Mechanistic Evidence of Andrographis paniculata (Burm. f.) Wall. ex Nees, Pelargonium sidoides DC., Echinacea Species and a Combination of Hedera helix L., Primula veris L./Primula elatior L. and Thymus vulgaris L./Thymus zygis L. in the Treatment of Acute, Uncomplicated Respiratory Tract Infections: A Systematic Literature Review and Expert Interviews
Abstract
1. Introduction
1.1. Clinical Evidence for TCIH Treatments in URTI
1.2. Study Aims
1.3. Research Questions
- What are the direct antibacterial and antiviral effects of each treatment?
- What are the effects on the immune system of each of these treatments?
- What are the effects on expectoration and disease management of each of these herbs?
- Are there effects resulting only from the combination of at least two of ivy, primrose, and thyme?
2. Materials and Methods
2.1. Search Strategy
2.1.1. Search Terms
2.1.2. Sources
2.2. Eligibility
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Selection and Extraction
2.4. Interviews
2.5. Analysis
3. Results
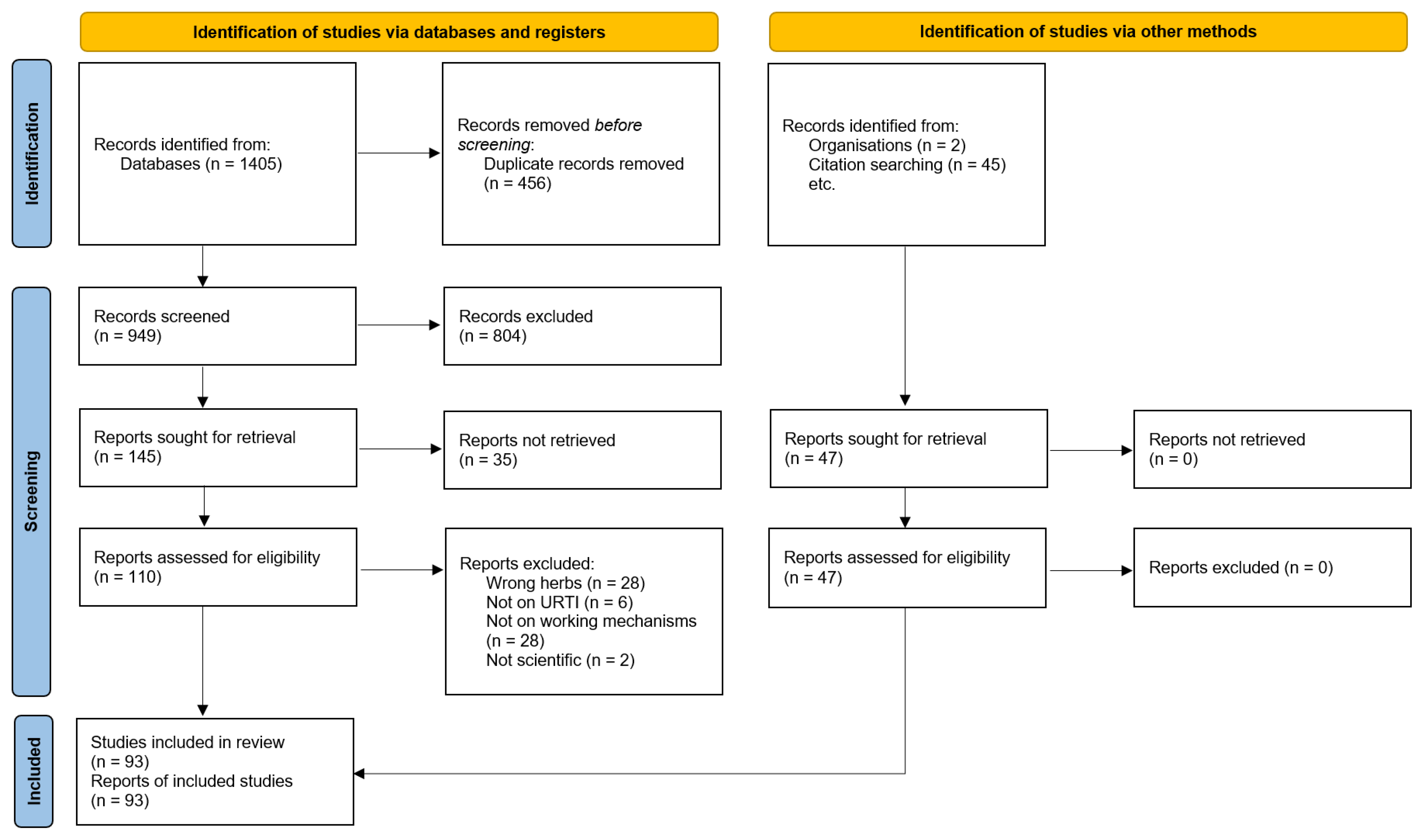
3.1. Search Results
3.2. Andrographis paniculata (Burm. F) Nees
3.2.1. Antibacterial Effects
Restoring Antibiotic Sensitivity and Reducing Biofilm
Reducing Bacterial Adhesion
3.2.2. Antiviral Effects
3.2.3. Immunomodulatory Effects
Regulation of Macrophage Functioning
3.2.4. Antipyretic Effects
3.3. Pelargonium sidoides (Thunb.) R. Knuth
3.3.1. Antibacterial Effects
3.3.2. Antiviral Effects
3.3.3. Immunomodulatory Effects
Effects on Macrophages
Cytokine Production and Effects on Other Immune Cells
Antimicrobial Proteins
3.3.4. Expectorant Activity
3.3.5. Expert Opinion
3.4. Echinacea Species
3.4.1. Antibacterial Effects
3.4.2. Antiviral Effects
3.4.3. Immunomodulatory Effects
Immunostimulatory Effects
Anti-Inflammatory Effects
Evidence from Mechanistic Clinical Trials
Compounds Involved and Differences between Species
3.4.4. Expectorant Effects
3.4.5. Expert Opinion
3.5. Hedera helix L.
3.5.1. Antibacterial and Antiviral Effects
3.5.2. Anti-Inflammatory Effects
3.5.3. Bronchospasmolytic and Secretolytic Effects
3.6. Primula veris L. and Primula elatior L.
3.7. Thymus vulgaris L. and Thymus zygis L.
3.7.1. Antibacterial Effects
Ethanolic Extracts
Essential Oils
| Bacterial Species | Method | MIC | |
|---|---|---|---|
| Acinetobacter baumanii | Agar dilution | 0.12% (v/v) | [111] |
| Enterococcus faecalis | Agar dilution | 0.5% (v/v) | [111] |
| Aeromonas sobria | Agar dilution | 0.12% (v/v) | [111] |
| Escherichia coli | Agar dilution | 0.12% (v/v) | [111] |
| Broth microdilution | 0.03% (v/v) | [111] | |
| Microwell dilution | 62.5 µg/mL | [112] | |
| Klebsiella pneumoniae | Agar dilution | 0.25% (v/v) | [111] |
| Microwell dilution | 500 µg/mL | [112] | |
| 0.025 mL/mL | [107] | ||
| Pseudomonas aeruginosa | Agar dilution | >2.0% (v/v) | [111] |
| Microwell dilution | >500 µg/mL | [112] | |
| Salmonella typhimurium | Agar dilution | >2.0% (v/v) | [111] |
| Microwell dilution | 125 µg/mL | [112] | |
| Serratia marcescens | Agar dilution | 0.25% (v/v) | [111] |
| Staphylococcus aureus | Agar dilution | 0.25% (v/v) | [111] |
| Broth microdilution | 0.03% (v/v) | [111] | |
| 0.0125 mL/mL | [107] | ||
| Microwell dilution | 31.2 µg/mL | [112] | |
| Streptococcus pyogenes | Broth microdilution | 0.43 mg/mL | [113] |
| 0.0125 mL/mL | [107] | ||
| Streptococcus pneumoniae | Broth microdilution | 0.11 mg/mL | [113] |
| 0.00625 mL/mL | [107] | ||
| Streptococcus mutans | Broth microdilution | 0.04 mg/mL | [113] |
| Haemophilius influenzae | Broth microdilution | 0.11 mg/mL | [113] |
| 0.00625 mL/mL | [107] | ||
| Haemophilius parainfluenzae | Broth microdilution | 0.11 mg/mL | [113] |
| Moraxella catarrhalis | Broth microdilution | 0.09 mg/mL | [113] |
| Meticillin-resistant Staphylococcus aureus (MRSA) | Tube dilution | 0.4 mg/mL | [108] |
| Pseudomonas aeruginosa | Tube dilution | 1.4 mg/mL | [108] |
| Bacillus cereus | Microwell dilution | 15.6 µg/mL | [112] |
| Proteus vulgaris | Microwell dilution | 31.2 µg/mL | [112] |
| Salmonella typhi | Microwell dilution | 250 µg/mL | [112] |
| Streptococcus agalactiae | Broth microdilution | 0.00625 mL/mL | [107] |
| Stenotrophomonas maltophilia | Broth microdilution | 0.003125 mL/mL | [107] |
| Extended-spectrum beta-lactamase (ESBL)-producing K. pneumoniae | Broth microdilution | 3.6 mg/mL | [109] |
| New Delhi metallo-beta-lactamase (MBL)-1-producing K. pneumoniae | Broth microdilution | 5.4 mg/mL | [109] |
Compounds
3.7.2. Antiviral Effects
3.7.3. Immunomodulatory Effects
3.8. Combinations of Ivy, Primrose, and Thyme
Expert Opinion
4. Discussion
4.1. Andrographis paniculata
4.2. Pelargonium sidoides
4.3. Echinacea Species
4.4. Ivy, Primrose, and Thyme
4.5. Practical Implications
4.6. Strengths and Limitations
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Study Characteristics
| Study | Methods | Population | Intervention | Outcomes |
| Perić et al. (2020) [66] | Prospective case–control study | 26 patients with acute post-viral rhinosinusitis | 3×/day 20 mg oral EPs 7630 tablets for 10 days | Chemokine levels in nasal secretions, nasal symptoms and endoscopic findings |
| Perić et al. (2021) [65] | Randomized prospective study | 78 patients with uncomplicated acute bacterial rhinosinusitis | During 10 days: 26 patients received 3 × 20 mg/day EPs 7630 tablets; 26 patients received 2 × 150 mg/day roxithromysin tablets | Chemokine levels in nasal secretion and clinical parameters were compared on day 0 and day 10 |
| Goel et al. (2005) [81] | Randomized, double-blind, placebo controlled trial | 150 volunteers, aged 18–65 years, with a history of two or more infections of the common cold in the previous year | At the onset of a cold, on day 1 eight doses of 5 mL EchinilinTM (‘Factors R&D Technologies’, Burnaby, BC, Canada) were taken, and on the 6 consecutive days three doses/day | Self-assessed severity of cold symptoms during the 7 days |
| Dapas et al. (2014) [82] | Pilot study | 10 healthy volunteers | 10 mL Polinacea® (Indena, Milano, Italy) syrup/day during 1 month | Expression levels of IL-2, IL-8, IL-6, and TNF-α in lympho-monocytes and plasma samples |
| Ritchie et al. (2011) [83] | Intervention study | 30 healthy volunteers | Five days of 4 × 1 mL, followed by 3 days of 10 × 1 mL Echinaforce® | Elastase, IL-1β, IL-6, IL-8, IL-10, IL-12 MCP-1, TNF-α, and IFN-γ in daily taken blood samples |
| Kim et al. (2002) [84] | Preliminary, randomized, double-blind, placebo-controlled trial | 48 healthy female volunteers, aged 22–51 years | Daily, a combination of E. purpurea and E. angustifolia (E. purpurea whole herb extract 4% (908 mg/day), E. purpurea whole herb (464 mg/day) or E. angustifolia root (36 mg/day) during 4 weeks | Complement properdin, white blood cell count, neutrophils, lymphocytes, monocytes, quality of life assessment |
| Sheeja et al. (2006) [46] | Animal study | 12 BALB/c mice | 5 doses of 10 mg methanolic extract of A. paniculata for 5 days | PMA-induced superoxide and nitric oxide formation |
| Wang et al. (2010) [42] | Animal study | 12 BALB/c mice | Intraperitoneal administration of 1 mg/kg/day andrographolide, during 7 days | Antibodies and IL-4-producing splenocytes |
| Mishra et al. (2013) [34] | In vitro | E. coli, P. aeruginosa, M. tuberculosis, S. Aureus, MRSA, and methicillin-resistant E. faecalis | Agar well diffusion with A. paniculata | MIC |
