Abstract
Metabolic-Associated Fatty Liver Disease (MAFLD) is a clinical–pathological scenario that occurs due to the accumulation of triglycerides in hepatocytes which is considered a significant cause of liver conditions and contributes to an increased risk of death worldwide. Even though the possible causes of MAFLD can involve the interaction of genetics, hormones, and nutrition, lifestyle (diet and sedentary lifestyle) is the most influential factor in developing this condition. Polyphenols comprise many natural chemical compounds that can be helpful in managing metabolic diseases. Therefore, the aim of this review was to investigate the impact of oxidative stress, inflammation, mitochondrial dysfunction, and the role of polyphenols in managing MAFLD. Some polyphenols can reverse part of the liver damage related to inflammation, oxidative stress, or mitochondrial dysfunction, and among them are anthocyanin, baicalin, catechin, curcumin, chlorogenic acid, didymin, epigallocatechin-3-gallate, luteolin, mangiferin, puerarin, punicalagin, resveratrol, and silymarin. These compounds have actions in reducing plasma liver enzymes, body mass index, waist circumference, adipose visceral indices, lipids, glycated hemoglobin, insulin resistance, and the HOMA index. They also reduce nuclear factor-KB (NF-KB), interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), blood pressure, liver fat content, steatosis index, and fibrosis. On the other hand, they can improve HDL-c, adiponectin levels, and fibrogenesis markers. These results show that polyphenols are promising in the prevention and treatment of MAFLD.
1. Introduction
Metabolic-Associated Fatty Liver Disease (MAFLD) is a clinical–pathological scenario that occurs due to the accumulation of triglycerides in hepatocytes which is considered a significant cause of liver conditions and contributes to an increased risk of death worldwide [,,]. It has different stages, starting with a simple accumulation of triglycerides (non-alcoholic steatohepatitis—NASH), which can progress to inflammation and later to fibrosis, cirrhosis, or hepatocarcinoma. The pathogenesis of MAFLD has not been fully understood. Still, there is evidence that insulin resistance (IR) and associated subclinical inflammation, obesity, and metabolic syndrome are recognized origins in the course of this condition [,] and affect up to 30% of the world’s population [,,].
Recently, the term non-alcoholic fatty liver disease (NAFLD) was changed to MAFLD, since this one better identifies patients at a higher risk of liver fibrosis and progression of the condition []. Moreover, other researchers proposed the term Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD) to include at least one of five cardiometabolic risk factors. However, some authors investigated MAFLD and MASLD as predictors of an augmented risk of atherosclerotic cardiovascular disease. The authors included more than six thousand people that participated in the National Health and Nutrition Examination Survey cohort. Their results showed that MAFLD and MASLD were related to different risks for atherosclerotic cardiovascular disease. Notwithstanding, MAFLD predicted the risk of this condition more than MASLD [,,,].
MAFLD is normally directly related to dyslipidemia, metabolic syndrome, obesity, and diabetes []. This condition causes the dysregulation of the brain–intestine–liver axis, and as a result, people with MAFLD tend to have greater cardiovascular risks and more severe fatty liver disease [,,]. Even though the possible causes of MAFLD can involve the interaction of genetics, hormones, and nutrition, lifestyle (diet and sedentary lifestyle) is the most influential factor in developing this condition. Furthermore, if body weight decreases from 7% to 10%, MAFLD can be reversed in adults and children [,,,]. Figure 1 shows some aspects of MAFLD pathogenesis.
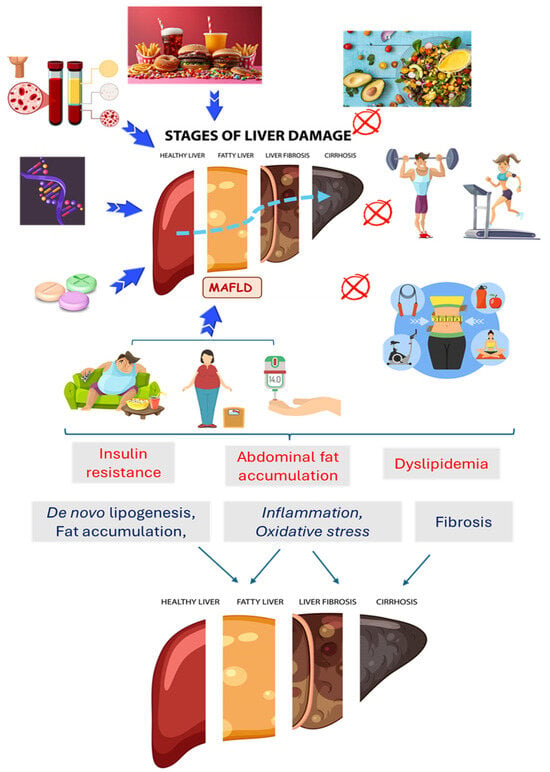
Figure 1.
Factors related to the occurrence of Metabolic-Associated Fatty Liver Disease (MAFLD) and the possibility of the inhibition of this condition. An unhealthy diet, sedentary lifestyle, obesity, insulin resistance/diabetes, dyslipidemia, genetics, and excessive drug consumption are related to the pathogenesis of MAFLD and its progression to fibrosis, cirrhosis, and cancer. A healthy diet, physical exercise, and weight loss can improve metabolic conditions and can prevent or reduce MAFLD.
Diet can profoundly influence metabolic diseases. Food diets rich in fats, sugar, and ultra-processed foods are related to inflammatory and oxidative processes. A diet abundant in fruit and vegetables can reduce risk factors such as dyslipidemia, hyperglycemia, hypertension, obesity, inflammation, and oxidative stress [,,,,,,,], which are essential in the development and progression of MAFLD [,,,]. Phytocompounds comprise many natural chemical compounds that are beneficial to counter metabolic diseases [,,,,,,,,,,]. Polyphenols are part of this group, and more than a thousand have been identified. Significant components of this class are phenols, polyphenols, carotenoids, phytosterols, isoprenoids, saponins, and dietary fibers [].
Some polyphenols can reverse part of the liver damage related to inflammation, oxidative stress, or mitochondrial dysfunction, acting directly on the functioning, synthesis, and degradation of mitochondria and optimizing the functions of these cellular organelles. Among these polyphenols are anthocyanin, baicalin, catechin, curcumin, chlorogenic acid, didymin, epigallocatechin-3-gallate, luteolin, mangiferin, puerarin, punicalagin, resveratrol, and silymarin [,,,,,,,,,,,]. The incidence of liver disorders and associated conditions such as overweight/obesity, diabetes, and metabolic syndrome has grown exponentially. For these reasons, more research must be conducted to propose ways to mitigate risk factors for these conditions []. Therefore, this review aims to investigate the impact of oxidative stress, inflammation, mitochondrial dysfunction, and the role of phenolic compounds in managing MAFLD.
2. Discussion
2.1. Metabolic-Associated Fatty Liver Disease: General Aspects
As pointed out above, the modification of the term NAFLD to MAFLD was proposed due to the augmented knowledge regarding the pathological disease scenario and new therapeutic approaches for individuals, not only in non-alcoholic contexts but all patients presenting fatty liver dysfunction, correlating this dysfunction with other conditions related to metabolic deregulation []. Moreover, the definition of MAFLD can recognize hepatic fibrosis better than NAFLD [,,].
MAFLD is profoundly linked to lipid metabolism which involves two pathways, starting with the exogenous pathway, in which the body’s first contact with fat is ingested in the diet [,]. Through chylomicrons produced by enterocytes, these lipids are transported through the lymphatic ducts until they reach the bloodstream and then continue the endogenous route through the encountering of these lipoproteins with hepatocytes and the deposition of lipoprotein content in these cells. Then, the triglycerides synthesized in hepatocytes are secreted as VLDL (very-low-density lipoprotein). As it reaches extrahepatic tissues, it loses lipid content and is transformed into IDL (intermediate-density lipoprotein) and LDL-c (low-density lipoprotein cholesterol) [,,,]. It is possible to better understand the relationship between MAFLD and other diseases through understanding various metabolic processes that occur in mitochondria, such as the tricarboxylic acid cycle (TCA), the β-oxidation of fatty acids, urea synthesis, and respiratory chain [,]. When using fuels such as glucose and fatty acids to obtain adenosine triphosphate (ATP), any disturbance in one of these mechanisms can cause severe damage to the cell and, consequently, to the tissue. Mitochondrial changes include a reduction in mitochondrial DNA (mtDNA), structural lesion formation, reduced activity of respiratory chain complexes, and damage to β-oxidation [,,]; therefore, the metabolic repercussions of these changes can be devastating for the body [,,,,].
2.2. Metabolic-Associated Fatty Liver Disease and Lipid Metabolism
De novo lipogenesis (DNL) is an essential component of the lipid cross-talk between the liver and adipose tissues, maintaining metabolic homeostasis. Imbalance between these tissues is a common feature of conditions associated with obesity, metabolic syndrome, and MAFLD, indicating how important it is to understand how this metabolic pathway contributes to cellular function. In addition, targeting this pathway shows clinical promise in MAFLD treatment [,,,,].
On the other hand, excess triglycerides in the liver can decrease VLDL secretion through negative feedback []. Increased liver fat accumulation also accelerates inflammatory processes contributing to oxidative stress so that the structure of cell membranes, proteins, and mitochondria can be compromised, reducing the production of this VLDL and balanced distribution of lipids throughout the body [,,,].
The result of the above-mentioned factors, associated with insulin resistance and hyperglycemia, is liver steatosis. It is related to the abnormal accumulation of triglycerides within parenchymal cells, mainly in the liver [,,,]. Steatosis occurs when there is a dysfunction in the transport of lipids, with a consequent excessive accumulation of fat in hepatocytes [], a condition that has a direct link with dyslipidemia and type 2 diabetes mellitus (T2DM) [], as adipose tissue releases pro-inflammatory cytokines that can interfere with insulin signal transduction pathways. It is also possible to establish a relationship between VLDL levels and DNL, proving that MAFLD is closely related to eating habits [,,,,]. The natural process of lipogenesis corresponds to the synthesis and storage of lipids. At the same time, DNL generally occurs in response to excess calories in the diet, synthesizing fatty acids and triglycerides from non-lipid sources such as carbohydrates and proteins. When there is an excess of calories, the liver can increase the production of fatty acids, which are then incorporated into VLDL for transport to other tissues [,,,,,,,,].
The deposit of fats in hepatocytes, the main characteristic of MAFLD, poses a risk of developing NASH [,], leading to fibrosis and eventual liver cirrhosis. In this case, normal tissue is replaced by scar tissue, interfering with liver functions, which can result in liver failure and an increased risk of cancer []. This is a more advanced condition of steatosis, characterized by the presence of inflammation and damage to liver cells, and deserves attention, as in milder cases, this disease does not cause symptoms, and when more advanced, the most common symptoms are ascites, encephalopathy, mental confusion, bleeding, and a drop in the number of platelets [,]. Low levels of albumin, increased amounts of bilirubin, and changes in clotting factors may also indicate liver problems, which can help diagnose steatosis [,,,,,,,,].
In addition to the possible progression to fibrosis and its relation to metabolic syndrome, dyslipidemia, coronary artery disease, inflammation, and oxidative stress, MAFLD may be related to mitochondrial dysfunction, as the pro-inflammatory state caused by an augmented provision of lipids to the liver causes fatty infiltration in hepatocytes, which induces lipid peroxidation and mitochondrial dysfunction [,,,,].
Polyphenols such as anthocyanin, baicalin, catechin, chlorogenic acid, cichoric acid, curcumin, didymin, ellagic acid, epigallocatechin-3-gallate, gallic acid, hydroxytirosol, kaempferol, luteolin, mangiferin, puerarin, punigalin, quercetin, resveratrol, salvianolic acid, rosmarinic acid, and silymarin can target a variety of pathways related to the physio-pathogenesis of MAFLD pathways and may work as therapeutically significant compounds [,,,,,,,].
2.3. Metabolic-Associated Fatty Liver Disease, Insulin Resistance, and Oxidative Stress
Cellular respiration is a naturally oxidative process. It occurs through the respiratory chain in mitochondria and is responsible for the transport of electrons and the oxidation of coenzymes in order to produce ATP []. The metabolization of fatty acids consequently also occurs through oxidation, in so-called β-oxidation, giving rise to Acetyl-CoA, a molecule responsible for adding acetyl groups in biochemical reactions to metabolize carbohydrates, lipids, and proteins in the production of ATP. When the oxidative process occurs, reactive oxygen species (ROS) are produced []. The body has its own mechanism to regulate the amount of ROS produced through compensation by antioxidant enzymes (such as catalase, superoxide dismutase, and glutathione peroxidase) [,,,,], in addition to the use of antioxidants such as polyphenols. The mitochondria have an antioxidant system, including enzymes that neutralize part of the ROS produced in respiration [,,,]. However, when free radicals accumulate, oxidative stress can occur. Fat accumulation is a cause of oxidative stress, especially in visceral tissues [,,,,,].
In MAFLD, chronic inflammation and oxidative stress synergize the occurrence of insulin resistance [,]. Liver inflammation caused by NASH, associated with the presence of pro-inflammatory mediators such as leptin, resistin, IL-6, and tumor necrosis factor-alpha (TNF-α) and intestinal lipopolysaccharides (bacterial endotoxins), creates an obstacle to insulin signaling pathways, impairing the insulin uptake of glucose in peripheral tissues and the inhibition of liver glucose production. Furthermore, oxidative stress resulting from the accumulation of free fatty acids and lipids in the liver contributes to mitochondrial dysfunction and the activation of inflammatory signaling pathways, increasing insulin resistance [,,]. The elevated production of ROS leads to the oxidation of nucleic acids, proteins, and lipids, compromising cellular function and inducing the production of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and TGF-β) [,,]. This inflammatory and stressful environment interferes with the insulin signaling cascade, resulting in an attenuated response of target tissues to insulin and, consequently, the maintenance of hyperglycemia and hyperinsulinemia [,].
Insulin resistance can increase the production of advanced glycation ends (AGEs), which aggravate oxidative stress and pro-inflammatory pathways [,,,]. These pro-oxidant mechanisms also end up contributing to cardiovascular diseases as LDL-c undergoes oxidation, becoming more prone to forming atheroma plaques [,]. The heart muscle can also suffer from the dysfunctions mentioned above, which lead to failure and heart tissue damage [], as inflammatory responses have pathogenic importance by stimulating the production and liberation of inflammatory biomarkers such as IL-6, monocyte chemoattractant protein-1 (MCP-1), and matrix-9 metallopeptidase (MMP-9) [,,,].
2.4. Metabolic-Associated Fatty Liver Disease and Inflammation
The relationship between MAFLD and inflammation is of utmost importance in understanding this multifaceted liver condition. Chronic inflammation is essential in the progression of MAFLD, a crucial point of therapeutic intervention. The activation of inflammatory pathways, together with the imbalance of pro- and anti-inflammatory adipokines, contributes to the pathogenesis and transition from simple hepatic steatosis to more severe forms of the disease. Therefore, understanding these inflammatory mechanisms is essential for developing targeted therapeutic strategies, thus mitigating the intensity and preventing the progression of MAFLD [,,,].
Oxidative stress, as previously mentioned, is closely linked to MAFLD since it triggers pro-inflammatory pathways that can lead to liver diseases or are caused by the evolution of this condition. ROS production can trigger inflammation by activating pro-inflammatory signaling pathways as a homeostatic response to damage and modifications caused to cellular structures in an attempt to repair what has been injured [,,]. In this way, transcription factors are activated, such as nuclear factor kappa B (NF-kB) and mitogen-activated protein kinase (MAPK), responsible for regulating the expression of inflammatory genes []. At the same time, hepatocytes and non-parenchymal cells of the liver express Toll-like receptors (TLRs), which recognize molecular patterns associated with lipids and fatty acids. The activation of these receptors start the release of pro-inflammatory cytokines and chemokines, attracting immune system cells to the hepatic region [,,,,,,].
Oxidative damage also induces the release of cytokines such as pro-inflammatory interleukins, chemokines, and prostaglandins, along with the activation and migration of immune cells to sites that have suffered damage []. TNF-α is responsible for the induction of the synthesis of more cytokines, in addition to stimulating the expression of adhesion molecules on endothelial cells. Therefore, the migration of immune system cells to the site of inflammation is favored. IL-6 stimulates the immune response, promoting the activation and differentiation of cells such as T and B lymphocytes and Natural Killer (NK) cells, helping both the innate and acquired immune responses, which, depending on the progression of the condition, can trigger chronic inflammation in the liver tissue, characterized precisely by the presence of macrophages and T lymphocytes, responsible for eliminating inflammatory agents and releasing cytokines [,,,,,].
Inflammation becomes chronic due to the persistence of the aggressor stimulus, which can be ROS or growth factors such as transforming growth factor beta (TGF-β), which is responsible for fibroblast proliferation and extracellular matrix deposition [,,]. Fibroblasts produce large amounts of collagen and other proteins, leading to the progression of fibrous tissue. The fibrosis resulting from this process reduces the organ’s original functions due to the replacement of the original tissue with fibrous tissue, mostly composed of collagen, which can thus reduce liver function and cause liver failure [,,,]. Figure 2 shows a scenario of liver ROS production, inflammation, and mitochondrial dysfunction.
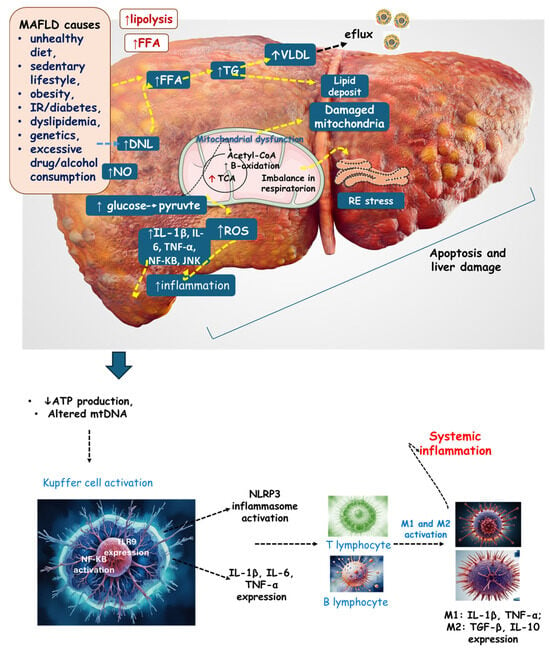
Figure 2.
The liver in the context of MAFLD. Lifestyle and metabolic alterations lead to an increased lipolysis of visceral adipose tissue, stimulating de novo lipogenesis, and an increase in FFA and VLDL (and a consequent efflux of this lipoprotein). Increased glucose intake results in increased pyruvate and Acetyl-CoA production, leading to increased TCA activity. Furthermore, there is augmented β-oxidation resulting in mitochondrial dysfunction. The consequences are mitochondrial dysfunction, altered mtDNA, an imbalance in respiration (reduction in ATP production), and RE stress. All these events are related to increased inflammation and ROS, which results in apoptosis and liver damage. Systemic inflammation occurs due to Kupffer cell activation. DNL: de novo lipogenesis; FFA: free fatty acid; IL: interleukin; JNK: c-Jun N-terminal kinase; M2: macrophage; mtDNA: mitochondrial DNA; NF-KB: nuclear factor-KB, NO: nitric acid; NLRP3: NLR family pyrin domain-containing 3; ROS: reactive oxygen species; VLDL: very-low-density lipoprotein; TG: triglyceride; TNF-α: tumor necrosis factor-α; TCA: tricarboxylic acid cycle.
It is also important to note that inflammation markers are critical in assessing and monitoring MAFLD, as they can suggest both the presence and severity of the disease. These include IL-6, as mentioned above, and the production of C-reactive protein (CRP), which occurs through the binding of IL-6 to specific hepatocyte receptors, a process triggering the stimulation of intracellular signaling pathways, so that the transcription of the CRP gene is induced []. In addition to connecting to damaged cells and modulating the immune response due to the activation of the complement system, CRP is an important marker for inflammatory activity [,,,].
Ferritin also can be considered a marker of inflammation. This protein is related to storing and releasing iron. It can interfere with oxygen transport, energy production, and DNA synthesis. In patients with MAFLD, serum ferritin may be increased because hepatocytes and hepatic macrophages, known as Kupffer cells, in this pro-inflammatory scenario, increase the production of proteins, including ferritin, as an attempt to prevent tissue injury [,]. At the same time, this protein helps protect cells from oxidative damage, so its quantity is increased in the abnormal presence of ROS [,,,].
TNF-α is also important in this topic, and high levels are associated with chronic inflammatory diseases. It can trigger insulin resistance by interfering with the correct signaling of this hormone. As already discussed above, this condition is closely related to MAFLD, as the liver’s glucose production through gluconeogenesis increases, contributing to hyperglycemia [,,]. This insulin resistance increases lipolysis in adipocytes, causing an increase in fatty acids in the bloodstream. At the same time, the availability of fatty acids is elevated in the liver, leading to greater hepatic lipogenesis and the accumulation of more lipids. Furthermore, TNF-α can stimulate hepatic stellate cells, responsible for excessive extracellular matrix production and TGF-β activation, leading to liver fibrosis [,,,].
