Effects of the N-Butanol Extract of Pulsatilla Decoction on Neutrophils in a Mouse Model of Ulcerative Colitis
Abstract
1. Introduction
2. Results
2.1. Main Components of BEPD
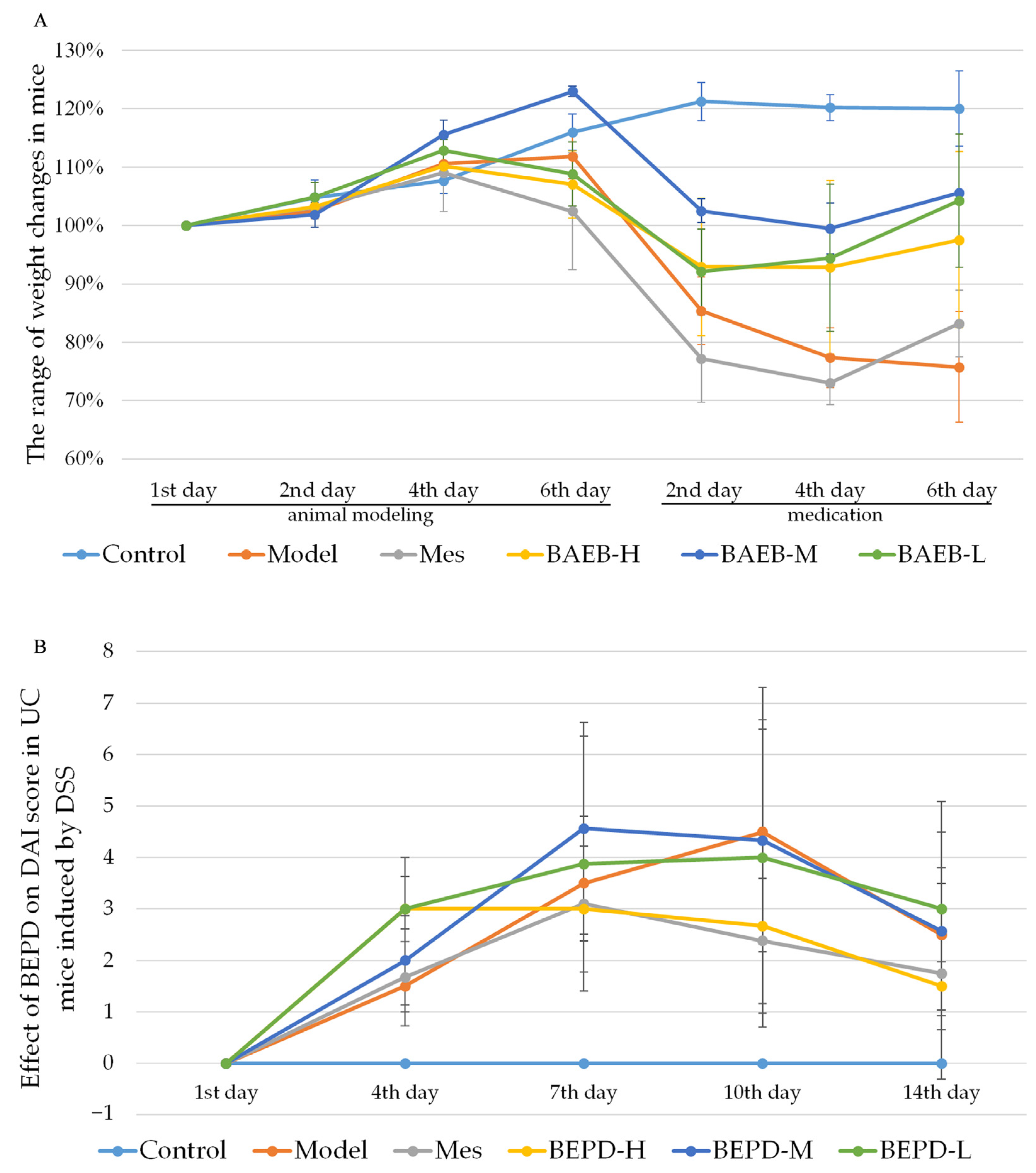
2.2. BEPD Significantly Improves the General Condition and Disease Activity Index (DAI) Scores of Mice with UC
2.3. Impact of BEPD on UC Lesions
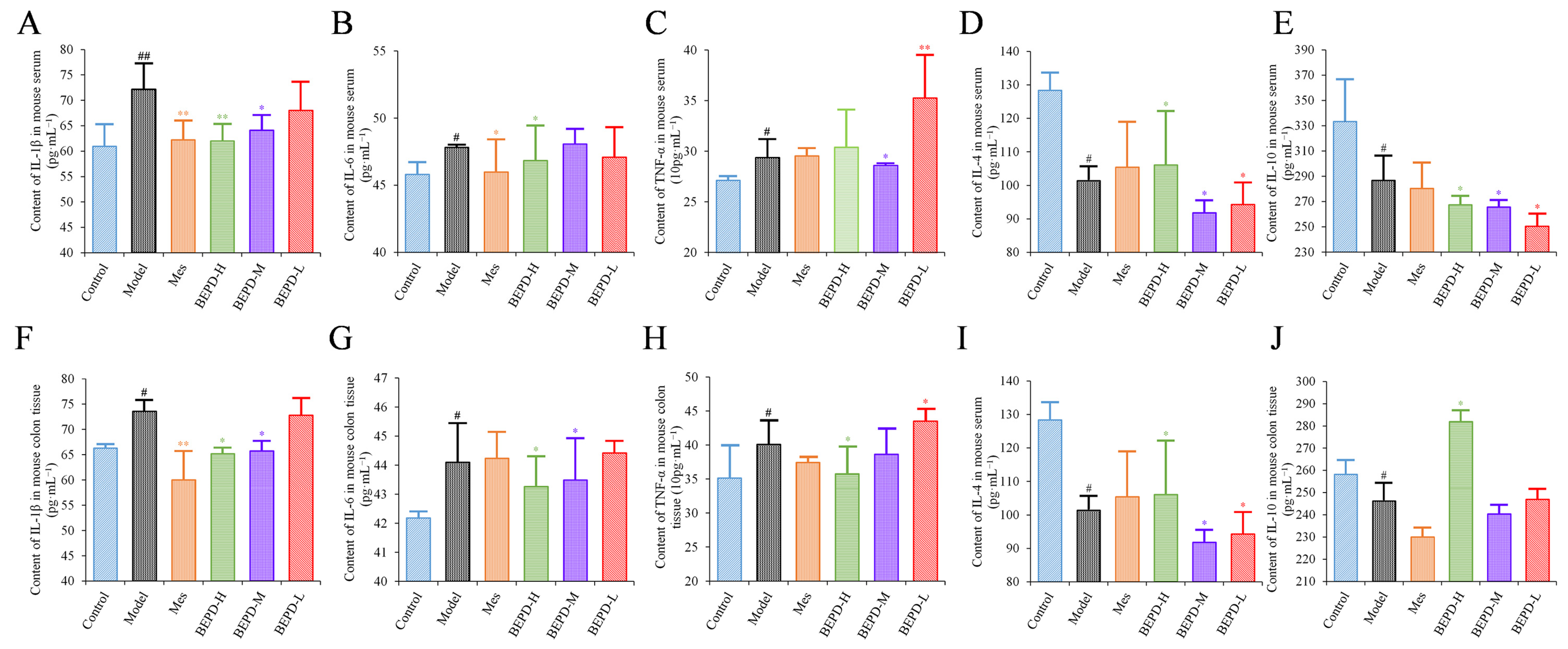
2.4. Effects of BEPD on Cytokine Production
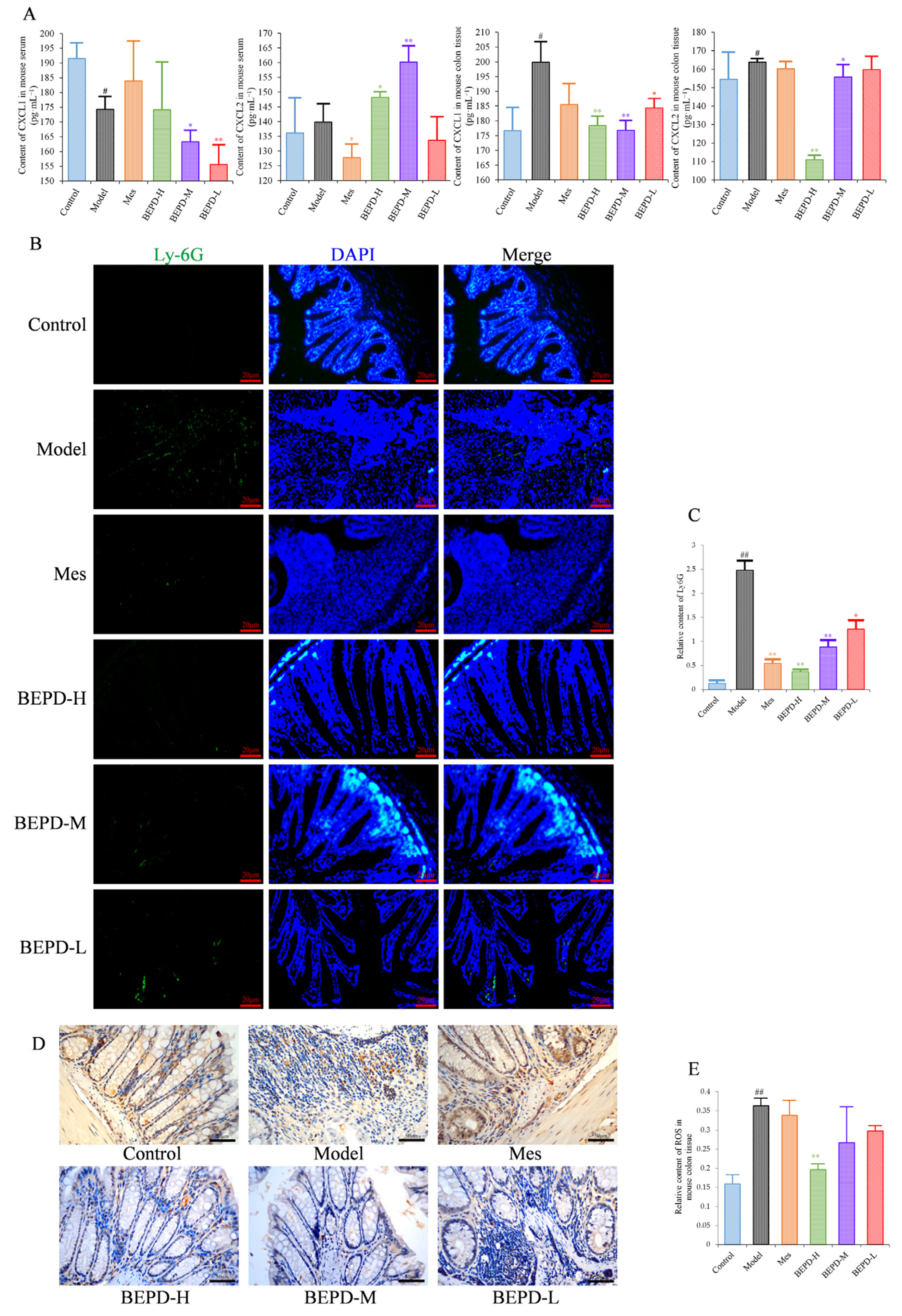
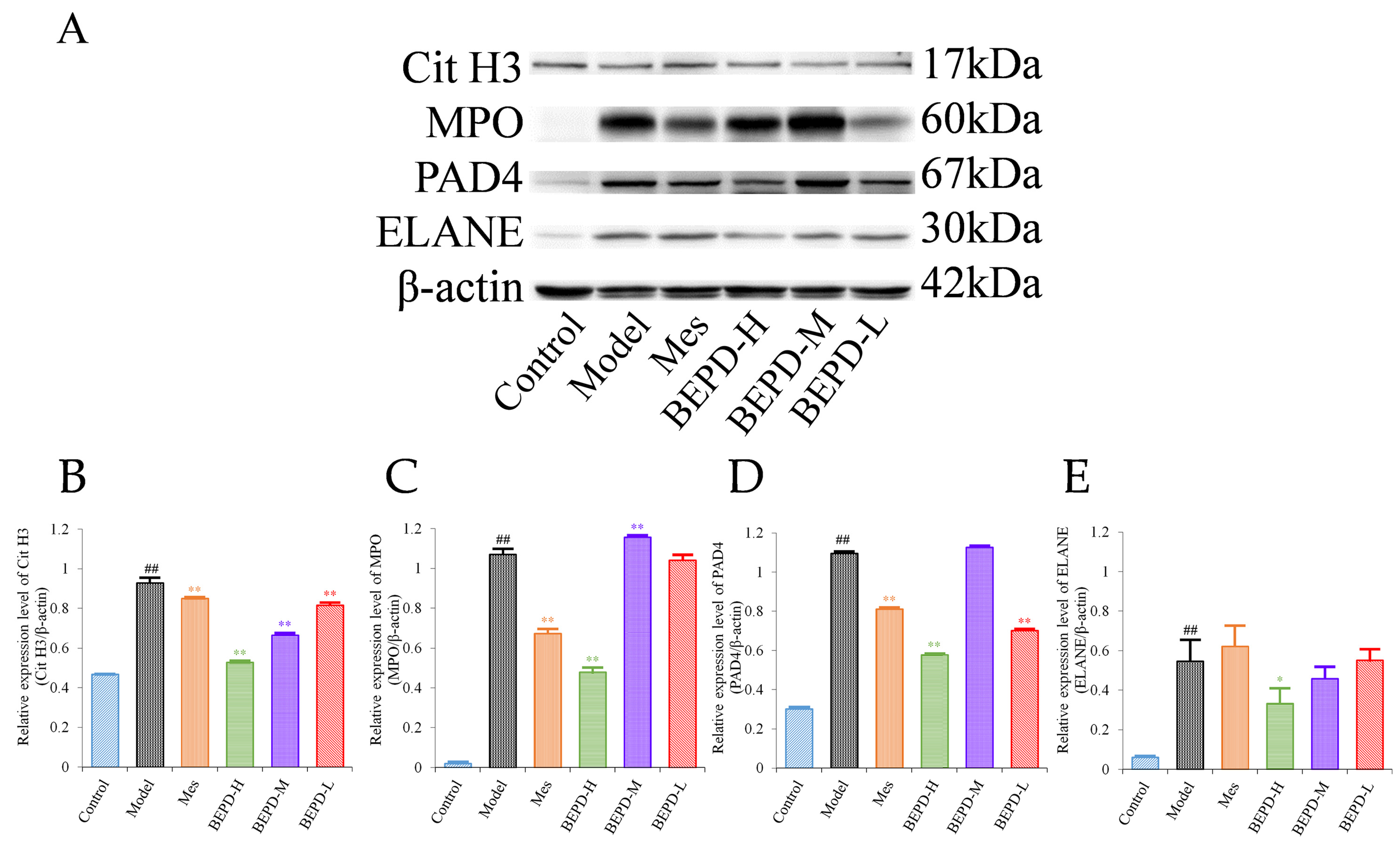
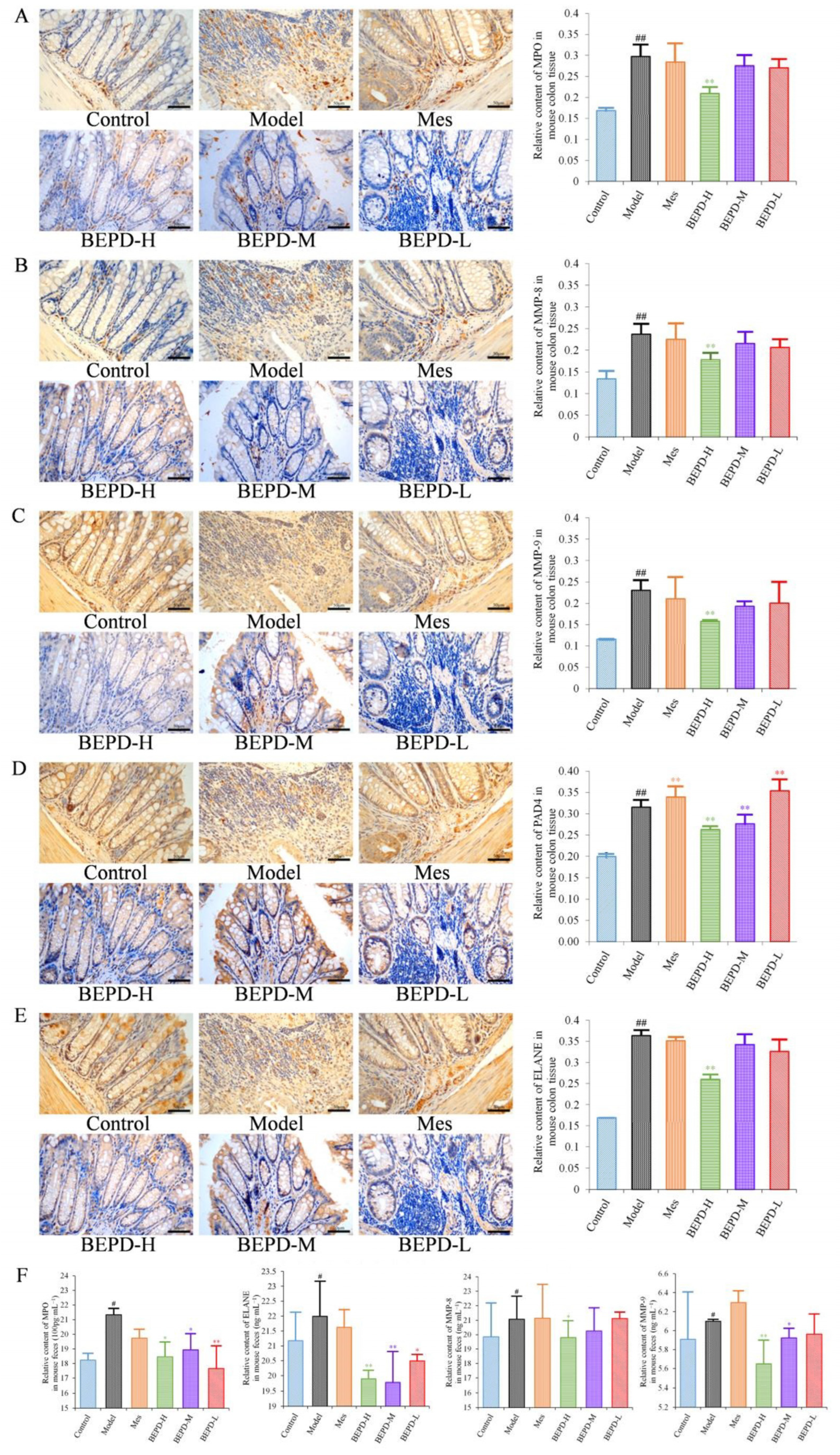
2.5. Effects of BEPD on Chemokine Production and PMN Infiltration and Activation
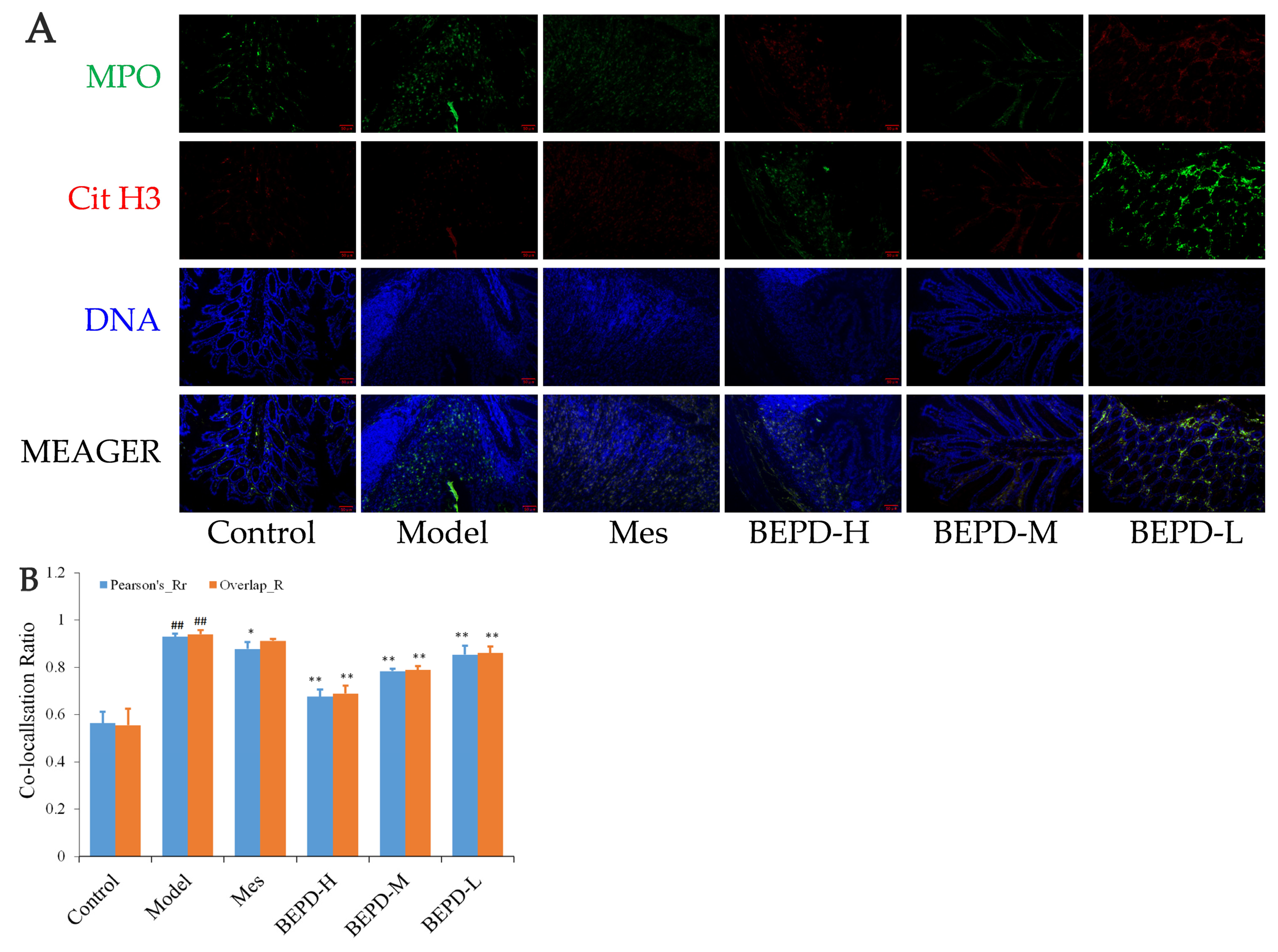
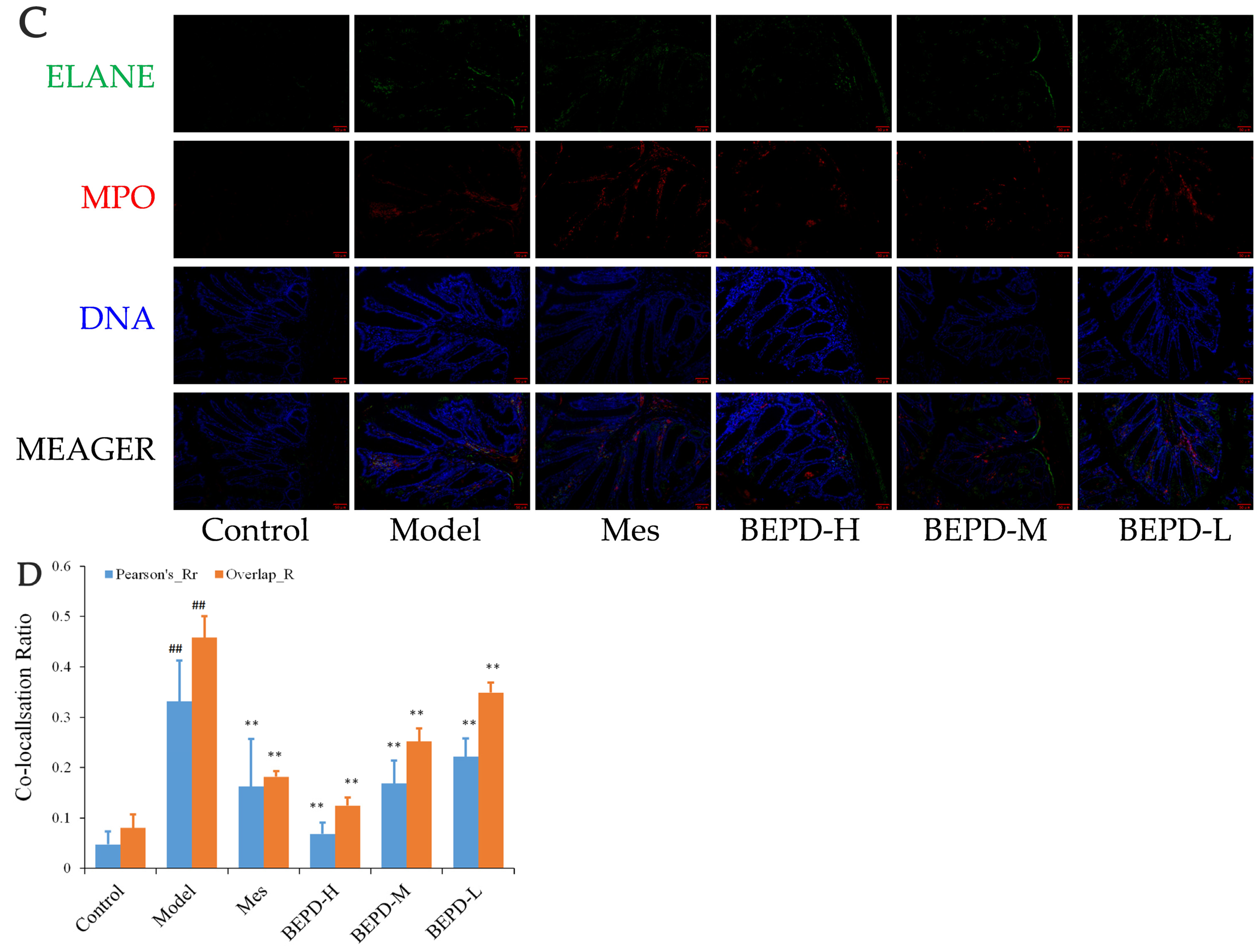
2.6. Effects of BEPD on the Formation and Release of NETs
2.7. Effect of BEPD on Extracellular NET
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Qualitative Analysis of BEPD Extract via HPLC
4.3. Establishment and Treatment of the UC Model
4.4. General Condition of Mice and Disease Activity Index Scores
4.5. Colonic Mucosal Permeability Detection
4.6. Histopathology
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Immunohistochemistry
4.9. Immunofluorescence
4.10. Western Blotting
4.11. NET Visualization
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patnaude, L.; Mayo, M.; Mario, R.; Wu, X.M.; Knight, H.; Creamer, K.; Wilson, S.; Pivorunas, V.; Karman, J.; Phillips, L.; et al. Mechanisms and regulation of IL-22-mediated intestinal epithelial homeostasis and repair. Life Sci. 2021, 271, 119195. [Google Scholar] [CrossRef]
- Xu, N.; Bai, X.L.; Cao, X.L.; Yue, W.J.; Jiang, W.W.; Yu, Z.H. Changes in intestinal microbiota and correlation with TLRs in ulcerative colitis in the coastal area of northern China. Microb. Pathog. 2021, 150, 104707. [Google Scholar] [CrossRef]
