Development of a Benzophenone-Free Red Propolis Extract and Evaluation of Its Efficacy against Colon Carcinogenesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Obtaining the Standard Compounds and the Development and Validation of the RP-HPLC-PDA Method for the BFRP Analysis
2.2. Toxicity Assessment
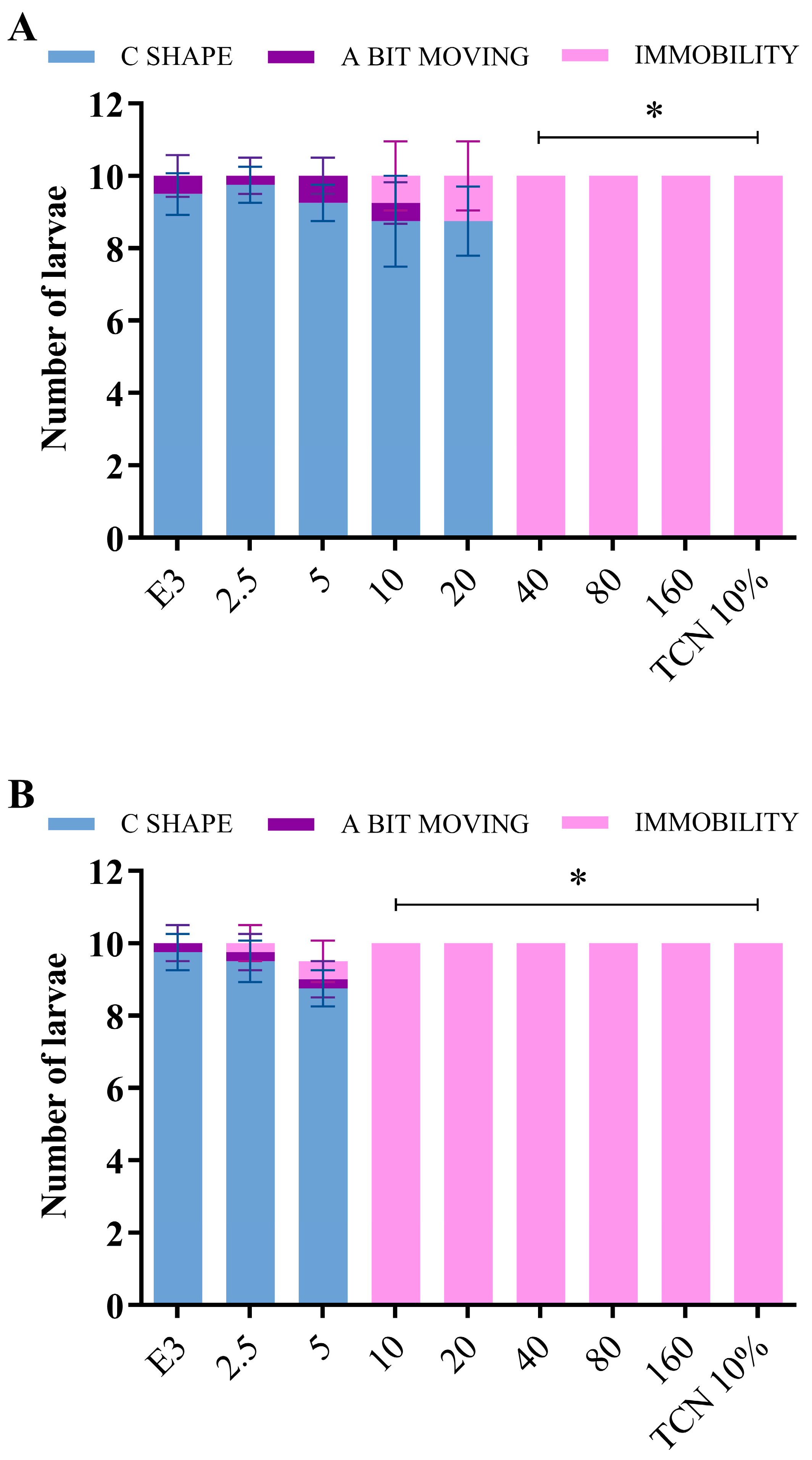
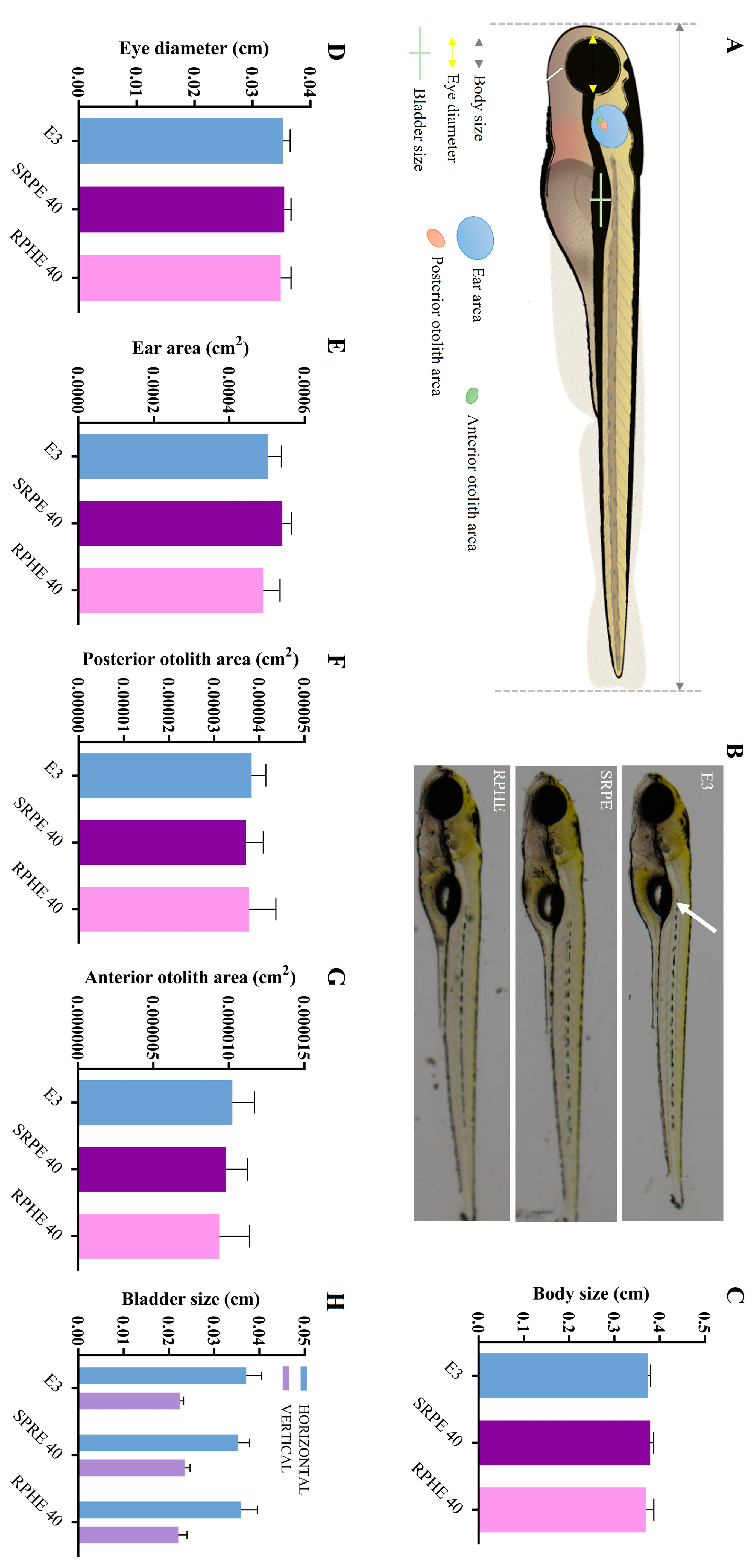
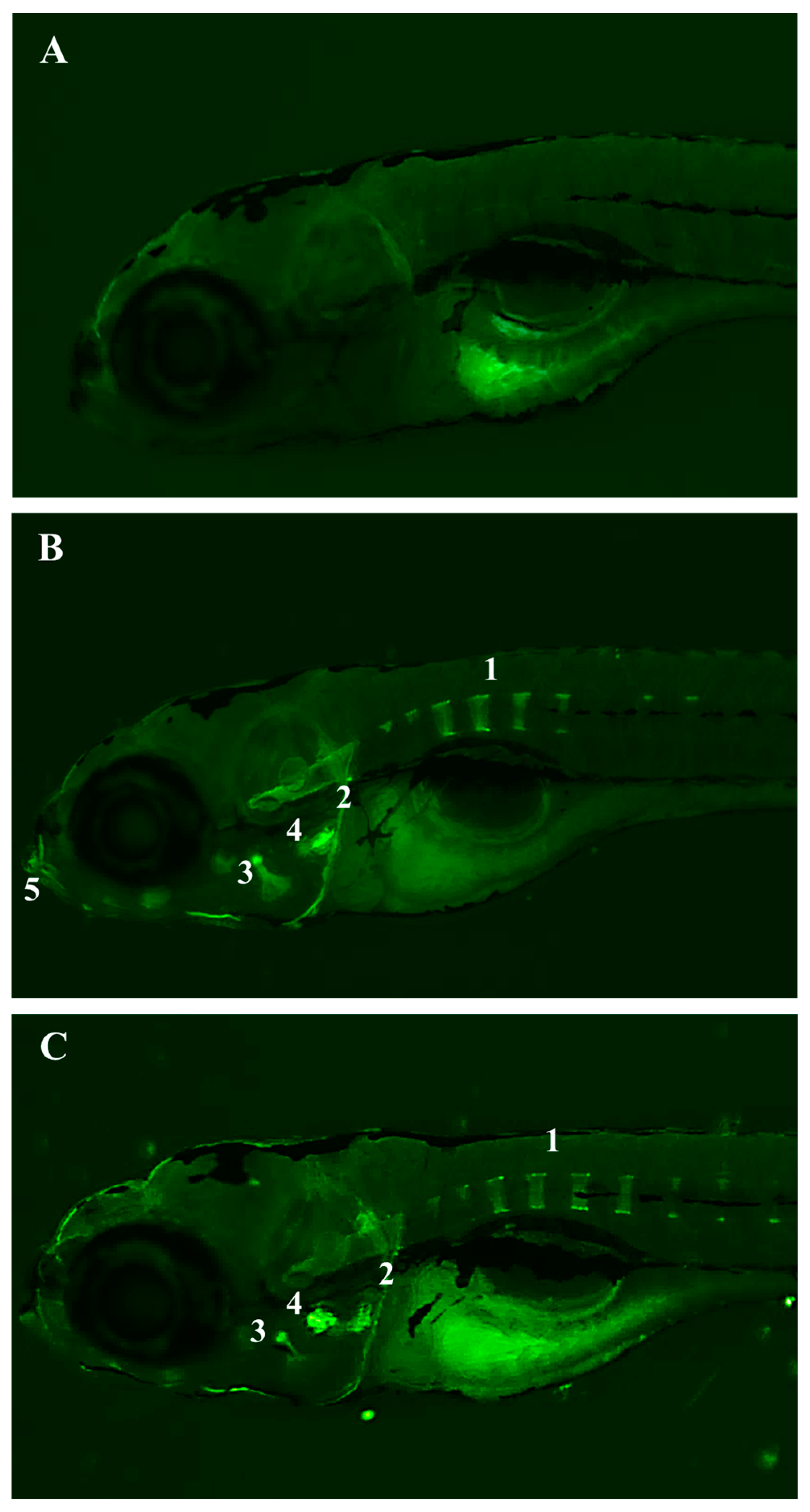
2.2.1. Fish Toxicity Test
2.2.2. Genetic Toxicology Test
2.3. Evaluation of the Effect of BFRP on Preneoplastic Colon Lesions
3. Material and Methods
3.1. Obtaining the Hydroalcoholic Extract of Red Propolis (RPHE) and the Benzophenone-Free Red Propolis Extract (BFRP)
3.2. Isolation and Identification of the Standard Compounds
3.3. RP-HPLC-PDA Method for BFRP Analysis
3.4. Validation of the Method
3.4.1. Selectivity
3.4.2. Linearity, Limit of Detection, and Limit of Quantification
3.4.3. Precision and Accuracy
3.4.4. Recovery
3.4.5. Robustness
3.5. Toxicity Assessment
3.5.1. Fish Toxicity Test
Zebrafish Husbandry
Vibration Startle Response Assay
Morphological Analyses
3.5.2. Genetic Toxicology Test
3.6. Evaluation of the Effect of BFRP on Preneoplastic Colon Lesions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid.-Based Complement. Altern. Med. 2006, 5, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A. Perspectives for uses of propolis in therapy against infectious diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef] [PubMed]
- Rufatto, L.C.; Luchtenberg, P.; Garcia, C.; Thomassigny, C.; Bouttier, S.; Henriques, J.A.P.; Roesch-Ely, M.; Dumas, F.; Moura, S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, I.C.G.; Porto, I.C.; do Nascimento, T.G.; de Souza, N.S.; Oliveira, J.M.; Arruda, R.E.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Souza Silva, T.; Silva, J.M.; Braun, G.H.; Mejia, J.A.; Ccapatinta, G.V.; Santos, M.F.C.; Tanimoto, M.H.; Bastos, J.K.; Parreira, R.L.T.; Orenha, R.P.; et al. Green and red brazilian propolis: Antimicrobial potential and anti-virulence against ATCC and clinically isolated multidrug-resistant bacteria. Chem. Biodivers. 2021, 18, e2100307. [Google Scholar] [CrossRef]
