Plants’ Impact on the Human Brain—Exploring the Neuroprotective and Neurotoxic Potential of Plants
Abstract
1. Introduction
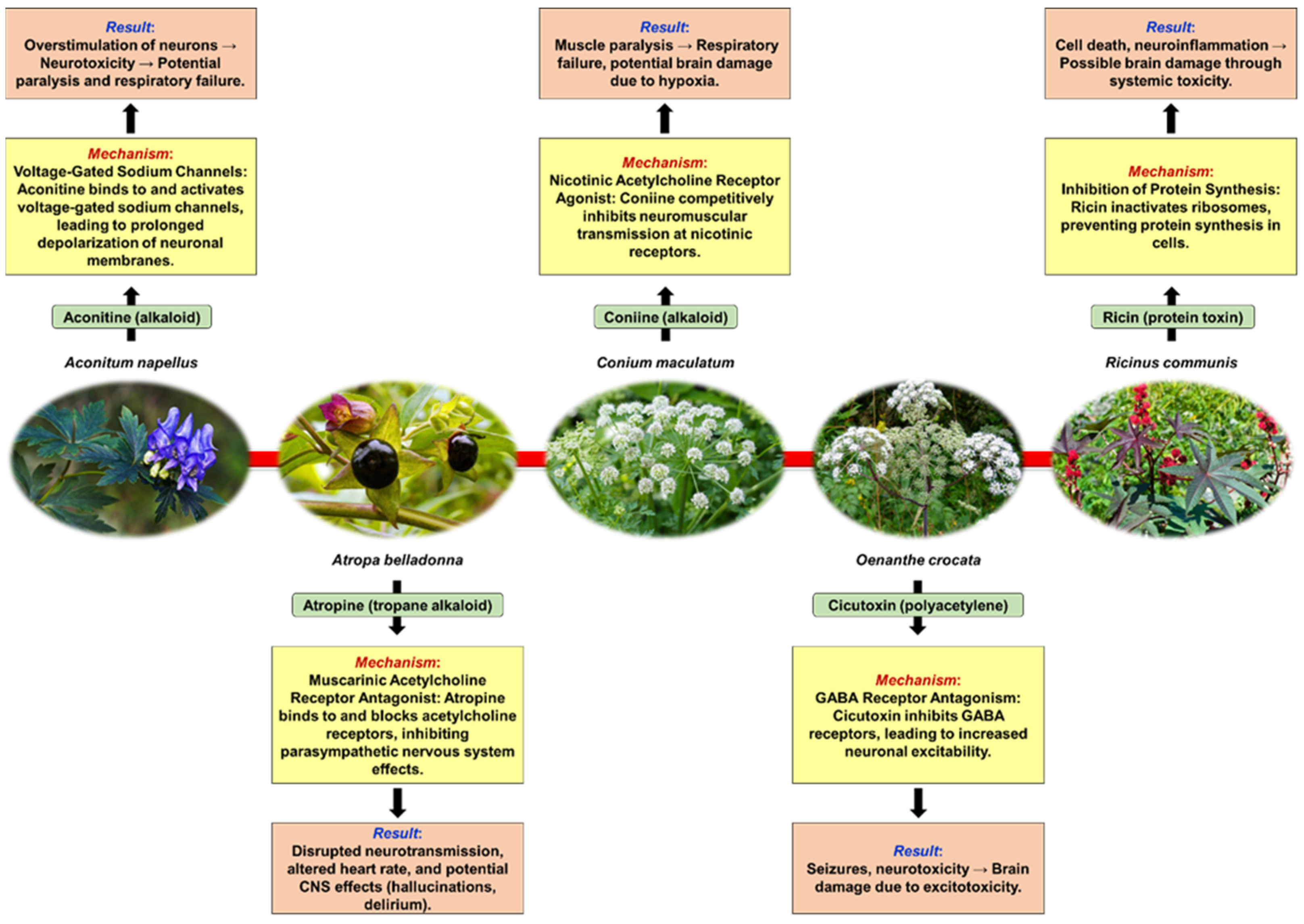
2. Neurotoxic Plants
2.1. Defining Neurotoxicity and Some Mechanisms of Plant-Induced Neurotoxicity
2.2. Common Neurotoxic Plants—Mechanisms of Neurotoxicity, Symptoms of Neurotoxicity, Management of Neurotoxic Plant Poisoning
2.2.1. Aconitum Species (Monkshood)
Historical Background
Relevant Toxic Chemical Compounds, Mechanism of Toxicity
Detoxification Process for Toxic Chemical Compounds, Mechanism of Toxicity
Poisoning
General Conclusion
2.2.2. Atropa belladonna (Deadly Nightshade)
Historical Background
Relevant Toxic Chemical Compounds, Mechanism of Toxicity
Poisoning
General Conclusion
2.2.3. Conium maculatum (Hemlock)
Historical Background
Relevant Toxic Chemical Compounds, Mechanism of Toxicity
Poisoning
General Conclusion
2.2.4. Oenanthe crocata (Hemlock Water-Dropwort)
Historical Background
Relevant Toxic Chemical Compounds, Mechanism of Toxicity
Poisoning
General Conclusion
2.2.5. Ricinus communis (Castor Plant)
Historical Background
Relevant Toxic Chemical Compounds, Mechanism of Toxicity
Poisoning
General Conclusion
| Plant, Family | Used Parts | Toxic Secondary Metabolites | In Vivo Studies and Toxic Dosage | Outcomes | Refs. | |
|---|---|---|---|---|---|---|
| Aconitum napellus (monkshood), Ranunculaceae | Roots, leaves | diterpenoid alkaloids: aconitine, mesaconitine, hypaconitine, benzoylaconitine, benzoylmesaconine, benzoylhypaconine, lappaconitine, jesaconitine, songorine, heteratisine, napelline | Aconitine | Mouse: 1.8 mg/kg (p.o.), 0.24–0.55 mg/kg (s.c.), 0.28–0.34 mg/kg (i.p.), 0.12 mg/kg (i.v.) | Aconitum poisoning disrupts the neural transmission pathway, preventing normal neuronal signals from traversing the synapse, leading to abnormal limb control, paralysis, dizziness, speech difficulties, muscle weakness, twitching, and spasms. Furthermore, the toxic compounds can inflict damage on the central nervous system, potentially causing both temporary and long-lasting neurological disorders, and in severe cases, this condition may culminate in shock or coma. | [51,104] |
| Rat: 0.102- 0.112 mg/kg (i.v.) | ||||||
| Cat: 0.07–0.13 mg/kg (i.v.) | ||||||
| Dog: 0.5 mg/kg (i.v.) | ||||||
| Human: 2–5 mg (p.o.) | ||||||
| Mesaconitine | Mouse: 1.9 mg/kg (p.o.), 0.19–0.25 mg/kg (s.c.), 0.20–0.23 mg/kg (i.p.), 0.068–0.10 mg/kg (i.v.) | |||||
| Rat: 0.158 mg/kg (i.v.) | ||||||
| Hypaconitine | Mouse: 5.8 mg/kg (p.o.), 1.1–1.9 mg/kg (s.c.), 1.0–1.2 mg/kg (i.p.), 0.47 mg/kg (i.v.) | |||||
| Rat: 0.291 mg/kg (i.v.) | ||||||
| Benzoylaconine | Mouse: 70 mg/kg (i.p.), 23 mg/kg (i.v.) | |||||
| Rat: > 20.2 mg/kg (i.v.) | ||||||
| Benzoylmesaconine | Mouse: 810 mg/kg (p.o.), 230 mg/kg (s.c.), 240 mg/kg (i.p.), 21 mg/kg (i.v.) | |||||
| Rat: > 18.7 mg/kg (i.v.) | ||||||
| Benzoylhypaconine | Mouse: 830 mg/kg (p.o.), 130 mg/kg (s.c.), 120 mg/kg (i.p.), 23 mg/kg (i.v.) | |||||
| Rat: > 20.4 mg/kg (i.v.) | ||||||
| Lappaconitine | Mouse: 5.9–11.5 mg/kg (i.v.) | |||||
| Jesaconitine | Mouse: 0.23 mg/kg (s.c.) | |||||
| Songorine | Mouse: 106 mg/kg (i.v.) | |||||
| Heteratisine | Mouse: 147 mg/kg (i.v.) | |||||
| Napelline | Mouse: > 147 mg/kg (i.v.) | |||||
| Atropa belladonna (deadly nightshade), Solanaceae | Leaves, roots, berries | tropane alkaloids: hyoscyamine, atropine, scopolamine | The lethal quantity: >2–4 mg/day Children = 2–5 berries with 2 mg of atropine each (0.2 mg/kg of atropine) Adults = 10–20 berries with 2 mg of atropine each | The anti-muscarinic effects of Atropa belladonna poisoning result in a range of symptoms, including confusion, incomprehensible speech, delirium, lethargy, coma, palpitations, flushing, mydriasis, blurred vision, and hallucinations. The anticholinergic properties of these compounds inhibit the action of acetylcholine at muscarinic receptors, leading to the aforementioned neurological and cardiovascular disturbances. | [81,105,106] | |
| Conium maculatum (hemlock), Apiaceae | Leaves, seeds | piperidine alkaloids: coniine, γ-coniceine | Rat: >50 mg/kg (p.o.) | Conium maculatum poisoning presents with a sequence of clinical manifestations, beginning with heightened activity of motor nerve endings and the central nervous system. This initial stimulation is succeeded by paralysis and a state of depression, leading to impaired movement characterized by slowness and weakness. Subsequently, affected individuals may experience a rapid pulse, hyperventilation, increased urination, respiratory paralysis, and ultimately progress to coma and death. In the initial phase of poisoning, symptoms such as ataxia and headache are evident, whereas heightened salivation, rapid heart rate, and dilation of the pupils arise as a result of the plant’s impact on the autonomic ganglia. | [107,108,109] | |
| Coniine | Mouse: 100 mg/kg (p.o.), 19 mg/kg (i.v.); 80 mg/kg (s.c.) | |||||
| γ-coniceine | Mouse: 12 mg/kg (p.o.), 2.6 mg/kg (i.v.); 12 mg/kg (s.c.) | |||||
| Oenanthe crocata (hemlock water-dropwort), Apiaceae | Tubers, leaves, seeds | polyacetylenes: oenanthotoxin, dihydrooenanthotoxin, cicutoxin | Mouse: 17 mg/kg for tubers, 1320 mg/kg for green seeds Goat: 1–2 fresh small tubers or 1 larger tuber, 0.25 g/kg BW (p.o.) | The toxicity of Oenanthe crocata is associated with the induction of respiratory paralysis and convulsions, characterized by seizures that occur alongside a depression of motor functions within the central nervous system. This dual manifestation highlights the compound’s detrimental effects on both respiratory and neurological systems. | [110,111,112] | |
| Ricinus communis (castor plant), Euphorbiaceae | Seeds, leaves | ricin | Mouse: 30 mg/kg (p.o.), 22 mg/kg (i.p.); 2–10 mg/kg (i.v.), 3.5 µg crude ricin/kg BW (intranasal)—24 μg/kg (injection or inhalation), 20–30 mg/kg (seed ingestion) Rat: 20–30 mg/kg (i.p.) Human: 3–10 μg/kg BW (inhalation—solid or liquid particles), 1–10 μg/kg BW (injection into muscle or vein), 1–20 mg ricin/kg BW = equivalent to approximately 8 seeds (p.o.) Adults: 10–20 seeds Children: 1–6 seeds | Ricin exerts significant neurotoxic effects primarily through its mechanism of action as an inhibitor of protein synthesis. Upon exposure, ricin can lead to a range of neurological symptoms, including seizures, ataxia, and altered mental status. The toxin disrupts cellular function by enzymatically inactivating ribosomal RNA, which impairs protein synthesis and ultimately results in cell death, particularly in neurons. Additionally, ricin can induce inflammation and oxidative stress within the central nervous system, contributing to neuronal damage. The severity of these neurotoxic effects is dose-dependent, with higher exposures leading to more pronounced neurological deficits and potential respiratory failure due to central respiratory depression. | [113,114,115] | |
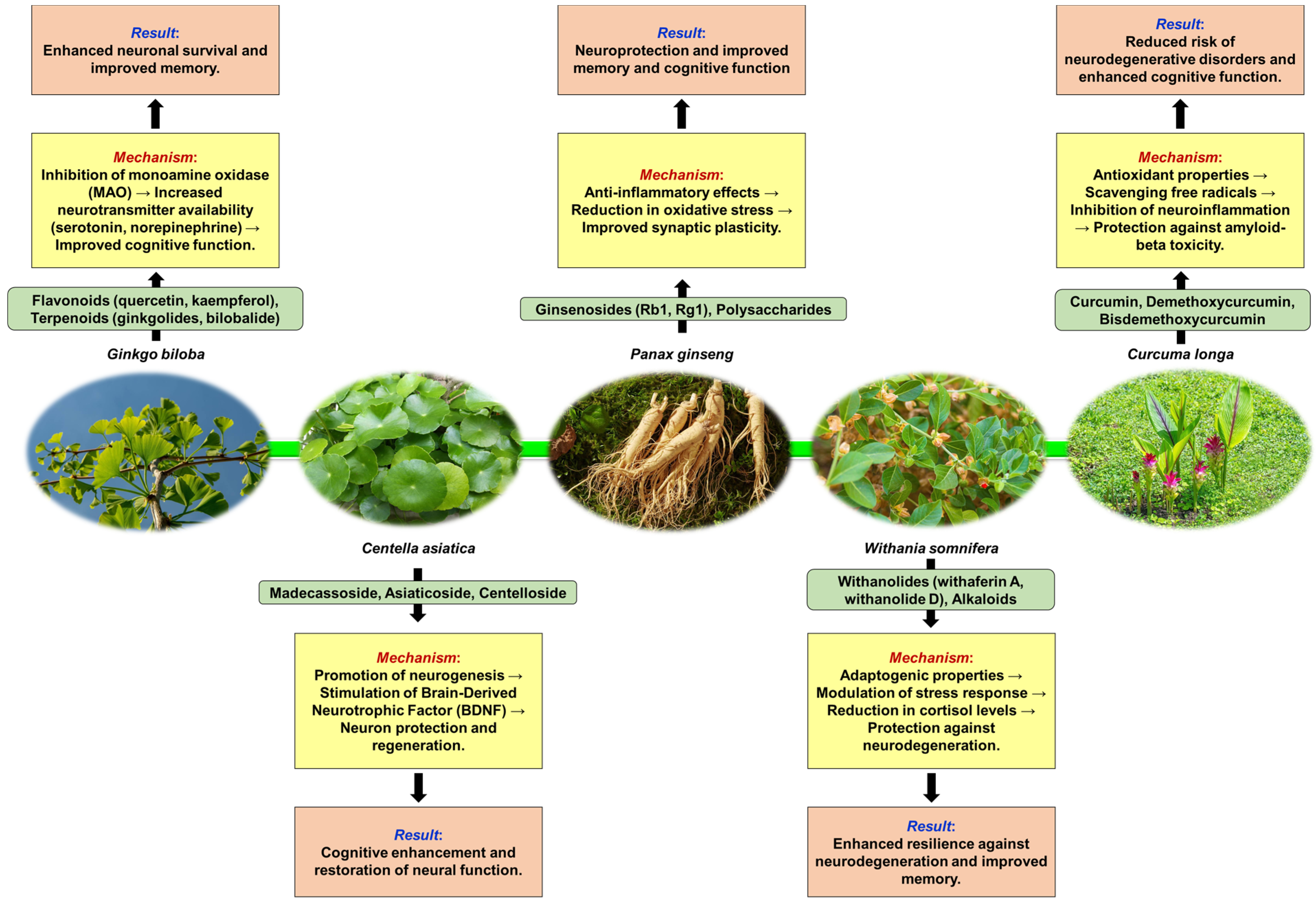
3. Neuroprotective Plants
3.1. Defining Neuroprotection and Some Mechanisms of Plant-Induced Neuroprotection
3.2. Common Neuroprotective Plants—Mechanisms of Neuroprotection, Neurological Benefits, Clinical Applications, and Research Findings
3.2.1. Ginkgo biloba (Ginkgo)
Historical Background
Neuroprotective Chemical Metabolites
Neuroprotective Properties
Clinical Applications
General Conclusion
3.2.2. Centella asiatica (Gotu Kola)
Historical Background
Neuroprotective Chemical Metabolites
Neuroprotective Properties
Clinical Applications
General Conclusion
3.2.3. Panax ginseng (Ginseng)
Historical Background
Neuroprotective Chemical Metabolites
Neuroprotective Properties
Clinical Applications
General Conclusion
3.2.4. Withania somnifera (Ashwagandha)
Historical Background
Neuroprotective Chemical Metabolites
Clinical Applications
Neuroprotective Properties
General Conclusion
3.2.5. Curcuma longa (Turmeric)
Historical Background
Neuroprotective Chemical Metabolites
Neuroprotective Properties
Clinical Applications
General Conclusion
4. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharm. Sin. B 2023, 13, 3208–3237. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Wali, A.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lyu, Y.; Lee, M.; Kang, H. Neuroprotective effect of some plant extracts in cultured CT105-induced PC12 cells. Biol. Pharm. Bull. 2006, 29, 2021–2024. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Nadège, K.; Sylviane, D.; Agathe, F.; Clarisse, M.; Germain, T.; David, P.; Simon, P.; Stephanie, M.; Elisabeth, N. Antioxidant properties of Dichrocephala integrifolia (Asteraceae) in a mouse model of monosodium glutamate-induced neurotoxicity. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 147–155. [Google Scholar] [CrossRef][Green Version]
- Lahane, V.D.; Katekar, L. Exploring the neuroprotective potential of herbal plants: A comprehensive review. GSC Biol. Pharm. Sci. 2024, 27, 141–148. [Google Scholar] [CrossRef]
- Oliveira, A.; Pinho, C.; Sarmento, B.; Dias, A. Neuroprotective activity of Hypericum perforatum and its major components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Khalil, H.; Henafy, H.; Khalil, I.; Bakr, A.; Fahmy, M.; Younis, N.; El-Shiekh, R. Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats. Toxics 2023, 11, 159. [Google Scholar] [CrossRef]
