Abstract
Phosphodiesterase 5 (PDE5) inhibitors presented themselves as important players in the nitric oxide/cGMP pathway, thus exerting a profound impact on various physiological and pathological processes. Beyond their well-known efficacy in treating male erectile dysfunction (ED) and pulmonary arterial hypertension (PAH), a plethora of studies have unveiled their significance in the treatment of a myriad of other diseases, including cognitive functions, heart failure, multiple drug resistance in cancer therapy, immune diseases, systemic sclerosis and others. This comprehensive review aims to provide an updated assessment of the crucial role played by PDE5 inhibitors (PDE5-Is) as disease-modifying agents taking their limiting side effects into consideration. From a medicinal chemistry and drug discovery perspective, the published PDE5-Is over the last 10 years and their binding characteristics are systemically discussed, and advancement in properties is exposed. A persistent challenge encountered with these agents lies in their limited isozyme selectivity; considering this obstacle, this review also highlights the breakthrough development of the recently reported PDE5 allosteric inhibitors, which exhibit an unparalleled level of selectivity that was rarely achievable by competitive inhibitors. The implications and potential impact of these novel allosteric inhibitors are meticulously explored. Additionally, the concept of multi-targeted ligands is critically evaluated in relation to PDE5-Is by inspecting the broader spectrum of their molecular interactions and effects. The objective of this review is to provide insight into the design of potent, selective PDE5-Is and an overview of their biological function, limitations, challenges, therapeutic potentials, undergoing clinical trials, future prospects and emerging uses, thus guiding upcoming endeavors in both academia and industry within this domain.
1. Introduction
PDE5 inhibitors (PDE5-Is) are groundbreaking medications for treating ED. They increase cGMP levels, causing muscle relaxation and vasodilation in the penis, leading to erections. Their therapeutic potential extends beyond ED, with clinical approval for treating PAH, BPH, and LUTS. Research highlights their potential in diseases like cancer, neurological disorders, cystic fibrosis, and diabetes. Promisingly, this has incited the development of new PDE5-Is with higher potency, selectivity, and improved pharmacokinetics for enhanced efficacy. This review provides an update on the approved and potential uses, side effects, and published inhibitors, covering binding properties, selectivity, pharmacokinetics, and in vivo efficacy over the past decade, as well as the most recent clinical studies.
2. Classification of Phosphodiesterases
PDE superfamily comprises 11 families (PDE1–PDE11) that are encoded by 21 different genes, whose expression are modulated via multiple promotors and messenger RNA (mRNA) alternative splicing generating more than 50 isoforms [1,2]. It is worth noting that PDE12, which cleaves 2′,5′-phosphodiester bond linking adenosines of the 5′-triphosphorylated oligoadenylates, belongs to the C–C chemokine receptor 4 (CCR4)/nocturin family [3] and is not a member of the cyclic nucleotide PDE superfamily. PDE isoforms are classified based on their amino acid sequences, substrate specificities, catalytic and cofactor requirements, kinetic properties, regulatory mechanisms, and tissue distributions [1]. Some PDEs are selective for the hydrolysis of cAMP (PDE 4, 7, and 8) or cGMP (PDE 5, 6 and 9), while others can hydrolyze both cAMP and cGMP (PDE 1, 2, 3, 10 and 11) [4]. PDEs share a conserved catalytic domain (C domain) but differ significantly in their N-terminal regulatory domains. PDEs are mainly regulated via (i) binding of Ca2+/calmodulin (PDE1), (ii) phosphorylation/dephosphorylation events (PDE1, 3, 4 and 5) and (iii) allosteric binding of cGMP via GAF domains (PDE2, 5, 6, 10 and 11) [1]. The description of diverse tissue distribution/cell expression and functional significance of PDE isoenzymes is detailed in [5] and is beyond the scope of this review. Notably, such tissue/cellular compartmentalization allows selective PDE inhibitors to exert their effects almost exclusively on the target tissue.
Our focus herein is on the PDE5 family, which is generated by one gene, PDE5A, and has three alternative spliced variants, PDE5A1, 5A2 and 5A3. The three human PDE5 isoforms differ only in the 5’-end of the mRNA and the corresponding N-terminal of the protein. These isoforms have similar phosphorylation sites, allosteric cGMP-binding sites, catalytic domain and cGMP binding and hydrolysis activities [6]. However, PDE5A1 was reported to be more resistant to chemical inhibition than PDE5A2 or PDE5A3. PDE5A1 and PDE5A2 are widely distributed in nearly all tissues, whereas PDE5A3 is confined to vascular smooth muscle cells [2].
3. Tissues and Organs of High Expression for PDE5
PDE5 is present in virtually all cell types, tissues and organs. PDE5 is highly expressed in the smooth muscle cells of the peripheral arteries and venous vessels and in coronary and pulmonary arteries [2]. In addition, PDE5 is expressed in the vascular smooth muscle cells of the corpora cavernosa of the penis besides spermatozoa, peritubular myoid of Leydig cells and vas deferens in males [7]. PDE5 is widely distributed in the cytoplasmic cell compartment in myometrial cells, endothelial cells and peripheral blood mononuclear cells. It is also expressed in skeletal muscles, cardiomyocytes, platelets, lung, spinal cord, cerebellum, retina, pancreas, prostate, urethra and bladder [1,2,8]. PDE5A1 and PDE5A2 are further expressed in renal vessels, glomeruli, tubular epithelial cells of the renal proximal tubule and medullary collecting duct [9]. Consequently, PDE5 isoforms exhibit diverse and numerous functions both in physiological and pathological conditions.
4. PDE5 Physiological Role
Nitric oxide (NO) is synthesized from the precursor L-arginine through the activities of different NO synthases (neuronal, inducible or endothelial NOS). Intracellularly, NO binds to and activates soluble guanylyl cyclase (sGC), promoting the conversion of guanosine triphosphate (GTP) to the second messenger cyclic guanosine monophosphate (cGMP) [10,11]. Thereafter, cGMP activates protein kinase G (PKG), whose phosphorylation mediates activities of various membrane channels/pumps, leading to decreased calcium influx through L-type calcium channels and increased calcium sequestration, resulting in smooth muscle relaxation and vascular tone modulation [12]. PKG-dependent phosphorylation of other various downstream proteins can regulate further pivotal physiological functions, such as cell differentiation and proliferation, endothelial permeability, ion transport, secretion and gene transcription [13].
Given the broad expression and the ability of PDE5 to specifically hydrolyze cGMP, controlling its cellular levels, PDE5 has been proposed as a crucial player in many NO/cGMP/PKG-dependent biological processes such as smooth muscle relaxation, heart muscle contraction, platelet activation/aggregation and immune response [14].
PDE5 inhibition was found to enhance smooth muscle relaxation and vasodilation, which in the penis corpus cavernosum favors erection, in the pulmonary vasculature decreases pulmonary vessels’ pressure, and in the systemic circulation decreases arterial blood pressure [15].
In addition, PDE5 is an important regulator of platelet function, whose inhibition increases platelet cGMP levels and augments the ability of NO to inhibit platelet aggregation and activation [16].
Furthermore, PDE5 governs fundamental physiological processes in the kidney. It can regulate renal vascular blood flow by hampering cGMP-mediated vascular relaxation. PDE5 is also a negative regulator for cGMP-dependent natriuresis. Moreover, it increases renin synthesis by degrading cGMP in juxtaglomerular cells [17].
5. PDE5 as a Drug Target for Disease Treatment
Competitive PDE5-Is reported so far exclusively bind to the catalytic domain, preventing cGMP hydrolysis and elevating its levels in cells of various tissues [18]. The subsequent activation or restoration of normal NO/cGMP/PKG signaling cascade prompted the use of these inhibitors as therapeutics for several clinical indications. Food and Drug Administration (FDA)-approved PDE5-Is (Figure 1) include (i) sildenafil (approved in 1998 for erectile dysfunction (ED) as Viagra®, and in 2005 for pulmonary arterial hypertension (PAH, WHO Group I) as Revatio®), (ii) vardenafil (approved in 2003 for ED as Levitra®), (iii) tadalafil (approved in 2003 for ED as Cialis®, in 2009 for PAH (WHO Group I) as Adcirca® and in 2011 for lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH) with or without ED and the most recent (iv) avanafil (approved in 2012 for ED as Stendra®) [19,20].
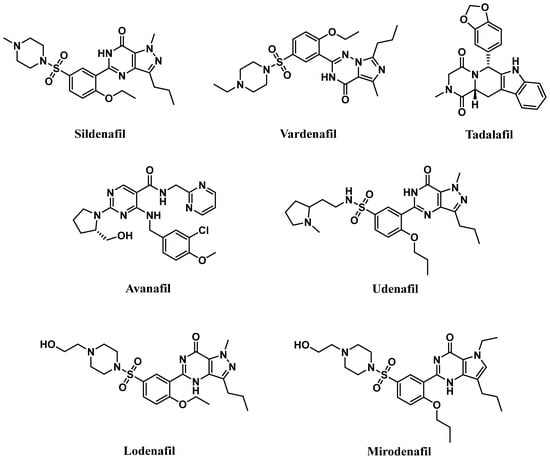
Figure 1.
Chemical structures of marketed PDE5 inhibitors.
These inhibitors differ in their selectivity, potency, onset and duration of action, cost, administration considerations, precautions, and adverse effects profiles. The relative potency of vardenafil for PDE5 was reported to be the highest (PDE5 IC50 of 0.1–0.4 nM), followed by tadalafil (PDE5 IC50 of 2 nM), and then sildenafil and avanafil (PDE5 IC50 of 4 nM and 4.3–5.2 nM, respectively). They all share a quick onset time in the range of 11–16 min after oral administration, with avanafil advertised as the fastest-acting. Plasma half-lives of sildenafil and vardenafil are similar, about 4 h, and their efficacy of action lasts up to 12 h. Avanafil half-life is shorter, about 3 h, with a maximal duration of action of 6 h. Tadalafil’s half-life is the longest, 17.5 h, with an efficacy maintained for up to 36 h [18,20,21,22].
A new generation of PDE5-Is, namely lodenafil, udenafil, and mirodenafil are also available in Brazil and Korea for ED treatment (Figure 1), but none of them have been FDA-approved, yet [23].
Aside from the three FDA-approved clinical indications, PDE5-Is have been intensively investigated for their potential use in the treatment of various emerging indications, such as cancer, central nervous system (CNS) and cardiovascular system (CVS) related diseases, kidney diseases, cystic fibrosis and diabetes, all of which will be discussed herein.
5.1. Approved Clinical Uses of PDE5 Inhibitors
5.1.1. Erectile Dysfunction
In the corpora cavernosa, parasympathetic stimulation and sexual arousal induce the release of NO from endothelial cells and nitrergic neurons surrounding the arteries and sinusoids, leading to increased cGMP synthesis. PDE5-Is can slow the degradation of penile connective tissue cGMP. This leads to a drop in the intracellular Ca2+ levels in the corpus cavernosum smooth muscles, causing their relaxation and a reduction in arterial blood drainage, providing a sufficient degree of penile tumescence and sustaining penile erection (Figure 2). Accordingly, it can be deduced that the action of PDE5-Is requires normal neuronal input into the erectile tissues, as well as unimpaired cavernous endothelial structures [24,25].
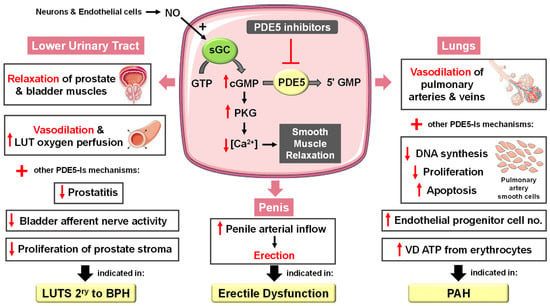
Figure 2.
Approved clinical uses of PDE5 inhibitors. Nitric oxide (NO) is produced by neurons and endothelial cells. Inside smooth muscle cells, NO activates soluble guanylyl cyclase (sGC), promoting the conversion of guanosine triphosphate (GTP) to the second messenger cyclic guanosine monophosphate (cGMP). Thereafter, cGMP activates protein kinase G (PKG), whose phosphorylation mediates activities of various membrane channels/pumps, leading to decreased intracellular calcium levels resulting in smooth muscle relaxation (SMR). Phosphodiesterase 5 (PDE5) regulates cGMP levels by degrading it into inactive 5′ guanosine monophosphate (5′ GMP). PDE5-Is can thus enhance the cGMP/PKG pathway, boosting the relaxation of various smooth muscles. In the penis corpus cavernosum, SMR favors erection due to increased penile arterial inflow, and thus PDE5-Is are approved for the treatment of erectile dysfunction. In the lungs, PDE5-Is lead to vasodilation of pulmonary vasculature, which, along with other mechanisms, such as suppressed DNA synthesis and proliferation and enhanced apoptosis of pulmonary artery cells, increased endothelial progenitor cell number, and enhanced release of vasodilating adenosine triphosphate (ATP) from erythrocytes culminate in effectiveness in the treatment of pulmonary arterial hypertension (PAH). In the lower urinary tract (LUT), PDE5-Is mediate prostate and bladder SMR, vasodilation and increased LUT oxygen perfusion. In addition, PDE5-Is could suppress prostatitis, bladder afferent nerve activity and prostate stroma cell proliferation, and thus indicated in the treatment of LUT symptoms secondary to benign prostatic hyperplasia (BPH).
PDE5-Is are thus considered the first-line choice for on-demand and chronic treatment of most ED cases. The efficacy and safety of the four FDA-approved PDE5-Is (sildenafil, vardenafil, tadalafil and avanafil) have been confirmed by a multitude of worldwide clinical trials involving thousands of ED patients with diverse etiologies that were documented by several reviews [19,26,27,28,29,30].
It is worth noting that the few differences between sildenafil, tadalafil and vardenafil pharmacokinetics allow tadalafil, with a longer half-life, to be superior in a number of sexual intercourses per pill, while vardenafil and sildenafil exhibited privilege whenever duration of erection, or vascular efficacy and penile hardness are explored [31,32].
5.1.2. Pulmonary Arterial Hypertension
PAH is a disease associated with endothelial dysfunction, vascular remodeling and fibrosis that causes gradual progression of pulmonary vascular resistance, ultimately leading to right heart failure. Accordingly, PAH therapies usually aim to enhance vasodilation, suppress cellular hyperproliferation and induce apoptosis [33].
PDE5 is highly expressed in the lung vasculature [34]. The fact that lung endothelial NOS is reduced [35] and PDE5 is upregulated in the remodeled pulmonary artery during PAH has proposed PDE5-Is as a potential PAH treatment [36]. A plethora of PDE5 inhibition-mediated mechanisms have been documented (Figure 2) including (i) activation of the NO/cGMP/PKG pathway, resulting in decreased calcium influx through L-type calcium channels and increased calcium sequestration, inducing vasorelaxation [37], (ii) suppression of DNA synthesis and cell proliferation and stimulation of apoptosis of pulmonary artery smooth cells whose proliferation is involved in the pathogenesis of intimal hyperplasia and major vascular lesions in PAH [38], and (iii) increasing circulating endothelial progenitor cell (EPC) number [39].
Several clinical studies confirmed the potential of PDE5-Is to improve several hemodynamic and clinical parameters in PAH patients [40,41,42,43], such as diminishing pulmonary artery systolic and mean artery pressure, dyspnea score and gas transfer, pulmonary vascular resistance and cardiac output [44]. Furthermore, PDE5-Is could improve ventilatory efficiency and oxygen uptake kinetics and prevent exercise-induced pulmonary edema [45]. Vardenafil usually exhibits the most rapid effect on pulmonary vasorelaxation, while sildenafil and tadalafil are more selective for pulmonary circulation. Substantial enhancement of arterial oxygenation is mainly observed with sildenafil [46].
Sildenafil, in 2005, and, thereafter, tadalafil have been FDA approved and became first-line therapies for PAH [47], primary or secondary to other connective tissue diseases, such as scleroderma (SSc) or systemic lupus erythematosus (SLE) [34].
PDE5-Is can also be used as combination therapy with other PAH-targeted treatments. The combination of sildenafil and long-term intravenous epoprostenol therapy was superior to epoprostenol monotherapy regarding improved exercise capacity, hemodynamic measurements and prolonged time to clinical worsening [48]. Other combinations, such as tadalafil with the endothelin receptor antagonist ambrisentan and sildenafil with systemic nitrates [49], were proven safe and effective in potentiating vasodilation and reducing mortality in PAH patients. Moreover, combined prostacyclin analogs and PDE5-Is were reported to synergistically enhance the release of the potent vasodilator ATP from PAH erythrocytes [50].
PDE5 inhibition has also emerged as a therapeutic strategy for high-altitude PAH where sildenafil’s ability to reverse hypoxia-mediated pulmonary vasoconstriction was proved to mediate positive results on exercise performance and lung hemodynamics [51,52].
5.1.3. Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia
Several studies have established an association between ED and BPH-related LUTS where alterations in the NO/cGMP pathway, alterations in RhoA/Rho kinase/endothelin signaling, pelvic atherosclerosis, autonomic adrenergic hyperactivity, inflammatory pathways, sex hormones and psychological factors were the major contributing factors [53,54]. Accordingly, attention was drawn towards the development of a single therapy to treat both conditions.
The clinical benefits of chronic PDE5 inhibition on LUTS secondary to BPH, regardless of whether these symptoms are associated with ED, are well documented [55]. These beneficial effects have been correlated to several mechanisms (Figure 2), including (i) stromal smooth muscle relaxation of the prostate and bladder due to modulation of the NO/cGMP pathway in the nitrinergic innervated organs or enhanced generation of relaxing hydrogen sulfide, (ii) significant cGMP-mediated dilatation of local blood vessels, (iii) enhanced LUT oxygen perfusion, (iv) inhibition of afferent nerve activity of bladder, (v) down-regulation of prostate inflammation and (vi) negative regulation of proliferation and trans-differentiation of the prostate stroma [54,56,57].
Many preclinical studies of PDE5 and its inhibitors in the prostate and bladder (reviewed in [58]) could validate the role of PDE5-Is in relaxing prostatic tissue, improving the severity of urinary symptoms, reducing bladder overactivity, decreasing indicators of bladder ischemia, normalizing changes in NOS activity and preventing the accumulation of collagen [59].
Several clinical trials demonstrated that the use of PDE5-Is alone could ameliorate LUTS in the first 12 weeks of treatment, where sildenafil [60], tadalafil [61,62,63] and vardenafil [64] led to a decrease, at different degrees, in the International Prostate Symptom Score (IPPS) scale. In particular, the effects of tadalafil 5 mg once daily versus placebo on LUTS/BPH have been extensively investigated (reviewed by Gacci et al. [65]). Only tadalafil (5 mg once daily) has been licensed for the treatment of LUTS with or without ED.
The combined administration of sildenafil, tadalafil or vardenafil with the α1-adrenoceptor antagonists alfuzosin or tamsulosin for the treatment of LUTS/BPH has also been evaluated and was confirmed to often outperform either type of monotherapy [66,67,68,69,70]. Interestingly, a very recent meta-analysis of randomized clinical trials demonstrated that tadalafil could be superior to tamsulosin in treating LUTS/BPH when associated with ED [71].
5.2. Emerging and Future Uses of PDE5 Inhibitors
5.2.1. Cancer
Numerous studies have reported the role of cGMP in suppressing cell growth and inducing apoptosis and that elevated PDE5 expression is involved in the progression of various tumor types, such as chronic lymphocytic leukemia, colon adenocarcinoma, bladder squamous carcinoma, human papillary thyroid carcinomas, metastatic breast, prostate, pancreatic and lung cancers [72,73,74]. Accordingly, PDE5 has gained attention as a promising target for anticancer drug discovery. Over the last two decades, several pre-clinical and clinical studies revealed potential anti-cancer effects of PDE5-Is [75,76] that were mediated via different mechanisms of action discussed herein (Figure 3).
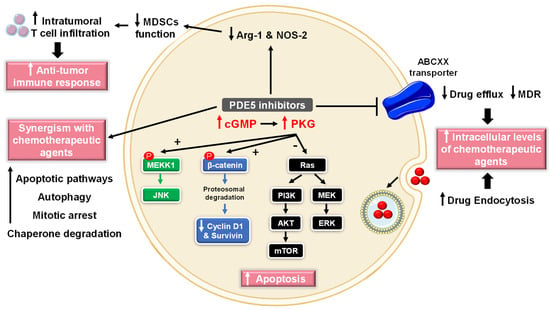
Figure 3.
Anti-cancer mechanisms of PDE5 inhibitors. Via activation of the cGMP/PKG signaling cascade, PDE5-Is can induce apoptosis in cancer cells via various pathways; activation of c-Jun NH2-terminal kinase (JNK) via phosphorylation of mitogen-activated protein kinase kinase kinase 1 (MEKK1), phosphorylation of β-catenin and inducing its proteosomal degradation which leads to decreased expression of Wnt/β-catenin regulated proteins, such as cyclin D1 and survivin in addition to blocking the phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathways. PDE5-Is could also increase intracellular levels of other chemotherapeutic agents via inhibition of the ATP-binding cassette (ABC) transporter-mediated drug efflux, averting multidrug resistance (MDR) in addition to increasing cellular drug uptake via enhancing endocytosis. Moreover, PDE5-Is synergize with other chemotherapeutic agents via boosting various apoptotic, autophagy, mitotic arrest and chaperone degradation pathways. PDE5-Is can also abrogate the function of myeloid-derived suppressor cells (MDSCs) via suppression of arginase-1 (Arg-1) and nitric oxide synthase–2 (NOS-2) production. This results in enhanced intratumoral T-cell infiltration and activation and restores both systemic and tumor-specific immunity. P = phosphorylation.
- (1)
- Cell growth arrest and induction of apoptosis
Sildenafil and vardenafil were reported to induce caspase-dependent apoptosis and antiproliferative effects in B-cell chronic lymphatic leukemia [77]. Moreover, sildenafil was shown to boost intracellular reactive oxygen species (ROS) levels, induce cell cycle arrest, and suppress cell proliferation in colorectal cancer cells [78]. In addition, multiple studies have validated the proapoptotic effects of exisulind (sulindac sulfone) and sulindac sulfide (SS), two metabolites of the non-steroidal anti-inflammatory drug (NSAID) sulindac, in breast, colorectal and metastatic prostate cancers. Exisulind or SS increases the activation of cGMP-dependent PKG, triggering a series of signaling events (Figure 3), including (i) phosphorylation of β-catenin and inducing its proteosomal degradation which leads to decreased expression of Wnt/β-catenin regulated proteins, such as cyclin D1 and survivin, (ii) activation of c-Jun NH2-terminal kinase (JNK) via phosphorylation of mitogen-activated protein kinase kinase kinase 1 (MEKK1), and (iii) blocking the phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathways, all of which culminate in triggering apoptosis cascade [79,80,81,82,83,84].
- (2)
- Chemotherapy sensitization
Several studies provided evidence that PDE5-Is can increase cellular concentrations of standard chemotherapeutic drugs or even enhance their efficacy within certain tumor cells where a combination of potential agents allows the reduction of dose levels and, consequently, of toxic side effects (Figure 3) [76,85,86,87].
One of the major causes of chemotherapy failure in cancer treatment is multidrug resistance (MDR) attributed to overexpression of the ATP-binding cassette (ABC) transporters, such as P-glycoprotein (ABCB1/P-gp/MDR1), multidrug-resistance proteins (ABCCs/MRPs) and breast cancer resistant protein (ABCG2/BCRP). These transporters actively expel chemotherapeutic agents out of the cancer cell, ameliorating their cellular efficacy [88]. Vardenafil was reported to inhibit the drug efflux in ABCB1-overexpressing cells [89], while sildenafil was effective in opposing the activity of ABCB1 and ABCG2, both attenuating MDR in tumor cells [90].
Another study showed that PDE5-Is can increase cellular uptake of structurally diverse compounds into lung cancer cells both in vitro and in vivo via modulation of endocytosis [85]. Moreover, oral administration of sildenafil and vardenafil was found to actively enhance blood tumor barrier (BTB) permeability and boost the efficacy of chemotherapy in a rat brain tumor model [91]. Vardenafil could also enhance the delivery and therapeutic efficacy of herceptin monoclonal antibodies in mouse models of metastatic HER2/neu-positive brain tumors through stimulating caveolae-mediated endocytosis and micropinocytosis [92].
Besides augmenting the delivery of chemotherapeutic agents, PDE5-Is can suppress tumor growth and induce cell death by synergizing with current chemotherapy medications in treating a wide range of cancers (Figure 3).
Celecoxib and PDE5-Is synergize in a NOS-dependent cyclooxygenase (COX)-independent fashion to kill multiple tumor cell types, including human glioma cells, as well as their associated activated microglia in vitro and could suppress the growth of mammary tumors in vivo. The drug combination increased the levels of autophagy by inactivating mTOR and inducing endoplasmic reticulum (ER) stress responses in these cells [93].
A combination of sildenafil with various standard chemotherapy agents was proved effective in various gastrointestinal/genitourinary cancers, such as bladder and colon cancers [87]. A combination of the topoisomerase inhibitor doxorubicin and sildenafil resulted in increased efficacy against prostate cancer cells through ROS generation and subsequent upregulation of pro-apoptotic proteins Bad and Bax and downregulation of anti-apoptotic proteins Bcl-2 and Bcl-xL, amplifying caspase-mediated apoptotic death [94]. In a later study, sildenafil and vardenafil but not tadalafil were found to induce PDE5-independent apoptotic sensitization to doxorubicin in castration-resistant prostate cancer (CRPC) cells through impairment of both homologous recombination (HR) and non-homologous end joining (NHEJ) DNA repair pathways [95]. Furthermore, both in vitro and in vivo studies suggested that sildenafil could synergistically potentiate vincristine-induced mitotic arrest signaling and sensitize caspase-dependent apoptosis in CRPC cells via a mitochondrial damage pathway [96].
The multi-kinase inhibitors sorafenib/regorafenib in combination with sildenafil were reported to suppress xenograft tumor growth using liver and colon cancer cells in a greater than additive manner via various autophagy and intrinsic and extrinsic apoptotic pathways [97]. In multiple genetically diverse lung cancer cell lines, sildenafil increased the lethality of pemetrexed and sorafenib combination via fully inactivating signaling by multiple cytoprotective proteins, including the AKT/ERK pathways, nuclear factor-κB (NF-κB) and STAT3/STAT5 besides enhancing death receptor expression and activation [98].
Treatment of stem-like glioblastoma cells with a combination of OSU-03012 (a non-COX-2 inhibiting derivative of celecoxib) and sildenafil abolished the expression of multiple oncogenic growth factor receptors and plasma membrane drug efflux pumps and caused rapid degradation of glucose-regulated protein (GRP78) and other chaperones in tumor cells. This downregulates key oncogenic kinases, including PI3K/AKT signaling, leading to tumoricidal effects [99]. Similarly, sildenafil alone or in combination with the heat shock protein 90 (HSP90) inhibitor PU-H71 could alter the expression of HSP90 chaperone followed by degradation of the oncogenic protein kinase D2 impairing proliferation and viability of various tumor cell lines [100]. These studies suggest a combination of PDE5 and chaperone inhibitors as a novel, promising strategy for targeting cancer.
