Abstract
Prostatitis, a prevalent urinary tract disorder in males, has a complex etiology that leads to severe clinical discomfort. Pule’an Tablets, a classic single-component formulation primarily based on rapeseed pollen, have been clinically proven to have a beneficial therapeutic effect on both prostatitis and benign prostatic hyperplasia. However, there is currently a lack of research on the chemical composition and mechanisms of action of Pule’an Tablets in treating prostatitis. In this study, using liquid chromatography–mass spectrometry (LC-MS), a total of 53 compounds in Pule’an Tablets were identified, including flavonoids, phenylpropionamides, lipids, glucosinolates, and nucleic acids. Subsequently, through a network pharmacology analysis, potential target genes and their mechanisms of action were predicted accordingly. The results suggested that genes such as LPAR5, LPAR6, LPAR4, LPAR3, LPAR2, LPAR1, F2, ENPP2, MMP9, and TNF, along with pathways like prostate cancer, endocrine resistance, bladder cancer, and the IL-17 signaling pathway, may represent potential pathways involved in the therapeutic effects of Pule’an Tablets. This study represents the first systematic investigation into the chemical composition of Pule’an Tablets, shedding light on the potential mechanisms underlying their efficacy in treating prostatitis. These findings could serve as a valuable reference for future pharmacological research on Pule’an Tablets.
1. Introduction
Prostatitis is a common urological disorder and one of the most prevalent clinical conditions in males. According to an epidemiological survey, the prevalence of prostatitis ranged from 2% to 9.7% (the prevalence of different types is different), and the average prevalence was 8.2% [1]. Prostatitis has a complex etiology and leads to severe clinical discomfort, significantly affecting the quality of life for those who suffer from it. In recent years, increasing evidence suggests that prostatitis is closely associated with the occurrence, development, and prognosis of prostate cancer, calling for timely and effective therapeutic interventions [2,3].
Based on the pathological manifestations, the National Institutes of Health (NIH) proposed a classification for prostatitis, the most common of which is chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) (Table 1) [4,5]. Specifically, CP/CPPS is characterized by symptoms of chronic pelvic pain in the absence of a urinary tract infection. However, the etiology of CP/CPPS has not yet been established.

Table 1.
Classification of prostatitis syndromes.
Common treatments for CP/CPPS include antibiotics, painkillers, alpha-blockers, physical therapy, prostate massage, lifestyle changes, and traditional Chinese medicine.
Alpha-blockers act on alpha-receptors on the surface of the smooth muscle of the prostate and bladder neck, relax the smooth muscle, and relieve the symptoms of pressure on the urethra, thereby improving urination symptoms [6]. Antibiotics are more suitable for treatment when the causative agents of prostatitis are identified. Although alpha-blockers and antibiotics have certain effects on alleviating prostatic symptoms, they may be accompanied by adverse reactions [7]. In addition, alpha-blockers and antibiotics only treat the symptoms not the underlying conditions, so it is necessary to find more effective treatments for prostatitis.
Pule’an Tablets (Qianliekang) are a classic pollen preparation capable of tonifying and strengthening the kidney. The kidney is related to the growth and reproduction of the body in Chinese medicine. In the WHO’s international standard terminologies for traditional Chinese medicine (S1), qi represents the intangible, high-mobility nutritive substance that maintains vital activities. Kidney qi deficiency syndrome refers to the decline of growth and reproductive function due to kidney qi deficiency [8]. The indications of Pule’an Tablets include kidney qi deficiency, waist and knee weakness, urine leakage or incontinence, chronic prostatitis, and prostatic hyperplasia. It has also been reported that Pule’an Tablets are effective in the treatment of type III prostatitis. Yang et al. [9] evaluated the clinical efficacy of Pule’an Tablets in treating type III prostatitis according to the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) scores, a widely used chronic prostatitis symptom scoring system. A total of 105 patients with type III prostatitis were administered with Pule’an Tablets, and the NIH-CPSI score and the number of leukocytes in the prostatic secretions were significantly decreased, which proved that the effect of Pule’an Tablets was clear in treating type III prostatitis. Clinical data also showed that Pule‘an Tablets have high efficacy in the treatment of prostate hyperplasia, with an effective cure rate of 92% [10].
Chronic inflammation plays an important role in the progression of most chronic prostatitis, evident from the benign prostatic hyperplasia where the infiltration of inflammatory cells is frequently observed in the prostate. Persistent inflammatory stimulation reshapes the prostate environment and alters the gene expression of prostate-constituting cells, which, in turn, further exacerbates inflammation, consequently leading to chronic inflammation. In animal experiments, the administration of anti-inflammatory drugs to rats having chronic prostatitis prevented the infiltration of inflammatory cells [11]. Therefore, inflammatory intervention might be an option to treat CP/CPPS. Of note, Pule’an Tablets have been reported to be effective in the treatment of chronic prostatitis and benign prostatic hyperplasia in both clinical and animal research, which can possibly be achieved by promoting local blood circulation in the prostate gland, improving microcirculation, enhancing metabolism, adjusting the physiological function of the prostate gland, and reducing inflammation. In addition, animal experiments also proved that Pule’an Tablets can significantly inhibit benign prostatic hyperplasia in rats [12]. Obviously, the therapeutic benefits of Pule’an Tablets for chronic prostatitis and prostatic hyperplasia have been widely recognized, but their chemical composition analysis and mechanism of action studies are still quite insufficient. For better understanding and more rational administration of Pule’an Tablets, there is an urgent need to carry out research on the pharmacological and material basis in the treatment of prostatitis.