| Abubacker and Vasantha (2010) [36] | In vitro | E. coli, K. pneumoniae, P. vulgaris, and S. pneumoniae | Disc diffusion assay with A. paniculata | Inhibition zone |
| Rajalakshmi and Cathrine (2016) [33] | In vitro | E. coli, S. aureus, B. subtilis | Screening for phytochemical components of A. paniculata and disc diffusion assay | Inhibition zone |
| Xu et al. (2012) [37] | In vitro | S. epidermidis, P. aeruginosa, B. subtilis | Microtitre plate broth dilution with noriridoids from the roots of A. paniculata | MIC |
| Chandrasekaran et al. (2010) [44] | In vitro | J774A.1 murine macrophages | Effect of an extract of A. paniculata leaves on inflammatory response to LPS | Inhibition of NO, PGE2, IL-1β, and IL-6 |
| Chandrasekaran et al. (2011) [43] | In vitro | J774A.1 macrophages | Effect of 7 phytoconstituents (andrographolide, neoandrographolide, isoandrographolide, andrograpanin, 14-deoxy-11,12-didehydroandrographolide, 7-O-methylwogonin, and skullcapflavone-I) isolated from A. paniculata on inflammatory mediators | NO, PGE2, IL-1β, IL-6, LTB4, TXB2, and histamine |
| Parichatikanond et al. (2010) [45] | In vitro | Ionophore A23 187-induced human platelets | The effect of diterpenoids from A. paniculata on the production of inflammatory cytokines and COX activities | COX-2 activity, TNF-α, IL-6, IL-1β, and IL-10, gene expression of cytokines and cytokine receptors |
| Liu et al. (2007) [41] | In vitro | L-929 cells and bone-marrow-derived macrophages from BALB-c mice | The effect of andrograpanin from A. paniculata on overproduction of NO, pro-inflammatory cytokines, and involved pathways | NO, TNF-, IL-6, and IL-12p70 |
| Kumar et al. (2004) [40] | In vitro | Peripheral blood lymphocytes of healthy volunteers | Different fractions of methanolic extract of A. paniculata were screened for immunostimulatory effects | IL-2 |
| Chen et al. (2009) [38] | Animal and in vitro study | BALB/c mice | Mice were infected with avian influenza A/chicken/Guang-dong/96 (H9N2) virus and A/duck/Guangdong/99 (H5N1) virus and different doses of A. paniculata were administered | LD50 of influenza virus in mice, lung virus titer, in vivo and in vitro antiviral evaluation, toxicity of A. paniculata |
| Conrad et al. (2007) [49] | In vitro | Human peripheral blood | The effect of EPs 7630 on the activity of human peripheral blood cells | Phagocytosis and oxidative burst |
| Janecki et al. (2011) [49] | In vitro | Human HEp-2 epithelial cells and group A-streptococci (GAS) | The effect of EPs 7630, the methanol and non-methanol fraction, on GAS adhesion to Hep-2 cells | Percentage of change in adhesion |
| Neugebauer et al. (2005) [57] | In vitro | Ciliated cell cultures of human epithelium | The effect of EPs 7630 on ciliary beat frequency | Percentage of change in ciliary beat frequency |
| Koch and Wohn (2007) [62] | In vitro | Human neutrophils and granulocytes | The effects of EPs 7630 in concentrations between 0.3 and 30 µg/mL after 5 h incubation on antibacterial peptide production | Antibacterial protein’s neutrophil peptides 1–3 and bactericidal/permeablity-increasing protein |
| Kayser et al. (2001) [59] | In vitro | Leishmania donovani, murine macrophages | Effects of EPs 7630 in in vitro models for intracellular infection with Leishmania parasites, an extracellular Leishmania growth assay, a fibroblast-virus protection assay (IFN activity), a fibroblast-lysis assay (TNF activity) and a biochemical assay for inorganic nitric oxides (iNO) were employed | TNF release, IFN induction, NO |
| Thäle et al. (2008) [60] | In vitro | Bone-marrow-derived mouse macrophages, Listeria monocytogenes | Effects of EPs 7630 on immunomodulators | NO, IL-1, IL-12, TNF-α, CD40, CD119 |
| Trun et al. (2006) [64] | In vitro | Non-infected and Leishmania-infected RAW 264.7 cells | Effect of EPs 7630 on cytokine production | Gene expression of NO synthase and IL-1, IL-12, IL-18, TNF-α, IFN-α, and IFN-γ |
| Kolodziej and Kinderlen (2007) [69] | In vitro | Non-infected and Leishmania-infected murine macrophages | Antibacterial effects of EPs 7630 and their effects on non-specific immune functions | iNOS, IFN-α, IFN-γ, TNF-α, IL-1, IL-10, IL-12, and IL-18 |
| Nöldner and Koch (2004) [55] | Animal study | NMRI mice | Effect of oral administration of a P. sidoides extract (100–400 mg/kg); 1 h later sickness behaviour was induced by intraperitoneal injection of LPS (400 μg/kg) | One hour later, behavioural effects were examined in the ‘light/dark box model’ by monitoring exploratory activity for 3 min |
| Nöldner and Schötz (2007) [56] | Animal study | NMRI mice | Effect of oral administration of a EPs 7630 extract (100–400 mg/kg) and subfractions; 1 h later sickness behaviour was induced by intraperitoneal injection of LPS (400 μg/kg) | Time spent in light compartment, number of changes |
| Walther et al. (2020) [50] | In vitro | MDCK and HeLa cells | Antiviral effects of P. sidoides against different types of rhinovirus and influenza virus | Cytotoxicity, viral plaque production, viral neuraminidase activity |
| Bao et al. (2015) [54] | Animal study | ICR mice SPF-class and guinea pigs | Antitussive, secretolytic, and anti-inflammatory effects of EPs 7630 were assessed in animal experiments following oral administration at human equivalent doses | Degree of tracheal and bronchial lesions, bronchosecretolytic (phenol red), concentrations of SOD and MDA in serum |
| Roth et al. (2019) [52] | In vitro | Human bronchial cells from patients with severe asthma (n = 6), moderate COPD (n = 6) and non-diseased controls (n = 6) | The effect of EPs 7630 on human bronchial cells was assessed with western blot, immunofluorescence, and polymerase chain reaction | Protein expression, cell survival |
| Roth et al. (2021) [53] | In vitro | Human bronchial epithelial cells | The effect of EPs 7630 on human bronchial cells was assessed with western blot and immunofluorescence | Protein expression, intracellular signalling, antiviral effect |
| Witte et al. (2015) [63] | In vitro | Human monocytes (PBMCs), isolated from healthy donors | The effects of EPs 7630 on human monocytes was assessed by ELISA, western blot, and flow cytometry analyses | TNF-α, IL-6, and IL-10 |
| Witte et al. (2020) [61] | In vitro | PBMCs | The effects of EPs 7630 on antimicrobial airway defense through monocytes | IL-22, IL-17, IFN-γ, IL-1, IL-23 |
| Vimalanathan et al. (2017) [70] | In vitro | MDCK and BEAS-2B (human) cells. Influenza virus A and H. influenzae and S. aureus | Effect of an E. purpurea extract (EchinaforceTM) on H3N2-induced adhesion of live H. influenzae and S. aureus | Cytotoxicity, bacterial adhesion, expression of ICAM-1, fibronectin, PAFr, TLR4 and NFkB p65, IL-6 and IL-8 |
| Fonseca et al. (2014) [80] | In vitro | Human Jurkat T-cells | Effect of different concentrations of E. purpurea extract (0, 10, 25, 100 and 250 μg/mL) on human T-cells was assessed | IL-2, IFN-γ, CD25 expression |
| Gertsch et al. (2004) [85] | In vitro | Human monocytes/macrophages | Echinacea alkylamides from EchinaforceTM | TNF-α protein and mRNA, cAMP, p38/MAPK, and JNK signaling, as well as NF-jB and ATF-2/CREB-1 |
| Classen et al. (2006) [86] | In vitro | Mouse spleen cell cultures and mouse macrophages | Effects of different arabinogalactan-proteins from E. paalida were assessed | Proliferation, IgM production, induction of IL-6 and nitrite production |
| Keyhanmanesch et al. (2015) [93] | Animal study | Dunkin–Hartley guinea pigs | Intraperitoneal injection of different doses of α-hederin | IL-4, IFN-γ, and IL-17 levels in blood and histopathological evaluation of lungs and trachea |