- Pereira, R.; Fachinetto, R.; Prestes, A.; Puntel, R.; Silva, G.; Heinzmann, B.; Boschetti, T.; Athayde, M.; Burger, M.; Morel, A.; et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita, and Cymbopogon citratus. Neurochem. Res. 2008, 34, 973–983. [Google Scholar] [CrossRef]
- Kumar, G.; Raghavan, A.; Naveen, S. Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn. J. 2014, 7, 1–17. [Google Scholar] [CrossRef]
- Turkez, H.; Arslan, M.; Stefano, A.; Cacciatore, I.; Mardinoglu, A. Nonpharmacological treatment options for Alzheimer’s disease: From animal testing to clinical studies. Turk. J. Zool. 2020, 44, 81–89. [Google Scholar] [CrossRef]
- Popescu, R.; Daliborca, C.V.; Cimporescu, A.; Vlad, C.S.; Verdes, D.; Botau, D.; Filimon, M.; Pauliuc, I.; Citu, C.; Dumitrascu, V. Chemical Properties and In vitro Antitumor Effects of Momordica charantia Extracts in Different Solvents. Rev. Chim. 2016, 67, 69–73. [Google Scholar]
- Mioc, M.; Prodea, A.; Racoviceanu, R.; Mioc, A.; Ghiulai, R.; Milan, A.; Voicu, M.; Mardale, G.; Soica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). Int. J. Mol. Sci. 2022, 23, 8896. [Google Scholar] [CrossRef]
- Dereli-Caliskan, N.; Husunet, M.; Ila, H.; Karagoz, I. Determination of potential anti-Alzheimer activity of gentiopicroside and isoorientin using molecular docking studies. Eurasia Proc. Sci. Technol. Eng. Math. 2021, 12, 106–112. [Google Scholar] [CrossRef]
- Daliborca, C.V.; Dumitrascu, V.; Popescu, R.; Cimporescu, A.; Vlad, C.S.; Flangea, C.; Grecu, D.S.; Vagvolgyi, C.; Papp, T.; Horhat, F. Gas Chromatography–mass Spectrometry Evidences for New Chemical Insights of Momordica charantia. Rev. Chim. 2015, 66, 1914–1920. [Google Scholar]
- Codocedo, J.; Allard, C.; Godoy, J.; Varela-Nallar, L.; Inestrosa, N. SIRT1 regulates dendritic development in hippocampal neurons. PLoS ONE 2012, 7, e47073. [Google Scholar] [CrossRef]
- Sakarwal, A.; Sen, K.; Ram, H.; Chowdhury, S.; Kashyap, P.; Shukla, S.; Panwar, A. Neuroprotective Efficacy of Phytoconstituents of Methanolic Shoots Extract of Calligonum polygonoides L. in Hypercholesterolemia-associated Neurodegenerations. Endocr. Metab. Immune Disord.-Drug Targets 2024, 24, e020424228551. [Google Scholar] [CrossRef]
- Lucena, G.; Franco, J.; Ribas, C.; Azevedo, M.; Meotti, F.; Gadotti, V.; Dafre, A.; Santos, A.; Farina, M. Cipura Paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin. Pharmacol. Toxicol. 2007, 101, 27–131. [Google Scholar] [CrossRef]
- Ye, C.; Wu, Y.; Shen, S.; Liu, X.; Guo, J. A structure-activity relationship between the Veratrum alkaloids on the antihypertension and DNA damage activity in mice. Chem. Biodivers. 2020, 17, e1900473. [Google Scholar] [CrossRef]
- Gao, X.; Hu, J.; Zhang, X.; Zuo, Y.; Wang, Y.; Zhu, S. Research progress of aconitine toxicity and forensic analysis of aconitine poisoning. Forensic Sci. Res. 2018, 5, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Mohanta, G.; Valliappan, K.; Manavalan, R. Lathyrus and lathyrism: A review. Int. J. Food Prop. 1999, 2, 197–203. [Google Scholar] [CrossRef]
- Rao, S.; Adiga, P.; Sarma, P. The isolation and characterization of β-n-oxalyl-l-α,β-diaminopropionic acid: A neurotoxin from the seeds of Lathyrus sativus. Biochemistry 1964, 3, 432–436. [Google Scholar] [CrossRef]
- Wei, X.; Ruan, W.; Vrieling, K. Current knowledge and perspectives of pyrrolizidine alkaloids in pharmacological applications: A mini-review. Molecules 2021, 26, 1970. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Ni, X.; Rigui, Y.; Bai, Y.; Xu, L.; Yang, J.; Zhang, X.; Deng, J.; Li, J.; et al. Aconitine disrupts serotonin neurotransmission via 5-hydroxytryptamine receptor in zebrafish embryo. J. Appl. Toxicol. 2020, 41, 483–492. [Google Scholar] [CrossRef]
- Spencer, P.; Palmer, V. Direct and indirect neurotoxic potential of metal/metalloids in plants and fungi used for food, dietary supplements, and herbal medicine. Toxics 2021, 9, 57. [Google Scholar] [CrossRef]
- Nissim, I.; Dagan-Wiener, A.; Niv, M. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef]
- Grandjean, P. Paracelsus revisited: The dose concept in a complex world. Basic Clin. Pharmacol. Toxicol. 2016, 119, 126–132. [Google Scholar] [CrossRef]
- Elyasi, L.; Rosenholm, J.; Jamshidi, F.; Jahanshahi, M. The antioxidative effects of picein and its neuroprotective potential: A review of the literature. Molecules 2022, 27, 6189. [Google Scholar] [CrossRef]
- Bauer, N. Unraveling the interplay of dopamine, carbon monoxide, and heme oxygenase in neuromodulation and cognition. ACS Chem. Neurosci. 2024, 15, 400–407. [Google Scholar] [CrossRef]
- Calabrese, E. The emergence of the dose-response concept in biology and medicine. Int. J. Mol. Sci. 2016, 17, 2034. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, F.; Tajbakhsh, E. Neurotoxicity mechanism of Ochratoxin A. Qual. Assur. Saf. Crops Foods 2021, 13, 34–45. [Google Scholar] [CrossRef]
- Fang, C. Network pharmacology study on the neurotoxic mechanism of Acorus tatarinowii. Adv. Eng. Technol. Res. 2023, 7, 647. [Google Scholar] [CrossRef]
- Gaitonde, B.; Joglekar, S. Mechanism of neurotoxicity of cardiotonic glycosides. Br. J. Pharmacol. 1977, 59, 223–229. [Google Scholar] [CrossRef]
- Tichvon, C. Synthesis of bufadienolide cinobufagin via late-stage singlet oxygen oxidation/rearrangement approach. Org. Lett. 2024, 26, 2445–2450. [Google Scholar] [CrossRef]
- Spochacz, M.; Chowański, S.; Walkowiak-Nowicka, K.; Szymczak, M.; Adamski, Z. Plant-derived substances used against beetles–pests of stored crops and food–and their mode of action: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1339–1366. [Google Scholar] [CrossRef]
- Xu, X.; Jia, L.; Ma, X.; Li, H.; Sun, C. Application potential of plant-derived medicines in prevention and treatment of platinum-induced peripheral neurotoxicity. Front. Pharmacol. 2022, 12, 792331. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Zhao, Y. Phthalates induce neurotoxicity by disrupting the MFN2-PERK axis-mediated endoplasmic reticulum–mitochondria interaction. J. Agric. Food Chem. 2024, 72, 7411–7422. [Google Scholar] [CrossRef]
- Guo, W.; Xing, Y.; Luo, X.; Li, F.; Ren, M.; Liang, Y. Reactive Oxygen Species: A Crosslink between Plant and Human Eukaryotic Cell Systems. Int. J. Mol. Sci. 2023, 24, 13052. [Google Scholar] [CrossRef]
- Povšnar, M.; Koželj, G.; Kreft, S.; Lumpert, M. Rare tradition of the folk medicinal use of Aconitum spp. is kept alive in Solčavsko, Slovenia. J. Ethnobiol. Ethnomed. 2017, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Shyaula, S. Phytochemicals, traditional uses and processing of Aconitum species in Nepal. Nepal J. Sci. Technol. 2012, 12, 171–178. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Liao, T.; Zhang, C.; Chen, K.; Huang, Q. Advances on pharmacology and toxicology of aconitine. Fundam. Clin. Pharmacol. 2022, 36, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Eric, N.; Xu, Y.; Li, Y.; Wang, Y.; Agyemang, K.; Zhang, Y. A review on phytochemistry, pharmacology and toxicology studies of Aconitum. J. Pharm. Pharmacol. 2014, 67, 1–19. [Google Scholar] [CrossRef]
- Chou, P.; Wang, C.; Tai, C.; Yang, T.; Tang, Y. Bradycardia and hypotension from improper use of aconite root: A case report and brief review. Complement. Med. Res. 2018, 25, 338–343. [Google Scholar] [CrossRef]
- Chan, T. Aconite poisoning presenting as hypotension and bradycardia. Hum. Exp. Toxicol. 2009, 28, 795–797. [Google Scholar] [CrossRef]
- Feng, C.; Li, H.; Liu, M. Clinical effect of a temporary pacemaker on an electrical storm induced by severe acute aconitine poisoning. World Acad. Sci. J. 2022, 4, 142. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Park, H.; Yoon, C.; Park, S.; Baek, S.; Cho, S. Development and validation of q-tof/ms method for the rapid and simultaneous quantification of three aconitum alkaloids in food. Anal. Methods 2015, 7, 7733–7740. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, Z.; Yin, L.; Ma, L.; Wang, X.; Fu, X. Anti-inflammatory and antinociceptive activities of non-alkaloids fractions from Aconitum flavum in vivo. Rev. Bras. Farmacogn. 2015, 25, 47–52. [Google Scholar] [CrossRef]
- Bonanno, G.; Ippolito, M.; Moscarelli, A.; Misseri, G.; Caradonna, R.; Accurso, G.; Cortegiani, A.; Giarratano, A. Accidental poisoning with Aconitum: Case report and review of the literature. Clin. Case Rep. 2020, 8, 696–698. [Google Scholar] [CrossRef]
- Chan, Y.; Wang, N.; Feng, Y. The toxicology and detoxification of Aconitum: Traditional and modern views. Chin. Med. 2021, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fang, Y. Relative quantification of the metabolite of aconitine in rat urine by LC-ESI-MS/MS and its application to pharmacokinetics. Anal. Sci. 2012, 28, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, J.; Xu, H.; Du, Y.; Li, Q.; Bi, K. Simultaneous determination of benzoylmesaconine and piperine in rat plasma after oral administration of naru-3 by an ultra fast liquid chromatography-tandem mass spectrometry method and its application in a comparative pharmacokinetic study. Anal. Methods 2014, 6, 3420–3428. [Google Scholar] [CrossRef]
