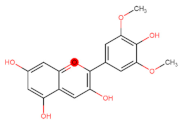
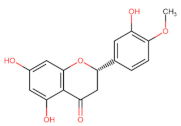
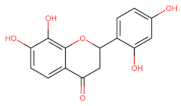
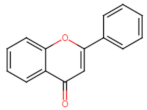
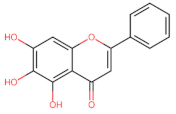
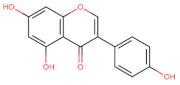
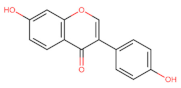
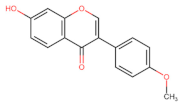
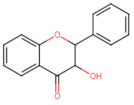
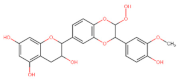
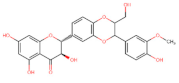
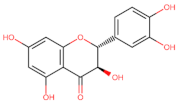
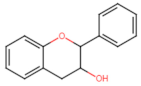
Abstract
Natural compounds with pharmacological activity, flavonoids have been the subject of an exponential increase in studies in the field of scientific research focused on therapeutic purposes due to their bioactive properties, such as antioxidant, anti-inflammatory, anti-aging, antibacterial, antiviral, neuroprotective, radioprotective, and antitumor activities. The biological potential of flavonoids, added to their bioavailability, cost-effectiveness, and minimal side effects, direct them as promising cytotoxic anticancer compounds in the optimization of therapies and the search for new drugs in the treatment of cancer, since some extensively antineoplastic therapeutic approaches have become less effective due to tumor resistance to drugs commonly used in chemotherapy. In this review, we emphasize the antitumor properties of tangeretin, a flavonoid found in citrus fruits that has shown activity against some hallmarks of cancer in several types of cancerous cell lines, such as antiproliferative, apoptotic, anti-inflammatory, anti-metastatic, anti-angiogenic, antioxidant, regulatory expression of tumor-suppressor genes, and epigenetic modulation.
1. Introduction
Cancer cells possess biological properties that confer the ability to develop and become malignant. The spread of these cells occurs through a variety of tumor physiological strategies, including the maintenance of proliferative signaling, evasion of growth suppressor genes, evasion of immune destruction, induction of replicative immortality, activation of invasion and metastasis, promotion of angiogenesis, resistance to cell death, deregulation of cellular energy and metabolism, unlocking of phenotypic plasticity, and cellular senescence. These properties are acquired at different stages of neoplasia in diverse types of cancer. This ability is triggered by strong genomic instability caused by successive mutations of regulatory genes, the infiltration of tumor-promoting immune cells, non-mutational epigenetic reprogramming, and polymorphic microbiomes [1,2,3]. Elucidating these “hallmarks” of cancer is the subject of intense experimentation to explore cancer therapies, as the effective intervention of any of these tumor characteristics can potentially improve and refine anticancer therapeutic treatments against cancer.
Within the therapeutic approaches to various types of cancers, the resistance to multiple drugs (MDRs) exhibited by tumor cells is considered the main cause of chemotherapy effectiveness failure. This occurs due to cellular physiological responses triggered by the tumor, including the evasion of drug-induced apoptosis, activation of detoxification pathways, reduction in drug uptake, and activation of DNA repair mechanisms [4]. From this perspective, the use of natural products in clinical trials has been instrumental in suppressing resistance mechanisms, and hence of utmost importance in the search for new genotoxic therapeutic approaches against tumors. These products have enabled the development of more effective strategic combinations with fewer side effects for the treatment of various types of cancer, in addition to improving our understanding of cancer cell defense and resistance mechanisms [5]. The alteration of gene expression patterns in tumor cells is linked to genetic and epigenetic events. Aberrant epigenetic modifications through DNA methylation, nucleosome remodeling, histone modifications, and non-coding microRNAs play a crucial role in tumor initiation and uncontrolled cellular progression. The understanding and discovery of drugs capable of restoring or inhibiting these abnormal epigenetic mechanisms represent a significant advance in the means of cancer control [6,7,8,9]. In this context, natural phenolic compounds have gained prominence in anticancer pharmaceutical studies. These compounds, found in plants and fruits, are described as potent epigenetic agents that regulate DNA methylation, histone modification, and microRNAs in cancer therapy. They have shown effectiveness when combined with chemotherapy drugs or even when used in combination with other natural compounds. These promising findings have driven research and the development of new therapeutic strategies for cancer treatment [10,11]. This review article aims to provide an overview of the main anticancer properties of flavonoids demonstrated in various scientific studies, considering the prominent characteristics of cancer, with an emphasis on tangeretin.
2. Polyphenols
Polyphenols constitute a diverse group of phytochemicals associated with secondary metabolism in plants. They have antidiabetic, antiosteoporotic, cardioprotective, neuroprotective, antioxidant, anti-inflammatory, antimicrobial, immunomodulatory, and anticancer properties [12,13]. They protect plants from ultraviolet radiation and microbial infections, serve as signaling molecules during the pollination process, and modulate plant growth hormones [14,15,16]. Based on their structure, polyphenols are classified into non-flavonoids (curcuminoids, lignans, stilbenes, and tannins) and flavonoids [17] (Figure 1).
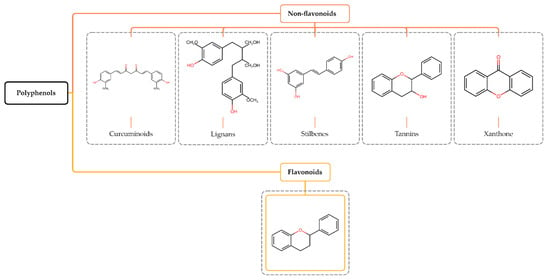
Figure 1.
Structure and classification of polyphenols. Polyphenols are phytochemical compounds found in plants, fruits, and natural compounds, divided into non-flavonoids and flavonoids.
Flavonoids (or bioflavonoids) represent an extensive class with over 10,000 described subtypes of compounds [18,19,20]. They are the most abundant phenolic compounds in the human diet, ubiquitously found in fruits, seeds, roots, cereals, teas, and wines [21,22,23,24]. Although some are colorless, their etymology is derived from the Latin word “flavus,” which means yellow [25]. In addition, flavonoids exist in various derived forms, including glycosylated, acetylated, methylated, and sulfated aglycones [20,26,27].
3. Structure and Classification of Flavonoids
Structurally, flavonoids have fifteen carbons in their chemical structure (C6-C3-C6), consisting of two benzene rings (A and B) connected by a heterocyclic pyran ring (C) (2-phenyl-1,4-benzopyran) [28,29] (Figure 2).
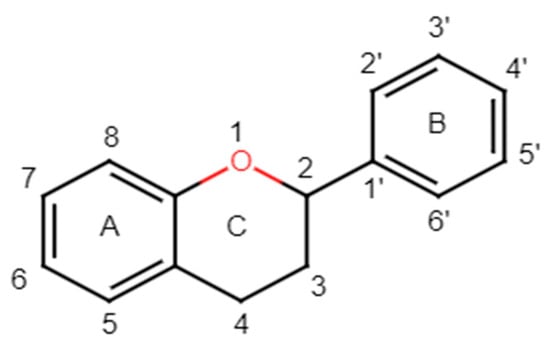
Figure 2.
Main molecular structure of flavonoids.