| Sieben et al. (2009) [97] | In vitro | Human airway smooth muscle cells (HASM) | Cells were pre-treated with α-hederin, hederacoside, and hederagenin | β2 adrenergic receptor density, intracellular cAMP |
| Gepdiremen et al. (2005) [99] | Animal study | Wistar albino rats | Anti-inflammatory potential of a-hederin (monodesmoside) and hederasaponin-C from H. helix was assessed in carrageenan-induced acute paw edema in rats | Edema rate percentage |
| Wolf et al. (2011) [95] | In vitro | Bovine tracheal smooth muscle strips | Effects of the three main saponins of ivy, α-hederin, hederacoside C, and hederagenin, on the contraction and relaxation behaviour of isolated bovine tracheal smooth muscle strips by isometric tension measurements | Histamine-, methacholine-, and isoprenaline-induced relaxation of precontracted muscle strips |
| Hocauglu et al. (2012) [94] | Animal study | BALB/c mice | Mice were challenged with ovalbumin after which they received either a placebo, H. helix extract, or dexamethasone. | Goblet cell numbers, thickness of basement membrane, epithelium, and subepithelial smooth muscle layers |
| Saadat et al. (2015) [92] | Animal study | Dunkin–Hartley guinea pigs | Guinea pigs were sensitized with ovalbumin, after which a low (0.3 mg/kg) or high (3.0 mk/kg) dose of α-hederin was given | Tracheal response to methacholine, histamine and ovalbumin, white blood cell count |
| Shulte-Michels et al. (2016) [90] | In vitro | HEK293 cells | To assess the effect of H. helix extract on protein kinase A and G protein-coupled receptor kinases phosphorylation of β2 adrenergic receptors, an in-cell western was performed | Protein kinase A-mediated phosphorylation, G coupled receptor kinases phosphorylation |
| Greunke et al. (2015) [96] | In vitro | Human airway smooth muscle cells | The effect of an ivy leaf extract (EA 575) on human airway smooth muscle cells was assessed using live cell imaging, fluorescence correlation spectroscopy, and a cAMP assay | cAMP, internalization and binding of β-adrenergic receptors |
| Orhan et al. (2012) [88] | In vitro | S. pneumonia, S. pyogenes, S. aureus, S. epidermidis, M. tuberculosis, K. pneumoniae, H. influenzae, P. auruginosa, A. baumannii | Antibacterial effects of H. helix were assessed | DPPH inhibition, MIC |
| Schulte-Michels et al. (2019) [91] | In vitro | Mouse macrophages (J774.2), human embryonic kidney cells (HEK 293), acute monocytic leukemia cells (THP-1), and human lung epithelium-derived cells (A549) | Effect of EA 575 on transcriptional activity of NFkB was assessed | NFkB, IkBa and RelA, TNF-α |
| Schulte-Michels et al. (2016) [90] | In vitro | Murine macrophages (J774.2) | Different concentrations of EA 575 were tested for their effect on IL-6 release in macrophages | IL-6 |
| Ünsal et al. (2010) [89] | Field study and in vitro study | S. aureus, S. epidermidis, E. coli, K. pneumoniae | During a three-month field study, 64 plant samples of plants that are traditionally used in common infections were collected and tested for antimicrobial activity | MIC |
| Acs et al. (2016) [108] | In vitro | Methicillin-resistant Staphylococcus aureus and the antibiotic-resistant Pseudomonas aeruginosa | With a tube dilution test, antimicrobial activity of thyme was assessed | MIC |
| Acs et al. (2018) [113] | In vitro | S. pneumoniae, S. mutans, S. pyogenes, H. influenzae, H. parainfluenzae, and M. catarrhalis | With broth microdilution test and vapor phase test, antimicrobial activity of thyme was assessed | MIC and MBC |
| Al-Bayati et al. (2008) [112] | In vitro | S. aureus, B. cereus, E. coli, P. vulgaris, P. mirabilis, S. typhi, S. typhimurium, K. pneumoniae, and P. aeruginosa | With microwell dilution method, the antimicrobial activity of T. vulgaris was assessed | MIC |
| Csikos et al. (2020) [117] | Animal study | C57BL/6J mice | Acute airway inflammation was induced in mice, essential oil of thyme was inhaled by one group of the animals | Airway function, hyperresponsiveness, and myeloperoxidase activity |
| Dahiya and Purkayastha (2012) [101] | In vitro | MDR clinical isolates of S. aureus (3 isolates), S. aureus (MRSA), Escherichia coli (3 isolates), K. pneumoniae (2 isolates), and P. mirabilis | With the agar well diffusion method, the antimicrobial activity of thyme was assessed | MIC |
| Fabio et al. (2007) [107] | In vitro | S. pyogenes, S. agalactiae, S. Pneumoniae, K. pneumoniae, H. influenzae, S. aureus, S. maltophilia | With the Kirby–Bauer paper method | Cytotoxicity, MIC, and MBC |
| Hammer et al. (1999) [111] | In vitro | Aer. Sobria, Candida albica, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, S. enterica, S. marcescens, and S. aureu | With the agar dilution method and the broth dilution method, the antimicrobial activity of essential oil of T. vulgaris was assessed | MIC and MCC |
| Hoferl et al. (2009) [114] | In vitro | S. aureus, E. coli, P. aeruginosa, P. vulgaris, K. pneumoniae, and S. sp. | With agar diffusion disc method and agar dilution method, the antimicrobial activity of thyme was assessed | MIC and inhibition zone (IZ) |
| Inouye et al. (2001) [103] | In vitro | H. influenzae, S. aureus, S. pneumoniae, E. coli, and S. pyogenes | A minimum inhibitory dose was established to assess the antimicrobial activity of thyme | Minimum inhibitory dose (MID) |
| Iseppe et al. (2020) [104] | In vitro | E. coli, K. pneumoniae, P. aeruginosa | With agar diffusion disc method, the antimicrobial activity of essential oil of thyme was assessed | MIC |
| Javed et al. (2018) [100] | In vitro | S. aureus, P. aeruginosa, E. coli, P. vulgaris, K. pneumoniae | With the disk diffusion method, the antibacterial activity of T. vulgaris was assessed | MIC |
| Kwiatkowski et al. (2018) [109] | In vitro | K. pneumoniae | With broth microdilution method, the antimicrobial activity of essential oil of T. vulgaris was assessed | MIC, MBC |
| Lelesius et al. (2019) [116] | In vitro | Avian infectious bronchitis virus (IBV)/Vero Cells | Antiviral effects of T. vulgaris were assessed | Cytotoxicity, antiviral effect against IBV, and prior to infection in Vero cells |
| Man et al. (2016) [106] | In vitro | S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa | Antibacterial effects of T. vulgaris were assessed | MIC, MBC |
| Marchese et al. (2016) [32] | Review | n.a. | Antibacterial and antifungal activities of thymol are researched in the literature | Chemistry, bioavailability, safety assessment, biological activity, antibacterial, and antifungal activity |
| Mohamed et al. (2019) [115] | In vitro | K. pneumoniae | Assessment of antibacterial effects of thymol, also in combination with ampicillin with disc diffusion assay and checkerboard assay | MIC, IZ |
| Mohammed et al. (2018) [105] | In vitro | K. pneumoniae | Assessment of antibacterial effects of essential oil of thyme, also in combination with ciprofloxacin with disc diffusion assay and checkerboard assay | MIC, MBC |
| Seibel et al. (2015) [120] | Animal study | Wistar rats | Bronchoalveolitis was induced in rats, which were then given different doses of Bronchipret (combination of ivy/primrose/thyme) | Granulocyte infiltration, effects on leukocytes, goblet cells in bronchial tissue, and leukotriene production and 5-LO enzyme activity |