Focusing on the issues mentioned above, a UPLC-Triple-TOF/MS analysis method was developed to obtain the mass spectrum data of Pule’an Tablets. A total of 53 compounds in Pule’an Tablets were identified, including 22 flavonoids, 15 phenylpropionamides, five lipids, six glucosinolates, and five nucleic acids. Subsequently, potential target genes and mechanisms of action were predicted by network pharmacology analysis. The present study may shed light on the rational use of Pule’an Tablets in prostatitis treatment and provide clues for the deep development of other botanical drugs.
2. Results
2.1. Identification of Compounds in Pule’an Tablets Based on LC-MS
Pule’an Tablets are a single preparation made from rapeseed pollen. An analytical method was established based on LC-MS technology for the chemical composition analysis of this botanical drug. Figure 1 illustrates base peak chromatograms (BPCs) of Pule’an Tablets.
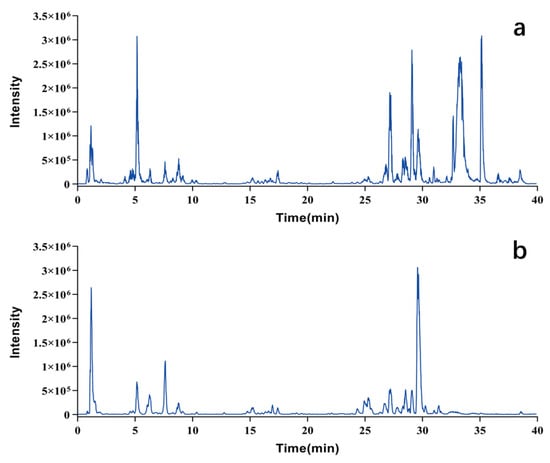
Figure 1.
Base peak chromatograms (BPC) of Pule’an Tablets. (a) Positive ion mode; (b) negative ion mode.
The structural classes of Pule’an Tablets were preliminarily analyzed. Based on the results, the identified compounds were divided into five types, including flavonoids, phenylpropionamides, lipids, glucosinolates, and nucleic acids. Characterization methods were established for different types of chemical compounds (Section 4.4), and the five classes of identified compounds were successfully enriched and characterized in Pule’an Tablets (Figure 2).
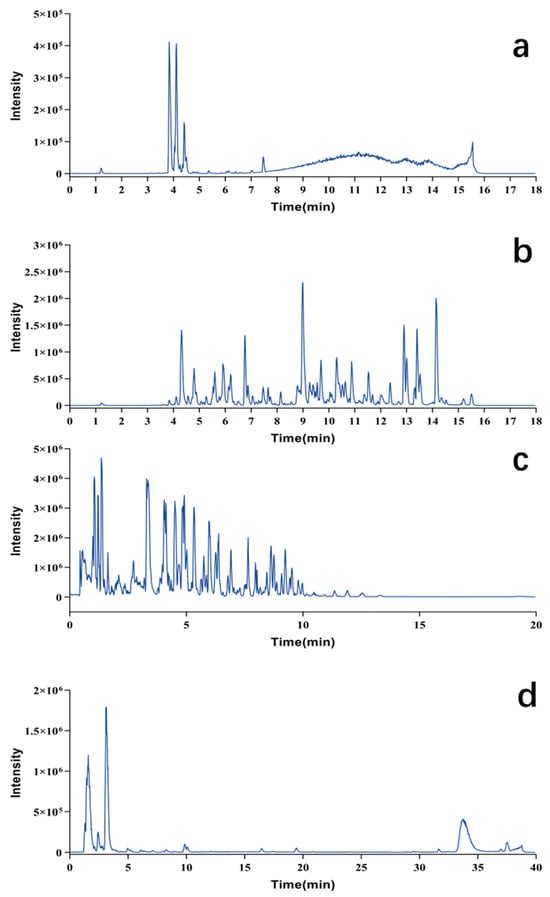
Figure 2.
Chromatograms for chemical composition analysis of Pule’an Tablets. (a) Flavonoids of alcoholic extract of tablet (254 nm); (b) phenylpropanoids of alcoholic extract of tablet (254 nm); (c) lipids of alcoholic extract of tablet; (d) glucosinolates and nucleic acids of aqueous extract of tablet (254 nm).
According to the literature survey and comparison with reference materials, a total of 53 chemical compounds of Pule’an Tablets were identified. Briefly, 22 flavonoids, 15 phenylpropionamides, five lipids, six glucosinolates, and five nucleic acids were identified, as shown in Table 2. The data, in positive and negative ion modes, were analyzed by Peakview software 1.2. Please refer to Table 2 and the Supplementary Material S1 (Figures S1–S28) for more information regarding the structure elucidation.

Table 2.
The identified compounds of Pule’An Tablets based on UPLC coupled with quadrupole TOF-MS (UPLC-Q-TOF-MS).
2.2. Network Pharmacological Analysis of Identified Compounds
The obtained compounds were imported into SwissTargetPrediction [31,32] to predict potential targets with probability > 0. A total of 327 targets from 53 compounds of Pule’an Tablets were obtained, and duplicate targets were removed, as shown in Table 3.

