Synergistic Antihyperglycemic and Antihyperlipidemic Effect of Polyherbal and Allopolyherbal Formulation
Abstract
:1. Introduction
2. Results and Discussions
2.1. Phytochemical Analysis of Polyherbal Formulation (PHF)
2.2. Quantitative Estimation of Total Phenolic, Flavonoid, and Tannins Content in PHF
2.2.1. Total Phenolic Content
2.2.2. Total Flavonoid Content
2.2.3. Total Tannin Content
2.3. Compatibility Study by Differential Scanning Calorimetry (DSC)
2.4. Acute Toxicity Study of Polyherbal Formulation (PHF)
2.5. In Vivo Antidiabetic and Antihyperlipidemic Activity
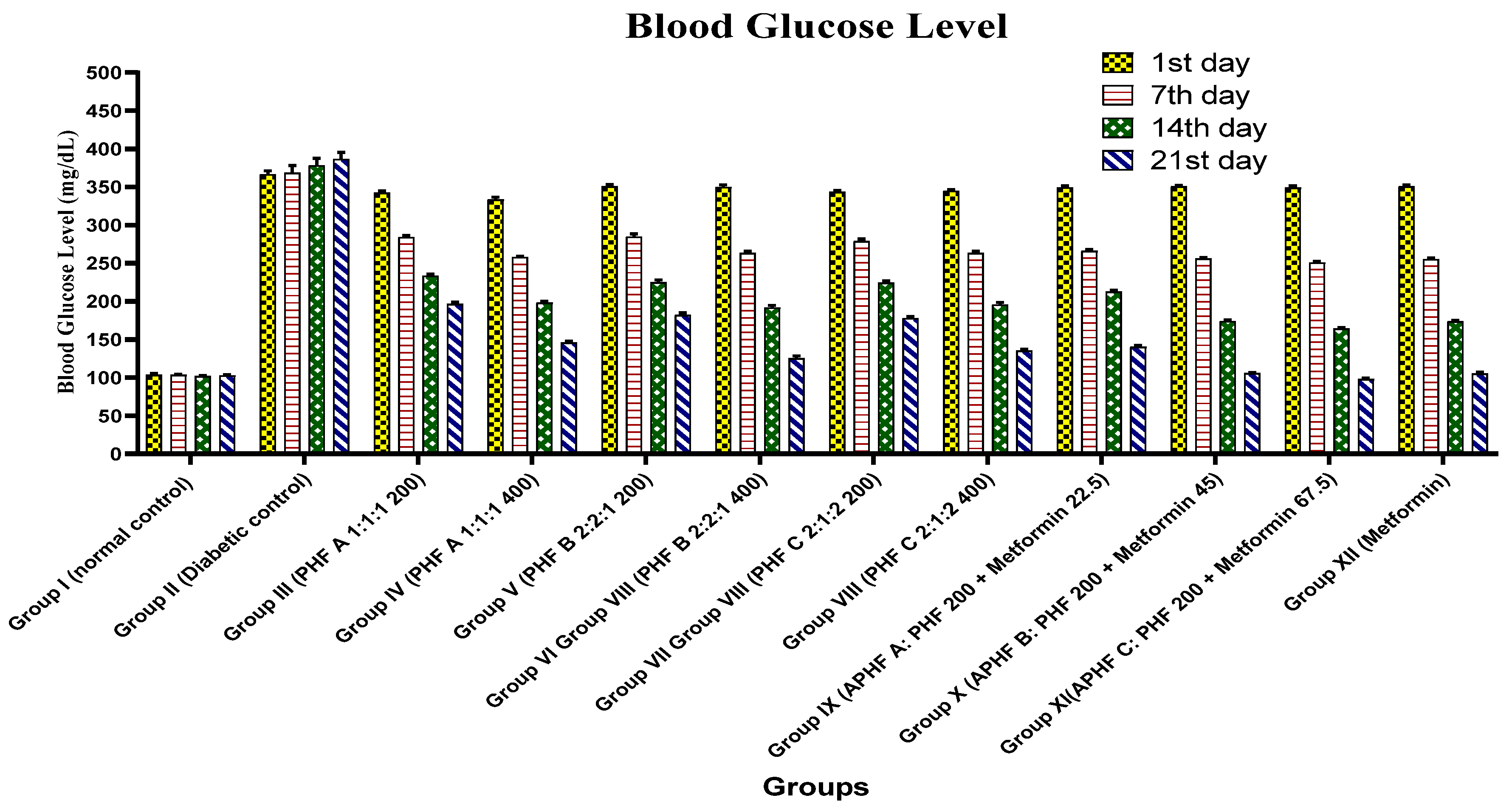
2.5.1. Effect on Blood Glucose Level
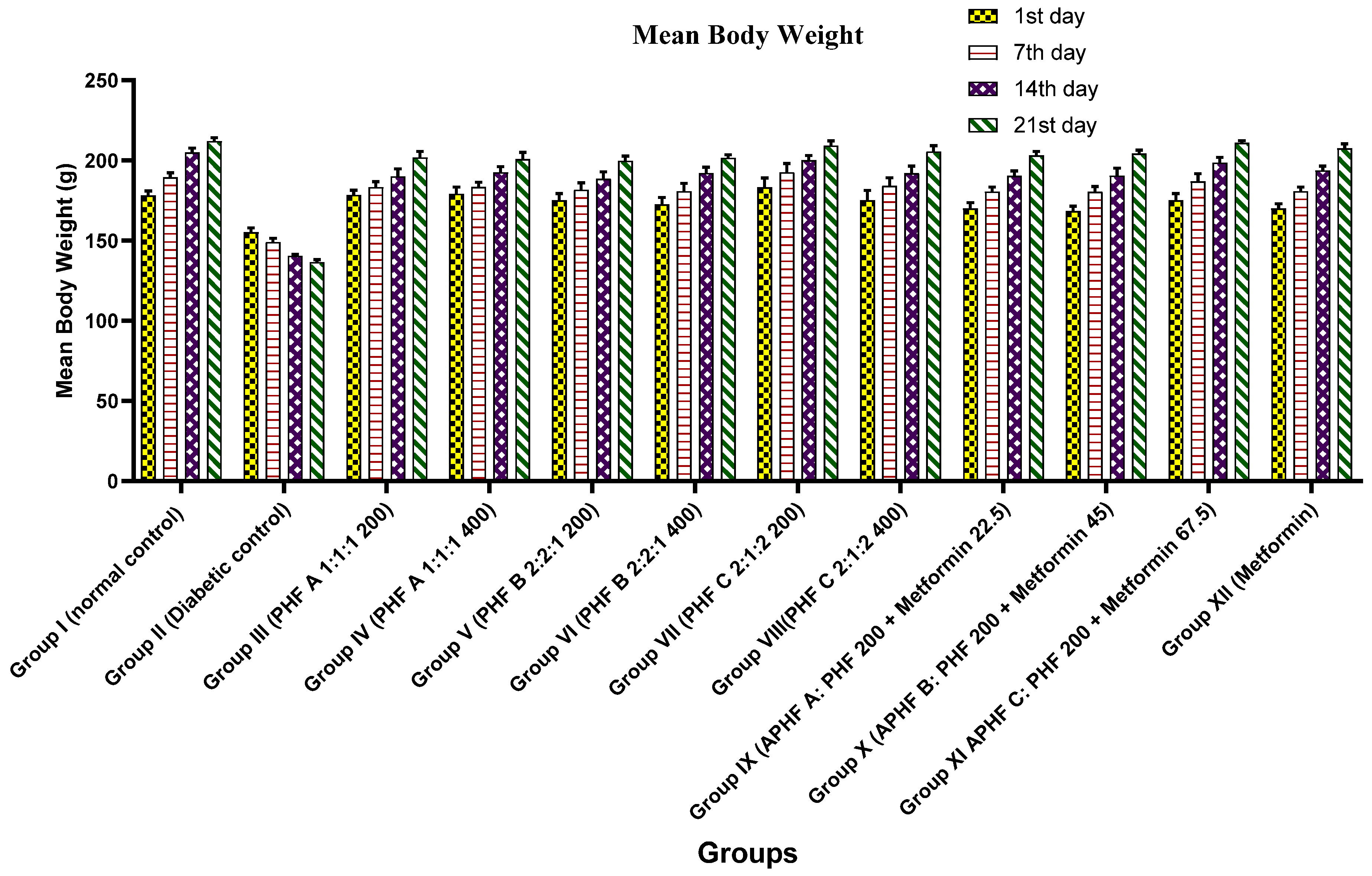
2.5.2. Effect on Bodyweight
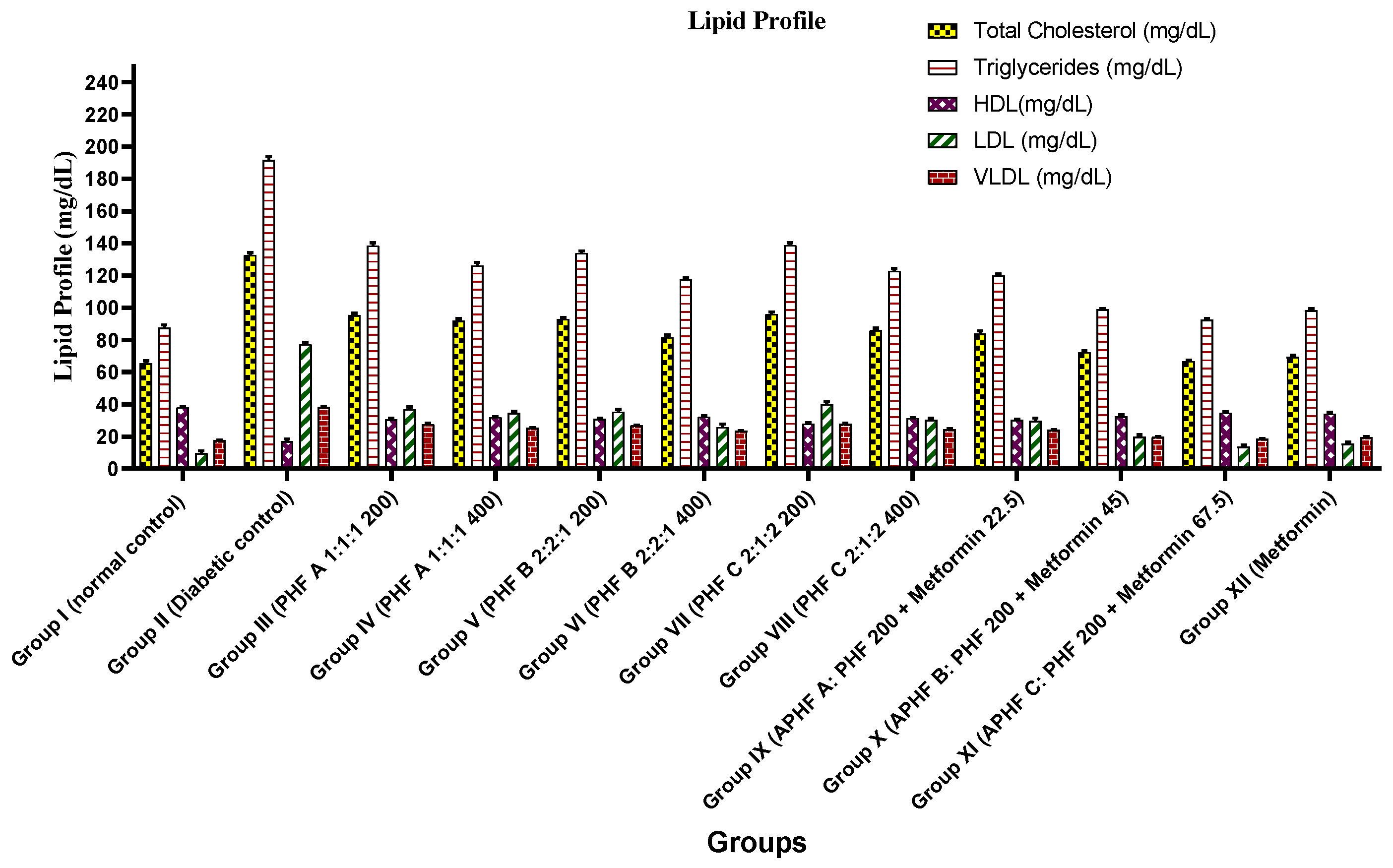
2.5.3. Effect on Lipid Profile
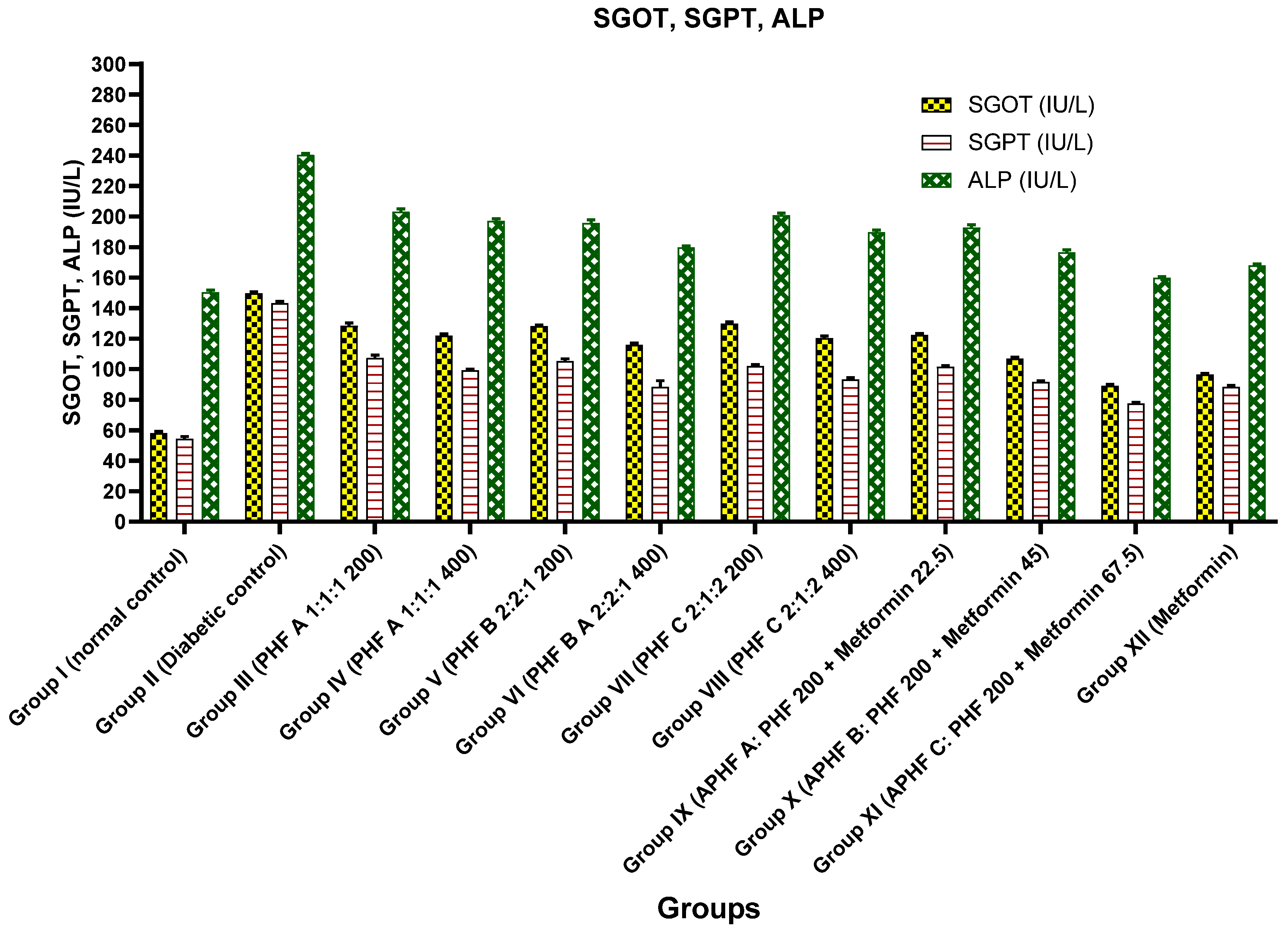
2.5.4. Effect on SGOT, SGPT, and ALP Levels
2.5.5. Effect on Total and Differential White Blood Cell Count
2.5.6. Effect on HbA1c, Urea, Creatinine, Total Protein, and Albumin
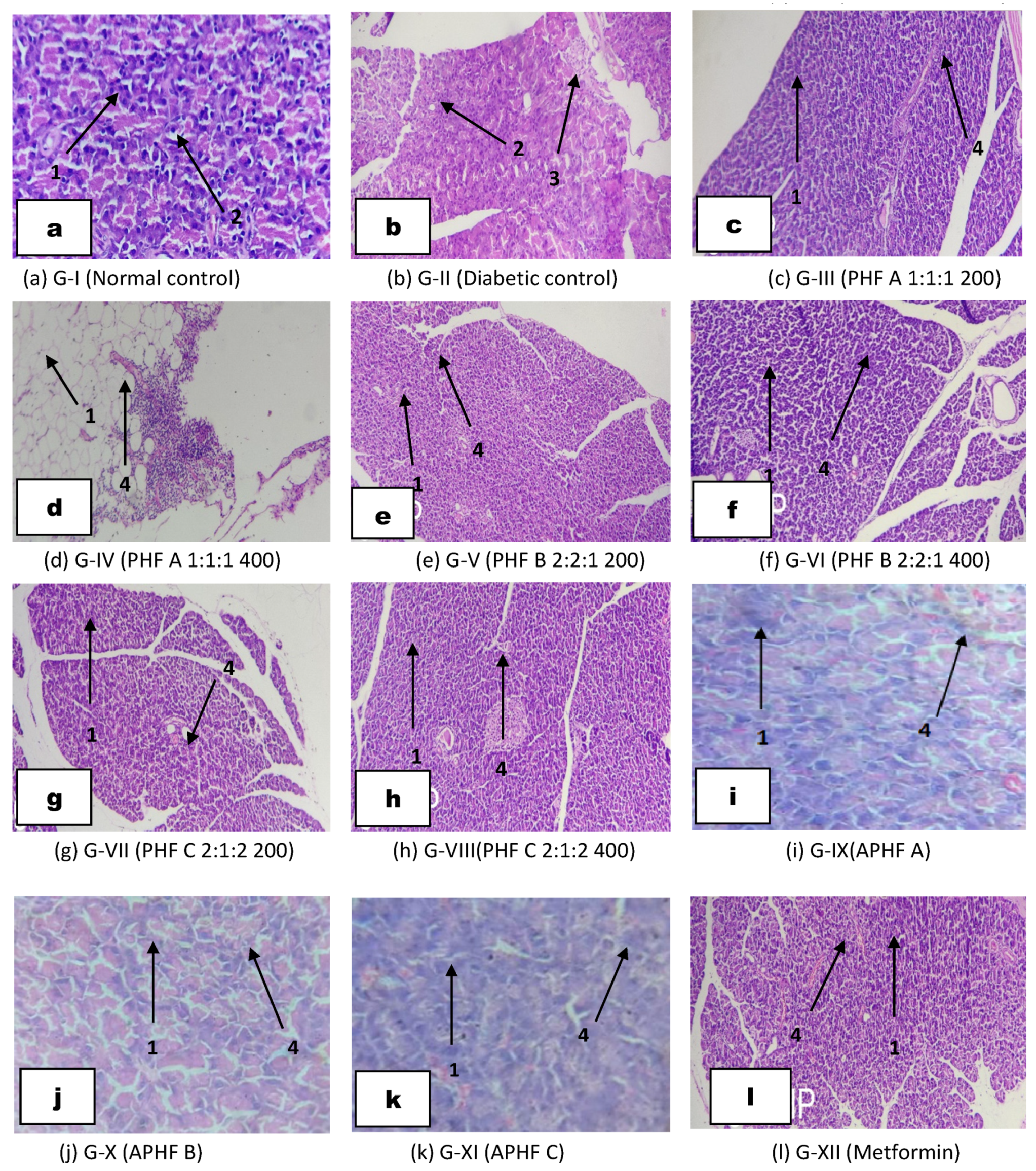
2.5.7. Histopathological Studies of Liver Cells
2.5.8. Histopathological Studies of Pancreatic Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of Extract
3.4. Preparation of Polyherbal and Allopolyherbal Formulations
- APHF A: PHF B (200 mg/kg b.w) + 25% of metformin standard dose (22.5 mg/kg b.w)
- APHF B: PHF B (200 mg/kg b.w) + 50% of metformin standard dose (45 mg/kg b.w)
- APHF C: PHF B (200 mg/kg b.w) + 75% of metformin standard dose (67.5 mg/kg b.w)
3.5. Preliminary Phytochemical Investigation of Polyherbal Formulation
3.6. Quantitative Estimation of Total Phenolic, Flavonoid, and Tannin Content
3.6.1. Estimation of Total Phenolic Content
3.6.2. Estimation of Total Flavonoid Content
3.6.3. Estimation of Total Tannin Content
3.7. Compatibility Study of APHF by Differential Scanning Calorimetry (DSC)
3.8. InVivo Antidiabetic Activity
3.8.1. Experimental Animals
3.8.2. Acute Toxicity Study of Polyherbal Formulation
3.8.3. Induction of Diabetes
3.8.4. Experimental Design
- Group I: Served as a normal control (2.5% acacia suspension b.w, p.o)
- Group II: Served as a diabetic control (Streptozotocin 55 mg/kg b.w, i.p)
- Group III: Treatment with PHF A—1:1:1 (200 mg/kg b.w, p.o)
- Group IV: Treatment with PHF A—1:1:1 (400 mg/kg b.w, p.o)
- Group V: Treatment with PHF B—2:2:1 (200 mg/kg b.w, p.o)