| Van Vuuren et al. (2008) [121] | In vitro | S. aureus, K. pnuemoniae, C. albicans | Essential oil of T. vulgaris was assessed for antimicrobial activity, also in combination with conventional antimicrobials | MIC |
| Vazquez-Ucha et al. (2020) [110] | In vitro | A. baumannii and K. pneumoniae | Combined disk diffusion test and checkerboard assays were performed with the essential oil of T. zygis in combination with colistin | MIC |
| Wani et al. (2021) [102] | Review | n.a. | Literature review of antiviral potential of essential oils (including thyme) | Chemical composition, antiviral effects |
| Samuel and Priyadarshoni (2019) [75] | Review | n.a. | Literature review on biologic effects of Echinacea species | Active components, pharmacological actions, medicinal effects |
| Rondanelli et al. (2018) [76] | Review | n.a. | Literature review on the use of Echinacea in self-care for common colds | Effects on cellular immunity and adaptive immunity |
| Roxas and Jurenka (2007) [79] | Review | n.a. | Literature review on the use of Echinacea in treatment of the common cold | Incidence, signs and symptoms, potential complications, conventional treatment, alternative treatments |
| Barnes et al. (2005) [72] | Review | n.a. | Literature review on E. angustifolia, E. pallida, and E. purpurea | Chemistry, pharmacology, and clinical properties |
| Islam and Carter (2005) [87] | Review | n.a. | Literature review on the use of Echinacea in upper respiratory tract infections | Qualitative and quantitative properties, mechanism of action, clinical trials, and its effect on the immune system |
| Barret (2003) [73] | Review | n.a. | Literature review on the medicinal properties of Echinacea | Pharmacology, safety, contraindications, risk of adverse events, phytochemistry |
| Bash et al. (2005) [74] | Review | n.a. | Natural standard review on E. angustifolia, E. pallida, and E. purpurea | Toxicology, dosing, interactions, mechanism of action |
| Borchers et al. (2000) [77] | Review | n.a. | Literature review on Echinacea species | Anti-inflammatory activities, Echinacea species |
| Moyo and Van Staden (2014) [26] | Review | n.a. | Literature review on the uses and mechanism of action of P. sidoides | Phytochemistry, pharmacological properties, commercial potential, biotechnology application |
| Kolodziej et al. (2011) [67] | Review | n.a. | Literature review on the antimicrobial, antiviral, and immunomodulatory activities of P. sidoides | Respiratory tract related antibacterial activity, effect on mucociliary system, antiviral effects, immunomodulatory effects |
| Brendler et al. (2008) [68] | Review | n.a. | Literature review on pre-clinical and clinical studies of P. sidoides | Historical and commercial perspectives, scientific perspectives |
| Hossain et al. (2021) [35] | Review | n.a. | Literature review on A. paniculata | Phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy |
| Jiang et al. (2021) [39] | Review | n.a. | Literature review on A. paniculata and andrographolide | Ethnopharmacology, phytochemicals, antiviral properties, toxicology |
| Kumar et al. (2021) [24] | Review | n.a. | Literature review on A. paniculata | Traditional uses, phytochemistry, pharmacology, and quality control |
| Okhuarobo et al. (2014) [47] | Review | n.a. | Literature review on A. paniculata | Phytochemistry and pharmacology |
Appendix B. Search Terms
Appendix B.1. Search 1: Reviews on All Herbs
Appendix B.2. Search 2: Mechanistic studies on Ivy, Primrose, and Thyme
Appendix B.3. Search 3: Recent Studies on Pelargonium
References
- Ikuta, K.S.; Swetschinski, L.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; Johnson, S.C.; Fell, F.; Hackett, S.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- WHO. Global Action Plan on Antimicrobial Resistance, 1st ed.; WHO Document Production Services: Geneva, Switzerland, 2015; pp. 1–19. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU and EEA: Annual Epidemiological Report 2019; ECDC: Solna, Sweden, 2019; pp. 1–25. [Google Scholar]
- Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Actual versus ‘Ideal’ Antibiotic Prescribing for Common Conditions in English Primary Care. J. Antimicrob. Chemother. 2018, 73, 19–26. [Google Scholar] [CrossRef]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper Respiratory Tract Infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef]
- Nicholson, K.G.; Kent, J.; Hammersley, V.; Cancio, E. Acute Viral Infections of Upper Respiratory Tract in Elderly People Living in the Community: Comparative, Prospective, Population Based Study of Disease Burden. BMJ 1997, 315, 1060–1064. [Google Scholar] [CrossRef]
- Kardos, P.; Malek, F. Common Cold—An Umbrella Term for Acute Infections of Nose, Throat, Larynx and Bronchi. Pneumologie 2016, 71, 221–226. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Peterson, K.; Winthrop, K.; Cantor, A.; Lazur, B.H.; Buckley, D.I. Interventions to Reduce Inappropriate Prescribing of Antibiotics for Acute Respiratory Tract Infections: Summary and Update of a Systematic Review. J. Int. Med. Res. 2018, 46, 3337–3357. [Google Scholar] [CrossRef]
- Baars, E.W.; Belt-Van Zoen, E.; Willcox, M.; Huber, R.; Hu, X.; van der Werf, E.T. CAM Treatments for Cough and Sore Throat as Part of an Uncomplicated Acute Respiratory Tract Infection: A Systematic Review of Prescription Rates and a Survey among European Integrative Medical Practitioners. Eur. J. Integr. Med. 2020, 39, 101194. [Google Scholar] [CrossRef]
- van der Werf, E.T.; Duncan, L.J.; Flotow, P.; Baars, E.W. Do NHS GP Surgeries Employing GPs Additionally Trained in Integrative or Complementary Medicine have Lower Antibiotic Prescribing Rates? Retrospective Cross-Sectional Analysis of National Primary Care Prescribing Data in England in 2016. BMJ Open 2018, 8, e020488. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Bégaud, B.; Rossignol, M.; Avouac, B.; Lert, F.; Rouillon, F.; Bénichou, J.; Massol, J.; Duru, G.; Magnier, A.; et al. Management of Upper Respiratory Tract Infections by Different Medical Practices, Including Homeopathy, and Consumption of Antibiotics in Primary Care: The EPI3 Cohort Study in France 2007–2008. PLoS ONE 2014, 9, e89990. [Google Scholar] [CrossRef]
- Hamre, H.J.; Glockmann, A.; Schwarz, R.; Riley, D.S.; Baars, E.W.; Kiene, H.; Kienle, G.S. Antibiotic use in Children with Acute Respiratory or Ear Infections: Prospective Observational Comparison of Anthroposophic and Conventional Treatment Under Routine Primary Care Conditions. Evid. Based Complement. Altern. Med. 2014, 2014, 243801. [Google Scholar] [CrossRef]
- Baars, E.W.; Zoen, E.B.; Breitkreuz, T.; Martin, D.; Matthes, H.; Schoen-Angerer, T.v.; Soldner, G.; Vagedes, J.; Wietmarschen, H.v.; Patijn, O.; et al. The Contribution of Complementary and Alternative Medicine to Reduce Antibiotic use: A Narrative Review of Health Concepts, Prevention, and Treatment Strategies. Evid. Based Complement. Altern. Med. 2019, 2019, 5365608. [Google Scholar] [CrossRef]
- Dantzer, R.; Cohen, S.; Russo, S.J.; Dinan, T.G. Resilience and Immunity. Brain Behav. Immun. 2018, 74, 28–42. [Google Scholar] [CrossRef]
- Baars, E.W.; Belt-Van Zoen, E.; Hu, X.Y.; Lai, L.; Willcox, M.; Huber, R.; Roberts, N.; Huntley, A.; van Wietmarschen, H.; van der Werf, E.T. Can Complementary and Alternative Medicine Treatment Strategies Control Symptoms of Uncomplicated Acute RTIs and Reduce Antibiotic use? A Systematic Review of Systematic Reviews Examining Observational Studies and Clinical Trials. Louis Bolk Institute, Bunnik, The Netherlands. 2023, in preparation.