Table 3.
Number of predicted targets for different kinds of chemical compounds.
The distribution of the Venny diagram (Figure 3) [33] shows that there is little overlap among the predicted targets of the different classes of compounds, suggesting that the different classes of compounds may synergistically play the role of action on prostatitis through different targets. By utilizing Cytoscape 3.6.1 software and the collected data, the relationship between the compounds and the targets of Pule’an Tablets was visually analyzed, and classes-compounds-targets network of Pule’an Tablets was constructed (Figure 4).
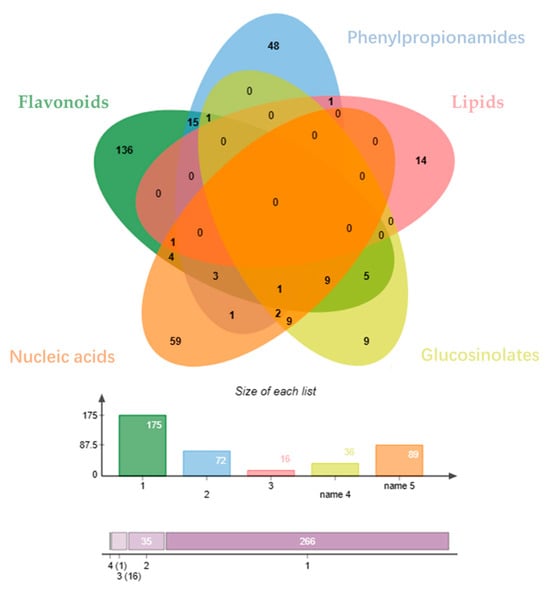
Figure 3.
Venny diagram of targets for different compound: flavonoids (green), phenylpropionamides (blue), lipids (red), glucosinolates (yellow), and nucleic acids (orange).
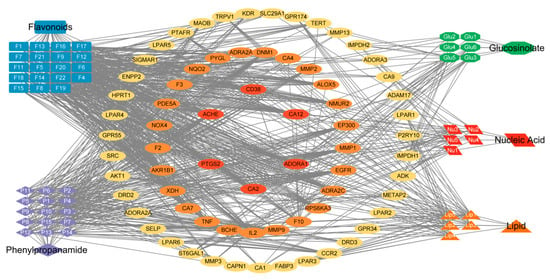
Figure 4.
The classes-compounds-targets network of Pule’an Tablets: orange (lipid compounds), blue (flavonoids), red (glucosinolate compounds), purple (phenylpropionamide compounds), and green (nucleic acid compounds). In the middle, targets with a degree greater than 5 are screened. Use different colors to represent different degrees of gene. From the outside to the inside, the degrees of each circle are 5–14 (yellow), 15–24 (orange) and above 25 respectively(red). Please also see Supplementary Material S2 for more information about the matchups between the identified compounds and the targets.
Targets related to chronic prostatitis, an increased frequency of micturition, and prostatic hyperplasia were collected through GeneCards [34] and Disgenet [35]. Then, 1263 targets associated with prostatitis, an increased frequency of micturition, and prostatic hyperplasia were screened out. A total of 122 targets were consistent among prostatitis, an increased frequency of micturition, prostatic hyperplasia, and Pule’an Tablets using a Venny diagram, as shown in Figure 5.
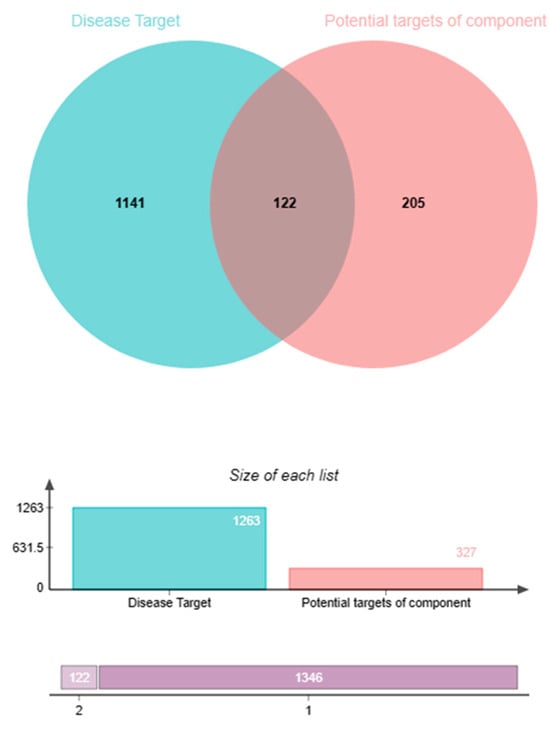
Figure 5.
Venny diagram of the common targets of Pule’an Tablets and diseases (prostatitis, increased frequency of micturition, and prostatic hyperplasia).
The results suggested that these consistent targets might be related to the mechanism of disease action. The screened targets were imported into the String platform [36] and a protein-protein interaction network with a confidence of >0.7 was constructed, as shown in Figure 6. The PPI network containing 54 nodes and 102 edges was downloaded and optimized via Cytoscape 3.6.1 software. The PPI network was analyzed using the cytohubba plugin in conjunction with the MCC algorithm to sort out the top 10 genes in terms of weight share, forming 29 edges of 10 nodes, as shown in Figure 7. In order of degree, the top 10 are LPAR5, LPAR6, LPAR4, LPAR3, LPAR2, LPAR1, F2, ENPP2, MMP9, and TNF.
