Exploring Deep Eutectic Solvents as Pharmaceutical Excipients: Enhancing the Solubility of Ibuprofen and Mefenamic Acid
Abstract
1. Introduction
2. Results and Discussions
2.1. Composition and Rationale for DES Selection
| Comp. | Code | 2D Chemical Structure | Electrostatic Potential (ESP) Surface Map | Mw (g/mol) | HBD Count | HBA Count | Density (g/cm3) | Tm (°C) | Tb (°C) | Tg (°C) | logP * | ST * (mN/m) | MV * (cm3) | TPSA * (Å) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ibuprofen | IBU |  | 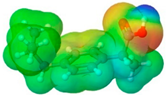 | 206.28 | 1 | 2 | 1.03 [123] | 75 | 157 [123] | −44 [124] | 3.72 | 38.1 ± 3.0 | 200.34 | 37.30 |
| Mefenamic acid | MFA |  | 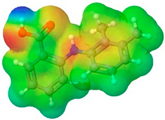 | 241.29 | 2 | 3 | 1.20 [125] | 230 [126] | 398.8 [125] | - | 5.33 | 51.8 ± 3.0 | 200.55 | 49.33 |
| Choline chloride | ChCl | 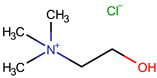 |  | 139.62 | 1 | 2 | ~1.205 (solid), ~1.1 (sol. 70%) [127] | 302–305 (dec.) [20,128] | - | - | −5.16 | - | - | 20.23 |
| (±)-menthol | MNT | 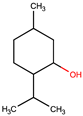 |  | 156.26 | 1 | 1 | 0.89 [90] | 42.5 [126] | 212 [129] | −54.3 [130] | 3.20 | 29.7 ± 3.0 | 175.54 | 20.23 |
| L-Arginine | Arg |  |  | 174.20 | 4 | 4 | 1.42 [126] | 244 (dec.) [126] | - | −4.2 | 66.1 ± 7.0 | 118.72 | 125.22 | |
| L-Cysteine | Cys |  |  | 121.16 | 3 | 3 | 1.66 [126] | 220–240 (dec.) [126] | - | - | 0.23 | 59.0 ± 3.0 | 90.78 | 102.12 |
| Glycolic acid | Gla |  |  | 76.05 | 2 | 3 | 1.26 [126] | 79.5 [126] | 100 (dec.) [126] | - | −1.05 | 61.4 ± 3.0 | 53.68 | 57.53 |
| Oxalic acid | Oxa |  |  | 90.03 | 2 | 4 | 1.90 [126] | 185–189.5 (dec.) [126] | - (sublimes) | - | −1.19 | 87.4 ± 3.0 | 50.80 | 74.60 |
| L-lactic acid | Lac |  |  | 90.08 | 2 | 3 | 1.25 [129] | 53 [126] | 125–140 [131] | - | −0.70 | 49.8 ± 3.0 | 70.56 | 57.53 |
| Glycerol | Gly |  |  | 92.09 | 3 | 3 | 1.26 [129] | 17.9–18.2 [126] | 290.0 ± 0.0 [129] | −83.15 [132] | −2.32 | 62.0 ± 3.0 | 70.95 | 60.69 |
| D-(+)-glucose | Glu |  |  | 180.15 | 5 | 6 | 1.5620 [126] | 146–165 [126,133] | - | 38.87 ± 0.15 [134] | −3.17 | 92.1 ± 3.0 | 113.93 | 118.22 |
| D-(−)-fructose | Fru |  |  | 180.16 | 5 | 6 | 1.60 [126] | 91–185 (dec.) [126] | - | 16–25 [135] | −1.47 | 92.7 ± 3.0 | 106.29 | 110.38 |
| D-(−)-sorbitol | Sor |  |  | 182.17 | 6 | 6 | 1.47 [129] | 98 – 100 hydrated, 111 anhidrous [136] | 295 [129] | −4.15 [132] | −4.67 | 99.9 ± 3.0 | 114.12 | 121.38 |
| Xylitol | Xyl |  |  | 152.15 | 5 | 5 | 1.50 [137] | 95.9 [126] | 380 [126] | −23.15 [132] | −3.77 | 89.7 ± 3.0 | 99.73 | 101.15 |
| Decanoic acid (capric acid) | Dec |  |  | 172.27 | 1 | 2 | 0.89 [126] | 30.41 [138] | 270 [126] | - | 3.97 | 33.2 ± 3.0 | 188.23 | 37.30 |
| Oleic acid | OLA |  |  | 282.47 | 1 | 2 | 0.89 [126] | 13–14 [126] | 360 [139] | - | 7.7 | 33.9 ± 3.0 | 313.90 | 37.30 |
| Medium chain tryglicerides (Miglyol® 812) | MCT |  R1, R2, R3: -H, -C2H5 |  | 498.75 | 0 | 6 | 0.93–0.96 [139] | 6.0 [139] | - | - | 10.39 | 34.7 ± 3.0 | 518.05 | 78.90 |
| (R)-(+)-limonene | Lim |  | 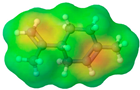 | 136.24 | 0 | 0 | 0.84 [126] | −96.9 [129] | 195 [129] | - | 4.45 | 25.9 ± 3.0 | 163.26 | 0.00 |
| Polyethylene glycol 400 | PEG 400 | 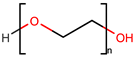 |  | 380–420 | 2 | 10 | 1.12 [126] | 5.8 [140] | 240–250 [126] | −81 [124] | −4.02 | 40.8 ± 3.0 | 371.57 | 114.30 |
| Isoporpyl miristate | IPM |  | 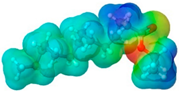 | 270.46 | 0 | 2 | 0.85 [126] | 3.0 [126] | 193 [126] | - | 7.43 | 29.7 ± 3.0 | 313.01 | 26.30 |
| Propylene glycol | PG |  | 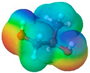 | 76.10 | 2 | 2 | 1.04 [126] | −60 [126] | 187.3 [126] | −101.15 [141] | −1.34 | 38.0 ± 3.0 | 73.44 | 40.46 |
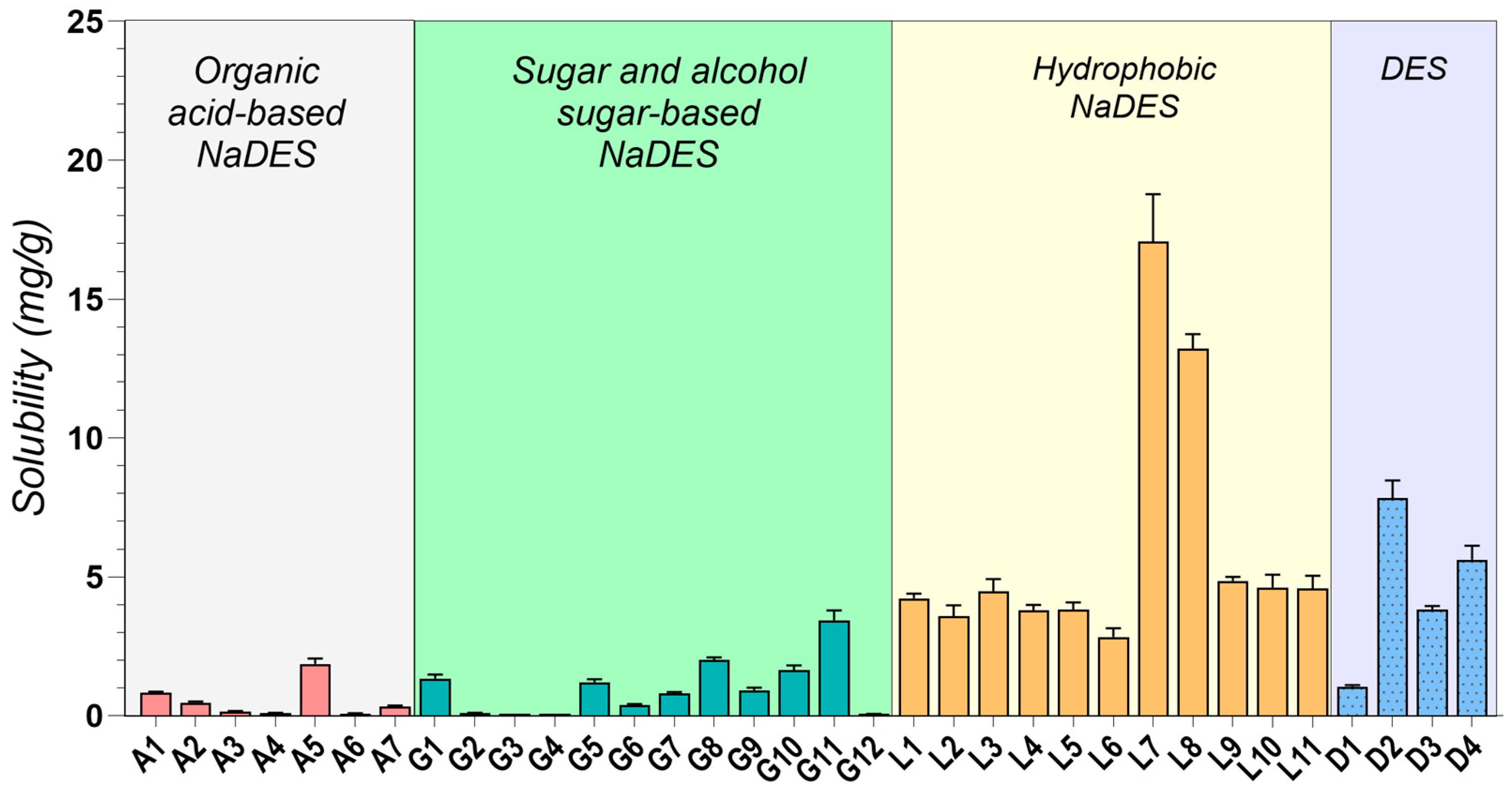
| Code | Components | Density (g/cm3) | Solubility (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Molar Ratio | IBU | MF | ||
| A1 | ChCl | Gla | - | 1:1 | 1.2117 ± 0.0314 | 8.610 ± 0.067 | 0.832 ± 0.033 |
| A2 | ChCl | Gla | - | 1:2 | 1.2597 ± 0.0218 | 3.992 ± 0.043 | 0.481 ± 0.043 |
| A3 | ChCl | Oxa | - | 1:1 | 1.2814 ± 0.0222 | 1.512 ± 0.016 | 0.158 ± 0.016 |
| A4 | ChCl | Lac | - | 1:1 | 1.2366 ± 0.0748 | 8.463 ± 0.011 | 0.104 ± 0.006 |
| A5 | Arg | Gla | - | 1:8 | 1.4479 ± 0.0876 | 48.695 ± 0.112 | 1.861 ± 0.205 |
| A6 | Arg | Lac | - | 1:8 | 1.2372 ± 0.0428 | 3.850 ± 0.005 | 0.087 ± 0.005 |
| A7 | Cys | Gla | - | 1:4 | 1.4821 ± 0.0641 | 16.827 ± 0.020 | 0.341 ± 0.027 |
| G1 | ChCl | Gly | - | 1:2 | 1.1821 ± 0.0920 | 132.330 ± 0.081 | 1.344 ± 0.148 |
| G2 | ChCl | Glu | - | 1:1 | 1.3082 ± 0.0565 | 0.543 ± 0.01 | 0.106 ± 0.003 |
| G3 | ChCl | Sor | - | 1:1 | 1.2792 ± 0.0774 | 2.758 ± 0.005 | 0.082 ± 0.002 |
| G4 | ChCl | Xyl | - | 1:1 | 1.2718 ± 0.0220 | 1.382 ± 0.005 | 0.083 ± 0.002 |
| G5 | ChCl | Sor | Gly | 2:1:1 | 1.2330 ± 0.0853 | 54.619 ± 0.110 | 1.217 ± 0.097 |
| G6 | ChCl | Glu | Gly | 2:1:1 | 1.2409 ± 0.0322 | 12.286 ± 0.028 | 0.396 ± 0.028 |
| G7 | ChCl | Xyl | Gly | 2:1:1 | 1.2241 ± 0.0529 | 32.423 ± 0.057 | 0.812 ± 0.049 |
| G8 | ChCl | Glu | water | 1:1:1 | 1.2829 ± 0.0998 | 38.084 ± 0.160 | 2.006 ± 0.100 |
| G9 | ChCl | Sor | water | 1:1:1 | 1.2632 ± 0.0983 | 17.958 ± 0.055 | 0.919 ± 0.092 |
| G10 | ChCl | Xyl | water | 1:1:1 | 1.2582 ± 0.0326 | 7.018 ± 0.148 | 1.650 ± 0.165 |
| G11 | ChCl | Fru | water | 1:1:1 | 1.2982 ± 0.0673 | 48.944 ± 0.310 | 3.445 ± 0.344 |
| G12 | Glu | Lac | - | 1:5 | 1.2773 ± 0.0552 | 49.098 ± 0.004 | 0.066 ± 0.003 |
| L1 | MNT | Dec | - | 1:1 | 0.8924 ± 0.0694 | 330.347 ± 33.035 | 4.230 ± 0.169 |
| L2 | MNT | Dec | - | 2:1 | 0.8872 ± 0.0383 | 302.445 ± 9.073 | 3.579 ± 0.394 |
| L3 | MNT | Dec | - | 1:2 | 0.8972 ± 0.062 | 356.299 ± 24.941 | 4.469 ± 0.447 |
| L4 | MNT | Ola | - | 1:1 | 0.8887 ± 0.0538 | 279.169 ± 11.167 | 3.794 ± 0.190 |
| L5 | MNT | Ola | - | 1:2 | 0.884 ± 0.0229 | 319.254 ± 9.578 | 3.814 ± 0.267 |
| L6 | MNT | Ola | - | 2:1 | 0.8813 ± 0.0457 | 272.940 ± 10.918 | 2.836 ± 0.312 |
| L7 | MNT | MCT | - | 1:1 | 0.8824 ± 0.0686 | 225.197 ± 13.512 | 17.068 ± 1.707 |
| L8 | MNT | MCT | - | 1:2 | 0.8803 ± 0.0380 | 234.432 ± 11.722 | 13.208 ± 0.528 |
| L9 | MNT | Lim | - | 1:1 | 0.8760 ± 0.0606 | 263.229 ± 7.897 | 4.854 ± 0.146 |
