Abstract
A strong synthetic tool for many naturally occurring chemicals, polymers, and pharmaceutical substances is transition metal-catalyzed synthesis. A serious concern to human health is the emergence of bacterial resistance to a broad spectrum of antibacterial medications. The synthesis of chemical molecules that are potential antibacterial candidates is underway. The main contributions to medicine are found to be effective in transition metal catalysis and heterocyclic chemistry. This review underlines the use of heterocycles and certain effective transition metals (Pd, Cu, and Ni) as catalysts in chemical methods for the synthesis of antibacterial compounds. Pharmaceutical chemists might opt for clinical exploration of these techniques due to their potential.
1. Introduction
Over time, bacterial illnesses have grown to be a serious hazard, with elevated rates of death and morbidity globally [1]. Antimicrobial agents combat pathogenic bacteria by either eliminating or drastically lowering their metabolic activity. When antibiotics were initially developed in the 1900s, people believed that the battle against microbes had been won. However, it was quickly found that the microbes may become resistant to any medication that was being offered. Antibiotics that we currently have in stock are rapidly losing their effectiveness, and there is no indication that they will be sufficiently restocked soon [2,3]. An increasing number of bacterial infections resist conventional treatment and are challenging, if not untreatable, to cure. Globally, resistance to several antibiotics is becoming more widespread, and there are an increasing number of reports of treatment failures and rising expenses, particularly in the hospital setting [4,5]. Based on their ability to withstand these antimicrobial drugs, bacteria can be classified into two categories: drug-resistant bacteria and multiple drug-resistant bacteria. Bacteria that have developed a resistance to specific medications are known as multidrug-resistant, meaning that the bacteria can no longer be controlled or killed by the antibiotics. More importantly, the emergence of these multi-drug-resistant bacteria is one of the biggest hazards to public health and the whole economy. The main causes of multiple drug-resistant bacteria are overexpression of the efflux pump, bacterial gene mutation, and bacterial adaptability to antibiotics [6]. Most of the world’s countries have abused antibacterial agents, which complicates this issue. Between 1983 and 2012, the FDA approved fewer new systemic antibacterial medications, while the phenomenon of bacterial resistance was becoming even worse [2]. Antibiotic resistance is a global health concern that poses a danger to the effectiveness of traditional antibiotics against common bacterial diseases. In 2022, the WHO released the Global Antimicrobial Resistance and Use Surveillance System (GLASS) report, which reveals resistance rates among common bacterial infections. In 2019, the Centers for Disease Control and Prevention published an overview of risks posed by antibiotic resistance in the US, highlighting the “potentially catastrophic consequences of inaction”. To synthesize novel antibacterial drugs in response, new mechanisms are incredibly needed [7].
Small drug molecules have historically been the most often utilized medicines in antibacterial therapy [8,9]. The antibacterial properties of heterocyclic organic compounds are mostly mediated by metal ions [10,11]. Some natural products, including alkaloids such as morphine, vinblastine, and reserpine, and antibiotics such as cephalosporin and penicillin, include a heterocyclic moiety. Aromatic heterocyclic derivatives, such as β-lactam derivatives, are a key component of the chemical structure of antibiotics. These demonstrate the significant role that heterocyclic compounds play in the formation of antibacterial drugs when they combine with other molecules. Additional methods such as the azide-alkyne reaction and click reaction are also employed in the synthesis of antibiotics. Therefore, chemists must combine heterocycles with other small compounds and copper-catalyzed reactions, such as the azide-alkyne reaction and click reaction, to develop new antibacterial agents. FDA-approved different antibacterial drugs are synthesized by using different transition metals as catalysts [12].
Thus, it is necessary to alter the medications that are now on the market to develop brand-new antibiotics that are impervious to germs. It is now clear that there is an immediate requirement for new antibacterial medications that do not show resistance to currently used antibiotics, as well as for increased efforts to promote the prudent and responsible use of antibiotics [13,14] and improved infection control practices. The ability of organic synthesis to synthesize compounds that are primarily carbon-based, including those found in living things, was a key factor in this revolution. With time, pharmacists and chemists produced new medications by utilizing different approaches and techniques; however, these medications are insufficient. More precisely, the best chance for the quick discovery and development of novel antibiotics in the near future is for us to provide workable, diversifiable, fully synthetic pathways to antibiotic scaffolds that are not now accessible [15].
Drugs synthesized via metal catalysis are valsartan [16], losartan [17], irbesartan [18], atazanavir [19], ruxolitinib derivatives [20], etoricoxib [21], lapatinib [22], etc. Some marketed antibacterial drugs have also been synthesized by using transition metal catalysis, e.g., penicillin [23], cephalosporin [24], linezolid [25], vancomycin [26], and tetracycline derivatives [27]. Microwave Ullmann reaction conditions were used by Sun and colleagues (2012) to synthesize macrocyclic diaryl ethers, including diarylheptanoids [28]. Significant antibacterial activity was shown by the 15-membered p-fluorophenethylamine derivative and the geranylamine derivative, which were produced by the Ullmann process [29,30]. This review highlights the chemical approaches that might be used in the future to synthesize marketed drugs against antibacterial infections. This review provides insight into research areas of heterocyclic chemistry, transition metal catalysis, and medicinal chemistry. Pd, Cu, and Ni are widely used in synthetic chemistry because of their efficiency.
In this study, we compiled research from the previous five years that discussed the role of transition metals as catalysts in the synthesis of crucial small organic compounds and antibacterial agents. The research data have been organized specifically for efficient Pd, Cu, and Ni-catalyzed antibacterial molecule synthesis. Subdivision according to cross-coupling and named reactions is also being completed. The WHO has designated certain bacterial infections as “critical priorities”, and the rate of death from these diseases has increased in humans as well as in plants and animals. As a result, rising rates of antibiotic resistance are widely regarded as an impending problem. Studying all antibacterial compounds made with transition metal as a catalyst is therefore imperative to combat bacterial resistance to several drugs, reduce the mortality rate in living things, and combat multidrug-resistant germs.
2. Palladium-Catalyzed Synthesis
Currently, one of the most adaptable and practical methods for performing organic synthesis in both academic labs and industrial production facilities is cross-couplings and related reactions. For all these reactions to continue at a synthetically useful pace, a transition metal catalyst is typically required. Transition metal complexes find extensive uses in photochemistry, biological systems, material production, and catalysis.
Palladium is a key component in cross-coupling reactions, even though other metal centers might theoretically catalyze the different stages of these processes. Cross-coupling reactions in which carbon-carbon bonds are formed by using palladium as a catalyst include those of Suzuki, Sonogashira, Heck, Negishi, Stille, Corriu-Kumada, Ullmann, Tsuji-Trost, and Hiyama. Pd salts or complexes, either prepared or generated in situ upon the addition of a ligand, are popular Pd sources for cross-couplings and related processes. Palladium (II) species are typically selected as starting materials due to their greater stability. Following their in-situ reduction, these substances become palladium (0) species, which then add to the catalytic cycle. It is interesting to note that Pd metal has been used as a catalyst for these reactions for almost as long as the reactions themselves: the Heck reaction was successfully used for the first time in the early 1970s [31,32], and Julia and colleagues’ revolutionary research was the first to apply supported Pd metal [33,34]. Up to now, Pd metal-based catalytic systems for these reactions have been continually developed, yielding catalysts with good activity, selectivity, and durability.
2.1. Suzuki Cross-Coupling
Suzuki cross-coupling is a significant synthetic reaction utilized in laboratories and industries for combinatorial and synthetic objectives [35]. Palladium-catalyzed Suzuki-Miyaura coupling reaction, was employed by Mujahid et al. to produce thiophene carboxylate derivatives of 5 and 6 with moderate to acceptable yields. In contrast, compound 3 was synthesized in the presence of DCC and DMAP via Steglich esterification of carboxylic acid 1 with 2-bromo-4-chlorophenol 2. Compound 6a exhibited the highest potential value, possessing the strongest binding affinities and the largest potential utility. It was demonstrated as an antibacterial agent against E. coli with a minimum inhibitory concentration (MIC) of 50 mg/mL. Antibacterial effectiveness in vitro was assessed for the active lead molecules; compounds 5a, 6a, and 3 exhibited activities against bacteria (Scheme 1) [36].
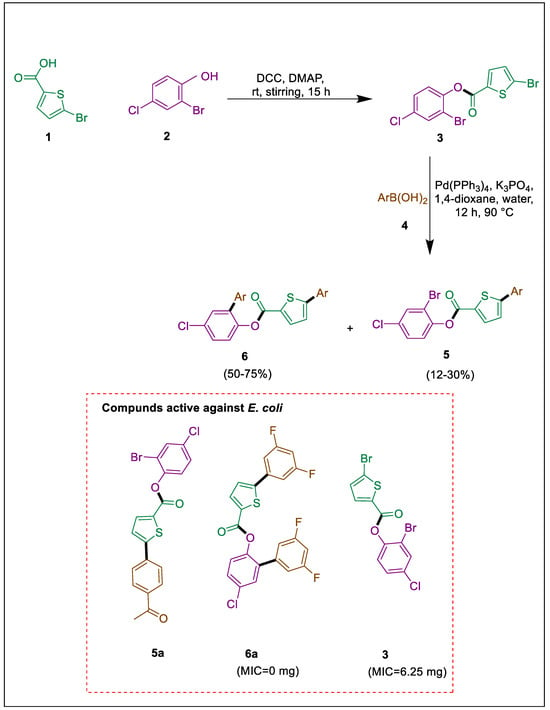
Scheme 1.
Synthesis of thiophene carboxylates by using Suzuki-Miyaura cross-coupling.
The number of antibiotic-resistant microbes has increased due to the recent overuse of medications [37]. All these advancements challenge scientific research regarding the synthesis of novel antimicrobial and anticancer agents. The noteworthy inhibitory effects of bromoindanol[1,2-b]quinolineamine compounds motivate us to examine their potential anticancer properties against various cancer cells and demonstrate their ability to kill bacteria. In their attempt to synthesize amine derivatives of indenol[1,2-b], Aydin et al. employed the Friedlander reaction. With MIC values ranging from 15.62–250 μg/mL, the monosubstituted indenol[1,2-b]quinolines (9 and 11) exhibit significantly enhanced antiproliferative and antimicrobial activity, according to the present investigation. The compound 11 (96% yield) was produced via Suzuki-Miyaura cross-coupling and possesses potent anticancer and antibacterial properties. Several bromo indan-1-ones 8 and 2-aminobenzonitrile 7 undergo a cyclodehydration reaction under toluene reflux, with Lewis’s acid serving as a catalyst and bromo indan-1-ones 8 treated with 2-amino-3,5-dibromobenzonitrile 7 to produce monobromo compound 9. Subsequently, one equivalent of phenylboronic acid 10 was coupled with compound 9a to produce 1-phenyl-5-indenol[1,2-b]quinoline amines 11 by using a Suzuki-Miyaura coupling reaction. The growth of all bacteria that are gram-positive was effectively halted by derivatives 9 and 11. At the same time, moderate antibacterial effects of compound 9a against E. coli and P. aeruginosa compound 9c against only E. coli were observed (Scheme 2) [38].
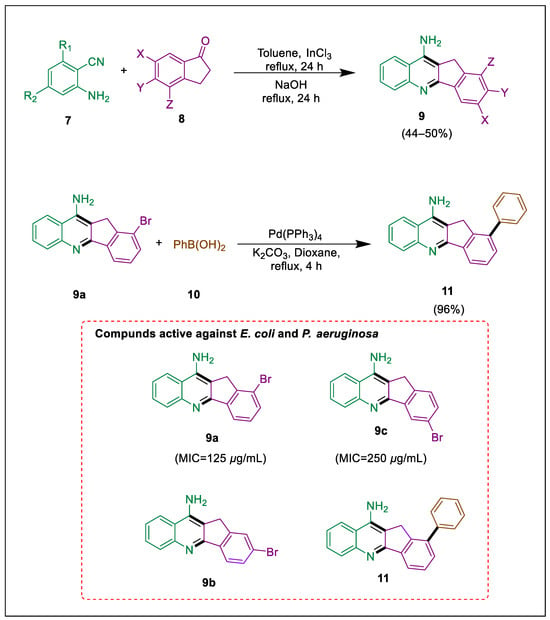
Scheme 2.
Synthesis of indenoquinoline amine by using Suzuki-Miyaura cross-coupling.
Types that are extraordinarily resistant to drugs, for which virtually no antibiotic is effective. Staphylococcus aureus is a bacterium that can cause disease [39]. The increasing resistance of S. aureus to antibacterial agents poses a significant clinical challenge in treating such infections. Natural bindles exhibit cytostatic and antifungal properties but no antibacterial activity by synthesizing natural compounds into pharmacophores, which have enhanced antibacterial activity compared to their parent molecules [40]. Rehberg et al. used the Masuda borylation-Suzuki coupling pathway to produce a library comprised of natural product-derived and artificial (di)azine-bridged bisindoles because unmodified bisindoles had no antibacterial action. 5,5′-chloro derivatives have remarkably significant action against other gram-positive bacteria as well as methicillin-resistant S. aureus (MRSA). Compounds 17a and 17b demonstrated significant in vivo effectiveness against MRSA in a wound infection test on mice. Natural Bisnode alkaloids, exemplified by Hyrtinadine A and Alocasin A, offer chemical scaffolds for drug development with considerable promise due to their recognized versatility in bioactivity. Desired product bisindoles 14 or 17 were obtained in high yield through the Masuda borylation of indoles 12 or 15 in dry 1,4-dioxane in an argon environment at 80–100 °C with water-free triethylamine and base cesium carbonate as carbonate/methanol mixtures for Suzuki coupling processes at elevated temperatures, followed by the addition of dithiatopazines or heteroaryl halides utilized in a mixture containing water as a co-solvent during the Suzuki coupling process. Compounds 17a, 17b, and 17c demonstrated broad-spectrum antibacterial activity against every gram-positive nosocomial pathogen examined. The bactericidal activity of compounds 14a, 14b, 17j, 17b, 17c, and 14c was significant (Scheme 3) [41].
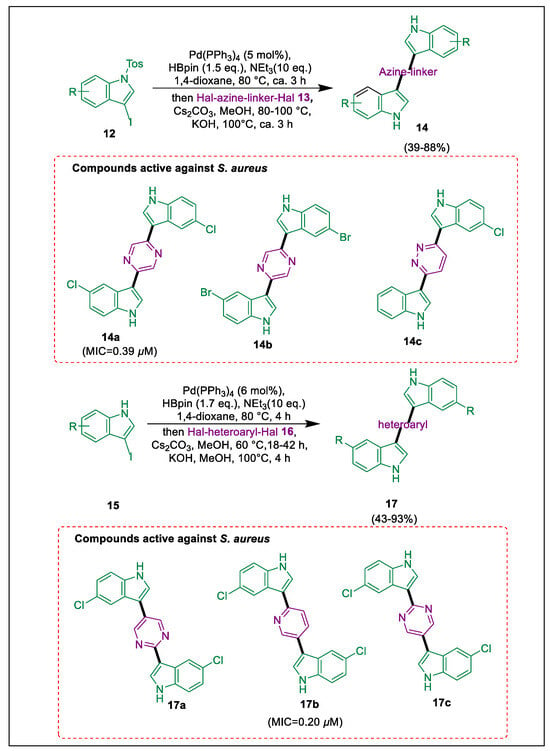
Scheme 3.
Synthesis of bisindoles derivatives by using Masuda borylation Suzuki coupling.
Exploring novel heterocycles that exhibit efficacy against numerous biological indicators continues to be a captivating technological pursuit. It has been documented that compounds comprising a quinazoline moiety reveal diverse natural and therapeutic characteristics. 1,3,4-Oxadiazole as a possible physical component. Phenylpiperazine bases that have been N-substituted exhibit various pharmacological effects [42,43,44,45,46]. A series of piperazine-fused and oxadiazole-fused quinazoline derivatives were produced by Patel et al. After an effective Suzuki cross-coupling on the quinazoline ring, 1,3,4-oxadiazole intermediates are generated as part of the synthetic protocol. In methanol, these oxadiazoles are treated with piperazine bases, while formalin is present to produce the final N-Mannich compounds. A considerable proportion of these compounds exhibit formidable antibacterial properties when tested against diverse strains of gram-positive bacteria. The synthesis of benzonitrile 19 involves the reaction of dichlorquinazoline 18 with 4-hydroxybenzonitrile under basic conditions. This molecule 19 then undergoes a Suzuki coupling with the phenyl ring of the boronic acid pinacol ester, resulting in the formation of quinazoline benzoate 20. Subsequently, hydrazide derivative 21 was obtained by treating the compound 20 with hydrazine hydrate in ethanol. Hydrazinecarbodithioate salt 22 was produced by cyclizing analogue 21 with carbon disulfide in ethanolic KOH. Subsequently, sulfuric acid and HCl were utilized to convert it into the comparable 1,3,4-oxadiazole derivatives 23, which were reacted with various piperazines in the presence of formalin in methanol to obtain Mannich bases 24. In contrast, the quinazoline derivative 24b exhibited more encouraging efficacy against S. aureus. Furthermore, the activity of 24a, 24c, and 24d against S. aureus was observed to be half-fold lower than that of 24b. Notably, the methoxy piperazine-fused derivative 24d exhibited activity against S. aureus as well. It was discovered that 24b, 24c, and 24d were effective against quinolone-resistant S. aureus (Scheme 4) [47].
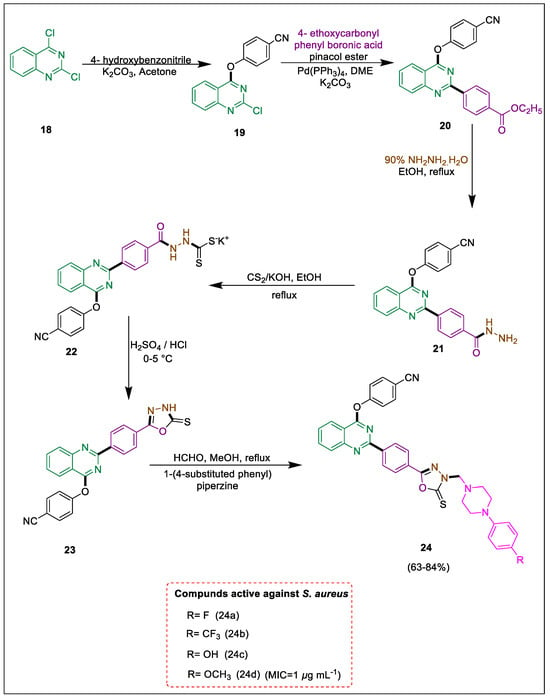
Scheme 4.
Synthesis of Mannich bases by using Suzuki cross-coupling.
Tetrazole is an essential structure of numerous drug molecules [48,49,50,51,52,53,54]. For the manufacturing of biphenyls and polyaryls, a Suzuki cross-coupling reaction catalyzed by palladium is utilized due to the importance of aryl-aryl bond formation in organic synthesis [55,56,57]. In organic synthesis, using microwave irradiation [58] is advantageous due to its eco-friendliness and increased product yield, and the solvent used for the reaction is water. An eco-friendly approach was devised by Ashok et al. to produce substituted methanone scaffolds. This was accomplished by utilizing a Suzuki interaction reaction in an aqueous solution, employing conventional heating methods and microwaves. In vitro, the evaluation of synthesized frameworks for antifungal and antibacterial properties was performed. Using microwave irradiation increases the reaction rate, decreases by-products, and uses water as a solvent. 1-(o-tolyl)ethan-1-one 25 reacted with tetrazole benzaldehyde 26 to form compound 27, which underwent a reaction with various substituted phenacyl bromides 28 used solvent dry acetone and anhydrous K2CO3 as a base to intermediate 29, which was subsequently subjected to treatment with replaced arylboronic acids 4 in the presence of a Pd catalyst and Na2CO3 to yield tetrazole derivatives 30. Compounds 30a, 30b, 30c, and 30d demonstrated the most significant zone of inhibition and superior activity against all bacterial organisms. The compounds 30e, 30f, and 30g exhibit a moderate zone of prevention against bacterial strains but demonstrate limited activity (Scheme 5) [49].
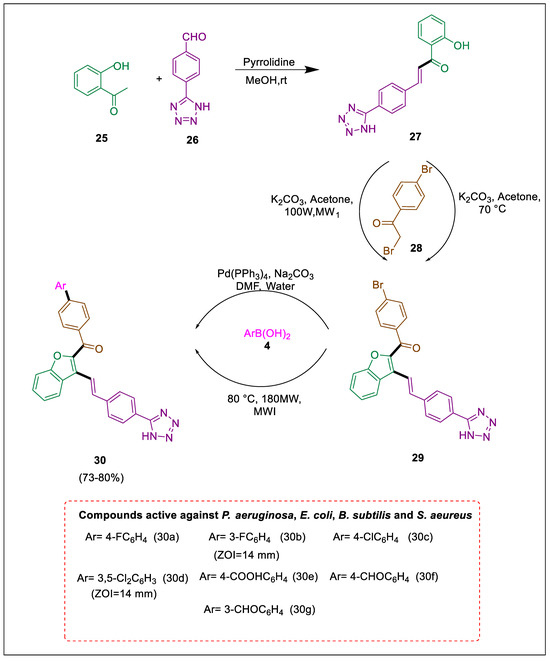
Scheme 5.
Synthesis of tetrazole derivatives by using Suzuki cross-coupling.
It has been noticed that products of various heterocycles, which include quinoline and 1,3,4-oxadiazole, contain an extensive array of biologically efficacious molecules [59,60,61,62,63]. Both quinoline and oxadiazoles are widely recognized heterocycles that exhibit a substantial biochemical profile. The synthesis, evaluation, and antibacterial and antifungal effects of derivatives based on quinoline and oxadiazole are detailed in this article. Ten novel oxadiazole derivatives were formed by Thummar and Bhatt utilizing 4,7-dichloroquinoline in sequential reactions with various moieties, including phenylboronic acid and 1,3,4-oxadiazole, which produced the desired target with an exceptional yield. The antibacterial activity of the synthesized substances against gram-positive and gram-negative microorganisms was assessed in vitro. Oxadiazole derivatives were synthesized via a Pd-catalyzed Suzuki coupling reaction and a nucleophilic substitution reaction. Arylhydrazide 31 and CS2 in KOH form 1,3,4-oxadizole derivatives 32, which were treated with 4,7-dichloroquinoline to afford a nucleophilic substitution product on the fourth position of 4,7-dichloroquinoline 33. The desired oxadiazole derivatives 34 resulted from the palladium-catalyzed reaction of the -Cl remaining at position 7 of 33 with phenylboronic acid in an N2 atmosphere. Compounds 34 were assessed for antimicrobial capacity at 50 to 500 µg/mL concentrations. Compounds 34a, 34b, and 34c exhibited MIC values of 50, 50, and 70 µg/mL, respectively (Scheme 6) [64].
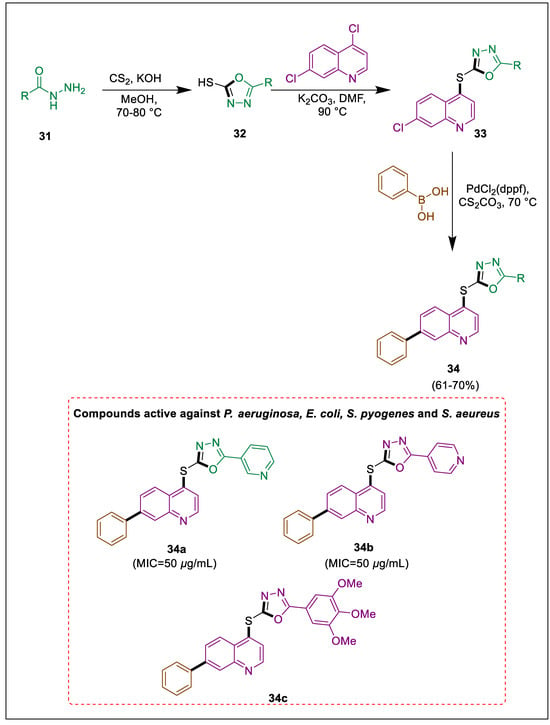
Scheme 6.
Synthesis of oxadiazole derivatives by using Suzuki cross-coupling.
The requirement for novel antimicrobial pharmaceuticals persists due to the diminishing efficacy of antibiotics that are presently accessible. With numerous biological functions, the pyrazole skeleton is a prominent structure in the pharmaceutical sector [65,66,67]. The initial identification of New-Delhi metallo-b-lactamase (NDM-1) occurred in 2009 in clinical isolates of E. coli and K. pneumoniae obtained from a patient in Sweden [68]. Ahmad et al. conducted a direct amination of protected amine 37 to produce benzamide 39. The pyrazole amide derivatives of interest 40 were formed through a Suzuki coupling of the intermediate molecule 39 with various boronic acids catalyzed by palladium. The antibacterial activity of newly synthesized analogues 40 against NDM-1-positive A. baumannii and K. pneumoniae was assessed. Protection of amine 35 by di-tert-butyl tricarbonate 36 to synthesize carboxylate 37 and its one pot condensation with benzoic acid 38 in pyridine to form intermediate 39, which undergo Suzuki coupling with different boronic acids to obtain pyrazole hybrids 40. Compounds 40a and 40b exhibited the most significant zone of suppression towards NDM-positive A. baumannii compared to other compounds (Scheme 7) [69].
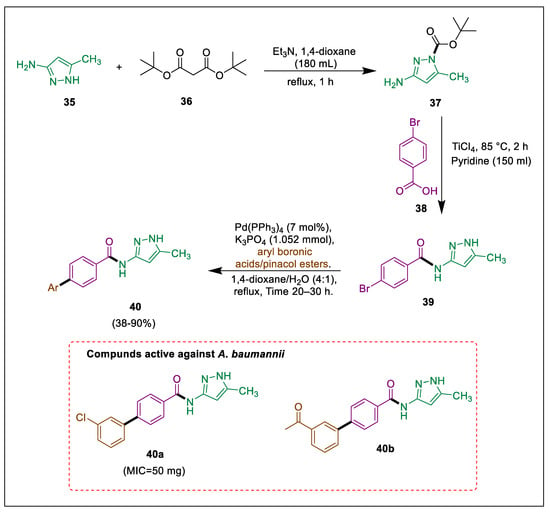
Scheme 7.
Synthesis of pyrazole amide derivatives by using metal catalysts through Suzuki cross-coupling.
Globally, antibiotic-resistant infections are regarded as the primary cause of mortality due to antibacterial resistance. Heterocycles with nitrogen have numerous biological and chemical applications; for instance, pyrazole synthesized numerous biologically active compounds. Furthermore, natural compounds incorporating the pyrazole moiety exhibit highly potent physical properties [70,71,72,73]. In their study, Salman et al. produced an innovative sequence of bispyrazole derivatives with yields ranging from 68 to 83%, which can be classified as moderate to outstanding. The synthesized compounds were screened against gram-positive and gram-negative bacteria. Each newly synthesized compound demonstrated antibacterial activity ranging from excellent to moderate. Compound 47d significantly inhibited the growth of all microorganisms that were tested. Using Suzuki coupling, bispyrazole derivatives are produced with a high yield [74]. Compounds bromophenylethane-1-one 41 and phenyl hydrazine 42 react to form compound 43, which forms compound 4-(4-bromophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 44 in the presence of DMF and POCl3. Compound 44 then reacts with p-methoxyacetophenone using NaOH and ethanol, followed by a dehydration reaction to form oxadiazole derivative 45, which forms bispyrazole 46 in ethanol with some drops of glacial acetic acid under reflux [75]. Suzuki cross-coupling reaction [76,77,78,79,80] between bispyrazole 46 and various arylboronic acids 4 using [Pd(PPh3)], K3PO4, and dioxane at a temperature of 95 °C for 10 h for the formation of bispyrazole derivatives 47. In vitro evaluation was conducted to determine the antimicrobial potency of target compounds 47; compound 47b demonstrated activity against all bacterial isolates. Compounds 47a and 47c exhibited significant inhibitory activity when tested towards B. subtilis and S. aureus (Scheme 8) [74].
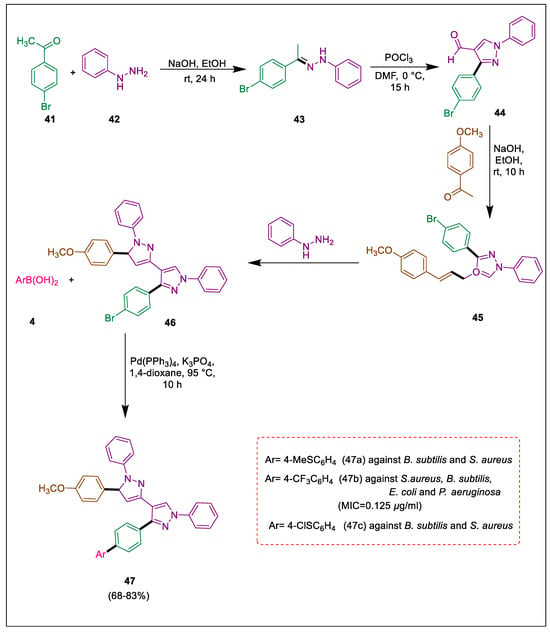
Scheme 8.
Synthesis of bispyrazole derivatives by using Suzuki cross-coupling reaction.
Natural goods often contain nitrogen-containing heterocyclic compounds, such as the pyrazoline scaffold, which have demonstrated exceptional utility in medicinal chemistry [81]. Substances comprising a 2-pyrazoline moiety exhibit biological activity [82,83,84,85]. Preliminary antibacterial activity of 2-pyrazoline derivatives was determined, which were produced via arylation, by using Suzuki-Miyaura reactions. In their study, Karim et al. utilized one equivalent of arylboronic acid in a Suzuki-Miyaura reaction with 51 to produce 3-(biphenyl)-1,5-diphenylpyrazoline. In contrast, two equivalents of aryl boronic acids with 53 via Suzuki-Miyaura reactions formed 3,5-bis(biphenyl)-1-phenylpyrazoline. Mono-coupling compound 52 demonstrated negligible antibacterial activity in contrast to the di-coupling compound 54, which exhibited antibiotic activity against all bacterial strains. p-Bromoacetophenone 48 is condensed with benzaldehyde 49 in the presence of ethanol and NaOH to get substituted chalcone 50. In the presence of glacial acetic acid, this compound interacts with phenyl hydrazine to generate derivatives of pyrazoline (51, 53). In the presence of Pd catalyst, dioxane, and K3PO4, the SM reaction between 51 and arylboronic acids 4 results in pyrazoline derivative 52. Suzuki-Miyaura reaction between 53 with arylboronic acid 4 formed 3,5-bis(biphenyl)-1-phenylpyrazoline 54 in the presence of Pd catalyst, K2CO3, 1,4-dioxane, at 100 °C temperature. Di-coupling compounds 54 demonstrated high bioactivity against four bacteria in comparison to trimethoprim, while mono-coupling compounds 52 showed slight activity against both gram-positive and negative bacteria, of which 52a,b, and 54a–d were more potent and showed antibacterial activity (Scheme 9) [86].
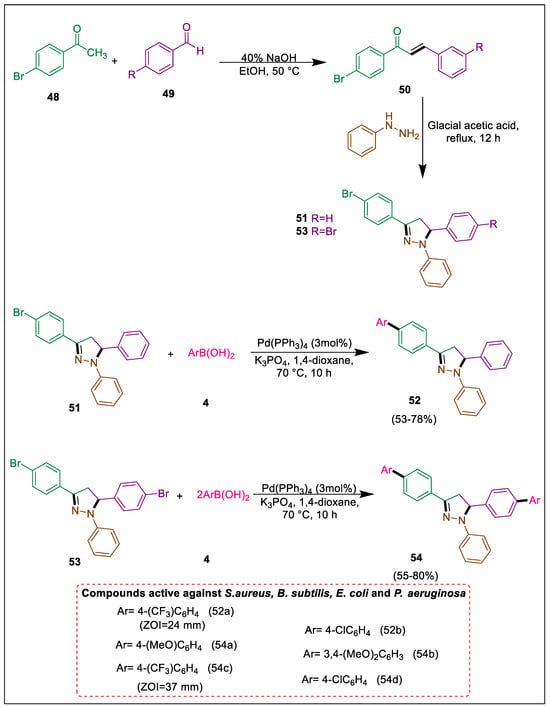
Scheme 9.
Synthesis of pyrazoline derivatives by using Suzuki cross-coupling reaction.
It is known that quinolone scaffolds inhibit bacterial proliferation. An efficient artificial pathway for the synthesis of E-styryl quinolones [87,88] was documented by Silva et al. [89]. By using the slow-release method of the Suzuki-Miyuara and Sonogashira reactions, novel 3-substituted-4-quinolones were synthesized. Mohamed et al. performed a Suzuki coupling reaction in the presence of Pd as a catalyst by using N-methyl-quinolone with boronic acids to obtain aza-isoflavone derivatives. All produced compounds were tested for their antibacterial properties against a variety of bacterial strains. Commercially available 2-aminoacetophenone 55 is cyclized using dimethylformamide dimethyl acetal (DMFDMA) at 95 °C for three hours by using toluene as solvent and iodine in the presence of Na2CO3 to yield 3-iodoquinolone 56. Compound 57 is then obtained by methylation, which undergoes SMC using the slow-release methodology with commercially available boronic acids 58 to form corresponding azaisoflavone analogues 59. By reacting terminal alkynes 60 with iodoquinolone, a set of quinolone derivatives 61 were produced. An in vitro antibacterial assessment of each quinoline and aza isoflavone derivative (59 and 61) was conducted compared to ciprofloxacin, norfloxacin, and ampicillin, indicating that compounds 59a and 59b are the most active antibacterial agents (Scheme 10) [90].
