Exploring the Chemical Properties and Medicinal Applications of Tetramethylthiocycloheptyne Sulfoximine Used in Strain-Promoted Azide–Alkyne Cycloaddition Reactions
Abstract
1. Introduction
2. Results and Discussion
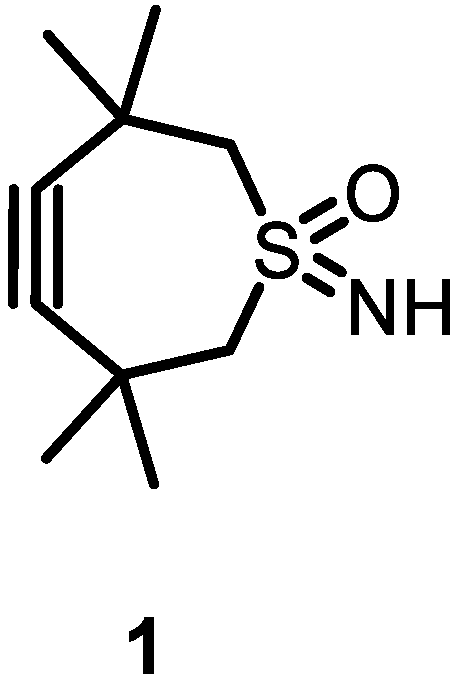
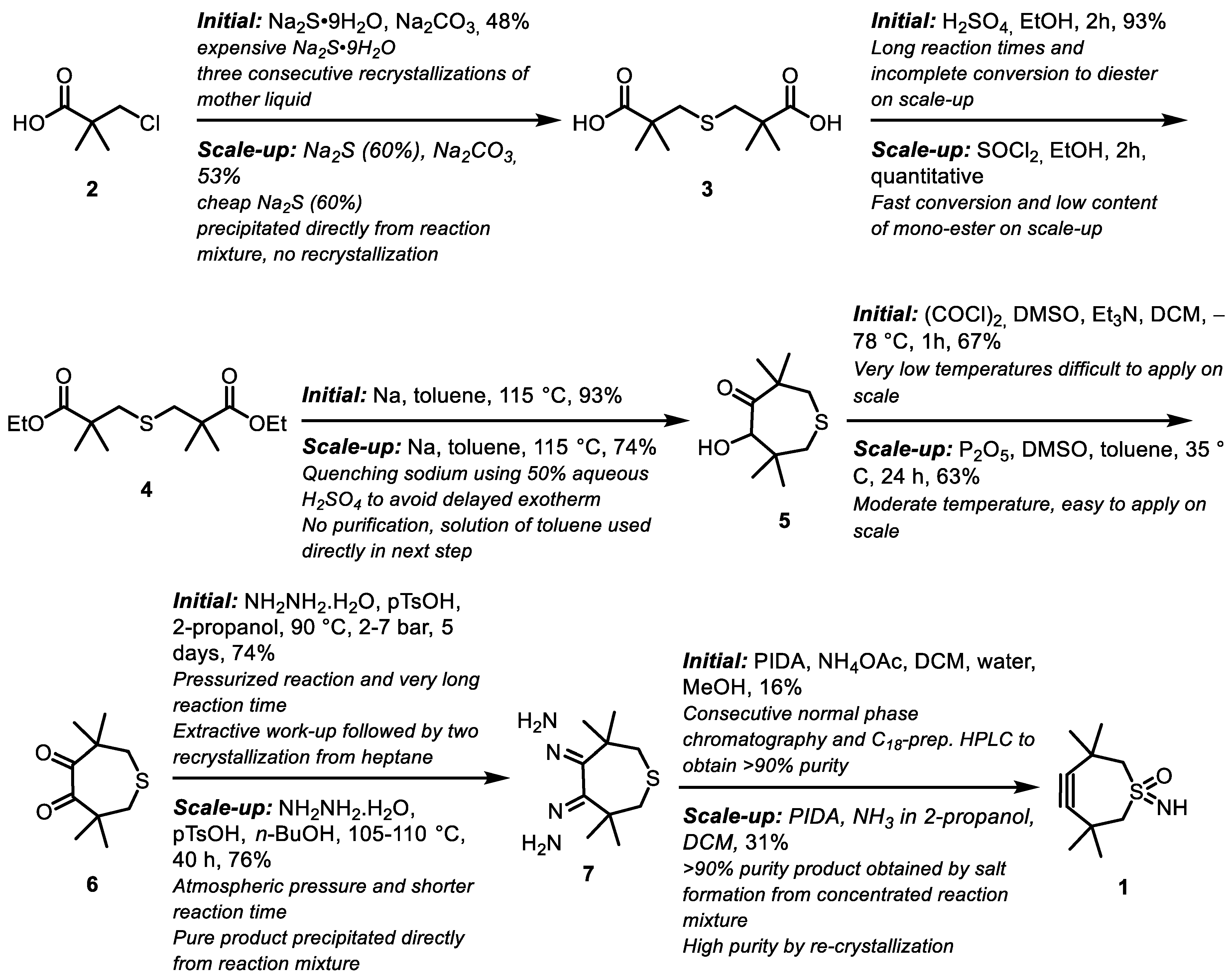
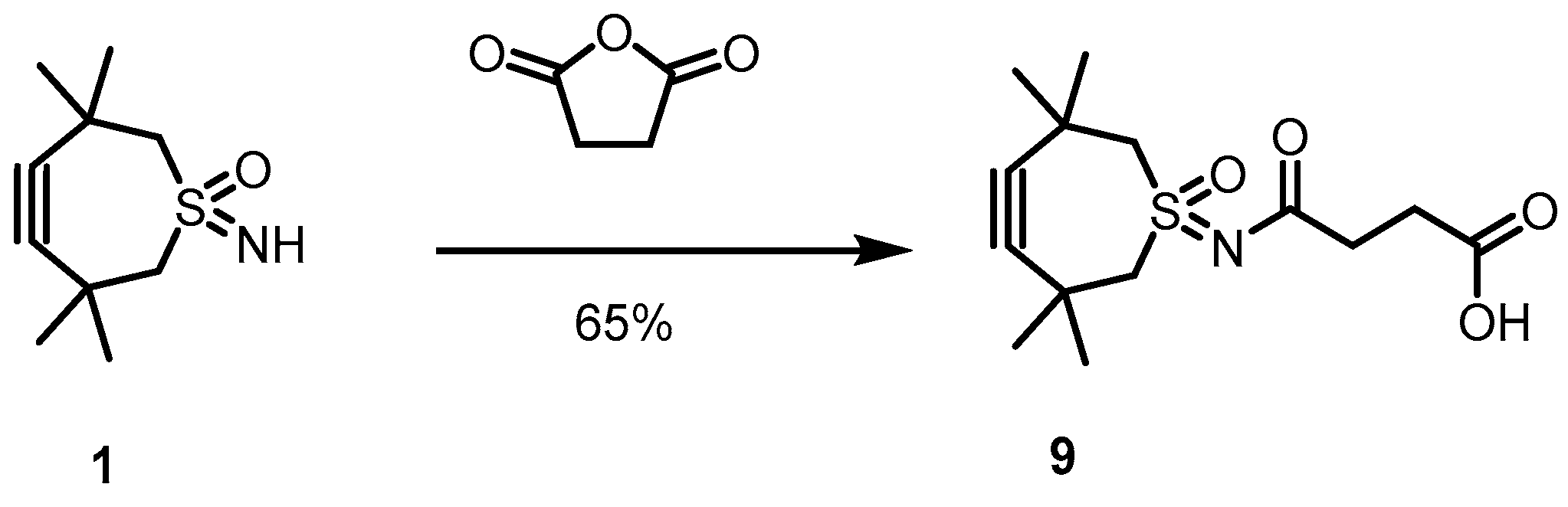
2.1. Large-Scale Production of TMTHSI
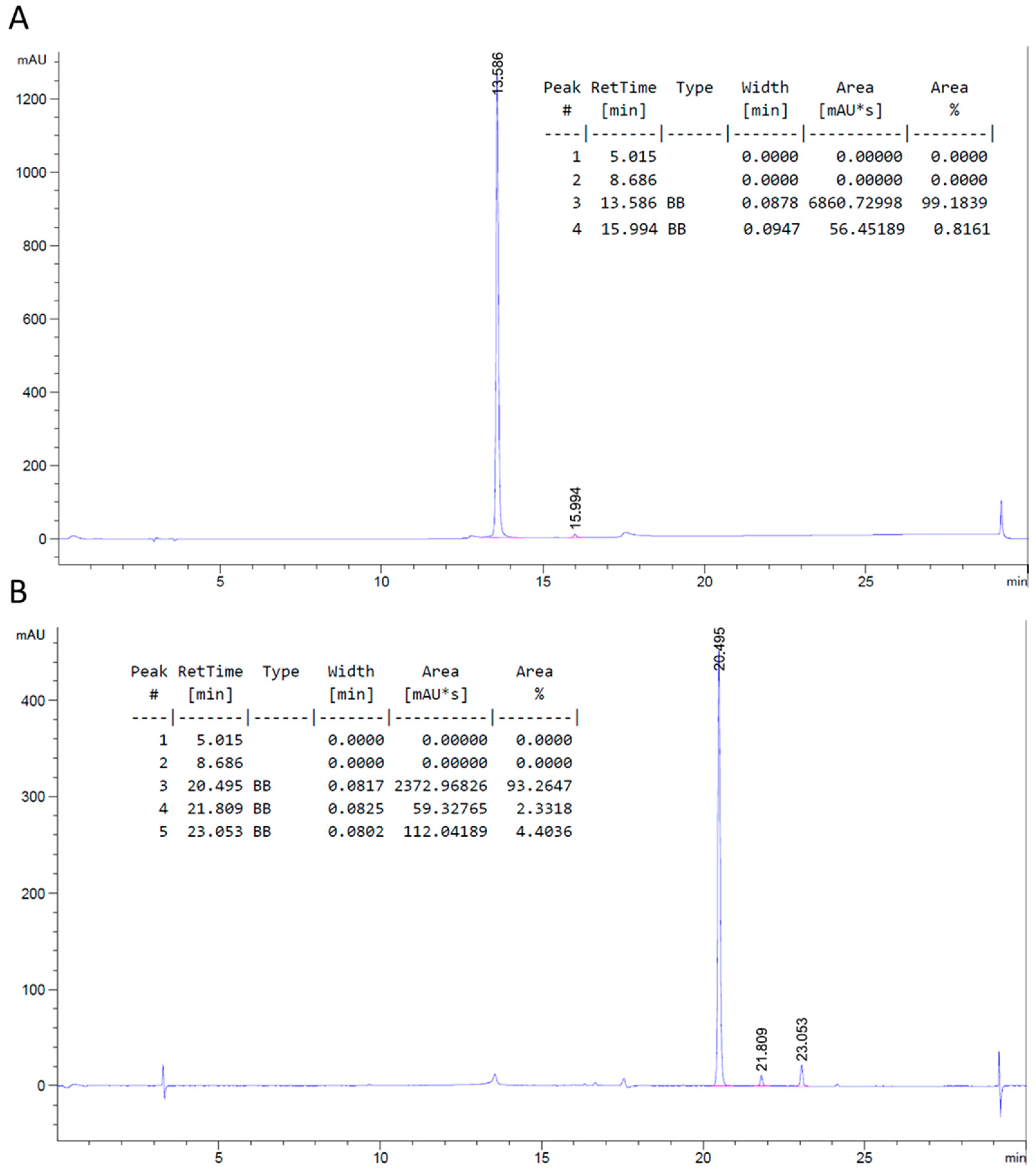
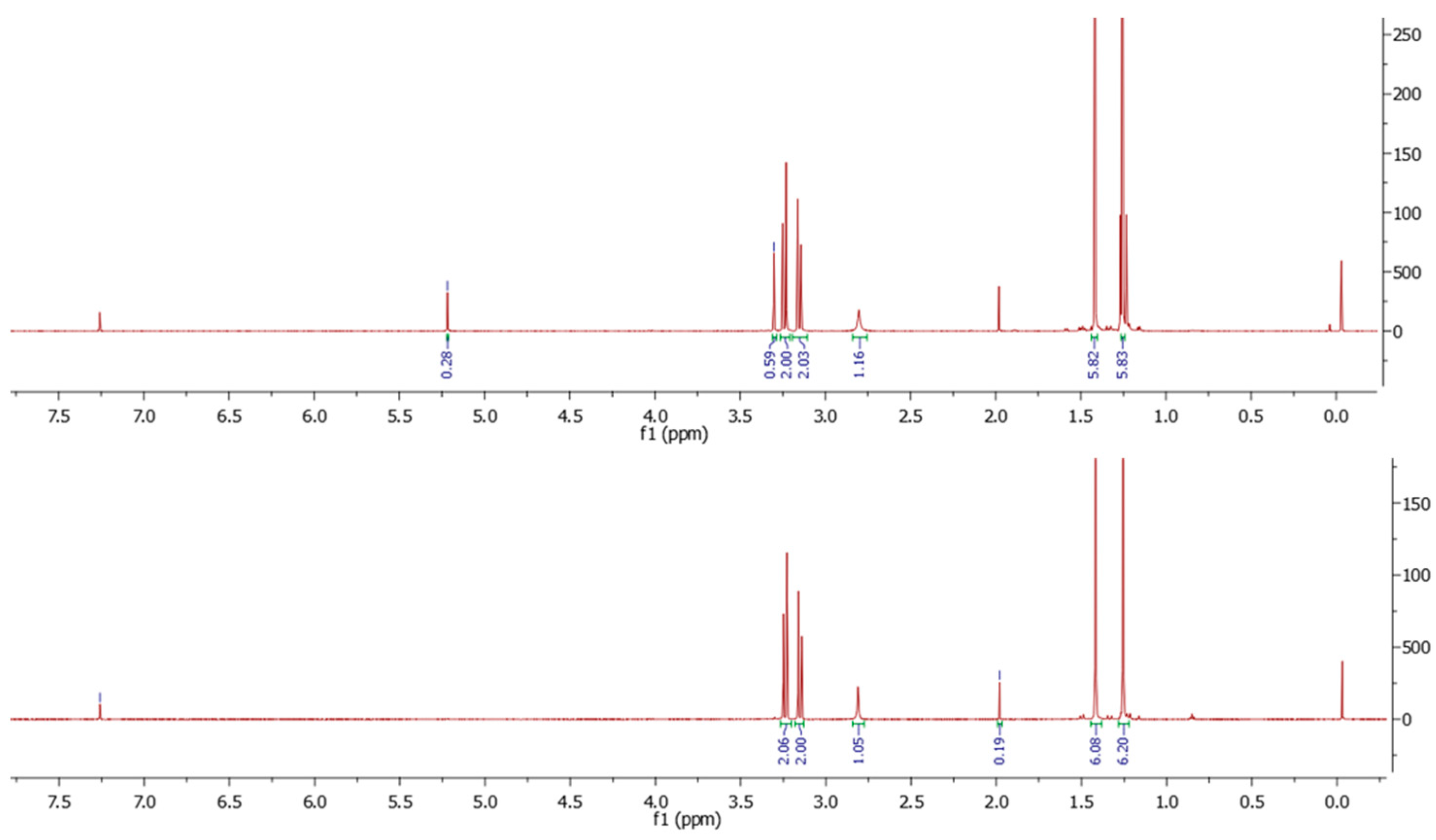
2.2. TMTHSI Stability
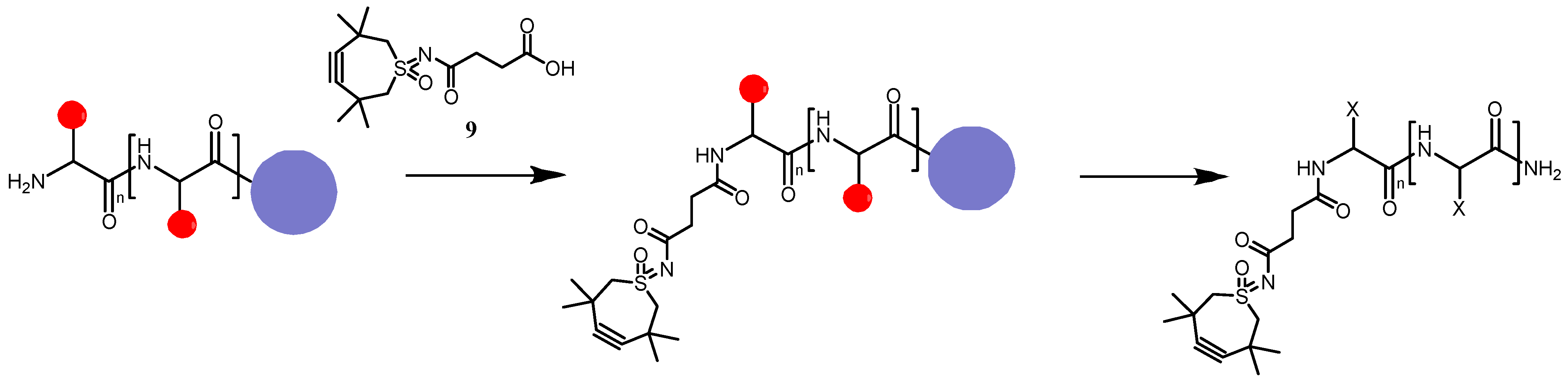
2.3. Antibody Functionalization Using TMTHSI Derivatives
2.3.1. Functionalization of TMTHSI–β-Alanine with FITC
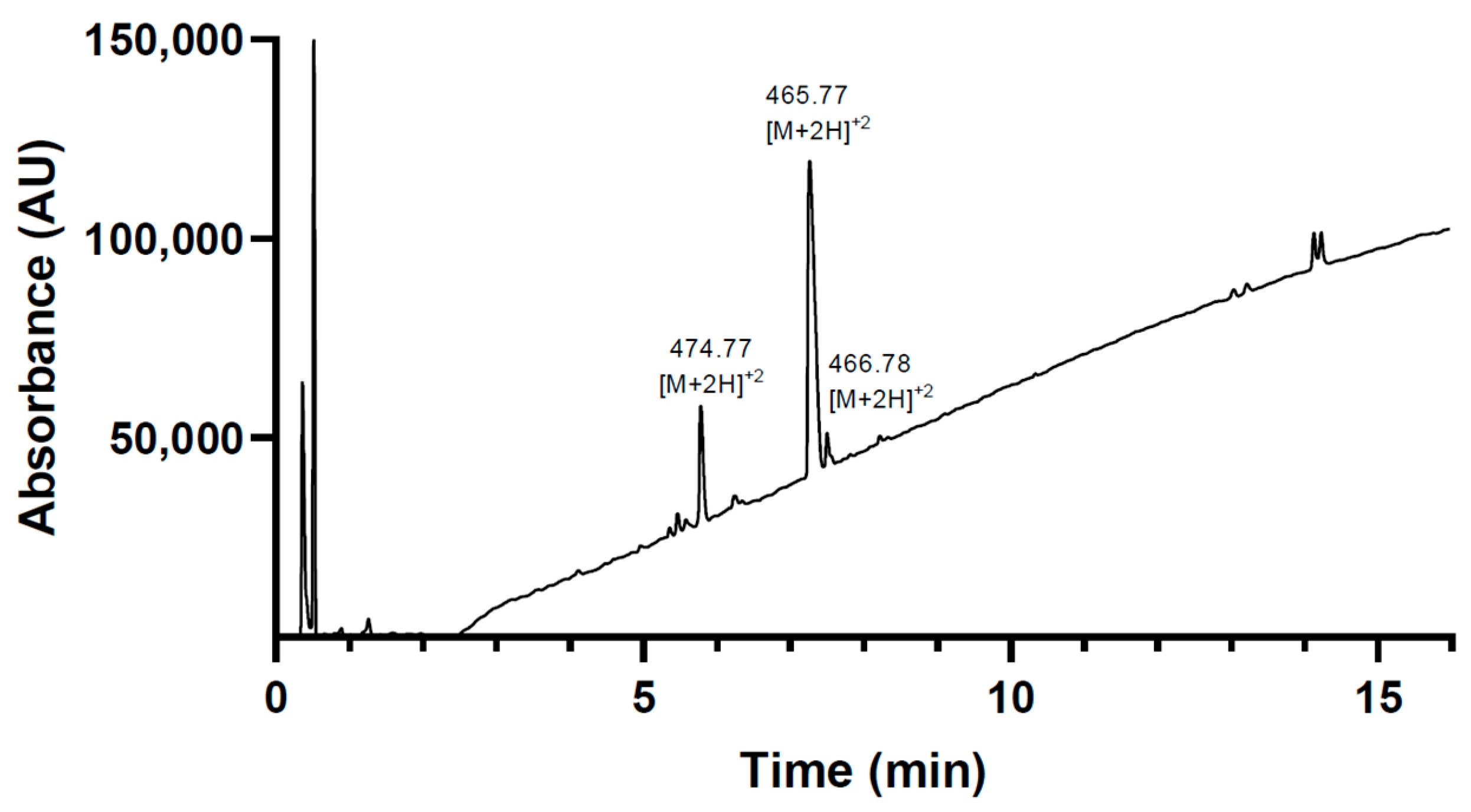
