Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents
Abstract
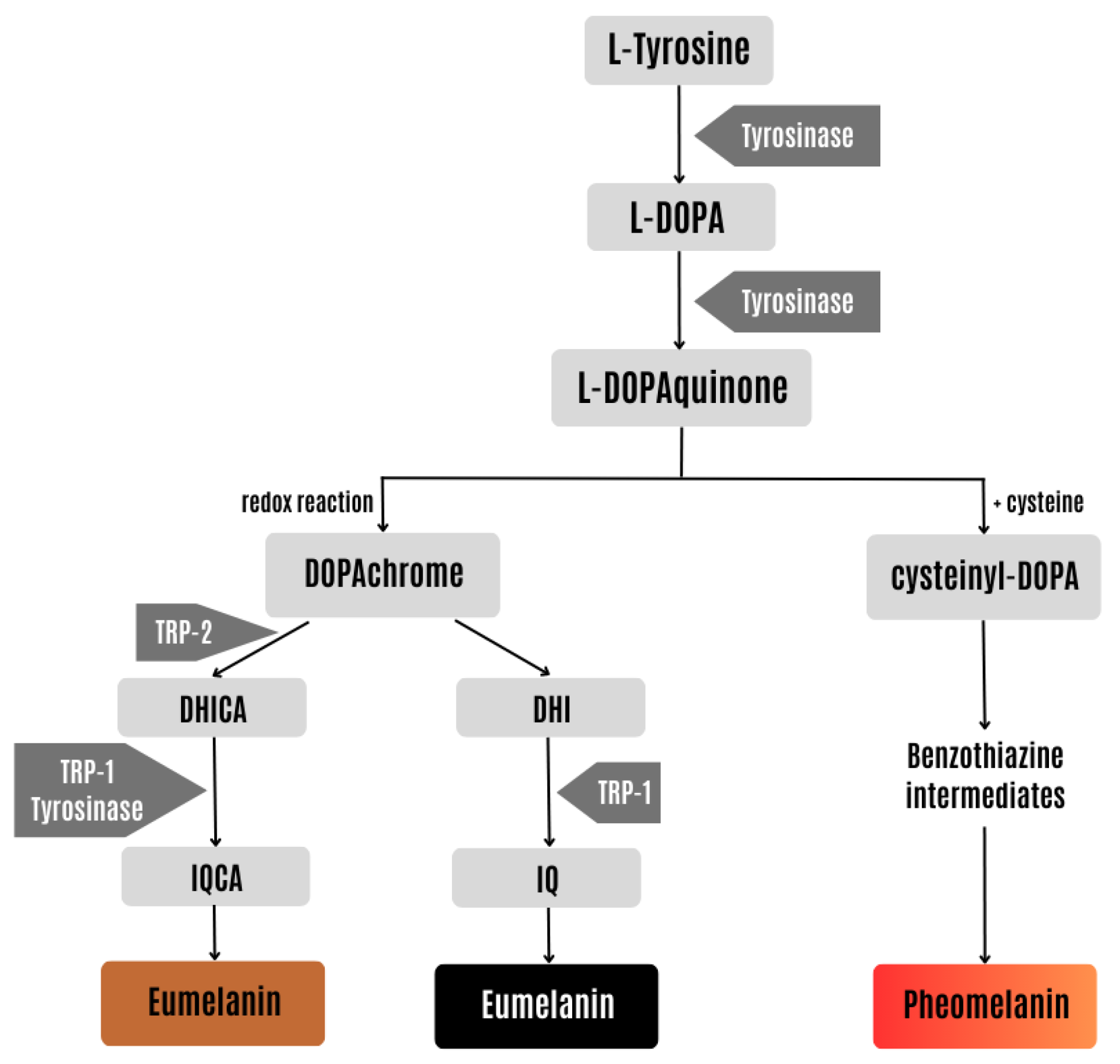
:1. Introduction
2. Results and Discussion
2.1. UV-Visible Spectra
2.2. ROS Assay
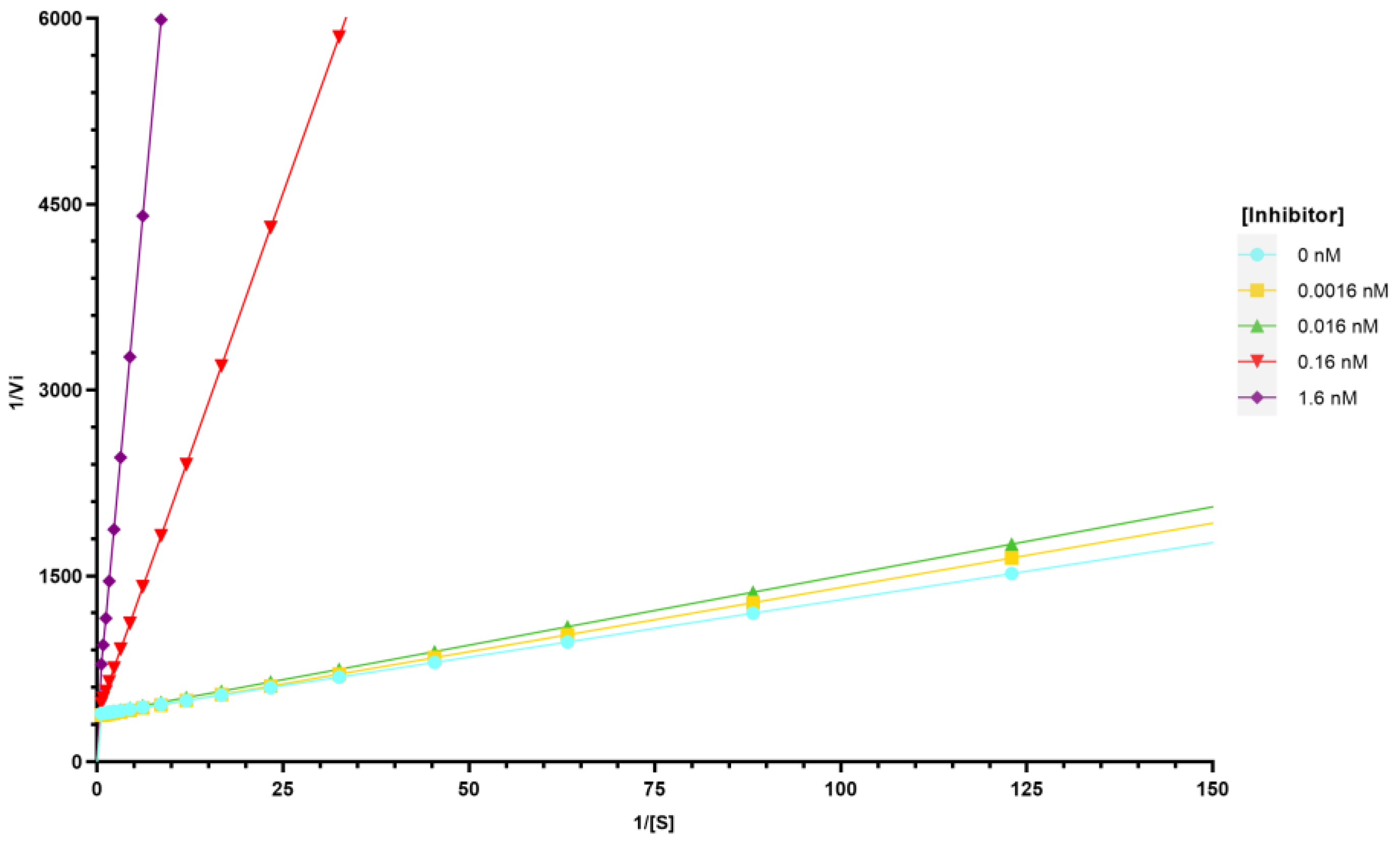
2.3. Inhibition of Tyrosinase Activity
3. Materials and Methods
3.1. Depigmenting Agents
3.2. Chemicals
3.3. UV-Visible Spectral Analysis
3.4. ROS Assay
3.5. Tyrosinase Inhibition Assay
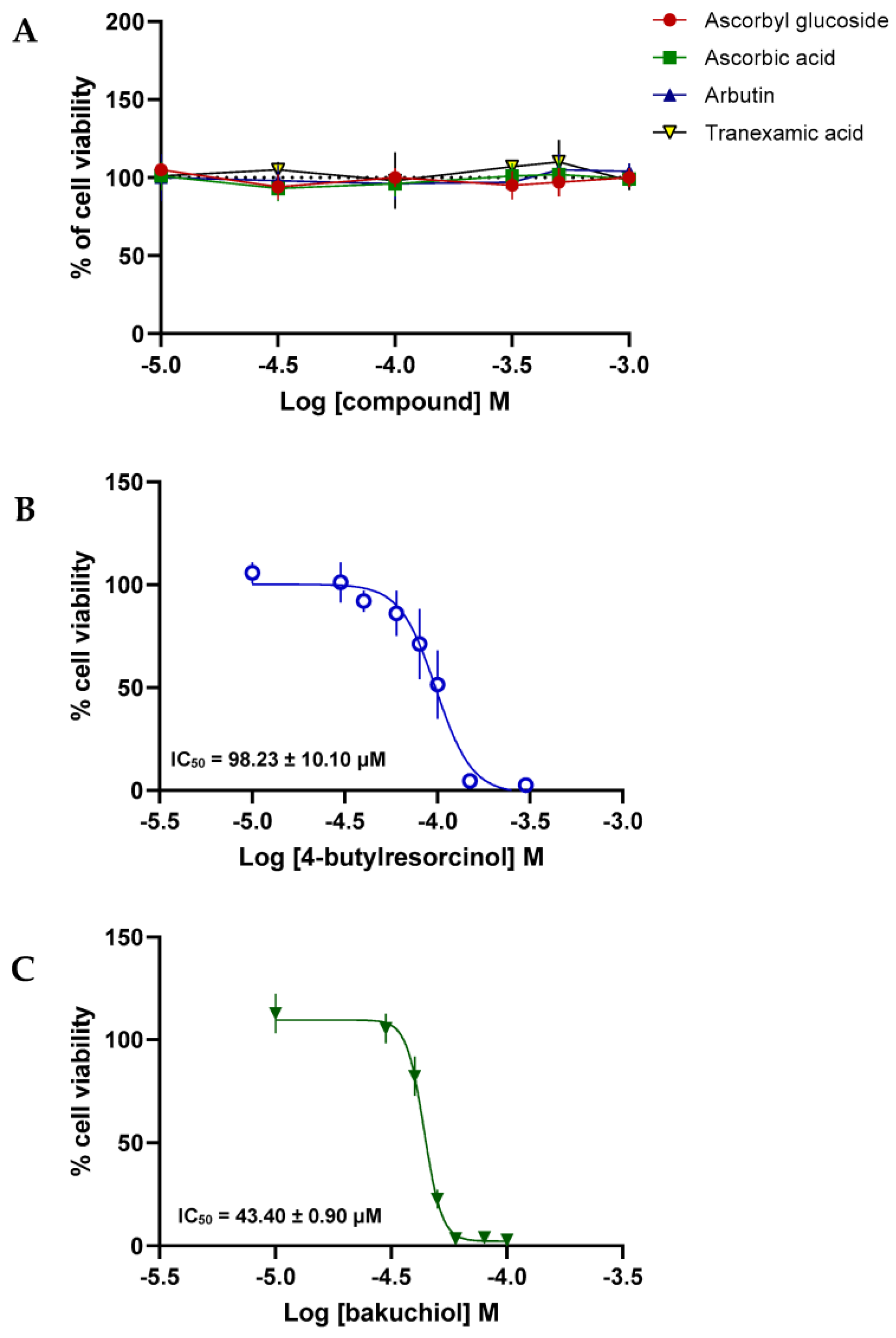
3.6. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, A.D.; De La Garza, H.; Maymone, M.B.C.; Johansen, V.M.; Vashi, N.A. Skin-Lightening Products: Consumer Preferences and Costs. Cureus 2021, 13, e17245. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Pokhrel, D.B. Assessment and comparison of quality of life in patients with Melasma and Vitiligo. Kathmandu Univ. Med. J. 2019, 17, 114–118. [Google Scholar]
- Research, G.V. Skin Lightening Products Market Size, Share & Trends Analysis Report by Product (Creams, Cleanser, Mask), by Nature, by Region, and Segment Forecasts, 2022–2030; Grand View Research Inc.: San Francisco, CA, USA, 2022. [Google Scholar]
- Research and Markets. Europe Skin Lightening Products Market Size, Share & Industry Trends Analysis Report by Product (Creams, Cleanser, Mask, and Others), by Nature (Synthetic, Natural, and Organic), by Country and Growth Forecast 2022–2028. Available online: https://www.researchandmarkets.com/reports/5636733/europe-skin-lightening-products-market-size#product--toc (accessed on 15 October 2023).