- Group VI: Treatment with PHF B—2:2:1 (400 mg/kg b.w, p.o)
- Group VII: Treatment with PHF C—2:1:2 (200 mg/kg b.w, p.o)
- Group VIII: Treatment with PHF C—2:1:2 (400 mg/kg b.w, p.o)
- Group IX: APHF A (200 mg/kg PHF B+22.5 mg/kg metformin b.w, p.o)
- Group X: APHF B (200 mg/kg PHF B+45 mg/kg metformin b.w, p.o)
- Group XI: APHF C (200 mg/kg PHF B+67.5 mg/kg metformin b.w, p.o
- Group XII: Treatment with metformin (90 mg/kg b.w, p.o)
3.8.5. Body Weight (b.w) Analysis
3.8.6. Blood Glucose Level
3.8.7. Biochemical Analysis
3.8.8. Histopathological Analysis
3.8.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motevalli, S.; Salahshour, H.M.; Bailey, R.P. The mediating role of cognitive flexibility in the relationship between cognitive emotion regulation strategies and mindfulness in patients with type 2 diabetes. J. Affect. Disord. 2023, 339, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal plants with potential antidiabetic activity—A review of ten years of herbal medicine research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Petchi, R.R.; Vijaya, C.; Parasuraman, S. Antidiabetic activity of Polyherbal Formulation in Streptozotocin—Nicotinamide induced diabetic wistar rats. J. Tradit. Complement. Med. 2014, 4, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bisht, L.; Ram, V. Allopolyherbal Formulations and their Strategies. J. Phytochem. Biochem. 2017, 1, 101. [Google Scholar]
- Kumar, R.; Arora, V.; Ram, V.; Bhandari, A.; Vyas, P. Hypoglycemic and hypolipidemic effect of Allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. Int. J. Diabetes Mellit. 2015, 3, 45–50. [Google Scholar] [CrossRef]
- DeRuiter, J. Overview of the Antidiabetic Agents. Endocr. Pharmacother. Modul. 2003, 3, 45–50. [Google Scholar]
- Kalaivani, A.; Umamaheswari, A.; Vinayagam, A.; Kalaivani, K. Anti-hyperglycemic and antioxidant properties of Cassia auriculata leaves and flowers on alloxan induced diabetic rats. Pharmacologyonline 2008, 1, 204–217. [Google Scholar]
- Pari, L.; Latha, M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore. Med. J. 2002, 43, 617–621. [Google Scholar]
- Thangavelu, L.; Kumar, R.; Arivarasu, L.; Aravindan, M. Cytotoxic Effect and Antibacterial Activity of Ethanolic Extract of Cassia auriculata. J. Pharm. Res. Int. 2021, 33, 456–461. [Google Scholar]
