Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges
Abstract
:1. Introduction
2. Results
2.1. The Synthetic TRβ Ligand TG68 Efficiently Induces OPC Differentiation from NSCs
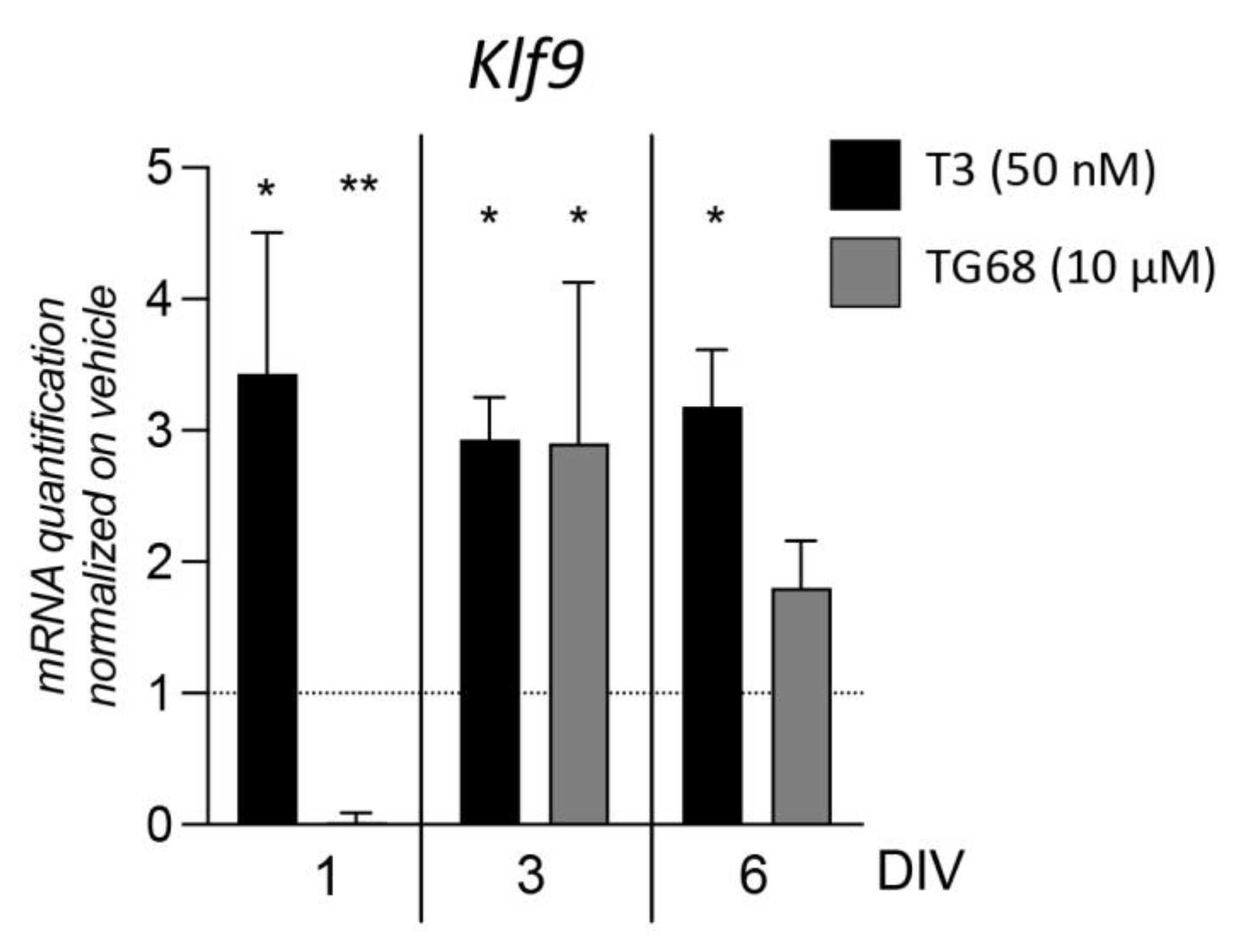
2.2. TRβ Downstream Signaling by TG68 and Other TR Ligands
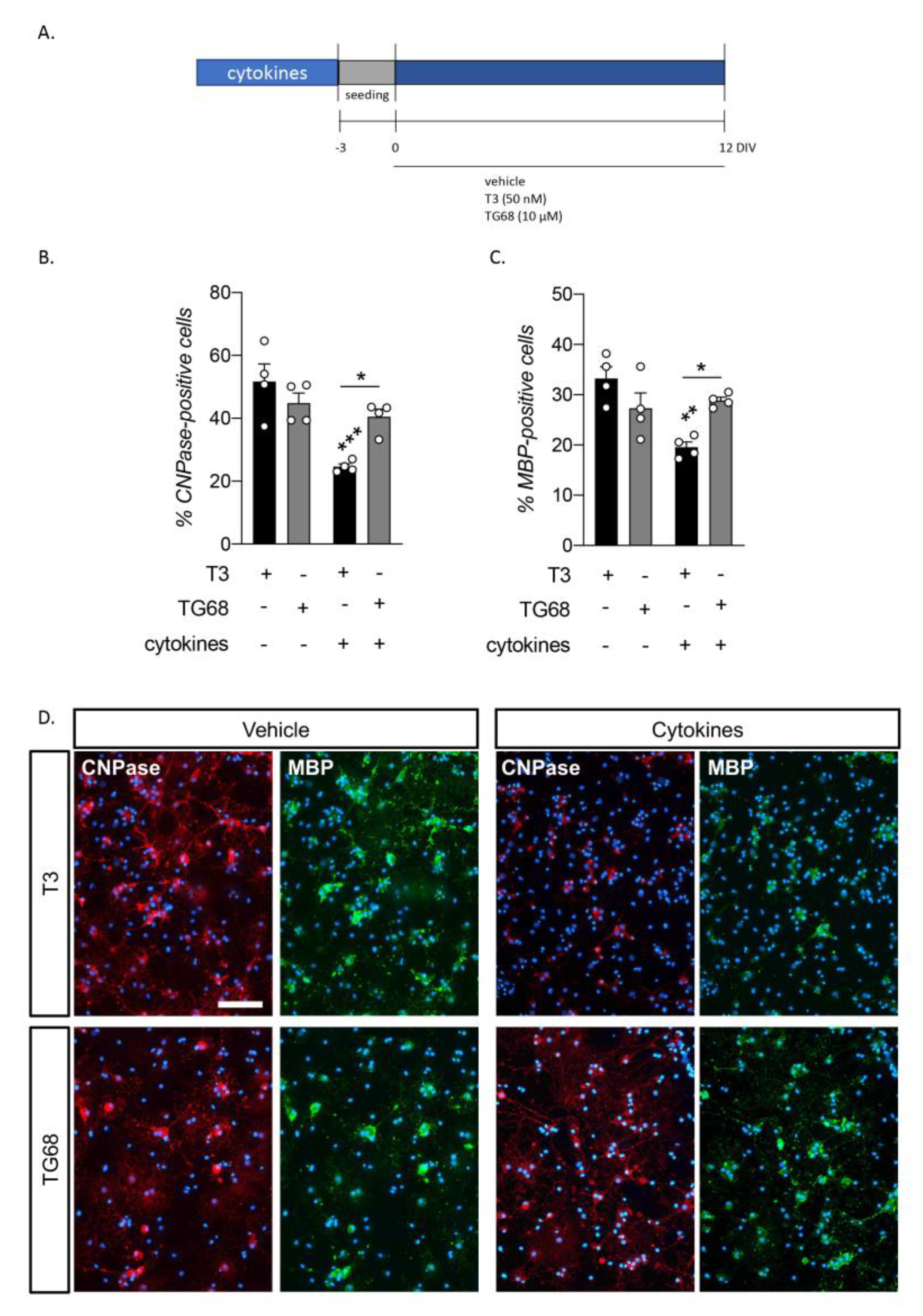
2.3. The Synthetic TRβ Ligand TG68 Reverts Cytokine-Induced OPC Differentiation Impairment More Efficiently Than T3
3. Discussion
3.1. TRβ1 Agonists and OPC Differentiation
3.2. Implications for CNS Lesion Repair and Neurodegenerative Diseases
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Cytokine Mix Exposure
4.3. RNA Extraction and Gene Expression Analysis
4.4. Immunocytochemistry and Image Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef] [PubMed]
- Bercury, K.K.; Macklin, W.B. Dynamics and mechanisms of CNS myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Young, K.M. White matter plasticity in adulthood. Neuroscience 2014, 276, 148–160. [Google Scholar] [CrossRef]
- Bando, Y. Mechanism of demyelination and remyelination in multiple sclerosis. Clin. Exp. Neuroimmunol. 2020, 11, 14–21. [Google Scholar] [CrossRef]
- Gruchot, J.; Weyers, V.; Göttle, P.; Förster, M.; Hartung, H.-P.; Küry, P.; Kremer, D. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells 2019, 8, 825. [Google Scholar] [CrossRef]
- Fancy, S.P.; Kotter, M.R.; Harrington, E.P.; Huang, J.K.; Zhao, C.; Rowitch, D.H.; Franklin, R.J. Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp. Neurol. 2010, 225, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Mi, S. Dissecting demyelination. Nat. Neurosci. 2007, 10, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Miron, V.; Cuo, Q.; Wegner, C.; Antel, J.; Bruck, W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Wu, D.; Xu, Y. ROR nuclear receptors: Structures, related diseases, and drug discovery. Acta Pharmacol. Sin. 2015, 36, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Aktas, O.; Hartung, H.P.; Küry, P. The complex world of oligodendroglial differentiation inhibitors. Ann. Neurol. 2011, 69, 602–618. