Derivation and Clinical Utility of Safety Targets for Linezolid-Related Adverse Events in Drug-Resistant Tuberculosis Treatment
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Exposure–Safety Relationship
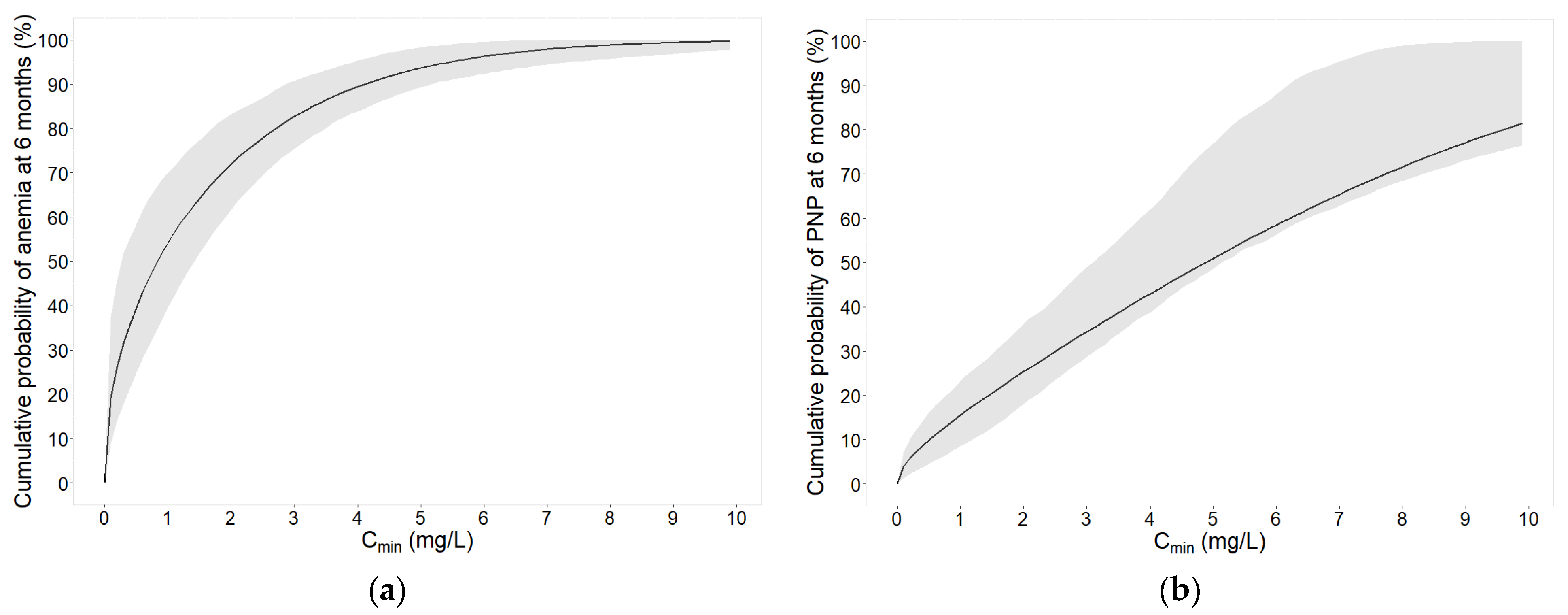
2.2.1. Cmin for Anemia and Peripheral Neuropathy