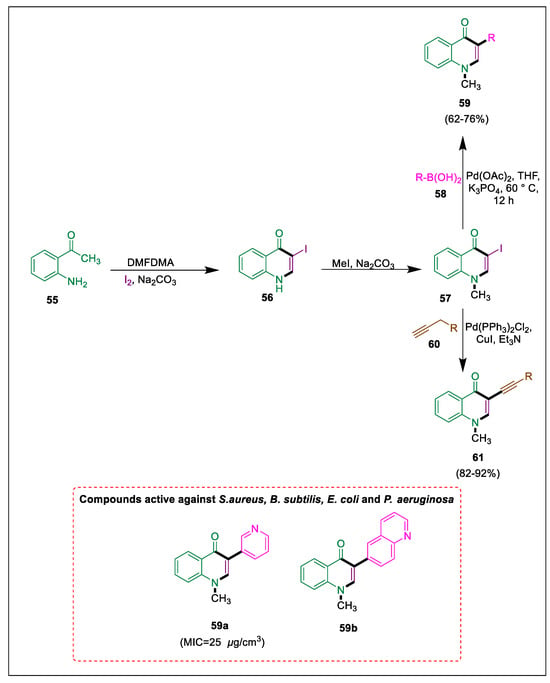
Scheme 10.
Synthesis of quinoline and aza-isoflavone derivatives by using Suzuki-Miyuara coupling.
Infections that are resistant to antibiotics are becoming more frequent and severe globally. Infections caused by extended-spectrum β-lactamase (ESBL) are classified as “High-Priority Pathogens” by the WHO [91]. Specifically, ESBL-producing Escherichia coli infections require treatment. Carboxamide serves as an essential scaffold due to its antibacterial attributes. Thiophene-carboxamides 65 were formed by Ahmad et al., and activity against ESBL-producing E. coli ST131 strains was evaluated. Compounds 65a and 65b are more potent inhibitors against the selected target enzymes (6N9K and 7BDS) of β-lactamase E. coli in comparison to the remaining carboxamide analogues. Compound 64 was formed by the reaction of carboxylic acid 62 with methylpyridine 63 in the presence of TiCl4 and pyridine. It then goes through a Suzuki-Miyaura reaction with different arylboronic acids 4, using a commercially available palladium-tetrakis catalyst, resulting in thiophene-carboxamides 65. A newly synthesized compound 65 was evaluated against ESBL-producing E. coli at five concentrations; compounds 65a and 65b exhibited the most significant zone of inhibition (13 ± 2 and 15 ± 2, respectively) in comparison to the other compounds that also demonstrated good activity (Scheme 11) [92].
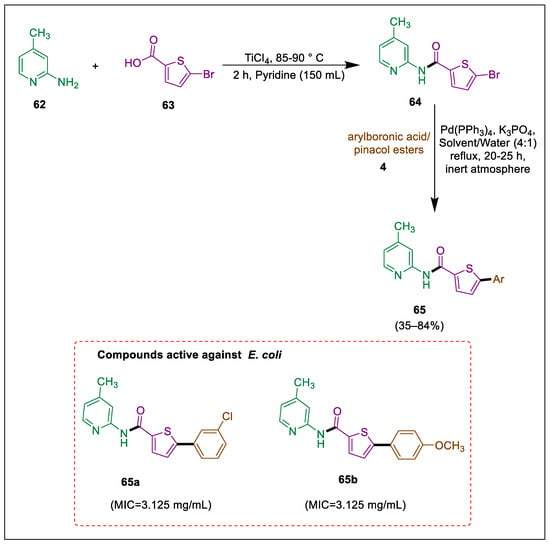
Scheme 11.
Synthesis of carboxamide derivatives by using Suzuki-Miyuara coupling.
Around the world, public healthcare systems are seriously threatened by the emergence of extensively drug-resistant (XDR) microorganisms [93]. The WHO listed infections that are resistant to carbapenem on its “Critical Pathogens List”. Numerous natural products, synthetic precursors, and pharmaceuticals contain oxygen-heterocycles [94,95,96,97]. Antibacterial properties may be exhibited by carboxyamide, an essential scaffold. By reacting furan derivative 66 with aniline 67 in the presence of Et3N, Siddiqa et al. synthesized carboxamide derivatives 68. Carboxamide analogues 69 were obtained by arylating the carboxamide 68 using Pd as a catalyst and K3PO4 as a base, and then in vitro antibacterial properties were examined. Carboxamide derivatives 69 were obtained by using Pd as a catalyst and K3PO4 as a base, allowing a Suzuki coupling reaction between boronic acids 4 and carboxamide 68, which was formed by the reaction of furan 66 and aniline 67 in triethylamine base and dry dichloromethane (DCM). At different concentrations (10, 20, 30, 40, and 50 mg/well), molecules 68 and 69 were evaluated for antibacterial activity against XDR pathogens. However, compound 68 demonstrated exceptional activity, as evidenced by its MIC value of 6.25 mg and MBC value of 12.5 mg. Antibacterial properties have also been observed in compounds 69a and 69b (Scheme 12) [98].
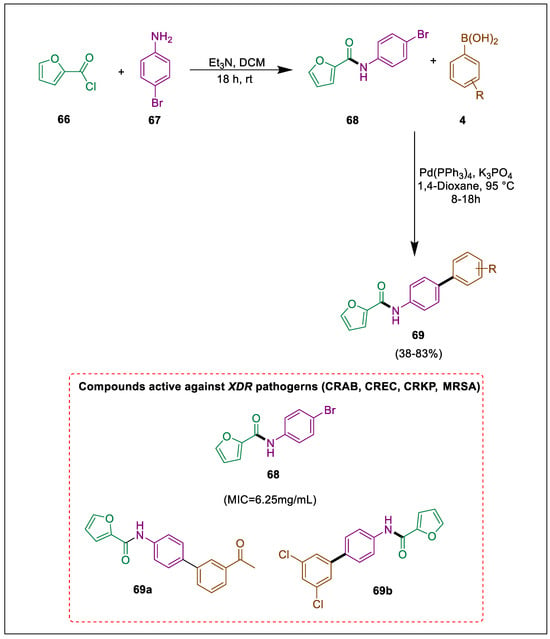
Scheme 12.
Synthesis of carboxamide derivatives by using Suzuki-Miyuara coupling.
Several thiophene-based heterocycles, including biaryl thiophenes, exhibit a wide range of pharmacological actions [99,100,101,102,103,104]. It has been observed that 2,5-dibromo-3-hexylthiophene undergoes regioselective Suzuki cross-coupling; coupling ideally takes place at the C-5 position [105]. The lone pair on the heteroatom (O, S, and N) is donated to the ring in heterocycle substitution processes. By using the Suzuki coupling procedure, Rizwan et al. produced new compounds of 2,5-dibromo-3-methylthiophene 71 in moderate yields. Every examined substance exhibited encouraging biological activity. Utilizing the Suzuki reaction between 2,5-dibromo-3-methylthiophene 70 and arylboronic acid 4 in the presence of Pd catalyst, K3PO4 (2 eq), 1,4-dioxane, and 90 °C under argon, then aryl thiophenes 71 were synthesized. At 50 μg/mL, synthesized compounds 71a, 71e, 71f, and 71g displayed the highest percentage of inhibition against P. aeruginosa (67.3, 50.5, and 41.1%), while compounds 71b, 71c, 71d, and 71h showed moderate activity (39.2, 37.6, 34.9, and 20.8% inhibition). Compounds 71a, 71e, and 71g demonstrated exceptional efficacy against E. coli, exhibiting 94.5, 72.5, and 70.4% inhibition, whereas compounds 71b, 71d, and 71h showed a moderate inhibitory effect against the same strain of bacteria (Scheme 13) [106].
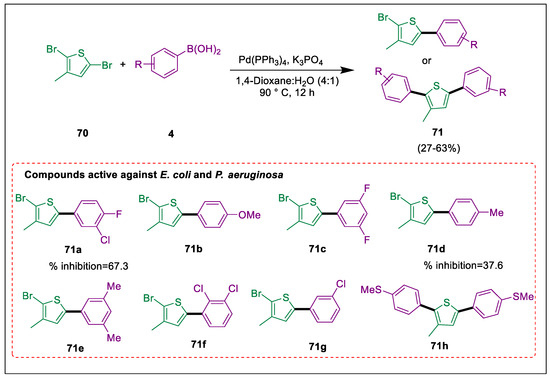
Scheme 13.
Synthesis of aryl thiophene derivatives via Pd-catalyzed Suzuki coupling reaction.
Drug-resistant infections are referred to as “superbugs” because they pose a serious threat to human society due to microbial resistance [107,108,109]. Nitrogen-containing heterocyclic chemicals are quinolone and its fluorine derivatives. Hydantoin, a heterocyclic chemical molecule, is recognized for its biological activities [110]. In medicine design, halogens, especially fluorine substituents, are the focus of extensive investigation. Mahajan et al. synthesized a range of novel trifluoromethyl-substituted quinolone and hydantoin hybrids and tested them against gram-positive and gram-negative bacteria. Compound 79a, with the propene group on the quinolone ring, demonstrated comparable activity to conventional medication (chloramphenicol), with MIC values of 50 µg/mL against P. aeruginosa and S. aureus. After treating urea 72 with chloroacetyl chloride in the presence of toluene, chloroacetamide 73 was produced. This was then refluxed with cyclopropylamine in DMF for 16 h to produce dione 74, and after that, compound 75 was produced by treating its solution with 1,2-dibromoethane in the presence of K2CO3. Subsequently, compound 77 was formed via the intermolecular condensation of aniline 76 and ethyl-4,4,4-trifluoro-3-oxobutanoate in polyphosphoric acid at 140 °C for two hours. In the presence of K2CO3, triethylamine, and DMF compound 77 reacted with 75 at 80 °C for 48 h to produce compound 78, which in the presence of a Pd catalyst, DCM, and potassium carbonate in DMF at 80 °C undergoes Suzuki coupling with boronic ester/boronic acid 4, then quinolone and hydantoin hybrids 79 were obtained. Three compounds 79a–c had equivalent inhibition zones to chloramphenicol, whereas 79d and 79e had comparable antibacterial activity against S. aureus (Scheme 14) [111].
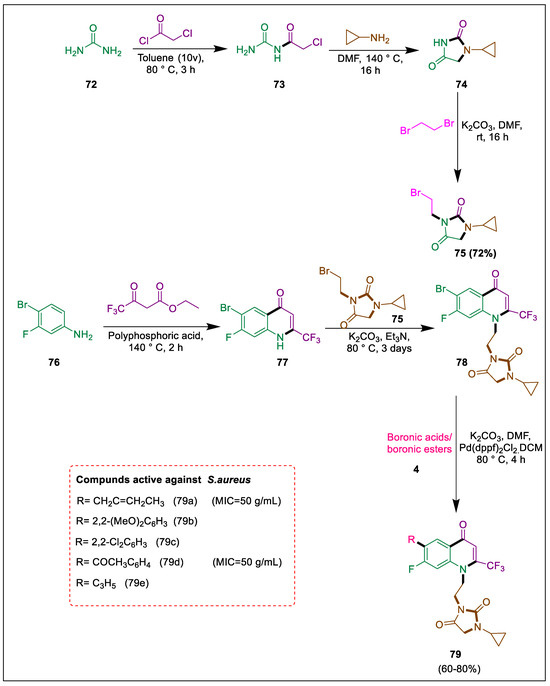
Scheme 14.
Synthesis of quinolone and hydantoin derivatives by Suzuki cross-coupling.
Because of their biological action, 1,3-thiazolidine, imidazolidine, and their derivatives, including rhodanine or 2-2-thiohydantoin, have been studied [112]. Toma et al. also reported that an increase in antimicrobial activity resulted from adding a benzylidene substituent to rhodamine at position C-5 [113]. Tejchman et al. investigated the biological properties of twelve newly synthesized heterocyclic derivatives. The compounds under examination are based on 2-thiohydantoin and rhodanine cores that have carboxymethyl or hydrogen substituents. These cores are connected to a triphenylamine moiety through spacers at the C-5 position of the heterocycles, which are 1,4-phenylene, 1,9-anthracenylene, and 1,4-naphthalenylene. The compounds exhibit antibacterial properties. In the presence of PhCH3 and ethanol in H2O, an SMC Pd-catalyzed reaction occurs between 4-(diphenylamino)phenylboronic acid 80 and bromoarenecarbaldehydes 81 to form aldehydes 82, which then go through Knoevenagel condensation with rhodanine 83 to form compound 84 and with 2-thiohydantoins 85 to form compound 86. Rhodamine derivative 84 exhibited a greater capacity to impede bacterial growth than 2-thiohydantoin derivative 86, which demonstrated greater bactericidal activity against M. luteus (Scheme 15) [114].
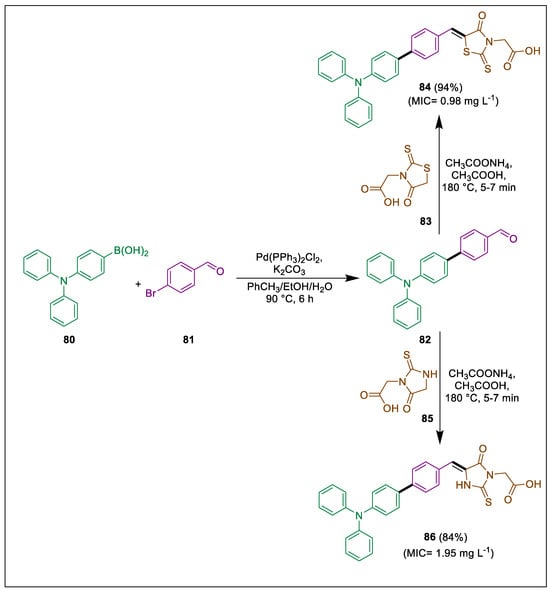
Scheme 15.
Synthesis of rhodanine and 2-thiohydantoin derivatives by using Suzuki cross-coupling.
1,8-Naphthalimide dyes are biologically active and employed as chemosensors, liquid crystal additives, and cell imaging agents [115,116,117]. A Suzuki-Miyaura (SM) cross-coupling process is one method using the naphthalene ring to form novel structures. Using an SM coupling process, a fluorescent chemosensor molecule with rhodamine and naphthalimide attached was synthesized [118]. Türkmen et al. used the catalyst NHC-Pd (II) complex and potassium carbonate in isopropyl alcohol (IPA) under modest conditions to manufacture 4-phenyl-1,8-naphthalimide derivatives from compound 89 via Suzuki coupling reactions. By calculating MIC values, the antimicrobial properties of the synthetic dyes were assessed against a set of six microorganisms. Naphthalic anhydride 87 and cyclohexylamine 88 in EtOH refluxed for seven hours, then 4-bromo-N-(cyclohexyl)-1,8-naphthalimide 89 was formed, which synthesized new 1,8-naphthalimide dyes 90 using a Suzuki coupling process with boronic acids 4 in the presence of a Pd catalyst. Compared to alternative dyes, 90a shows high antibacterial activity against P. aureginosa with a MIC value of 0.095 μg/mL (Scheme 16) [119].
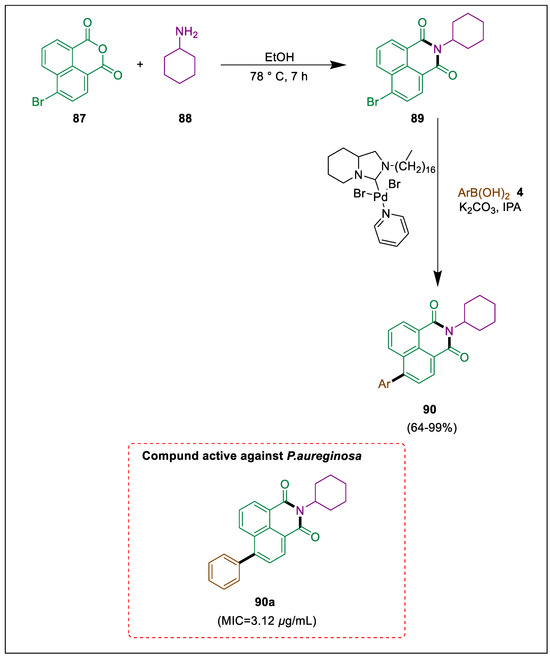
Scheme 16.
Synthesis of naphthalimide derivatives by using Suzuki-Miyaura cross-coupling.
Cancer and malaria are two serious health problems that claim millions of lives every year. Apart from its critical role in the synthesis of therapeutic chemicals, the quinoline core is a structural motif that finds extensive application in the fields of material science and the dye industry [120]. Indoloquinoline natural components represent an ideal class of bioactive compounds. Håheim et al. synthesized a sequence of novel quinoline-based tetracyclic ring systems and assessed them in vitro for their biological activities. The pyridophenanthridine 94 is also active against some strains of gram-positive and gram-negative bacteria, alongside the compound 94b being active against E. coli (MIC = 50 µM) and S. agalactiae (MIC = 75 µM). Compounds quinoline 91 and boronic esters 92 cross-couple via the Suzuki-Miyaura process to generate biaryl compounds 93. These compounds then move via pathway A to form pyridophenanthridine scaffolds 94, which N-methylate using excess iodomethane in refluxing acetonitrile to form pyridophenanthridines 95. Derivative pyridocarbazole 97 is formed when pathway B is followed. 97a exhibited bacteriostatic characteristics against Gram-(+) bacteria and showed modest efficacy against S. agalactiae (MIC = 100 µM). excluding P. aeruginosa. While 94b was only marginally effective against E. coli, 94a and 95a were both effective against S. aureus and E. faecalis (Scheme 17) [121].
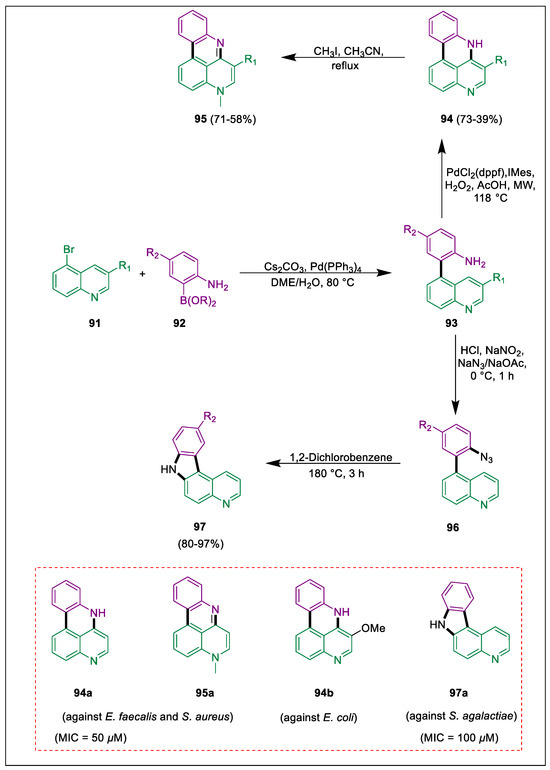
Scheme 17.
Synthesis of pyridophenanthridines and pyridocarbazole derivatives by using Suzuki cross-coupling.
Several heterocyclic substructures are employed in the formation of physiologically active substances [122]. A pharmacophore used in the synthesis of strong, physiologically active substances is the thieno nucleus [123,124]. A library of thieno nucleus-fused heterocyclic compounds containing two or three bioactive pharmacophores in a single molecule was developed using Suzuki coupling. Using bis(triphenylphosphine)palladium(II) dichloride, Sain et al. produced thieno nucleus-adorned trinuclear and tetranuclear nitrogen heteroaryl via the Suzuki cross-coupling reaction. In vitro antibacterial studies of synthesized compounds against gram-positive and negative strains were determined. In the presence of [Pd(PPh3)2Cl2], a mixture of 2-formyl-3-thienylboronic acid 99 with heteroaryl iodides (98, 101, 103, 105, 107, 109) undergoes Suzuki coupling in 1,4-dioxane stirred for 30 min. After that, the mixture is degassed, Na2CO3 solution is added, the mixture refluxes under a nitrogen atmosphere, and the coupling products (100, 102, 104, 106, 108, 110) are obtained by evaporation of methanol. Test compounds 102 and 104 demonstrated significant inhibitory efficacy against all targeted bacterial strains except pseudomonas (Scheme 18) [125].
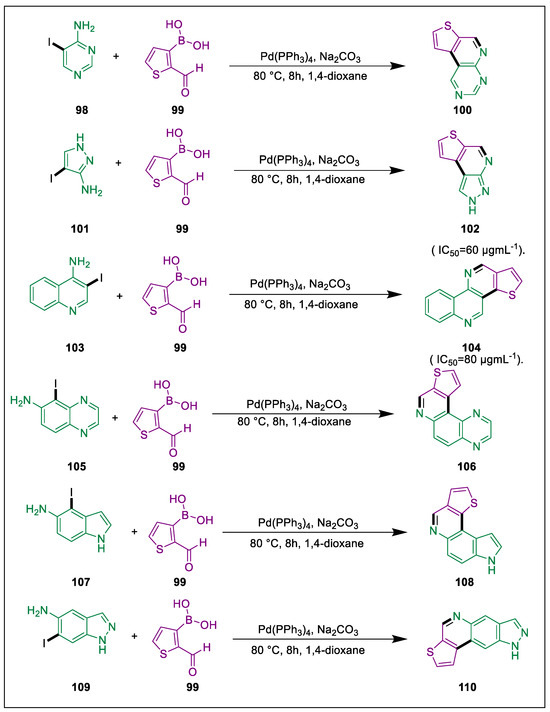
Scheme 18.
Synthesis of thieno nucleus containing hetero-aryls by using Suzuki cross-coupling.
Because of their biological characteristics, a novel class of ruthenium complexes has been discovered to represent intriguing pharmacological possibilities [126]. Pyrazolone ligands exhibit impressive performance with Ru-II, and when combined with 5,7-dibromo-8-hydroxyquinoline, then this complex shows increased antibacterial activity. The synthesis of 5,7-dihalogenated-2-methyl-8-quinolinol and its equipotent anticancer efficacy were documented by Meng et al. [127]. Perez et al. used a Suzuki cross-coupling reaction to synthesize eight arylmethylquinolin ligands 113 from precursors brominated at positions 5 and 7. These ligands were converted into ruthenium(II)-p-cymene 114 novel complexes. All compounds were examined for their cytotoxic and antibacterial properties. Compared to ruthenium complexes (EC50 = 5.2–7.8 μM), ligands exhibited greater cytotoxicity (EC50 = 3.1–4.8 μM). Tert-butyldimethylsilyl chloride (TBSCl) was used to treat 2-methyl-quinoline-8-ol 111 in the presence of imidazole. The next step was adding bromine to form arylbromide 112, which then produced eight arylquinoline intermediates when it interacted with different aryl boronic acids in the presence of a Pd(PPh3)4 catalyst. After treating these intermediates with acid to eliminate the TBS group, the required product 113 was obtained. Handling of [RuCymCl2]Cl2 (ruthenium dimer) along with each derivative 113 of 2-methylquinolin-8-ol [6] produced the required complexes 114. Compound 122a was capable of inhibiting S. aureus. Complexes 114 exhibit IC50 values ranging from 4.64 to 146.15 μgmL−1, indicating more activity against S. Typhimurium. The most effective compounds against S. Typhimurium and S. aureus are 114a and 114b, while compounds 113a, 113b, and 114c are active against B. cereus (Scheme 19) [128].
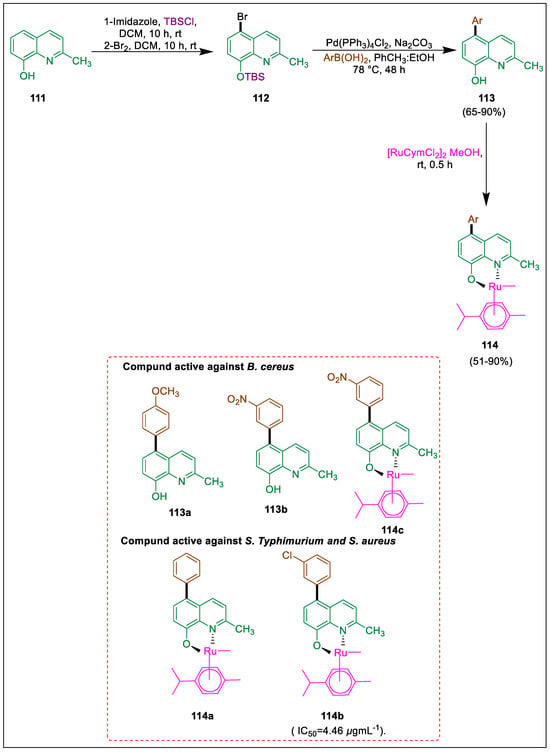
Scheme 19.
Synthesis of arylmethylquinolin ligands and their ruthenium complexes by using Suzuki cross-coupling.
Marine natural materials are employed to produce lead compounds in medication development because of their uniqueness and diversity [129]. A marine β-carboline alkaloid called facsaplysin was taken out of a sponge Fijian. Numerous biological actions of fascaplysin have been documented, including the potency of amide-modified derivatives of fascaplysin against MRSA [130]. Suzuki-Miyaura coupling forms carbon-carbon bonds by using palladium as a catalyst [131]. Jiang et al. developed a novel synthesis process for fascaplysin derivatives, which is produced using Suzuki-Miyaura coupling that is regioselective and then quaternized. Particularly against the gram-negative bacterium E. coli, certain synthetic fascaplysin derivatives showed stronger antibacterial activity in comparison to pristine fascaplysin. By using regioselective Suzuki-Miyaura cross-coupling, bromo-chloro β-carboline 115 couples with other boronic acids 4 in the presence of Na2CO3, Pd-catalyst, and THF/H2O to synthesize fascaplysin precursors 116. The fascaplysin A-ring derivatives 117 were synthesized by employing a few compounds that have typical characteristic functional groups. Compound 117c, in combination with 2,4-difluorophenyl, demonstrated the greatest antibacterial activity against MRSA with MIC values of 0.20 μg/mL, whereas compounds 117a–d showed increased anti-MRSA activity. With a MIC value of 1.56 μg/mL, compounds 117c and 117d had the strongest antibacterial activity against E. coli (Scheme 20) [132].
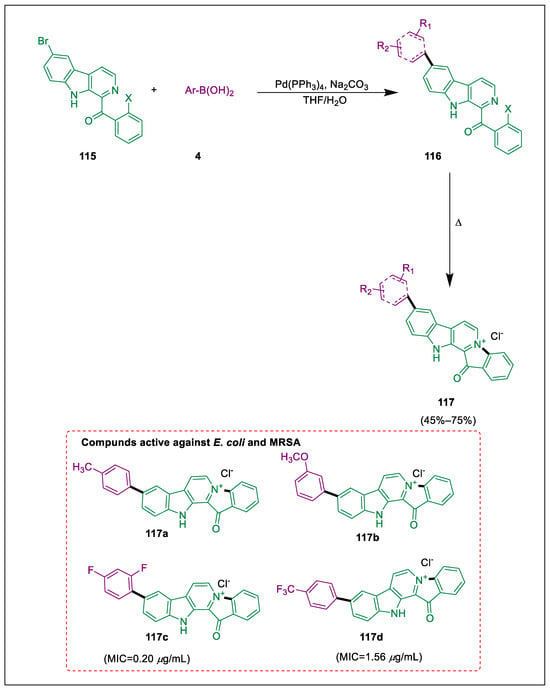
Scheme 20.
Synthesis of fascaplysin structural hybrids by using Suzuki-Miyaura cross coupling.
Since quinoline derivatives have well-established therapeutic benefits, they have been designated as synthetic intermediates in drug research [133,134], and the quinoline moiety can be found in several scaffolds that are both pharmaceutically and physiologically significant. The derivatives of halogenated tetrahydroquinoline are significant intermediates [135,136,137,138]. The Suzuki-Miyaura cross-coupling process works well for making arylated quinolones. By treating bromo tetrahydroquinoline with boronic acids in the presence of a Pd catalyst, Koçyiğit et al. produced phenyl tetrahydroquinolines 120a and 121a in high yields. Next, a new 8-bromo-6-pheyltetrahydroquinoline was produced by bromination of diphenyl tetrahydroquinolines 121a. Bromo tetrahydroquinolines 118 and 119 undergo Suzuki-Miyaura cross-coupling with boronic acids 4 synthesized 6-aryl substituted 120 and 6,8-diaryl substituted tetrahydroquinolines 121. By brominating 6,8-diphenyl-1,2,3,4-tetrahydroquinoline 121a, compound 122 (3-bromo-6,8-diphenylquinoline) was synthesized. With MIC values ranging from 125–250 μg/mL, two compounds 120a and 122 have moderately reduced the proliferation of all gram-positive bacteria (Scheme 21) [139].
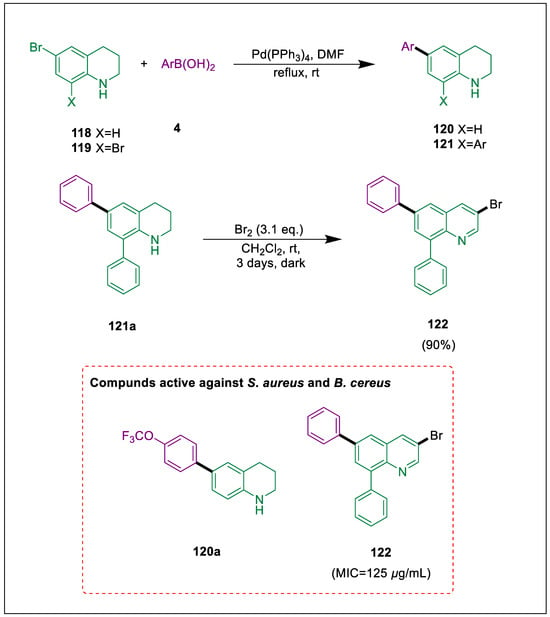
Scheme 21.
Synthesis of tetrahydroquinolines derivatives by using Suzuki-Miyaura cross-coupling.
N-acyl sulfonamide (sulfacetamide) is a common basic structural motif [140]. This is found as a functional group in many different therapies [141]. According to several pharmaceutical patents, aryl sulfonamides have a broad spectrum of biological properties and could be used as medicinal agents [142] and have huge significance in drug chemistry because of their several biological activities. Noreen et al. using Pd-catalyzed Suzuki cross-coupling processes, produced a range of new 5-arylthiophenes 126 with sulphonylacetamide (sulfacetamide) groups in significant yields. The agar-well diffusion method is used to assess the antibacterial activities of these compounds, whereas the indophenol method is used to determine their anti-urease activities. After 2-bromo-thiophene 123 reacts with PCl5 and chlorosulfonic acid, it forms 5-bromothiophene-2-sulfonamide 124. This compound then reacts with ethanoic anhydride in acetonitrile with a few drops of H2SO4 to form acetamide 125, which then undergoes the Suzuki reaction with various arylboronic acids 4 and boronic esters to produce compound 126. Compounds 126c displayed a high activity against S. typhaes, 126f showed the highest activity against Bacillus subtitles, and 126e demonstrated activity against Pseudomonas aeruginosa. Compounds 126a,b and 126d show the highest activity against E. coli and also demonstrated the highest activity against P. aeruginosa (Scheme 22) [143].
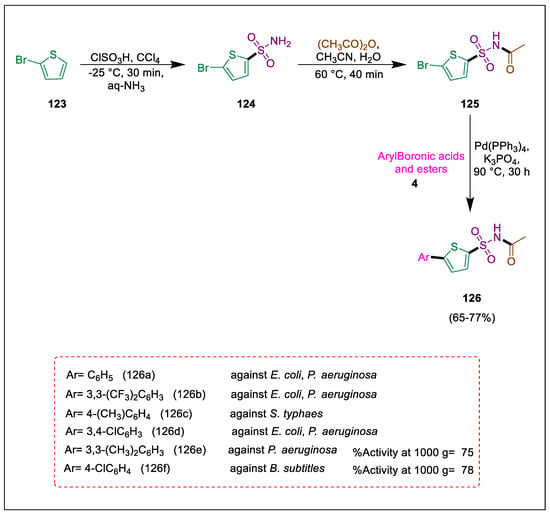
Scheme 22.
Synthesis of sulphonyl acetamide derivatives by using Suzuki-Miyaura cross-coupling.
Heterocycles exhibit biological activity and are important pharmacophoric components in both synthetic and natural medicinal compounds. Heterocyclic compounds that include nitrogen, such as phthalazine derivatives, provide the structural framework for biologically active molecules [144,145,146,147]. Derivatives of 1,2,4-trizole also exhibit a wide range of biological functions. HCT116 is one of the most extensively researched in vitro colorectal cancer cell lines. Using Suzuki coupling, Kumar et al. synthesized several novel N-aryl substituted phenyl acetamide analogues phthalazines. The process began with readily accessible, reasonably priced phthalic anhydride. These substances were tested for their capacity to inhibit the HCT116 cancer cell line using the MIT assay. Additionally, compounds were examined for antibacterial properties. Under reflux conditions, commercially available phthalic anhydride 127 reacted with hydrazine hydrate in methane carboxylic acid to yield phthalazine 128, which was then treated with phosphorus oxychloride to produce 1,4-dichlorophthalazine 129. This compound was then treated with hydrazine hydrate to produce 1-choro-4-hydrazinophthalazine 130, which dissolved in dioxane, triethylamine, and acetyl chloride to obtain the compound 131, and it was then treated with Boronic acid in the presence of (Pd2(dba)2) to obtain ester derivatives 132 through the Suzuki reaction, which undergo hydrolysis by using KOH in methanol to form acid derivative 133, which reacts with various aromatic amines and propyl phosphonic anhydride (T3P) reagent to yield phthalazine derivatives 134. Compared to tetracycline, compounds 134a and 134c were shown to be moderately active against S. aureus, whereas compounds 133 and 134b were also moderately active against E. coli (Scheme 23) [148].
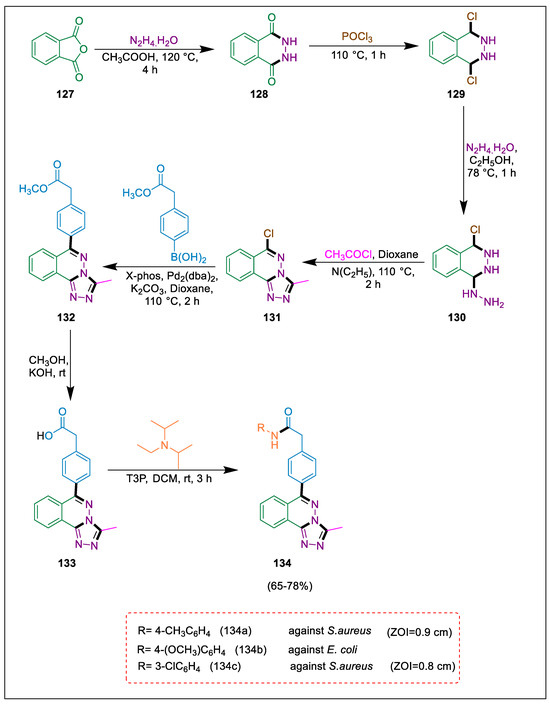
Scheme 23.
