Abstract
Background: This research centers on the development and spectroscopic characterization of new quinazolin-4(3H)-one-isoxazole derivatives (5a–e). The aim was to investigate the regioselectivity of the 1,3-dipolar cycloaddition involving arylnitriloxides and N-propargylquinazolin-4(3H)-one, and to assess the antioxidant properties of the synthesized compounds. The synthetic approach started with the alkylation of quinazolin-4(3H)-one using propargyl bromide, followed by a 1,3-dipolar cycloaddition reaction. Methods: The structural identification of the products was performed using various spectroscopic methods, such as IR, 1H, 13C, and HMBC NMR, HRMS, and single-crystal X-ray diffraction. To further examine the regioselectivity of the cycloaddition, Density Functional Theory (DFT) calculations at the B3LYP/6-31G(d) level were employed. Additionally, the antioxidant potential of the compounds was tested in vitro using DPPH (2,2-Diphenyl-1-picrylhydrazyl)radical scavenging assays. The reaction selectively produced 3,5-disubstituted isoxazoles, with the regiochemical outcome being independent of the substituents on the phenyl ring. Results: Theoretical calculations using DFT were in agreement with the experimental results, revealing activation energies of −81.15 kcal/mol for P-1 and −77.32 kcal/mol for P-2, favoring the formation of P-1. An analysis of the Intrinsic Reaction Coordinate (IRC) confirmed that the reaction proceeded via a concerted but asynchronous mechanism. The antioxidant tests demonstrated that the synthesized compounds exhibited significant radical scavenging activity, as shown in the DPPH assay. The 1,3-dipolar cycloaddition of arylnitriloxides with N-propargylquinazolin-4(3H)-one successfully resulted in novel 3,5-disubstituted isoxazoles. Conclusions: The experimental findings were well-supported by theoretical predictions, and the antioxidant assays revealed strong activity, indicating the potential for future biological applications of these compounds.
1. Introduction
The body’s natural antioxidant defense system plays a vital role in maintaining physiological functions and protecting against the harmful effects of reactive oxygen species (ROS) and other oxidants. Excessive ROS are linked to the development of various oxidative stress-related diseases, including cardiovascular disorders [1], Alzheimer’s disease [2] and cancer [3]. Consequently, antioxidants are crucial in both the prevention and treatment of these conditions. Recent studies suggest that single-target drugs are increasingly prone to resistance, leading to the diminished efficacy of many promising drug candidates. It is now widely recognized that drugs designed to act on multiple targets or various sites within the same target tend to be more effective than those focused on a single target [4,5,6,7,8].
In light of this observation, the design of new, more effective antioxidant agents has become of great importance. Over the past decade, particular attention has been paid to the synthesis and discovery of more efficient antioxidant agents. A variety of heterocyclic compounds, including heteroatoms such as nitrogen, sulfur, and oxygen, have been explored [9,10].
Quinazolin-4-one is a structurally important motif found in a variety of synthetic derivatives with pharmaceutical purposes and in numerous natural alkaloids. In fact, it is an essential scaffold found in about 150 natural alkaloids and drugs [11]. The quinazolin-4-one skeleton is also crucial in the prevention and treatment of agricultural diseases, as seen in commercial fungicides such as proquinazid and fluquinconazole [12,13] (Figure 1a).
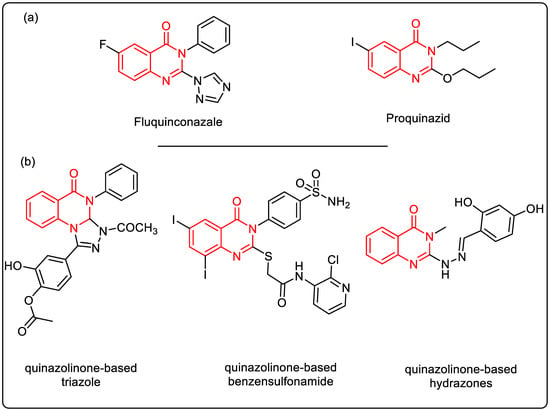
Figure 1.
(a) Chemical structures of commercial drugs containing the quinazolinone backbone; (b) representative examples of diverse quinazolin4(3H)-ones with antioxidant activity. The quinazoline nucleus in red indicates its presence in drug structures and as an antioxidant.
Compounds containing the quinazolin-4-one backbone are versatile molecules with diverse pharmacological activities, making it a promising candidate for the development of various drugs and therapeutic agents [14,15]. Such compounds have garnered significant interest from researchers because of their antioxidant properties [16,17,18,19,20] (Figure 1b) and the simplicity of their synthesis.
Besides the quinazolin-4-one motif, the isoxazole ring is recognized as one of the most important heterocycles for the discovery of new agents in medicinal chemistry. Several derivatives of the isoxazole ring serve as basic structures for many drugs, including immunomodulatory [21,22], antimicrobial [23], antiviral [24], anticancer [22], antiplatelet, antithrombotic, triglyceride suppressing [25], antidiabetic [26], analgesic [27], and anti-Alzheimer [28]. They are also used in the production of pesticides and insecticides [29,30]. Some examples of isoxazole ring-based molecules with antioxidant activity are shown in Figure 2 [31,32,33].
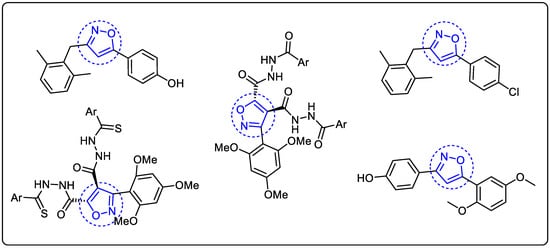
Figure 2.