- Ye, B.; Lai, L.Q. Yu Shi An Chang Fang Ameliorates TNBS-Induced Colitis in Mice by Reducing Inflammatory Response and Protecting the Intestinal Mucosal Barrier. Evid.-Based Complement. Altern. Med. 2021, 2021, 8870901. [Google Scholar] [CrossRef]
- Formiga, R.d.O.; Alves Júnior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araújo, A.; Carvalho, T.G.d.; Garcia, V.B.; de Araújo Junior, R.F.; Gadelha, F.A.A.F.; Vieira, G.C.; et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Nyström, E.E.L.; Martinez-Abad, B.; Arike, L.; Birchenough, G.M.H.; Nonnecke, E.B.; Castillo, P.A.; Svensson, F.; Bevins, C.L.; Hansson, G.C.; Johansson, M.E.V. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 2021, 372, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choe, J.; Kim, S.O.; Lee, S.H.; Lee, H.J.; Seo, H.; Kim, G.U.; Seo, M.; Song, E.M.; Hwang, S.W.; et al. Overall and cause-specific mortality in Korean patients with inflammatory bowel disease: A hospital-based cohort study. J. Gastroen Hepatol. 2017, 32, 782–788. [Google Scholar] [CrossRef]
- Kassam, Z.; Belga, S.; Roifman, I.; Hirota, S.; Jijon, H.; Kaplan, G.G.; Ghosh, S.; Beck, P.L. Inflammatory Bowel Disease Cause-specific Mortality: A Primer for Clinicians. Inflamm. Bowel Dis. 2014, 20, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Witte, J.; Shivananda, S.; Lennard-Jones, J.E.; Beltrami, M.; Politi, P.; Bonanomi, A.; Tsianos, E.V.; Mouzas, I.; Schulz, T.B.; Monteiro, E.; et al. Disease outcome in inflammatory bowel disease: Mortality, morbidity and therapeutic management of a 796-person inception cohort in the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Scand. J. Gastroenterol. 2000, 35, 1272–1277. [Google Scholar] [CrossRef]
- Lavie, L.; Dyugovskaya, L.; Polyakov, A.; Rogovoy, O.; Leder, E. Development and Identification of a Novel Subpopulation of Human Neutrophil-derived Giant Phagocytes In Vitro. Jove J. Vis. Exp. 2017, 119, e54826. [Google Scholar] [CrossRef]
