Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Physicochemical Analysis of ACPs
2.2. Spectroscopic Analysis of ACPs
2.2.1. Monosaccharides by HPLC-FID
2.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.3. Infra-Red Spectroscopic Analysis
2.3. In Vitro Biological Activities of ACPs
2.3.1. Effect of ACPs on DPPH Scavenging Activity
2.3.2. Scavenging Effect of ACPs on Nitric Oxide Radical
2.3.3. ABTS Radical Scavenging Activity of ACPs
2.3.4. Ferric Reducing Antioxidant Power (FRAP)
2.3.5. Antimicrobial Activity of ACPs
2.4. Phytotoxicity Essay of ACPs
2.5. In Vivo Biological Activities of ACPs
2.5.1. Effect of ACPs on Toxicity Biomarkers in Hepatic Tissue
2.5.2. Effect of ACPs on the Antioxidant Statute in Liver Tissue
2.5.3. Effect of ACPs on Liver Biochemical Markers
2.5.4. Effect of ACPs on Plasma Lipid Levels
2.5.5. Histopathological Analysis of Liver Tissue
3. Materials and Methods
3.1. Source of Alga-Derived Polysaccharides
3.2. Extraction of Sulfated Polysaccharides (ACPs)
3.3. Extraction Yield and Physicochemical Analysis of ACPs
3.4. Spectroscopic Analysis
3.4.1. Monosaccharide Analysis by HPLC-FID
3.4.2. Fourier Transmission-Infra Red (FT-IR) Spectral Analysis
3.4.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.5. In Vitro Biological Activities of ACPs
3.5.1. DPPH Radical-Scavenging Assay
3.5.2. ABTS Radical Scavenging Assay
3.5.3. Ferric Reducing Activity Power (FRAP)
3.5.4. Nitric Oxide Radical Scavenging Activity
3.5.5. In Vitro Evaluation of Antimicrobial Activity
Microbial Strains and Growth Conditions
Disk Diffusion Method
Microdilution Method
3.6. ACPs Phytotoxicity Analysis
3.7. In Vivo Antioxidant Activities of ACPs
3.7.1. Tebuconazole Presentation
3.7.2. Animal Diet and Tissue Preparation
3.7.3. Liver Protein Quantification
3.7.4. Determination of Oxidative Stress Markers
3.7.5. Biochemical Index Measurements
3.7.6. Lipid Profile in Plasma
3.7.7. Histological Examination
3.8. Computational Analysis and Interactions Assay
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; White, D.W.; on Behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur. Heart. J. 2007, 28, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, E.G.; Escrig, A.J.; Ruperez, P. Dietary fibre and physicochemical properties of several edible seaweeds from northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Marine Nutraceuticals: Prospects and Perspectives. Eur. J. Neur. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Tehila, T.S.; Margalit, B.; Dorit, M.; Shlomo, G.; Shoshana, A. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, K.N.; Lee, S.H. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS stimulated RAW macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Dore, C.M.; Alves, M.G.C.F.; Will, L.S. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Kozlova, L.V.; Mikshina, P.V. Spatial structure of plant cell wall polysaccharides and its functional significance. Biochemistry 2013, 78, 836–853. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Tully, D.B.; Bao, W.; Goetz, A.K.; Blystone, C.R.; Ren, H.; Schmid, J.E.; Strader, L.F.; Wood, C.R.; Best, D.S.; Narotsky, M.G.; et al. Gene expression profiling in liver and testis of rats to characterize the toxicity of triazole fungicides. Toxicol. Appl. Pharmacol. 