- Liyanage, A.; Liyanage, G.; Sirimanna, G.; Schürer, N. Comparative Study on Depigmenting Agents in Skin of Color. J. Clin. Aesthetic Dermatol. 2022, 15, 12–17. [Google Scholar]
- Rendon, M.I.; Gaviria, J.I. Review of skin-lightening agents. Dermatol. Surg. 2005, 31, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Skin Depigmenting Agents in Anti-Aging Cosmetics: A Medicinal Perspective on Emerging Ingredients. Appl. Sci. 2022, 12, 775. [Google Scholar] [CrossRef]
- Juliano, C.C.A. Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Appl. Sci. 2022, 12, 3177. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. Alpha Arbutin as a Skin Lightening Agent: A Review. Int. J. Pharm. Res. 2021, 13, 3502–3510. [Google Scholar] [CrossRef]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18000. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.R.; Rodríguez-López, J.N.; García-Cánovas, F. Effect of l-ascorbic acid on the monophenolase activity of tyrosinase. Biochem. J. 1993, 295, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Asmaro, K.; Li, G.; Palomino, E. Redox cycling of quinones reduced by ascorbic acid. Chem.-Biol. Interact. 2023, 373, 110397. [Google Scholar] [CrossRef] [PubMed]
- Correia, G.; Magina, S. Efficacy of topical vitamin C in melasma and photoaging: A systematic review. J. Cosmet. Dermatol. 2023, 22, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef]
- Tan, L.F.; Mogana, R.; Chinnappan, S.; Venkatalakshmi, R.; Yap, V.L. Various Plants and Bioactive Constituents for Pigmentation Control: A Review. Res. J. Pharm. Technol. 2021, 14, 6106–6112. [Google Scholar] [CrossRef]
- Brown, A.; Furmanczyk, M.; Ramos, D.; Ribes, A.; Pons, L.; Bustos, J.; de Henestrosa, A.R.F.; Granger, C.; Jourdan, E. Natural Retinol Analogs Potentiate the Effects of Retinal on Aged and Photodamaged Skin: Results from In Vitro to Clinical Studies. Dermatol. Ther. 2023, 13, 2299–2317. [Google Scholar] [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chem. 2023, 405, 134953. [Google Scholar] [CrossRef] [PubMed]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11, 2021-11-2. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Tomita, Y. Mechanism of the Inhibitory Effect of Tranexamic Acid on Melanogenesis in Cultured Human Melanocytes in the Presence of Keratinocyte-conditioned Medium. J. Health Sci. 2007, 53, 389–396. [Google Scholar] [CrossRef]