Isoxazole ring-based molecules with antioxidant activity. The isoxazole core in blue indicates its presence in the antioxidant agent structures.
To achieve the expected objectives, molecular hybridization technique is emerging as a new alternative [34,35,36]. This strategy involves the fusion of two or more distinct pharmacophores to create a new hybrid molecule that combines the properties of each pharmacophore. Currently, this strategy is widely used in the development of new drugs targeting various targets. It helps to minimize the risks associated with multiple side effects, which are characteristic of traditional treatments [37,38].
In the present work, we provide an overview of a series of quinazolin-4-one-isoxazole hybrids synthesized via N-alkylation and 1,3-dipolar cycloaddition reactions. A DFT study was conducted to rationalize the experimental findings and to gain deeper insight into the regioselectivity of the 1,3-dipolar cycloaddition between propargylated quinazolin-4(3H)-one and nitrile oxides, using the B3LYP/6-31G(d) computational level. Furthermore, the antioxidant properties of the synthesized compounds were assessed in vitro using the DPPH radical scavenging assay.
2. Results and Discussion
2.1. Synthesis and Spectral Analysis
3-(prop-2-yn-1-yl)quinazoline-4(3H)-one 3, used as a dipolarophile in this work, was prepared according to the strategy described in Scheme 1. Quinazoline-4(3H)-one 2 was prepared according to the procedure described in the literature from anthranilic acid (1) and formamide [39]. It was then alkylated with propargyl bromide under phase transfer catalysis (PTC) in the presence of tetra-n-butylammonium bromide (TBAB) [40].
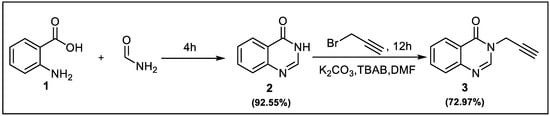
Scheme 1.
Synthesis of the dipolarophile 3.
The quinazolinone-isoxazole hybrids 5a–e were prepared by the 1,3-dipolar cycloaddition reaction of N-propargyl quinazoline-4(3H)-one 3 and suitably substituted nitrile oxides. The nitrile oxides were generated in situ by the action of triethylamine as a base on their hydroxamoyl chloride precursors 4a–e [41].
The 1,3-cycloaddition reactions of N-propargyl quinazoline-4(3H)-one 3 were performed in chloroform at room temperature for 6 to 24 h, depending on the dipole used, yielding the 3,5-disubstituted compounds (5) as the sole regioisomers, with good yields and no traces of the (5′) regioisomers (Scheme 2). The observed regioselectivity aligns well with both NMR results and literature reports [32,33], which may indicate that the possible reaction mechanism for the 1,3-dipolar reaction between dipolarophile 3 and the nitrile oxide derivatives 4a–h is as presented in Scheme 3.
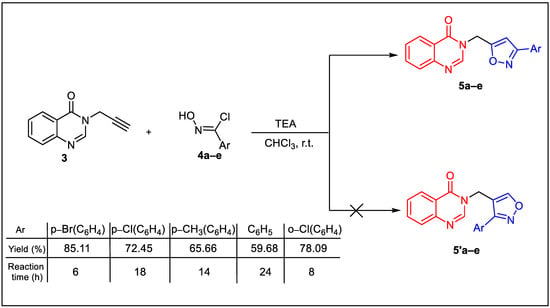
Scheme 2.
Synthesis procedure of the novel hybrid molecules (5). The quinazolinone core in red and the isoxazole core in blue to visualize their assembly within the same molecular fragment.
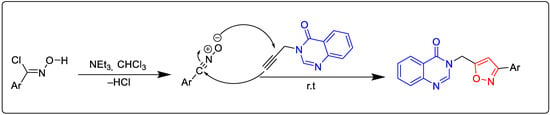
Scheme 3.
Possible reaction mechanism for the synthesis of new isoxazoles. The quinazolinone core in red and the isoxazole core in blue to visualize their assembly within the same molecular fragment.
The structures of the cycloadducts were confirmed through IR spectroscopy, 1H and 13C NMR, 2D HMBC NMR, HRMS, and single-crystal X-ray diffraction. The physical properties and spectroscopic data for compounds 5a–e, while detailed spectral information can be found in the Supplementary Information.
High-resolution mass spectrometry (HRMS) confirmed the exact masses of the [M + H]+ ions for the synthesized hybrid compounds, supporting the proposed molecular structures. For instance, compound 5a exhibited a molecular ion peak at m/z 383.021, matching the calculated mass for the molecular formula C18H13N3O2Br [M + H]. The spectrum also shows the presence of two isotopic peaks [M + H]+ at 382.018 and [M + H]+2 at 384.016 with approximatively the same intensity (100.00% and 98.10%), attesting the presence of the bromine atom. These findings are further supported by IR spectroscopy. The IR spectrum of compound 5a displays a strong absorption band at 1672 cm−1, corresponding to the stretching vibration of the C=O bond in the quinazolinone carbonyl group. Additionally, bands at 1159 cm−1 and 1612 cm−1 are consistent with the stretching vibrations of the C–O and C=N bonds within the isoxazole ring, respectively.
The 1H and 13C NMR spectra of the hybrid compounds 5a–e were recorded in DMSO-d6 or CDCl3, based on the solubility of each compound. The 1H NMR spectra display singlet peaks between 5.35 and 5.45 ppm, corresponding to the two protons of the methylene group (N–H2), and singlets within the 6.67–7.10 ppm range, which can be attributed to the methine protons (CH) in the isoxazole ring. Additionally, singlet signals observed between 7.57 and 8.59 ppm can be assigned to the methine protons (N=CH) of the quinazolinone ring.
