Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications
Abstract
1. Introduction
2. Intervention of Hypoxia-Mediated Pathways and Cancer Therapy
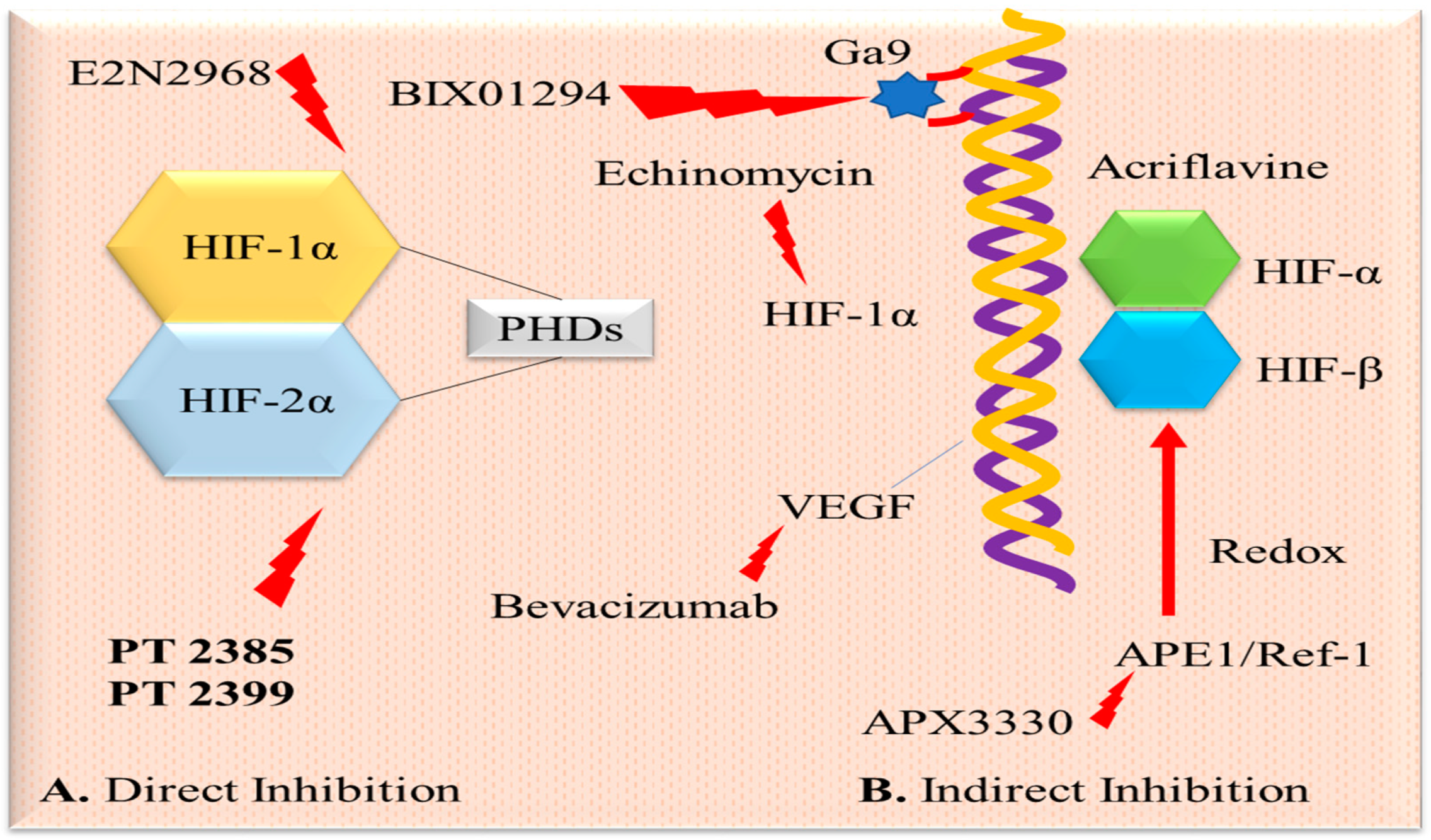
2.1. Hypoxia-Inducible Factor-1 and Targeted Therapy
2.2. Metabolic Reprogramming by Hypoxia and Its Targets
2.3. Hypoxia-Mediated Adaptation to Apoptosis and Its Therapeutic Target
2.4. Genomic Instability and Its Target
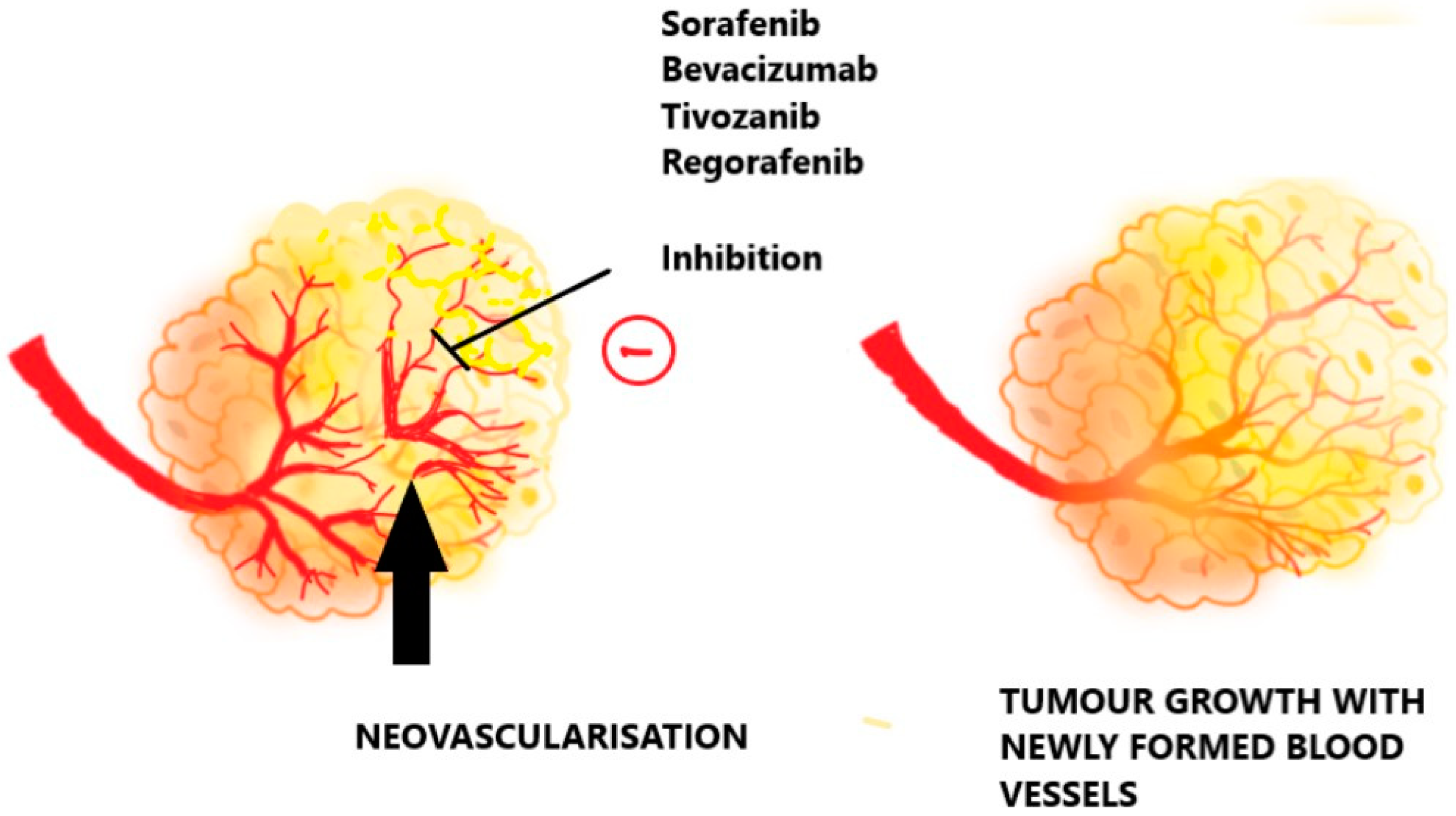
2.5. Angiogenesis and Possible Targets
2.6. Inhibition of PI3K/AKT/mTOR Pathway
3. Classification of Anti-Hypoxic Drugs
3.1. Hypoxia Inhibitor
3.2. Hypoxia-Activated Prodrugs
3.3. Agents for Molecular Targeting
3.4. Supplemental Oxygenation
3.5. Recombinant Anaerobic Bacteria
3.6. Mitochondrial Electron Transport Chain Inhibitors
3.7. Inhibitors of the Mitochondrial Electron Transport Chain
3.8. Hypoxia-Targeted Immunotherapy
3.9. Small Interfering RNA-Mediated Gene Silencing
4. Drawback of Current Treatment with Anti-Hypoxic Drugs
4.1. Assessment of In Vivo Tumor Hypoxia
4.1.1. Lack of Validated Hypoxia Diagnostic Modalities
4.1.2. Limitations of Positron Emission Tomography Scan
4.2. Challenges in Hypoxia-Activated Prodrug Delivery
4.2.1. Tumor Interstitial Fluid Pressure
4.2.2. The Disruption of the Vasculature
4.2.3. The pH of the Microenvironment
4.3. Hypersensitivity Towards HAPs
4.4. Compensatory Upregulation
4.5. Targeting Specific Compartments
4.6. Challenges of a 3D Microsystem
4.7. Toxicities
5. Intervention of Nanoparticulate-Based Anti-Hypoxic Drug Delivery
5.1. Lipoidal Nanoparticles
5.2. Polymeric Nanoparticles
5.3. Metal Nanoparticles
6. Scope of Nanoparticle-Based Targeted Delivery of Anti-Hypoxic Drugs
6.1. Lungs Targeted
6.2. Liver Targeted
6.3. Colon Targeted
6.4. Breast Targeted
6.5. Brain Targeted
6.6. Pancreas Targeted
7. Conclusions
7.1. Impact on Real-World Outcomes
7.2. Key Areas for Improvement
7.3. Potential of Further Research
7.4. Future Directions and Evolution of the Field
7.5. Speculative Viewpoint for the Next Five Years
Author Contributions
Funding
Conflicts of Interest
References
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, C.; Man, Y.K.S.; Chisholm, J.C.; Lepicard, E.Y.; Robinson, S.P.; Shipley, J.M. Hypoxia and Its Therapeutic Possibilities in Paediatric Cancers. Br. J. Cancer 2020, 124, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Su, K.; Zhao, D.; Lu, A.; Zhong, C. Nanomaterials for Tumor Hypoxia Relief to Improve the Efficacy of ROS-Generated Cancer Therapy. Front. Chem. 2021, 9, 649158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, W.; Niu, M.; Tian, J.; Xu, K. Hypoxia-Active Nanoparticles Used in Tumor Theranostic. Int. J. Nanomed. 2019, 14, 3705–3722. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Tirpe, A.; Gulei, D.; Ciortea, S.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Albadari, N.; Deng, S.; Li, W. The Transcriptional Factors HIF-1 and HIF-2 and Their Novel Inhibitors in Cancer Therapy. Expert Opin. Drug Discov. 2019, 14, 667. [Google Scholar] [CrossRef]