The classification of flavonoids is based on the arrangement of the hydroxyl groups, the degree of unsaturation, and the oxidation of the heterocyclic C-ring. The main subclasses include flavones, flavonols, flavanones, flavanonols, flavanols, isoflavones, anthocyanidins, and chalcones [30,31,32,33,34] (Figure 3). Flavanones and flavanonols show a saturated benzopyran ring, the difference between them being the presence of a hydroxyl group on carbon number three of the benzopyran ring in flavanols. Similarly, flavanols also have a saturated benzopyran ring and hydroxyl groups on carbon number three; however, they differ in the absence of a carbonyl group on carbon number four of the benzopyran ring. Anthocyanins are hydroxylated at carbon number three and have two double bonds. Isoflavones have a double bond between carbon numbers two and three of the benzopyran ring, with the phenyl group attached to carbon number three. Flavonoids that do not have the benzopyran ring are called minor flavonoids. This is true for chalcones, characterized by the absence of the heterocyclic benzopyran ring with oxygen [35,36,37].
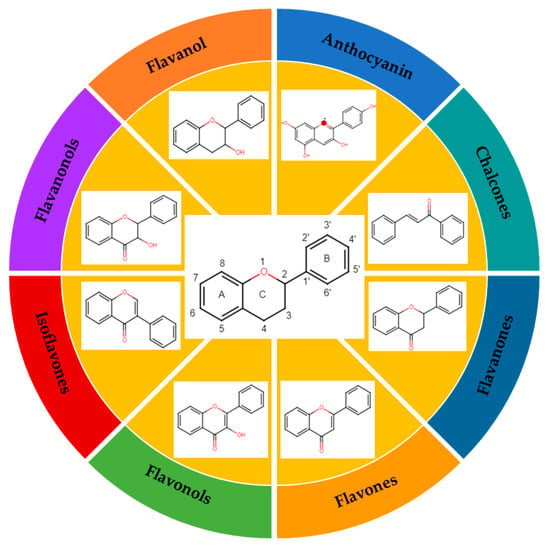
Figure 3.
Subclassification of flavonoids.
4. Antitumor Activity of Flavonoids
These bioactive compounds exhibit many biological properties, including antioxidant, antiviral, antifungal, antibacterial, anti-inflammatory, antidiabetic, anti-obesity, antimutagenic, cardioprotective, and anticancer activities [38,39,40,41,42]. Concerning their anticancer activities, the recognized importance of flavonoids has led to efforts and challenges to elucidate the molecular and cellular mechanisms of antitumor effects [43]. This awareness has been accompanied by an increasing number of scientific publications comparing the human health benefits of flavonoids in the field of oncology with those of other medical specialties, such as endocrinology, cardiology, and neurology [44]. Epidemiological studies support the chemopreventive benefits of flavonoids when included in the human diet, with their intake correlated with a lower risk of developing some tumors, such as gastric, breast, prostate, and colorectal cancers [45,46]. Flavonoids mediate anti-neoplastic mechanisms by modulating reactive oxygen species (ROS) levels in tumor cells, inhibiting carcinogens, pro-inflammatory pathways, angiogenesis, autophagy, inducing apoptosis, and inhibiting tumor proliferation and invasion [47,48,49,50,51,52,53,54,55] (Figure 4).
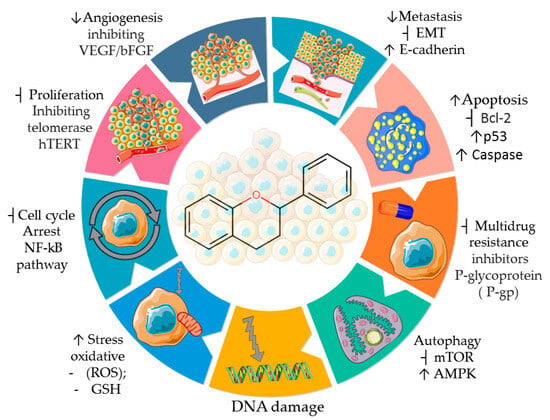
Figure 4.
Properties and some anticancer action mechanisms of flavonoids. Parts of the figure are drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ accessed on 12 August 2023).
Even though the anticancer efficacy of flavonoids is described in the literature, the pharmacological activity of these compounds may be limited due to their water insolubility. The low solubility of flavonoids presents a double-edged sword in the therapeutic field. On one hand, their reduced absorption due to low solubility does not confer toxicity to the organism. On the other hand, it also becomes a problem as it may reduce their chemosensitizing effectiveness due to inefficient absorption [55,56].
In order to overcome this disadvantage, nanoparticle-based delivery systems have been developed aiming to improve the bioavailability and absorption of drugs in cancer therapy. These drug-carrying nanocarriers, such as polymeric micelles, liposomes, dendrimers, and carbon nanotubes, have been extensively investigated to ensure the chemotherapeutic and chemosensitizing effectiveness of drugs targeted to cancer cells [57,58]. In this context, the production of flavonoid-loaded phytoparticles has added advantages to the treatment, prevention, and clinical perspectives of cancer. These phytoparticles increase the bioavailability of compounds with low solubility, prolong the half-life of drugs, improve blood absorption, and reduce gastrointestinal degradation. Moreover, this delivery system allows for lower quantities of flavonoids to be used, thereby decreasing the risk of toxicity in non-tumor cells [59,60,61,62].
As an example, the effect of an oral nanoparticle delivery system of chitosan containing an encapsulated epigallocatechin-3-O-gallate (EGCG) flavonoid has been described as excellent in vitro in human melanoma cells and in vivo in melanoma tumor xenografts. It promotes cell growth inhibition and the induction of apoptosis in vivo, showing enhanced effectiveness in vitro when compared to native EGCG treatment [63]. These results stem from efforts to improve the bioavailability of EGCG based on previous research focusing on melanoma cancer, aiming to optimize the anticancer effects of antiproliferation and pro-apoptosis physiologically [64]. These findings reaffirm that the encapsulation (nanochemoprevention) of substances with chemopreventive activity in EGCG nanoparticles can be an efficient alternative in cancer treatment [65].
In the same way, treatment with EGCG nano-emulsion (nano-EGCG) in lung cancer cells showed the anti-tumor effects between EGCG and nano-EGCG groups. Both treatment groups blocked tumor cell growth. Importantly, the nano-EGCG treatment inhibited cell migration and invasion in a dose-dependent manner, achieved through the stimulation of the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [66]. This pathway is altered in the metabolic reprogramming of cancer cells and is responsible for conferring resistance to cancer-fighting drugs, preventing the autophagy of cancer cells [67,68].
Moderate levels of reactive oxygen species (ROS) resulting from mitochondrial activity act as redox signaling molecules in growth, differentiation, and cell proliferation pathways. However, excessive levels of ROS induce DNA mutations, protein and lipid damage, and stimulate pro-oncogenic signaling pathways, thus contributing to carcinogenesis [69,70]. Tumor cells have significantly higher ROS levels in the tumor microenvironment compared to the homeostatic conditions of non-tumor cells. However, excess ROS can be harmful to cancer cells, leading to cell death. Consequently, tumor cells develop adaptive detoxification mechanisms in response to excessive ROS [71,72]. As the elevation of ROS can trigger apoptosis in cancer cells, therapeutic strategies aimed at modulating ROS levels in cancer treatment have shown the efficacy of anticancer drugs [73,74,75]. In this sense, flavonoids are described to exhibit antioxidant biological activity in non-tumor cells and pro-oxidant activity by inducing increased oxidative stress in cancer cells, thereby inhibiting cell proliferation signaling, suppressing pro-inflammatory cytokines, promoting apoptosis, necrosis, and autophagy activation [28]. The ability to scavenge oxygen reactive species is related to the presence of a large number of phenolic hydroxyl groups in the molecular structure of flavonoids, where intense electron exchange facilitates substitution reactions with free radicals, forming a more stable compound. Therefore, the higher the number of hydroxyl groups, the greater the oxidant and pro-oxidant capacities of the flavonoid [76,77]. Ovarian cancer cells treated with flavonoids apigenin, luteolin, and myricetin showed an intracellular increase in ROS levels in a dose-dependent manner compared to untreated control cells, resulting in the activation of the intrinsic apoptotic pathway, cell cycle arrest, and anti-invasion [78]. Similarly, it was described that the flavonoid quercetin triggered cell death in cancer cells by positively regulating ROS levels [79]. The expression of the transglutaminase 2 (TGM2) gene is generally associated with poor prognosis in pancreatic cancer and is involved in its initiation, inflammation, and progression, making it a target marker in studies analyzing drugs with chemosensitizing activity [80,81,82]. Treatment with kaempferol suppressed pancreatic cancer growth in vivo and in vitro. It was observed that treated cells had decreased TGM2 expression, and the increase in ROS induced apoptosis through the Akt/mTOR signaling pathway [83]. The therapeutic potential of flavonoids in modulating ROS demonstrates that their pro-oxidant activity can positively contribute to anticancer research.
