Therapeutic Drug Monitoring and Pharmacogenetic Testing as Guides to Psychotropic Drug Dose Adjustment: An Observational Study
Abstract
:1. Introduction
2. Results
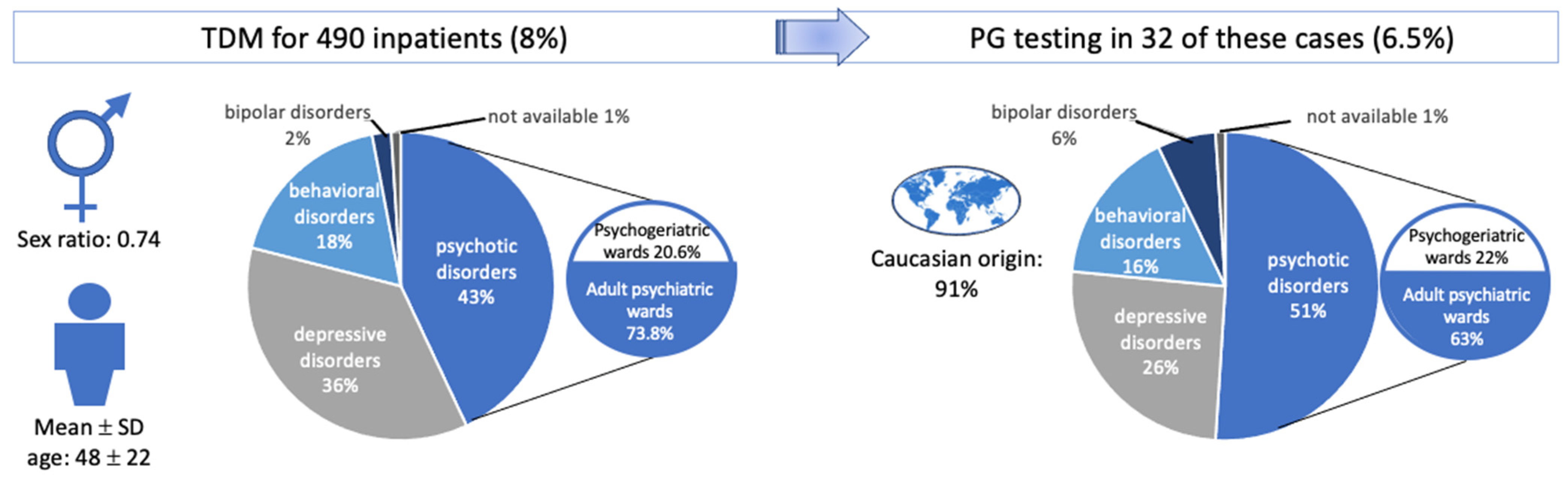
2.1. Characteristics of the Study Population
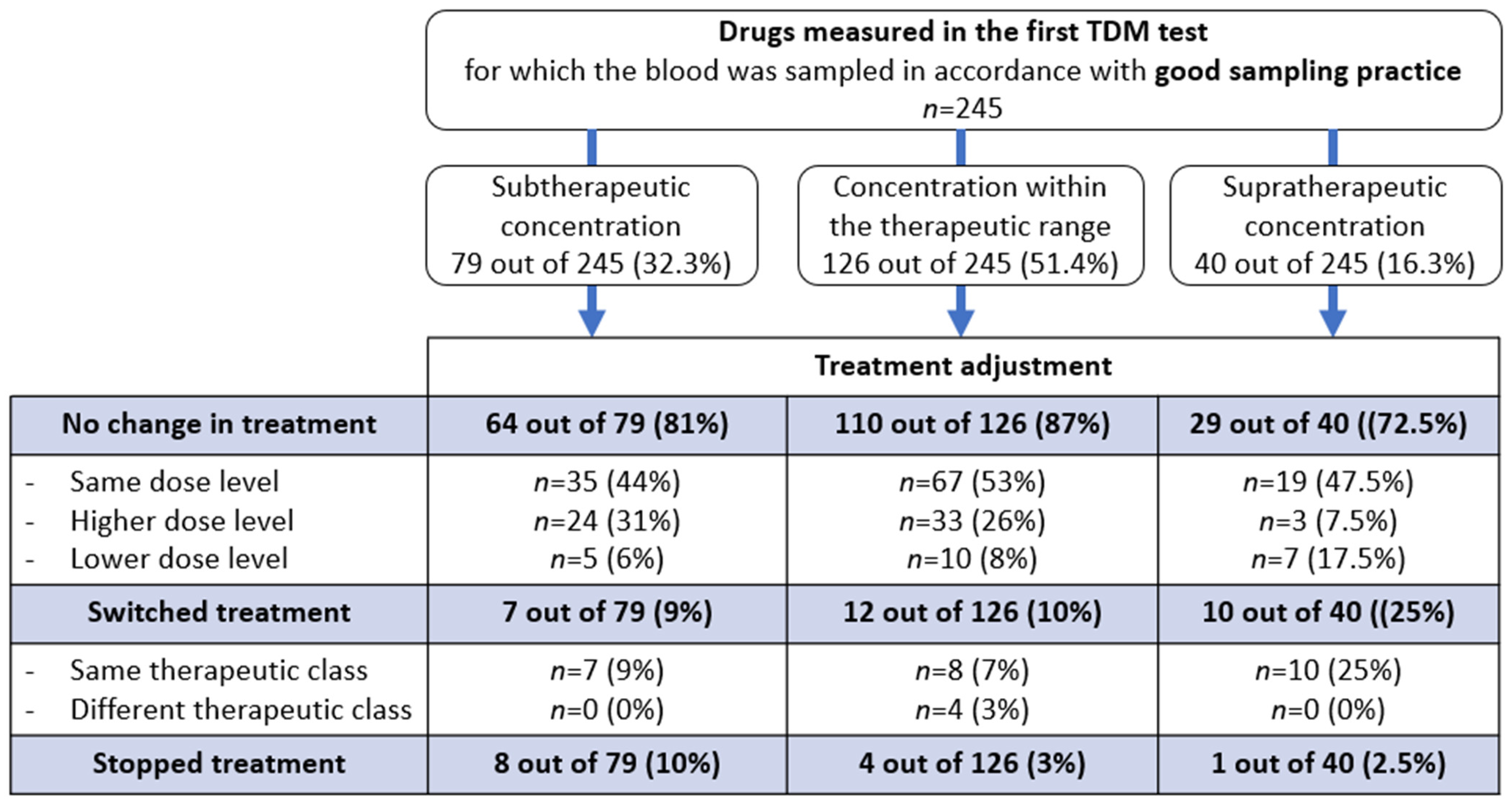
2.2. The Drug Management Process after TDM
2.3. The Drug Management Process after PG Testing
2.4. Use of the DDI-Predictor Tool to Adjust the Psychotropic Drug Regimen to the Patient’s Metabolic Status
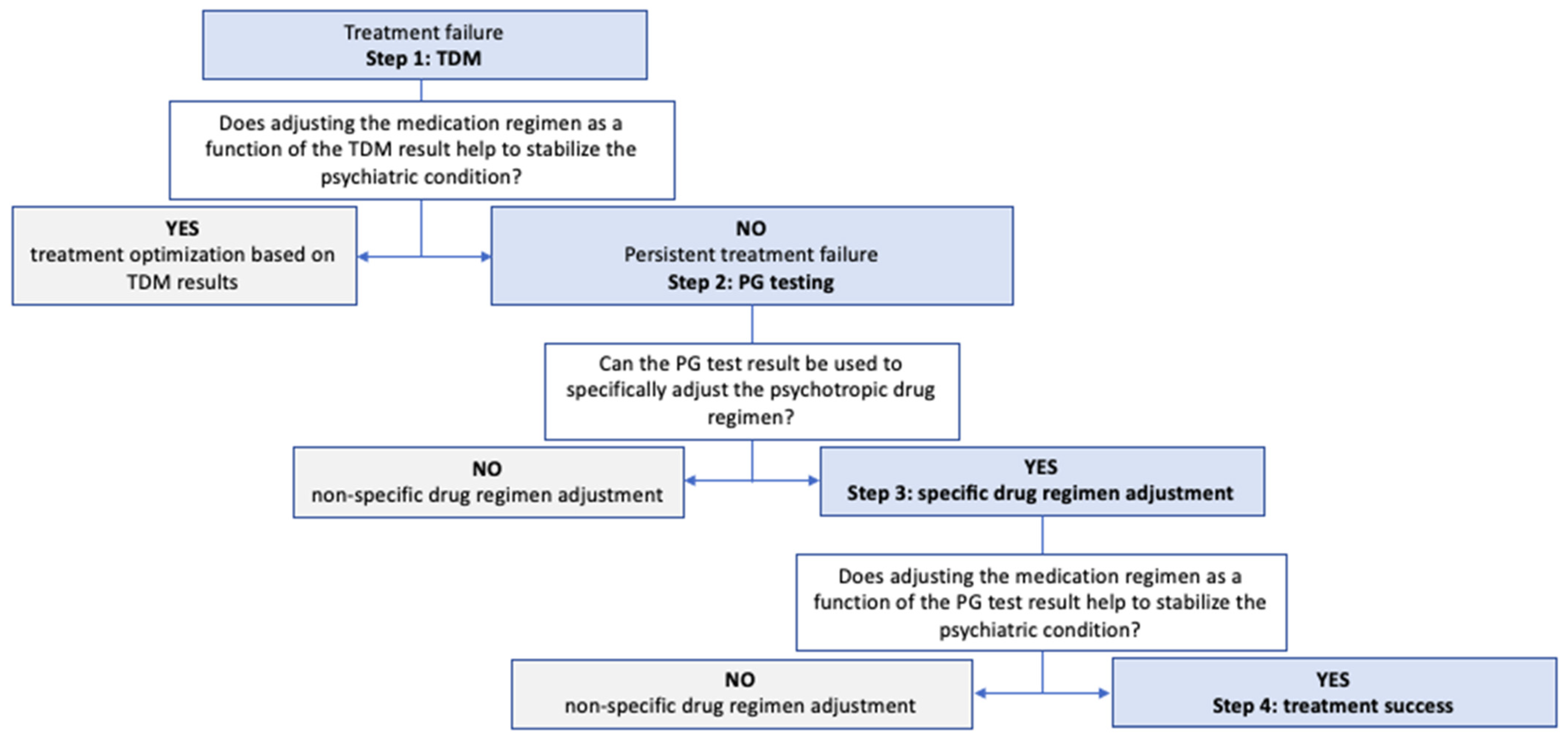
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Context
4.3. Therapeutic Drug Monitoring and PG Testing
4.4. Data Collection
4.5. Primary Objective
4.6. Secondary Objective
4.7. Data Presentation
4.8. Ethics Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGNP | Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie |
| AP | antipsychotic |
| ATDs | antidepressants |
| AUC | area under the curve |
| CYP | cytochrome P450 superfamily |
| DDI | drug–drug interaction |
| EM | extensive metabolizer |
| IM | intermediate metabolizer |
| PG | pharmacogenetic |
| PM | poor metabolizer |
| TDM | therapeutic drug monitoring |
| UM | ultra-rapid metabolizer |
References
- Uher, R. Genes, environment, and individual differences in responding to treatment for depression. Harv. Rev. Psychiatry 2011, 19, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Grolleau, A.; Cougnard, A.; Bégaud, B.; Verdoux, H. Usage et congruence diagnostique des traitements à visée psychotrope: Résultats de l’enquête santé mentale en population générale en France métropolitaine. Encephale 2008, 34, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Tebeka, S.; Airagnes, G.; Limosin, F. When and how prescribe antipsychotics? Rev. Med. Interne. 2017, 38, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hoehe, M.R.; Morris-Rosendahl, D.J. The role of genetics and genomics in clinical psychiatry. Dialogues Clin. Neurosci. 