- Somchit, M.N.; Sulaiman, M.R.; Zuraini, A.; Samsuddin, L.; Somchit, N.; Israf, D.A.; Moin, S. Antinociceptive and antiinflammatory effects of Centella asiatica. Indian. J. Pharmacol. 2004, 36, 377. [Google Scholar]
- Arora, D.; Kumar, M.; Dubey, S.D. Centella asiatica-A Review of it’s Medicinal Uses and Pharmacological Effects. J. Nat. Remedies. 2002, 2, 143–149. [Google Scholar]
- Akhani, S.P.; Vishwakarma, S.L.; Goyal, R.K. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2004, 56, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An Herbal Medicinal Product with Broad Anti-Inflammatory Actions. J. Med. Food. 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, S.B.; Bansal, S.K.; Prabhu, K.M. Antihyperglycemic and hypolipidemic activity of aqueous extract of Cassia auriculata L. leaves in experimental diabetes. J. Ethnopharmacol. 2009, 123, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.U.; Samad, M.B.; D’Costa, N.M.; Akhter, F.; Ahmed, A.; Hannan, J.M. Anti- hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC. Complement. Med. 2014, 14, 31. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Kumar, M.R. Elucidation of Antidiabetic Mechanism of Centella asiatica and Zingiber officinale-An In-vitro and In-vivo approach. J. Res. Pharm. 2023, 27, 420–431. [Google Scholar]
- Weli, A.M.; Al-Salmi, S.; Al Hoqani, H.; Hossain, M.A. Biological and phytochemical studies of different leaves extracts of Pteropyrum scoparium. Beni-Suef. Univ. J. Basic. Appl. Sci. 2018, 7, 481–486. [Google Scholar]
- Asha, L.; Londonkar, R.L.; Bankalgi, S.K. Standardization of extraction process for Rumex vesicarius L. Int. J. Res. Sci. Eng. 2014, 5, 1061–1064. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhle, S.B. Khandelwal. In Practical Pharmacognosy; Nirali Publications: Pune, India, 2005; pp. 140–143. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhle, S.B. Practical Pharmacognosy; Nirali Prakashan: Pune, India, 2005; pp. 108–111. [Google Scholar]
- Mahdi-Pour, B.; Jothy, S.L.; Latha, L.Y.; Chen, Y.; Sasidharan, S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac. J. Trop Biomed. 2012, 2, 960–965. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Bhat, R.; Tze, L.M.; Karim, A.A.; Mansor, S.M. The free radical scavenging and antioxidant activities of pod and seed extract of Clitoria fairchildiana (Howard)-an underutilized legume. J. Food Sci. Technol. 2013, 50, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Burns, R.E. Method for estimation of tannin in grain sorghum. Agron. J. 1971, 63, 511–512. [Google Scholar] [CrossRef]