In order for excessive cell growth to be achieved, cancer cells reprogram their energy metabolism. This reprogramming is directly related to the maintenance and aggressiveness of neoplastic cells [84]. In this sense, glutathione is a ubiquitous endogenous antioxidant tripeptide (γ-Glu-Cys-Gly; GSH) found in eukaryotic cells, being responsible for maintaining cellular redox homeostasis by eliminating reactive oxygen species (ROS), a cellular metabolic byproduct [85,86,87]. Glutathione (GSH) metabolism has been investigated in tumor progression and explored as a targeted therapeutic strategy for cancer [87,88]. The positive modulation of GSH levels is directly related to the response to cellular detoxification mechanisms. This provides advantages to various types of cancers, as it is crucial for the elimination and detoxification of certain chemotherapeutic agents, thus conferring therapeutic resistance. Moreover, high GSH levels contribute to tumor development and increase metastasis events [89]. On the other hand, the reduction (depletion) in GSH levels leads to certain types of cell death, such as apoptosis, necroptosis, ferroptosis, and autophagy, providing a foundation for studies exploring the suppression of GSH levels in chemosensitization approaches in cancer therapies, making tumor cells prone to the cytotoxic and cytoprotective effects of antineoplastic substances [90]. In this direction, it has been observed that tangeretin is able to reduce oxidative stress in human hepatocellular carcinoma induced by tert-Butyl Hydroperoxide (t-BHP) by inhibiting GSH depletion in the cell [91]. Similarly, in cisplatin-induced liver lesions in rats treated with tangeretin, protective activity against cellular oxidative stress was observed, and an increase in antioxidant defense was also observed, as evidenced by elevated GSH levels [92]. Hence, this flavonoid is capable of reducing cellular stress and restoring the antioxidant defense system.
Epigenetic mechanisms are commonly associated with cancer development. In breast cancer, the expression pattern of certain tumor suppressor genes is related to methylation patterns. DNA methylation plays a critical role in controlling gene activity and nuclear architecture, being the most extensively studied epigenetic modification in humans. It is involved in the regulation of various biological processes, such as cell differentiation, embryogenesis, X-chromosome inactivation, microRNA expression, suppression of transposable elements, and genomic imprinting [93,94,95]. Hence, DNA methylation is an epigenetic mark associated with gene silencing, as it affects chromatin structure and blocks the access of binding factors, preventing the expression of the genes. This pattern can be stably maintained throughout life or undergo changes during aging [96]. Hypermethylation of CpG islands in the promoter region of tumor suppressor genes is an early event in various types of cancer. Consequently, CpG island hypermethylation in the promoter region can affect genes involved in cell control, DNA repair, apoptosis, and angiogenesis. In breast and ovarian cancers, hypermethylation is found in the promoter region of the BRCA1 gene, which acts as a tumor suppressor and is responsible for preventing the uncontrolled proliferation of cells [97,98]. Hypomethylation of DNA also triggers neoplastic transformations when it causes chromosomal instability, thus reactivating or activating oncogenes [99]. The literature highlights flavonoids as epigenetic modifiers in breast cancer. Epigallocatechin-3-gallate (EGCG), genistein, daidzein, resveratrol, and quercetin are capable of restoring the expression pattern of silenced tumor suppressor genes, such as BRCA1 and BRCA2, by inhibiting the enzymes called DNA methyltransferases (DNMTs). These enzymes are responsible for catalyzing the gene silencing process in the promoter region of the genes [99,100,101]. The restauration of the original expression patterns of these suppressor genes by the flavonoids was observed in different breast cancer cells, resulting in decreased proliferation and cancer cell migration [100]. The knowledge of the antitumor properties and ability of flavonoid subclasses (anthocyanidin—delphinidin, flavones—apigenin, luteolin, tangeretin, isoflavones—genistein, flavanones—hesperetin, silibinin, flavanol—EGCG, flavonols—quercetin, kaempferol, and fisetin) to modulate epigenetic enzymes, such as DNA methyltransferases (DNMTs), acetyltransferases (HATs), histone methyltransferases (HMTs), and histone deacetylases (HDACs), reinforce the incentive for research on therapeutic combination approaches involving these natural compounds that alter the epigenetic marks related to cancer development and progression along with drugs already used for cancer treatment [102].
The study of the mechanisms of action of apoptotic caspases in cancer has been explored through the use of antineoplastic drugs as a therapeutic strategy to overcome resistance and control the proliferation of cancer cells. The modulation of apoptosis under the action of natural products has demonstrated efficacy in inducing neoplastic cell death, representing an additional alternative to common chemotherapeutic agents employed in cancer treatment. It opens up a path for the development of new antineoplastic drugs, focusing on the apoptotic events executed by caspases [103,104,105,106,107]. The deregulation of the caspase cascade is implicated in the disruption of programmed cell death and directly related to the pathophysiology of cancer (evasion of apoptotic programming). The apoptotic imbalance resulting from negative caspase regulation is considered one of the causes of the resistance to tumor death found in cancer treatment [108,109,110]. The apoptotic proteolytic activation of caspases is executed through intrinsic (mitochondrial) and extrinsic (cytoplasmic) pathways. The intrinsic pathway is activated as feedback in response to cellular stress caused by cytotoxic substances, DNA mutations, hypoxia, cytoskeletal disruption, etc. [111,112,113]. In lung cancer cells treated with the flavonoid hesperetin, cell death by apoptosis was induced through the extrinsic pathway by increasing the expression levels of death domains genes, such as FADD, caspase-8, and FAS. The same study also mentioned that increased cell death occurred independently of the suppressor protein p53 and the pro-apoptotic protein Bax [114]. Treatment with malvidin and an analysis through flow cytometry showed that apoptotic activity was triggered by increased effector caspase-3 in myeloid and lymphoid leukemia cells in a dose-dependent manner, resulting in cell death [115]. Another study, also using flow cytometry, as well as Western blot and real-time PCR, showed the result of cell death by apoptosis in gastric cancer cells, where silibinin increased the level of caspases-3 and -9, followed by the inhibition of the transducer of signaling and activator of transcription 3 (STAT3) pathway, which is related to tumor growth and metastasis [116].
The molecular protective effect of flavonoids on DNA reduces the damage caused by carcinogens and promotes cellular genomic stability, allowing the development of strategies to treat neoplasms [19]. Table 1 presents the developed studies that describe the antitumor properties of flavonoids in various types of cancers.

Table 1.
Subclasses of flavonoids and their compounds with antitumor activity described in cancer cell lines.