- (3)
- Modulation of antitumor immune response
PDE5 inhibition contrasts tumor-induced immunosuppressive mechanisms and generates a measurable antitumor-immune response that significantly delays tumor progression. Both sildenafil and tadalafil could abrogate the function of myeloid-derived suppressor cells (MDSCs) via suppression of arginase-1 (Arg-1) and nitric oxide synthase–2 (NOS-2) production. This resulted in enhanced intratumoral T-cell infiltration and activation and restored both systemic and tumor-specific immunity in multiple myeloma and head and neck cancer patients (Figure 3) [101,102].
- (4)
- Chemopreventive mechanisms
A nationwide population-based study in Sweden suggested that the use of PDE5-Is was associated with a lower risk of colorectal cancer among male patients with benign colorectal neoplasm [103]. Moreover, two very recent studies provided evidence that sildenafil was more effective than tadalafil in preventing the development and progression of aflatoxin B1-induced hepatocellular carcinoma. This beneficial effect was attributed to a plethora of mechanisms, including (i) improved enzymatic antioxidant system capacity with a concomitant decline in the level of lipid peroxidation, (ii) increase in activity of glutathione S-transferase, (iii) downregulation of glucose transporter 1 (GLUT1) restoring normal declined blood glucose levels in tumor cells, (iv) inhibition of lactate dehydrogenase dependent glycolytic machinery, (v) vasodilation of blood vessels resulting in decreased tumor hypoxia and downregulation of the angiogenesis markers; hypoxia-inducible factor 1-alpha (HIF-1α), transforming growth factor-beta 1 (TGF-β1) and vascular endothelial growth factor A (VEGFA) [104,105]. PDE5-Is have also been shown to suppress the stemness of PC3-derived cancer stem cells (PCSCs) that were confirmed essential for the initiation, progression and recurrence of prostate cancer. cGMP-dependent PKG promotes mammalian sterile 20-like kinase/large tumor suppressor (MST/LATS) kinases, leading to cytosolic degradation of the oncogenic protein Tafazzin (TAZ) and the activation of the Hippo pathway, a crucial player in modulating stemness of PCSCs [106].
5.2.2. CNS Diseases
cGMP/PKG signaling has been regarded as a central mechanism of neuroinflammation, neurodegeneration and cognitive disorders [106,107]. Accordingly, PDE5-Is have gained growing attention as potential therapeutic agents for the treatment of several CNS-related diseases, such as Alzheimer’s disease (AD), cognitive deficits, strokes, multiple sclerosis (MS), depression, noise-induced hearing loss (NIHL) and neuropathic pain that will all be discussed in this section (Figure 4).
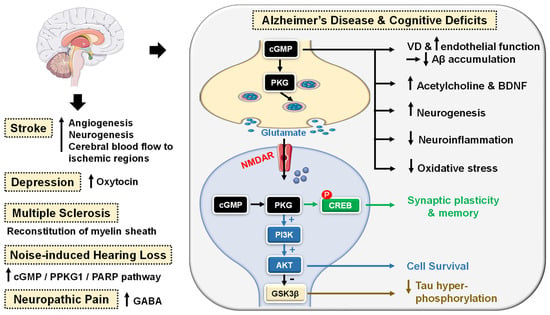
Figure 4.
Emerging central nervous system (CNS)-related indications of PDE5 inhibitors. In Alzheimer’s disease (AD) and cognitive deficiency disease models, PDE5 inhibition increases presynaptic cGMP levels, which, through PKG activation, enhances the release of glutamate and activates N-methyl-D-aspartate receptors (NMDAR). On the other hand, postsynaptic PKG activates transcription factor cyclic adenosine monophosphate (cAMP) response element-binding element (CREB), promoting neurotransmission, synaptic plasticity and memory consolidation. PKG also activates the PI3K/AKT signaling pathway that mediates neuroprotection via the inhibition of apoptosis and also suppresses tau hyper-phosphorylation via inhibition of glycogen synthase kinase-3 beta (GSK3β). Elevated cGMP levels exhibit other cognitive enhancement mechanisms, such as vasodilation, which improves or maintains cerebrovascular endothelial function, preventing Aβ amyloid accumulation, rise in acetylcholine (ACh) and brain-derived neurotrophic factor (BDNF) levels in the cortex, striatum, and other areas of the brain, facilitation of neurogenesis, suppression of neuroinflammation and oxidative stress, all averting neuronal loss. In strokes, PDE5-Is could induce angiogenesis and neurogenesis and enhance cerebral blood flow to ischemic regions. PDE5-Is have anxiolytic effects in part due to enhanced oxytocin release. Moreover, PDE5-Is can promote efficient reconstitution of the myelin sheath and govern the Inflammatory processes involved in demyelination models of multiple sclerosis. PDE5-Is are also beneficial in noise-induced hearing loss via activating cGMP/protein kinase cGMP-dependent 1/poly (ADP-ribose) polymerase (cGMP/PRKG1/PARP) signaling in response to traumas in cochlea sensory cells. PDE5-Is exhibit pain-relieving effects in neuropathic pain models via enhanced release of gamma-aminobutyric acid (GABA). P = phosphorylation.
PDE5 inhibition increases presynaptic cGMP levels, which, through PKG activation, enhances the release of glutamate and activates N-methyl-D-aspartate (NMDA) receptors. On the other hand, postsynaptic PKG activates transcription factor cyclic adenosine monophosphate (cAMP) response element-binding element (CREB), promoting neurotransmission, synaptic plasticity and memory consolidation [108,109]. PKG also activates the PI3K/AKT signaling pathway that mediates neuroprotection via the inhibition of apoptosis (Figure 4) [110].
The upregulation of PDE5 expression in the brains of AD patients and the subsequent drop in cGMP levels have been linked to the elevation of Aβ amyloid peptide, whose deposition in the brain is the main hallmark of AD [111]. Sabayan et al. described PDE5-Is as disease-modifying agents against AD and proposed three main mechanisms for their action: (i) vasodilation, which improves or maintains cerebrovascular endothelial function preventing Aβ amyloid accumulation; (ii) cGMP-dependent rise in acetylcholine (ACh) levels in the cortex, striatum, and other areas of the brain, reversing low-ACh associated memory and cognitive deficits in AD, and finally (iii) inhibition of apoptosis and facilitation of neurogenesis averting neuronal loss (Figure 4) [112].
For example, chronic administration of sildenafil completely reversed cognitive impairment in Tg2576 transgenic mice without changing Aβ load. The underlying mechanism involved suppression of tau hyperphosphorylation and inhibition of glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (CDK5) [113]. In addition, Puzzo et al. and Zhang et al. showed that chronic administration of sildenafil in amyloid precursor protein/presenilin-1 (APP/PS1) transgenic mice could reverse AD-related cognitive deficits and synaptic dysfunction via improving cGMP/PKG/CREB signaling, inhibiting neuroinflammation and reducing hippocampal Aβ levels [114,115].
Chronic treatment with tadalafil even exhibited a higher beneficial effect, probably due to its longer half-life and could improve spatial memory in the J20 mouse model of AD by decreasing tau protein via the activation of the AKT/GSK3β pathway [116]. Most recently, mirodenafil was reported to ameliorate Aβ-induced AD pathology and improve cognitive behavior in the APP-C105 mouse model through the modulation of the cGMP/PKG/CREB signaling pathway, GSK-3β activity, glucocorticoid receptor transcriptional activity and Wnt/β-catenin signaling in neuronal cells (Figure 4) [107].
Preclinical studies proved that PDE5-Is could boost memory and synaptic plasticity by augmenting the NO/cGMP/PKG pathway [107,117]. In mouse models with induced cognitive deficits, sildenafil could improve novel object recognition, ameliorate cognitive impairment and upregulate the brain-derived neurotrophic factor (BDNF), contributing to neuroprotective effects [118,119]. Another study showed the potential of sildenafil to defy neurological stress, increase neuroprotection and restore cognitive functions in the hippocampus region of noise alone-induced mice via modulation of cGMP/PKG/CREB and p25/CDK5 pathways and induction of various free radical scavengers in the brain of stressed mice [120]. A similar alleviation n of oxidative stress in the hippocampus of aged mice has been observed upon chronic tadalafil administration as well (Figure 4) [121].
Very recent reviews by Liu et al. [122] and Zuccarello et al. [123] summarized clinical trials of PDE5-Is in cognition and AD. However, none of the investigated drugs has reached the market for those indications so far.
Numerous animal models investigated the potential role of PDE5 inhibition in stroke. In these studies, PDE5-Is could induce angiogenesis, enhance cerebral blood flow to the ischemic region, increase neurogenesis and advanced functional post-stroke recovery [124,125,126]. In particular, sildenafil treatment for two weeks (25 mg daily) was proven safe in patients who suffered mild to moderate strokes [127]. Additionally, tadalafil could attenuate ischemia-induced short-term memory impairment by suppressing ischemia-induced neuronal apoptosis [128].
Further mechanisms for PDE5 inhibition-induced neurogenesis have been reported and include AKT/GSK3β phosphorylation [129] or triggering proliferation of neural stem cells (NSC) via a mitogen-activated protein kinase (MAPK) dependent signaling cascade [130].
Moreover, preclinical studies have provided further evidence of sildenafil’s neuroprotective potential observed against Aβ amyloid-induced mitochondrial toxicity [131]. Additionally, 3-nitropropionic acid-induced behavioral and biochemical toxicities in a Huntington’s disease rat model [132].
Interestingly, a clinical study showed that single-dose sildenafil could improve regional cerebrovascular reactivity deficits in chronic traumatic brain injury patients as well [133].
Sildenafil has also been reported to promote efficient reconstitution of the myelin sheath and govern the inflammatory processes involved in demyelination models of MS [134]. Sildenafil could also normalize experimental autoimmune encephalomyelitis in MS mouse models [135].
Administration of sildenafil or tadalafil could yield significant anxiolytic-like effects in rodent genetic models of depression as well due to chronic activation of the NO/cGMP system [136,137]. Another reported mechanism for the antidepressant-like effect of sildenafil involved the activation of the oxytocin [138].
Jaumann et al. unveiled a potential protective role of activated cGMP/protein kinase cGMP-dependent 1/poly (ADP-ribose) polymerase (cGMP/PRKG1/PARP) signaling in response to traumas in cochlea sensory cells of various animal models. These data suggested PDE5 as a valid target for the improvement of NIHL. In particular, treatment of rodent models with vardenafil before or 6 h after acoustic trauma was shown to diminish auditory-evoked brain stream response thresholds in all frequency ranges tested [139].
Several animal studies have also proposed a beneficial pain-relieving effect of PDE5-Is in models of lesional [140,141] or metabolic neuropathic pain [142]. Sildenafil could ameliorate neuropathic pain symptoms in patients with diabetic peripheral neuropathy [143] and showed an antinociceptive effect in Sprague–Dawley male rats’ neuropathic pain models [144]. Mechanistically, this analgesic effect has been correlated to cGMP-dependent enhanced release of gamma-aminobutyric acid (GABA) [144].
5.2.3. Cardiovascular Diseases
Cardiomyocytes normally express a minimal basal level of PDE5. However, cardiac PDE5 expression was reported to be upregulated in hypertrophic, dilated, and ischemic cardiomyopathy and in congestive heart failure [47,145,146]. The protective effects of PDE5-Is against myocardial infarction (MI), cardiac ischemic and reperfusion (I/R) injury were validated in many in vitro studies with sildenafil [147], tadalafil [148,149], and vardenafil [150]. When given either prior to occlusion or at reperfusion, these PDE5-Is could reduce infarct size, attenuate cardiac hypertrophy, improve left ventricular (LV) function and prevent progression to heart failure.
In a mouse model, sildenafil exhibited a preconditioning effect to protect the heart against necrosis and apoptosis [151]. Another study suggested that the cardioprotective effect of sildenafil in female mice is estrogen-dependent as ovariectomy suppressed its anti-hypertrophic effect [152].
Intramyocardial transplantation of human adipose stem cells (ASCs) is regarded as a potential treatment for post-ischemic heart failure. Hoke et al. showed that preconditioning of ASCs with sildenafil could trigger the release of significantly high levels of pro-angiogenic or pro-survival growth factors, which enhance ASCs survival and therapeutic efficacy in cardiac ischemic microenvironment, allowing successful cardiac regeneration [153].
Tadalafil also showed cardioprotective effects via PKG-dependent generation of hydrogen sulfide [154]. Moreover, tadalafil was suggested to be clinically beneficial in metabolic syndrome (MetS) patients who are at high risk for CVS diseases where it improved insulin sensitivity, lowered circulating lipids, improved LV diastolic dysfunction and protected against I/R injury in MetS mice [155].
PDE5-Is manifested more significant protective effects against advanced heart failure (HF) with reduced ejection fraction than in HF with preserved ejection fraction [156]. Sildenafil could suppress chamber and myocyte hypertrophy and reverse preestablished hypertrophy in mice exposed to chronic pressure overload. This anti-hypertrophic effect was mediated by the deactivation of multiple signaling pathways, including the calcineurin/nuclear factor of activated T-cells (NFAT), PI3K/AKT, and ERK1/2 signaling pathways [157]. Furthermore, several clinical studies have confirmed the potential role of sildenafil in improving cardiac output, endothelial function, muscle perfusion, and exercise ventilatory and aerobic efficiencies in systolic HF patients [158,159,160].
Moreover, prophylactic treatment with either sildenafil or tadalafil improved cardiac contractile function and survival by attenuating doxorubicin-induced apoptosis and cardiac oxidative stress without interfering with the antitumor efficacy of doxorubicin in both in vitro and in vivo tumor models [161,162].
PDE5 inhibition could govern two crucial vascular manifestations of essential hypertension as well via diminishing blood pressure and improving arterial stiffness and endothelial dysfunction [163].
In addition, sildenafil elicited a significant decrease in inducible ventricular tachycardia and ventricular fibrillation in animal models and demonstrated protection against ventricular arrhythmias associated with the early stages of cardiac ischemia or following MI [164,165].
PDE5-Is could also inhibit platelet activation and aggregation [166,167]. Sildenafil, in particular, was demonstrated to (i) improve coronary patency in an animal model [168], (ii) reduce thrombosis, thromboembolic events, and the risk of thrombotic strokes in a clinical study [169], and (iii) potentiate the anti-aggregation effect of NO donors via cGMP-dependent and independent pathways [170].
Owing to their vasoactive effects, both sildenafil and tadalafil showed advantages in minimizing skin flap necrosis and in preventing extremity and flap ischemia in patients with Raynaud’s phenomenon and with scleroderma [171,172].
Kloner et al. thoroughly investigated the cardiovascular safety profile of PDE5-Is published in the last two decades and confirmed their safety [173].
Cardio protection achieved by PDE5-Is is mainly attributed to restoring high cGMP levels in cardiomyocytes that govern diverse cardioprotective mechanisms as follows (Figure 5): (i) vascular tone regulation and release of endogenous cardioprotective molecules, such as adenosine, bradykinin and phenylephrine from endothelial cells [174], (ii) PKG-dependent opening of mitochondrial and sarcolemmal ATP-sensitive potassium channels modulating calcium homeostasis and survival of cardiomyocytes, preventing post-infarct LV remodeling and reducing infarct size [175,176], (iii) PKG-dependent suppression of adrenergic drive which reduces nerve growth factor leading to anti-arrhythmic effects [164], (iv) ischemic post-conditioning protection against MI via PKG-dependent enhancement of Na+/K+-ATPase activity [177] and inhibition of Na+/H+-exchanger, delaying normalization of pH during reperfusion [178], (v) suppression of protein kinase C (PKC) and calcineurin culminating in improved contractility and protection against HF [179], (vi) improving mitochondrial ultrastructure and function via increased sirtuin-3 (Sirt3) protein expression and decreased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) acetylation protecting against post-infarction HF [180], and (vii) inhibition of RhoA/Rho-kinase pathway [181].
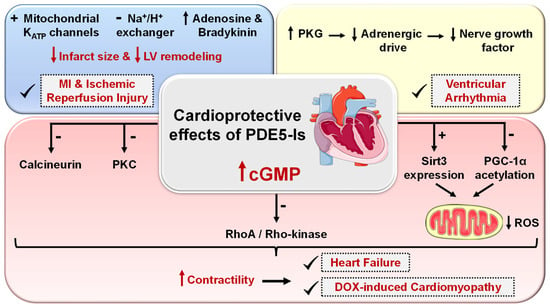
Figure 5.
Cardioprotective effects of PDE5 inhibitors. PDE5-Is restore high cGMP levels in cardiomyocytes that govern diverse downstream cardioprotective mechanisms: (i) PKG-dependent opening of mitochondrial and sarcolemmal ATP-sensitive potassium channels, inhibition of Na+/H+-exchanger and release of endogenous cardioprotective molecules, such as adenosine, bradykinin from endothelial cells; resulting in reduced infarct size and hampered post-infarct left ventricular (LV) remodeling. All are beneficial for ischemic post-conditioning protection against myocardial infarction (MI) and ischemic reperfusion (I/R) injury, (ii) PKG-dependent suppression of adrenergic drive which reduces nerve growth factor leading to anti-arrhythmic effects, (iii) suppression of protein kinase C (PKC), calcineurin and RhoA/Rho-kinase pathways and (vi) suppression of oxidative stress and improving mitochondrial ultrastructure and function via increased sirtuin-3 (Sirt3) protein expression and decreased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) acetylation, all culminating in improved cardiac contractility and protection against heart failure (HF) and doxorubicin(dox)-induced cardiomyopathy.
5.2.4. Kidney Diseases
Coskuner and coauthor [17] and Afsar et al. [182] thoroughly investigated the renoprotective benefits of PDE5-Is in kidney-related clinical conditions, such as diabetic or nephrotoxic nephropathy, renal ischemia/reperfusion injury, renovascular hypertension and chronic kidney disease. Most reported preclinical studies highlighted a promising potential of PDE5-Is to improve renal function and histopathological changes via collaborative mechanisms, including antioxidative, anti-inflammatory, anti-apoptotic, antifibrotic pathways along with suppression of DNA damage and improving renal blood flow, NOS levels, endothelial function and mitochondrial biogenesis. Most recently, tadalafil was also reported to avert the onset of ureter inflammation and urothelial degeneration in a unilateral ureteral obstruction animal model via modulation of various histopathologic and biochemical changes [183].
5.2.5. Cystic Fibrosis
Cystic fibrosis (CF) is a disease that is caused by a mutation in the CF transmembrane conductance regulator (CFTR) gene “F508del allele” that encodes the main chloride channel expressed in epithelia, which leads to a reduced transepithelial chloride transport in multiple organs, such as pancreas, intestine, kidney, liver and most significantly lungs. This results in abnormal mucociliary clearance and endosomal hyper-acidification along with obstruction, infection and excessive proinflammatory responses that progressively damage the respective organ function and structure [184].
Several preclinical and clinical studies highlighted that PDE5-Is can correct the majority of the known pathological defects in CF, where tadalafil showed the highest efficacy, while vardenafil granted prolonged effects after a single therapeutic dose [185,186]. The efficacy of PDE5-Is in CF could be correlated to one or more of the following mechanisms: (i) correction of the mislocalization of the mutant CFTR protein, restoring normal transepithelial chloride transport [187,188,189], (ii) normalizing the excessive proinflammatory responses via downregulation of M1 markers, tumor necrosis factor (TNF)-α and inducible NOS-2 [190,191], (iii) reversing endosomal hyper-acidification via elevating cGMP levels [192], (iv) improving endothelial function via promoting NOS-3 phosphorylation in endothelial cells [193], and (v) reducing adhesion of bacterial pathogens to respiratory epithelial cells [190].
5.2.6. Diabetes
Das et al. have summarized the potential protective roles of PDE5-Is against several diabetes-related pathologies including (i) prevention of diabetic neuropathy and vasculopathy via improving endothelial function, (ii) protection against I/R injury in diabetic heart via an AMP-activated protein kinase/Sirt1/PGC-1α (AMPK/Sirt1/PGC-1α) cytoprotective signaling cascade, along with (iii) antioxidant and anti-inflammatory effects in diabetic hearts [86].
A meta-analysis of randomized controlled trials has also validated PDE5-Is as effective and safe medications for the treatment of sexual dysfunction in patients with diabetes mellitus suffering from ED [194].
Most recently, a combination of tadalafil and hydrochloroquine successfully improved several Type 2 diabetes-related clinical parameters, including a drop in fasting blood glucose and lipid levels, a rise in plasma insulin and insulin-like growth factor-1 levels and improved insulin sensitivity. Interestingly, pretreatment with the same combination showed a potential to diminish the rate and severity of COVID-19 infection in vulnerable diabetic patients [195].
5.2.7. Miscellaneous Indications
Several studies have demonstrated the efficacy of the combined administration of sildenafil with selective serotonin reuptake inhibitors (SSRIs), such as paroxetine and sertraline, for the treatment of premature ejaculation [196]. Moreover, PDE5-Is prompted penile rigidity and recovery of erections in the post-ejaculatory period [197]. Details of related preclinical and clinical trials were further elaborated by the reviews [23,198].
Long-term chronic administration of PDE5-Is could also avert the progression of fibrotic plaques and halt corporal fibrosis in animal models of Peyronie’s disease [199,200].
In addition, prolonged administration of low-dose PDE5-Is exhibited a promising beneficial effect in the treatment of male infertility. Sildenafil and vardenafil, in particular, could enhance Leydig cells’ secretory and steroidogenic functions, augmenting sperm concentration and the percentages of motile and morphologically normal sperm [201,202,203]. An increase in serum testosterone levels by both inhibitors has been reported as well [204].
Interestingly, tadalafil was proven safe to improve selective fetal growth restriction, a condition of twin pregnancy in which the development of one fetus is restricted, without severe side effects in the mothers or neonates [205]. Most recently, Isidori et al. collaborated evidence possibly linking the NO/cGMP/PDE5 axis to the pathophysiology of coronavirus disease (COVID-19) and suggested the repurposing of PDE5-Is as a treatment strategy to halt the progression of COVID-19 via diverse immunomodulatory mechanisms [206]. All reported FDA-approved and emerging uses of PDE5-Is are summarized in Figure 6.
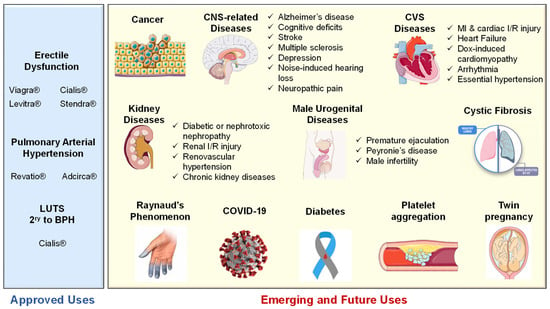
Figure 6.
Summary of approved and emerging/future uses of PDE5 inhibitors.
5.3. Side Effects and Contraindications of PDE5 Inhibitors
The use of PDE5-Is is usually associated with some common side effects, which include headache, flushing, dyspepsia, visual disturbances, back pain, myalgia, tachycardia, and nasal congestion [207]. Most of these side effects are due to the inhibition of PDEs other than PDE5, visual disturbances are associated with PDE6 inhibition and back pain and myalgia are attributed to the inhibition of PDE11. Nevertheless, these side effects rarely led to discontinuation of the treatment.
Other less known, seldom encountered serious side effects have been reported concomitant to the use of PDE5-Is are highlighted in the following lines.
- (i)
- Although PDE5 is reported as a promising target for anti-cancer therapy, as explained earlier, the prolonged use of PDE5-Is has been linked to an increased risk of melanoma. Lie and co-workers reported an association between sildenafil use and an increased risk of melanoma in a prospective cohort study conducted on 25,848 men [208]. Several other cohorts and case-control studies have also reported a correlation between the use of sildenafil and tadalafil and the increased risk of melanoma [209,210]. However, this association between the prolonged use of PDE5-Is and the development of cancer was only reported for melanoma; even the risk of other types of skin cancer, such as squamous cell carcinoma and basal cell carcinoma, was not correlated to the use of PDE5-Is [211].
- (ii)
- Visual disturbances have been usually reported with the use of PDE5-Is because of PDE6 inhibition. However, several studies have reported more serious ophthalmologic side effects associated with the use of PDE5-Is, which include non-arteritic anterior ischemic optic neuropathy (NAION), which may eventually lead to vision loss [212]. Two case-crossover studies have shown a two-fold increase in the risk of NAION in men using PDE5-Is, and currently, all PDE5Is (Viagra®, Cialis®, Levitra® and Spedra®) mention NAION as a caution in their summary of product characteristics [213,214].
- (iii)
- Moreover, sensorineural hearing loss (SSHL) has been associated with the prolonged use of PDE5-Is. Two in vivo studies have shown that the prolonged use of sildenafil could lead to hearing loss in mice and rats [215,216]; in addition, published trials and pharmacovigilance agencies reported 47 cases of SSHL as a result of prolonged administration of sildenafil [217], and more specifically, Maddox et al. reported two cases of SSHL due to daily use of tadalafil 10 mg and sildenafil 50 mg + tadalafil 10 mg use where both patients did not recover after a follow-up [218]. Both NAION and SSHL are of unknown pathophysiology.
- (iv)
- Priapism (prolonged erection of the penis) is another less common side effect reported with the prolonged use of PDE5-Is, as only a few cases have been reported for priapism associated with the use of PDE5-Is [219]. The risk of priapism increases in the case of concomitant use of other ED medications along with the PDE5-Is.
Not only can these side effects potentially restrict the utilization of PDE5-Is, but PDE5-Is are also contradicted in the presence of various cardiovascular disorders. Given that approximately one out of every thirteen individuals is estimated to have a cardiovascular disorder, and considering that there are around 620 million people globally living with cardiovascular conditions, it becomes evident that this is a significant concern. Clinical guidelines dictate that the use of PDE5-Is is not recommended in cases of advanced congestive heart failure, unstable or treatment-resistant angina pectoris, recent myocardial infarction, high-risk arrhythmias, obstructive hypertrophic cardiomyopathy, and severe valve diseases, particularly aortic stenosis [220].
6. PDE5 Inhibitors
The PDE5 enzyme is a homodimer that includes three main sites [221]: (i) an allosteric site, which consists of two regulatory GAF domains (GAF-A and GAF-B), both are regarded as allosteric binding regions for cGMP, (ii) a phosphorylation site (at Ser92) which plays a role in enzyme activation, and (iii) a catalytic site, which is located at the C-terminal end of the protein (amino acid residues: 535–860) and contains the divalent metal (Zn2+ and possibly, Mg2+) and the active site of PDE5.
The catalytic site of PDE11 is the most similar one to that of PDE5 among all other PDEs, while PDE6 shares a similar amino acid sequence and a secondary structure of the catalytic site to PDE5; therefore, PDE6 and PDE11 are the two most common off-targets for PDE5-Is [221]. PDE6 is a key effector enzyme for the phototransduction cascade in the rod and cone segments of the retina in the mammalian eyes. It has a function in visual transduction and response to light [221]. As for PDE11, not much information is available about its physiological functions. However, it is reported to be localized in skeletal muscles, prostate, and the testes [222].