- Ren, J.; Yan, D.; Wang, Y.; Zhang, J.; Li, M.; Xiong, W.; Jing, X.; Li, P.; Zhao, W.; Xiong, X.; et al. Inhibitor of Differentiation-2 Protein Ameliorates DSS-Induced Ulcerative Colitis by Inhibiting NF-κB Activation in Neutrophils. Front. Immunol. 2021, 12, 760999. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, I.; Chrysanthopoulou, A.; Mitsios, A.; Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Ritis, D.; Tsironidou, V.; Moschos, I.; Dalla, V.; et al. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hafez, A.; Mohamed, A.S.; Shehta, A.; Sheta, H. Neutrophil extracellular traps-associated protein peptidyl arginine deaminase 4 immunohistochemical expression in ulcerative colitis and its association with the prognostic predictors. Pathol. Res. Pract. 2020, 216, 153102. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Szabo, A.; Hsu, C.L.; Krier-Burris, R.A.; Schroeder, H.A.; Wang, M.Y.; Carter, R.G.; Velez, T.E.; Aguiniga, L.M.; Brown, J.B.; et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal Immunol. 2018, 11, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.Q.; Liu, J.; Zhang, S.H.; Fei, J.X.; Li, J.; Zhang, T.; Wang, J.D.; Park, P.W.; Chen, Y. Cell surface-anchored syndecan-1 ameliorates intestinal inflammation and neutrophil transmigration in ulcerative colitis. J. Cell Mol. Med. 2017, 21, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.F.; Blank, A.; Reardon, C.A.; et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 2021, 184, 3163–3177. [Google Scholar] [CrossRef] [PubMed]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Bottcher, G.; Clausen, I.G.; Karrman Mardh, C.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.F.; Liu, D.; Zhang, T.T.; You, Q.; Huang, F.J.; Wu, J. Oral delivery of staphylococcal nuclease ameliorates DSS induced ulcerative colitis in mice via degrading intestinal neutrophil extracellular traps. Ecotoxicol. Environ. Safe 2021, 215, 112161. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosamnn, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Ramos, A.D.; Viana, G.C.S.; Brigido, M.M.; Almeida, J.F. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets. Pharmacol. Res. 2021, 171, 105779. [Google Scholar] [CrossRef]