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Morrison, V.E.; Gross, P.S.; Huang, J.K. Extrinsic Factors Driving Oligodendrocyte Lineage Cell Progression in CNS Development and Injury. Neurochem. Res. 2020, 45, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Flagelli, A.; Sannia, M.; Calzà, L. Nuclear receptors and differentiation of oligodendrocyte precursor cells. In Vitamins and Hormones; Academic Press Inc.: Cambridge, MA, USA, 2021; pp. 389–407. [Google Scholar]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Hughes, E.G.; Stockton, M.E. Premyelinating Oligodendrocytes: Mechanisms Underlying Cell Survival and Integration. Front. Cell Dev. Biol. 2021, 9, 714169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Petratos, S. Thyroid Hormone Signaling in Oligodendrocytes: From Extracellular Transport to Intracellular Signal. Mol. Neurobiol. 2016, 53, 6568–6583. [Google Scholar] [CrossRef]
- Pagnin, M.; Kondos-Devcic, D.; Chincarini, G.; Cumberland, A.; Richardson, S.J.; Tolcos, M. Role of thyroid hormones in normal and abnormal central nervous system myelination in humans and rodents. Front. Neuroendocr. 2021, 61, 100901. [Google Scholar] [CrossRef]
- Emamnejad, R.; Dass, M.; Mahlis, M.; Bozkurt, S.; Ye, S.; Pagnin, M.; Theotokis, P.; Grigoriadis, N.; Petratos, S. Thyroid hormone-dependent oligodendroglial cell lineage genomic and non-genomic signaling through integrin receptors. Front. Pharmacol. 2022, 13, 934971. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.C.; Tai, Y.C.; Speed, T.P.; Ngai, J.; Barres, B.A. Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 2006, 26, 10967–10983. [Google Scholar] [CrossRef] [PubMed]
- Zekri, Y.; Guyot, R.; Flamant, F. An Atlas of Thyroid Hormone Receptors’ Target Genes in Mouse Tissues. Int. J. Mol. Sci. 2022, 23, 11444. [Google Scholar] [CrossRef]
- Grøntved, L.; Waterfall, J.J.; Kim, D.W.; Baek, S.; Sung, M.-H.; Zhao, L.; Park, J.W.; Nielsen, R.; Walker, R.L.; Zhu, Y.J.; et al. Transcriptional activation by the thyroid hormone receptor through ligand-dependent receptor recruitment and chromatin remodelling. Nat. Commun. 2015, 6, 7048. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Fernandez, M.; Giuliani, A.; Aloe, L.; Giardino, L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA 2002, 99, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Mikita, J.; Boiziau, C.; Chassande, O.; Fliers, E.; Petry, K.G. Type 3 deiodinase expression in inflammatory spinal cord lesions in rat experimental autoimmune encephalomyelitis. Thyroid 2009, 19, 1401–1406. [Google Scholar] [CrossRef]