Prior to 2012, the three most important PDE5-Is were sildenafil, vardenafil and tadalafil. Sildenafil was discovered based on the optimization of zaprinast (an anti-allergic drug), which was one of the first PDE5-Is to be reported in the literature; however, it showed only moderate activity against PDE5 (IC50 = 2000 nM), as well as low selectivity for PDE5 over PDE1 (SI = 4.7). Several rounds of optimization led to the discovery of sildenafil with a much-improved potency over PDE5 (IC50 = 3.6 nM), higher selectivity for PDE5 over PDE1 than zaprinast (SI = 72.2), and improved solubility and in vivo characteristics (Figure 7) [223]. Isosteric replacement of the pyrazolopyrimidinone core with an imidazotriazinone scaffold led to the discovery of vardenafil; vardenafil was more potent (IC50 = 0.7 nM) and selective (SI = 257) for PDE5 over PDE1 than sildenafil (Figure 7) [224]. Both sildenafil and vardenafil were reported to prompt visual disorders, such as functional blindness, blue (cynopsia), blurred vision and enhanced light sensitivity, all attributed to the cross-reactivity with the PDE6 catalytic site [225,226].
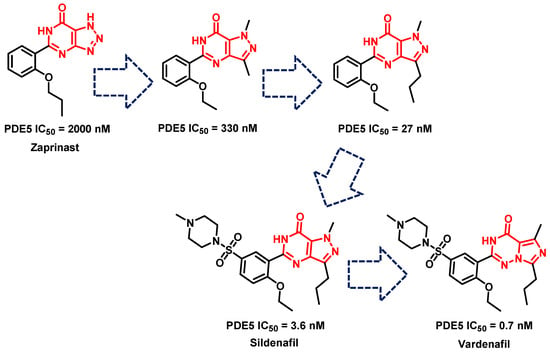
Figure 7.
The discovery of sildenafil and vardenafil.
Tadalafil’s development was based on the β-carboline scaffold. The discovery of tadalafil started from the ethyl β-carboline-3-carboxylate (I) that displayed a moderate PDE5 inhibitory activity (IC50 = 800 nM) [227]. Reduction of the β-carboline scaffold of cpd. I to a tetahydrabetacarboline (THβC) and extending the structure with a hydantoin ring (cpd. II) improved the PDE5 inhibitory activity (IC50 = 300 nM), Figure 8 [227]. Adopting various substituted phenyls at position 6 of the tetrahydro-β-carboline scaffold of cpd. II led to the discovery of a highly potent and selective PDE5 inhibitor (cpd. III, Figure 8) with an IC50 of 5 nM and a selectivity index of more than 2000 for PDE5 over PDEs 1–4, with the ability to increase intracellular cGMP levels in rat smooth muscle cells (EC50 = 1 µM). However, cpd. III displayed poor hypotensive activity in spontaneous hypertensive rats model after oral administration (30 mg/kg), indicating its poor oral absorption [227]. The modification of the hydantoin ring of cpd. III to a piperazinedione, as well as incorporating the 1,3-benzodioxole moiety at position 6 of the THβC scaffold led to the discovery of tadalafil which displayed higher cellular activity in rat smooth muscle cells (increased intracellular cGMP with an EC50 of 0.15 µM), and long-lasting blood pressure lowering activity in the spontaneously hypertensive rat model lasting for 7 h after an oral dose of 5 mg/kg [228].
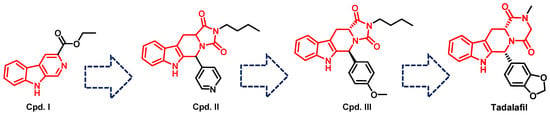
Figure 8.
The discovery of tadalafil.
Due to these adverse effects reported for sildenafil, vardenafil and tadalafil that are related mainly to their cross-reactivity with other PDEs, great attention has grown recently towards the design of more selective PDE5-Is.
In the coming sections, we discuss PDE5-Is reported since 2012, including PDE5-Is with dual pharmacological activities and those developed for radiodiagnosis.
6.1. PDE5 Inhibitors
6.1.1. Pyrimidinones
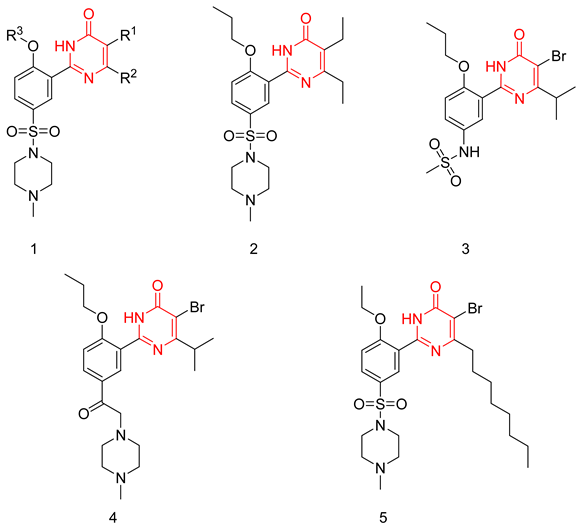
Wang et al. applied a structure simplification strategy to sildenafil to produce several pyrimidine-4(3H)-one derivative as PDE5-Is with a general scaffold (1). Hydrophobic groups were a much more preferred substitution at R2 than hydrophilic ones, with ethyl and isopropyl being the best substituents. The introduction of a small aliphatic group or a halogen atom except fluorine at R1 led to a huge boost in PDE5 inhibitory activity. At R3, an n-propyl group was optimum for activity. Compound 2 was the most potent PDE5 inhibitor of the series with an IC50 of 1.6 nM (2.5 times more potent than sildenafil) [229].
Compound 2 was further tested against 11 PDE isoforms to evaluate its selectivity. It showed no significant inhibition against PDE2, PDE3, PDE4, PDE7A1, PDE8A1, PDE9A2, and PDE10A2 at 10 μM, and selectivity factors of 2127, 469 and 29 for PDE5 over PDE11A4, PDE1 and PDE6C, respectively. In comparison to sildenafil, compound 2 showed a slightly better selectivity profile.
Compound 2 was further evaluated in vivo. Despite having a low oral bioavailability (23%), which is 10% lower than that of sildenafil, it showed good efficacy in a rat model of erection. After 30 min of an oral administration of a dose of 10 mg/kg, both intracavernous pressure and arterial blood pressure were significantly elevated in the rat model [229].
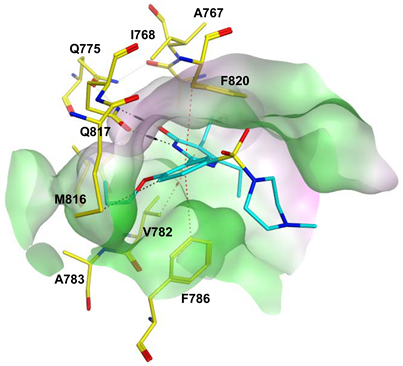
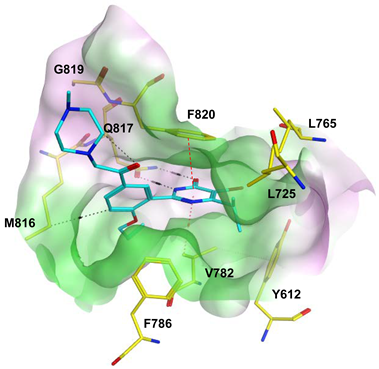
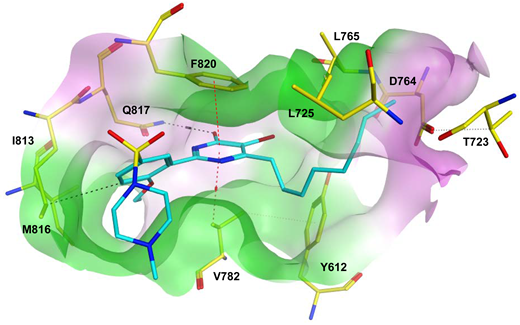
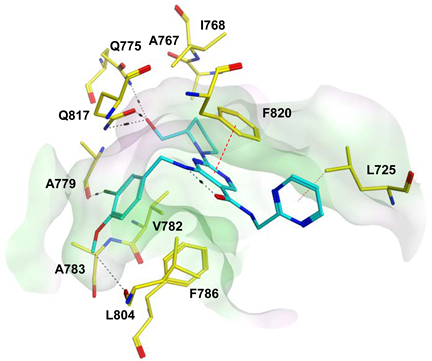
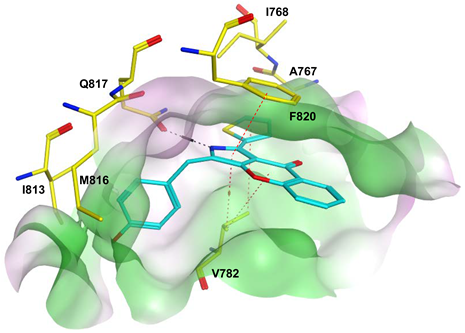
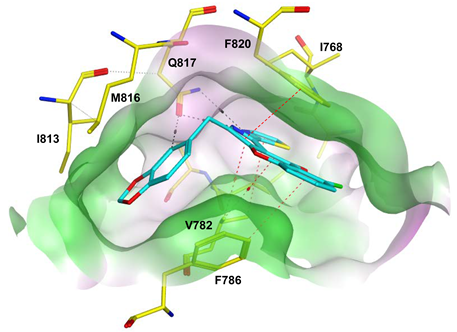
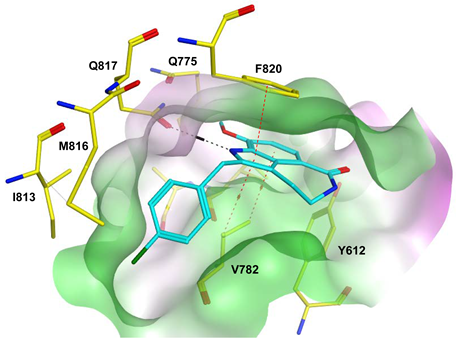
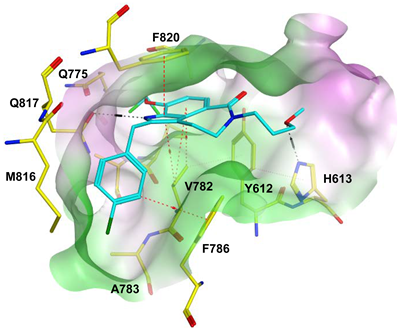
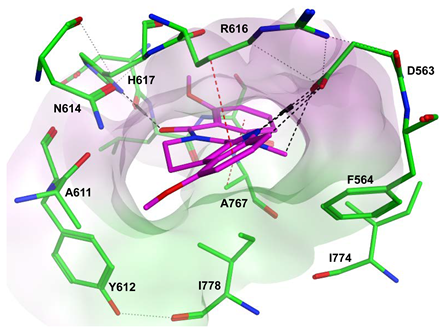
To improve the selectivity of this class of compounds, specifically over PDE6, Gong et al. focused on structural modifications involving the sulfamide part of the molecule. Despite being equipotent to compound 2, compound 3 (IC50 = 1.7 nM) was far more selective for PDE5 over PDE6 with a selectivity factor of 941. However, the in vivo efficacy of compound 3 was not evaluated [230]. Moreover, Gong et al. explored the different binding modes of the pyrimidinone scaffold through cocrystal structures of cpds. 4 (PDB: 4I9Z) and 5 (PDB: 4IA0) with the PDE5 active site [230]. See the crystal structures/docking section.
6.1.2. Aminopyrimidines
The aminopyrimidine scaffold was extensively explored by Sakamoto et al., aiming to produce potent and selective PDE5-Is [231,232]. The synthesized derivatives had a general structure (6), where their potency was tested on a PDE5 enzyme isolated from a canine lung, their selectivity was evaluated through testing on a light-activated bovine retina PDE6, and their in vivo efficacy was evaluated through testing their ability to induce relaxant effects on an isolated rabbit corpus cavernosum [231,232].
The first series of compounds developed by Sakamoto et al. were 5-(3,4,5-trimethoxybenzoyl)-4-aminopyrimidine derivatives. T-6932 (7) was the standout compound of the series with a PDE5 IC50 of 0.13 nM and a selectivity factor of 2400 over PDE6; however, compound 7 had a moderate in vivo efficacy with an EC30 of 53 nM, which was explained by its high clogP value (4.58). On the contrary, compound 8 was the most effective in vivo with an EC30 of 3.1 nM (clogP = 3.63). In comparison to sildenafil (EC30 = 8.7 nM), it is three times more effective in vivo. However, it was 26 times less potent and four times less selective than 7.
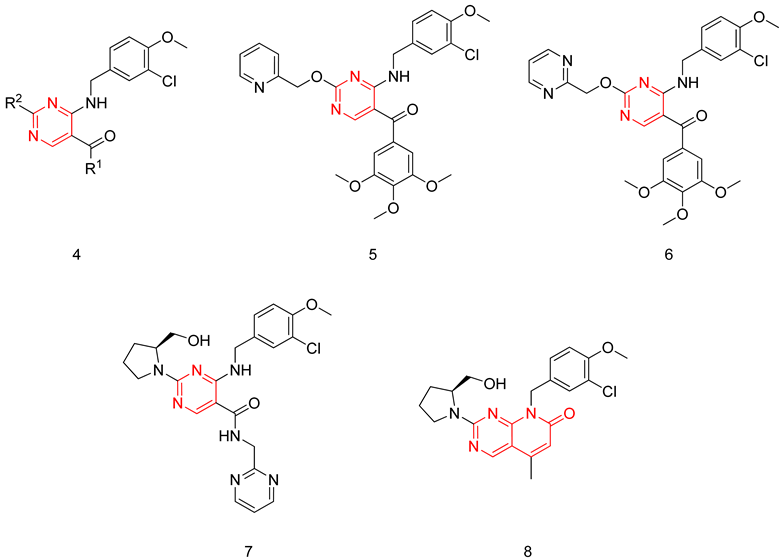
In the next stage of compound development, Sakamoto et al. focused on improving the in vivo efficacy of T-6932 by reducing its lipophilicity. This was done by replacing the 3,4,5-trimethoxyphenyl moiety at R1 with several heteroarylmethylamino and hydroxylamine groups, as it was proven in the same study that the 3,4,5-trimethoxyphenyl at R1 is not crucial for PDE5 inhibitory activity. Incorporation of these substituents together with a 2-pyridylmethyloxy group at R2 maintained the potency against PDE5 but had a negative impact on the selectivity. This was overcome by the introduction of several secondary amines having hydroxyl groups at R2. The incorporation of an (S)-2-hydroxymethyl-pyrrolidin-1-yl group at R2 led to the discovery of avanafil (9) with an IC50 of 5.2 nM and a selectivity factor of 4000 for PDE5 over PDE6. Avanafil had a much more improved clogP value (2.36) in comparison to T-6932, which could explain its remarkable in vivo efficacy (EC30 = 2.1 nM) [232].
Avanafil was further tested on the other PDE isoforms, where it showed an excellent selectivity profile, with a selectivity factor of 121 for PDE5 over trypsin-activated PDE6, which is higher than that of sildenafil (16) and vardenafil (21) but lower than that of tadalafil (550). However, avanafil holds the advantage over tadalafil with respect to the selectivity over PDE11 as it has a selectivity factor of more than 19,231, while tadalafil has a selectivity factor of only 25. Moreover, avanafil showed a selectivity factor of more than 1000 over all other PDE isoforms [232].
Avanafil also demonstrated an excellent pharmacokinetic profile, where it possessed a faster onset of action than sildenafil, as well as a short duration of action, improving the tolerability of the drug [232]. The high potency and in vivo efficacy, together with the excellent selectivity and pharmacokinetic profiles, granted avanafil FDA approval for the treatment of male ED [232].
6.1.3. Pyrido-Pyrimidines
Sakamoto et al. used the same general structure (6) reported in [231,232] and performed a cyclization between the substituents at positions 4 and 5 of the pyrimidine ring, thus developing a new series of 8-(3-chloro-4-methoxybenzyl)-8H-pyrido[2,3-d]pyrimidin-7-one derivatives having the (S)-2-hydroxymethyl-pyrrolidin-1-yl group at R2 similar to avanafil [233]. The potency, selectivity and in vivo efficacy of the synthesized compounds were evaluated using the same methods described in [231,232]. The standout compound of this series was compound (10), showing the highest PDE5 inhibitory potency (IC50 = 0.86 nM), the highest selectivity (selectivity factor of 2300 over PDE6) and the highest in vivo efficacy (EC30 = 0.85 nM) [233].
6.1.4. Pyrazolopyrimidinones
The pyrazolopyrimidinone scaffold is considered a privileged scaffold when it comes to designing potent PDE5-Is, as sildenafil possesses the same scaffold. However, it retains the main disadvantage of sildenafil, which is cross-reactivity with PDE6.
Sawant et al. focused on modifying the methyl piperazine part of sildenafil, replacing it with various open-chain substitutions at the N-terminal of the sulfonamide. Substituents with an aliphatic side chain having a terminal hydroxy group or a terminal morpholine group were better than other adopted substituents. Compound 11 was the most potent PDE5 inhibitor of the series; it was twice as active as sildenafil with an IC50 of 1.5 nM [234]. Upon evaluating compound 11 against PDEs 1–11, it showed a similar selectivity profile to sildenafil and, most importantly, a poor selectivity over PDE6 [234]. Compound 11 exhibited 1.5 times better in vivo efficacy than sildenafil in a conscious rabbit model, however, with a poor pharmacokinetic profile (rapid metabolism by mice liver microsomes and six times higher efflux ratio in a Caco2 permeability model) [234].
In a later study, Sawant and Co. replaced the methyl piperazine moiety of sildenafil with various substituted piperidine and piperazine moieties. A piperidone moiety was optimum for PDE5 inhibitory activity, resulting in compound 12 with an IC50 of 0.8 nM (7 times more active than sildenafil) and a much-improved selectivity than both sildenafil and cpd. 11, with a selectivity factor of 20 for PDE5 over PDE6 [235].
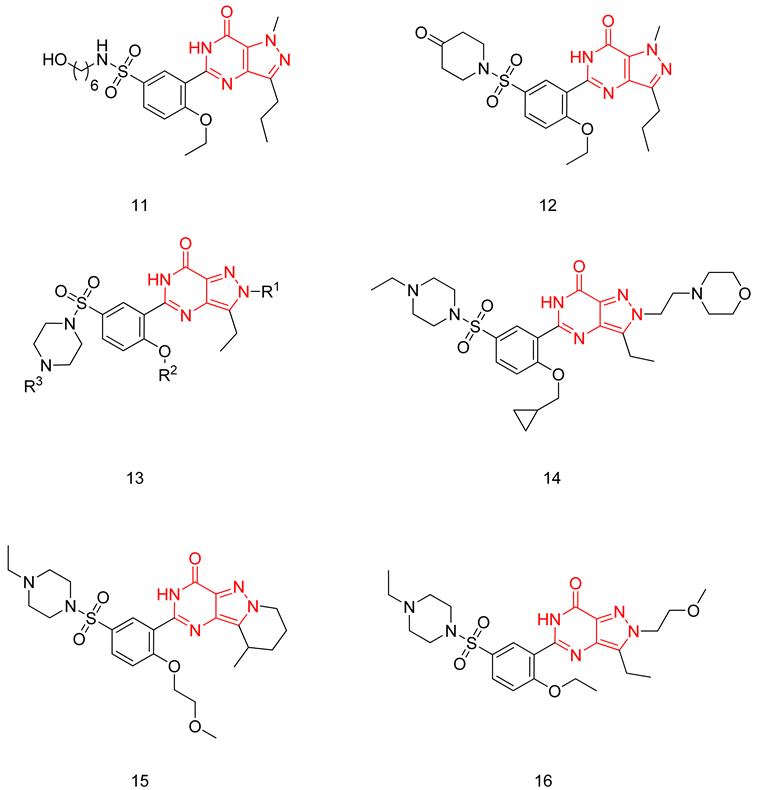
Compound 12 has revealed similar efficacy to sildenafil in maintaining penile erection in the rabbit model, with improved efflux ratio, as well as similar metabolic stability against mice liver microsomes to sildenafil [235].
Rawson et al. made a more extensive exploration of the pyrazolopyrimidines, introducing structural modifications at different positions of the scaffold, summarized in general structure 13. The potency of the synthesized derivatives was evaluated using a PDE5 enzyme derived from the human corpus carvernosum, while selectivity was evaluated through testing against PDE6 derived from a dog’s retina [236].
At R3, an ethyl group was optimum for PDE5 inhibitory activity. Different substituents with variations in size and chain length were all well tolerated at R1. However, the best substituents were either a methoxyethyl or a morpholinoethyl group. Several substituents were also well tolerated at R2, but using a cyclopropylmethyl moiety at R2 produced the most potent PDE5 inhibitor of the series (14) with an IC50 of 0.26 nM. Compound 14 was the most potent but not the most selective compound of the series. Cyclizing the N2 alkyl and the C3 to form a 3rd ring fused to the pyrazolopyrimidinone scaffold was the key structure modification to boost the selectivity for PDE5 over PDE6. Compound 15 held the highest selectivity factor over PDE6 (490) among all the synthesized analogs, with a PDE5 inhibitory activity of 1.96 nM [236]. However, it showed a relatively low oral bioavailability (18%) upon testing its pharmacokinetic properties in dogs. Compound 16 (PDE5 IC50 = 1.23 nM) showed the best pharmacokinetic profile among the other tested PDE5-Is with an oral bioavailability of 61%, 55% and 34% in rats, dogs and humans, respectively [236].
6.1.5. Tetrahydro-β-Carbolines (THβCs)
THβCs have been extensively explored as a prominent scaffold for PDE5-Is, inspired and guided by the discovery of the FDA-approved tadalafil [237]. Abadi and co. reported the synthesis of tadalafil analogs with a tetrahydro-β-carboline-imidazolidinedione and tetrahydro-β-carboline-piperazinedione scaffolds, having different substituents at the nitrogen of the terminal ring, as well as different pedant aryl moieties at C5/C6 of the scaffold. The synthesized derivatives were tested against recombinant human PDE5 enzyme [238,239].
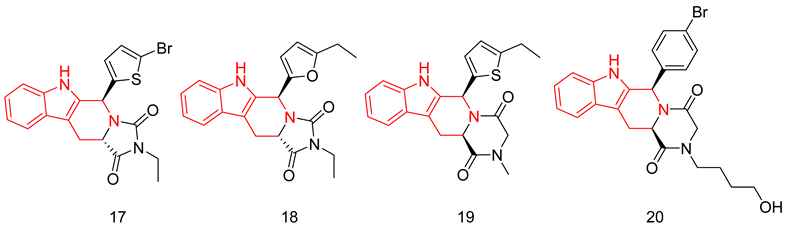
With a 5-bromo-2-thienyl substituent at C5/C6, the hydantoin scaffold was superior to the piperazinedione; the N-ethyl substitution was the best among all tried N-alkyl moieties, with S configuration at C5 essential for PDE5 inhibitory activity. The most potent compound of this series (17) was 53 times less potent than tadalafil (IC50 = 160 nM) [238], with higher selectivity than tadalafil for PDE5 over PDE11) selectivity factor of 49 vs. 13 for tadalafil) [238].
In a later study, Zheng et al. explored the use of different substituted thienyl and furyl moieties other than the 5-bromo-2-thienyl group at C5 of the THβC imidazolidinedione scaffold while keeping the other structural features of compound 17 (an S-configuration at C5 and an ethyl substituent at the terminal nitrogen), the 5-ethyl-2-furyl group in compound 18 showed the highest activity with equal potency to tadalafil (IC50 of 2.92 nM). Compound 18 was more selective than both compound 17 and tadalafil for PDE5 over PDE6 and PDE11 with selectivity factors of 43 and ˃342, respectively. As for the other PDEs, 18 showed no inhibition towards PDE1-3, PDE4 and PDE7-10 at screening doses of 20 μM, 10 μM and 3 μM, respectively [240].
In the same study, Zheng et al. employed various substituted thienyl and furyl moieties at the C6 of the THβC-piperazinedione scaffold while keeping an S-configuration at C6, and a methyl substituent at the terminal nitrogen of the piperazinedione ring, the 5-ethyl-thienyl group granted the most PDE5 inhibitor of the series (19) with an IC50 of 3.87 nM (equipotent to tadalafil). 19 was more selective than tadalafil, 17 and 18 for PDE5 over PDE6 with a selectivity factor of ˃258, but less selective than 18 for PDE5 over PDE11 with a selectivity factor of 70, which is still higher than both tadalafil and 17. Similar to 18, 19 showed no inhibition towards PDE1-3, PDE4 and PDE7–10 at screening doses up to 20 μM [240].
The in vitro vasorelaxant activities of 18 and 19 were evaluated in a rat 3rd order mesenteric arteries pre-contracted by 20 µM norepinephrine; both compounds showed a stronger vasodilatory effect than tadalafil (EC50 = 78 nM) with EC50 values of 30 and 63 nM, respectively [240].
SAR of the THβC derivatives was altered when a 4-chloro or a 4-bromo substituent was employed at C5/C6 of the scaffold; the piperazinedione scaffold was found to be superior to the hydantoin scaffold, the ethyl and butyl groups were the best among the other tried N-alkyl moieties, and an R configuration at C6 was essential for PDE5 inhibitory activity [241,242].
Abadi and co. explored the effect of adding a terminal amino or a hydroxyl group to the N-ethyl and N-butyl moieties of the 4-chloro and 4-bromo THβC analogs, but the attempted structural modification led to a huge reduction in PDE5 inhibitory activity. The most potent compound of the series (20) (IC50 = 100 nM) was 11 times less potent than its N-n-butyl congener (IC50 = 9 nM) [241] and 33 times less potent than tadalafil [239].
6.1.6. Quinazolines
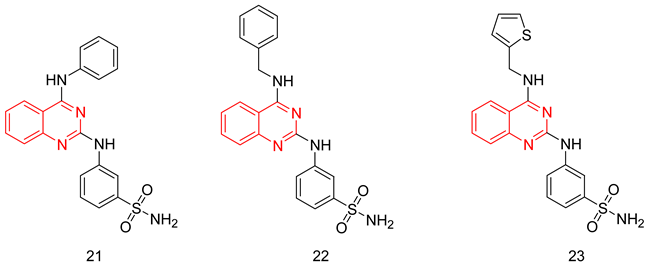
The quinazoline scaffold has been used by several researchers to obtain PDE5-Is, such as the 4-substituted variants of Watanabe [243] and the 2 & 4-substituted variants of Lee et al. [244] Gleeson and co. reported the synthesis of N2 and N4-diaminoquinazolines as PDE5-Is. At the N2 amino group, several substituted phenyls were employed, mainly a sulfonamide or an N-methylpiperazine-1-sulfonamide at either the meta or the para positions. On the N4 amino group, the substituents were either a benzyl, a substituted piperidyl or an alkyl group [245,246].
The best compound of the series(21) showed moderate PDE5 inhibitory potency with an IC50 of 72 nM (36 times less active than sildenafil) [246]. However, it showed good selectivity over PDE1, with a selectivity factor of 164 [245], as well as good efficacy when tested in an ex vivo vasodilatation model with an EC50 of 1.63 µM [246]. In addition to its moderate PDE5 potency, major drawbacks could be highlighted for 21; its selectivity for PDE5 over PDE6 was less than that of sildenafil with a selectivity factor of only 4.61, besides showing high cytotoxicity in human alveolar basal epithelial cell line (ATCC CCL-185) with an IC50 of 11.1 µM [246].