- Li, T.; Mao, C.; Wang, X.; Shi, Y.; Tao, Y. Epigenetic Crosstalk between Hypoxia and Tumor Driven by HIF Regulation. J. Exp. Clin. Cancer Res. 2020, 39, 224. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Moghadaszadeh Ardebili, S.; Baghi Moornani, M.; Masjedi, A.; Atyabi, F.; Kiani, M.; Namdar, A.; Karpisheh, V.; Izadi, S.; Baradaran, B.; et al. Silencing of HIF-1α/CD73 Axis by SiRNA-Loaded TAT-Chitosan-Spion Nanoparticles Robustly Blocks Cancer Cell Progression. Eur. J. Pharmacol. 2020, 882, 173235. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Pliszka, M.; Szablewski, L. Glucose Transporters as a Target for Anticancer Therapy. Cancers 2021, 13, 4184. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.H.; Vassileva, V.; Acedo, P.; Damink, S.W.M.O.; Malago, M.; Dhar, D.K.; Pereira, S.P. Targeting Pyruvate Kinase M2 and Lactate Dehydrogenase A Is an Effective Combination Strategy for the Treatment of Pancreatic Cancer. Cancers 2019, 11, 1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate Dehydrogenase Kinases (PDKs): An Overview toward Clinical Applications. Biosci. Rep. 2021, 41, 20204402. [Google Scholar] [CrossRef]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic Dysregulation and Emerging Therapeutical Targets for Hepatocellular Carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, J.; Zhang, Q.; Wang, Z.; Wang, N.; He, S.; Zhao, Y.; Naman, C.B. Progress in the Development of Eukaryotic Elongation Factor 2 Kinase (EEF2K) Natural Product and Synthetic Small Molecule Inhibitors for Cancer Chemotherapy. Int. J. Mol. Sci. 2021, 22, 2408. [Google Scholar] [CrossRef]
- Kunder, R.; Velyunskiy, M.; Dunne, S.F.; Cho, B.K.; Kanojia, D.; Begg, L.; Orriols, A.M.; Fleming-Trujillo, E.; Vadlamani, P.; Vialichka, A.; et al. Synergistic PIM Kinase and Proteasome Inhibition as a Therapeutic Strategy for MYC-Overexpressing Triple-Negative Breast Cancer. Cell Chem. Biol. 2022, 29, 358–372. [Google Scholar] [CrossRef]
- Stoica, A.-F.; Chang, C.-H.; Pauklin, S. Molecular Therapeutics of Pancreatic Ductal Asdenocarcinoma: Targeted Pathways and the Role of Cancer Stem Cells. Trends Pharmacol. Sci. 2020, 41, 977–993. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N.; Guo, X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 719836. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Md Hashim, N.F.; Ammar, A.; Zakuan, N.M. An Insight into the Anti-Angiogenic and Anti-Metastatic Effects of Oridonin: Current Knowledge and Future Potential. Molecules 2021, 26, 775. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, C.; Zhang, Y.; Li, A.; Sun, C.; Li, R.; Xing, Y.; Shi, M.; Wang, Q. PKI-587 Enhances Radiosensitization of Hepatocellular Carcinoma by Inhibiting the PI3K/AKT/MTOR Pathways and DNA Damage Repair. PLoS ONE 2021, 16, e0258817. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, S.V.; Reginato, M.J. Progress toward Overcoming Hypoxia-Induced Resistance to Solid Tumor Therapy. Cancer Manag. Res. 2015, 7, 264. [Google Scholar] [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.-M. An Overview of the Recent Development of Anticancer Agents Targeting the HIF-1 Transcription Factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef]
- D’Oto, A.; Tian, Q.; Davidoff, A.M.; Yang, J. Histone Demethylases and Their Roles in Cancer Epigenetics. J. Med. Oncol. Ther. 2016, 1, 40. [Google Scholar] [CrossRef]
- Ikeda, H.; Kakeya, H. Targeting Hypoxia-Inducible Factor 1 (HIF-1) Signaling with Natural Products toward Cancer Chemotherapy. J. Antibiot. 2021, 74, 687–695. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.F. Targeting Hypoxia: Hypoxia-Activated Prodrugs in Cancer Therapy. Front. Oncol. 2021, 11, 700407. [Google Scholar] [CrossRef]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic Targeting of the Hypoxic Tumour Microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef]
- Anduran, E.; Dubois, L.J.; Lambin, P.; Winum, J.-Y. Hypoxia-Activated Prodrug Derivatives of Anti-Cancer Drugs: A Patent Review 2006–2021. Expert Opin. Ther. Pat. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- de la Torre, P.; Perez-Lorenzo, M.J.; Alcazar-Garrido, A.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef] [PubMed]
- Zaccagna, F.; Grist, J.T.; Quartuccio, N.; Riemer, F.; Fraioli, F.; Caracò, C.; Halsey, R.; Aldalilah, Y.; Cunningham, C.H.; Massoud, T.F.; et al. Imaging and Treatment of Brain Tumors through Molecular Targeting: Recent Clinical Advances. Eur. J. Radiol. 2021, 142, 109842. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting Hypoxia in the Tumor Microenvironment: A Potential Strategy to Improve Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 24. [Google Scholar] [CrossRef] [PubMed]
- Ferenz, K.B.; Steinbicker, A.U. Artificial Oxygen Carriers—Past, Present, and Future—A Review of the Most Innovative and Clinically Relevant Concepts. J. Pharmacol. Exp. Ther. 2019, 369, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Winslow, R.M. Blood Substitutes. Curr. Opin. Hematol. 2002, 9, 146–151. [Google Scholar] [CrossRef]
- Mowday, A.M.; van de Laak, J.M.; Fu, Z.; Henare, K.L.; Dubois, L.; Lambin, P.; Theys, J.; Patterson, A.V. Tumor-Targeting Bacteria as Immune Stimulants—The Future of Cancer Immunotherapy? Crit. Rev. Microbiol. 2024, 50, 1–16. [Google Scholar] [CrossRef]
- Drozdz, M.; Makuch, S.; Cieniuch, G.; Wozniak, M.; Ziołkowski, P. Obligate and Facultative Anaerobic Bacteria in Targeted Cancer Therapy: Current Strategies and Clinical Applications. Life Sci. 2020, 261, 118296. [Google Scholar] [CrossRef]