- D’intino, G.; Lorenzini, L.; Fernandez, M.; Taglioni, A.; Perretta, G.; Del Vecchio, G.; Villoslada, P.; Giardino, L.; Calzà, L. Triiodothyronine Administration Ameliorates the Demyelination/Remyelination Ratio in a Non-Human Primate Model of Multiple Sclerosis by Correcting Tissue Hypothyroidism. J. Neuroendocr. 2011, 23, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Caraffi, R.; Lorenzini, L.; Ottonelli, I.; Sannia, M.; Alastra, G.; Baldassarro, V.A.; Giuliani, A.; Duskey, J.T.; Cescatti, M.; et al. “Combo” Multi-Target Pharmacological Therapy and New Formulations to Reduce Inflammation and Improve Endogenous Remyelination in Traumatic Spinal Cord Injury. Cells 2023, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Baldassarro, V.A.; Sivilia, S.; Giardino, L.; Calzà, L. Inflammation severely alters thyroid hormone signaling in the central nervous system during experimental allergic encephalomyelitis in rat: Direct impact on OPCs differentiation failure. Glia 2016, 64, 1573–1589. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Marchesini, A.; Giardino, L.; Calzà, L. Differential effects of glucose deprivation on the survival of fetal versus adult neural stem cells-derived oligodendrocyte precursor cells. Glia 2020, 68, 898–917. [Google Scholar] [CrossRef]
- Baxi, E.G.; Schott, J.T.; Fairchild, A.N.; Kirby, L.A.; Karani, R.; Uapinyoying, P.; Pardo-Villamizar, C.; Rothstein, J.R.; Bergles, D.E.; Calabresi, P.A. A selective thyroid hormone β receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia 2014, 62, 1513–1529. [Google Scholar] [CrossRef]
- Runfola, M.; Sestito, S.; Bellusci, L.; La Pietra, V.; D’Amore, V.M.; Kowalik, M.A.; Rapposelli, S. Design, synthesis and biological evaluation of novel TRβ selective agonists sustained by ADME-toxicity analysis. Eur. J. Med. Chem. 2020, 188, 112006. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef]
- Caddeo, A.; Serra, M.; Sedda, F.; Bacci, A.; Manera, C.; Rapposelli, S.; Columbano, A.; Perra, A.; Kowalik, M.A. Potential use of TG68-A novel thyromimetic-for the treatment of non-alcoholic fatty liver (NAFLD)-associated hepatocarcinogenesis. Front. Oncol. 2023, 13, 1127517. [Google Scholar] [CrossRef]