Later, Chatturong et al. evaluated the PDE5 inhibitory potency of derivatives possessing the same scaffold against HEK293-extracted PDE5. Compounds 22 and 23 were the two most potent PDE5-Is with an IC50 value of 5 nM (2.5 times less potent than sildenafil) [247]. Both compounds showed a good vasorelaxant effect against isolated intrapulmonary arteries with EC50 values of 0.94 and 1.03 µM, respectively. Despite showing a less potent vasorelaxant effect than sildenafil (EC50 = 0.05 µM), their vasorelaxant effect was more selective for pulmonary arteries over the thoracic aorta. Both compounds potentiated the vasorelaxant effect of sodium nitroprusside in endothelium-denuded pulmonary arteries, and the vasorelaxant effect of both compounds was reduced upon treatment with a guanylyl cyclase inhibitor (ODQ); both results confirm that the vasorelaxant effect of both compounds is related to their PDE5 inhibitory activity. The hepatotoxicity of both compounds was evaluated in rat hepatocytes where more than 80% of the cells were viable at a test concentration of 10 µM for both compounds [247].
6.1.7. Quinazolinedihydro-β-Carbolines
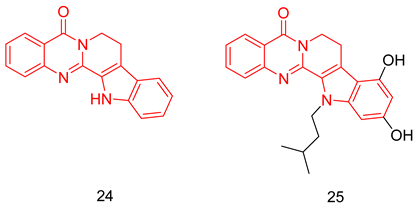
Rutaecarpine (24) is a quanzolinocarboline alkaloid reported to have vasodilation, anti-inflammation, and neuroprotective effects. Huang et al. introduced rutaecarpine (PDE5 IC50 = 1.23 µM) [248] as a lead for the development of PDE5-Is for the treatment of AD. Structural modifications were aimed at the indole part of the scaffold, with compound 25 showing the highest PDE5 inhibitory activity (IC50 = 86 nM). Twenty-five showed a better selectivity profile than sildenafil, as it showed a selectivity factor of 500 folds for PDE5 over PDE6, as well as showing no inhibition against PDE2, 4 and 9 at 500 µM. The in vivo efficacy of 25 was tested in scopolamine-induced cognitive deficit mice, where it showed relief in the learning and memory defects at a dose of 5 mg/kg [248].
6.1.8. Pyrroloquinolones
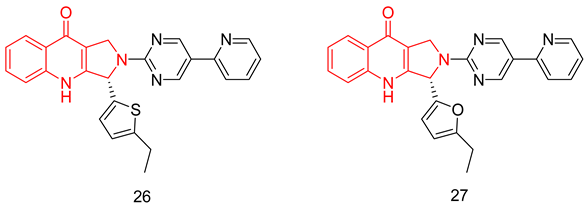
Zheng et al. succeeded in modulating the THβC scaffold into novel pyrroloquinolones with different substituted furyl and thienyl moieties at C3. The two most potent inhibitors were compounds (S)-26 and (S)-27 with IC50 values of 0.52 and 0.39 nM, respectively [249].
Both compounds showed acceptable to good oral bioavailability values (F = 24% and 66%, respectively). Moreover, both compounds showed superior in vitro vasorelaxant effects at a dose of 1 µM, as both induced almost complete relaxation in an isolated rabbit thoracic aorta contracted by norepinephrine [249]. In vivo studies in an anesthetized male New Zealand rabbits’ model showed the ability of both compounds to increase the intracavernosal pressure of electrically stimulated rabbits with ED50 values of 21.68 and 24.21 µg/kg, respectively, which are comparable to that of sildenafil (14.25 µg/kg) [249].
6.1.9. Quinolines
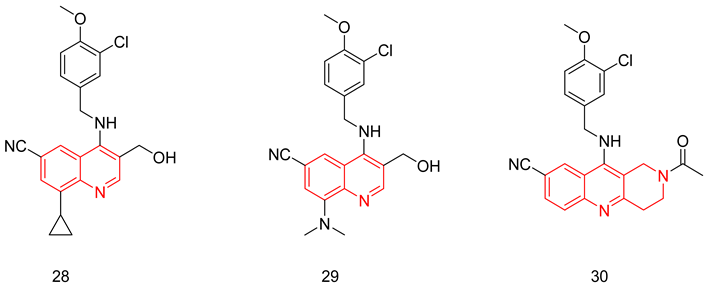
Fiorito et al. reported the synthesis of 4-(3-chloro-4-methoxybenzylamino) quinoline derivatives as potent and selective PDE5-Is for the treatment of AD. All the synthesized analogs had a hydroxymethyl at C3, a benzylamino at C4 and a cyano group at C7 of the quinoline scaffold, all essential for PDE5 inhibitory activity. The PDE5 potency was evaluated using PBS PDE assay kits. The two most potent derivatives were compounds 28 and 29 with IC50 values of 0.27 and 0.4 nM, respectively [250]. Both compounds showed excellent selectivity profiles, showing a selectivity factor of 1256 and 12,750 over PDE6, respectively, and showing no inhibition against the other PDEs (PDE1-PDE11) at a screening dose of 10 μM [250].
Compound 28 was then chosen to be further evaluated in vivo. Upon testing compound 28 in a male BALB/c model, it exhibited a good pharmacokinetic profile, reaching the maximum plasma concentration in 30 min, besides showing a fast distribution to the brain as the Tmax values in the brain and plasma were similar. Compound 28 was able to elevate the cGMP levels in the hippocampus of adult mice after administration of a 3 mg/kg dose followed by a foot shock after 30 min. Moreover, 28 was tested in mice treated with oligomers of Aβ42 that are known to induce loss in memory and hippocampal long-term potentiation and were found to restore the long-term potentiation effect, as well as treating the behavioral defects in mice caused by the loss of memory [250].
6.1.10. Tetrahydrobenzo[b][1,6]Naphthyridine
In a later study, Fiorito et al. further optimized their quinoline derivatives to improve their water solubility. The next scaffold was obtained by locking the rotatable bonds of the hydroxymethyl group of compounds 28 and 29 into a ring to give tetrahydrobenzo[b][1,6]naphthyridine scaffold [251]. This rigidification strategy led to the discovery of compound 30, which elicited higher potency against PDE5 (IC50 = 0.056 nM) and improved aqueous solubility. Additionally, compound 30 exhibited more than 500-fold selectivity for PDE5 vs. PDE6. However, the selectivity profile vs. other PDE isoforms, especially PDE11, was not presented. In a mouse model of AD, 30 improved learning and memory impairments by raising cGMP levels in the hippocampus. The very low microsomal metabolic stability was one of the major drawbacks of this very potent class of compounds that needs further optimization [251].
6.1.11. Chromenopyrrolones
Luo group reported the synthesis of chromeno [2,3-c]pyrrol-9(2H)-ones as PDE5-Is with a general structure (31) [252]. From the inhibitory activity data of the compounds, the following SAR could be concluded: PDE5 inhibition is hugely affected by the substitution at R3 and R4; at R3, using 5-membered heterocycles was preferred to substituted phenyl moieties, biphenyl, and naphthalene rings while the best substitution at R4 was a p-hydroxy benzyl moiety. The two most potent inhibitors were compounds 32 and 33, with IC50s of 17 and 18 nM, respectively, against PDE5 [252].
Compound 32 was further tested against PDE1, 4, 7, 8, 9 and 10. Despite showing good selectivity for PDE5 over the tested kinases (IC50s ˃ 750 nM), its overall selectivity couldn’t be judged as its activity against the two most common off-targets, PDE6 and PDE11 was not evaluated. One drawback of compound 32 is the relatively weak pharmacokinetic properties with an oral bioavailability of 4.9% [252].
In a later study, 32 was further optimized using the structure-based approach, where the thiophane ring at C1 was converted to thiazole, aiming at establishing a key bidentate H-bond interaction with Gln817 involving the thiazole nitrogen and the NH of pyrrole (colored in blue). This successfully led to compound 34 with an IC50 of 5.4 nM. It is worth mentioning that the other six and five-membered heterocycles at C1 did not lead to the same improvement in potency. In order to improve the metabolic stability and PK properties, the 4-hydroxybenzyl group in 34 was replaced with benzodioxole moiety to give compound 35 with a highly improved PK profile (F = 63.4%). In addition, 35 exhibited good drug-like properties, such as human liver microsomal stability, low cytochrome inhibition, low hERG inhibition, and pharmacological safety. When tested in the PAH in vivo model, 35 exhibited higher efficacy than sildenafil. Generally, 35 exhibited good isoform selectivity for PDE5. However, it had only selectivity indices of 10 and 27 for PDE6 and PDE10A, respectively, while selectivity against PDE11 was not presented by the authors [253]. Finally, the Luo group crowned their efforts in developing this class by further synthesis of nineteen analogs, which all showed IC50 values towards PDE5 of less than 10 nM [254]. In these analogs, compound 35 was further optimized on two stages, (i) through adding mono/di substituents at positions 5, 6, 7 and 8, yielding several more potent compounds like compounds 36 and 37 (IC50 = 1.41 and 1.05 nM, receptively). (ii) cocrystal of 34 with PDE5 catalytic domain (PDB code 5ZZ2, see binding modes section)) has guided the synthesis of compound 38 with a sub-nanomolar IC50 against PDE5 (0.32 nM). Thirty-eight exhibited a high selectivity index (SI) vs. many other PDE isoforms; however, compared to compound 35, the SI for PDE5 vs. PDE6 was compromised from 10 to 4 (comparable to sildenafil), while SI vs. PDE11 was disclosed to be 122 (SI of tadalafil = 20). Similar to compound 35, 37 exhibited good drug-like properties; the higher in vitro potency was correlated to a higher pharmacodynamic effect in vivo than 33 and sildenafil in the PAH model [254].
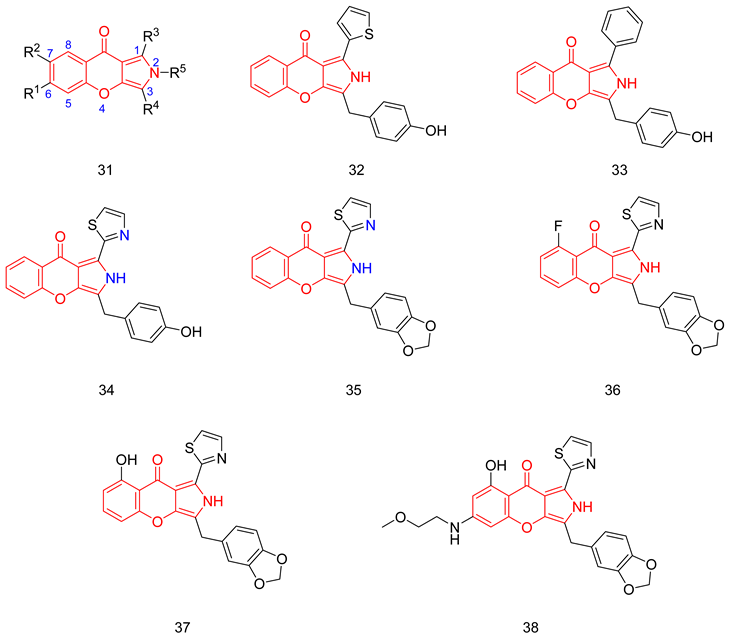
6.1.12. Azepinoindolones
Based on the structures of tadalafil and the chromenopyrrolone derivative 35, Luo and co-workers used a free energy perturbation-guided scaffold hopping strategy to identify 39; a 2,3,4,6-tetrahydro-1H-azepino[5,4,3-cd]indol-1-one derivative with a PDE5 inhibitory activity of 55 nM. Structural modifications on 39 led to the discovery of 40, a potent PDE5 inhibitor with an IC50 of 8.3 nM. 40 showed a 20 folds better selectivity for PDE5 over PDE11 than tadalafil, but almost the same selectivity for PDE5 over PDE6 as sildenafil. No significant inhibition was observed against other PDEs [255].
The in vivo efficacy of 40 was evaluated using a monocrotaline-induced pulmonary arterial hypertension rat model. Treatment of rats with a 2.5 mg/kg intraperitoneal dose of 40 for three weeks gave an effect comparable to that produced upon oral administration of a 10 mg/kg dose of sildenafil citrate for the same period. Furthermore, no acute toxicity was observed on the first day upon oral administration of a 1.5 mg/kg dose of 40 in male rats [255].
6.1.13. Pyridopyrazinones
Pyridopyrazinone-based derivatives have a wide spectrum of biological activities, such as CRF-R1 (corticotropin-releasing factor receptor 1) antagonists, PI3K inhibitors and antiproliferative agents [256]. In 2009, Pfizer Global R&D reported a new series of tri-substituted pyridopyrazinone derivatives as PDE5-Is [257,258]. This encouraged Amin et al. to evaluate their previously reported anti-cancer agents bearing a mono-substituted pyrido[2,3-b]pyrazinone scaffold against PDE5. In silico studies and in vitro biological testing disclosed compound 41 as the most potent PDE5 inhibitor, with an IC50 of 18.13 nM (9 times less potent than sildenafil). Selectivity testing against other members of the PDE family and in vivo studies were not reported for 41 [256].
6.1.14. Thienopyrimidines
Abadi and coworkers reported a series of 4-substituted thienopyrimidines fused to cyclopentene or cycloheptene. Several amnio substituents were tried at position 4, including aryl/acetyl/methyl piperazino groups, cyclohexlmethyl amino and arylhydrazones. Among more than 50 presented analogs, compounds 42 and 43 showed submicromolar potency against PDE5 with IC50 values of 0.42 and 0.19 µM, respectively. Selectivity was only presented vs. PDE7 and PDE9, where the two compounds weakly inhibited both enzymes at 25 µM [259].
6.1.15. PDE5 Allosteric Inhibitors
Targeting non-active site regions, which are less conserved, may offer a better chance to obtain selective PDE5-Is. This will be discussed in the next section.
Evodiamine Derivatives
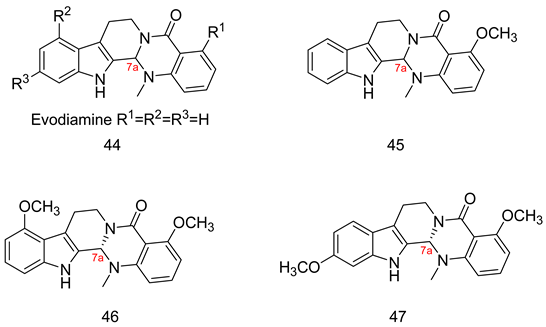
Evodiamine (44) is a natural product that was shown to inhibit PDE5 with an IC50 of 2.1 µM. In their efforts to discover PDE5 allosteric inhibitors with improved selectivity profiles, the Luo group characterized allosteric pockets 468 Å3 on the PDE5 catalytic domain using Allofinder [260]. After virtual screening of their natural product library followed by in vitro enzyme assay, compound 45 and its S enantiomer were identified as PDE5-Is with IC50 values of 340 and 110 nM, respectively, with 5–10 fold better selectivity profile vs. PDE6 than sildenafil. A small library of compounds was synthesized guided by molecular docking to reduce synthetic efforts. Regarding the chiral center at position 7a, the activity of the S isomer was higher than the racemate, while the R enantiomer was much less active. At R1, the methoxy > hydroxy > hydrogen regarding the potency. Having a 2nd methoxy group at R2 or R3 further increased the potency to yield compounds 46 and 47 with IC50 values of 35 and 42 nM, respectively. However, 47 exhibited better oral bioavailability (14%) compared to 46 (1%).
Enzyme kinetics revealed non-competitive behavior for 46; this is unlike all the previously reported PDE5-Is, which were shown to be substrate-competitive. Additionally, a cocrystal of 46 with PDE5 catalytic domain (PDB 6VBI) showed that the compound binds to a novel allosteric pocket on the catalytic domain, designated as EVO pocket. (See the crystal structures/docking section). Due to their allosteric nature, 46 and 47 exhibited excellent selectivity profiles with more than 570-fold selectivity over other PDEs except for PDE10 (20–30 folds). Compound 47 exhibited comparable in vivo efficacy to sildenafil in the pulmonary hypertension mouse model.
Trisubstituted Pyrazolines
Celecoxib (48), the selective COX-2 inhibitor, was shown to have an off-target activity towards PDE5 with an IC50 of 37 µM. Unlike the approved PDE5-Is, its PDE5 inhibitory activity was found to be against the full-length enzyme but not the PDE5 catalytic domain, indicating that its activity is strictly dependent on the presence of PDE5 regulatory domain, giving promise to develop a class with higher selectivity than the conventional PDE5-Is [261]. Abdel-Halim et al. reported a selective optimization of side activities (SOSA) approach to enhance the PDE5 inhibitory activity of celecoxib and abolish the COX-2 inhibition. The major structural variations introduced to celecoxib were (i) the replacement of the sulfonamide group with a carboxylic acid group, (ii) using the non-planar pyrazoline core instead of the pyrazole, (iii) using the t-Bu instead of the trifluoromethyl group and (iv) trying different substituents at the 5-phenyl. This resulted in compound 49 with an IC50 of 2 µM against PDE5 and diminished activity against COX-2 [261]. Further structure-based optimization via systematic modifications for 49 led to compounds 50 and 51 with an IC50 of 4 and 1 nM, respectively. Incorporation of the carboxylic acid functional group in an amide bond with methyl piperazine and difluorination at meta or ortho and meta positions of the 5-phenyl were key changes that achieved this huge boost in PDE5 inhibition. Fifty-one exhibited an unprecedented selectivity profile, almost approaching 15,000 fold toward all PDE isoforms, including PDE6 and PDE11 [261]. Similar to celecoxib, 51 was only active against the PDE5 full-length enzyme and not the PDE5 catalytic domain, justifying the unprecedented selectivity profile. Until now, only the PDE5 catalytic domain could be crystalized with several inhibitors. Thus, a cocrystal of 51 with a PDE5 full-length enzyme was not yet feasible. As expected, the study of PDE5 enzyme kinetics with 51 ruled out the competitive mode of inhibition, with no clear tendency towards a non- or uncompetitive mode of inhibition. The authors suggested that, presumably, 51 has a mixed mode of inhibition, with a preferred binding to the apoenzyme or to the substrate-bound form. As for the binding site, it might be located in the interface between the active site of PDE5 and the GAF-B domain, or it might be an allosteric binding to the regulatory domain with allosteric modulation of the active site. Keeping in mind that till now, the full-length PDE5 enzyme could not be crystallized, it remains an important question to be answered: Where do these intriguing pyrazolines exactly bind?
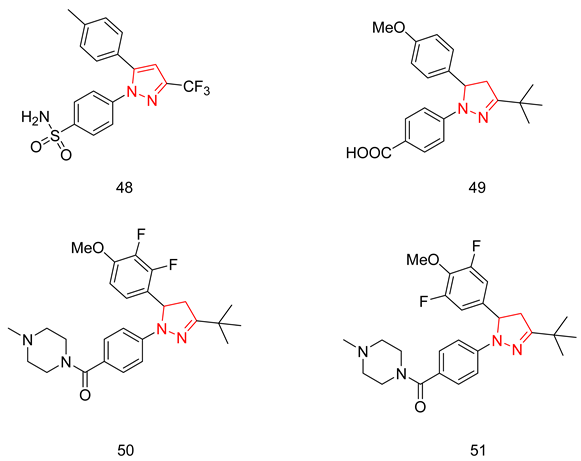
6.2. PDE5 in the Context of Dual Inhibitors
6.2.1. Compounds with Dual PDE5 and HDAC Inhibitory Activities
PDE5 and HDAC (histone deacetylase) were reported as therapeutic targets in AD. Oyarzabal and Co. aimed at designing dual PDE5 and HDAC inhibitors as multitarget directed ligands for the treatment of AD, where the complex etiology of the disease suggests higher efficacy than the classical ‘one molecule-one target approach’. Despite accumulated evidence that targeting HDAC6 specifically is beneficial for treating AD, Oyarzabal and co. designed three classes of inhibitors with different HDAC inhibitory profiles: class A, having a pan-HDAC inhibitory activity; class B, having selectivity for HDAC6 over class I HDACs; and finally, class C, having selectivity for class I HDACs over HDAC6. For these inhibitors to be successful, they were planned to have moderate inhibition for HDAC class I (HDAC1, 2, 3 and 8) to avoid the possible toxicity but potent PDE5 inhibition to provide the synergistic effect needed for a cellular and an in vivo functional response, as well as CNS-penetration. In their studies, structural modifications were made to replace the piperazine sulfonamide part of sildenafil and vardenafil with groups that have a terminal zinc binding group ZBG (a hydroxamic acid or an ortho-aminoanilide moiety), which is essential for HDAC inhibition. They also employed similar modifications at the piperidinedione nitrogen of tadalafil [262,263,264,265].
In their first report, Oyarzabal and Co. focused on developing class A inhibitors using the sildenafil core, together with a wide range of substituents having a terminal hydroxamic acid group (a ZBG essential for HDAC inhibitory activity) and linked to the 5′position of the phenyl ring of sildenafil via a carbon, nitrogen, or an oxygen atom [262].
Despite being a 7-fold less potent PDE5 inhibitor than sildenafil, compound 52 (CM-414) was the best pan-HDAC inhibitor. The cytotoxicity of 52 was evaluated in THLE-2 cells and primary neuronal cultures of glia cells and showed moderate cytotoxicity in THLE-2 cells and low cytotoxicity in glia cells, with an LC50 of 7.2 µM and 17.7 µM, respectively [262].
52 was further studied in vivo, where it increased the acetylation of histone by 98% and increased the phosphorylation of CREB by 148% in the hippocampus of mice 30 min post a 40 mg/kg intraperitoneal injection [262]. It was reported in another related study that chronic treatment of Tg2576 mice with 52 resulted in a huge decrease in the brain Aβ and pTau levels through favoring the inactive form of GSK3β, reverted the decrease in dendritic spine density on hippocampal neurons, and reversed the memory impairment in mice through inducing the expression of genes related to synaptic transmission [266]. Furthermore, 52 succeeded in decreasing the expression of fibrogenic markers and collagen deposition in Mdr2-KO mice (a clinically relevant model of liver inflammation and fibrosis), impeding the progression of chronic liver disease in this type of mice [267].
Despite showing a good functional response both in vitro and in vivo, some drawbacks of 52 could be highlighted: its moderate PDE5 potency in comparison to other synthesized derivatives; it was highly potent against PDE6 (IC50 = 2.6 nM), and finally, its poor in vitro pharmacokinetics, attributed to its low permeation (Pe value = 15.7 nm/s in a PAMPA assay), as well as its high efflux ratio (41.3 in a Caco-2 permeability assay) [262].
The vardenafil-matched pair of 52 was reported in another study carried out by Oyarzabal and co [263]. Despite being a more potent PDE5 inhibitor than 52, compound 53 showed moderate cytotoxicity upon its evaluation in THLE-2 cells, primary neuronal cultures of glia cells and peripheral blood mononuclear cells (PBMCs) with LC50 values of 9.37 µM, 6.1 µM and 6.2 µM, respectively. It can be thus concluded that 53 has a narrow therapeutic window, given the fact that 400 nM was needed to elicit a significant in vitro cellular response. Moreover, 53 showed poor in vivo efficacy, producing only a 25% increase in the levels of phosphorylated CREB in the hippocampus of mice after 1 h of a 40 mg/kg intraperitoneal injection [263].
Following this study, Oyarzabal and co. focused on developing inhibitors of class B, using both the sildenafil and vardenafil cores. Different phenyl, substituted phenyl, thienyl and furyl moieties linked to a terminal hydroxamic acid group were employed at the 5′position of the phenyl ring of the core. Compound 54 was the most potent HDAC6 inhibitor, with excellent selectivity over class I HDACs, in addition to having potent PDE5 inhibition. Fifty-four showed moderate cytotoxicity in THLE-2 cells and low cytotoxicity in primary neuronal cultures of glial cells, with an LC50 of 6.81 µM and 46.3 µM, respectively [265].
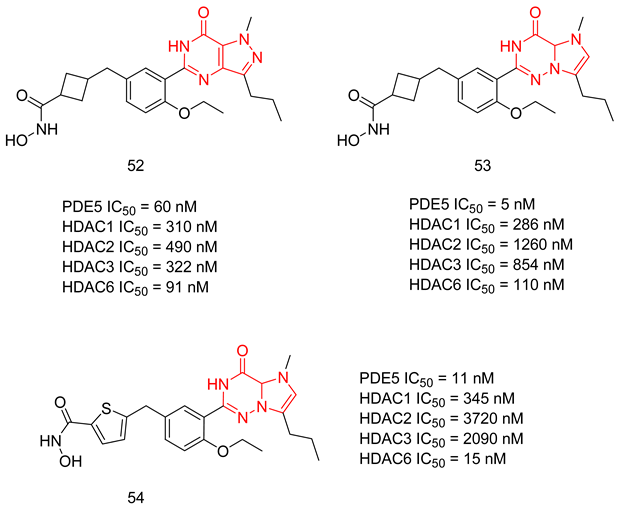
Despite showing a good functional response in vitro, 54 was able to increase the level of phosphorylated CREB by only 48% in the hippocampus of mice after 30 min of a 40 mg/kg intraperitoneal injection. In addition, no significant improvement in the memory of Tg2576 mice was observed following a two-week treatment with 54 [265].
Finally, Oyarzabal and co. aimed at designing inhibitors of class C, using both the sildenafil and vardenafil cores. A wide range of phenyl and cycloalkyl substituents were employed with an ortho-aminoanilide moiety as the ZBG instead of the hydroxamic acid group, a structural modification that led to the masking of the HDAC6 inhibitory activity. Compound 55, having a vardenafil core, was the most potent dual PDE5/class I HDAC inhibitor [263]. The second-best class I HDAC inhibitor was compound 56, which, despite having a moderate PDE5 inhibitory activity, was the compound of choice for further in vivo testing [264]. Fifty-six showed a low cytotoxicity profile, with an LC50 of 11,700 nM in THLE-2 cells, while no effect was observed in PBMCs at a screening dose of 100 µM. Despite showing an excellent pharmacokinetic profile, with a Pe value of 82.9 nm/s in the PAMPA assay and a low efflux ratio (0.86), 56 failed to produce a significant reversing effect for the memory impairment of Tg2576 mice after administration of a 20 mg/kg dose for two weeks [264].
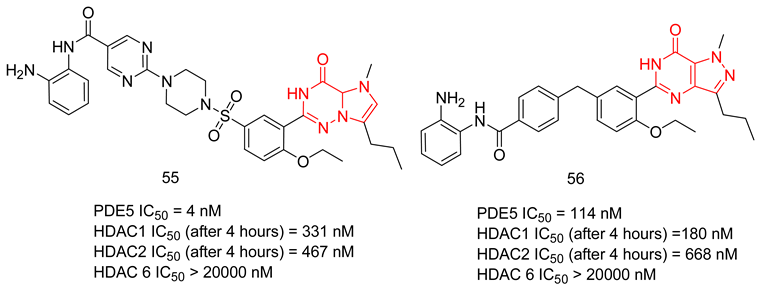
Upon comparing the three classes of inhibitors reported by Oyarzabal and co., inhibitors of class A employed on a sildenafil core provided the best pharmacological profile needed for AD treatment, as it was the only inhibitors class whose stand-out compound, despite having poor pharmacokinetics, showed memory restoration in mice AD model.
A few substituents were employed by Oyarzabal and Co. on the tadalafil core, specifically on the terminal nitrogen of the piperazinedione ring of tadalafil. The reported compounds were either inactive or much less active than their sildenafil and vardenafil matched pairs [263,264].