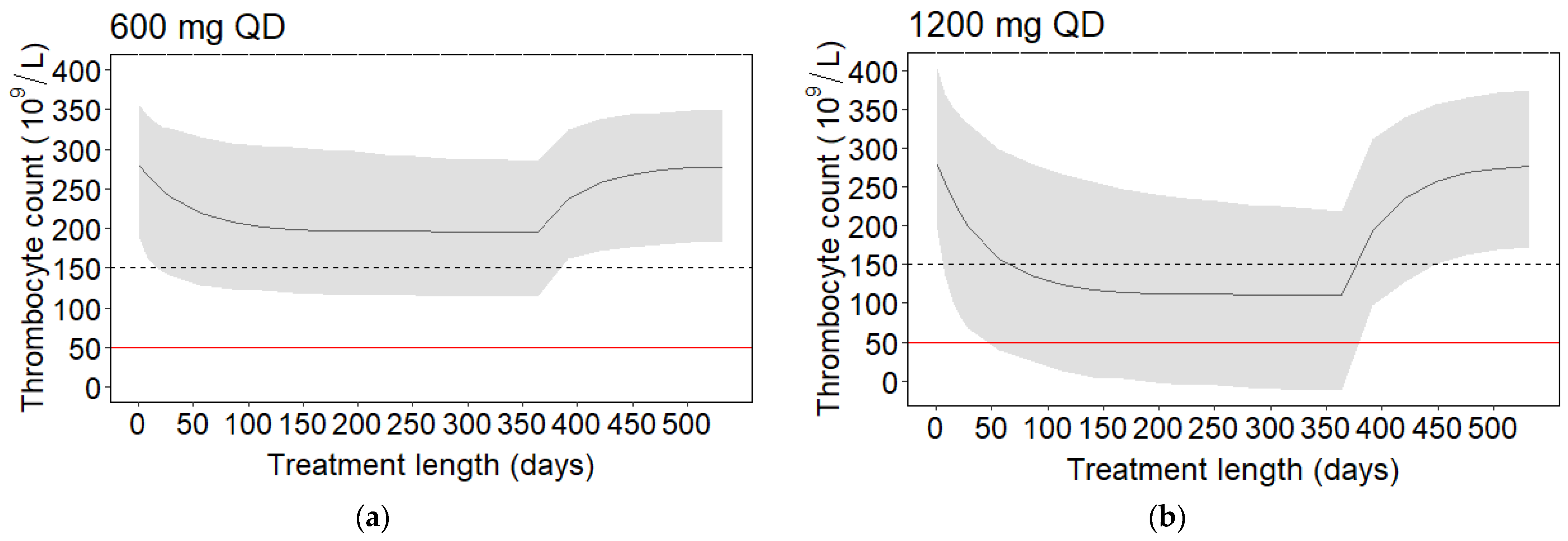
2.2.2. AUC0–24h for Thrombocytopenia
2.2.3. Dropout
2.2.4. Safety Targets and Simulations
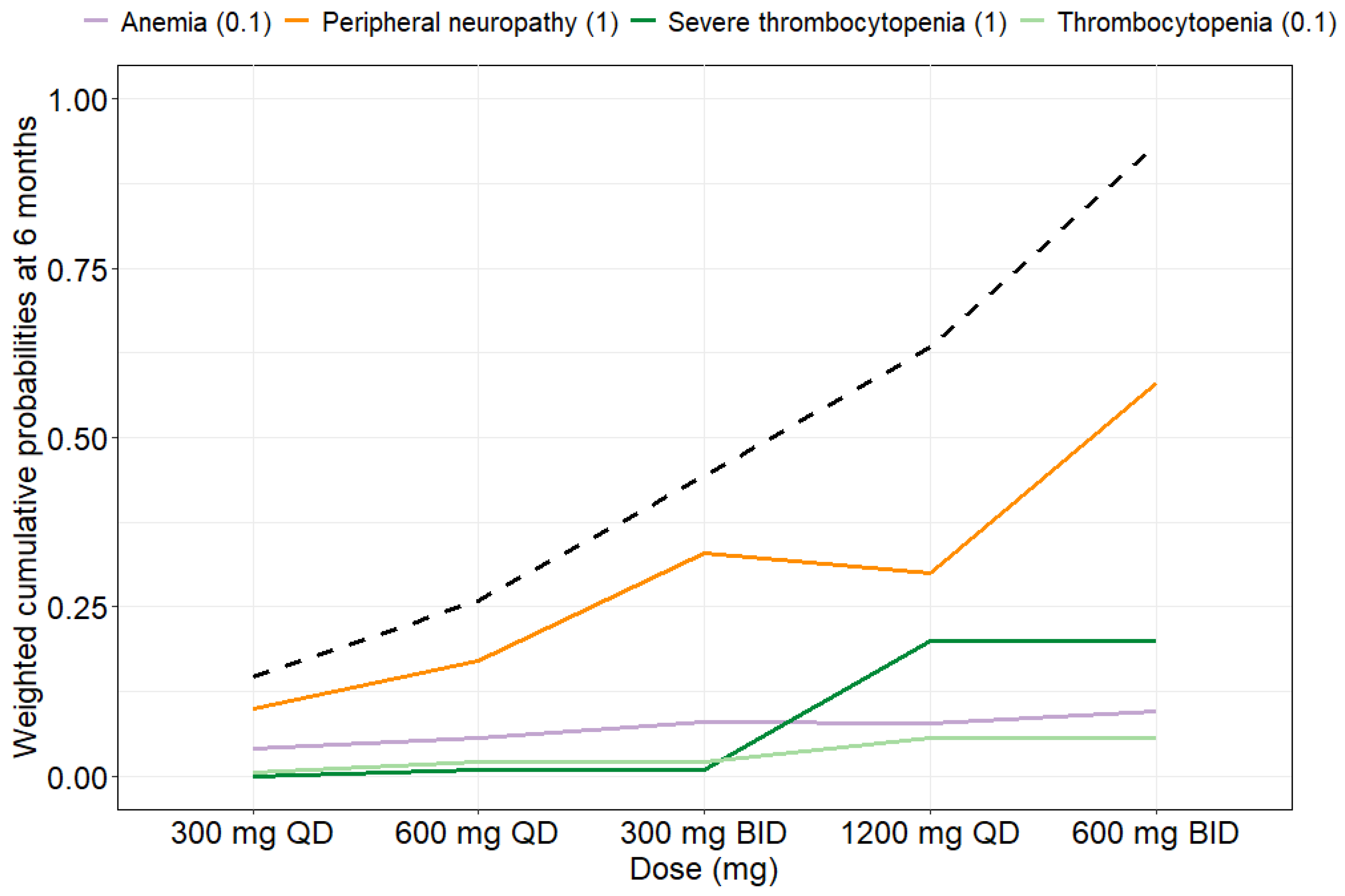
2.2.5. Clinical Utility Assessment
3. Discussion
4. Materials and Methods
4.1. Patients and Data
4.2. Pharmacokinetic Data and Exposure Indices