In the 13C NMR spectra, signals for the quaternary carbons of the isoxazole ring are noted between 165.25 and 169.12 ppm for C3, and between 161.10 and 162.87 ppm for C5. Signals for the isoxazole carbons within the C–H group are found around 100 ppm. The carbonyl and imine carbons of the quinazolinone ring have corresponding signals at approximately 160 ppm and 148 ppm, respectively, while the aromatic carbons are found between 120 and 146.99 ppm.
The structural assignment of the 5a–e hybrids is consistent with the literature data concerning the compounds obtained through 1,3-dipolar cycloaddition of arylnitriloxides to terminal alkynes [42]. According to the literature, only one 3,5-disubstituted regioisomer was obtained. This structure was characterized by the presence of a single singlet signal in the 1H NMR spectrum between 6.16–6.85 ppm assigned to the C4 carbon proton, and a signal around 100 ppm for the C4 carbon in the 13C NMR spectrum. In the case of the 3,4-disubstituted regioisomer, one would expect to observe highly deshielded signals for the C5 carbon atom and the corresponding proton bonded to this carbon atom [42,43].
To confirm that the synthesized cycloadducts are indeed 3,5-disubstituted regioisomers, we used HMBC 2D NMR spectroscopy. The HMBC spectra enable the detection of C–H interactions over three bonds. To demonstrate how this technique can distinguish between 3,5- and 3,4-disubstituted isomers, an HMBC analysis was performed for compound 5a and the important assignments are shown in Figure 3.
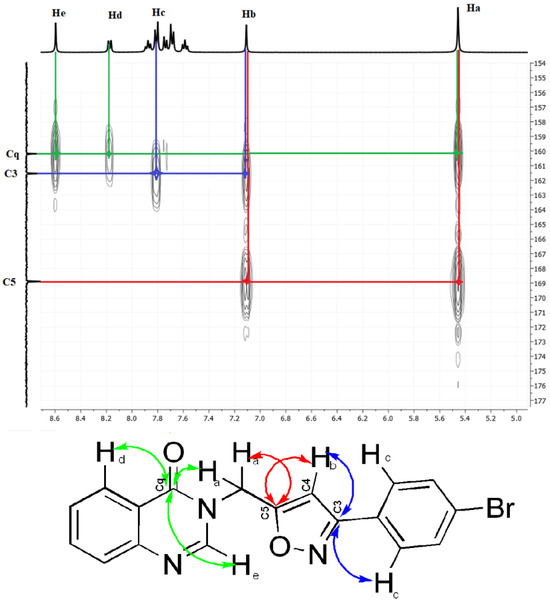
Figure 3.
HMBC (1H-13C) spectrum of compound 5a.
It is noteworthy that the signal in the 13C NMR at 169.1 ppm, corresponding to C5, correlates with two signals in the 1H NMR, one corresponding to a proton in the methine group (Hb), at 7.1 ppm, with 2JCH coupling, the other to a proton in the methylene group (Ha), at 5.45 ppm, with 2JCH coupling. Subsequently, the carbon atom at 161.8 ppm is correlated with two protons: one resonating at 7.85 ppm, representing the aromatic proton Hc (3JCH coupling), and the other signal at 7.1 ppm, corresponding to a proton in the methine group (Hb), showing a 2JCH coupling. These observations confirm that the signal at 161.8 ppm is for carbon atom C3 of the isoxazole ring. Finally, the carbon atom at 160.4 ppm is correlated with three signals at 8.59 ppm, 8.19 ppm, and 5.45 ppm, respectively, corresponding to the methine group (He) of the quinazolinone ring (3JCH coupling), the aromatic proton (Hd) of the same ring (3JCH coupling), and the methylene group (Ha) that connects the two nuclei (3JCH coupling). These confirm that compound 5a is the 3,5-disubstituted regioisomer and not the 3,4-disubstituted regioisomer.
2.2. X-Ray Diffraction Data and Crystal Structures of the Two Compounds 5a and 5c
Compound 5a crystallizes in the monoclinic Pc space group, with two molecules per unit cell. The asymmetric unit (Figure 4) shows the expected quinazoline and isoxazole moieties linked through a methylene bridge.
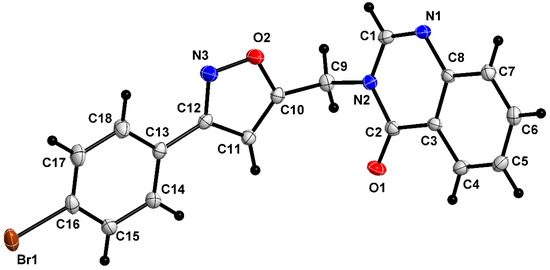
Figure 4.
The asymmetric unit of 5a, shown with 50% probability ellipsoids. Grey indicates carbon atom, Black indicates hydrogen atom, Red indicates Oxygen atom, and Blue indicates Nitrogen atom.
Compound 5c crystallizes in the orthorhombic Pbca space group, with eight molecules per unit cell. The asymmetric unit of 5c (Figure 5) is similar to that of 5a. Table 1 presents the crystallographic data, experimental details of the data collection and structure refinements.
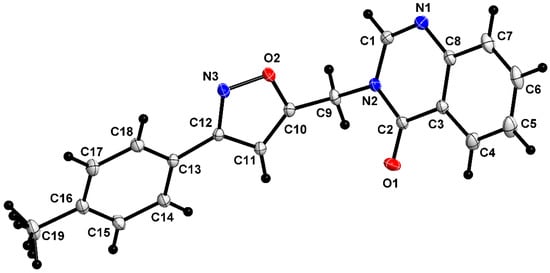
Figure 5.