2018, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Turpeinen, M.; Klein, K.; Schwab, M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008, 392, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Samer, C.F.; Lorenzini, K.I.; Rollason, V.; Daali, Y.; Desmeules, J.A. Applications of CYP450 testing in the clinical setting. Mol. Diagn. Ther. 2013, 17, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; de Leon, J. Clinical applications of CYP genotyping in psychiatry. J. Neural Transm. 2015, 122, 5–28. [Google Scholar] [CrossRef]
- Murray, M. Role of CYP pharmacogenetics and drug-drug interactions in the efficacy and safety of atypical and other antipsychotic agents. J. Pharm. Pharmacol. 2006, 58, 871–885. [Google Scholar] [CrossRef]
- Crettol, S.; de Leon, J.; Hiemke, C.; Eap, C.B. Pharmacogenomics in psychiatry: From therapeutic drug monitoring to genomic medicine. Clin. Pharmacol. Ther. 2014, 95, 254–257. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; A Snyder, K.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; A Mrazek, D. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Hiemke, C.; Schmidt, L.G.; Bates, D.W. Appropriateness of therapeutic drug monitoring for antidepressants in routine psychiatric inpatient care. Ther. Drug Monit. 2006, 28, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017, a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Brøsen, K.; Dahl, M.L.; Gram, L.F.; Kasper, S.; Roots, I.; Sjöqvist, F.; Spina, E.; Brockmöller, J. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: A first step towards subpopulation-specific dosages. Acta Psychiatr. Scand. 2001, 104, 173–192, Erratum in: Acta Psychiatr. Scand. 2001, 104, 475. [Google Scholar] [CrossRef]
- Steimer, W. Pharmacogenetics and psychoactive drug therapy: Ready for the patient? Ther. Drug Monit. 2010, 32, 381–386. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; Llerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar]