2006, 215, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, W.; Liu, D. Stereo selective Degradation of Tebuconazole in Rat Liver Microsomes. Chirality 2012, 24, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Petricca, S.; Flati, V.; Celenza, G.; Di Gregorio, J.; Rita Lizzi, A.; Luzi, C.; Cristiano, L.; Cinque, B.; Rossi, G.; Festuccia, C. Tebuconazole and Econazole Act Synergistically in Mediating Mitochondrial Stress, Energy Imbalance, and Sequential Activation of Autophagy and Apoptosis in Mouse Sertoli TM4 Cells: Possible Role of AMPK/ULK1 Axis. Toxicol. Sci. 2019, 169, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Yargholin, M.; Esfehani Tahere, A.; Sadat Musavi, S.; Mehdi Erfani, A.; Salar Amoli, J. Poisoning of dog by tebuconazole fungicide—A case report. Iran. Vet. Rep. 2016, 11, 114–119. [Google Scholar]
- Sancho, E.; Villarroel, M.J.; Andreu, E.; Ferrando, M.D. Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 2009, 74, 1171–1178. [Google Scholar] [CrossRef]
- Ben Saad, H.; Kharrat, N.; Krayem, N. Biological properties of Alsidium corallinum and its potential protective effects against damage caused by potassium bromate in the mouse liver. Environ. Sci. Pollut. Res. 2015, 23, 3809–3823. [Google Scholar] [CrossRef]
- Pereira, M.S.; Vilela-Silva, A.-C.E.S.; Valente, A.-P.; Mourao, P.A.S. A 2-sulfated, 3- linked α-l-galactan is an anticoagulant polysaccharide. Carbohydr. Res. 2002, 337, 2231–2238. [Google Scholar] [CrossRef]
- Kurup, G.M.; Jose, M.G. In Vitro Antioxidant Properties of Edible Marine Algae. Pharm. Bioprocess. 2016, 4, 100–108. [Google Scholar]
- Hamzaoui, A.; Ghariani, M.; Sallem, I. Extraction characterization and biological properties of polysaccharide derived from green seaweed “Chaetomorpha linum” and its potential application in Tunisian beef sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef]
- Ktari, N.; Trabelsi, I.; Bardaa, S. Effects in rat cutaneous wound healing of a novel polysaccharide from fenugreek (Trigonella foenum- graecum) seeds. Int. J. Biol. Macromol. 2017, 95, 625–634. [Google Scholar] [CrossRef]
- Hao, H.; Han, Y.; Yang, L.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macrom. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Fleury, N.; Lahaye, M. Chemical and physico-chemical characterisation of fibres from Laminaria digitata (kombu breton): A physiological approach. J. Sci. Food Agric. 1991, 55, 389–400. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Chen, L. Chemical composition and bioactivities of a water-soluble polysaccharide from the endodermis of shaddock. Int. J. Biol. Macrom. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.A.; Kim, K.N. Antioxidative and antimicrobial activities of Sargassum muticum extracts. J. Korean Soc. Food. Sci. Nutr. 2007, 36, 663–669. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef]
- Hammed, A.; Irwandi, J.; Senay, S.; Azura, A.; Zahangir, A. Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata). Int. J. Food. Prop. 2017, 20, 1457–1469. [Google Scholar]
- Robic, A.; Gaillard, C.D.; Sassi, J.F.O.; Lerat, Y.; Lahaye, M. Ultrastructureof ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef]
- Phyo, P.; Wang, T.; Xiao, C.; Anderson, C.T.; Hong, M. Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: Insights from solid-state NMR. Biomacromolecules 2017, 18, 2937–2950. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Krichen, F.; Karoud, W.; Sila, A. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macrom. 2015, 75, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Ox. Med. Cell. Long. 2016, 2016, 5692852. [Google Scholar]