5. Antineoplastic Activity of Tangeretin
The flavonoid tangeretin (5,6,7,8,4′-pentamethoxyflavone) is found in the peels of citrus fruits, especially oranges and tangerines. Studies have reported the beneficial bioactivities of this flavonoid, including its anti-asthmatic, antioxidant, anti-teratogenic, anti-inflammatory, neuroprotective, and anticancer properties [143,144,145,146]. Citrus flavonoids have demonstrated their potential anticarcinogenic activity both in in vivo and in vitro experiments by targeting cancer-related cellular processes, such as carcinogen bioactivation, cell signaling, cell cycle regulation, inflammation, and angiogenesis [147,148]. Tangeretin exhibits pharmacological properties, such as antiproliferative, anti-invasive, and anti-metastatic, and can induce apoptosis in specific cancers [149,150,151,152,153] (Figure 5). Experimental molecular analyses have focused on exploring and elucidating the cellular pathways involved in the metabolic activity of these flavonoids, further supporting their chemotherapeutic potential [147].
Using a proteomic approach, Yumnam et al. [154] investigated the effect of tangeretin on a human gastric cancer cell line. They observed that the treatment inhibited the activity of markers (PKCs, MAPK4, PI4K, PARP14) associated with poor prognosis in various cancers related to cell migration, proliferation, chemoresistance, the suppression of apoptosis, and differentiation. Remarkably, this study also sheds light on the importance of the PKC family as a novel biomarker in gastric cancer, as the overexpression of one of its members, PKCε, known for its anti-apoptotic functions, was inhibited by tangeretin treatment, which ultimately induced apoptosis in the gastric cell line. These findings highlight the potential of the PKC family as a promising marker and therapeutic target for treating gastric cancer with tangeretin.
Gliomas are responsible for originating the majority of brain tumors, presenting a high mortality rate, infiltrative growth, and low early detection. Despite intense conventional therapeutic advancements in gliomas, a cure for these tumors is still considered distant [155]. Increasing clinical data and research demonstrate that natural compounds emerge as promising agents in therapies aimed at combating GBM [156]. The potential antineoplastic effect of tangeretin was demonstrated by inducing cell cycle arrest and cell death in GBM. Tangeretin treatment positively modulated the expression of the PTEN gene and cell cycle regulating genes, and induced cell cycle arrest in G2/M and apoptosis. This suggests that tangeretin can be used as a chemopreventive agent in treating GBM. This assay reinforces the importance of further studies on the antitumor activity of tangeretin in nervous system tumors [157].
In in vivo experiments conducted on rat mammary carcinogenesis, tangeretin exhibited promising results. After cancer induction by 7,12-dimethylbenz(α)anthracene, oral treatment with this flavonoid affected markers associated with uncontrolled cell growth (PCNA, COX-2, and Ki-67). It effectively arrested the division of tumor cells at the G1/S phase by positively regulating the p53/p21 genes. Additionally, tangeretin demonstrated remarkable antimetastatic and antiangiogenic activities by inhibiting matrix metalloproteinases (MMPs) MMP-2/MMP-9 and the vascular endothelial growth factor (VEGF), respectively [158]. Sangavi and Langeswaran, using in silico approaches [159], investigated the inhibitory effect of natural compounds on liver cancer, targeting cyclooxygenase 2 (COX-2), an enzyme associated with inflammatory and carcinogenic processes (angiogenesis, metastasis, and apoptosis resistance). They found that tangeretin exhibited efficacy, showing a favorable pharmacokinetic profile for absorption, distribution, metabolism, excretion, and toxicity. These properties are essential for synthesizing new antineoplastic drugs and confirming the antitumor activity of this compound on the target cyclooxygenase 2 (COX-2) in hepatocellular carcinoma (HCC). The suppression of cyclooxygenase 2 (COX-2) was also observed in the epidermal cells of mice exposed to ultraviolet-B radiation (UVB). This occurred by blocking mitogen-activated protein kinase (MAPK) signaling and NF-kB activation and inhibiting the increase in ROS levels in cells upon UVB exposure, providing cellular protection against oxidative stress. These results suggest that the anti-inflammatory and modulatory effects of tangeretin may have a chemopreventive effect on skin cancer [160].
In macrophage cells, the process of inflammation induced by lipopolysaccharide (LPS) triggered a substantial increase in pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) that were activated by the messenger molecule nitric oxide (NO). After incubation with tangeretin, the activation of anti-inflammatory cytokines (IL-4, IL-13, TNF-β, and IL-10) was observed, along with significant inhibition of inducible nitric oxide synthase (iNOS) and COX-2 [161]. As LPS is responsible for promoting inflammation and cell migration in certain types of cancer [162,163,164,165], the control of cellular inflammation described by the action of tangeretin may contribute to cancer treatment.
Antineoplastic agents administered as part of therapies can induce apoptosis in cancer cells. However, these agents often cause cytotoxicity in noncancerous cells, particularly immature immune system cells (myelocytes) and leukocytes (lymphocytes). In this context, the use of tangeretin in human leukemic cells (HL-60) from promyelocytic leukemia inhibited their growth by inducing apoptosis without promoting cytotoxicity or other side effects in immune system cells [128]. Combining antineoplastic agents (synergistic therapy) has resulted in more effective therapeutic strategies and the mitigation of side effects associated with chemotherapeutic agents commonly used for cancer treatment. When tangeretin was combined with the synthetic 5-fluorouracil (5-FU) and administered in treating certain solid tumors, significant antitumor activity was observed in colon cancer cells. This co-exposure decreased the antioxidant levels in tumor cells, resulting in oxidative stress through the accumulation of reactive oxygen species (ROS), triggering a DNA damage response and directing the cells toward apoptosis via c-Jun N-terminal kinases (JNKs). Significantly, tangeretin synergistically intensified the induction of apoptosis by 5-FU. Similarly, the co-treatment also caused a decrease in mitochondrial activity [166].
Another well-known chemotherapeutic agent commonly used to treat various human cancers is cisplatin, or cis-diamindichloroplatin (II). Cisplatin proves its effectiveness by causing DNA damage in tumor cells, leading to apoptosis. However, the side effects, such as kidney problems, weakened immunity, gastrointestinal problems, bleeding, and hearing damage, limit its applicability and effectiveness [167,168]. The use of tangeretin in acute liver injury caused by cisplatin in rats showed a protective effect against these histopathological deformations, underscoring its effect on one of the severe side effects of cisplatin treatment. Moreover, tangeretin reduced inflammatory mechanisms by neutralizing tumor necrosis factor-alpha (TNF-α) and stimulating interleukin-10 (IL-10) [92].
The expression of cell division-retarding tumor suppressor proteins, such as p21, p53, and p27, was increased in colorectal carcinoma cells when treated with tangeretin, thus promoting the inhibition of cell growth by triggering the blocking of enzymes responsible for regulating cell cycle progression and cyclin-dependent kinases (CDK2) and (CDK4) [125]. The elevation of tumor suppressor protein levels, followed by the inhibition of CDK, shows important anticancer effects, preventing neoplastic cells from entering division and ensuring the evasion of the suppression mechanism targeted against carcinogenesis. Considering the anticancer activities exhibited by citrus flavonoids, a study of the effects of a synthetic derivative of tangeretin (5,4’-didemethyltangeretin (PMF2)) in human prostate cancer cells demonstrated the restoration of P21 gene expression through epigenetic mechanisms of demethylation, followed by the blocking of DNMT 3B and HDACs protein expressions, thereby inhibiting cell proliferation [167].
Given the properties demonstrated for the different tumor characteristics in various types of cancer, tangeretin presents itself as a promising agent in the development of anticancer therapeutic strategies.