| L10 | MNT | Lim | - | 1:2 | 0.8732 ± 0.0302 | 232.844 ± 20.956 | 4.620 ± 0.462 |
| L11 | MNT | Lim | - | 2:1 | 0.8816 ± 0.0533 | 275.228 ± 11.009 | 4.579 ± 0.458 |
| D1 | ChCl | PG | - | 1:2 | 1.1294 ± 0.0586 | 204.520 ± 8.181 | 1.060 ± 0.042 |
| D2 | ChCl | PG | - | 1:3 | 1.0623 ± 0.0367 | 196.468 ± 17.682 | 7.848 ± 0.628 |
| D3 | MNT | PEG 400 | - | 1:1 | 1.0293 ± 0.0178 | 379.685 ± 22.781 | 3.838 ± 0.115 |
| D4 | MNT | IPM | - | 1:1 | 0.8672 ± 0.0225 | 269.091 ± 26.909 | 5.617 ± 0.506 |
| W | Water | - | - | - | - | 0.056 ± 0.003 | 0.042 ± 0.002 |
2.1.1. Organic Acids-Based NADESs
2.1.2. Sugar- and Sugar Alcohol-Based NADESs
2.1.3. Hydrophobic MNT-Based NADESs (HNADESs)
2.1.4. DESs with Synthetic Common Pharmaceutical Excipients
2.2. Drug Solubility Evaluation
2.3. Density Evaluation
2.4. Additional Physicochemical Evaluation
2.4.1. Rheological Study
2.4.2. Surface Properties
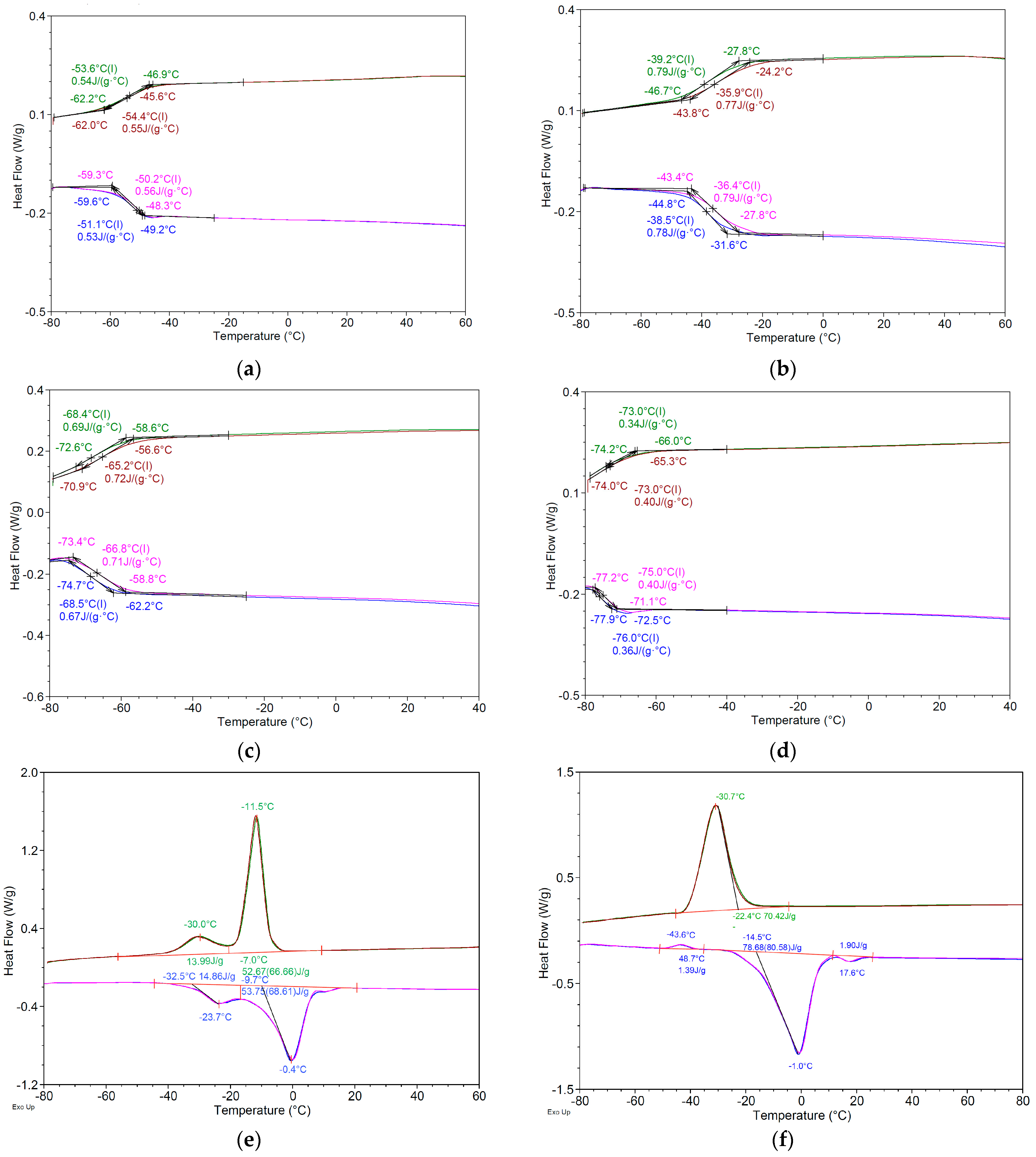
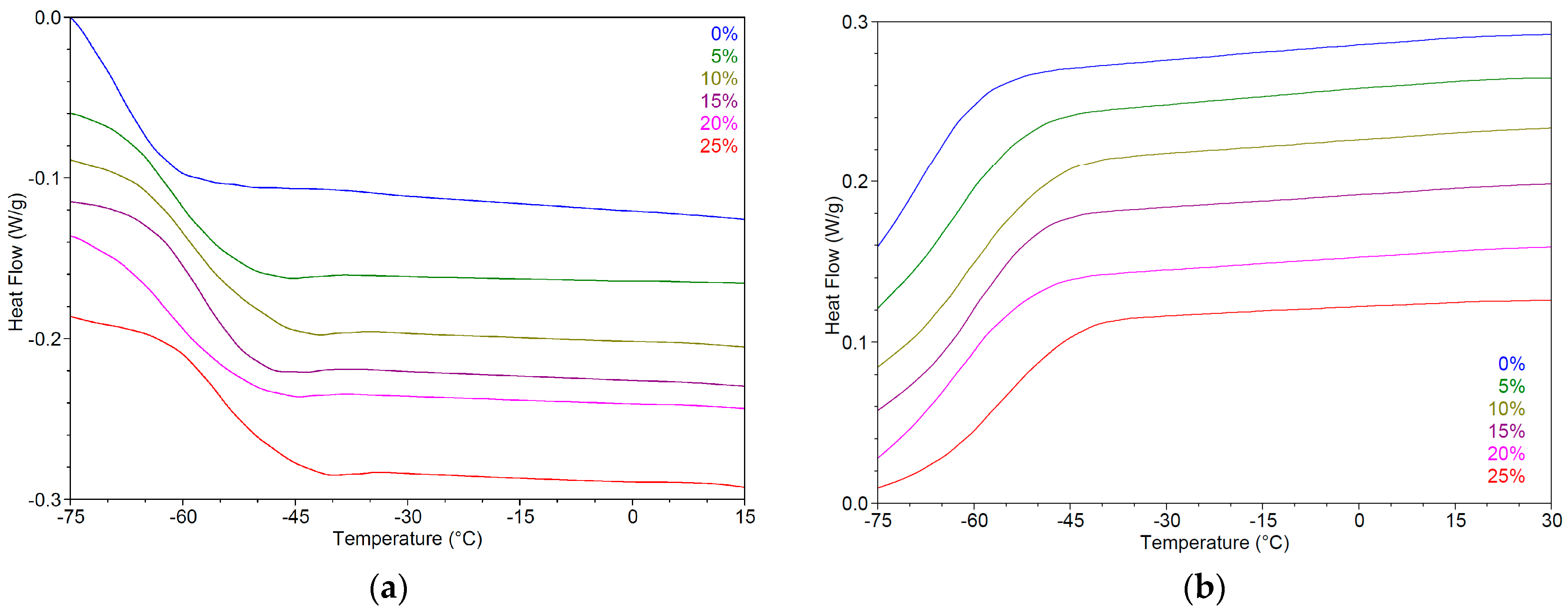
2.4.3. Thermal Analysis
2.5. Impact of Water on Physicochemical Properties of Hydrophilic NADESs
3. Materials and Methods
3.1. Materials
3.2. Preparation of DESs
3.3. Equilibrium Solubility Studies
3.4. Drug Quantification
3.5. Measurement of DESs Density
3.6. Rheological Evaluation
3.7. Surface Properties
3.8. Thermal Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Sharma, D.; Patel, P.; Shah, M. A comprehensive study on Industry 4.0 in the pharmaceutical industry for sustainable development. Environ. Sci. Pollut. Res. 2023, 30, 90088–90098. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.L. Comparative dissolution study of a solid pharmaceutical form containing nanostructured lipid carrier (NLC) incorporating diosgenin—Conventional vs. biorelevant dissolution media. Farmacia 2023, 71, 116–129. [Google Scholar] [CrossRef]
- Mircioiu, C.; Anuta, V.; Mircioiu, I.; Nicolescu, A.; Fotaki, N. In Vitro–In Vivo Correlations Based on In Vitro Dissolution of Parent Drug Diltiazem and Pharmacokinetics of Its Metabolite. Pharmaceutics 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Choudhari, Y.; Patel, P.; Das Gupta, G.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Das Kurmi, B. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, Y.; Zhang, J.; Qian, S.; Gao, Y.; Heng, W. Are all poorly soluble drugs dissolved in deep eutectic solvents true solutions? J. Colloid Interface Sci. 2023, 645, 813–822. [Google Scholar] [CrossRef]
- Dangre, P.V.; Borase, H.P.; Gunde, M.C.; Pethe, A.M.; Borkar, M.R. Deep Eutectic Solvents: Fundamental Aspect, Characterizations and Applications. Recent Adv. Drug Deliv. Formul. 2023, 17, 3–12. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Wei, C.; Wang, H.; Gao, H. Deep Eutectic Solvents as Active Pharmaceutical Ingredient Delivery Systems in the Treatment of Metabolic Related Diseases. Front. Pharmacol. 2021, 12, 794939. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Altamash, T.; Amhamed, A.; Aparicio, S.; Atilhan, M. Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes 2020, 8, 1533. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Atilhan, M.; Aparicio, S. Behavior of Antibiotics in Natural Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 4669–4683. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, R.; Han, H.; Wu, K.; Wang, B.; Liu, Y.; Zhu, Y.; Lu, H.; Liang, B. Preparation strategy and stability of deep eutectic solvents: A case study based on choline chloride-carboxylic acid. J. Clean. Prod. 2022, 345, 131028. [Google Scholar] [CrossRef]
- Pour, S.B.; Sardroodi, J.J.; Ebrahimzadeh, A.R.; Pazuki, G.; Rezvan, V.H. A comparative study of deep eutectic solvents based on fatty acids and the effect of water on their intermolecular interactions. Sci. Rep. 2024, 14, 1763. [Google Scholar] [CrossRef]
- Rozas, S.; Zamora, L.; Benito, C.; Atilhan, M.; Aparicio, S. A study on monoterpenoid-based natural deep eutectic solvents. Green Chem. Eng. 2023, 4, 99–114. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2018, 18, 31–36. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P.; Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; et al. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, M.S.T.; Esfandiari, Z.; Rostamabadi, H.; Nodeh, H.R. Application of amino acid-based natural deep eutectic solvents in extraction of different analytes: A review study. Trends Food Sci. Technol. 2024, 147, 104448. [Google Scholar] [CrossRef]
- Pereira, J.; Castro, M.M.; Santos, F.; Jesus, A.R.; Paiva, A.; Oliveira, F.; Duarte, A.R.C. Selective terpene based therapeutic deep eutectic systems against colorectal cancer. Eur. J. Pharm. Biopharm. 2022, 175, 13–26. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Syed Putra, S.S.; Khan, H.W.; Azmi, N.A.N.; Sani, M.S.A.; Abllah, N.; Hayyan, A.; Jewaratnam, J.; Basirun, W.J. Menthol and Fatty Acid-Based Hydrophobic Deep Eutectic Solvents as Media for Enzyme Activation. Processes 2023, 11, 547. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.-S. Toxicity test profile for deep eutectic solvents: A detailed review and future prospects. Chemosphere 2024, 350, 141097. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Duque, A.; Sanjuan, A.; Bou-Ali, M.M.; Alonso, R.M.; Campanero, M.A. Physicochemical characterization of hydrophobic type III and type V deep eutectic solvents based on carboxylic acids. J. Mol. Liq. 2023, 392, 123431. [Google Scholar] [CrossRef]
- Palmelund, H.; Andersson, M.P.; Asgreen, C.J.; Boyd, B.J.; Rantanen, J.; Löbmann, K. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents (DES). Int. J. Pharm. X 2019, 1, 100034. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Siciliano, C.; Procopio, D.; Curcio, F.; Laganà, A.S.; Di Gioia, M.L.; Cassano, R. Deep Eutectic Solvents for Improving the Solubilization and Delivery of Dapsone. Pharmaceutics 2022, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Marullo, S.; Meli, A.; Giannici, F.; D’aNna, F. Supramolecular Eutecto Gels: Fully Natural Soft Materials. ACS Sustain. Chem. Eng. 2018, 6, 12598–12602. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; della Cuna, F.S.R.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.d.L.; Gomez, F.J.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Jahanbakhsh-Bonab, P.; Pazuki, G.; Sardroodi, J.J.; Dehnavi, S.M. Assessment of the properties of natural-based chiral deep eutectic solvents for chiral drug separation: Insights from molecular dynamics simulation. Phys. Chem. Chem. Phys. 2023, 25, 17547–17557. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, B.; Chen, M.; Ye, J.; Chu, Q. Deep eutectic solvents combined with beta-cyclodextrin derivatives for chiral separation of typical adrenergic receptor agonists by capillary electrophoresis with amperometric detection. J. Pharm. Biomed. Anal. 2023, 236, 115748. [Google Scholar] [CrossRef] [PubMed]