Synthesis of triazolo phthalazine derivatives by using Suzuki cross-coupling.
Reducing the emergence of microbial resistance and developing new antibacterial medications are vital because antimicrobial drugs make it more difficult to treat evolving MDR infections using currently available drugs [149]. They can be treated only with antibiotics such as colistin, which is very harmful to the human body [150]. Nicotinic acid derivatives show anti-tubular [151], anti-lipolytic [152], and anti-viral activities [153]. Fischer esterification was utilized by Naheed et al. to synthesize butyl-2-bromoisonicotinate, which was subsequently arylated to produce arylated butyl 2-bromoisonicotinates using a palladium catalyst. In vitro antibacterial activities of molecules (137, 138) were screened against clinically isolated ESBL E. coli ST405 and MRSA. Using a catalytic amount of sulphuric acid (H2SO4), commercially available isonicotinic acid 135 reacts with butanol 136 to form butyl 2-bromoisonicotinate 137. This compound then reacts with various boronic acids 4 in the presence of K3PO4 and palladium catalyst to form arylated butyl 2-bromoisonicotinate 138 via SMC. The results showed that, with a 29 mm zone of inhibition against the ESBL-producing E. coli ST405, molecule 137 was the most effective, while molecules 138a and 138b were the most effective against the MRSA strain, exhibiting 24 mm and 21 mm zones of inhibition, respectively (Scheme 24) [154].
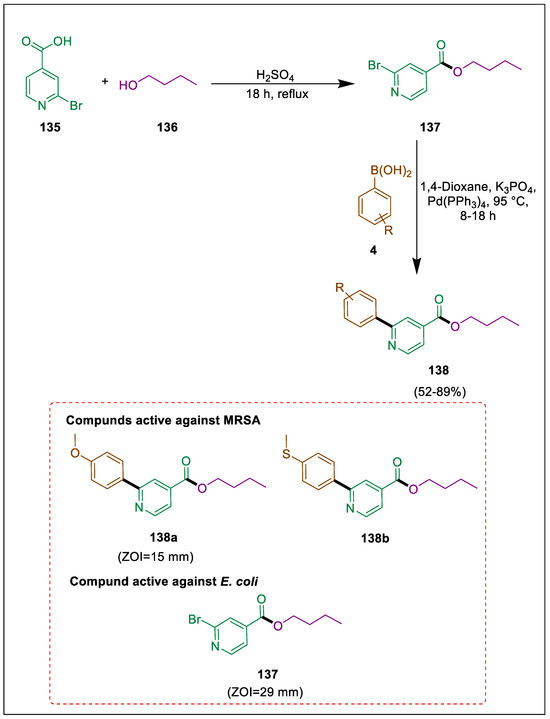
Scheme 24.
Synthesis of arylated butyl bromoisonicotinate derivatives by using Suzuki-Miyaura cross-coupling.
Heterocyclic compounds are synthesized in labs to be used as therapeutic agents and play a vital role in medicinal chemistry [155]. Heterocyclic ring systems, alongside particular substitutions, behave like strong scaffolds for several biological activities [156]. Benzo[b]thiophene is a special type of heterocyclic based on sulfur that is found in bioactive molecules; its diaryl derivatives are not as well documented as they could be. Using Suzuki coupling processes, Sial et al. produced a range of 2,3-diaryldibenzo[b]thiophene derivatives in moderate to good yields. The hemolytic potential, biofilm inhibition, and antithrombolytic properties of the produced compounds were assessed. Every compound had considerable biological potential. Benzo[b]thiophene 139 was brominated, and 2,3-dibromobenzo[b]thiophene 140 was the intermediate chemical that was produced. This compound reacted with different boronic acids/esters 4 in the presence of a palladium catalyst, resulting in the synthesis of the benzothiophene derivatives 141 via the Suzuki reaction. All compounds are active to varied degrees, ranging from moderate 141c to very high 141a, 141b. Compound 141b, which is most active with 99.64% biofilm suppression of B. subtilis, with cyano and trifluoromethyl groups (Scheme 25) [156].
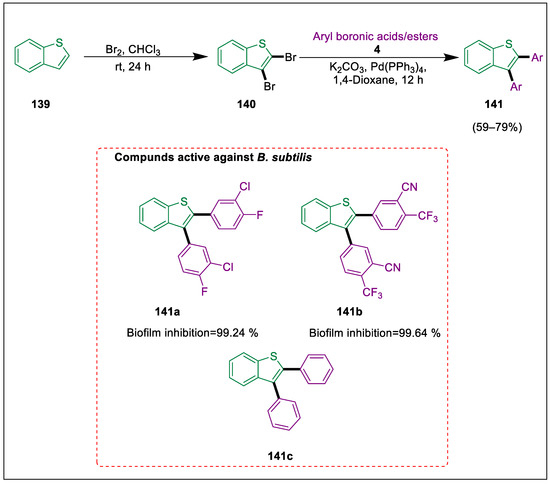
Scheme 25.
Synthesis of benzo-thiophene derivatives by using Suzuki-Miyaura cross-coupling.
Because of their possible biological activity, imidazo pyrazines, a family of nitrogen-containing bridgehead moieties, have drawn a lot of attention [157]. 2,3-Diarylimidazo pyridazine is a well-known molecule with a variety of biological characteristics. The most popular techniques for arylating these scaffolds are palladium-catalyzed direct arylations and Suzuki cross-couplings [158,159]. Two effective synthetic methods for the synthesis of diarylimidazo pyrazines have been synthesized by Soltani et al. The steps include a direct arylation at position 3 catalyzed by a palladium Suzuki cross-coupling reaction. The produced compounds have moderate to good antibacterial properties, according to measurements. Direct arylation and Suzuki cross-coupling of imidazo-pyrazines/pyridazines 142 with a range of boronic acids 4 and aryl halides 143 to obtain the required products diarylimidazo pyrazines 144. Compounds 144a–f showed high effectiveness against B. subtilis and S. aureus (Scheme 26) [160].
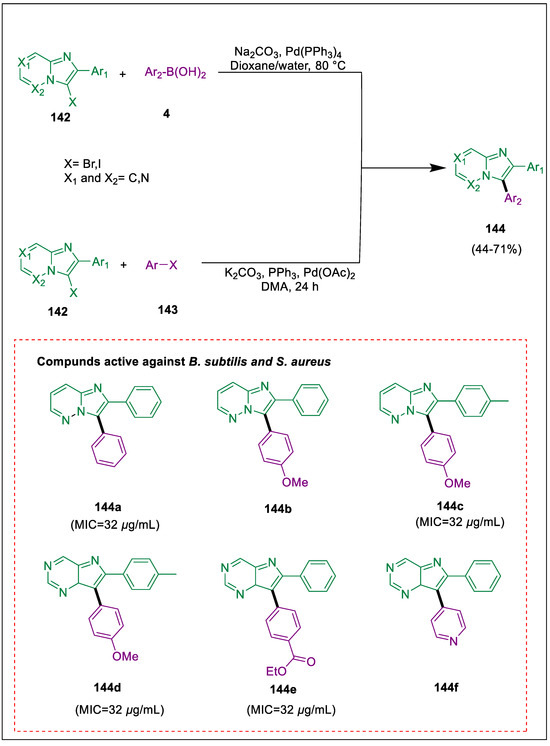
Scheme 26.
Synthesis of diarylimidazo pyrazine derivatives by using Suzuki-Miyaura cross-coupling.
Antibiotics have revolutionized medicine, and once-fatal infections may now be treated easily. Antibacterial resistance is induced by antibiotics, even at trace levels [161,162,163]. Thiophenes, which are heterocyclic chemicals, are significant because of their pharmacological characteristics and ease of synthesis [164,165,166]. Additionally, furan-containing compounds are used in many different therapeutic applications [167]. Hurtado et al. used SMC and microwave irradiation to synthesize thiophene, furan, and thiazole derivatives. Numerous substances exhibit widespread antibacterial action. Significant antibacterial activity was demonstrated by 151g against Salmonella enterica having a MIC value of 0.78 µg/mL and Streptococcus pyogenes having a MIC value of 0.097 µg/mL. Compounds 151 are synthesized using the SMC process and microwave irradiation in the presence of palladium catalyst, base, and solvent from bromo-heterocycles 145–150 and boronic acids 4. Nine compounds, such as 151a–j, displayed growth inhibition, but only compounds 151d and 151f–i showed inhibition rings like ampicillin (10 µg/dis) control. 151g is most active against both gram-positive and gram-negative bacteria (Scheme 27) [168].
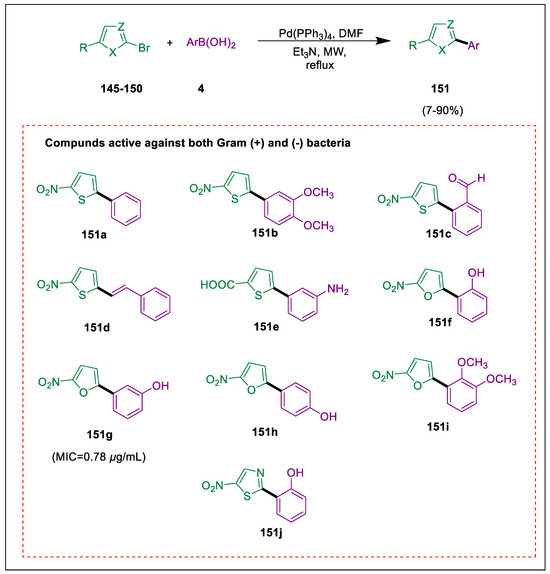
Scheme 27.
Synthesis of thiophene, furan, and thiazole derivatives by using Suzuki cross-coupling.
Axial chiral phosphoric acids, such as those formed from BINOL, have demonstrated their usefulness as catalysts in a variety of processes. Similarly, 3,3′-disubstituted phosphoric acids derived from BINOL are employed in highly enantioselective reactions. As a result, organocatalysis is of great interest for the synthesis of new Bronsted catalysts having chirality and for improving their manufacturing process [169,170]. Using the BINOL derivative, which is not protected Konda et al. synthesized a new class of 3,3′-disubstituted chiral (R)-BINOL-derived phosphoric acid derivatives and optimized them by catalyzing with Pd/C in the SMC. The antibacterial and α-glucosidase inhibitory properties of the target compounds have been evaluated. ortho-lithiation of (R)-binaphthalene diol 152 in the presence of tetramethylethylenediamine followed by reaction with iodine produced (R)-diiodo binaphthalene diol 153, which underwent Suzuki coupling with aryl boronic acids to yield 3,3′-disubstituted chiral (R)-BINOL derivatives 154. One of the derivatives 154 was produced via the double ortho-lithiation of two in the presence of tetramethylethylenediamine and the subsequent reaction with diphenylphosphinous chloride. 154 were phosphorylated with POCl3 in pyridine, which formed 3,3′-disubstituted (R)-BINOL-derived phosphoric acids 155 having chirality. Compound 155d showed the highest level of activity, with MIC values ranging from 1.17 to 2.34 μg/mL. Among the compounds, 155a–d showed strong antibacterial activity against both gram-positive and gram-negative pathogens (Scheme 28) [171].
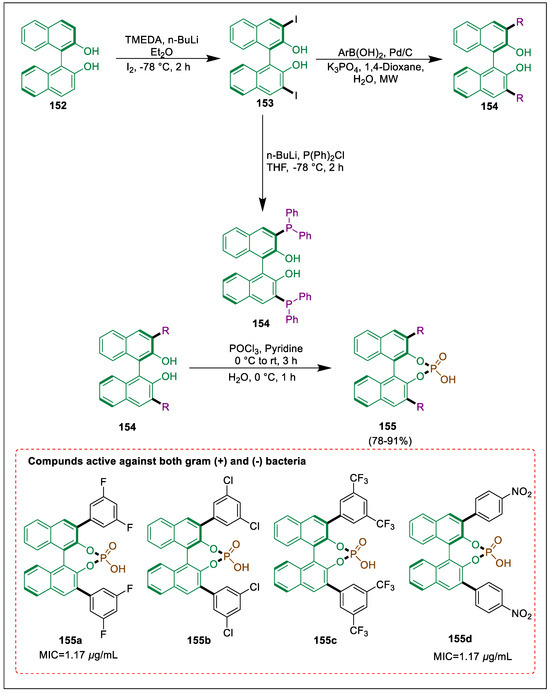
Scheme 28.
Synthesis of BINOL-derived phosphoric acid derivatives by using SMC.
The development of novel antimicrobial agents has become imperative due to the proliferation of antibiotic resistance [172]. An essential enzyme, thymidylate monophosphate kinase (TMPK), could be a potential target for novel antibiotics [173]. Selective inhibition is possible because the bacterial and human TMPKs differ sufficiently in their sequences. In recent years, the emergence of other classes of inhibitors, such as the imidazopyridinone developed by Choi et al. [174], evaluated for biological activity. Imidazopyridinones are an important class of heterocyclic compounds synthesized by Blindheim et al. The inhibitory effects of the imidazopyridine on the E. coli thymidylate monophosphate kinase were significant. Using an amide coupling between bromobenzoic acid 156 and 3-phenyl-1-propylamine 157 were assembled by using HATU and DIPEA to form boronic acid 158 following a Suzuki coupling with Pd(dppf)Cl2, and analog 159 produced the protected analog 160, and the target compound benzamide derivative imidazopyridine 161 was obtained in a 58% yield after deprotection with TFA in CH2Cl2. [175] Compound 161 has been identified as an inhibitor of TMPK in E. coli. At 8.3 μM, single-point inhibition measurements determined that imidazopyridinones 161 exhibited a good effect (Scheme 29) [175].
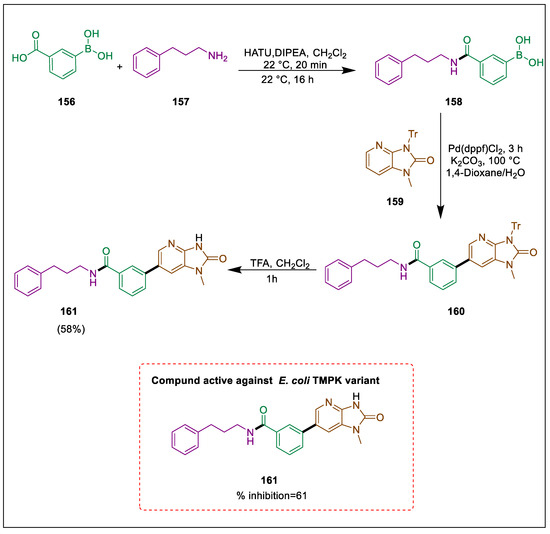
Scheme 29.
Synthesis of benzamide derivative by using Suzuki cross-coupling.
Pd catalyst was employed in Heck, Stille, Suzuki, Sonogashira, and Buchwald-Hartwig couplings to generate carbon-carbon and carbon-heteroatom bonds. These reactions are significant in the production of medicinal agrochemicals. The research on pyridine and its derivatives shows that several of these substances are used for the treatment of many illnesses [176,177,178]. Through carbon-carbon coupling, Ghiasuddin et al. synthesized two novel pyridine derivatives: 164 and 165. The experimental activity of compounds 164 and 165 in terms of zones of inhibition against fungus and bacteria has validated their bioactivity. In the presence of K3PO4, Pd catalyst, and dioxane, a one-pot reaction combining 3,5-dibromopyridine 162 and 1-naphthyleneboronic acid 163a yields pyridine derivative 164, whereas the use of 2,5-difluorophenylboronic acid 163b yields pyridine derivative 165. Compound 165 demonstrated maximum zone of inhibition of 12 mm against S. aureus. Likewise, compound 164 showed the highest value of inhibition of 12.5 mm against E. coli (Scheme 30) [179].
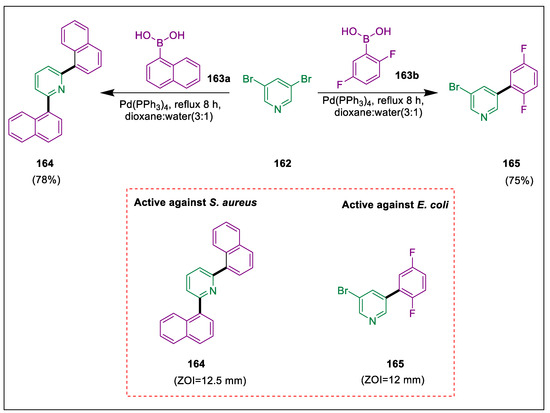
Scheme 30.
Synthesis of pyridine derivatives by using Suzuki cross-coupling.
Morbidity and death are significantly increased by infectious diseases [180]. Comparing oxazolidinones to other antibacterial medications, they have a distinct mechanism and low cross-resistance. Analogs of biarylloxazolidinone having a hydrazone moiety exhibited remarkable antibacterial activity [181]. To assess their antibacterial efficacy, Xu et al. synthesized several new biaryloxazolidinone derivatives with amide and acrylamide structures. Whereas most compounds showed strong antibacterial activity overall. Using 4-bromobenzaldehyde 166 as the starting material, a two-step reaction yields intermediate 169, which is then Suzuki coupled with compound 170 to produce intermediate 171. This intermediate is then treated with TFA to produce 172 and its condensation with different amines to make the target product acetamide derivative 173. Compound 173 demonstrated good antibacterial activity, with MIC values of 0.5 μg/mL against MRSA, S. aureus, and MSSA (Scheme 31) [182].
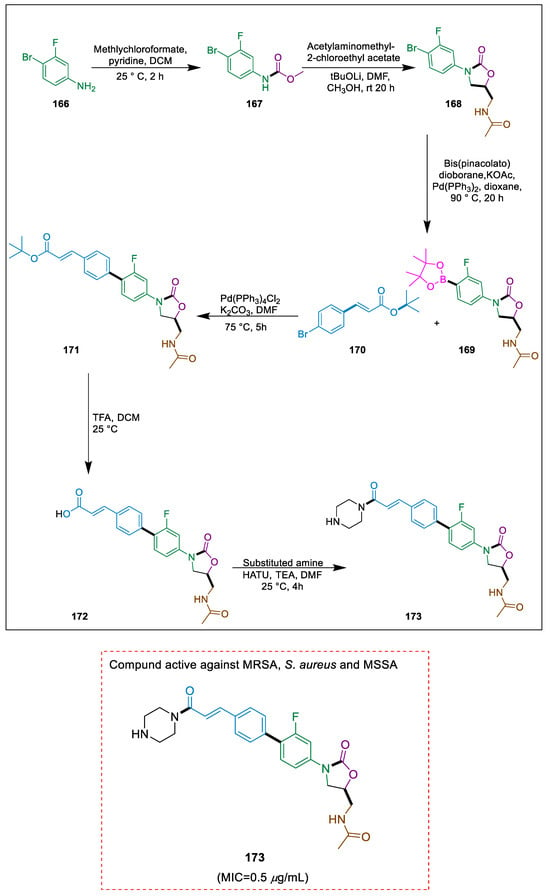
Scheme 31.
Synthesis of acetamide derivatives by using Suzuki cross-coupling.
2.2. Sonogashira Cross-Coupling
A palladium complex conventionally facilitates Sonogashira coupling, while copper iodide serves as the co-catalyst when an amine is present [183,184]. Numerous coupling reactions devoid of copper have been documented [185]. Oxazoles of 2-aryl are the most important heterocycles because of their wide range of physical and biological properties [186]. Quinoxaline derivatives hold significant importance as heterocyclic molecules due to their utility as intermediates in organic chemistry and their manifestation of natural properties. In their study, Keivanloo et al. employed a copper-free Sonogashira coupling reaction to produce oxazole-quinoxaline amine hybrids by using 2-amine substituted chloroquinoxalines, prop-2-yn-1-amine, and benzoyl chloride compounds that catalyzed this reaction by using a Pd catalyst. The ligand utilized was TBTA, an effective ligand in ethanol. All prepared compounds underwent testing against Micrococcus luteus and Pseudomonas aeruginosa, two bacterial strains. Sonogashira coupling without copper is utilized in the synthesis of oxazole-quinoxaline amine hybrids. The desired product was produced by combining benzene carbonyl chloride 174 and propargylamine 175 in the presence of trimethylamine in ethanol at ambient temperature for two hours, followed by the addition of 2-amine substituted chloroquinoxalines 176 and Pd catalyst. The mixture was stirred for an additional eight hours to obtain oxazole-quinoxaline amine hybrids 177. The antibacterial activities of compounds 177a–g are superior against Micrococcus luteus and Pseudomonas aeruginosa to those of the other compounds. Furthermore, the antibacterial properties of 177a, 177b, 177d, and 177f exhibited a suppression level equivalent to amoxicillin (Scheme 32) [187].
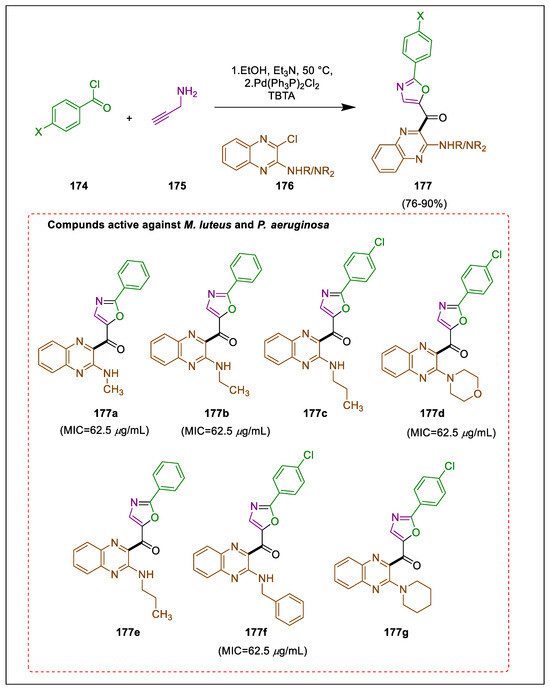
Scheme 32.
Synthesis of quinoxaline amine derivatives via Sonogashira cross-coupling.
Quinoxalines are an essential class of heterocyclic compounds having nitrogen atoms because of their important biological activities alongside pharmacological activities [188]. The Sonogashira coupling is a significant reaction utilized for carbon-carbon bond formation [189,190]. However, multi-component reactions (MCRs) are strong and prevailing chemical ways for the formation of important organic compounds [191,192,193]. To synthesize new 3-(3-(aminoquinoxalin-2-yl) prop-2-yn-1-yl carboxylates, Abbaspour et al. used a multi-component, copper-free Sonogashira coupling. In this reaction, carboxylic acids react with propargyl bromide and various amine-substituted chloroquinoxalines while using a Pd catalyst. The antibacterial properties of each newly synthesized compound were examined. 3-(3-(diethylamino)quinoxalin-2-yl)prop-2-yn-1-yl carboxylates 181 were generated via the multicomponent reaction of chloroquinoxalines amines 178, carboxylic acids 179, and propargylbromide 180, which was catalyzed by Pd(PPh3)2Cl2 in DMF in the presence of Et3N and Cs2CO3. Compounds 181a–g against M. luteus while 181b, 181c, and 181g against P. aeruginosa showed superior antibacterial activity when compared to the other derivatives, according to the MIC and MBC values (Scheme 33) [194].
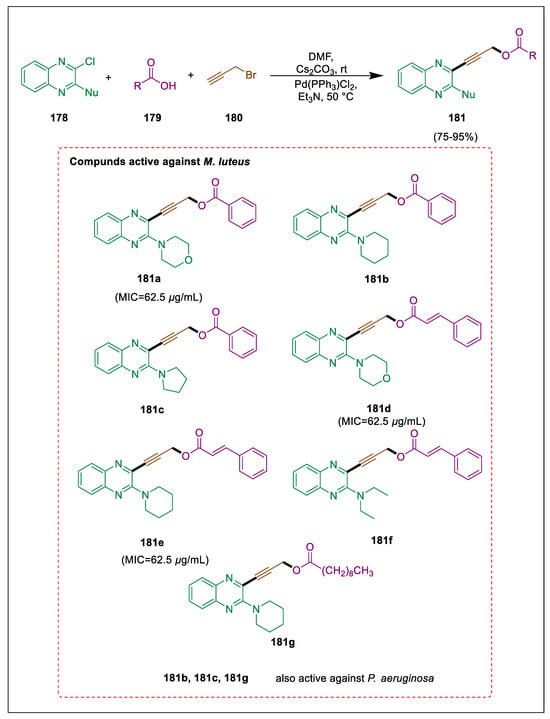
Scheme 33.
Synthesis of carboxylate derivatives by using Sonogashira cross-coupling.
Products from the Sonogashira coupling are widely employed in the production of heterocyclic compounds, agrochemicals, and medicines [195]. The benzoheterocycle quinoxaline and its derivatives constitute an important class with a broad spectrum of biological action. Pyrroloquinoxalines act as bioactive compounds [196]. The synthesis of disubstituted pyrrolo quinoxalines has been described by Cacchi and colleagues. By reacting N-alkyl chloroquinoxaline amines with propargylic alcohols in the absence of copper but in the presence of Pd(PPh3)2Cl2, Fakharian et al. produced alkanol-substituted pyrrolo quinoxalines. Additionally, the three bacterial strains were tested against the produced pyrroloquinoxaline derivatives [197]. N-alkyl-3-chloroquinoxaline-4-amines 182 synthesized [198,199]. They were followed by a reaction at room temperature with propargylic alcohols 183, catalytic quantities of Pd(PPh3)2Cl2, morpholine, and MeCN to generate 2-alkanol substituted pyrrolo[2,3-b]quinoxalines 185. However, when morpholine was not employed in the first step, intermediate 184 was produced, which in the presence of morpholine and CH3CN synthesized derivatives 185. While compounds 185 were assessed for their antibacterial activity at a concentration of 1000 μg/mL in methyl sulfoxide. Compound 185a demonstrated antibacterial properties against both B. subtilis and M. luteus, and compound 185b was active against P. aeruginos better than penicillin (Scheme 34) [197].
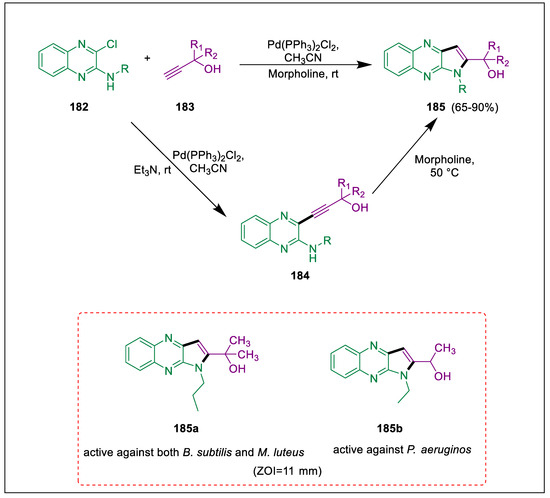
Scheme 34.
Synthesis of pyrrolo quinoxaline derivatives by using Sonogashira cross-coupling.
Because thiophene and its derivatives have so many different biological actions, they have been studied as an essential class of heterocyclic chemicals [200]. Thieno[a]dibenzothiophene derivatives are normally synthesized by Pd as a catalyst in cycloaddition reactions between alkynes and halo-benzothiophenes [201]. A range of highly substituted thieno dibenzothiophenes were produced by Konus et al. via a cascade electrophilic cyclization procedure. The reaction allowed a range of molecules to be examined for antimicrobial and antifungal activities. The reaction between bromothiophene 186 and ethynyl(trimethyl)silane 187 by using Sonogashira coupling to prepare ((3-bromothiophen-2-yl)ethynyl)trimethylsilane 188, which again undergoes Sonogashira coupling to obtain the desired compound silane 189, which undergoes desilation for the synthesize of 2-ethynyl-3-(aryl/alkyl ethynyl)thiophenes 190, which react with 2-iodothioanisol by Sonogashira reaction to form thiophene derivatives 191. Compound 191a demonstrated antibacterial activity against S. aureus, while compound 189a was the only one to show some degree of activity against P. aeruginosa (Scheme 35) [202].
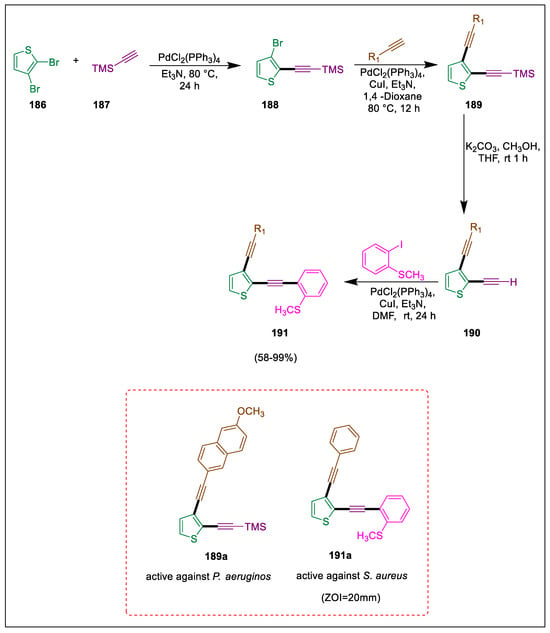
Scheme 35.
Synthesis of thiophene derivatives by using Sonogashira cross-coupling.
Multidrug-resistant bacteria pose a serious risk, necessitating the synthesis of new antibiotics [203]. Natural antibacterial substances provide great starting points for the development of innovative treatments to identify drug-resistant microorganisms. Overall Synthesis of molecules that occur naturally offers a chance to overcome these problems [204]. Kukla et al. produced and studied eighteen analogs of the natural product anaephene. It was discovered that the antibacterial activity of these analogs against MRSA and MSSA varied. Compound 199 discovered that, in comparison to anaphase B, an internal alkyne with no extra unsaturations in the alkyl chain increases antibacterial efficacy against MRSA. Compounds 194 are obtained by Sonogashira coupling of tert-butyl-(3-iodanylphenoxy)-dimethyl-silane 192 with either 1-undecyne or pent-4-yn-1-ol 193. After treating compound 194a with THF to eliminate the TBS-protecting group, alcohol 195 was produced. Under hydrogenation conditions, compound 195 yields alcohol derivatives 196, and other derivatives, such as 199, were also synthesized but do not undergo hydrogenation. Alcohol 194b oxidized to aldehyde 197, then underwent sulfone-mediated Julia-Kocienski olefination, and finally, TBAF deprotection produced compound 198. Compound 195a is more potent than compound 193 with MIC values of 2 μg/mL, although compound 198a is equivalent to the natural product anaphase B. Compounds 199a–c exhibit action against S. aureus that is resistant to methicillin (Scheme 36) [205].
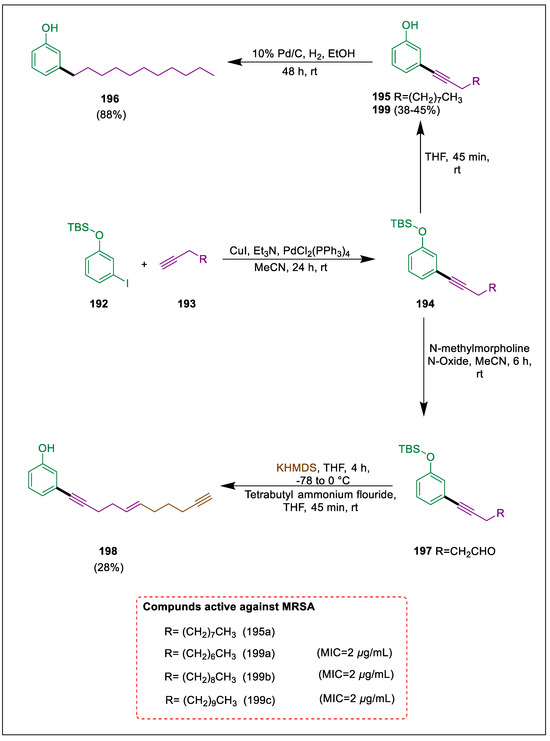
Scheme 36.
Synthesis of anaephene derivatives by using Sonogashira cross-coupling.
Engineering the surface of cells is crucial for controlling how cells behave, including shielding them from hostile surroundings [206,207] and improving biosynthesis efficiency [208,209]. Numerous functional materials are used as coating materials on the surface of cells, including metal/semiconductor nanoparticles [210,211], polymers [212,213], and metal complexes [214]. Live cells synthesized by Pd-mediated reactions have recently been subjected to the in-situ polymerization technique. Using a cell-generated bio-palladium catalyst and a Sonogashira polymerization process, Qi et al. formed photoactive polyphenylene ethylene (PPE) on the cell surface. The in-situ generated PPE has excellent light-harvest capacity and blue fluorescence on the surfaces of E. coli. Additionally, PPE shows increased antibacterial activity against E. coli. Cationic 1,4-bis(oxyhexamethylene-trimethylammonium bromide)-2,5-diiodobenzene 200 and 1,4-bis(oxyhexamethylene-trimethylammonium bromide)-2,5-diethynylbenzene 201 choose as monomers and Sonogashira polymerizations take place in aqueous solution, catalyzed by the in-situ produced surface bio-palladium catalysts to form photoactive polyphenyleneethynylene (PPE) 202. In-situ synthesized photoactive polyphenyleneethynylene (PPE) 202 exhibits good antibacterial capacity (Scheme 37) [215].
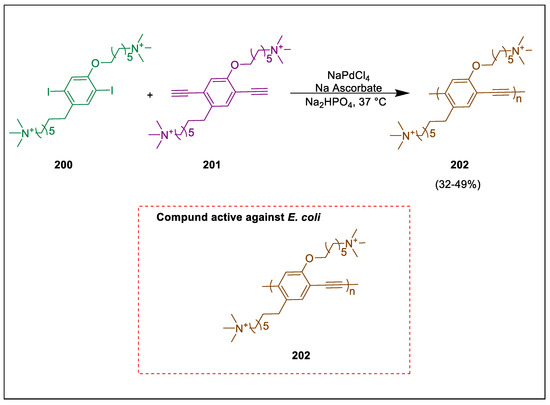
Scheme 37.
Synthesis of photoactive polyphenylene ethylene (PPE) derivatives by using Sonogashira polymerization.