- Li, T.; Gong, N.; Wang, Y. Ester hydrolysis differentially reduces aconitine-induced anti-hypersensitivity and acute neurotoxicity: Involvement of spinal microglial dynorphin expression and implications for Aconitum processing. Front. Pharmacol. 2016, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Zhang, N.; Wang, C.; Wang, X.; Huang, W.; Peng, C.; He, G.; Han, B. Aconitine induces cardiomyocyte damage by mitigating BNIP3-dependent mitophagy and the TNFA-NLRP3 signaling axis. Cell Prolif. 2019, 53, e12701. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Li, Q.; Liu, H.; Zhang, X.; Xie, K.; Zhu, Z.; Miao, Q.; Su, S. Protective effect of berberine on Aconite-induced myocardial injury and the associated mechanisms. Mol. Med. Rep. 2018, 18, 4468–4476. [Google Scholar] [CrossRef]
- Ameri, A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998, 56, 211–235. [Google Scholar] [CrossRef]
- Wu, J.; Lin, N.; Li, F.; Zhang, G.; He, S.; Zhu, Y.; Ou, R.; Li, N.; Liu, S.; Feng, L.; et al. Induction of P-glycoprotein expression and activity by Aconitum alkaloids: Implication for clinical drug–drug interactions. Sci. Rep. 2016, 6, 25343. [Google Scholar] [CrossRef]
- Sheikh-Zade, Y.R.; Cherednik, I.L.; Galenko-Yaroshevskii, P.A. Peculiarities of cardiotropic effect of aconitine. Bull. Exp. Biol. Med. 2000, 129, 365–366. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, J.; Wu, Z.; Shi, Y.; Chen, M.; Li, M.; Hu, H.; Tian, K.; Lv, X.; Li, C.; et al. Involvement of autophagy in mesaconitine-induced neurotoxicity in HT22 cells revealed through integrated transcriptomic, proteomic, and m6A epitranscriptomic profiling. Front. Pharmacol. 2024, 15, 1393717. [Google Scholar] [CrossRef]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.; Pyne, S.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O- acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 12845. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Seth, A.; Laloo, D.; Singh, N.; Gautam, D.; Singh, A. Śodhana: An ayurvedic process for detoxification and modification of therapeutic activities of poisonous medicinal plants. Anc. Sci. Life 2015, 34, 188–197. [Google Scholar] [CrossRef]
- Rabb, U. Pharmacological review on purification of visha dravyas (poisonous plants) according to Ayurveda. Int. J. Curr. Sci. Res. Rev. 2022, 5, 3265–3272. [Google Scholar] [CrossRef]
- Pal, P.; Nandi, M.; Singh, N. Detoxification of Croton tiglium L. seeds by ayurvedic process of śodhana. Anc. Sci. Life 2014, 33, 157–161. [Google Scholar] [CrossRef]
- Arya, N.; Kumar, H. Comparative physico-chemical profile of ‘vatsanabha’ (Aconitum ferox, Ranunculaceae) mula processed through cow’s urine and cow’s milk. Int. J. Res. Ayurveda Pharm. 2017, 8, 217–222. [Google Scholar] [CrossRef]
- Mitra, S.; Shukla, V.; Acharya, R. Effect of shodhana (processing) on kupeelu (Strychnos nux-vomica Linn.) with special reference to strychnine and brucine content. AYU Int. Q. J. Res. Ayurveda 2011, 32, 402–407. [Google Scholar] [CrossRef]
- Deore, S.; Moon, K.; Khadabadi, S.; Deokate, U.; Baviskar, B. Evaluation of toxicity of ‘vatsanabha’ (Aconitum ferox, Ranunculaceae) before and after shodhana. J. Young Pharm. 2013, 5, 3–6. [Google Scholar] [CrossRef]
- Vyas, K.; Shukla, V.; Ruknuddin, G.; Prajapati, P. Pharmaceutical standardization of guggulu śodhana. J. Ayurveda Med. Sci. 2017, 2, 165–173. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhu, S.; Liu, Q. Case reports of Aconite poisoning in mainland China from 2004 to 2015: A retrospective analysis. J. Forensic Leg. Med. 2016, 42, 68–73. [Google Scholar] [CrossRef]
- Chan, T.Y. Aconite poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- Danaie, E.; Masoudi, S.; Masnabadi, N. Chemical composition analysis of Atropa belladonna Grown in Iran and Evaluation of Antibacterial properties of Extract-loaded Nanofibers. Iran. J. Pharm. Res. 2023, 22, e137839. [Google Scholar] [CrossRef] [PubMed]
- Rajput, H. Effects of Atropa belladonna as an anti-cholinergic. Nat. Prod. Chem. Res. 2014, 1, 1000104. [Google Scholar] [CrossRef]
- Hasgül, B.; Çatak, M.; Çatak, A.; Karaman, S. Case series with Atropa belladonna (deadly nightshade) intoxication and experience of physostigmin. J. Emerg. Med. Case Rep. 2022, 13, 25–28. [Google Scholar] [CrossRef]
- Schoen-Angerer, T.; Madeleyn, R.; Kiene, H.; Kienle, G.; Vagedes, J. Improvement of asthma and gastroesophageal reflux disease with oral pulvis stomachicus cum belladonna, a combination of Matricaria recutita, Atropa belladonna, bismuth, and antimonite: A pediatric case report. Glob. Adv. Health Med. 2016, 5, 107–111. [Google Scholar] [CrossRef]
- Bektaş, M.; Aktar, F.; Güneş, A.; Uluca, Ü.; Gülşen, S.; Karaman, K. Atropa belladonna (deadly nightshade) poisoning in chilhood. West Indian Med. J. 2016, 69, 230–234. [Google Scholar] [CrossRef]
- Maurya, V.; Kumar, S.; Kabir, R.; Shrivastava, G.; Shanker, K.; Nayak, D.; Khurana, A.; Manchanda, R.; Gadugu, S.; Kar, S.; et al. Dark classics in chemical neuroscience: An evidence-based systematic review of belladonna. ACS Chem. Neurosci. 2020, 11, 3937–3954. [Google Scholar] [CrossRef]
- Ashtiania, F. Tropane alkaloids of Atropa belladonna L. and Atropa acuminata Royle ex Miers plants. J. Med. Plants Res. 2011, 5, 6515–6522. [Google Scholar] [CrossRef][Green Version]
- Yılmaz, Y.; Kara, F.; Aydın, S.; Ozlece, H.; Can, S.; Üstebay, S. Poisoning with Atropa belladonna in childhood. Iran. J. Pediatr. 2018, 28, e7865. [Google Scholar] [CrossRef]
- Kwakye, G.F.; Jiménez, J.; Jiménez, J.A.; Aschner, M. Atropa belladonna neurotoxicity: Implications to neurological disorders. Food Chem. Toxicol. 2018, 116, 346–353. [Google Scholar] [CrossRef]
- Rastakhiz, N.; Azar, P.; Tehrani, M.; Moradalizadeh, M.; Larijani, K. Chemical constituents comparison of essential oils of aerial parts of Conium maculatum L. growing wild in Iran by hydrodistillation, microwave-assisted hydrodistillation and solid phase microextraction methods. Int. J. Life Sci. 2015, 9, 48–50. [Google Scholar] [CrossRef]
- Erenler, A.; Baydın, A.; Duran, L.; Yardan, T.; Türköz, B. A case of respiratory failure due to poison hemlock poisoning presented to an emergency department. Hong Kong J. Emerg. Med. 2011, 18, 235–238. [Google Scholar] [CrossRef]
- Kulaksiz, F.; Erenler, A.; Ozden, H.; Ay, M.; Yastı, A. Hemlock poisoning in emergent patients. Glob. J. Rare Dis. 2016, 1, 004–006. [Google Scholar] [CrossRef]
- Grosu, E.; Ichim, M. Turning Meadow Weeds Into Valuable Species for the Romanian Ethnomedicine While Complying With the Environmentally Friendly Farming Requirements of the European Union’s Common Agricultural Policy. Front. Pharmacol. 2020, 1, 529. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Mukherjee, A.; Paul, A.; Khuda-Bukhsh, A. Homeopathic mother tincture of Conium initiates reactive oxygen species mediated DNA damage and makes HeLa cells prone to apoptosis. Tang Humanit. Med. 2012, 2, 26.1–26.5. [Google Scholar] [CrossRef]
- Salem, M.; Mohamed, A.; Ali, H.; Farraj, D. Characterization of phytoconstituents from alcoholic extracts of four woody species and their potential uses for management of six Fusarium oxysporum isolates identified from some plant hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Madaan, R.; Kumar, S. Screening of alkaloidal fraction of Conium maculatum L. aerial parts for analgesic and antiinflammatory activity. Indian J. Pharm. Sci. 2012, 74, 457. [Google Scholar] [CrossRef]
- Heinle, H.; Tober, C.; Zhang, D.; Jaggi, R.; Kuebler, W. The low-dose combination preparation vertigoheel activates cyclic nucleotide pathways and stimulates vasorelaxation. Clin. Hemorheol. Microcirc. 2010, 46, 23–35. [Google Scholar] [CrossRef]
- Lee, S.; Green, B.; Welch, K.; Jordan, G.; Zhang, Q.; Panter, K.; Hughes, D.; Chang, C.-W.; Pfister, J.; Gardner, D. Stereoselective potencies and relative toxicities of γ-coniceine and n-methylconiine enantiomers. Chem. Res. Toxicol. 2013, 26, 616–621. [Google Scholar] [CrossRef]
- Green, B.; Lee, S.; Welch, K.; Pfister, J.; Panter, K. Fetal muscle-type nicotinic acetylcholine receptor activation in TE-671 cells and inhibition of fetal movement in a day 40 pregnant goat model by optical isomers of the piperidine alkaloid coniine. J. Pharmacol. Exp. Ther. 2012, 344, 295–307. [Google Scholar] [CrossRef]
- Pallares, J.; Sabán, J.; Bouza, C.; Diaz, J.; Rodríguez, R.; Morena, J.; Liste, D.; Serrano-Rios, M. Reversible autonomic dysfunction in Oenanthe crocata poisoning evaluated by simple bedside tests. Hum. Toxicol. 1985, 4, 521–526. [Google Scholar] [CrossRef]
- Banerjee, S.; Manisha, C.; Murugan, D.; Justin, A. Natural Products Altering GABAergic Transmission. In Natural Medicinal Plants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Ruekberg, B. A chemistry tidbit for batman fans. J. Chem. Educ. 2010, 87, 1017–1018. [Google Scholar] [CrossRef]
- Appendino, G.; Pollastro, F.; Verotta, L.; Ballero, M.; Romano, A.; Wyrembek, P.; Szczuraszek, K.; Mozrzymas, J.; Taglialatela-Scafati, O. Polyacetylenes from Sardinian Oenanthe fistulosa: A molecular clue to risus sardonicus. J. Nat. Prod. 2009, 72, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Gonçalves, M.; Lopes, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Wyrembek, P.; Negri, R.; Kaczor, P.; Czyżewska, M.; Appendino, G.; Mozrzymas, J. Falcarindiol allosterically modulates GABAergic currents in cultured rat hippocampal neurons. J. Nat. Prod. 2012, 75, 610–616. [Google Scholar] [CrossRef]
- Mitchell, M.; Routledge, P. Hemlock water dropwort poisoning—A review. Clin. Toxicol. 1978, 12, 417–426. [Google Scholar] [CrossRef]
- Ai, T.; Phien, H.; Men, T. Phytochemical constituents and toxicity of the ethanol extract of Ricinus communis (L.) in Drosophila melanogaster. Asian J. Biol. 2021, 13, 12–21. [Google Scholar] [CrossRef]