For all these reasons, treating MAFLD with therapeutic interventions should include changes in diet, the intake of antioxidants and phytochemicals, physical exercise, and the use of medications, which would help decrease inflammatory activity and improve the bad clinical scenario [,,,,,,,,,].
2.5. Metabolic-Associated Fatty Liver Disease and Mitochondrial Dysfunction
Mitochondria are essential organelles for eukaryotic cells, performing the function of energy production and various metabolic processes. Proper mitochondrial functioning is necessary for cellular homeostasis, which is critical in cellular respiration and ATP production [,,]. Therefore, their structure or function can be related to several consequences (metabolic dysfunction, oxidative stress, and cell death). Mitochondria can have a critical role in the progression or regression of MAFLD [,,].
Some authors have shown that in patients with MAFLD, mitochondria had an abnormally activated mitochondrial permeability transition pore, keeping the organelle membranes open for longer due to the intracellular accumulation of free fatty acids []. The increase in membrane permeability causes the loss of Ca2+ ions, reducing the number of protons that participate in the electron transport chain, resulting in an insufficient production of ATP and an increase in the cytoplasmic concentration of Ca2+. This change in gradient concentration inside and outside the organelle can cause changes in the structure of mitochondria and even their destruction, in addition to causing the loss of cytochrome C and coenzyme Q, participants in the respiratory chain [,,,,,].
The loss of membrane potential caused by free radicals also causes a greater quantity of fatty acids to enter the mitochondria, decreasing the activity of proteins in oxidative phosphorylation [,] and β-oxidation, resulting in lipotoxic accumulation associated with MAFLD. One of the mechanisms of the self-regulation of metabolic activity is the formation of new mitochondria through the fission and fusion of these organelles [,,]. The first is the process by which mitochondria divide into two units (enabling the exchange of genetic material and the restoration of damaged mitochondria), allowing for the reproduction and renewal of these organelles, an essential process for the production of an adequate supply of mitochondria and sufficient production of energy for the body’s metabolism, in addition to serving as a mechanism for regulating the size and shape of mitochondria, to maintain homeostasis [,]. It is possible to observe that in hepatic steatosis, there is an increase in fission and a decrease in fusion, which could be beneficial in normal health conditions [,]. However, fission, affected by oxidative stress, accelerates the fragmentation of mitochondrial DNA and stimulates the production of ROS, further contributing to the evolution of MAFLD. Moreover, it has been observed that the new mitochondria formed under these conditions are defective [,]. Therefore, it is possible to diagnose and monitor hepatic steatosis through markers of mitochondrial enzymatic activities, ATP production levels, and gene expression related to the fission of new organelles and lipid metabolization capacity so that it is possible from the onset of the disease, carrying out therapeutic interventions in order to reduce the progression of the condition [,,,,,,].
Another element in the mitochondrial dysfunction mechanism is nutrition overload, which accelerates fatty acid oxidation through the TCA and causes ROS overproduction. Excess ROS damages the mitochondrial electron transfer chain (ETC), promoting mitochondrial dysfunction and further cellular apoptosis, inflammation, and liver fibrosis. Beyond that, increased inflammatory mediators, such as NF-κB, IL-6, and TNF-α (related to ROS and inflammation excess), increase the risk of atherosclerosis injuries and damage in the liver vessels [,,,].
The mitophagy pathway, which is beneficial for removing problematic mitochondria and oxidative toxic byproducts (mt-ROS), is inhibited in MAFLD. In this case, mt-ROS probably increases its concentration inside the cell, promoting a higher level of release of cytochrome C due to ETC activity, causing apoptosis and worsening oxidative stress [,,,,].
Excessive free radical production in mitochondria can also trigger a condition known as mitochondrial permeability transition (MPT). In this process, several proteins from the inner mitochondrial membrane, such as the phosphate carrier and the adenine nucleotide translocator (ANT), along with the matrix chaperone cyclophilin D, form a supramolecular structure that acts as a non-specific pore [,]. These MPT pores are responsible for dissipating the mitochondrial membrane potential and losing ATP synthesis capacity. It is not only MAFLD that can cause mitochondrial defects; mitochondrial defects can also contribute to MAFLD. For instance, defects or polymorphisms in mitochondrial DNA, like mutations in the gene encoding mitochondrial isobutyryl-coA dehydrogenase or mitochondrial DNA depletion syndromes, can result in excessive lipid accumulation in hepatocytes and the loss of the sirtuin 3 mediator, which can lead to reduced resistance to oxidative stress, and these are some of the various mechanisms that can contribute to the pathology [,,].
In MAFLD, besides the dysfunction in mitochondrial metabolism during fat accumulation, the endoplasmic reticulum (ER) also plays a role in metabolite exchange through complex polymeric protein structures such as mitochondrial-associated membrane proteins (MAM) [,,,,]. When there is an imbalance in ER homeostasis or energy deficiency, the ER is activated by the unfolded protein response (UPR), leading to a reduction in glutathione (GSH). This imbalance in the distribution between GSH and oxidized glutathione (GSSH) induces mitochondrial stress and results in an impaired regulation of mt-ROS production, leading to an increase in its concentration, which is a crucial factor in the enhancement in oxidative stress [,].
Figure 3 summarizes the mechanisms of mitochondrial dysfunction.
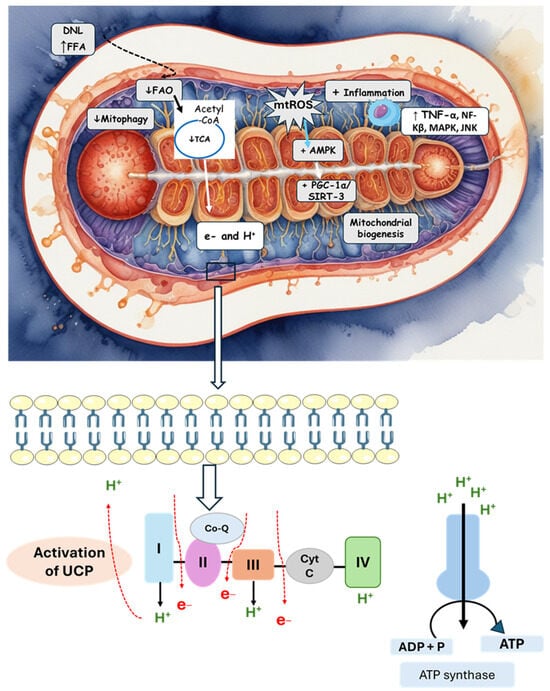
Figure 3.
The activation of DNL and an increase in FFAs lead to mitochondrial alterations and an increase in oxidative stress and inflammation. The stimulation of the mitochondrial membrane permeability transition pore is also observed by mitochondrial alterations and the deposit of fatty acids. There is stimulation in the activity of inner membrane proteins, leading to a reduction in ATP production. Mitochondrial gene mutation (mt-DNA) also activates uncoupling proteins. AMPK: AMP-activated protein kinase; CoQ: coenzyme Q; Cyt C: cytochrome C; DNL: de novo lipogenesis; FAO: fatty acid oxidation; FFA: free fatty acid; PGC1α: peroxisome proliferator-activated receptor-γ coactivator 1-α; JNK: c-Jun N-terminal kinase; NF-KB: nuclear factor kappa B; SIRT3: sirtuin 3; TCA: tricarboxylic acid cycle; TNF-α: tumor necrosis factor-α; UCP: uncoupling protein.
2.6. Polyphenols and Metabolic-Associated Fatty Liver Disease
Polyphenols are bioactive compounds of plant origin that, when ingested, act as natural antioxidants [,,,,,,,,,,,,,,]. They are present in foods according to color and are responsible for vegetables’ characteristic aromas and flavors. In plants, phytochemicals have the role of resistance to infections by bacteria, fungi, and viruses, as well as the consumption of insects and other animals. For humans, they are known for their antioxidant and anti-inflammatory actions. Among these plant biocompounds, some phenols and polyphenols can also help prevent several health conditions related to oxidative or inflammatory processes. Dietary polyphenols can include phenolic acids, flavonoids, and non-flavonoids. The most known flavonoids are anthocyanins, flavones, flavanol, and isoflavones. Among non-flavonoids are stilbenes and lignans (Figure 4). These compounds are also known to strengthen immunity, regulate the body’s hormonal activity, and promote mitochondrial health [,,,,,,,]. Table 1 shows the main polyphenols related to benefits for liver conditions.
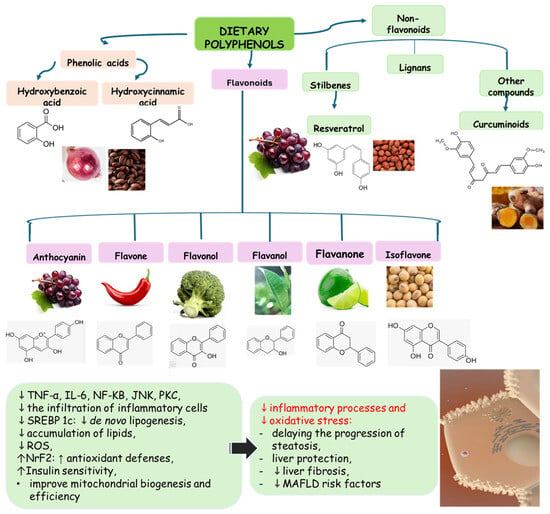
Figure 4.
Polyphenols: classification and origin. Polyphenols are found in many fruits and vegetables and can be separated into phenolic acids, flavonoids, and non-flavonoids. Phenolic acids can be found in onion, tea, and coffee; flavonoids in grapes, pepper, broccoli, green tea, lemon, and soy; and non-flavonoids in grapes, peanut skin, and Curcuma longa. These compounds can protect the liver since they can reduce the risks for MAFLD, such as oxidative stress, inflammation, and lipid deposits. IL: interleukin; JNK: c-Jun N-terminal kinase; MAFLD: Metabolic-Associated Fatty Liver Disease; NF-KB: nuclear factor kappa B; Nrf2: nuclear factor erythroid 2-related factor 2, PKC: protein kinase C; ROS: reactive oxygen species; SREBP-1c: Sterol regulatory element-binding protein 1c.
Polyphenols can protect mitochondria from oxidative stress due to the potential antioxidant effects essential for the proper functioning of these organelles. Furthermore, some interfere with metabolic processes related to energy production by regulating the activity of enzymes involved in the formation of ATP and promoting the process of mitochondrial renewal and biogenesis [,,,,,,].
Natural or processed products, when added with polyphenols, can increase their antioxidant and anti-inflammatory power, bringing benefits to the consumer in terms of preventing health conditions of an oxidative or pro-inflammatory nature such as cardiovascular diseases, inflammatory diseases, and cancer [].
However, the bioavailability of these polyphenols may be insufficiently low to reach an effective plasma level to produce the desired effects. Therefore, new pharmaceutical formulations have been developed, such as nanoparticles, nanoemulsions, nanomicelles, and preparations that increase absorption [,,].

Table 1.
Some polyphenols related to the improvement in MAFLD risk factors.
Table 1.
Some polyphenols related to the improvement in MAFLD risk factors.
| Bioactive Compound | Molecular Structures | Plant Rich in the Biocompound | Part of the Plant | Effects | References |
|---|---|---|---|---|---|
| Anthocyanin | 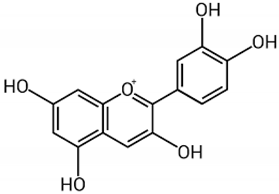 | Berries, strawberries, and grapes | Leaves, flowers, fruits, and roots | Antioxidant and anti-inflammatory, lipolysis induction, modulation of lipoprotein metabolism and PPARs | [,,] |
| Baicalin | 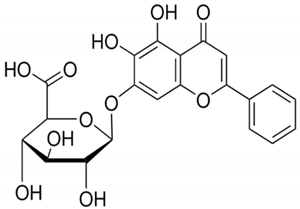 | Scutellaria baicalensis | Roots | Anti-inflammatory, antioxidant, and hepatoprotective | [,,] |
| Catechin | 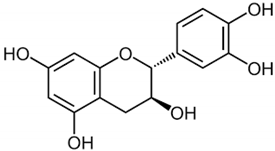 | Camellia sinensis | Leaves | Anti-inflammatory, antioxidant, and hepatoprotective | [,] |
| Chlorogenic acid | 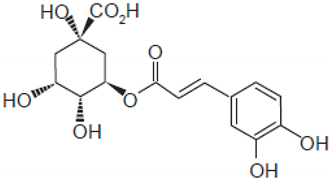 | Green coffee | Roots | Anti-inflammatory, antioxidant, and hepatoprotective | [,,] |
| Cichoric acid | 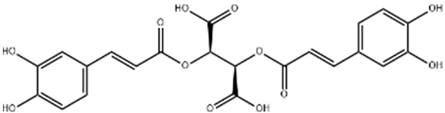 | Cichorium intybus | Leaves, flowers, and roots | Antilipogenesis, prevention of lipid accumulation and fibrosis | [,,] |
| Curcumin | 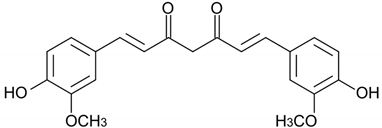 | Curcuma longa | Rhizome | Anti-inflammatory and antioxidant | [,,,,,,,,,,] |
| Didymin | 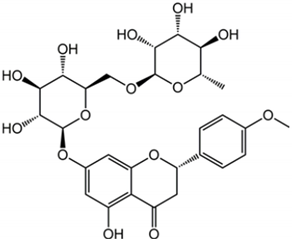 | Citrus fruits | Fruit | Anti-inflammatory and antioxidant | [,,] |
| Epigallocatechin-3 gallate | 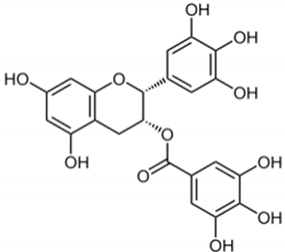 | Camellia sinensis | Leaves | Antioxidant and anti-inflammatory | [,] |
| Kaempferol | 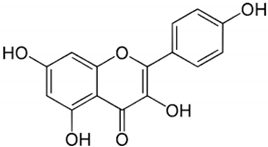 | Leaves and stem | Antioxidant, anti-inflammatory, and improves insulin resistance | [,] | |
| Luteolin | 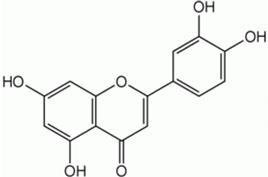 | Celery, peppers, carrots | Leaves and seeds | Antioxidant and anti-inflammatory | [,] |
| Mangiferin | 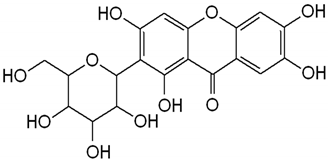 | Mango | Leaves, roots, and stem | Regulation of glucose and lipids metabolism | [,,] |
| Puerarin | 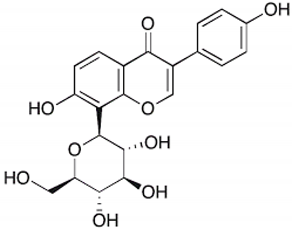 | Pueraria lobata | Roots | Antioxidant and anti-inflammatory | [,] |
| Punicalagin |  | Punica galum | Shells and seeds | Decreases lipid accumulation and increases gene expression levels of fatty acid beta-oxidation pathways | [,] |
| Quercetin |  | Evodiae fructus | Bark, leaves, flowers, seeds, and shoots | Improvement in insulin resistance, modulation of lipid metabolism, reduces inflammation and oxidative stress | [,,] |
| Resveratrol |  | Red grapes and peanuts | Grape and peanut skin | Mitochondrial biogenesis and synthesis; antioxidant and anti-inflammatory | [,,,] |
| Rosmarinic acid |  | Salvia rosmarinus | Leaves | Antioxidant and anti-inflammatory | [,] |
| Silymarin | 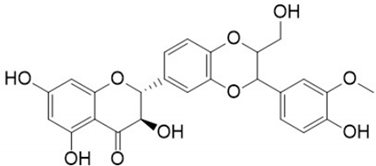 | Silybum marianum | Leaves, fruit, and seeds | Reduction in liver injury and lipid accumulation; insulin resistance improvement | [,,,] |
2.6.1. Anthocyanins
Anthocyanins are flavonoids responsible for the reddish color of foods such as red fruits and vegetables such as red cabbage, purple potatoes, and eggplant. Studies carried out on cells derived from hepatocellular carcinoma indicate that they can reduce the accumulation of lipids in hepatocytes by inhibiting lipogenesis. At the same time, they promote lipolysis and reduce oxidative stress through the activation of AMPK, being able to act agonistically on peroxisome proliferator-activated receptors (PPARs) in liver cells. PPARs act as transcription factors of lipid metabolism, especially PPAR-α, widely found in the liver, which regulates mitochondrial and peroxisome β-oxidation and plays an important role in lipoprotein synthesis [,,].
2.6.2. Baicalin
By modifying signaling pathways, baicalin (a flavonoid derived from Scutellaria baicalensis) can potentially reduce NAH, hepatic steatosis, and MAFLD. It acts on nuclear factor kappa B (NF-κB), thus reducing inflammation in the liver, a crucial factor in the development of liver diseases. This polyphenol can also act on transforming growth factor beta 1 (TGF-β1)/SMAD3, reducing liver fibrosis. Baicalin intensifies sirtuin 1 (SIRT1) by upregulating lipid metabolism. In addition, it inhibits p38/MAPK and has the ability to reduce oxidative stress and programmed hepatocyte death [,]. Baicalin regulates MERTK +/hi M2c derived from mononuclear cells (MNCs), demonstrating a role in modulating the liver’s immune response and attenuating chronic hepatic inflammation. The interaction of baicalin with the enzyme carnitine palmitoyltransferase 1 (CPT1) promotes the oxidation of fatty acids, favoring the reduction in lipid accumulation in the liver [].
2.6.3. Catechin
Catechins, largely found in Curcuma longa (saffron) and Camellia sinensis (green tea) [], act similarly to anthocyanins through an indirect activation of PPARα [], as they inhibit oxidative and inflammatory activity, responsible for decreasing the expression of this receptor; they can also elevate the gene expression of proteins involved in lipid metabolism and modulate signaling pathways, such as the AMPK pathway []. Their antioxidant activity occurs through the neutralization of free radicals by donating electrons while at the same time having the property of binding to metal ions, preventing Fenton reactions—the decomposition of hydrogen peroxides catalyzed by FeII and the production of HO radicals and the generation of FeIV, highly oxidizing products []. Catechin may also increase the activity of antioxidant enzymes by interfering with nuclear factor erythroid 2-related factor 2 (Nrf-2) translocation [].
2.6.4. Chlorogenic Acid
Chlorogenic acid, found in green tea, fruits, and green coffee [,,], is capable of acting on the intestine–liver axis; it has antilipogenic and anti-inflammatory action and helps regulate the intestinal microbiota. It may be related to the degradation of fatty acids through the activation of hepatic autophagy, binding to ALKBH5 (demethylase Alk B homolog 5) and preventing its action of removing methyl groups from position 6 of the adenine of messenger RNA (m6A). This process compromises the gene expression of liver cells, promoting autophagy to reduce hepatic steatosis []. It is also related to the improvement in the expression of carnitine palmitoyltransferase (CPT-1), responsible for conjugating long-chain fatty acids to carnitine in the mitochondria so that β-oxidation occurs [,].
2.6.5. Cichoric Acid
The chicory plant (Cichorium intybus), Astraceae family, is a source of vitamins, phenolic acids, and cichoric acid) []. Cichoric acid plays an important role in reducing hepatic steatosis, as it reduces the expression of lipogenic actors, such as SREBP-1c, DGAT1, FAS, and SCD-1, and also of inflammatory factors such as IL-6, IL-1b, NF-κB, and TNF-α. It is known that advanced steatosis can cause fibrosis, and cichoric acid also prevents TGF-β and the development of type I and type III collagen in the liver []. In a study where HePG2 cells were treated with palmitate, it was observed that fish oil, together with cichoric acid, significantly reduced lipid accumulation through the AMPK-mediated stimulation of PPAR-α [].
2.6.6. Curcumin
Curcumin is a polyphenol belonging to curcuminoids, which are compounds of the ginger family. It is found in the rhizome of Curcuma longa. Curcumin is associated with a reduction in body weight, improves insulin resistance, reduces lipid levels, reduces inflammation and oxidative stress, and can improve liver disease (showing a decrease in hepatic fat levels and a reduction in serum aspartate aminotransferase and alanine aminotransferase levels) [,,,]. It can inhibit cytotoxins and cyclooxygenase (COX) and lipoxygenase (LOX) enzymes [,], responsible for producing prostaglandins and leukotrienes, respectively, which are mediators that contribute to the inflammatory process. At the same time, curcumin also plays a role in reducing the production of free radicals in some ways, such as donating electrons to these radicals to become stable or increasing the activity of the body’s natural antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase. In this way, they can contribute to the treatment of MAFLD along with lifestyle changes [] and may also prevent the development of liver fibrosis, resulting in a 3- to 5-fold higher chance of resolution in hepatic steatosis [].