- Oliveira Neto, N.F.; Bonvicini, J.F.S.; de Souza, G.L.; Santiago, M.B.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K.; Silva, M.J.B.; Martins, C.H.G.; Moura, C.C.G.; et al. Antibacterial activity of Brazilian red propolis and in vitro evaluation of free radical production. Arch. Oral Biol. 2022, 143, 105520. [Google Scholar] [CrossRef]
- Ripari, N.; Pereira, A.F.M.; Júnior, A.F.; Rall, V.L.M.; A Aldana-Mejía, J.; Bastos, J.K.; Sforcin, J.M. Brazilian red propolis in combination with β-lactams exerts an efficient antibacterial action over methicillin-resistant Staphylococcus aureus(MRSA) strains. J. Appl. Microbiol. 2022, 134, lxac080. [Google Scholar] [CrossRef]
- Silva, N.B.S.; de Souza, J.H.; Santiago, M.B.; da Silva Aguiar, J.R.; Martins, D.O.S.; da Silva, R.A.; Santos, I.d.A.; Aldana-Mejía, J.A.; Jardim, A.C.G.; Pedroso, R.d.S.; et al. Potential in vitro anti-periodontopathogenic, anti-Chikungunya activities and in vivo toxicity of Brazilian red propolis. Sci. Rep. 2022, 12, 21165. [Google Scholar] [CrossRef]
- Conceição, M.; Gushiken, L.F.S.; Aldana-Mejía, J.A.; Tanimoto, M.H.; Ferreira, M.V.d.S.; Alves, A.C.M.; Miyashita, M.N.; Bastos, J.K.; Beserra, F.P.; Pellizzon, C.H. Histological, immunohistochemical and antioxidant analysis of skin wound healing influenced by the topical application of brazilian red propolis. Antioxidants 2022, 11, 2188. [Google Scholar] [CrossRef]
- Squarisi, I.; De Freitas, K.S.; Lemes, D.C.; Ccapatinta, G.V.C.; Mejía, J.A.A.; Bastos, J.K.; Veneziani, R.C.S.; Ambrosio, S.R.; Tavares, D.C. Evaluation of the antiproliferative activity of red propolis hydroalcoholic extract and its fractions obtained by partition. Biofarmasi J. Nat. Prod. Biochem. 2020, 18, 70–73. [Google Scholar] [CrossRef]
- Santiago, M.B.; Leandro, L.F.; Rosa, R.B.; Silva, M.V.; Teixeira, S.C.; Servato, J.P.S.; Ambrósio, S.R.; Veneziani, R.C.S.; Aldana-Mejía, J.A.; Bastos, J.K.; et al. Brazilian red propolis presents promising anti-H. pylori activity in in vitro and in vivo assays with the ability to modulate the immune response. Molecules 2022, 27, 7310. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.B.; Rodrigues, J.C.Z.; de Oliveira Cardoso, E.; Conte, F.L.; Tasca, K.I.; Romagnoli, G.G.; Aldana-Mejía, J.A.; Bastos, J.K.; Sforcin, J.M. Brazilian red propolis exerts a cytotoxic action against prostate cancer cells and upregulates human monocyte functions. Phytother. Res. 2022, 37, 399–409. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, K.S.; da Silva, L.H.D.; Squarisi, I.S.; de Souza Oliveira, L.T.; Ribeiro, A.B.; Alves, B.S.; Esperandim, T.R.; de Melo, M.R.S.; Ozelin, S.D.; Lemes, D.C.; et al. Red propolis exhibits chemopreventive effect associated with antiproliferative and anti-inflammatory activities. Toxicol. Res. 2022, 11, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; Mejía, J.A.A.; Ccana-Ccapatinta, G.V.; Mariott, M.; Melo Vilhena de Andrade Fonseca Da Silva, R.C.; de Souza, P.; Mariano, L.N.B.; Oliveira, G.R.; da Rocha, I.M.; da Costa, G.A.; et al. The gastroprotective effect of red propolis extract from Northeastern Brazil and the role of its isolated compounds. J. Ethnopharmacol. 2020, 267, 113623. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; de Oliveira, B.M.M.; Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Venzon, L.; Cury, B.J.; França, T.C.S.; de Souza, P.; Junior, W.A.R.; da Silva, L.M.; et al. Brazilian red propolis accelerates gastric healing and reduces gastric submucosal layer inflammation in ultrasound-monitored rats. Chem. Biodivers. 2022, 20, e202200992. [Google Scholar] [CrossRef]
- Silva, L.H.D.; Squarisi, I.S.; de Freitas, K.S.; Barcelos Ribeiro, A.; Ozelin, S.D.; Aldana-Mejía, J.A.; de Oliveira, L.T.S.; Rodrigues, T.E.; de Melo, M.R.S.; Nicolella, H.D.; et al. Toxicological and chemoprevention studies of Dalbergia ecastaphyllum (L.) Taub. stem, the botanical source of Brazilian red propolis. J. Pharm. Pharmacol. 2022, 74, 740–749. [Google Scholar] [CrossRef]
- Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Squarisi, I.S.; Nascimento, S.; Tanimoto, M.H.; Ribeiro, V.P.; Arruda, C.; Nicolella, H.; Esperandim, T.; Ribeiro, A.B.; et al. Nonclinical toxicological studies of brazilian red propolis and its primary botanical source Dalbergia ecastaphyllum. Chem. Res. Toxicol. 2021, 34, 1024–1033. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; de Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The botanical sources of isoflavonoids and benzophenones in brazilian red propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Russo, G.L.; Spagnuolo, C.; Russo, M. Reassessing the role of phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2024, 228, 116165. [Google Scholar] [CrossRef]