- Bavandpour, R.; Rajabi, M.; Asghari, A. Electrochemical determination of epirubicin in the presence of topotecan as essential anti-cancer compounds using paste electrode amplified with Pt/SWCNT nanocomposite and a deep eutectic solvent. Chemosphere 2022, 289, 133060. [Google Scholar] [CrossRef] [PubMed]
- Svigelj, R.; Zanette, F.; Toniolo, R. Electrochemical Evaluation of Tyrosinase Enzymatic Activity in Deep Eutectic Solvent and Aqueous Deep Eutectic Solvent. Sensors 2023, 23, 3915. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O. Natural Deep Eutectic Solvents in the Synthesis of Inorganic Nanoparticles. Materials 2023, 16, 627. [Google Scholar] [CrossRef]
- Wysokowski, M.; Luu, R.K.; Arevalo, S.; Khare, E.; Stachowiak, W.; Niemczak, M.; Jesionowski, T.; Buehler, M.J. Untapped Potential of Deep Eutectic Solvents for the Synthesis of Bioinspired Inorganic–Organic Materials. Chem. Mater. 2023, 35, 7878–7903. [Google Scholar] [CrossRef]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep Eutectic Solvents for Biotechnology Applications. Biochemistry 2023, 88, S150–S175. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. Adv. Biochem. Eng. Biotechnol. 2019, 168, 31–59. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- van Osch, D.J.; Zubeir, L.F.; Bruinhorst, A.v.D.; Rocha, M.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Valente, S.; Oliveira, F.; Ferreira, I.J.; Paiva, A.; Sobral, R.G.; Diniz, M.S.; Gaudêncio, S.P.; Duarte, A.R.C. Hydrophobic DES Based on Menthol and Natural Organic Acids for Use in Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2023, 11, 9989–10000. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Moral, R.; Thakuria, S.; Mitra, A.; Paul, S. Hydrophobic Deep Eutectic Solvents as Greener Substitutes for Conventional Extraction Media: Examples and Techniques. ACS Omega 2023, 8, 9702–9728. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Bahiraei, M.; Jafari, F. A green hydrophobic deep eutectic solvent for extraction of phenol from aqueous phase. Sci. Rep. 2023, 13, 17449. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, X.; Chen, B.; Mao, Y.; Shao, P. Lycopene in hydrophobic deep eutectic solvent with natural catalysts: A promising strategy to simultaneously promote lycopene Z-isomerization and extraction. Food Chem. 2023, 426, 136627. [Google Scholar] [CrossRef] [PubMed]
- Panbachi, S.; Beranek, J.; Kuentz, M. Hydrophobic deep eutectic solvent (HDES) as oil phase in lipid-based drug formulations. Int. J. Pharm. 2024, 661, 124418. [Google Scholar] [CrossRef]
- Daadoue, S.; Al-Remawi, M.; Al-Mawla, L.; Idkaidek, N.; Khalid, R.M.; Al-Akayleh, F. Deep eutectic liquid as transdermal delivery vehicle of Risperidone. J. Mol. Liq. 2022, 345, 117347. [Google Scholar] [CrossRef]
- Kalantri, S.; Vora, A. Eutectic solutions for healing: A comprehensive review on therapeutic deep eutectic solvents (TheDES). Drug Dev. Ind. Pharm. 2024, 50, 387–400. [Google Scholar] [CrossRef]
- Abdelquader, M.M.; Li, S.; Andrews, G.P.; Jones, D.S. Therapeutic deep eutectic solvents: A comprehensive review of their thermodynamics, microstructure and drug delivery applications. Eur. J. Pharm. Biopharm. 2023, 186, 85–104. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Sultan, M.H.; Almoshari, Y.; Sivadasan, D.; Alqahtani, S.S.; Madkhali, O.A.; Ahsan, W. Pharmaceutical applications of therapeutic deep eutectic systems (THEDES) in maximising drug delivery. Heliyon 2024, 10, e29783. [Google Scholar] [CrossRef]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chormale, J.H.; Bansal, A.K. Deep eutectic systems: An overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021, 610, 121203. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, K.; Huang, C.; Hu, Y.; Ji, H.; Liu, S.; Gao, J. Improvement of solubility, stability and antioxidant activity of carotenoids using deep eutectic solvent-based microemulsions. Colloids Surf. B Biointerfaces 2022, 217, 112591. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. MedChemComm 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Nerurkar, J.; Beach, J.; Park, M.; Jun, H. Solubility of (±)-ibuprofen and S (+)-ibuprofen in the presence of cosolvents and cyclodextrins. Pharm. Dev. Technol. 2005, 10, 413–421. [Google Scholar] [PubMed]
- Li, Z.; Lee, P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharm. 2016, 505, 283–288. [Google Scholar] [CrossRef]
- Asghar, S.Z.; Kaviani, R.; Shayanfar, A. Solubility of Some Drugs in Aqueous Solutions of Choline Chloride-Based Deep Eutectic Solvent Systems: Experimental Data, Modeling, and the Impact of Solution pH. Iran. J. Pharm. Res. 2023, 22, e137011. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mokhtarpour, M.; Faraji, S. Deep eutectic solvents for antiepileptic drug phenytoin solubilization: Thermodynamic study. Sci. Rep. 2021, 11, 24081. [Google Scholar] [CrossRef]
- Sarraguça, M.C.; Ribeiro, P.R.S.; Nunes, C.; Seabra, C.L. Solids Turn into Liquids—Liquid Eutectic Systems of Pharmaceutics to Improve Drug Solubility. Pharmaceuticals 2022, 15, 279. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hatakeyama, T.; Hatakeyama, H. Phenomenological theory describing the behaviour of non-freezing water in structure formation process of polysaccharide aqueous solutions. Carbohydr. Polym. 2000, 41, 91–95. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Soares, B.; Silvestre, A.J.D.; Pinto, P.C.R.; Freire, C.S.R.; Coutinho, J.A.P. Hydrotropy and Cosolvency in Lignin Solubilization with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 12485–12493. [Google Scholar] [CrossRef]