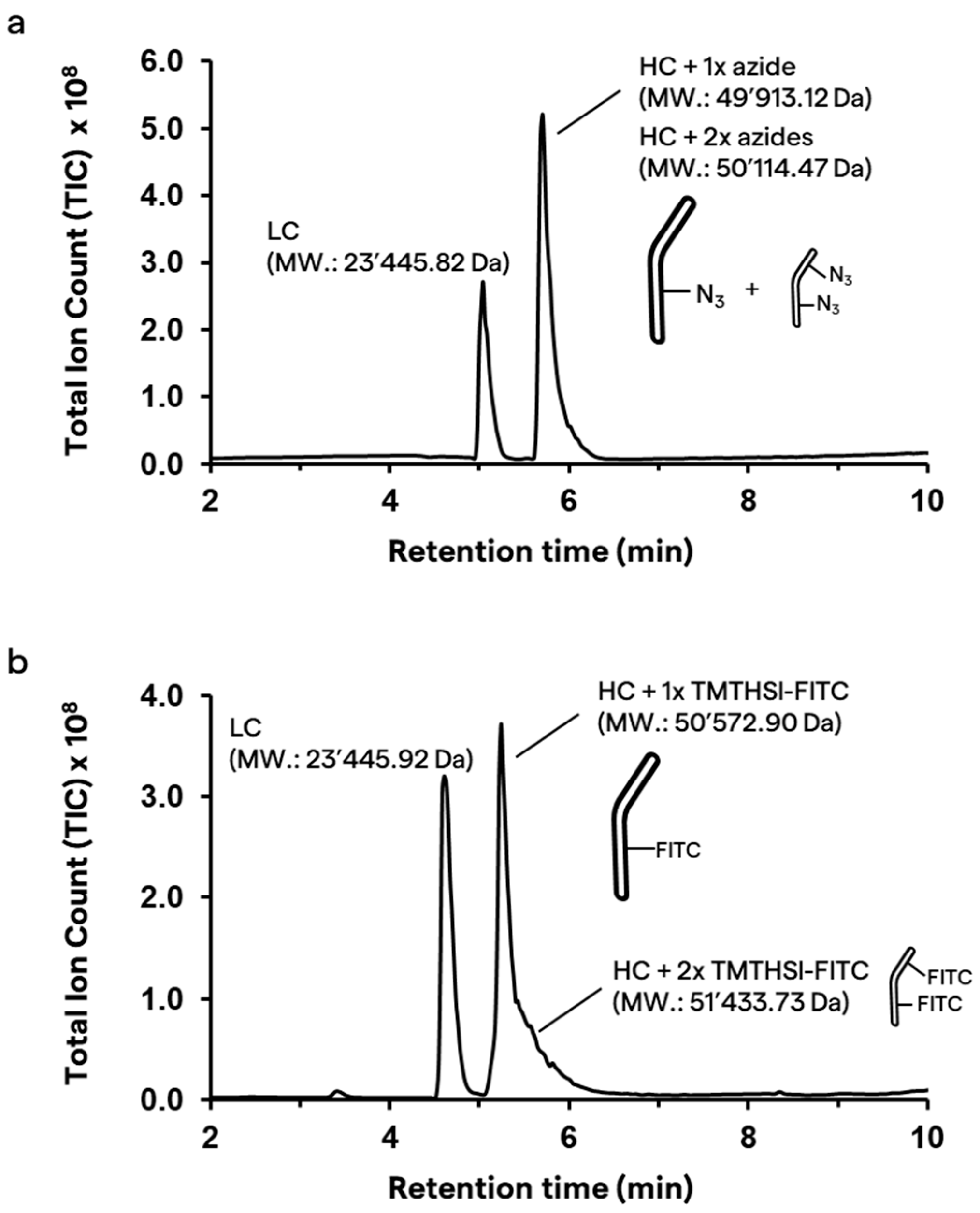
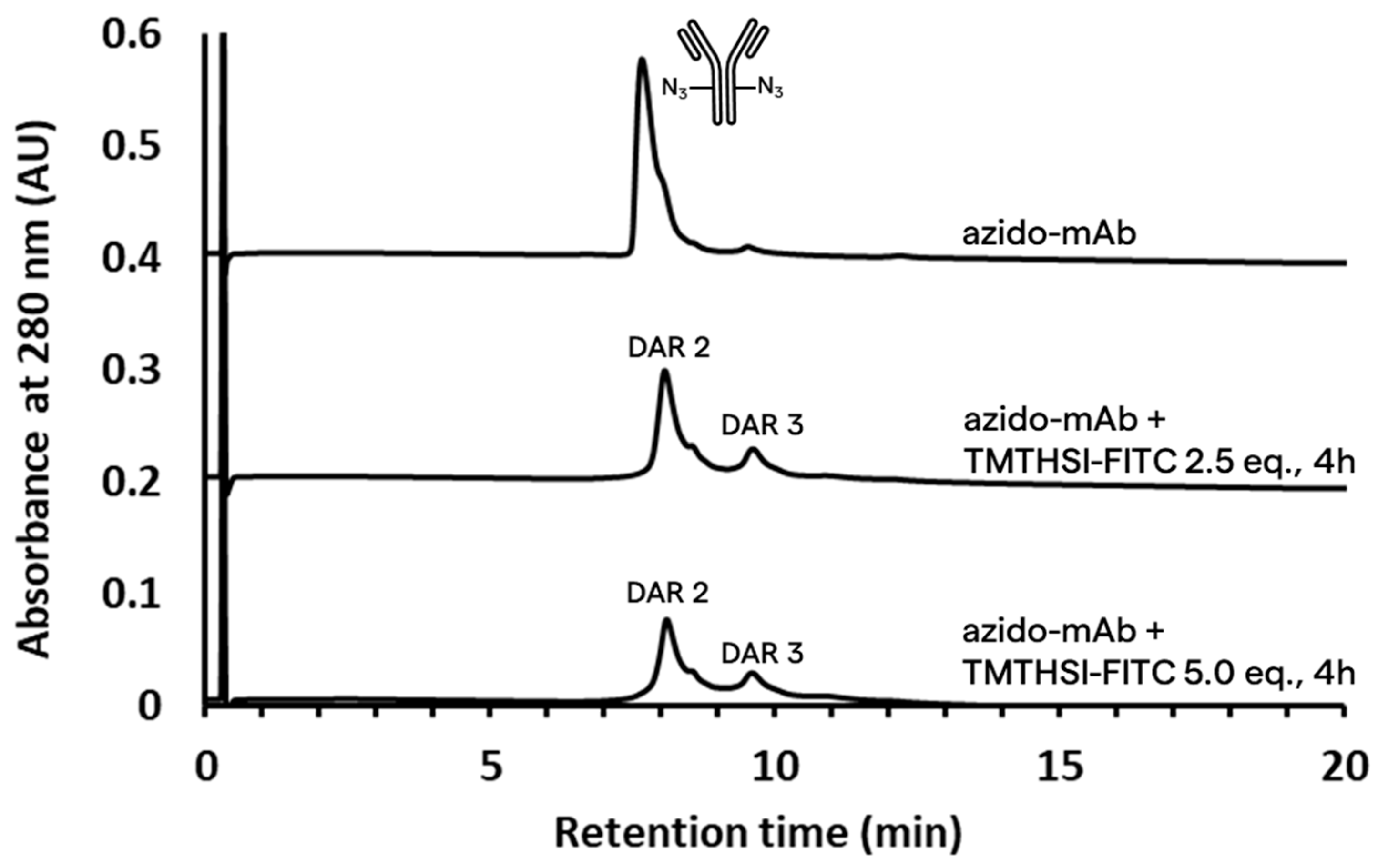
2.3.2. Site-Selective Antibody Functionalization with Azide Moieties
2.4. Click Reaction with TMTHSI–FITC
3. Conclusions
4. Materials and Methods
4.1. General
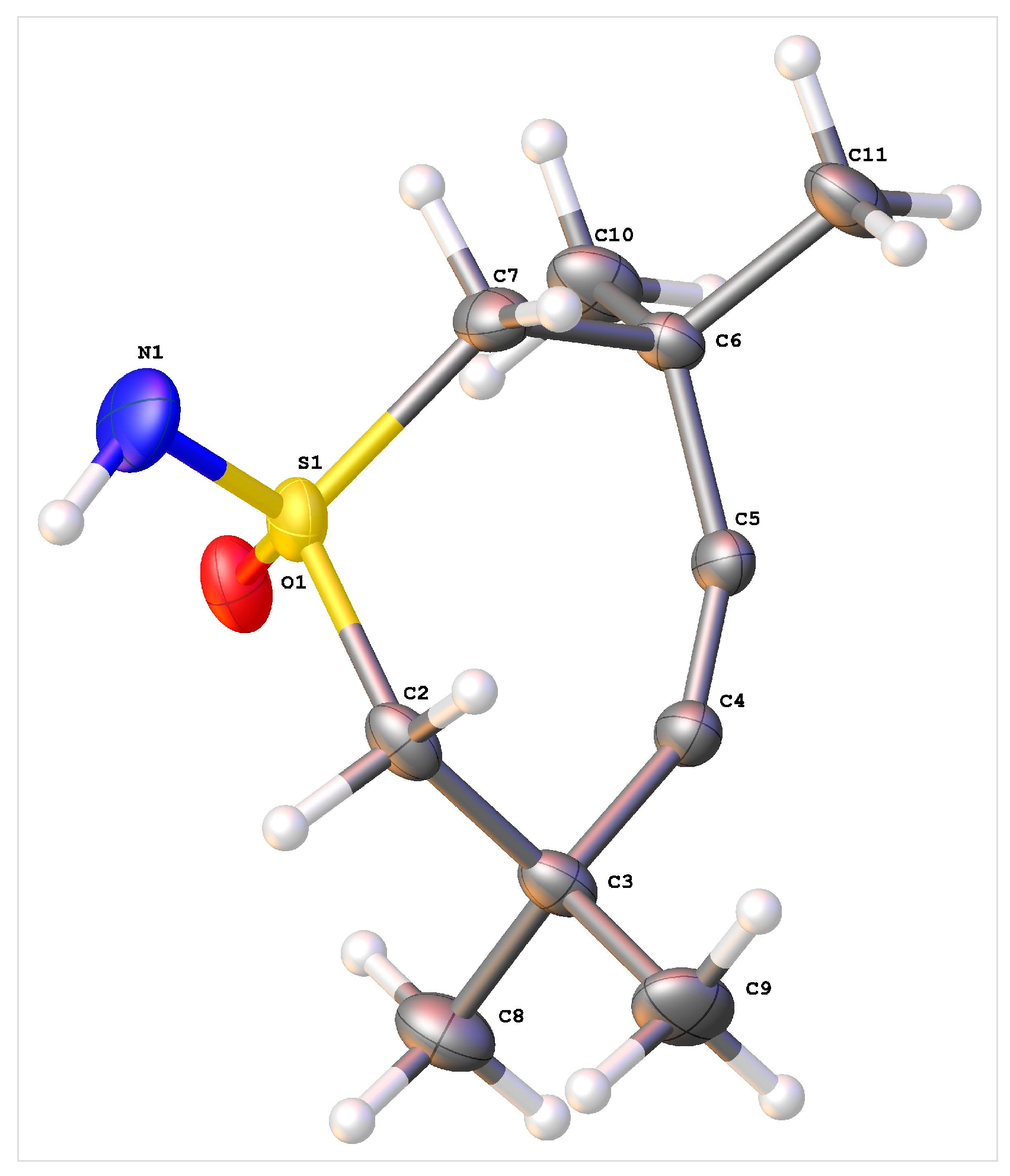
4.2. X-Ray Crystallography
4.3. Synthesis of 9
4.4. Peptide Coupling with 9 and Cleavage
4.5. Synthesis of 11
4.6. Synthesis of 12
4.7. Antibody Modification and Conjugation
4.8. Click Reaction of TMTHSI–FITC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem.—Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Debets, M.F.; Van Berkel, S.S.; Schoffelen, S.; Rutjes, F.P.J.T.; Van Hest, J.C.M.; Van Delft, F.L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Dommerholt, J.; Schmidt, S.; Temming, R.; Hendriks, L.J.A.; Rutjes, F.P.J.T.; Van Hest, J.C.M.; Lefeber, D.J.; Friedl, P.; Van Delft, F.L. Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew. Chem. Int. Ed. 2010, 49, 9422–9425. [Google Scholar] [CrossRef]
- Stump, B. Click Bioconjugation: Modifying Proteins Using Click-Like Chemistry. ChemBioChem 2022, 23, e202200016. [Google Scholar] [CrossRef]
- Liu, D.; Cohen, J.; Turkman, N. PEG2000-DBCO surface coating increases intracellular uptake of liposomes by breast cancer xenografts. Sci. Rep. 2022, 12, 10564. [Google Scholar] [CrossRef]
- Gai, M.; Simon, J.; Lieberwirth, I.; Mailänder, V.; Morsbach, S.; Landfester, K. A bio-orthogonal functionalization strategy for site-specific coupling of antibodies on vesicle surfaces after self-assembly. Polym. Chem. 2020, 11, 527–540. [Google Scholar] [CrossRef]
- Weterings, J.; Rijcken, C.J.F.; Veldhuis, H.; Meulemans, T.; Hadavi, D.; Timmers, M.; Honing, M.; Ippel, H.; Liskamp, R.M.J. TMTHSI, a superior 7-membered ring alkyne containing reagent for strain-promoted azide–alkyne cycloaddition reactions. Chem. Sci. 2020, 11, 9011–9016. [Google Scholar] [CrossRef] [PubMed]
