Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i)
Abstract
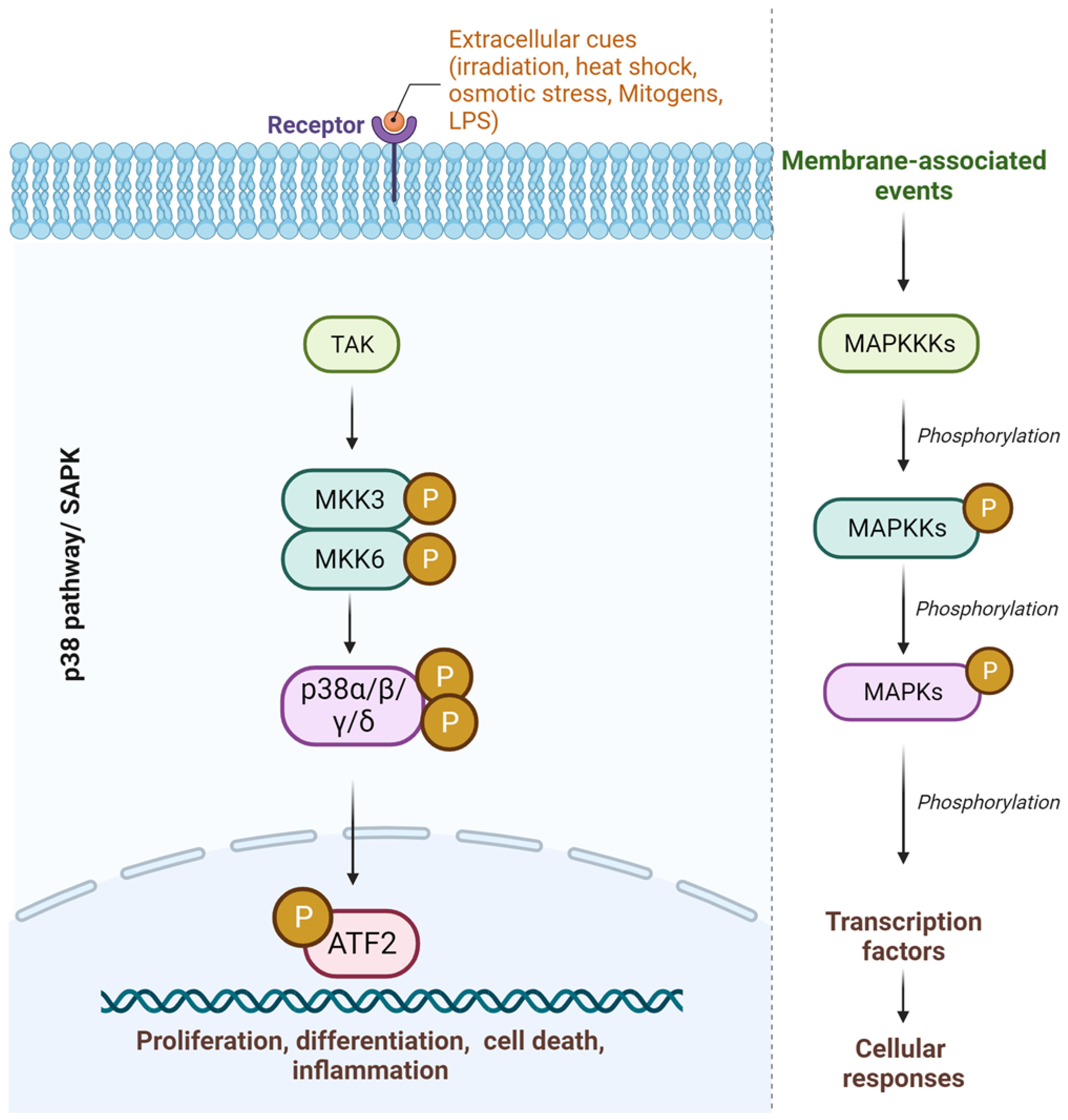
1. Introduction
2. Emergence of New Targets Downstream of p38-MAPK: MK2 Inhibition
3. Inflammatory Arthritis and MK2 Inhibitors
4. Challenges, Conclusions, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.D.; Abell, A.N.; Johnson, G.L. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 2007, 26, 3159–3171. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2023, 12, 9–18. [Google Scholar] [CrossRef]
- Tanoue, T.; Nishida, E. Molecular recognitions in the MAP kinase cascades. Cell. Signal. 2003, 15, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Meloche, S.; Pouysségur, J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007, 26, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Pua, L.J.W.; Mai, C.-W.; Chung, F.F.-L.; Khoo, A.S.-B.; Leong, C.-O.; Lim, W.-M.; Hii, L.-W. Functional Roles of JNK and p38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Schett, G.; Zwerina, J.; Firestein, G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909–916. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, J.-D.; Bibbs, L.; Ulevitch, R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994, 265, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Mbalaviele, G.; Anderson, G.; Jones, A.; De Ciechi, P.; Settle, S.; Mnich, S.; Thiede, M.; Abu-Amer, Y.; Portanova, J.; Monahan, J. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J. Pharmacol. Exp. Ther. 2006, 317, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.L.E.; Brook, M.; Clark, A.R.; Saklatvala, J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 1999, 274, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Underwood, D.C.; Osborn, R.R.; Bochnowicz, S.; Webb, E.F.; Rieman, D.J.; Lee, J.C.; Romanic, A.M.; Adams, J.L.; Hay, D.W.P.; Griswold, D.E.; et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L895–L902. [Google Scholar] [CrossRef]
- Goedert, M. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997, 16, 3563–3571. [Google Scholar] [CrossRef]
- Escós, A.; Risco, A.; Alsina-Beauchamp, D.; Cuenda, A. p38γ and p38δ Mitogen Activated Protein Kinases (MAPKs), New Stars in the MAPK Galaxy. Front. Cell Dev. Biol. 2016, 4, 31. [Google Scholar] [CrossRef]
- Risco, A.; Cuenda, A. New Insights into the p38γ and p38δ MAPK Pathways. J. Signal Transduct. 2012, 2012, 520289. [Google Scholar] [CrossRef]
- Risco, A.; Martin-Serrano, M.A.; Barber, D.F.; Cuenda, A. p38γ and p38δ Are Involved in T Lymphocyte Development. Front. Immunol. 2018, 9, 65. [Google Scholar] [CrossRef]
- Ittner, A.; Block, H.; Reichel, C.A.; Varjosalo, M.; Gehart, H.; Sumara, G.; Gstaiger, M.; Krombach, F.; Zarbock, A.; Ricci, R. Regulation of PTEN activity by p38δ-PKD1 signaling in neutrophils confers inflammatory responses in the lung. J. Exp. Med. 2012, 209, 2229–2246. [Google Scholar] [CrossRef]
- Porras, A.; Zuluaga, S.; Black, E.; Valladares, A.; Alvarez, A.M.; Ambrosino, C.; Benito, M.; Nebreda, A.R. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol. Biol. Cell 2004, 15, 922–933. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Silke, J. An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research 2020, 9, 653. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Trempolec, N.; Dave-Coll, N.; Nebreda, A.R. SnapShot: p38 MAPK substrates. Cell 2013, 152, 924. [Google Scholar] [CrossRef] [PubMed]