As an anticancer agent, tadalafil is usually not used solely but rather in combination with other chemotherapeutic agents to enhance its cytotoxicity against numerous types of cancer cells [76]. HDAC inhibitors could be regarded as one of the most important chemotherapeutics that could be used in combination with tadalafil for cancer treatment. Noteworthy, pan-HDAC inhibitors like vorinostat (SAHA), belinostat and panobinostat were approved by the FDA for the treatment of some types of hematological cancers [268].
As mentioned previously, the incorporation of a terminal amino or a hydroxyl group to the N-ethyl and N-butyl moieties of the 4-chloro and 4-bromo THβC analogs by Abadi and co. led to a sharp decrease in the PDE5 inhibitory activity [239]. Therefore, in a more recent study, Abdel-Halim and co. incorporated a terminal ZBG (carboxylic acid or a hydroxamic acid group) instead and manipulated the spacer length between the nitrogen of the piperazinedione and the terminal ZBG. Inhibitors with a terminal carboxylic acid group were only active against PDE5, while those with a stereochemistry of 6S and 12aS were only active against HDAC. The most potent dual inhibitor was compound 57, with an IC50 of 46.3 against PDE5 and a pan-HDAC IC50 of 14.5 nM [269].
Fifty-seven had a moderate in vitro cellular potency against three types of colon cancer cell lines (HT-29, HCT-116 and SW-620), with GI50 values of 12.35, 7.19 and 11.79 µM, respectively. 57 was also tested against several types of cancer cell lines, showing a high cellular potency (<3 µM) against Molt 4 (acute lymphoblastic leukemia), Sup-T1 (T-cell lymphoblastic lymphoma), K562 (chronic myelogenous leukemia), as well as T47D (breast cancer cells), where it could induce apoptosis in Molt-4 cells. NCI-60 human tumor cell line screen showed a high selectivity of 57 for leukemia and solid tumor cell lines. Fifty-seven showed a good therapeutic window, as it did not show any significant inhibitory effect in the non-cancer cell line CCD966SK with a GI50 of 28.2 µM [269].
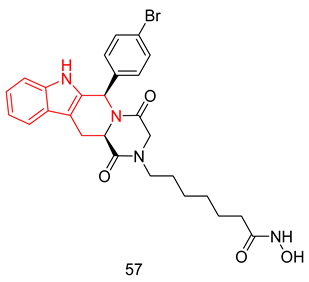
6.2.2. Compounds with Dual PDE5 and AchE Inhibitory Activities
Four of the clinically approved drugs against AD are AchE inhibitors. AchE inhibition has been shown to improve memory and cognitive functions in AD patients; therefore, designing MTDLs towards AchE and another target involved in AD pathology would produce a more significant reduction in AD symptoms. Mao et al. reported the synthesis of tadalafil analogs with varying substituents at the nitrogen of the terminal piperazinedione ring as dual AchE/PDE5-Is for the treatment of AD [270].
The two most interesting compounds of the series (58 and its diastereomer 59) were the most potent derivatives against aChE with IC50s of 36 and 32 nM with PDE5 inhibitory IC50s of 150 and 1530 nM, respectively. Further evaluation of 58 and 59 revealed their ability to cross the BBB upon testing them in a parallel artificial membrane permeation assay. 58 and 59 could inhibit PDE5 in vivo as they significantly enhanced the phosphorylation of CREB. Finally, at a dose of 10 mg/kg, 59 was more effective than 58 at improving the cognitive functions of scopolamine-induced cognitive deficit mice [270].
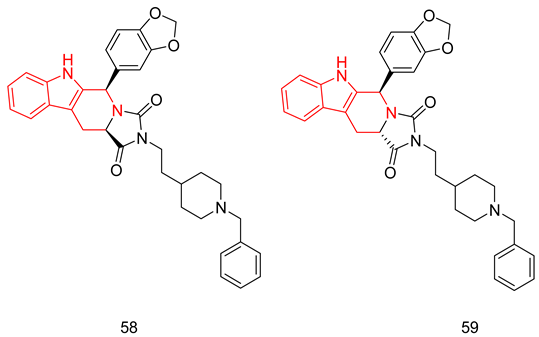
6.2.3. Dual PDE5 Inhibitor and NO Donor
Topical or systemic application of sildenafil has been shown to be beneficial for wound healing through increasing cGMP levels. The significant role of NO in wound repair has been clearly observed in mice deficient for inducible or endothelial NO synthase, where they showed delayed wound closure and impaired angiogenesis. The in vitro and in vivo effects of NO donors and PDE5-Is on the different cell types involved in wound repair, as well as the potential additive effect of NO supplementation and PDE5 inhibition, were not tested until Greenwald et al. reported the synthesis of TOP-N53 (60), a dual-acting NO donor and PDE5 inhibitor, having wound healing effects in both normal mice and mice with diabetes mellitus [271].
Sixty showed a PDE5 inhibitory potency of 1.6 nM, and it significantly increased the levels of NO in human keratinocytes after 24 h from the treatment with a 10 µM dose of 60 and up to 72 h [271].
The wound healing effects of 60 were tested in both healthy mice and mice with diabetes mellitus. Treatment with 60 led to the closure of 26% of the wounds in normal mice on the 5th day of treatment, where it induced keratinocyte proliferation, angiogenesis, and collagen maturation in wounded skin without enhancing the normal wound inflammatory response. A similar effect was seen in mice with diabetes mellitus, where 60 promoted re-epithelization, as well as angiogenesis, without enhancing the normal inflammatory response [271].
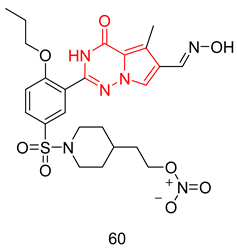
6.2.4. Compounds with Dual PDE5 and Topoisomerase 2 Inhibitory Activities
Abadi and co. tried to combine the anticancer properties of PDE5 and Topoisomerase II inhibitors in a single molecule through the synthesis of several 9-benzylaminoacridine derivatives. They presented dual PDE5-Topoisomerase 2 inhibition as a potential strategy against colorectal cancer. The most potent PDE5 inhibitor of the series 61 (IC50 = 0.83 µM) exhibited lower growth inhibitory activity against colorectal cells (HCT-116). On the other hand, the three most potent topoisomerase two inhibitors, 62, 63 and 64, showed low micromolar PDE5 inhibitory activity and significant growth inhibitory effects against HCT-116 cells. However, 64 was shown to have less selective anticancer activity, where it showed a relatively high growth inhibition against the non-malignant dermal fibroblast CCD-966SK cells (IC50 = 4.67 µM) [272].
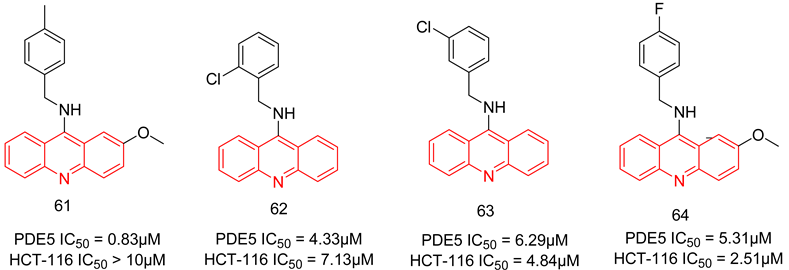
6.3. PDE5 Inhibitors for Radiodiagnosis
Positron emission tomography (PET) is one of the most sensitive in vivo molecular imaging modalities that are based on utilizing radiotracers labeled with short-lived positron-emitting radionuclides [273]. Lately, there is growing interest in the design and biological evaluation of PDE5-specific PET radiotracers, which would facilitate the non-invasive evaluation of PDE5 expression levels in vivo. Using PDE5 radiotracers with optimal pharmacokinetic profiles in combination with high accuracy would likely address multiple issues facing clinical investigators, such as (i) providing valuable diagnostic information regarding the localization, extent and severity of impairments and/or deregulation of the cGMP/PDE5 pathway, thus permitting an early identification of patients that would benefit from treatment with PDE5-Is, (ii) quantification of changes in PDE5 expression during disease progression, and (iii) assessment of occupancy by PDE5-Is in target tissues enabling physicians to tailor optimal dose and dosage regimens on an individual basis [274,275,276].
Despite the availability of many specific and high-affinity PDE5-Is, only a few radiotracers have been evaluated for PET imaging of this enzyme until now. The first PDE5 radiotracers were reported by Chekol et al., who developed the carbon-11 (65) and fluorine-18 labeled (66) vardenafil-based PDE5 radioligands [274]. Both radiotracers exhibited high retention in the lungs, and their specific inhibition of PDE5 was proven via a pre-blocking study in mice by tadalafil. However, none of those ligands demonstrated significant brain uptake [274]. A later report by the same group revealed that a pyridopyrazinone based 18F-labeled derivative (67) exhibited the highest PDE5-specific retention in the lungs of wild-type mice and in the myocardium of transgenic mice with cardiomyocyte-specific PDE5 over-expression. Although (67) readily entered the brain, its radioactivity uptake was found not specific toward the PDE5 [275]. More recently, a 4(3H)-pyrimidinone compound (2) developed for the treatment of PAH was 11C-radiolabeled at the N-methyl of its piperazine ring. However, the authors did not perform a biodistribution study or an in vitro assessment of tracer inhibitory activity to ensure that the radiotracer’s binding ability to PDE5 was unaffected [277]. Very recently, a 14C-radiolabeled derivative of (2) was used to assess its pharmacokinetics where (2) exhibited rapid absorption in humans (Tmax = 0.67 h) and t1/2 of 9.9 h, besides extensive metabolism into 22 metabolites in human plasma, urine and feces [278].
Several studies by the research group of Liu, Wenzel and coworkers have focused on the development of fluorinated quinoline derivatives as brain-specific PDE5 tracers. However, all promising candidates showed high non-specific retention in the brain besides their fast metabolism in vivo, forming brain penetrable radio metabolites [273,276,279]. The challenging design of PDE5-specific radiotracers in the brain is likely attributed to the substantially low PDE5 expression in the brain with only nanomolar density. Accordingly, a radioligand with at least sub-nanomolar PDE5 potency is needed for the quantification of brain PDE5 [280]. This belief guided Dong et al. to 11C-radiolabel the O-methyl of the previously reported picomolar potent PDE5-Is (28 and 30) for the treatment of AD. However, neither good brain penetration of those radiolabeled tracers nor their specific PDE5 binding in vivo was validated by authors, requiring further preclinical investigations [280].
In 2022, avanafil (9) was successfully labeled with iodine-125 via an electrophilic substitution reaction of its methoxy-activated aromatic ring. An in vitro stability study and evaluation of the tracer’s PDE5 inhibitory activity, in addition to biodistribution and clearance studies in rat models of ED, have verified the applicability of radiolabeled avanafil as a promising tracer for ED [281].
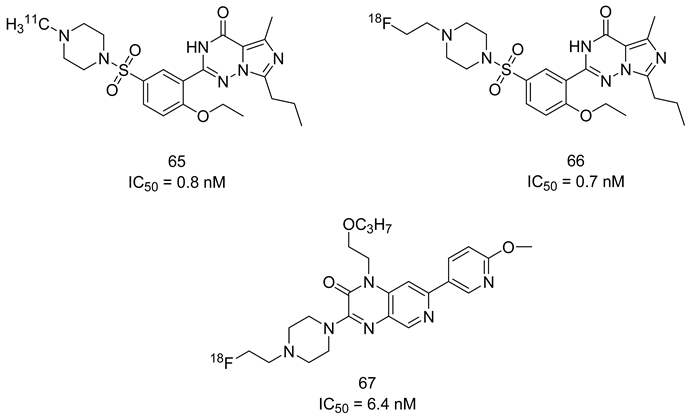
7. Binding Modes of PDE5 Inhibitors
7.1. The PDE5 Active Site
The catalytic domain of PDE5 has three helical subdomains: an N-terminal cyclin-fold region, a linker region, and a C-terminal helical bundle. The active site of PDE5 is located at the center of the C-terminal helical bundle domain, and it can be divided into five subsites: [241,282]
- A metal-binding site (M site) that contains Zn+2 and Mg+2 ions, together with several aspartate and histidine residues.
- A core pocket (Q pocket) lined by Gln817, Phe820, Val782, and Tyr612.
- A hydrophobic pocket (H pocket).
- The Q2-pocket, which is lined by Phe786, Phe787, Leu804, Ile813, Met816.
- The Lid region consists of Tyr664, Met816, Ala823 and Gly819.
A deep insight into the X-ray cocrystal structures of PDE5 enzyme with inhibitors resolved to date provides comprehensive data about the key interactions involved in inhibitors binding and reveals that inhibitors binding to PDE5 active site mainly adopt either a sildenafil/vardenafil-like binding mode or a tadalafil-like binding mode (summarized in Table 1).

Table 1.
Summary of the reported cocrystal structures for PDE5 inhibitors and their key interactions with the PDE5 active site.
Sildenafil is stabilized in the active site of the PDE5 through (a) a bidentate hydrogen bond with the amide group of Gln817, (b) hydrophobic interactions of the pyrazolopyrimidinone core with the side chains of Val782, Leu785, Tyr612, and Phe820, including a face-to-face pi–pi interaction with the phenyl ring of Phe820. In addition, the N2 of the pyrazole forms water-mediated interactions with the Zn+2 in the M site and the side chain of Tyr612. Moreover, the ethoxyphenyl group of sildenafil fits into the Q2 pocket, and finally, the methylpiperazine group of sildenafil is embedded in the L-region [282]. Vardenafil shows a very similar binding mode to sildenafil where no extra interactions are observed for the ethylpiperazine of vardenafil in comparison to the methylpiperazine moiety of sildenafil [282].
The binding mode of tadalafil shows some similarities to that of sildenafil, where the beta-carboline core of tadalafil forms CH-pi interactions with Phe820 and Val782, as well as hydrophobic interactions with Val782 and Tyr612. Moreover, the benzodioxole moiety resides in the Q2 pocket, similar to the ethoxyphenyl group of sildenafil, showing interactions with the residues lining the pocket (mainly Phe786 and Leu804). However, three major differences could be highlighted between the binding modes of sildenafil and tadalafil: (a) tadalafil binds to the conserved Gln817 residue via a monodentate hydrogen bond; (b) tadalafil shows no interactions with the M site; (c) tadalafil shows no interactions with the L-region [241,282].
7.2. The Evo Pocket
Zhang et al. identified a novel site for the binding within the catalytic domain of PDE5 other than the competitive binding site targeted by sildenafil, vardenafil and tadalafil, designated as the EVO pocket. The EVO pocket is located at the back of the active site and is isolated from it by Tyr612, His 613, His617, and Leu781. It is composed of a hydrophobic wall (Phe564, Ile778, Leu781, and Tyr612), a polar wall (Asp563, Arg616, and Asn620), and a polar bottom (Asp764 and His617) [260].
Compound 46 reported by Zhang et al. as a potent allosteric inhibitor for PDE5 with an IC50 of 0.035 µM was co-crystallized with the PDE5 enzyme (PDB ID: 6VIB), and it was shown to be anchored in the EVO pocket via (i) three H-bonds with the side chains of Asp563, Asn614, and His617, (ii) a water-mediated H-bond with Ala767 and Asp764, in addition to (iii) numerous van der Waals interactions with residues Ile778, Leu781, and Phe564 (Table 1) [260].
8. Recent Update on Clinical Trials Involving PDE5 Inhibitors
A plethora of preclinical and clinical studies were conducted over the past 20 years to evaluate the role of PDE5-Is either as a single treatment or in combination therapy for various FDA-approved or emerging clinical conditions. These studies have been extensively discussed in several previous reviews [17,23,76,122,123,283,284,285,286,287]; thus, we herein present an update of the clinical trials status of PDE5-Is in the past five years (summarized in Table 2). Focus was mainly directed to registered trials in ClinicalTrials.gov (accessed on 1 August 2023) that are complete with published results. Moreover, a summary of clinical trials that are currently open (recruiting or soon to commence recruitment) or ongoing is presented in Table S1 (Supplementary Materials), providing a comprehensive overview of the current status of drug discovery efforts involving PDE5 as a therapeutic target. It is worth noting that current clinical trials assessing PDE5-Is in CVS diseases are mainly focused on right ventricular dysfunction, congenital heart disease, or cystic fibrosis, so further trials are yet needed to explore the efficacy of PDE5-Is on cardiac outcomes in myocardial infarction, coronary artery disease, heart failure, and ventricular arrhythmia. Similarly, the potential utility of PDE5-Is as reno-protective agents in AD or in non-ED urological diseases requires further support by carefully designed dose-dependent and time-course animal and clinical studies. On the other hand, a plausible number of ongoing trials are investigating the effects of PDE5-Is in unconventional clinical conditions, such as obesity, retinitis, scleroderma, liver fibrosis, depression, Duchenne muscular dystrophy and fetal hypoxia Table S1 (Supplementary Materials). Accordingly, revealing novel clinical applications for PDE5-Is could be anticipated in the near future.

Table 2.
Recent clinical trials investigating the effects of PDE5 inhibitors in various diseases.
9. Conclusions
The evidence presented in this review underscores the pivotal role of PDE5-Is as disease-modifying agents, not only in the treatment of erectile dysfunction and pulmonary hypertension but also in the treatment of a wide array of other diseases, ranging from cognitive impairments to immune disorders and beyond. Most of the emerging uses of PDE5-Is arise from their abilities to modulate the level of the universal cellular secondary messenger cGMP with its implication in various pathological and physiological conditions. This is expected to provide new approved and off-label uses for this class of medications.
The primary challenge in the development of PDE5-Is as potential drug candidates isn’t necessarily their potency. Instead, various other obstacles impede this progress. These include insufficient specificity for PDE5 compared to other PDEs, unfavorable pharmacokinetics, limited in vivo effectiveness, and a lack of well-defined safety profiles for many of the inhibitors reported.
The recent advancements in reporting novel chemical scaffolds as allosteric PDE5-Is with high potency and isozyme selectivity represent an exciting avenue for further research and optimization of their therapeutic benefits. Regardless of the limitations, the balance between benefits and risks highly favors the advantageous utilization of PDE5-Is, and their use continues to rise. Although non-selective targeting of isozymes could result in undesired side effects, the existence of PDE5-Is with dual impacts on factors contributing to the same pathological condition, particularly multi-factorial diseases, could potentially offer an advantage over the traditional single target-one disease approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16091266/s1, Table S1: Current on-going clinical trials investigating the effects of PDE5 inhibitors in various diseases.
Author Contributions
A.K.E.: writing, review and editing. D.S.E.-G.: writing, review editing and figures preparation. M.A.-H.: writing, review, editing, conceptualization, supervision. A.H.A.: writing, review, editing, conceptualization, supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ABC: ATP-binding cassette; Ach: Acetylcholine; AChE: Acetylcholine esterase; AD: Alzheimer’s disease; Ala: Alanine; AMP: Adenosine monophosphate; AMPK: AMP-activated protein kinase; APP: Amyloid precursor protein; Arg-1: Arginase 1; ASCs: Adipose stem cells; ATP: Adenosine triphosphate; BBB: Blood brain barrier; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma extra-large; BDNF: Brain-derived neurotrophic factor; BID: twice a day; BPH: Benign prostatic hyperplasia; BTB: Blood tumor barrier; cAMP: Cyclic adenosine monophosphate; CDK5: Cyclin-dependent kinase 5; CF: Cystic fibrosis; CFTR: CF transmembrane conductance regulator; cGMP: Cyclic guanosine monophosphate; CNS: Central nervous system; COVID-19: Coronavirus disease of 2019; COX: Cyclooxygenase; CREB: cAMP response element-binding element; CRF-R1: Corticotropin-releasing factor receptor 1; CRPC: Castration-resistant prostate cancer; CVS: Cardiovascular system; DNA: Deoxyribonucleic acid; dox: Doxorubicin; EC30: 30% of maximal effective concentration; EC50: Half maximal effective concentration; ED: Erectile dysfunction; EMEA: European Medicines Agency; EPC: Endothelial progenitor cell; ER: Endoplasmic reticulum; ERK: Extracellular signal-regulated kinase; FDA: Food and Drug Administration; GABA: Gamma-aminobutyric acid; Gln: Glutamine; GLUT1: Glucose transporter 1; GRP: Glucose regulated protein; GSK3β: Glycogen synthase kinase 3β; HDAC: Histone deacetylase; HEK293: Human embryonic kidney cells 293; HER2: Human epidermal growth factor receptor 2; HF: Heart failure; Hgf: Hepatocyte growth factor; HIF-1α: Hypoxia-inducible factor 1-alpha; His: Histidine; HR: Homologous recombination; HSP90: Heat shock protein 90; I/R: Ischemic and reperfusion; IC50: Half maximal inhibitory concentration; IPPS: International Prostate Symptom Score; JNK: Jun N-terminal kinase; Kg: Kilogram; LC50: Half maximal lethal concentration; Leu: Leucine; LUTS: Lower urinary tract symptoms; LV: Left ventricular; MAPK: Mitogen-activated protein kinase; MDR: Multidrug resistance; MDSCs: Myeloid-derived suppressor cells; MEKK1: Mitogen-activated protein kinase kinase kinase 1; Met: Methionine; MetS: Metabolic syndrome; mg: Milligrams; MI: Myocardial infraction; mRNA: Messenger ribonucleic acid; MS: Multiple sclerosis; MTDLs: Multitarget-directed ligands; mTOR: Mammalian target of rapamycin; NFAT: Nuclear factor of activated T-cells; NFκB: Nuclear factor-κB; NHEJ: Non-homologous end joining; NIHL: Noise-induced hearing loss; NMDA: N-methyl-D-aspartate; NMDAR: N-methyl-D-aspartate receptors; NO: Nitric oxide; NOS: Nitric oxide synthase; NSAID: Non-steroidal anti-inflammatory drug; NSC: Neural stem cells; PAH: Pulmonary arterial hypertension; PAMPA: Parallel Artificial Membrane Permeability Assay; PARP: Poly (ADP-ribose) polymerase; PBMCs: Peripheral blood mononuclear cells; PBS: Phosphate buffer saline; PCSCs: PC3-derived cancer stem cells; PDE: Phosphodiesterase; PDE5-Is: Phosphodiesterase 5 inhibitors; PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha: Phe: Phenylalanine; PI3K: Phosphoinositide 3-kinase; PK: Pharmacokinetic; PKC: Protein kinase C; PKG: Protein kinase G; PS1: Presenilin-1; R&D: Research and development; ROS: Reactive oxygen species; SAR: Structure activity relationship; sGC: Soluble guanylyl cyclase; SI: Selectivity index; Sirt3: Sirtuin-3; SLE: Systemic lupus erythematosus; SMR: Smooth muscle relaxation; SOSA: Selective optimization of side activities; SS: Sulindac sulfide; SSc: Scleroderma; SSRIs: Selective serotonin reuptake inhibitors; TAZ: Tafazzin; TID: three times daily; TGF-β1: Transforming growth factor beta 1; THβC: Tetrahydro-beta-carbolines; TNF: Tumor necrosis factor; Tyr: Tyrosine; Val: Valine; VEGFA: Vascular endothelial growth factor A; Vegfa: Vascular endothelial growth factor; ZBG: Zinc binding group.