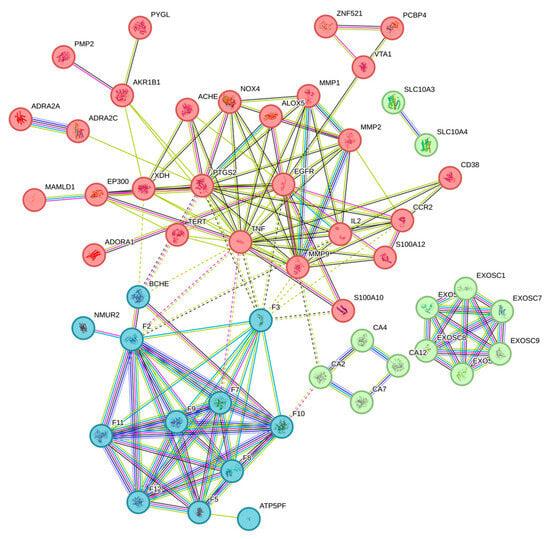
Figure 6.
Protein-protein interaction and enrichment analysis. The same color denotes that the proteins in the cluster have similar functions.
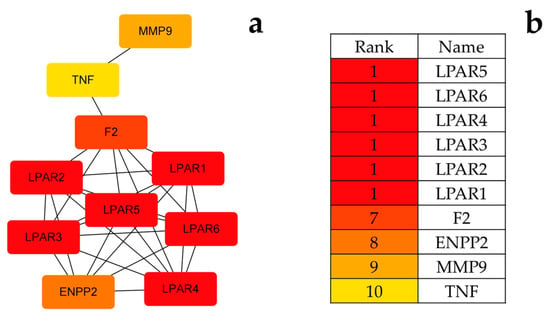
Figure 7.
(a) PPI network optimized via Cytoscape 3.6.1 software; (b) sequencing of the top 10 target proteins.
The main collected targets were imported into the DAVID database [37,38] for further bioinformatics analysis, and the potential molecular mechanisms and biological processes of the main compounds of Pule’an Tablets were explored. The GO biological process (GO-BP), the GO cellular component (GO-CC), and GO molecular function (GO-MF) term analysis were performed on the David platform. The top five terms from the GO-BP, GO-CC, and GO-MF enrichment analyses, according to the false discovery rate (FDR), are shown in Figure 8. The results showed that the chemical compounds of Pule’an Tablets have a high correlation with the GO molecular function, e.g., endopeptidase activity and serine-type endopeptidase activity. They also involve more genes in the biological processes, namely, response to xenobiotic stimulus, positive regulation of cell growth, and extracellular matrix disassembly. Due to the low FDR of these results, they can be used as key biological processes.
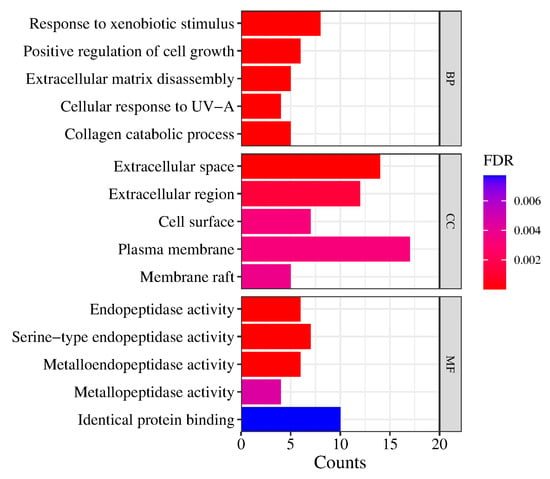
Figure 8.
The top 5 terms from the GO-BP, GO-CC, and GO-MF enrichment analyses.
For the KEGG analysis, the top 10 items are shown in Figure 9. Excluding broad-spectrum pathways such as the pathways in cancer, the results showed that prostate cancer, endocrine resistance, lipids and atherosclerosis, bladder cancer, and the IL-17 signaling pathway function in the regulation of the main components of Pule’an Tablets, which can provide reference for the subsequent study of the pharmacodynamic mechanism.
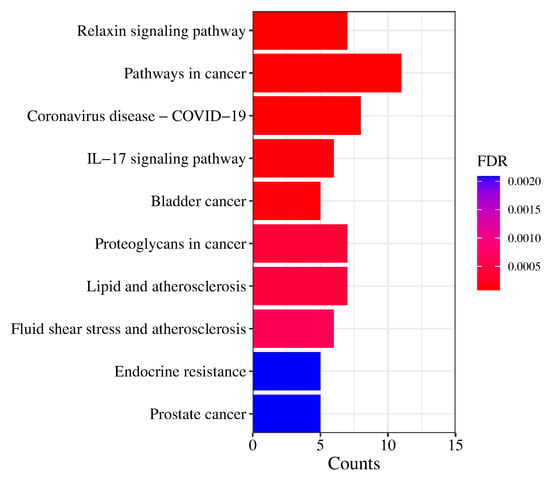
Figure 9.