- Niu, S.J.; Zhang, X.Y.; Ding, X.K.; Shi, H.Y.; Guo, X.J. The Review of Etiology and Pathogenesis of Ulcerative Colitis. Henan Tradit. Chin. Med. 2019, 39, 799–801. [Google Scholar] [CrossRef]
- Liu, X.Y.; He, S.W.; Li, Q.Y.; Mu, X.; Hu, G.; Dong, H. Comparison of the gut microbiota between pulsatilla decoction and levofloxacin hydrochloride therapy on Escherichia coli infection. Front. Cell. Infect. Microbiol. 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zou, M.; Han, Q.; Deng, L.R.; Weinshilboum, R.M. Therapeutic potential of triterpenoid saponin anemoside B4 from Pulsatilla chinensis. Pharmacol. Res. 2020, 160, 105079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Jiang, H.; Yang, J.; Wang, Q.; Du, Y.; Xu, H. Integrated strategy for accurately screening biomarkers based on metabolomics coupled with network pharmacology. Talanta 2020, 211, 120710. [Google Scholar] [CrossRef]
- Wang, Y.D.; Xu, Z.Q.; Xia, D.; Zhang, M.X.; Wang, T.M.; Shao, J.; Wang, C.Z. Mechanism of butyl alcohol extract of Baitouweng Decoction on ulcerative colitis in mice colonized by Candida albicans based on NLRP3 inflammasome. Chin. Tradit. Herb. Drugs 2022, 53, 3997–4006. [Google Scholar]
- Jiang, X.J.; Wang, Y.D.; Sun, J.; Ma, K.L.; Wu, D.Q.; Shao, J.; Wang, T.M.; Wang, C.Z. Mechanism of n-butanol extract of Pulsatilla Decoction in treating ulcerative colitis by activating BMP signaling pathway. China J. Chin. Mater. Medica 2024, 49, 1762–1773. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating Intestinal Inflammation in DSS-induced Model of IBD. Jove-J. Vis. Exp. 2012, 60, e3678. [Google Scholar] [CrossRef]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohns Colitis 2021, 16, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, Y.; Uchiyama, K.; Takagi, T.; Mizushima, K.; Asaeda, K.; Mariko Kajiwara-Kubota, M.; Kashiwagi, S.; Hotta, Y.; Tanaka, M.; Inoue, K.; et al. Retrospective investigation of mesalamine intolerance in patients with ulcerative colitis. J. Clin. Biochem. Nutr. 2022, 71, 249–254. [Google Scholar] [CrossRef]
- Hiraoka, S.; Fujiwara, A.; Toyokawa, T.; Higashi, R.; Moritou, Y.; Takagi, S.; Matsueda, K.; Suzuki, S.; Miyaike, J.; Inokuchi, T.; et al. Multicenter survey on mesalamine intolerance in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2021, 36, 137–143. [Google Scholar] [CrossRef]
- Xie, C.; Quan, R.; Hong, F.; Zou, K.F.; Yan, W.; Fu, Y. The culprit of mesalamine intolerance: Case series and literature review. BMC Gastroenterol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Kucharzik, T. Living Guideline Colitis ulcerosa. Chirurg 2022, 93, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Calafat, M.; Mañosa, M.; Cañete, F.; Domènech, E. Clinical Considerations Regarding the Use of Thiopurines in Older Patients with Inflammatory Bowel Disease. Drugs Aging 2021, 38, 193–203. [Google Scholar] [CrossRef]
- Farkas, K.; Molnár, T. A Review on Biosimilar Infliximab, CT-P13, in the Treatment of Inflammatory Bowel Disease. Immunotherapy 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Niu, C.; Hu, X.L.; Yuan, Z.W.; Xiao, Y.; Ji, P.; Wei, Y.M.; Hua, Y.L. Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity. J. Ethnopharmacol. 2023, 300, 115741. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, J.X.; Shen, H.; Tang, X.D. Experts consensus on traditional Chinese medicine diagnosis and treatment of ulcerative colitis(2023). Chin. J. Tradit. Chin. Med. Pharm. 