It has also been demonstrated that the association of curcumin with resveratrol has led to a synergistic effect by attenuating MAFLD, and this result may be, at least in part, associated with the modulation of the Hypoxia-inducible factor 1 (HIF-1) signaling pathway. HIF can modulate lipid metabolism in a particular way in the liver tissue by sensing the cellular microenvironment under different conditions. In a low-oxygen environment, HIF-1 stimulates the uptake and utilization of fatty acids and can elevate lipogenic gene expression, therefore augmenting lipid accumulation in the liver [].
2.6.7. Didymin
Didymin, a flavonoid identified in citrus fruits, has antioxidant and anti-inflammatory action, making it suitable for use in MAFLD as a therapeutic intervention. In experiments, it was noted that didymin results in the activation of Sirt1, a sirtuin that regulates energy metabolism and the inflammatory response. Sirt1 activation is linked to the inhibition of the TLR4/NF-κB pathway (an inflammatory pathway that determines the progression of MAFLD), showing that didymin can attenuate hepatic inflammation and oxidative stress []. Furthermore, it can suppress the PI3K/Akt pathway, demonstrated by the decrease in the phosphorylation levels of PI3K and Akt, which modulates insulin resistance and lipid accumulation in hepatocytes correlated with MAFLD. Thus, its therapeutic role is a natural intermediary one that adds to existing therapeutic strategies [].
2.6.8. Epigallocatechin-Gallate (EGCG)
EGCG is the major active compound found in green tea and has been linked to a reduction in obesity and an improvement in metabolic parameters. A study aiming to evaluate the effects of this compound on lipolysis, obesity, and the browning of human white adipocytes showed that EGCG can significantly reduce systolic and diastolic blood pressure (p < 0.05), fasting plasma triglyceride levels (p < 0.05), and serum kisspeptin levels (p < 0.05) after eight weeks of supplementation [].
2.6.9. Kaempferol
Kaempferol, a flavonoid found in foods such as broccoli, kale, green tea, and apples, has therapeutic properties in the fight against numerous liver diseases. This polyphenol promotes antioxidant and anti-inflammatory results, which are essential for liver protection [,]. It intensifies the action of superoxide dismutase, an antioxidant enzyme, and catalase []. This compound acts on PI3K/AKT signaling, improving insulin receptivity. Kaempferol can prevent H2O2-induced oxidative stress in the production of nitric oxide (NO) coordinated by HepG2 and lipopolysaccharides (LPSs) in RAW264.7 cells. It also acts by reducing the production of free oxygen radicals, rebuilding the redox balance, and preventing the production of exaggerated NO, a mediator of inflammation caused by exposure to LPSs []. Kaempferol intensifies the action of the activated protein kinase AMPK, favoring beta-oxidation, which reduces the formation of lipids in the hepatic region []. These consequences are important to prevent the progression of MAFLD to NASH. The compound has antiapoptotic and anti-necroptotic capabilities, protecting hepatocytes from predisposed death. Additionally, kaempferol is related to the restriction of cyclooxygenase and lipoxygenase enzymes [].
2.6.10. Luteolin
Luteolin, a flavonoid identified in vegetables (celery, peppers, carrots, and some medicinal herbs), has been highlighted for its therapeutic role in liver diseases. It exhibits anti-inflammatory and antioxidant results, which are essential in protecting the liver against oxidative and inflammatory damage related to fat accumulation []. Studies show that luteolin can significantly reduce the infiltration of inflammatory cells in liver tissue, in addition to attenuating the amount of liver enzymes and lipids in the liver, conditions that influence the progression of MAFLD. Luteolin prevents oxidative damage by neutralizing ROS and enhancing the functioning of endogenous antioxidant enzymes, which detoxify free radicals []. Furthermore, luteolin improves insulin sensitivity by regulating the PI3K/AKT/FoxO1 signaling pathway, which is necessary for glucose capture by hepatocytes and muscle cells []. The intensification of this pathway exacerbates the translocation of cell membrane proteins, helping the intake of glucose and thus reducing blood glucose. Simultaneously, luteolin intensifies the oxidation of fatty acids by activating AMP-activated protein kinase (AMPK), an enzyme that allows for the beta-oxidation of fatty acids in mitochondria, improving mitochondrial functioning and decreasing hepatic lipogenesis [].
2.6.11. Mangiferin
Mangiferin, found especially in mangoes and other plants, has antioxidant properties, which is why it helps improve the condition of MAFLD []. This polyphenol can reverse the translocation of GLUT4 in the membrane, consequently interfering in the modulation of liver glucose and lipid metabolism, especially in MAFLD []. Mangiferin influences the AMPK protein, causing the activation of AKT phosphorylation. This activation is related to the regulation of pantothenate and CoA biosynthesis, which is essential for hepatic lipid metabolism [] and can also modulate the NLRP3 inflammasome (a protein complex involved in chronic liver inflammation in MAFLD), suppressing its activation. These findings show that mangiferin acts on dysfunctional metabolic aspects characteristic of NASH and in the control of hepatic inflammation [].
2.6.12. Puerarin
Puerarin, a bioactive compound found in Pueraria lobata roots, aroused interest as a potential therapy for MAFLD []. Researchers suggest that puerarin has antioxidant and anti-inflammatory properties that may help reduce fat accumulation in the liver and mitigate liver inflammation, two crucial components of MAFLD [,]. Furthermore, preclinical studies have indicated that puerarin can regulate lipid and glucose metabolism, helping to improve insulin sensitivity and reduce triglyceride and cholesterol levels, factors that are often dysregulated in patients with MAFLD [].
In a Salmonella enterica-infected chick model, puerarin protected against infection and improved liver morphology, inflammatory indices, and antioxidant capacity in chicks. Moreover, it significantly decreased the levels of hepatocellular carcinoma markers in the liver []. In rats, puerarin reduced liver fibrosis through the signaling pathway mediated by TGF-β/extracellular signal-regulated kinase ½ (ERK1/2), inhibiting hepatic stellate cell stimulation and excessive collagen deposition in liver fibrosis [].
2.6.13. Punicalagin
Punicalagin is a flavonoid found mainly in Punica galum [] and positively affects the functioning of mitochondria. A study carried out on maturing adipocytes showed that the presence of punicalagin decreased lipid accumulation and significantly increased the gene expression levels of fatty acid beta-oxidation pathways such as peroxisome proliferator-activated receptor γ (PPARγ)C1α, uncoupling protein-1 (UCP-1), and PR domain-containing 16 (PRDM-16), increasing mitochondrial efficiency []. Thus, the increase in lipolysis and the decrease in hypertrophic adipocytes reduce the secretion of adipokines, associated with obesity and the inflammation of vascular cells [].
2.6.14. Quercetin
Quercetin, found in the rhizome of Evodiae fructus, is a phytochemical that helps treat MAFLD and reduce cancer []. This flavonoid acts in terms of AMPK, improving insulin resistance and helping with lipid metabolism to reduce liver fat []. Quercetin may also reduce inflammation caused by MAFLD by inhibiting the release of inflammatory biomarkers such as TNF-α and IL-6 [], along with antioxidant actions, neutralizing free radicals and reducing oxidative stress [].
There is a combination of different clinical and biochemical factors that lead to metabolic dysregulation. Quercetin intake can significantly decrease fasting blood glucose and systolic blood pressure [].
2.6.15. Resveratrol
A well-known example is resveratrol, which comes from grapes, red fruits, peanuts, and wine. It exhibits antioxidant properties associated with the production of mitochondria. Resveratrol is a non-flavonoid polyphenol [] capable of improving mitochondrial biogenesis, acting on the main effectors of biogenesis, such as the peroxisome proliferator-activated coactivator γ-1α (PGC-1α), sirtuin 1 (SIRT1), adenosine monophosphate protein kinase (AMPK), α-related receptor estrogen (ERR-α), telomerase reverse transcriptase (TERT), mitochondrial transcription factor A (TFAM), and nuclear respiration factors 1 and 2 (NRF-1, NRF-2) [,,]. A study carried out in mice also revealed that resveratrol supplementation significantly increased the activity of SIRT1 and PGC-1, improving the efficiency of mitochondrial synthesis [].
2.6.16. Rosmarinic Acid
Rosmarinic acid, found in Salvia rosmarinus (rosemary) and Prunella vulgaris [,], has antioxidant effects through the modulation of signaling pathways. This polyphenol acts on MAPKs, reducing oxidative stress and hepatic inflammation. It acts on the activation of quinone acceptor oxidoreductase 1 (NQO1) and Nrf2. The increase in MAPKs and Nrf2 reduces the effects related to liver disease. Rosmarinic acid acts on the negative regulation of YAP1 and TAZ, related to the activation of PPARγ and PGC-1α, regulating lipid metabolism and providing hepatic homeostasis [,,].
2.6.17. Silymarin
Silymarin, a group of flavonolignans extracted from milk thistle (Silybum marianum), has motivated scientific interest due to its therapeutic capacity in several liver diseases, including MAFLD and NASH). Silymarin has beneficial effects through its antioxidant and anti-inflammatory characteristics. Studies indicate that silymarin improves liver function by reducing oxidative stress and inflammation through the formation of glutathione peroxidase, which reduces glutathione to hydrogen peroxide and water, providing the recovery of damaged liver cells [].
In some animal models and clinical investigations, it was observed that silymarin can reduce the accumulation of lipids in the liver, improve insulin sensitivity, and modulate metabolic pathways related to lipid and glucose metabolism []. These results are important for treating MAFLD, in which insulin resistance and metabolic dysfunction play central roles. By improving liver integrity and function, silymarin, in addition to delaying the progression of steatosis to more severe forms, such as NASH, can also reverse initial liver disorders, proving to be a promising treatment for controlling these chronic liver diseases []. Figure 4 shows the main polyphenols that can be obtained from the diet, and Figure 5 shows the primary mechanism of action of these compounds in the liver.
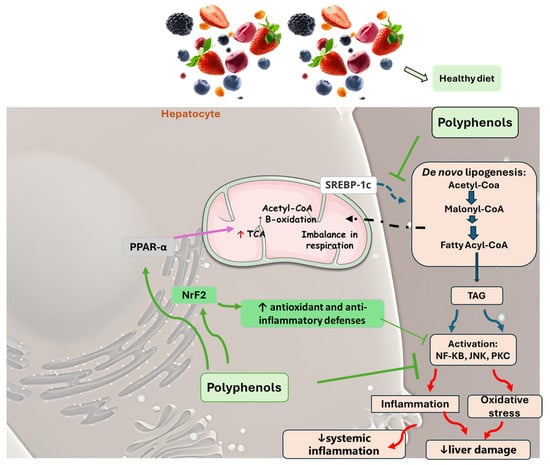
Figure 5.
The main mechanisms of action promoted by phenols in MAFLD. A salubrious diet with an increased consumption of fruits and vegetables elevates the intake of polyphenols. These phytochemicals can inhibit liver cellular damage associated with MAFLD through varied mechanisms that may include a decrease in de novo lipogenesis due to the downregulation of SREBP-1c, elevating β-fatty acid oxidation through PPAR α upregulation, ameliorating insulin sensitivity, and reducing oxidative stress and inflammation processes. This scenario is related to a reduction in liver damage and systemic inflammation. JNK: c-Jun N-terminal kinase; NF-KB: nuclear factor kappa B; Nrf2: nuclear factor erythroid 2-related factor 2, PKC: protein kinase C; PPAR-α: peroxisome proliferator-activated receptor gamma; SREBP-1c: Sterol regulatory element-binding protein 1c; TCA: tricarboxylic acid cycle; TAG: triglyceride.
2.7. Effects of Polyphenols in MAFLD: Results of Clinical Trials
Table 2 shows some clinical trials that investigated the effects of polyphenols in MAFLD. Although some results are controversial, in general, they showed that these compounds can be effective in reducing or preventing risk factors for liver conditions.

Table 2.
Clinical trials showing the effects of some polyphenols in liver conditions (MAFLD).
Polyphenols are normally related to antioxidant and anti-inflammatory effects; thus, they can be related to different actions in several metabolic and physiological pathways, leading to characteristics that can improve liver tissue damage and function which are observed in MAFLD and its complications.
Besides the suggestion that other clinical trials with a more homogeneous population and a higher number of participants should be performed with the phytocompounds considered in this review, we can say that these phytocompounds can act as preventive compounds or can provide natural treatment and complement existing treatments for MAFLD and other liver conditions such as steatosis. In summary, the results of the included clinical trials show that these compounds, in comparison to placebo, can achieve the following:
- Significantly reduce gamma-GT, AST, and ALT;
- Reduce body weight, BMI, waist circumference, adipose visceral indices, and visceral and subcutaneous abdominal fat mass;
- Decrease plasma total cholesterol, triglycerides, and LDL-c;
- Reduce the plasma concentrations of atherogenic oxidized LDL-c;
- Reduce glycated hemoglobin, glycemia, insulin resistance, and the HOMA index;
- Reduce plasma leptin levels (as well as the leptin–adiponectin ratio);
- Reduce urinary 8-isoprostane excretion;
- Reduce the induction of NFKB;
- Reduce serum cytokeratin-18 and kisspeptin levels;
- Reduce the levels of pro-inflammatory interleukins such as IL-1β, IL-6, IL-18, and TNF-α;
- Decrease the mRNA expression of NLRP3 inflammasome (caspase-1, IL-1β, and IL-18) in peripheral blood mononuclear cells;
- Reduce systolic and diastolic blood pressure;
- Improve HDL-c and adiponectin levels;
- Improve the liver-to-spleen computed tomography attenuation ratio;
- Improve flow-mediated dilation and carotid intima–media thickness;
- Decrease liver fat content, the steatosis index, and the level of fibrosis;
- Improve fibrogenesis markers.
3. Conclusions and Future Directions
For MAFLD, the cornerstone of current treatment strategies involves significant lifestyle modifications. These typically include dietary changes and increased physical activity. While these approaches can be effective, the incorporation of polyphenols into these strategies offers exciting potential for enhancing therapeutic outcomes. Polyphenols, known for their antioxidant and anti-inflammatory properties, can complement traditional interventions and provide additional benefits in managing MAFLD (In patients with hypercholesteremia, 70% of them do not respond adequately to statins. For these reasons, using polyphenols in these conditions may bring to light a new direction [,]). However, to fully realize the potential of polyphenols in this context, future research needs to explore their impact on various metabolic pathways and liver function biomarkers more comprehensively. Understanding how polyphenols affect these processes could reveal mechanisms through which they influence liver health, potentially leading to novel therapeutic strategies. Moreover, while the Phytochemical Index serves as a valuable tool for assessing dietary polyphenol content, there is an opportunity to refine and enhance its application in clinical settings. By developing more precise and clinically relevant measures of polyphenol intake and their biological effects, we can better guide dietary interventions and tailor recommendations for individuals with MAFLD.
Firstly, focusing on the bioavailability and metabolic conversion of polyphenols is essential. Understanding how different polyphenols are absorbed, metabolized, and converted into their active forms will provide a more accurate reflection of their potential benefits for liver health. Since polyphenols vary significantly in these aspects, this refinement will ensure that the index accounts for not just the quantity of polyphenols consumed but also their efficacy within the body. Expanding the index to include a broader range of polyphenol compounds is also crucial. By developing a detailed profile that encompasses a variety of polyphenols known to impact liver health, the index can offer a more comprehensive measure of dietary intake. Employing advanced analytical techniques like liquid chromatography–mass spectrometry (LC-MS) will enhance the accuracy of these measurements and help identify which specific polyphenols are the most beneficial for MAFLD management.
Additionally, personalizing the Phytochemical Index based on individual genetic and microbiome profiles is another important step. Variations in genetic makeup and gut microbiota can significantly influence polyphenol metabolism and efficacy. By integrating these personalized data into the index, dietary recommendations can be tailored to individual needs, optimizing the benefits of polyphenols for managing MAFLD.
To ensure that the refined index is practical and reliable, it should also be validated through rigorous clinical trials. These studies would assess how polyphenol intake, as guided by the index, impacts liver biomarkers and clinical outcomes in MAFLD patients. Clinical validation will provide the necessary evidence to support the index’s effectiveness and its integration into standard clinical practice. Furthermore, developing practical measurement tools will enhance the application of the Phytochemical Index in everyday settings. For example, mobile health apps or digital platforms could be designed to track polyphenol intake and provide real-time feedback. Such tools would help patients adhere to dietary recommendations and make informed choices about their diet, facilitating a better management of MAFLD.
Several critical research areas also warrant exploration to fully harness the potential of polyphenols. As an example, research into polyphenols and their effects on immune system function could provide significant benefits for managing MAFLD. Understanding how polyphenols influence immune cell markers in subjects with MAFLD could lead to strategies for reducing liver inflammation in these patients, potentially slowing or reversing liver damage. Studying how polyphenols affect immune cell activation in MAFLD could reveal methods to prevent or reduce liver damage caused by immune responses. Genetic research could also identify polymorphisms that affect individual responses to polyphenols, potentially through genome-wide association studies (GWASs). This research would facilitate personalized nutrition approaches by tailoring polyphenol interventions based on genetic predispositions, optimizing therapeutic outcomes.
In this scenario, RNA-based assays, such as transcriptomic studies using RNA sequencing, could illuminate the molecular mechanisms by which polyphenols alter gene expression in the MAFLD liver. This research could uncover specific genes and pathways influenced by polyphenols, providing a clearer understanding of their role in cholesterol metabolism and liver health. Advancements in nanotechnology also hold promise for enhancing polyphenol delivery and effectiveness. Developing nanocarriers for controlled release and targeted action could improve the bioavailability of polyphenols, maximize their therapeutic benefits, and minimize potential side effects.
Moreover, exploring the synergistic effects of polyphenols in combination with established medications could also lead to novel treatment strategies against MAFLD. Research could focus on how polyphenols interact with statins, other lipid-lowering agents, or diabetes medications in the context of MAFLD to enhance efficacy or reduce adverse effects, offering new insights into optimizing combination therapies.
However, since all novel interventions start with preclinical research, clinical trials are essential to translate these findings into practical clinical applications. These trials should include diverse populations and consider long-term outcomes to assess efficacy, safety, and optimal dosages. Evaluating different forms of polyphenol intake—such as supplements, functional foods, or fortified diets—will also be crucial. Integrating metabolomic and proteomic analyses into research could provide a comprehensive understanding of how polyphenols influence metabolic pathways and protein expression in the realm of clinical research.
Research should also consider how environmental factors and lifestyle choices interact with polyphenol consumption in MAFLD conditions. Cohort studies exploring the effects of diet, microbiome composition, and exposure to environmental toxins on polyphenol efficacy could provide additional insights into optimizing their use against MAFLD.
In summary, while current evidence supports the beneficial role of polyphenols in managing cholesterol and metabolic diseases, advancing our understanding through targeted research is essential. By employing advanced technologies, conducting rigorous clinical trials, and exploring synergistic effects, we can unlock the full potential of polyphenols. This comprehensive approach promises to enhance patient outcomes and contribute significantly to advancements in public health.