- Quaranta, S.; Dupouey, J.; Colle, R.; Verstuyft, C. Pharmacogénétique des médicaments antidépresseurs: État des connaissances et des pratiques—Recommandations du Réseau national de pharmacogénétique (RNPGx). Therapies 2017, 72, 301–309. [Google Scholar] [CrossRef]
- Moreau, F.; Simon, N.; Walther, J.; Dambrine, M.; Kosmalski, G.; Genay, S.; Perez, M.; Lecoutre, D.; Belaiche, S.; Rousselière, C.; et al. Does DDI-Predictor Help Pharmacists to Detect Drug-Drug Interactions and Resolve Medication Issues More Effectively? Metabolites 2021, 11, 173. [Google Scholar] [CrossRef]
- Vuille, F.; Amey, M.; Baumann, P. Use of plasma level monitoring of antidepressants in clinical practice. Towards an analysis of clinical utility. Pharmacopsychiatry 1991, 24, 190–195. [Google Scholar] [CrossRef]
- Zernig, G.; Lechner, T.; Kramer-Reinstadler, K.; Hinterhuber, H.; Hiemke, C.; Saria, A. What the clinician still has to be reminded of. Ther. Drug Monit. 2004, 26, 582. [Google Scholar] [CrossRef] [PubMed]
- Almohammde, S.; Alhodian, H.; Almofareh, S.; Alshehri, S.; Almasri, D.M.; Ghoneim, R.H. A survey of therapeutic drug monitoring in a teaching hospital. Saudi J. Biol. Sci. 2021, 28, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Tod, M.; Bourguignon, L.; Bleyzac, N.; Goutelle, S. A model for predicting the interindividual variability of drug-drug interactions. AAPS J. 2017, 19, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G.; et al. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry 2011, 44, 195–235. [Google Scholar] [CrossRef]
- Tod, M.; Nkoud-Mongo, C.; Gueyffier, F. Impact of genetic polymorphism on drug-drug interactions mediated by cytochromes: A general approach. AAPS J. 2013, 15, 1242–1252. [Google Scholar] [CrossRef]
- Henriques, B.C.; Yang, E.H.; Lapetina, D.; Carr, M.S.; Yavorskyy, V.; Hague, J.; Aitchison, K.J. How Can Drug Metabolism and Transporter Genetics Inform Psychotropic Prescribing? Front. Genet. 2020, 11, 491895. [Google Scholar] [CrossRef]