KEGG pathway analysis of potential targets of Pule’an Tablets.
3. Discussion
Pule’an Tablets have been used in the clinical treatment of prostatitis and benign prostatic hyperplasia and proved to be effective. However, the chemical composition and the corresponding targets remain unknown, which impedes the pharmacological and clinical research of this botanical drug as well as the screening of lead compounds. LC-MS uses LC as the separation system and MS as the detection system, which has the advantages of the separation ability of LC and the high sensitivity and resolution of MS, andn at the same time, MS can also provide the fragmentation information for the determination of the structure [39]. Therefore, LC-MS has been widely used in the field of traditional Chinese medicine analysis in recent years [40,41].
In this study, therefore, the chemical profiles of Pule’an Tablets were characterized by the LC-MS method. It was found that the main components in Pule’an Tablets were flavonoids, phenylpropionamides, and lipids. Collectively, a total of 53 chemical components in Pule’an Tablets were identified, including 22 flavonoids, 15 phenylpropionamides, five lipids, six glucosinolates, and five nucleic acids.
Obviously, the chemical composition of Pule’an Tablets is complex, even if they are made of only a single ingredient, rapeseed pollen. To clarify the mechanism underlying the effect of Pule’an Tablets, a protein-protein interaction network and KEGG and GO pathway analyses were carried out. The results suggested that LPAR5, LPAR6, LPAR4, LPAR3, LPAR2, LPAR1, F2, ENPP2, MMP9, and TNF are potential targets for prostatitis. In addition, the main compounds in Pule’an Tablets might exert the therapeutical effect through the signaling pathways of prostate cancer, endocrine resistance, fluid shear stress and atherosclerosis, lipids and atherosclerosis, proteoglycans in cancer, bladder cancer, the IL-17 signaling pathway, the pathways in cancer, and relaxin signaling. According to the literature, MMP may play a significant role in the development of cancer [42]. Extracellular matrix (ECM) degradation plays an important role in tumor invasion and migration [43]. It is worth noting that MMP is closely related to ECM degradation and remodeling [43]. The mRNA expression of MMP1/11 in bladder cancer samples was significantly higher than that in normal bladder tissues. Although some studies showed that prostatitis is associated with prostate cancer, their relationship is still not clear. Our network pharmacological prediction results provide an idea for the application of MMP in the treatment of prostatitis and prostate cancer. Another pathway deserving of attention is the leukocyte-17 (IL-17) signaling pathway. IL-17, a pro-inflammatory cytokine that acts on various cellular targets, leads to cell activation. IL-17’s action on endothelial cells leads to inflammation and pro-coagulant activity, and it is also involved in innate and adaptive immune responses and has been proved to be associated with a variety of diseases, such as infectious diseases, autoimmune diseases, and cancer. Studies indicated that the occurrence of prostatitis may be related to inflammation and the immune reaction, and IL17A- and IFN-γ-expressing cells induce chronic pelvic pain [44]. These findings suggest that the IL17 pathway can be a potential pathway for the treatment of prostatitis.
Studies have proved that Chinese medicine has the characteristics of a multi-component, multi-target, and complex mechanism of action, and different compounds may synergistically or antagonistically act on the same target [45]. The complexity of the system of traditional Chinese medicine presents great difficulties for its in-depth study. Systemic pharmacology is a product of multidisciplinary intersection, including pharmacology, structural biology, bioinformatics, and so on. Systematic pharmacology can be used to predict pharmacokinetic and pharmacodynamic models and evaluate drug therapy mechanisms through the network analysis of databases [46,47]. The systemic pharmacology of traditional Chinese medicine provides a new idea and perspective for the study of traditional Chinese medicine. Meanwhile, it also provides ideas for us to carry out follow-up pharmacological research on Pule’an Tablets.
Collectively, these results could provide important references for subsequent pharmacological studies on the mechanism of action of Pule’an Tablets for prostatitis and also give guidance for the clinical use of Pule’an Tablets.
4. Materials and Methods
4.1. Chemicals and Reagents
Pule’an Tablets were produced by Zhejiang CONBA Pharmaceutical Co., Ltd. (Hangzhou, China). Acetonitrile and formic acid were obtained from Merk KGaA (Darmstadt, Germany). Ultrapure water was prepared by Millipore plus system.
4.2. Sample Preparation
4.2.1. Sample Preparation for Flavonoid Analysis
First, 0.595 g of powder of crushed Pule’an Tablets was extracted by ultrasonication with 5.95 mL of 70% ethanol for half an hour at 25 °C. The extract was centrifuged, and the supernatant was collected for subsequent analysis.
4.2.2. Sample Preparation for Phenylpropionamide Analysis
First, 0.599 g of powder of crushed Pule’an Tablets was extracted by ultrasonication with 5.99 mL of 70% ethanol for half an hour at 25 °C. The extract was centrifuged, and the supernatant was collected for subsequent analysis.
4.2.3. Sample Preparation for Lipid Analysis
First, 0.597 g of powder of crushed Pule’an Tablets was extracted by ultrasonication with 5.97 mL of chloroform and methanol (chloroform:methanol = 1:1) for half an hour at 25 °C. The extract was centrifuged, and the supernatant was collected for subsequent analysis.