- Perra, A.; Kowalik, M.A.; Cabras, L.; Runfola, M.; Sestito, S.; Migliore, C.; Giordano, S.; Chiellini, G.; Rapposelli, S.; Columbano, A. Potential role of two novel agonists of thyroid hormone receptor-β on liver regeneration. Cell Prolif. 2020, 53, e12808. [Google Scholar] [CrossRef]
- Baldassarro, V.A. High-content screening differentiation and maturation analysis of fetal and adult neural stem cell-derived oligodendrocyte precursor cell cultures. J. Vis. Exp. 2021, 2021, e61988. [Google Scholar]
- Baldassarro, V.A.; Krężel, W.; Fernández, M.; Schuhbaur, B.; Giardino, L.; Calzà, L. The role of nuclear receptors in the differentiation of oligodendrocyte precursor cells derived from fetal and adult neural stem cells. Stem Cell Res. 2019, 37, 101443. [Google Scholar] [CrossRef]
- Trost, S.U.; Swanson, E.; Gloss, B.; Wang-Iverson, D.B.; Zhang, H.; Volodarsky, T.; Grover, G.J.; Baxter, J.D.; Chiellini, G.; Scanlan, T.S.; et al. The thyroid hormone receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 2000, 141, 3057–3064. [Google Scholar] [CrossRef]
- Dugas, J.C.; Ibrahim, A.; Barres, B.A. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol. Cell Neurosci. 2012, 50, 45–57. [Google Scholar] [CrossRef]
- Long, K.L.P.; Breton, J.M.; Barraza, M.K.; Perloff, O.S.; Kaufer, D. Hormonal regulation of oligodendrogenesis i: Effects across the lifespan. Biomolecules 2021, 11, 283. [Google Scholar] [CrossRef]
- Chang, A.; Tourtellotte, W.W.; Rudick, R.; Trapp, B.D. Premyelinating Oligodendrocytes in Chronic Lesions of Multiple Sclerosis. N. Engl. J. Med. 2002, 346, 165–173. [Google Scholar] [CrossRef]
- Boyd, A.; Zhang, H.; Williams, A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 2013, 3, 841–859. [Google Scholar] [CrossRef]
- Starost, L.; Lindner, M.; Herold, M.; Xu, Y.K.T.; Drexler, H.C.A.; Heß, K.; Ehrlich, M.; Ottoboni, L.; Ruffini, F.; Stehling, M.; et al. Extrinsic immune cell-derive, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 2020, 140, 715–736. [Google Scholar] [CrossRef]
- Makino, A.; Wang, H.; Scott, B.T.; Yuan, J.X.J.; Dillmann, W.H. Thyroid hormone receptor-α and vascular function. Am. J. Physiol.-Cell Physiol. 2012, 302, C1346. [Google Scholar] [CrossRef]
