Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Information Search
2.2. Population, Interventions, Control, and Outcome Strategy (Inclusion Criteria)
2.3. Exclusion Criteria
2.4. Assessment of Bias
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
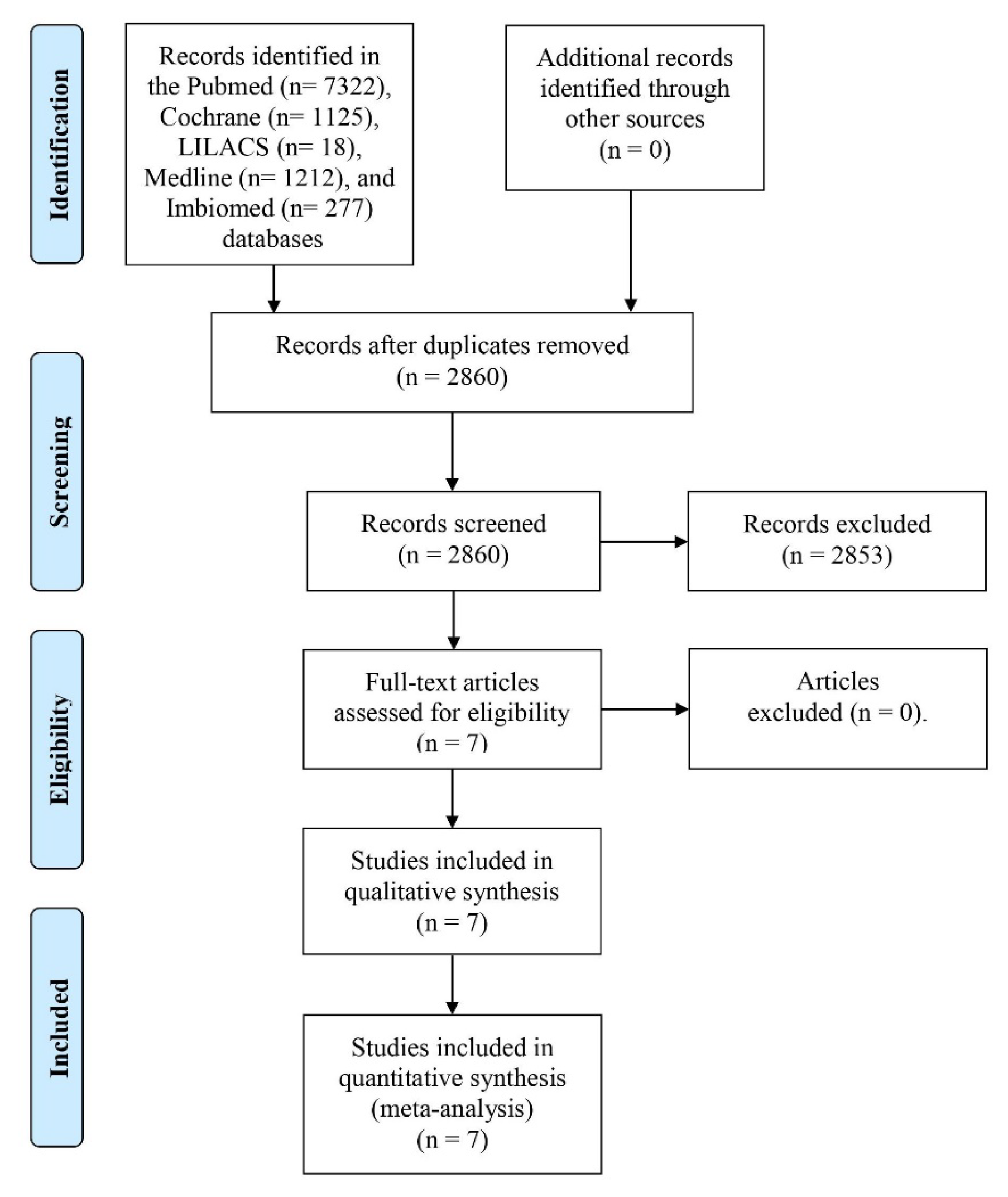
3.1. Information Search and Bias
3.2. Qualitative Assessment
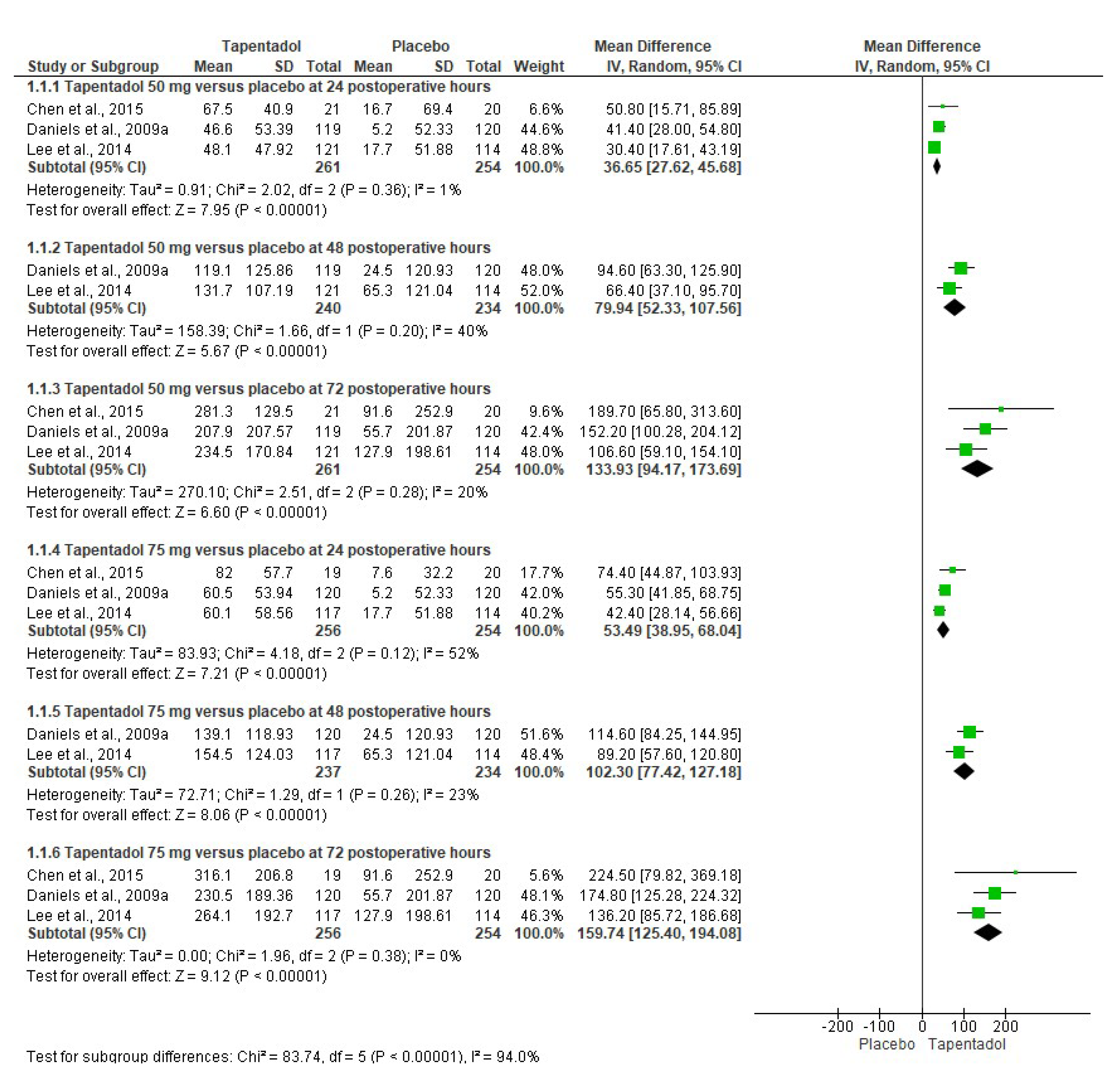
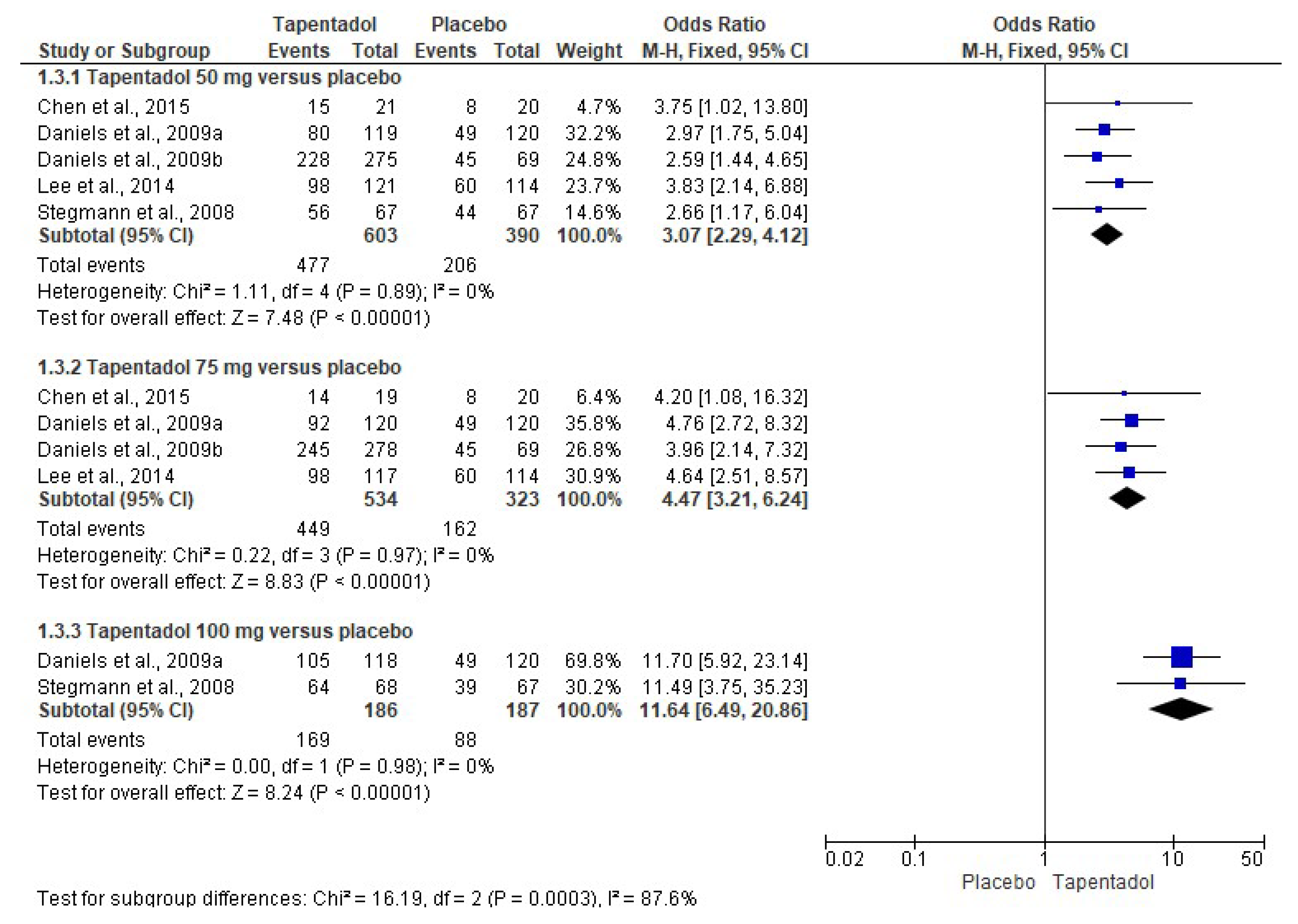
3.3. Quantitative Evaluation
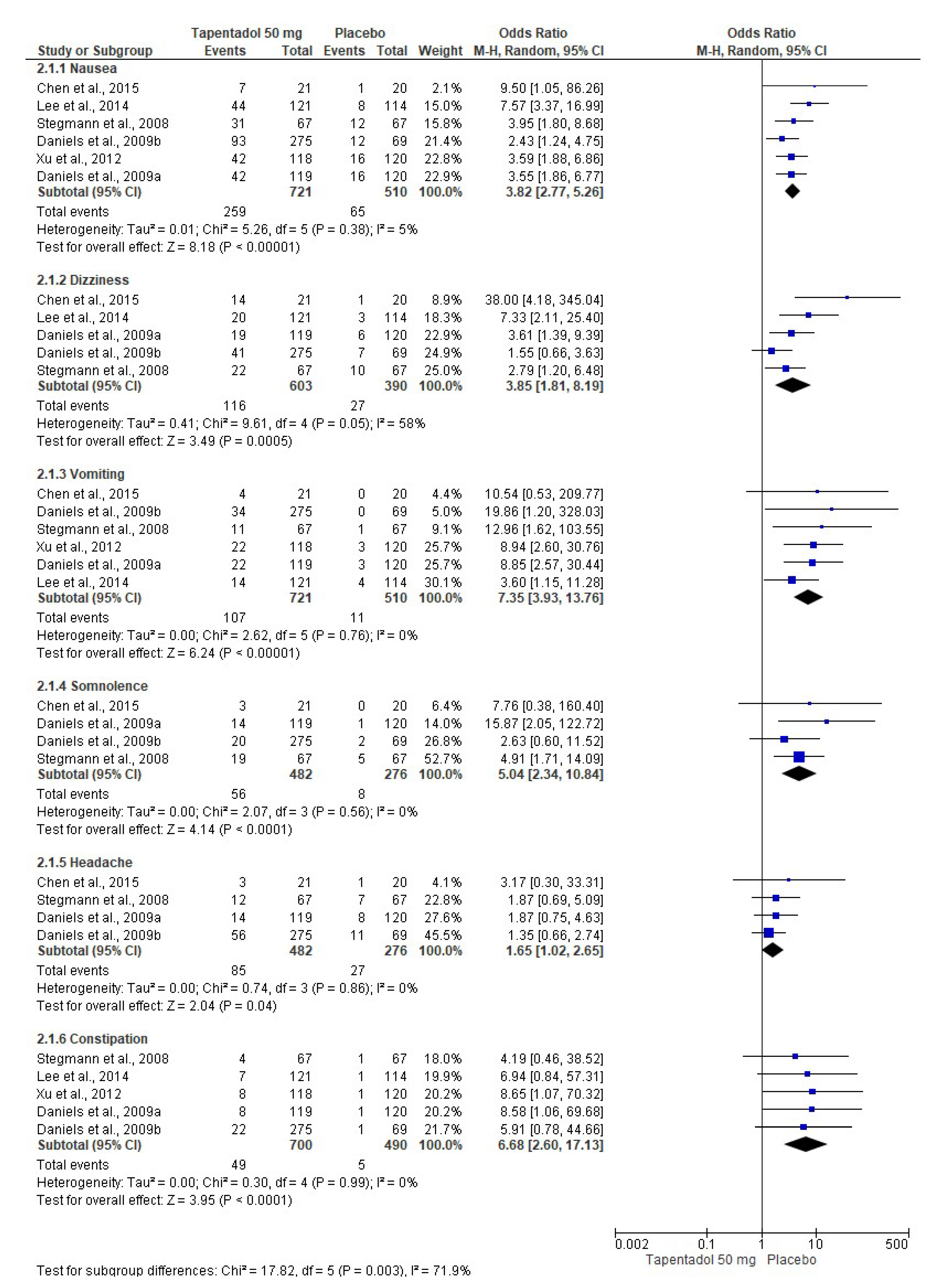
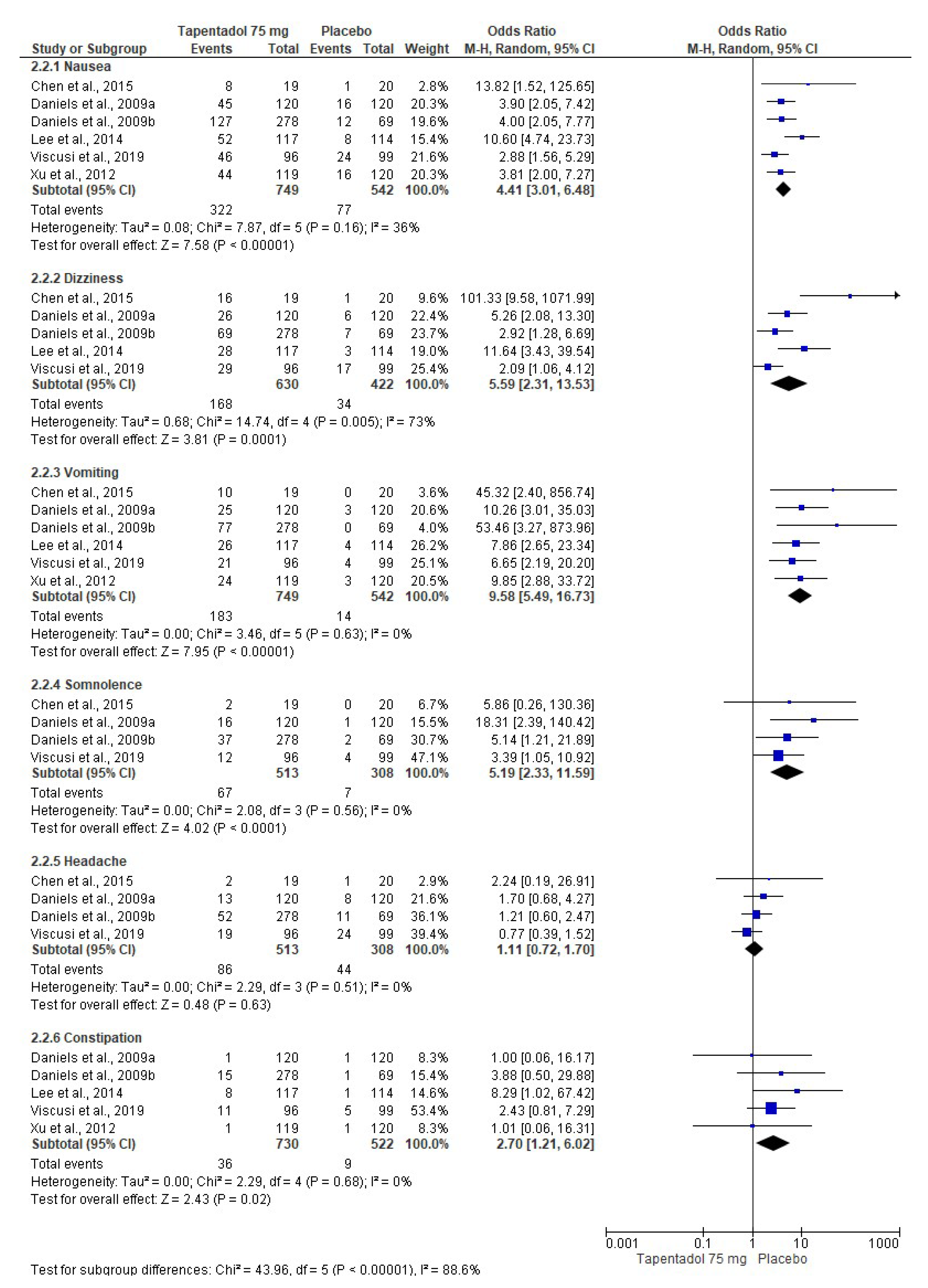
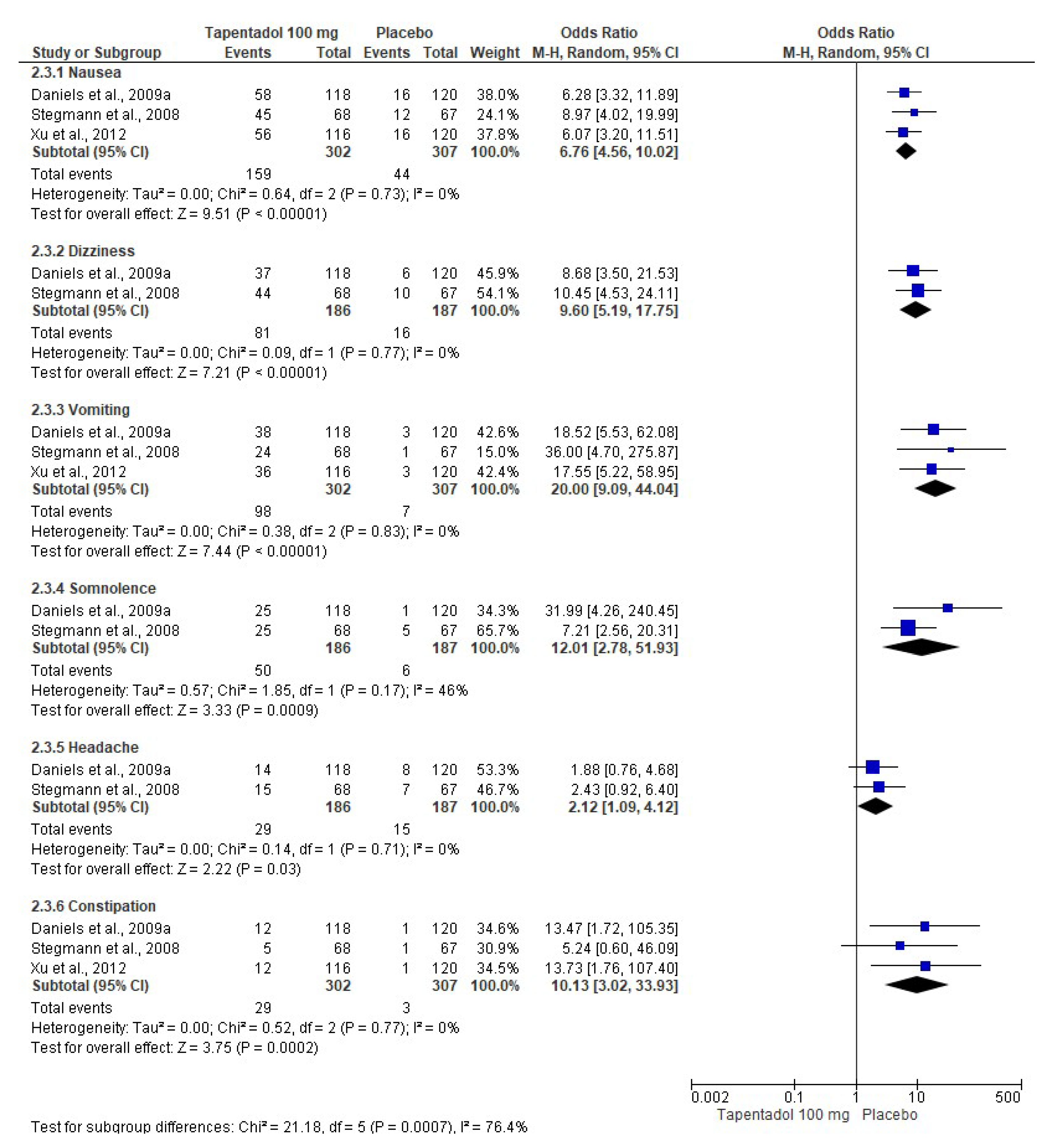
3.4. Adverse Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Gargano, C.; Lukac, S.; Jackson, A.; Beals, C.; Smiley, P.; Drexel, M.; Ruddy, M.; Herman, G.; Johnson-Levonas, A.O.; et al. An