (HMPA) | Room temperature | [63] |
| Acyl Chloride Method | Caffeic acid, phenethyl alcohol, SOCl2, pyridine | Nitrobenzene | Refluxing temperature | [66] |
| Witting Reaction | 3,4-Dihydroxy benzaldehyde, triphenylphosphonic acid phenylethanol ester chloride, potassium carbonate | CHCl3/dioxane (1:1) | Room temperature | [67,68] |
| Malonic Acid Monoester Method | Malonic acid, phenethyl alcohol, DPAT, 3,4-dihydroxy benzaldehyde | Toluene | 80 °C | [69] |
| One Pot Method | Isopropylidene malonate, phenethyl alcohol, 3,4-dihydroxy benzaldehyde, pyridine, piperidine | Toluene | 60 °C–room temperature | [70] |
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 3a | OCH3 | OH | CH3 |
| 3b | OCH3 | OH | CH(CH3)2 |
| 3c | OCH3 | OH | CH2CH(CH3)2 |
| 3d | OCH3 | OOCCH3 | CH3 |
| 3e | OCH3 | OOCCH3 | CH(CH3)2 |
| 3f | OCH3 | OOCCH3 | CH2CH(CH3)2 |
| 3g | OH | OH | CH3 |
| 3h | OH | OH | CH(CH3)2 |
| 3i | OH | OH | CH2CH(CH3)2 |
| 3j | OH | OH | CH(CH3)CH2CH3 |
| 3k | OH | OH | H |
| 3l | OH | OH | CH2C6H6 |
| Compound | R1 | R2 | R3 | R4 | R5 | X |
|---|---|---|---|---|---|---|
| 4a | CH2CH(CH3)2 | OCH3 | OH | OH | H | CH |
| 4b | CH2CH(CH3)2 | NH2 | OH | OH | H | CH |
| 4c | CH2CH(CH3)2 | SCH3 | OH | OH | H | CH |
| 4d | CH2CH(CH3)2 | OCH3 | OH | OH | H | N |
| 4e | CH2CH2CH3 | OCH3 | OH | OH | H | CH |
| 4′a | CH2CH(CH3)2 | OCH3 | OH | OH | H | CH |
| 4′b | CH2CH(CH3)2 | NH2 | OH | OH | H | CH |
| 4′c | CH2CH(CH3)2 | SCH3 | OH | OH | H | CH |
| 4′d | CH2CH(CH3)2 | OCH3 | OH | OH | H | N |
| 4′e | CH2CH2CH3 | OCH3 | OH | OH | H | CH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, Y.; Wen, S.; Sha, H.-K.; Zhao, Y.; Gui, L.-J.; Wang, Y.; Jiang, Z.-Y. Discovery and Development of Caffeic Acid Analogs as Versatile Therapeutic Agents. Pharmaceuticals 2024, 17, 1403. https://doi.org/10.3390/ph17101403
Mou Y, Wen S, Sha H-K, Zhao Y, Gui L-J, Wang Y, Jiang Z-Y. Discovery and Development of Caffeic Acid Analogs as Versatile Therapeutic Agents. Pharmaceuticals. 2024; 17(10):1403. https://doi.org/10.3390/ph17101403
Chicago/Turabian StyleMou, Yi, Shuai Wen, Hong-Kai Sha, Yao Zhao, Li-Juan Gui, Yan Wang, and Zheng-Yu Jiang. 2024. "Discovery and Development of Caffeic Acid Analogs as Versatile Therapeutic Agents" Pharmaceuticals 17, no. 10: 1403. https://doi.org/10.3390/ph17101403
APA StyleMou, Y., Wen, S., Sha, H.-K., Zhao, Y., Gui, L.-J., Wang, Y., & Jiang, Z.-Y. (2024). Discovery and Development of Caffeic Acid Analogs as Versatile Therapeutic Agents. Pharmaceuticals, 17(10), 1403. https://doi.org/10.3390/ph17101403