The asymmetric unit of 5c, shown with 50% probability ellipsoids. Grey indicates the carbon atom, Black indicates the hydrogen atom, Red indicates the Oxygen atom, and Blue indicates the Nitrogen atom.

Table 1.
Crystal data and refinement details for compounds 5a and 5c.
2.3. Antioxidant Activity
Given the significant damage inflicted by reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the human body, it is crucial to identify new therapeutic agents that offer improved efficacy compared to current natural and synthetic antioxidants.
Quinazolin-4(3H)-ones and isoxazole are known for their large spectrum of biological activities; combining the two active pharmacophores may enhance the biological activity, in particular the antioxidant activity. In general, quinazolinone and isoxazole are not considered to possess significant antioxidant activity against free radicals such as DPPH, since they are relatively stable and do not contain functional groups capable of readily surrendering electrons or hydrogen atoms to reduce a free radical [44,45]. In this study, all synthesized hybrid compounds have shown moderate anti-DPPH activity (Figure 6), but these activities are lower than that of the positive control. Indeed, at a concentration of 400 µg/mL, the products inhibit DPPH radicals with a percentage which varies between 16.88 and 41.16%. These findings indicate that the IC50 values are greater than 400 µg/mL.
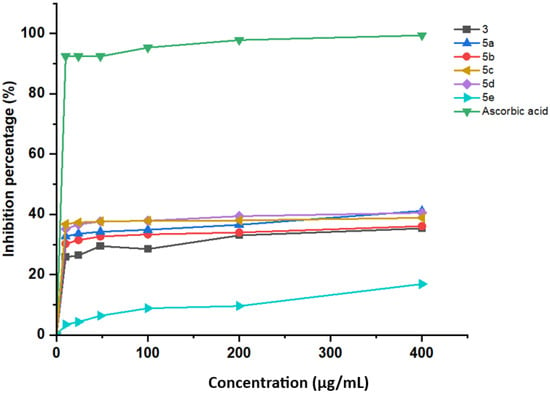
Figure 6.
DPPH radical scavenging activity of products and ascorbic acid.
2.4. DFT Study
2.4.1. Analysis Based on the Global Reactivity Indexes
To understand the mechanism and predict the reactivity of the 1,3-dipolar cycloaddition reaction under investigation, global indices such as chemical potential (μ), chemical hardness (η), electrophilicity (ω), and nucleophilicity (N) were calculated and are summarized in Table 2.

Table 2.
B3LYP/6-31G(d) electronic chemical potential µ, chemical hardness η, electrophilicity ω, nucleophilicity N, in eV, of the two reagents.
According to the data in Table 2, it can be observed that the electronic chemical potential of compound 3 (µ = −3.76 eV) is higher than that of compound 4d (µ = −3.83 eV), this suggesting that during the 1,3-dipolar cycloaddition reaction, charge transfer is likely to occur from compound 3 to compound 4e. Additionally, the nucleophilicity and electrophilicity indices for compound 3 are 2.80 eV and 2.77 eV, respectively, whereas those for compound 4e are 2.78 eV and 2.91 eV. Thus, both reagents are categorized as moderate nucleophiles and strong electrophiles, within the electrophilicity and nucleophilicity scales [46,47]. Since these reagents display similar characteristics, and considering the earlier discussion about charge transfer from compound 3 to compound 4e, we can infer that in this reaction, compound 3 acts as a nucleophile while compound 4e functions as an electrophile.
The difference in energy between the HOMO orbital of compound 3 and the LUMO orbital of 4e (ΔE1) is on the order of 5.01 eV, lower than that between the HOMO orbital of 4e and the LUMO orbital of compound 3 (ΔE2) which is on the order of 5.14 eV (Figure 7), indicating that this reaction can be classified as an inverse-electron demand (IED) 1,3-dipolar cycloaddition reaction, and it is controlled by charge transfer; this transfer will occur from compound 3 to 4e, confirming the results described earlier.
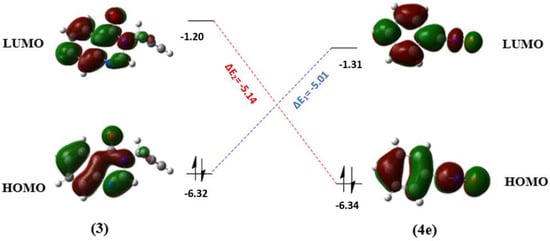
Figure 7.
Interactions between the frontier molecular orbitals of 3 and 4e involved in the 1,3-dipolar cycloaddition reaction, from a quantum calculation by DFT/6-31G(d) (energy gaps ΔE in eV).
2.4.2. Energetic Study
Because of the asymmetry of both reactants, the 1,3-dipolar cycloaddition reaction between compounds 3 and 4e can occur via two competitive reactive pathways, corresponding to two regioisomeric approach modes, P-1 involving the formation of single bonds C1–O5 and C2–C3, and P-2 implicating the formation of single bonds C1–C3 and C2–O5. This occurs through two distinct transition states, TS-1 and TS-2, as shown in Scheme 4.
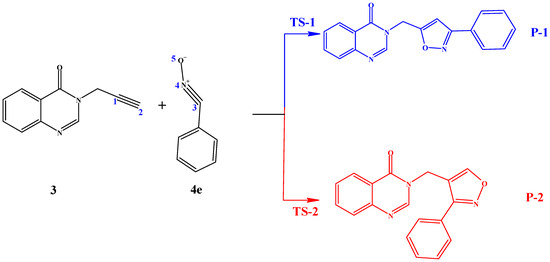
Scheme 4.