- Hoefen, R.J.; Berk, B.C. The role of MAP kinases in endothelial activation. Vasc. Pharmacol. 2002, 38, 271–273. [Google Scholar] [CrossRef]
- Roussel, L.; Houle, F.; Chan, C.; Yao, Y.; Bérubé, J.; Olivenstein, R.; Martin, J.G.; Huot, J.; Hamid, Q.; Ferri, L.; et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J. Immunol. 2010, 184, 4531–4537. [Google Scholar] [CrossRef]
- Ramalingam, P.; Poulos, M.G.; Lazzari, E.; Gutkin, M.C.; Lopez, D.; Kloss, C.C.; Crowley, M.J.; Katsnelson, L.; Freire, A.G.; Greenblatt, M.B.; et al. Chronic activation of endothelial MAPK disrupts hematopoiesis via NFKB dependent inflammatory stress reversible by SCGF. Nat. Commun. 2020, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A.J. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef]
- Peroval, M.Y.; Boyd, A.C.; Young, J.R.; Smith, A.L. A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PLoS ONE 2013, 8, e51243. [Google Scholar] [CrossRef]
- Fillatreau, S.; Manfroi, B.; Dörner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 17, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Larionova, R.V.; Brooks, W.H.; Bettacchioli, E.; Renaudineau, Y. Toll-Like Receptors, Infections, and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2019, 58, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Schindler, J.; Monahan, J.; Smith, W. p38 pathway kinases as anti-inflammatory drug targets. J. Dent. Res. 2007, 86, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, V.A.; Hinton, H.J.; Eftychi, C.; Apostolaki, M.; Armaka, M.; Darragh, J.; McIlrath, J.; Carr, J.M.; Armit, L.J.; Clacher, C.; et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol. Cell. Biol. 2005, 25, 10454–10464. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Yamamoto, T.; Maeda, R.; Nishida, E. A Novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38 alpha and beta MAPKs. J. Biol. Chem. 2001, 276, 26629–26639. [Google Scholar] [CrossRef]
- Yong, H.-Y.; Koh, M.-S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Giacomelli, R.; Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: New players and therapeutic targets. BMC Rheumatol. 2017, 1, 3. [Google Scholar] [CrossRef]
- Dayer, J.-M.; Oliviero, F.; Punzi, L. A Brief History of IL-1 and IL-1 Ra in Rheumatology. Front. Pharmacol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Ruscitti, P.; Masedu, F.; Alvaro, S.; Airò, P.; Battafarano, N.; Cantarini, L.; Cantatore, F.P.; Carlino, G.; D’Abrosca, V.; Frassi, M.; et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 2019, 16, 1002901. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Cantarini, L.; Liakouli, V.; Vitale, A.; Carubbi, F.; Berardicurti, O.; Galeazzi, M.; Valenti, M.; Giacomelli, R. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: Two case reports and review of the literature. J. Med. Case Rep. 2015, 9, 123. [Google Scholar] [CrossRef]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell. Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Fragiadaki, K.; Konsta, M.; Bratengeier, C.; Papatheodorou, A.; Sfikakis, P.P. Early effects of IL-6 receptor inhibition on bone homeostasis: A pilot study in women with rheumatoid arthritis. Clin. Exp. Rheumatol. 2011, 29, 921–925. [Google Scholar] [PubMed]