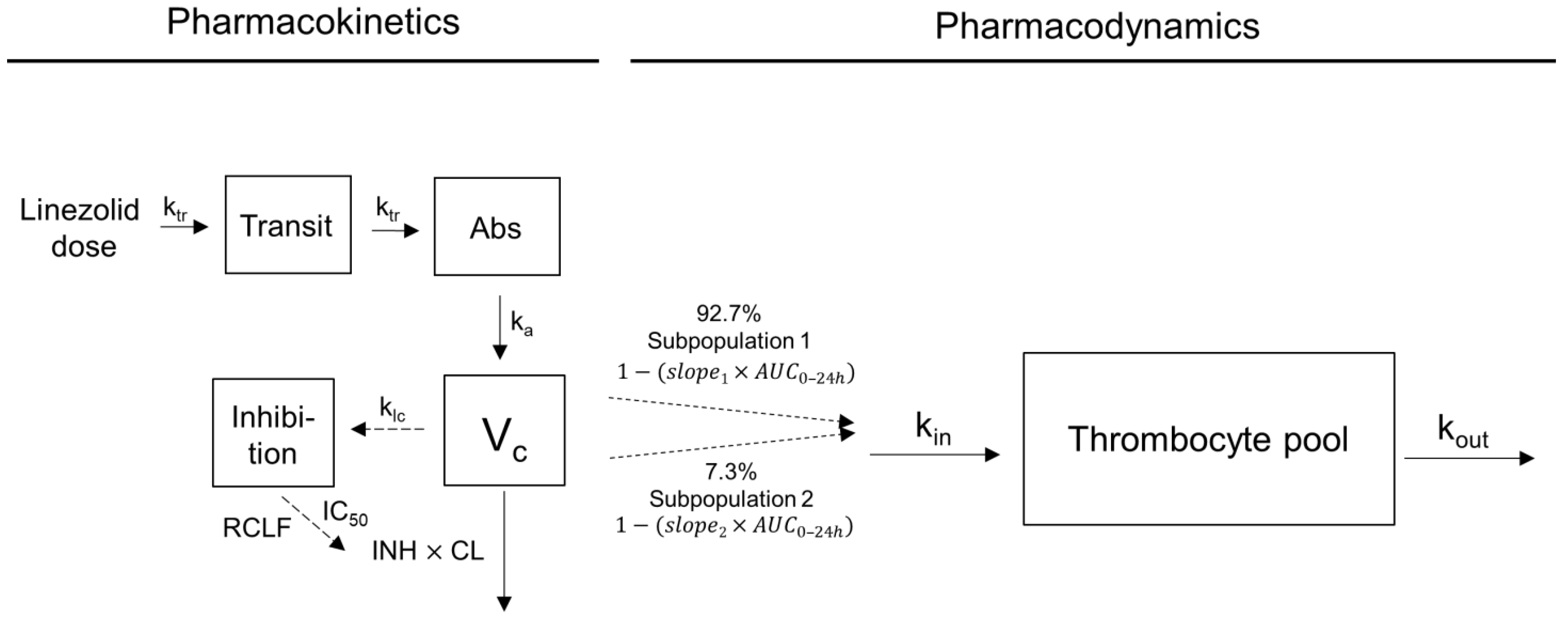
4.3. Exposure–Response Analysis
4.3.1. TTE Modelling
4.3.2. Indirect Response Modelling
4.4. Dropout Model
4.5. Model Evaluation
4.6. Safety Target Derivation
4.7. Clinical Utility Index
4.8. Software
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 7 June 2023).