- Yu, B.; Yang, M.; Shi, L.; Yao, Y.; Jiang, Q.; Li, X.; Tang, L.H.; Zheng, B.J.; Yuen, K.Y.; Smith, D.K.; et al. Explicit Hypoxia Targeting with Tumor Suppression by Creating an “Obligate” Anaerobic Salmonella Typhimurium Strain. Sci. Rep. 2012, 2, 436. [Google Scholar] [CrossRef]
- Tao, C.; Miao, X.; Yan, J.; Xiao, X.; Wu, R.; Cao, Q.; Wang, Z.; Lv, R.; Ge, T.; Liu, J. Hypoxia-Targeted and Spatial-Selective Tumor Suppression by near Infrared Nanoantenna Sensitized Engineered Bacteria. Acta Biomater. 2023, 170, 442–452. [Google Scholar] [CrossRef]
- Siles, R. Design, Synthesis and Biological Evaluation of New Anti-Cancer Nitrogen-Containing Combretastatins and Novel Cysteine Protease Inhibitors for the Treatment of Chagas. Available online: https://baylor-ir.tdl.org/handle/2104/3018 (accessed on 8 November 2021).
- Wang, S.; Zhou, X.; Zeng, Z.; Sui, M.; Chen, L.; Feng, C.; Huang, C.; Yang, Q.; Ji, M.; Hou, P. Atovaquone-HSA Nano-Drugs Enhance the Efficacy of PD-1 Blockade Immunotherapy by Alleviating Hypoxic Tumor Microenvironment. J. Nanobiotechnol. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Vito, A.; El-Sayes, N.; Mossman, K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhang, J.; Wang, J. The Immune Regulatory Role of Adenosine in the Tumor MicroenvironmentNo Title. Int. J. Mol. Sci. 2023, 24, 14928. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Sitkovsky, M.V. Antihypoxic Oxygenation Agents with Respiratory Hyperoxia to Improve Cancer Immunotherapy. J. Clin. Investig. 2020, 130, 5629–5637. [Google Scholar] [CrossRef] [PubMed]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of Tumor Hypoxia for Radiotherapy: Current Status and Future Directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef]
- Castellano, A.; Bailo, M.; Cicone, F.; Carideo, L.; Quartuccio, N.; Mortini, P.; Falini, A.; Cascini, G.L.; Minniti, G. Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Semenova, N.; Tuchin, V. V 3D Models of the Dynamics of Cancer Cells under External Pressure. Chaos 2021, 31, 083122. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Ivan, M.; Fishel, M.L.; Tudoran, O.M.; Pollok, K.E.; Wu, X.; Smith, P.J. Hypoxia Signaling: Challenges and Opportunities for Cancer Therapy. Semin. Cancer Biol. 2021, 85, 185–195. [Google Scholar] [CrossRef]
- Kumar, K.; Wigfield, S.; Gee, H.E.; Devlin, C.M.; Singleton, D.; Li, J.-L.; Buffa, F.; Huffman, M.; Sinn, A.L.; Silver, J.; et al. Dichloroacetate Reverses the Hypoxic Adaptation to Bevacizumab and Enhances Its Antitumor Effects in Mouse Xenografts. J. Mol. Med. 2013, 91, 758. [Google Scholar] [CrossRef]
- Ma, L.; Li, G.; Zhu, H.; Dong, X.; Zhao, D.; Jiang, X.; Li, J.; Qiao, H.; Ni, S.; Sun, X. 2-Methoxyestradiol Synergizes with Sorafenib to Suppress Hepatocellular Carcinoma by Simultaneously Dysregulating Hypoxia-Inducible Factor-1 and -2. Cancer Lett. 2014, 355, 96–105. [Google Scholar] [CrossRef]