| Indication for TDM | TDM Result | |||
|---|---|---|---|---|
| Total (n = 27) | Subtherapeutic Drug Level (n = 15) | Optimal Drug Level (n = 2) | Supratherapeutic Drug Level (n = 10) | |
| Lack of a response at the therapeutic dose (suggested dose adjustment or drug switch) | 18 (67%) | 11 (61%) | 1 (6%) | 6 (33%) |
| Potentially poor adherence | 5 (18%) | 3 (75%) | 0 (0%) | 2 (25%) |
| Suboptimal drug tolerance | 4 (15%) | 1 (25%) | 1 (25%) | 2 (50%) |
| Orally Administered Psychotropic Drugs Related to the PG Testing | Reason for PG Testing | CYP Isoform Studied and Phenotype | Expected Plasma Concentration, According to the Genotype Analyzed | Dose/Drug Adjustment after the Metabolic Status Result | Major CYP Isoform Responsible for Metabolism of New Treatment |
|---|---|---|---|---|---|
| The metabolic status could explain the treatment failure in 12 of the 30 cases (40%) | |||||
| Clozapine | High drug concentration (n = 1) | CYP2D6 IM, CYP1A2 IM | Elevated plasma concentration | * Dose reduction (from 200 mg to 175 mg) | |
| Lack of a response at the therapeutic dose (n = 1) | CYP1A2 UM | Low plasma concentration | * Switch to haloperidol (SC) | 3A4; 2D6 | |
| Clozapine and aripiprazole | Suboptimal tolerance; high concentration of aripiprazole (n = 1) | CYP2D6 IM, CYP4A5 IM | Elevated plasma concentration | No change | |
| Olanzapine | Low drug concentration (n = 1) | CYP1A2 UM, CYP2D6 IM | Low plasma concentration | Dose increase (from 40 mg to 60 mg) | |
| Olanzapine and aripiprazole | Suboptimal tolerance; high concentration (n = 1) | CYP2D6 IM, CYP4A5 IM | Elevated plasma concentration | Split the daily dose (from 20 mg 1/d to 10 mg × 2/d) | |
| Paroxetine | High drug concentration (n = 4) | CYP2D6 PM (n = 1) | Elevated plasma concentration | Switch to sertraline | 2C19 |
| CYP2D6 IM (n = 3) | Dose reduction (from 60 mg to 40 mg) | ||||
| * Switch to citalopram (1/4) | 2C19 | ||||
| * Switch to sertraline (1/4) | 2C19 | ||||