- Leong, L.P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Khaskheli, M.; Arain, M.A.; Chaudhry, S.; Soomro, A.H.; Qureshi, T.A. PhysicoChemical Quality of Camel Milk. J. Agric. Soc. Sci. 2005, 1, 164–166. [Google Scholar]
- Ruperez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucusve siculosus. J. Agric. Food. Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Huimin, Q.; Tingting, Z.; Quanbin, Z.; Zhien, L.; Zengqin, Z.; Ronge, X. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A. High molecular weight plant phenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macrom. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Mayer, A.; Hamann, M. Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. J. Comp. Biochem. Physiol. 2005, 140, 265–286. [Google Scholar] [CrossRef]
- Kaewsrithong, J.; Intarak, K.; Longpol, T.; Chairgulprasert, V.; Prasertsongsakun, S.; Chotimakorn, C.; Ohshima, T. Antibacterial activity and bioactive compounds of some brown algae from Thailand. In JSPS-NRCT International Symposium Joint Seminar; Kasetsart University: Bangkok, Thailand, 2007; pp. 608–613. [Google Scholar]
- Belhaj, D.; Frikha, D.; Athmouni, K. Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, in vitro antioxidant and antimicrobial activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef]
- Belhaj, D.; Elloumi, N.; Jerbi, B. Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ. Sci. Pollut. Res. 2016, 23, 20168–20177. [Google Scholar] [CrossRef]
- Bissell, M. Chronic liver injury, TGF-β, and cancer. Experim. Mol. Med. 2001, 33, 179–190. [Google Scholar] [CrossRef]
- Sanchez, V.; Valle, C.; Chavez-Tapia, N.; Uribe, M.; Mendez-Sanchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Kamel, J.; Mellouli, L.; Nasri, M.; Ben Amara, I. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Locckie, L.Z.; Meerman, J.H.N.; Commander, J.N.M.; Vermeulen, N.P.E. Biomarkers of free radical damage: Application in experimental animals and in human. Free Rad. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Ben Saad, H.; Driss, D.; Ellouz-Chaabouni, S. Vanillin mitigates potassium bromate-induced molecular, biochemical and histopathological changes in the kidney of adult mice. Chem. Biol. Inter. 2016, 252, 102–113. [Google Scholar] [CrossRef]
- Knebe, C.; Neeb, J.; Zahn, E.F. Propiconazole, Tebuconazole, and Their Mixture on the Receptors CAR and PXR in Human Liver Cells. Toxicol. Sci. 2018, 163, 170–181. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Hu, R.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Fan, Y.; Yan, P.; Li, S.; Zhou, X. The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem. Biol. Inter. 2019, 308, 61–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Hu, T. Metabonomic profiling in study hepatoprotective effect of polysaccharides from Flammulina velutipes on carbon tetrachloride-induced acute liver injury rats using GC–MS. Int. J. Biol. Macromol. 2017, 12, 149. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Arun, A.R.; Muraleedhara, K.G. Sulfated polysaccharides from the edible marine algae Padina tetrastromatica attenuates isoproterenol-induced oxidative damage via activation of PI3K/ Akt/Nrf2 signaling pathway—An in vitro and in vivo approach. Chem. Biol. Inter. 2019, 308, 258–268. [Google Scholar] [CrossRef]