- Aigner, Z.; Heinrich, R.; Sipos, E.; Farkas, G.; Ciurba, A.; Berkesi, O.; Szabó-Revesz, P. Compatibility studies of aceclofenac with retard tablet excipients by means of thermal and FT-IR spectroscopic methods. J. Therm. Anal. Calorim. 2011, 104, 265–271. [Google Scholar] [CrossRef]
- de Andrade, F.H.; de Araujo Batista, R.S.; Melo, T.B.; Fernandes, F.H.; Macedo, R.O.; de Souza, F.S.; Wanderley, A.G. Characterization and compatibility of dry extract from Annona muricata L. and pharmaceutical excipients. J. Therm. Anal. Calorim. 2019, 143, 237–246. [Google Scholar] [CrossRef]
- Costa, R.S.D.; Negrão, C.A.; Camelo, S.R.; Ribeiro-Costa, R.M.; Barbosa, W.L.; Costa, C.E.; Júnior, J.O. Investigation of thermal behavior of Heliotropium indicum L. lyophilized extract by TG and DSC. J. Therm. Anal. Calorim. 2013, 111, 1959–1964. [Google Scholar] [CrossRef]
- Sasikala, S.; Lakshminarasaiah, S.; Naidu, M.D. Antidiabetic activity of Centella asiatica on streptozotocin induced diabetic male albino rats. World. J. Pharm. Sci. 2015, 3, 1701–1705. [Google Scholar]
- John, A.O. Ojewole. Analgesic, Antiinflammatory and Hypoglycaemic Effects of Ethanol Extract of Zingiber officinale (Roscoe) Rhizomes (Zingiberaceae) in Mice and Rats. Phyther. Res. 2006, 20, 764–772. [Google Scholar]
- Manik, S.; Gauttam, V.; Kalia, A.N. Anti-diabetic and antihyperlipidemic effect of allopolyherbal formulation in OGTT and STZ-induced diabetic rat model. Indian. J. Exp. Biol. 2013, 51, 702–708. [Google Scholar]
- Nagaraja, S.; Ahmed, S.S.; Bharathi, D.R.; Goudanavar, P.; Fattepur, S.; Meravanige, G.; Shariff, A.; Shiroorkar, P.N.; Habeebuddin, M.; Telsang, M. Green Synthesis and Characterization of Silver Nanoparticles of Psidium guajava Leaf Extract and Evaluation for Its Antidiabetic Activity. Molecules 2022, 27, 4336. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The current and future perspectives of zinc oxide nanoparticles in the treatment of DM. Life Sci. 2019, 239, 117011. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar] [PubMed]
- Prabhu, S.; Vinodhini, S.; Elanchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2018, 10, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Madić, V.; Petrović, A.; Jušković, M.; Jugović, D.; Djordjević, L.; Stojanović, G.; Vasiljević, P. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J. Ethnopharmacol. 2021, 265, 113210. [Google Scholar] [CrossRef] [PubMed]