- Zarei, A.; Haghbakhsh, R.; Raeissi, S. Overview and thermodynamic modelling of deep eutectic solvents as co-solvents to enhance drug solubilities in water. Eur. J. Pharm. Biopharm. 2023, 193, 1–15. [Google Scholar] [CrossRef]
- Olivares, B.; Martínez, F.; Rivas, L.; Calderón, C.; Munita, J.M.; Campodonico, P.R. A Natural Deep Eutectic Solvent Formulated to Stabilize β-Lactam Antibiotics. Sci. Rep. 2018, 8, 14900. [Google Scholar] [CrossRef]
- Zhang, H.; Vicent-Luna, J.M.; Tao, S.; Calero, S.; Riobóo, R.J.J.; Ferrer, M.L.; del Monte, F.; Gutiérrez, M.C. Transitioning from Ionic Liquids to Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2022, 10, 1232–1245. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Rengstl, D. Choline as a Cation for the Design of Low-Toxic and Biocompatible Ionic Liquids, Surfactants, and Deep Eutectic Solvents. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2014. [Google Scholar]
- Crucean, D.; Pontoire, B.; Debucquet, G.; Le-Bail, A.; Le-Bail, P. The use of choline chloride for salt reduction and texture enhancement in bread. Appl. Food Res. 2023, 3, 100371. [Google Scholar] [CrossRef]
- Gallo, M.; Gámiz, F. Choline: An Essential Nutrient for Human Health. Nutrients 2023, 15, 2900. [Google Scholar] [CrossRef]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Silva, E.; Matias, A.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C. Menthol-based deep eutectic systems as antimicrobial and anti-inflammatory agents for wound healing. Eur. J. Pharm. Sci. 2023, 182, 106368. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Pirbaluti, M.; Motaghi, E.; Bozorgi, H. The effect of menthol on acute experimental colitis in rats. Eur. J. Pharmacol. 2017, 805, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.; Bastaki, M.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Mint, buchu, dill and caraway derived flavoring ingredients. Food Chem. Toxicol. 2020, 135, 110870. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Taylor, R., Jr.; LeQuang, J.-A.; Raffa, R.B.; The NEMA Research Group. The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef]
- Shahoei, R.; Tajkhorshid, E. Menthol Binding to the Human α4β2 Nicotinic Acetylcholine Receptor Facilitated by Its Strong Partitioning in the Membrane. J. Phys. Chem. B 2020, 124, 1866–1880. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; de Souza, E.O.; Hussni, C.A.; Martinez, E.R.M.; Nóbrega, R.H.; Pellizzon, C.H. The Use of Menthol in Skin Wound Healing—Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization. Pharmaceutics 2021, 13, 1902. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F. The permeability enhancing mechanism of menthol on skin lipids: A molecular dynamics simulation study. J. Mol. Model. 2017, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Wang, Y.; Li, Y.; Li, Q.; Zhang, L. The distinctive role of menthol in pain and analgesia: Mechanisms, practices, and advances. Front. Mol. Neurosci. 2022, 15, 1006908. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, I.; Anuta, V.; Purcaru, S.O.; Radulescu, F.; Miron, D.; Dumitrescu, I.B.; Ibrahim, N.; Mircioiu, C. In vitro dissolution of poorly soluble drugs in the presence of surface active agents—In vivo pharmacokinetics correlations. II. Nimesulide. Farmacia 2013, 61, 88–102. [Google Scholar]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2014, 31, 1082–1095. [Google Scholar] [CrossRef]
- Afrose, A.; White, E.T.; Rashid, M.A.; Howes, T.; Alhamhoom, Y. Tailoring of solubility of ibuprofen in the presence of hydrophilic excipients in water-ethanol mixtures by crystallization method. Int. J. Appl. Pharm. 2022, 14, 102–109. [Google Scholar] [CrossRef]
- Bolten, D.; Lietzow, R.; Türk, M. Solubility of Ibuprofen, Phytosterol, Salicylic Acid, and Naproxen in Aqueous Solutions. Chem. Eng. Technol. 2013, 36, 426–434. [Google Scholar] [CrossRef]
- Dittanet, P.; Phothipanyakun, S.; Charoenchaitrakool, M. Co-precipitation of mefenamic acid−polyvinylpyrrolidone K30 composites using Gas Anti-Solvent. J. Taiwan Inst. Chem. Eng. 2016, 63, 17–24. [Google Scholar] [CrossRef]
- Calatayud, S.; Esplugues, J.V. Chemistry, Pharmacodynamics, and Pharmacokinetics of NSAIDs. In NSAIDs and Aspirin: Recent Advances and Implications for Clinical Management; Lanas, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–16. [Google Scholar]
- Hussain, A.; Smith, G.; Khan, K.A.; Bukhari, N.I.; Pedge, N.I.; Ermolina, I. Solubility and dissolution rate enhancement of ibuprofen by co-milling with polymeric excipients. Eur. J. Pharm. Sci. 2018, 123, 395–403. [Google Scholar] [CrossRef]
- Naik, J.; Rajput, R.; Singh, M.K. Development and Evaluation of Ibuprofen Loaded Hydrophilic Biocompatible Polymeric Nanoparticles for the Taste Masking and Solubility Enhancement. BioNanoScience 2021, 11, 21–31. [Google Scholar] [CrossRef]
- Nascimento, A.L.C.S.; Fernandes, R.P.; Charpentier, M.D.; ter Horst, J.H.; Caires, F.J.; Chorilli, M. Co-crystals of non-steroidal anti-inflammatory drugs (NSAIDs): Insight toward formation, methods, and drug enhancement. Particuology 2021, 58, 227–241. [Google Scholar] [CrossRef]
- Sarafska, T.; Ivanova, S.; Dudev, T.; Tzachev, C.; Petrov, V.; Spassov, T. Enhanced Solubility of Ibuprofen by Complexation with β-Cyclodextrin and Citric Acid. Molecules 2024, 29, 1650. [Google Scholar] [CrossRef] [PubMed]
- Talianu, M.-T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuţa, V.; Jinga, V.; Popa, L. Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology. Nanomaterials 2020, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Q.; Ayoub, R.; Moshawih, S.; Jarrar, Y.; Jilani, J. Synthesis and Biological Evaluation of Hydroxypropyl Ester of Mefenamic Acid as a Promising Prodrug. Lett. Drug Des. Discov. 2023, 20, 144–152. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and sustainable solvents of the future: Deep eutectic solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- FDA. GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 15 August 2024).