- Guo, T.; Xu, H.; Zhang, L. In vivo protective effect of Porphyra yezoensis polysaccharide against carbon tetrachloride induced hepatotoxicity in mice. Regul. Toxicol. Pharmacol. 2007, 49, 101–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Badraoui, R.; Saoudi, M.; Hamadou, W.S.; Elkahoui, S.; Siddiqui, A.J.; Alam, J.A. Antiviral effects of Artemisinin and its derivatives against SARS-CoV-2 main protease: Computational evidences and interactions with ACE2 allelic variants. Pharmaceuticals 2022, 15, 129. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Struct. Dynam. 2022, 40, 4341–4351. [Google Scholar] [CrossRef]
- Rahmouni, F.; Badraoui, R.; Ben-Nasr, H.; Bardakci, F.; Elkahoui, S. Pharmacokinetics and therapeutic potential of Teucrium polium against liver damage associated hepatotoxicity and oxidative injury in rats: Computational, biochemical and histological studies. Life 2022, 12, 1092. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Issaoui, N.; Hamadou, W.S.; Alam, J.M.; Elhadi, A.S.; Adnan, M. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. ChemSelect 2022, 7, e202103592. [Google Scholar] [CrossRef]
- Alreshidi, M.; Badraoui, R.; Adnan, M.; Patel, M.; Alotaibi, A.; Saeed, M. Phytochemical profiling, antibacterial, and antibiofilm activities of Sargassum sp. (brown algae) from the Red Sea: ADMET prediction and molecular docking analysis. Algal Res. 2023, 69, 102912. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A. Thymus musilii Velen. Methanolic Extract: In Vitro and In Silico Screening of Its Antimicrobial, Antioxidant, Anti-Quorum Sensing, Antibiofilm, and Anticancer Activities. Life 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jin, C.; Tong, Z. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockyville, MD, USA, 2005. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 29, 21770–21776. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, F.J.; Du, L.; Li, G.Y.; Takahashi, K.; Xue, Y. Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. Int. J. Biol. Macromol. 2014, 66, 354–361. [Google Scholar] [CrossRef]
- Van, V.F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends. Food Sci. Technol. 2002, 13, 73–92. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Huang, S.S.; Huang, G.J.; Lin, Y.H. Antioxidant and antiproliferative activities of the four Hydrocotyle species from Taiwan. Bot. Stud. 2008, 49, 311–322. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J. A new method for measuring antioxidant activity. Biochem. Soc. Trans. 1993, 21, 95S. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L.; Theron, K.I. Chemical and phytochemical properties and antioxidant activities of three pomegranate cultivars grown in South Africa. Food Bioprocess Technol. 2012, 5, 2934–2940. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Nilsson-Ehle, P.; Carlstrom, S.; Belfrage, P. Rapid effects on lipoprotein lipase activity in adipose tissue of humans after carbohydrate and lipid intake: Time course and relation to plasma glycerol, triglyceride, and insulin levels. Scand. J. Clin. Lab. Invest. 1975, 35, 373–378. [Google Scholar] [CrossRef]
- Bassole, I.; Ouattara, A.; Nebie, R. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003, 62, 209–212. [Google Scholar] [CrossRef]
- Zucconi, F.A.; Monaco, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publication: New York, NY, USA, 1985; pp. 73–86. [Google Scholar]