enhanced bunionectomy model as a potential tool for early decision-making in the development of new analgesics. Adv. Ther. 2010, 27, 963–980. [Google Scholar] [CrossRef] [PubMed]
- Willens, J.S.; Bucior, I.; Bujanover, S.; Mehta, N. Assessment of rescue opioid use in patients with post-bunionectomy pain treated with diclofenac potassium liquid-filled capsules. J. Pain Res. 2015, 8, 53–62. [Google Scholar] [CrossRef]
- Gottlieb, I.J.; Tunick, D.R.; Mack, R.J.; McCallum, S.W.; Howard, C.P.; Freyer, A.; Du, W. Evaluation of the safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate-to-severe pain after bunionectomy. J. Pain Res. 2018, 11, 383–393. [Google Scholar] [CrossRef]
- Ravanbod, H.R. Analgesic efficacy of local versus proximal nerve blocks after hallux valgus surgery: A systematic review. J. Foot Ankle Res. 2022, 15, 78. [Google Scholar] [CrossRef]
- Isiordia-Espinoza, M.A.; Sánchez-Prieto, M.; Tobías-Azúa, F.; Reyes-García, J.G.; Granados-Soto, V. Pre-emptive analgesia with the combination of tramadol plus meloxicam for third molar surgery: A pilot study. Br. J. Oral Maxillofac. Surg. 2012, 50, 673–677. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chiang, C.C.; Huang, P.J.; Huang, J.; Karcher, K.; Li, H. Tapentadol immediate-release for acute postbunionectomy pain: A phase 3, randomized, double-blind, placebo-controlled, parallel-group study in Taiwan. Curr. Med. Res. Opin. 2015, 31, 2001–2009. [Google Scholar] [CrossRef]
- Daniels, S.E.; Upmalis, D.; Okamoto, A.; Lange, C.; Häeussler, J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr. Med. Res. Opin. 2009, 25, 765–776. [Google Scholar] [CrossRef]