- Aldana-Mejía, J.A.; de Miranda, A.M.; Ccana-Ccapatinta, G.V.; de Araújo, L.S.; Ribeiro, V.P.; Arruda, C.; Nascimento, S.; Squarisi, I.; Esperandim, T.; de Freitas, K.S.; et al. Genotoxicity and toxicological evaluations of Brazilian red propolis oral ingestion in a preclinical rodent model. J. Ethnopharmacol. 2023, 303, 115920. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Resolução da Diretoria Colegiada—RCD No. 166; 24 July 2017; Ministério da Saúde—MS: Brasilia, Brazil, 2017; p. 21. [Google Scholar]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.d.S.; da Silva, J.; Garcia, A.L.H.; Boaretto, F.B.M.; Grivicich, I.; Conter, L.U.; Salvi, A.d.O.; Reginatto, F.H.; Vencato, S.B.; Ferraz, A.d.B.F.; et al. Evaluation of DNA damage in HepG2 cells and mutagenicity of garcinielliptone FC, A bioactive benzophenone. Basic Clin. Pharmacol. Toxicol. 2017, 120, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Choodej, S.; Koopklang, K.; Raksat, A.; Chuaypen, N.; Pudhom, K. Bioactive xanthones, benzophenones and biphenyls from mangosteen root with potential anti-migration against hepatocellular carcinoma cells. Sci. Rep. 2022, 12, 8605. [Google Scholar] [CrossRef]
- Crouzier, L.; Diez, C.; Richard, E.M.; Cubedo, N.; Barbereau, C.; Rossel, M.; Delaunay, T.; Maurice, T.; Delprat, B. Loss of Pde6a induces rod outer segment shrinkage and visual alterations in pde6aQ70X mutant zebrafish, a relevant model of retinal dystrophy. Front. Cell Dev. Biol. 2021, 9, 675517. [Google Scholar] [CrossRef]
- Forero, L.L.; Narayanan, R.; Huitema, L.F.; VanBergen, M.; Apschner, A.; Peterson-Maduro, J.; Logister, I.; Valentin, G.; Morelli, L.G.; Oates, A.C.; et al. Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 2018, 7, e33843. [Google Scholar] [CrossRef]
- Shimojo, H.; Kageyama, R. Making waves toward the shore by synchronicity. Dev. Cell 2016, 36, 358–359. [Google Scholar] [CrossRef][Green Version]