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P.J.C.C.A. Molecular surfaces, van der Waals radii and electrostatic potentials in relation to noncovalent interactions. Croat. Chem. Acta 2009, 82, 267–275. [Google Scholar]
- Lemaoui, T.; Darwish, A.S.; Attoui, A.; Abu Hatab, F.; Hammoudi, N.E.H.; Benguerba, Y.; Vega, L.F.; Alnashef, I.M. Predicting the density and viscosity of hydrophobic eutectic solvents: Towards the development of sustainable solvents. Green Chem. 2020, 22, 8511–8530. [Google Scholar] [CrossRef]
- Fan, C.; Sebbah, T.; Liu, Y.; Cao, X. Terpenoid-capric acid based natural deep eutectic solvent: Insight into the nature of low viscosity. Clean. Eng. Technol. 2021, 3, 100116. [Google Scholar] [CrossRef]
- Alasalvar, H.; Yildirim, Z.; Yildirim, M. Development and characterization of sustainable active pectin films: The role of choline chloride/glycerol-based natural deep eutectic solvent and lavender extracts. Heliyon 2023, 9, e21756. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Lapeña, D.; Lomba, L.; Artal, M.; Lafuente, C.; Giner, B. The NADES glyceline as a potential Green Solvent: A comprehensive study of its thermophysical properties and effect of water inclusion. J. Chem. Thermodyn. 2019, 128, 164–172. [Google Scholar] [CrossRef]
- Uzochukwu, M.I.; Oyegoke, T.; Momoh, R.O.; Isa, M.T.; Shuwa, S.M.; Jibril, B.Y. Computational insights into deep eutectic solvent design: Modeling interactions and thermodynamic feasibility using choline chloride & glycerol. Chem. Eng. J. Adv. 2023, 16, 100564. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Sailau, Z.; Almas, N.; Aldongarov, A.; Toshtay, K. Studying the Formation of Choline Chloride- and Glucose-Based Natural Deep Eutectic Solvent at the Molecular Level. J. Mol. Model. 2022, 28, 235. [Google Scholar] [CrossRef]
- Moradi, M.; Jafari, P.; Rahimpour, E.; Shayanfar, A.; Acree, W.E.; Jouyban, A. Interactions between components of choline chloride/propylene glycol or ethylene glycol deep eutectic solvents in the presence of water by determination of amlodipine besylate solubility profile. J. Drug Deliv. Sci. Technol. 2023, 90, 105143. [Google Scholar] [CrossRef]
- Rogošić, M.; Kučan, K.Z. Deep eutectic solvent based on choline chloride and propylene glycol as a potential medium for extraction denitrification of hydrocarbon fuels. Chem. Eng. Res. Des. 2020, 161, 45–57. [Google Scholar] [CrossRef]
- Bello, O.S.; Alagbada, T.C.; Alao, O.C.; Olatunde, A.M. Sequestering a non-steroidal anti-inflammatory drug using modified orange peels. Appl. Water Sci. 2020, 10, 172. [Google Scholar] [CrossRef]
- Haddadin, R.; Qian, F.; Desikan, S.; Hussain, M.; Smith, R.L. Estimation of Drug Solubility in Polymers via Differential Scanning Calorimetry and Utilization of the Fox Equation. Pharm. Dev. Technol. 2008, 14, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Schrimpl, L.; Schmidt, P.C. Some Physicochemical Properties of Mefenamic Acid. Drug Dev. Ind. Pharm. 2000, 26, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M.E. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP). Scientific Opinion on safety and efficacy of choline chloride as a feed additive for all animal species. EFSA J. 2011, 9, 2353. [Google Scholar] [CrossRef]
- Al-Risheq, D.I.M.; Nasser, M.S.; Qiblawey, H.; Hussein, I.A.; Benamor, A. Choline chloride based natural deep eutectic solvent for destabilization and separation of stable colloidal dispersions. Sep. Purif. Technol. 2021, 255, 117737. [Google Scholar] [CrossRef]
- Brunton, L.L.; Hilal-Dandan, R.; Knollmann, B.C. Lange’s Handbook of Chemistry, 17th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Cordeiro, T.; Castiñeira, C.; Mendes, D.; Danède, F.; Sotomayor, J.; Fonseca, I.M.; da Silva, M.G.; Paiva, A.; Barreiros, S.; Cardoso, M.M.; et al. Stabilizing Unstable Amorphous Menthol through Inclusion in Mesoporous Silica Hosts. Mol. Pharm. 2017, 14, 3164–3177. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Hernández, J.G.; Sánchez-Ramírez, E.; Ramírez-Márquez, C.; Contreras-Zarazúa, G. Chapter 11—Lactic acid. In Improvements in Bio-Based Building Blocks Production through Process Intensification and Sustainability Concepts; Segovia-Hernández, J.G., Sánchez-Ramírez, E., Ramírez-Márquez, C., Contreras-Zarazúa, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–226. [Google Scholar]
- Chen, Z.; Huang, C.; Yao, X.; Benmore, C.J.; Yu, L. Structures of glass-forming liquids by x-ray scattering: Glycerol, xylitol, and D-sorbitol. J. Chem. Phys. 2021, 155, 244508. [Google Scholar] [CrossRef] [PubMed]
- Hurtta, M.; Pitkänen, I.; Knuutinen, J. Melting behaviour of d-sucrose, d-glucose and d-fructose. Carbohydr. Res. 2004, 339, 2267–2273. [Google Scholar] [CrossRef]
- Schugmann, M.; Foerst, P. Systematic Investigation on the Glass Transition Temperature of Binary and Ternary Sugar Mixtures and the Applicability of Gordon–Taylor and Couchman–Karasz Equation. Foods 2022, 11, 1679. [Google Scholar] [CrossRef]
- Truong, V.; Bhandari, B.R.; Howes, T.; Adhikari, B. Glass transition behaviour of fructose. Int. J. Food Sci. Technol. 2004, 39, 569–578. [Google Scholar] [CrossRef]
- Katona, G.; Szalontai, B.; Budai-Szűcs, M.; Csányi, E.; Szabó-Révész, P.; Jójárt-Laczkovich, O. Formulation of paracetamol-containing pastilles with in situ coating technology. Eur. J. Pharm. Sci. 2016, 95, 54–61. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A Review on Bioproduction, Application, Health Benefits, and Related Safety Issues. Crit. Rev. Food Sci. Nutr. 2013, 55, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, M.; Mailhé, C.; Doppiu, S.; Dauvergne, J.-L.; Santos-Moreno, S.; Godin, A.; Fleury, G.; Rouault, F.; del Barrio, E.P. Characterization of Fatty Acids as Biobased Organic Materials for Latent Heat Storage. Materials 2021, 14, 4707. [Google Scholar] [CrossRef] [PubMed]
- MIGLYO®812 N Safety Data Sheet. Available online: https://tr.organic-materials.com/cms/wp-content/uploads/2020/09/MIGLYOL_812_NF_MSDS_GB.pdf (accessed on 29 July 2024).
- Paberit, R.; Rilby, E.; Göhl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling Stability of Poly(ethylene glycol) of Six Molecular Weights: Influence of Thermal Conditions for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Kojima, S. Low frequency Raman scattering study of liquid-glass transition in propylene glycol. J. Mol. Struct. 1993, 294, 197–200. [Google Scholar] [CrossRef]
- White, T.W.; Duncan, D.A.; Fortuna, S.; Wang, Y.-L.; Moreton, B.; Lee, T.-L.; Blowey, P.; Costantini, G.; Woodruff, D.P. A structural investigation of the interaction of oxalic acid with Cu(110). Surf. Sci. 2018, 668, 134–143. [Google Scholar] [CrossRef]
- Grąz, M. Role of oxalic acid in fungal and bacterial metabolism and its biotechnological potential. World J. Microbiol. Biotechnol. 2024, 40, 178. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 106, pp. 49–77. [Google Scholar]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Karwal, K.; Mukovozov, I. Topical AHA in Dermatology: Formulations, Mechanisms of Action, Efficacy, and Future Perspectives. Cosmetics 2023, 10, 131. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef]
- Mizukoshi, K. Effects of lactic acid on the flexibility of the stratum corneum. Ski. Res. Technol. 2020, 26, 599–607. [Google Scholar] [CrossRef]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vanella, V.V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Sasaki, D.; Aizawa, R.; Yamamoto, M.; Yaegashi, T.; Irié, T.; Sasaki, M. The Role of Lactic Acid on Wound Healing, Cell Growth, Cell Cycle Kinetics, and Gene Expression of Cultured Junctional Epithelium Cells in the Pathophysiology of Periodontal Disease. Pathogens 2021, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Aparicio, S.; Atilhan, M. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients. Phys. Chem. Chem. Phys. 2019, 21, 10621–10634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, H.; Qin, X.; Liu, Y.; Tian, S.; Yang, Y.; Wang, S. Cheap and biodegradable amino acid-based deep eutectic solvents for radioactive iodine capture via halogen bonds. J. Mol. Liq. 2020, 303, 112615. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Cao, B.; Jiang, J.; Zhao, W.; Wang, J.; Mu, T. Highly efficient I2 capture by simple and low-cost deep eutectic solvents. Green Chem. 2016, 18, 2522–2527. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-based natural deep eutectic solvent (NADES): Physicochemical properties, antimicrobial activity, toxicity, biodegradability and potential use as green extraction media for phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Lomba, L.; Werner, Á.; Giner, B.; Lafuente, C. Deep Eutectic Solvents Formed by Glycerol and Xylitol, Fructose and Sorbitol: Effect of the Different Sugars in Their Physicochemical Properties. Molecules 2023, 28, 6023. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2020, 22, 4. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L.; Duarte, A.R.C. A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain. Chem. Pharm. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Czyrski, G.S.; Kirkensgaard, J.J.; Rønholt, S.; Rades, T.; Heinz, A. Terpene-based eutectic mixtures for cutaneous delivery: Eutectic point vs. molar ratio—Which matters more? J. Mol. Liq. 2024, 411, 125726. [Google Scholar] [CrossRef]
- Araki, Y.; Hamada, Y.; Imamura, N.; Yamasaka, K.; Sakuragi, M. Evaluation of terpene-based hydrophobic deep eutectic solvents as skin permeation enhancers. Jpn. J. Appl. Phys. 2023, 62, 015003. [Google Scholar] [CrossRef]
- Zhang, M.; Ouyang, J.; Fu, L.; Xu, C.; Ge, Y.; Sun, S.; Li, X.; Lai, S.; Ke, H.; Yuan, B.; et al. Hydrophobicity Determines the Bacterial Killing Rate of α-Helical Antimicrobial Peptides and Influences the Bacterial Resistance Development. J. Med. Chem. 2022, 65, 14701–14720. [Google Scholar] [CrossRef] [PubMed]
- Křížek, T.; Bursová, M.; Horsley, R.; Kuchař, M.; Tůma, P.; Čabala, R.; Hložek, T. Menthol-based hydrophobic deep eutectic solvents: Towards greener and efficient extraction of phytocannabinoids. J. Clean. Prod. 2018, 193, 391–396. [Google Scholar] [CrossRef]
- Song, Y. Solubility and Mass Transfer Performance of Ethane and n-Butane in Menthol and Decanoic Acid Deep Eutectic Solvent. ACS Omega 2024, 9, 30935–30944. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Atef, B.; Ishak, R.A.; Badawy, S.S.; Osman, R. Exploring the potential of oleic acid in nanotechnology-mediated dermal drug delivery: An up-to-date review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- Supaweera, N.; Chulrik, W.; Jansakun, C.; Bhoopong, P.; Yusakul, G.; Chunglok, W. Therapeutic deep eutectic solvent-based microemulsion enhances anti-inflammatory efficacy of curcuminoids and aromatic-turmerone extracted from Curcuma longa L. RSC Adv. 2022, 12, 25912–25922. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- de Araújo-Filho, H.G.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytotherapy Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Pour, S.B.; Sardroodi, J.J.; Ebrahimzadeh, A.R.; Avestan, M.S. Structural and dynamic properties of eutectic mixtures based on menthol and fatty acids derived from coconut oil: A MD simulation study. Sci. Rep. 2022, 12, 5153. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, F.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C. Untangling the bioactive properties of therapeutic deep eutectic solvents based on natural terpenes. Curr. Res. Chem. Biol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, F.; Silva, J.M.; Matias, A.; Reis, R.L.; Duarte, A.R.C. Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen. Pharmaceutics 2020, 12, 1121. [Google Scholar] [CrossRef]
- Lázaro-Rangel, J.M.; Sánchez, A.; Inoue, M.; Olivares-Romero, J.L.; Meza-Gordillo, R.; Salas-Reyes, M.; Virués, C.; Domínguez, Z. Natural Eutectic Solvent Mixture of l-Menthol/Malonic acid (4:1) and Its Ibuprofen Solution: Thermal and Physical Characterizations and NMR Studies of Molecular Interaction. Eur. J. Org. Chem. 2023, 26, e202201326. [Google Scholar] [CrossRef]
- Phaechamud, T.; Tuntarawongsa, S.; Charoensuksai, P. Evaporation Behavior and Characterization of Eutectic Solvent and Ibuprofen Eutectic Solution. AAPS PharmSciTech 2016, 17, 1213–1220. [Google Scholar] [CrossRef]
- Lomba, L.; Garralaga, M.P.; Werner, Á.; Giner, B.; Baptista, P.M.; Sánchez-Romero, N. Ibuprofen solubility and cytotoxic study of deep eutectic solvents formed by xylitol, choline chloride and water. J. Drug Deliv. Sci. Technol. 2023, 82, 104327. [Google Scholar] [CrossRef]
- Joules, A.; Burrows, T.; Dosa, P.I.; Hubel, A. Characterization of eutectic mixtures of sugars and sugar-alcohols for cryopreservation. J. Mol. Liq. 2023, 371, 120937. [Google Scholar] [CrossRef]
- Pedro, S.N.; Mendes, M.S.M.; Neves, B.M.; Almeida, I.F.; Costa, P.; Correia-Sá, I.; Vilela, C.; Freire, M.G.; Silvestre, A.J.D.; Freire, C.S.R. Deep Eutectic Solvent Formulations and Alginate-Based Hydrogels as a New Partnership for the Transdermal Administration of Anti-Inflammatory Drugs. Pharmaceutics 2022, 14, 827. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Mai, A.; Kułak, J. Experimental and Machine-Learning-Assisted Design of Pharmaceutically Acceptable Deep Eutectic Solvents for the Solubility Improvement of Non-Selective COX Inhibitors Ibuprofen and Ketoprofen. Molecules 2024, 29, 2296. [Google Scholar] [CrossRef]
- FDA. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry: Testing of Glycerin, Propylene Glycol, Maltitol Solution, Hydrogenated Starch Hydrolysate, Sorbitol Solution, and other High-Risk Drug Components for Diethylene Glycol and Ethylene Glycol; FDA: Silver Spring, MD, USA, 2023.