- Daniels, S.; Casson, E.; Stegmann, J.U.; Oh, C.; Okamoto, A.; Rauschkolb, C.; Upmalis, D. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr. Med. Res. Opin. 2009, 25, 1551–1561. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ko, J.S.; Rhim, H.Y.; Lee, E.J.; Karcher, K.; Li, H.; Shapiro, D.; Lee, H.S. Acute postoperative pain relief with immediate-release tapentadol: Randomized, double-blind, placebo-controlled study conducted in South Korea. Curr. Med. Res. Opin. 2014, 30, 2561–2570. [Google Scholar] [CrossRef]
- Stegmann, J.U.; Weber, H.; Steup, A.; Okamoto, A.; Upmalis, D.; Daniels, S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr. Med. Res. Opin. 2008, 24, 3185–3196. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Allard, R.; Sohns, M.; Eerdekens, M. Tapentadol immediate release for moderate to severe acute post-surgery pain. J. Opioid Manag. 2019, 15, 51–67. [Google Scholar] [CrossRef]
- Xu, X.S.; Etropolski, M.; Upmalis, D.; Okamoto, A.; Lin, R.; Nandy, P. Pharmacokinetic and pharmacodynamic modeling of opioid-induced gastrointestinal side effects in patients receiving tapentadol IR and oxycodone IR. Pharm. Res. 2012, 29, 2555–2564. [Google Scholar] [CrossRef]
- Leonardo, R. PICO: Model for clinical questions. Evid. Based Med. Pract. 2008, 3, 2. [Google Scholar]
- Higgins, P.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011], The Cochrane Collaboration, Oxford. 2011. Available online: https://www.cochrane-handbook.org/ (accessed on 25 April 2023).