- Fleming, A.; Keynes, R.; Tannahill, D. A central role for the notochord in vertebral patterning. Development 2004, 131, 873–880. [Google Scholar] [CrossRef]
- Grotmol, S.; Kryvi, H.; Nordvik, K.; Totland, G.K. Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat. Embryol. 2003, 207, 263–272. [Google Scholar] [CrossRef]
- Wang, S.; Kryvi, H.; Grotmol, S.; Wargelius, A.; Krossøy, C.; Epple, M.; Neues, F.; Furmanek, T.; Totland, G.K. Mineralization of the vertebral bodies inAtlantic salmon (Salmo salar L.) is initiated segmentally in the form of hydroxyapatite crystal accretions in the notochord sheath. Am. J. Anat. 2013, 223, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Inohaya, K.; Takano, Y.; Kudo, A. The teleost intervertebral region acts as a growth center of the centrum: In vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev. Dyn. 2007, 236, 3031–3046. [Google Scholar] [CrossRef] [PubMed]
- Renn, J.; Büttner, A.; To, T.T.; Chan, S.J.H.; Winkler, C. A col10a1:nlGFP transgenic line displays putative osteoblast precursors at the medaka notochordal sheath prior to mineralization. Dev. Biol. 2013, 381, 134–143. [Google Scholar] [CrossRef]
- Angeline, P.; Thomas, A.; Sankaranarayanan, S.A.; Rengan, A.K. Effect of pH on Isoliquiritigenin (ISL) fluorescence in lipo- polymeric system and metallic nanosystem. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119545. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, Y.; Zhang, H.; Rajendran, R.S.; Wang, R.; Hsiao, C.D.; Li, J.; Xia, Q.; Liu, K. Isoliquiritigenin triggers developmental toxicity and oxidative stress–mediated apoptosis in zebrafish embryos/larvae via Nrf2-HO1/JNK-ERK/mitochondrion pathway. Chemosphere 2020, 246, 125727. [Google Scholar] [CrossRef]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Hagmar, L.; Strömberg, U.; Tinnerberg, H.; Mikoczy, Z. Epidemiological evaluation of cytogenetic biomarkers as potential surrogate end-points for cancer. IARC Sci. Publ. 2004, 157, 207–215. [Google Scholar]
- ICH Topic S2[R1]. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use; Published by the authority of the Minister of Health; Health Products and Food Branch: London, UK, 2016. [Google Scholar]