References
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lin, G.; Xin, Z.-C.; Lue, T.F. Expression, distribution and regulation of phosphodiesterase 5. Curr. Pharm. Des. 2006, 12, 3439–3457. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.R.; Bledsoe, R.; Chai, J.; Daka, P.; Deng, H.; Ding, Y.; Harris-Gurley, S.; Kryn, L.H.; Nartey, E.; Nichols, J. The role of phosphodiesterase 12 (PDE12) as a negative regulator of the innate immune response and the discovery of antiviral inhibitors. J. Biol. Chem. 2015, 290, 19681–19696. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cruz, J.; Cabrera-Leon, A.; Martın-Morales, A.; Fernandez, A.; Burgos, R.; Rejas, J. Male erectile dysfunction and health-related quality of life. Eur. Urol. 2003, 44, 245–253. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Di Luigi, L.; Lenzi, A.; Crescioli, C. Phosphodiesterase type 5 inhibitors: Back and forward from cardiac indications. J. Endocrinol. Investig. 2016, 39, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Loughney, K.; Hill, T.R.; Florio, V.A.; Uher, L.; Rosman, G.J.; Wolda, S.L.; Jones, B.A.; Howard, M.L.; McAllister-Lucas, L.M.; Sonnenburg, W.K. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′, 5′-cyclic nucleotide phosphodiesterase. Gene 1998, 216, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Tsounapi, P.; Saito, M.; Watanabe, T.; Sylakos, A.; Tsabalas, S.; Miyagawa, I.; Sofikitis, N. Is there a role for PDE5 inhibitors in the management of male infertility due to defects in testicular or epididymal function? Curr. Pharm. Des. 2009, 15, 3506–3520. [Google Scholar] [CrossRef]
- Ückert, S.; Kuczyk, M.A. Cyclic nucleotide metabolism including nitric oxide and phosphodiesterase-related targets in the lower urinary tract. Urin. Tract 2011, 2011, 527–542. [Google Scholar]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the⋅ NO/cGMP signaling pathway. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Murad, F. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Schlossmann, J.; Ammendola, A.; Ashman, K.; Zong, X.; Huber, A.; Neubauer, G.; Wang, G.X.; Allescher, H.D.; Korth, M.; Wilm, M.; et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 2000, 404, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Ammendola, A.; Schlossmann, J. Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 2000, 113, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Strada, S.J. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr. Top. Med. Chem. 2007, 7, 437–454. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 2001, 91, 1421–1430. [Google Scholar] [CrossRef]
- Rondina, M.T.; Weyrich, A.S. Targeting phosphodiesterases in anti-platelet therapy. Antiplatelet Agents 2012, 210, 225–238. [Google Scholar]
- Coskuner, E.R.; Ozkan, B. Reno-protective effects of Phosphodiesterase 5 inhibitors. Clin. Exp. Nephrol. 2021, 25, 585–597. [Google Scholar] [CrossRef]
- Blount, M.A.; Beasley, A.; Zoraghi, R.; Sekhar, K.R.; Bessay, E.P.; Francis, S.H.; Corbin, J.D. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol. Pharmacol. 2004, 66, 144–152. [Google Scholar] [CrossRef]
- Bruzziches, R.; Francomano, D.; Gareri, P.; Lenzi, A.; Aversa, A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin. Pharmacother. 2013, 14, 1333–1344. [Google Scholar] [CrossRef]
- Kedia, G.T.; Ückert, S.; Assadi-Pour, F.; Kuczyk, M.A.; Albrecht, K. Avanafil for the treatment of erectile dysfunction: Initial data and clinical key properties. Ther. Adv. Urol. 2013, 5, 35–41. [Google Scholar] [CrossRef]
- Schellack, N.; Agoro, A. A review of phosphodiesterase type 5 inhibitors. S. Afr. Fam. Pract. 2014, 56, 96–101. [Google Scholar] [CrossRef]
- Gupta, M.; Kovar, A.; Meibohm, B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J. Clin. Pharmacol. 2005, 45, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Ückert, S.; Kuczyk, M.A.; Oelke, M. Phosphodiesterase inhibitors in clinical urology. Expert Rev. Clin. Pharmacol. 2013, 6, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Corbin, J.D. Phosphodiesterase-5 inhibition: The molecular biology of erectile function and dysfunction. Urol. Clin. 2005, 32, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Francis, S.H. Molecular Biology and Pharmacology of PDE-5—Inhibitor Therapy for Erectile Dysfunction. J. Androl. 2003, 24, S38–S41. [Google Scholar] [CrossRef]
- Carson, C.C.; Burnett, A.L.; Levine, L.A.; Nehra, A. The efficacy of sildenafil citrate (Viagra®) in clinical populations: An update. Urology 2002, 60, 12–27. [Google Scholar] [CrossRef]
- Hellstrom, W.J.; Gittelman, M.; Karlin, G.; Segerson, T.; Thibonnier, M.; Taylor, T.; Padma-Nathan, H.; Group, V.S. Sustained efficacy and tolerability of vardenafil, a highly potent selective phosphodiesterase type 5 inhibitor, in men with erectile dysfunction: Results of a randomized, double-blind, 26-week placebo-controlled pivotal trial. Urology 2003, 61, 8–14. [Google Scholar] [CrossRef]
- Brock, G.B.; McMahon, C.G.; Chen, K.; Costigan, T.; Shen, W.; Watkins, V.; Anglin, G.; Whitaker, S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: Results of integrated analyses. J. Urol. 2002, 168, 1332–1336. [Google Scholar] [CrossRef]
- Goldstein, I.; McCullough, A.R.; Jones, L.A.; Hellstrom, W.J.; Bowden, C.H.; DiDonato, K.; Trask, B.; Day, W.W. A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J. Sex. Med. 2012, 9, 1122–1133. [Google Scholar] [CrossRef]
- Cui, Y.-S.; Li, N.; Zong, H.-T.; Yan, H.-L.; Zhang, Y. Avanafil for male erectile dysfunction: A systematic review and meta-analysis. Asian J. Androl. 2014, 16, 472. [Google Scholar]
- Jannini, E.A.; DeRogatis, L.R.; Chung, E.; Brock, G.B. How to evaluate the efficacy of the phosphodiesterase type 5 inhibitors. J. Sex. Med. 2012, 9, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mirone, V.; Fusco, F.; Rossi, A.; Sicuteri, R.; Montorsi, F. Tadalafil and vardenafil vs sildenafil: A review of patient-preference studies. BJU Int. 2009, 103, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.K.; Eickelberg, O.; Voelkel, N.F. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43, S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Beasley, A.; Blount, M.A.; Francis, S.H. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem. Biophys. Res. Commun. 2005, 334, 930–938. [Google Scholar] [CrossRef]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef]
- Shenoy, P.; Agarwal, V. Phosphodiesterase inhibitors in the management of autoimmune disease. Autoimmun. Rev. 2010, 9, 511–515. [Google Scholar] [CrossRef]
- Nguyen, H.; Amanullah, A.M. Therapeutic potentials of phosphodiesterase-5 inhibitors in cardiovascular disease. Rev. Cardiovasc. Med. 2014, 15, 158–167. [Google Scholar] [CrossRef]
- Yamamura, A.; Fujitomi, E.; Ohara, N.; Tsukamoto, K.; Sato, M.; Yamamura, H. Tadalafil induces antiproliferation, apoptosis, and phosphodiesterase type 5 downregulation in idiopathic pulmonary arterial hypertension in vitro. Eur. J. Pharmacol. 2017, 810, 44–50. [Google Scholar] [CrossRef]
- Foresta, C.; De Toni, L.; Di Mambro, A.; Garolla, A.; Ferlin, A.; Zuccarello, D. BASIC SCIENCE: The PDE5 Inhibitor Sildenafil Increases Circulating Endothelial Progenitor Cells and CXCR4 Expression. J. Sex. Med. 2009, 6, 369–372. [Google Scholar] [CrossRef]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; Galiè, N.; Black, C.M.; Keogh, A.; Pulido, T.; Frost, A.; Roux, S.; Leconte, I. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002, 346, 896–903. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Gilbert, C.; Collings, L.; Brown, M.C. Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest 2008, 133, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Brundage, B.H.; Galiè, N.; Ghofrani, H.A.; Simonneau, G.; Botros, F.T.; Chan, M.; Beardsworth, A.; Barst, R.J.; PHIRST Study Group. Tadalafil for the treatment of pulmonary arterial hypertension: A double-blind 52-week uncontrolled extension study. J. Am. Coll. Cardiol. 2012, 60, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Beardsworth, A.; Chan, M.; Angalakuditi, M. Tadalafil therapy and health-related quality of life in pulmonary arterial hypertension. Curr. Med. Res. Opin. 2009, 25, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C.; Salloum, F.N.; Das, A.; Koka, S.; Ockaili, R.A.; Xi, L. Emerging new uses of phosphodiesterase-5 inhibitors in cardiovascular diseases. Exp. Clin. Cardiol. 2011, 16, e30. [Google Scholar] [PubMed]
- Silvera, F.; Blasina, M.; Vaamonde, L.; Tellechea, S.; Godoy, C.; Zabala, S.; Mañana, G.; Martell, M.; Olivera, W. Sildenafil prevents the increase of extravascular lung water and pulmonary hypertension after meconium aspiration in newborn piglets. Braz. J. Med. Biol. Res. 2011, 44, 778–785. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Voswinckel, R.; Reichenberger, F.; Olschewski, H.; Haredza, P.; Karadaş, B.; Schermuly, R.T.; Weissmann, N.; Seeger, W.; Grimminger, F. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: A randomized prospective study. J. Am. Coll. Cardiol. 2004, 44, 1488–1496. [Google Scholar]
- Schwartz, B.G.; Levine, L.A.; Comstock, G.; Stecher, V.J.; Kloner, R.A. Cardiac uses of phosphodiesterase-5 inhibitors. J. Am. Coll. Cardiol. 2012, 59, 9–15. [Google Scholar] [CrossRef][Green Version]
- Simonneau, G.; Rubin, L.J.; Galie, N.; Barst, R.J.; Fleming, T.R.; Frost, A.E.; Engel, P.J.; Kramer, M.R.; Burgess, G.; Collings, L. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann. Intern. Med. 2008, 149, 521–530. [Google Scholar] [CrossRef]
- Stehlik, J.; Movsesian, M.A. Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J. Card. Fail. 2009, 15, 31–34. [Google Scholar] [CrossRef]
- Bowles, E.A.; Moody, G.N.; Yeragunta, Y.; Stephenson, A.H.; Ellsworth, M.L.; Sprague, R.S. Phosphodiesterase 5 inhibitors augment UT-15C-stimulated ATP release from erythrocytes of humans with pulmonary arterial hypertension. Exp. Biol. Med. 2015, 240, 121–127. [Google Scholar] [CrossRef]
- Zhao, L.; Mason, N.A.; Morrell, N.W.; Kojonazarov, B.; Sadykov, A.; Maripov, A.; Mirrakhimov, M.M.; Aldashev, A.; Wilkins, M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 2001, 104, 424–428. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Gratadour, P.; Robach, P.; Pham, I.; Déchaux, M.; Joncquiert-Latarjet, A.; Mollard, P.; Brugniaux, J.; Cornolo, J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005, 171, 275–281. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; McKenna, K.E. The relationship between erectile dysfunction and lower urinary tract symptoms: Epidemiological, clinical, and basic science evidence. Curr. Prostate Rep. 2004, 2, 71–77. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Uher, E.; Wiesinger, G.; Kaider, A.; Ebenbichler, G.; Nicolakis, P.; Kollmitzer, J.; Preisinger, E.; Fialka-Moser, V. Reliability of pelvic floor muscle strength measurement in elderly incontinent women. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2002, 21, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Lawrentschuk, N.; Bolton, D.M. Phosphodiesterase 5 inhibitors in the management of benign prostatic hyperplasia and erectile dysfunction: The best of both worlds. Curr. Opin. Urol. 2009, 19, 7–12. [Google Scholar] [CrossRef]
- Fusco, F.; di Villa Bianca, R.D.E.; Mitidieri, E.; Cirino, G.; Sorrentino, R.; Mirone, V. Sildenafil effect on the human bladder involves the L-cysteine/hydrogen sulfide pathway: A novel mechanism of action of phosphodiesterase type 5 inhibitors. Eur. Urol. 2012, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.A.; dos Gomes, F.O.S. The role of phosphodiesterase-5 inhibitors in prostatic inflammation: A review. J. Inflamm. 2015, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Mónica, F.Z.; De Nucci, G. Tadalafil for the treatment of benign prostatic hyperplasia. Expert Opin. Pharmacother. 2019, 20, 929–937. [Google Scholar] [CrossRef]
- Nomiya, M.; Burmeister, D.M.; Sawada, N.; Campeau, L.; Zarifpour, M.; Keys, T.; Peyton, C.; Yamaguchi, O.; Andersson, K.-E. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J. Urol. 2013, 189, 754–761. [Google Scholar] [CrossRef]
- Sairam, K.; Kulinskaya, E.; McNicholas, T.; Boustead, G.; Hanbury, D. Sildenafil influences lower urinary tract symptoms. BJU Int. 2002, 90, 836–839. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Kaminetsky, J.C.; Auerbach, S.M.; Wachs, B.; Young, J.M.; Esler, A.; Sides, G.D.; Denes, B.S. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2007, 177, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; Kaminetsky, J.C.; Auerbach, S.M.; Montelongo, R.M.; Elion-Mboussa, A.; Viktrup, L. Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment. BJU Int. 2010, 105, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R.; Roehrborn, C.; Klise, S.; Xu, L.; Kaminetsky, J.; Kraus, S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: A randomized, placebo controlled 12-week clinical trial. J. Urol. 2010, 183, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Stief, C.G.; Porst, H.; Neuser, D.; Beneke, M.; Ulbrich, E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 2008, 53, 1236–1244. [Google Scholar] [CrossRef]
- Gacci, M.; Andersson, K.-E.; Chapple, C.; Maggi, M.; Mirone, V.; Oelke, M.; Porst, H.; Roehrborn, C.; Stief, C.; Giuliano, F. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 2016, 70, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Trombetta, C.; De Giorgi, G.; Pomara, G.; Maio, G.; Vecchio, D.; Ocello, G.; Ollandini, G.; Bucci, S.; Belgrano, E. Efficacy and Safety of Combined Oral Therapy with Tadalafil andAlfuzosin: An Integrated Approach to the Management of Patientswith Lower Urinary Tract Symptoms and Erectile Dysfunction. Preliminary Report. J. Sex. Med. 2009, 6, 544–552. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Gonzalez, R.R.; Te, A.E. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur. Urol. 2007, 51, 1717–1723. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Salvi, M.; Vignozzi, L.; McVary, K.T.; Kaplan, S.A.; Roehrborn, C.G.; Serni, S.; Mirone, V.; Carini, M.; et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2012, 61, 994–1003. [Google Scholar] [CrossRef]
- Tuncel, A.; Nalcacioglu, V.; Ener, K.; Aslan, Y.; Aydin, O.; Atan, A. Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J. Urol. 2010, 28, 17–22. [Google Scholar] [CrossRef]
- Bechara, A.; Romano, S.; Casabé, A.; Haime, S.; Dedola, P.; Hernández, C.; Rey, H. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J. Sex. Med. 2008, 5, 2170–2178. [Google Scholar] [CrossRef]
- Guo, B.; Chen, X.; Wang, M.; Hou, H.; Zhang, Z.; Liu, M. Comparative Effectiveness of Tadalafil versus Tamsulosin in Treating Lower Urinary Tract Symptoms Suggestive of Benign Prostate Hyperplasia: A Meta-Analysis of Randomized Controlled Trials. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923179-1–e923179-8. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. Phosphodiesterase type 5 and cancers: Progress and challenges. Oncotarget 2017, 8, 99179. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Campana, A.; Giordano, C.; Győrffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Ferraro, A.; Romeo, F.; Lanzino, M. Expression and Function of Phosphodiesterase Type 5 in Human Breast Cancer Cell Lines and Tissues: Implications for Targeted TherapyPDE5 Enhances Breast Cancer Cell Invasive Potential. Clin. Cancer Res. 2016, 22, 2271–2282. [Google Scholar] [CrossRef]
- Sponziello, M.; Verrienti, A.; Rosignolo, F.; De Rose, R.F.; Pecce, V.; Maggisano, V.; Durante, C.; Bulotta, S.; Damante, G.; Giacomelli, L. PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine 2015, 50, 434–441. [Google Scholar] [CrossRef]
- Peak, T.C.; Richman, A.; Gur, S.; Yafi, F.A.; Hellstrom, W.J. The role of PDE5 inhibitors and the NO/cGMP pathway in cancer. Sex. Med. Rev. 2016, 4, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Sukhatme, V.; Crispino, S.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing drugs in oncology (ReDO)—Selective PDE5 inhibitors as anti-cancer agents. Ecancermedicalscience 2018, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, M.; Mateo, V.; Baudet, S.; Rubio, M.; Fernandez, C.; Davi, F.; Binet, J.L.; Delic, J.; Merle-Beral, H. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 2003, 101, 265–269. [Google Scholar] [CrossRef]
- Mei, X.-L.; Yang, Y.; Zhang, Y.-J.; Li, Y.; Zhao, J.-M.; Qiu, J.-G.; Zhang, W.-J.; Jiang, Q.-W.; Xue, Y.-Q.; Zheng, D.-W. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am. J. Cancer Res. 2015, 5, 3311. [Google Scholar]
- Li, H.; Liu, L.; David, M.L.; Whitehead, C.M.; Chen, M.; Fetter, J.R.; Sperl, G.J.; Pamukcu, R.; Thompson, W.J. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve β-catenin and cyclin D1 down-regulation. Biochem. Pharmacol. 2002, 64, 1325–1336. [Google Scholar] [CrossRef]
- Tinsley, H.N.; Gary, B.D.; Keeton, A.B.; Lu, W.; Li, Y.; Piazza, G.A. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin–mediated transcription in human breast tumor cells. Cancer Prev. Res. 2011, 4, 1275–1284. [Google Scholar] [CrossRef]
- Rice, P.L.; Beard, K.S.; Driggers, L.J.; Ahnen, D.J. Inhibition of extracellular-signal regulated kinases 1/2 is required for apoptosis of human colon cancer cells in vitro by sulindac metabolites. Cancer Res. 2004, 64, 8148–8151. [Google Scholar] [CrossRef]
- Aono, Y.; Horinaka, M.; Iizumi, Y.; Watanabe, M.; Taniguchi, T.; Yasuda, S.; Sakai, T. Sulindac sulfone inhibits the mTORC1 pathway in colon cancer cells by directly targeting voltage-dependent anion channel 1 and 2. Biochem. Biophys. Res. Commun. 2018, 505, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.J.; Piazza, G.A.; Li, H.; Liu, L.; Fetter, J.; Zhu, B.; Sperl, G.; Ahnen, D.; Pamukcu, R. Exisulind induction of apoptosis involves guanosine 3′, 5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Cancer Res. 2000, 60, 3338–3342. [Google Scholar]
- Soh, J.-W.; Mao, Y.; Kim, M.-G.; Pamukcu, R.; Li, H.; Piazza, G.A.; Thompson, W.J.; Weinstein, I.B. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase. Clin. Cancer Res. 2000, 6, 4136–4141. [Google Scholar] [PubMed]
- Li, Q.; Shu, Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm. Res. 2014, 31, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Salloum, F.N.; Xi, L.; Kukreja, R.C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2015, 147, 12–21. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.; Cruickshanks, N.; Conley, A.; Durrant, D.; Das, A.; Fisher, P.; Kukreja, R.; Grant, S.; Poklepovic, A. PDE5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharmacol. 2014, 85, 408–419. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Sim, W.-S.; Lee, I.-K.; Seu, Y.-B.; Kim, I.-H. Decursinol angelate: A cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med. 1997, 63, 360–361. [Google Scholar] [CrossRef]
- Ding, P.-R.; Tiwari, A.K.; Ohnuma, S.; Lee, J.W.; An, X.; Dai, C.-L.; Lu, Q.-S.; Singh, S.; Yang, D.-H.; Talele, T.T. The phosphodiesterase-5 inhibitor vardenafil is a potent inhibitor of ABCB1/P-glycoprotein transporter. PLoS ONE 2011, 6, e19329. [Google Scholar] [CrossRef]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Singh, S.; Kim, I.-W.; Bates, S.E.; Peng, X.; Abraham, I.; Ambudkar, S.V. Sildenafil reverses ABCB1-and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71, 3029–3041. [Google Scholar] [CrossRef]
- Black, K.L.; Yin, D.; Ong, J.M.; Hu, J.; Konda, B.M.; Wang, X.; Ko, M.K.; Bayan, J.-A.; Sacapano, M.R.; Espinoza, A. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008, 1230, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ljubimova, J.Y.; Inoue, S.; Konda, B.; Patil, R.; Ding, H.; Espinoza, A.; Wawrowsky, K.A.; Patil, C.; Ljubimov, A.V. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS ONE 2010, 5, e10108. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.; Roberts, J.L.; Cruickshanks, N.; Tavallai, S.; Webb, T.; Samuel, P.; Conley, A.; Binion, B.; Young, H.F.; Poklepovic, A. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J. Cell. Physiol. 2015, 230, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Mitchell, C.; Mayton, E.; Hoke, N.N.; Salloum, F.N.; Park, M.A.; Qureshi, I.; Lee, R.; Dent, P. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 18202–18207. [Google Scholar] [CrossRef]
- Chang, J.-F.; Hsu, J.-L.; Sheng, Y.-H.; Leu, W.-J.; Yu, C.-C.; Chan, S.-H.; Chan, M.-L.; Hsu, L.-C.; Liu, S.-P.; Guh, J.-H. Phosphodiesterase type 5 (PDE5) inhibitors sensitize topoisomerase II inhibitors in killing prostate cancer through PDE5-independent impairment of HR and NHEJ DNA repair systems. Front. Oncol. 2019, 8, 681. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Leu, W.-J.; Hsu, L.-C.; Ho, C.-H.; Liu, S.-P.; Guh, J.-H. Phosphodiesterase Type 5 Inhibitors Synergize Vincristine in Killing Castration-Resistant Prostate Cancer Through Amplifying Mitotic Arrest Signaling. Front. Oncol. 2020, 10, 1274. [Google Scholar] [CrossRef]
- Tavallai, M.; Hamed, H.A.; Roberts, J.L.; Cruickshanks, N.; Chuckalovcak, J.; Poklepovic, A.; Booth, L.; Dent, P. Nexavar/Stivarga and viagra interact to kill tumor cells. J. Cell. Physiol. 2015, 230, 2281–2298. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.L.; Poklepovic, A.; Dent, P. PDE5 inhibitors enhance the lethality of [pemetrexed+ sorafenib]. Oncotarget 2017, 8, 13464. [Google Scholar] [CrossRef]
- Chuckalovcak, J.; Carter, J.; Poklepovic, A.; Dent, P. OSU-03012 and Viagra treatment inhibits the activity of multiple chaperone proteins and disrupts the blood brain barrier: Implications for anti-cancer therapies. J. Cell. Physiol. 2015, 230, 1982–1998. [Google Scholar]
- Chen, L.; Liu, Y.; Becher, A.; Diepold, K.; Schmid, E.; Fehn, A.; Brunner, C.; Rouhi, A.; Chiosis, G.; Cronauer, M. Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2. Carcinogenesis 2020, 41, 1421–1431. [Google Scholar] [CrossRef]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.A.; Khan, Z.; Noonan, K.A.; Rudraraju, L.; Zhang, Z.; Wang, H.; Goodman, S.; Gourin, C.G.; Ha, P.K.; Fakhry, C. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Use of phosphodiesterase 5 inhibitors is associated with lower risk of colorectal cancer in men with benign colorectal neoplasms. Gastroenterology 2019, 157, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, S.K.; Rawat, D.; Koiri, R.K. Protective and therapeutic effects of sildenafil and tadalafil on aflatoxin B1-induced hepatocellular carcinoma. Mol. Cell. Biochem. 2021, 476, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, S.K.; Rawat, D.; Koiri, R.K. Repurposing PDE5 inhibitor tadalafil and sildenafil as anticancer agent against hepatocellular carcinoma via targeting key events of glucose metabolism and multidrug resistance. J. Biochem. Mol. Toxicol. 2022, 36, e23100. [Google Scholar] [CrossRef]
- Liu, N.; Mei, L.; Fan, X.; Tang, C.; Ji, X.; Hu, X.; Shi, W.; Qian, Y.; Hussain, M.; Wu, J. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett. 2016, 378, 38–50. [Google Scholar] [CrossRef]
- Puzzo, D.; Sapienza, S.; Arancio, O.; Palmeri, A. Role of phosphodiesterase 5 in synaptic plasticity and memory. Neuropsychiatr. Dis. Treat. 2008, 4, 371–387. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Kandel, E.R.; Hawkins, R.D. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J. Neurosci. 1999, 19, 10250–10261. [Google Scholar] [CrossRef]
- Ben Aissa, M.; Lee, S.H.; Bennett, B.M.; Thatcher, G.R.J. Targeting NO/cGMP signaling in the CNS for neurodegeneration and Alzheimer’s disease. Curr. Med. Chem. 2016, 23, 2770–2788. [Google Scholar] [CrossRef]
- Kawasaki, K.; Smith Jr, R.S.; Hsieh, C.-M.; Sun, J.; Chao, J.; Liao, J.K. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol. Cell. Biol. 2003, 23, 5726–5737. [Google Scholar] [CrossRef]
- García-Osta, A.; Cuadrado-Tejedor, M.; García-Barroso, C.; Oyarzabal, J.; Franco, R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Zamiri, N.; Farshchizarabi, S.; Sabayan, B. Phoshphodiesterase-5 inhibitors: Novel weapons against Alzheimer’s disease? Int. J. Neurosci. 2010, 120, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Tejedor, M.; Hervias, I.; Ricobaraza, A.; Puerta, E.; Pérez-Roldán, J.; García-Barroso, C.; Franco, R.; Aguirre, N.; García-Osta, A. Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br. J. Pharmacol. 2011, 164, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Staniszewski, A.; Deng, S.X.; Privitera, L.; Leznik, E.; Liu, S.; Zhang, H.; Feng, Y.; Palmeri, A.; Landry, D.W. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-β load in an Alzheimer’s disease mouse model. J. Neurosci. 2009, 29, 8075–8086. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Zhao, X.; Chen, Z.; Wang, G.; Liu, A.; Wang, Q.; Zhou, W.; Xu, Y.; Wang, C. Phosphodiesterase-5 inhibitor sildenafil prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in APP/PS1 transgenic mice. Behav. Brain Res. 2013, 250, 230–237. [Google Scholar] [CrossRef]
- García-Barroso, C.; Ricobaraza, A.; Pascual-Lucas, M.; Unceta, N.; Rico, A.J.; Goicolea, M.A.; Sallés, J.; Lanciego, J.L.; Oyarzabal, J.; Franco, R. Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology 2013, 64, 114–123. [Google Scholar] [CrossRef]