- Krawczyk, M.S.; Sroka, A.; Majerz, I. The Crystal Structure and Intermolecular Interactions in Fenamic Acids–Acridine Complexes. Molecules 2021, 26, 2956. [Google Scholar] [CrossRef] [PubMed]
- Sid, D.; Baitiche, M.; Elbahri, Z.; Djerboua, F.; Boutahala, M.; Bouaziz, Z.; Le Borgne, M. Solubility enhancement of mefenamic acid by inclusion complex with β-cyclodextrin: In silico modelling, formulation, characterisation, and in vitro studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Vaksler, Y.; Benedis, D.; Dyshin, A.; Oparin, R.; Correia, N.; Capet, F.; Shishkina, S.; Kiselev, M.; Idrissi, A. Spectroscopic characterization of single co-crystal of mefenamic acid and nicotinamide using supercritical CO2. J. Mol. Liq. 2021, 334, 116117. [Google Scholar] [CrossRef]
- Prasad, E.; Robertson, J.; Halbert, G.W. Mefenamic acid solid dispersions: Impact of formulation composition on processing parameters, product properties and performance. Int. J. Pharm. 2022, 616, 121505. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Yeow, A.T.; Hayyan, A.; Hayyan, M.; Junaidi, M.U.M.; Saleh, J.; Basirun, W.J.; Nor, M.R.M.; Al Abdulmonem, W.; Salleh, M.Z.M.; Zuki, F.M.; et al. A comprehensive review on the physicochemical properties of deep eutectic solvents. Results Chem. 2024, 7, 101378. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Luhaibi, D.K.; Ali, H.H.M.; Al-Ani, I.; Shalan, N.; Al-Akayleh, F.; Al-Remawi, M.; Nasereddin, J.; Qinna, N.A.; Al-Adham, I.; Khanfar, M. The Formulation and Evaluation of Deep Eutectic Vehicles for the Topical Delivery of Azelaic Acid for Acne Treatment. Molecules 2023, 28, 6927. [Google Scholar] [CrossRef] [PubMed]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Annaland, M.v.S.; Tuinier, R.; van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; et al. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Xia, H.; Ren, M.; Zou, Y.; Qin, S.; Zeng, C. Novel Biocompatible Polysaccharide-Based Eutectogels with Tunable Rheological, Thermal, and Mechanical Properties: The Role of Water. Molecules 2020, 25, 3314. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, Z.; Yan, L. Deep eutectic solvents eutectogels: Progress and challenges. Green Chem. Eng. 2021, 2, 359–367. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. Chemphyschem 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, Z.; de María, P.D. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Rukmani, S.J.; Doherty, B.W.; Acevedo, O.; Colina, C.M. Molecular simulations of deep eutectic solvents: A perspective on structure, dynamics, and physical properties. In Reviews in Computational Chemistry; Wiley: Hoboken, NJ, USA, 2022; Volume 32, pp. 135–216. [Google Scholar]
- Kadhom, M.A.; Abdullah, G.H.; Al-Bayati, N. Studying Two Series of Ternary Deep Eutectic Solvents (Choline Chloride–Urea–Glycerol) and (Choline Chloride–Malic Acid–Glycerol), Synthesis and Characterizations. Arab. J. Sci. Eng. 2017, 42, 1579–1589. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine—Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Paveglio, G.; Milani, F.; Sauer, A.; Roman, D.; Meyer, A.; Pizzuti, L. Structure-Physical Properties Relationship of Eutectic Solvents Prepared from Benzyltriethylammonium Chloride and Carboxylic Acids. J. Braz. Chem. Soc. 2021, 32, 542–551. [Google Scholar] [CrossRef]
- Jangir, A.K.; Sethy, P.; Verma, G.; Bahadur, P.; Kuperkar, K. An inclusive thermophysical and rheology portrayal of deep eutectic solvents (DES) for metal oxides dissolution enhancement. J. Mol. Liq. 2021, 332, 115909. [Google Scholar] [CrossRef]
- Elhamarnah, Y.; AlRasheedi, M.; AlMarri, W.; AlBadr, A.; AlMalki, A.; Mohamed, N.; Fatima, I.; Nasser, M.; Qiblawey, H. An Experimental Investigation on the Thermo-Rheological Behaviors of Lactic Acid-Based Natural Deep Eutectic Solvents. Materials 2022, 15, 4027. [Google Scholar] [CrossRef]
- D’sOuza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Hussain, Y.; Cui, J.; Dormocara, A.; Khan, H. The most recent advances in liquisolid technology: Perspectives in the pharmaceutical industry. Pharm. Sci. Adv. 2024, 2, 100038. [Google Scholar] [CrossRef]
- Albertini, B.; Bertoni, S.; Sangiorgi, S.; Nucci, G.; Passerini, N.; Mezzina, E. NaDES as a green technological approach for the solubility improvement of BCS class II APIs: An insight into the molecular interactions. Int. J. Pharm. 2023, 634, 122696. [Google Scholar] [CrossRef]
- Jha, D.K.; Shah, D.S.; Amin, P.D. Thermodynamic aspects of the preparation of amorphous solid dispersions of Naringenin with enhanced dissolution rate. Int. J. Pharm. 2020, 583, 119363. [Google Scholar] [CrossRef]
- Hornberger, K.; Li, R.; Duarte, A.R.C.; Hubel, A. Natural deep eutectic systems for nature-inspired cryopreservation of cells. AIChE J. 2021, 67, e17085. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, Y.; Sun, X.; Pi, F. Numerical modeling of polymorphic transformation of oleic acid via near-infrared spectroscopy and factor analysis. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 197, 153–158. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, H.; Lou, X.; Wu, X.; Wang, Y.; Zhao, K.; Du, X.; Xia, X. Analysis of NADES and its water tailoring effects constructed from inulin and L-proline based on structure, physicochemical and antifreeze properties. Int. J. Biol. Macromol. 2024, 277, 134049. [Google Scholar] [CrossRef]
- Lorenzetti, A.S.; Fiego, M.J.L.; Silva, M.F.; Domini, C.; Gomez, F.J. Water behavior study for tailoring fructose-citric acid based natural deep eutectic solvent properties towards antibiotics solubilization. J. Mol. Liq. 2022, 363, 119917. [Google Scholar] [CrossRef]
- Sothornvit, R.; Reid, D.S.; Krochta, J.M. Plasticizer effect on the glass transition temperature of beta–lactoglobulin films. Trans. ASAE 2002, 45, 1479. [Google Scholar] [CrossRef]
- Tian, H.; Liu, D.; Yao, Y.; Ma, S.; Zhang, X.; Xiang, A. Effect of Sorbitol Plasticizer on the Structure and Properties of Melt Processed Polyvinyl Alcohol Films. J. Food Sci. 2017, 82, 2926–2932. [Google Scholar] [CrossRef]
- ICH. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R2) Step 5 Version 1. 2024. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 12 June 2024).