- Anheyer, D.; Cramer, H.; Lauche, R.; Saha, F.J.; Dobos, G. Children with Respiratory Tract Infection: Systematic Review and Meta-Analysis. Acad. Pediatr. 2018, 18, 8–19. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.; Moore, M.; Shepherd, J.; et al. Andrographis Paniculata (Chuān Xīn Lián) for Symptomatic Relief of Acute Respiratory Tract Infections in Adults and Children: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0181780. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Guo, R.; Ernst, E. Pelargonium Sidoides for Acute Bronchitis: A Systematic Review and Meta-Analysis. Phytomedicine 2008, 15, 378–385. [Google Scholar] [CrossRef]
- Kamin, W.; Funk, P.; Seifert, G.; Zimmermann, A.; Lehmacher, W. EPs 7630 is Effective and Safe in Children under 6 Years with Acute Respiratory Tract Infections: Clinical Studies Revisited. Curr. Med. Res. Opin. 2018, 34, 475–485. [Google Scholar] [CrossRef]
- Wagner, L.; Cramer, H.; Klose, P.; Lauche, R.; Gass, F.; Dobos, G.; Langhorst, J. Herbal Medicine for Cough: A Systematic Review and Meta-Analysis. Complement. Med. Res. 2015, 22, 359–368. [Google Scholar] [CrossRef]
- Timmer, A.; Günther, J.; Motschall, E.; Rücker, G.; Antes, G.; Kern, W.V.; Timmer, A. Pelargonium Sidoides Extract for Treating Acute Respiratory Tract Infections. Cochrane Database Syst. Rev. 2013, 2013, CD006323. [Google Scholar] [CrossRef]
- Holzinger, F.; Chenot, J. Systematic Review of Clinical Trials Assessing the Effectiveness of Ivy Leaf (Hedera Helix) for Acute Upper Respiratory Tract Infections. Evid. Based Complement. Altern. Med. 2011, 2011, 382789. [Google Scholar] [CrossRef]
- Schaefer, A.; Kehr, M.S.; Giannetti, B.M.; Bulitta, M.; Staiger, C. A Randomized, Controlled, Double-Blind, Multi-Center Trial to Evaluate the Efficacy and Safety of a Liquid Containing Ivy Leaves Dry Extract (EA 575®) vs. Placebo in the Treatment of Adults with Acute Cough. Pharmazie 2016, 71, 504–509. [Google Scholar]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis Paniculata (Burm.F.) Nees: Traditional Uses, Phytochemistry, Pharmacological Properties and Quality Control/Quality Assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Zaini Asmawi, M.; Sadikun, A. Bitter Plant with a Sweet Future? A Comprehensive Review of an Oriental Medicinal Plant: Paniculata. Phytochem. Rev. 2012, 11, 39–75. [Google Scholar]
- Moyo, M.; Van Staden, J. Medicinal Properties and Conservation of Pelargonium Sidoides DC. J. Ethnopharmacol. 2014, 152, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea Biotechnology: Advances, Commercialization and Future Considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Völk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K.; Karsch-Völk, M. Echinacea for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2014, 2014, CD000530. [Google Scholar] [CrossRef]
- Lang, C.; Röttger-Lüer, P.; Staiger, C. A Valuable Option for the Treatment of Respiratory Diseases: Review on the Clinical Evidence of the Ivy Leaves Dry Extract EA 575. Planta Med. 2015, 81, 968–974. [Google Scholar] [CrossRef]
- Colombo, P.S.; Flamini, G.; Rodondi, G.; Giuliani, C.; Santagostini, L.; Fico, G. Phytochemistry of European Primula Species. Phytochemistry 2017, 143, 132–144. [Google Scholar] [CrossRef]
- Jaric, S.; Pavlovic, P.; Mitrovic, M. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Rajalakshmi, V.; Cathrine, L. Phytochemical Screening and Antimicrobial Activity of Ethanolic Extract of Andrographis paniculata. J. Pharmacogn. Phytochem. 2016, 5, 175–177. [Google Scholar]
- Mishra, P.K.; Singh, R.K.; Gupta, A.; Chaturvedi, A.; Pandey, R.; Tiwari, S.P.; Mohapatra, T.M. Antibacterial Activity of Andrographis Paniculata (Burm. F.) Wall Ex Nees Leaves Against Clinical Pathogens. J. Pharm. Res. 2013, 7, 459–462. [Google Scholar] [CrossRef]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis Paniculata (Burm. F.) Wall. Ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Vasantha, S. Antibacterial Activity of Ethanolic Leaf Extract of Andrographis Paniculata Nees (Acanthaceae) and its Bioactive Compound Andrographolide. Drug Invent. Today 2010, 2, 440–442. [Google Scholar]
- Xu, C.; Chou, G.; Wang, C.; Wang, Z. Rare Noriridoids from the Roots of Andrographis paniculata. Phytochemistry 2012, 77, 275–279. [Google Scholar] [CrossRef]
- Chen, J.; Xue, H.; Ye, W.; Fang, B.; Liu, Y.; Yuan, S.; Yu, P.; Wang, Y. Activity of Andrographolide and its Derivatives against Influenza Virus in Vivo and in Vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis Paniculata (Burm.F.) Nees and its Major Constituent Andrographolide as Potential Antiviral Agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Ajaya Kumar, R.; Sridevi, K.; Vijaya Kumar, N.; Nanduri, S.; Rajagopal, S. Anticancer and Immunostimulatory Compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Ge, B. Andrograpanin, Isolated from Andrographis Paniculata, Exhibits Anti-Inflammatory Property in Lipopolysaccharide-Induced Macrophage Cells through Down-Regulating the p38 MAPKs Signaling Pathways. Int. Immunopharmacol. 2008, 8, 951–958. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Dong, S.; Liu, C.; Italiani, P.; Sun, S.; Xu, J.; Boraschi, D.; Ma, S.; Qu, D. Immunomodulatory Activity of Andrographolide on Macrophage Activation and Specific Antibody Response. Acta Pharmacol. Sin. 2010, 31, 191–201. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Thiyagarajan, P.; Deepak, H.B.; Agarwal, A. In Vitro Modulation of LPS/Calcimycin Induced Inflammatory and Allergic Mediators by Pure Compounds of Andrographis Paniculata (King of Bitters) Extract. Int. Immunopharmacol. 2011, 11, 79–84. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Gupta, A.; Agarwal, A. Effect of an Extract of Andrographis Paniculata Leaves on Inflammatory and Allergic Mediators in Vitro. J. Ethnopharmacol. 2010, 129, 203–207. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Suthisisang, C.; Dhepakson, P.; Herunsalee, A. Study of Anti-Inflammatory Activities of the Pure Compounds from Andrographis Paniculata (Burm.F.) Nees and their Effects on Gene Expression. Int. Immunopharmacol. 2010, 10, 1361–1373. [Google Scholar]
- Sheeja, K.; Shihab, P.K.; Kuttan, G. Antioxidant and Anti-Inflammatory Activities of the Plant Andrographis Paniculata Nees. Immunopharmacol. Immunotoxicol. 2006, 28, 129–140. [Google Scholar] [CrossRef]
- Okhuarobo, A.; Ehizogie Falodun, J.; Erharuyi, O.; Imieje, V.; Falodun, A.; Langer, P. Harnessing the Medicinal Properties of Andrographis Paniculata for Diseases and Beyond: A Review of its Phytochemistry and Pharmacology. Asian Pac. J. Trop. Dis. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Conrad, A.; Jung, I.; Tioua, D.; Lallemand, C.; Carrapatoso, F.; Engels, I.; Daschner, F.D.; Frank, U. Extract of Pelargonium Sidoides (EPs ® 7630) Inhibits the Interactions of Group A-Streptococci and Host Epithelia in Vitro. Phytomedicine 2007, 14, 52–59. [Google Scholar] [CrossRef]