- Silici, S.; Demiray, S.; Okan, A.; Ertuğrul, S.; Alizada, S.; Doğanyiğit, Z. Effects of short- and long-term use of propolis extracts on liver and kidney in rats. Food Sci. Nutr. 2024, 12, 5538–5547. [Google Scholar] [CrossRef]
- Bhargava, P.; Mahanta, D.; Kaul, A.; Ishida, Y.; Terao, K.; Wadhwa, R.; Kaul, S.C. Experimental Evidence for Therapeutic Potentials of Propolis. Nutrients 2021, 13, 2528. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Vinayagam, R.; Anand, M.A.V.; Isa, N.M.; Ponnaiyan, R. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: A review. Toxicol. Res. 2020, 9, 2–18. [Google Scholar] [CrossRef]
- Darband, S.G.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020, 253, 117584. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.B. Effect of oral Lactococcus lactis containing endostatin on 1, 2-dimethylhydrazine-induced colon tumor in rats. World J. Gastroenterol. 2005, 11, 7242. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Kamal, R.; Kanwar, S.S.; Dhawan, D.K. Cyclooxygenase as a target in chemoprevention by probiotics during 1,2-dimethylhydrazine induced colon carcinogenesis in rats. Nutr. Cancer 2015, 67, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wang, X.; Hu, Y.; Su, S.; Li, W.; Li, S. Editorial: Network pharmacology and traditional medicine. Front. Pharmacol. 2020, 11, 1194. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Santos, F.F.; Morais-Urano, R.P.; Cunha, W.R.; de Almeida, S.G.; Cavallari, P.S.D.S.R.; Manuquian, H.A.; Pereira, H.A.; Furtado, R.; Santos, M.F.C.; Amdrade, E.; et al. A review on the anti-inflammatory activities of Brazilian green, brown and red propolis. J. Food Biochem. 2022, 46, e14350. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book; A guide for the laboratory use of zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Hayot, G.; Marcato, D.; von Clausbruch, C.A.C.; Pace, G.; Strähle, U.; Colbourne, J.K.; Pylatiuk, C.; Peravali, R.; Weiss, C.; Scholz, S.; et al. Evaluating Toxicity of Chemicals using a Zebrafish Vibration Startle Response Screening System. J. Vis. Exp. 2024, 203, e66153. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Furtado, R.A.; Oliveira, B.R.; Silva, L.R.; Cleto, S.S.; Munari, C.C.; Cunha, W.R.; Tavares, D.C. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. Eur. J. Cancer Prev. 2015, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