- Koga, T.; Kawakami, A. Interleukin-6 inhibition in the treatment of autoinflammatory diseases. Front. Immunol. 2022, 13, 956795. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2022, 23, 289–303. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Robert, M.; Miossec, P. IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front. Med. 2019, 5, 364. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, M.; Liu, M.; Zhou, E.; Ren, T.; Chang, X.; He, M.; Zeng, K.; Guo, Y.; Wu, J. Efficacy and safety of IL-17 inhibitors for the treatment of ankylosing spondylitis: A systematic review and meta-analysis. Arthritis Res. Ther. 2020, 22, 111. [Google Scholar] [CrossRef]
- Wang, E.A.; Suzuki, E.; Maverakis, E.; Adamopoulos, I.E. Targeting IL-17 in psoriatic arthritis. Eur. J. Rheumatol. 2017, 4, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Parhar, K.; Ray, A.; Steinbrecher, U.; Nelson, C.; Salh, B. The p38 mitogen-activated protein kinase regulates interleukin-1beta-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells. Immunology 2003, 108, 502–512. [Google Scholar] [CrossRef]
- Mack, M.; Brühl, H. Il-3 Inhibitors in Use for Treatment of Rheumatoid Arthritis in an Early Stage. U.S. Patent No 13/132,754, 16 February 2012. [Google Scholar]
- Kim, L.; Del Rio, L.; Butcher, B.A.; Mogensen, T.H.; Paludan, S.R.; Flavell, R.A.; Denkers, E.Y. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 2005, 174, 4178–4184. [Google Scholar] [CrossRef]
- Li, J.-K.; Nie, L.; Zhao, Y.-P.; Zhang, Y.-Q.; Wang, X.; Wang, S.-S.; Liu, Y.; Zhao, H.; Cheng, L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J. Transl. Med. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, A.; Yannoni, Y.; Fritz, S.; Laaß, K.; Telliez, J.-B.; Pitman, D.; Lin, L.-L.; Gaestel, M. Distinct cellular functions of MK2. Mol. Cell. Biol. 2002, 22, 4827–4835. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.J.; Ballon, D.R.; Thorner, J. When the stress of your environment makes you go HOG wild. Science 2004, 306, 1511–1512. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 469. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Gu, Z.; Li, L.; Liu, Y.; Wang, L.; Su, L. p38 MAPK-MK2 pathway regulates the heat-stress-induced accumulation of reactive oxygen species that mediates apoptotic cell death in glial cells. Oncol. Lett. 2018, 15, 775–782. [Google Scholar] [CrossRef]
- Damjanov, N.; Kauffman, R.S.; Spencer-Green, G.T. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: Results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009, 60, 1232–1241. [Google Scholar] [CrossRef]
- Goldstein, D.M.; Kuglstatter, A.; Lou, Y.; Soth, M.J. Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J. Med. Chem. 2010, 53, 2345–2353. [Google Scholar] [CrossRef]
- Chopra, P.; Kanoje, V.; Semwal, A.; Ray, A. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin. Investig. Drugs 2008, 17, 1411–1425. [Google Scholar] [CrossRef]
- Genovese, M.C.; Cohen, S.B.; Wofsy, D.; Weinblatt, M.E.; Firestein, G.S.; Brahn, E.; Strand, V.; Baker, D.G.; Tong, S.E. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J. Rheumatol. 2011, 38, 846–854. [Google Scholar] [CrossRef]
- Cohen, S.B.; Cheng, T.-T.; Chindalore, V.; Damjanov, N.; Burgos-Vargas, R.; DeLora, P.; Zimany, K.; Travers, H.; Caulfield, J.P. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009, 60, 335–344. [Google Scholar] [CrossRef]