- Janecki, A.; Conrad, A.; Engels, I.; Frank, U.; Kolodziej, H. Evaluation of an Aqueous-Ethanolic Extract from Pelargonium Sidoides (EPs ® 7630) for its Activity against Group A-Streptococci Adhesion to Human HEp-2 Epithelial Cells. J. Ethnopharmacol. 2011, 133, 147–152. [Google Scholar] [CrossRef]
- Walther, C.; Döring, K.; Schmidtke, M. Comparative in Vitro Analysis of Inhibition of Rhinovirus and Influenza Virus Replication by Mucoactive Secretolytic Agents and Plant Extracts. BMC Complement. Altern. Med. 2020, 20, 380. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M.; et al. Botanical Drugs and Supplements Affecting the Immune Response in the Time of COVID-19: Implications for Research and Clinical Practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Roth, M.; Fang, L.; Stolz, D.; Tamm, M. Pelargonium Sidoides Radix Extract EPs 7630 Reduces Rhinovirus Infection through Modulation of Viral Binding Proteins on Human Bronchial Epithelial Cells. PLoS ONE 2019, 14, e0210702. [Google Scholar] [CrossRef]
- Roth, M.; Sun, Q.; Tamm, M. Up-Regulated Vitamin D Receptor by Pelargonium Sidoides Extract EPs® 7630 Contributes to Rhinovirus Defense in Bronchial Epithelial Cells. Pharmaceuticals 2021, 14, 172. [Google Scholar] [CrossRef]
- Bao, Y.; Gao, Y.; Koch, E.; Pan, X.; Jin, Y.; Cui, X. Evaluation of Pharmacodynamic Activities of EPs® 7630, a Special Extract from Roots of Pelargonium Sidoides, in Animals Models of Cough, Secretolytic Activity and Acute Bronchitis. Phytomedicine 2015, 22, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Nöldner, M.; Koch, E. Inhibition of Endotoxin-induced Sickness Behaviour in Mice by an Extract from Roots of Pelargonium Sidoides (Umckaloabo). Focus Altern. Complement. Ther. 2004, 9, 20. [Google Scholar] [CrossRef]
- Noldner, M.; Schotz, K. Inhibition of Lipopolysaccharid-Induced Sickness Behavior by a Dry Extract from the Roots of Pelargonium Sidoides (EPs ® 7630) in Mice. Phytomedicine 2007, 14, 27–31. [Google Scholar] [CrossRef]
- Neugebauer, P.; Mickenhagen, A.; Siefer, O.; Walger, M. A New Approach to Pharmacological Effects on Ciliary Beat Frequency in Cell Cultures—Exemplary Measurements under Pelargonium Sidoides Extract (EPs 7630). Phytomedicine 2005, 12, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Hansmann, C.; Engels, I.; Daschner, F.D.; Frank, U. Extract of Pelargonium Sidoides (EPs ® 7630) Improves Phagocytosis, Oxidative Burst, and Intracellular Killing of Human Peripheral Blood Phagocytes in Vitro. Phytomedicine 2007, 14, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H.; Kiderlen, A.F. Immunomodulatory Principles of Pelargonium Sidoides. Phytother. Res. 2001, 15, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Thale, C.; Kiderlen, A.; Kolodziej, H. Anti-Infective Mode of Action of EPs 7630 at the Molecular Level. Planta Med. 2008, 74, 675–681. [Google Scholar] [CrossRef]
- Witte, K.; Koch, E.; Volk, H.; Wolk, K.; Sabat, R. The Herbal Extract EPs® 7630 Increases the Antimicrobial Airway Defense through Monocyte-Dependent Induction of IL-22 in T Cells. J. Mol. Med. 2020, 98, 1493–1503. [Google Scholar] [CrossRef]
- Koch, E.; Wohn, C. Pelargonium Sidoides Root Extract EPs® 7630 Stimulates Release of Antimicrobial Peptides from Neutrophil Granulocytes in Human Whole Blood. Planta Med. 2007, 73, P_072. [Google Scholar] [CrossRef]
- Witte, K.; Koch, E.; Volk, H.; Wolk, K.; Sabat, R. The Pelargonium Sidoides Extract EPs 7630 Drives the Innate Immune Defense by Activating Selected MAP Kinase Pathways in Human Monocytes. PLoS ONE 2015, 10, e0138075. [Google Scholar] [CrossRef]
- Trun, W.; Kiderlen, A.F.; Kolodziej, H. Nitric Oxide Synthase and Cytokines Gene Expression Analyses in Leishmania-Infected RAW 264.7 Cells Treated with an Extract of Pelargonium Sidoides (Eps ® 7630). Phytomedicine 2006, 13, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Perić, A.; Vezmar Kovačević, S.; Barać, A.; Perić, A.V.; Vojvodić, D. Effects of Pelargonium Sidoides Extract vs. Roxithromycin on Chemokine Levels in Nasal Secretions of Patients with Uncomplicated Acute Rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2021, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Perić, A.; Vezmar Kovačević, S.; Barać, A.; Gaćeša, D.; Perić, A.V.; Vojvodić, D. Effects of Pelargonium Sidoides Extract on Chemokine Levels in Nasal Secretions of Patients with Non-Purulent Acute Rhinosinusitis. J. Drug Assess. 2020, 9, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium Sidoides (EPs® 7630) in the Context of Health Promotion. Pharmaceuticals 2011, 4, 1295–1314. [Google Scholar] [CrossRef]
- Brendler, T.; van Wyk, B.E. A Historical, Scientific and Commercial Perspective on the Medicinal use of Pelargonium Sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. In Vitro Evaluation of Antibacterial and Immunomodulatory Activities of Pelargonium Reniforme, Pelargonium Sidoides and the Related Herbal Drug Preparation EPs ® 7630. Phytomedicine 2007, 14, 18–26. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of Influenza Virus Induced Bacterial Superinfection by Standardized Echinacea Purpurea, via Regulation of Surface Receptor Expression in Human Bronchial Epithelial Cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef]
- Hudson, J.; Vimalanathan, S. Echinacea—A Source of Potent Antivirals for Respiratory Virus Infections. Pharmaceuticals 2011, 4, 1019–1031. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea Species (Echinacea Angustifolia (DC.) Hell., Echinacea Pallida (Nutt.) Nutt., Echinacea Purpurea (L.) Moench): A Review of their Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Barrett, B. Medicinal Properties of Echinacea: A Critical Review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef]
- Basch, E.; Ulbricht, C.; Basch, S.; Dalton, S.; Ernst, E.; Foppa, I.; Szapary, P.; Tiffany, N.; Orlando, C.W.; Vora, M. An Evidence-Based Systematic Review of Echinacea (E. Angustifolia DC, E. Pallida, E. Purpurea) by the Natural Standard Research Collaboration. J. Herb. Pharmacother. 2005, 5, 57–88. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.S.; Priyadarshoni, S.P. Echinacea Purpurea—A Potent Medicinal Herb. Drug Invent. Today 2019, 11, 448–452. [Google Scholar]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds—Practical Advice on Dosages and on the Time to Take these Nutrients/Botanicals in Order to Prevent or Treat Common Colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- Borchers, A.T.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Inflammation and Native American Medicine: The Role of Botanicals1,2,3. Am. J. Clin. Nutr. 2000, 72, 339–347. [Google Scholar] [CrossRef]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-Viral Properties and Mode of Action of Standardized Echinacea Purpurea Extract against Highly Pathogenic Avian Influenza Virus (H5N1, H7N7) and Swine-Origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef]
- Roxas, M.; Jurenka, J. Colds and influenza: A review of diagnosis and conventional, botanical, and nutritional considerations. Altern. Med. Rev. J. Clin. Ther. 2007, 12, 25–48. [Google Scholar]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea Purpurea (L.) Moench Modulates Human T-Cell Cytokine Response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef]