Author Contributions
Conceptualization, R.B.T., P.T.G., E.P.d.L., R.D. and S.M.B.; methodology, R.B.T., P.T.G., E.P.d.L., R.d.A.G., E.L.G., C.C.T.N., A.M.R.F., L.F.L., M.D.B. and S.M.B.; formal analysis, R.B.T., P.T.G., E.P.d.L., R.d.A.G., E.L.G., V.E.V., L.F.L., M.D.B. and S.M.B.; investigation, R.B.T., P.T.G., E.P.d.L., R.d.A.G., E.L.G., L.F.L., M.D.B., J.L.Y.J., N.M.-S., L.A., R.D. and C.C.T.N.; data curation, R.B.T., P.T.G., E.L.G., A.M.R.F., M.D.B., J.L.Y.J., R.D. and S.M.B.; writing—original draft preparation, R.B.T., P.T.G., E.P.d.L., R.d.A.G., E.L.G., J.L.Y.J., N.M.-S., L.A. and S.M.B.; writing—review and editing, R.B.T., P.T.G., E.P.d.L., N.M.-S., L.A. and S.M.B.; visualization, R.B.T., P.T.G., E.P.d.L., N.M.-S., L.A. and S.M.B.; supervision, N.M.-S., L.A., S.M.B. and R.D.; project administration, S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Free Pick was used to build the figures (https://www.freepik.com/).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramos-Lopez, O. Multi-Omics Nutritional Approaches Targeting Metabolic-Associated Fatty Liver Disease. Genes 2022, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review from NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–531. [Google Scholar] [CrossRef] [PubMed]
- Khaznadar, F.; Khaznadar, O.; Petrovic, A.; Hefer, M.; Gjoni, F.; Gjoni, S.; Steiner, J.; Smolic, M.; Bojanic, K. MAFLD Pandemic: Updates in Pharmacotherapeutic Approach Development. Curr. Issues Mol. Biol. 2024, 46, 6300–6314. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8850. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Huang, Y.; Zhou, C.; Wang, N.; Zhang, L.; Zhang, Z.; Li, B.; He, X.; Wang, K.; et al. Metabolomics analyses reveal the liver-protective mechanism of Wang’s metabolic formula on metabolic-associated fatty liver disease. Heliyon 2024, 10, e33418. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Argenziano, M.E.; Kim, M.N.; Montori, M.; Di Bucchianico, A.; Balducci, D.; Ahn, S.H.; Svegliati Baroni, G. Epidemiology, pathophysiology and clinical aspects of Hepatocellular Carcinoma in MAFLD patients. Hepatol. Int. 2024, 18, 922–940. [Google Scholar] [CrossRef]
- Ramírez-Mejía, M.M.; Jiménez-Gutiérrez, C.; Eslam, M.; George, J.; Méndez-Sánchez, N. Breaking new ground: MASLD vs. MAFLD-which holds the key for risk stratification? Hepatol. Int. 2024, 18, 168–178. [Google Scholar] [CrossRef]
- Pan, Z.; Shiha, G.; Esmat, G.; Méndez-Sánchez, N.; Eslam, M. MAFLD predicts cardiovascular disease risk better than MASLD. Liver Int. Off. J. Int. Assoc. Study Liver 2024, 44, 1567–1574. [Google Scholar] [CrossRef]
- Zhang, H.; Targher, G.; Byrne, C.D.; Kim, S.U.; Wong, V.W.; Valenti, L.; Glickman, M.; Ponce, J.; Mantzoros, C.S.; Crespo, J.; et al. A global survey on the use of the international classification of diseases codes for metabolic dysfunction-associated fatty liver disease. Hepatol. Int. 2024, 18, 1178–1201. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Brouwer, W.P.; Lammert, F.; Yilmaz, Y. Metabolic dysfunction associated fatty liver disease in healthy weight individuals. Hepatol. Int. 2024, 18, 884–896. [Google Scholar] [CrossRef] [PubMed]
- De Cól, J.P.; de Lima, E.P.; Pompeu, F.M.; Cressoni Araújo, A.; de Alvares Goulart, R.; Bechara, M.D.; Laurindo, L.F.; Méndez-Sánchez, N.; Barbalho, S.M. Underlying Mechanisms behind the Brain-Gut-Liver Axis and Metabolic-Associated Fatty Liver Disease (MAFLD): An Update. Int. J. Mol. Sci. 2024, 25, 3694. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Méndez-Sánchez, N.; Fornari Laurindo, L. AdipoRon and ADP355, adiponectin receptor agonists, in Metabolic-associated Fatty Liver Disease (MAFLD) and Nonalcoholic Steatohepatitis (NASH): A systematic review. Biochem. Pharmacol. 2023, 218, 115871. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef]
- Fouda, S.; Jeeyavudeen, M.S.; Pappachan, J.M.; Jayanthi, V. Pathobiology of Metabolic-Associated Fatty Liver Disease. Endocrinol. Metab. Clin. N. Am. 2023, 52, 405–416. [Google Scholar] [CrossRef]
- Soysouvanh, F.; Rousseau, D.; Bonnafous, S.; Bourinet, M.; Strazzulla, A.; Patouraux, S.; Machowiak, J.; Farrugia, M.A.; Iannelli, A.; Tran, A.; et al. Osteopontin-driven T-cell accumulation and function in adipose tissue and liver promoted insulin resistance and MAFLD. Obesity (Silver Spring Md.) 2023, 31, 2568–2582. [Google Scholar] [CrossRef]
- Rupasinghe, K.; Hind, J.; Hegarty, R. Updates in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Children. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 583–591. [Google Scholar] [CrossRef]
- Abenavoli, L.; Gambardella, M.L.; Scarlata, G.G.M.; Lenci, I.; Baiocchi, L.; Luzza, F. The Many Faces of Metabolic Dysfunction-Associated Fatty Liver Disease Treatment: From the Mediterranean Diet to Fecal Microbiota Transplantation. Medicina 2024, 60, 563. [Google Scholar] [CrossRef]
- de Lima, E.P.; Moretti, R.C., Jr.; Torres Pomini, K.; Laurindo, L.F.; Sloan, K.P.; Sloan, L.A.; Castro, M.V.M.; Baldi, E., Jr.; Ferraz, B.F.R.; de Souza Bastos Mazuqueli Pereira, E.; et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology 2024, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Girotto, O.S.; Furlan, O.O.; Moretti Junior, R.C.; Goulart, R.A.; Baldi Junior, E.; Barbalho-Lamas, C.; Fornari Laurindo, L.; Barbalho, S.M. Effects of apples (Malus domestica) and their derivatives on metabolic conditions related to inflammation and oxidative stress and an overview of by-products use in food processing. Crit. Rev. Food Sci. Nutr. 2024, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.F.D.; Bragante, W.R.; Junior, R.C.M.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; Nicolau, C.C.T.; Oshiiwa, M.; Lima, E.P.; et al. Effects of Smallanthus sonchifolius Flour on Metabolic Parameters: A Systematic Review. Pharmaceuticals 2024, 17, 658. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Rodrigues, V.D.; Minniti, G.; de Carvalho, A.C.A.; Zutin, T.L.M.; DeLiberto, L.K.; Bishayee, A.; Barbalho, S.M. Pomegranate (Punica granatum L.) phytochemicals target the components of metabolic syndrome. J. Nutr. Biochem. 2024, 131, 109670. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Maio, M.C.; Lemes, M.A.; Laurindo, L.F.; Haber, J.; Bechara, M.D.; Prado, P.S.D., Jr.; Rauen, E.C.; Costa, F.; Pereira, B.C.A.; et al. Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Ota, T. Impact of Glucoraphanin-Mediated Activation of Nrf2 on Non-Alcoholic Fatty Liver Disease with a Focus on Mitochondrial Dysfunction. Int. J. Mol. Sci. 2019, 20, 5920. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Santos, G.O.; Machado, N.M.; Otoboni, A.; Laurindo, L.F.; Bishayee, A.; Fimognari, C.; Bishayee, A.; Barbalho, S.M. Peanut (Arachis hypogaea L.) seeds and by-products in metabolic syndrome and cardiovascular disorders: A systematic review of clinical studies. Phytomed. Int. J. Phytother. Phytopharm. 2024, 123, 155170. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Llavero, C.; Rodriguez-Ortega, M.; De Castro, N.M.; Martín-Crespo, M.C.; Escobar-Aguilar, G.; Martin-Nieto, A.; Gonzalez, G. Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients 2024, 16, 2280. [Google Scholar] [CrossRef]
- Beygi, M.; Ahi, S.; Zolghadri, S.; Stanek, A. Management of Metabolic-Associated Fatty Liver Disease/Metabolic Dysfunction-Associated Steatotic Liver Disease: From Medication Therapy to Nutritional Interventions. Nutrients 2024, 16, 2220. [Google Scholar] [CrossRef]
- Keating, S.E.; Chawla, Y.; De, A.; George, E.S. Lifestyle intervention for metabolic dysfunction-associated fatty liver disease: A 24-h integrated behavior perspective. Hepatol. Int. 2024, 18, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Minniti, G.; Laurindo, L.F.; Machado, N.M.; Duarte, L.G.; Guiguer, E.L.; Araujo, A.C.; Dias, J.A.; Lamas, C.B.; Nunes, Y.C.; Bechara, M.D.; et al. Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life 2023, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Collignon, T.E.; Webber, K.; Piasecki, J.; Rahman, A.S.W.; Mondal, A.; Barbalho, S.M.; Bishayee, A. Avocado (Persea americana Mill) and its phytoconstituents: Potential for cancer prevention and intervention. Crit. Rev. Food Sci. Nutr. 2023, 1–21. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Figueira, M.E.; Minniti, G.; de Carvalho, G.M.; de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; de Góes Corrêa, N.; Rodrigues, V.D.; de Alvares Goulart, R.; et al. Medicinal Plants, Phytochemicals and Regulation of the NLRP3 Inflammasome in Inflammatory Bowel Diseases: A Comprehensive Review. Metabolites 2023, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, É.L.; Nicolau, C.C.T.; Goulart, R.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Direito, R.; Bueno Otoboni, A.M.; Goulart, R.A.; Quesada, K.; Barbalho, S.M. Grape processing waste: Effects on inflammatory bowel disease and colorectal cancer. Food Rev. Int. 2024, 40, 336–369. [Google Scholar] [CrossRef]
- Bosso, H.; Barbalho, S.M.; de Alvares Goulart, R.; Otoboni, A. Green coffee: Economic relevance and a systematic review of the effects on human health. Crit. Rev. Food Sci. Nutr. 2023, 63, 394–410. [Google Scholar] [CrossRef]
- Bosso, H.; Soares Arantes, G.E.P.; Barbalho, S.M.; Guiguer, É.L.; de Souza, M.; Bueno, P.; Chies, A.B.; Oliveira, P.B.; Mendes, C.G.; Araújo, A.C. Effects of Green and Ripe Coffee in the Metabolic Profile and Muscle Enzymes in Animals Practicing Physical Exercise. J. Med. Food 2019, 22, 416–420. [Google Scholar] [CrossRef]
- Paravati, M.R.; Procopio, A.C.; Milanović, M.; Scarlata, G.G.M.; Milošević, N.; Ružić, M.; Milić, N.; Abenavoli, L. Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study. Nutrients 2024, 16, 1226. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Pawase, P.A.; Shams, R.; Pandey, V.K.; Tripathi, A.; Rustagi, S.; Darshan, G. A Conceptual Review on Classification, Extraction, Bioactive Potential and Role of Phytochemicals in Human Health. Future Foods 2024, 9, 100313. [Google Scholar]
- Kung, H.C.; Lin, K.J.; Kung, C.T.; Lin, T.K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Lone, Z.R.; Bharati, P.; Mahapatra, S.; Rai, P.; Khandelwal, N.; Gaikwad, A.N.; Jagavelu, K.; Hanif, K. Pyrroloquinoline quinone (PQQ) improves pulmonary hypertension by regulating mitochondrial and metabolic functions. Pulm. Pharmacol. Ther. 2022, 76, 102156. [Google Scholar] [CrossRef]
- Zou, X.; Yan, C.; Shi, Y.; Cao, K.; Xu, J.; Wang, X.; Chen, C.; Luo, C.; Li, Y.; Gao, J.; et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid Redox Signal 2014, 21, 1557–1570. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxid Med. Cell Longev. 2019, 2019, 8409329. [Google Scholar] [CrossRef]
- Yong, Z.; Ruiqi, W.; Hongji, Y.; Ning, M.; Chenzuo, J.; Yu, Z.; Zhixuan, X.; Qiang, L.; Qibing, L.; Weiying, L.; et al. Mangiferin Ameliorates HFD-Induced NAFLD through Regulation of the AMPK and NLRP3 Inflammasome Signal Pathways. J. Immunol. Res. 2021, 2021, 4084566. [Google Scholar] [CrossRef]
- Jinato, T.; Chayanupatkul, M.; Dissayabutra, T.; Chutaputti, A.; Tangkijvanich, P.; Chuaypen, N. Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients 2022, 14, 2921. [Google Scholar] [CrossRef]
- Fang, X.; Cao, J.; Tao, Z.; Yang, Z.; Dai, Y.; Zhao, L. Hydroxytyrosol attenuates ethanol-induced liver injury by ameliorating steatosis, oxidative stress and hepatic inflammation by interfering STAT3/iNOS pathway. Redox Rep. Commun. Free. Radic. Res. 2023, 28, 2187564. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Y.; Di, C.; Zeng, Y.; Zhou, Y.; Chen, Y.; Pan, Z.; Li, Z.; Ling, W. Supplementation of Silymarin Alone or in Combination with Salvianolic Acids B and Puerarin Regulates Gut Microbiota and Its Metabolism to Improve High-Fat Diet-Induced NAFLD in Mice. Nutrients 2024, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, L.; Xiong, Z.; Huang, C.; Yong, Q.; Fang, D.; Fu, Y.; Gu, S.; Chen, C.; Li, J.; et al. Ursolic acid targets secreted phosphoprotein 1 to regulate Th17 cells against non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2024, 30, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, W.; Zhang, G.; Liu, L.; Wang, L.; Wu, D.; Tao, J.; Yue, H.; Zhang, D.; Zhao, X. Extract of Silphium perfoliatum L. improve lipid accumulation in NAFLD mice by regulating AMPK/FXR signaling pathway. J. Ethnopharmacol. 2024, 327, 118054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Q.; Yang, X.; Zhang, Z.; Wang, D.; Hu, D.; Huang, Y.; Sheng, J.; Wang, X. Gallic Acid Can Promote Low-Density Lipoprotein Uptake in HepG2 Cells via Increasing Low-Density Lipoprotein Receptor Accumulation. Molecules 2024, 29, 1999. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Chen, M.; Zuo, Y.; Liu, F.; Yang, Y.; Li, J.; Zhou, X.; Li, M.; Huang, J.A.; Liu, Z.; et al. Tea Polyphenol Epigallocatechin Gallate Protects Against Nonalcoholic Fatty Liver Disease and Associated Endotoxemia in Rats via Modulating Gut Microbiota Dysbiosis and Alleviating Intestinal Barrier Dysfunction and Related Inflammation. J. Agric. Food Chem. 2024, 72, 9067–9086. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Keramat, M.; Golmakani, M.T. Effects of rosmarinic acid esters on the oxidation kinetic of organogel and emulsion gel. Food Chem. X 2024, 22, 101343. [Google Scholar] [CrossRef]
- Yamamura, S.; Eslam, M.; Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Yoshinaga, S.; Takahashi, H.; Anzai, K.; George, J.; Torimura, T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020, 40, 3018–3030. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Casares, M.; Gómez, C.; Ugarriza, L.; Tur, J.A.; Sureda, A. Effects of a Two-Year Lifestyle Intervention on Intrahepatic Fat Reduction and Renal Health: Mitigation of Inflammation and Oxidative Stress, a Randomized Trial. Antioxidants 2024, 13, 754. [Google Scholar] [CrossRef]
- Zhou, X.D.; Cai, J.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Sung, K.C.; Somers, V.K.; Chahal, C.A.A.; George, J.; Chen, L.L.; et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022, 21, 270. [Google Scholar] [CrossRef]
- Koperska, A.; Moszak, M.; Seraszek-Jaros, A.; Bogdanski, P.; Szulinska, M. Does berberine impact anthropometric, hepatic, and metabolic parameters in patients with metabolic dysfunction-associated fatty liver disease? Randomized, double-blind placebo-controlled trial. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2024, 75, 291. [Google Scholar] [CrossRef]
- Vaz, K.; Kemp, W.; Majeed, A.; Lubel, J.; Magliano, D.J.; Glenister, K.M.; Bourke, L.; Simmons, D.; Roberts, S.K. NAFLD and MAFLD independently increase the risk of major adverse cardiovascular events (MACE): A 20-year longitudinal follow-up study from regional Australia. Hepatol. Int. 2024, 18, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xiao, X.; Deng, S.; Kuang, J.; Li, Y. HRD1-mediated ubiquitination of HDAC2 regulates PPARα-mediated autophagy and alleviates metabolic-associated fatty liver disease. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119765. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Charbe, N.B.; Lagos, C.F.; Ortiz, C.A.V.; Tambuwala, M.; Palakurthi, S.S.; Zacconi, F.C. PCSK9 conjugated liposomes for targeted delivery of paclitaxel to the cancer cell: A proof-of-concept study. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 153, 113428. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Christensen, J.J.; Formo, G.A.; Holven, K.B.; Ulven, S.M. A comprehensive metabolic profiling of the metabolically healthy obesity phenotype. Lipids Health Dis. 2020, 19, 90. [Google Scholar] [CrossRef]
- Wang, C.H.; Wei, Y.H. Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5266. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Yan, L.; Wu, Y.; Zhang, L.; Li, B.; Tong, H.; Lin, X. Molecular Mechanisms of Fucoxanthin in Alleviating Lipid Deposition in Metabolic Associated Fatty Liver Disease. J. Agric. Food Chem. 2024, 72, 10391–10405. [Google Scholar] [CrossRef]
- Jin, C.; Felli, E.; Lange, N.F.; Berzigotti, A.; Gracia-Sancho, J.; Dufour, J.F. Endoplasmic Reticulum and Mitochondria Contacts Correlate with the Presence and Severity of NASH in Humans. Int. J. Mol. Sci. 2022, 23, 8348. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Wang, Z.; Liu, Z.; Yi, X.; Wu, J. Mettl3-m6A-YTHDF1 axis promotion of mitochondrial dysfunction in metabolic dysfunction-associated steatotic liver disease. Cell. Signal. 2024, 121, 111303. [Google Scholar] [CrossRef]
- Anwar, S.D.; Foster, C.; Ashraf, A. Lipid Disorders and Metabolic-Associated Fatty Liver Disease. Endocrinol. Metab. Clin. N. Am. 2023, 52, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Clare, K.; Dillon, J.F.; Brennan, P.N. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022, 10, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Lința, A.V.; Lolescu, B.M.; Ilie, C.A.; Vlad, M.; Blidișel, A.; Sturza, A.; Borza, C.; Muntean, D.M.; Crețu, O.M. Liver and Pancreatic Toxicity of Endocrine-Disruptive Chemicals: Focus on Mitochondrial Dysfunction and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 7420. [Google Scholar] [CrossRef]
- Chi, W.Y.; Lee, G.H.; Tang, M.J.; Chen, B.H.; Lin, W.L.; Fu, T.F. Disturbed intracellular folate homeostasis impairs autophagic flux and increases hepatocytic lipid accumulation. BMC Biol. 2024, 22, 146. [Google Scholar] [CrossRef]
- Mollet, I.G.; Macedo, M.P. Pre-Diabetes-Linked miRNA miR-193b-3p Targets PPARGC1A, Disrupts Metabolic Gene Expression Profile and Increases Lipid Accumulation in Hepatocytes: Relevance for MAFLD. Int. J. Mol. Sci. 2023, 24, 3875. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, P.; Lu, S.; Ma, W. METTL3 inhibitor STM2457 improves metabolic dysfunction-associated fatty liver disease by regulating mitochondrial function in mice. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2023, 43, 1689–1696. [Google Scholar] [CrossRef]
- Wallace, M.; Metallo, C.M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 2020, 108, 65–71. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metab. Clin. Exp. 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Talari, N.K.; Mattam, U.; Kaminska, D.; Sotomayor-Rodriguez, I.; Rahman, A.P.; Péterfy, M.; Pajukanta, P.; Pihlajamäki, J.; Chella Krishnan, K. Hepatokine ITIH3 protects against hepatic steatosis by downregulating mitochondrial bioenergetics and de novo lipogenesis. iScience 2024, 27, 109709. [Google Scholar] [CrossRef]
- Yao, W.; Fan, M.; Qian, H.; Li, Y.; Wang, L. Quinoa Polyphenol Extract Alleviates Non-Alcoholic Fatty Liver Disease via Inhibiting Lipid Accumulation, Inflammation and Oxidative Stress. Nutrients 2024, 16, 2276. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, J.; Xie, W.; Ni, Y.; Fang, X.; Liu, M.; Peng, X.; Wang, J.; Dai, Y.; Zhou, Y. Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018. Nutrients 2022, 14, 4505. [Google Scholar] [CrossRef] [PubMed]