- Jones, A.; Steel, D. Evaluating the quality of medical evidence in real-world contexts. J. Eval. Clin. Pract. 2018, 24, 950–956. [Google Scholar] [CrossRef]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Huynh, T.M.; Marret, E.; Bonnet, F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur. J. Anaesthesiol. 2015, 32, 751–758. [Google Scholar] [CrossRef]
- Whitley, E.; Ball, J. Statistics review 3: Hypothesis testing and P values. Crit. Care 2002, 6, 222–225. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Risk Reduction Calculator. Available online: http://araw.mede.uic.edu/cgi-bin/nntcalc.pl (accessed on 17 May 2023).
- Xiao, J.P.; Li, A.L.; Feng, B.M.; Ye, Y.; Wang, G.J. Efficacy and safety of tapentadol immediate release assessment in treatment of moderate to severe pain: A systematic review and meta-analysis. Pain Med. 2017, 18, 14–24. [Google Scholar] [CrossRef]
- Rico-Plantón, J.M. Systematic review for analgesics treatments for the postoperative in foot and ankle surgeries. Rev. Española De Podol. 2016, 27, 18–26. [Google Scholar]
- Beverly, A.; Kaye, A.D.; Ljungqvist, O.; Urman, R.D. Essential elements of multimodal analgesia in enhanced recovery after surgery (ERAS) guidelines. Anesthesiol. Clin. 2017, 35, e115–e143. [Google Scholar] [CrossRef]
- Gelman, D.; Gelmanas, A.; Urbanaitė, D.; Tamošiūnas, R.; Sadauskas, S.; Bilskienė, D.; Naudžiūnas, A.; Širvinskas, E.; Benetis, R.; Macas, A. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina 2018, 54, 20. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Swisher, M.W.; Sztain, J.F.; Furnish, T.J.; Ilfeld, B.M.; Said, E.T. State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin. Pharmacother. 2019, 20, 949–961. [Google Scholar] [CrossRef]
- Buvanendran, A.; Kroin, J.S. Multimodal analgesia for controlling acute postoperative pain. Curr. Opin. Anaesthesiol. 2009, 22, 588–593. [Google Scholar] [CrossRef]
- Elvir-Lazo, O.L.; White, P.F. Postoperative pain management after ambulatory surgery: Role of multimodal analgesia. Anesthesiol. Clin. 2010, 28, 217–224. [Google Scholar] [CrossRef]
- Koh, W.S.; Leslie, K. Postoperative analgesia for complex spinal surgery. Curr. Opin. Anaesthesiol. 2022, 35, 543–548. [Google Scholar] [CrossRef]
- Polomano, R.C.; Fillman, M.; Giordano, N.A.; Vallerand, A.H.; Nicely, K.L.; Jungquist, C.R. Multimodal analgesia for acute postoperative and trauma-related pain. Am. J. Nurs. 2017, 117 (Suppl. 1), S12–S26. [Google Scholar] [CrossRef]
- Singla, N.K.; Pollak, R.; Gottlieb, I.; Leiman, D.; Minkowitz, H.; Zimmerman, J.; Harnett, M.; Ryan, M.; Lu, L.; Reines, S. Efficacy and safety of intravenously administered tramadol in patients with moderate to severe pain following bunionectomy: A randomized, double-blind, placebo-controlled, dose-finding study. Pain Ther. 2020, 9, 545–562. [Google Scholar] [CrossRef]
- Viscusi, E.R.; de Leon-Casasola, O.; Cebrecos, J.; Jacobs, A.; Morte, A.; Ortiz, E.; Sust, M.; Vaqué, A.; Gottlieb, I.; Daniels, S.; et al. Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: A phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial. Pain Pract. 2023, 23, 8–22. [Google Scholar] [CrossRef]
- Richards, P.; Riff, D.; Kelen, R.; Stern, W. A phase 3, randomized, double-blind comparison of analgesic efficacy and tolerability of Q8003 vs oxycodone or morphine for moderate-to-severe postoperative pain following bunionectomy surgery. Pain Med. 2013, 14, 1230–1238. [Google Scholar] [CrossRef]
- Singla, N.; Barrett, T.; Sisk, L.; Kostenbader, K.; Young, J. Assessment of the safety and efficacy of extended-release oxycodone/acetaminophen, for 14 days postsurgery. Curr. Med. Res. Opin. 2014, 30, 2571–2578. [Google Scholar] [CrossRef]
- Daniels, S.E.; Spivey, R.J.; Singla, S.; Golf, M.; Clark, F.J. Efficacy and safety of oxycodone HCl/niacin tablets for the treatment of moderate-to-severe postoperative pain following bunionectomy surgery. Curr. Med. Res. Opin. 2011, 27, 593–603. [Google Scholar] [CrossRef]
- Thipphawong, J.B.; Babul, N.; Morishige, R.J.; Findlay, H.K.; Reber, K.R.; Millward, G.J.; Otulana, B.A. Analgesic efficacy of inhaled morphine in patients after bunionectomy surgery. Anesthesiology 2003, 99, 693–700, discussion 6A. [Google Scholar] [CrossRef]
- Webster, L.; Richards, P.; Stern, W.; Kelen, R.; MoxDuo Study Group. A double-blind, placebo-controlled study of dual-opioid treatment with the combination of morphine plus oxycodone in patients with acute postoperative pain. J. Opioid Manag. 2010, 6, 329–340. [Google Scholar] [CrossRef]