The powerful and essential approach to forming C-C bonds and aryl-alkynes is known as Sonogashira coupling [216,217], and it is used to make biologically active compounds in the pharmaceutical industry [218]. Important nitrogen heterocyclic compounds in the organic synthesis and pharmaceutical industry are hydantoins. Important nitrogen heterocyclic molecules in organic synthesis and medicinal chemistry are hydantoins. Compounds 207 were synthesized by Keivanloo et al. by reacting diphenyl imidazolidine 2,4-dione with ArI2 in CH3CN at normal temperature with a palladium catalyst. Pseudomonas aeruginosa and Micrococcus luteus were the two bacterial strains against which all produced compounds were tested. Diphenylimidazolidine-2,4-dione 203 was produced as a starting material by the reaction of urea and benzil in alkaline EtOH. This compound then reacted with 3-bromo-prop-1-yne 204 in the presence of potassium carbonate in DMF as a solvent to yield 5,5-diphenyl-3-(prop-2-yn-1-yl) imidazolidine-2,4-dione 205, which was coupled with ArI2 206 in the presence of a palladium catalyst and CuI in CH3CN at room temperature to synthesize derivatives 207. Compounds 207a–d exhibit superior inhibitory activity against M. luteus, while compounds 207b and 207d demonstrate superior inhibitory activity against P. aeruginosa (Scheme 38) [219].
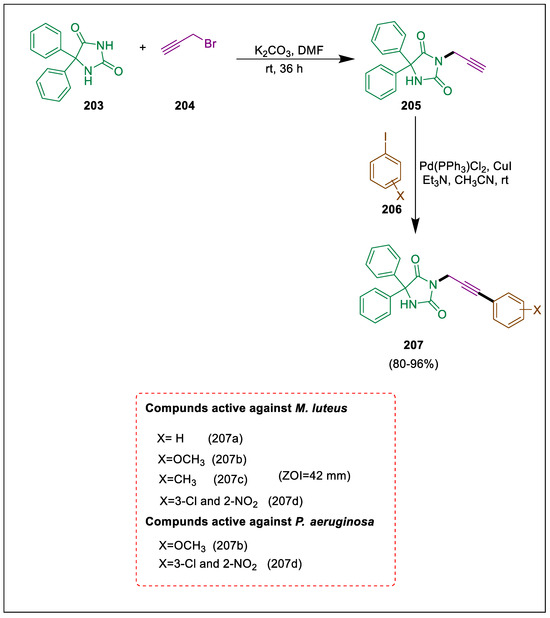
Scheme 38.
Synthesis of dione derivatives by using Sonogashira cross-coupling.
2.3. Stille Cross-Coupling
The indole and patterns are frequently observed in most compounds with biological activity, including medications and alkaloids [220]. Numerous significant medical properties have been attributed to indole derivatives, including anti-inflammatory [221], antiallergic, anti-viral [222], anti-tumor [223], antimicrobial [224], antihypertensive [225], and antioxidant activities [226]. Konus et al. conducted electrophilic substitution reactions and Pd-catalyzed Stille coupling to produce novel indole derivatives containing mono- and di-thiophene groups 211 and 212. Compound 211 exhibited no notable cytotoxic, antioxidant, or antimicrobial properties. In contrast, compound 212 demonstrated substantial reducing and exceptionally potent antibacterial activity. Compound 212 is also a potentially effective malignancy therapeutic agent. By treating 1-ethyl-2-phenylindole1 208 with NBS to form 3-bromo-1-ethyl-1H-indole 209, which undergoes an electrophilic aromatic substitution reaction with 2-(tributylstannyl) thiophene in the presence of a Pd catalyst to form compound 210. Following the characterization process, a second electrophilic aromatic substitution reaction was conducted to produce 1-phenyl-1H-indole 211, which had a 92% yield. In the end, 3-([2,2′-bithiophen]-5-yl)-1-ethyl-2-phenyl-1H-indole 212 was synthesized using a Stille coupling reaction between 2-(tributylstannyl) thiophene and 1-(5-bromothiophen-2-yl)-1-ethyl-2-phenyl-1H-indole 211 in the presence of a Pd catalyst while under prolonged reflux. The antimicrobial activity of compound 212 (250 μg) towards three gram-positive bacteria was found. However, it exhibited exceptionally potent antibacterial activity against E. faecalis ATCC 29212, with a MIC of 4 μg/mL (Scheme 39) [227].
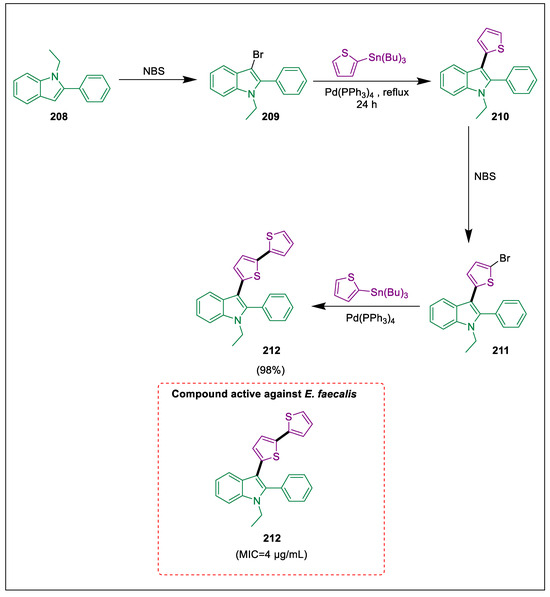
Scheme 39.
Synthesis of indole derivatives via Pd-catalyzed Stille cross-coupling.
Important biological actions are displayed by indole and indole-based compounds [228,229]. Indoles have the potential to function as a crucial structural class in synthetic medications, including sumatriptan, indomethacin, pindolol, indolmycin, and reserpine [230]. The prevalence of multidrug-resistant microbial infections has increased substantially, posing an additional risk to human health. Konus et al. used a Pd catalyst in cross-coupling reactions and iodocyclization reactions to synthesize different derivatives (215–219). Moreover, compounds 215 and 219 had antifungal and antibacterial action, suggesting they were superior medications. After undergoing an electrophilic substitution reaction, indole 213 was converted to 3-bromo-1-ethyl-2-phenyl-1H-indole 214. This allowed it to react with tributylthiophen-2-ylstannane in the presence of palladium as a catalyst to form compound 215, which with bromide undergoes Stille coupling, resulting in 3-(5-bromothiophen-2-yl)-1-ethyl-2-phenyl-1H-indole 216. Conversely, a novel synthetic method was used to synthesize polyheteroaromatic compound 217 in the presence of I2 by using compound 215. After that, compound 218 was formed through a Sonogashira coupling reaction with terminal alkyne. Next, compound 214 underwent the Stille coupling procedure, and furanyl-substituted indole derivative 219 was formed. In terms of inhibitory zones against S. aureus ATCC 25,923 (25 mm), P. aeruginosa ATCC 27,853 (25 mm), B. subtilis ATCC 6633 (19.5 mm), and compound 219 was the most powerful. Subsequently, compound 215 also showed strong antimicrobial activity (Scheme 40) [231].
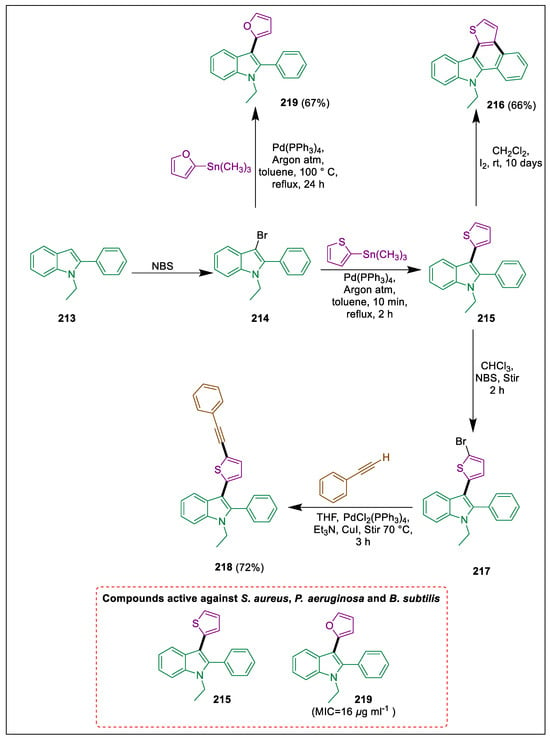
Scheme 40.
Synthesis of indole derivatives by using Stille and Sonogashira cross-coupling.
2.4. Buchwald-Hartwig Coupling
Numerous uses in therapy have been attributed to natural and synthetic varieties of coumarin-containing amines and amides, which are reportedly potent antibacterial [232], anti-inflammatory [233], and antiviral agents [234], and show various applications in medicinal chemistry. Certain pharmaceuticals available for purchase contain the coumarin moiety [235]. Our investigation into the pharmacological potential of coumarins coupled with various amides and amines was motivated by the preceding findings of coumarins and amines. Joy et al. synthesized methyl amido/amino coumarins by coupling a variety of coumarins with amides and amines via a palladium-catalyzed Buchwald crossing reaction. There are intentions to investigate the antioxidant and antimicrobial characteristics of the synthesized methyl amino/amido coumarins. The antibacterial capacity of molecule 224h was like that of the standard ciprofloxacin. The hydroxy coumarin 222 was produced through an adapted Pechmann cyclization of resorcinol 220 with ethyl acetoacetate 221. This coumarin was subsequently converted to the nonaflate 223, which underwent a Buchwald-Hartwig cross-coupling reaction with different amines and pyridines at –10 °C to synthesize methyl amino/amido coumarins 224. The catalyst for this coupling was Pd2(dba)3, and the ligands consisted of Xanthos. Compounds 224a–h exhibited comparable and favorable activity to ciprofloxacin. In contrast, compounds 224g and 224h demonstrated potent activity towards every bacterial strain (S. aureus, B. subtilis, and E. coli). The antibacterial effect of compounds 224o–u (amides) was superior to that of 224a–t (amines) (Scheme 41) [236].
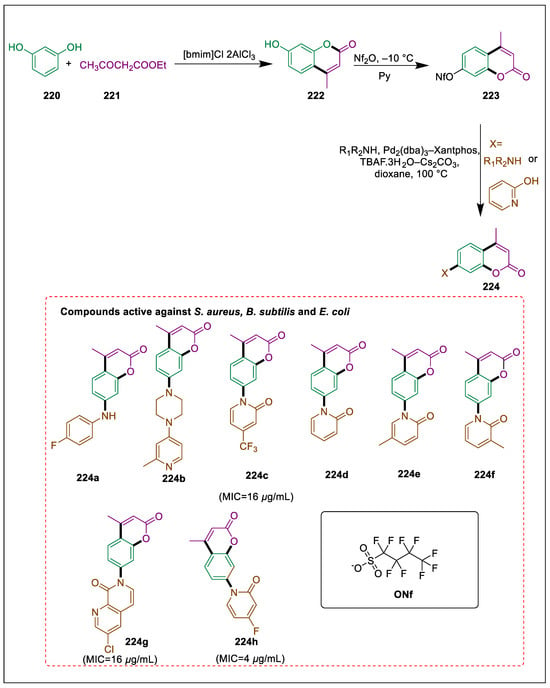
Scheme 41.
Synthesis of amino/amido coumarins by using Buchwald-Hartwig coupling.
To stop the proliferation of bacteria that are resistant to antibiotics, new antibacterial medications with unique modes of action must be developed [237]. Unfortunately, because there are currently no biofilm-eradicating medicines available, biofilm-associated illnesses remain an unsolved clinical concern. Phenazine 2-bromo-1-hydroxyphenazine, one analog that has been halogenated (HP), exhibits significant antibacterial and biofilm-destructive properties. Halogenated phenazine (HP) analogs that exhibit biofilm-eradicating activity against priority pathogens were identified by Garrison et al. The synthesis of 1-methylphenazine scaffolds via Buchwald-Hartwig cross-coupling and reductive cyclization facilitated the rapid identification of strong HPs that destroy biofilms. By employing 6 mol% Pd2(dba3) and 18 mol% (±)rac-BINAP as a ligand, anilines 225 were coupled with 226 to produce 2-series diarylamines 227 with an average yield of 71% and inverted 3-series with an average yield of 25%. The 2-series diarylamines underwent a smooth two-step Buchwald-Hartwig/reductive cyclization, yielding 1-methoxyphenazines 228. However, the 3-series diarylamines did not yield any desired 1-1-methoxyphenazine products. Thirteen of these intermediates were then demethylated using boron tribromide to yield 1-hydroxyphenazine, and its dibromination produced HP target structures analogs 229, while bromination at position 4 also produced other derivatives 229. For instance, halogenated phenazines 229a and 229b report MIC activity of less than 0.1μM. The MICs of 6.25–50 μM for monohalogenated HPs 229c–f were merely fair to moderately active against gram-positive bacteria (Scheme 42) [238].
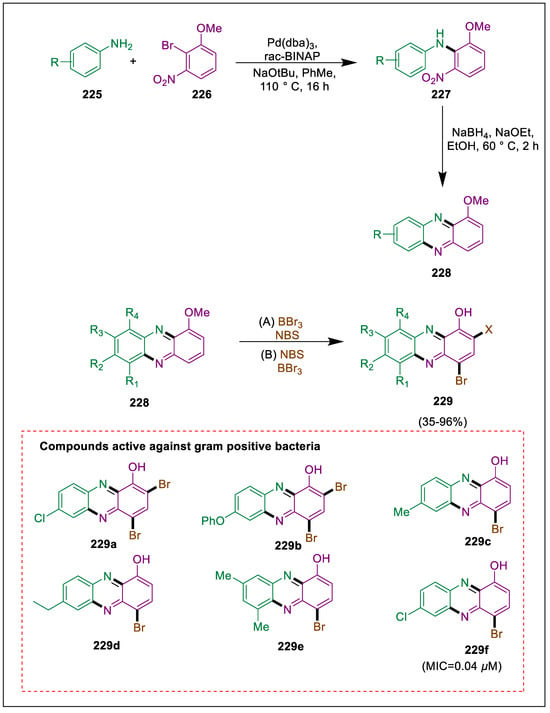
Scheme 42.
Synthesis of phenazine derivatives by using Buchwald-Hartwig reaction.
Caffeine, a naturally occurring 1,3,7-trimethyl xanthine, has biological action and is employed as a biostimulant in food and medicine [239,240]. As bactericidal agents, caffeine and its derivatives that have been 8-alkoxy substitution are recognized [241,242,243]. Utilizing cross-coupling reactions to introduce substituents into the xanthine skeleton at the C-8 position is a crucial technique. Reshetnikov et al. cross-coupled bromocaffeine with α-, β-, or ω-amino-acid of methyl or t-butyl ester hydrochlorides in toluene in the presence of a Pd catalyst and cesium carbonate with microwave activation, which results in the synthesis of xanthine derivatives containing amino-acid fragments in the C-8 position and study their antibacterial activities. Bromo caffeine 230 reacts with methyl or t-butyl ester hydrochlorides of α, β, or ω amino acids 231a–c in dry toluene with microwave heating to synthesize synthetic xanthine derivatives 232a–c, including amino-acid fragments, in the presence of a Pd catalyst and Cs2CO3. Compounds 232a–c demonstrated decreased minimum inhibitory concentrations (MIC) for B. cereus growth suppression and were active at 150 ± 25 μg/mL. These compounds inhibited the growth of S. aureus at concentrations greater than 500 μg/mL (Scheme 43) [244].
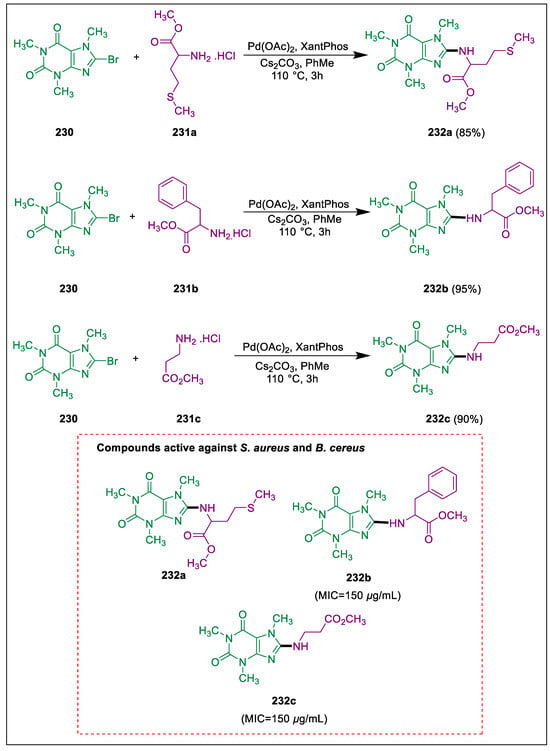
Scheme 43.
Synthesis of xanthine derivatives by using Buchwald-Hartwig reaction.
2.5. Heck Reaction
Quinoxaline derivatives have highly intriguing biological characteristics [245,246]. Cross-coupling reactions of aryl halides with organometallic reagents catalyzed by palladium to produce C-C bonds, which are utilized to synthesize medicinal and agrochemical compounds [247]. Because of the biological and pharmacological characteristics of pyrroloquinoxalines, several straightforward techniques have been established for their production. Seidani et al. synthesized quinoxaline derivatives by reacting N-alkyl/benzyl-3-chloroquinoxaline-2-amines with chalcones catalyzed by palladium with the addition of potassium tert-butoxide as the base in DMSO. The MIC and MBC values indicated these compounds might be employed in future research for additional antibiotic synthesis. The intermolecular Heck reactions were employed to synthesize trisubstituted pyrrole quinoxalines 235 from N-alkyl/benzyl-3-chloroquinoxaline-2-amines 233 and chalcones 234 in the presence of Pd catalyst, KOtBu, NaOAc, and DMSO. Compounds 235a and 235b showed stronger anti-bacterial activities against M. luteus and P. aeruginos than the other derivatives when both the MIC and MBC values were taken into consideration. Anti-bacterial activity of 235a was comparable to tetracycline with strong inhibition (Scheme 44) [248].
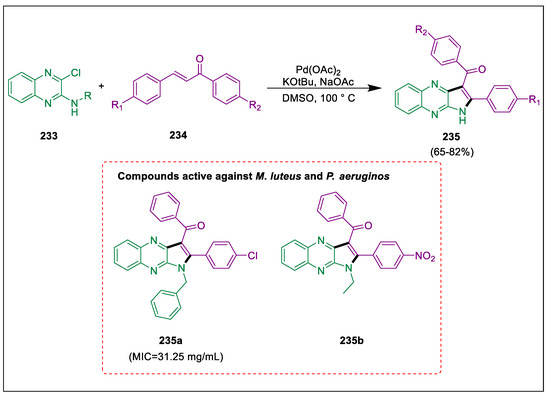
Scheme 44.
Synthesis of quinoxaline derivatives via Heck reaction catalyzed by palladium.
Numerous pharmacologically active substances have sulfonamide moiety. Important pharmacophores with a broad spectrum of bioactivity are cyclic imides. The bicyclic structure of norbornane [bicyclo(2.2.1) heptane] is bridged, and its derivatives show some biological characteristics [249,250]. Synthesis of medicines and agrochemicals is possible by using the Heck reaction. By reacting endo-endic anhydride with sulfa medications and then reductive Heck reactions of these products, Bagdatli and Cil produced novel sulfa drug-substituted norbornyl imides as potential bioactive scaffolds. The antibacterial activity of all eight produced compounds, aryl aryl-substituted norbornyl imides, and two sulfa drug-based norbornenyl imides was assessed against nine different pathogens. Furan-2,5-dione 236 and freshly distilled cyclopenta-1,3-diene 237 react to form endogenic anhydride 238 in the presence of dry toluene. This endogenous anhydride then reacted with sulfa drugs, specifically sulfamethoxypyridazine and pyridazine in acetic acid, to form sulfa drug-based norbornenylimides 239, which undergo reductive Heck reactions with aryl iodide 240, yielding sulfa-based norbornenylimide derivatives 241. For M. luteus, M. abscessus, and S. murinus, MIC results from samples 241a and 241b were superior to those from other investigated samples. Among the actinobacteria under study, 239a had a MIC of 7.0–14.0 mg/mL and produced better results than other compounds against bacterial strains (Scheme 45) [251].
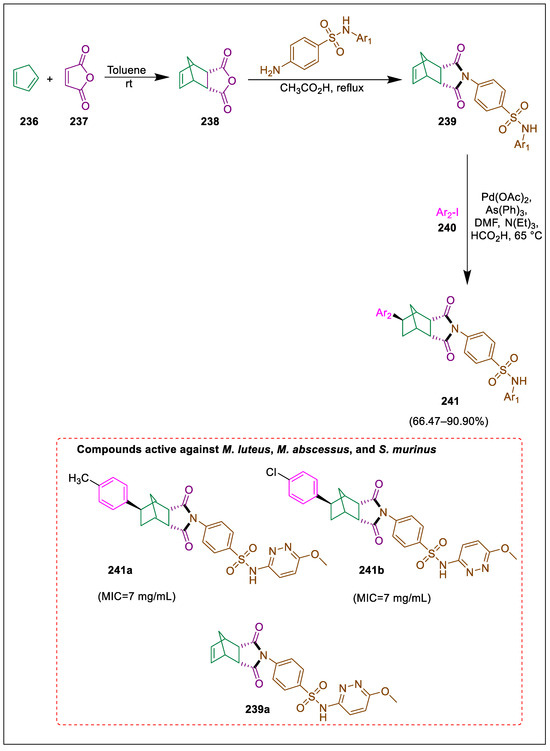
Scheme 45.
Synthesis of sulfa drug-based norbornenylimide derivatives by using reductive Heck reaction.
2.6. 1,4-Addition to Dienes
Bacterial leaf blight (BLB) is a serious global disease caused by the bacterium Xanthomonas oryzae pv. Oryzae (Xoo) that severely decreases rice productivity. Implementing pharmacological or biological agents is of the utmost importance to mitigate the detrimental effects of the disease [252,253,254,255]. It is extremely advantageous to incorporate fluoroalkyl motifs into organic frameworks and their application in pharmaceutical compounds. Through the coupling of 1,3-dienes, amines, and fluoroalkyl iodides, Shi et al. produced a series of photoinduced selective 1,3-diene fluoroalkyl amination derivatives. The bactericidal activity of all the synthesized compounds against Xoo was assessed. Compound E14 among them exhibited notable efficacy against Xanthomonas oryzae pv. Oryzae (Xoo). In the presence of a palladium catalyst with xantphos as ligand, Cs2CO3 as a base, and DCM, 1,3-dienes 242, amines 243, and fluoroalkyl iodides 244 combine to form 1,3-diene-selective fluoroalkyl amination derivatives 245. By using fluoroalkyl iodide 244a, 1,3-diene 242a, and amines 243a, we synthesized the compounds listed in the title, which are amine derivatives, 245E1–E5. Compounds 245E1–E5 displayed outstanding antibacterial activity against Xoo, with 245E5 having a MIC of 12.5 μmol/mL (Scheme 46) [256].
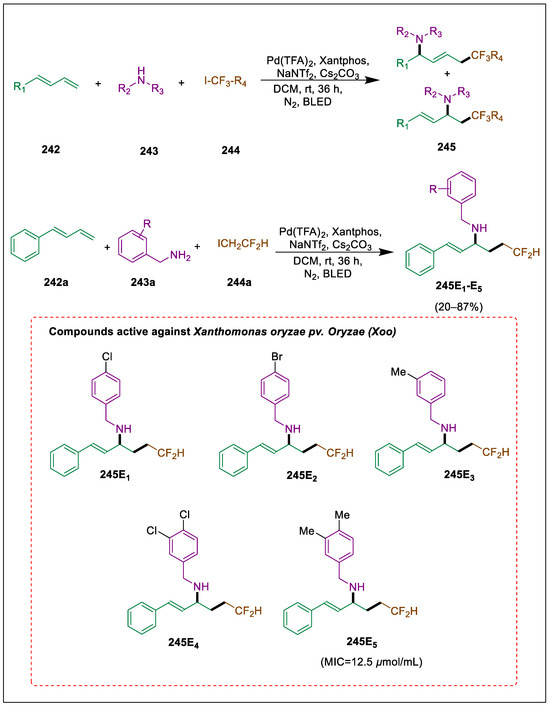
Scheme 46.
Synthesis of amine derivatives by using 1,4-addition to dienes.
3. Copper-Catalyzed Synthesis
Cross-coupling reactions are effective methods for forming carbon-carbon (C-C) bonds and are frequently employed in the synthesis of a variety of chemicals [257,258,259]. Pd complexes are usually the catalysts for these reactions. Much work has recently been put into developing Cu as a Pd substitute for these kinds of processes [260]. Concerns over the long-term sustainability of cross-couplings, mainly related to the high cost and poor natural availability of Pd, are the driving force behind efforts to develop Cu-based catalytic procedures.
Even though it can be challenging to accomplish Cu-catalyzed cross-couplings, Cu-salts are, however, quite effective in forming C-C bonds in a variety of procedures. Excellent yields and milder reaction conditions are two benefits of using copper catalysts. Among other transition-metal-mediated reactions to form carbon-carbon and carbon-heteroatom bonds, copper catalysis is used in Ullmann reactions, Diels-Alder reactions, ring expansions, and a notable variation of the Huisgen 1,3-dipolar cycloaddition that uses a Cu(I) catalyst that was independently developed by Meldal and Sharpless. Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC) is a reaction that forms a triazole by combining an azide and a terminal alkyne. The production of biological molecules and medicinal materials depends extensively on copper’s capability to catalyze carbon-carbon and carbon-heteroatom bonds [261].
3.1. Click Reaction
Varieties of groups comprise a quinoxaline, which shows biological activity [262]. Significant heterocyclic compounds, triazoles, have applications in organic chemistry, medicinal chemistry, and drug discovery [263,264,265,266]. Transition metal complexes derived from Schiff bases have valuable catalytic transformations [267,268]. Therefore, triazoles-based 3-substituted thioquinoxalines were synthesized using Cu(II) salen complex. The Cu(II) salen complex catalyzed a click reaction between 2-propargythioquinoxalines and aryl azides; the resulting triazole-linked thioquinoxaline derivatives were formed by Keivanloo et al. The in vitro antibacterial activities of these compounds indicated that Bacillus subtilis and Micrococcus luteus bacteria are susceptible to the effects of compounds 252a, 252b, and 252c. By using the salen Cu (II) complex as a catalyst, the loading of the hazardous copper species was decreased, and the reactivity rate was increased. The reaction between dichloroquinoxaline 246 and morpholine 247 produced chloroquinoxaline amines 248 when combined with Na2S.9H2O in dimethyl sulfoxide (DMF) at ambient temperature. Subsequently, substituted thioquinoxalines 249 were made, which reacted with propargyl bromide in the presence of sodium methoxide in methanol, resulting in the formation of propargyl thioquinoxalines 250 in a quantitative yield, which reacted with azide 251 in ethanol, then added the salen-Cu(II) complex and sodium ascorbate, and stirred the mixture at 50 °C for 3h to obtain the pure product 252 obtained by crystallization from ethanol. By using a good diffusion method, the antibacterial activities of the newly synthesized molecules against M. bacteria (DSM 6887), P. aeruginosa (ATCC 27853), and M. luteus (ATCC 4698) were assessed. Antibacterial properties have been observed in molecules 252a–c against M. luteus and B. subtilis (Scheme 47) [269].
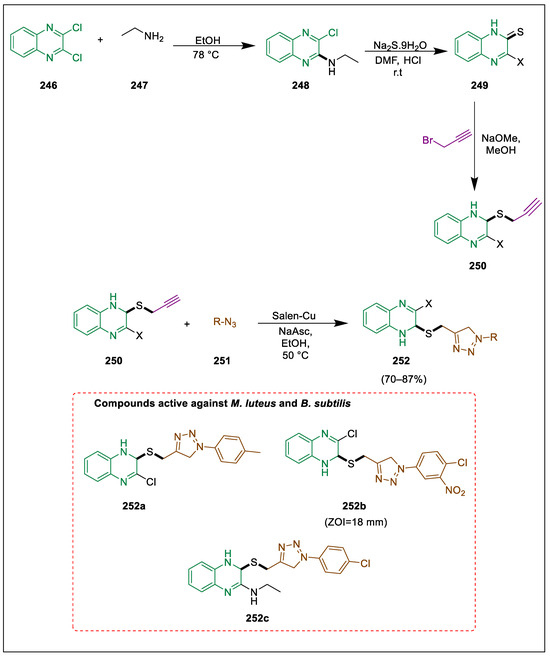
Scheme 47.
Synthesis of triazole-linked thioquinoxaline derivatives via Cu salen complex-catalyzed click reaction.
3.2. Ullman Cross-Coupling
Because there are no effective antibiotics for microbial diseases, they have become a global health concern. Heterocyclic compounds nitrogen-containing are the most prevalent and exhibit both biochemical and economic uses [270]. Hoemann et al. discovered that 2-(1H-indol-3-yl) quinolines were efficacious towards S. aureus, which resists methicillin [271]. Kung et al. found that compounds having quinazoline possess potent antibacterial properties [272]. Nandwana et al. performed a Cu-catalyzed Ullmann-type C-N coupling to synthesize fused quinazolines, followed by an intramolecular cross-dehydrogenative coupled process. The in vitro antibacterial activity of these compounds was evaluated against three gram-negative bacteria and two gram-positive bacteria. Compared to the beneficial control ciprofloxacin, the compounds with the lowest inhibitory concentrations against all bacterial strains examined were 256a, 256b, and 256c. A series of bromoaryl imidazoles 253 were produced by reacting benzyl and a related dicarbonyl compound, 2-bromoaryl aldehyde, and NH4OAc in the presence of L-proline/MeOH. Similarly, one of the derivatives 2-(2-bromophenyl)-1H-benzo[d]imidazole 253 was produced by reacting a bromoaryl aldehyde with diaminobenzene in the presence of H2O2/CAN. Aryl modification at the C-4 and C-5 positions of compound 253 when reacted with various azoles 254 in the presence of CuI and potassium carbonate in DMF at 150 °C and produced intermediates 255 followed by Ullmann-type coupling catalyzed by Cu(OAc)2·H2O resulted in the fused imidazo/benzimidazo quinazolines 256. Using ciprofloxacin as a standard drug, an antibacterial evaluation of imidazole/benzimidazole quinazoline derivatives was conducted against a panel of three gram-negative and two gram-positive strains. Compounds 256a, 256b, and 256c were determined to exhibit the highest efficacy and antibacterial activity (Scheme 48) [273].
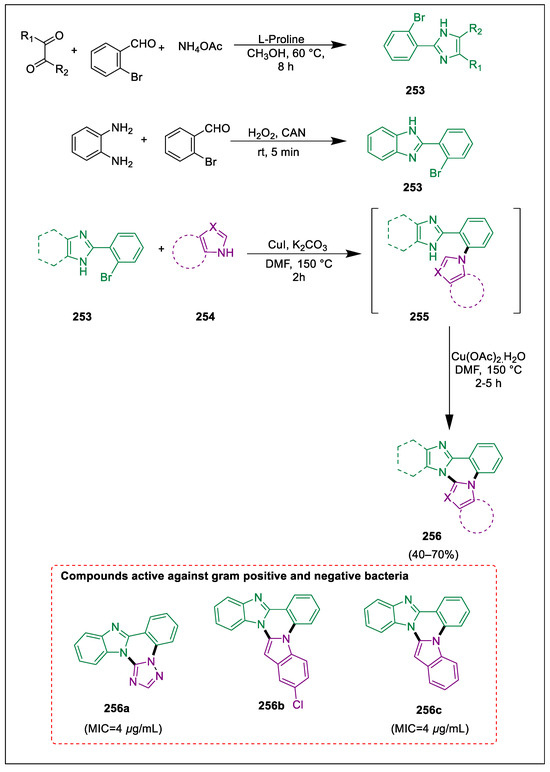
Scheme 48.
Synthesis of imidazo/benzimidazo quinazolines by using Ullman cross coupling.
3.3. Chan-Lam Cross-Coupling
ESBL E. coli and methicillin-resistant Staphylococcus aureus become resistant to β-lactam inhibitors and β-lactamase quinoline due to the accumulation of numerous antimicrobial-resistant genes [68]. Quinoline is a biologically active heterocyclic molecule that is present in pharmacological dynamics [274]. The synthesis of a novel 4-aryl-3,4-dihydro-2H-[1,3]oxazino[5,6-h]quinolin-2-one, exhibiting antibacterial action, was reported by Rbaa and colleagues. Using Chan-Evans-Lam coupling, Arshad et al. synthesized novel 6-Bromoquinolin-4-ol derivatives 258 by using 6-bromoquinolin-4-ol, which react with different boronic acids in the presence of a copper catalyst and various protic, aprotic, and mixed solvents. These derivatives show antibacterial activity against MRSA and ESBL E. coli. By using the Chan-Lam coupling process, between 6-bromoquinolin-4-ol 257 and with arylboronic acid 4 in the presence of Et3N, Cu catalyst, and molecular sieves of 4 Å in the aerobic atmosphere yields derivatives of 6-bromoquinolin-4-ol 258. Compound 258a possesses the largest zone of inhibition, with MIC and MBC values of 6.25 and 12.5 mg against ESBL-producing E. coli and 3.125 and 6.5 mg against MRSA, respectively (Scheme 49) [275].
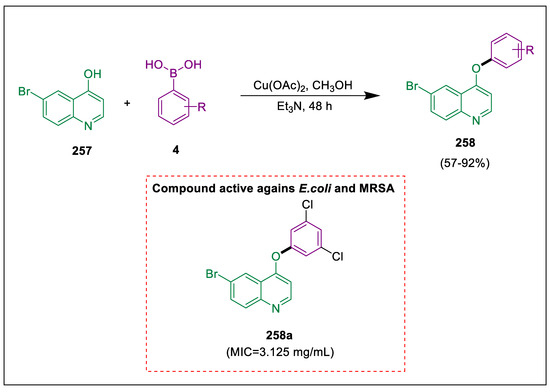
Scheme 49.
Synthesis of bromoquinolin-4-ol derivatives by using Chan-Evans-lam coupling.