- Devan, B.D.; Sierra-Mercado, D., Jr.; Jimenez, M.; Bowker, J.L.; Duffy, K.B.; Spangler, E.L.; Ingram, D.K. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacol. Biochem. Behav. 2004, 79, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Reneerkens, O.A.; Rutten, K.; Akkerman, S.; Blokland, A.; Shaffer, C.L.; Menniti, F.S.; Steinbusch, H.W.; Prickaerts, J. Phosphodiesterase type 5 (PDE5) inhibition improves object recognition memory: Indications for central and peripheral mechanisms. Neurobiol. Learn. Mem. 2012, 97, 370–379. [Google Scholar] [CrossRef]
- Orejana, L.; Barros-Miñones, L.; Jordán, J.; Puerta, E.; Aguirre, N. Sildenafil ameliorates cognitive deficits and tau pathology in a senescence-accelerated mouse model. Neurobiol. Aging 2012, 33, 625.e611–625.e620. [Google Scholar] [CrossRef]
- Sikandaner, H.E.; Park, S.Y.; Kim, M.J.; Park, S.N.; Yang, D.W. Neuroprotective effects of sildenafil against oxidative stress and memory dysfunction in mice exposed to noise stress. Behav. Brain Res. 2017, 319, 37–47. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Hasan, S.N.; Alam, T.; Hasan, A.T.; Hossain, I.; Didar, R.R.; Alam, M.A.; Rahman, M.M. Tadalafil enhances working memory, and reduces hippocampal oxidative stress in both young and aged mice. Eur. J. Pharmacol. 2014, 745, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, H.; Ding, S.; Wang, D.; Song, G.; Huang, X. Phosphodiesterase 5 inhibitors as novel agents for the treatment of Alzheimer’s disease. Brain Res. Bull. 2019, 153, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, E.; Acquarone, E.; Calcagno, E.; Argyrousi, E.K.; Deng, S.-X.; Landry, D.W.; Arancio, O.; Fiorito, J. Development of novel phosphodiesterase 5 inhibitors for the therapy of Alzheimer’s disease. Biochem. Pharmacol. 2020, 176, 113818. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Zhang, R.L.; Cui, Y.; LaPointe, M.C.; Silver, B.; Chopp, M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006, 1118, 192–198. [Google Scholar] [CrossRef]
- Mendes Soares, L.; Prickaerts, J.; Milani, H.; Del Bel, E.; Wilhelm Maria Steinbusch, H.; Maria Weffort de Oliveira, R. Phosphodiesterase inhibition as a therapeutic target for brain ischemia. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2015, 14, 1012–1023. [Google Scholar]
- Ölmestig, J.N.; Marlet, I.R.; Hainsworth, A.H.; Kruuse, C. Phosphodiesterase 5 inhibition as a therapeutic target for ischemic stroke: A systematic review of preclinical studies. Cell. Signal. 2017, 38, 39–48. [Google Scholar] [CrossRef]
- Silver, B.; McCarthy, S.; Lu, M.; Mitsias, P.; Russman, A.N.; Katramados, A.; Morris, D.C.; Lewandowski, C.A.; Chopp, M. Sildenafil treatment of subacute ischemic stroke: A safety study at 25-mg daily for 2 weeks. J. Stroke Cerebrovasc. Dis. 2009, 18, 381–383. [Google Scholar] [CrossRef]
- Ozdegirmenci, O.; Kucukozkan, T.; Akdag, E.; Topal, T.; Haberal, A.; Kayir, H.; Oter, S.; Akyol, M.; Uzbay, T. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J. Matern.-Fetal Neonatal Med. 2011, 24, 317–323. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.G.; Zhang, R.L.; Chopp, M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J. Cereb. Blood Flow Metab. 2005, 25, 1150–1158. [Google Scholar] [CrossRef]
- Santos, A.I.; Carreira, B.P.; Nobre, R.J.; Carvalho, C.M.; Araújo, I.M. Stimulation of neural stem cell proliferation by inhibition of phosphodiesterase 5. Stem Cells Int. 2014, 2014, 878397. [Google Scholar] [CrossRef]
- Son, Y.; Kim, K.; Cho, H.-R. Sildenafil protects neuronal cells from mitochondrial toxicity induced by β-amyloid peptide via ATP-sensitive K+ channels. Biochem. Biophys. Res. Commun. 2018, 500, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Sharma, S.; Kumar, K.; Deshmukh, R.; Sharma, P.L. Neuroprotective role of PDE4 and PDE5 inhibitors in 3-nitropropionic acid induced behavioral and biochemical toxicities in rats. Eur. J. Pharmacol. 2013, 714, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kenney, K.; Amyot, F.; Moore, C.; Haber, M.; Turtzo, L.C.; Shenouda, C.; Silverman, E.; Gong, Y.; Qu, B.X.; Harburg, L. Phosphodiesterase-5 inhibition potentiates cerebrovascular reactivity in chronic traumatic brain injury. Ann. Clin. Transl. Neurol. 2018, 5, 418–428. [Google Scholar] [CrossRef]
- de Santana Nunes, A.K.; Rapôso, C.; de Oliveira, W.H.; Thomé, R.; Verinaud, L.; Tovar-Moll, F.; Peixoto, C.A. Phosphodiesterase-5 inhibition promotes remyelination by MCP-1/CCR-2 and MMP-9 regulation in a cuprizone-induced demyelination model. Exp. Neurol. 2016, 275, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pifarre, P.; Prado, J.; Baltrons, M.A.; Giralt, M.; Gabarro, P.; Feinstein, D.L.; Hidalgo, J.; Garcia, A. Sildenafil (Viagra) ameliorates clinical symptoms and neuropathology in a mouse model of multiple sclerosis. Acta Neuropathol. 2011, 121, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, N.; Harvey, B.H.; Brand, L.; Wegener, G.; Brink, C.B. Chronic treatment with the phosphodiesterase type 5 inhibitors sildenafil and tadalafil display anxiolytic effects in Flinders Sensitive Line rats. Metab. Brain Dis. 2012, 27, 337–340. [Google Scholar] [CrossRef]
- Otari, K.; Upasani, C. Antidepressant-like effect of tadalafil, a phosphodiesterase type 5 inhibitor, in the forced swim test: Dose and duration of treatment dependence. Neurochem. J. 2015, 9, 306–310. [Google Scholar] [CrossRef]
- Matsushita, H.; Matsuzaki, M.; Han, X.-J.; Nishiki, T.-I.; Ohmori, I.; Michiue, H.; Matsui, H.; Tomizawa, K. Antidepressant-like effect of sildenafil through oxytocin-dependent cyclic AMP response element-binding protein phosphorylation. Neuroscience 2012, 200, 13–18. [Google Scholar] [CrossRef]
- Jaumann, M.; Dettling, J.; Gubelt, M.; Zimmermann, U.; Gerling, A.; Paquet-Durand, F.; Feil, S.; Wolpert, S.; Franz, C.; Varakina, K. cGMP-Prkg1 signaling and Pde5 inhibition shelter cochlear hair cells and hearing function. Nat. Med. 2012, 18, 252–259. [Google Scholar] [CrossRef]
- Huang, L.J.; Yoon, M.H.; Choi, J.I.; Kim, W.M.; Lee, H.G.; Kim, Y.O. Effect of sildenafil on neuropathic pain and hemodynamics in rats. Yonsei Med. J. 2010, 51, 82–87. [Google Scholar] [CrossRef]
- Vieira, M.C.; de Monte, F.B.M.; Eduardo Dematte, B.; Montagnoli, T.L.; Montes, G.C.; da Silva, J.S.; Mendez-Otero, R.; Trachez, M.M.; Sudo, R.T.; Zapata-Sudo, G. Antinociceptive Effect of Lodenafil Carbonate in Rodent Models of Inflammatory Pain and Spinal Nerve Ligation-Induced Neuropathic Pain. J. Pain Res. 2021, 14, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chopp, M.; Szalad, A.; Liu, Z.; Bolz, M.; Ãlvarez, F.M.; Lu, M.; Zhang, L.; Cui, Y.; Zhang, R.L. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience 2011, 193, 399–410. [Google Scholar] [CrossRef]
- Hackett, G. PDE5 inhibitors in diabetic peripheral neuropathy. Int. J. Clin. Pract. 2006, 60, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. Chronic neuropathic pain: Mechanisms, drug targets and measurement. Fundam. Clin. Pharmacol. 2007, 21, 129–136. [Google Scholar] [CrossRef]
- Wallis, R.M.; Corbin, J.D.; Francis, S.H.; Ellis, P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am. J. Cardiol. 1999, 83, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz-Icöz, S.; Radovits, T.; Szabó, G. Targeting phosphodiesterase 5 as a therapeutic option against myocardial ischaemia/reperfusion injury and for treating heart failure. Br. J. Pharmacol. 2018, 175, 223–231. [Google Scholar] [CrossRef]
- du Toit, E.F.; Rossouw, E.; Salie, R.; Opie, L.H.; Lochner, A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc. Drugs Ther. 2005, 19, 23–31. [Google Scholar] [CrossRef]
- Sesti, C.; Florio, V.; Johnson, E.; Kloner, R. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int. J. Impot. Res. 2007, 19, 55–61. [Google Scholar] [CrossRef]
- Ahmad, N.; Wang, Y.; Ali, A.K.; Ashraf, M. Long-acting phosphodiesterase-5 inhibitor, tadalafil, induces sustained cardioprotection against lethal ischemic injury. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H387–H391. [Google Scholar] [CrossRef]
- Maas, O.; Donat, U.; Frenzel, M.; Rütz, T.; Kroemer, H.; Felix, S.; Krieg, T. Vardenafil protects isolated rat hearts at reperfusion dependent on GC and PKG. Br. J. Pharmacol. 2008, 154, 25–31. [Google Scholar] [CrossRef]
- Das, A.; Xi, L.; Kukreja, R.C. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: Essential role of nitric oxide signaling. J. Biol. Chem. 2005, 280, 12944–12955. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nagayama, T.; Blanton, R.M.; Seo, K.; Zhang, M.; Zhu, G.; Lee, D.I.; Bedja, D.; Hsu, S.; Tsukamoto, O. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J. Clin. Investig. 2014, 124, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Hoke, N.N.; Salloum, F.N.; Kass, D.A.; Das, A.; Kukreja, R.C. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells 2012, 30, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Salloum, F.N.; Chau, V.Q.; Hoke, N.N.; Abbate, A.; Varma, A.; Ockaili, R.A.; Toldo, S.; Kukreja, R.C. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G–dependent generation of hydrogen sulfide. Circulation 2009, 120, S31–S36. [Google Scholar] [CrossRef]
- Koka, S.; Xi, L.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: Role of nitric oxide. Mol. Cell. Biochem. 2020, 468, 47–58. [Google Scholar] [CrossRef]
- Hutchings, D.C.; Anderson, S.G.; Caldwell, J.L.; Trafford, A.W. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart 2018, 104, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, J.; Archer, S.L.; Soliman, D.; Gurtu, V.; Moudgil, R.; Haromy, A.; St. Aubin, C.; Webster, L.; Rebeyka, I.M.; Ross, D.B. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007, 116, 238–248. [Google Scholar] [CrossRef]
- Lewis, G.D.; Lachmann, J.; Camuso, J.; Lepore, J.J.; Shin, J.; Martinovic, M.E.; Systrom, D.M.; Bloch, K.D.; Semigran, M.J. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007, 115, 59–66. [Google Scholar] [CrossRef]
- Guazzi, M.; Casali, M.; Berti, F.; Rossoni, G.; D’Gennaro Colonna, V.; Guazzi, M. Endothelium-mediated modulation of ergoreflex and improvement in exercise ventilation by acute sildenafil in heart failure patients. Clin. Pharmacol. Ther. 2008, 83, 336–341. [Google Scholar] [CrossRef]
- De Vecchis, R.; Cesaro, A.; Ariano, C.; Giasi, A.; Cioppa, C. Phosphodiesterase-5 inhibitors improve clinical outcomes, exercise capacity and pulmonary hemodynamics in patients with heart failure with reduced left ventricular ejection fraction: A meta-analysis. J. Clin. Med. Res. 2017, 9, 488. [Google Scholar] [CrossRef]
- Fisher, P.W.; Salloum, F.; Das, A.; Hyder, H.; Kukreja, R.C. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 2005, 111, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Das, A.; Zhu, S.-G.; Durrant, D.; Xi, L.; Kukreja, R.C. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J. Pharmacol. Exp. Ther. 2010, 334, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Yaguas, K.; Bautista, R.; Quiroz, Y.; Ferrebuz, A.; Pons, H.; Franco, M.; Vaziri, N.D.; Rodriguez-Iturbe, B. Chronic sildenafil treatment corrects endothelial dysfunction and improves hypertension. Am. J. Nephrol. 2010, 31, 283–291. [Google Scholar] [CrossRef]
- Lee, T.-M.; Chen, C.-C.; Chung, T.-H.; Chang, N.-C. Effect of sildenafil on ventricular arrhythmias in post-infarcted rat hearts. Eur. J. Pharmacol. 2012, 690, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Nagy, O.; Hajnal, Á.; Parratt, J.R.; Végh, Á. Sildenafil (Viagra) reduces arrhythmia severity during ischaemia 24 h after oral administration in dogs. Br. J. Pharmacol. 2004, 141, 549–551. [Google Scholar] [CrossRef]
- De Bon, E.; Bonanni, G.; Saggiorato, G.; Bassi, P.; Cella, G. Effects of tadalafil on platelets and endothelium in patients with erectile dysfunction and cardiovascular risk factors: A pilot study. Angiology 2010, 61, 602–606. [Google Scholar] [CrossRef]
- Toque, H.; Teixeira, C.; Priviero, F.; Morganti, R.; Antunes, E.; De Nucci, G. Vardenafil, but not sildenafil or tadalafil, has calcium-channel blocking activity in rabbit isolated pulmonary artery and human washed platelets. Br. J. Pharmacol. 2008, 154, 787–796. [Google Scholar] [CrossRef]
- Lewis, G.D.; Witzke, C.; Colon-Hernandez, P.; Guerrero, J.L.; Bloch, K.D.; Semigran, M.J. Sildenafil improves coronary artery patency in a canine model of platelet-mediated cyclic coronary occlusion after thrombolysis. J. Am. Coll. Cardiol. 2006, 47, 1471–1477. [Google Scholar] [CrossRef]
- Saeed, O.; Rangasamy, S.; Selevany, I.; Madan, S.; Fertel, J.; Eisenberg, R.; Aljoudi, M.; Patel, S.R.; Shin, J.; Sims, D.B. Sildenafil is associated with reduced device thrombosis and ischemic stroke despite low-level hemolysis on Heart Mate II support. Circ. Heart Fail. 2017, 10, e004222. [Google Scholar] [CrossRef]
- Gudmundsdóttir, I.J.; McRobbie, S.J.; Robinson, S.D.; Newby, D.E.; Megson, I.L. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem. Biophys. Res. Commun. 2005, 337, 382–385. [Google Scholar] [CrossRef]
- Shenoy, P.D.; Kumar, S.; Jha, L.K.; Choudhary, S.K.; Singh, U.; Misra, R.; Agarwal, V. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: A double-blind randomized cross-over trial. Rheumatology 2010, 49, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Griffiths, B.; Allen, J. Thermographic and symptomatic effect of a single dose of sildenafil citrate on Raynaud’s phenomenon in patients with systemic sclerosis: A potential treatment. J. Rheumatol. 2006, 33, 1918–1919. [Google Scholar] [PubMed]
- Kloner, R.A.; Goldstein, I.; Kirby, M.G.; Parker, J.D.; Sadovsky, R. Cardiovascular safety of phosphodiesterase type 5 inhibitors after nearly 2 decades on the market. Sex. Med. Rev. 2018, 6, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R. Cardiovascular protection with sildenafil following chronic inhibition of nitric oxide synthase. Br. J. Pharmacol. 2007, 150, 538–540. [Google Scholar] [CrossRef]
- Salloum, F.N.; Ockaili, R.A.; Wittkamp, M.; Marwaha, V.R.; Kukreja, R.C. Vardenafil: A novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP channels in rabbits. J. Mol. Cell. Cardiol. 2006, 40, 405–411. [Google Scholar] [CrossRef]
- Wang, X.; Fisher, P.W.; Xi, L.; Kukreja, R.C. Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 105–113. [Google Scholar] [CrossRef]
- Madhani, M.; Hall, A.R.; Cuello, F.; Charles, R.L.; Burgoyne, J.R.; Fuller, W.; Hobbs, A.J.; Shattock, M.J.; Eaton, P. Phospholemman Ser69 phosphorylation contributes to sildenafil-induced cardioprotection against reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H827–H836. [Google Scholar] [CrossRef]
- Inserte, J.; Barba, I.; Poncelas-Nozal, M.; Hernando, V.; Agulló, L.; Ruiz-Meana, M.; Garcia-Dorado, D. cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J. Mol. Cell. Cardiol. 2011, 50, 903–909. [Google Scholar] [CrossRef]
- Nagayama, T.; Hsu, S.; Zhang, M.; Koitabashi, N.; Bedja, D.; Gabrielson, K.L.; Takimoto, E.; Kass, D.A. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J. Am. Coll. Cardiol. 2009, 53, 207–215. [Google Scholar] [CrossRef]
- Li, N.; Yuan, Y.; Li, S.; Zeng, C.; Yu, W.; Shen, M.; Zhang, R.; Li, C.; Zhang, Y.; Wang, H. PDE5 inhibitors protect against post-infarction heart failure. Front. Biosci.-Landmark 2016, 21, 1194–1210. [Google Scholar]
- Chau, V.Q.; Salloum, F.N.; Hoke, N.N.; Abbate, A.; Kukreja, R.C. Mitigation of the progression of heart failure with sildenafil involves inhibition of RhoA/Rho-kinase pathway. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H2272–H2279. [Google Scholar] [CrossRef]
- Afsar, B.; Ortiz, A.; Covic, A.; Gaipov, A.; Esen, T.; Goldsmith, D.; Kanbay, M. Phosphodiesterase type 5 inhibitors and kidney disease. Int. Urol. Nephrol. 2015, 47, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, S.; Benli, E.; Ayyıldız, A.; Cırrık, S.; Çetinkol, Y.; Ayyıldız, S.N.; Noyan, T. Positive outcomes of phosphodiesterase type 5 inhibitor on histopathologic and biochemical changes induced by ureteral obstruction. Rev. Da Assoc. Médica Bras. 2019, 65, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Dhooghe, B.; Leal, T. PDE5 inhibitors as potential tools in the treatment of cystic fibrosis. Front. Pharmacol. 2012, 3, 167. [Google Scholar] [CrossRef] [PubMed]
- Lubamba, B.; Lebacq, J.; Reychler, G.; Marbaix, E.; Wallemacq, P.; Lebecque, P.; Leal, T. Inhaled phosphodiesterase type 5 inhibitors restore chloride transport in cystic fibrosis mice. Eur. Respir. J. 2011, 37, 72–78. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Ishii, H.; Seigler, N.; Crandall, R.; Thomas, J.; Forseen, C.; McKie, K.T.; Harris, R.A. Sildenafil improves exercise capacity in patients with cystic fibrosis: A proof-of-concept clinical trial. Ther. Adv. Chronic Dis. 2019, 10, 2040622319887879. [Google Scholar] [CrossRef]
- Dormer, R.L.; Harris, C.M.; Clark, Z.; Pereira, M.M.C.; Doull, I.J.M.; Norez, C.; Becq, F.; McPherson, M.A. Sildenafil (Viagra) corrects ΔF508-CFTR location in nasal epithelial cells from patients with cystic fibrosis. Thorax 2005, 60, 55–59. [Google Scholar] [CrossRef][Green Version]
- Lubamba, B.; Lecourt, H.; Lebacq, J.; Lebecque, P.; De Jonge, H.; Wallemacq, P.; Leal, T. Preclinical evidence that sildenafil and vardenafil activate chloride transport in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 506–515. [Google Scholar] [CrossRef]
- Dhooghe, B.; Noël, S.; Bouzin, C.; Behets-Wydemans, G.; Leal, T. Correction of chloride transport and mislocalization of CFTR protein by vardenafil in the gastrointestinal tract of cystic fibrosis mice. PLoS ONE 2013, 8, e77314. [Google Scholar] [CrossRef]
- Poschet, J.F.; Timmins, G.S.; Taylor-Cousar, J.L.; Ornatowski, W.; Fazio, J.; Perkett, E.; Wilson, K.R.; Yu, H.D.; de Jonge, H.R.; Deretic, V. Pharmacological modulation of cGMP levels by phosphodiesterase 5 inhibitors as a therapeutic strategy for treatment of respiratory pathology in cystic fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L712–L719. [Google Scholar] [CrossRef]
- Noel, S.; Panin, N.; Beka, M.; Dhooghe, B.; Huaux, F.; Leal, T. Vardenafil reduces macrophage pro-inflammatory overresponses in cystic fibrosis through PDE5-and CFTR-dependent mechanisms. Clin. Sci. 2017, 131, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Poschet, J.F.; Fazio, J.A.; Timmins, G.S.; Ornatowski, W.; Perkett, E.; Delgado, M.; Deretic, V. Endosomal hyperacidification in cystic fibrosis is due to defective nitric oxide–cylic GMP signalling cascade. EMBO Rep. 2006, 7, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Miguelez, P.; Lee, N.; Tucker, M.A.; Csányi, G.; McKie, K.T.; Forseen, C.; Harris, R.A. Sildenafil improves vascular endothelial function in patients with cystic fibrosis. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1486–H1494. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int. J. Impot. Res. 2004, 16, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C.; Wang, R.; Koka, S.; Das, A.; Samidurai, A.; Xi, L. Treating diabetes with combination of phosphodiesterase 5 inhibitors and hydroxychloroquine—A possible prevention strategy for COVID-19? Mol. Cell. Biochem. 2023, 479, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Maga, T.; Colombo, R.; Scattoni, V.; Briganti, A.; Cestari, A.; Guazzoni, G.; Rigatti, P.; Montorsi, F. A prospective study comparing paroxetine alone versus paroxetine plus sildenafil in patients with premature ejaculation. J. Urol. 2002, 168, 2486–2489. [Google Scholar] [CrossRef]
- Gökçe, A.; Halis, F.; Demirtas, A.; Ekmekcioglu, O. The effects of three phosphodiesterase type 5 inhibitors on ejaculation latency time in lifelong premature ejaculators: A double-blind laboratory setting study. BJU Int. 2011, 107, 1274–1277. [Google Scholar] [CrossRef]
- Mcmahon, C.G.; Mcmahon, C.N.; Leow, L.J.; Winestock, C.G. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: A systematic review. BJU Int. 2006, 98, 259–272. [Google Scholar] [CrossRef]
- Ferrini, M.G.; Kovanecz, I.; Nolazco, G.; Rajfer, J.; Gonzalez-Cadavid, N.F. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie’s disease. BJU Int. 2006, 97, 625–633. [Google Scholar] [CrossRef]
- Gonzalez-Cadavid, N.F.; Rajfer, J. Treatment of Peyronie’s disease with PDE5 inhibitors: An antifibrotic strategy. Nat. Rev. Urol. 2010, 7, 215–221. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Giannakis, D.; Pardalidis, N.; Zikopoulos, K.; Paraskevaidis, E.; Giotitsas, N.; Kalaboki, V.; Tsounapi, P.; Baltogiannis, D.; Georgiou, I. Effects of phosphodiesterase 5 inhibitors on sperm parameters and fertilizing capacity. Asian J. Androl. 2008, 10, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Lombardo, F.; Salacone, P.; Gandini, L.; Lenzi, A. Treatment of sexual dysfunctions secondary to male infertility with sildenafil citrate. Fertil. Steril. 2004, 81, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Andric, S.A.; Janjic, M.M.; Stojkov, N.J.; Kostic, T.S. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E544–E550. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.M.; Stojkov, N.J.; Bjelic, M.M.; Mihajlovic, A.I.; Andric, S.A.; Kostic, T.S. Transient rise of serum testosterone level after single sildenafil treatment of adult male rats. J. Sex. Med. 2012, 9, 2534–2543. [Google Scholar] [CrossRef]
- Magawa, S.; Nii, M.; Tanaka, H.; Furuhashi, F.; Maki, S.; Kubo, M.; Tanaka, K.; Kondo, E.; Ikeda, T. Phase-1 clinical study of tadalafil administered for selective fetal growth restriction in twin pregnancy. J. Matern.-Fetal Neonatal Med. 2021, 34, 1075–1082. [Google Scholar] [CrossRef]
- Isidori, A.M.; Giannetta, E.; Pofi, R.; Venneri, M.A.; Gianfrilli, D.; Campolo, F.; Mastroianni, C.M.; Lenzi, A.; d’Ettorre, G. Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. The DEDALO project. Andrology 2021, 9, 33–38. [Google Scholar] [CrossRef]
- Zurawin, J.L.; Stewart, C.A.; Anaissie, J.E.; Yafi, F.A.; Hellstrom, W.J. Avanafil for the treatment of erectile dysfunction. Expert Rev. Clin. Pharmacol. 2016, 9, 1163–1170. [Google Scholar] [CrossRef]
- Li, W.-Q.; Qureshi, A.A.; Robinson, K.C.; Han, J. Sildenafil use and increased risk of incident melanoma in US men: A prospective cohort study. JAMA Intern. Med. 2014, 174, 964–970. [Google Scholar] [CrossRef]
- Loeb, S.; Folkvaljon, Y.; Lambe, M.; Robinson, D.; Garmo, H.; Ingvar, C.; Stattin, P. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA 2015, 313, 2449–2455. [Google Scholar] [CrossRef]
- Pottegård, A.; Schmidt, S.A.J.; Olesen, A.B.; Achacoso, N.; Van Den Eeden, S.K.; Hallas, J.; Sørensen, H.T.; Friis, S.; Habel, L.A. Use of sildenafil or other phosphodiesterase inhibitors and risk of melanoma. Br. J. Cancer 2016, 115, 895–900. [Google Scholar] [CrossRef]
- Gul, M.; Serefoglu, E.C. An update on the drug safety of treating erectile dysfunction. Expert Opin. Drug Saf. 2019, 18, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.M.; Danesh-Meyer, H.V. Phosphodiesterase inhibitors and the eye. Clin. Exp. Ophthalmol. 2009, 37, 514–523. [Google Scholar] [CrossRef]
- Campbell, U.B.; Walker, A.M.; Gaffney, M.; Petronis, K.R.; Creanga, D.; Quinn, S.; Klein, B.E.; Laties, A.M.; Lewis, M.; Sharlip, I.D. Acute nonarteritic anterior ischemic optic neuropathy and exposure to phosphodiesterase type 5 inhibitors. J. Sex. Med. 2015, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Flahavan, E.M.; Li, H.; Gupte-Singh, K.; Rizk, R.T.; Ruff, D.D.; Francis, J.L.; Kinchen, K.S. Prospective case-crossover study investigating the possible association between nonarteritic anterior ischemic optic neuropathy and phosphodiesterase type 5 inhibitor exposure. Urology 2017, 105, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.N.; Yi, T.H.; Kim, S.Y.; Kang, T.H. High dosage sildenafil induces hearing impairment in mice. Biol. Pharm. Bull. 2008, 31, 1981–1984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bakir, S.; Firat, U.; Gün, R.; Bozkurt, Y.; Yorgancilar, E.; Kiniş, V.; Penbegül, N.; Gökalp, O.; Topçu, İ. Histopathologic results of long-term sildenafil administration on rat inner ear. Am. J. Otolaryngol. 2012, 33, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Sheikh, Z.; Khan, S.; Dwivedi, R.; Benjamin, E. Viagra deafness—Sensorineural hearing loss and phosphodiesterase-5 inhibitors. Laryngoscope 2011, 121, 1049–1054. [Google Scholar] [CrossRef]
- Maddox, P.T.; Saunders, J.; Chandrasekhar, S.S. Sudden hearing loss from PDE-5 inhibitors: A possible cellular stress etiology. Laryngoscope 2009, 119, 1586–1589. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kadioglu, A.; Bivalacqua, T.J.; Ghanem, H.; Nehra, A.; Shamloul, R. Priapism: Pathogenesis, epidemiology, and management. J. Sex. Med. 2010, 7, 476–500. [Google Scholar] [CrossRef]
- Nehra, A.; Jackson, G.; Miner, M.; Billups, K.L.; Burnett, A.L.; Buvat, J.; Carson, C.C.; Cunningham, G.R.; Ganz, P.; Goldstein, I. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin. Proc. 2012, 87, 766–778. [Google Scholar] [CrossRef]