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.; Dübel, S.; Thullier, P. Isolation of a human-like antibody fragment (SCFV) that neutralizes ricin biological activity. BMC Biotechnol. 2009, 9, 60. [Google Scholar] [CrossRef]
- Chouhan, H.; Swarnakar, G.; Jogpal, B. Medicinal Properties of Ricinus communis: A Review. Int. J. Pharm. Sci. Res. 2021, 12, 3632–3642. [Google Scholar] [CrossRef]
- Istaufa, F.; Subagio, Y.; Suswati, I. Castor plant (Ricinus communis L.) leaf extract as potential antibacterial against the growth of Mycobacterium tuberculosis. Folia Medica Indones. 2022, 58, 371–376. [Google Scholar] [CrossRef]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.; Schaer, M.; Dorner, M.; Dorner, B. Ricinus communis intoxications in human and veterinary medicine—A summary of real cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef]
- Citores, L.; Ferreras, J.; Iglesias, R.; Carbajales, M.; Arias, F.; Jiménez, P.; Rojo, M.; Girbes, T. Molecular mechanism of inhibition of mammalian protein synthesis by some four-chain agglutinins. FEBS Lett. 1993, 329, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Pastor, A.; Delgado-García, J. The neurotoxic effects of Ricinus communis agglutinin-II. J. Toxicol. Toxin Rev. 1995, 14, 1–46. [Google Scholar] [CrossRef]
- Almehmady, A.M.; Alhakamy, N.A.; Alharbi, W.S. Screening of Herbal Medicines for Neurotoxicity: Principles and Methods. In Medicinal Herbs and Fungi; Springer: Singapore, 2021; p. 355. [Google Scholar] [CrossRef]
- Williamson, E.M. Herbal Neurotoxicity: An Introduction to Its Occurrence and Causes. In Toxicology of Herbal Products; Pelkonen, O., Duez, P., Vuorela, P., Vuorela, H., Eds.; Springer Int. Publ.: Cham, Switzerland, 2017; pp. 345–362. [Google Scholar] [CrossRef]
- Milanlioglu, A. Toxic encephalopathy after Atropa belladonna poisoning. Pak. J. Med. Sci. 2011, 27, 926–928. [Google Scholar]
- Radulović, N.; Đorđević, N.; Denić, M.; Pinheiro, M.M.G.; Fernandes, P.D.; Boylan, F. A novel toxic alkaloid from poison hemlock (Conium maculatum L., Apiaceae): Identification, synthesis and antinociceptive activity. Food Chem. Toxicol. 2012, 50, 274–279. [Google Scholar] [CrossRef]
- Sharma, S.; Raina, A.; Agrawal, D.C.; Dhar, M.K.; Kaul, S. Neurotoxic Medicinal Plants of Indian Himalayan Regions: An Overview. In Medicinal Herbs and Fungi; Springer: Singapore, 2021; pp. 469–493. [Google Scholar] [CrossRef]
- Kharchoufa, L.; Merrouni, I.A.; Yamani, A.; Elachouri, M. Profile on medicinal plants used by the people of North Eastern Morocco: Toxicity Concerns. Toxicon 2018, 154, 90–113. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, P.; Sharma, B.; Guerrero-Perilla, C.; Coy-Barrera, E. Analysis of polyacetylenes. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 707–722. [Google Scholar] [CrossRef]
- Orlando-Goulart, C.F.; Welch, K.D.; Pfister, J.A.; Goulart, D.S.; Damasceno, A.D.; Lee, S.T. Neurobehavioral evaluation of mice dosed with water hemlock green seeds and tubers. Poisonous Plant Res. PPR 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Welch, K.D.; Stonecipher, C.A.; Lee, S.T.; Cook, D. The acute toxicity of water hemlock (Cicuta douglasii) in a goat model. Toxicon 2020, 176, 55–58. [Google Scholar] [CrossRef]
- Gal, Y.; Sapoznikov, A.; Lazar, S.; Shoseyov, D.; Aftalion, M.; Gutman, H.; Evgy, Y.; Gez, R.; Nevo, R.; Falach, R. Long-Term Pulmonary Damage in Surviving Antitoxin-Treated Mice following a Lethal Ricin Intoxication. Toxins 2024, 16, 103. [Google Scholar] [CrossRef]
- Franke, H.; Scholl, R.; Aigner, A. Ricin and Ricinus communis in pharmacology and toxicology-from ancient use and “Papyrus Ebers” to modern perspectives and “poisonous plant of the year 2018”. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1181–1208. [Google Scholar] [CrossRef]
- Jung, H.; Son, H.; Jin, G.; Park, Y. Preventive role of PD-1 on MPTP-induced dopamine depletion in mice. Cell. Biochem. Funct. 2010, 28, 217–223. [Google Scholar] [CrossRef]
- Khazdair, M.; Kianmehr, M.; Anaeigoudari, A. Effects of medicinal plants and flavonoids on Parkinson’s disease, a review on basic and clinical evidences. Adv. Pharm. Bull. 2020, 11, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Dempsey, R.; Vemuganti, R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Sulthana, A.; Muthusamy, G.; Elangovan, N. Progress in the development of naturally derived active metabolites-based drugs: Potential therapeutics for Alzheimer’s disease. Biotechnol. Appl. Biochem. 2022, 69, 2713–2732. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A. Medicinal plants with neuroprotective effects. GSC Biol. Pharm. Sci. 2021, 17, 213–231. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.; Docea, A.; Mahomoodally, M.; Lobine, D.; Chazot, P.; Kurt, B.; Tumer, T.; Moreira, A.; et al. Impact of natural compounds on neurodegenerative disorders: From preclinical to pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.; An, S. Mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease. Antioxidants 2021, 10, 1571. [Google Scholar] [CrossRef]
- Moura, M. Neuroprotective effects of crude extracts, compounds, and isolated molecules obtained from plants in the central nervous system injuries: A systematic review. Front. Neurosci. 2023, 17, 1249685. [Google Scholar] [CrossRef]
- He, X.; Zhou, Y.; Sheng, S.; Li, J.; Wang, G.; Zhang, F. Ellagic acid protects dopamine neurons via inhibition of NLRP3 inflammasome activation in microglia. Oxidative Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef]
- Ansari, M.; Abdul, H.; Joshi, G.; Opii, W.; Butterfield, D. Protective effect of quercetin in primary neurons against aβ(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef]
- Tian, J.; Wang, X.; Tian, Z. Focusing on formononetin: Recent perspectives for its neuroprotective potentials. Front. Pharmacol. 2022, 13, 905898. [Google Scholar] [CrossRef]
- Krasieva, T.; Ehren, J.; O’Sullivan, T.; Tromberg, B.; Maher, P. Cell and brain tissue imaging of the flavonoid fisetin using label-free two-photon microscopy. Neurochem. Int. 2015, 89, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Wang, Y.; Tian, T.; Wu, W.; Zhou, M.; Meng, X.; Ruan, H. Neuroprotective lignans from the fruits of Schisandra bicolor var. tuberculata. J. Nat. Prod. 2017, 80, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Z.; Sun, B.; Liu, J.; Meng, F.; Liu, Y.; Tian, T.; Jin, A.; Ruan, H. Lignans from the fruit of Schisandra glaucescens with antioxidant and neuroprotective properties. J. Nat. Prod. 2014, 77, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhou, Y.; Zeng, Y.; Lin, Y.; Li, Y.; Zhong, Z.; Chan, W. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS ONE 2012, 7, e29641. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Guo, J.; Xu, C.; Cao, Y.; Fang, Z.; Wang, Q. Pharmacological mechanisms underlying the neuroprotective effects of Alpinia oxyphylla Miq. on Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 2071. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Gu, X.; Ding, F. Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell. Mol. Neurobiol. 2008, 28, 1067–1078. [Google Scholar] [CrossRef]
- Bozorgi, M.; Najafi, Z.; Omidpanah, S.; Sadri, A.; Narimani, Z.; Moghadam, F.; Edraki, N.; Akbarzadeh, T.; Saeedi, M. Investigation of anti-Alzheimer’s activity of aqueous extract of Areca nuts (Areca catechu L.): In vitro and in vivo studies. Bol. Latinoam. Caribe Plantas Med. Aromat. 2021, 20, 406–415. [Google Scholar] [CrossRef]
- Sun, A.; Wang, Q.; Simonyi, Á.; Sun, G. Botanical phenolics and brain health. Neuromol. Med. 2008, 10, 259–274. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, M.; Zhang, Y.; Yu, L.; Wu, J.; Tang, Y.; Ai, W.; Zhu, F.; Law, B.; Chen, Q.; et al. Dietary plant polyphenols as the potential drugs in neurodegenerative diseases: Current evidence, advances, and opportunities. Oxidative Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef]
- Mohsenpour, H.; Pesce, M.; Patruno, A.; Bahrami, A.; Pour, P.; Farzaei, M. A review of plant extracts and plant-derived natural compounds in the prevention/treatment of neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2021, 22, 833. [Google Scholar] [CrossRef]
- Zain, M.; Shamsuddin, N.; Noorden, M. The effects of Centella asiatica extract (CAE) on methamphetamine-induced neurotoxicity via human neuroblastoma cell line. ASM Sci. J. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.; Kumar, M.; Radha; Ghosh, A.; Prockow, J.; Lastra, J.; Dey, A. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: A review. J. Cell. Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Neuroprotective phenolics in medicinal plants. Arch. Pharmacal Res. 2010, 33, 1611–1632. [Google Scholar] [CrossRef]
- Tran, N. Neuroprotective potential of pyranocoumarins from Angelica gigas Nakai on glutamate-induced hippocampal cell death. Antioxidants 2023, 12, 1651. [Google Scholar] [CrossRef]
- Carrera, I.; Cacabelos, R. Current drugs and potential future neuroprotective compounds for Parkinson’s disease. Curr. Neuropharmacol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Al-Khazaleh, A. The neurotherapeutic arsenal in Cannabis sativa: Insights into anti-neuroinflammatory and neuroprotective activity and potential entourage effects. Molecules 2024, 29, 410. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the NRF2/are pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef]