Regioisomeric reactive pathways associated with the 1,3-dipolar cycloaddition reaction between (3) and (4e). 1 = C1, 2 = C2, 3 = C3, 4 = N4, 5 = O5; Transition state-1 in blue, and transition state-2 in red.
The energies and relative energies were calculated and summarized in Table 3, and the energy profiles of the reaction paths associated with the 1,3-dipolar cycloaddition reaction between compounds 3 and 4e are presented in Figure 8.

Table 3.
B3LYP/6-31G(d) total energy (E, in a.u.) and relative energy (ΔE, in kcal/mol), for the species involved in the 1,3-dipolar cycloaddition reaction between 3 and 4e.
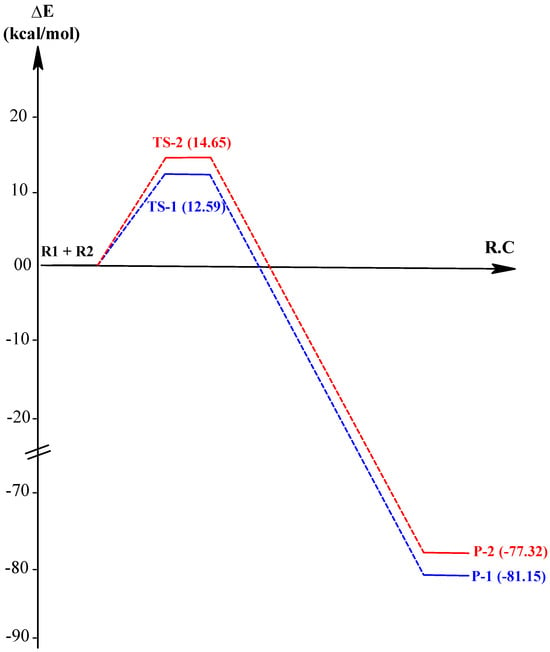
Figure 8.
Energy profiles for the studied reaction paths of the 1,3-dipolar cycloaddition reaction between 3 and 4e. Red indicates transition state-2, and Blue indicates transition state-1.
According to the results presented in Table 3 and Figure 8, the 1,3-dipolar cycloaddition reaction between 3 and 4e exhibits an exothermic character with −81.15 and −77.32 kcal. mol−1 for products P-1 and P-2, respectively, indicating that the formation of these products is solely under kinetic control. The activation energies associated with TS-1 and TS-2 of this reaction are 12.59 and 14.65 kcal.mol−1, respectively, which shows that the formation of product P-1 is kinetically favored over P-2. Consequently, P-1 is kinetically and thermodynamically favored, which is consistent with the experimental results, obtaining compound 5 instead of 5′ (Scheme 2).
A comparative analysis of the geometric parameters of the transition states has been conducted. For TS-1, the bond lengths for the formed bonds C1–O5 and C2–C3 are 2.004 and 1.993 Å, respectively, and for TS-2, the bond lengths for the formed bonds C1–C3 and C2–O5 were 2.103 and 1.998 Å, respectively. This indicates that the new single bonds are formed asynchronously. The geometries of the transition states associated with the two reaction pathways are presented in Figure 9.
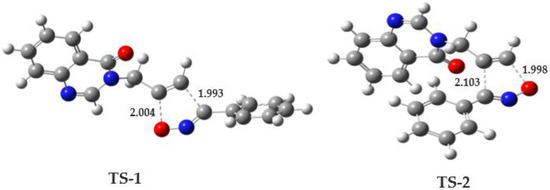
Figure 9.
B3LYP/6-31G(d) geometries of the TSs involved in the regioisomeric pathways associated between 3 and 4e. Distances are given in Angstroms Å. Grey indicates the carbon atom, Red indicates the Oxygen atom, and Blue indicates the Nitrogen atom.
The Intrinsic Reaction Coordinate (IRC) curve is essential for elucidating reaction mechanisms, as it illustrates the progression of the molecular structure from the initial to the final state, traversing through the transition state. Analysis of the IRC curve for our 1,3-dipolar cycloaddition reaction, specifically for the favored transition state TS-1, reveals that the reaction proceeds in a single step without the formation of intermediates, indicating an asynchronous concerted mechanism.
3. Materials and Methods
3.1. Chemistry
The new compounds 5a–e were obtained according to the protocols described in the Supplementary Materials, which also provides detailed information on the reagents, solvents, synthesis methods and analysis techniques used.
3.2. Crystallographic Study
Single crystals of 5a and 5c were obtained at room temperature by vapor diffusion between a dichloromethane solution of the respective compound and pentane. The crystals of 5a and 5c, were mounted on MicroMount cryoloops (MiTeGen, Ithaca, NY, USA) and data were collected on a Bruker D8 VENTURE diffractometer (Karlsruhe, Germany) using Mo-Kα radiation (λ = 0.71073 Å) from a IμS 3.0 micro focus source with multilayer optics, at low temperature (100 K). For structure solving and refinement the Bruker APEX4 software package was used [48]. The structures were solved by dual methods (SHELXT-2018/2) [49] and refined by full matrix least-squares procedures based on F2 with all measured reflections (SHELXL-2019/1) [50]. The structures were refined with anisotropic thermal parameters for non-H atoms. Hydrogen atoms were placed in fixed, idealized positions and refined with a riding model and a mutual isotropic thermal parameter.
Further details on the data collection and refinement methods can be found in Table 1 in the main text. The drawings were created with the Diamond program (version 5.02) [51]. The CCDC reference numbers are 2371662 (5a), 2371661 (5c). The supplementary crystallographic data for this paper can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/ (accessed on 25 September 2024).