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Nyang’wa, B.-T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Solodovnikova, V.; Liverko, I.; Moodliar, R.; Dodd, M.; et al. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update. Available online: https://www.who.int/publications-detail-redirect/9789240063129 (accessed on 25 July 2023).
- Millard, J.; Pertinez, H.; Bonnett, L.; Hodel, E.M.; Dartois, V.; Johnson, J.L.; Caws, M.; Tiberi, S.; Bolhuis, M.; Alffenaar, J.-W.C.; et al. Linezolid Pharmacokinetics in MDR-TB: A Systematic Review, Meta-Analysis and Monte Carlo Simulation. J. Antimicrob. Chemother. 2018, 73, 1755–1762. [Google Scholar] [CrossRef]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.-W.; Marriott, D.J.E.; Neely, M. Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics. Ther. Drug Monitor. 2020, 42, 83–92. [Google Scholar] [CrossRef]
- Song, T.; Lee, M.; Jeon, H.-S.; Park, Y.; Dodd, L.E.; Dartois, V.; Follman, D.; Wang, J.; Cai, Y.; Goldfeder, L.C.; et al. Linezolid Trough Concentrations Correlate with Mitochondrial Toxicity-Related Adverse Events in the Treatment of Chronic Extensively Drug-Resistant Tuberculosis. EBioMedicine 2015, 2, 1627–1633. [Google Scholar] [CrossRef]
- Leach, K.L.; Swaney, S.M.; Colca, J.R.; McDonald, W.G.; Blinn, J.R.; Thomasco, L.M.; Gadwood, R.C.; Shinabarger, D.; Xiong, L.; Mankin, A.S. The Site of Action of Oxazolidinone Antibiotics in Living Bacteria and in Human Mitochondria. Mol. Cell 2007, 26, 393–402. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Van Coster, R.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.-J.; Ceuterick-de Groote, C.; Vandecasteele, S.; et al. Linezolid-Induced Inhibition of Mitochondrial Protein Synthesis. Clin. Infect. Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Cojutti, P.; Del Pin, B.; Zamparini, E.; Furlanut, M. Therapeutic Drug Monitoring May Improve Safety Outcomes of Long-Term Treatment with Linezolid in Adult Patients. J. Antimicrob. Chemother. 2012, 67, 2034–2042. [Google Scholar] [CrossRef]
- Nukui, Y.; Hatakeyama, S.; Okamoto, K.; Yamamoto, T.; Hisaka, A.; Suzuki, H.; Yata, N.; Yotsuyanagi, H.; Moriya, K. High Plasma Linezolid Concentration and Impaired Renal Function Affect Development of Linezolid-Induced Thrombocytopenia. J. Antimicrob. Chemother. 2013, 68, 2128–2133. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shigemi, A.; Takeshita, A.; Watanabe, E.; Yokoyama, Y.; Ikawa, K.; Morikawa, N.; Takeda, Y. Analysis of Thrombocytopenic Effects and Population Pharmacokinetics of Linezolid: A Dosage Strategy According to the Trough Concentration Target and Renal Function in Adult Patients. Int. J. Antimicrob. Agents 2014, 44, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Jaspard, M.; Butel, N.; El Helali, N.; Marigot-Outtandy, D.; Guillot, H.; Peytavin, G.; Veziris, N.; Bodaghi, B.; Flandre, P.; Petitjean, G.; et al. Linezolid-Associated Neurologic Adverse Events in Patients with Multidrug-Resistant Tuberculosis, France. Emerg. Infect. Dis. 2020, 26, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Graciaa, D.S.; Kipiani, M.; Magee, M.J.; Mikiashvili, L.; Barbakadze, K.; Bablishvili, N.; Auld, S.C.; Alghamdi, W.A.; Alshaer, M.H.; Peloquin, C.A.; et al. Linezolid Exposure Is Associated with Cytopenias in Patients Treated for Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2022, 66, e0040822. [Google Scholar] [CrossRef] [PubMed]