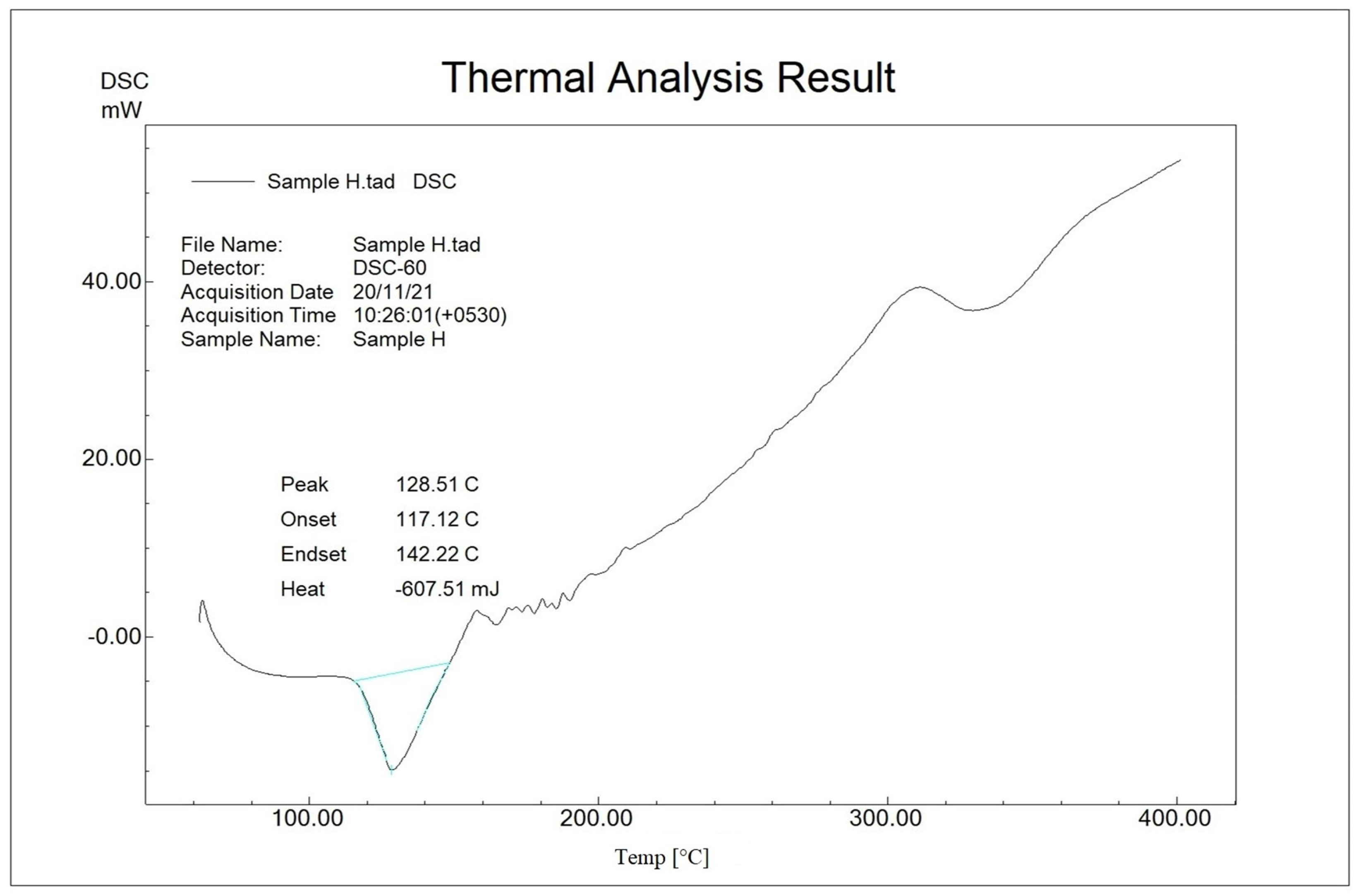


| SL. NO | MATERIALS | PEAK (°C) | ONSET (°C) | ENDSET (°C) | HEAT (mJ) |
|---|---|---|---|---|---|
| 1 | Metformin (Figure 1) | 244.2 | 234.7 | 251.4 | −2.0 |
| 2 | Polyherbal extract (Figure 2) | 128.5 | 117.1 | 142.2 | −607.5 |
| 3 | Allopolyherbal formulation (Polyherbal + metformin) (Figure 3) | 132.4 224.4 | 124.7 225.7 | 103.5 232.1 | −299.7 −608.5 |
| Group | Blood Glucose Levels (mg/dL) (Mean ± SEM) | |||
|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | 21st Day | |
| Group I (Normal control) | 103.8 ± 1.6 | 103.6 ± 0.7 | 102.0 ± 0.9 | 103.0 ± 1.0 |
| Group II (Diabetic control) | 366.6 ± 4.6 | 368.8 ± 9.5 | 378.3 ± 9.2 | 386.6 ± 8.8 |
| Group III (PHF A 1:1:1 200) | 342.5 ± 2.6 * | 284.5 ± 2.1 *** | 233.8 ± 2.1 *** | 196.6 ± 2.4 *** |
| Group IV (PHF A 1:1:1 400) | 333.6 ± 3.0 * | 258.1 ± 1 *** | 198.5 ± 1.8 *** | 146.0 ± 2.0 *** |
| Group V (PHF B 2:2:1 200) | 351.0 ± 2.5 * | 285.0 ± 3.5 *** | 225.0 ± 3.2 *** | 182.1 ± 2.8 *** |
| Group VI (PHF B 2:2:1 400) | 350.1 ± 2.4 * | 263.5 ± 2.07 *** | 191.8 ± 2.9 *** | 125.3 ± 3.2 *** |
| Group VII (PHF C 2:1:2 200) | 343.5 ± 1.7 * | 279.1 ± 2.7 *** | 224.5 ± 2.0 *** | 178.0 ± 2.3 *** |
| Group VIII (PHF C 2:1:2 400) | 345.0 ± 1.7 * | 263.5 ± 2.1 *** | 195.8 ± 2.8 *** | 135.5 ± 2.1 *** |
| Group IX APHF A | 349.6 ± 1.8 * | 266.3 ± 2.0 *** | 212.6 ± 1.8 *** | 140.3 ± 2.2 *** |
| Group X APHF B | 351 ± 1.1 * | 256.1 ± 1.3 *** | 174.1 ± 1.3 *** | 105.8 ± 0.9 *** |
| Group XI APHF C | 349.3 ± 2.2 * | 250.8 ± 1.4 *** | 164.3 ± 1.5 *** | 98.3 ± 1.3 *** |
| Group XII (Metformin) | 350.8 ± 1.7 * | 255.5 ± 1.1 *** | 173.8 ± 1.49 *** | 105.3 ± 1.4 *** |
| Group | Mean Body Weight (g) (Mean ± SEM) | |||
|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | 21st Day | |
| Group I (Normal control) | 178.1 ± 2.7 | 189.5 ± 2.7 | 205 ± 2.6 *** | 212 ± 2.0 *** |
| Group II (Diabetic control) | 155.1 ± 2.6 | 149 ± 2.3 | 140.5 ± 0.8 *** | 136.5 ± 1.5 *** |
| Group III (PHF A 1:1:1 200) | 178.3 ± 3.0 *** | 183.3 ± 3.3 *** | 190 ± 4.6 *** | 201.8 ± 3.7 *** |
| Group IV (PHF A 1:1:1 400) | 179.1 ± 4.1 *** | 183.5 ± 2.8 *** | 192.5 ± 3.5 *** | 200.8 ± 4.1 *** |