4.2.4. Sample Preparation for Glucosinolate and Nucleic Acids Compounds
First, 0.595 g of powder of crushed Pule’an Tablets was extracted by ultrasonication with 5.95 mL of pure water for half an hour at 25 °C. The extract was centrifuged, and the supernatant was collected for subsequent analysis.
4.3. LC-MS System and Apparatus
LC-MS and MS/MS analyses were carried out on a Acquity UPLC system, which was equipped with a binary solvent delivery system, autosampler, and photodiode-array detection (DAD) system, combined with a Triple TOF 5600+ Mass Spectrometer (AB SCIEX, Singapore).
4.4. LC-MS Analytical Methods
4.4.1. Test Conditions for Flavonoids
UPLC conditions: Sample separation was achieved using a Waters CSH-C18 (1.7 μm, 2.1 mm × 150 mm, Waters, Wexford, Ireland) at 50 °C. The flow rate was 0.35 mL/min. The injection volume was 3 μL for each run. The mobile phase was composed of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B) with a gradient program: 0–20 min, 10–35% B; 20–35 min, 35–95% B; 35–37 min, 95% B. The detection wavelength was 254 nm.
TOF-MS conditions: Electrospray ionization source (ESI), scanning mode: positive and negative ions; GAS 1: 50 psi; GAS 2: 50 psi; curtain gas (CUS): 35 psi; desolvation gas temperature: (TEM): 550 °C (negative) and 600 °C (positive); ion source voltage (IS): −4500 V (negative) and 5500 V (positive). The mass range for data acquisition was set to m/z: 100 to 1500. The MS1 parameters were as follows: declustering potential (DP): 100 V; collision energies (CE): 10 V. Using TOF MS-Product Ion-IDA mode to record the MS2 information, collision-induced dissociation: ±40 ± 20 eV. Before injection, the mass axis correction was completed with a CDS pump to make the mass axis error less than 2 ppm.
4.4.2. Test Conditions for Phenylpropionamides
UPLC conditions were in line with Section 4.4.1.
TOF-MS conditions were in line with Section 4.4.1.
4.4.3. Test Conditions for Lipids
UPLC conditions: Sample separation was achieved using a Waters HSS T3-C18 (1.8 μm, 3.0 mm × 50 mm, Waters, Wexford, Ireland) at 40 °C. The flow rate was 0.5 mL/min. The injection volume was 3 μL for each run. The mobile phase was composed of 90% methanol in water (A) and Isopropanol:Acetonitrile = 1:1 (B) with a gradient program: 0–10 min, 0–11% B; 10–18 min, 100% B. The detection wavelength was 254 nm.
TOF-MS conditions were consistent with Section 4.4.1.
4.4.4. Test Conditions for Glucosinolates and Nucleic Acids
UPLC conditions: Sample separation was achieved using a ThermoFisher HYPERCARB (2.1 mm × 100 mm, Los Angeles, CA, USA) at 50 °C. The flow rate was 0.25 mL/min. The injection volume was 3 μL for each run. The mobile phase was composed of 0.3% ammonia and 0.1% ammonium acetate in water (A) acetonitrile (B) with a gradient program: 0–2 min, 2–7% B; 2–25 min, 7–25% B; 25–37 min, 25–95% B. The detection wavelength was 254 nm.
TOF-MS conditions were in line with Section 4.4.1.
4.5. Targeted Network Pharmacology Analysis
The components obtained by inference or prediction from LC-MS analysis were imported into SwissTargetPrediction to predict the corresponding targets, and targets with a possibility > 0 were collected for prediction. Disease targets were acquired in the DisGeNet database and Genecards database. Chronic prostatitis, prostate hyperplasia, and urinary-frequency disease genes were searched, and genes with a relevance score greater than 10 were selected, while duplicate targets were screened out. Cytoscape 3.6.1 was used for visualization analysis to screen the gene targets with degree > 5. PPI analysis was performed through the STRING platform. Cytoscape software (Version 3.6.1) was used to further analyze the PPI network, and the cytoHubba plug-in was applied to screen the most core targets in the network according to the MCC algorithm. GO analysis and KEGG analysis were performed using the DAVID platform.
5. Conclusions
In conclusion, while the biological activity and potential phytotherapeutic role of the compounds require further study, the present work systematically investigated the chemical composition of Pule’an Tablets. A total of 53 compounds encompassing five structural classes were identified. In addition, potential pathways and bioactive compounds involved in the therapeutic effects of this botanical drug were studied through a network pharmacology analysis. Collectively, the findings based on both wet-and dry-lab experiments in this research could shed light on the potential mechanisms underlying their efficacy in treating prostatitis, can serve as a valuable reference for future pharmacological research on Pule’an Tablets, and are of guiding significance for the further screening and subsequent development of active substances in other botanical drugs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph17010056/s1, Supplementary Material S1: Identified structure in Pule’an Tablets. Supplementary Material S2: The identified compounds of Pule’an Tablets and corresponding targets.
Author Contributions
Original draft preparation, visualization, H.Z.; methodology, funding acquisition, Z.G.; formal analysis, J.W.; resources, J.Y.; resources, J.H.; writing—review and editing, conceptualization, project administration, funding acquisition, Y.W. (Yi Wang), Y.W. (Yingchao Wang) and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Key Research and Development Program of China (grant no. 2022YFC3501802), the National Natural Science Foundation of China (82204614), the “Pioneer” and “Leading Goose” R&D Programs of Zhejiang (grant no. 2023C03004), and the Experimental Technology Research Project of Zhejiang University (grant no. szd202104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available within the article and Supplementary Materials.