Much study has been conducted on the medical uses of azauracil. The potency of 6-azauracil is enhanced by attaching a phenyl side chain at position N-1; thus, derivative products of azauracil consisting of N-aryl groups are used to form biologically active compounds [276,277,278,279]. Historically, synthesizing N-aryl 6-azauracil derivatives [280] involved four stages. A viable approach for synthesizing N-aryl 6-azauracil derivatives via Chan-Lam interaction mediated through copper. To synthesize N-arylated-6-azauracil derivatives, Gulipalli et al. conducted Chan-Lam coupling between azauracil and haloboronic acids in the presence of copper catalyst and pyridine. The synthesized compounds’ antimicrobial capacity was evaluated through the effective diffusion of agar. A monoarylated compound is produced when 6-azauracil 259 is treated with arylboronic acid 4 in the presence of diverse copper catalysts and base pyridine used to synthesize azauracil derivatives 260. Compounds bearing trifluoromethyl groups, namely 260b, 260f, and 260h, exhibited noteworthy antibiotic activity. The activity of substances with a chloro group attached to the phenyl rings 260a, 260c, 260g, and 260i was greater than that of products possessing fluorine 260d and 260e against gram-positive and gram-negative strains. In contrast, the activity of the remaining compounds was found to be moderate when compared with the drug chloramphenicol (Scheme 50) [281].
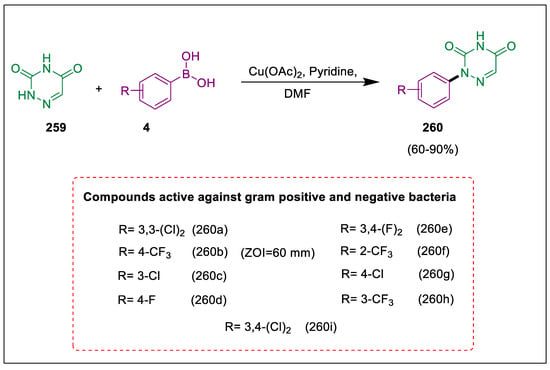
Scheme 50.
Synthesis of azauracil derivatives via Cu-catalyzed Chan-Lam cross-coupling.
Significant precursors for the synthesis of physiologically active aromatic compounds are imidazoles, nitroimidazoles, and benzimidazoles. Heteroatoms can be coupled to arenes via the Chan-Evans-Lam (CEL) coupling technique [282]. Cu(II)-catalyzed or copper(II)/cobalt(II) co-catalyzed carbon-nitrogen cross-coupling of pyrrole and boronic acid has been reported by Batey [283], Ghanbari [284], Aberi [285], and Allahresani [286]. Raju et al. used Cu-catalyzed CEL coupling to a couple of boronic acids with poorly activated imidazoles to produce targeted C-N bonds. Azomycin and many recently arylated derivatives were tested against S. pneumoniae; the lowest minimum inhibitory concentration (MIC) was shown by 262d. Arylboronic acid 4 and imidazole 261 are coupled by ligands L1–L4 and a few other classical bidentate ligand systems to support Cu (II) to generate C-N coupling product 262. Reactant demonstrates antibacterial action against several species. At 500 μM, all compounds 261, 262a–k showed an impact on the growth of S. pneumoniae cultures (Scheme 51) [287].
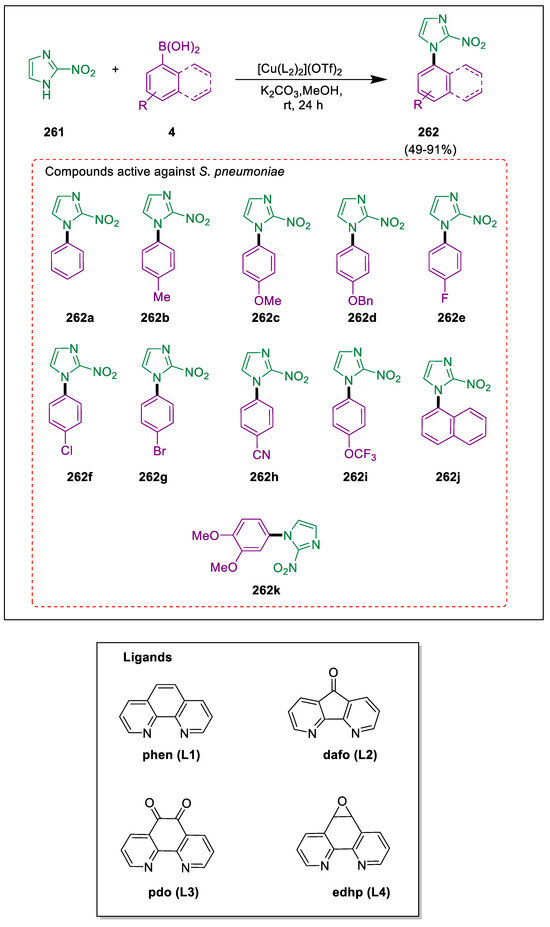
Scheme 51.
Synthesis of azomycin derivatives by using Chan-Evans-Lam cross-coupling.
3.4. Knoevenagel and Michael’s Addition Reaction
A significant component of many types of naturally occurring compounds, benzopyran (also known as chromene) has biological activity [288,289,290]. Pyrimidine and its analogs have a wide range of biological and medicinal uses [291]. The synthesis of pyrano-pyrimidine derivatives in the presence of bases has been reported using several techniques; however, the yield was low. Using Knoevenagel and Michael’s addition by using nano CuO-Ag as a catalyst, Poola et al. devised a highly effective and environmentally friendly process for the synthesis of pyranopyrimidine derivatives. The antibacterial and antifungal properties of the produced target compounds 267 were examined. Using substituted aldehydes 263, malononitrile 264, and resorcinol 265 in the presence of ethanol and DBU, perform a one-pot, three-component domino Knoevenagel–Michael addition reaction to obtain pyran derivatives 266. These derivatives were then cyclized in the presence of (Diethoxymethoxy)ethane, ammonium ethanoate, and a nano CuO–Ag catalyst to yield pyrano [2,3–d] pyrimidine derivatives 267. Compared to the normal tetracycline, compounds 267a–c showed greater activity on Gram(+ve) bacteria and 267a–d on Gram(-ve) bacteria (Scheme 52) [292].
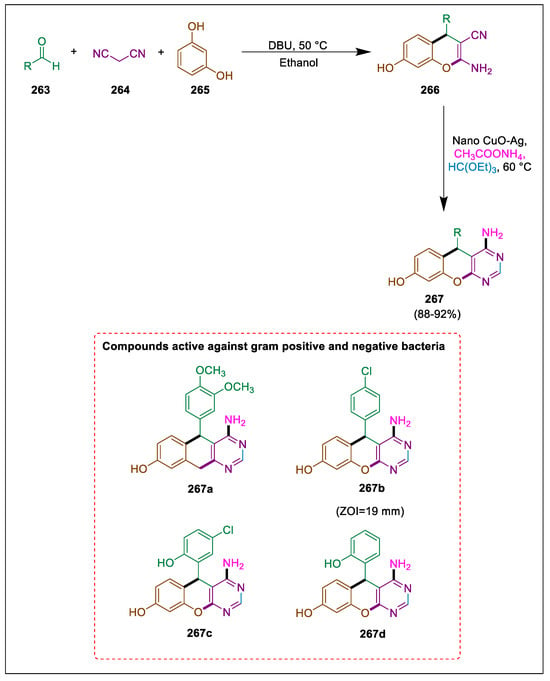
Scheme 52.
Synthesis of pyranopyrimidine derivatives by using Knoevenagel and Michael addition reactions.
3.5. 1,3-Dipolar Cycloaddition Reaction
Several extremely resilient bacterial infections have developed innovative defense mechanisms to counteract the effects of several medicines [293]. The third most often isolated nosocomial pathogen, Enterococcus, exhibits resistance to a wide range of antibiotics [294]. Because of their extensive spectrum of biological properties, coumarins and 1,2,3-triazoles are significant structures. Using a Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition process of corresponding O-propargylated coumarin 271 or N-propargylated coumarin 275 with azides, Rojas et al. synthesized a new family of coumarin-triazole conjugates. Six out of twenty-six compounds exhibited notable antibacterial activity against E. faecalis. After 4-hydroxy-coumarin 268 was treated with propargyl bromide 269 using K2CO3 in anhydrous acetone, O-propargylated coumarin 270 was obtained, and N-propargylated coumarin 274 was obtained when 4-bromo-coumarin 272 underwent nucleophilic replacement with 2-propyn-1-amine 273 in DMF. The corresponding coumarins 270 and 274 undergo copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reactions with azides 251 to provide 4-substituted 1,2,3-triazole-coumarin derivatives 271 or 275. At MICs ranging from 12.5 to 50.0 µg/mL, compounds 271a–c and 275a,b showed encouraging action against Enterococcus faecalis (Scheme 53) [295].
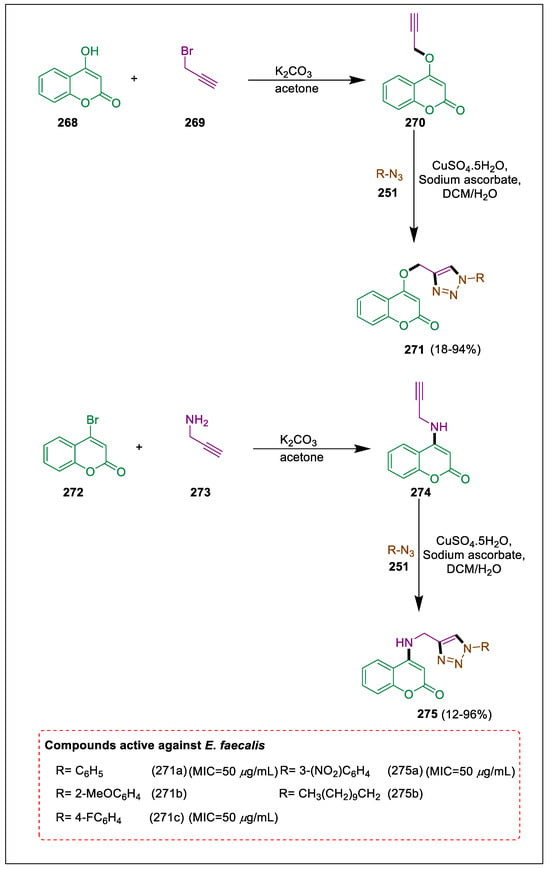
Scheme 53.
Synthesis of triazole-coumarin derivatives by using Huisgen 1,3-dipolar cycloaddition reaction.
Drug resistance to Mycobacterium tuberculosis (Mtb) evolved with the evolution of drugs. The synthesis of novel and highly effective therapeutic compounds has faced difficult challenges due to the rise of XDR and MDR tuberculosis strains [296,297] (+)-usnic acid shows biological characteristics. The usage of 1,2,3-triazoles in medical chemistry is common. Bangalore et al. synthesized novel 1,2,3-triazoles (303B1–B35) conjugated with enaminone and used them as antimycobacterial drugs. Usnic acid enaminone 303B1 with a terminal ethynyl moiety was obtained by condensing usnic acid with propargyl amine. Triazoles 284 were produced via a subsequent reaction with different azides A1–A35 that was catalyzed by copper. Aryl methyl ketones 276 were dissolved in either methanol or a CHCl3-EtOAc combination and then α-brominated to form synthetic acyl bromides 277. Sodium azide was then added, and the mixture was agitated for a whole night and obtained azide 278. To get one of azide 278, stepwise reactions in the presence of dibromoethane in DMF and NaN3 were carried out with saccharin sodium salt 279. To produce other azides 278, N-substituted piperazines 281 reacted with chloroacetyl chloride in the presence of triethyl amine using CH2Cl2 to form intermediate 282, then this mixture was dissolved in acetone-water and catalytic NaI and sodium azide were added and was agitated overnight. By interacting 283 with the produced azides 251 in the t-BuOH-H2O system in the presence of a copper catalyst and sodium-L-ascorbate, compounds 284 were synthesized using the 1,3-dipolar cycloaddition method. 284f and 284g, two compounds, exhibited a moderate level of antibacterial activity. Compound 284e demonstrated a 13 mm diametrical inhibitory zone on B. subtilis in addition to its antitubercular activity, and compound 284c demonstrated the highest antibacterial potency with moderate antitubercular activity. Additionally, it was discovered that active antitubercular compounds 284a, 284b, and 284d had an antibacterial impact on B. subtilis (Scheme 54) [252].
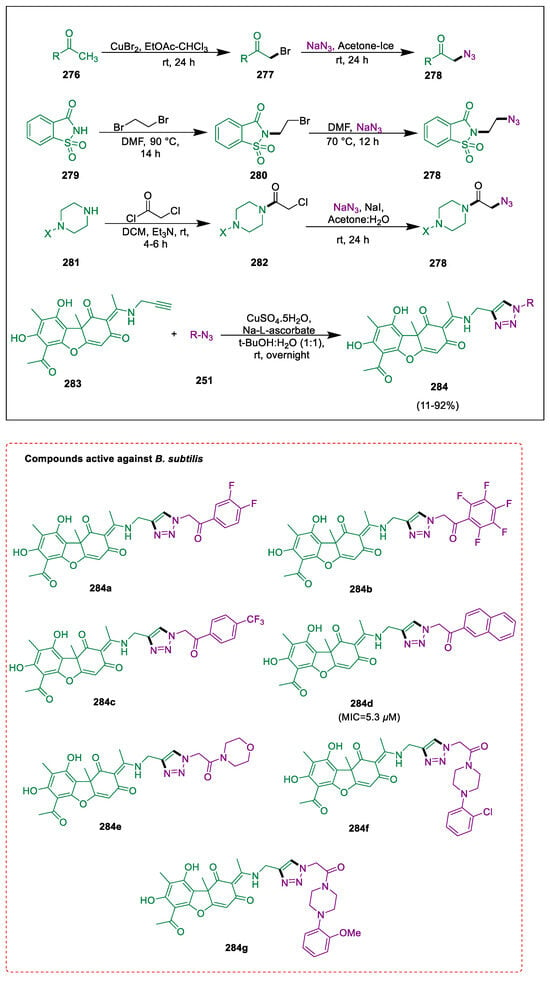
Scheme 54.
Synthesis of triazole derivatives by using 1,3-dipolar cycloaddition reaction.
1,3-Thiazine and benzothiazine heterocycles are noteworthy because of their diverse biological properties [298,299,300]. Fused triazoles and their hybrids have pharmacological characteristics [301]. We synthesized several completely decorated 1,2,3-triazoles employing a cheap Cu catalyst and sequential 1,3-dipolar cycloaddition followed by intramolecular arylation under microwave irradiation. Sucharitha et al. performed a one-pot reaction of three components that included copper-catalyzed arylation after Cu-catalyzed 1,3-dipolar cycloaddition (CuAAC) of the resultant triazole under microwave circumstances in an atmosphere of an ionizing liquid BF4. The novel manufactured compounds were tested against bacterial strains. Fused triazole and its derivatives 287 or 288 are produced by reacting either chloromethyl phenyl sulfur 285a or chloromethyl phenyl sulfone 285b with 1-(iodoethynyl)-4-substituted benzene 286 in the presence of sodium azide, CuI with 2 equivalents of t-BuOK in [Emim]BF4 under MWI at 200 W. Compounds 287a, 288a–c exhibited considerable bacterial inhibitory action against all tested positive strains and E. coli with MICs ranging from 3.12 to 12.5 µg/mL, and compound 288c also demonstrated much more inhibitory activity than conventional ciprofloxacin (Scheme 55) [302].
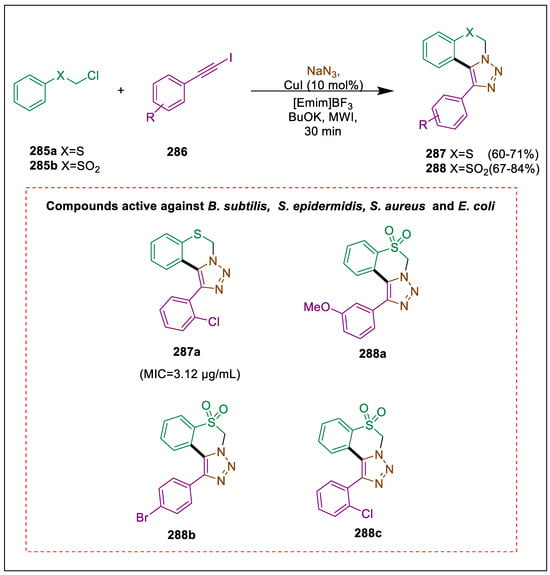
Scheme 55.
Synthesis of triazole derivatives by using Cu-catalyzed 1,3-dipolar cycloaddition (CuAAC).
3.6. Copper-Catalyzed Azide-Alkyne Cycloaddition
In organic and pharmaceutical chemistry, transition metal-catalyzed coupling reactions are crucial. Triazoles have a huge variety of applications in synthetic, medicinal, and material chemistry [303,304]. Cu-catalyzed azide-alkyne cycloaddition (CuAAC) reactions are considered the most suitable method for the preparation of 1,4-disubstituted triazoles. Naphthoquinone, alongside its derivatives, shows broad biological activities. Abbaspour et al. performed the reaction of naphthoquinone with the aromatic azides in the presence of a lower Cu catalyst loading, which provided phenyl naphthoquinone, individually and screened for antibacterial activity. The reaction of dichloronaphthalene-1,4-dione 289 with propynol 290 in the presence of t-BuOK and DMF afforded 2-chloro-3-(prop-2-yn-1-yloxy)-1,4-naphthoquinone 291 when two equiv. of propynol 290 were employed, 2,3-bis(prop-2-yn-1-yloxy)-1,4-naphthoquinone 294 was produced. Subsequently, compound 291 and compound 294 react with various aromatic azides 292 in ethanol (EtOH) with salophen copper (II) complex and sodium ascorbate to produce the intended aryl substituted 1,4-naphthoquinone 293 and 295. Compounds 295a–c showed advanced anti-bacterial activities as compared to the other derivatives (MIC, MBC = 62.5 μg/mL). The anti-bacterial activity of 293a was approximate to that of penicillin with a strong inhibition (MIC = 500 μg/mL); however, other compounds show more enhanced activity than penicillin (Scheme 56) [1].
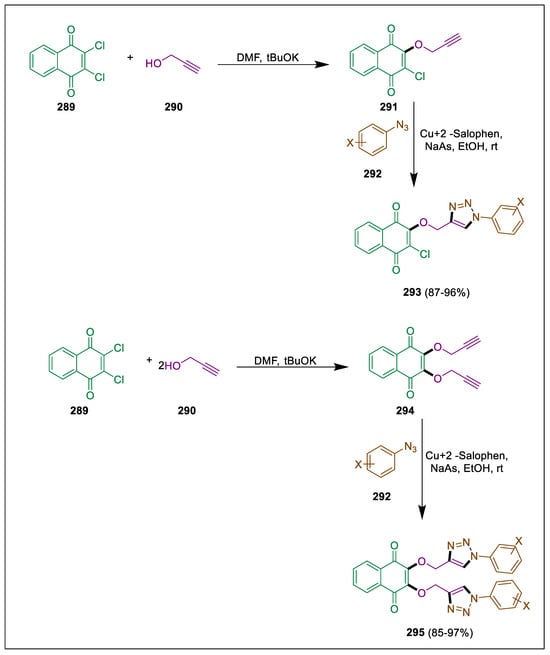
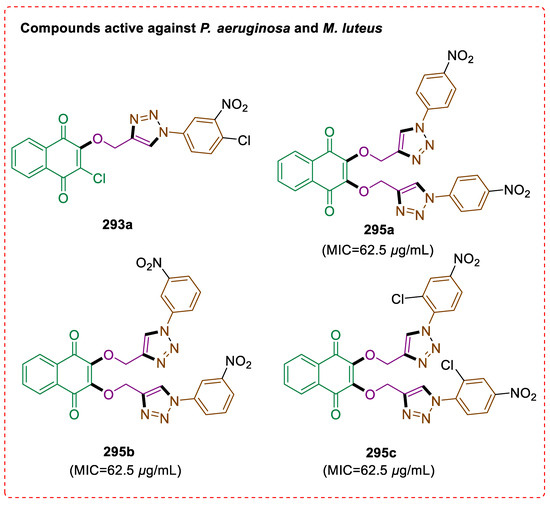
Scheme 56.
Synthesis of naphthoquinone derivatives by using copper-catalyzed azide-alkyne cycloaddition.
Presently accessible antimicrobial drugs come with several disadvantages. By using click chemistry, heterocyclic compounds with triazole moiety can be synthesized. The click reaction known as the 1,3-dipolar cycloaddition of alkynes and azides, which is catalyzed by Cu, provides a productive approach to synthesizing 1,2,3-triazoles [305]. These compounds are utilized in drug production and exhibit a wide range of biological activity. An essential role played by benzoxazepines in medicinal chemistry [306]. A unique series of dihydrobenzoxepin connected to triazole scaffolds (300a–f) was synthesized by Gandham et al. along with their pharmacological activity against various strains of fungi, bacteria Gram-(+ve) and Gram-(–ve). According to biological investigations, compounds 300a-f have zones of inhibition that range from 33–30 mm to 32–28 mm, making them good antibacterial and antifungal agents. N-phenyl-2-(prop-2-yn-1-ylamino)acetamide 296 and liquid terminal alkyne 297 undergo CuAAC reaction in the presence of pre-catalyst CuSO4⋅5H2O and sodium-ascorbate in DMF to yield 298, which then reacts with corresponding cyclic, alicyclic amines 299 to yield triazole derivatives 300. Triazoles 300a, 300b, 300e, and 300f showed moderate to better antibacterial activity against S. pyogenes and S. aureus, whereas compounds 300c, 300d, 300e, and 300f showed greater activity with MIC values of 1.2, 0.85, 2.5, and 2.9 μg/mL (Scheme 57) [307].
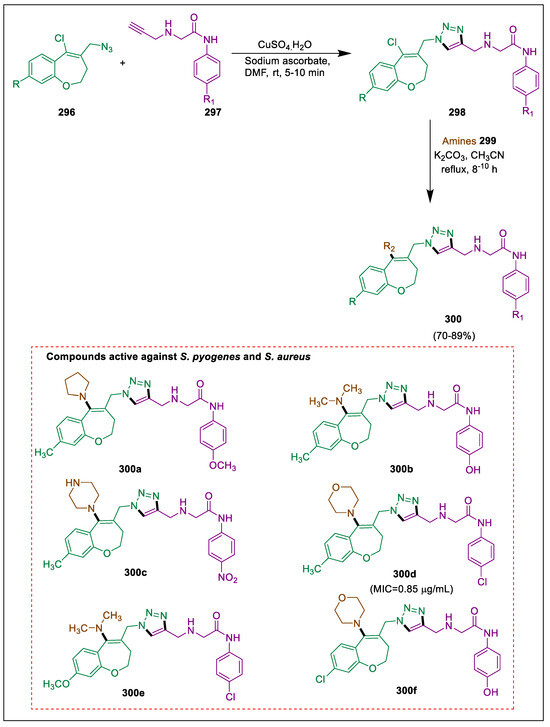
Scheme 57.
Synthesis of dihydrobenzoxepin connected to triazole derivatives by using CuAA.
3.7. Oxidative Cross-Dehydrogenative Coupling
The overuse of antibiotics is contributing to antibiotic resistance, which is a serious problem that is endangering public health [308]. To solve these problems, it may be essential to find novel antibiotics made from heterocyclic compounds, particularly those that include nitrogen that are well-known for their pharmacological characteristics. The use of triazoles in pharmaceuticals has increased. Turkmen et al. employed a one-pot Cu-Catalyzed Oxidative Cross-Dehydrogenative Coupling/Oxidative Cycloaddition technique to manufacture a novel series of triazoyl arylmethanone derivatives. The most effective compounds with lower minimum inhibitory concentrations (306a, 306c, and 306d) demonstrated high antimycobacterial activity of the aryl methanone derivatives against Mycobacterium tuberculosis. The synthesis of organic azides 304 involves three phases, which undergo a one-pot copper-catalyzed [3 + 2] cycloaddition reaction using ketone derivatives 305 in the presence of a copper catalyst to synthesize 4-(1,2,3-triazoyl)arylmethanone derivatives 306. Comparing the compounds 306a, 306c, and 306d (MIC = 4 µg/mL [12.8, 11.7, and 12.8 µM, respectively]) to the standard medication, they showed good inhibition; in contrast, compounds 306b and 306e (MIC = 8 µg/mL [25.7 and 27.7 µM, respectively]) demonstrated moderate inhibition (Scheme 58) [309].
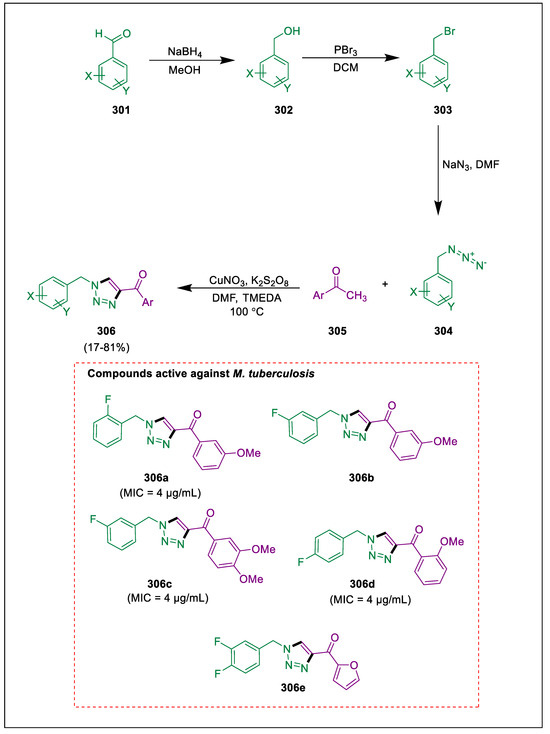
Scheme 58.
Synthesis of arylmethanone derivatives by using oxidative cross-dehydrogenative coupling.
3.8. Cyclization of Thioureas
Novel antibacterial drugs are synthesized using derivatives of benzothiazoles. The condensation reactions of 2-aminothiophenol with derivatives of carboxylic acids are part of the conventional pathway for BTA scaffolding [310]. Although palladium [311], copper(II) [312], ruthenium [313], and iron [312] catalysts are used for the synthesis of 2-aminobenzothiazoles by intramolecular cyclization of thioureas. Using copper iodide/oxone as a catalyst, Doğan et al. devised a practical, quick, and effective approach for the ring closure reaction of thioureas to generate 2-aminobenzothiazole derivatives. The antimicrobial properties of compounds were evaluated using a range of microbial strains. The thiourea-based necessary cyclization precursors 309 were synthesized by reacting 3-methylpyridin-2-amine 307 with an equivalent quantity of different isothiocyanate derivatives 308 in toluene at 80 °C. CuI/oxone was then used to catalyze the cyclization of these precursors 309 to produce 2-aminobenzothiazole derivatives 310. Apart from compounds 310a and 310b, all compounds exhibited antibacterial activity against all tested bacteria, with MIC values ranging from 32 to 1024 μg/mL. While 310b had a MIC value of 32 μg/mL against three gram-positive bacteria, making it more effective against bacteria. Moreover, 310a,b attracts interest with a MIC value of 64 μg/mL against MRSA and S. aureus (Scheme 59) [314].
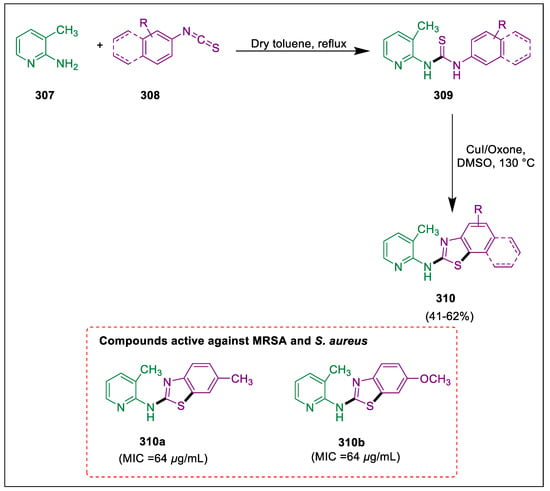
Scheme 59.
Synthesis of aminobenzothiazole derivatives by using CuI/oxone as a catalyst for cyclization of thioureas.
3.9. Copper-Catalyzed 1,2-Addition
Many bioactive natural products have skeletons of p-quinol [315,316,317,318], and their glycosides have been shown to have analgesic properties [319]. An effective technique for separating p-quinol derivatives containing aryl groups and testing them against pathogenic strains of E. coli. And overcome the limitations where p-quinols are obtained on the basis of dearomatization [320]. p-quinols were synthesized in an aqueous medium by Koszelewski et al. The antibacterial properties of the p-quinols were evaluated against E. coli, and the additives played an important role in the copper-catalyzed addition of aryl and heteroaryl boronic acids to produce benzoquinones. To synthesize p-quinols 312, a model addition reaction involving 1 mmol of arylboronic acid 4 and 1 mmol of benzoquinone 311 was carried out in distilled H2O at 20 °C with atm using a CuI-PVP catalytic system. Based on the MIC and MBC values, synthesized compounds 312a–c showed high antimicrobial activity (Scheme 60) [321].
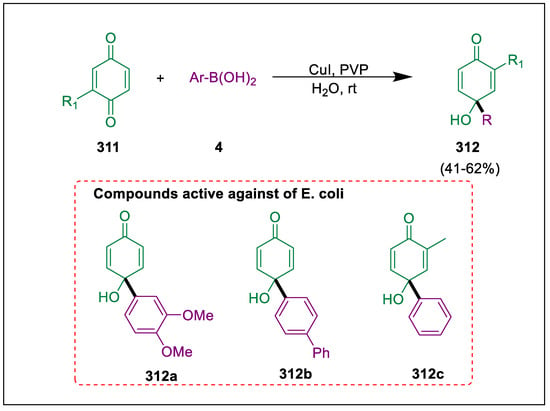
Scheme 60.
Synthesis of p-quinol derivatives by using copper-catalyzed 1,2-addition.
4. Nickel-Catalyzed Synthesis
The focus of academia and industry has recently shifted to efficient chemistry, which uses inexpensive and environmentally friendly reagents in transformations designed with high atom economy, starting from feedstocks sourced from biofuels and going beyond small molecules in strategies to produce supramolecular combinations [322,323] and designed macromolecular materials [324]. Numerous procedures have been studied for the synthesis of relatively big organic compounds and polymers, but often the linking of prefabricated components together is more expeditious [325,326,327]. Early transition metals such as Ni were discovered to be helpful reagents and catalysts early in the development of homocoupling, the combining of chemical fragments, and cross-coupling.
For cross-coupling processes, inexpensive and readily available nickel (Ni) catalysts are a good substitute for precious metal catalysts such as Pd. Furthermore, Ni demonstrates distinct reactivity, resulting in various pathways. In 1972, a nickel catalyst was used to perform the cross-coupling of organic halides with Grignard reagents; this reaction is now referred to as the Kumada-Tamao-Corriu process. Ni catalysts perform better in some reactions if the substrates are heteroaryl [328]. Therefore, one of the most effective synthetic techniques is Ni-catalyzed cross-coupling, which is widely utilized to generate complex compounds, active medicinal components, and precursors for materials chemistry [329,330].
4.1. Kumada Cross-Coupling
The incidence of antimicrobial resistance (AMR) persistently increases, creating opportunities for developing novel antibacterial agents [272,331,332]. Quinazoline derivatives are utilized in many medicinal contexts [333,334,335,336,337,338,339,340,341,342,343,344,345]. The synthetic routes for the quinazoline derivatives, which yield the desired compounds in five steps requiring column extraction for each step’s product, have been previously documented by a group [346]. Considering this, the two-step synthesis of quinazoline derivatives is described in this article. In their investigation, Ankireddy et al. utilized a nickel-catalyzed Kumada cross-coupling process to produce substituted-2-morpholino-N-(pyridin-2-ylmethyl)quinazoline-4-amine derivatives 315. Antibacterial activity tests were conducted on all the target compounds against gram-positive and gram-negative bacteria. The findings showed compounds 315e–i demonstrated significant antibacterial efficacy. 4-Chloroanthranilic acid 313 treated with morpholine-4-carbonyl chloride in tetrahydrofuran stirred at room temperature for 1 h. Acetic anhydride was added and stirred at 80 °C for 1 h, add NH3 solution was stirred at 100 °C for 2 h, then phosphoryl trichloride was stirred at 80 °C for 1 h, then pyridine-2-yl-methanamine was added and stirred at 90 °C for 1 h to obtain compound 314, which undergoes Kumada cross-coupling reaction with various aryl bromides by using five Ni catalysts to obtain quinazoline derivatives 315. 315e–i exhibited comparable antibacterial activity against susceptible bacteria but were noticeably more effective against resistant ones, whereas 315a–d, which has an electronegative atom attached to the aromatic group, demonstrates moderate antibacterial effectiveness (Scheme 61) [346].
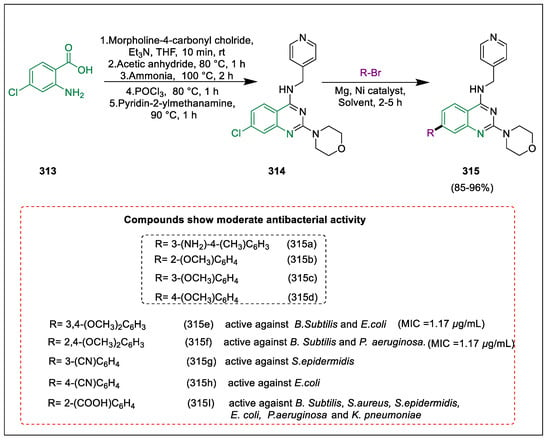
Scheme 61.
Ni-catalyzed Kumada cross-coupling to synthesize quinazoline amine and its derivatives.