3.3. Antioxidant Assessment
The antioxidant test was carried out using the 2.2-diphenyl-1-picrylhydrazyl radical (DPPH) method as reported in our previous study [52]. Thus, 2.5 mL of each compound at different concentrations (10–400 μg/mL) was added to 0.5 mL of a methanolic solution of DPPH (0.2 mM). Absorbance is read against a blank prepared for each concentration at 517 nm after 30 min incubation in the dark at room temperature on a UV-VIS spectrophotometer. The positive control was a solution of a standard antioxidant, ascorbic acid, whose absorbance was measured under the same conditions as the samples. For each concentration, the test was repeated 3 times. Results were expressed as percent inhibition (I%).
where Abs control is the absorbance of the negative control, and Abs tested is Absorbance of the test sample at 30 min. The tests were done in triplicate and the half maximal inhibitory concentration (IC50) values were reported as means ± SD.
I% = [(Abs control − Abs tested)/Abs control] ∗ 100
3.4. Computational Methods
DFT calculations were performed using the B3LYP functional [53] combined with the 6-31G(d) basis set [54]. Optimization was achieved through the Berny analytical gradient method [55]. Stationary points were verified by frequency calculations to ensure that transition states (TSs) exhibited precisely one imaginary frequency. Intrinsic Reaction Coordinate (IRC) paths [56] were mapped to illustrate the energy profiles connecting each TS to the corresponding minima [57]. All computations were carried out with the Gaussian 09 suite of programs [58]. The global electrophilicity index ω [59] was calculated using the formula ω = (μ2/2η), where μ represents the electronic chemical potential and η denotes the chemical hardness. These quantities are derived from the one-electron energies of the frontier molecular orbitals, HOMO and LUMO, with μ defined as (EHOMO − ELUMO)/2 and η (ELUMO − EHOMO), respectively [60]. We also introduced an empirical nucleophilicity index (N) [61], based on HOMO energies within the Kohn-Sham framework [62], defined as N = EHOMO(Nu) − EHOMO(TCE). The nucleophilicity is referenced to tetracyanoethylene (TCE), which has the lowest HOMO energy among a wide range of organic molecules considered [61]. This reference allows for a convenient positive nucleophilicity scale.
4. Conclusions
In conclusion, we have successfully synthesized a new series of quinazolin-4(3H)-one-isoxazole molecules 5a–e, confirming their structures by characterization techniques such as infrared spectroscopy (IR), proton and carbon-13 nuclear magnetic resonance, 2D HMBC NMR, mass spectrometry, and corroborated by crystallographic studies. Preliminary studies on antioxidant activity show that the newly synthesized compounds do not exhibit significant efficacy as antioxidant agents. These results indicate that, although the basic structure is promising, further modifications are required to enhance their biological activity. The regioselectivity and mechanism of the 1,3-dipolar cycloaddition reaction between compound 3 and compound 4e were studied using DFT calculation methods at the B3LYP/6-31G(d) level. The study of the energy profiles associated with this reaction demonstrated that the formation of the two products P-1 and P-2 is exothermic by −81.15 and −77.32 kcal.mol−1, respectively, and indicating that P-1 is kinetically and thermodynamically favorable, in good agreement with the experimental results. Additionally, the IRC curve showed that the reaction follows an asynchronous concerted mechanism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17101390/s1. Reference [39] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.R., R.S. and A.A.; methodology, Y.R.; software, B.R. and S.C.; validation, Y.R., M.L. and K.H.; formal analysis, N.D.; investigation, A.N.; resources, B.E.-S.; data curation, A.S.; writing—original draft preparation, Y.R.; writing—review and editing, N.D. and M.M.A.; visualization, N.D.; supervision, M.E.Y.; project administration, M.E.Y. and G.N.; funding acquisition, M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Researchers Supporting Project number (RSPD2024R628), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Acknowledgments
N.D. thanks for the financial support received from a Grant for Young Researchers (SRG-UBB 32934/22.06.2023) funded by the Babes-Bolyai University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med. 2004, 36, 718–744. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Lu, T.; Chen, Z.; Ma, L.; Xu, Z.; Schaffer, P.; Lu, G. Design, synthesis and anti-mycobacterial activity evaluation of benzofuran-isatin hybrids. Eur. J. Med. Chem. 2018, 159, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Wang, X.; Wang, Y.; Tang, W.J.; Shi, J.B.; Liu, H. Novel curcumin analogue hybrids: Synthesis and anticancer activity. Eur. J. Med. Chem. 2018, 156, 493–509. [Google Scholar] [CrossRef]
- Maestro, A.; Martín-Encinas, E.; Alonso, C.; de Marigorta, E.M.; Rubiales, G.; Vicario, J.; Palacios, F. Synthesis of novel antiproliferative hybrid bis-(3-indolyl)methane phosphonate derivatives. Eur. J. Med. Chem. 2018, 158, 874–883. [Google Scholar] [CrossRef]