Acknowledgments
We extend our thanks to Zhejiang CONBA Pharmaceutical Co., Ltd. for providing the Pule’an Tablets.
Conflicts of Interest
J.Y. and J.H. were employed by the company Zhejiang CONBA Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Krieger, J.N.; Lee, S.W.H.; Jeon, J.; Cheah, P.Y.; Liong, M.L.; Riley, D.E. Epidemiology of prostatitis. Int. J. Antimicrob. Agents 2008, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Kramer, G.; Marberger, M.; Montironi, R.; Nelson, W.; Schröder, F.; Sciarra, A.; Tubaro, A. The Controversial Relationship Between Benign Prostatic Hyperplasia and Prostate Cancer: The Role of Inflammation. Eur. Urol. 2011, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.N.; Nyberg, L., Jr.; Nickel, J.C. NIH Consensus Definition and Classification of Prostatitis. JAMA 1999, 282, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mikrani, R.; Xie, D.; Wazir, J.; Shrestha, S.; Ullah, R.; Baig, M.M.F.A.; Ahmed, A.; Srivastava, P.K.; Thapa, K.B.; et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: Study of immune cells and cytokines. Fundam. Clin. Pharmacol. 2020, 34, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Sugaya, K. Mechanisms of action for α1-adrenoceptor blockers in storage symptoms with new insights into the micturition reflex. Life Sci. 2017, 191, 90–96. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, J.; Tian, J.; Zhou, J.; Liu, Y.; Liu, Z. Network Meta-Analysis of the Efficacy of Acupuncture, Alpha-blockers and Antibiotics on Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Sci. Rep. 2016, 6, 35737. [Google Scholar] [CrossRef]
- Li, X.-T. Introductory Chapter: Therapies Based on Kidney Essence and Qi in Chinese Medicine. In Chinese Medical Therapies for Diabetes, Infertility, Silicosis and the Theoretical Basis; IntechOpen: London, UK, 2017; ISBN 978-953-51-2914-1. [Google Scholar]
- Yang, F.; Xiao, F.; Yao, W.; Gao, Y. Clinical effcacy of Qianliekang tablets on type Ⅲ prostatitis. Chin. J. Clin. Pharmacol. 2014, 30, 765–766. [Google Scholar] [CrossRef]
- Sha, J. Comparative study of Qianlie Kang and Prostat Tablets in the treatment of prostatic hyperplasia. Electron. J. Clin. Med. Lit. 2016, 3, 10828. [Google Scholar] [CrossRef]
- Tsunemori, H.; Sugimoto, M. Effects of inflammatory prostatitis on the development and progression of benign prostatic hyperplasia: A literature review. Int. J. Urol. 2021, 28, 1086–1092. [Google Scholar] [CrossRef]
- Yang, C.; Yao, J.; Chen, T. The effect of Qianliekang tablets on the clinical efficacy, immune function, and inflammatory factor levels in the prostatic fluid of elderly chronic prostatitis patients. Am. J. Transl. Res. 2021, 13, 7363–7369. [Google Scholar] [PubMed]
- Sayed, A.M.E.; Omar, F.A.; Emam, M.M.A.-A.; Farag, M.A. UPLC-MS/MS and GC-MS based metabolites profiling of Moringa oleifera seed with its anti-Helicobacter pylori and anti-inflammatory activities. Nat. Prod. Res. 2022, 36, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lai, J.; Wu, Y.; Fang, S.; Liang, X. Investigation on Antioxidant Activity and Different Metabolites of Mulberry (Morus spp.) Leaves Depending on the Harvest Months by UPLC–Q-TOF-MS with Multivariate Tools. Molecules 2023, 28, 1947. [Google Scholar] [CrossRef] [PubMed]
- De Lima Júnior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, N.; Zhang, J.; Huang, Y.; Xiang, Q.; Wen, J.; Chen, Y.; Hu, T.; Qiuyan, L.; Rao, C. Neurotoxicity study of ethyl acetate extract of Zanthoxylum armatum DC. on SH-SY5Y based on ROS mediated mitochondrial apoptosis pathway. J. Ethnopharmacol. 2024, 319, 117321. [Google Scholar] [CrossRef] [PubMed]
- De Paula Menezes, R.; de Bessa, M.A.S.; de Siqueira, C.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef]
- Aguila-Muñoz, D.G.; Jiménez-Montejo, F.E.; López-López, V.E.; Mendieta-Moctezuma, A.; Rodríguez-Antolín, J.; Cornejo-Garrido, J.; Cruz-López, M.C. Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L. Molecules 2023, 28, 5760. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, H.-D.; Lee, S.; Lee, A.Y. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2024, 319, 117105. [Google Scholar] [CrossRef]
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen. Food Chem. 2023, 405, 134800. [Google Scholar] [CrossRef]