| Quetiapine | Low drug concentration (n = 1) | CYP3A5 EM (partial explanation) | Low plasma concentration | No change | |
| Risperidone | Suboptimal tolerance (n = 1) | CYP2D6 IM | Elevated plasma concentration | Dose reduction (from 2 mg to 1.5 mg) | |
| Venlafaxine | Suboptimal tolerance (n = 1) | CYP2D6 IM | Elevated plasma concentration | Switch to escitalopram | 2C19 |
| The metabolic status could not explain the treatment failure and TDM results in 18 of the 30 cases (60%) | |||||
| Clozapine | Lack of a response at the therapeutic dose and high drug concentration (n = 1) | CYP1A2 UM (smoker) | Low plasma concentration | Tobacco stop suggested | |
| Non-response at therapeutic doses (n = 1) | CYP1A2 UM (non smoker) | Low plasma concentration | No change | ||
| Clozapine and escitalopram | Low drug concentration (n = 1) | CYP2D6 IM CYP3A5 IM | Elevated plasma concentration | Switch to clozapine and clomipramine | 1A2; 3A4 |
| Fluoxetine (+1: unknown) | Lack of a response at the therapeutic dose (n = 2) | None (n = 2) | No variation | Switch to amitriptyline | 2C19; 2D6 |
| Switch to clomipramine | 2C19 | ||||
| Paroxetine | High drug concentration (n = 1) | None | No variation | * Switch to venlafaxine | 2D6 |
| Quetiapine | Low drug concentration (n = 6) | CYP2D6 IM | Elevated plasma concentration | No change (n = 2) | |
| CYP2D6 IM, CYP2C19 IM, CYP3A5 IM | * Switch to lithium carbonate | none | |||
| None (n = 3) | No variation | * Switch to amisulpride | none | ||
| Switch to olanzapine | 1A2 | ||||
| Switch to amisulpride | none | ||||
| Risperidone | Lack of a response at the therapeutic dose (n = 2) | CYP1A2 UM (smoker) CYP2D6 IM | Elevated plasma concentration | * Switch to paliperidone (IM) | none |
| None | No variation | * Switch to paliperidone (IM) | none | ||
| Low drug concentration (n = 4) | CYP2D6 IM (n = 2) | Elevated plasma concentration | No change | ||
| CYP2D6 EM (n = 2) | No variation | Switch to olanzapine IM | 1A2 | ||