- Eimer, J.; Fréchet-Jachym, M.; Le Dû, D.; Caumes, E.; El-Helali, N.; Marigot-Outtandy, D.; Mechai, F.; Peytavin, G.; Pourcher, V.; Rioux, C.; et al. Association Between Increased Linezolid Plasma Concentrations and the Development of Severe Toxicity in Multidrug-Resistant Tuberculosis Treatment. Clin. Infect. Dis. 2023, 76, e947–e956. [Google Scholar] [CrossRef]
- Wasserman, S.; Brust, J.C.M.; Abdelwahab, M.T.; Little, F.; Denti, P.; Wiesner, L.; Gandhi, N.R.; Meintjes, G.; Maartens, G. Linezolid Toxicity in Patients with Drug-Resistant Tuberculosis: A Prospective Cohort Study. J. Antimicrob. Chemother. 2022, 77, 1146–1154. [Google Scholar] [CrossRef]
- Deshpande, D.; Srivastava, S.; Pasipanodya, J.G.; Bush, S.J.; Nuermberger, E.; Swaminathan, S.; Gumbo, T. Linezolid for Infants and Toddlers With Disseminated Tuberculosis: First Steps. Clin. Infect. Dis. 2016, 63, S80–S87. [Google Scholar] [CrossRef][Green Version]
- Bolhuis, M.S.; Akkerman, O.W.; Sturkenboom, M.G.G.; Ghimire, S.; Srivastava, S.; Gumbo, T.; Alffenaar, J.-W.C. Linezolid-Based Regimens for Multidrug-Resistant Tuberculosis (TB): A Systematic Review to Establish or Revise the Current Recommended Dose for TB Treatment. Clin. Infect. Dis. 2018, 67, S327–S335. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Miettinen, O.S. Survival Analysis: Up from Kaplan-Meier-Greenwood. Eur. J. Epidemiol. 2008, 23, 585–592. [Google Scholar] [CrossRef]
- Mockeliunas, L.; Keutzer, L.; Sturkenboom, M.G.G.; Bolhuis, M.S.; Hulskotte, L.M.G.; Akkerman, O.W.; Simonsson, U.S.H. Model-Informed Precision Dosing of Linezolid in Patients with Drug-Resistant Tuberculosis. Pharmaceutics 2022, 14, 753. [Google Scholar] [CrossRef]
- Keizer, R.J.; Heine, R.t.; Frymoyer, A.; Lesko, L.J.; Mangat, R.; Goswami, S. Model-Informed Precision Dosing at the Bedside: Scientific Challenges and Opportunities. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Merelli, M.; Bassetti, M.; Pea, F. Proactive Therapeutic Drug Monitoring (TDM) May Be Helpful in Managing Long-Term Treatment with Linezolid Safely: Findings from a Monocentric, Prospective, Open-Label, Interventional Study. J. Antimicrob. Chemother. 2019, 74, 3588–3595. [Google Scholar] [CrossRef]