- Goel, V.; Lovlin, R.; Chang, C.; Slama, J.V.; Barton, R.; Gahler, R.; Bauer, R.; Goonewardene, L.; Basu, T.K. Proprietary Extract from the Echinacea Plant (Echinacea Purpurea) Enhances Systemic Immune Response during a Common Cold. Phytother. Res. 2005, 19, 689–694. [Google Scholar] [CrossRef]
- Dapas, B.; Dall’Acqua, S.; Bulla, R.; Agostinis, C.; Perissutti, B.; Invernizzi, S.; Grassi, G.; Voinovich, D. Immunomodulation Mediated by a Herbal Syrup Containing a Standardized Echinacea Root Extract: A Pilot Study in Healthy Human Subjects on Cytokine Gene Expression. Phytomedicine 2014, 21, 1406–1410. [Google Scholar] [CrossRef]
- Ritchie, M.R.; Gertsch, J.; Klein, P.; Schoop, R. Effects of Echinaforce® Treatment on Ex Vivo-Stimulated Blood Cells. Phytomedicine 2011, 18, 826–831. [Google Scholar] [CrossRef]
- Kim, L.S.; Waters, R.F.; Burkholder, P.M. Immunological Activity of Larch Arabinogalactan and Echinacea: A Preliminary, Randomized, Double-Blind, Placebo-Controlled Trial. Altern. Med. Rev. 2002, 7, 138–149. [Google Scholar]
- Gertsch, J.; Schoop, R.; Kuenzle, U.; Suter, A. Echinacea Alkylamides Modulate TNF-A Gene Expression via Cannabinoid Receptor CB2 and Multiple Signal Transduction Pathways. FEBS Lett. 2004, 577, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Classen, B.; Thude, S.; Blaschek, W.; Wack, M.; Bodinet, C. Immunomodulatory Effects of Arabinogalactan-Proteins from Baptisia and Echinacea. Phytomedicine 2006, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Carter, R. Use of Echinacea in Upper Respiratory Tract Infection. South. Med. J. 2005, 98, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Hoşbaş, S.; Vural, M. Assessment of Antioxidant, Antibacterial, Antimycobacterial, and Antifungal Activities of some Plants used as Folk Remedies in Turkey against Dermatophytes and Yeast-Like Fungi. Turk. J. Biol. 2012, 36, 672–686. [Google Scholar] [CrossRef]
- Ünsal, C.; Vural, H.; Sariyar, G.; Özbek, B.; Ötük, G. Traditional Medicine in Bilecik Province (Turkey) and Antimicrobial Activities of Selected Species. Turk. J. Pharm. Sci. 2010, 7, 139–150. [Google Scholar]
- Schulte-Michels, J.; Runkel, F.; Gokorsch, S.; Häberlein, H. Ivy Leaves Dry Extract EA 575® Decreases LPS-Induced IL-6 Release from Murine Macrophages. Pharmazie 2016, 71, 158–161. [Google Scholar]
- Schulte-Michels, J.; Keksel, C.; Häberlein, H.; Franken, S. Anti-Inflammatory Effects of Ivy Leaves Dry Extract: Influence on Transcriptional Activity of NFκB. Inflammopharmacology 2019, 27, 339–347. [Google Scholar] [CrossRef]
- Saadat, S.; Mohammadi, M.; Fallahi, M.; Keyhanmanesh, R.; Aslani, M.R. The Protective Effect of A-Hederin, the Active Constituent of Nigella Sativa, on Tracheal Responsiveness and Lung Inflammation in Ovalbumin-Sensitized Guinea Pigs. J. Physiol. Sci. 2015, 65, 285–292. [Google Scholar] [CrossRef]
- Keyhanmanesh, R.; Saadat, S.; Mohammadi, M.; Shahbazfar, A.; Fallahi, M. Protective Effect of α-Hederin, the Active Constituent of Nigella Sativa, on Lung Inflammation and Blood Cytokines in Ovalbumin Sensitized Guinea Pigs. Phytother. Res. 2015, 29, 1761–1767. [Google Scholar] [CrossRef]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Kivcak, B.; Bagriyanik, H.A.; Uzuner, N. Effect of Hedera Helix on Lung Histopathology in Chronic Asthma. Iran. J. Allergy Asthma Immunol. 2012, 11, 316–323. [Google Scholar] [PubMed]
- Wolf, A.; Gosens, R.; Meurs, H.; Häberlein, H. Pre-Treatment with A-Hederin Increases Β-Adrenoceptor Mediated Relaxation of Airway Smooth Muscle. Phytomedicine 2011, 18, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Greunke, C.; Hage-Hülsmann, A.; Sorkalla, T.; Keksel, N.; Häberlein, F.; Häberlein, H. A Systematic Study on the Influence of the Main Ingredients of an Ivy Leaves Dry Extract on the Β2 -Adrenergic Responsiveness of Human Airway Smooth Muscle Cells. Pulm. Pharmacol. Ther. 2015, 31, 92–98. [Google Scholar] [CrossRef][Green Version]
- Sieben, A.; Prenner, L.; Sorkalla, T.; Wolf, A.; Jakobs, D.; Runkel, F.; Häberlein, H. A-Hederin, but Not Hederacoside C and Hederagenin from Hedera Helix, Affects the Binding Behavior, Dynamics, and Regulation of Β 2-Adrenergic Receptors. Biochemistry 2009, 48, 3477–3482. [Google Scholar] [CrossRef]
- Runkel, F.; Prenner, L.; Häberlein, H. Ein Beitrag Zum Wirkmechanismus Von Efeu. Pharm. Ztg. 2005, 150, 19–25. [Google Scholar]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute Anti-Inflammatory Activity of Four Saponins Isolated from Ivy: Alpha-Hederin, Hederasaponin-C, Hederacolchiside-E and Hederacolchiside-F in Carrageenan-Induced Rat Paw Edema. Phytomedicine 2005, 12, 440–444. [Google Scholar] [CrossRef]
- Javed, H.; Tabassum, S.; Erum, S.; Murtaza, I.; Muhammad, A.; Amin, F.; Nisar, M.F. Screening and Characterization of Selected Drugs having Antibacterial Potential. Pak. J. Pharm. Sci. 2018, 31, 933–939. [Google Scholar]
- Dahiya, P.; Purkayastha, S. Phytochemical Screening and Antimicrobial Activity of some Medicinal Plants against Multi-Drug Resistant Bacteria from Clinical Isolates. Indian J. Pharm. Sci. 2012, 74, 443–450. [Google Scholar]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An Updated and Comprehensive Review of the Antiviral Potential of Essential Oils and their Chemical Constituents with Special Focus on their Mechanism of Action against various Influenza and Coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Fabio, A.; Cermelli, C.; Fabio, G.; Nicoletti, P.; Quaglio, P. Screening of the Antibacterial Effects of a Variety of Essential Oils on Microorganisms Responsible for Respiratory Infections. Phytother. Res. 2007, 21, 374–377. [Google Scholar] [CrossRef]
- Ács, K.; Bencsik, T.; Böszörményi, A.; Kocsis, B.; Horváth, G. Essential Oils and their Vapors as Potential Antibacterial Agents Against Respiratory Tract Pathogens. Nat. Prod. Commun. 2016, 11, 1709–1712. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary Study on the Antibacterial Activity of Essential Oils Alone and in Combination with Gentamicin Against Extended-Spectrum Β-Lactamase-Producing and New Delhi Metallo-Β-Lactamase-1-Producing Klebsiella Pneumoniae Isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Conde-Pérez, K.; Arca-Suárez, J.; Álvarez-Fraga, L.; Pérez, A.; Crecente-Campo, J.; Alonso, M.J.; Bou, G.; et al. Syzygium Aromaticum (Clove) and Thymus Zygis (Thyme) Essential Oils Increase Susceptibility to Colistin in the Nosocomial Pathogens Acinetobacter Baumannii and Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 130, 110606. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Synergistic Antibacterial Activity between Thymus Vulgaris and Pimpinella Anisum Essential Oils and Methanol Extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Ács, K.; Balázs, V.L.; Kocsis, B.; Bencsik, T.; Böszörményi, A.; Horváth, G. Antibacterial Activity Evaluation of Selected Essential Oils in Liquid and Vapor Phase on Respiratory Tract Pathogens. BMC Complement. Altern. Med. 2018, 18, 227. [Google Scholar] [CrossRef]