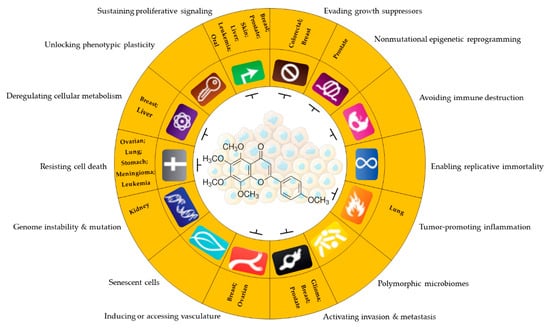
Figure 5.
Antitumor potential of tangeretin. Anticancer activity of tangeretin under some cancer characteristics promotes uncontrolled cell progression and resistance to therapies in different types of tumors. Sustaining proliferative signaling: [127,152,160,161,167,168]; evading growth suppressors: [127,167]; nonmutational epigenetic reprogramming: [167]; tumor-promoting inflammation: [161,169]; activating invasion and metastasis: [151,170,171]; inducing or accessing vasculature: [170,172]; genome instability and mutation: [143]; resisting cell death: [128,129,173]; deregulating cellular metabolism: [92,174]. Parts of the figure are drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ accessed on 12 August 2023).
6. Conclusions and Future Perspectives
The biological potential, bioavailability, cost-effectiveness, and minimal side effects of flavonoids position them as promising cytotoxic anticancer compounds in the optimization of therapies and in the search for new drugs for the treatment of cancer. However, it is crucial to address the challenges that limit the effectiveness of flavonoids in the field of oncology, including pharmacokinetics (low solubility and stability, interaction with intestinal microflora, and metabolic interaction with receptors), pharmacodynamics, epidemiological studies (long duration, delays in data collection and categorization, absence of participant data, and exposure to heterogeneous factors), and isolation/purification of their natural sources. Among citrus flavonoids, tangeretin exhibited antitumor activities against cell proliferation. In addition, it also synergistically promoted improvements in reducing the side effects and yield when combined with some traditional chemotherapy drugs already implemented in cancer treatments. In order to provide more robust scientific knowledge about the antineoplastic activity of flavonoids, further studies are needed to examine the dosage, bioavailability, efficacy, and safety to establish the clinical use of these promising anticancer therapeutic agents.
Author Contributions
Conceptualization, F.C.F.d.L.; writing—original draft preparation, F.C.F.d.L.; writing—review and editing, F.C.F.d.L., W.A.S.F., S.M.M.C. and E.H.C.d.O.; visualization, supervision, W.A.S.F., S.M.M.C. and E.H.C.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Evandro Chagas Institute (Brazil)/SVSA/MS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Authors would like to thank Pro-reitoria de pesquisa e pós-graduação (PROPESP/UFPA, Brazil) for financial support (publication fees). (Edital 02/2023-PAPQ).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Biological Hallmarks of Cancer. In Holland-Frei Cancer Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Sulaiman, A.A.; Balch, C.; Chauhan, H.; Alhadidi, Q.M.; Tiwari, A.K. Natural Polyphenols in Cancer Chemoresistance. Nutr. Cancer 2016, 68, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jaitak, V. Natural Products as Multidrug Resistance Modulators in Cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Nirmaladevi, R. Epigenetic Alterations in Cancer. Front. Biosci. 2020, 25, 4847. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, R.; Gupta, S. Epigenetic Modifications in Cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Gao, P. Metabolic Reprogramming and Epigenetic Modifications on the Path to Cancer. Protein Cell 2022, 13, 877–919. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The Epigenomics of Cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Sandu, M.; Bîrsă, L.M.; Bahrin, L.G. Flavonoids–Small Molecules, High Hopes. Acta Chem. Iasi 2017, 25, 6–23. [Google Scholar] [CrossRef][Green Version]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef] [PubMed]
- Afshari, K.; Haddadi, N.; Haj-Mirzaian, A.; Farzaei, M.H.; Rohani, M.M.; Akramian, F.; Naseri, R.; Sureda, A.; Ghanaatian, N.; Abdolghaffari, A.H. Natural Flavonoids for the Prevention of Colon Cancer: A Comprehensive Review of Preclinical and Clinical Studies. J. Cell. Physiol. 2019, 234, 21519–21546. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant Flavonoids in Cancer Chemoprevention: Role in Genome Stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural Polyphenols as Sirtuin 6 Modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Vishwavidyalaya, D.A.; Pradesh, M. Flavonoids Impact on Prevention and Treatment of Obesity and Related. Int. J. Pharm. Sci. Res. 2019, 10, 4420–4429. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; De Souza, M.d.F.V. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C.; Grayer, R.J. Flavonoids and Their Glycosides, Including Anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Brodowska, K.M. European Journal of Biological Research Natural Flavonoids: Classification, Potential Role, and Application of Flavonoid Analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar] [CrossRef]
- Khajuria, R.; Singh, S.; Bahl, A. General Introduction and Sources of Flavonoids. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Springer: Singapore, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Xie, M. Flavonoids: Recent Advances as Anticancer Drugs. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 152–164. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Symonowicz, M.; Kolanek, M. Flavonoids and Their Properties to Form Chelate Complexes. Biotechnol. Food Sci. 2012, 76, 35–41. [Google Scholar]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and Gut Health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J.; et al. Antidiabetic Potential of Flavonoids from Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Sharma, N.; Dobhal, M.; Joshi, Y.; Chahar, M. Flavonoids: A Versatile Source of Anticancer Drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Fraga, C.G. Research Trends in Flavonoids and Health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Mohana, S.; Ganesan, M.; Rajendra Prasad, N.; Ananthakrishnan, D.; Velmurugan, D. Flavonoids Modulate Multidrug Resistance through Wnt Signaling in P-Glycoprotein Overexpressing Cell Lines. BMC Cancer 2018, 18, 1168. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Poursoleiman, F.; Biregani, A.N.; Esmailzadeh, A. Flavonoids Target Different Molecules of Autophagic and Metastatic Pathways in Cancer Cells. Cancer Cell Int. 2023, 23, 114. [Google Scholar] [CrossRef]
- De Sousa Silva, G.V.; Lopes, A.L.V.F.G.; Viali, I.C.; Lima, L.Z.M.; Bizuti, M.R.; Haag, F.B.; Tavares de Resende e Silva, D. Therapeutic Properties of Flavonoids in Treatment of Cancer through Autophagic Modulation: A Systematic Review. Chin. J. Integr. Med. 2023, 29, 268–279. [Google Scholar] [CrossRef]
- Parekh, N.; Garg, A.; Choudhary, R.; Gupta, M.; Kaur, G.; Ramniwas, S.; Shahwan, M.; Tuli, H.S.; Sethi, G. The Role of Natural Flavonoids as Telomerase Inhibitors in Suppressing Cancer Growth. Pharmaceuticals 2023, 16, 605. [Google Scholar] [CrossRef]
- Rahman, N.; Khan, H.; Zia, A.; Khan, A.; Fakhri, S.; Aschner, M.; Gul, K.; Saso, L. Bcl-2 Modulation in P53 Signaling Pathway by Flavonoids: A Potential Strategy towards the Treatment of Cancer. Int. J. Mol. Sci. 2021, 22, 11315. [Google Scholar] [CrossRef]