- Xu, W.; Li, R.; Quasie, O.; Yang, M.; Kong, L.; Luo, J. Polyprenylated tetraoxygenated xanthones from the roots of Hypericum monogynum and their neuroprotective activities. J. Nat. Prod. 2016, 79, 1971–1981. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; Martino, L.; Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by AΒ1–42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Rawa, M.; Mazlan, M.; Ahmad, R.; Nogawa, T.; Wahab, H. Roles of Syzygium in anti-cholinesterase, anti-diabetic, anti-inflammatory, and antioxidant: From Alzheimer’s perspective. Plants 2022, 11, 1476. [Google Scholar] [CrossRef]
- Elufioye, T.; Berida, T.; Habtemariam, S. Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. Evid.-Based Complement. Altern. Med. 2017, 2017, 3574012. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; Martino, L.; Feo, V.; Nazzaro, F. Anti-cholinesterase and anti-α-amylase activities and neuroprotective effects of carvacrol and p-cymene and their effects on hydrogen peroxide-induced stress in SH-SY5Y cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef] [PubMed]
- Parasram, K. Flavonoids and diarylheptanoids: Neuroprotective activities of phytochemicals. Int. J. Pharmacol. Phytochem. Ethnomed. 2017, 6, 82–86. [Google Scholar] [CrossRef]
- Omoruyi, S.; Ibrakaw, A.; Ekpo, O.; Boatwright, J.; Cupido, C.; Hussein, A. Neuroprotective activities of Crossyne flava bulbs and Amaryllidaceae alkaloids: Implications for Parkinson’s disease. Molecules 2021, 26, 3990. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Q.; Brinton, R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med. 2002, 227, 509–519. [Google Scholar] [CrossRef]
- Oluwatunase, G.; Enemali, F.; Okwute, P.; Asafa, O.; Ajimoh, F. Comparative study of the effect of Ginkgo biloba extract and Vitamin C on lead-induced hippocampal toxicity in adult male Wistar rats. Anat. J. Afr. 2023, 12, 2327–2336. [Google Scholar] [CrossRef]
- Pilija, V.; Radenković, M.; Brenesel, M.; Popović, M.; Ivetic, V.; Trivić, S. Inhibitory effect of Ginkgo biloba extract on the tonus 1250 of the small intestine and the colon of rabbits. Molecules 2010, 15, 2079–2086. [Google Scholar] [CrossRef]
- Mazza, M.; Capuano, A.; Bria, P.; Mazza, S. Ginkgo biloba and donepezil: A comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur. J. Neurol. 2006, 13, 981–985. [Google Scholar] [CrossRef]
- Nikmahzar, E.; Jahanshahi, M.; Babakordi, F. Ginkgo biloba extract decreases scopolamine-induced congophilic amyloid plaques accumulation in male rat’s brain. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e69143. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Menghini, L.; Ferrante, C.; Chiavaroli, A.; Vacca, M. Ginkgo biloba leaf extract reverses amyloid β-peptide-induced isoprostane production in rat brain in vitro. Planta Medica 2006, 72, 1296–1299. [Google Scholar] [CrossRef]
- Nathan, P.; Ricketts, E.; Wesnes, K.; Mrazek, L.; Greville, W.; Stough, C. The acute nootropic effects of Ginkgo biloba in healthy older human subjects: A preliminary investigation. Hum. Psychopharmacol. Clin. Exp. 2002, 17, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Niederhofer, H. Ginkgo biloba treating patients with attention-deficit disorder. Phytother. Res. 2009, 24, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, T.; Lovato, J.; Arnold, A.; Furberg, C.; Kuller, L.; Burke, G.; Nahin, R.; Lopez, O.; Yasar, S.; Williamson, J. Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. Am. J. Hypertens. 2010, 23, 528–533. [Google Scholar] [CrossRef]
- Helal, N.; Ibrahim, F.; Safia, H.; Shamy, A.; Ibrahim, H.; AboHashish, N.; Elbastawesy, S. The nephrotoxic effect of chloroquine, the off-label anti-COVID 19 and possible protective role of Ginkgo biloba extract in male albino rats. Egypt. J. Forensic Sci. Appl. Toxicol. 2023, 23, 49–59. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Ohta, Y.; Shimizu, M.; Maruyama, J.; Mochizuki, M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015, 1, 14. [Google Scholar] [CrossRef]
- Johnson, W.; Bergfeld, W.; Belsito, D.; Hill, R.; Klaassen, C.; Liebler, D.; Marks, J.; Shank, R.; Slaga, T.; Snyder, P.; et al. Safety assessment of Centella asiatica-derived ingredients as used in cosmetics. Int. J. Toxicol. 2023, 42, 5S–22S. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Arfian, N.; Fitriawan, A.; Yuniartha, R.; Sari, D. Ethanolic extract of Centella asiatica treatment in the early stage of hyperglycemia condition inhibits glomerular injury and vascular remodeling in diabetic rat model. Evid.-Based Complement. Altern. Med. 2021, 2021, 6671130. [Google Scholar] [CrossRef]
- Gupta, R.; Flora, S. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J. Appl. Toxicol. 2006, 26, 213–222. [Google Scholar] [CrossRef]
- Widiyastuti, T. The effect of Centella asiatica extract in zebrafish (Danio rerio) larvae as a chronic constant hypoxia (CCH) model. Pediatr. Sci. J. 2022, 1, 20–26. [Google Scholar] [CrossRef]
- Choi, M.; Zheng, H.; Kim, J.; Lee, K.; Park, Y.; Lee, D. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Mol. Med. Rep. 2016, 14, 4521–4528. [Google Scholar] [CrossRef]
- Kuswati, K. The effect of Centella asiatica ethanolic extract on caspase-3 expression in prefrontal cortex of chronic restraint stress induced Sprague Dawley rat. J. Kedokt. Dan Kesehat. Indones. 2015, 7, 65–72. [Google Scholar] [CrossRef]
- Ariani, A. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Vet. J. 2024, 14, 1154. [Google Scholar] [CrossRef] [PubMed]
- KK, A. Centella asiatica (L.) urban: A review on panoramic exploration of medicinal marvels for health and healing. Int. J. Adv. Acad. Stud. 2020, 2, 369–372. [Google Scholar] [CrossRef]
- Hussin, H.; Lawi, M.; Haflah, N.; Kassim, A.; Idrus, R.; Lokanathan, Y. Centella asiatica (L.)-neurodifferentiated mesenchymal stem cells promote the regeneration of peripheral nerve. Tissue Eng. Regen. Med. 2020, 17, 237–251. [Google Scholar] [CrossRef]
- Roushan, R.; Tiwari, S.; Ayurveda, P.; Sansthan, C. Response of Centella asiatica in the management of age-related problems among elderly with special reference to cognitive problems as per Prakriti. Int. J. Res. Ayurveda Pharm. 2013, 4, 163–167. [Google Scholar] [CrossRef]
- Kannoor, A.; Ramani, P. Effect of ethanolic extract of Centella asiatica on pentylene tetrazol induced seizure in albino mice. Int. J. Basic Clin. Pharmacol. 2020, 9, 1583. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, G.; Lv, J.; Chen, H.; Lin, J.; Fan, G.; Ding, X. Identification of Centella asiatica’s effective ingredients for inducing the neuronal differentiation. Evid.-Based Complement. Altern. Med. 2016, 2016, 9634750. [Google Scholar] [CrossRef]
- Xiang, Y.; Shang, H.; Gao, X.; Zhang, B. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. 2008, 22, 851–858. [Google Scholar] [CrossRef]
- Huang, X.; Li, N.; Pu, Y.; Zhang, T.; Bing, W. Neuroprotective effects of Ginseng phytochemicals: Recent perspectives. Molecules 2019, 24, 2939. [Google Scholar] [CrossRef]
- Boța, M.; Vlaia, L.; Jîjie, A.R.; Marcovici, I.; Crişan, F.; Oancea, C.; Dehelean, C.A.; Mateescu, T.; Moacă, E.A. Exploring Synergistic Interactions between Natural Compounds and Conventional Chemotherapeutic Drugs in Preclinical Models of Lung Cancer. Pharmaceuticals 2024, 17, 598. [Google Scholar] [CrossRef]
- Kim, K.; Lee, D.; Lee, H.; Kim, C.; Jung, K.; Kang, K. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dik, B.; Avci, O.; Dik, I. In vitro antiviral and antioxidant activities of silymarin and Panax ginseng on vero cells infected with bovine ephemeral fever virus and blue tongue virus. Acta Pol. Pharm.-Drug Res. 2019, 76, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Gu, J.; Zhao, B.; Wang, S.; Yuan, J.; Wang, C.; Chen, J.; Liu, J.; Feng, L.; Jia, X. Ginseng improves cognitive deficit via the rage/NF-ΚB pathway in advanced glycation end product-induced rats. J. Ginseng Res. 2015, 39, 116–124. [Google Scholar] [CrossRef]
- Gong, L.; Yin, J.; Ren, H.; Lou, Y.; Jiang, H.; Sun, L.; Jia, J.; Zeng, X. Neuroprotective mechanisms of Ginsenoside RB1 in central nervous system diseases. Front. Pharmacol. 2022, 13, 914352. [Google Scholar] [CrossRef]
- Lee, S.; Chu, K.; Sim, J.; Heo, J.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Mahour, K. Mercury retention after Panax ginseng treatment against mercuric chloride intoxication in hepato-haemato indices in albino rats. World J. Biol. Pharm. Health Sci. 2023, 13, 345–349. [Google Scholar] [CrossRef]
- Yang, K.; Ryu, T.; Chung, B. A meta-analysis of preclinical studies to investigate the effect of Panax ginseng on alcohol-associated liver disease. Antioxidants 2023, 12, 841. [Google Scholar] [CrossRef]
- Cristina-Souza, G.; Santos-Mariano, A.; Lima-Silva, A.; Costa, P.; Domingos, P.; Silva, S.; Abreu, W.; De-Oliveira, F.; Osiecki, R. Panax ginseng supplementation increases muscle recruitment, attenuates perceived effort, and accelerates muscle force recovery after an eccentric-based exercise in athletes. J. Strength Cond. Res. 2020, 36, 991–997. [Google Scholar] [CrossRef]
- Singh, M.; Jayant, K.; Singh, D.; Bhutani, S.; Poddar, N.; Chaudhary, A.; Khan, S.; Adnan, M.; Siddiqui, A.; Hassan, M.; et al. Withania somnifera (L.) dunal (ashwagandha) for the possible therapeutics and clinical management of SARS-COV-2 infection: Plant-based drug discovery and targeted therapy. Front. Cell. Infect. Microbiol. 2022, 12, 933824. [Google Scholar] [CrossRef]