- Etropolski, M.; Kuperwasser, B.; Flügel, M.; Häufel, T.; Lange, B.; Rauschkolb, C.; Laschewski, F. Safety and tolerability of tapentadol extended release in moderate to severe chronic osteoarthritis or low back pain management: Pooled analysis of randomized controlled trials. Adv. Ther. 2014, 31, 604–620. [Google Scholar] [CrossRef]
- Santos, J.; Alarcão, J.; Fareleira, F.; Vaz-Carneiro, A.; Costa, J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2015, 2015, CD009923. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Naessens, K.; Bell, R.F. Oral tapentadol for cancer pain. Cochrane Database Syst Rev 2015, 2015, CD011460. [Google Scholar] [CrossRef]
- Meng, Z.; Yu, J.; Acuff, M.; Luo, C.; Wang, S.; Yu, L.; Huang, R. Tolerability of opioid analgesia for chronic pain: A network meta-analysis. Sci. Rep. 2017, 17, 1995. [Google Scholar] [CrossRef]
- Panic, N.; Leoncini, E.; de Belvis, G.; Ricciardi, W.; Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013, 8, e83138. [Google Scholar] [CrossRef]
- Argimon-Pallás, J.M.; Jiménez-Villa, J. Métodos de Investigación Clínica y Epidemiológica, 5th ed.; Elsevier: Barcelona, Spain, 2019. [Google Scholar]
- Dawson, B.; Trapp, R.G. Bioestadística Médica, 4th ed.; Editorial El Manual Moderno: Mexico City, Mexico, 2005. [Google Scholar]

| ID Study and Study Design | Treatments (n) | Details of Patients, Surgical Procedure, and Evaluation | Authors’ Conclusion |
|---|---|---|---|
| Chen et al., 2015 [6]. Phase 3, randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial. | Group A: Tapentadol IR 50 mg (n = 21). Group B: Tapentadol IR 75 mg (n = 19). Group C: Placebo (n = 20). The treatments were given orally every 4–6 h over a 72 h period. | Healthy or medically stable patients aged 20 to 80 years needing a first metatarsal bunionectomy were included. The bunionectomy was made using standardized surgical procedures. The continuous popliteal sciatic block was used. The taking of drugs that interfered with the perception of postoperative pain, such as opioid analgesics or other analgesic or sedative medications, was not allowed during the treatment phase. Subjects who took a different drug than the study drug were discontinued from the clinical trial because of a lack of analgesic efficacy. Pain intensity, pain relief, patient global assessment, SPID, TOTPAR, pain intensity difference (SPRID), and adverse effects were evaluated. | Tapentadol relieved moderate to severe acute pain and had an acceptable safety profile compared to the placebo. |
| Daniels et al., 2009 [7]. Phase 3, randomized, double-blind, placebo-and-active-controlled, parallel-group, multicenter clinical assay. | Group A: Tapentadol IR 50 mg (n = 119). Group B: Tapentadol IR 75 mg (n = 120). Group C: Tapentadol IR 100 mg (n = 118). Group D: Oxycodone HCl IR 15 mg (n = 125). Group E: Placebo (n = 120). The treatments were given orally every 4–6 h over a 72 h period. | ASA-1-to-3 patients aged 18 to 80 years who had a pain score ≥ 4 after the bunionectomy were eligible. The continuous popliteal sciatic block was used. Rescue medication with acetaminophen, ketorolac, and/or hydrocodone/acetaminophen combination was allowed. SPID, TOTPAR, patient global evaluation, and adverse effects were evaluated. | Tapentadol had better analgesic efficacy and lower adverse effects when compared to the placebo. Tapentadol 100 mg had similar analgesic activity to 15 mg of oxycodone. |
| Daniels et al., 2009 [8]. Phase 3, randomized, double-blind, placebo-and-active-controlled, parallel-group, multicenter study. | Group A: Tapentadol IR 50 mg (n = 275). Group B: Tapentadol IR 75 mg (n = 278). Group C: Oxycodone HCl IR 10 mg (n = 278). Group D: Placebo (n = 69). The treatments were given orally every 4–6 h over a 72 h period. | ASA-1 and ASA-2 patients aged 18 to 80 years who had a pain score ≥ 4 after the bunionectomy were eligible. The continuous popliteal sciatic block was used. Patients were allowed up to 2 g of acetaminophen after receipt of the first dose of study medication. SPID, TOTPAR, patient global evaluation, and adverse effects were evaluated. | Tapentadol relieved moderate to severe acute pain and had an acceptable safety profile compared to the placebo. Furthermore, both doses of tapentadol were not inferior to oxycodone. |
| Lee et al., 2014 [9]. Phase 3, randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial. | Group A: Tapentadol IR 50 mg (n = 121). Group B: Tapentadol IR 75 mg (n = 117). Group C: Placebo (n = 114). The treatments were given orally every 4–6 h over a 72 h period. | Healthy or medically stable patients aged 20 to 80 years needing a first metatarsal bunionectomy were included. The 0.5% mepivacaine continuous popliteal sciatic block was used. Acetaminophen at a dose of 650 mg taken orally (PO) and/or ketorolac at a dose of 30 mg IV every 4–6 h, or alternatively fentanyl at a dose of 100 μg IV were used as supplemental analgesia until the start of the qualifying period. SPID, TOTPAR, SPRID, global evaluation, and adverse effects were evaluated. | Tapentadol relieved the acute pain when compared to the placebo. |