- Zhao, D.; Zhai, B.; He, C.; Tan, G.; Jiang, X.; Pan, S.; Dong, X.; Wei, Z.; Ma, L.; Qiao, H.; et al. Upregulation of HIF-2α Induced by Sorafenib Contributes to the Resistance by Activating the TGF-α/EGFR Pathway in Hepatocellular Carcinoma Cells. Cell. Signal. 2014, 26, 1030–1039. [Google Scholar] [CrossRef]
- Thiepold, A.-L.; Lorenz, N.I.; Foltyn, M.; Engel, A.L.; Divé, I.; Urban, H.; Heller, S.; Bruns, I.; Hofmann, U.; Dröse, S.; et al. Mammalian Target of Rapamycin Complex 1 Activation Sensitizes Human Glioma Cells to Hypoxia-Induced Cell Death. Brain 2017, 140, 2623–2638. [Google Scholar] [CrossRef] [PubMed]
- Shelton, S.E.; Nguyen, H.T.; Barbie, D.A.; Kamm, R.D. Engineering Approaches for Studying Immune-Tumor Cell Interactions and Immunotherapy. iScience 2021, 24, 101985. [Google Scholar] [CrossRef] [PubMed]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-Responsive Nanoparticle Based Drug Delivery Systems in Cancer Therapy: An up-to-Date Review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Zhao, G.; Zhang, L.; Zhao, Z.; Wang, Z.; Zhao, L.; Hua, L.; Naveena, K.; Lu, J.; et al. Hypoxia-Responsive Lipid–Polymer Nanoparticle-Combined Imaging-Guided Surgery and Multitherapy Strategies for Glioma. ACS Appl. Mater. Interfaces 2020, 12, 52319–52328. [Google Scholar] [CrossRef]
- Liu, L.-H.; Zhang, Y.-H.; Qiu, W.-X.; Zhang, L.; Gao, F.; Li, B.; Xu, L.; Fan, J.-X.; Li, Z.-H.; Zhang, X.-Z. Dual-Stage Light Amplified Photodynamic Therapy against Hypoxic Tumor Based on an O2 Self-Sufficient Nanoplatform. Small 2017, 13, 1701621. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wu, L.; Tian, X.; Shen, S. Lipid Nanoparticles for the Controlled Generation of Free Radicals and Effective Treatment of Hypoxic Cancer. Chem. Lett. 2020, 49, 817–819. [Google Scholar] [CrossRef]
- Chen, H.; He, W.; Guo, Z. An H2O2-Responsive Nanocarrier for Dual-Release of Platinum Anticancer Drugs and O2: Controlled Release and Enhanced Cytotoxicity against Cisplatin Resistant Cancer Cells. Chem. Commun. 2014, 50, 9714–9717. [Google Scholar] [CrossRef]
- Europe I de Cancérologie, S. Study of Preoperative Radiation Therapy with Concomitant Liposomal Transcrocetin (L-TC) in Soft Tissue Sarcomas. Available online: https://clinicaltrials.gov/study/NCT06476704?cond=Hypoxia,cancer&page=23&rank=221 (accessed on 8 October 2024).
- Mertes, P.M.; Collange, O.; Coliat, P.; Banerjee, M.; Diringer, M.C.; Roche, A.; Delabranche, X.; Chaban, V.; Voegelin, M.; Bernard, A.; et al. Liposomal Encapsulation of Trans-Crocetin Enhances Oxygenation in Patients with COVID-19-Related ARDS Receiving Mechanical Ventilation. J. Control. Release 2021, 336, 252–261. [Google Scholar] [CrossRef]