- Chen, X.; Zhu, Q.; Li, X.; Huang, T.; Wang, S.; Wang, Y.; Chen, X.; Lin, Z.; Ge, R. Pubertal exposure to tebuconazole increases testosterone production via inhibiting testicular aromatase activity in rats. Chemosphere 2019, 230, 519–526. [Google Scholar] [CrossRef]
- Kammoun, I.; Bkhairia, I.; Ben Abdallah, F.; Jaballi, I.; Ktari, N.; Boudawara, O.; Nasri, M.; Gharsallah, N.; Hakim, A.; Ben Amara, I. Potential protective effects of polysaccharide extracted from Ulva lactuca against male reprotoxicity Induced by thiacloprid. Arch. Physiol. Biochem. 2017, 123, 334–343. [Google Scholar] [CrossRef]
- Council of European Communities Council Directive 86/609/EEC of 24 November, on the Approximation of Laws, Regulations and Administrative Provisions of the Member States regarding the Protection of Animals Used for Experimental and Other Scientific Purposes; Food and Agriculture Organization of the United Nations: Rome, Italy, 1986; Volume 358, pp. 1–18.
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Chem. Biol. 1951, 193, 65–275. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods. Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Wolff, S.P. A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J. Biochem. Biophys. Methods 1996, 31, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Witko, V.; Nguyen, A.T.; Descamps-Latscha, B. Microtiter plate assay for phagocyte-derived taurine chloramines. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improve assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillete, J.R. Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4 bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Gabe, M. Techniques Histologiques; Masson: Paris, France, 1968; pp. 838–879. [Google Scholar]
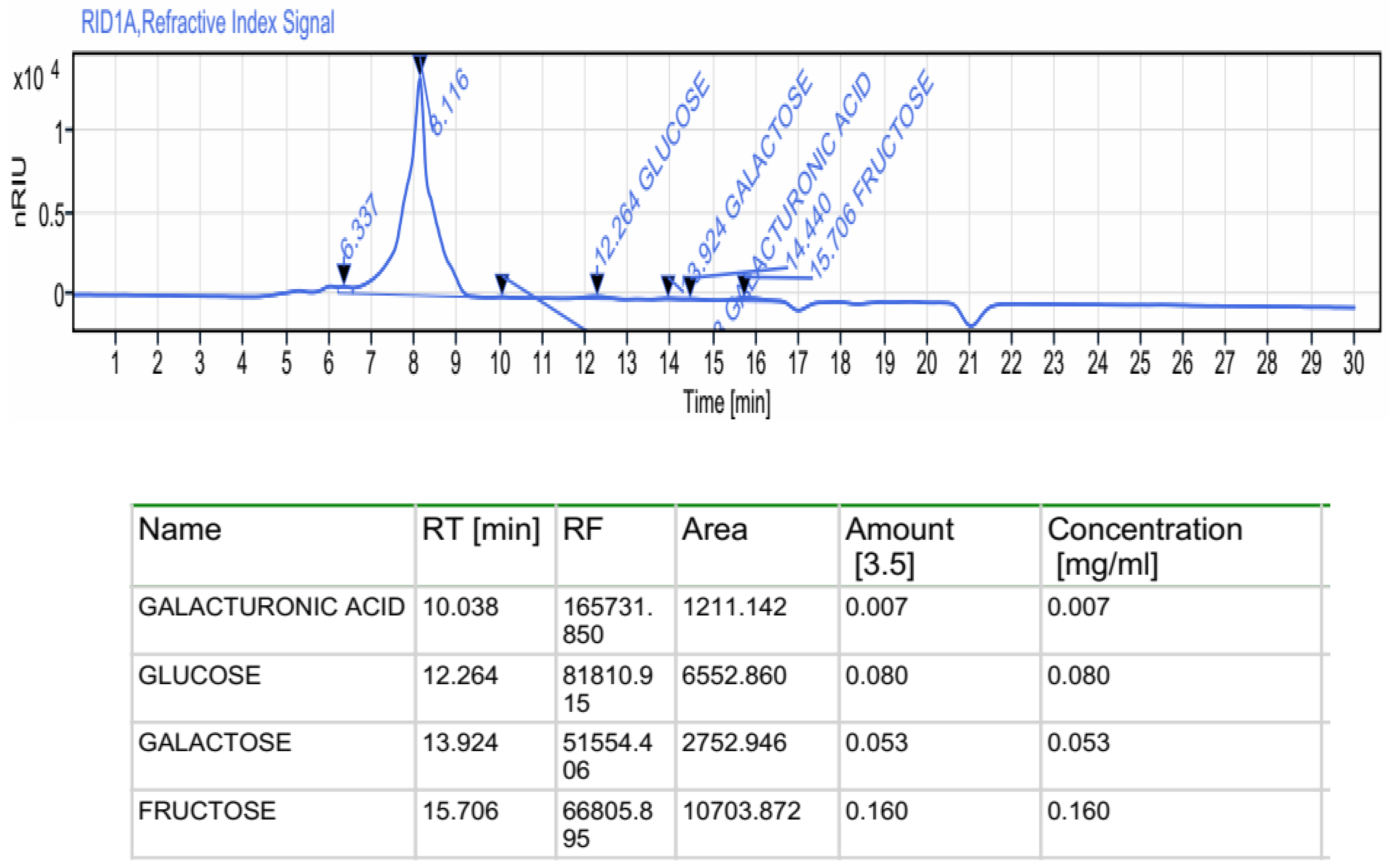







 vascular congestion;
vascular congestion;  inflammation;
inflammation;  infiltration;
infiltration;  focal hepatic hemorrhage. CV: central vein. Different superscript letters (a,b,c) in the same row indicate significant differences at p < 0.05.
focal hepatic hemorrhage. CV: central vein. Different superscript letters (a,b,c) in the same row indicate significant differences at p < 0.05.
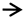 vascular congestion;
vascular congestion;  inflammation;
inflammation;  infiltration;
infiltration;  focal hepatic hemorrhage. CV: central vein. Different superscript letters (a,b,c) in the same row indicate significant differences at p < 0.05.
focal hepatic hemorrhage. CV: central vein. Different superscript letters (a,b,c) in the same row indicate significant differences at p < 0.05.




| Parameters | ACPs |
|---|---|
| Yield (%) | 12.63 ± 1.39 |
| pH | 6.20 ± 0.20 |
| Moisture (%) | 2.96 ± 0.09 |
| Ash (%) | 3.00 ± 0.08 |
| Proteins (%) | 1.94 ± 0.07 |
| Uronic acid (%) | 12.06 ± 1.74 |
| Carbohydrates (%) | 66.06 ± 4.69 |
| Microorganisms | DD (mm) ACPs | DD (10 μg/disk) | MIC (µg/mL) | MBC (µg/mL) | R |
|---|---|---|---|---|---|
| E. coli | 9.66 ± 0.50 | 28 | 100 | 50 | 2 |
| S. enterica | 9.33 ± 0.70 | 21 | 100 | 100 | 1 |
| P. aeruginosa | 9.55 ± 1.00 | 39 | 100 | 100 | 1 |
| M. luteus | 9.66 ± 0.50 | 15 | 100 | 100 | 1 |
| L. invanovii | 9.00 ± 1.00 | 25 | 100 | 100 | 1 |
| S. aureus | 10.66 ± 0.10 | 23 | 50 | 100 | 2 |
| Parameters and Treatments | Control | TEB | TEB + ACPs | ACPs |
|---|---|---|---|---|