- Kim, M.H. Flavonoids Inhibit VEGF/BFGF-Induced Angiogenesis in Vitro by Inhibiting the Matrix-Degrading Proteases. J. Cell. Biochem. 2003, 89, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.M.; Šupolíková, L.; Molčanová, L.; Šmejkal, K.; Bednar, D.; Slaninová, I. Screening of Natural Compounds as P-Glycoprotein Inhibitors against Multidrug Resistance. Biomedicines 2021, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement Strategies for the Oral Bioavailability of Poorly Water-Soluble Flavonoids: An Overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an Effective Sensitizer for Anti-Cancer Therapy: Insights into Multi-Faceted Mechanisms and Applicability towards Individualized Patient Profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2019, 69, 200–211. [Google Scholar] [CrossRef]
- Wei, Q.Y.; He, K.M.; Chen, J.L.; Xu, Y.M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent Anti-Proliferative and pro-Apoptotic Effects of (−)-Epigallocatechin-3-Gallate Encapsulated in Chitosan Nanoparticles on Human Melanoma Cell Growth Both in Vitro and in Vivo. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G.S. Anti-Proliferative and Proapoptotic Effects of (−)-Epigallocatechin-3-Gallate on Human Melanoma: Possible Implications for the Chemoprevention of Melanoma. Int. J. Cancer 2005, 114, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with Green Tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hsieh, C.; Tsai, S.; Wang, C.-Y.; Wang, C. Anticancer Effects of Epigallocatechin-3-Gallate Nanoemulsion on Lung Cancer Cells through the Activation of AMP-Activated Protein Kinase Signaling Pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Shaw, R.J. AMPK: Restoring Metabolic Homeostasis over Space and Time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Chang, Y.Z. Role of AMPK and Its Molecular Intermediates in Subjugating Cancer Survival Mechanism. Life Sci. 2019, 227, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Huang, M.Z.; Li, J.Y. Physiological Regulation of Reactive Oxygen Species in Organisms Based on Their Physicochemical Properties. Acta Physiol. 2020, 228, e13351. [Google Scholar] [CrossRef]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a Novel Indicator to Predict Anticancer Drug Efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Tavsan, Z.; Kayali, H.A. Flavonoids Showed Anticancer Effects on the Ovarian Cancer Cells: Involvement of Reactive Oxygen Species, Apoptosis, Cell Cycle and Invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Kim, W.J.; Demircan, B.; Dyer, L.M.; Bray, K.J.; Skehan, R.R.; Massoll, N.A.; Brown, K.D. The Transglutaminase 2 Gene (TGM2), a Potential Molecular Marker for Chemotherapeutic Drug Sensitivity, Is Epigenetically Silenced in Breast Cancer. Carcinogenesis 2008, 29, 510–518. [Google Scholar] [CrossRef]
- Eckert, R.L. Transglutaminase 2 Takes Center Stage as a Cancer Cell Survival Factor and Therapy Target. Mol. Carcinog. 2019, 58, 837–853. [Google Scholar] [CrossRef]
- Mehta, K.; Han, A. Tissue Transglutaminase (TG2)-Induced Inflammation in Initiation, Progression, and Pathogenesis of Pancreatic Cancer. Cancers 2011, 3, 897–912. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol Induces ROS-Dependent Apoptosis in Pancreatic Cancer Cells via TGM2-Mediated Akt/MTOR Signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in Cancer Cell Death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, A.; Dalirsani, Z.; Hashemy, S.I.; Ghazi, A.; Mostaan, L.V.; Anvari, K.; Pour, A.M. Evaluation of Serum Levels of Oxidized and Reduced Glutathione and Total Antioxidant Capacity in Patients with Head and Neck Squamous Cell Carcinoma. J. Cancer Res. Ther. 2018, 14, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione Metabolism in Cancer Progression and Treatment Resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Jung, E.; Noh, J.; Hyun, H.; Seon, S.; Hong, S.; Kim, D.; Lee, D. Glutathione-Depleting Pro-Oxidant as a Selective Anticancer Therapeutic Agent. ACS Omega 2019, 4, 10070–10077. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef]
- Liang, F.; Fang, Y.; Cao, W.; Zhang, Z.; Pan, S.; Xu, X. Attenuation of tert-Butyl Hydroperoxide (t-BHP)-Induced Oxidative Damage in HepG2 Cells by Tangeretin: Relevance of the Nrf2-ARE and MAPK Signaling Pathways. J. Agric. Food Chem. 2018, 66, 6317–6325. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.R.; Arab, H.H.; Arafa, E.S.A. Tangeretin Alleviates Cisplatin-Induced Acute Hepatic Injury in Rats: Targeting MAPKs and Apoptosis. PLoS ONE 2016, 11, e0151649. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic Modifications and Human Disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Hernando-Herraez, I.; Garcia-Perez, R.; Sharp, A.J.; Marques-Bonet, T. DNA Methylation: Insights into Human Evolution. PLoS Genet. 2015, 11, e1005661. [Google Scholar] [CrossRef] [PubMed]
- Parry, L.; Clarke, A.R. The Roles of the Methyl-CpG Binding Proteins in Cancer. Genes Cancer 2011, 2, 618–630. [Google Scholar] [CrossRef]
- Bergman, Y.; Cedar, H. DNA Methylation Dynamics in Health and Disease. Nat. Struct. Mol. Biol. 2013, 20, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Li, W.; Zhao, W.; Wang, R. Targeting Epigenetic Regulations in Cancer. Acta Biochim. Biophys. Sin. 2015, 48, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, O.A.; Moran, S.; Gomez, A.; Sayols, S.; Arribas-Jorba, C.; Sandoval, J.; Hilmarsdottir, H.; Olafsdottir, E.; Tryggvadottir, L.; Jonasson, J.G.; et al. A DNA Methylation-Based Definition of Biologically Distinct Breast Cancer Subtypes. Mol. Oncol. 2015, 9, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, S. Alcohol Metabolism and Epigenetics Changes. Alcohol Res. 2013, 35, 6–16. [Google Scholar] [PubMed]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Cadet, J.; McCoy, M.; Jayanthi, S. Epigenetics and Addiction. Clin. Pharmacol. Ther. 2016, 99, 502–511. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Bhattacharya, A.; Koney, N.K.K.; Husain, K.; Abbas, A.; Ansari, R.A. Role of Flavonoids as Epigenetic Modulators in Cancer Prevention and Therapy. Front. Genet. 2021, 12, 758733. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; Abualmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Tewary, P.; Gunatilaka, A.A.L.; Sayers, T.J. Using Natural Products to Promote Caspase-8-Dependent Cancer Cell Death. Cancer Immunol. Immunother. 2017, 66, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.N.T.; Yu, R.; Chen, C.; Mandlekar, S.; Primiano, T. Signal Transduction Events Elicited by Natural Products: Role of MAPK and Caspase Pathways in Homeostatic Response and Induction of Apoptosis. Arch. Pharm. Res. 2000, 23, 1–16. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Hashemi, M.; Ande, S.R.; Yeganeh, B.; Xiao, W.; Eshraghi, M.; Bus, C.J.; Kadkhoda, K.; Wiechec, E.; Halayko, A.J.; et al. Apoptosis and Cancer: Mutations within Caspase Genes. J. Med. Genet. 2009, 46, 497–510. [Google Scholar] [CrossRef]