- Kim, K.M.; Lim, H.W. The uses of tranexamic acid in dermatology: A review. Int. J. Dermatol. 2023, 62, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, B.; Carmo, H.; Garrido, J.; Sousa Lobo, J.M.; Almeida, I.F. In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules 2021, 27, 189. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 495: Ros (Reactive Oxygen Species) Assay for Photoreactivity; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Rotava, P.A.; Favero, J.S.; Garcia, K.R.; Angeli, V.W. Profile of depigmenting cosmetics and dermatological products on the market. Rev. Cienc. Farm. Basica Apl. 2020, 41, 1–11. [Google Scholar] [CrossRef]
- Imanaga, Y. Autooxidation of L-Ascorbic Acid and Imidazole Nucleus: II. Decomposition Products of Imidazole Derivatives Present in the Autooxidation Mixture. J. Biochem. 1955, 42, 669–676. [Google Scholar] [CrossRef]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef]
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702. [Google Scholar] [CrossRef]
- Kaewdaungdee, S.; Chaveerach, P.D.A.; Tanee, T.; Siripiyasing, P.; Runglawan, S. Effect of dried ethanol extract of arbutin-containing leaves from Artocarpus on tyrosinase inhibition and postharvest preservation. ScienceAsia 2020, 46, 420. [Google Scholar] [CrossRef]
- Nokinsee, D.; Shank, L.; Lee, V.S.; Nimmanpipug, P. Estimation of Inhibitory Effect against Tyrosinase Activity through Homology Modeling and Molecular Docking. Enzym. Res. 2015, 2015, 262364. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Bang, S.H.; Kim, J.-H.; Shin, H.-J.; Choi, J.-H.; Chang, S.E. Tranexamic Acid Diminishes Laser-Induced Melanogenesis. Ann. Dermatol. 2015, 27, 250–256. [Google Scholar] [CrossRef] [PubMed]
- 김성우, 정.; 조병기. Cosmetic Composition for Skin-Whitening Comprising Ascorbic Acid 2-Glucoside as Active Ingredient. KR Patent 20030080869A, 11 April 2002. [Google Scholar]