- Murphy, D.A.; Cheng, H.; Yang, T.; Yan, X.; Adjei, I.M. Reversing Hypoxia with PLGA-Encapsulated Manganese Dioxide Nanoparticles Improves Natural Killer Cell Response to Tumor Spheroids. Mol. Pharm. 2021, 18, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Wu, X.; Guo, L.; Wang, F.; Xia, G.; Chen, B.; Yin, H.; Wang, Y.; Li, X. Tf-PEG-PLL-PLGA Nanoparticles Enhanced Chemosensitivity for Hypoxia-Responsive Tumor Cells. Onco. Targets. Ther. 2016, 9, 5049–5059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3 Nanoparticles for Timely Supply of Oxygen under near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ji, C.; Liang, C.; Song, X.; Yi, X.; Dong, Z.; Yang, K.; Liu, Z. TaOx Decorated Perfluorocarbon Nanodroplets as Oxygen Reservoirs to Overcome Tumor Hypoxia and Enhance Cancer Radiotherapy. Biomaterials 2017, 112, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, E.J.; Kim, J.W.; Chung, U.S.; Koh, W.G.; Keum, K.C.; Koom, W.S. Gold Nanoparticles Enhance Anti-Tumor Effect of Radiotherapy to Hypoxic Tumor. Radiat. Oncol. J. 2016, 34, 230. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon Nanoparticles Enhance Reactive Oxygen Levels and Tumour Growth Inhibition in Photodynamic Therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to Metallic Nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 289. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor Microenvironment in Glioblastoma: Current and Emerging Concepts. Neuro-Oncology Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Feczko, T. Polymeric Nanotherapeutics Acting at Special Regions of Body. J. Drug Deliv. Sci. Technol. 2021, 64, 102597. [Google Scholar] [CrossRef]
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Red Blood Cell-Membrane-Coated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Enhanced Chemo- and Hypoxia-Activated Therapy. ACS Appl. Bio Mater. 2019, 2, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Targeted Nanotherapeutics for Respiratory Diseases: Cancer, Fibrosis, and Coronavirus. Adv. Ther. 2021, 4, 2000203. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Pec, M.; Zhai, K.; Smejkal, K.; Mirzaei, S.; et al. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Batenburg, M.C.T.; van den Bongard, H.J.G.D.; Kleynen, C.E.; Maarse, W.; Witkamp, A.; Ernst, M.; Doeksen, A.; van Dalen, T.; Sier, M.; Schoenmaeckers, E.J.P.; et al. Assessing the Effect of Hyperbaric Oxygen Therapy in Breast Cancer Patients with Late Radiation Toxicity (HONEY Trial): A Trial Protocol Using a Trial within a Cohort Design. Trials 2020, 21, 980. [Google Scholar] [CrossRef]
- Sulaiman, A.; McGarry, S.; Chambers, J.; Al-Kadi, E.; Phan, A.; Li, L.; Mediratta, K.; Dimitroulakos, J.; Addison, C.; Li, X.; et al. Targeting Hypoxia Sensitizes TNBC to Cisplatin and Promotes Inhibition of Both Bulk and Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 5788. [Google Scholar] [CrossRef]
- Jin, M.Z.; Wang, X.P. Immunogenic Cell Death-Based Cancer Vaccines. Front. Immunol. 2021, 12, 2030. [Google Scholar] [CrossRef]