- Lazcano, I.; Hernández-Puga, G.; Robles, J.P.; Orozco, A. Alternative ligands for thyroid hormone receptors. Mol. Cell Endocrinol. 2019, 493, 110448. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, S.; Yan, Y.; Zhang, S.; Liu, S.; Tang, Z.; Yu, J.; Ma, M.; Niu, Z.; Li, Z.; et al. Adipocyte Thyroid Hormone β Receptor–Mediated Hormone Action Fine-tunes Intracellular Glucose and Lipid Metabolism and Systemic Homeostasis. Diabetes 2023, 72, 562–574. [Google Scholar] [CrossRef]
- Grover, G.J.; Mellstrom, K.; Malm, J. Development of the thyroid hormone receptor β-subtype agonist KB-141: A strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovasc. Drug Rev. 2005, 23, 133–148. [Google Scholar] [CrossRef]
- Sarliève, L.L.; Rodríguez-Peña, A.; Langley, K. Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem. Res. 2004, 29, 903–922. [Google Scholar] [CrossRef]
- Barres, B.A.; Lazar, M.A.; Raff, M.C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 1994, 120, 1097–1108. [Google Scholar] [CrossRef]
- Gao, F.B.; Apperly, J.; Raff, M. Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev. Biol. 1998, 197, 54–66. [Google Scholar] [CrossRef]
- Billon, N.; Tokumoto, Y.; Forrest, D.; Raff, M. Role of Thyroid Hormone Receptors in Timing Oligodendrocyte Differentiation. Dev. Biol. 2001, 235, 110–120. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marracci, G.; Calkins, E.; Pocius, E.; Bensen, A.; Scanlan, T.; Emery, B.; Bourdette, D. Thyroid hormone and thyromimetics inhibit myelin and axonal degeneration and oligodendrocyte loss in EAE. J. Neuroimmunol. 2021, 352, 577468. [Google Scholar] [CrossRef]
- Genin, E.C.; Gondcaille, C.; Trompier, D.; Savary, S. Induction of the adrenoleukodystrophy-related gene (ABCD2) by thyromimetics. J. Steroid Biochem. Mol. Biol. 2009, 116, 37–43. [Google Scholar] [CrossRef]
- Peixoto, C.A.; de Oliveira, W.H.; da Racho Araújo, S.M.; Nunes, A.K.S. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol. 2017, 298, 31–41. [Google Scholar] [CrossRef]
- Houshmand, F.; Barati, M.; Golab, F.; Ramezani-Sefidar, S.; Tanbakooie, S.; Tabatabaei, M.; Amiri, M.; Sanadgol, N. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. DARU J. Pharm. Sci. 2019, 27, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Z.; Qin, H.; Yao, Z. Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination. Mol. Neurobiol. 2016, 53, 4406–4416. [Google Scholar] [CrossRef]