- Reis-Costa, A.; Belew, G.D.; Viegas, I.; Tavares, L.C.; Meneses, M.J.; Patrício, B.; Gastaldelli, A.; Macedo, M.P.; Jones, J.G. The Effects of Long-Term High Fat and/or High Sugar Feeding on Sources of Postprandial Hepatic Glycogen and Triglyceride Synthesis in Mice. Nutrients 2024, 16, 2186. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; La Russa, D.; Lascala, A.; Crupi, F.; Riillo, C.; Fotschki, B.; Mollace, V.; Janda, E. Dramatic Suppression of Lipogenesis and No Increase in Beta-Oxidation Gene Expression Are among the Key Effects of Bergamot Flavonoids in Fatty Liver Disease. Antioxidants 2024, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.; Lofaro, D.; Lupinacci, S.; Toteda, G.; Curti, A.; Urso, A.; Bonofiglio, R.; Pellegrino, D.; Brunetti, A.; Greco, E.; et al. Proinflammatory profile of visceral adipose tissue and oxidative stress in severe obese patients carrying the variant rs4612666 C of NLRP3 gene. Minerva Endocrinol. 2021, 46, 309–316. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Q.; Li, R.; Seshadri, V.D. Nigericin Abrogates Maternal and Embryonic Oxidative Stress in the Streptozotocin-Induced Diabetic Pregnant Rats. Appl. Biochem. Biotechnol. 2023, 195, 801–815. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Chen, S.; Dli, H.; Zhao, J.; Sun, X.; Yang, P.; Mao, Q.; Xia, H. BI-7273, a BRD9 inhibitor, reduces lipid accumulation by downregulating the AKT/mTOR/SREBP1 signaling pathway. Biochem. Pharmacol. 2024, 226, 116412. [Google Scholar] [CrossRef]
- Zhang, Z.; TeSlaa, T.; Xu, X.; Zeng, X.; Yang, L.; Xing, G.; Tesz, G.J.; Clasquin, M.F.; Rabinowitz, J.D. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat. Metab. 2021, 3, 1608–1620. [Google Scholar] [CrossRef]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef]
- Ding, L.; Sun, W.; Balaz, M.; He, A.; Klug, M.; Wieland, S.; Caiazzo, R.; Raverdy, V.; Pattou, F.; Lefebvre, P.; et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat. Metab. 2021, 3, 1648–1661. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Meng, Y.; Song, X.; Li, X.; Liu, Z.; Gu, C.; Zheng, X.; Jing, Y.; Cai, W.; Pinyopornpanish, K.; et al. Artificial Intelligence in Liver Diseases: Recent Advances. Adv. Ther. 2024, 41, 967–990. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Zheng, J.; Wang, M.; Goh, G.B.; Lin, S. The prognostic role of diet quality in patients with MAFLD and physical activity: Data from NHANES. Nutr. Diabetes 2024, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Liu, J.; Peng, H.; Wu, X.; Zheng, X.; Yao, S.; Xu, C. Dietary composition and its association with metabolic dysfunction-associated fatty liver disease among Chinese adults: A cross-sectional study. Arab. J. Gastroenterol. Off. Publ. Pan-Arab. Assoc. Gastroenterol. 2024, 25, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ohene-Marfo, P.; Nguyen, H.V.M.; Mohammed, S.; Thadathil, N.; Tran, A.; Nicklas, E.H.; Wang, D.; Selvarani, R.; Farriester, J.; Varshney, R.; et al. Non-Necroptotic Roles of MLKL in Diet-Induced Obesity, Liver Pathology, and Insulin Sensitivity: Insights from a High Fat, High Fructose, High Cholesterol Diet Mouse Model. Int. J. Mol. Sci. 2024, 25, 2813. [Google Scholar] [CrossRef]
- Xia, Q.; Lu, F.; Chen, Y.; Li, J.; Huang, Z.; Fang, K.; Hu, M.; Guo, Y.; Dong, H.; Xu, L.; et al. 6-Gingerol regulates triglyceride and cholesterol biosynthesis to improve hepatic steatosis in MAFLD by activating the AMPK-SREBPs signaling pathway. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 170, 116060. [Google Scholar] [CrossRef]
- Zhu, X.; Qucuo, N.; Zhang, N.; Tang, D.; Hu, Y.; Xie, X.; Zhao, X.; Meng, Q.; Chen, L.; Jiang, X.; et al. Dietary patterns and metabolic dysfunction-associated fatty liver disease in China’s multi-ethnic regions. J. Health Popul. Nutr. 2023, 42, 141. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Z.; Yang, L.; Zheng, W.; Chen, Y.; Qu, F.; Crabbe, M.J.C.; Zhang, Y.; Andersen, M.E.; Zheng, Y.; et al. Disinfection Byproducts of Haloacetaldehydes Disrupt Hepatic Lipid Metabolism and Induce Lipotoxicity in High-Fat Culture Conditions. Environ. Sci. Technol. 2024, 58, 12356–12367. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, S.J.; Lee, K.J.; Song, A.R.; Park, H.J.; Kang, J.S.; Cha, J.Y.; Han, Y.H. The Root Extract of Rosa multiflora Ameliorates Nonalcoholic Steatohepatitis Development via Blockade of De Novo Lipogenesis and Inflammation. Curr. Issues Mol. Biol. 2024, 46, 5881–5893. [Google Scholar] [CrossRef]
- Boleti, A.P.D.A.; Cardoso, P.H.D.O.; Frihling, B.E.F.; e Silva, P.S.; de Moraes, L.F.R.; Migliolo, L. Adipose tissue, systematic inflammation, and neurodegenerative diseases. Neural Regen. Res. 2023, 18, 38–46. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.F.; Ruan, X.Z.; Lau, C.W.; Wang, L.; Huang, Y. The role of KLF2 in regulating hepatic lipogenesis and blood cholesterol homeostasis via the SCAP/SREBP pathway. J. Lipid Res. 2024, 65, 100472. [Google Scholar] [CrossRef] [PubMed]
- Angelico, F.; Baratta, F.; Coronati, M.; Ferro, D.; Del Ben, M. Diet and metabolic syndrome: A narrative review. Intern. Emerg. Med. 2023, 18, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; El-Shabrawi, M.; Baur, L.A.; Byrne, C.D.; Targher, G.; Kehar, M.; Porta, G.; Lee, W.S.; Lefere, S.; Turan, S.; et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med 2024, 5, 797–815.E2. [Google Scholar] [CrossRef] [PubMed]
- Medina-Julio, D.; Ramírez-Mejía, M.M.; Cordova-Gallardo, J.; Peniche-Luna, E.; Cantú-Brito, C.; Mendez-Sanchez, N. From Liver to Brain: How MAFLD/MASLD Impacts Cognitive Function. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e943417. [Google Scholar] [CrossRef]
- Guo, W.; Ge, X.; Lu, J.; Xu, X.; Gao, J.; Wang, Q.; Song, C.; Zhang, Q.; Yu, C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients 2022, 14, 5335. [Google Scholar] [CrossRef]
- Tang, L.J.; Li, G.; Eslam, M.; Zhu, P.W.; Chen, S.D.; Leung, H.H.; Huang, O.Y.; Wong, G.L.; Zhou, Y.J.; Karsdal, M.; et al. N-terminal propeptide of type 3 collagen-based sequential algorithm can identify high-risk steatohepatitis and fibrosis in MAFLD. Hepatol. Int. 2023, 17, 190–201. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Vali, Y.; Alzoubi, O.; Staufer, K.; Trauner, M.; Paternostro, R.; Stauber, R.E.; Holleboom, A.G.; van Dijk, A.M.; et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: An individual participant data meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 704–713. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Choudhury, A.; Arora, V.; Rela, M.; Jothimani, D.K.; Mahtab, M.A.; Devarbhavi, H.; Eapen, C.E.; Goel, A.; et al. Impact of Diabetes, Drug-Induced Liver Injury, and Sepsis on Outcomes in MAFLD-Related Acute-on-Chronic Liver Failure. Am. J. Gastroenterol. 2024. [Google Scholar] [CrossRef]
- Patel, V.S.; Mahmood, S.F.; Bhatt, K.H.; Khemkar, R.M.; Jariwala, D.R.; Harris, B.; George, M.M.; Kurudamannil, R.A.; Anyagwa, O.E.; Tak, R.S.; et al. Ursodeoxycholic Acid’s Effectiveness in the Management of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Euroasian J. Hepato-Gastroenterol. 2024, 14, 92–98. [Google Scholar] [CrossRef]
- Naskar, A.; Mondal, A.; Chatterjee, R.; De, R.D.; Roy, S. Assessing Liver Fibrosis in Type 2 Diabetes Mellitus Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: The Role of Non-invasive Scoring Systems and Associated Factors. Cureus 2024, 16, e62405. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Chou, L.J.; Huang, S.C.; Chu, T.W.; Lee, S.S. Machine learning-based comparison of factors influencing estimated glomerular filtration rate in Chinese women with or without non-alcoholic fatty liver. World J. Clin. Cases 2024, 12, 2506–2521. [Google Scholar] [CrossRef] [PubMed]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.M.; Gilmanshin, T.R.; Zainullin, R.M.; Kazakbaeva, G.M.; Iakupova, E.M.; Fakhretdinova, A.A.; Tuliakova, A.M.; Panda-Jonas, S.; Gilemzianova, L.I.; Khakimov, D.A.; et al. Prevalence of non-alcoholic fatty liver disease in the Russian Ural Eye and Medical Study and the Ural Very Old Study. Sci. Rep. 2022, 12, 7842. [Google Scholar] [CrossRef] [PubMed]
- Labayen, I.; Cadenas-Sánchez, C.; Idoate, F.; Medrano, M.; Tobalina, I.; Villanueva, A.; Rodríguez-Vigil, B.; Álvarez de Eulate, N.; Osés, M.; Cabeza, R. Liver Fat, Bone Marrow Adipose Tissue, and Bone Mineral Density in Children with Overweight. J. Clin. Endocrinol. Metab. 2023, 109, e253–e258. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Ruli, T.; Buttar, D.; Shoreibah, M.; Gray, M. Metabolic dysfunction associated steatotic liver disease: Current practice, screening guidelines and management in the primary care setting. Am. J. Med. Sci. 2024, 367, 77–88. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Bishayee, A.; Kavalakatt, J.; Sunkara, C.; Johnson, O.; Zinzuwadia, S.S.; Collignon, T.E.; Banerjee, S.; Barbalho, S.M. Litchi (Litchi chinensis Sonn.): A comprehensive and critical review on cancer prevention and intervention. Food Chem. 2024, 457, 140142. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Fujihara, K.; Yamada-Harada, M.; Mitsuma, Y.; Sato, T.; Yaguchi, Y.; Osawa, T.; Yamamoto, M.; Kitazawa, M.; Yamada, T.; et al. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc. Diabetol. 2022, 21, 90. [Google Scholar] [CrossRef]
- Banerjee, T.; Sarkar, A.; Ali, S.Z.; Bhowmik, R.; Karmakar, S.; Halder, A.K.; Ghosh, N. Bioprotective Role of Phytocompounds Against the Pathogenesis of Non-alcoholic Fatty Liver Disease to Non-alcoholic Steatohepatitis: Unravelling Underlying Molecular Mechanisms. Planta Medica 2024, 90, 675–707. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Sandeman, S.; Dymond, M.; Rogers, C.; Ostler, E.L.; Faragher, R.G. Resveralogues protect HepG2 cells against cellular senescence induced by hepatotoxic metabolites. Mech. Ageing Dev. 2024, 219, 111938. [Google Scholar] [CrossRef] [PubMed]
- Bueno, P.; Barbalho, S.M.; Guiguer, É.L.; Souza, M.; Medeiros, I.R.A.; Zattiti, I.V.; Bueno, M.D.S.; Nutels, G.S.; Goulart, R.A.; Araújo, A.C. Effects of Green Wheat (Triticum turgidum) and Common Wheat (Triticum aestivum) on the Metabolic Profile of Wistar Rats. J. Med. Food 2019, 22, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Bang, V.M.J.; Aranão, A.L.C.; Nogueira, B.Z.; Araújo, A.C.; Bueno, P.; Barbalho, S.M.; de Souza, M.; Guiguer, E.L. Effects of Rhodiola rosea and Panax ginseng on the Metabolic Parameters of Rats Submitted to Swimming. J. Med. Food 2019, 22, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Li, Z.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol. Cell 2021, 81, 691–707.e696. [Google Scholar] [CrossRef]
- Bar, S.; Kara, M. Linalool exerts antioxidant activity in a rat model of diabetes by increasing catalase activity without antihyperglycemic effect. Exp. Ther. Med. 2024, 28, 359. [Google Scholar] [CrossRef]
- Thongrong, S.; Promsrisuk, T.; Sriraksa, N.; Surapinit, S.; Jittiwat, J.; Kongsui, R. Alleviative effect of scopolamine-induced memory deficit via enhancing antioxidant and cholinergic function in rats by pinostrobin from Boesenbergia rotunda (L.). Biomed. Rep. 2024, 21, 130. [Google Scholar] [CrossRef]
- Saka, W.A.; Adeogun, A.E.; Adisa, V.I.; Olayioye, A.; Igbayilola, Y.D.; Akhigbe, R.E. L-arginine attenuates dichlorvos-induced testicular toxicity in male Wistar rats by suppressing oxidative stress-dependent activation of caspase 3-mediated apoptosis. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 178, 117136. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kurokawa, H.; Tokinoya, K.; Matsui, H. Monascus pigment prevent the oxidative cytotoxicity in myotube derived hydrogen peroxide. J. Clin. Biochem. Nutr. 2024, 75, 33–39. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, L.; Fang, X.; Du, S.; Mauck, J.; Loor, J.J.; Sun, X.; Jia, H.; Xu, C.; Xu, Q. Role of hypoxia-inducible-factor-1α (HIF-1α) in ferroptosis of adipose tissue during ketosis. J. Dairy Sci. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Y.; Du, H.; Lu, Y.; Jiang, Y.; Ding, M.; Sheng, X. Vascular endothelial cells of Mongolian gerbils are resistant to cholesterol-induced mitochondrial dysfunction and oxidative damage. Exp. Ther. Med. 2024, 28, 356. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Imai, H. Oxidative Metabolism as a Cause of Lipid Peroxidation in the Execution of Ferroptosis. Int. J. Mol. Sci. 2024, 25, 7544. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, L.; Jiang, X.; Wang, J. Glutathione dynamics in subcellular compartments and implications for drug development. Curr. Opin. Chem. Biol. 2024, 81, 102505. [Google Scholar] [CrossRef] [PubMed]
- Wołosowicz, M.; Dajnowicz-Brzezik, P.; Łukaszuk, B.; Żebrowska, E.; Maciejczyk, M.; Zalewska, A.; Kasacka, I.; Chabowski, A. Diverse impact of N-acetylcysteine or alpha-lipoic acid supplementation during high-fat diet regime on fatty acid transporters in visceral and subcutaneous adipose tissue. Adv. Med. Sci. 2022, 67, 216–228. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, Z.; Zhang, H.; Wang, P.; Wang, F.; Zhang, J. Pea Albumin Extracted from Pea (Pisum sativum L.) Seeds Ameliorates High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease by Regulating Lipogenesis and Lipolysis Pathways. Nutrients 2024, 16, 2232. [Google Scholar] [CrossRef]
- Tsou, S.H.; Lin, S.C.; Chen, W.J.; Hung, H.C.; Liao, C.C.; Kornelius, E.; Huang, C.N.; Lin, C.L.; Yang, Y.S. Hydrogen-Rich Water (HRW) Reduces Fatty Acid-Induced Lipid Accumulation and Oxidative Stress Damage through Activating AMP-Activated Protein Kinase in HepG2 Cells. Biomedicines 2024, 12, 1444. [Google Scholar] [CrossRef]
- Wang, T.; Tyler, R.E.; Ilaka, O.; Cooper, D.; Farokhnia, M.; Leggio, L. The crosstalk between fibroblast growth factor 21 (FGF21) system and substance use. iScience 2024, 27, 110389. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A. Adipose organ dysfunction and type 2 diabetes: Role of nitric oxide. Biochem. Pharmacol. 2024, 221, 116043. [Google Scholar] [CrossRef]
- Sapatini, L.R.L.; Calsa, B.; Marim, L.J.; Helaehil, J.V.; Chiarotto, G.B.; Corezola do Amaral, M.E. Caloric restriction prevents inflammation and insulin dysfunction in middle-aged ovariectomized mice. Mol. Biol. Rep. 2023, 50, 5675–5685. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Zou, X.; Liu, S.; Guan, C.; Shi, W.; Gao, J.; Zhong, X.; Jiang, X. Forkhead box O1 in metabolic dysfunction-associated fatty liver disease: Molecular mechanisms and drug research. Front. Nutr. 2024, 11, 1426780. [Google Scholar] [CrossRef] [PubMed]
- Imi, Y.; Ogawa, W.; Hosooka, T. Insulin resistance in adipose tissue and metabolic diseases. Diabetol. Int. 2023, 14, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A. Alanine aminotransferase predicts incident steatotic liver disease of metabolic etiology: Long life to the old biomarker! World J. Gastroenterol. 2024, 30, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Trochimczyk, K.; Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Lebensztejn, A.; Wojtkowska, M.; Jamiołkowski, J.; Lebensztejn, D.M. Biochemical and Anthropometric Indices of Insulin Resistance in Obese and Overweight Children with Metabolic Dysfunction-Associated Fatty Liver Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e943375. [Google Scholar] [CrossRef]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease-The Transition from an Adipocentric to Liver-Centric Approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef]
- Wang, W.; Guo, X.L.; Qiu, X.P.; Yu, Y.J.; Tu, M. Systemic immune-inflammation index mediates the association between metabolic dysfunction-associated fatty liver disease and sub-clinical carotid atherosclerosis: A mediation analysis. Front. Endocrinol. 2024, 15, 1406793. [Google Scholar] [CrossRef]
- He, Q.; He, W.; Dong, H.; Guo, Y.; Yuan, G.; Shi, X.; Wang, D.; Lu, F. Role of liver sinusoidal endothelial cell in metabolic dysfunction-associated fatty liver disease. Cell Commun. Signal. CCS 2024, 22, 346. [Google Scholar] [CrossRef]
- Pal, S.C.; Méndez-Sánchez, N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J. Gastroenterol. 2023, 29, 3999–4008. [Google Scholar] [CrossRef]
- Castro-Sepulveda, M.; Fernández-Verdejo, R.; Zbinden-Foncea, H.; Rieusset, J. Mitochondria-SR interaction and mitochondrial fusion/fission in the regulation of skeletal muscle metabolism. Metab. Clin. Exp. 2023, 144, 155578. [Google Scholar] [CrossRef]
- Yang, C.; Yu, Y.; An, J. Effect of High-Sucrose Diet on the Occurrence and Progression of Diabetic Retinopathy and Dietary Modification Strategies. Nutrients 2024, 16, 1393. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; El Zahraa Sayed Bokhary, F.; Ismail, S.; Mahmoud Abd El Hameed, W. Predictive value of the glycated albumin versus glycosylated hemoglobin in follow-up of glucose homeostasis in hemodialysis-maintained type-2 diabetic patients. Endocr. Regul. 2022, 56, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Walke, P.B.; Bansode, S.B.; More, N.P.; Chaurasiya, A.H.; Joshi, R.S.; Kulkarni, M.J. Molecular investigation of glycated insulin-induced insulin resistance via insulin signaling and AGE-RAGE axis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166029. [Google Scholar] [CrossRef] [PubMed]
- Legaki, A.I.; Moustakas, I.I.; Sikorska, M.; Papadopoulos, G.; Velliou, R.I.; Chatzigeorgiou, A. Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease. Curr. Obes. Rep. 2022, 11, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Verma, H.K.; Lakkakula, S.; Merchant, N.; Kadir, F.; Rahman, S.; Jeffree, M.S.; Lakkakula, B.; Rao, P.V. Biomarkers of Oxidative Stress Tethered to Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 9154295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. = Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Wu, D.; Eeda, V.; Maria, Z.; Rawal, K.; Herlea-Pana, O.; Undi, R.B.; Lim, H.Y.; Wang, W. Targeting IRE1α improves insulin sensitivity and thermogenesis and suppresses metabolically active adipose tissue macrophages in obesity. bioRxiv 2024. [Google Scholar] [CrossRef]
- Alam, N.; Jia, L.; Cheng, A.; Ren, H.; Fu, Y.; Ding, X.; Haq, I.U.; Liu, E. Global research trends on gut microbiota and metabolic dysfunction-associated steatohepatitis: Insights from bibliometric and scientometric analysis. Front. Pharmacol. 2024, 15, 1390483. [Google Scholar] [CrossRef]
- Ding, Z.; Pothineni, N.V.K.; Goel, A.; Lüscher, T.F.; Mehta, J.L. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 2020, 116, 908–915. [Google Scholar] [CrossRef]
- Wang, D.; Baghoomian, A.; Zhang, Z.; Cui, Y.; Whang, E.C.; Li, X.; Fraga, J.; Spellman, R.; Dong, T.S.; Li, W.; et al. Hepatic lipopolysaccharide binding protein partially uncouples inflammation from fibrosis in MAFLD. J. Clin. Investig. 2024, 134, e179752. [Google Scholar] [CrossRef]
- Rajendran, R.; Suman, S.; Divakaran, S.J.; Swatikrishna, S.; Tripathi, P.; Jain, R.; Sagar, K.; Rajakumari, S. Sesaminol alters phospholipid metabolism and alleviates obesity-induced NAFLD. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23835. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Marjanovic-Haljilji, M.; Mijac, D.; Lukic, S.; Kapor, S.; Kapor, S.; Starcevic, A.; Popovic, D.; Djokovic, A. Molecular Aspects of MAFLD-New Insights on Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2023, 45, 9132–9148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Xiao, J.; Wang, H.; Guo, Y.; Mao, X.; Shi, P.; Hou, Y.; Zhang, X.; Zhao, N.; et al. Unique DUOX2(+)ACE2(+) small cholangiocytes are pathogenic targets for primary biliary cholangitis. Nat. Commun. 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Biao, Y.; Li, D.; Zhang, Y.; Gao, J.; Xiao, Y.; Yu, Z.; Li, L. Wulingsan Alleviates MAFLD by Activating Autophagy via Regulating the AMPK/mTOR/ULK1 Signaling Pathway. Can. J. Gastroenterol. Hepatol. 2024, 2024, 9777866. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; McCaughan, G.; Grønbæk, H. Role of gut microbiota and immune cells in metabolic-associated fatty liver disease: Clinical impact. Hepatol. Int. 2024, 18, 861–872. [Google Scholar] [CrossRef]