| No change | |||||
| Psychotic Drug | Genotype *X/*X | Rd | RAUC with Tolerance Interval (5th to 95th Percentiles) |
|---|---|---|---|
| Paroxetine | CYP2D6*1/*4 or *2/*4 | 60/40 = 1.5 | 1.6 [1.09–2.36] |
| Quetiapine | CYP2D6*1/*2 | 1200/1200 = 1 | 1 [0.76–1.32] |
| CYP2D6*1/*4 or *2/*4 | 900/900 = 1 | 1.09 [0.82–1.45] | |
| Risperidone | CYP2D6*1/*2 | 4/4 = 1 | 1 [0.76–1.32] |
| CYP2D6*1/*4 or *2/*4 | 2/1.5 = 1.33 | 1.66 [1.11–2.48] | |
| Olanzapine and aripiprazole | CYP2D6*1/*4 or *2/*4 | 20/20 = 1 20/20 = 1 | 1.09 [0.82–1.45] 1.22 [0.9–1.66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuvelier, E.; Khazri, H.; Lecluse, C.; Hennart, B.; Amad, A.; Roche, J.; Tod, M.; Vaiva, G.; Cottencin, O.; Odou, P.; et al. Therapeutic Drug Monitoring and Pharmacogenetic Testing as Guides to Psychotropic Drug Dose Adjustment: An Observational Study. Pharmaceuticals 2024, 17, 21. https://doi.org/10.3390/ph17010021
Cuvelier E, Khazri H, Lecluse C, Hennart B, Amad A, Roche J, Tod M, Vaiva G, Cottencin O, Odou P, et al. Therapeutic Drug Monitoring and Pharmacogenetic Testing as Guides to Psychotropic Drug Dose Adjustment: An Observational Study. Pharmaceuticals. 2024; 17(1):21. https://doi.org/10.3390/ph17010021
Chicago/Turabian StyleCuvelier, Elodie, Houda Khazri, Cloé Lecluse, Benjamin Hennart, Ali Amad, Jean Roche, Michel Tod, Guillaume Vaiva, Olivier Cottencin, Pascal Odou, and et al. 2024. "Therapeutic Drug Monitoring and Pharmacogenetic Testing as Guides to Psychotropic Drug Dose Adjustment: An Observational Study" Pharmaceuticals 17, no. 1: 21. https://doi.org/10.3390/ph17010021
APA StyleCuvelier, E., Khazri, H., Lecluse, C., Hennart, B., Amad, A., Roche, J., Tod, M., Vaiva, G., Cottencin, O., Odou, P., Allorge, D., Décaudin, B., & Simon, N. (2024). Therapeutic Drug Monitoring and Pharmacogenetic Testing as Guides to Psychotropic Drug Dose Adjustment: An Observational Study. Pharmaceuticals, 17(1), 21. https://doi.org/10.3390/ph17010021