- Sankar, S.; Manivasagam, T.; Krishnamurti, A.; Ramanathan, M. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: An analysis of behavioral and biochemical variables. Cell. Mol. Biol. Lett. 2007, 12, 473–481. [Google Scholar] [CrossRef]
- Sandhu, J.; Shah, B.; Shenoy, S.; Chauhan, S.; Lavekar, G.; Padhi, M. Effects of Withania somnifera (ashwagandha) and Terminalia arjuna (arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.; Chauhan, M.; Chauhan, S. Protective role of Withania somnifera root extract on lipid peroxidation of erythrocytes. Univ. J. Phytochem. Ayurvedic Heights 2022, II, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Wakabayashi, Y.; Wakamatsu, K.; Imokawa, G. An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Phytother. Res. 2011, 25, 1398–1411. [Google Scholar] [CrossRef]
- Chaudhari, K.; Tiwari, R.; Chaudhari, S.; Joshi, S.; Singh, H. Withania somnifera as an adjunctive treatment for refractory restless legs syndrome in Parkinson’s disease: A case report. Cureus 2021, 13, e20775. [Google Scholar] [CrossRef]
- Pingali, U.; Pilli, R.; Fatima, N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacogn. Res. 2014, 6, 12–18. [Google Scholar] [CrossRef]
- Basudkar, V. Emerging vistas for the nutraceutical Withania somnifera in inflammaging. Pharmaceuticals 2024, 17, 597. [Google Scholar] [CrossRef]
- Seth, S.; Agarwal, K.; Rahman, A. Curcumin: A review of its effects on human health. Int. Healthc. Res. J. 2022, 5, RV1–RV4. [Google Scholar] [CrossRef]
- Han, S.; Xu, J.; Guo, X.; Huang, M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin. Exp. Pharmacol. Physiol. 2017, 45, 84–93. [Google Scholar] [CrossRef]
- Tomeh, M.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, C.; Qu, S.; Dong, S.; Ma, Q.; Hao, Y.; Liu, Z.; Wang, S.; Zhao, H.; Shi, Y. Chinese Herbal Extracts Exert Neuroprotective Effect in Alzheimer’s Disease Mouse Through the Dopaminergic Synapse/Apoptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 817213. [Google Scholar] [CrossRef]
- Paek, S.; Han, S.; Mun, S. Effects of Curcuma longa on status epilepticus induced by pilocarpine in rats. Int. J. Clin. Exp. Physiol. 2023, 9, 112–114. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and safety of curcumin and Curcuma longa extract in the treatment of arthritis: A systematic review and meta-analysis of randomized controlled trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Akhter, Q.; Nahar, S. Cardioprotective effect of Curcuma longa (turmeric) on serum cardiac marker enzymes in isoproterenol induced myocardial infarction in rats. World J. Adv. Res. Rev. 2022, 13, 98–103. [Google Scholar] [CrossRef]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabianska, M.; Wrobel, M.; Solek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front Pharmacol 2021, 12, 775034. [Google Scholar] [CrossRef]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef]
- Montes, P.; Ruiz-Sanchez, E.; Rojas, C.; Rojas, P. Ginkgo biloba extract 761: A review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets CNS Neurol. Disord. 2015, 14, 132–149. [Google Scholar] [CrossRef]
- El Tabaa, M.M.; Sokkar, S.S.; Ramadan, E.S.; Abd El Salam, I.Z.; Zaid, A. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: An animal model study. Neurochem. Int. 2017, 108, 199–212. [Google Scholar] [CrossRef]
- Jana, U.; Sur, T.K.; Maity, L.N.; Debnath, P.K.; Bhattacharyya, D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Med. Coll. J. 2010, 12, 8–11. [Google Scholar]
- Tabassum, R.; Vaibhav, K.; Shrivastava, P.; Khan, A.; Ahmed, M.; Javed, H.; Islam, F.; Ahmad, S.; Siddiqui, M.; Safhi, M.; et al. Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol. Sci. 2013, 34, 925–933. [Google Scholar] [CrossRef]
- Amjad, S.; Umesalma, S. Protective Effect of Centella asiatica against Aluminium-Induced Neurotoxicity in Cerebral Cortex, Striatum, Hypothalamus and Hippocampus of Rat Brain- Histopathological, and Biochemical Approach. J. Mol. Biomark. Diagn. 2015, 6, 1000212. [Google Scholar] [CrossRef]
- Boondam, Y.; Tantisira, M.H.; Tilokskulchai, K.; Tapechum, S.; Pakaprot, N. Acute enhancing effect of a standardized extract of Centella asiatica (ECa 233) on synaptic plasticity: An investigation via hippocampal long-term potentiation. Pharm. Biol. 2021, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Teerapattarakan, N.; Benya-Aphikul, H.; Tansawat, R.; Wanakhachornkrai, O.; Tantisira, M.; Rodsiri, R. Neuroprotective effect of a standardized extract of Centella asiatica ECa233 in rotenone-induced parkinsonism rats. Phytomedicine 2018, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wanakhachornkrai, O.; Pongrakhananon, V.; Chunhacha, P.; Wanasuntronwong, A.; Vattanajun, A.; Tantisira, B.; Chanvorachote, P.; Tantisira, M. Neuritogenic effect of standardized extract of Centella asiatica ECa233 on human neuroblastoma cells. BMC Complement. Altern. Med. 2013, 13, 204. [Google Scholar] [CrossRef]
- Jantwal, A.; Durgapal, S.; Upadhyay, J.; Rana, M.; Tariq, M.; Dhariwal, A.; Joshi, T. Centella asiatica. In Naturally Occurring Chemicals against Alzheimer’s Disease; Academic Press: Cambridge, MA, USA, 2021; pp. 257–269. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Mourad, I.M.; Mohammed, H.S.; Noor, N.A.; Ezz, H.S.A. A study on the possible therapeutic role of Panax ginseng extract against a rat model of Parkinson’s disease induced by intrastriatal rotenone injection. Int. J. Clin. Exp. Med. 2016, 9, 3831–3841. [Google Scholar]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Luo, H.; Hu, J.; Wang, Y.; Chen, Y.; Zhu, D.; Jiang, R.; Qiu, Z. In vivo and in vitro neuroprotective effects of Panax ginseng glycoproteins. Int. J. Biol. Macromol. 2018, 113, 607–615. [Google Scholar] [CrossRef]
- Sun, X.C.; Ren, X.F.; Chen, L.; Gao, X.Q.; Xie, J.X.; Chen, W.F. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 2016, 155, 94–103. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Oh, S. Neuroprotective effect of Korean Red Ginseng against single prolonged stress-induced memory impairments and inflammation in the rat brain associated with BDNF expression. J. Ginseng Res. 2022, 46, 435–443. [Google Scholar] [CrossRef]
- Singh, M.; Ramassamy, C. In Vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J. Nutr. Sci. 2017, 6, e54. [Google Scholar] [CrossRef]
- Pandey, A.; Bani, S.; Dutt, P.; Kumar Satti, N.; Avtar Suri, K.; Nabi Qazi, G. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef]
- Elhadidy, M.E.; Sawie, H.G.; Meguid, N.A.; Khadrawy, Y.A. Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats. Asian Pac. J. Trop. Biomed. 2018, 8, 59–66. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Al Hilo, A.S.; Al-Balas, Q.A.; El-Salem, K.; El-Elimat, T.; Alali, F.Q. Withania somnifera root powder protects againist post-traumatic stress disorder-induced memory impairment. Mol. Biol. Rep. 2019, 46, 4709–4715. [Google Scholar] [CrossRef] [PubMed]
- Auddy, B.; Hazra, J.; Mitra, A.; Abedon, B.; Ghosal, S. A standardized Withania somnifera extract significantly reduces stress related parameters in chronically stressed humans: A double blind randomized placebo controlled study. J. Am. Med. 2008, 11, 51–57. [Google Scholar]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N.A. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef]
- Rajakrishnan, V.; Viswanathan, P.; Rajasekharan, K.N.; Menon, V.P. Neuroprotective role of curcumin from Curcuma longa on ethanol-induced brain damage. Phytother. Res. 1999, 13, 571–574. [Google Scholar] [CrossRef]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.; Yoon, Y.; et al. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef]
- He, H.J.; Xiong, X.; Zhou, S.; Zhang, X.R.; Zhao, X.; Chen, L.; Xie, C.L. Neuroprotective effects of curcumin via autophagy induction in 6-hydroxydopamine Parkinson’s models. Neurochem. Int. 2022, 15, 105297. [Google Scholar] [CrossRef]
- Akter, F.; Haque, M.; Islam, J.; Rahaman, A.; Bhowmick, S.; Hossain, S. Chronic Administration of Curcuma longa Extract Improves Spatial Memory-Related Learning Ability in Aged Rats by Inhibiting Brain Cortico-Hippocampal Oxidative Stress and TNFα. Adv. Alzheimer’s Dis. 2015, 4, 78–89. [Google Scholar] [CrossRef][Green Version]
| Botanical Name of the Plant | Name of the Secondary Metabolites | Chemical Structures of the Metabolites | Structural Diversity |
|---|---|---|---|
| Aconitum napellus | Aconitine |  | Structurally, aconitine (C34H47NO11) features a bicyclic skeleton with a long aliphatic chain, a methoxy group, and a hydroxyl group. The molecular architecture contributes to its cardiotoxicity and neurotoxicity. |
| Mesaconitine | 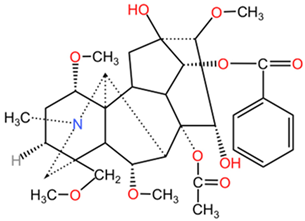 | Mesaconitine (C34H47NO10) and hypaconitine (C34H47NO9) differ from aconitine through minor modifications in their functional groups and the presence of different hydroxy and methoxy groups, which impact their pharmacological profiles. | |
| Hypaconitine | 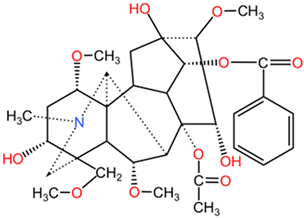 | ||
| Benzoylaconine | 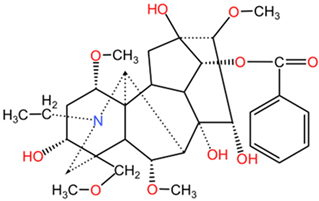 | Benzoylaconine (C32H45NO10), benzoylmesaconine (C31H43NO9), and benzoylhypaconine (C31H43NO10) feature benzoyl group substitutions, which affect solubility and bioactivity. The incorporation of aromatic moieties tends to enhance their lipophilicity, potentially leading to improved cell membrane permeability. | |