- Cheng, Z.; Chu, H.; Seki, E.; Lin, R.; Yang, L. Hepatocyte programmed cell death: The trigger for inflammation and fibrosis in metabolic dysfunction-associated steatohepatitis. Front. Cell Dev. Biol. 2024, 12, 1431921. [Google Scholar] [CrossRef]
- Malladi, N.; Lahamge, D.; Somwanshi, B.S.; Tiwari, V.; Deshmukh, K.; Balani, J.K.; Chakraborty, S.; Alam, M.J.; Banerjee, S.K. Paricalcitol attenuates oxidative stress and inflammatory response in the liver of NAFLD rats by regulating FOXO3a and NFκB acetylation. Cell. Signal. 2024, 121, 111299. [Google Scholar] [CrossRef]
- Orhan, S.; Turkmen, R.; Demirel, H.H.; Akosman, M.S.; Turkmen, T.; Fırat, F. Chlorogenic acid mitigates potassium dichromate-induced acute hepato-nephrotoxicity by attenuating the NF-κB signalling pathway. Mol. Biol. Rep. 2024, 51, 798. [Google Scholar] [CrossRef]
- Hao, J.; Jin, X.; Li, Z.; Zhu, Y.; Wang, L.; Jiang, X.; Wang, D.; Qi, L.; Jia, D.; Gao, B. Anti-Obesity Activity of Sanghuangporus vaninii by Inhibiting Inflammation in Mice Fed a High-Fat Diet. Nutrients 2024, 16, 2159. [Google Scholar] [CrossRef]
- Elsayed Abouzed, D.E.; Ezelarab, H.A.A.; Selim, H.; Elsayed, M.M.A.; El Hamd, M.A.; Aboelez, M.O. Multimodal modulation of hepatic ischemia/reperfusion-induced injury by phytochemical agents: A mechanistic evaluation of hepatoprotective potential and safety profiles. Int. Immunopharmacol. 2024, 138, 112445. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Yang, L.; Guo, R. Alkannin reverses lipopolysaccharides-induced inflammatory responses by suppressing mitogen-activated protein kinase and nuclear factor kappa-B signalling. Bioengineered 2022, 13, 14936–14946. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.R. TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism. Front. Endocrinol. 2020, 11, 613639. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Chu, Q.; Ma, X.; Wang, J.; Chen, C.; Guan, J.; Ren, Y.; Wu, S.; Zhu, H. New insights into iNKT cells and their roles in liver diseases. Front. Immunol. 2022, 13, 1035950. [Google Scholar] [CrossRef]
- Akkız, H.; Gieseler, R.K.; Canbay, A. Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells. Int. J. Mol. Sci. 2024, 25, 7873. [Google Scholar] [CrossRef]
- Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Potup, P.; Thongsri, Y.; Daowtak, K.; Ferrante, A.; Usuwanthim, K. Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model. Biomolecules 2024, 14, 800. [Google Scholar] [CrossRef]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.F.; Li, Y.; Gupta, V.; Li, C.; et al. CD8(+) T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef]
- Sarmadi, N.; Poustchi, H.; Ali Yari, F.; Radmard, A.R.; Karami, S.; Pakdel, A.; Shabani, P.; Khaleghian, A. Anti-inflammatory function of apolipoprotein B-depleted plasma is impaired in non-alcoholic fatty liver disease. PLoS ONE 2022, 17, e0266227. [Google Scholar] [CrossRef]
- Convertini, P.; Santarsiero, A.; Todisco, S.; Gilio, M.; Palazzo, D.; Pappalardo, I.; Iacobazzi, D.; Frontuto, M.; Infantino, V. ACLY as a modulator of liver cell functions and its role in Metabolic Dysfunction-Associated Steatohepatitis. J. Transl. Med. 2023, 21, 568. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, K.; Wang, X.; Zhang, X.; Qian, D.; Meng, X.; Yu, L.; Yan, X.; He, Z. Self-Sulfhydrated, Nitro-Fixed Albumin Nanoparticles as a Potent Therapeutic Agent for the Treatment of Acute Liver Injury. ACS Nano 2024, 18, 20772–20791. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. BioMed Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.M.; Abdelhamid, A.M.; El Sayed, N.M.; Radwan, A. Alpha-Asarone attenuates alcohol-induced hepatotoxicity in a murine model by ameliorating oxidative stress, inflammation, and modulating apoptotic-Autophagic cell death. Toxicol. Appl. Pharmacol. 2024, 490, 117041. [Google Scholar] [CrossRef] [PubMed]
- Nasr, H.E.; Hegazy, A.M.; El-Shaer, N.O.; El-Shafey, R.S.; Elgendy, S.A.; Elnoury, H.A.; Gazzar, W.B.E.; Mohammed, L.A. Ameliorative effects of sildenafil against carbon tetrachloride induced hepatic fibrosis in rat model through downregulation of osteopontin gene expression. Sci. Rep. 2024, 14, 16902. [Google Scholar] [CrossRef] [PubMed]
- Laib, I.; Ali, B.D.; Alsalme, A.; Croun, D.; Bechelany, M.; Barhoum, A. Therapeutic potential of silver nanoparticles from Helianthemum lippii extract for mitigating cadmium-induced hepatotoxicity: Liver function parameters, oxidative stress, and histopathology in wistar rats. Front. Bioeng. Biotechnol. 2024, 12, 1400542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Wang, D.; Huang, Z.; Xiao, X.; Zheng, Q.; Li, S.; Long, D.; Feng, L. Mitochondrial Dysfunction in Metabolic Dysfunction Fatty Liver Disease (MAFLD). Int. J. Mol. Sci. 2023, 24, 17514. [Google Scholar] [CrossRef]
- Gong, H.; He, Q.; Zhu, L.; Feng, Z.; Sun, M.; Jiang, J.; Yuan, X.; Shen, Y.; Di, J. Associations between systemic inflammation indicators and nonalcoholic fatty liver disease: Evidence from a prospective study. Front. Immunol. 2024, 15, 1389967. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, Y.; Peng, J.; Wang, S.; Chen, G.; Sun, J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines 2023, 11, 2711. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Youn, J.; Song, S.; Chung, G.E.; Yang, S.Y.; Kim, Y.S.; Lee, J.E. Predicted pro-inflammatory hs-CRP score and non-alcoholic fatty liver disease. Gastroenterol. Rep. 2023, 11, goad059. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Cheng, L.; Gao, J.; Li, J.; Geng, C. Mendelian randomization explores the causal relationships between obesity, diabetes, inflammation and nonalcoholic fatty liver disease. Medicine 2023, 102, e34638. [Google Scholar] [CrossRef]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef]
- Li, J.H.; Ma, X.Y.; Yi, Y.; Li, L.R.; Xu, Z.Y.; Chang, Y. Association between Serum Ferritin Levels and Metabolic-associated Fatty Liver Disease in Adults: A Cross-sectional Study Based on the NHANES. Curr. Med. Sci. 2024, 44, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Lian, L.Y.; Zhang, H.; Chen, S.D.; Jin, X.Z.; Zhang, N.; Ye, C.H.; Chen, W.Y.; Bee, G.G.B.; Wang, F.D.; et al. A Population-Based and Clinical Cohort Validation of the Novel Consensus Definition of Metabolic Hyperferritinemia. J. Clin. Endocrinol. Metab. 2024, 109, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Balcar, L.; Wernly, S.; Völkerer, A.; Semmler, L.; Hauptmann, L.; Wernly, B.; Aigner, E.; Niederseer, D.; Datz, C. Insulin resistance and central obesity determine hepatic steatosis and explain cardiovascular risk in steatotic liver disease. Front. Endocrinol. 2023, 14, 1244405. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, L.; Qin, T.; Luo, Y.; Song, C.; Chen, X.; Duan, H.; Jiang, Y.; Zeng, H.; Wan, H.; et al. Associations of Serum Iron Status with MAFLD and Liver Fibrosis in the USA: A Nationwide Cross-Section Study. Biol. Trace Elem. Res. 2024, 202, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.H.; Chen, L.W.; Lin, C.L.; Hu, C.C.; Chien, C.H. Serum Uric Acid but Not Ferritin Level Is Associated with Hepatic Fibrosis in Lean Subjects with Metabolic Dysfunction-Associated Fatty Liver Disease: A Community-Based Study. J. Pers. Med. 2022, 12, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Jiang, Y.; Wu, D.; Cai, J.; Jiang, Z.; Zhou, Z.; Liu, L.; Ling, Q.; Wang, Q.; Zhao, G. Atractylodin alleviates nonalcoholic fatty liver disease by regulating Nrf2-mediated ferroptosis. Heliyon 2023, 9, e18321. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, C.; Yu, B. White adipose tissue in metabolic associated fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102336. [Google Scholar] [CrossRef]
- Xin, M.; Wang, H.; Wang, M.; Yang, B.; Liang, S.; Xu, X.; Dong, L.; Cai, T.; Huang, Y.; Wang, Q.; et al. Attenuating effect of Polygala tenuifolia Willd. seed oil on progression of MAFLD. Front. Pharmacol. 2023, 14, 1253715. [Google Scholar] [CrossRef]
- Lodge, M.; Dykes, R.; Kennedy, A. Regulation of Fructose Metabolism in Nonalcoholic Fatty Liver Disease. Biomolecules 2024, 14, 845. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Wang, M.; Xiong, T.; Song, X.; Sun, W.; Li, J. Oroxin B improves metabolic-associated fatty liver disease by alleviating gut microbiota dysbiosis in a high-fat diet-induced rat model. Eur. J. Pharmacol. 2023, 951, 175788. [Google Scholar] [CrossRef]
- López-Sánchez, G.N.; Montalvo-Javé, E.; Domínguez-Perez, M.; Antuna-Puente, B.; Beltrán-Anaya, F.O.; Hidalgo-Miranda, A.; Chávez-Tapia, N.C.; Uribe, M.; Nuño-Lámbarri, N. Hepatic mir-122-3p, mir-140-5p and mir-148b-5p expressions are correlated with cytokeratin-18 serum levels in MAFLD. Ann. Hepatol. 2022, 27, 100756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, M.X.; Meng, X.X.; Zhu, J.; Wang, J.J.; He, Y.F.; Li, Y.H.; Zhao, S.C.; Shi, Z.M.; Zheng, L.N.; et al. Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways. Mil. Med. Res. 2023, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Zhu, X.; Ren, D.; Ky, A.; MacDougald, O.A.; O’Rourke, R.W.; Rui, L. Adipose METTL14-Elicited N(6)-Methyladenosine Promotes Obesity, Insulin Resistance, and NAFLD Through Suppressing β Adrenergic Signaling and Lipolysis. Adv. Sci. 2023, 10, e2301645. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Othman, Z.A.; Suleiman, J.B.; Che Jalil, N.A.; Ghazali, W.S.W.; Nna, V.U.; Mohamed, M. Hepatoprotective Effect of Bee Bread in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Rats: Impact on Oxidative Stress and Inflammation. Antioxidants 2021, 10, 2031. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Yeewa, R.; Xiaochen, K.; Taychaworaditsakul, W.; Naiki, T.; Kato, H.; Nagayasu, Y.; Chewonarin, T.; Takahashi, S. Hexane insoluble fraction from purple rice extract improves steatohepatitis and fibrosis via inhibition of NF-κB and JNK signaling. Food Funct. 2024, 15, 8562–8571. [Google Scholar] [CrossRef]
- Służały, P.; Paśko, P.; Galanty, A. Natural Products as Hepatoprotective Agents-A Comprehensive Review of Clinical Trials. Plants 2024, 13, 1985. [Google Scholar] [CrossRef]
- Lee, J.A.; Gu, M.J.; Lee, Y.R.; Kim, Y.; Choi, I.; Kim, D.; Ha, S.K. Lindera obtusiloba Blume Alleviates Non-Alcoholic Fatty Liver Disease Promoted by N(ε)-(carboxymethyl)lysine. Nutrients 2024, 16, 2330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Feng, Y.; Wu, L.; Jia, Y.; Zhao, R. Alisma Orientalis Extract Ameliorates Hepatic Iron Deregulation in MAFLD Mice via FXR-Mediated Gene Repression. Nutrients 2024, 16, 2272. [Google Scholar] [CrossRef]
- Afarin, R.; Hatami, M.; Monjezi, S.; Bineshfar, F.; Ahangarpour, A. Suppression of TGF-β/Smad3 signaling pathway by Capparis spinosa and quercetin in a rat model of nonalcoholic steatohepatitis. Iran. J. Basic Med. Sci. 2024, 27, 1096–1104. [Google Scholar] [CrossRef]
- Zhang, H.; You, Y.; Xu, J.; Jiang, H.; Jiang, J.; Su, Z.; Chao, Z.; Du, Q.; He, F. New sesquiterpenes and viridin derivatives from Penicillium sp. Ameliorates NAFLD by regulating the PINK1/Parkin mitophagy pathway. Bioorgan. Chem. 2024, 151, 107656. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.C.; Deng, B. Protective Effects of Berberine on Nonalcoholic Fatty Liver Disease in db/db Mice via AMPK/SIRT1 Pathway Activation. Curr. Med. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, G.; Perez-Diaz-Del-Campo, N.; Rosso, C.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 2027. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, T.; Ridho, M.F.; Santoso, P.T.R.; Setiawan, S.; Goenawan, H.; Tarawan, V.M. Effects of Moringa oleifera Leaf Extract on Liver Histopathology: A Systematic Review. J. Nutr. Metab. 2024, 2024, 6815993. [Google Scholar] [CrossRef] [PubMed]
- Vera-Vives, A.M.; Novel, P.; Zheng, K.; Tan, S.L.; Schwarzländer, M.; Alboresi, A.; Morosinotto, T. Mitochondrial respiration is essential for photosynthesis-dependent ATP supply of the plant cytosol. New Phytol. 2024, 243, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Makio, T.; Simmen, T. The Discovery of Mitochondria-Endoplasmic Reticulum Contact Sites (MERCs) as Mitochondria-Associated Membranes (MAMs). Contact 2024, 7, 25152564241261228. [Google Scholar] [CrossRef]
- Jabůrek, M.; Klöppel, E.; Průchová, P.; Mozheitova, O.; Tauber, J.; Engstová, H.; Ježek, P. Mitochondria to plasma membrane redox signaling is essential for fatty acid β-oxidation-driven insulin secretion. Redox Biol. 2024, 75, 103283. [Google Scholar] [CrossRef]
- Yamada, A.; Watanabe, A.; Nara, A.; Ishimaru, N.; Maeda, K.; Ido, Y.; Kotake, K.; Asano, M.; Shinohara, Y.; Yamamoto, T. Longitudinal Analysis of Mitochondrial Function in a Choline-Deficient L-Amino Acid-Defined High-Fat Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis Mouse Model. Int. J. Mol. Sci. 2024, 25, 6193. [Google Scholar] [CrossRef]
- Fan, H.; Tan, Y. Lipid Droplet-Mitochondria Contacts in Health and Disease. Int. J. Mol. Sci. 2024, 25, 6878. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, M.; Zheng, Y.; Wang, M.; Gao, Y.; Wang, X.; Liu, X.; Lv, W.; Zeng, X.; Belosludtsev, K.N.; et al. α-Ketoglutarate prevents hyperlipidemia-induced fatty liver mitochondrial dysfunction and oxidative stress by activating the AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 2024, 74, 103230. [Google Scholar] [CrossRef]
- Tong, L.; Chen, Z.; Li, Y.; Wang, X.; Yang, C.; Li, Y.; Zhu, Y.; Lu, Y.; Liu, Q.; Xu, N.; et al. Transketolase promotes MAFLD by limiting inosine-induced mitochondrial activity. Cell Metab. 2024, 36, 1013–1029.e1015. [Google Scholar] [CrossRef]
- Yuan, S.; Schmidt, H.M.; Wood, K.C.; Straub, A.C. CoenzymeQ in cellular redox regulation and clinical heart failure. Free. Radic. Biol. Med. 2021, 167, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Su, W.; Zhang, H.; Wei, X.; Ma, X.; Gong, S.; Qu, G.; Zhang, L.; Xu, H.; et al. The mitochondria-targeted antioxidant MitoQ ameliorates inorganic arsenic-induced DCs/Th1/Th2/Th17/Treg differentiation partially by activating PINK1-mediated mitophagy in murine liver. Ecotoxicol. Environ. Saf. 2024, 277, 116350. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, Q.Y.; Li, H.; Wang, H.Y.; Fan, C.X.; Dong, Q.Y.; Pan, B.C.; Ji, Z.L.; Li, J.Y. ROS-induced oxidative stress is a major contributor to sperm cryoinjury. Hum. Reprod. 2024, 39, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Miah, R.; Nina, S.; Murate, T.; Kataoka, N.; Matsutani, M.; Ano, Y.; Matsushita, K.; Yakushi, T. Dissection and Reconstitution Provide Insights into Electron Transport in the Membrane-Bound Aldehyde Dehydrogenase Complex of Gluconacetobacter diazotrophicus. J. Bacteriol. 2022, 204, e0055821. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, N.; Olenin, A.; Beloborodova, N. Influence of Microbial Metabolites on the Nonspecific Permeability of Mitochondrial Membranes under Conditions of Acidosis and Loading with Calcium and Iron Ions. Biomedicines 2021, 9, 558. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Yang, M.; Wei, L.; Wu, H.; Wang, Q.; Shi, H. Physiological evidence of mitochondrial permeability transition pore opening caused by lipid deposition leading to hepatic steatosis in db/db mice. Free. Radic. Biol. Med. 2021, 162, 523–532. [Google Scholar] [CrossRef]
- Fromenty, B.; Robin, M.A.; Igoudjil, A.; Mansouri, A.; Pessayre, D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004, 30, 121–138. [Google Scholar] [CrossRef]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anti-Cancer Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Li, Q.; Guo, P.; Wang, S.; Su, L.; Yu, W.; Guo, J.; Hu, L.; Zhang, H.; Pan, J.; Tang, Z.; et al. Drp1 Aggravates Copper Nanoparticle-Induced ER-Phagy by Disturbing Mitochondria-Associated Membranes in Chicken Hepatocytes. J. Agric. Food Chem. 2024, 72, 16506–16518. [Google Scholar] [CrossRef]
- Sami Alkafaas, S.; Obeid, O.K.; Ali Radwan, M.; Elsalahaty, M.I.; Samy ElKafas, S.; Hafez, W.; Janković, N.; Hessien, M. Novel insight into mitochondrial dynamin-related protein-1 as a new chemo-sensitizing target in resistant cancer cells. Bioorgan. Chem. 2024, 150, 107574. [Google Scholar] [CrossRef]
- Jiang, Y.; Krantz, S.; Qin, X.; Li, S.; Gunasekara, H.; Kim, Y.M.; Zimnicka, A.; Bae, M.; Ma, K.; Toth, P.T.; et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission—Fusion dynamics and mitophagy. Redox Biol. 2022, 52, 102304. [Google Scholar] [CrossRef] [PubMed]
- Chevrollier, A.; Boursier, J.; Desquiret-Dumas, V. Food perception induces fast fragmentation of hepatic mitochondria. Trends Endocrinol. Metab. TEM 2024, 35, P680–P682. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, K.; Nishiyama, K.; Kato, Y.; Mi, X.; Ito, T.; Azuma, Y.T.; Nishimura, A.; Nishida, M. Inhibition of Drp1-Filamin Protein Complex Prevents Hepatic Lipid Droplet Accumulation by Increasing Mitochondria-Lipid Droplet Contact. Int. J. Mol. Sci. 2024, 25, 5446. [Google Scholar] [CrossRef] [PubMed]
- Henschke, S.; Nolte, H.; Magoley, J.; Kleele, T.; Brandt, C.; Hausen, A.C.; Wunderlich, C.M.; Bauder, C.A.; Aschauer, P.; Manley, S.; et al. Food perception promotes phosphorylation of MFFS131 and mitochondrial fragmentation in liver. Science 2024, 384, 438–446. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Wu, J.; Wang, Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: New insights from pathogenic mechanisms to clinically targeted therapy. J. Transl. Med. 2023, 21, 510. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.J.; Chen, S.Q.; Chen, Z.; Zhang, C.; Ying, R.; Liu, H.B.; Chen, L.W.; Tang, Y.H.; Lu, Z.Q.; et al. Mutual promotion of mitochondrial fission and oxidative stress contributes to mitochondrial-DNA-mediated inflammation and epithelial-mesenchymal transition in paraquat-induced pulmonary fibrosis. World J. Emerg. Med. 2023, 14, 209–216. [Google Scholar] [CrossRef]
- He, J.; Qian, Y.C.; Yin, Y.C.; Kang, J.R.; Pan, T.R. Polydatin: A potential NAFLD therapeutic drug that regulates mitochondrial autophagy through SIRT3-FOXO3-BNIP3 and PINK1-PRKN mechanisms—A network pharmacology and experimental investigation. Chemico-Biol. Interact. 2024, 398, 111110. [Google Scholar] [CrossRef]
- Dai, L.; Jiang, R.; Zhan, Z.; Zhang, L.; Qian, Y.; Xu, X.; Yang, W.; Zhang, Z. Machine learning-based algorithm identifies key mitochondria-related genes in non-alcoholic steatohepatitis. Lipids Health Dis. 2024, 23, 137. [Google Scholar] [CrossRef]
- Nakamura, H.; Matsui, T.; Shinozawa, T. Triclocarban induces lipid droplet accumulation and oxidative stress responses by inhibiting mitochondrial fatty acid oxidation in HepaRG cells. Toxicol. Lett. 2024, 396, 11–18. [Google Scholar] [CrossRef]
- Gnoni, A.; Di Chiara Stanca, B.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Damiano, F. Quercetin Reduces Lipid Accumulation in a Cell Model of NAFLD by Inhibiting De Novo Fatty Acid Synthesis through the Acetyl-CoA Carboxylase 1/AMPK/PP2A Axis. Int. J. Mol. Sci. 2022, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef] [PubMed]