- Kayık, G.; Tüzün, N.Ş.; Durdagi, S. Investigation of PDE5/PDE6 and PDE5/PDE11 selective potent tadalafil-like PDE5 inhibitors using combination of molecular modeling approaches, molecular fingerprint-based virtual screening protocols and structure-based pharmacophore development. J. Enzym. Inhib. Med. Chem. 2017, 32, 311–330. [Google Scholar] [CrossRef]
- Padma-Nathan, H.; Giuliano, F. Oral drug therapy for erectile dysfunction. Urol. Clin. N. Am. 2001, 28, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Terrett, N.K.; Bell, A.S.; Brown, D.; Ellis, P. Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg. Med. Chem. Lett. 1996, 6, 1819–1824. [Google Scholar] [CrossRef]
- Haning, H.; Niewöhner, U.; Schenke, T.; Es-Sayed, M.; Schmidt, G.; Lampe, T.; Bischoff, E. Imidazo [5, 1-f], triazin-4 (3H)-ones, a new class of potent PDE 5 inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.C., III. Phosphodiesterase type 5 inhibitors: State of the therapeutic class. Urol. Clin. N. Am. 2007, 34, 507–515. [Google Scholar] [CrossRef]
- Briganti, A.; Salonia, A.; Gallina, A.; Saccà, A.; Montorsi, P.; Rigatti, P.; Montorsi, F. Drug insight: Oral phosphodiesterase type 5 inhibitors for erectile dysfunction. Nat. Clin. Pract. Urol. 2005, 2, 239–247. [Google Scholar] [CrossRef]
- Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. The discovery of Tadalafil: A novel and highly selective PDE5 inhibitor. 1: 5, 6, 11, 11a-Tetrahydro-1 H-imidazo [1′, 5′: 1, 6] pyrido [3, 4-b] indole-1, 3 (2 H)-dione analogues. J. Med. Chem. 2003, 46, 4525–4532. [Google Scholar] [CrossRef]
- Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Linget, J.M.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. The discovery of tadalafil: A novel and highly selective PDE5 inhibitor. 2: 2, 3, 6, 7, 12, 12a-hexahydropyrazino [1′, 2′: 1, 6] pyrido [3, 4-b] indole-1, 4-dione analogues. J. Med. Chem. 2003, 46, 4533–4542. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Chen, T.; Wang, Z.; Yang, H.; Zheng, M.; Ren, J.; Tian, G.; Yang, X.; Li, L. Design, synthesis, and pharmacological evaluation of monocyclic pyrimidinones as novel inhibitors of PDE5. J. Med. Chem. 2012, 55, 10540–10550. [Google Scholar] [CrossRef]
- Gong, X.; Wang, G.; Ren, J.; Liu, Z.; Wang, Z.; Chen, T.; Yang, X.; Jiang, X.; Shen, J.; Jiang, H. Exploration of the 5-bromopyrimidin-4 (3H)-ones as potent inhibitors of PDE5. Bioorg. Med. Chem. Lett. 2013, 23, 4944–4947. [Google Scholar] [CrossRef]
- Sakamoto, T.; Koga, Y.; Hikota, M.; Matsuki, K.; Murakami, M.; Kikkawa, K.; Fujishige, K.; Kotera, J.; Omori, K.; Morimoto, H. Design and synthesis of novel 5-(3, 4, 5-trimethoxybenzoyl)-4-aminopyrimidine derivatives as potent and selective phosphodiesterase 5 inhibitors: Scaffold hopping using a pseudo-ring by intramolecular hydrogen bond formation. Bioorg. Med. Chem. Lett. 2014, 24, 5175–5180. [Google Scholar] [CrossRef]
- Sakamoto, T.; Koga, Y.; Hikota, M.; Matsuki, K.; Murakami, M.; Kikkawa, K.; Fujishige, K.; Kotera, J.; Omori, K.; Morimoto, H. The discovery of avanafil for the treatment of erectile dysfunction: A novel pyrimidine-5-carboxamide derivative as a potent and highly selective phosphodiesterase 5 inhibitor. Bioorg. Med. Chem. Lett. 2014, 24, 5460–5465. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Koga, Y.; Hikota, M.; Matsuki, K.; Mochida, H.; Kikkawa, K.; Fujishige, K.; Kotera, J.; Omori, K.; Morimoto, H. 8-(3-Chloro-4-methoxybenzyl)-8H-pyrido [2, 3-d] pyrimidin-7-one derivatives as potent and selective phosphodiesterase 5 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.D.; Reddy, G.L.; Dar, M.I.; Srinivas, M.; Gupta, G.; Sahu, P.K.; Mahajan, P.; Nargotra, A.; Singh, S.; Sharma, S.C. Discovery of novel pyrazolopyrimidinone analogs as potent inhibitors of phosphodiesterase type-5. Bioorg. Med. Chem. 2015, 23, 2121–2128. [Google Scholar] [CrossRef]
- Reddy, G.L.; Dar, M.I.; Hudwekar, A.D.; Mahajan, P.; Nargotra, A.; Baba, A.M.; Nandi, U.; Wazir, P.; Singh, G.; Vishwakarma, R.A. Design, synthesis and biological evaluation of pyrazolopyrimidinone based potent and selective PDE5 inhibitors for treatment of erectile dysfunction. Bioorg. Chem. 2019, 89, 103022. [Google Scholar] [CrossRef]
- Rawson, D.J.; Ballard, S.; Barber, C.; Barker, L.; Beaumont, K.; Bunnage, M.; Cole, S.; Corless, M.; Denton, S.; Ellis, D. The discovery of UK-369003, a novel PDE5 inhibitor with the potential for oral bioavailability and dose-proportional pharmacokinetics. Bioorg. Med. Chem. 2012, 20, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.S. Tadalafil: 15 years’ journey in male erectile dysfunction and beyond. Drug Dev. Res. 2019, 80, 683–701. [Google Scholar] [CrossRef]
- El-Gamil, D.S.; Ahmed, N.S.; Gary, B.D.; Piazza, G.A.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Design of novel β-carboline derivatives with pendant 5-bromothienyl and their evaluation as phosphodiesterase-5 inhibitors. Arch. Der Pharm. 2013, 346, 23–33. [Google Scholar] [CrossRef]
- Elhady, A.K.; Sigler, S.C.; Noureldin, N.; Canzoneri, J.C.; Ahmed, N.S.; Piazza, G.A.; Abadi, A.H. Structure-based design of novel tetrahydro-beta-carboline derivatives with a hydrophilic side chain as potential phosphodiesterase inhibitors. Sci. Pharm. 2016, 84, 428–446. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, Y.; Sun, B.; Cheng, C.; Qiao, Y.; Jiang, Y.; Zhao, S.; Xie, Z.; Tan, J.; Lou, H. Discovery of furyl/thienyl β-carboline derivatives as potent and selective PDE5 inhibitors with excellent vasorelaxant effect. Eur. J. Med. Chem. 2018, 158, 767–780. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Ali, A.H.; El-Nashar, S.M.; Gary, B.D.; Fajardo, A.M.; Tinsley, H.N.; Piazza, G.A.; Negri, M.; Abadi, A.H. Exploring the PDE5 H-pocket by ensemble docking and structure-based design and synthesis of novel β-carboline derivatives. Eur. J. Med. Chem. 2012, 57, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.S.; Gary, B.D.; Tinsley, H.N.; Piazza, G.A.; Laufer, S.; Abadi, A.H. Design, Synthesis and Structure–Activity Relationship of Functionalized Tetrahydro-β-carboline Derivatives as Novel PDE5 Inhibitors. Arch. Der Pharm. 2011, 344, 149–157. [Google Scholar] [CrossRef]
- Takase, Y.; Saeki, T.; Watanabe, N.; Adachi, H.; Souda, S.; Saito, I. Cyclic GMP phosphodiesterase inhibitors. 2. Requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. J. Med. Chem. 1994, 37, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Konishi, Y.; Yu, D.T.; Miskowski, T.A.; Riviello, C.M.; Macina, O.T.; Frierson, M.R.; Kondo, K.; Sugitani, M. Discovery of potent cyclic GMP phosphodiesterase inhibitors. 2-Pyridyl-and 2-imidazolylquinazolines possessing cyclic GMP phosphodiesterase and thromboxane synthesis inhibitory activities. J. Med. Chem. 1995, 38, 3547–3557. [Google Scholar] [CrossRef]
- Somnarin, T.; Pobsuk, N.; Chantakul, R.; Panklai, T.; Temkitthawon, P.; Hannongbua, S.; Chootip, K.; Ingkaninan, K.; Boonyarattanakalin, K.; Gleeson, D. Computational design, synthesis and biological evaluation of PDE5 inhibitors based on N2, N4-diaminoquinazoline and N2, N6-diaminopurine scaffolds. Bioorg. Med. Chem. 2022, 76, 117092. [Google Scholar] [CrossRef] [PubMed]
- Pobsuk, N.; Paracha, T.U.; Chaichamnong, N.; Salaloy, N.; Suphakun, P.; Hannongbua, S.; Choowongkomon, K.; Pekthong, D.; Chootip, K.; Ingkaninan, K. Design, synthesis and evaluation of N2, N4-diaminoquinazoline based inhibitors of phosphodiesterase type 5. Bioorg. Med. Chem. Lett. 2019, 29, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Chatturong, U.; Martin, H.; Totoson, P.; Ingkaninan, K.; Temkitthawon, P.; Sermsenaphorn, S.; Somarin, T.; Konsue, A.; Gleeson, M.P.; Demougeot, C. Quinazoline-based human phosphodiesterase 5 inhibitors exhibited a selective vasorelaxant effect on rat isolated pulmonary arteries involving NO-sGC-cGMP pathway and calcium inhibitory effects. Vasc. Pharmacol. 2022, 147, 107111. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Dong, Y.-H.; Wang, J.-H.; Ke, H.-M.; Song, G.-Q.; Xu, D.-F. Novel PDE5 inhibitors derived from rutaecarpine for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2020, 30, 127097. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Sun, B.; Gao, Y.; Song, W.; Zhao, X.; Gao, Y.; Xie, Z.; Zhang, N.; Ji, J. Design and synthesis of furyl/thineyl pyrroloquinolones based on natural alkaloid perlolyrine, lead to the discovery of potent and selective PDE5 inhibitors. Eur. J. Med. Chem. 2018, 150, 30–38. [Google Scholar] [CrossRef]
- Fiorito, J.; Saeed, F.; Zhang, H.; Staniszewski, A.; Feng, Y.; Francis, Y.I.; Rao, S.; Thakkar, D.M.; Deng, S.-X.; Landry, D.W. Synthesis of quinoline derivatives: Discovery of a potent and selective phosphodiesterase 5 inhibitor for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 60, 285–294. [Google Scholar] [CrossRef]
- Fiorito, J.; Vendome, J.; Saeed, F.; Staniszewski, A.; Zhang, H.; Yan, S.; Deng, S.-X.; Arancio, O.; Landry, D.W. Identification of a novel 1, 2, 3, 4-tetrahydrobenzo [b][1, 6] naphthyridine analogue as a potent phosphodiesterase 5 inhibitor with improved aqueous solubility for the treatment of Alzheimer’s disease. J. Med. Chem. 2017, 60, 8858–8875. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.-N.; Shao, Y.-X.; Cai, Y.-H.; Guan, M.; Huang, M.; Cui, W.; He, L.; Yu, Y.-J.; Huang, L.; Li, Z. Discovery of 3-(4-hydroxybenzyl)-1-(thiophen-2-yl) chromeno [2, 3-c] pyrrol-9 (2H)-one as a phosphodiesterase-5 inhibitor and its complex crystal structure. Biochem. Pharmacol. 2014, 89, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, T.; Chen, Y.; Huang, Y.; Geng, H.; Yu, Y.; Zhang, C.; Lai, Z.; Wu, Y.; Guo, X. Discovery and optimization of chromeno [2, 3-c] pyrrol-9 (2 H)-ones as novel selective and orally bioavailable phosphodiesterase 5 inhibitors for the treatment of pulmonary arterial hypertension. J. Med. Chem. 2017, 60, 6622–6637. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, Y.; Chen, Y.; Huang, Y.-Y.; Geng, H.; Zhang, T.; Zhang, C.; Li, Z.; Guo, L.; Chen, J. Optimization of Chromeno [2, 3-c] pyrrol-9 (2 H)-ones as Highly Potent, Selective, and Orally Bioavailable PDE5 Inhibitors: Structure–Activity Relationship, X-ray Crystal Structure, and Pharmacodynamic Effect on Pulmonary Arterial Hypertension. J. Med. Chem. 2018, 61, 8468–8473. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, X.; Liu, R.; Li, Z.; Jiang, Z.; Zhou, Q.; Huang, Y.; Wu, X.-N.; Zhang, C.; Huang, Y.-Y. Free energy perturbation (FEP)-guided scaffold hopping. Acta Pharm. Sin. B 2022, 12, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.M.; El-Badry, O.M.; Abdel Rahman, D.E.; Abdellattif, M.H.; Abourehab, M.A.; El-Maghrabey, M.H.; Elsaid, F.G.; El Hamd, M.A.; Elkamhawy, A.; Ammar, U.M. Scaffold repurposing reveals new nanomolar phosphodiesterase type 5 (PDE5) inhibitors based on pyridopyrazinone scaffold: Investigation of in vitro and in silico properties. Pharmaceutics 2022, 14, 1954. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Walker, J.K.; Jacobsen, E.J.; Freskos, J.N.; Hughes, R.O.; Brown, D.L.; Bell, A.S.; Brown, D.G.; Phillips, C.; Mischke, B.V. Identification, synthesis and SAR of amino substituted pyrido [3, 2b] pyrazinones as potent and selective PDE5 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 4088–4091. [Google Scholar] [CrossRef][Green Version]
- Hughes, R.O.; Walker, J.K.; Cubbage, J.W.; Fobian, Y.M.; Rogier, D.J.; Heasley, S.E.; Blevis-Bal, R.M.; Benson, A.G.; Owen, D.R.; Jacobsen, E.J. Investigation of aminopyridiopyrazinones as PDE5 inhibitors: Evaluation of modifications to the central ring system. Bioorg. Med. Chem. Lett. 2009, 19, 4092–4096. [Google Scholar] [CrossRef]
- El-Sharkawy, L.Y.; El-Sakhawy, R.A.; Abdel-Halim, M.; Lee, K.; Piazza, G.A.; Ducho, C.; Hartmann, R.W.; Abadi, A.H. Design and synthesis of novel annulated thienopyrimidines as phosphodiesterase 5 (PDE5) inhibitors. Arch. Der Pharm. 2018, 351, 1800018. [Google Scholar] [CrossRef]
- Zhang, T.; Lai, Z.; Yuan, S.; Huang, Y.-Y.; Dong, G.; Sheng, C.; Ke, H.; Luo, H.-B. Discovery of evodiamine derivatives as highly selective PDE5 inhibitors targeting a unique allosteric pocket. J. Med. Chem. 2020, 63, 9828–9837. [Google Scholar] [CrossRef]
- Abdel-Halim, M.; Sigler, S.; Racheed, N.A.; Hefnawy, A.; Fathalla, R.K.; Hammam, M.A.; Maher, A.; Maxuitenko, Y.; Keeton, A.B.; Hartmann, R.W. From celecoxib to a novel class of phosphodiesterase 5 inhibitors: Trisubstituted pyrazolines as novel phosphodiesterase 5 inhibitors with extremely high potency and phosphodiesterase isozyme selectivity. J. Med. Chem. 2021, 64, 4462–4477. [Google Scholar] [CrossRef] [PubMed]
- Rabal, O.; Sánchez-Arias, J.A.; Cuadrado-Tejedor, M.; de Miguel, I.; Perez-Gonzalez, M.; García-Barroso, C.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Sáez, E.; Espelosin, M. Design, synthesis, and biological evaluation of first-in-class dual acting histone deacetylases (HDACs) and phosphodiesterase 5 (PDE5) inhibitors for the treatment of Alzheimer’s disease. J. Med. Chem. 2016, 59, 8967–9004. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arias, J.A.; Rabal, O.; Cuadrado-Tejedor, M.; de Miguel, I.; Pérez-González, M.; Ugarte, A.; Saez, E.; Espelosin, M.; Ursua, S.; Haizhong, T. Impact of scaffold exploration on novel dual-acting histone deacetylases and phosphodiesterase 5 inhibitors for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2017, 8, 638–661. [Google Scholar] [CrossRef]
- Rabal, O.; Sánchez-Arias, J.A.; Cuadrado-Tejedor, M.; de Miguel, I.; Pérez-González, M.; García-Barroso, C.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Sáez, E.; Espelosin, M. Discovery of in vivo chemical probes for treating Alzheimer’s disease: Dual phosphodiesterase 5 (PDE5) and class I histone deacetylase selective inhibitors. ACS Chem. Neurosci. 2018, 10, 1765–1782. [Google Scholar] [CrossRef] [PubMed]
- Rabal, O.; Sánchez-Arias, J.A.; Cuadrado-Tejedor, M.; de Miguel, I.; Pérez-González, M.; García-Barroso, C.; Ugarte, A.; de Mendoza, A.E.-H.; Sáez, E.; Espelosin, M. Design, synthesis, biological evaluation and in vivo testing of dual phosphodiesterase 5 (PDE5) and histone deacetylase 6 (HDAC6)-selective inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 150, 506–524. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Garcia-Barroso, C.; Sánchez-Arias, J.A.; Rabal, O.; Pérez-González, M.; Mederos, S.; Ugarte, A.; Franco, R.; Segura, V.; Perea, G. A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5 and that rescues hippocampal synaptic impairment in Alzheimer’s disease mice. Neuropsychopharmacology 2017, 42, 524–539. [Google Scholar] [CrossRef]
- Claveria-Cabello, A.; Colyn, L.; Uriarte, I.; Latasa, M.U.; Arechederra, M.; Herranz, J.M.; Alvarez, L.; Urman, J.M.; Martinez-Chantar, M.L.; Banales, J.M. Dual Pharmacological Targeting of HDACs and PDE5 Inhibits Liver Disease Progression in a Mouse Model of Biliary Inflammation and Fibrosis. Cancers 2020, 12, 3748. [Google Scholar] [CrossRef]
- Ma, N.; Luo, Y.; Wang, Y.; Liao, C.; Ye, W.-C.; Jiang, S. Selective histone deacetylase inhibitors with anticancer activity. Curr. Top. Med. Chem. 2016, 16, 415–426. [Google Scholar] [CrossRef]
- ElHady, A.K.; Shih, S.-P.; Chen, Y.-C.; Liu, Y.-C.; Ahmed, N.S.; Keeton, A.B.; Piazza, G.A.; Engel, M.; Abadi, A.H.; Abdel-Halim, M. Extending the use of tadalafil scaffold: Development of novel selective phosphodiesterase 5 inhibitors and histone deacetylase inhibitors. Bioorg. Chem. 2020, 98, 103742. [Google Scholar] [CrossRef]
- Mao, F.; Wang, H.; Ni, W.; Zheng, X.; Wang, M.; Bao, K.; Ling, D.; Li, X.; Xu, Y.; Zhang, H. Design, synthesis, and biological evaluation of orally available first-generation dual-target selective inhibitors of acetylcholinesterase (AChE) and phosphodiesterase 5 (PDE5) for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 328–345. [Google Scholar] [CrossRef]
- Greenwald, M.B.-Y.; Tacconi, C.; Jukic, M.; Joshi, N.; Hiebert, P.; Brinckmann, J.; Tenor, H.; Naef, R.; Werner, S. A dual-acting nitric oxide donor and phosphodiesterase 5 inhibitor promotes wound healing in normal mice and mice with diabetes. J. Investig. Dermatol. 2021, 141, 415–426. [Google Scholar] [CrossRef]
- Ammar, L.; Lin, H.-Y.; Shih, S.-P.; Tsai, T.-N.; Syu, Y.-T.; Abdel-Halim, M.; Hwang, T.-L.; Abadi, A.H. Novel 9-Benzylaminoacridine Derivatives as Dual Inhibitors of Phosphodiesterase 5 and Topoisomerase II for the Treatment of Colon Cancer. Molecules 2023, 28, 840. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wenzel, B.; Dukic-Stefanovic, S.; Teodoro, R.; Ludwig, F.-A.; Deuther-Conrad, W.; Schröder, S.; Chezal, J.-M.; Moreau, E.; Brust, P. Development of a new radiofluorinated quinoline analog for PET imaging of phosphodiesterase 5 (PDE5) in brain. Pharmaceuticals 2016, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Chekol, R.; Gheysens, O.; Cleynhens, J.; Pokreisz, P.; Vanhoof, G.; Ahamed, M.; Janssens, S.; Verbruggen, A.; Bormans, G. Evaluation of PET radioligands for in vivo visualization of phosphodiesterase 5 (PDE5). Nucl. Med. Biol. 2014, 41, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Chekol, R.; Gheysens, O.; Ahamed, M.; Cleynhens, J.; Pokreisz, P.; Vanhoof, G.; Janssens, S.; Verbruggen, A.; Bormans, G. Carbon-11 and fluorine-18 radiolabeled pyridopyrazinone derivatives for positron emission tomography (PET) imaging of phosphodiesterase-5 (PDE5). J. Med. Chem. 2017, 60, 486–496. [Google Scholar] [CrossRef]
- Liu, J.; Maisonial-Besset, A.; Wenzel, B.; Canitrot, D.; Baufond, A.; Chezal, J.-M.; Brust, P.; Moreau, E. Synthesis and in vitro evaluation of new fluorinated quinoline derivatives with high affinity for PDE5: Towards the development of new PET neuroimaging probes. Eur. J. Med. Chem. 2017, 136, 548–560. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, L.; Liu, W.; Li, W.; Song, Y.; Zheng, Q.-H. Radiosynthesis of a carbon-11 labeled PDE5 inhibitor [11C] TPN171 as a new potential PET heart imaging agent. Appl. Radiat. Isot. 2020, 162, 109190. [Google Scholar] [CrossRef]
- He, Y.-F.; Liu, Y.; Yu, J.-H.; Cheng, H.; Odilov, A.; Yang, F.-P.; Tian, G.-H.; Yao, X.-M.; Duan, H.-Q.; Yu, C.-Y. Pharmacokinetics, mass balance, and metabolism of [14C] TPN171, a novel PDE5 inhibitor, in humans for the treatment of pulmonary arterial hypertension. Acta Pharmacol. Sin. 2023, 44, 221–233. [Google Scholar] [CrossRef]
- Wenzel, B.; Liu, J.; Dukic-Stefanovic, S.; Deuther-Conrad, W.; Teodoro, R.; Ludwig, F.-A.; Chezal, J.-M.; Moreau, E.; Brust, P.; Maisonial-Besset, A. Targeting cyclic nucleotide phosphodiesterase 5 (PDE5) in brain: Toward the development of a PET radioligand labeled with fluorine-18. Bioorg. Chem. 2019, 86, 346–362. [Google Scholar] [CrossRef]
- Dong, F.; Du, J.; Miao, C.; Jia, L.; Li, W.; Wang, M.; Zheng, Q.-H.; Xu, Z. Radiosynthesis of carbon-11 labeled PDE5 inhibitors as new potential PET radiotracers for imaging of Alzheimer’s disease. Appl. Radiat. Isot. 2019, 154, 108873. [Google Scholar] [CrossRef]
- El-Kawy, O.; Ibrahim, I.; Shewatah, H.; El-Azony, K. Preparation and evaluation of radioiodinated avanafil: A novel potential radiopharmaceutical for the diagnostic evaluation of erectile dysfunction. Appl. Radiat. Isot. 2022, 183, 110160. [Google Scholar] [CrossRef] [PubMed]
- Zoraghi, R.; Francis, S.H.; Corbin, J.D. Critical amino acids in phosphodiesterase-5 catalytic site that provide for high-affinity interaction with cyclic guanosine monophosphate and inhibitors. Biochemistry 2007, 46, 13554–13563. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.A.; Philip, K.J.; Schwarz, E.R. The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc. Health Risk Manag. 2010, 6, 273–280. [Google Scholar]
- Miller, M.S. Role of phosphodiesterase type 5 inhibitors for lower urinary tract symptoms. Ann. Pharmacother. 2013, 47, 278–283. [Google Scholar] [CrossRef]
- Gur, S.; Kadowitz, P.J.; Can Serefoglu, E.; Hellstrom, W.J.G. PDE5 inhibitor treatment options for urologic and non-urologic indications: 2012 update. Curr. Pharm. Des. 2012, 18, 5590–5606. [Google Scholar] [CrossRef]
- Sanati, M.; Aminyavari, S.; Mollazadeh, H.; Bibak, B.; Mohtashami, E.; Afshari, A.R. How do phosphodiesterase-5 inhibitors affect cancer? A focus on glioblastoma multiforme. Pharmacol. Rep. 2022, 74, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kloner, R.A.; Salloum, F.N.; Jovin, I.S. Cardiac effects of phosphodiesterase-5 inhibitors: Efficacy and safety. Cardiovasc. Drugs Ther. 2021, 37, 793–806. [Google Scholar] [CrossRef]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M. Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction: A randomized clinical trial. JAMA Netw. Open 2020, 3, e205323. [Google Scholar] [CrossRef]
- Behr, J.; Nathan, S.D.; Wuyts, W.A.; Bishop, N.M.; Bouros, D.E.; Antoniou, K.; Guiot, J.; Kramer, M.R.; Kirchgaessler, K.-U.; Bengus, M. Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 85–95. [Google Scholar] [CrossRef]
- Kuwana, M.; Blair, C.; Takahashi, T.; Langley, J.; Coghlan, J.G. Initial combination therapy of ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) in the modified intention-to-treat population of the AMBITION study: Post hoc analysis. Ann. Rheum. Dis. 2020, 79, 626–634. [Google Scholar] [CrossRef]
- Luginbuhl, A.J.; Johnson, J.M.; Harshyne, L.A.; Linnenbach, A.J.; Shukla, S.K.; Alnemri, A.; Kumar, G.; Cognetti, D.M.; Curry, J.M.; Kotlov, N. Tadalafil enhances immune signatures in response to neoadjuvant nivolumab in resectable head and neck squamous cell carcinoma. Clin. Cancer Res. 2022, 28, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Song, S.; Chang, S.-A.; Kim, H.-K.; Jung, H.-O.; Choi, J.-H.; Lee, J.S.; Kim, K.-H.; Jeong, J.-O.; Lee, J.H. Efficacy and safety of udenafil for the treatment of pulmonary arterial hypertension: A placebo-controlled, double-blind, phase IIb clinical trial. Clin. Ther. 2019, 41, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, M.G.; Beddings, I.; Lomakin, F.M.; Boisier Riscal, D.; Gutierrez Claveria, M.; Vidal Marambio, J.; Retamal Baez, N.; Pavez Novoa, C.; Reyes Allende, C.; Ferreira Perey, P. Sildenafil for treating patients with COVID-19 and perfusion mismatch: A pilot randomized trial. Crit. Care 2022, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hubers, S.A.; Wan, S.H.; Adel, F.W.; Benike, S.L.; Burnett, J.C., Jr.; Scott, C.; Chen, H.H. Cardiorenal Effects of Long-Term Phosphodiesterase V Inhibition in Pre–Heart Failure. J. Am. Heart Assoc. 2022, 11, e022126. [Google Scholar] [CrossRef]
- Ferguson-Sells, L.; Velez de Mendizabal, N.; Li, B.; Small, D. Population pharmacokinetics of tadalafil in pediatric patients with pulmonary arterial hypertension: A combined adult/pediatric model. Clin. Pharmacokinet. 2022, 61, 249–262. [Google Scholar] [CrossRef]
- Omarjee, L.; Le Pabic, E.; Custaud, M.-A.; Fontaine, C.; Locher, C.; Renault, A.; Jaquinandi, V.; Azzola, V.; Barbeau-Terrier, C.; Laporte, I. Effects of sildenafil on maximum walking time in patients with arterial claudication: The ARTERIOFIL study. Vasc. Pharmacol. 2019, 118, 106563. [Google Scholar] [CrossRef] [PubMed]
- Greutmann, M.; Tobler, D.; Engel, R.; Heg, D.; Mueller, C.; Frenk, A.; Gabriel, H.; Rutz, T.; Buechel, R.R.; Willhelm, M. Effect of phosphodiesterase-5 inhibition on SystEmic Right VEntricular size and function—A multi-center, double-blind, randomized, placebo-controlled trial–SERVE. Eur. J. Heart Fail. 2023, 25, 1105–1114. [Google Scholar] [CrossRef]
- Jagdish, R.K.; Kamaal, A.; Shasthry, S.M.; Benjamin, J.; Maiwall, R.; Jindal, A.; Choudhary, A.; Rajan, V.; Arora, V.; Bhardwaj, A. Tadalafil improves erectile dysfunction and quality of life in men with cirrhosis: A randomized double blind placebo controlled trial. Hepatol. Int. 2021, 17, 434–451. [Google Scholar] [CrossRef]
- Di Maria, M.V.; Goldberg, D.J.; Zak, V.; Hu, C.; Lubert, A.M.; Dragulescu, A.; Mackie, A.S.; McCrary, A.; Weingarten, A.; Parthiban, A. Impact of Udenafil on Echocardiographic Indices of Single Ventricle Size and Function in FUEL Study Participants. Circ. Cardiovasc. Imaging 2022, 15, e013676. [Google Scholar] [CrossRef]
- Andersen, A.; Waziri, F.; Schultz, J.G.; Holmboe, S.; Becker, S.W.; Jensen, T.; Søndergaard, H.M.; Dodt, K.K.; May, O.; Mortensen, U.M. Pulmonary vasodilation by sildenafil in acute intermediate-high risk pulmonary embolism: A randomized explorative trial. BMC Pulm. Med. 2021, 21, 72. [Google Scholar] [CrossRef]
- Pierce, C.M.; Zhang, M.H.; Jonsson, B.; Iorga, D.; Cheruvu, N.; Balagtas, C.C.; Steinhorn, R.H. Efficacy and safety of IV sildenafil in the treatment of newborn infants with, or at risk of, persistent pulmonary hypertension of the newborn (PPHN): A multicenter, randomized, placebo-controlled trial. J. Pediatr. 2021, 237, 154–161.e153. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Miguelez, P.; Seigler, N.; Ishii, H.; Crandall, R.; McKie, K.T.; Forseen, C.; Harris, R.A. Exercise intolerance in cystic fibrosis: Importance of skeletal muscle. Med. Sci. Sports Exerc. 2021, 53, 684. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).