- Talianu, M.-T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuţa, V.; Prisada, R.M.; Popa, L. Development and Characterization of New Miconazole-Based Microemulsions for Buccal Delivery by Implementing a Full Factorial Design Modeling. Pharmaceutics 2024, 16, 271. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Kaya, M.G.A.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C.; et al. Design and evaluation of new wound dressings based on collagen-cellulose derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Anicescu, M.-C.; Dinu-Pîrvu, C.-E.; Talianu, M.-T.; Ghica, M.V.; Anuța, V.; Prisada, R.-M.; Nicoară, A.C.; Popa, L. Insights from a Box–Behnken Optimization Study of Microemulsions with Salicylic Acid for Acne Therapy. Pharmaceutics 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Musat, G.C.; Mihai, P.R.; Ghica, M.V.; Dinu-Pirvu, C.E.; Anuta, V.; Velescu, B.S.; Popa, L. Contributions on formulation and preliminary evaluation of ocular colloidal systems of chitosan and Poloxamer 407 with bupivacaine hydrochloride. Farmacia 2019, 67, 702–708. [Google Scholar] [CrossRef]
- Zamora, L.; Benito, C.; Gutiérrez, A.; Alcalde, R.; Alomari, N.; Al Bodour, A.; Atilhan, M.; Aparicio, S. Nanostructuring and macroscopic behavior of type V deep eutectic solvents based on monoterpenoids. Phys. Chem. Chem. Phys. 2021, 24, 512–531. [Google Scholar] [CrossRef]

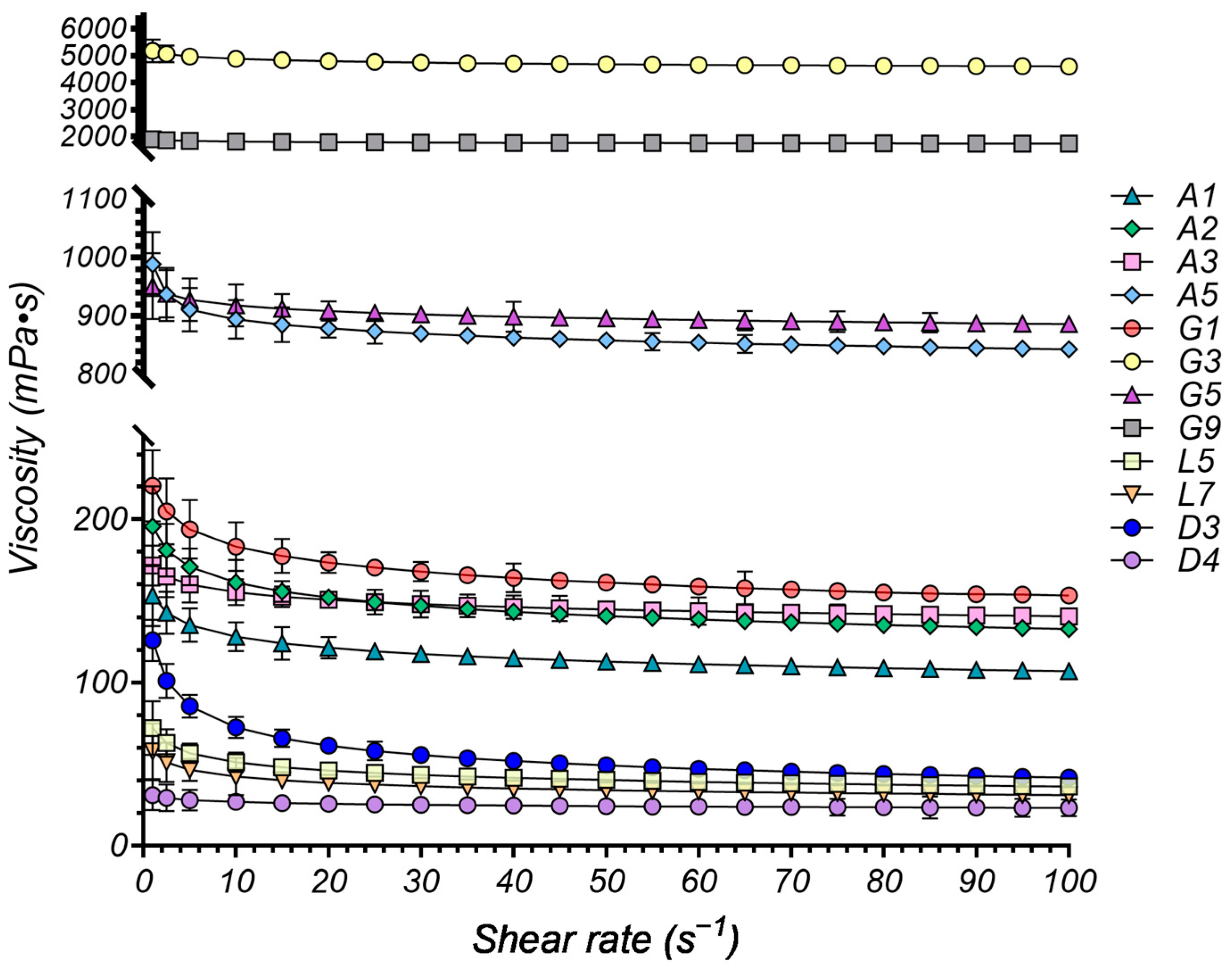
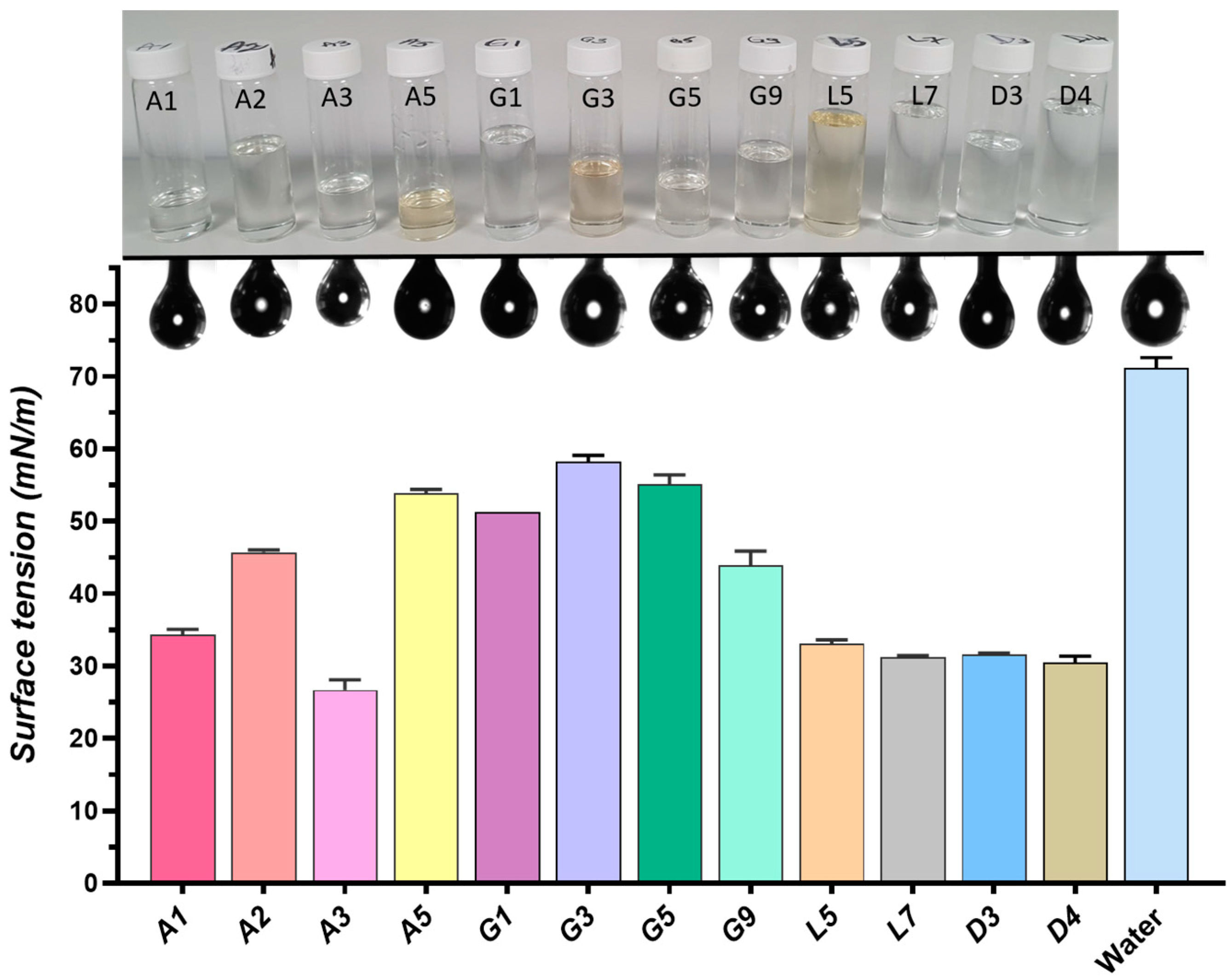

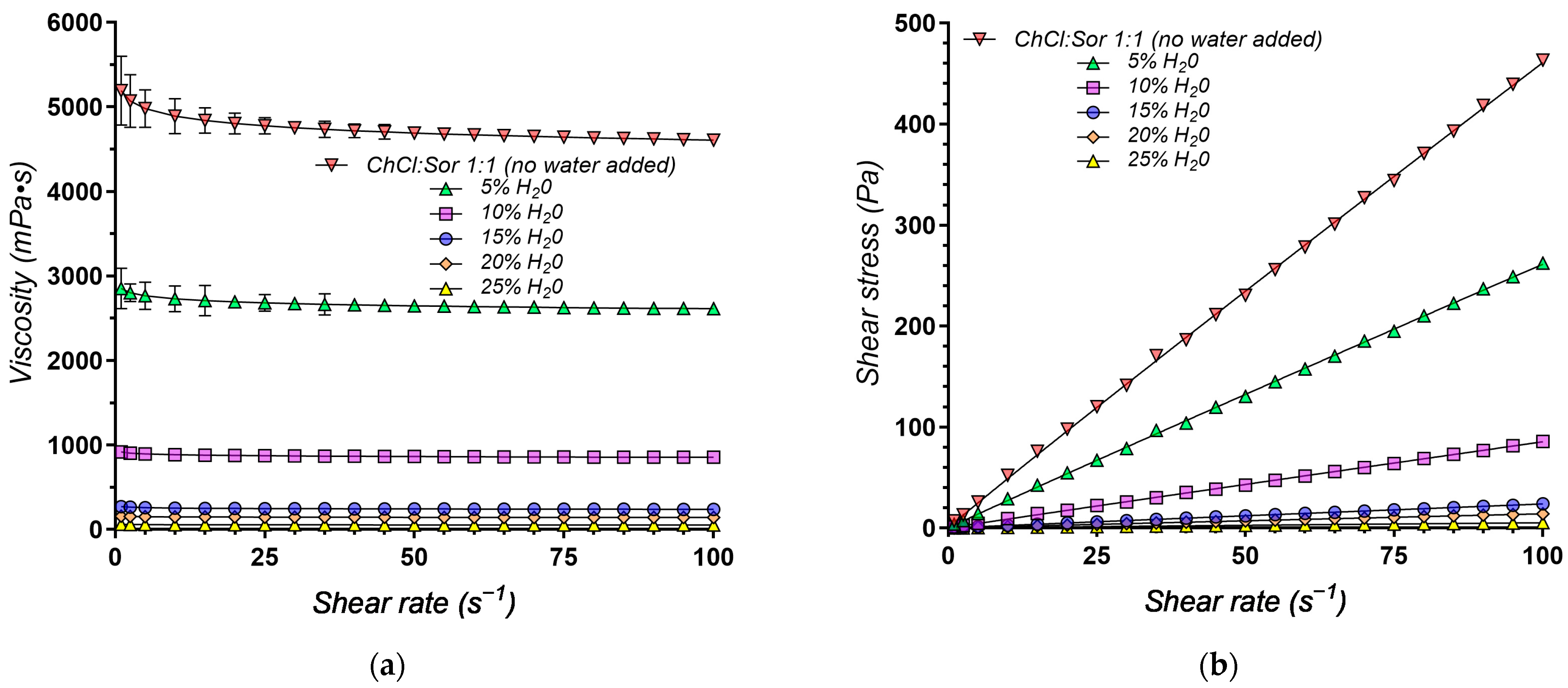
| Sample | Components | K (Pa·sn) | n | R2 | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Molar Ratio | ||||
| A1 | ChCl | Gla | - | 1:1 | 0.154 | 0.9216 | 0.9994 |
| A2 | ChCl | Gla | - | 1:2 | 0.196 | 0.9159 | 0.9985 |
| A3 | ChCl | Oxa | 1:1 | 0.172 | 0.9566 | 0.9971 | |
| A5 | Arg | Gla | 1:8 | 0.949 | 0.9743 | 0.9994 | |
| G1 | ChCl | Gly | 1:2 | 0.221 | 0.9200 | 0.9983 | |
| G3 | ChCl | Sor | 1:1 | 5.191 | 0.9741 | 0.9993 | |
| G5 | ChCl | Glu | Gly | 2:1:1 | 0.951 | 0.9846 | 0.9997 |
| G9 | ChCl | Sor | Water | 1:1:1 | 1.901 | 0.9810 | 0.9982 |
| L5 | MNT | Ola | - | 1:2 | 0.072 | 0.8513 | 0.9968 |
| L7 | MNT | MCT | - | 1:1 | 0.058 | 0.8651 | 0.9974 |
| D3 | MNT | PEG 400 | - | 1:1 | 0.126 | 0.7603 | 0.9989 |
| D4 | MNT | IPM | - | 1:1 | 0.031 | 0.9380 | 0.9976 |
| First Heating | First Cooling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ton (°C) | Tm (°C) | ΔH J/g | Ton (°C) | Tg (°C) | ΔC J/(g·°C) | Ton (°C) | Tc (°C) | ΔH J/g | Ton (°C) | Tg (°C) | ΔC J/(g·°C) | |
| A1 | - | - | - | −78.5 ± 0.8 | −70.7 ± 0.6 | 0.48 ± 0.02 | - | - | - | −76.4 ± 0.6 | −67.1 ± 0.6 | 0.51 ± 0.02 |
| A2 | - | - | - | −71.8 ± 0.5 | −65.7 ± 0.4 | 0.51 ± 0.01 | - | - | - | −73.9 ± 0.4 | −65.7 ± 0.4 | 0.55 ± 0.03 |
| A3 | - | - | - | −59.7 ± 0.8 | −51.0 ± 0.5 | 0.53 ± 0.02 | - | - | - | −46.8 ± 0.3 | −53.5 ± 0.3 | 0.53 ± 0.02 |
| A5 | - | - | - | −44.6 ± 0.5 | −38.3 ± 0.1 | 0.76 ± 0.03 | - | - | - | −27.6 ± 0.5 | −39.1 ± 0.5 | 0.78 ± 0.03 |
| G1 | - | - | - | - | - | - | - | - | - | - | - | - |
| G3 | - | - | - | −74.5 ± 0.7 | −68.3 ± 0.5 | 0.65 ± 0.03 | −58.4 ± 0.5 | −68.3 ± 0.3 | 0.68 ± 0.03 | |||
| G5 | - | - | - | −77.7 ± 1.0 | −76.1 ± 0.3 | 0.37 ± 0.02 | - | - | - | −66.2 ± 0.8 | −73.2 ± 0.5 | 0.34 ± 0.01 |
| G9 | - | - | - | −70.6 ± 0.9 | −62.3 ± 0.4 | 0.58 ± 0.02 | −54.7 ± 0.9 | −63.7 ± 0.6 | 0.59 ± 0.02 | |||
| L5 | −32.7 ± 1.1 | −23.6 ± 0.4 | 14.84 ± 0.12 | −7.0 ± 0.3 | −11.5 ± 0.2 | 52.60 ± 1.02 | ||||||
| −9.7 ± 0.4 | −0.4 ± 0.2 | 53.70 ± 0.91 | −22.1 ± 0.3 | −30.0 ± 0.1 | 13.90 ± 0.34 | |||||||
| L7 | −17.8 ± 0.4 | −10.3 ± 0.3 | 45.13 ± 0.65 | −42.0 ± 0.3 | −48.8 ± 0.3 | 27.99 ± 0.92 | ||||||
| D3 | −14.7 ± 0.7 | −1.1 ± 0.4 | 78.74 ± 1.02 | - | - | - | −22.2 ± 0.7 | −30.7 ± 0.3 | 70.42 ± 1.26 | - | - | - |