2024, 39, 288–296. [Google Scholar]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Choteau, L.; Vancraeyneste, H.; le Roy, D.; Dubuquoy, L.; Romani, L.; Jouault, T.; Poulain, D.; Sendid, B.; Calandra, T.; Roger, T.; et al. Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination. Gut Pathog. 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.L.; Han, Z.J.; Pan, M.; Chen, M.L.; Ge, Y.Z.; Shao, J.; Wu, D.Q.; Wang, T.M.; Yan, G.M.; Wang, C.Z. Therapeutic effect of cinnamaldehyde on ulcerative colitis in mice induced bydextran sulfate sodium with Candida albicans colonization and its effect ondectin-1/TLRs/NF-κB signaling pathway. China J. Chin. Mater. Medica 2020, 45, 3211–3219. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETS in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Hu, K.F.; Zhang, H.; Shi, G.X.; Wang, B.F.; Wu, D.Q.; Shao, J.; Wang, T.M.; Wang, C.Z. Effects of n-butanol extract of Pulsatilla decoction on the NLRP3 inflammasome in macrophages infectedwith Candida albicans. J. Ethnopharmacol. 2023, 304, 116041. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Jeong, Y.J.; Lee, J.W.; Jhun, J.Y.; Na, H.S.; Cho, K.H.; Kim, S.J.; Cho, M.L.; Heo, T.H. Tannic acid, an IL-1β-direct binding compound, ameliorates IL-1β-induced inflammation and cartilage degradation by hindering IL-1β-IL-1R1 interaction. PLoS ONE 2023, 18, e0281834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Yao, Z.X.; Chen, L.; Li, J.H.; Chen, S.; Fan, C.Y. Preclinical assessment of IL-1β primed human umbilical cord mesenchymal stem cells for tendon functional repair through TGF-β/IL-10 signaling. Heliyon 2023, 9, e21411. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, Y.Y.; Zhu, X.D.; Yang, J.C.; Liu, Z.H.; Chen, Y.X.; Wang, J.; Shang, R.Y.; Zheng, W.X.; Zhang, X.R.; et al. P311 Promotes IL-4 Receptor-Mediated M2 Polarization of Macrophages to Enhance Angiogenesis for Efficient Skin Wound Healing. J. Investig. Dermatol. 