- Xu, D.C.; Arthurton, L.; Baena-Lopez, L.A. Learning on the Fly: The Interplay between Caspases and Cancer. Biomed Res. Int. 2018, 2018, 5473180. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Lomonosova, E.; Chinnadurai, G. BH3-Only Proteins in Apoptosis and beyond: An Overview. Oncogene 2008, 27, S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Athinarayanan, J.; Subbarayan, V.P.; Lei, D.K.Y.; Alshatwi, A.A. Hesperetin Induces an Apoptosis-Triggered Extrinsic Pathway and a P53-Independent Pathway in Human Lung Cancer H522 Cells. J. Asian Nat. Prod. Res. 2018, 20, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Dahlawi, H. Effect of Malvidin on Induction of Apoptosis and Inhibition of Cell Proliferation on Myeloid and Lymphoid Leukemia. Sch. J. Appl. Med. Sci. 2022, 10, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.X.; Cai, H.; Jiang, G.; Zhou, T.B.; Wu, H. Silibinin Inhibits Proliferation, Induces Apoptosis and Causes Cell Cycle Arrest in Human Gastric Cancer MGC803 Cells via STAT3 Pathway Inhibition. Asian Pac. J. Cancer Prev. 2014, 15, 6791–6798. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, D.; Hao, Y.; Liu, Q.; Wu, Y.; Liu, X.; Luo, J.; Zhou, T.; Sun, B.; Luo, X.; et al. Cyanidin Curtails Renal Cell Carcinoma Tumorigenesis. Cell. Physiol. Biochem. 2018, 46, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin Chloride Induces Apoptosis by Inhibiting NF-κB Signaling through Activation of Nrf2 in Colorectal Cancer Cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhu, Y.; Han, B.; Peng, J.; Deng, X.; Chen, W.; Du, J.; Ou, Y.; Peng, X.; Yu, X. Delphinidin Induces Cell Cycle Arrest and Apoptosis in HER-2 Positive Breast Cancer Cell Lines by Regulating the NF-κB and MAPK Signaling Pathways. Oncol. Lett. 2021, 22, 832. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, C.-Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.-H. Phloretin Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species. Pathol. Oncol. Res. 2020, 26, 977–984. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, L.; Zhang, Y. Naringin Inhibits Thyroid Cancer Cell Proliferation and Induces Cell Apoptosis through Repressing PI3K/AKT Pathway. Pathol. Res. Pract. 2019, 215, 152707. [Google Scholar] [CrossRef]
- He, M.; Qiu, C.; Wang, J.; Li, B.; Wang, G.; Ji, X. Naringin Targets Zeb1 to Suppress Osteosarcoma Cell Proliferation and Metastasis. Aging 2018, 10, 4141–4151. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Yumnam, S.; Saralamma, V.V.G.; Ha, S.E.; Lee, W.S.; Kim, G.S. Naringin Induces Lysosomal Permeabilization and Autophagy Cell Death in AGS Gastric Cancer Cells. Am. J. Chin. Med. 2020, 48, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Ni, H. Basic Research Eriodictyol Exerts Potent Anticancer Activity against A549 Human Lung Cancer Cell Line by Inducing Mitochondrial-Mediated Apoptosis, G2/M Cell Cycle Arrest and Inhibition of m-TOR/PI3K/Akt Signalling Pathway. Arch. Med. Sci. 2020, 16, 446–452. [Google Scholar] [CrossRef]
- Li, W.; Du, Q.; Li, X.; Zheng, X.; Lv, F.; Xi, X.; Huang, G. Eriodictyol Inhibits Proliferation, Metastasis and Induces Apoptosis of Glioma Cells via PI3K/Akt/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein Induces Apoptosis and Autophagy of Breast Cancer Cells via Inhibiting PI3K/AKT Pathway In Vivo and Vitro. Drug Des. Dev. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chen, W.J.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Tangeretin Induces Cell-Cyle G1 Arrest through Inhibiting Cyclin-Dependent Kinases 2 and 4 Activities as Well as Elevating Cdk Inhibitors P21 and P27 in Human Colorectal Carcinoma Cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Abe, K.; Gotoh, M. Citrus Flavone Tangeretin Inhibits Leukaemic HL-60 Cell Growth Partially through Induction of Apoptosis with Less Cytotoxicity on Normal Lymphocytes. Br. J. Cancer 1995, 72, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a Citrus Polymethoxyflavonoid, Induces Apoptosis of Human Gastric Cancer AGS Cells through Extrinsic and Intrinsic Signaling Pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin Promotes Apoptotic Cell Death via Upregulation of Nrf2 Expression by DNA Demethylase and the Interaction of Nrf2 with P53 in Human Colon Cancer Cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Cross, N.A.; Mahy, N.J. The Effect of Apigenin and Chemotherapy Combination Treatments on Apoptosis-Related Genes and Proteins in Acute Leukaemia Cell Lines. Sci. Rep. 2022, 12, 8858. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, G.; Weng, D. Low Concentration of Quercetin Antagonizes the Cytotoxic Effects of Anti-Neoplastic Drugs in Ovarian Cancer. PLoS ONE 2014, 9, e100314. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic Interaction of Rutin and Silibinin on Human Colon Cancer Cell Line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fan, J.; Cheng, L.; Hu, P.; Liu, R. The Anticancer Activity of Genistein Is Increased in Estrogen Receptor Beta 1-Positive Breast Cancer Cells. OncoTargets. Ther. 2018, 11, 8153–8163. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-Rich Iso Fl Avones Aglycone Inhibits Lung Cancer Growth through Inhibition of NF-κB Signaling Pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, F.; Li, W.; Zhou, L.; Liu, W.; Li, M. Formononetin Inhibits Tumor Growth by Suppression of EGFR-Akt-Mcl-1 Axis in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 62. [Google Scholar] [CrossRef] [PubMed]
- Won, D.; Kim, L.; Jang, B.; Yang, I.; Kwon, H.; Jin, B.; Oh, S.H.; Kang, J. In Vitro and in Vivo Anti-Cancer Activity of Silymarin on Oral Cancer. Tumor Biol. 2018, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The Anti-Tumor Effect of Taxifolin on Lung Cancer via Suppressing Stemness and Epithelial-Mesenchymal Transition in Vitro and Oncogenesis in Nude Mice. Ann. Transl. Med. 2020, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a Natural Flavonoid Interacts with Cell Cycle Regulators Causes Cell Cycle Arrest and Causes Tumor Regression by Activating Wnt/β-Catenin Signaling Pathway. BMC Cancer 2018, 18, 1043. [Google Scholar] [CrossRef]
- Silva, C.; Correia-Branco, A.; Andrade, N.; Ferreira, A.C.; Soares, M.L.; Sonveaux, P.; Stephenne, J.; Martel, F. Selective Pro-Apoptotic and Antimigratory Effects of Polyphenol Complex Catechin:Lysine 1:2 in Breast, Pancreatic and Colorectal Cancer Cell Lines. Eur. J. Pharmacol. 2019, 859, 172533. [Google Scholar] [CrossRef]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells Is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Li, J.; Zhang, Q.; Zhong, P.; Teng, T.; Chen, M.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Inhibits the Growth and Increases the Apoptosis of Human Thyroid Carcinoma Cells through Suppression of EGFR/RAS/RAF/MEK/ERK Signaling Pathway. Cancer Cell Int. 2019, 19, 43. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S.P. Tangeretin Ameliorates Oxidative Stress in the Renal Tissues of Rats with Experimental Breast Cancer Induced by 7,12-Dimethylbenz[a]anthracene. Toxicol. Lett. 2014, 229, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective Effects of Citrus Fruit-Derived Flavonoids, Nobiletin and Tangeretin in Alzheimer’s and Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Y.; Wu, M.Y.; Lo, Y.C. Tangeretin Sensitizes SGS1-Deficient Cells by Inducing DNA Damage. J. Agric. Food Chem. 2013, 61, 6376–6382. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.S.A.; Shurrab, N.T.; Buabeid, M.A. Therapeutic Implications of a Polymethoxylated Flavone, Tangeretin, in the Management of Cancer via Modulation of Different Molecular Pathways. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 4709818. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Ortuno, A.; Benavente-Garcia, O.; Castillo, J.; Alcaraz, M.; Vicente, V.; Del Rio, J. Beneficial Action of Citrus Flavonoids on Multiple Cancer-Related Biological Pathways. Curr. Cancer Drug Targets 2007, 7, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Kandaswami, C.; Perkins, E.; Soloniuk, D.S.; Drzewiecki, G.; Middleton, E. Antitproliferative Effects of Citrus Flavonoids on a Human Squamous Cell Carcinoma in Vitro. Cancer Lett. 1991, 56, 147–152. [Google Scholar] [CrossRef]