- Cattaneo, D.; Gervasoni, C.; Cozzi, V.; Castoldi, S.; Baldelli, S.; Clementi, E. Therapeutic Drug Management of Linezolid: A Missed Opportunity for Clinicians? Int. J. Antimicrob. Agents 2016, 48, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, M.S.; van der Werf, T.S.; Kerstjens, H.A.M.; de Lange, W.C.M.; Alffenaar, J.-W.C.; Akkerman, O.W. Treatment of Multidrug-Resistant Tuberculosis Using Therapeutic Drug Monitoring: First Experiences with Sub-300 Mg Linezolid Dosages Using in-House Made Capsules. Eur. Respir. J. 2019, 54, 1900580. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, M.S.; Akkerman, O.W.; Sturkenboom, M.G.G.; de Lange, W.C.M.; van der Werf, T.S.; Alffenaar, J.-W.C. Individualized Treatment of Multidrug-Resistant Tuberculosis Using Therapeutic Drug Monitoring. Int. J. Mycobacteriol. 2016, 5, S44–S45. [Google Scholar] [CrossRef]
- Srivastava, S.; Magombedze, G.; Koeuth, T.; Sherman, C.; Pasipanodya, J.G.; Raj, P.; Wakeland, E.; Deshpande, D.; Gumbo, T. Linezolid Dose That Maximizes Sterilizing Effect While Minimizing Toxicity and Resistance Emergence for Tuberculosis. Antimicrob. Agents Chemother. 2017, 61, 8. [Google Scholar] [CrossRef]
- Cui, D.; Hu, X.; Shi, L.; Wang, D.; Chen, G. Linezolid-Related Adverse Effects in the Treatment of Rifampicin Resistant Tuberculosis: A Retrospective Study. J. Chemother. 2023, 35, 404–410. [Google Scholar] [CrossRef]
- Ouellet, D. Benefit-Risk Assessment: The Use of Clinical Utility Index. Expert. Opin. Drug Saf. 2010, 9, 289–300. [Google Scholar] [CrossRef]
- Nyberg, J.; Karlsson, K.; Jönsson, S.; Yin, O.; Miller, R.; Karlsson, M.; Simonsson, U. Edoxaban Exposure-Response Analysis and Clinical Utility Index Assessment in Patients With Symptomatic Deep-Vein Thrombosis or Pulmonary Embolism. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 222–232. [Google Scholar] [CrossRef]
- Alghamdi, W.A.; Al-Shaer, M.H.; An, G.; Alsultan, A.; Kipiani, M.; Barbakadze, K.; Mikiashvili, L.; Ashkin, D.; Griffith, D.E.; Cegielski, J.P.; et al. Population Pharmacokinetics of Linezolid in Tuberculosis Patients: Dosing Regimens Simulation and Target Attainment Analysis. Antimicrob. Agents Chemother. 2020, 64, 10. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Cojutti, P.; Cristini, F.; Zamparini, E.; Franceschi, L.; Viale, P. Therapeutic Drug Monitoring of Linezolid: A Retrospective Monocentric Analysis. Antimicrob. Agents Chemother. 2010, 54, 4605–4610. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, M.A.; Friberg, L.E.; Simonsson, U.S.H. Performance of Nonlinear Mixed Effects Models in the Presence of Informative Dropout. AAPS J. 2014, 17, 245–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siddiqui, O.; Hung, H.M.J.; O’Neill, R. MMRM vs. LOCF: A Comprehensive Comparison Based on Simulation Study and 25 NDA Datasets. J. Biopharm. Stat. 2009, 19, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Inference and Missing Data. Biometrika 1976, 63, 581–592. [Google Scholar] [CrossRef]
- Laird, N.M. Missing Data in Longitudinal Studies. Stat. Med. 1988, 7, 305–315. [Google Scholar] [CrossRef]
- Pharmacia & Upjohn Company Clinical Pharmacology and Biopharmaceutics Review(s). Zyvox Tablets, I.V. & Oral Suspensions (Linezolid) 1999. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-130S003_Zyvox_biopharmr.PDF (accessed on 3 September 2023).