2023, 143, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Tian, G.; Duan, Q.J.; Wu, D.Q.; Shao, J.; Wang, T.M.; Wang, C.Z. Therapeutic potential of n-butanol extract of Pulsatilla decoction in a murine model of ulcerative colitis induced by DSS combined with Candida albicans colonization. China J. Chin. Mater. Medica 2018, 43, 2979–2984. [Google Scholar] [CrossRef]
- Guo, W.J.; Liu, W.; Jin, B.; Geng, J.; Li, J.; Ding, H.Q.; Wu, X.F.; Xu, Q.; Sun, Y.; Gao, J. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2015, 24, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Du, L.D.; Ma, Q.L.; Wu, G.T.; Shao, J.; Cao, R.B.; Zang, K.H.; Ren, Y. Mechanism Study of Wumeiwan in the Treatment of Ulcerative Colitis ViaIKKα/NF-κB/COX-2 Signaling Pathways in the Colon tissue of Rats. Pharmacol. Clin. Chin. Mater. Medica 2021, 37, 3–7. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Xu, Y.S.; Tang, M.; Xin, W.F. The effect of olsalazine of chinese generic drugs on ulcerative colitis induced by dextran sulfate sodium salt in BALB/c mice. Acta Cir. Bras. 2023, 38, e382923. [Google Scholar] [CrossRef]
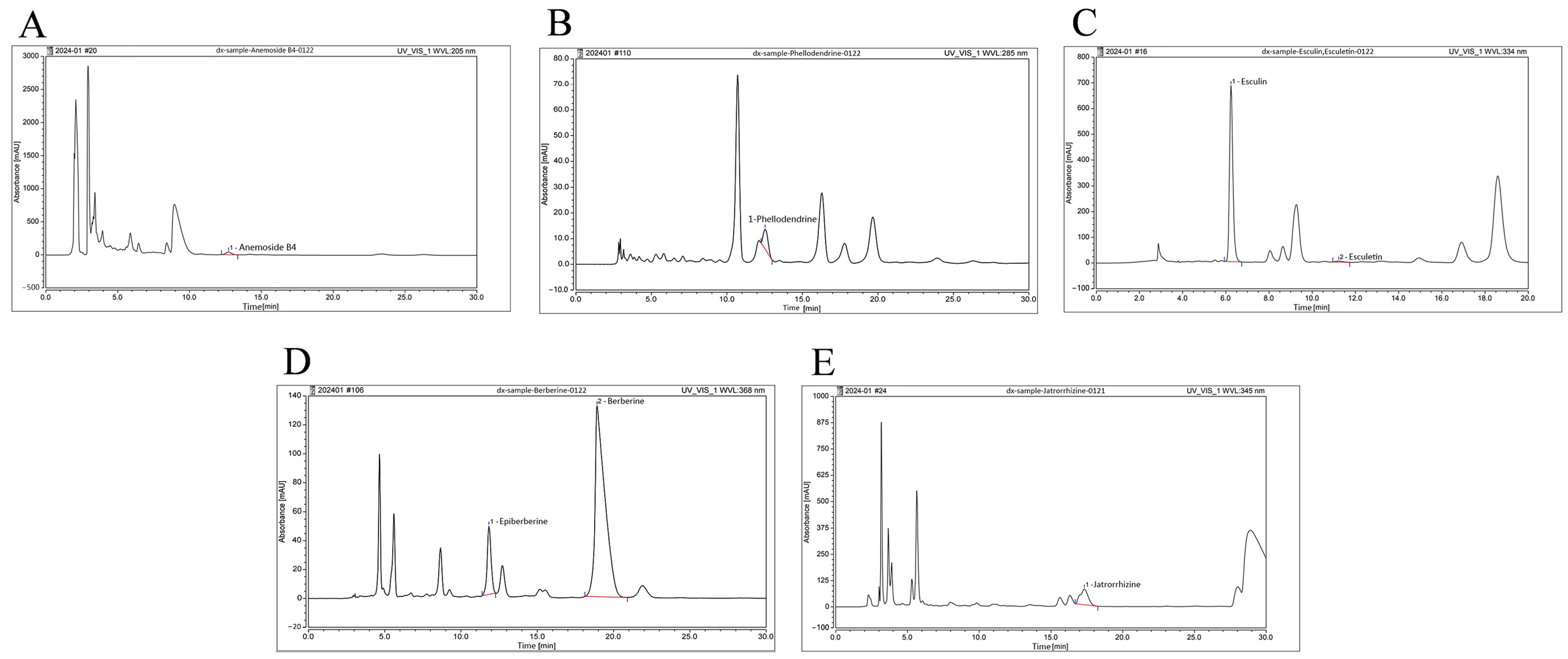

| Main Components | Retention Time (min) | Peak Area (mAU × min) | Content (μg/g) |
|---|---|---|---|
| Anemoside B4 | 12.711 | 17.506 | 78,116.529 |
| Phellodendrine | 12.530 | 2.828 | 3294.236 |
| Berberine | 18.913 | 99.167 | 65,110.351 |
| Epiberberine | 11.833 | 13.923 | 4177.739 |
| Esculin | 6.238 | 124.418 | 59,261.456 |
| Esculetin | 11.223 | 1.531 | 514.884 |
| Jatrorrhizine | 17.328 | 47.883 | 14,949.716 |
| Score | Weight Loss (%) | Stool Condition | Fecal Blood |
|---|---|---|---|
| 0 | n ≤ 1% | formed, moderate hardness | no abnormalities |
| 1 | 1% < n ≤ 5% | formed, soft, not adhering to the perianal area | feces with dark red spots |
| 2 | 5% < n ≤ 10% | soft and sticky around the anus | feces with dark red spots and visible bleeding around the anus |
| 3 | n > 10% | unformed, adhering to the perianal area, diarrhea | deep red feces with adhesion of perianal blood |