- Shimada, Y.; Tai, H.; Tanaka, A.; Ikezawa-Suzuki, I.; Takagi, K.; Yoshida, Y.; Yoshie, H. Effects of ascorbic acid on gingival melanin pigmentation in vitro and in vivo. J. Periodontol. 2009, 80, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Review, C.I. Amended Safety Assessment of Hydroquinone as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2014. [Google Scholar]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Rosa, G.P.; Palmeira, A.; Resende, D.; Almeida, I.F.; Kane-Pagès, A.; Barreto, M.C.; Sousa, E.; Pinto, M.M.M. Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity. Bioorg. Med. Chem. 2021, 29, 115873. [Google Scholar] [CrossRef]
- Presern, A.; Pharm, M.; Glavac, N.K. Bakuchiol: A promising ingredient to help slow down time. Cos ACTIVE J. 2022, 2, 8–14. [Google Scholar]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
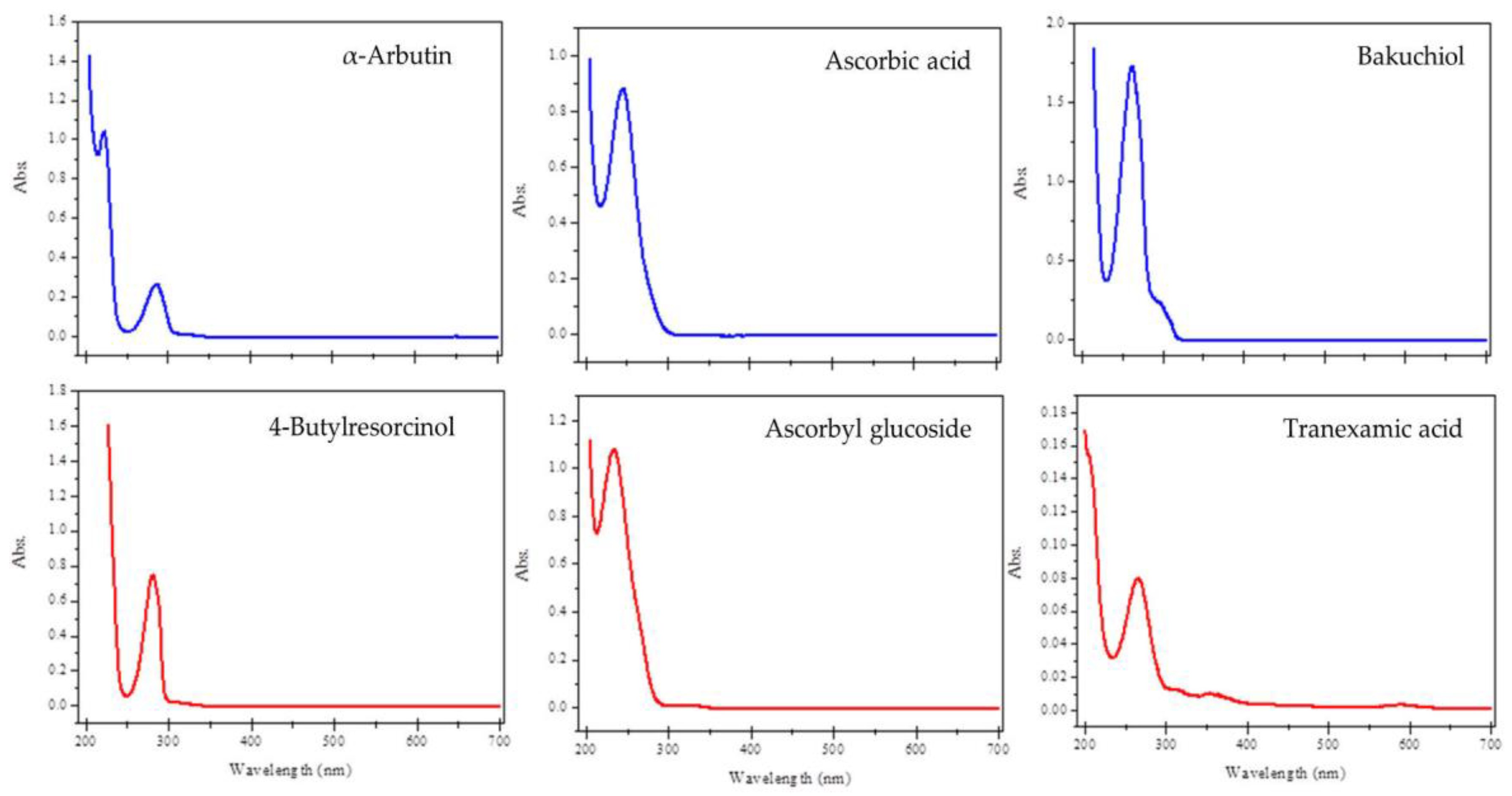
| Compound | Absorbance (10 µg mL−1) | MEC (L mol−1 cm−1) |
|---|---|---|
| ABT | 0.0736 | ε = 2004 |
| AA | 0.1586 | ε = 2793 |
| AA-2G | 0.0303 | ε = 1025 |
| BAK | 0.2203 | ε = 5648 |
| 4BR | 0.2088 | ε = 3471 |
| TA | 0.0242 | ε = 380 |
| Compound | 1O2 | O2•− |
|---|---|---|
| ABT | 19 ± 1 | 0 ± 0 |
| AA | 671 | 26 |
| AA-2G | 1 ± 1 | 1 ± 0 |
| BAK | inconclusive | inconclusive |
| 4BR | 1 ± 0 | 13 ± 1 |
| Compound | % Inhib (150 µM) | SD | IC50 (µM) | SD |
|---|---|---|---|---|
| TA | 13.20 | 1.99 | - | - |
| BAK | 52.59 | 2.04 | - | - |
| AA-2G | 11.17 | 0.84 | - | - |
| ABT | 27.04 | 1.74 | - | - |
| 4BR * | 100 | n.d. | 5.8 × 10−5 | 0.8 × 10−5 |
| Kojic acid ** | 99.15 | 0.64 | 12.80 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, S.; Rosa, G.P.; Barreto, M.C.; Garrido, J.; Sousa, E.; Cruz, M.T.; Almeida, I.F.; Quintas, C. Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents. Pharmaceuticals 2024, 17, 55. https://doi.org/10.3390/ph17010055
Mota S, Rosa GP, Barreto MC, Garrido J, Sousa E, Cruz MT, Almeida IF, Quintas C. Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents. Pharmaceuticals. 2024; 17(1):55. https://doi.org/10.3390/ph17010055
Chicago/Turabian StyleMota, Sandra, Gonçalo P. Rosa, Maria Carmo Barreto, Jorge Garrido, Emília Sousa, Maria T. Cruz, Isabel F. Almeida, and Clara Quintas. 2024. "Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents" Pharmaceuticals 17, no. 1: 55. https://doi.org/10.3390/ph17010055
APA StyleMota, S., Rosa, G. P., Barreto, M. C., Garrido, J., Sousa, E., Cruz, M. T., Almeida, I. F., & Quintas, C. (2024). Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents. Pharmaceuticals, 17(1), 55. https://doi.org/10.3390/ph17010055