- Schreiber, S.; Feagan, B.; D’haens, G.; Colombel, J.; Geboes, K.; Yurcov, M.; Isakov, V.; Golovenko, O.; Bernstein, C.N.; Ludwig, D.; et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2006, 4, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Bajpai, M.; Bughani, U.; Dastidar, S.G.; Ray, A.; Chopra, P. MK2: A novel molecular target for anti-inflammatory therapy. Expert Opin. Ther. Targets 2008, 12, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Hedström, U.; Norberg, M.; Evertsson, E.; Lever, S.R.; Rosenschöld, M.M.A.; Lönn, H.; Bold, P.; Käck, H.; Berntsson, P.; Vinblad, J.; et al. An Angle on MK2 Inhibition-Optimization and Evaluation of Prevention of Activation Inhibitors. ChemMedChem 2019, 14, 1701–1709. [Google Scholar] [CrossRef]
- Beamer, E.; Corrêa, S.A.L. The p38MAPK-MK2 Signaling Axis as a Critical Link Between Inflammation and Synaptic Transmission. Front. Cell Dev. Biol. 2021, 9, 635636. [Google Scholar] [CrossRef]
- Hitti, E.; Iakovleva, T.; Brook, M.; Deppenmeier, S.; Gruber, A.D.; Radzioch, D.; Clark, A.R.; Blackshear, P.J.; Kotlyarov, A.; Gaestel, M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol. Cell. Biol. 2006, 26, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.B.; Gaestel, M. MK2-TNF-Signaling Comes Full Circle. Trends Biochem. Sci. 2018, 43, 170–179. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R. The p38 MAPK Pathway in Rheumatoid Arthritis: A Sideways Look. Open Rheumatol. J. 2012, 6, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Blank, M. Targeting p38 MAP kinase signaling in cancer through post-translational modifications. Cancer Lett. 2017, 384, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, D.; Fan, Z.; Yuan, Y.; Shao, M.; Hou, J.; Zhu, Y.; Wei, R.; Zhu, Y.; Qian, J.; et al. Targeting MK2 Is a Novel Approach to Interfere in Multiple Myeloma. Front. Oncol. 2019, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef]
- Soni, S.; Anand, P.; Padwad, Y.S. MAPKAPK2: The master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J. Exp. Clin. Cancer Res. 2019, 38, 121. [Google Scholar] [CrossRef]
- O’Bak, R.; Mikkelsen, J.G. Regulation of cytokines by small RNAs during skin inflammation. J. Biomed. Sci. 2010, 17, 53–119. [Google Scholar] [CrossRef]
- Corre, I.; Paris, F.; Huot, J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef]
- Hedges, J.C.; Dechert, M.A.; Yamboliev, I.A.; Martin, J.L.; Hickey, E.; Weber, L.A.; Gerthoffer, W.T. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 1999, 274, 24211–24219. [Google Scholar] [CrossRef] [PubMed]
- Ben-Levy, R.; Hooper, S.; Wilson, R.; Paterson, H.F.; Marshall, C.J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 1998, 8, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Hellriegel, E.T.; Hope, H.R.; Burt, D.; Monahan, J.B. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the MK2 Inhibitor ATI-450 in Healthy Subjects: A Placebo-Controlled, Randomized Phase 1 Study. Clin. Pharmacol. Adv. Appl. 2021, 13, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Sodhi, R.; Sharma, S.; Dastidar, S.G.; Tandon, R. Targeting MAPAKAP2(MK2) to combat inflammation by avoiding the differential regulation of anti-inflammatory genes by p38 MAPK inhibitors. bioRxiv 2022. 2022.07. [Google Scholar] [CrossRef]
- Hegen, M.; Gaestel, M.; Nickerson-Nutter, C.L.; Lin, L.-L.; Telliez, J.-B. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J. Immunol. 2006, 177, 1913–1917. [Google Scholar] [CrossRef]