- Sabouny, R.; Shutt, T.E. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem. Sci. 2020, 45, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Cai, J.; Zhang, X.-J.; Zhang, P.; She, Z.-G.; Chen, S.; Li, H. NAFLD as a continuous driver in the whole spectrum of vascular disease. J. Mol. Cell. Cardiol. 2022, 163, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Yang, Y.J.; Meng, X.Y.; Lin, R.H.; Tian, X.Y.; Zhang, Y.; Lai, W.F.; Yang, C.; Ma, X.Q.; Huang, M.Q. Oxysophoridine inhibits oxidative stress and inflammation in hepatic fibrosis via regulating Nrf2 and NF-κB pathways. Phytomed. Int. J. Phytother. Phytopharm. 2024, 132, 155585. [Google Scholar] [CrossRef] [PubMed]
- Arconzo, M.; Piccinin, E.; Pasculli, E.; Cariello, M.; Loiseau, N.; Bertrand-Michel, J.; Guillou, H.; Matrella, M.L.; Villani, G.; Moschetta, A. Hepatic-specific Pgc-1α ablation drives fibrosis in a MASH model. Liver Int. Off. J. Int. Assoc. Study Liver 2024. early view. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.; Schindler, F.; Afjehi-Sadat, L.; Montsch, B.; Heffeter, P.; Heiss, E.H.; Weckwerth, W. Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin. J. Agric. Food Chem. 2024, 72, 13039–13053. [Google Scholar] [CrossRef]
- Tsuji, A.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. Tactics with Prebiotics for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease via the Improvement of Mitophagy. Int. J. Mol. Sci. 2023, 24, 5465. [Google Scholar] [CrossRef]
- Yu, L.P.; Li, Y.Q.; Li, Y.J.; Zi, L.; Tao, Y.X.; Hao, J.J.; Zhang, M.; Gu, W.; Zhang, F.; Yu, J.; et al. In vivo identification of the pharmacodynamic ingredients of Polygonum cuspidatum for remedying the mitochondria to alleviate metabolic dysfunction-associated fatty liver disease. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 156, 113849. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, Y.; Lin, Z.; Hu, X.; Zhu, Y.; Xiao, W.; Jia, X.; Chen, W.; Lu, G.; Gong, W. Decrease in UCP1 by sustained high lipid promotes NK cell necroptosis to exacerbate nonalcoholic liver fibrosis. Cell Death Dis. 2024, 15, 518. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Jain, S.M.; Blot-Chabaud, M.; Pathak, S.; Banerjee, A.; Rawat, S.; Sharma, N.R.; Duttaroy, A.K. Mitochondrial dysfunction and its association with age-related disorders. Front. Physiol. 2024, 15, 1384966. [Google Scholar] [CrossRef] [PubMed]
- Sodeinde, K.O.; Adeoye, A.O.; Adesipo, A.; Adeniyi, A.A.; Falode, J.A.; Obafemi, T.O.; Olusanya, S.O.; Twigge, L.; Conradie, J.; Mosaku, T.O. Isolation, characterization and modulatory potentials of β-stigmasterol, ergosterol and xylopic acid from Anchomanes difformis on mitochondrial permeability transition pore in vitro. Chin. Herb. Med. 2023, 15, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.-H.; Portincasa, P. Role of mitochondria in nonalcoholic fatty liver disease-from origin to propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Quan, S.; Schell, J.; Gao, Y.; Varmazyad, M.; Sreenivas, P.; Cruz, D.; Jiang, H.; Pan, M.; Han, X.; et al. Mitochondrial ACSS1-K635 acetylation knock-in mice exhibit altered metabolism, cell senescence, and nonalcoholic fatty liver disease. Sci. Adv. 2024, 10, eadj5942. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, D.; Nikolic, D.; Paparella, R.R.; Sabbà, C.; Mazzocca, A. Crithmum maritimum Extract Restores Lipid Homeostasis and Metabolic Profile of Liver Cancer Cells to a Normal Phenotype. Plant Foods Hum. Nutr. 2024, 79, 417–424. [Google Scholar] [CrossRef]
- Andres, M.; Hennuyer, N.; Zibar, K.; Bicharel-Leconte, M.; Duplan, I.; Enée, E.; Vallez, E.; Herledan, A.; Loyens, A.; Staels, B.; et al. Insulin-degrading enzyme inhibition increases the unfolded protein response and favours lipid accumulation in the liver. Br. J. Pharmacol. 2024, 181, 3610–3626. [Google Scholar] [CrossRef]
- Peyman, M.; Babin-Ebell, A.; Rodríguez-Rodríguez, R.; Rigon, M.; Aguilar-Recarte, D.; Villarroya, J.; Planavila, A.; Villarroya, F.; Palomer, X.; Barroso, E.; et al. SIRT1 regulates hepatic vldlr levels. Cell Commun. Signal. CCS 2024, 22, 297. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, X.; Zhao, X.; He, T.; Li, X.; Du, H.; Zhao, M.; Zhu, R.; Li, M.; Zhang, Z.; et al. Polystyrene Nanoplastics Induce Lipid Metabolism Disorder by Activating the PERK-ATF4 Signaling Pathway in Mice. ACS Appl. Mater. Interfaces 2024, 16, 34524–34537. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Wu, M.; Chen, Y.; Wang, X.; Li, X.; Liu, F. The role of taurine through endoplasmic reticulum in physiology and pathology. Biochem. Pharmacol. 2024, 226, 116386. [Google Scholar] [CrossRef]
- Kanoni, S.; Kumar, S.; Amerikanou, C.; Kurth, M.J.; Stathopoulou, M.G.; Bourgeois, S.; Masson, C.; Kannt, A.; Cesarini, L.; Kontoe, M.S.; et al. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients with NAFLD. Front. Immunol. 2021, 12, 683028. [Google Scholar] [CrossRef] [PubMed]
- Seidita, A.; Cusimano, A.; Giuliano, A.; Meli, M.; Carroccio, A.; Soresi, M.; Giannitrapani, L. Oxidative Stress as a Target for Non-Pharmacological Intervention in MAFLD: Could There Be a Role for EVOO? Antioxidants 2024, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-González, V.; Rojas-Solé, C.; Gómez-Hevia, F.; González-Fernández, T.; Cereceda-Cornejo, A.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Tapping into Nature’s Arsenal: Harnessing the Potential of Natural Antioxidants for Human Health and Disease Prevention. Foods 2024, 13, 1999. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Vela, J.; Sánchez-Oliver, A.J.; Huertas, J.R.; Casuso, R.A. Does curcumin improve liver enzymes levels in nonalcoholic fatty liver disease? A systematic review, meta-analysis, and meta-regression. Phytother. Res. PTR 2024, 38, 4261–4271. [Google Scholar] [CrossRef] [PubMed]
- Ghani, I.; An, Y.; Qiao, Q.; He, S.; Li, Z. Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice. Foods 2024, 13, 1683. [Google Scholar] [CrossRef]
- Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. [Google Scholar] [CrossRef]
- Rahimlou, M.; Baghdadi, G.; Khodi, A.; Rahimi, Z.; Saki, N.; Banaei Jahromi, N.; Cheraghian, B.; Tavasolian, R.; Hosseini, S.A. Polyphenol consumption and Nonalcoholic fatty liver disease risk in adults. Sci. Rep. 2024, 14, 6752. [Google Scholar] [CrossRef]
- Tian, X.; Wang, X.; Xu, W.; Gong, M.; Zhou, C.; Jiang, E.; Tang, Y.; Jia, L.; Zeng, L.; Deng, S.; et al. Penthorum chinense Pursh leaf tea debittering mechanisms via green tea manufacturing process and its influence on NAFLD-alleviation activities. Food Chem. 2024, 445, 138715. [Google Scholar] [CrossRef]
- Thilakarathna, W.; Rupasinghe, H.P.V. Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction. Molecules 2024, 29, 709. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, X.; Heiyan-Perhat, S.U.; Yang, P.; Han, H.; Li, Y.; Gao, J.; He, E.; Li, Y. Therapeutic Role of Polyphenol Extract from Prunus cerasifera Ehrhart on Non-Alcoholic Fatty Liver. Plants 2024, 13, 288. [Google Scholar] [CrossRef]
- Yu, C.; Guo, X.; Cui, X.; Su, G.; Wang, H. Functional Food Chemical Ingredient Strategies for Non-alcoholic Fatty Liver Disease (NAFLD) and Hepatic Fibrosis: Chemical Properties, Health Benefits, Action, and Application. Curr. Nutr. Rep. 2024, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hua, J.; Huang, Z. Effects of beer, wine, and baijiu consumption on non-alcoholic fatty liver disease: Potential implications of the flavor compounds in the alcoholic beverages. Front. Nutr. 2022, 9, 1022977. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Cui, Y.; Hu, X.; Chen, F.; Ma, C. Alleviation Effects of Microbial Metabolites from Resveratrol on Non-Alcoholic Fatty Liver Disease. Foods 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Abenavoli, L. Mediterranean diet in liver steatosis: The role of polyphenols. Minerva Gastroenterol. Dietol. 2018, 64, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Luzza, F.; Boccuto, L.; De Lorenzo, A. Polyphenols Treatment in Patients with Nonalcoholic Fatty Liver Disease. J. Transl. Intern. Med. 2017, 5, 144–147. [Google Scholar] [CrossRef]
- Vicknasingam, B.; Karunakaran, T.; Chawarski, M.C. Research and publication gaps on kratom and kratom products: A scoping review of current literature. Curr. Opin. Psychiatry 2024, 37, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yabalak, E.; Bahadırlı, N.P.; Yetkin, D.; Yaldız, F.D.; Han Türkseven, Ç. Unlocking nature’s potential: Anticancer potential of Helichrysum sanguineum (L.) Kostel on breast cancer cells and its chemical composition. Int. J. Environ. Health Res. 2024, 1–13. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary polyphenols and their relationship to the modulation of non-communicable chronic diseases and epigenetic mechanisms: A mini-review. Food Chem. Mol. Sci. 2023, 6, 100155. [Google Scholar] [CrossRef]
- Ferro, Y.; Maurotti, S.; Mazza, E.; Pujia, R.; Sciacqua, A.; Musolino, V.; Mollace, V.; Pujia, A.; Montalcini, T. Citrus Bergamia and Cynara Cardunculus Reduce Serum Uric Acid in Individuals with Non-Alcoholic Fatty Liver Disease. Medicina 2022, 58, 1728. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Kitayama, E.; Oba, S.; Takeishi, N.; Chiba, H.; Hui, S.P. Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts. Antioxidants 2024, 13, 513. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Yi, D.; Qiu, F.; Wu, L.; Tang, Y.; Wang, N. Mediterranean diet affects the metabolic outcome of metabolic dysfunction-associated fatty liver disease. Front. Nutr. 2023, 10, 1225946. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Tkacz, K.; Turkiewicz, I.P.; Santos, I.; Camoesas, E.S.M.; Lima, A.; Sousa, I. Exploring the Bioactive Properties and Therapeutic Benefits of Pear Pomace. Antioxidants 2024, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Valle, M.S.; D’Angeli, F.; Surdo, S.; Malaguarnera, L. Resveratrol and Vitamin D: Eclectic Molecules Promoting Mitochondrial Health in Sarcopenia. Int. J. Mol. Sci. 2024, 25, 7503. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, W.; Hu, X.; Liu, Z.; Wang, F.; Wang, J. The role of polyphenols in modulating mitophagy: Implications for therapeutic interventions. Pharmacol. Res. 2024, 207, 107324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Yu, Z.L.; Chung, S.K.; Xu, B. The roles of dietary polyphenols at crosstalk between type 2 diabetes and Alzheimer’s disease in ameliorating oxidative stress and mitochondrial dysfunction via PI3K/Akt signaling pathways. Ageing Res. Rev. 2024, 99, 102416. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Nesci, S.; Spagnoletta, A. Mitochondrial sirtuin 3 and role of natural compounds: The effect of post-translational modifications on cellular metabolism. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 199–220. [Google Scholar] [CrossRef]
- Jamali, Z.; Salimi, A.; Khezri, S.; Norozi, P.; Garmabi, B.; Khaksari, M. Protective Role of Ellagic Acid Against Ethanol-Induced Neurodevelopmental Disorders in Newborn Male Rats: Insights into Maintenance of Mitochondrial Function and Inhibition of Oxidative Stress. J. Stud. Alcohol Drugs 2024. [Google Scholar] [CrossRef]
- Dong, L.; Luo, L.; Wang, Z.; Lian, S.; Wang, M.; Wu, X.; Fan, J.; Zeng, Y.; Li, S.; Lv, S.; et al. Targeted degradation of NDUFS1 by agrimol B promotes mitochondrial ROS accumulation and cytotoxic autophagy arrest in hepatocellular carcinoma. Free. Radic. Biol. Med. 2024, 220, 111–124. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Huo, J.; Li, S. Study on the mechanism of polyphenols regulating the stability of pea isolate protein formed Pickering emulsion based on interfacial effects. Food Chem. 2024, 463, 141423. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Jana, S.; Swarnakar, S.; Das, N. Neuro-protective role of nanocapsulated curcumin against cerebral ischemia-reperfusion induced oxidative injury. Brain Res. 2019, 1704, 164–173. [Google Scholar] [CrossRef] [PubMed]
- de Brito Alves, J.L.; de Sousa, V.P.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.A.; da Costa-Silva, J.H.; Leandro, C.G.; Vidal, H.; Pirola, L. New Insights on the Use of Dietary Polyphenols or Probiotics for the Management of Arterial Hypertension. Front. Physiol. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Riso, P.; Mazzocchi, A.; Porrini, M.; Fargion, S.; Agostoni, C. Dietary anthocyanins as nutritional therapy for nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2013, 2013, 145421. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhang, J.; Zeng, F.Y.; Zhu, Y.Z. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol. Res. 2023, 192, 106786. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, M.; Luo, Y.; Liu, H.; Ling, H.; He, Y.; Lu, Y. PPARα is one of the key targets for dendrobine to improve hepatic steatosis in NAFLD. J. Ethnopharmacol. 2024, 323, 117684. [Google Scholar] [CrossRef]
- Ganguly, R.; Gupta, A.; Pandey, A.K. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review. World J. Gastroenterol. 2022, 28, 3047–3062. [Google Scholar] [CrossRef]
- Junior; Lai, Y.S.; Nguyen, H.T.; Salmanida, F.P.; Chang, K.T. MERTK(+/hi) M2c Macrophages Induced by Baicalin Alleviate Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 604. [Google Scholar] [CrossRef]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S.; et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef]
- Marinovic, M.P.; Sousa-Filho, C.P.B.; Batista, F.A.H.; Avelino, T.M.; Cogliati, B.; Figueira, A.C.M.; Otton, R.; Rodrigues, A.C. Green tea extract increases adiponectin and PPAR α levels to improve hepatic steatosis. J. Nutr. Biochem. 2022, 103, 108957. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Trepiana, J.; Macarulla, M.T.; Gómez-Zorita, S.; Arellano-García, L.; Fernández-Quintela, A.; Portillo, M.P. Potential usefulness of Mediterranean diet polyphenols against COVID-19-induced inflammation: A review of the current knowledge. J. Physiol. Biochem. 2023, 79, 371–382. [Google Scholar] [CrossRef]
- Ma, K.; Sheng, W.; Song, X.; Song, J.; Li, Y.; Huang, W.; Liu, Y. Chlorogenic Acid from Burdock Roots Ameliorates Oleic Acid-Induced Steatosis in HepG2 Cells through AMPK/ACC/CPT-1 Pathway. Molecules 2023, 28, 7257. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Song, C.; Liu, J.; Chen, F.; Zhu, Y.; Fang, X.; Cao, Q.; Ma, D.; Wang, Y.; Zhang, C. Chlorogenic Acid Modulates Autophagy by Inhibiting the Activity of ALKBH5 Demethylase, Thereby Ameliorating Hepatic Steatosis. J. Agric. Food Chem. 2023, 71, 15073–15086. [Google Scholar] [CrossRef] [PubMed]
- Bacil, G.P.; Romualdo, G.R.; Rodrigues, J.; Barbisan, L.F. Indole-3-carbinol and chlorogenic acid combination modulates gut microbiome and attenuates nonalcoholic steatohepatitis in a murine model. Food Res. Int. 2023, 174, 113513. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ren, X.; Ma, L.; Liu, J.; Yuan, S.; Wang, G. Pharmacokinetics and antioxidant activity of dihydrocaffeic acid grafted chitosan nanomicelles loaded with chicoric acid in broilers. Poult. Sci. 2024, 103, 103776. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Kalinowska, M.; Świderski, G.; Krętowski, R.; Naumowicz, M.; Kondzior, P.; Cechowska-Pasko, M.; Lewandowski, W. The Influence of Mesotrione on Human Colorectal Adenocarcinoma Cells and Possibility of Its Toxicity Mitigation by Cichoric Acid. Int. J. Mol. Sci. 2024, 25, 5655. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Guo, H.; Li, X.; Wang, Y.; Song, Y.; Wang, H.; Ma, D. Cichoric acid improves isoproterenol-induced myocardial fibrosis via inhibition of HK1/NLRP3 inflammasome-mediated signaling pathways by reducing oxidative stress, inflammation, and apoptosis. Food Sci. Nutr. 2024, 12, 180–191. [Google Scholar] [CrossRef]
- Calvi, P.; Terzo, S.; Amato, A. Betalains: Colours for human health. Nat. Prod. Res. 2023, 37, 1746–1765. [Google Scholar] [CrossRef]
- Luo, X.; Liu, P.; Ye, X.; He, J.; Lai, Y.; Lv, Y.; Wu, X.; Liu, Y.; Zhang, Q.; Yang, H.; et al. Curcumin improves atrial fibrillation susceptibility by regulating tsRNA expression in aging mouse atrium. PeerJ 2024, 12, e17495. [Google Scholar] [CrossRef]
- Martins, A.; Araújo, O.R.P.; Gomes, A.D.S.; Araujo, F.L.C.; Oliveira Junior, J.; Vasconcelos, J.K.G.; Rodrigues Junior, J.I.; Cerqueira, I.T.; Lins Neto MÁ, F.; Bueno, N.B.; et al. Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2024, 17, 849. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.; Carvalho, A.C.A.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M.; Lima, V.M.; Souza, G.A.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; Carvalho, A.C.A.; et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2021, 24, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Cunha Neto, F.; Marton, L.T.; de Marqui, S.V.; Lima, T.A.; Barbalho, S.M. Curcuminoids from Curcuma Longa: New adjuvants for the treatment of crohn’s disease and ulcerative colitis? Crit. Rev. Food Sci. Nutr. 2019, 59, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Akuri, M.C.; Barbalho, S.M.; Val, R.M.; Guiguer, E.L. Reflections about Osteoarthritis and Curcuma longa. Pharmacogn. Rev. 2017, 11, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Mazieiro, R.; Frizon, R.R.; Barbalho, S.M.; Goulart, R.A. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food 2018, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M.; Rubira, C.J.; Araújo, A.C.; Lima, V.M.; Rogerio Leoni, B.; Guiguer, E.L. Curcumin therapy for ulcerative colitis remission: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1171–1179. [Google Scholar] [CrossRef]
- Matias, J.N.; Achete, G.; Campanari, G.; Guiguer, É.L.; Araújo, A.C.; Buglio, D.S.; Barbalho, S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry 2021, 55, 451–462. [Google Scholar] [CrossRef]
- Feng, Z.; Pang, L.; Chen, S.; Pang, X.; Huang, Y.; Qiao, Q.; Wang, Y.; Vonglorkham, S.; Huang, Q.; Lin, X.; et al. Didymin ameliorates dexamethasone-induced non-alcoholic fatty liver disease by inhibiting TLR4/NF-κB and PI3K/Akt pathways in C57BL/6J mice. Int. Immunopharmacol. 2020, 88, 107003. [Google Scholar] [CrossRef]
- Mohammadi, M.; Abbasalipourkabir, R.; Ziamajidi, N. Fish oil and chicoric acid combination protects better against palmitate-induced lipid accumulation via regulating AMPK-mediated SREBP-1/FAS and PPARα/UCP2 pathways. Arch. Physiol. Biochem. 2023, 129, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; RuXian, G. Didymin, a natural flavonoid, relieves the progression of myocardial infarction via inhibiting the NLR family pyrin domain containing 3 inflammasome. Pharm. Biol. 2022, 60, 2319–2327. [Google Scholar] [CrossRef]
- Xie, L.W.; Cai, S.; Zhao, T.S.; Li, M.; Tian, Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free. Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef] [PubMed]
- Oanh, H.T.; Hoai Thu, N.T.; Van Hanh, N.; Hoang, M.H.; Minh Hien, H.T. Co-encapsulated astaxanthin and kaempferol nanoparticles: Fabrication, characterization, and their potential synergistic effects on treating non-alcoholic fatty liver disease. RSC Adv. 2023, 13, 35127–35136. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Cao, J.; Wang, Y.; Anwar, N.; Zhang, Z.; Zhang, D.; Ma, Y.; Xiao, Y.; Xiao, L.; et al. The role of autophagy in bone metabolism and clinical significance. Autophagy 2023, 19, 2409–2427. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jiao, G.; Pang, T.; Wen, T.; He, Z.; Han, J.; Zhang, F.; Chen, W. Ameliorative effects of mangiferin derivative TPX on insulin resistance via PI3K/AKT and AMPK signaling pathways in human HepG2 and HL-7702 hepatocytes. Phytomed. Int. J. Phytother. Phytopharm. 2023, 114, 154740. [Google Scholar] [CrossRef]