The medical management of diseases induced by numerous gram-positive and gram-negative bacteria has proven difficult due to their resistance to microorganisms [346,347]. Given these factors, there is an ongoing demand for novel chemical entities that can effectively tackle the problem of multidrug resistance. A variety of chiral macrocycles and antibacterial substances were synthesized through the utilization of BINOL derivatives. In their study, Ankireddy et al. utilized the Kumada and SMC reactions to produce a novel series of disubstituted chiral (S)-BINOL derivatives 320. Additionally, the authors assessed the antibacterial activity of these compounds against gram-positive and gram-negative bacteria. Compounds 320a–d have been located to possess the most potent antibacterial activity. The Kumada coupling yield and reaction time are more favorable than the Suzuki coupling [348]. After BINOL 316 was treated with potassium carbonate and MeI, (S)-2,2′-dimethoxy-1,1-binaphthalene 317 was produced. This compound was then treated with n-butyllithium, N, N, N′, N′-tetramethylethylenediamine, and dibromo tetrachloroethane, resulting in the formation of (S)-dibromodimethoxy binaphthalene 318 [349], and it undergoes the Kumada coupling reaction [350], giving the corresponding products 319 [351]. Then subjected to demethylation under the action of BBr3 at room temperature [352] or AlCl3 upon heating and MW irradiation synthesized compound 320. All the synthesized compounds were subjected to in vitro antibacterial efficacy testing [353] and were found to be exceedingly potent. The compounds that exhibited the highest level of antibacterial capability against every type of bacteria were identified as 320a–e, with MIC values ranging from 1.17 to 4.68 μg/mL (Scheme 62) [348].
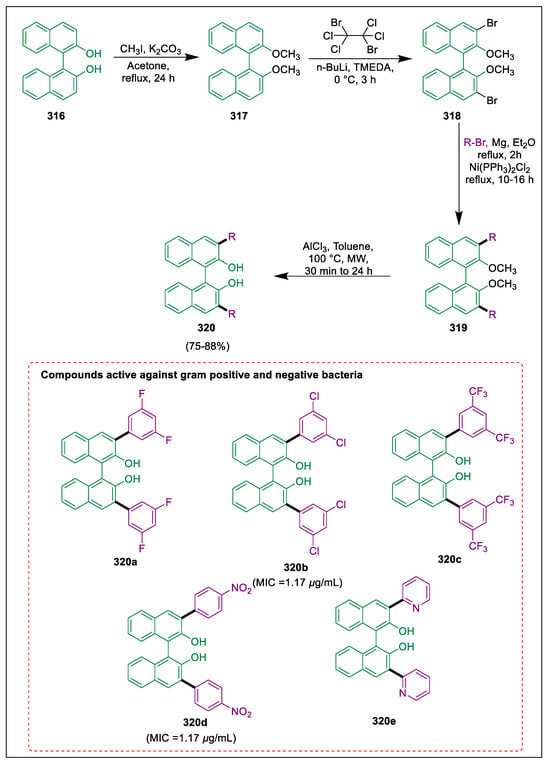
Scheme 62.
Synthesis of BINOL derivatives by using Suzuki and Kumada cross-coupling.
4.2. Buchwald-Hartwig Reaction
The synthesis of sulphonamides is an interesting topic in medicinal chemistry [354]. Sulphonamides are commonly employed in medicinal chemistry due to their high biological activity. Nassir et al. described a variety of bis-imidazole and bis-benzimidazole sulphonamides that were proven effective against bacteria. The synthesis of the C-N moiety in compounds by tandem reactions catalyzed by nickel is not well documented. Izuchukwu et al. effectively synthesized methylbenzensulphonamide and its Ni-catalyzed transformation to N-aryl substituted p-toluene sulphonamides 324 using the Buchwald-Hartwig tandem amidation technique. These compounds were tested for antibacterial and antifungal activity. Some of the substances showed greater efficacy when compared to the standard drugs (ketoconazole and ciprofloxacin). After 8 min of stirring 4-methylbenzene sulphonyl chloride 321 with ammonium hydroxide 322 in water and 5 min of heating, 4-methylbenzenesulphonamide 323 was obtained. This reacted with the appropriate aryl halides in the presence of Ni catalyst, and a solvent of t-butanol and water was pre-activated. After 5 min of stirring and heating at 80 °C for 90 min, K2CO3 was added along with additional t-butanol, and after refluxing with stirring for 1 h at 110 °C, corresponding N-aryl substituted methyl benzenesulfonamide 324 was obtained. The antibacterial activity of these compounds was compared to the standard medication ciprofloxacin. The MIC of most of the sulphonamides was found to be higher than the standard. 324a–c are the most effective antibacterial compounds (Scheme 63) [355].
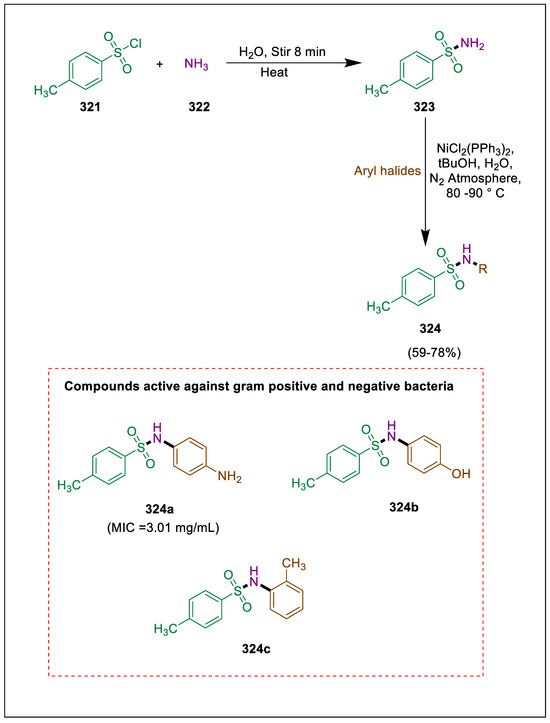
Scheme 63.
Synthesis of sulphonamide derivatives by using Buchwald-Hartwig reaction.
4.3. Photocatalytic Cross-Coupling
Because of their possible pharmacological properties, medicinal chemistry researchers are paying more attention to heterocyclic compounds that include imidazole pyridines [356,357,358]. A multitude of synthetic techniques have been established for the synthesis of imidazopyridines [359], which find application in pharmaceuticals. It has been demonstrated that imidazo [4,5-b] pyridine, one of the isomers, and its derivatives show a variety of biological functions. Under mild conditions, Jebamani et al. reported an effective approach for photocatalytically producing novel 7-aryl-1H-imidazo[4,5-b]pyridines 350 using the C-C cross-coupling process. Some compounds showed strong antimicrobial activity against both antibacterial and antifungal strains when compounds 370 were tested. After refluxing 4-bromopyridine-2,3-diamine 347 with triethyl-ortho-formate, the scaffold 7-bromo-1H-imidazo[4,5-b]pyridine 348 was produced. This scaffold then underwent a photocatalytic cross-coupling approach with the use of dual catalytic and single electron-transfer mechanisms with heteroaryl bromides 349 to produce 7-aryl-1H-imidazo[4,5-b] pyridines 350. Compounds 350a and 350c were identified as effective bactericides towards S. faecalis and B. subtilis, respectively, and consequently moderate responses towards K. pneumonia, E. coli, and P. aeruginosa. Compounds 350b and 350d demonstrated remarkable anti-bacterial responses against gram-positive and gram-negative bacteria (Scheme 64) [360].
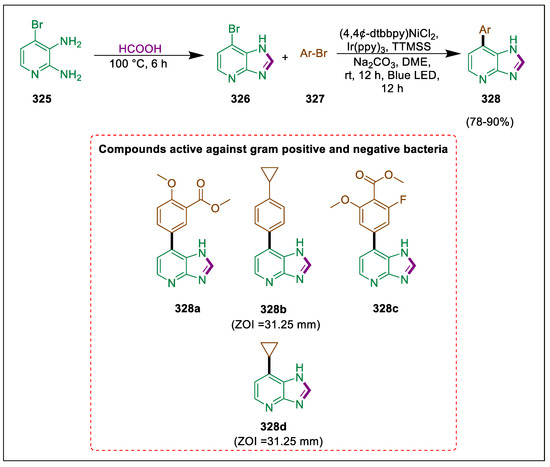
Scheme 64.
Synthesis of imidazo pyridine derivatives by using photocatalytic cross-coupling.
5. Conclusions
Transition metal-catalyzed reactions are employed in synthetic and pharmaceutical sectors. These reactions have enabled the synthesis of complicated pharmaceutical scaffolds, while catalysis with transition metals such as Pd, Ni, and Cu has brought efficiency to the synthesis of approved drug candidates that are now being developed in labs all over the world [361,362,363,364,365]. The last five years’ research on the synthesis of antibacterial molecules via Pd, Cu, and Ni-catalyzed reactions is provided in a comprehensible manner, keeping in mind that these synthetic approaches may inspire pharmaceutical researchers. Since it is quite challenging to design a drug without knowing its chemical structures, which in return provide a gateway of compatibility with the target protein inside the human body. Thus, it is essential to have knowledge about some bioactive molecules that may prove to be the next most effective drug. Scheme numbers with their methods and against which these compounds show activity summarized in Table 1.

Table 1.
Key synthetic methods and antibacterial compounds.
Author Contributions
A.Z., writing—original draft, writing—review and editing; S.K., visualization, writing—review and editing; N.R., supervision, project administration; N.M., conceptualization; M.I., review editing; C.M., data curation; S.I.T., project administration; O.A., visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
There are no conflicts of interest.
Abbreviations
| Full Form | Abbreviation |
| Global Antimicrobial Resistance and Use Surveillance System | GLASS |
| Antimicrobial resistance | AMR |
| Dimethylformamide dimethyl acetal | DMFDMA |
| Extended-spectrum β-lactamase | ESBL |
| Extensively drug-resistant | XDR |
| Dichloromethane | DCM |
| Thymidylate monophosphate kinase | TMPK |
| photoactive polyphenylene ethynylene | PPE |
| Minimum inhibitory concentrations | MIC |
| Bacterial leaf blight | BLB |
| Chan-Evans-Lam | CEL |
| Minimum Inhibitory Concentration | MIC |
| Cu-catalyzed 1,3-dipolar cycloaddition | CuAAC |
References
- Abbaspour, S.; Keivanloo, A.; Bakherad, M.; Sepehri, S. Salophen copper (II) complex-assisted click reactions for fast synthesis of 1, 2, 3-triazoles based on naphthalene-1,4-dione scaffold, antibacterial evaluation, and molecular docking studies. Chem. Biodivers. 2019, 16, e1800410. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K., Jr.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D.; Infectious Diseases Society of America. 10ב20 progress-development of new drugs active against Gram-negative bacilli: An update from the infectious diseases society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 2013, 66, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.D. Daptomycin: A new drug class for the treatment of Gram-positive infections. Drugs Today 2005, 41, 81–90. [Google Scholar] [CrossRef]
- Solomon, S.; Horan, T.; Andrus, M.; Edwards, J.; Fridkin, S.; Koganti, J.; Peavy, G.; Tolson, J. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 2003, 31, 481–498. [Google Scholar]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; Kashef, H.E.; Lancelot, J.C.; Rault, S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of NO production and antiproliferative activities of some indole derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar] [CrossRef]
- Parisi, O.I.; Fiorillo, M.; Caruso, A.; Cappello, A.R.; Saturnino, C.; Puoci, F.; Panno, A.; Dolce, V.; El-Kashef, H.; Sinicropi, M.S. Enhanced cellular uptake by “pharmaceutically oriented devices” of new simplified analogs of linezolid with antimicrobial activity. Int. J. Pharm. 2014, 461, 163–170. [Google Scholar] [CrossRef]
- Iacopetta, D.; Mariconda, A.; Saturnino, C.; Caruso, A.; Palma, G.; Ceramella, J.; Muià, N.; Perri, M.; Sinicropi, M.S.; Caroleo, M.C. Novel gold and silver carbene complexes exert antitumor effects triggering the reactive oxygen species dependent intrinsic apoptotic pathway. ChemMedChem 2017, 12, 2054–2065. [Google Scholar] [CrossRef]
- Khan, R.A.; Tăbăcaru, A.; Ali, F.; Koo, B.H. Anticancer and antimicrobial properties of inorganic compounds/nanomaterials. Bioinorg. Chem. Appl. 2019, 2019, 6019632. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Droc, G.; Stefan, G. FDA approved antibacterial drugs: 2018–2019. Discoveries 2019, 7, e102. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Emonet, S. Navigating the world wide web in search of resources on antimicrobial resistance. Clin. Infect. Dis. 2006, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Coulborn, R.M.; Clayton, E.R.; Aiello, A.E. Pharmaceutical and personal care products in the environment and potential risks of emerging antibiotic resistance. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; ACS Publications: Washington, DC, USA, 2010; pp. 367–382. [Google Scholar]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef] [PubMed]
- Goossen, L.J.; Melzer, B. Synthesis of valsartan via decarboxylative biaryl coupling. J. Org. Chem. 2007, 72, 7473–7476. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.D.; King, A.O.; Chen, C.Y.; Corley, E.G.; Foster, B.S.; Roberts, F.E.; Yang, C.; Lieberman, D.R.; Reamer, R.A.; Tschaen, D.M. Efficient synthesis of losartan, a nonpeptide angiotensin II receptor antagonist. J. Org. Chem. 1994, 59, 6391–6394. [Google Scholar] [CrossRef]
- Cousaert, N.; Willand, N.; Gesquière, J.C.; Tartar, A.; Déprez, B.; Deprez-Poulain, R. Original loading and Suzuki conditions for the solid-phase synthesis of biphenyltetrazoles application to the first solid-phase synthesis of irbesartan. Tetrahedron Lett. 2008, 49, 2743–2747. [Google Scholar] [CrossRef]
- Vijayalakshmi, C.; Prasad, M.G.; Katari, N.K.; Reddy, P.N. A scalable synthesis of biaryl unit of the HIV protease inhibitor atazanavir. Lett. Org. Chem. 2020, 17, 68–72. [Google Scholar] [CrossRef]
- Gehringer, M.; Forster, M.; Laufer, S.A. Solution-phase parallel synthesis of ruxolitinib-derived janus kinase inhibitors via copper-catalyzed azide-alkyne cycloaddition. ACS Comb. Sci. 2015, 17, 5–10. [Google Scholar] [CrossRef]
- Zhang, E.; Tang, J.; Li, S.; Wu, P.; Moses, J.E.; Sharpless, K.B. Chemoselective synthesis of polysubstituted pyridines from heteroaryl fluorosulfates. Chem. Eur. J. 2016, 22, 5692–5697. [Google Scholar] [CrossRef]
- Erickson, G.; Guo, J.; McClure, M.; Mitchell, M.; Salaun, M.C.; Whitehead, A. Synthesis of lapatinib via direct regioselective arylation of furfural. Tetrahedron Lett. 2014, 55, 6007–6010. [Google Scholar] [CrossRef]
- Lau, R.M.; van Eupen, J.T.; Schipper, D.; Tesser, G.I.; Verweij, J.; de Vroom, E. Synthesis of penicillin N and isopenicillin N. Tetrahedron 2000, 56, 7601–7606. [Google Scholar] [CrossRef]
- Farina, V.; Baker, S.R.; Benigni, D.A.; Hauck, S.I.; Sapino, C., Jr. Palladium catalysis in cephalosporin chemistry: General methodology for the synthesis of cephem side chains. J. Org. Chem. 1990, 55, 5833–5847. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Wang, S.; Zhang, Q. A convenient synthesis of linezolid through Buchwald-Hartwig amination. Tetrahedron Lett. 2022, 105, 154050. [Google Scholar] [CrossRef]
- Nicolaou, K.; Li, H.; Boddy, C.N.; Ramanjulu, J.M.; Yue, T.Y.; Natarajan, S.; Chu, X.J.; Bräse, S.; Rübsam, F. Total synthesis of vancomycin-part 1: Design and development of methodology. Chem. Eur. J. 1999, 5, 2584–2601. [Google Scholar] [CrossRef]
- Nelson, M.L.; Ismail, M.Y.; McIntyre, L.; Bhatia, B.; Viski, P.; Hawkins, P.; Rennie, G.; Andorsky, D.; Messersmith, D.; Stapleton, K. Versatile and facile synthesis of diverse semisynthetic tetracycline derivatives via Pd-catalyzed reactions. J. Org. Chem. 2003, 68, 5838–5851. [Google Scholar] [CrossRef]
- Shen, L.; Simmons, C.J.; Sun, D. Microwave-assisted synthesis of macrocycles via intramolecular and/or bimolecular Ullmann coupling. Tetrahedron Lett. 2012, 53, 4173–4178. [Google Scholar] [CrossRef]
- Lin, H.; Bruhn, D.F.; Maddox, M.M.; Singh, A.P.; Lee, R.E.; Sun, D. Synthesis and antibacterial evaluation of macrocyclic diarylheptanoid derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 4070–4076. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent advances in the synthesis of diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Mizoroki, T.; Mori, K.; Ozaki, A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971, 44, 581. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J., Jr. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Julia, M.; Duteil, M. Condensation des halogenureś aromatiques avec les olefine catalyseés par le palladium. Bull. Soc. Chim. Fr. 1973, 2790. [Google Scholar]
- Julia, M.; Duteil, M.; Grard, C.K.E.; Kunz, E. Etude de la condesation de chlorures aromatiques aves les olefines catalisee par le palladium. Bull. Soc. Chim. Fr. 1973, 9–10, 2791–2794. [Google Scholar]
- Batey, R.A.; Quach, T.D. Synthesis and cross-coupling reactions of tetraalkylammonium organotrifluoroborate salts. Tetrahedron Lett. 2001, 42, 9099–9103. [Google Scholar] [CrossRef]
- Mujahid, A.; Rasool, N.; Qamar, M.U.; Zubair, M.; Ahmad, F.; Altaf, A.A.; Akhtar, A.; Shah, S.A.A.; Alqahtani, F.; Alsanea, S. Arylation of halogenated thiophene carboxylate via Suzuki-Miyaura reaction: Anti-bacterial study against clinically isolated extensively drug resistant Escherichia coli sequence type 405 and computational investigation. Arab. J. Chem. 2022, 15, 103662. [Google Scholar] [CrossRef]
- Dheilly, N.M.; Ewald, P.W.; Brindley, P.J.; Fichorova, R.N.; Thomas, F. Parasite-microbe-host interactions and cancer risk. PLoS Pathog. 2019, 15, e1007912. [Google Scholar] [CrossRef]
- Aydın, A.; Ökten, S.; Erkan, S.; Bulut, M.; Özcan, E.; Tutar, A.; Eren, T. In-vitro anticancer and antibacterial activities of brominated indeno[1, 2-b]qinolineamines supported with molecular docking and MCDM. ChemistrySelect 2021, 6, 3286–3295. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Zhu, L.H.; Chen, C.; Wang, H.; Ye, W.C.; Zhou, G.X. Indole alkaloids from alocasia macrorrhiza. Chem. Pharm. Bull. 2012, 60, 670–673. [Google Scholar] [CrossRef]
- Rehberg, N.; Sommer, G.A.; Drießen, D.; Kruppa, M.; Adeniyi, E.T.; Chen, S.; Wang, L.; Wolf, K.; Tasch, B.O.; Ioerger, T.R.; et al. Nature-inspired (di) azine-bridged bisindole alkaloids with potent antibacterial in vitro and in vivo efficacy against methicillin-resistant Staphylococcus aureus. J. Med. Chem. 2020, 63, 12623–12641. [Google Scholar] [CrossRef]
- Ly, K.S.; Letavic, M.A.; Keith, J.M.; Miller, J.M.; Stocking, E.M.; Barbier, A.J.; Bonaventure, P.; Lord, B.; Jiang, X.; Boggs, J.D.; et al. Synthesis and biological activity of piperazine and diazepane amides that are histamine H3 antagonists and serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; del Carmen Nuñez, M.; Morelli, A.; Falzoni, S.; Di Virgilio, F.; Romagnoli, R. Synthesis and biological activity of N-arylpiperazine-modified analogues of KN-62, a potent antagonist of the purinergic P2X7 receptor. J. Med. Chem. 2003, 46, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, Y.; Zhan, Y.; Zhang, L.; Zhang, Y.; Wang, L.; Zhang, X.; Li, Y.; Li, Z.; Li, B. Synthesis and biological activity of novel furan/thiophene and piperazine-containing (bis)-1, 2, 4-triazole Mannich bases. Chin. J. Chem. 2015, 33, 1124–1134. [Google Scholar] [CrossRef]
- Tataringa, G.; Stan, C.D.; Zbancioc, A.M.; Jitareanu, A.; Tuchilus, C. Preliminary screening of biological activities of some new schiff bases of isatins. Farmacia 2014, 62, 14–22. [Google Scholar]
- Chhatriwala, N.M.; Patel, A.B.; Patel, R.V.; Kumari, P. In vitro biological investigations of novel piperazine based heterocycles. J. Chem. Res. 2014, 38, 611–616. [Google Scholar] [CrossRef]
- Patel, A.B. Investigation of the antibacterial activity of new quinazoline derivatives against methicillin and quinolone resistant Staphylococcus aureus. J. Chem. Res. 2020, 44, 315–321. [Google Scholar] [CrossRef]
- Ashok, D.; Nagaraju, N.; Lakshmi, B.V.; Sarasija, M. Microwave assisted synthesis of 5-[4-(3-phenyl-4,5-dihydro-1 H-pyrazol-5-yl) phenyl]-1H-tetrazole derivatives and their antimicrobial activity. Russ. J. Gen. Chem. 2019, 89, 1905–1910. [Google Scholar] [CrossRef]
- Ashok, D.; Nagaraju, N.; Reddy, M.R.; Dharavath, R.; Ramakrishna, K.; Sarasija, M. Microwave-assisted synthesis of tetrazole based biphenyls derivatives and their antimicrobial activity. Russ. J. Chem. 2020, 13, 601–609. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Murugesan, S.; AnandaRajagopal, K. Antibacterial, antifungal and anticonvulsant evaluation of novel newly synthesized 1-[2-(1H-tetrazol-5-yl) ethyl]-1 H-benzo [d][1, 2, 3] triazoles. Arch. Pharm. Res. 2006, 29, 535–540. [Google Scholar] [CrossRef]
- Adamec, J.; Waisser, K.; Kuneš, J.; Kaustova, J. A note on the antitubercular activities of 1-aryl-5-benzylsulfanyltetrazoles. Int. J. Pharm. 2005, 338, 385–389. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Salvador, M.K.; Preti, D.; Aghazadeh Tabrizi, M.; Brancale, A.; Fu, X.H.; Li, J.; Zhang, S.Z.; Hamel, E.; et al. Synthesis and evaluation of 1, 5-disubstituted tetrazoles as rigid analogues of combretastatin A-4 with potent antiproliferative and antitumor activity. J. Med. Chem. 2012, 55, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Le Bourdonnec, B.; Cauvin, C.; Meulon, E.; Yous, S.; Goossens, J.F.; Durant, F.; Houssin, R.; Hénichart, J.P. Comparison of 3D structures and AT1 binding properties of pyrazolidine-3, 5-diones and tetrahydropyridazine-3,6-diones with parent antihypertensive drug irbesartan. J. Med. Chem. 2002, 45, 4794–4798. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Nagaraju, N.; Sarasija, M.; Lakshmi, V.B. Microwave assisted synthesis of 1H-tetrazole-based flavonoid derivatives and their antimicrobial activity. J. Serb. Chem. Soc. 2018, 83, 1305–1313. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Marco, M.J. Ligand-free palladium catalysis of the Suzuki reaction in water using microwave heating. Org. Lett. 2002, 4, 2973–2976. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, Y. Phosphine-free palladium acetate catalyzed Suzuki reaction in water. J. Org. Chem. 2005, 70, 6122–6125. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. An efficient protocol for palladium-catalyzed ligand-free Suzuki-Miyaura coupling in water. Green Chem. 2012, 14, 1873–1876. [Google Scholar] [CrossRef]
- Chavan, O.S.; Jadhav, S.A.; Shioorkar, M.G.; Chavan, S.B.; Baseer, M.A.; Shinde, D.B. Microwave assisted solvent free synthesis of coumarins using Zn [(L)-Proline] 2 catalyst. Rasayan J. Chem 2015, 8, 194–197. [Google Scholar]
- Li, Z.; Zhan, P.; Liu, X. 1, 3, 4-Oxadiazole: A privileged structure in antiviral agents. Mini Rev. Med. Chem. 2011, 11, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, K.M.; Hagrs, M.S.; Abulkhair, H.S. Synthesis, characterization and antimicrobial evaluation of some novel quinoline derivatives bearing different heterocyclic moieties. Bull. Fac. Pharm. Cairo Univ. 2016, 54, 263–273. [Google Scholar] [CrossRef][Green Version]
- Sindhe, M.A.; Bodke, Y.D.; Kenchappa, R.; Telkar, S.; Chandrashekar, A. Synthesis of a series of novel 2, 5-disubstituted-1, 3, 4-oxadiazole derivatives as potential antioxidant and antibacterial agents. J. Chem. Biol. 2016, 9, 79–90. [Google Scholar] [CrossRef]
- Makane, V.B.; Krishna, V.S.; Krishna, E.V.; Shukla, M.; Mahizhaveni, B.; Misra, S.; Chopra, S.; Sriram, D.; Azger Dusthackeer, V.N.; Rode, H.B. Novel 1, 3, 4-oxadiazoles as antitubercular agents with limited activity against drug-resistant tuberculosis. Future Med. Chem. 2019, 11, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Akhter, M.; Shaquiquzzaman, M. A review exploring therapeutic worth of 1, 3, 4-oxadiazole tailored compounds. Mini Rev. Med. Chem. 2019, 19, 477–509. [Google Scholar] [CrossRef] [PubMed]
- Thummar, S.N.; Bhatt, V.D. Palladium-catalyzed Suzuki coupling synthesis and biological activities of ten new 1, 3, 4-oxadiazole derivatives. Indian J. Heterocycl. Chem. 2020, 30, 49–54. [Google Scholar]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- El-Emary, T.I. Synthesis and biological activity of some new pyrazolo[3, 4-b]pyrazines. J. Chin. Chem. Soc. 2006, 53, 391–401. [Google Scholar] [CrossRef]
- Goda, F.; Maarouf, A.; El-Bendary, E. Synthesis and antimicrobial evaluation of new isoxazole and pyrazole derivatives. Saudi Pharm. J. 2003, 11, 111–117. [Google Scholar]
- Qamar, M.U.; Saleem, S.; Toleman, M.A.; Saqalein, M.; Waseem, M.; Nisar, M.A.; Khurshid, M.; Taj, Z.; Jahan, S. In vitro and in vivo activity of manuka honey against NDM-1-producing Klebsiella pneumoniae ST11. Future Microbiol. 2018, 13, 13–26. [Google Scholar] [CrossRef]
- Ahmad, G.; Rasool, N.; Qamar, M.U.; Alam, M.M.; Kosar, N.; Mahmood, T.; Imran, M. Facile synthesis of 4-aryl-N-(5-methyl-1H-pyrazol-3-yl)benzamides via Suzuki Miyaura reaction: Antibacterial activity against clinically isolated NDM-1-positive bacteria and their docking studies. Arab. J. Chem. 2021, 14, 103270. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Isloor, A.M.; Kalluraya, B.; Shetty, P. Regioselective reaction: Synthesis, characterization and pharmacological studies of some new mannich bases derived from 1, 2, 4-triazoles. Eur. J. Med. Chem. 2009, 44, 3784–3787. [Google Scholar] [CrossRef] [PubMed]
- Ali Salman, G.; Zinad, D.S.; Mahal, A.; Rizki Fadhil Pratama, M.; Duan, M.; Alkhouri, A.; Alamiery, A. Synthesis, antibacterial activity, and molecular docking study of bispyrazole-based derivatives as potential antibacterial agents. ChemistrySelect 2022, 7, e202103901. [Google Scholar] [CrossRef]
- Kumar, G.; Tanwar, O.; Kumar, J.; Akhter, M.; Sharma, S.; Pillai, C.; Alam, M.M.; Zama, M. Pyrazole-pyrazoline as promising novel antimalarial agents: A mechanistic study. Eur. J. Med. Chem. 2018, 149, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Eleya, N.; Mahal, A.; Hein, M.; Villinger, A.; Langer, P. Synthesis of arylated quinolines by chemo- and site- selective Suzuki-Miyaura reactions of 5, 7-dibromo-8-(trifluoromethanesulfonyloxy)quinoline. Adv. Synth. Catal. 2011, 353, 2761–2774. [Google Scholar] [CrossRef]
- Ibad, M.F.; Eleya, N.; Obaid-Ur-Rahman, A.; Mahal, A.; Hussain, M.; Villinger, A.; Langer, P. Site-selective Suzuki-Miyaura reactions of 1, 4-and 3, 5-bis(trifluoromethylsulfonyloxy)-2-naphthoates. Synthesis 2011, 13, 2101–2116. [Google Scholar] [CrossRef]
- Salman, G.A.; Mahal, A.; Shkoor, M.; Hussain, M.; Villinger, A.; Langer, P. Regioselective Suzuki–Miyaura reactions of the bis(triflate) of 1, 2, 3, 4-tetrahydro-9, 10-dihydroxyanthracen-1-one. Tetrahedron Lett. 2011, 52, 392–394. [Google Scholar] [CrossRef]
- Mahal, A.; Villinger, A.; Langer, P. Synthesis of 1, 2-diarylanthraquinones by site-selective Suzuki-Miyaura reactions of the bis(triflate) of alizarin. Synlett 2010, 2010, 1085–1088. [Google Scholar] [CrossRef]
- Mahal, A.; Villinger, A.; Langer, P. Site-selective arylation of alizarin and purpurin based on Suzuki-Miyaura cross-coupling reactions. Chem. Eur. 2011, 2011, 2075–2087. [Google Scholar] [CrossRef]
- Johnson, M.; Younglove, B.; Lee, L.; LeBlanc, R.; Holt, H., Jr.; Hills, P.; Mackay, H.; Brown, T.; Mooberry, S.L.; Lee, M. Design, synthesis, and biological testing of pyrazoline derivatives of combretastatin-A4. Bioorg. Med. Chem. Lett. 2007, 17, 5897–5901. [Google Scholar] [CrossRef]
- Solankee, A.; Kapadia, K.; Ćirić, A.; Soković, M.; Doytchinova, I.; Geronikaki, A. Synthesis of some new S-triazine based chalcones and their derivatives as potent antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamledesai, S.; Thippeswamy, A.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.; Vélez, I.D.; Upegui, Y.; Nogueras, M.; Cobo, J. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl) amino] chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.C.; Li, H.Q.; Sun, J.; Zhou, Y.; Zhu, H.L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Biorg. Med. Chem. 2010, 18, 4606–4614. [Google Scholar] [CrossRef]
- Karim, D.K.; Salman, G.A.; Al-Mansury, S.; Jinzeel, N.A. Synthesis and antimicrobial evaluation of arylated 1, 3, 5-triphenyl pyrazoline derivatives using Suzuki-Miyaura reactions. Egypt. J. Chem. 2021, 64, 2469–2481. [Google Scholar]
- Jeffery, T. Highly stereospecific palladium-catalysed vinylation of vinylic halides under solid-liquid phase transfer conditions. Tetrahedron Lett. 1985, 26, 2667–2670. [Google Scholar] [CrossRef]
- Jeffery, T. Palladium-catalyzed vinylation of acetylenic iodides under solid-liquid please-transfer conditions. Synthesis 1987, 1987, 70–71. [Google Scholar] [CrossRef]
- Almeida, A.I.; Silva, A.M.; Cavaleiro, J.A. Reactivity of 3-iodo-4-quinolones in Heck reactions: Synthesis of novel (E)-3-styryl-4-quinolones. Synlett 2010, 2010, 462–466. [Google Scholar] [CrossRef]
- Mohamed, Y.M.; Solum, E.J.; Eweas, A.F. Synthesis, antibacterial evaluation, and docking studies of azaisoflavone analogues generated by palladium-catalyzed cross-coupling. Monatsh. Chem. 2018, 149, 1857–1864. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO global priority pathogens list: A bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019, 34, 184. [Google Scholar] [CrossRef]
- Ahmad, G.; Khalid, A.; Qamar, M.U.; Rasool, N.; Saadullah, M.; Bilal, M.; Bajaber, M.A.; Obaidullah, A.J.; Alotaibi, H.F.; Alotaibi, J.M. Antibacterial efficacy of N-(4-methylpyridin-2-yl) thiophene-2-carboxamide analogues against extended-spectrum-β-lactamase producing clinical strain of Escherichia coli ST 131. Molecules 2023, 28, 3118. [Google Scholar] [CrossRef] [PubMed]
- Usman Qamar, M.; Lopes, B.S.; Hassan, B.; Khurshid, M.; Shafique, M.; Atif Nisar, M.; Mohsin, M.; Nawaz, Z.; Muzammil, S.; Aslam, B. The present danger of New delhi metallo-β-lactamase: A threat to public health. Future Microbiol. 2020, 15, 1759–1778. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Ieri, F.; Goretti, M.; Branda, E.; Mulinacci, N.; Romani, A. Catechins and proanthocyanidins: Naturally occurring o-heterocycles with antimicrobial activity. Bioact. Heterocycles IV 2007, 10, 239–263. [Google Scholar]
- Donnelly, D.; Boland, G. Isoflavonoids and neoflavonoids: Naturally occurring o-heterocycles. Nat. Prod. Rep. 1995, 12, 321–338. [Google Scholar] [CrossRef]
- Kumawat, M.K. Thiazole containing heterocycles with antimalarial activity. Curr. Drug Discov. Technol. 2018, 15, 196–200. [Google Scholar] [CrossRef]
- Siddiqa, A.; Zubair, M.; Bilal, M.; Rasool, N.; Qamar, M.U.; Khalid, A.; Ahmad, G.; Imran, M.; Mahmood, S.; Ashraf, G.A. Synthesis of functionalized N-(4-bromophenyl) furan-2-carboxamides via Suzuki-Miyaura cross-coupling: Anti-bacterial activities against clinically isolated drug resistant A. baumannii, K. pneumoniae, E. cloacae and MRSA and its validation via a computational approach. Pharmaceuticals 2022, 15, 841. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Jha, K.; Kumar, S. Biological diversity of thiophene: A review. J. Adv. Sci. Res. 2012, 3, 3–10. [Google Scholar]
- Mohan, C.; Bhargava, G.; Bedi, P.M. Thieno[3, 2-d]pyrimidin-4-one derivatives as potential antibacterial agents. J. Life Sci. 2009, 1, 97–101. [Google Scholar] [CrossRef]
- Folkes, A.J.; Ahmadi, K.; Alderton, W.K.; Alix, S.; Baker, S.J.; Box, G.; Chuckowree, I.S.; Clarke, P.A.; Depledge, P.; Eccles, S.A. The identification of 2-(1 H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3, 2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef]