- Mourey, R.J.; Burnette, B.L.; Brustkern, S.J.; Daniels, J.S.; Hirsch, J.L.; Hood, W.F.; Meyers, M.; Mnich, S.J.; Pierce, B.S.; Saabye, M.J.; et al. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor alpha production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J. Pharmacol. Exp. Ther. 2010, 333, 797–807. [Google Scholar] [CrossRef]
- Malona, J.; Chuaqui, C.; Seletsky, B.M.; Beebe, L.; Cantin, S.; VAN Kalken, D.; Fahnoe, K.; Wang, Z.; Browning, B.; Szabo, H.; et al. Discovery of CC-99677, a selective targeted covalent MAPKAPK2 (MK2) inhibitor for autoimmune disorders. Transl. Res. J. Lab. Clin. Med. 2022, 249, 49–73. [Google Scholar] [CrossRef]
- Gaur, R.; Mensah, K.A.; Stricker, J.; Adams, M.; Parton, A.; Cedzik, D.; Connarn, J.; Thomas, M.; Horan, G.; Schafer, P.; et al. CC-99677, a novel, oral, selective covalent MK2 inhibitor, sustainably reduces pro-inflammatory cytokine production. Arthritis Res. Ther. 2022, 24, 199. [Google Scholar] [CrossRef]
- W.H.O. Rheumatoid Srthritis—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/rheumatoid-arthritis (accessed on 22 August 2023).
- Cai, Y.; Zhang, J.; Liang, J.; Xiao, M.; Zhang, G.; Jing, Z.; Lv, L.; Nan, K.; Dang, X. The Burden of Rheumatoid Arthritis: Findings from the 2019 Global Burden of Diseases Study and Forecasts for 2030 by Bayesian Age-Period-Cohort Analysis. J. Clin. Med. 2023, 12, 1291. [Google Scholar] [CrossRef]
- Papakonstantinou, D. Work disability and rheumatoid arthritis: Predictive factors. Work 2021, 69, 1293–1304. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Wu, O.; Geue, C.; McIntosh, E.; McInnes, I.B.; Siebert, S. Economic burden of rheumatoid arthritis: A systematic review of literature in biologic era. Ann. Rheum. Dis. 2020, 79, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C. Effect and Treatment of Chronic Pain in Inflammatory Arthritis. Curr. Rheumatol. Rep. 2012, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Ward, M.M. Advances in the treatment of inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 2012, 26, 251–261. [Google Scholar] [CrossRef]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.-L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Bahrami, A.A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Martinez, J.M.; Dawson, J.R.D.; Flores, J.; Gabriel, M.; Garcia, G.; Guevara, A.; Murray, K.; Pacifici, N.; Vargas, M.V.; et al. The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front. Pharmacol. 2021, 12, 680043. [Google Scholar] [CrossRef]
- Gordon, D.; Kivitz, A.; Singhal, A.; Burt, D.; Bangs, M.C.; Huff, E.E.; Hope, H.R.; Monahan, J.B. Selective Inhibition of the MK2 Pathway: Data from a Phase IIa Randomized Clinical Trial in Rheumatoid Arthritis. ACR Open Rheumatol. 2023, 5, 63–70. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Glintborg, B.; Svensson, A.L.; McMaster, C.; Robinson, P.C.; Deleuran, B.; Liew, D.F. Waiting for JAK inhibitor safety data. RMD Open 2022, 8, e002236. [Google Scholar] [CrossRef]
- US_FDA. FDA Requires Warnings about Increased Risk of Serious Heart-Related Events, Cancer, Blood Clots, and Death for JAK Inhibitors That Treat Certain Chronic Inflammatory Conditions|FDA. Available online: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (accessed on 10 August 2023).