- Harmelink, I.M.; Alffenaar, J.-W.; Wessels, A.M.A. A Rapid and Simple Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Linezolid in Human Serum. Eur. J. Hosp. Pharm. 2008, 14, 3–7. [Google Scholar]
- Trotti, A.; Colevas, A.D.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N.; et al. CTCAE v3.0: Development of a Comprehensive Grading System for the Adverse Effects of Cancer Treatment. Semin. Radiat. Oncol. 2003, 13, 176–181. [Google Scholar] [CrossRef]
- Sasaki, T.; Takane, H.; Ogawa, K.; Isagawa, S.; Hirota, T.; Higuchi, S.; Horii, T.; Otsubo, K.; Ieiri, I. Population Pharmacokinetic and Pharmacodynamic Analysis of Linezolid and a Hematologic Side Effect, Thrombocytopenia, in Japanese Patients. Antimicrob. Agents Chemother. 2011, 55, 1867–1873. [Google Scholar] [CrossRef]
- Boak, L.M.; Rayner, C.R.; Grayson, M.L.; Paterson, D.L.; Spelman, D.; Khumra, S.; Capitano, B.; Forrest, A.; Li, J.; Nation, R.L.; et al. Clinical Population Pharmacokinetics and Toxicodynamics of Linezolid. Antimicrob. Agents Chemother. 2014, 58, 2334–2343. [Google Scholar] [CrossRef]
- Tsuji, Y.; Holford, N.H.G.; Kasai, H.; Ogami, C.; Heo, Y.; Higashi, Y.; Mizoguchi, A.; To, H.; Yamamoto, Y. Population Pharmacokinetics and Pharmacodynamics of Linezolid-induced Thrombocytopenia in Hospitalized Patients. Br. J. Clin. Pharmacol. 2017, 83, 1758–1772. [Google Scholar] [CrossRef]
- Pascoalinho, D.; Vilas, M.J.; Coelho, L.; Moreira, P. Linezolid-Related Immune-Mediated Severe Thrombocytopenia. Int. J. Antimicrob. Agents 2011, 37, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, W.B.; Trotta, R.F.; Rector, J.T.; Tjaden, J.A.; Barile, A.J. Mechanisms for Linezolid-Induced Anemia and Thrombocytopenia. Ann. Pharmacother. 2003, 37, 517–520. [Google Scholar] [CrossRef]
- Beal, S.; Sheiner, L.; Boeckmann, A.; Bauer, R. NONMEM 7.4 Users Guides; ICON plc: Gaithersburg, MD, USA, 1989. [Google Scholar]
- Carlsson, K.C.; Savic, R.M.; Hooker, A.C.; Karlsson, M.O. Modeling Subpopulations with the $MIXTURE Subroutine in NONMEM: Finding the Individual Probability of Belonging to a Subpopulation for the Use in Model Analysis and Improved Decision Making. AAPS J. 2009, 11, 148–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keizer, R.J.; Karlsson, M.O.; Hooker, A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e50. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]

| Parameter | Unit | All Patients |
|---|---|---|
| N | 75 | |
| Mean weight (range) | kg | 60.7 (35.3–88.9) |
| Mean height (range) | m2 | 1.69 (1.50–1.93) |
| Mean creatinine clearance (range) | (mL/min) | 116.1 (40.7–150.0) a,b |
| Mean age (range) | years | 32 (15–70) |
| Mean body mass index (range) | kg/m2 | 21.2 (15.5–32.6) |
| No. of male sex | n (%) | 42 (56) |
| No. with HIV | n (%) | 5 (7) |
| No. with Diabetes | n (%) | 9 (12) |
| No. Smoking | n (%) | 28 (38) |
| No. Alcohol abuse | n (%) | 6 (8) |
| No. Pregnancy | n (%) | 4 (5) |
| No. from indicated WHO region | n (%) | |
| African Region | 11 (15) | |
| Region of the Americas | 2 (3) | |
| South-East Asia Region | 6 (8) | |
| European Region | 28 (37) | |
| Eastern Mediterranean Region | 17 (23) | |
| Western Pacific Region | 11 (15) |
| Safety Endpoint | No. of Excluded Patients Due to Missing Information | No. of Excluded Patients Due to Adverse Event at Start of Treatment | Patients at Risk | No. of First Events (%) |
|---|---|---|---|---|
| Anemia a | 0 | 37 | 38 | 22 (58) |