| 12.7 ± 0.5 | 17.6 ± 0.3 | 1.90 ± 0.04 | - | - | - | - | - | - | - | - | - | |
| D4 | −6.5 ± 0.3 | −0.9 ± 0.2 | 113.40 ± 3.28 | −6.1 ± 0.3 | −16.7 ± 0.2 | 136.97 ± 4.25 | ||||||
| ChCl | 63.4 ± 0.8 | 68.7 ± 0.8 | 112.41 ± 2.93 | - | - | - | - | - | - | - | - | - |
| 179.0 ± 0.5 | 182.8 ± 0.5 | 103.71 ± 3.12 | - | - | - | - | - | - | - | - | - | |
| MNT | 31.2 ± 0.4 | 36.1 ± 0.3 | 87.33 ± 1.56 | - | - | - | 20.7 ± 0.4 | 17.2 ± 0.3 | 62.96 ± 1.67 | - | - | - |
| Gla | - | - | - | - | - | - | ||||||
| Oxa | 186.0 ± 0.3 | 191.2 ± 0.5 | 69.44 ± 1.3 | - | - | - | - | - | - | - | - | - |
| Arg | 212.0 ± 0.5 | 221.3 ± 0.7 * | - | - | - | - | - | - | - | - | - | - |
| Gly | - | - | - | −84.4 ± 0.8 | −82.7 ± 0.6 | 0.37 ± 0.01 | - | - | - | - | - | - |
| Sor | 94.4 ± 0.2 | 99.2 ± 0.2 | 169.33 ± 0.32 | - | - | - | - | - | - | 1.3 ± 0.8 | −2.6 ± 0.7 | 1.02 ± 0.02 |
| Ola | −25.6 ± 0.5 | −18.5 ± 0.3 | 12.41 ± 0.41 | - | - | - | 0.5 ± 0.2 | −2.8 ± 0.3 | 91.43 ± 1.42 | - | - | - |
| −0.1 ± 0.3 | 6.4 ± 0.2 | 88.80 ± 0.71 | - | - | - | −17.3 ± 0.3 | −23.0 ± 0.2 | 11.63 ± 0.32 | ||||
| MCT | −11.0 ± 0.5 | −1.9 ± 0.3 | 76.07 ± 0.94 | - | - | - | −37.8 ± 0.2 | −42.1 ± 0.3 | 54.46 ± 0.81 | - | - | - |
| PEG 400 | −12.4 ± 0.6 | 5.0 ± 0.5 | 92.68 ± 1.78 | - | - | - | −13.7 ± 0.5 | −20.4 ± 0.5 | 82.94 ± 1.59 | - | - | - |
| Code | Components | K (Pa·sn) | n | R2 | ||
|---|---|---|---|---|---|---|
| 1 | 2 | Weight Ratio | ||||
| G3-0 | G3 | - | - | 5.191 | 0.9741 | 0.9993 |
| G3-5 | G3 | water | 95:5 | 2.851 | 0.9810 | 0.9991 |
| G3-10 | G3 | water | 90:10 | 0.917 | 0.9846 | 0.9989 |
| G3-15 | G3 | water | 85:15 | 0.270 | 0.9727 | 0.9978 |
| G3-20 | G3 | water | 80:20 | 0.158 | 0.9748 | 0.9993 |
| G3-25 | G3 | water | 75:25 | 0.061 | 0.9687 | 0.9984 |
| Sample Code | First Heating | First Cooling | ||||
|---|---|---|---|---|---|---|
| Ton (°C) | Tg (°C) | ΔC J/(g·°C) | Ton (°C) | Tg (°C) | ΔC J/(g·°C) | |
| G3-0 | −74.5 ± 0.7 | −68.3 ± 0.5 | 0.65 ± 0.03 | −58.4 ± 0.5 | −68.3 ± 0.3 | 0.68 ± 0.03 |
| G3-5 | −68.4 ± 0.6 | −60.6 ± 0.4 | 0.59 ± 0.02 | −52.2 ± 0.4 | −61.4 ± 0.2 | 0.60 ± 0.02 |
| G3-10 | −67.6 ± 0.6 | −59.9 ± 0.2 | 0.64 ± 0.02 | −49.2 ± 0.5 | −62.1 ± 0.3 | 0.70 ± 0.02 |
| G3-15 | −65.4 ± 0.5 | −57.2 ± 0.2 | 0.64 ± 0.02 | −50.5 ± 0.5 | −60.5 ± 0.3 | 0.63 ± 0.01 |
| G3-20 | −70.0 ± 0.4 | −62.7 ± 0.3 | 0.61 ± 0.01 | −51.3 ± 0.4 | −59.8 ± 0.2 | 0.62 ± 0.02 |
| G3-25 | −63.5 ± 0.7 | −55.1 ± 0.5 | 0.61 ± 0.02 | −44.6 ± 0.4 | −58.1 ± 0.2 | 0.59 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, M.-A.; Anuța, V.; Nicolae, C.A.; Popa, L.; Ghica, M.V.; Cocoș, F.-I.; Dinu-Pîrvu, C.-E. Exploring Deep Eutectic Solvents as Pharmaceutical Excipients: Enhancing the Solubility of Ibuprofen and Mefenamic Acid. Pharmaceuticals 2024, 17, 1316. https://doi.org/10.3390/ph17101316
Nica M-A, Anuța V, Nicolae CA, Popa L, Ghica MV, Cocoș F-I, Dinu-Pîrvu C-E. Exploring Deep Eutectic Solvents as Pharmaceutical Excipients: Enhancing the Solubility of Ibuprofen and Mefenamic Acid. Pharmaceuticals. 2024; 17(10):1316. https://doi.org/10.3390/ph17101316
Chicago/Turabian StyleNica, Mihaela-Alexandra, Valentina Anuța, Cristian Andi Nicolae, Lăcrămioara Popa, Mihaela Violeta Ghica, Florentina-Iuliana Cocoș, and Cristina-Elena Dinu-Pîrvu. 2024. "Exploring Deep Eutectic Solvents as Pharmaceutical Excipients: Enhancing the Solubility of Ibuprofen and Mefenamic Acid" Pharmaceuticals 17, no. 10: 1316. https://doi.org/10.3390/ph17101316
APA StyleNica, M.-A., Anuța, V., Nicolae, C. A., Popa, L., Ghica, M. V., Cocoș, F.-I., & Dinu-Pîrvu, C.-E. (2024). Exploring Deep Eutectic Solvents as Pharmaceutical Excipients: Enhancing the Solubility of Ibuprofen and Mefenamic Acid. Pharmaceuticals, 17(10), 1316. https://doi.org/10.3390/ph17101316