| Group V (PHF B 2:2:1 200) | 175 ± 4.2 ** | 181.6 ± 4.3 *** | 188.5 ± 4.3 *** | 199.6 ± 2.9 *** |
| Group VI (PHF B 2:2:1 400) | 172.5 ± 4.4 ** | 180.8 ± 4.8 *** | 192.1 ± 3.6 *** | 201.6 ± 1.8 *** |
| Group VII (PHF C 2:1:2 200) | 183.1 ± 5.8 *** | 192.5 ± 5.4 *** | 200.1 ± 2.8 *** | 209.1 ± 3.0 *** |
| Group VIII (PHF C 2:1:2 400) | 175 ± 6.1 ** | 184.1 ± 5.0 *** | 192 ± 4.4 *** | 205.5 ± 3.6 *** |
| Group IX APHF A | 170 ± 3.6 ** | 180.5 ± 2.8 *** | 190.3 ± 3.0 *** | 203 ± 2.5 *** |
| Group X APHF B | 168.3 ± 3.0 ** | 180.3 ± 3.4 *** | 190.5 ± 4.6 *** | 204.3 ± 2.0 *** |
| Group XI APHF C | 175 ± 4.2 ** | 187 ± 4.6 *** | 198.5 ± 3.4 *** | 211 ± 1.3 *** |
| Group XII (Metformin) | 170 ± 2.8 * | 180.8 ± 2.5 *** | 193.6 ± 2.7 *** | 207.5 ± 2.8 *** |
| Group | Lipid Profile (mg/dL) | ||||
|---|---|---|---|---|---|
| TC | TG | HDL | LDL | VLDL | |
| Group I (Normal control) | 65.3 ± 1.5 *** | 87.6 ± 1.7 *** | 38 ± 0.3 *** | 9.8 ± 1.1 *** | 17.5 ± 0.3 *** |
| Group II (Diabetic control) | 132.8 ± 1.3 *** | 191.6 ± 2.0 *** | 17.1 ± 1.1 *** | 77.3 ± 1.2 *** | 38.3 ± 0.4 *** |
| Group III (PHF A 1:1:1 200) | 95.3 ± 1.3 *** | 138.6 ± 1.6 *** | 30.6 ± 0.4 *** | 36.9 ± 1.3 *** | 27.7 ± 0.3 *** |
| Group IV (PHF A 1:1:1 400) | 91.8 ± 1.3 *** | 126.1 ± 1.9 *** | 32 ± 0.3 *** | 34.6 ± 1.1 *** | 25.2 ± 0.3 *** |
| Group V (PHF B 2:2:1 200) | 93 ± 0.9 *** | 134 ± 1.3 *** | 30.8 ± 0.6 *** | 35.3 ± 1.4 *** | 26.8 ± 0.2 *** |
| Group VI (PHF B 2:2:1 400) | 81.6 ± 1.3 *** | 117.6 ± 0.7 *** | 32.3 ± 0.5 *** | 25.8 ± 1.8 *** | 23.5 ± 0.1 *** |
| Group VII (PHF C 2:1:2200) | 96 ± 1.2 *** | 139 ± 1.3 *** | 28 ± 0.6 *** | 40.2 ± 1.3 *** | 27.8 ± 0.2 *** |
| Group VIII (PHF C 2:1:2400) | 86.1 ± 1.1 *** | 122.8 ± 1.4 *** | 31.3 ± 0.4 *** | 30.2 ± 0.8 *** | 24.5 ± 0.2 *** |
| Group IX APHF A | 83.8 ± 1.6 *** | 120 ± 0.9 *** | 30.1 ± 0.4 *** | 29.6 ± 1.8 *** | 24 ± 0.1 *** |
| Group XAPHF B | 72.3 ± 0.8 *** | 99 ± 0.5 *** | 32.5 ± 1.0 *** | 20.0 ± 1.0 *** | 19.8 ± 0.1 *** |
| Group XI APHF C | 66.8 ± 0.6 *** | 92.5 ± 0.7 *** | 34.6 ± 0.7 *** | 13.6 ± 1.0 *** | 18.5 ± 0.1 *** |
| Group XII (Metformin) | 69.5 ± 0.9 *** | 98.3 ± 0.8 *** | 34.1 ± 0.7 *** | 15.6 ± 1.0 *** | 19.6 ± 0.1 *** |
| Group | SGOT Level (IU/L) | SGPT Level (IU/L) | ALP Level (IU/L) |
|---|---|---|---|
| Group I (Normal control) | 58 ± 1.2 | 54.3 ± 1.5 | 150.3 ± 1.3 |
| Group II (Diabetic control) | 149.8 ± 0.7 | 143.1 ± 1.2 | 240.3 ± 0.8 |