| Benzoylmesaconine | 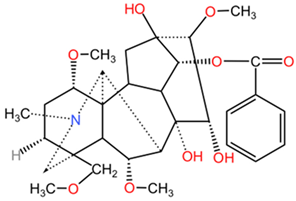 | ||
| Benzoylhypaconine | 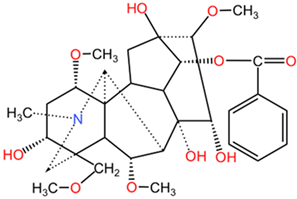 | ||
| Atropa belladonna | Hyoscyamine |  | Hyoscyamine (C17H23NO3) is a naturally occurring tropane alkaloid, structurally characterized by a tropan ring fused with a cyclic ether. Its epimer, atropine (C17H23NO3), exhibits similar structural elements but differs in the spatial arrangement of its atoms, influencing its pharmacological efficacy and selectivity at muscarinic receptors. |
| Atropine | 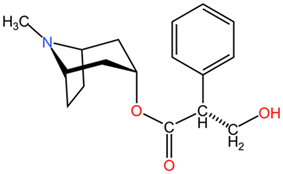 | ||
| Scopolamine | 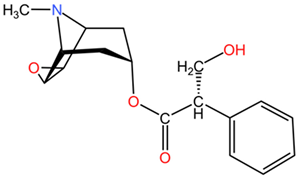 | Scopolamine (C17H21NO4), another tropane derivative, features an additional hydroxyl group compared to hyoscyamine, imparting distinct central nervous system effects. | |
| Conium maculatum | Coniine | 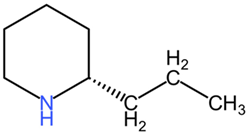 | Coniine (C8H17N) and γ-coniceine (C8H15N) are piperidine derivatives, with structural simplicity relative to the previously mentioned compounds. Coniine consists of a saturated piperidine ring, while γ-coniceine has a double bond, influencing their activity and toxicity. |
| γ-coniceine | 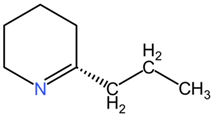 | ||
| Oenanthe crocata | Oenanthotoxin | 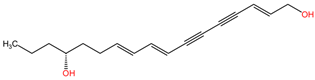 | Oenanthotoxin and cicutoxin are structurally complex compounds that exhibit potent neurotoxic properties. Oenanthotoxin has a tricyclic structure with multiple functional groups, while cicutoxin contains an alkaloid structure with unsaturation, highlighting the versatility of alkaloid chemistry. |
| Cicutoxin |  | ||
| Ricinus communis | Ricinine | 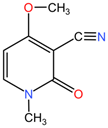 | Ricinine (C12H17N3O4) differs significantly from the previous alkaloids, featuring a distinct tricyclic structure with nitrogen atoms in different orientations. Its diverse functional groups suggest varied biological activities compared to the other compounds. |
| Plant, Family | Used Parts | Beneficial Secondary Metabolites/Extract Type | In Vivo/In Vitro Studies and Recommended Effective Dosage | Outcomes | Refs. |
|---|---|---|---|---|---|
| Ginkgo biloba, Ginkgoaceae | Leaves | EGb 761 extract (the standardized extract of dried leaves made with acetone 60% (w/w) as the extraction solvent): 22–27% flavonoids and 5–7% terpene lactones, consisting of 2.8–3.4% A, B and C ginkgolides and 2.6–3.2% bilobalide | Randomized, placebo-controlled trials: 240 mg/day (for 12–26 weeks), 2 × 120 mg/day (for 22–24 weeks) | Neuroprotection can be achieved through several mechanisms, which include the suppression of inflammation and apoptosis, a reduction in amyloid precursor protein levels, particularly Amyloid β, and the promotion of cell proliferation within the hippocampus. At the cellular level, the neuroprotective actions encompass the elimination of free radicals, enhancement of mitochondrial performance, a decrease in blood viscosity, modulation of serotonin concentrations across different brain regions, and an elevation of dopamine levels in the prefrontal cortex. These processes collectively contribute to the preservation of neuronal health and function. | [199,200] |
| Mice, rats: 50–100 mg/kg/day for 14 days–7 months | Antistress effects, decreasing anxiety level and MAO activity. | [201] | |||
| Mice: 10 mg/kg/day (i.p.) for 17 days | Reduction in lipid peroxidation and superoxide radical production, and modulation of serotonergic and dopaminergic neurotransmission → antidepressant-like effect. | [201] | |||
| Randomized, placebo-controlled clinical trials evaluating efficacy in dementia: 120 mg/day (52–26 weeks), 240 mg/day (12–24 weeks) | Significant improvement in cognitive function. | [199,201] | |||
| Rats: 4 mg/kg BW (for 8 weeks) | The extract promotes the secretion of estrogen-dependent biogenic amines within the hippocampus. Additionally, it mitigates neuronal damage in the hippocampus through its antioxidant properties and its ability to stimulate adiponectin release. Furthermore, it has the potential to manage memory and learning impairments effectively. | [202] | |||
| Healthy volunteers (18–26 years): 120, 240, 360 mg/day—single doses; 120 mg/day for 6 weeks | Improved cognitive performance, self-esteem, and mood, increasing attention efficiency and improving memory quality. | [199] | |||
| Patients with mild to moderate dementia, Alzheimer’s disease (50–70 years): 240 mg/day for 24 weeks | Overall improvement in cognitive functions and general condition. | [199] | |||
| Centella asiatica, Apiaceae | The aerial parts (leaves and stems) | 70% hydro-ethanolic extract | Clinical study (patients 18–60 years old): 500 mg/capsule, twice daily for 60 days | Overall improvement in stress, anxiety, depression, adjustment, and attention. | [203] |
| Ethanolic extract | Rats: 200, 300 mg/kg BW/day (p.o.) for 21 days | Improved neurobehavioral activity and reduced tissue death due to lack of oxygen. Additionally, there was considerable safeguarding against neuronal damage, as indicated by the preservation of hippocampal and cortical neurons. | [204] | ||
| Aqueous leaf extract | Rats: 500 mg/kg BW (p.o.) | The extract reduced behavioral impairments and enhanced learning and memory performance. | [205] | ||
| ECa233 (standardized extract): 85% triterpenoid glycoside, divided into madecassoside (53.1%) and asiaticoside (32.3%) in a ratio of 1.5:0.5; 15% triterpenoid saponins, of which less than 1% are triterpenic acid metabolites (madecassic acid and asiatic acid) | Rats: 30 mg/kg (p.o.) | Significantly enhanced memory retention, with an increase in synaptic-related proteins in the hippocampus, leading to a strong synaptic plasticity enhancement. It also demonstrated an anxiolytic effect. | [206,207] | ||
| In vitro (neuroblastoma cell lines): 100 μg/mL | Significant stimulatory effects on the elongation of neuroblastoma cell neurites. | [208] | |||
| Extract containing tannic acid (29.9 mg/g), asiaticoside (1.09 mg/g), and asiatic acid (48.89 mg/g) | Randomized, double-blind, placebo-controlled clinical study (mean age: 65 years): 250, 500, 750 mg/day for 2 months | Enhanced overall cognitive functions, memory, and self-rated mood. | [209] | ||
| Clinical study (mean age: 65 years): 1000 mg/day for 6 months | Significant cognitive improvement. | ||||
| Capsules of 70% hydro-ethanolic extract of dried aerial parts | Open-label (adults with generalized anxiety disorder, mean age 33 years): 500 mg twice daily for 2 months | The capsules demonstrated a multifaceted impact on psychological well-being, as they significantly lowered anxiety levels, increased self-perceived stress, decreased the depression index, enhanced the adjustment index, and elevated attention levels. | [203] | ||
| Panax ginseng, Araliaceae | Roots | Panax ginseng extract: ginsenosides (the main, 70–80%, found in fresh ginseng are Rb2, Rb1, Re, Rg1, Rc) | Rats: 100 mg/kg (p.o.) for 2 weeks | Enhanced alterations in the midbrain and striatum, as well as demonstrated a partial therapeutic effect in a rat model of Parkinson’s disease. | [179,210] |
| Total saponins | Mice: 50, 100, 200 mg/kg/day (p.o.) | Memory deterioration was mitigated through the enhancement of antioxidant levels in the hippocampus, alongside an increase in proteins associated with neural plasticity. | [211] | ||
| Glycoprotein PGL-1 | Rats: 40, 80, 160 mg/kg·d−1 (i.p.) for 35 days | The treatment significantly improved the learning and memory abilities in the Alzheimer’s disease rat model. The neuroprotective effects of PGL-1 may be associated with its ability to suppress nitric oxide synthesis. | [212] | ||
| Ginsenoside Rg1 | Mice: 10 mg/kg (i.p.) for 14 days | Ginsenoside Rg1 has demonstrated the ability to inhibit the activation of microglial cells triggered by lipopolysaccharides and to safeguard dopaminergic neurons within the nigrostriatal pathway. This suggests that Rg1 could serve as a potential therapeutic approach to mitigate inflammatory harm associated with chronic neurodegenerative disorders. | [213] | ||
| Korean red ginseng (KRG) | Rats: 20, 50, 100 mg/kg (i.p.) for 14 days | The administration of KRG has been shown to enhance cognitive functions and capabilities, including the processes of learning acquisition, memory consolidation, and the reduction in fear memory in the animal model exhibiting memory impairment due to SPS. These results indicate that KRG may serve as a beneficial alternative therapy for cognitive dysfunctions associated with traumatic stress, particularly in conditions like PTSD. | [214] | ||
| Withania somnifera, Solanaceae | Roots, leaves (occasionally) | A standardized extract: 2.6% withanolides including withanolides IV (0.87%) and V (0.65%), withaferin A (0.56%), withanolide A (0.20%) and B (0.06%), and 12-deoxy withastramonolide (0.26%) | In vitro (human neuroblastoma cell line): 12–50 µg/mL | The extract demonstrated neuroprotective activities through multiple mechanisms, including protection against Aβ and acrolein toxicity, reduction in oxidative stress, and inhibition of AChE activity. | [215] |
| Withanone extracted from the roots | Rats: 5, 10, 20 mg/kg (p.o.) for 21 days in vitro (PC-12 cell line) | Withanone exhibits a significant protective effect against amyloid β toxicity, which is crucial in the context of neurodegenerative diseases such as Alzheimer’s. Withanone not only enhances memory retention but also significantly mitigates cognitive impairment associated with Aβ accumulation. Furthermore, the compound effectively attenuates elevated levels of pro-inflammatory cytokines, suggesting a dual mechanism of action that involves both neuroprotection and anti-inflammatory effects. | [216] | ||