- Laddha, S.S.; Bhatnagar, S.P. A new therapeutic approach in Parkinson’s disease: Some novel quinazoline derivatives as dual selective phosphodiesterase 1 inhibitors and anti-inflammatory agents. Biorg. Med. Chem. 2009, 17, 6796–6802. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Raja Solomon, V.; Meenac, R.; Ramaseshu, K.; Thirumurugan, K.; Murugesan, S. Design and Synthesis of 2-Methylthio-3-substituted-5, 6-dimethylthieno [2, 3-d] pyrimidin-4 (3H)-ones as analgesic, anti-inflammatory and antibacterial agents. Med. Chem. 2007, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wardakhan, W.W.; Abdel-Salam, O.M.; Elmegeed, G.A. Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharm. 2008, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ikram, H.M.; Rasool, N.; Ahmad, G.; Chotana, G.A.; Musharraf, S.G.; Zubair, M.; Rana, U.A.; Zia-Ul-Haq, M.; Jaafar, H.Z. Selective C-arylation of 2, 5-dibromo-3-hexylthiophene via Suzuki cross-coupling reaction and their pharmacological aspects. Molecules 2015, 20, 5202–5214. [Google Scholar] [CrossRef]
- Rizwan, K.; Zubair, M.; Rasool, N.; Mahmood, T.; Ayub, K.; Alitheen, N.B.; Aziz, M.N.M.; Akhtar, M.N.; Nasim, F.-u.-H.; Bukhary, S.M. Palladium (0) catalyzed Suzuki cross-coupling reaction of 2, 5-dibromo-3-methylthiophene: Selectivity, characterization, DFT studies and their biological evaluations. Chem. Cent. J. 2018, 12, 49. [Google Scholar] [CrossRef]
- Chu, D.T.; Plattner, J.J.; Katz, L. New directions in antibacterial research. J. Med. Chem. 1996, 39, 3853–3874. [Google Scholar] [CrossRef] [PubMed]
- Beović, B. The issue of antimicrobial resistance in human medicine. Int. J. Food Microbiol. 2006, 112, 280–287. [Google Scholar] [CrossRef]
- Finch, R.; Hunter, P. Antibiotic resistance-action to promote new technologies: Report of an EU intergovernmental conference held in Birmingham, UK, 12–13 december 2005. J. Antimicrob. Chemother. 2006, 58, i3–i22. [Google Scholar] [CrossRef]
- McNaughton, B.R.; Miller, B.L. A mild and efficient one-step synthesis of quinolines. Org. Lett. 2003, 5, 4257–4259. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, H.; Singh, A.; Agrawal, D.K.; Arora, A.; Chundawat, T.S. Trifluoromethylated quinolone-hydantoin hybrids: Synthesis and antibacterial evaluation. Sci 2022, 4, 30. [Google Scholar] [CrossRef]
- Manjal, S.K.; Kaur, R.; Bhatia, R.; Kumar, K.; Singh, V.; Shankar, R.; Kaur, R.; Rawal, R.K. Synthetic and medicinal perspective of thiazolidinones: A review. Bioorg. Chem. 2017, 75, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, T.; Zidar, N.; Mueller-Premru, M.; Kikelj, D.; Mašič, L.P. Synthesis and antibacterial activity of 5-ylidenethiazolidin-4-ones and 5-benzylidene-4, 6-pyrimidinediones. Eur. J. Med. Chem. 2010, 45, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Tejchman, W.; Orwat, B.; Korona-Głowniak, I.; Barbasz, A.; Kownacki, I.; Latacz, G.; Handzlik, J.; Żesławska, E.; Malm, A. Highly efficient microwave synthesis of rhodanine and 2-thiohydantoin derivatives and determination of relationships between their chemical structures and antibacterial activity. RSC Adv. 2019, 9, 39367–39380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-J.; Zhou, Y.-J.; Wang, J.-H.; Mao, L.-F.; Li, W.; Xu, G.-Q. The synthesis and antitumor activity of 1, 8-naphthalimide derivatives linked 1, 2, 3-triazole. Front. Bioeng. Biotechnol. 2021, 9, 662432. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1, 8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef]
- Dong, H.Q.; Wei, T.B.; Ma, X.Q.; Yang, Q.Y.; Zhang, Y.F.; Sun, Y.J.; Shi, B.B.; Yao, H.; Zhang, Y.M.; Lin, Q. 1, 8-Naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, G.; Tripathi, A.K.; Seena, S.; Koh, J. Enhanced fluorescence norfloxacin substituted naphthalimide derivatives: Molecular docking and antibacterial activity. J. Mol. Struct. 2018, 1157, 292–299. [Google Scholar] [CrossRef]
- Türkmen, G. Pd catalyzed synthesis of 4-aryl 1, 8-naphthalimide dyes: Determining photophysical parameters and antimicrobial properties. J. Mol. Struct. 2022, 1266, 133448. [Google Scholar] [CrossRef]
- Madapa, S.; Tusi, Z.; Batra, S. Advances in the syntheses of quinoline and quinoline-annulated ring systems. Curr. Org. Chem. 2008, 12, 1116–1183. [Google Scholar] [CrossRef]
- Håheim, K.S.; Lindbäck, E.; Tan, K.N.; Albrigtsen, M.; Urdal Helgeland, I.T.; Lauga, C.; Matringe, T.; Kennedy, E.K.; Andersen, J.H.; Avery, V.M. Synthesis and evaluation of the tetracyclic ring-system of isocryptolepine and regioisomers for antimalarial, antiproliferative and antimicrobial activities. Molecules 2021, 26, 3268. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Meena, S.; Ramseshu, K.; Solomon, V.; Thirumurugan, K.; Dhanabal, K.; Murugan, M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5, 6, 7, 8-tetrahydrobenzo(b)thieno[2, 3-d]pyrimidin-4(3H)-ones. Eur. J. Med. Chem. 2006, 41, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Mane, V.; Baiju, T.V.; Namboothiri, I.N. Synthesis of functionalized thieno[2, 3-b]indoles via one-pot reaction of indoline-2-thiones with morita-baylis-hillman and rauhut-currier adducts of nitroalkenes. ACS Omega 2018, 3, 17617–17628. [Google Scholar] [CrossRef] [PubMed]
- Sain, S.; Jaiswal, S.; Jain, S.; Misra, N.; Srivastava, A.; Jendra, R.; Kishore, D.; Dwivedi, J.; Wabaidur, S.M.; Islam, M.A. Synthesis and theoretical studies of biologically active thieno nucleus incorporated tri and tetracyclic nitrogen containing heterocyclics scaffolds via Suzuki cross-coupling Reaction. Chem. Biodivers. 2022, 19, e202200540. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef]
- Meng, T.; Qin, Q.P.; Chen, Z.L.; Zou, H.H.; Wang, K.; Liang, F.P. Discovery of high in vitro and in vivo antitumor activities of organometallic ruthenium (II)-arene complexes with 5, 7-dihalogenated-2-methyl-8-quinolinol. Dalton Trans. 2019, 48, 5352–5360. [Google Scholar] [CrossRef]
- Nain-Perez, A.; Barbosa, L.C.; Araujo, M.H.; Martins, J.P.; Takahashi, J.A.; Oliveira, G.; Diniz, R.; Heller, L.; Hoenke, S.; Csuk, R. Antibacterial and cytotoxic activity of ruthenium-pcy-mene complexes with 2-methylquinolin-8-ol derivatives. ChemistrySelect 2021, 6, 2942–2950. [Google Scholar] [CrossRef]
- Jimenez, C. ACS Med. Chem. Lett 2018, 9, 959. [Google Scholar]
- Wang, X.; Qiu, H.; Yang, N.; Xie, H.; Liang, W.; Lin, J.; Zhu, H.; Zhou, Y.; Wang, N.; Tan, X. Fascaplysin derivatives binding to DNA via unique cationic five-ring coplanar backbone showed potent antimicrobial/antibiofilm activity against MRSA in vitro and in vivo. Eur. J. Med. Chem. 2022, 230, 114099. [Google Scholar] [CrossRef]
- El-Maiss, J.; Mohy El Dine, T.; Lu, C.S.; Karamé, I.; Kanj, A.; Polychronopoulou, K.; Shaya, J. Recent advances in metal-catalyzed alkyl-boron (C (sp3)–C (sp2)) Suzuki-Miyaura cross-couplings. Catalysts 2020, 10, 296. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, H.; Liang, W.; Lin, J.; Lin, J.; Liu, W.; Wang, X.; Cui, W.; Chen, X.; Wang, H. Derivatization of Marine-derived fascaplysin via highly regioselective Suzuki-Miyaura coupling contributing to the enhanced antibacterial activity. ChemistrySelect 2022, 7, e202201441. [Google Scholar] [CrossRef]
- El Azzaoui, B.; Rachid, B.; Doumbia, M.L.; Essassi, E.M.; Gornitzka, H.; Bellan, J.; Muscia, G.C.; Bollini, M.; Carnevale, J.P.; Bruno, A.M. Unexpected opening of benzimidazole derivatives during 1, 3-dipolar cycloaddition pp 8807–8810. Tetrahedron Lett. 2006, 47, 8807–8810. [Google Scholar] [CrossRef]
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ökten, S.; Aydın, A.; Koçyiğit, Ü.M.; Çakmak, O.; Erkan, S.; Andac, C.A.; Taslimi, P.; Gülçin, İ. Quinolin-based promising anticancer and antibacterial agents, and some metabolic enzyme inhibitors. Arch. Pharm. 2020, 353, 2000086. [Google Scholar] [CrossRef] [PubMed]
- Köprülü, T.K.; Ökten, S.; Atalay, V.E.; Tekin, Ş.; Çakmak, O. Biological activity and molecular docking studies of some new quinolines as potent anticancer agents. Med. Oncol. 2021, 38, 84. [Google Scholar] [CrossRef] [PubMed]
- Ökten, S.; Cakmak, O.; Erenler, R.; Şahin, Ö.Y.; Tekin, Ş. Simple and convenient preparation of novel 6, 8-disubstituted quinoline derivatives and their promising anticancer activities. Turk. J. Chem. 2013, 37, 896–908. [Google Scholar] [CrossRef]
- Ramesh, E.; Manian, R.D.R.; Raghunathan, R.; Sainath, S.; Raghunathan, M. Synthesis and antibacterial property of quinolines with potent DNA gyrase activity. Biorg. Med. Chem. 2009, 17, 660–666. [Google Scholar] [CrossRef]
- Koçyiğit, Ü.M.; Ökten, S.; Çakmak, O.; Burhan, G.; Ataş, M.; Taslimi, P.; Gülçin, İ. Arylated quinoline and tetrahydroquinolines: Synthesis, characterization and their metabolic enzyme inhibitory and antimicrobial activities. ChemistrySelect 2022, 7, e202203469. [Google Scholar] [CrossRef]
- Martin, M.T.; Roschangar, F.; Eaddy, J.F. Practical acid-catalyzed acylation of sulfonamides with carboxylic acid anhydrides. Tetrahedron Lett. 2003, 44, 5461–5463. [Google Scholar] [CrossRef]
- Berredjem, M.; Bouchareb, F.; Kaki, S.A.; Dekhil, M.; Aouf, N.-E. Synthesis and antibacterial activity of novel N-acylsulfonamides. Arab. J. Chem. 2017, 10, S1095–S1099. [Google Scholar] [CrossRef]
- Reitz, A.B.; Smith, G.R.; Parker, M.H. The role of sulfamide derivatives in medicinal chemistry: A patent review (2006–2008). Expert Opin. Ther. Pat. 2009, 19, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Noreen, M.; Rasool, N.; Gull, Y.; Zubair, M.; Mahmood, T.; Ayub, K.; Nasim, F.-u.-H.; Yaqoob, A.; Zia-Ul-Haq, M.; De Feo, V. Synthesis, density functional theory (DFT), urease inhibition and antimicrobial activities of 5-aryl thiophenes bearing sulphonylacetamide moieties. Molecules 2015, 20, 19914–19928. [Google Scholar] [CrossRef]
- Bold, G.; Altmann, K.H.; Frei, J.; Lang, M.; Manley, P.W.; Traxler, P.; Wietfeld, B.; Brüggen, J.; Buchdunger, E.; Cozens, R. New anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesis. J. Med. Chem. 2000, 43, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Strappaghetti, G.; Brodi, C.; Giannaccini, G.; Betti, L. New 4-(4-methyl-phenyl)phthalazin-1(2H)-one derivatives and their effects on α1-receptors. Bioorg. Med. Chem. Lett. 2006, 16, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Lebsack, A.D.; Gunzner, J.; Wang, B.; Pracitto, R.; Schaffhauser, H.; Santini, A.; Aiyar, J.; Bezverkov, R.; Munoz, B.; Liu, W. Identification and synthesis of [1, 2, 4]triazolo [3, 4-a]phthalazine derivatives as high-affinity ligands to the α2δ-1 subunit of voltage gated calcium channel. Bioorg. Med. Chem. Lett. 2004, 14, 2463–2467. [Google Scholar]
- Haack, T.; Fattori, R.; Napoletano, M.; Pellacini, F.; Fronza, G.; Raffaini, G.; Ganazzoli, F. Phthalazine PDE IV inhibitors: Conformational study of some 6-methoxy-1, 4-disubstituted derivatives. Biorg. Med. Chem. 2005, 13, 4425–4433. [Google Scholar] [CrossRef]
- Kumar, G.R.; Kurmarayuni, C.M.; Indupalli, M.; Pallapati, R.K.; Bollikolla, H.B. Synthesis of new analogs of 3-methyl-[1, 2,4]triazolo [3, 4-a]phthalazines via Suzuki coupling and evaluation of their anticancer and antimicrobial activity. Mediterr. J. Chem. 2019, 8, 261–269. [Google Scholar] [CrossRef][Green Version]
- Smith, R.D.; Coast, J. Antimicrobial resistance: A global response. Bull. World Health Organ. 2002, 80, 126–133. [Google Scholar]
- Ejaz, H.; Younas, S.; Qamar, M.U.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Alameen, A.A.M.; Elamir, M.Y.M.; Ahmad, N.; Hamam, S.S.M. Molecular epidemiology of extensively drug-resistant mcr encoded colistin-resistant bacterial strains co-expressing multifarious β-lactamases. Antibiotics 2021, 10, 467. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Santikul, M.; Smith, M.; Wong, M.Y.; Shryock, J.C.; Belardinelli, L. Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: Comparison with nicotinic acid. J. Pharmacol. Exp. Ther. 2007, 321, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med. Chem. Res. 2012, 21, 1557–1576. [Google Scholar] [CrossRef]
- Naheed, S.; Din, I.U.; Qamar, M.U.; Rasool, N.; Ahmad, M.; Bilal, M.; Khalid, A.; Ahmad, G.; Al-Hussain, S.A.; Zaki, M.E. Synthesis, anti-bacterial and molecular docking studies of arylated butyl 2-bromoisonicotinate against clinical isolates of ESBL-producing Escherichia coli ST405 and Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2023, 16, 5295–5308. [Google Scholar] [CrossRef] [PubMed]
- Clapp, L.B.; Katritzky, A.; Boulton, A. Advances in Heterocyclic Chemistry; Academic Press: New York, NY, USA, 1976; Volume 20, pp. 65–116. [Google Scholar]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef]
- Sayer, J.R.; Walldén, K.; Pesnot, T.; Campbell, F.; Gane, P.J.; Simone, M.; Koss, H.; Buelens, F.; Boyle, T.P.; Selwood, D.L. 2- and 3-substituted imidazo [1, 2-a]pyrazines as inhibitors of bacterial type IV secretion. Biorg. Med. Chem. 2014, 22, 6459–6470. [Google Scholar] [CrossRef]
- Chikhi, S.; Djebbar, S.; Soule, J.F.; Doucet, H. Environmentally-safe conditions for a palladium-catalyzed direct C3-arylation with high turn over frequency of Imidazo [1, 2-b] pyridazines using aryl bromides and chlorides. Chem. Asian J. 2016, 11, 2443–2452. [Google Scholar] [CrossRef]
- Marchand, P.; Bazin, M.A.; Pagniez, F.; Rivière, G.; Bodero, L.; Marhadour, S.; Nourrisson, M.R.; Picot, C.; Ruchaud, S.; Bach, S. Synthesis, antileishmanial activity and cytotoxicity of 2, 3-diaryl-and 2, 3, 8-trisubstituted imidazo[1, 2-a]pyrazines. Eur. J. Med. Chem. 2015, 103, 381–395. [Google Scholar] [CrossRef]
- Salek Soltani, S.; Farnia, S.M.F.; Foroumadi, A. A comparison between Suzuki cross-coupling reaction and direct arylation in the synthesis of new antibacterial imidazo-pyrazines/pyridazines. J. Heterocycl. Chem. 2020, 57, 1770–1780. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef]
- Tello, A.; Austin, B.; Telfer, T.C. Selective pressure of antibiotic pollution on bacteria of importance to public health. Environ. Health Perspect. 2012, 120, 1100–1106. [Google Scholar] [CrossRef]
- Wang, W.; Shangguan, S.; Qiu, N.; Hu, C.; Zhang, L.; Hu, Y. Design, synthesis and biological evaluation of novel 3, 4, 5-trisubstituted aminothiophenes as inhibitors of p53–MDM2 interaction. Part 1. Biorg. Med. Chem. 2013, 21, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Alsayari, A.; Muhsinah, A.B.; Asiri, Y.I.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Alatibi, F.; El-Sayed, N.N.E.; Mabkhot, Y.N. Synthesis of antimicrobial agents of tetra-substituted thiophenes from ethyl 5-(2-bromoacetyl)-4-phenyl-2-(phenylamino) thiophene-3-carboxylate. J. Heterocycl. Chem. 2020, 57, 2911–2922. [Google Scholar] [CrossRef]
- Boibessot, T.; Zschiedrich, C.P.; Lebeau, A.; Bénimèlis, D.; Dunyach-Remy, C.; Lavigne, J.P.; Szurmant, H.; Benfodda, Z.; Meffre, P. The rational design, synthesis, and antimicrobial properties of thiophene derivatives that inhibit bacterial histidine kinases. J. Med. Chem. 2016, 59, 8830–8847. [Google Scholar] [CrossRef] [PubMed]
- Nasr, T.; Bondock, S.; Eid, S. Design, synthesis, antimicrobial evaluation and molecular docking studies of some new thiophene, pyrazole and pyridone derivatives bearing sulfisoxazole moiety. Eur. J. Med. Chem. 2014, 84, 491–504. [Google Scholar] [CrossRef]
- De, B.; Sen, S.; Easwari, T. Chemistry and therapeutic aspect of furan: A short review. Asian J. Res. Chem. 2015, 8, 428–438. [Google Scholar] [CrossRef]
- Rosales-Hurtado, M.; Duvauchelle, V.; Bénimélis, D.; Ogawa-Okada, M.; Yamamoto, N.; Meffre, P.; Szurmant, H.; Benfodda, Z. Microwave-assisted synthesis of biodegradable and antibacterial thiophene, furan and thiazole derivatives. Environ. Chem. Lett. 2023, 21, 47–53. [Google Scholar] [CrossRef]
- Priya Rani, M.; Padmakumari, K.; Sankarikutty, B.; Lijo Cherian, O.; Nisha, V.; Raghu, K. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ajish, K.; Dhanya, B.; Joseph, N.; Rani, M.P.; Raghu, K.; Vineetha, V.; Radhakrishnan, K. Synthesis of novel zerumbone derivatives via regioselective palladium catalyzed decarboxylative coupling reaction: A new class of α-glucosidase inhibitors. Tetrahedron Lett. 2014, 55, 665–670. [Google Scholar] [CrossRef]
- Konda, Y.; Ankireddy, A.R.; Velavalapalli, V.M.; Paidikondala, K.; Pasula, A.; Gundla, R. Synthesis, alpha-glucosidase inhibition and antibacterial activities of the new chiral (R)-3, 3-disubstituted BINOL-phosphates. Russ. J. Gen. Chem. 2022, 92, 898–907. [Google Scholar] [CrossRef]
- Bax, R.; Mullan, N.; Verhoef, J. The millennium bugs-the need for and development of new antibacterials. Int. J. Antimicrob. Agents 2000, 16, 51–59. [Google Scholar] [CrossRef]
- Kawatkar, S.P.; Keating, T.A.; Olivier, N.B.; Breen, J.N.; Green, O.M.; Guler, S.Y.; Hentemann, M.F.; Loch, J.T.; McKenzie, A.R.; Newman, J.V. Antibacterial inhibitors of gram-positive thymidylate kinase: Structure-activity relationships and chiral preference of a new hydrophobic binding region. J. Med. Chem. 2014, 57, 4584–4597. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Plummer, M.S.; Starr, J.; Desbonnet, C.R.; Soutter, H.; Chang, J.; Miller, J.R.; Dillman, K.; Miller, A.A.; Roush, W.R. Structure guided development of novel thymidine mimetics targeting Pseudomonas aeruginosa thymidylate kinase: From hit to lead generation. J. Med. Chem. 2012, 55, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Blindheim, F.H.; Olsen, C.E.; Krogh Søgaard, C.; Otterlei, M.; Sundby, E.; Hoff, B.H. Synthetic strategies towards imidazopyridinones and 7-azaoxindoles and their evaluation as antibacterial agents. Eur. J. Org. Chem. 2021, 2021, 2701–2712. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Khan, F.A.K.; Chouthe, R.S.; Damale, M.G.; Shinde, D.B. Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H-1, 2, 4-triazol-3-yl) methyl)-4, 5, 6, 7-tetrahydrothieno [3, 2-c] pyridine as antifungal agents. Chin. Chem. Lett. 2014, 25, 1033–1038. [Google Scholar] [CrossRef]
- Groenhagen, U.; Maczka, M.; Dickschat, J.S.; Schulz, S. Streptopyridines, volatile pyridine alkaloids produced by streptomyces sp. Form5. Beilstein J. Org. Chem. 2014, 10, 1421–1432. [Google Scholar] [CrossRef]
- Movassaghi, M.; Hill, M.D.; Ahmad, O.K. Direct synthesis of pyridine derivatives. J. Am. Chem. Soc. 2007, 129, 10096–10097. [Google Scholar] [CrossRef]
- Akram, M.; Adeel, M.; Khalid, M.; Tahir, M.N.; Khan, M.U.; Asghar, M.A.; Ullah, M.A.; Iqbal, M. A combined experimental and computational study of 3-bromo-5-(2, 5-difluorophenyl)pyridine and 3, 5-bis(naphthalen-1-yl)pyridine: Insight into the synthesis, spectroscopic, single crystal XRD, electronic, nonlinear optical and biological properties. J. Mol. Struct. 2018, 1160, 129–141. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Synergistic interactions of methanolic extract of acacia mearnsii de wild with antibiotics against bacteria of clinical relevance. Int. J. Mol. Sci. 2012, 13, 8915–8932. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, X.; Ding, L.; Zhang, Y.; Cui, L.; Sun, L.; Li, W.; Wang, D.; Zhao, Y. Synthesis and antibacterial activity evaluation of novel biaryloxazolidinone analogues containing a hydrazone moiety as promising antibacterial agents. Eur. J. Med. Chem. 2018, 158, 247–258. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, J.; Qi, Y.; Ding, X.; Wu, Y.; Lei, H.; Zhao, Y. Design and synthesis of biaryloxazolidinone derivatives containing amide or acrylamide moiety as novel antibacterial agents against Gram-positive bacteria. Bioorg. Med. Chem. Lett. 2019, 29, 126747. [Google Scholar] [CrossRef]
- Hierso, J.C.; Fihri, A.; Amardeil, R.; Meunier, P.; Doucet, H.; Santelli, M.; Ivanov, V.V. Catalytic efficiency of a new tridentate ferrocenyl phosphine auxiliary: Sonogashira cross-coupling reactions of alkynes with aryl bromides and chlorides at low catalyst loadings of 10-1 to 10-4 mol%. Org. Lett. 2004, 6, 3473–3476. [Google Scholar] [CrossRef] [PubMed]
- Adjabeng, G.; Brenstrum, T.; Frampton, C.S.; Robertson, A.J.; Hillhouse, J.; McNulty, J.; Capretta, A. Palladium complexes of 1, 3, 5, 7-tetramethyl-2, 4, 8-trioxa-6-phenyl-6-phosphaadamantane: Synthesis, crystal structure and use in the Suzuki and Sonogashira reactions and the α-arylation of ketones. J. Org. Chem. 2004, 69, 5082–5086. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Li, L.; Wang, R. The copper-free Sonogashira cross-coupling reaction promoted by palladium complexes of nitrogen-containing chelating ligands in neat water at room temperature. Dalton Trans. 2014, 43, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Zificsak, C.A.; Hlasta, D.J. Current methods for the synthesis of 2-substituted azoles. Tetrahedron Lett. 2004, 60, 8991–9016. [Google Scholar] [CrossRef]
- Keivanloo, A.; Abbaspour, S.; Sepehri, S.; Bakherad, M. Synthesis, antibacterial activity and molecular docking study of a series of 1, 3-oxazole-quinoxaline amine hybrids. Polycycl. Aromat. Compd. 2022, 42, 2378–2391. [Google Scholar] [CrossRef]
- Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Chemistry, biological properties and SAR analysis of quinoxalinones. Mini Rev. Med. Chem. 2006, 6, 1179–1200. [Google Scholar] [CrossRef]
- Sonogashira, K. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Schwarzbaum, P.J.; Kaufman, S.B.; Rossi, R.C.; Garrahan, P.J. An unexpected effect of ATP on the ratio between activity and phosphoenzyme level of Na+/K+-ATPase in steady state. Biochim. Biophys. Acta Biomembr. 1995, 1233, 33–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, L. Multi-component reactions and evolutionary chemistry. Drug Discov. Today 2002, 7, 143–147. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. “Multi-component reactions: Emerging chemistry in drug discovery” ‘from xylocain to crixivan’. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef]
- Tempest, P.A. Recent advances in heterocycle generation using the efficient Ugi multiple-component condensation reaction. Curr. Opin. Drug Discov. Devel. 2005, 8, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, S.; Keivanloo, A.; Bakherad, M.; Sepehri, S. Design, synthesis, antibacterial evaluation and molecular docking study of new 3-aminoquinoxaline-2-alkynyl carboxylate esters. ChemistrySelect 2020, 5, 8701–8706. [Google Scholar] [CrossRef]
- Uenishi, J.i.; Matsui, K.; Ohmiya, H. Studies on stereoselective Sonogashira coupling of 1, 1-dibromo-1-alkene. J. Organomet. Chem. 2002, 653, 141–149. [Google Scholar] [CrossRef]
- Huang, A.; Ma, C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini Rev. Med. Chem. 2013, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, M.; Keivanloo, A.; Nabid, M.R. Rapid synthesis of 2-alkanol-substituted pyrrolo[2, 3-b] quinoxalines from propargylic alcohols via copper-free Sonogashira coupling reaction at room temperature. J. Heterocycl. Chem. 2018, 55, 1331–1338. [Google Scholar] [CrossRef]
- Keivanloo, A.; Bakherad, M.; Rahimi, A.; Taheri, S.A.N. One-pot synthesis of 1, 2-disubstituted pyrrolo[2, 3-b] quinoxalines via palladium-catalyzed heteroannulation in water. Tetrahedron Lett. 2010, 51, 2409–2412. [Google Scholar] [CrossRef]
- Keivanloo, A.; Bakherad, M.; Rahimi, A. Synthesis of unexpected pyrrolo[2, 3-b]quinoxaline-2-carbaldehydes via Sonogashira coupling reaction. Synthesis 2010, 10, 1599–1602. [Google Scholar] [CrossRef]
- Shelly, W.; Draper, M.W.; Krishnan, V.; Wong, M.; Jaffe, R.B. Selective estrogen receptor modulators: An update on recent clinical findings. Obstet. Gynecol. Surv. 2008, 63, 163–181. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b] thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Konus, M.; Algso, M.A.; Kavak, E.; Kurt-Kızıldoğan, A.; Yılmaz, C.; Kivrak, A. Design, synthesis, and in vitro evaluation of thieno[a]dibenzothiophene derivatives. ChemistrySelect 2020, 5, 3700–3709. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Kukla, D.L.; Canchola, J.; Rosenthal, J.D.; Mills, J.J. Design, synthesis, and structure-activity relationship studies of the anaephene antibiotics. Chem. Biol. Drug Des. 2021, 98, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Drachuk, I.; Shchepelina, O.; Harbaugh, S.; Kelley-Loughnane, N.; Stone, M.; Tsukruk, V.V. Cell surface engineering with edible protein nanoshells. Small 2013, 9, 3128–3137. [Google Scholar] [CrossRef]
- Simon, C.; Mathilde, D.; Thibaut, J.; Claude, N.; Mike, R.; Jean-Pierre, L.P.; Françoise, S.S. Synthesis, solubility and optical properties of π-conjugated oligoelectrolytes derived from bis-thiophene-dialkoxyphenylene derivatives. New J. Chem. 2023, 47, 1479–1487. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef]
- Meunier, C.F.; Rooke, J.C.; Léonard, A.; Van Cutsem, P.; Su, B.L. Design of photochemical materials for carbohydrate production via the immobilisation of whole plant cells into a porous silica matrix. J. Mater. Chem. 2010, 20, 929–936. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Tang, R. Improvement of biological organisms using functional material shells. Adv. Funct. Mater. 2016, 26, 1862–1880. [Google Scholar] [CrossRef]
- Bian, R.; Jiang, Y.; Wang, Y.; Sun, J.K.; Hu, J.; Jiang, L.; Liu, H. Highly boosted microbial extracellular electron transfer by semiconductor nanowire array with suitable energy level. Adv. Funct. Mater. 2018, 28, 1707408. [Google Scholar] [CrossRef]
- Wilson, J.T.; Krishnamurthy, V.R.; Cui, W.; Qu, Z.; Chaikof, E.L. Noncovalent cell surface engineering with cationic graft copolymers. J. Am. Chem. Soc. 2009, 131, 18228–18229. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef] [PubMed]
- Riccò, R.; Liang, W.; Li, S.; Gassensmith, J.J.; Caruso, F.; Doonan, C.; Falcaro, P. Metal-organic frameworks for cell and virus biology: A perspective. ACS Nano 2018, 12, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhao, H.; Zhou, X.; Liu, J.; Dai, N.; Zeng, Y.; Zhang, E.; Lv, F.; Huang, Y.; Liu, L. In situ synthesis of photoactive polymers on a living cell surface via bio-palladium catalysis for modulating biological functions. Angew. Chem. 2021, 133, 5823–5829. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Hundertmark, T.; Littke, A.F.; Buchwald, S.L.; Fu, G.C. Pd(PhCN)2Cl2/P(t-Bu)3: A versatile catalyst for Sonogashira reactions of aryl bromides at room temperature. Org. Lett. 2000, 2, 1729–1731. [Google Scholar] [CrossRef]
- De Kort, M.; Correa, V.; Valentijn, A.R.P.; Van Der Marel, G.A.; Potter, B.V.; Taylor, C.W.; Van Boom, J.H. Synthesis of Potent Agonists of the d-m yo-inositol 1, 4, 5-trisphosphate receptor based on clustered disaccharide polyphosphate analogues of adenophostin A. J. Med. Chem. 2000, 43, 3295–3303. [Google Scholar] [CrossRef]
- Keivanloo, A.; Lashkari, S.; Sepehri, S.; Bakherad, M.; Abbaspour, S. Synthesis of hydantoin alkynes through palladium-catalyzed reaction, antibacterial evaluation, and molecular docking studies. J. Chin. Chem. Soc. 2021, 68, 1317–1327. [Google Scholar] [CrossRef]
- Zora, M.; Kivrak, A.; Yazici, C. Synthesis of pyrazoles via electrophilic cyclization. J. Org. Chem. 2011, 76, 6726–6742. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J. Immunol. 2016, 196, 1108–1122. [Google Scholar] [CrossRef]
- Cihan-Üstündağ, G.; Gürsoy, E.; Naesens, L.; Ulusoy-Güzeldemirci, N.; Çapan, G.J.B.; Chemistry, M. Synthesis and antiviral properties of novel indole-based thiosemicarbazides and 4-thiazolidinones. Bioorg. Med. Chem. 2016, 24, 240–246. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, S.; Hu, J.; Huang, L.; Li, X. Synthesis, evaluation, and mechanism study of novel indole-chalcone derivatives exerting effective antitumor activity through microtubule destabilization in vitro and in vivo. J. Med. Chem. 2016, 59, 5264–5283. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, D.; Sundararajan, G.; Loganath, N.; Krishnasamy, K. Synthesis, molecular structure, DFT studies and antimicrobial activities of some novel 3-(1-(3, 4-dimethoxyphenethyl)-4, 5-diphenyl-1H-imidazol-2-yl)-1H-indole derivatives and its molecular docking studies. J. Mol. Struct. 2017, 1127, 597–610. [Google Scholar] [CrossRef]
- Bao, X.L.; Zhu, W.B.; Shan, T.L.; Wu, Z.; Zhang, R.J.; Liao, P.Y.; Zheng, M.Z.; Tang, H.S.; Yan, Y.J.; Chen, Z.L. Design, synthesis and evaluation of novel angiotensin II receptor 1 antagonists with antihypertensive activities. RSC Adv. 2017, 7, 26401–26410. [Google Scholar] [CrossRef]
- ElBordiny, H.S.; El-Miligy, M.M.; Kassab, S.E.; Daabees, H.; Ali, W.A.M.; El-Hawash, S.A.M. Design, synthesis, biological evaluation and docking studies of new 3-(4, 5-dihydro-1H-pyrazol/isoxazol-5-yl)-2-phenyl-1H-indole derivatives as potent antioxidants and 15-lipoxygenase inhibitors. Eur. J. Med. Chem. 2018, 145, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Konus, M.; Çetin, D.; Yılmaz, C.; Arslan, S.; Mutlu, D.; Kurt-Kızıldoğan, A.; Otur, Ç.; Ozok, O.; As Algso, M.; Kivrak, A.J.C. Synthesis, biological evaluation and molecular docking of novel thiophene-based indole derivatives as potential antibacterial, GST inhibitor and apoptotic anticancer agents. ChemistrySelect 2020, 5, 5809–5814. [Google Scholar] [CrossRef]