| Group III (PHF A 1:1:1 200) | 128.3 ± 1.9 *** | 107.1 ± 2.0 *** | 203.1 ± 1.8 *** |
| Group IV (PHF A 1:1:1 400) | 121.8 ± 1.2 *** | 99.2 ± 0.8 *** | 197.1 ± 1.4 *** |
| Group V (PHF B 2:2:1 200) | 128.1 ± 0.7 *** | 105.3 ± 1.4 *** | 195.8 ± 1.9 *** |
| Group VI (PHF B 2:2:1 400) | 115.8 ± 1.1 *** | 88.3 ± 4.1 *** | 179.8 ± 0.9 *** |
| Group VII (PHF C 2:1:2 200) | 129.8 ± 1.0 *** | 102 ± 1.0 *** | 200.8 ± 1.3 *** |
| Group VIII (PHF C 2:1:2 400) | 120.3 ± 1.3 *** | 93.1 ± 1.2 *** | 189.6 ± 1.4 *** |
| Group IX APHF A | 122.3 ± 1.1 *** | 101.5 ± 0.9 *** | 192.8 ± 1.7 *** |
| Group X APHF B | 106.8 ± 1.1 *** | 91.6 ± 0.6 *** | 176.5 ± 1.6 *** |
| Group XI APHF C | 89 ± 1.1 *** | 77.5 ± 0.7 *** | 159.8 ± 0.7 *** |
| Group XII (Metformin) | 96.4 ± 0.9 *** | 88.3 ± 1.0 *** | 168 ± 0.9 *** |
| Group | Leucocytes 103/Cumm | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) |
|---|---|---|---|---|---|
| Group I (Normal control) | 10.0 ± 0.1 | 61.1 ± 0.4 | 33.1 ± 0.6 | 4.1 ± 0.3 | 1.5 ± 0.4 |
| Group II (Diabetic control) | 5.2 ± 0.1 | 63.8 ± 0.5 | 29.8 ± 0.7 | 4.5 ± 0.4 | 1.8 ± 0.3 |
| Group III (PHF A 1:1:1 200) | 6.7 ± 0.1 ns | 62 ± 0.8 ns | 32 ± 0.6 * | 4.3 ± 0.3 ns | 1.6 ± 0.3 ns |
| Group IV (PHF A 1:1:1 400) | 7.8 ± 0.1 ** | 62 ± 0.9 ns | 32.5 ± 0.8 ** | 4.3 ± 0.3 ns | 1.1 ± 0.3 ns |
| Group V (PHF B 2:2:1 200) | 6.4 ± 0.1 ns | 62.1 ± 0.7 ns | 32.1 ± 0.7 * | 4.1 ± 0.4 ns | 1.5 ± 0.5 ns |
| Group VI (PHF B 2:2:1 400) | 7.7 ± 0.1 ** | 61.5 ± 0.6 * | 32.5 ± 0.7 ** | 4.3 ± 0.3 ns | 1.6 ± 0.4 ns |
| Group VII (PHF C 2:1:2 200) | 6.5 ± 0.0 ns | 62.1 ± 0.8 ns | 32.3 ± 0.9 ** | 4.1 ± 0.3 ns | 1.3 ± 0.4 ns |
| Group VIII (PHF C 2:1:2 400) | 7.9 ± 0.0 ** | 61.5 ± 0.4 * | 33.3 ± 0.7 *** | 4.1 ± 0.4 ns | 1 ± 0.4 ns |
| Group IX APHF A | 6.8 ± 0.0 ns | 61.1 ± 0.4 ** | 34 ± 0.2 *** | 4.1 ± 0.4 ns | 0.6 ± 0.2 ns |
| Group X APHF B | 7.1 ± 0.0 | 62.1 ± 0.4 ns | 32.5 ± 0.6 *** | 4.1 ± 0.5 ns | 1.1 ± 0.4 ns |
| Group XI APHF C | 7.9 ± 0.1 ** | 62.5 ± 0.7 ns | 32 ± 0.6 *** | 4 ± 0.5 ns | 1.5 ± 0.4 ns |
| Group XII (Metformin) | 8.0 ± 0.0 *** | 62.3 ± 0.9 ns | 32 ± 0.6 * | 4.1 ± 0.4 ns | 1.5 ± 0.4 ns |
| Group | HbA1c (%) | Urea (mg/dL) | Creatinine (mg/dL) | Total Protein (g/dL) | Albumin (mg/dL) |
|---|---|---|---|---|---|
| Group I (Normal control) | 5.2 ± 0.0 | 29.3 ± 1.1 | 0.8 ± 0.0 | 7.3 ± 0.1 | 5.4 ± 0.0 |