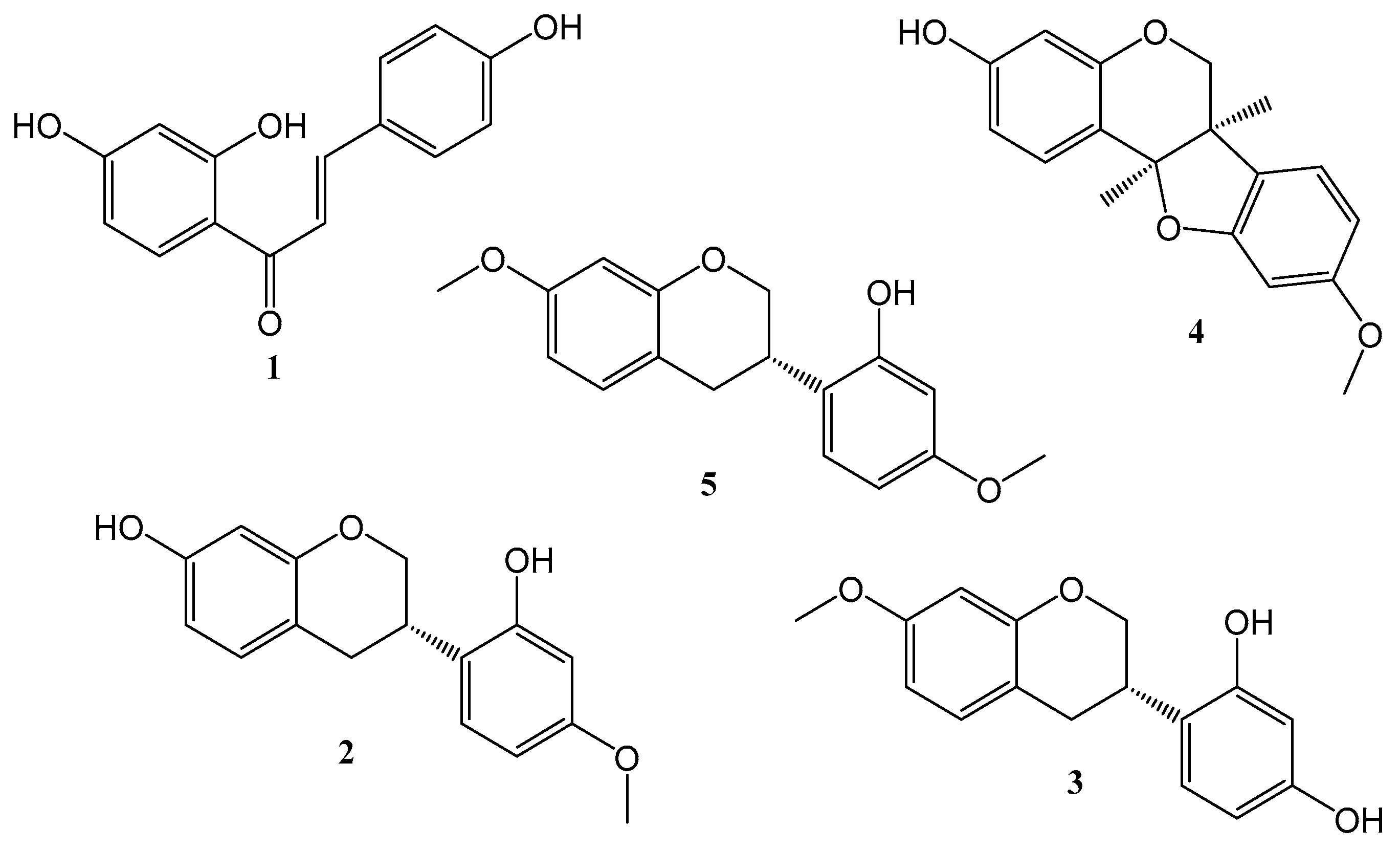
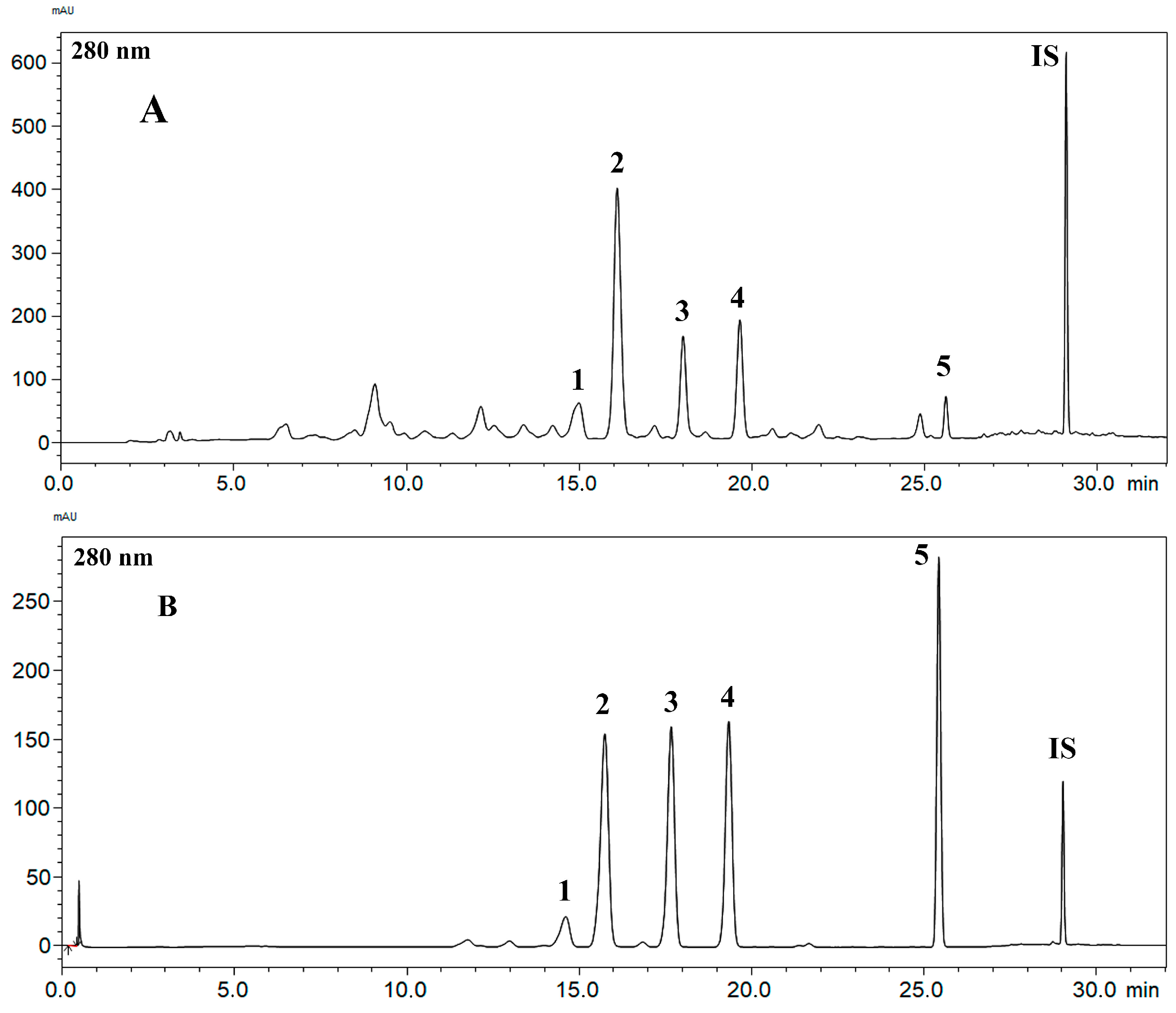

| Compound | Equation | R2 | R | LD | LQ | Minimum Observed Residual Value | Maximum Observed Residual Value | Lack of Fit p Value |
|---|---|---|---|---|---|---|---|---|
| (1) | y = 0.006x + 0.063 | 0.998 | 0.998 | 3.94 | 10.99 | −3.31823 | 3.23520 | 0.61 |
| (2) | y = 0.0356x + 0.3835 | 0.999 | 0.999 | 2.90 | 8.96 | −2.2265 | 2.2288 | 0.79 |
| (3) | y = 0.0313x + 0.4228 | 0.998 | 0.998 | 2.78 | 9.42 | −2.26225 | 1.62954 | 0.74 |
| (4) | y = 0.0293x + 0.4153 | 0.997 | 0.998 | 2.62 | 9.15 | −2.26638 | 1.84199 | 0.81 |
| (5) | y = 0.0292x + 0.4808 | 0.998 | 0.998 | 1.31 | 3.97 | −1.59636 | 1.26722 | 0.93 |
| Compound | Level | Precision (RSD) | Accuracy (%) | E (%) | Recovery (%) | |
|---|---|---|---|---|---|---|
| Intraday | Interday | |||||
| (1) | High | 0.08 | 2.13 | 100.89 ± 2.7 | −0.89 | 104.02 ± 1.2 |
| Medium | 0.79 | 1.38 | 113.93 ± 1.1 | 3.93 | 101.10 ± 2.8 | |
| (2) | Low | 0.66 | 2.97 | 97.93 ± 0.8 | 4.07 | 99.26 ± 1.9 |
| High | 0.35 | 1.65 | 98.49 ± 1.9 | −1.51 | 107.02 ± 0.7 | |
| Medium | 0.65 | 2.99 | 107.7 ± 10.2 | 4.71 | 106.55 ± 1.1 | |
| (3) | Low | 0.46 | 1.15 | 104.04 ± 1.1 | 3.96 | 96.67 ± 0.9 |
| High | 0.57 | 0.72 | 99.50 ± 0.2 | −0.50 | 108.69 ± 1.7 | |
| Medium | 0.61 | 0.44 | 110.92 ± 0.3 | −2.92 | 105.01 ± 1.6 | |
| (4) | Low | 0.39 | 1.25 | 96.05 ± 0.9 | 3.59 | 99.50 ± 2.1 |
| High | 0.51 | 0.76 | 106.38 ± 1.2 | 2.38 | 97.52 ± 1.3 | |
| Medium | 0.56 | 0.43 | 108.05 ± 0.8 | −1.35 | 100.94 ± 2.4 | |
| (5) | Low | 1.36 | 0.37 | 106.50 ± 1.2 | 2.65 | 102.86 ± 1.9 |
| High | 0.61 | 2.63 | 97.89 ± 2.5 | 2.11 | 99.65 ± 0.4 | |
| Medium | 0.44 | 0.43 | 101.94 ± 1.7 | −1.94 | 96.90 ± 1.1 | |
| I.S | Low | 0.43 | 0.97 | 98.94 ± 2.3 | −1.06 | 100.23 ± 2.2 |
| High | 100.98 ± 1.2 | |||||
| Medium | 96.71 ± 0.9 | |||||
| Low | 100.39 ± 1.6 | |||||
| Treatments (mg/kg) | MNPCEs | PCE/PCE + NCE |
|---|---|---|
| DMSO | 6.0 ± 2.0 | 0.04 ± 0.02 |
| 500 | 5.0 ± 1.9 | 0.04 ± 0.01 |
| 1000 | 7.8 ± 2.6 | 0.04 ± 0.01 |
| 2000 | 9.2 ± 1.3 | 0.05 ± 0.00 |
| PC | 30.3 ± 3.2 * | 0.03 ± 0.01 |