- Womeldorff, M.; Gillespie, D.; Jensen, R.L. Hypoxia-Inducible Factor-1 and Associated Upstream and Downstream Proteins in the Pathophysiology and Management of Glioblastoma. Neurosurg. Focus 2014, 37, E8. [Google Scholar] [CrossRef]
- Jin, J.; Bae, K.H.; Yang, H.; Lee, S.J.; Kim, H.; Kim, Y.; Joo, K.M.; Seo, S.W.; Park, T.G.; Nam, D.H. In Vivo Specific Delivery of C-Met SiRNA to Glioblastoma Using Cationic Solid Lipid Nanoparticles. Bioconjug. Chem. 2011, 22, 2568–2572. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of Hypoxia in Cancer Therapy by Regulating the Tumor Microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Li, X.X.; Liu, C.; Dong, S.L.; Ou, C.S.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Anticarcinogenic Potentials of Tea Catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]


| Pathways | Enzymes | Effects | Example | References |
|---|---|---|---|---|
| Glucose transport | GLUT1, GLUT3 | Helps transport glucose for tumor cell survival | GLUT1 inhibitor WZB117 | [12] |
| Glycolysis | Aldolase A, HK1, PKM2, and LDHA | Facilitates glycolysis in tumor cells | FX11 inhibits LDHA;pharmacological inhibition of PKM2 | [13] |
| Mitochondrial oxidative metabolism | PDK1 expression | PDK1 regulates PDH, the enzyme responsible for converting pyruvate to acetyl-coenzyme A, which enters the tricarboxylic acid cycle | Dichloroacetate is a potent PDK inhibitor that has been shown to trigger apoptosis in preclinical animals | [14] |
| Lipid metabolism | Lipin-1 | Absorption and lipid droplet accumulation | Numerous FAS inhibitors have demonstrated anticancer efficacy, including cerulenin, C75, orlistat, C93, and GSK837149A | [15] |
| Type | Nanoformulation | The Reason Behind the Development | Achievement |
|---|---|---|---|
| Lipid | LN [LN (DOX + ICG)] | Controls the invasiveness and aggressive growth of gliomas | Incorporation of four different functional constituents like targeted delivery to gliomas, rapid release of DOX, image-guided surgery, and inhibition of glioma growth [57] |
| LipoMB/CaO2 | Hypoxia limits the efficiency of traditional PDT | Due to O2’s self-sufficient property, LipoMB/CaO2 demonstrated dual-stage light-driven PDT [58] | |
| 2,2-azobis [2-(2-imidazoline-2-yl)propane] dihydrochloride (AIPH)-loaded lipid nanoparticles | A hypoxic environment reduces the therapeutic outcomes | Lipid nanoparticles deliver polymerization initiators that generate free radicals in an oxygen-dependent manner [59] | |
| Photosensitizer-loaded perfluorocarbon nanodroplets | Inadequate oxygen supply during PDT efficacy | Oxygen self-enriching photodynamic therapy accelerated the production of 1O2 and elevated cytotoxicity [60] | |