- Jamilloux, Y.; El Jammal, T.; Vuitton, L.; Gerfaud-Valentin, M.; Kerever, S.; Sève, P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019, 18, 102390. [Google Scholar] [CrossRef]
- Hoisnard, L.; Lebrun-Vignes, B.; Maury, S.; Mahevas, M.; El Karoui, K.; Roy, L.; Zarour, A.; Michel, M.; Cohen, J.L.; Amiot, A.; et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022, 12, 7140. [Google Scholar] [CrossRef]
- Celgene. A Study of CC-99677 in Participants with Active Ankylosing Spondylitis (AS SpA axSpA). Available online: https://clinicaltrials.gov/ (accessed on 23 August 2023).
- Fiore, M.; Forli, S.; Manetti, F. Targeting Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 (MAPKAPK2, MK2): Medicinal Chemistry Efforts to Lead Small Molecule Inhibitors to Clinical Trials. J. Med. Chem. 2016, 59, 3609–3634. [Google Scholar] [CrossRef] [PubMed]
- Luber, A.; Peterson, C.; Panitch, A.; Wetering, J.V.D.; Hoogdalem, E.; Nicholson, G.; Leaker, B.; Lander, C. MMI-0100, a Novel MAPKAP Kinase II (MK2) Inhibitor, Delivered Via Inhalation, Displays an Excellent Safety and Tolerability Profile in Three Phase 1 Clinical Trials. In Proceedings of the American Thoracic Society 2018 International Conference, San Diego, CA, USA, 18–23 May 2018. [Google Scholar]
- Evans, B.C.; Hocking, K.M.; Osgood, M.J.; Voskresensky, I.; Dmowska, J.; Kilchrist, K.V.; Brophy, C.M.; Duvall, C.L. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci. Transl. Med. 2015, 7, 291ra95. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M.; Anderson, B.D.; Brooks, N.A.; Brooks, H.B.; Chan, E.M.; De Dios, A.; Gilmour, R.; Graff, J.R.; Jambrina, E.; Mader, M.; et al. Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol. Cancer Ther. 2014, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Son, S.-H.; Kim, N.-J.; Im, D.-S. p38 MAPK Inhibitor NJK14047 Suppresses CDNB-Induced Atopic Dermatitis-Like Symptoms in BALB/c Mice. Biomol. Ther. 2022, 30, 501–509. [Google Scholar] [CrossRef]
- Hou, K.; Xiao, Z.-C.; Dai, H.-L. p38 MAPK Endogenous Inhibition Improves Neurological Deficits in Global Cerebral Ischemia/Reperfusion Mice. Neural Plast. 2022, 2022, 3300327. [Google Scholar] [CrossRef]


| ILs Regulated | Role in Inflammation | References | Drugs Targeted for Inflammatory Arthritis |
|---|---|---|---|
| IL-1s, IL-1β | pro-inflammatory | [33,53] | Anakinra, Canakinumab, Rilonacept |
| IL-2 | anti and pro-inflammatory | [33] | Baciliximab, Daclizumab for IL-2R |
| IL-3 | pro-inflammatory | [33] | IL-3 inhibitor patent [54] |
| IL-6 | pro-inflammatory | [7,33] | Tocilizuma, Sarilumab (IL-6R); Siltuximab |
| IL-8 | pro-inflammatory | [33,55] | NA |
| IL-12 | pro-inflammatory | [55] | Ustekinumab, Birankizumab |
| IL-17 | pro-inflammatory | [56] | Secukinumab, Izekizumab |
| IL-18 | pro-inflammatory | [57,58] | NA |
| IL-23p19 | pro-inflammatory | [59] | Rizankizumab, Guselkumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, P.; Macleod, T.; Wong, C.; Harland, M.; McGonagle, D. Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i). Pharmaceuticals 2023, 16, 1286. https://doi.org/10.3390/ph16091286
Ganguly P, Macleod T, Wong C, Harland M, McGonagle D. Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i). Pharmaceuticals. 2023; 16(9):1286. https://doi.org/10.3390/ph16091286
Chicago/Turabian StyleGanguly, Payal, Tom Macleod, Chi Wong, Mark Harland, and Dennis McGonagle. 2023. "Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i)" Pharmaceuticals 16, no. 9: 1286. https://doi.org/10.3390/ph16091286
APA StyleGanguly, P., Macleod, T., Wong, C., Harland, M., & McGonagle, D. (2023). Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i). Pharmaceuticals, 16(9), 1286. https://doi.org/10.3390/ph16091286