| Score | Severity of Inflammation | Range of Inflammation | Amount of Crypt Damage |
|---|---|---|---|
| 0 | none | none | none |
| 1 | mild | mucosa | 1/3 damaged |
| 2 | moderate | mucosa and submucosa | 2/3 damaged, and epithelial surface present |
| 3 | severe | transmural | crypts and epithelial surface lost |
| Score | Mucosal Injury Degree |
|---|---|
| 0 | normal intestinal mucosa |
| 1 | mucosa congestion without ulcer lesions and bleeding |
| 2 | sporadic mucosal ulcer or slight bleeding |
| 3 | extensive ulcer necrosis or adhesion of intestinal mucosa and bleeding |
| 4 | severe bleeding and megacolon or stenosis or perforation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, H.; Sun, J.; Li, C.; Fang, Y.; Shi, G.; Ma, K.; Wu, D.; Shao, J.; Song, H.; et al. Effects of the N-Butanol Extract of Pulsatilla Decoction on Neutrophils in a Mouse Model of Ulcerative Colitis. Pharmaceuticals 2024, 17, 1077. https://doi.org/10.3390/ph17081077
Wang Y, Wu H, Sun J, Li C, Fang Y, Shi G, Ma K, Wu D, Shao J, Song H, et al. Effects of the N-Butanol Extract of Pulsatilla Decoction on Neutrophils in a Mouse Model of Ulcerative Colitis. Pharmaceuticals. 2024; 17(8):1077. https://doi.org/10.3390/ph17081077
Chicago/Turabian StyleWang, Yadong, Hui Wu, Juan Sun, Can Li, Ying Fang, Gaoxiang Shi, Kelong Ma, Daqiang Wu, Jing Shao, Hang Song, and et al. 2024. "Effects of the N-Butanol Extract of Pulsatilla Decoction on Neutrophils in a Mouse Model of Ulcerative Colitis" Pharmaceuticals 17, no. 8: 1077. https://doi.org/10.3390/ph17081077
APA StyleWang, Y., Wu, H., Sun, J., Li, C., Fang, Y., Shi, G., Ma, K., Wu, D., Shao, J., Song, H., Wang, T., & Wang, C. (2024). Effects of the N-Butanol Extract of Pulsatilla Decoction on Neutrophils in a Mouse Model of Ulcerative Colitis. Pharmaceuticals, 17(8), 1077. https://doi.org/10.3390/ph17081077