| Severe anemia b | 0 | 0 | 75 | 1 (1) |
| Thrombocytopenia c | 0 | 2 | 73 | 15 (21) |
| Severe thrombocytopenia d | 0 | 2 | 73 | 1 (1) |
| Peripheral neuropathy | 25 | 0 | 50 | 13 (26) |
| Optic neuritis | 0 | 0 | 75 | 1 (1) |
| Anemia | Peripheral Neuropathy | ||
|---|---|---|---|
| Parameter | Description | Estimate (90% CI) a | Estimate (90% CI) a |
| λ (day−1) | Scale factor of the Gompertz distribution | 0.004 (0.002–0.009) | 0.0006 (0.0002–0.0013) |
| γ | Shape factor of the Gompertz distribution | −0.0056 (−0.015–−0.001) | −0.0011 (−0.0060–0.0032) |
| βER·(Cmin) [(mg/L)−1] | Coefficient describing the exposure–response relationship as risk factor | 0.417 (0.258–0.631) | 0.464 (0.355–0.751) |
| Parameter | Description | Typical Value (90% CI) a | IIV b (90% CI) a |
|---|---|---|---|
| kin (109/L/day) | Zero-order input rate | 6.72 (0.60–12.83) | 1.82 (0.86–2.78) |
| base (109/L) | Baseline thrombocyte count | 279 (261.87–296.14) | 0.0789 (0.05–0.11) |
| Slope1 (mg−1·L·h−1) | Linear drug effect for patients belonging to subpopulation 1 | 0.0022 (0.001–0.003) | 0.18 (0.04–0.32) |
| Slope2 (mg−1·L·h−1) | Linear drug effect for patients belonging to subpopulation 2 | −0.0027 (−0.004–−0.001) | 0.18 (0.04–0.32) |
| βAge | Age effect on slope | 0.0206 (0–0.048) | - |
| p | Proportion of patients belonging to subpopulation 2 | 0.073 (0–0.17) | - |
| ϵ (%) | Proportional residual error | 2.07 (1.58–2.57) | 0.103 (0.05–0.15) |
| Parameter | Description | Estimate (90% CI) a |
|---|---|---|
| µ | Mean of the log-normal hazard model for dropout | 5.22 (4.83–5.60) |
| σ | SD of the log-normal hazard model for dropout | 0.983 (0.786–1.180) |
| βER (Cmin) [(mg/L)−1] | Coefficient describing the exposure–response relationship as risk factor | 0.251 (0.119–0.383) |
| Treatment Length | Anemia | Peripheral Neuropathy | Thrombocytopenia | Severe Thrombocytopenia |
|---|---|---|---|---|
| Target Cmin (mg/L) | Target Cmin (mg/L) | Target AUC0–24h (h·mg/L) | Target AUC0–24h (h·mg/L) | |
| 6 months | <0.11 | <1.4 | <111 | <270 |
| 9 months | <0.08 | <1.0 | <110 | <270 |
| 12 months | <0.06 | <0.7 | <106 | <255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keutzer, L.; Mockeliunas, L.; Sturkenboom, M.G.G.; Bolhuis, M.S.; Akkerman, O.W.; Simonsson, U.S.H. Derivation and Clinical Utility of Safety Targets for Linezolid-Related Adverse Events in Drug-Resistant Tuberculosis Treatment. Pharmaceuticals 2023, 16, 1575. https://doi.org/10.3390/ph16111575
Keutzer L, Mockeliunas L, Sturkenboom MGG, Bolhuis MS, Akkerman OW, Simonsson USH. Derivation and Clinical Utility of Safety Targets for Linezolid-Related Adverse Events in Drug-Resistant Tuberculosis Treatment. Pharmaceuticals. 2023; 16(11):1575. https://doi.org/10.3390/ph16111575
Chicago/Turabian StyleKeutzer, Lina, Laurynas Mockeliunas, Marieke G. G. Sturkenboom, Mathieu S. Bolhuis, Onno W. Akkerman, and Ulrika S. H. Simonsson. 2023. "Derivation and Clinical Utility of Safety Targets for Linezolid-Related Adverse Events in Drug-Resistant Tuberculosis Treatment" Pharmaceuticals 16, no. 11: 1575. https://doi.org/10.3390/ph16111575
APA StyleKeutzer, L., Mockeliunas, L., Sturkenboom, M. G. G., Bolhuis, M. S., Akkerman, O. W., & Simonsson, U. S. H. (2023). Derivation and Clinical Utility of Safety Targets for Linezolid-Related Adverse Events in Drug-Resistant Tuberculosis Treatment. Pharmaceuticals, 16(11), 1575. https://doi.org/10.3390/ph16111575