| Stegmann et al., 2008 [10]. Phase II, randomized, double-blind, placebo-controlled, parallel-group, multiple-dose clinical assay. | Group A: Tapentadol IR 50 mg (n = 67). Group B: Tapentadol IR 100 mg (n = 68). Group C: Oxycodone HCl IR 10 mg (n = 67). Group D: Placebo (n = 67). The treatments were given orally every 4–6 h over a 72 h period. | Healthy or medically stable patients aged 18 to 65 years needing a first metatarsal bunionectomy were included. The 0.5% mepivacaine sciatic block was used. The use of analgesics, sedatives, and narcotic drugs 12 h before surgery was not allowed. Ibuprofen, ketorolac, and the hydrocodone–acetaminophen combination as rescue medication were used. SPID, TOTPAR, analgesic consumption, global evaluation, and adverse effects were evaluated. | Both tapentadol and oxycodone were effective in postoperative pain control. |
| Viscusi et al., 2019 [11]. Phase 3, randomized, double-blind, placebo-and-active-controlled, parallel-group, multicenter trial. | Group A: Tapentadol IR 75 mg (n = 96). Group B: Morphine 30 mg (n = 96). Group C: Placebo (n = 99). The treatments were given orally every 4–6 h over a 72 h period. | Patients aged 18 to 65 years needing a first metatarsal bunionectomy were included. The 0.5% mepivacaine continuous popliteal sciatic block was used. Paracetamol–hydrocodone combination rescue medication was used. SPID, the first intake of the investigational medicinal product, global evaluation, and adverse effects were evaluated. | Tapentadol was effective in postoperative pain control and was well-tolerated. |
| Xu et al., 2012 [12]. Randomized, double-blind, placebo-and-active-controlled, parallel-group, clinical trial. | Group A: Tapentadol IR 50 mg (n = 118). Group B: Tapentadol IR 75 mg (n = 119). Group C: Tapentadol IR 100 mg (n = 116). Group D: Oxycodone HCl IR 15 mg (n = 123). Group E: Placebo (n = 120). The treatments were given orally every 4–6 h over a 72 h period. | Healthy patients with moderate to severe pain following the bunionectomy. Data for regional anesthesia and rescue medication were not included. The adverse effects—nausea, vomiting, and constipation—were evaluated. | A decrease in the adverse effects of tapentadol compared to oxycodone was observed. |
| Tapentadol 50 mg | Tapentadol 75 mg | Tapentadol 100 mg | ||||
|---|---|---|---|---|---|---|
| NNH | 95% CI | NNH | 95% CI | NNH | 95% CI | |
| Nausea | 4.3 | 3.5 to 5.4 | 3.4 | 2.9 to 4.1 | 2.5 | 2 to 3.2 |
| Dizziness | 8.1 | 6.1 to 12.1 | 5.4 | 4.4 to 7 | 2.9 | 2.3 to 3.7 |
| Vomiting | 8.3 | 6.6 to 11.2 | 4.4 | 3.8 to 5.3 | 3.2 | 2.6 to 4.1 |
| Somnolence | 11.5 | 8.2 to 19.1 | 9.3 | 7.1 to 13.5 | 4.2 | 3.3 to 5.9 |
| Headache | 12.7 | 7.9 to 33.7 | 40.3 | 13.2 to infinity | 13.2 | 7.1 to 94.1 |
| Constipation | 16.8 | 12.1 to 27.5 | 26.8 | 16.6 to 69.2 | 12.4 | 8 to 27.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-de la Torre, L.; Gómez-Sánchez, E.; Aragon-Martinez, O.H.; Hernández-Gómez, A.; Franco-González, D.L.; Guzmán-Flores, J.M.; Alonso-Castro, A.J.; Granados-Soto, V.; Isiordia-Espinoza, M.A. Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis. Pharmaceuticals 2023, 16, 1287. https://doi.org/10.3390/ph16091287
Franco-de la Torre L, Gómez-Sánchez E, Aragon-Martinez OH, Hernández-Gómez A, Franco-González DL, Guzmán-Flores JM, Alonso-Castro AJ, Granados-Soto V, Isiordia-Espinoza MA. Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis. Pharmaceuticals. 2023; 16(9):1287. https://doi.org/10.3390/ph16091287
Chicago/Turabian StyleFranco-de la Torre, Lorenzo, Eduardo Gómez-Sánchez, Othoniel Hugo Aragon-Martinez, Adriana Hernández-Gómez, Diana Laura Franco-González, Juan Manuel Guzmán-Flores, Angel Josabad Alonso-Castro, Vinicio Granados-Soto, and Mario Alberto Isiordia-Espinoza. 2023. "Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis" Pharmaceuticals 16, no. 9: 1287. https://doi.org/10.3390/ph16091287
APA StyleFranco-de la Torre, L., Gómez-Sánchez, E., Aragon-Martinez, O. H., Hernández-Gómez, A., Franco-González, D. L., Guzmán-Flores, J. M., Alonso-Castro, A. J., Granados-Soto, V., & Isiordia-Espinoza, M. A. (2023). Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis. Pharmaceuticals, 16(9), 1287. https://doi.org/10.3390/ph16091287