| Treatments (mg/kg) | Initial Weight (g) | Final Weight (g) | Weight Gain (g) | Water Consumption (mL/Animal/Day) |
|---|---|---|---|---|
| DMSO | 225.8 ± 5.2 | 352.8 ± 2.7 | 128.0 ± 5.3 | 53.9 ± 18.1 |
| 24 | 229.3 ± 4.0 | 350.3 ± 5.5 | 121.0 ± 4.6 | 50.7 ± 12.1 |
| DMH | 227.0 ± 7.0 | 296.0 ± 9.5 | 89.8 ± 10.4 * | 65.9 ± 22.2 |
| DMSO + DMH | 230.6 ± 7.0 | 295.7 ± 8.1 | 76.8 ± 14.0 * | 49.0 ± 12.6 |
| 3 + DMH | 219.8 ± 6.6 | 340.8 ± 6.2 | 122.2 ± 7.7 | 63.2 ± 12.5 |
| 6 + DMH | 218.8 ± 5.5 | 341.7 ± 9.8 | 121.4 ± 6.9 | 52.2 ± 13.3 |
| 12 + DMH | 225.8 ± 6.9 | 348.2 ± 7.2 | 122.4 ± 8.4 | 73.6 ± 23.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squarisi, I.S.; Ribeiro, V.P.; Ribeiro, A.B.; de Souza, L.T.M.; Junqueira, M.d.M.; de Oliveira, K.M.; Hayot, G.; Dickmeis, T.; Bastos, J.K.; Veneziani, R.C.S.; et al. Development of a Benzophenone-Free Red Propolis Extract and Evaluation of Its Efficacy against Colon Carcinogenesis. Pharmaceuticals 2024, 17, 1340. https://doi.org/10.3390/ph17101340
Squarisi IS, Ribeiro VP, Ribeiro AB, de Souza LTM, Junqueira MdM, de Oliveira KM, Hayot G, Dickmeis T, Bastos JK, Veneziani RCS, et al. Development of a Benzophenone-Free Red Propolis Extract and Evaluation of Its Efficacy against Colon Carcinogenesis. Pharmaceuticals. 2024; 17(10):1340. https://doi.org/10.3390/ph17101340
Chicago/Turabian StyleSquarisi, Iara Silva, Victor Pena Ribeiro, Arthur Barcelos Ribeiro, Letícia Teixeira Marcos de Souza, Marcela de Melo Junqueira, Kátia Mara de Oliveira, Gaelle Hayot, Thomas Dickmeis, Jairo Kenupp Bastos, Rodrigo Cassio Sola Veneziani, and et al. 2024. "Development of a Benzophenone-Free Red Propolis Extract and Evaluation of Its Efficacy against Colon Carcinogenesis" Pharmaceuticals 17, no. 10: 1340. https://doi.org/10.3390/ph17101340
APA StyleSquarisi, I. S., Ribeiro, V. P., Ribeiro, A. B., de Souza, L. T. M., Junqueira, M. d. M., de Oliveira, K. M., Hayot, G., Dickmeis, T., Bastos, J. K., Veneziani, R. C. S., Ambrósio, S. R., & Tavares, D. C. (2024). Development of a Benzophenone-Free Red Propolis Extract and Evaluation of Its Efficacy against Colon Carcinogenesis. Pharmaceuticals, 17(10), 1340. https://doi.org/10.3390/ph17101340