- Chopra, R.; Chibale, K.; Singh, K. Pyrimidine-chloroquinoline hybrids: Synthesis and antiplasmodial activity. Eur. J. Med. Chem. 2018, 148, 39–53. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, M.; Nepali, K.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Mohinder, P.; Bedi, S. Triazole tethered C 5-curcuminoid-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. Eur. J. Med. Chem. 2016, 116, 102–115. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L.; Bian, Y.; Li, Y.; Qu, J.; Song, F. The Antibacterial Activity of Quinazoline and Quinazolinone Hybrids. Curr. Top. Med. Chem. 2022, 22, 1035–1044. [Google Scholar] [CrossRef]
- Kalirajan, R.; Rafick, M.H.M.; Sankar, S.; Jubie, S. Characterization and Evaluation of Their Antioxidant and Cytotoxic Activities of Some Novel Isoxazole-Substituted 9-Anilinoacridine Derivatives. Sci. World J. 2012, 2012, 165258. [Google Scholar] [CrossRef]
- Kshirsagar, U.A. Organic & Biomolecular Chemistry Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Mercader, J.V.; Parra, J.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Ready access to proquinazid haptens via cross-coupling chemistry for antibody generation and immunoassay development. PLoS ONE 2015, 10, e0134042. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Cao, X.; Liu, F.; Wang, Q.; Liu, L.; Xue, W. Design, Synthesis, Antibacterial Activity, Antiviral Activity, and Mechanism of Myricetin Derivatives Containing a Quinazolinone Moiety. ACS Omega 2021, 6, 30826–30833. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- ElZahabi, H.S.A.; Nafie, M.S.; Osman, D.; Elghazawy, N.H.; Soliman, D.H.; EL-Helby, A.A.H.; Arafa, R.K. Design, synthesis and evaluation of new quinazolin-4-one derivatives as apoptotic enhancers and autophagy inhibitors with potent antitumor activity. Eur. J. Med. Chem. 2021, 222, 113609. [Google Scholar] [CrossRef] [PubMed]
- Alamshany, Z.M.; Tashkandi, N.Y.; Othman, I.M.M.; Ishak, E.A.; Gad-Elkareem, M.A.M. Synthesis, antimicrobial and antioxidant activities of some new pyrazolo[1,5-a]pyrimidine and imidazo[1,2-b]pyrazole derivatives based isoxazole. Synth. Commun. 2023, 53, 1451–1467. [Google Scholar] [CrossRef]
- Eid, A.M.; Hawash, M.; Amer, J.; Jarrar, A.; Qadri, S.; Alnimer, I.; Sharaf, A.; Zalmoot, R.; Hammoudie, O.; Hameedi, S.; et al. Synthesis and Biological Evaluation of Novel Isoxazole-Amide Analogues as Anticancer and Antioxidant Agents. Biomed. Res. Int. 2021, 2021, 6633297. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Mekkawy, M.H.; Karam, H.M.; Higgins, M.; Dinkova-Kostova, A.T.; Ghorab, M.M. Novel iodinated quinazolinones bearing sulfonamide as new scaffold targeting radiation induced oxidative stress. Bioorganic Med. Chem. Lett. 2021, 42, 128002. [Google Scholar] [CrossRef]
- Zicane, D.; Tetere, Z.; Mierina, I.; Turks, M.; Ravina, I.; Leonciks, A. Synthesis of quinazolinone-1, 3, 4-oxadiazole conjugates and studies of their antibacterial and antioxidant activity. J. Chem. Pharm. Res 2014, 6, 1153–1158. [Google Scholar]
- El-Sayed, A.; Ismail, M.; Amr, A.; Molecules, A.N. Synthesis, Antiproliferative, and Antioxidant Evaluation of 2-Pentylquinazolin-4(3H)-one(thione) Derivatives with DFT Study. Molecules 2019, 24, 3787. [Google Scholar] [CrossRef]
- Abdelall, E.K.A. Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg. Chem. 2020, 94, 103441. [Google Scholar] [CrossRef] [PubMed]
- Pedada, S.R.; Yarla, N.S.; Tambade, P.J.; Dhananjaya, B.L.; Bishayee, A.; Arunasree, K.M.; Philip, G.H.; Dharmapuri, G.; Aliev, G.; Putta, S.; et al. Synthesis of new secretory phospholipase A2-inhibitory indole containing isoxazole derivatives as anti-inflammatory and anticancer agents. Eur. J. Med. Chem. 2016, 112, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Savaliya, K.R. Design, synthesis and characterization of novel isoxazole-quinazolinone linked analogues as an antimicrobial agent. World J. Pharm. Res. 2023, 12, 1208. [Google Scholar]
- Yang, Z.B.; Li, P.; He, Y.J. Design, synthesis, and bioactivity evaluation of novel isoxazole-amide derivatives containing an acylhydrazone moiety as new active antiviral agents. Molecules 2019, 24, 3766. [Google Scholar] [CrossRef]
- Batra, S.; Srinivasan, T.; Rastogi, S.K.; Kundu, B.; Patra, A.; Bhaduri, A.P.; Dikshit, M. Combinatorial synthesis and biological evaluation of isoxazole-based libraries as antithrombotic agents. Bioorg. Med. Chem. Lett. 2002, 12, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.Y.; Caldwell, R.D.; Caravella, J.A.; Chen, L.; Creech, K.L.; Deaton, D.N.; Madauss, K.P.; Marr, H.B.; Mcfadyen, R.B.; Miller, A.B.; et al. Substituted isoxazole analogs of farnesoid X receptor (FXR) agonist GW4064. Bioorg. Med. Chem. Lett. 2009, 19, 2969–2973. [Google Scholar] [CrossRef]
- Badio, B.; Garraffo, H.M.; Plummer, C.V.; Padgett, W.L.; Daly, J.W. Synthesis and nicotinic activity of epiboxidine: An isoxazole analogue of epibatidine. Eur. J. Pharmacol. 1997, 321, 189–194. [Google Scholar] [CrossRef]
- Narlawar, R.; Pickhardt, M.; Leuchtenberger, S.; Baumann, K.; Krause, S.; Dyrks, T.; Weggen, S.; Mandelkow, E.; Schmidt, B. Curcumin-Derived Pyrazoles and Isoxazoles: Swiss Army Knives or Blunt Tools for Alzheimer’s Disease? ChemMedChem 2008, 3, 165–172. [Google Scholar] [CrossRef]