| Group II (Diabetic control) | 15.0 ± 0.3 | 47.3 ± 0.6 | 2.0 ± 0.0 | 4.5 ± 0.0 | 2.9 ± 0.0 |
| Group III (PHF A 1:1:1 200) | 8.4 ± 0.0 *** | 36.3 ± 1.0 *** | 1.1 ± 0.0 ns | 6.1 ± 0.0 * | 3.7 ± 0.0 ns |
| Group IV (PHF A 1:1:1 400) | 6.7 ± 0.0 *** | 36.3 ± 0.8 *** | 0.9 ± 0.0 ns | 6.5 ± 0.0 ** | 4.0 ± 0.0 ns |
| Group V (PHF B 2:2:1 200) | 7.9 ± 0.0 *** | 38.1 ± 0.5 *** | 1.2 ± 0.0 ns | 6.2 ± 0.0 * | 4.0 ± 0.4 ns |
| Group VI (PHF B 2:2:1 400) | 5.9 ± 0.1 *** | 34.1 ± 1.0 *** | 1.1 ± 0.0 ns | 6.4 ± 0.0 ** | 4.1 ± 0.0 ns |
| Group VII (PHF C 2:1:2 200) | 7.8 ± 0.0 *** | 38.6 ± 0.8 *** | 1.2 ± 0.0 ns | 5.8 ± 0.0 ns | 3.8 ± 0.0 ns |
| Group VIII (PHF C 2:1:2 400) | 6.3 ± 0.0 *** | 32.3 ± 0.6 *** | 1.0 ± 0.0 ns | 6.1 ± 0.0 * | 4.0 ± 0.0 ns |
| Group IX APHF A | 6.5 ± 0.0 *** | 33.1 ± 0.9 *** | 1.0 ± 0.0 ns | 6.1 ± 0.0 * | 4.1 ± 0.0 ns |
| Group X APHF B | 5.3 ± 0.0 *** | 34.5 ± 0.7 *** | 0.9 ± 0.0 ns | 6.3 ± 0.0 ** | 4.4 ± 0.0 * |
| Group XI APHF C | 5.0 ± 0.0 *** | 29.8 ± 1.0 *** | 0.8 ± 0.0 ns | 6.8 ± 0.0 *** | 4.8 ± 0.0 ** |
| Group XII (Metformin) | 5.2 ± 0.0 *** | 30.3 ± 0.8 *** | 0.9 ± 0.0 ns | 6.7 ± 0.0 ** | 4.6 ± 0.1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamhoom, Y.; Ahmed, S.S.; M., R.K.; Salahuddin, M.; D. R., B.; Ahmed, M.M.; Farhana, S.A.; Rahamathulla, M. Synergistic Antihyperglycemic and Antihyperlipidemic Effect of Polyherbal and Allopolyherbal Formulation. Pharmaceuticals 2023, 16, 1368. https://doi.org/10.3390/ph16101368
Alhamhoom Y, Ahmed SS, M. RK, Salahuddin M, D. R. B, Ahmed MM, Farhana SA, Rahamathulla M. Synergistic Antihyperglycemic and Antihyperlipidemic Effect of Polyherbal and Allopolyherbal Formulation. Pharmaceuticals. 2023; 16(10):1368. https://doi.org/10.3390/ph16101368
Chicago/Turabian StyleAlhamhoom, Yahya, Syed Sagheer Ahmed, Rupesh Kumar M., MD. Salahuddin, Bharathi D. R., Mohammed Muqtader Ahmed, Syeda Ayesha Farhana, and Mohamed Rahamathulla. 2023. "Synergistic Antihyperglycemic and Antihyperlipidemic Effect of Polyherbal and Allopolyherbal Formulation" Pharmaceuticals 16, no. 10: 1368. https://doi.org/10.3390/ph16101368
APA StyleAlhamhoom, Y., Ahmed, S. S., M., R. K., Salahuddin, M., D. R., B., Ahmed, M. M., Farhana, S. A., & Rahamathulla, M. (2023). Synergistic Antihyperglycemic and Antihyperlipidemic Effect of Polyherbal and Allopolyherbal Formulation. Pharmaceuticals, 16(10), 1368. https://doi.org/10.3390/ph16101368