- Lin, H.; Teng, H.; Wu, W.; Li, Y.; Lv, G.; Huang, X.; Yan, W.; Lin, Z. Pharmacokinetic and metabolomic analyses of Mangiferin calcium salt in rat models of type 2 diabetes and non-alcoholic fatty liver disease. BMC Pharmacol. Toxicol. 2020, 21, 59. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, X.; Yu, Y.; Yang, L.; OuYang, J.; Bian, Y.; Liu, Y.; Li, G. Puerarin Alleviates Oxidized Oil-Induced Oxidative Injury and Inflammation via Inhibition of the Nrf2/Keap1 and HMGB1/TLR4/MAPK Signaling Pathways: An Investigation in a Chicken Model. Mol. Nutr. Food Res. 2023, 67, e2200663. [Google Scholar] [CrossRef]
- Yang, M.; Xia, L.; Song, J.; Hu, H.; Zang, N.; Yang, J.; Zou, Y.; Wang, L.; Zheng, X.; He, Q.; et al. Puerarin ameliorates metabolic dysfunction-associated fatty liver disease by inhibiting ferroptosis and inflammation. Lipids Health Dis. 2023, 22, 202. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Al-Numair, N.; Almuzaini, T.; Athinarayanan, J.; Alshatwi, A.A. Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes. Nanomaterials 2022, 12, 368. [Google Scholar] [CrossRef]
- Javadi-Farsani, F.; Karimi, A.; Razavi Nikoo, H.; Moradi, M.T.; Tabarraei, A. An in vitro antiviral evaluation of punicalagin toward influenza A virus. Avicenna J. Phytomed. 2024, 14, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Castañeta, G.; Simirgiotis, M.J.; Sepulveda, B.; Areche, C. Comprehensive phytochemical profile of leaves, stems and fruits from Orthopterygium huaucui (A. Gray) Hemsl. and their antioxidant activities. Chem. Biodivers. 2024. early view. [Google Scholar] [CrossRef] [PubMed]
- Kundrapu, D.B.; Chaitanya, A.K.; Manaswi, K.; Kumari, S.; Malla, R. Quercetin and taxifolin inhibits TMPRSS2 activity and its interaction with EGFR in paclitaxel-resistant breast cancer cells: An in silico and in vitro study. Chem. Biol. Drug Des. 2024, 104, e14600. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, Y.; Liu, S.; Ying, L.; Zhang, K.; Liang, N.; Li, H.; Luo, G.; Xiao, L. Quercetin Attenuates KLF4-Mediated Phenotypic Switch of VSMCs to Macrophage-like Cells in Atherosclerosis: A Critical Role for the JAK2/STAT3 Pathway. Int. J. Mol. Sci. 2024, 25, 7755. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liu, C.C.; Tsao, J.P.; Hsu, C.L.; Cheng, I.S. Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients 2020, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Santana, T.M.; Caria, S.J.; Carlini, G.C.G.; Rogero, M.M.; Donato, J., Jr.; Tavares, M.R.; Castro, I.A. Trans-resveratrol reduced hepatic oxidative stress in an animal model without inducing an upregulation of nuclear factor erythroid 2-related factor 2. J. Clin. Biochem. Nutr. 2024, 75, 40–45. [Google Scholar] [CrossRef]
- Saygun, I.; Slezovic, M.; Özkan, C.K.; Bengi, V.U.; Elçi, P.; Serdar, M.; Kantarci, A. Anti-proliferative impact of resveratrol on gingival fibroblasts from juvenile hyaline fibromatosis. Clin. Oral Investig. 2024, 28, 448. [Google Scholar] [CrossRef]
- Su, X.; Li, Q.; Yang, M.; Zhang, W.; Liu, X.; Ba, Y.; Deng, Q.; Zhang, Y.; Han, L.; Huang, H. Resveratrol protects against a high-fat diet-induced neuroinflammation by suppressing mitochondrial fission via targeting SIRT1/PGC-1α. Exp. Neurol. 2024, 380, 114899. [Google Scholar] [CrossRef]
- Luo, C.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Zhang, C.; Zhu, W.; Jin, Y. Rosmarinic acid exerts an antagonistic effect on nonalcoholic fatty liver disease by regulating the YAP1/TAZ-PPARγ/PGC-1α signaling pathway. Phytother. Res. PTR 2021, 35, 1010–1022. [Google Scholar] [CrossRef]
- Musolino, V.; Macrì, R.; Cardamone, A.; Tucci, L.; Serra, M.; Lupia, C.; Maurotti, S.; Mare, R.; Nucera, S.; Guarnieri, L.; et al. Salvia rosmarinus Spenn. (Lamiaceae) Hydroalcoholic Extract: Phytochemical Analysis, Antioxidant Activity and In Vitro Evaluation of Fatty Acid Accumulation. Plants 2023, 12, 3306. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Huang, Z.; Luo, S.; Zhang, X.; Sun, W.; Lan, T.; He, R. Determination of Flavonolignan Compositional Ratios in Silybum marianum (Milk Thistle) Extracts Using High-Performance Liquid Chromatography. Molecules 2024, 29, 2949. [Google Scholar] [CrossRef] [PubMed]
- Butanda-Nuñez, A.; Rodríguez-Cortés, O.; Ramos-Martínez, E.; Cerbón, M.A.; Escobedo, G.; Chavarría, A. Silybin restores glucose uptake after tumour necrosis factor-alpha and lipopolysaccharide stimulation in 3T3-L1 adipocytes. Adipocyte 2024, 13, 2374062. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.E.; Elieh-Ali-Komi, D.; Goudarzi, F.; Mohammadi Noori, E.; Assar, S.; Shavandi, M.; Kiani, A.; Elahi, H. Effects of silymarin as adjuvant drug on serum levels of CTRP3, anti-cyclic citrullinated peptide (CCP), and high-sensitivity C-reactive protein (hs-CRP) in rheumatoid arthritis patients. Mol. Biol. Res. Commun. 2024, 13, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.M.; Bairy, L.K.; Rehman, A.; Attia, M.; Ahmed, L.; Emad, K.; Jaafer, Y.; Bahaaeldin, A. Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology 2024, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Nalam, S.M.; Chintamaneni, P.K.; Saxena Pal, R.; Chaitanya, M.; Kumar Singh, S.; Saranya, P.; Arora, S.; Sharma, S.; Pandey, P.; Mazumder, A.; et al. From nature’s bounty to drug discovery: Leveraging phytochemicals and molecular approaches to combat multi-drug-resistant (MDR) tuberculosis. Indian J. Tuberc. 2024, 71 (Suppl. S1), S117–S129. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Oh, Y.; Kim, M.K.; Chong, Y. Green Tea Catechol (-)-Epigallocatechin Gallate (EGCG) Conjugated with Phenylalanine Shows Enhanced Autophagy Stimulating Activity in Human Aortic Endothelial Cells. Planta Medica 2023, 89, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Benítez, F.J.; Melín, V.; Perez-Gonzalez, G.; Henríquez, A.; Zarate, X.; Schott, E.; Contreras, D. The ferryl generation by fenton reaction driven by catechol. Chemosphere 2023, 335, 139155. [Google Scholar] [CrossRef]
- Aborziza, M.; Amalia, R.; Zuhrotun, A.; Ikram, N.K.K.; Novitasari, D.; Muchtaridi, M. Coffee Bean and Its Chemical Constituent Caffeine and Chlorogenic Acid as Promising Chemoprevention Agents: Updated Biological Studies against Cancer Cells. Molecules 2024, 29, 3302. [Google Scholar] [CrossRef]
- Singh, S.; Varshney, M. Exploring the Pharmacological Potential of Chlorogenic acid as an Anti-Cancer Agent and a Call for Advance Research. Comb. Chem. High Throughput Screen. 2024. [Google Scholar] [CrossRef]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural Compounds for Counteracting Nonalcoholic Fatty Liver Disease (NAFLD): Advantages and Limitations of the Suggested Candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Eltayeb, W.A.; Rakshit, G.; El-Arabey, A.A.; Khan, J.; Aldosari, S.M.; Alshehri, B.; Abdalla, M. Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies. Sci. Rep. 2023, 13, 8656. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Soni, U.; Varadwaj, P.K.; Misra, K.; Rizvi, S.I. Anti-inflammatory effect of curcumin in an accelerated senescence model of Wistar rat: An in vivo and in-silico study. J. Biomol. Struct. Dyn. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Różański, G.; Kujawski, S.; Newton, J.L.; Zalewski, P.; Słomko, J. Curcumin and Biochemical Parameters in Metabolic-Associated Fatty Liver Disease (MAFLD)—A Review. Nutrients 2021, 13, 2654. [Google Scholar] [CrossRef] [PubMed]
- Lukkunaprasit, T.; Tansawet, A.; Boonmanunt, S.; Sobhonslidsuk, A.; McKay, G.J.; Attia, J.; Thakkinstian, A. An updated meta-analysis of effects of curcumin on metabolic dysfunction-associated fatty liver disease based on available evidence from Iran and Thailand. Sci. Rep. 2023, 13, 5824. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced effect of curcumin and resveratrol on mitigating hepatic steatosis in metabolic associated fatty liver disease via the PI3K/AKT/mTOR and HIF-1/VEGF cascade. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef]
- Yang, J.W.; Zou, Y.; Chen, J.; Cui, C.; Song, J.; Yang, M.M.; Gao, J.; Hu, H.Q.; Xia, L.Q.; Wang, L.M.; et al. Didymin alleviates metabolic dysfunction-associated fatty liver disease (MAFLD) via the stimulation of Sirt1-mediated lipophagy and mitochondrial biogenesis. J. Transl. Med. 2023, 21, 921. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Hamouda, A.O.; Abdel-Hamed, A.R.; Abo-Elmatty, D.M.; Khedr, N.F.; Ghattas, M.H. Pentoxifylline and its association with kaempferol improve NASH-associated manifestation in mice through anti-apoptotic, anti-necroptotic, antioxidant, and anti-inflammatory mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8644–8659. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yin, L.; Shang, J.; Liang, M.; Liu, Z.; Yang, H.; Qiang, G.; Du, G.; Yang, X. Kaempferol attenuates nonalcoholic fatty liver disease in type 2 diabetic mice via the Sirt1/AMPK signaling pathway. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 165, 115113. [Google Scholar] [CrossRef] [PubMed]
- Chiș, A.; Noubissi, P.A.; Pop, O.L.; Mureșan, C.I.; Fokam Tagne, M.A.; Kamgang, R.; Fodor, A.; Sitar-Tăut, A.V.; Cozma, A.; Orășan, O.H.; et al. Bioactive Compounds in Moringa oleifera: Mechanisms of Action, Focus on Their Anti-Inflammatory Properties. Plants 2023, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Yang, J.W.; Dou, H.Y.; Li, G.Q.; Li, X.Y.; Shen, L.; Ji, H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorgan. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dai, S.; Wang, C.; Fu, K.; Wu, R.; Zhao, X.; Yao, Y.; Li, Y. Luteolin as a potential hepatoprotective drug: Molecular mechanisms and treatment strategies. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 167, 115464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.S.; Mohamed, H.E.; Farrag, M.A. Luteolin loaded on zinc oxide nanoparticles ameliorates non-alcoholic fatty liver disease associated with insulin resistance in diabetic rats via regulation of PI3K/AKT/FoxO1 pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221137435. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Q.; Cao, Y.; Zhang, C.; Chen, S.; Zhang, Y.; Liang, T. Luteolin Ameliorates Hepatic Steatosis and Enhances Mitochondrial Biogenesis via AMPK/PGC-1α Pathway in Western Diet-Fed Mice. J. Nutr. Sci. Vitaminol. 2023, 69, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, Y.; Liu, X.; Wang, M. Mangiferin for the Management of Liver Diseases: A Review. Foods 2023, 12, 2469. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Aldhahrani, A.; Soliman, M.M.; Zhang, L.; Zhou, F. Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front. Immunol. 2022, 13, 956688. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Wu, L.; Yang, K.; Yang, F.; Yang, J.; Hu, S.; Yao, Y.; Xia, X.; Liu, Y.; et al. Puerarin inhibits inflammation and lipid accumulation in alcoholic liver disease through regulating MMP8. Chin. J. Nat. Med. 2023, 21, 670–681. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, X.; Xu, F.; Wang, F.; Wu, H.; Bai, Y.; Li, W.; Zhang, G.; Yuan, J.; Pang, Q. Protective effects of puerarin on liver tissue in Salmonella-infected chicks: A proteomic analysis. Poult. Sci. 2024, 103, 103281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Pan, L.; Zou, H.; Miao, X.; Cheng, J.; Wu, Y. Puerarin alleviates liver fibrosis via inhibition of the ERK1/2 signaling pathway in thioacetamide-induced hepatic fibrosis in rats. Exp. Ther. Med. 2019, 18, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, L.; Deng, J.; Zhang, J.; Hu, X. Coptidis rhizoma and evodiae fructus against lipid droplet deposition in nonalcoholic fatty liver disease-related liver cancer by AKT. Chem. Biol. Drug Des. 2023, 102, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhang, Y.; Botchway, B.O.A.; Huang, M.; Lu, Q.; Liu, X. Quercetin activates the Sestrin2/AMPK/SIRT1 axis to improve amyotrophic lateral sclerosis. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 161, 114515. [Google Scholar] [CrossRef] [PubMed]
- Olla, S.; Siguri, C.; Fais, A.; Era, B.; Fantini, M.C.; Di Petrillo, A. Inhibitory Effect of Quercetin on Oxidative Endogen Enzymes: A Focus on Putative Binding Modes. Int. J. Mol. Sci. 2023, 24, 5391. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, N.; Bonyadian, A.; Hojati, A.; Abbasalizad-Farhangi, M.; Heidari, M.; Darzi, M.; Seyedhosseini-Ghaheh, H.; Tabrizi, F.P.F.; Vajdi, M.; Askari, G. The effect of quercetin supplementation on the components of metabolic syndrome in adults: A systematic review and dose–response meta-analysis of randomized controlled trials. J. Funct. Foods 2024, 116, 106175. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Javanmardi, S.; Moradi-Ozarlou, M.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S.; Garg, M. Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy: An updated review on resveratrol. Biosci. Rep. 2020, 40, 257. [Google Scholar] [CrossRef]
- Feng, S.; Gui, J.; Qin, B.; Ye, J.; Zhao, Q.; Guo, A.; Sang, M.; Sun, X. Resveratrol Inhibits VDAC1-Mediated Mitochondrial Dysfunction to Mitigate Pathological Progression in Parkinson’s Disease Model. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Xu, Z.; Shao, W.; Pang, X.; Li, D.; Huang, X.; Luo, W.; Du, Z.; Li, Y.; et al. Dietary resveratrol improves the flesh quality of Siberian sturgeon (Acipenser baerii) by enhancing myofiber growth, nutrient accumulation and antioxidant capacity. BMC Genom. 2024, 25, 514. [Google Scholar] [CrossRef]
- Shaito, A.; Al-Mansoob, M.; Ahmad, S.M.S.; Haider, M.Z.; Eid, A.H.; Posadino, A.M.; Pintus, G.; Giordo, R. Resveratrol-Mediated Regulation of Mitochondria Biogenesis-associated Pathways in Neurodegenerative Diseases: Molecular Insights and Potential Therapeutic Applications. Curr. Neuropharmacol. 2023, 21, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, D.; Tiwari, N.; Mittal, P.; Siddiqui, M.H.; Mittal, N. Exploring the therapeutic potential of rosemary compounds against Alzheimer’s disease through GC-MS and molecular docking analysis. Silico Pharmacol. 2024, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, Z.; Yue, Z.; Ding, L.; Zhou, Y.; Huang, Z.; Huang, H. Rosmarinic Acid Ameliorates H2O2-Induced Oxidative Stress in L02 Cells Through MAPK and Nrf2 Pathways. Rejuvenation Res. 2019, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Aghemo, A.; Alekseeva, O.P.; Angelico, F.; Bakulin, I.G.; Bakulina, N.V.; Bordin, D.; Bueverov, A.O.; Drapkina, O.M.; Gillessen, A.; Kagarmanova, E.M.; et al. Role of silymarin as antioxidant in clinical management of chronic liver diseases: A narrative review. Ann. Med. 2022, 54, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.F.; Ferreira, F.M.; Dias, B.V.; Azevedo, D.C.; de Souza, G.H.B.; Milagre, M.M.; de Lana, M.; Vieira, P.M.A.; Carneiro, C.M.; Paula-Gomes, S.; et al. Silymarin inhibits the lipogenic pathway and reduces worsening of non-alcoholic fatty liver disease (NAFLD) in mice. Arch. Physiol. Biochem. 2022, 130, 460–474. [Google Scholar] [CrossRef]
- Zhang, P.W.; Chen, F.X.; Li, D.; Ling, W.H.; Guo, H.H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lin, X.; Zhang, P.; Liu, Y.; Ling, W.; Guo, H. Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101843. [Google Scholar] [CrossRef]
- Sakata, R.; Nakamura, T.; Torimura, T.; Ueno, T.; Sata, M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: A double-blind placebo-controlled study. Int. J. Mol. Med. 2013, 32, 989–994. [Google Scholar] [CrossRef]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix(®) Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointest. Liver Dis. JGLD 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kelardeh, B.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M.A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes Obes. Metab. 2022, 24, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, X.; Li, Y.; Liang, Y.; Hong, T.; Yang, J.; Cao, Z.; Mai, H.; Yao, J.; Zhang, T.; et al. Curcumin supplementation alleviates hepatic fat content associated with modulation of gut microbiota-dependent bile acid metabolism in patients with nonalcoholic simple fatty liver disease: A randomized controlled trial. Am. J. Clin. Nutr. 2024, 120, 66–79. [Google Scholar] [CrossRef]
- Kwok, M.K.; Leung, G.M.; Xu, L.; Tse, H.F.; Lam, T.H.; Schooling, C.M. Effect of puerarin supplementation on cardiovascular disease risk factors: A randomized, double-blind, placebo-controlled, 2-way crossover trial. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 153, 113472. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Asghari-Jafarabadi, M.; Somi, M.H.; Ghavami, S.M.; Rafraf, M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Research. Int. Z. Fur Vitam.-Und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2020, 90, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ali Sangouni, A.; Abdollahi, S.; Mozaffari-Khosravi, H. Effect of resveratrol supplementation on hepatic steatosis and cardiovascular indices in overweight subjects with type 2 diabetes: A double-blind, randomized controlled trial. BMC Cardiovasc. Disord. 2022, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Trappoliere, M.; Federico, A.; Tuccillo, C.; de Sio, I.; Di Leva, A.; Niosi, M.; D’Auria, M.; Loguercio, C. Effects of a new pharmacological complex (silybin + vitamin-E + phospholipids) on some markers of the metabolic syndrome and of liver fibrosis in patients with hepatic steatosis. Preliminary study. Minerva Gastroenterol. Dietol. 2005, 51, 193–199. [Google Scholar]
- Federico, A.; Trappoliere, M.; Tuccillo, C.; de Sio, I.; Di Leva, A.; Del Vecchio Blanco, C.; Loguercio, C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: Preliminary observations. Gut 2006, 55, 901–902. [Google Scholar] [CrossRef]
- Loguercio, C.; Andreone, P.; Brisc, C.; Brisc, M.C.; Bugianesi, E.; Chiaramonte, M.; Cursaro, C.; Danila, M.; de Sio, I.; Floreani, A.; et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free. Radic. Biol. Med. 2012, 52, 1658–1665. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; Gómez, S.; Tafur, C.; González, G.; Berroa, E.; Mora, N.; González, J.M.; de Luis, D.A. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3118–3124. [Google Scholar]
- Sorrentino, G.; Crispino, P.; Coppola, D.; De Stefano, G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: A controlled clinical study. Drugs RD 2015, 15, 21–25. [Google Scholar] [CrossRef]
- Wah Kheong, C.; Nik Mustapha, N.R.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 1940–1949.e1948. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; Santamarina, A.B.; de Freitas, J.A.; Trarbach, E.B.; de Oliveira, D.R.; Palace-Berl, F.; de Souza, E.; de Miranda, D.A.; Escamilla-Garcia, A.; Otoch, J.P.; et al. Novel nutraceutical supplements with yeast β-glucan, prebiotics, minerals, and Silybum marianum (silymarin) ameliorate obesity-related metabolic and clinical parameters: A double-blind randomized trial. Front. Endocrinol. 2022, 13, 1089938. [Google Scholar] [CrossRef] [PubMed]
- Eilam, Y.; Pintel, N.; Khattib, H.; Shagug, N.; Taha, R.; Avni, D. Regulation of Cholesterol Metabolism by Phytochemicals Derived from Algae and Edible Mushrooms in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 3667. [Google Scholar] [CrossRef] [PubMed]
- Bakırhan, H.; Irgat, S. Dietary Patterns and Cardiovascular Risk: Are the Mediterranean Diet, the Heart-Healthy Diet, and Phytochemicals Associated with Lower Cardiovascular Risks? Iran. J. Public Health 2023, 52, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).