- Zucchi, R. Thyroid hormone analogues: An update. Thyroid 2020, 30, 1099–1105. [Google Scholar] [CrossRef]
- Cullen, C.L.; Young, K.M. Can Thyroid Hormone Analogues Be Used to Overcome Hypomyelination and Demyelination of the Central Nervous System? EBioMedicine 2017, 26, 15–16. [Google Scholar] [CrossRef]
- Sjouke, B.; Langslet, G.; Ceska, R.; Nicholls, S.J.; Nissen, S.E.; Öhlander, M.; Ladenson, P.W.; Olsson, A.G.; Hovingh, G.K.; Kastelein, J.J.P. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): A randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2014, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wooliscroft, L.; Altowaijri, G.; Hildebrand, A.; Samuels, M.; Oken, B.; Bourdette, D.; Cameron, M. Phase I randomized trial of liothyronine for remyelination in multiple sclerosis: A dose-ranging study with assessment of reliability of visual outcomes. Mult. Scler. Relat. Disord. 2020, 41, 102015. [Google Scholar] [CrossRef]
- Hartley, M.D.; Banerji, T.; Tagge, I.J.; Kirkemo, L.L.; Chaudhary, P.; Calkins, E.; Galipeau, D.; Shokat, M.D.; Debell, M.J.; Van Leuven, S.; et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight 2019, 4, e126329. [Google Scholar] [CrossRef]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193, 59–79. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassarro, V.A.; Quadalti, C.; Runfola, M.; Manera, C.; Rapposelli, S.; Calzà, L. Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges. Pharmaceuticals 2023, 16, 1207. https://doi.org/10.3390/ph16091207
Baldassarro VA, Quadalti C, Runfola M, Manera C, Rapposelli S, Calzà L. Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges. Pharmaceuticals. 2023; 16(9):1207. https://doi.org/10.3390/ph16091207
Chicago/Turabian StyleBaldassarro, Vito Antonio, Corinne Quadalti, Massimiliano Runfola, Clementina Manera, Simona Rapposelli, and Laura Calzà. 2023. "Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges" Pharmaceuticals 16, no. 9: 1207. https://doi.org/10.3390/ph16091207
APA StyleBaldassarro, V. A., Quadalti, C., Runfola, M., Manera, C., Rapposelli, S., & Calzà, L. (2023). Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges. Pharmaceuticals, 16(9), 1207. https://doi.org/10.3390/ph16091207