- Lin, J.J.; Huang, C.C.; Su, Y.L.; Luo, H.L.; Lee, N.L.; Sung, M.T.; Wu, Y.J. Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1017. [Google Scholar] [CrossRef]
- Rooprai, H.K.; Christidou, M.; Murray, S.A.; Davies, D.; Selway, R.; Gullan, R.W.; Pilkington, G.J. Inhibition of Invasion by Polyphenols from Citrus Fruit and Berries in Human Malignant Glioma Cells In Vitro. Anticancer Res. 2021, 41, 619–633. [Google Scholar] [CrossRef]
- Surichan, S.; Arroo, R.R.; Tsatsakis, A.M.; Androutsopoulos, V.P. Tangeretin Inhibits the Proliferation of Human Breast Cancer Cells via CYP1A1/CYP1B1 Enzyme Induction and CYP1A1/CYP1B1–Mediated Metabolism to the Product 4′ Hydroxy Tangeretin. Toxicol. Vitr. 2018, 50, 274–284. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a Ubiquitous Dietary Phenolic Subclass, Exert Extensive In Vitro Anti-Invasive and in Vivo Anti-Metastatic Activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Yumnam, S.; Raha, S.; Kim, S.; Venkatarame Gowda Saralamma, V.; Lee, H.; Ha, S.; Heo, J.; Lee, S.; Kim, E.; Lee, W.; et al. Identification of a Novel Biomarker in Tangeretin-induced Cell Death in AGS Human Gastric Cancer Cells. Oncol. Rep. 2018, 40, 3249–3260. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, Y.; Pal, I.; Banik, P.; Chakraborty, S.; Borkar, S.A.; Dey, G.; Mukherjee, A.; Mandal, M. Insights into Molecular Therapy of Glioma: Current Challenges and next Generation Blueprint. Acta Pharmacol. Sin. 2017, 38, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Erices, J.I.; Torres, Á.; Niechi, I.; Bernales, I.; Quezada, C. Current Natural Therapies in the Treatment against Glioblastoma. Phyther. Res. 2018, 32, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, D.; Yu, X.D.; Zhou, Y.L. Tangeretin Induces Cell Cycle Arrest and Apoptosis through Upregulation of PTEN Expression in Glioma Cells. Biomed. Pharmacother. 2016, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Sorimuthu Pillai, S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7,12-dimethylbenz(α)anthracene-induced rat mammary carcinoma. J. Nutr. Biochem. 2014, 24, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Sangavi, P.; Langeswaran, K. Anti-Tumorigenic Efficacy of Tangeretin in Liver Cancer—An in-Silico Approach. Curr. Comput.-Aided. Drug Des. 2020, 17, 337–343. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lim, T.G.; Lee, K.M.; Jeon, A.J.; Kim, S.Y.; Lee, K.W. Tangeretin Reduces Ultraviolet B (UVB)-Induced Cyclooxygenase-2 Expression in Mouse Epidermal Cells by Blocking Mitogen-Activated Protein Kinase (MAPK) Activation and Reactive Oxygen Species (ROS) Generation. J. Agric. Food Chem. 2011, 59, 222–228. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, Y.; Tan, C.; Sundararajan, B.; Li, Z.; Wang, D.; Zhou, Z. Comparative Effects of Five Polymethoxyflavones Purified from Citrus Tangerina on Inflammation and Cancer. Front. Nutr. 2022, 9, 963662. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, C.; Cheng, Z.; Wang, Q.; Hoffman, R.M.; Singh, S.R.; Huang, Y.; Zheng, W.; Yang, S.; Ye, J. TRAF6-Mediated Inflammatory Cytokines Secretion in LPS-Induced Colorectal Cancer Cells Is Regulated by MiR-140. Cancer Genom. Proteom. 2020, 17, 23–33. [Google Scholar] [CrossRef]
- Jain, S.; Dash, P.; Minz, A.P.; Satpathi, S.; Samal, A.G.; Behera, P.K.; Satpathi, P.S.; Senapati, S. Lipopolysaccharide (LPS) Enhances Prostate Cancer Metastasis Potentially through NF-κB Activation and Recurrent Dexamethasone Administration Fails to Suppress It In Vivo. Prostate 2019, 79, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, J.; Shen, W.; Gao, R.; Liu, Y.; Chen, Y.; Li, X.; Liu, C.; Xiang, R.; Luo, N. TLR4 Promotes Breast Cancer Metastasis via Akt/GSK3β/β-Catenin Pathway upon LPS Stimulation. Anat. Rec. 2017, 300, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, D.; Wang, Q.; Zheng, D.; Jiang, X.; Xu, L. LPS Induced miR-181a Promotes Pancreatic Cancer Cell Migration via Targeting PTEN and MAP2K4. Dig. Dis. Sci. 2014, 59, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic Therapy with Tangeretin and 5-Fluorouracil Accelerates the ROS/JNK Mediated Apoptotic Pathway in Human Colorectal Cancer Cell. Food Chem. Toxicol. 2020, 143, 111529. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.J.; Chao, Y.H.; Tung, Y.C.; Wu, T.Y.; Su, Z.Y. A Tangeretin Derivative Inhibits the Growth of Human Prostate Cancer LNCaP Cells by Epigenetically Restoring P21 Gene Expression and Inhibiting Cancer Stem-like Cell Proliferation. AAPS J. 2019, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.G.; Peacock, J.J.; Bourland, T.C.; Taylor, S.E.; Wright, J.M.; Patil, B.S.; Miller, E.G. Inhibition of Oral Carcinogenesis by Citrus Flavonoids. Nutr. Cancer 2007, 60, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Weng, M.-S.; Lin, J.-K. Tangeretin Suppresses IL-1β-Induced Cyclooxygenase (COX)-2 Expression through Inhibition of P38 MAPK, JNK, and AKT Activation in Human Lung Carcinoma Cells. Biochem. Pharmacol. 2007, 73, 215–227. [Google Scholar] [CrossRef]
- Zhu, W.B.; Xiao, N.; Liu, X.J. Dietary Flavonoid Tangeretin Induces Reprogramming of Epithelial to Mesenchymal Transition in Prostate Cancer Cells by Targeting the PI3K/Akt/MTOR Signaling Pathway. Oncol. Lett. 2018, 15, 433–440. [Google Scholar] [CrossRef]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting Bioactive Phenolic Compounds as Potential Agents to Inhibit Proliferation and VEGF Expression in Human Ovarian Cancer Cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef]
- Jaboin, J.; Iii, W.A.V.; Banik, N.L.; Giglio, P. A Novel Component from Citrus, Ginger, and Mushroom Family Exhibits Antitumor Activity on Human Meningioma Cells through Suppressing the Wnt/β-Catenin Signaling Pathway. Tumor Biol. 2022, 36, 7027–7034. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Ho, C.T.; Chang, Y.H.; Tan, K.T.; Chung, T.W.; Wang, B.Y.; Chen, Y.K.; Lin, C.C. Tangeretin Derivative, 5-Acetyloxy-6,7,8,4′-Tetramethoxyflavone Induces G2/M Arrest, Apoptosis and Autophagy in Human Non-Small Cell Lung Cancer Cells In Vitro and In Vivo. Cancer Biol. Ther. 2016, 17, 48–64. [Google Scholar] [CrossRef]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor Efficacy of Tangeretin by Targeting the Oxidative Stress Mediated on 7,12-Dimethylbenz(a) Anthracene-Induced Proliferative Breast Cancer in Sprague-Dawley Rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).