| Ethanolic extract | Rats: 200 mg/kg (p.o.) for 30 days | The extract exhibits properties that combat neurotoxicity through its antioxidant and anti-inflammatory actions, effectively mitigating oxidative stress, neuroinflammation, and excitotoxicity. Furthermore, it may help preserve cholinergic function by regulating acetylcholinesterase activity, suggesting its potential role as a cognitive enhancer. Consequently, ashwagandha extract could be considered a complementary treatment option for Alzheimer’s disease. | [217] | ||
| Hydro-ethanolic extract from roots | Rats: 500 mg/mL (p.o.) for 6 weeks | The extract may safeguard against memory deficits associated with PTSD, both in the short and long term, potentially by counteracting oxidative stress in the hippocampus. | [218] | ||
| Standardized ethanolic extract: 11.90% withanolide glycosides, 1.05% withaferin A, 40.25% oligosaccharides, 0.05% alkaloids, 3.44% polysaccharides. | Double-blind, randomized, placebo-controlled study (18–60 years): 12, 250 mg; 1000 mg/individual; 60 days | The administration of ashwagandha was associated with a decrease in subjective experiences of stress and anxiety in chronically stressed adults. Furthermore, it has been shown to lower serum levels of cortisol and C-reactive protein, as well as reduce pulse rate and blood pressure. Additionally, ashwagandha supplementation contributes to improvements in fasting blood glucose levels and lipid profiles. These findings suggest that ashwagandha may play a beneficial role in managing physiological and psychological stress responses, alongside enhancing metabolic health indicators. | [219,220] | ||
| Curcuma longa, Zingiberaceae | Rhizome | Curcumin | Rats: divided into two different groups: Group 1: rats were given only glucose solution Group 2: rats were treated with 25% alcohol for one month, then divided into two subgroups: (a) those who continued treatment with 25% alcohol only (b) those who received treatment with curcumin 80 mg/kg body combined with 25% ethanol | Alcohol treatment in the rats resulted in significant increases in cholesterol, phospholipids, free fatty acids, and TBARS levels in the brain compared to the control group, which received no alcohol at all. It was shown that administration of the alcoholic extract with curcumin led to a considerable decrease in cholesterol, phospholipids, and TBARS levels, and glutathione levels were partially restored. This supports the fact that curcumin has a protective effect on the brain, reducing oxidative stress and alleviating alcohol damage. | [221] |
| Alcoholic extract dissolved in Tween 20 before administration | Mice: 100 mg/kg (p.o.) for 13 weeks | Curcumin administration was effective in significantly improving memory, demonstrating once again its neuroprotective effect. | [222] | ||
| Curcumin | In vitro (rat adrenal pheochromocytoma PC12 cells): 0, 25, 50, 100, 150 and 200 μM for 24 h | In vitro treatment with curcumin has been shown to significantly reduce cellular damage and decrease 6-OHDA-induced mortality. | [223] | ||
| Aqueous extract | Rats: 100 mg/kg BW/day | Results of the in vivo study showed that Curcuma longa extract helped to improve learning ability in elderly rats, having a neuroprotective effect against cognitive impairment associated with aging or Alzheimer’s disease. | [224] |
| Botanical Name of the Plant | Name of the Secondary Metabolites | Chemical Structures of the Metabolites | Structural Diversity |
|---|---|---|---|
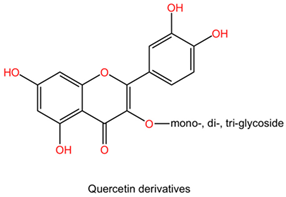
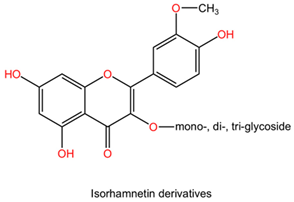
| Ginkgo biloba | Flavonoids (kaempferol, quercetin, isorhamnetin derivatives). | 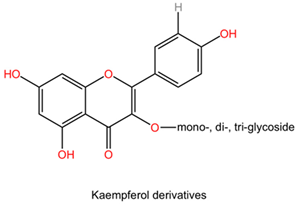 | Flavonoids are polyphenolic compounds characterized by a flavone backbone, featuring various substitutions that alter their chemical properties and biological activities. Kaempferol has multiple hydroxyl groups that can donate hydrogen and provide antioxidant properties. Quercetin possesses a similar structure but has an additional double bond and ketone, enhancing its bioactivity and solubility. Isorhamnetin is a methoxy derivative of quercetin that exhibits different pharmacokinetics and increased lipid solubility. |
 | |||
 | |||
| Terpene lactones: ginkgolides (A, B, C, J, M) and bilobalide | 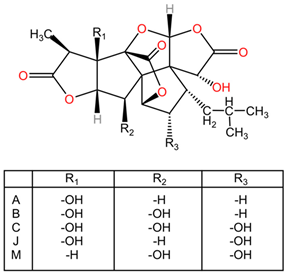 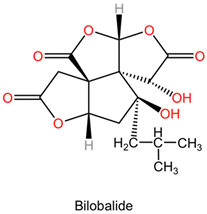 | Ginkgolides are a class of diterpene lactones found in Ginkgo biloba, exhibiting significant pharmacological properties. The major ginkgolides—A, B, C, J, and M—possess distinct structural features, including two terpene units and various functional groups that diversify their activity. Ginkgolide A and B are characterized by their unique bicyclic structures, impacting their receptor binding interactions. Bilobalide, another key compound, is a sesquiterpene derivative that complements the activity of ginkgolides. | |
| Centella asiatica | Triterpenoid glycoside: madecassoside, asiaticoside Triterpenoid saponins: madecassic acid, asiatic acid | 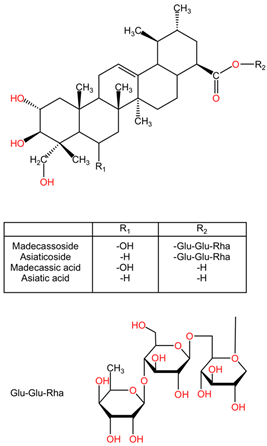 | Triterpenoid glycosides, such as madecassoside and asiaticoside, display a complex structure consisting of a triterpene backbone linked to sugar moieties. This glycosylation affects their solubility and bioavailability. Madecassoside exhibits a pentacyclic triterpene structure with various sugar attachments, increasing its medicinal properties. Asiaticoside, a closely related compound, differs from its sugar moieties, which contributes to its distinct pharmacological profile. Triterpenoid saponins, such as madecassic acid and asiatic acid, showcase a steroid-like structure that differs in functional group orientation and substitution patterns. |
| Panax ginseng | Ginsenosides (Rg1, Re, Rb1, Rc, Rd) | 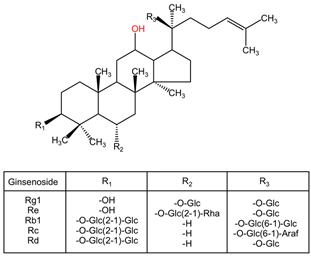 | Ginsenosides encompass a diverse range of triterpenoid saponins, including Rg1, Re, Rb1, Rc, and Rd. Each ginsenoside exhibits variability in sugar chains and structure, influencing its pharmacological properties. Rg1 has a protopanaxadiol framework that promotes nerve cell regeneration. Re and Rb1 contain additional sugar moieties that enhance their bioavailability and activity in modulating immune responses. |
| Withania somnifera | Withanolide A |  | Withanolides, derived from Withania somnifera, encompass a diverse group of steroidal lactones. Important representatives, including withanolide A, B, withanone, and withaferin A, exhibit variations in side chains and functional groups. Withanolide A features a complex cyclic structure with multiple hydroxyl substitutions, enhancing its bioactivity. Withaferin A has a highly oxygenated ring structure that contributes to its potent cytotoxicity against cancer cells. |
| Withanolide B |  | ||
| Withanone |  | ||
| Withaferin A |  | ||
| Withanoside IV |  | ||
| Withanoside V | 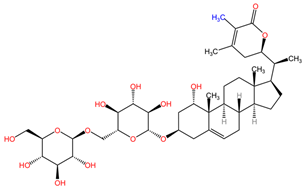 | ||
| Curcuma longa | Curcumin | 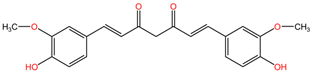 | Curcumin (C21H20O6) features a unique structure characterized by two feruloyl moieties connected by a methylene bridge. This structure is pivotal to curcumin’s interaction with biological targets. |
| Demethoxycurcumin | 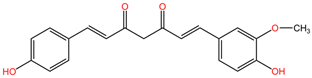 | Demethoxycurcumin (C20H18O5) is a derivative where one of the methoxy groups from curcumin is removed. This structural alteration has been shown to affect its binding affinity to various biological targets and its resultant therapeutic potential. | |
| Bisdemethoxycurcumin | 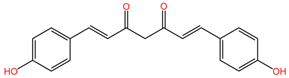 | Bisdemethoxycurcumin (C19H16O4) has both methoxy groups removed from the parent curcumin structure. This compound exhibits altered biological activities, showcasing how subtle changes in structure can lead to significant variations in pharmacodynamics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moise, G.; Jîjie, A.-R.; Moacă, E.-A.; Predescu, I.-A.; Dehelean, C.A.; Hegheș, A.; Vlad, D.C.; Popescu, R.; Vlad, C.S. Plants’ Impact on the Human Brain—Exploring the Neuroprotective and Neurotoxic Potential of Plants. Pharmaceuticals 2024, 17, 1339. https://doi.org/10.3390/ph17101339
Moise G, Jîjie A-R, Moacă E-A, Predescu I-A, Dehelean CA, Hegheș A, Vlad DC, Popescu R, Vlad CS. Plants’ Impact on the Human Brain—Exploring the Neuroprotective and Neurotoxic Potential of Plants. Pharmaceuticals. 2024; 17(10):1339. https://doi.org/10.3390/ph17101339
Chicago/Turabian StyleMoise, Georgiana, Alex-Robert Jîjie, Elena-Alina Moacă, Iasmina-Alexandra Predescu, Cristina Adriana Dehelean, Alina Hegheș, Daliborca Cristina Vlad, Roxana Popescu, and Cristian Sebastian Vlad. 2024. "Plants’ Impact on the Human Brain—Exploring the Neuroprotective and Neurotoxic Potential of Plants" Pharmaceuticals 17, no. 10: 1339. https://doi.org/10.3390/ph17101339
APA StyleMoise, G., Jîjie, A.-R., Moacă, E.-A., Predescu, I.-A., Dehelean, C. A., Hegheș, A., Vlad, D. C., Popescu, R., & Vlad, C. S. (2024). Plants’ Impact on the Human Brain—Exploring the Neuroprotective and Neurotoxic Potential of Plants. Pharmaceuticals, 17(10), 1339. https://doi.org/10.3390/ph17101339