- Williams, P.E.; Klein, D.R.; Greer, S.M.; Brodbelt, J.S. Pinpointing Double Bond and sn-Positions in Glycerophospholipids via Hybrid 193 nm Ultraviolet Photodissociation (UVPD) Mass Spectrometry. J. Am. Chem. Soc. 2017, 139, 15681–15690. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, C.; Wang, K.; Takahashi, S.; Krausz, K.W.; Lu, D.; Wang, Q.; Luo, Y.; Gong, X.; Mu, X.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand triptolide-induced liver injury in mice. Toxicol. Lett. 2020, 333, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Wang, M.; Zhou, Y.; Yuan, D.; Jian, Q.; Wu, S.; Liu, B.; Yang, Y. Deciphering the pharmacological mechanisms of Rostellularia procumbens (L) Nees. Extract alleviates adriamycin-induced nephropathy in vivo and in vitro. Phytomedicine 2023, 113, 154736. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jarrell, Z.R.; Smith, M.R.; Ly, V.T.; Liang, Y.; Orr, M.; Go, Y.-M.; Jones, D.P. Metabolomics of V2O5 nanoparticles and V2O5 nanofibers in human airway epithelial BEAS-2B cells. Toxicol. Appl. Pharmacol. 2023, 459, 116327. [Google Scholar] [CrossRef] [PubMed]
- Panara, A.; Aalizadeh, R.; Thomaidis, N.S. Chemical characterisation of Pelargonium sidoides root based on LC-QToF-MS non-target screening strategies. Phytochem. Anal. 2022, 33, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Bialecki, J.B.; Ruzicka, J.; Weisbecker, C.S.; Haribal, M.; Attygalle, A.B. Collision-induced dissociation mass spectra of glucosinolate anions. J. Mass. Spectrom. 2010, 45, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, Y.; Zhao, X.; Yang, Z. Tingli Dazao Decoction pretreatment ameliorates mitochondrial damage induced by oxidative stress in cardiomyocytes. J. Ethnopharmacol. 2023, 303, 115987. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Giorgi, G.; Cherubini, E.; Gervasi, P.G.; Della Croce, C.M.; Longo, V.; Bellani, L. Screening and identification of major phytochemical compounds in seeds, sprouts and leaves of Tuscan black kale Brassica oleracea (L.) ssp acephala (DC) var. sabellica L. Nat. Prod. Res. 2018, 32, 1617–1626. [Google Scholar] [CrossRef]
- Yu, X.; He, H.; Zhao, X.; Liu, G.; Hu, L.; Cheng, B.; Wang, Y. Determination of 18 Intact Glucosinolates in Brassicaceae Vegetables by UHPLC-MS/MS: Comparing Tissue Disruption Methods for Sample Preparation. Molecules 2022, 27, 231. [Google Scholar] [CrossRef]
- Đulović, A.; Usanović, K.; Kukoč Modun, L.; Blažević, I. Selenium Biofortification Effect on Glucosinolate Content of Brassica oleracea var. italic and Eruca vesicaria. Molecules 2023, 28, 7203. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Fishilevich, S.; Nudel, R.; Rappaport, N.; Hadar, R.; Plaschkes, I.; Iny Stein, T.; Rosen, N.; Kohn, A.; Twik, M.; Safran, M.; et al. GeneHancer: Genome-wide integration of enhancers and target genes in GeneCards. Database 2017, 2017, bax028. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Bi, J.-H.; Xie, M.; Zhang, H.; Shi, Z.-Q.; Guo, H.; Yin, H.-B.; Zhang, J.-N.; Xin, G.-Z.; Song, H.-P. Classification-based strategies to simplify complex traditional Chinese medicine (TCM) researches through liquid chromatography-mass spectrometry in the last decade (2011–2020): Theory, technical route and difficulty. J. Chromatogr. A 2021, 1651, 462307. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Y.; Lu, L.; Gu, F.; Chen, H. The Applications and Features of Liquid Chromatography-Mass Spectrometry in the Analysis of Traditional Chinese Medicine. Evid.-Based Complement. Altern. Med. 2016, 2016, e3837270. [Google Scholar] [CrossRef]
- Mustafa, S.; Mobashir, M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int. J. Biol. Macromol. 2020, 143, 11–29. [Google Scholar] [CrossRef]
- Shen, C.; Da, L.; Wu, Z.; Wang, Y.; Gao, S.; Tian, D.; Hu, H. Expression and Prognostic Significance of the MMP Family Molecules in Bladder Cancer. Comb. Chem. High Throughput Screen. 2021, 24, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Das, A.; Barai, A.; Sen, S. MMP Secretion Rate and Inter-invadopodia Spacing Collectively Govern Cancer Invasiveness. Biophys. J. 2018, 114, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Quick, M.L.; Wong, L.; Mukherjee, S.; Done, J.D.; Schaeffer, A.J.; Thumbikat, P. Th1-Th17 Cells Contribute to the Development of Uropathogenic Escherichia coli-Induced Chronic Pelvic Pain. PLoS ONE 2013, 8, e60987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Luther, M.A. Systems pharmacology and drug repositioning—An integrated approach to metabolic diseases. J. Transl. Med. 2012, 10, A18. [Google Scholar] [CrossRef][Green Version]
- Huang, C.; Zheng, C.; Li, Y.; Wang, Y.; Lu, A.; Yang, L. Systems pharmacology in drug discovery and therapeutic insight for herbal medicines. Brief. Bioinform. 2014, 15, 710–733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).