- Prashanthi, Y.; Kiranmai, K.; Subhashini, N. Synthesis, potentiometric and antimicrobial studies on metal complexes of isoxazole Schiff bases. Spectrochim. Acta Part A 2008, 70, 30–35. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; Van Almsick, A. Modern Agriculture 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors in Combination with Safeners: Solutions for Modern and Sustainable Agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef]
- Alshamari, A.; Al-Qudah, M.; Hamadeh, F.; Al-Momani, L.; Abu-Orabi, S. Synthesis, Antimicrobial and Antioxidant Activities of 2-Isoxazoline Derivatives. Molecules 2020, 25, 4271. [Google Scholar] [CrossRef] [PubMed]
- Fandakli, S. Synthesis of some new isoxazole compounds and their biological tyrosinase and antioxidant activities. Turk. J. Chem. 2022, 46, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Synthesis, V.O. Docking and Biological Evaluation of Novel Isoxazole Analogues as Anticancer and Antioxidant Agents. J. Pharm. Negat. Results 2022, 13, 2634–2642. [Google Scholar]
- Kanzouai, Y.; Chalkha, M.; Hadni, H.; Laghmari, M.; Bouzammit, R.; Nakkabi, A.; Benali, T.; Tüzün, B.; Akhazzane, M.; El Yazidi, M.; et al. Design, synthesis, in-vitro and in-silico studies of chromone-isoxazoline conjugates as anti-bacterial agents. J. Mol. Struct. 2023, 1293, 136205. [Google Scholar] [CrossRef]
- Chalkha, M.; Nour, H.; Chebbac, K.; Nakkabi, A.; Bahsis, L.; Bakhouch, M.; Akhazzane, M.; Bourass, M.; Chtita, S.; Jardan, Y.A.B.; et al. Synthesis, Characterization, DFT Mechanistic Study, Antimicrobial Activity, Molecular Modeling, and ADMET Properties of Novel Pyrazole-isoxazoline Hybrids. ACS Omega 2022, 7, 46731–46744. [Google Scholar] [CrossRef]
- Rhazi, Y.; Chalkha, M.; Nakkabi, A.; Hammoudan, I.; Akhazzane, M.; Bakhouch, M.; Chtita, S.; El Yazidi, M. Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry 2022, 4, 969–982. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Molecular Consortia—Various Structural and Synthetic Concepts for More Effective Therapeutics Synthesis. Int. J. Mol. Sci. 2018, 19, 1104. [Google Scholar] [CrossRef]
- Li, Y.; Touret, F.; de Lamballerie, X.; Nguyen, M.; Laurent, M.; Benoit-Vical, F.; Robert, A.; Liu, Y.; Meunier, B. Hybrid molecules based on an emodin scaffold. Synthesis and activity against SARS-CoV-2 and Plasmodium. Org. Biomol. Chem. 2023, 21, 7382–7394. [Google Scholar] [CrossRef]
- Hisano, T. Recent studies on the modified niementowski 4-quinazolone synthesis. A review. Org. Prep. Proced. Int. 1973, 5, 145–193. [Google Scholar] [CrossRef]
- Rammohan, A.; Zyryanov, G.V. Synthesis and characterization of 1,2,3-triazole integrated quinazolinone derivatives. AIP Conf. Proc. 2020, 2280, 040037. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Amaral, J.D.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Synthesis and evaluation of spiroisoxazoline oxindoles as anticancer agents. Bioorg. Med. Chem. 2014, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Aarjane, M.; Slassi, S.; Tazi, B.; Amine, A. Synthesis and biological evaluation of novel isoxazole derivatives from acridone. Arch. Pharm. 2021, 354, e2000261. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Alves, D.C.B.; Anjos, J.V.D.; Cavalcante, N.N.M.; Santos, G.K.N.; Navarro, D.M.D.A.F.; Srivastava, R.M. Larvicidal isoxazoles: Synthesis and their effective susceptibility towards Aedes aegypti larvae. Bioorg. Med. Chem. 2013, 21, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, M.K.; Revanasiddappa, H.D. Synthesis of some new glutamine linked 2,3-disubstituted quinazolinone derivatives as potent antimicrobial and antioxidant agents. Med. Chem. Res. 2013, 22, 2665–2676. [Google Scholar] [CrossRef]
- Arzine, A.; Abchir, O.; Chalkha, M.; Chebbac, K.; Rhazi, Y.; Barghady, N.; Yamari, I.; Moussaoui, A.E.L.; Nakkabi, A.; Akhazzane, M.; et al. Design, synthesis, In-vitro, In-silico and DFT studies of novel functionalized isoxazoles as antibacterial and antioxidant agents. Comput. Biol. Chem. 2024, 108, 107993. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- APEX4; Bruker AXS Inc.: Madison, WI, USA, 2023.
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization, Crystal Impact—H. Putz & K. Brandenburg GbR, Kreuzherrenstr. 102, D-53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 25 September 2024).
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea odorata subsp. Pectinata and Ruta Montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Hehre, W.J. Ab Initio Molecular Orbital Theory. Acc. Chem. Res. 1976, 9, 399–406. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Fukui, K. A formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Kawsar, S.M.A.; Hosen, M.A.; Ahmad, S.; El Bakri, Y.; Laaroussi, H.; Hadda, T.B.; Almalki, F.A.; Ozeki, Y.; Goumri-Said, S. Potential SARS-CoV-2 RdRp inhibitors of cytidine derivatives: Molecular docking, molecular dynamic simulations, ADMET, and POM analyses for the identification of pharmacophore sites. PLoS ONE 2022, 17, e0273256. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G.; Pearson, R.G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).