| MDA (µmol of MDA/mg protein) | 90.86 ± 16.47 a | 187.24 ± 6.10 c | 148.79 ± 11.54 b | 114.82 ± 15.83 a |
| H2O2 (µmol/mg protein) | 0.07 ± 0.01 | 0.12 ± 0.01 c | 0.09 ± 0.03 b | 0.08 ± 0.02 a |
| AOPPs (nmol/mg protein) | 0.59 ± 0.07 a | 0.86 ± 0.08 b | 0.77 ± 0.06 b | 0.63 ± 0.1 a |
| Parameters and Treatments | Control | TEB | TEB + ACPs | ACPs |
|---|---|---|---|---|
| GPx (nmol GSH/ min/mg protein) | 6.11 ± 1.13 b | 3.60 ± 0.59 a | 4.65 ± 0.97 ab | 5.64 ± 0.39 b |
| SOD (units/mg protein) | 23.34 ± 4.22 b | 12.76 ± 4.17 b | 18.43 ± 2.11 b | 25.62 ± 2.62 b |
| GSH (nmol/mg protein) | 20.35 ± 4.65 b | 11.38 ± 3.13 b | 14.35 ± 3.54 b | 18.08 ± 2.56 b |
| Monosaccharide (Ligand) | Intermolecular Interactions | |||
|---|---|---|---|---|
| Binding Affinity (kcal/mol) | No. H-Bond | Closest Interacting Residues (Distance, Å) | Closest Interacting Residue | |
| TyrRS from S. aureus (1JIJ) | ||||
| Glucuronic acid | −5.8 | 4 | Lys226 (2.155), Lys226 (2.112), Gly233 (2.446), Lys234 (2.721), Lys234 (3.468) | Lys226:HZ3 |
| Glucose | −7.0 | 8 | Asn124 (2.835), Gln174 (2.131), Asp80 (2.449), Gln196 (2.745), Asp40 (2.216), Tyr36 (2.241), Gln190 (2.511), Asp177 (1.898) | Asp177:OD1 |
| Galactose | −7.1 | 7 | Tyr170 (2.845), Tyr170 (2.678), Gln196 (2.478), Asp80 (2.185), Gln196 (2.197), Asp80 (2.039), Gln196 (2.565) | Asp80:OD2 |
| Fructose | −6.6 | 5 | Asp80 (2.752), Gln196 (2.824), Thr75 (2.171), Tyr170 (2.402), Tyr36 (2.647) | Tyr75:OG1 |
| Human peroxiredoxin (1HD2) | ||||
| Glucuronic acid | −5.3 | 6 | Asn21 (2.581), Asn21 (2.362), Arg86 (3.037), Arg86 (2.250), Gly92 (2.173), Leu96 (2.735), Gly82 (3.541), Glu91 (3.550) | Gly92:HN |
| Glucose | −5.4 | 4 | Asn76 (2.064), Asn122 (2.303), Asp77 (2.368), Asp77 (2.432) | Asn76:HD21 |
| Galactose | −5.1 | 5 | Asn76 (2.077), Asp77 (2.309), Arg124 (2.861), Arg124 (2.431), Asp77 (2.579), Asp77(2.488) | Asn76:HD21 |
| Fructose | −5.3 | 5 | Gly17 (3.034), Gly92 (2.823), Val94 (2.584), Thr81 (2.738), Leu96 (2.998), Glu16 (3.557) | Val94:O |
| Acyl-CoA: cholesterol acyltransferase (ACAT, 1WL4) | ||||
| Glucuronic acid | −5.2 | 3 | Asn68 (2.254), Ser87 (2.125), Ser87 (1.771), Gly66 (3.694) | Ser87:HG |
| Glucose | −4.9 | 5 | Thr36 (2.454), Asp32 (2.429), Asp32 (2.495), Asp32 (2.468), Leu206 (2.527), Ser35 (3.564), Gly76 (3.650) | Asp32:OD1 |
| Galactose | −4.9 | 7 | Arg223 (2.284), Arg223 (2.036), Asn227 (2.563), Asn227 (2.688), Met231 (2.864), Asn227 (2.369), Gly225 (2.553) | Arg223:HH21 |
| Fructose | −4.9 | 5 | Ser208 (1.870), Ser208 (2.117), Ser208 (1.880), Ser208 (3.072), Asp32 (1.916) | Ser208:HN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. https://doi.org/10.3390/ph16091305
Ben Saad H, Frikha D, Bouallegue A, Badraoui R, Mellouli M, Kallel H, Pujo JM, Ben Amara I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals. 2023; 16(9):1305. https://doi.org/10.3390/ph16091305
Chicago/Turabian StyleBen Saad, Hajer, Donyez Frikha, Amir Bouallegue, Riadh Badraoui, Manel Mellouli, Hatem Kallel, Jean Marc Pujo, and Ibtissem Ben Amara. 2023. "Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats" Pharmaceuticals 16, no. 9: 1305. https://doi.org/10.3390/ph16091305
APA StyleBen Saad, H., Frikha, D., Bouallegue, A., Badraoui, R., Mellouli, M., Kallel, H., Pujo, J. M., & Ben Amara, I. (2023). Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals, 16(9), 1305. https://doi.org/10.3390/ph16091305