| Liposomal trans-crocetin (L-TC) | A natural product that can help with hypoxia by increasing the diffusion of oxygen in plasma and tissues | Successfully used in phase 2 clinical trial for preoperative hypo-fractionated radiotherapy to localized or locally advanced soft-tissue sarcoma [61,62] | |
| Polymer | Fluorescent platinum complex encapsulated in Quencher-2 doped with PLGA | Increased production of H2O2 is associated with many cancers that are responsible for oxidative stress | Stimuli-responsive carriers release drugs and O2 sustainably [60] |
| MnO2 NPs encapsulated into PLGA to produce PLGA-MnO2 NPs | Hypoxia triggers immunosuppression in TME, resulting in the inhibition of the cytotoxic function of natural killer cells | PLGA-MnO2 NPs demonstrated O2 production and sustained high O2 tension; this nanocomposite reduces hypoxia after penetrating cancer spheroids [63] | |
| Polyethylene glycol (PEG)-Poly L-lysine (PLL)-Poly lactic-co-glycolic acid (PLGA)-based nanoparticles modified by transferrin-loaded daunorubicin (DNR-Tf-PEG-PLL-PLGA-NPs) | Hypoxia is a critical component of solid tumors and hampers cancer therapy | The downregulation of HIF-1α was observed with the DNR-Tf-NPs; additionally, significant induction of apoptosis was detected by overcoming the hypoxia [64] | |
| Metal | PEG-functionalized hollow Bi2Se3 nanoparticles (PEG-Bi2Se3@PFC@O2) | Hypoxia-associated resistance to radiotherapy is a big challenge | Hollow nanoparticles carried O2 to improve tumor oxygenation: an effective therapeutic outcome; O2-loaded perfluorocarbon nanodroplets enhance RT therapy [65]. |
| PEG-stabilized PFC nanodroplet decorated with TaOx nanoparticles (TaOx@PFC-PEG) | The absorption of radiation energy by tumor cells is less during hypoxic conditions | TaOx@PFC-PEG absorbs X-rays, increasing radiation energy in tumor cells, whereas PFC supplies O2 in the TME [66] | |
| Gold nanoparticle (GNPs) | Hypoxia impairs the therapeutic efficacy of radiotherapy | GNPs combined with radiotherapy improved the antitumor effect in hypoxic tumors [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, S.K.; Debnath, M.; Ghosh, A.; Srivastava, R.; Omri, A. Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications. Pharmaceuticals 2024, 17, 1389. https://doi.org/10.3390/ph17101389
Debnath SK, Debnath M, Ghosh A, Srivastava R, Omri A. Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications. Pharmaceuticals. 2024; 17(10):1389. https://doi.org/10.3390/ph17101389
Chicago/Turabian StyleDebnath, Sujit Kumar, Monalisha Debnath, Arnab Ghosh, Rohit Srivastava, and Abdelwahab Omri. 2024. "Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications" Pharmaceuticals 17, no. 10: 1389. https://doi.org/10.3390/ph17101389
APA StyleDebnath, S. K., Debnath, M., Ghosh, A., Srivastava, R., & Omri, A. (2024). Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications. Pharmaceuticals, 17(10), 1389. https://doi.org/10.3390/ph17101389