- Hoferl, M.; Buchbauer, G.; Jirovetz, L.; Schmidt, E.; Stoyanova, A.; Denkova, Z.; Slavchev, A.; Geissler, M. Correlation of Antimicrobial Activities of various Essential Oils and their Main Aromatic Volatile Constituents. J. Essent. Oil Res. 2009, 21, 459–463. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Khalil, M.S.; Azmy, M. In vitro Efficiency of Ampicillin, Thymol and Their Combinations against Virulence Strains of Klebsiella pneumoniae August 2019. Int. J. Pharm. Sci. 2019, 11, 315–321. [Google Scholar] [CrossRef]
- Lelešius, R.; Karpovaitė, A.; Mickienė, R.; Drevinskas, T.; Tiso, N.; Ragažinskienė, O.; Kubilienė, L.; Maruška, A.; Šalomskas, A. In Vitro Antiviral Activity of Fifteen Plant Extracts against Avian Infectious Bronchitis Virus. BMC Vet. Res. 2019, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Csikós, E.; Csekő, K.; Ashraf, A.R.; Kemény, Á.; Kereskai, L.; Kocsis, B.; Böszörményi, A.; Helyes, Z.; Horváth, G. Effects of Thymus Vulgaris, L., Cinnamomum Verum, J.Presl and Cymbopogon Nardus (L.) Rendle Essential Oils in the Endotoxin-Induced Acute Airway Inflammation Mouse Model. Molecules 2020, 25, 3553. [Google Scholar] [CrossRef]
- Seibel, J.; Kryshen, K.; Pongrácz, J.E.; Lehner, M.D. In Vivo and in Vitro Investigation of Anti-Inflammatory and Mucus-Regulatory Activities of a Fixed Combination of Thyme and Primula Extracts. Pulm. Pharmacol. Ther. 2018, 51, 10–17. [Google Scholar] [CrossRef]
- Seibel, J.; Wonnemann, M.; Werz, O.; Lehner, M.D. A Tiered Approach to Investigate the Mechanism of Anti-Inflammatory Activity of an Herbal Medicinal Product Containing a Fixed Combination of Thyme Herb and Primula Root Extracts. Clin. Phytosci. 2018, 4, 4. [Google Scholar] [CrossRef]
- Seibel, J.; Pergola, C.; Werz, O.; Kryshen, K.; Wosikowski, K.; Lehner, M.D.; Haunschild, J. Bronchipret® Syrup Containing Thyme and Ivy Extracts Suppresses Bronchoalveolar Inflammation and Goblet Cell Hyperplasia in Experimental Bronchoalveolitis. Phytomedicine 2015, 22, 1172–1177. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. Antimicrobial Activity of Four Commercial Essential Oils in Combination with Conventional Antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Oldfield, E.; Feng, X. Resistance-Resistant Antibiotics. Trends Pharmacol. Sci. 2014, 35, 664–674. [Google Scholar] [CrossRef]
- Sackett, D.L. Evidence Based Medicine, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 2000. [Google Scholar]
| Antibacterial | Antiviral | Immunomodulatory | Other |
|---|---|---|---|
| Direct effects against S. aureus, E. coli, S. typhimurium, B. subtilis, E. faecalis, K. pneumoniae, S. pneumoniae, P. vulgaris [24,33,34,35,36,37] | Against avian influenza A virus (H9N2, H5N1 and H1N1) [38,39] | Increased proliferation of white blood cells [40] | Antipyretic [24] |
| Increased antibiotic sensitivity and reduced biofilm formation [35] | Reduced inflammatory response macrophages [41,42,43,44] | ||
| Reduced bacterial adhesion to epithelium [35] | Anti-inflammatory [45,46] |
| Antibacterial | Antiviral | Immunomodulatory | Other |
|---|---|---|---|
| Reduced bacterial adhesion to epithelial cells [48,49] | Against influenza A virus (H1N1 and H3N2), respiratory syncytial virus (HCo-229E), parainfluenza virus type 3 and coxsackievirus A9 [50,51,52,53] | Reduced inflammatory damage [50,53,54,55,56] | Expectorant [54,57] |
| Increased bacterial adhesion to buccal cells [48] | Downregulation of docking proteins infected bronchial epithelial cells [51,52] | Increased macrophage functioning [58,59,60] | Antitussive [54] |
| Increased production of antimicrobial peptides [53,61,62] | Prevention of hemagglutination in human erythrocytes [50] | Increased inflammatory cytokines [60,61,63,64,65,66] | |
| Upregulation of vitamin D receptor on human epithelial cells [53] | Increased production of antimicrobial peptides [53,61,62] |
| Antibacterial | Antiviral | Immunomodulatory | Other |
|---|---|---|---|
| Reduced adhesion of S. aureus and H. influenzae to epithelial cells [70] | Against influenza virus A (both human and avian), influenza virus B, herpes simplex virus 1 and 2, respiratory syncytial virus and rhinoviruses [71] | Immunostimulatory effects:
| Expectorant [71] |
| Increased presentation of viral antigens by infected cells [73] | Anti-inflammatory effects:Inhibition of COX-1, COX-2 and 5-LOX [72,73,76] | ||
| Decreased viral binding [71,78] | |||
| Increased antibody-dependent and innate NK-mediated activity [73] |
| Antibacterial | Antiviral | Immunomodulatory | Other |
|---|---|---|---|
| Direct effects against S. pneumonia, S. pyogenes, S. aureus, S. epidermidis, M. tuberculosis, M. avium, H. influenzae, and A. baumannii [88,89] | No antiviral effects reported. | Anti-inflammatory effects [90,91,92,93] Reduced pathological changes to lung tissue [93,94] | Bronchospasmolytic and secretolytic [95,96,97,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldman, L.B.M.; Belt-Van Zoen, E.; Baars, E.W. Mechanistic Evidence of Andrographis paniculata (Burm. f.) Wall. ex Nees, Pelargonium sidoides DC., Echinacea Species and a Combination of Hedera helix L., Primula veris L./Primula elatior L. and Thymus vulgaris L./Thymus zygis L. in the Treatment of Acute, Uncomplicated Respiratory Tract Infections: A Systematic Literature Review and Expert Interviews. Pharmaceuticals 2023, 16, 1206. https://doi.org/10.3390/ph16091206
Veldman LBM, Belt-Van Zoen E, Baars EW. Mechanistic Evidence of Andrographis paniculata (Burm. f.) Wall. ex Nees, Pelargonium sidoides DC., Echinacea Species and a Combination of Hedera helix L., Primula veris L./Primula elatior L. and Thymus vulgaris L./Thymus zygis L. in the Treatment of Acute, Uncomplicated Respiratory Tract Infections: A Systematic Literature Review and Expert Interviews. Pharmaceuticals. 2023; 16(9):1206. https://doi.org/10.3390/ph16091206
Chicago/Turabian StyleVeldman, Liesbeth B. M., Eefje Belt-Van Zoen, and Erik W. Baars. 2023. "Mechanistic Evidence of Andrographis paniculata (Burm. f.) Wall. ex Nees, Pelargonium sidoides DC., Echinacea Species and a Combination of Hedera helix L., Primula veris L./Primula elatior L. and Thymus vulgaris L./Thymus zygis L. in the Treatment of Acute, Uncomplicated Respiratory Tract Infections: A Systematic Literature Review and Expert Interviews" Pharmaceuticals 16, no. 9: 1206. https://doi.org/10.3390/ph16091206
APA StyleVeldman, L. B. M., Belt-Van Zoen, E., & Baars, E. W. (2023). Mechanistic Evidence of Andrographis paniculata (Burm. f.) Wall. ex Nees, Pelargonium sidoides DC., Echinacea Species and a Combination of Hedera helix L., Primula veris L./Primula elatior L. and Thymus vulgaris L./Thymus zygis L. in the Treatment of Acute, Uncomplicated Respiratory Tract Infections: A Systematic Literature Review and Expert Interviews. Pharmaceuticals, 16(9), 1206. https://doi.org/10.3390/ph16091206