- Carbas, B.B.; Kivrak, A.; Kavak, E. Electrosynthesis of a new indole based donor-acceptor-donor type polymer and investigation of its electrochromic properties. Mater. Chem. Phys. 2017, 188, 68–74. [Google Scholar] [CrossRef]
- Gao, Y.; Shan, D.; Jia, Y. Intramolecular larock indole synthesis for the preparation of tricyclic indoles and its application in the synthesis of tetrahydropyrroloquinoline and fargesine. Tetrahedron 2014, 70, 5136–5141. [Google Scholar] [CrossRef]
- Lal, S.; Snape, T.J. 2-Arylindoles: A privileged molecular scaffold with potent, broad-ranging pharmacological activity. Curr. Med. Chem. 2012, 19, 4828–4837. [Google Scholar] [CrossRef]
- Konus, M.; Çetin, D.; Kızılkan, N.D.; Yılmaz, C.; Fidan, C.; Algso, M.; Kavak, E.; Kivrak, A.; Kurt-Kızıldoğan, A.; Otur, Ç. Synthesis and biological activity of new indole based derivatives as potent anticancer, antioxidant and antimicrobial agents. J. Mol. Struct. 2022, 1263, 133168. [Google Scholar] [CrossRef]
- Al-Haiza, M.A.; Mostafa, M.; El-Kady, M.J.M. Synthesis and biological evaluation of some new coumarin derivatives. Molecules 2003, 8, 275–286. [Google Scholar] [CrossRef]
- Symeonidis, T.; Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Synthesis and anti-inflammatory evaluation of novel angularly or linearly fused coumarins. Eur. J. Med. Chem. 2009, 44, 5012–5017. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Hussein, A.; Meyer, J.J.M. Antiviral and antituberculous activity of helichrysum melanacme constituents. Fitoterapia 2006, 77, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.W.; Hoeksema, H.; Caron, E.L.; Jackson, W.G. The partial structure of novobiocin (streptonivicin). 1 II. J. Am. Chem. Soc. 1956, 78, 1072–1074. [Google Scholar] [CrossRef]
- Joy, M.N.; Bodke, Y.D.; Telkar, S. 4-Methyl-7-amino/amido coumarin derivatives as potential antimicrobials and antioxidants. Chem. Nat. Compd. 2020, 56, 614–620. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Garrison, A.T.; Abouelhassan, Y.; Kallifidas, D.; Tan, H.; Kim, Y.S.; Jin, S.; Luesch, H.; Huigens, R.W., III. An efficient Buchwald-Hartwig/reductive cyclization for the scaffold diversification of halogenated phenazines: Potent antibacterial targeting, biofilm eradication, and prodrug exploration. J. Med. Chem. 2018, 61, 3962–3983. [Google Scholar] [CrossRef]
- Chrysant, S.G. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert Rev. Cardiovasc. Ther. 2017, 15, 151–156. [Google Scholar] [CrossRef]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit. Rev. Food Sci. Nutr. 2019, 59, 2597–2625. [Google Scholar] [CrossRef]
- Rao, F.V.; Andersen, O.A.; Vora, K.A.; DeMartino, J.A.; Van Aalten, D.M. Methylxanthine drugs are chitinase inhibitors: Investigation of inhibition and binding modes. Chem. Biol. 2005, 12, 973–980. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; Andersen, O.A.; Rao, F.V.; Allwood, M.; Lloyd, C.; Eggleston, I.M.; Van Aalten, D.M. Screening-based discovery and structural dissection of a novel family 18 chitinase inhibitor. J. Biol. Chem. 2006, 281, 27278–27285. [Google Scholar] [CrossRef]
- Kadi, A.A.; El-Tahir, K.E.; Jahng, Y.; Rahman, A.M. Synthesis, biological evaluation and structure activity relationships (SARs) study of 8-(substituted) aryloxycaffeine. Arab. J. Chem. 2019, 12, 2356–2364. [Google Scholar] [CrossRef]
- Reshetnikov, D.; Burova, L.; Rybalova, T.; Bondareva, E.; Patrushev, S.; Evstropov, A.; Shults, E. Synthesis and antibacterial activity of caffeine derivatives containing amino-acid fragments. Chem. Nat. Compd. 2022, 58, 908–915. [Google Scholar] [CrossRef]
- Harmenberg, J.; Åkesson-Johansson, A.; Gräslund, A.; Malmfors, T.; Bergman, J.; Wahren, B.; Åkerfeldt, S.; Lundblad, L.; Cox, S. The mechanism of action of the anti-herpes virus compound 2, 3-dimethyl-6 (2-dimethylaminoethyl)-6H-indolo-(2, 3-b)quinoxaline. Antivir. Res. 1991, 15, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.; Stephens, M.; Nolan, J.; Sutton, B.; Tocher, J.; Fielden, E.; Adams, G.; Stratford, I. Heterocyclic mono-N-oxides with potential applications as bioreductive anti-tumour drugs: Part 1. 8-Alkylamino-substituted phenylimidazo [1, 2-a]quinoxalines. Anti-Cancer Drug Des. 1993, 8, 439–461. [Google Scholar]
- Selvakumar, K.; Zapf, A.; Beller, M. New palladium carbene catalysts for the Heck reaction of aryl chlorides in ionic liquids. Org. Lett. 2002, 4, 3031–3033. [Google Scholar] [CrossRef]
- Besharati-Seidani, T.; Keivanloo, A.; Kaboudin, B.; Yoshida, A.; Yokomatsu, T. Regioselective synthesis of 2, 3-disubstituted 1-alkyl pyrrolo[2, 3-b]quinoxalines through palladium-catalyzed Heck reaction of chalcones and evaluation of their anti-bacterial activities. Tetrahedron 2018, 74, 2350–2358. [Google Scholar] [CrossRef]
- Tellier, F.; Acher, F.; Brabet, I.; Pin, J.P.; Azerad, R. Aminobicyclo [2.2.1.] heptane dicarboxylic acids (ABHD), rigid analogs of ACPD and glutamic acid: Synthesis and pharmacological activity on metabotropic receptors mGluR1 and mGluR2. Biorg. Med. Chem. 1998, 6, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Elz, S.; Zimmermann, H.; Rehse, K. Selectivity of sterically fixed tryptamine and 5-methoxytryptamine derivatives for serotonin receptor subtypes, ii: Structure-activity relationships and in vitro pharmacology of N-alkyl- and N, N-dialkyl-3-indolylbicyclo-[2.2.1]-heptane-2-amines. Arch. Pharm. 1993, 326, 893–899. [Google Scholar] [CrossRef]
- Bagdatli, E.; Cil, E. Sulfa drugs-based norbornenyl imides and reductive Heck reactions: Synthesis and antimicrobial screening. J. Heterocycl. Chem. 2022, 59, 264–274. [Google Scholar] [CrossRef]
- Bangalore, P.K.; Vagolu, S.K.; Bollikanda, R.K.; Veeragoni, D.K.; Choudante, P.C.; Misra, S.; Sriram, D.; Sridhar, B.; Kantevari, S. Usnic acid enaminone-coupled 1, 2, 3-triazoles as antibacterial and antitubercular agents. J. Nat. Prod. 2019, 83, 26–35. [Google Scholar] [CrossRef]
- Shi, J.; Luo, N.; Ding, M.; Bao, X. Synthesis, in vitro antibacterial and antifungal evaluation of novel 1, 3, 4-oxadiazole thioether derivatives bearing the 6-fluoroquinazolinylpiperidinyl moiety. Chin. Chem. Lett. 2020, 31, 434–438. [Google Scholar] [CrossRef]
- Teng, Y.; Qin, Y.; Song, D.; Liu, X.; Ma, Y.; Zhang, P.; Ma, S. A novel series of 11-o-carbamoyl-3-o-descladinosyl clarithromycin derivatives bearing 1, 2, 3-triazole group: Design, synthesis and antibacterial evaluation. Bioorg. Med. Chem. Lett. 2020, 30, 126850. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Lv, M.; Li, T.; Xu, H. Construction and agrochemical efficacy of novel indolo [2, 3-b] andrographolide derivatives. Adv. Agrochem. 2023, 2, 349–355. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Z.S.; Shao, J.; Fu, C.; Xiong, L.T.; Li, Z.D.; Cui, Z.N. Photoinduced palladium-catalyzed 1, 3-diene-selective fluoroalkylamination compounds as potential bactericidal agent against Xanthomonas oryzae pv. oryzae. Chin. Chem. Lett. 2024, 35, 108794. [Google Scholar] [CrossRef]
- Negishi, E.i.; Hu, Q.; Huang, Z.; Qian, M.; Wang, G.; Brown, H. Palladium-catalyzed alkenylation by the Negishi coupling. Aldrichim. Acta 2005, 38, 71–78. [Google Scholar] [CrossRef]
- Rouhi, A.M. Fine chemicals. Chem. Eng. News 2004, 82, 49–58. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Salih, K.S. Modern Development in Copper- and Nickel-catalyzed cross-coupling reactions: Formation of carbon-carbon and carbon-heteroatom bonds under microwave irradiation conditions. Asian J. Org. Chem. 2022, 11, e202200023. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Nagawade, R.R.; Shinde, D.B. Synthesis of novel 3-(1-(1-substituted piperidin-4-yl)-1H-1, 2, 3-triazol-4-yl)-1, 2, 4-oxadiazol-5 (4H)-one as antifungal agents. Bioorg. Med. Chem. Lett. 2009, 19, 3564–3567. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1, 3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Harkala, K.J.; Eppakayala, L.; Maringanti, T.C. Synthesis and biological evaluation of benzimidazole-linked 1, 2, 3-triazole congeners as agents. Org. Med. Chem. Lett. 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.M.A.; Yedla, P.; Babu, K.S.; Shaik, T.B.; Chityal, G.K.; Kamal, A. Synthesis and biological evaluation of 1, 2, 3-triazole tethered pyrazoline and chalcone derivatives. Chem. Biol. Drug Des. 2016, 88, 97–109. [Google Scholar] [CrossRef]
- Kumar, K.K.; Seenivasan, S.P.; Kumar, V.; Das, T.M. Synthesis of quinoline coupled [1, 2, 3]-triazoles as a promising class of anti-tuberculosis agents. Carbohydr. Res. 2011, 346, 2084–2090. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal (salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Katsuki, T. Catalytic asymmetric oxidations using optically active (salen) manganese (III) complexes as catalysts. Coord. Chem. Rev. 1995, 140, 189–214. [Google Scholar] [CrossRef]
- Keivanloo, A.; Fakharian, M.; Sepehri, S. 1, 2, 3-triazoles based 3-substituted 2-thioquinoxalines: Synthesis, anti-bacterial activities, and molecular docking studies. J. Mol. Struct. 2020, 1202, 127262. [Google Scholar] [CrossRef]
- Zhang, H.Z.; He, S.C.; Peng, Y.J.; Zhang, H.J.; Gopala, L.; Tangadanchu, V.K.R.; Gan, L.L.; Zhou, C.H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017, 136, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, M.Z.; Kumaravel, G.; Xie, R.L.; Rossi, R.F.; Meyer, S.; Sidhu, A.; Cuny, G.D.; Hauske, J.R. Potent in vitro methicillin-resistant Staphylococcus aureus activity of 2-(1H-indol-3-yl) quinoline derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 2675–2678. [Google Scholar] [CrossRef]
- Kung, P.P.; Casper, M.D.; Cook, K.L.; Wilson-Lingardo, L.; Risen, L.M.; Vickers, T.A.; Ranken, R.; Blyn, L.B.; Wyatt, J.R.; Cook, P.D. Structure-activity relationships of novel 2-substituted quinazoline antibacterial agents. J. Med. Chem. 1999, 42, 4705–4713. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Singh, R.P.; Patel, O.P.; Dhiman, S.; Saini, H.K.; Jha, P.N.; Kumar, A. Design and synthesis of imidazo/benzimidazo [1, 2-c]quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega 2018, 3, 16338–16346. [Google Scholar] [CrossRef] [PubMed]
- Özyanik, M.; Demirci, S.; Bektaş, H.; Demirbaş, N.; Demirbaş, A.; Karaoğlu, Ş.A. Preparation and antimicrobial activity evaluation of some quinoline derivatives containing an azole nucleus. Turk. J. Chem. 2012, 36, 233–246. [Google Scholar] [CrossRef]
- Arshad, M.; Rasool, N.; Qamar, M.U.; Shah, S.A.A.; Zakaria, Z.A. Facile Synthesis of functionalized phenoxy quinolines: Antibacterial activities against ESBL producing Escherichia coli and MRSA, docking studies, and structural features determination through computational approach. Molecules 2022, 27, 3732. [Google Scholar] [CrossRef] [PubMed]
- Saint Geme, J., Jr.; Martin, H.; Davis, C. Paramyxovirus-avian cell relationship: Discrepant impact of 6-azauridine on virus production by susceptible and less susceptible cells. Infect. Immun. 1975, 11, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Steffenhagen, K., Easterday, B., Galasso, G., Eds.; Evaluation of 6-azauridine and S-iododeoxyuridine in the treatment of experimental viral infections. J. Infect. Dis. 1976, 133, 603–612. [Google Scholar] [CrossRef]
- Li, J.J.; Mitchell, L.H.; Dow, R.L. Thyroid receptor agonists for the treatment of androgenetic alopecia. Bioorg. Med. Chem. Lett. 2010, 20, 306–308. [Google Scholar] [CrossRef]
- Vanparijs, O.; Hermans, L.; Van der Flaes, L.; Vlaminck, K.; Marsboom, R. Clazuril: A new anticoccidial agent for pigeons. Tijdschr. Diergeneeskd. 1988, 113, 190–194. [Google Scholar]
- Slouka, J. 1-Aryl-6-azauracile, 3. Mitt.: Die synthese von 1-phenyl-6-azauracil und einiger seiner derivate. Monatsh. Chem. Verw. Teile. Anderer. Wiss. 1965, 96, 134–137. [Google Scholar] [CrossRef]
- Gulipalli, K.C.; Bodige, S.; Ravula, P.; Bolla, R.S.; Endoori, S.; Cherukumalli, P.; Seelam, N. A mild and efficient copper-mediated N-arylation of 6-azauracil with corresponding boronic acids and their antibacterial activity. Asian J. Chem 2018, 30, 2495–2501. [Google Scholar] [CrossRef]
- Beaumier, E.P.; Pearce, A.J.; See, X.Y.; Tonks, I.A. Modern applications of low-valent early transition metals in synthesis and catalysis. Nat. Rev. Chem. 2019, 3, 15–34. [Google Scholar] [CrossRef]
- Quach, T.D.; Batey, R.A. Ligand-and base-free copper(II)-catalyzed C-N bond formation: Cross-coupling reactions of organoboron compounds with aliphatic amines and anilines. Org. Lett. 2003, 5, 4397–4400. [Google Scholar] [CrossRef] [PubMed]
- Molaei, H.; Ghanbari, M.M. Practical copper-catalyzed N-arylation of amines with 20% aqueous solution of n-Bu4NOH. Chin. Chem. Lett. 2012, 23, 301–304. [Google Scholar] [CrossRef]
- Sharghi, H.; Sepehri, S.; Aberi, M. Cu(II) complex of pyridine-based polydentate as a novel, efficient, and highly reusable catalyst in C-N bond forming reaction. Mol. Divers. 2017, 21, 855–864. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Rezazadeh, Z.; Kazemnejadi, M.; Allahresani, A. A Co-Cu bimetallic magnetic nanocatalyst with synergistic and bifunctional performance for the base-free Suzuki, Sonogashira, and C-N cross-coupling reactions in water. Dalton Trans. 2020, 49, 10645–10660. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Sheridan, P.E.; Hauer, A.K.; Garrett, A.E.; McConnell, D.E.; Thornton, J.A.; Stokes, S.L.; Emerson, J.P. Cu-catalyzed Chan-Evans-Lam coupling reactions of 2-nitroimidazole with aryl boronic acids: An Effort toward new bioactive agents against S. pneumoniae. Chem. Biodivers. 2022, 19, e202200327. [Google Scholar] [CrossRef] [PubMed]
- Eid, F.A.; Abd El-Wahab, A.H.; El-Hag Ali, G.A.; Khafagy, M.M. Synthesis and antimicrobial evaluation of naphtho [2, 1-b] pyrano [2, 3-d]pyrimidine and pyrano[3, 2-e][1, 2, 4] triazolo [1, 5-c]pyrimidine derivatives. Acta pharmaceutica 2004, 54, 13–26. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Kamdar, N.R.; Haveliwala, D.D.; Mistry, P.T.; Patel, S.K. Synthesis and evaluation of in vitro antitubercular activity and antimicrobial activity of some novel 4 H-chromeno [2, 3-d]pyrimidine via 2-amino-4-phenyl-4H-chromene-3-carbonitriles. Med. Chem. Res. 2011, 20, 854–864. [Google Scholar] [CrossRef]
- Mohamed, N.R.; El-Saidi, M.M.T.; Ali, Y.M.; Elnagdi, M.H. Utility of 6-amino-2-thiouracil as a precursor for the synthesis of bioactive pyrimidine derivatives. Biorg. Med. Chem. 2007, 15, 6227–6235. [Google Scholar] [CrossRef]
- Poola, S.; Shaik, M.S.; Sudileti, M.; Yakkate, S.; Nalluri, V.; Chippada, A.; Cirandur, S.R. Nano CuO-Ag-catalyzed synthesis of some novel pyrano [2, 3-d]pyrimidine derivatives and evaluation of their bioactivity. J. Chin. Chem. Soc. 2020, 67, 805–820. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A. Synthesis and antimicrobial activity of 4-substituted 1, 2, 3-triazole-coumarin derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [PubMed]
- Sacchettini, J.C.; Rubin, E.J.; Freundlich, J.S. Drugs versus bugs: In pursuit of the persistent predator Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2008, 6, 41–52. [Google Scholar] [CrossRef]
- Libardo, M.D.J.; de la Fuente-Nuñez, C.; Anand, K.; Krishnamoorthy, G.; Kaiser, P.; Pringle, S.C.; Dietz, C.; Pierce, S.; Smith, M.B.; Barczak, A. Phagosomal copper-promoted oxidative attack on intracellular Mycobacterium tuberculosis. ACS Infect. Dis. 2018, 4, 1623–1634. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Badshah, S.L.; Naeem, A. Bioactive thiazine and benzothiazine derivatives: Green synthesis methods and their medicinal importance. Molecules 2016, 21, 1054. [Google Scholar] [CrossRef]
- Afrough, T.; Bakavoli, M.; Eshghi, H.; Tajabadi, J.; Mague, J. Regioselective synthesis of new 5 H, 10 H-dipyrimido [2, 1-b: 4’, 5’-d][1, 3] thiazine: A combined experimental and computational study. J. Sulfur Chem. 2019, 40, 265–276. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1, 2, 3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Sucharitha, E.R.; Krishna, T.M.; Manchal, R.; Ramesh, G.; Narsimha, S. Fused benzo[1, 3]thiazine-1, 2, 3-triazole hybrids: Microwave-assisted one-pot synthesis, in vitro antibacterial, antibiofilm, and in silico ADME studies. Bioorg. Med. Chem. Lett. 2021, 47, 128201. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1, 2, 3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent advances of catalytic asymmetric 1, 3-dipolar cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, J.F.; Deville, M.; Dilly, S.; Lamy, C.; Mangin, F.; Résimont, M.; Tarazi, F.I. New pyridobenzoxazepine derivatives derived from 5-(4-methylpiperazin-1-yl)-8-chloro-pyrido [2, 3-b][1, 5] benzoxazepine (JL13): Chemical synthesis and pharmacological evaluation. J. Med. Chem. 2012, 55, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Gandham, S.K.; Kudale, A.A.; Rao Allaka, T.; Jha, A. Design and synthesis of benzoxepine-based 1, 2, 3-triazoles: Molecular docking and in vitro antimicrobial activity evaluation. ChemistrySelect 2022, 7, e202200683. [Google Scholar] [CrossRef]
- Burley, K.H.; Cuthbert, B.J.; Basu, P.; Newcombe, J.; Irimpan, E.M.; Quechol, R.; Foik, I.P.; Mobley, D.L.; Beste, D.J.; Goulding, C.W. Structural and molecular dynamics of Mycobacterium tuberculosis malic enzyme, a potential anti-TB drug target. ACS Infect. Dis. 2020, 7, 174–188. [Google Scholar] [CrossRef]
- Turkmen, Y.; Yagiz Erdemir, G.; Yuksel Mayda, P.; Akdemir, A.; Gunaydin Akyildiz, A.; Altundas, A. Synthesis, anti-TB activities, and molecular docking studies of 4-(1, 2, 3-triazoyl)arylmethanone derivatives. J. Biochem. Mol. Toxicol. 2022, 36, e22998. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Cheng, Y.; Peng, Q.; Fan, W.; Li, P. Room-temperature ligand-free Pd/C-catalyzed C-S bond formation: Synthesis of 2-substituted benzothiazoles. J. Org. Chem. 2014, 79, 5812–5819. [Google Scholar] [CrossRef]
- Banerjee, A.; Santra, S.K.; Rout, S.K.; Patel, B.K. A ligand free copper(II) catalyst is as effective as a ligand assisted Pd(II) catalyst towards intramolecular C-S bond formation via C-H functionalization. Tetrahedron 2013, 69, 9096–9104. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Pathare, R.S.; Maurya, A.K.; Gopal, K.; Roy, T.K.; Sawant, D.M.; Pardasani, R.T. Ruthenium catalyzed intramolecular C-S coupling reactions: Synthetic scope and mechanistic insight. Org. Lett. 2016, 18, 356–359. [Google Scholar] [CrossRef]
- Doğan, Ş.D.; Gündüz, M.G.; Uğur, S.B.; Doğan, H.; Özkul, C.; Çetinkaya, Y. Copper-oxone promoted oxidative C-H functionalization: Synthesis of 2-aminobenzothiazoles and evaluation of their antimicrobial activities. ChemistrySelect 2021, 6, 4382–4389. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Sugimoto, O.; Tanji, K.i.; Hirota, A. Identification of the quinol metabolite “sorbicillinol”, a key intermediate postulated in bisorbicillinoid biosynthesis. J. Am. Chem. Soc. 2000, 122, 12606–12607. [Google Scholar] [CrossRef]
- Urban, S.; Blunt, J.W.; Munro, M.H. Coproverdine, a novel, cytotoxic marine alkaloid from a New Zealand ascidian. J. Nat. Prod. 2002, 65, 1371–1373. [Google Scholar] [CrossRef]
- Chan, H.H.; Hwang, T.L.; Thang, T.D.; Leu, Y.L.; Kuo, P.C.; Nguyet, B.T.M.; Do Ngoc Dai, T.-S.W. Isolation and Synthesis of melodamide A, a new anti-inflammatory phenolic amide from the leaves. Planta Med. 2013, 79, 288–294. [Google Scholar]
- Li, H.X.; Xiao, C.J.; Wang, M.; Cui, S.J.; Li, H.F.; Wang, K.L.; Dong, X.; Jiang, B. Four new phenylethanoid glycosides from ternstroemia gymnanthera and their analgesic activities. Phytochem. Lett. 2019, 34, 25–29. [Google Scholar] [CrossRef]
- Yakura, T.; Omoto, M. Efficient synthesis of p-quinols using catalytic hypervalent iodine oxidation of 4-arylphenols with 4-iodophenoxyacetic acid and oxone. Chem. Pharm. Bull. 2009, 57, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Kowalczyk, P.; Samsonowicz-Górski, J.; Hrunyk, A.; Brodzka, A.; Łęcka, J.; Kramkowski, K.; Ostaszewski, R. Synthesis and antimicrobial activity of the pathogenic E. coli strains of p-quinols: Additive effects of copper-catalyzed addition of arylboronic acid to benzouinones. Int. J. Mol. Sci. 2023, 24, 1623. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Towards complex matter: Supramolecular chemistry and self-organization. Eur. Rev. 2009, 17, 263–280. [Google Scholar] [CrossRef]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar]
- Grimsdale, A.C.; Leok Chan, K.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [PubMed]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008, 41, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.C. The development of versatile methods for palladium-catalyzed coupling reactions of aryl electrophiles through the use of P(t-Bu)3 and PCy3 as ligands. Acc. Chem. Res. 2008, 41, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Hartwig, J.F. Highly reactive, single-component nickel catalyst precursor for Suzuk-Miyuara cross-coupling of heteroarylboronic acids with heteroaryl halides. Angew. Chem. 2012, 124, 13009–13013. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1, 5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Chevalier, J.; Mahamoud, A.; Baitiche, M.; Adam, E.; Viveiros, M.; Smarandache, A.; Militaru, A.; Pascu, M.L.; Amaral, L.; Pagès, J.M. Quinazoline derivatives are efficient chemosensitizers of antibiotic activity in enterobacter aerogenes, Klebsiella pneumoniae and Pseudomonas aeruginosa resistant strains. Int. J. Antimicrob. Agents 2010, 36, 164–168. [Google Scholar] [CrossRef]
- Rojas-Aguirre, Y.; Hernández-Luis, F.; Mendoza-Martínez, C.; Sotomayor, C.P.; Aguilar, L.F.; Villena, F.; Castillo, I.; Hernández, D.J.; Suwalsky, M. Effects of an antimalarial quinazoline derivative on human erythrocytes and on cell membrane molecular models. Biochim. Biophys. Acta Biomembr. 2012, 1818, 738–746. [Google Scholar] [CrossRef]
- Noolvi, M.N.; Patel, H.M.; Singh, N.; Gadad, A.K.; Cameotra, S.S.; Badiger, A. Synthesis and anticancer evaluation of novel 2-cyclopropylimidazo [2, 1-b][1, 3, 4]-thiadiazole derivatives. Eur. J. Med. Chem. 2011, 46, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.K.; Kim, Y.H.; Im, H.A.; Kim, J.Y.; Yoon, J.H.; Kim, A. Synthesis and antifungal activity of 6, 7-bis(arylthio)-quinazoline-5, 8-diones and furo [2, 3-f] quinazolin-5-ols. Bioorg. Med. Chem. Lett. 2012, 22, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Ganguly, S.; Veerasamy, R.; De Clercq, E. Synthesis, antiviral activity and cytotoxicity evaluation of schiff bases of some 2-phenyl quinazoline-4(3)H-ones. Eur. J. Med. Chem. 2010, 45, 5474–5479. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4, 6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef]
- Wang, H.J.; Wei, C.X.; Deng, X.Q.; Li, F.L.; Quan, Z.S. Synthesis and evaluation on anticonvulsant and antidepressant activities of 5-alkoxy-tetrazolo [1, 5-a]quinazolines. Int. J. Pharm. Med. Chem. 2009, 342, 671–675. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4 (3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef]
- Jatav, V.; Kashaw, S.; Mishra, P. Synthesis, antibacterial and antifungal activity of some novel 3-[5-(4-substituted phenyl)1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4 (3H)-ones. Med. Chem. Res. 2008, 17, 169–181. [Google Scholar] [CrossRef]
- Veerapandian, M.; Marimuthu, M.; Ilangovan, P.; Ganguly, S.; Yun, K.S.; Kim, S.; An, J. Analytical and biological characterization of quinazoline semicarbazone derivatives. Med. Chem. Res. 2010, 19, 283–298. [Google Scholar] [CrossRef]
- Ji, Q.G.; Yang, D.; Deng, Q.; Ge, Z.Q.; Yuan, L.J. Design, synthesis, and evaluation of novel 1-methyl-3-substituted quinazoline-2, 4-dione derivatives as antimicrobial agents. Med. Chem. Res. 2014, 23, 2169–2177. [Google Scholar] [CrossRef]
- Patel, A.B.; Chikhalia, K.H.; Kumari, P. Synthesis and biological evaluation of novel quinazoline derivatives obtained by Suzuki C-C coupling. Med. Chem. Res. 2014, 23, 2338–2346. [Google Scholar] [CrossRef]
- Sriram, D.; Bal, T.R.; Yogeeswari, P. Aminopyrimidinimino isatin analogues: Design and synthesis of novel non-nucleoside HIV-1 reverse transcriptase inhibitors with broad-spectrum anti-microbial properties. Med. Chem. Res. 2005, 1, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hong, W.D.; Cui, X.; Gao, H.; Wu, P.; Chen, Y.; Shen, D.; Yang, Y.; Zhang, B.; Taylor, M.J.; et al. Synthesis and structure-activity relationship of N 4-benzylamine-N 2-isopropyl-quinazoline-2, 4-diamines derivatives as potential antibacterial agents. RSC Adv. 2017, 7, 52227–52237. [Google Scholar] [CrossRef]
- Ankireddy, A.; Syed, R.; Gundla, R.; Manasa, K.; Reddy, C.; Yatam, S.; Paidikondala, K. Kumada cross coupling reaction for the synthesis of quinazoline derivatives, evaluation of their antibacterial activity and docking studies. Russ. J. Gen. Chem. 2019, 89, 2544–2557. [Google Scholar] [CrossRef]
- Ankireddy, A.R.; Paidikondala, K.; Gundla, R.; Balaraju, T.; Pagadala, R.; Banothu, V. Synthesis of new chiral (R)-binol derivatives under microwave irradiation and evaluation of their antibacterial and glucosidase inhibitory activity. ChemistrySelect 2019, 4, 5563–5569. [Google Scholar] [CrossRef]
- Ankireddy, A.R.; Paidikondala, K.; Syed, R.; Gundla, R.; Reddy, C.V.R.; Ganapathi, T. Synthesis of chiral 3, 3ʹ-disubstituted (S)-BINOL derivatives via the Kumada and Suzuki coupling and their antibacterial activity. Russ. J. Gen. Chem. 2020, 90, 1507–1517. [Google Scholar] [CrossRef]
- Wipf, P.; Jung, J.K. Formal total synthesis of (+)-diepoxin σ. J. Org. Chem. 2000, 65, 6319–6337. [Google Scholar] [CrossRef]
- Huang, J.; Nolan, S. Efficient cross-coupling of aryl chlorides with aryl grignard reagents (Kumada reaction) mediated by a palladium/imidazolium chloride system. J. Am. Chem. Soc. 1999, 121, 9889–9890. [Google Scholar] [CrossRef]
- Perez Garcia, P.M.; Di Franco, T.; Orsino, A.; Ren, P.; Hu, X. Nickel-catalyzed diastereoselective alkyl-alkyl Kumada coupling reactions. Org. Lett. 2012, 14, 4286–4289. [Google Scholar] [CrossRef]
- Lee, C.Y.; Cheon, C.H. Diastereomeric resolution of rac-1, 1,-bi-2-naphthol boronic acid with a chiral boron ligand and its application to simultaneous synthesis of (R)-and (S)-3, 3,-disubstituted 1, 1,-bi-2-naphthol derivatives. J. Org. Chem. 2013, 78, 7086–7092. [Google Scholar] [CrossRef]
- Battula, K.; Narsimha, S.; Nagavelli, V.R.; Srinivasa, R.M. Synthesis and biological evaluation of (3-arylisoxazol-5-yl) methyl 6-fluoro-4-oxo-4H-chromene-2-carboxylates as antioxidant and antimicrobial agents. J. Serb. Chem. Soc. 2017, 82, 1–12. [Google Scholar] [CrossRef]
- Bhat, M.; Imran, M.; Khan, S.; Siddiqui, N. Biological activities of sulfonamides. Indian J. Pharm. Sci. 2005, 67, 151–159. [Google Scholar]
- Izuchukwu, U.D.; Chris, O.U.; Izuchukwu, U.D. Synthesis of N-aryl substituted p-toluenesulphonamides via nickel catalyzed amidation reaction and their antibacterial, antifungal and antioxidant activities evaluation. Pak. J. Pharm. Sci. 2018, 31, 1209–1216. [Google Scholar]
- Wafford, K.; Van Niel, M.; Ma, Q.; Horridge, E.; Herd, M.; Peden, D.; Belelli, D.; Lambert, J. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology 2009, 56, 182–189. [Google Scholar] [CrossRef]
- Uemura, Y.; Tanaka, S.; Ida, S.; Yuzuriha, T. Pharmacokinetic study of loprinone hydrochloride, a new cardiotonic agent, in beagle dogs. J. Pharm. Pharmacol. 1993, 45, 1077–1081. [Google Scholar] [CrossRef]
- Hranjec, M.; Lučić, B.; Ratkaj, I.; Pavelić, S.K.; Piantanida, I.; Pavelić, K.; Karminski-Zamola, G. Novel imidazo [4, 5-b]pyridine and triaza-benzo[c] fluorene derivatives: Synthesis, antiproliferative activity and DNA binding studies. Eur. J. Med. Chem. 2011, 46, 2748–2758. [Google Scholar] [CrossRef]
- Temple, C., Jr.; Rose, J.D.; Comber, R.N.; Rener, G.A. Synthesis of potential anticancer agents: Imidazo[4, 5-c] pyridines and imidazo[4, 5-b]pyridines. J. Med. Chem. 1987, 30, 1746–1751. [Google Scholar] [CrossRef]
- Jebamani, J.; Jayachamarajapura Praneshm, S.; Shivalingappa, J.; Narayanarao, M.; Pasha, M.; Pawar, C. Synthesis and biological activities of novel 1H-Imidazo[4, 5-b]pyridine derivatives. ChemistrySelect 2023, 8, e202301239. [Google Scholar] [CrossRef]
- Blaser, H.U.; Federsel, H.J. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Buchwald, S.L.; Mauger, C.; Mignani, G.; Scholz, U. Industrial-scale palladium-catalyzed coupling of aryl halides and amines-a personal account. Adv. Synth. Catal. 2006, 348, 23–39. [Google Scholar] [CrossRef]
- Blaser, H.U.; Indolese, A.; Naud, F.; Nettekoven, U.; Schnyder, A. Industrial R&D on catalytic C-C and C-N coupling reactions: A personal account on goals, approaches and results. Adv. Synth. Catal. 2004, 346, 1583–1598. [Google Scholar]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Naso, F.; Babudri, F.; Farinola, G.M. Organometallic chemistry directed towards the synthesis of electroactive materials: Stereoselective routes to extended polyconjugated systems. Pure Appl. Chem. 1999, 71, 1485–1492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).