- Lücking, U. New Opportunities for the Utilization of the Sulfoximine Group in Medicinal Chemistry from the Drug Designer’s Perspective. Chem.—Eur. J. 2022, 28, e202201993. [Google Scholar] [CrossRef]
- Giagou, T.; Meyer, M.P. Mechanism of the Swern Oxidation: Significant Deviations from Transition State Theory. J. Org. Chem. 2010, 75, 8088–8099. [Google Scholar] [CrossRef][Green Version]
- Parikh, J.R.; Doering, W.v.E. Sulfur trioxide in the oxidation of alcohols by dimethyl sulfoxide. J. Am. Chem. Soc. 1967, 89, 5505–5507. [Google Scholar] [CrossRef]
- Damen, J.A.M.; Escorihuela, J.; Zuilhof, H.; Van Delft, F.L.; Albada, B. High Rates of Quinone-Alkyne Cycloaddition Reactions are Dictated by Entropic Factors. Chem.—Eur. J. 2023, 29, e202300231. [Google Scholar] [CrossRef] [PubMed]
- Chigrinova, M.; McKay, C.S.; Beaulieu, L.-P.B.; Udachin, K.A.; Beauchemin, A.M.; Pezacki, J.P. Rearrangements and addition reactions of biarylazacyclooctynones and the implications to copper-free click chemistry. Org. Biomol. Chem. 2013, 11, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Erickson, P.W.; Fulcher, J.M.; Spaltenstein, P.; Kay, M.S. Traceless Click-assisted native chemical ligation enabled by protecting dibenzocyclooctyne from acid-mediated rearrangement with copper (I). Bioconjug. Chem. 2021, 32, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Janson, N.; Krüger, T.; Karsten, L.; Boschanski, M.; Dierks, T.; Müller, K.M.; Sewald, N. Bifunctional Reagents for Formylglycine Conjugation: Pitfalls and Breakthroughs. ChemBioChem 2020, 21, 3580–3593. [Google Scholar] [CrossRef]
- La–Venia, A.; Dzijak, R.; Rampmaier, R.; Vrabel, M. An Optimized Protocol for the Synthesis of Peptides Containing trans-Cyclooctene and Bicyclononyne Dienophiles as Useful Multifunctional Bioorthogonal Probes. Chem.—Eur. J. 2021, 27, 13632–13641. [Google Scholar] [CrossRef]
- Dennler, P.; Chiotellis, A.; Fischer, E.; Brégeon, D.; Belmant, C.; Gauthier, L.; Lhospice, F.; Romagne, F.; Schibli, R. Transglutaminase-Based Chemo-Enzymatic Conjugation Approach Yields Homogeneous Antibody–Drug Conjugates. Bioconjug. Chem. 2014, 25, 569–578. [Google Scholar] [CrossRef]
- Dickgiesser, S.; Rieker, M.; Mueller-Pompalla, D.; Schröter, C.; Tonillo, J.; Warszawski, S.; Raab-Westphal, S.; Kühn, S.; Knehans, T.; Könning, D.; et al. Site-Specific Conjugation of Native Antibodies Using Engineered Microbial Transglutaminases. Bioconjug. Chem. 2020, 31, 1070–1076. [Google Scholar] [CrossRef]
- Marculescu, C.; Lakshminarayanan, A.; Gault, J.; Knight, J.C.; Folkes, L.K.; Spink, T.; Robinson, C.V.; Vallis, K.; Davis, B.G.; Cornelissen, B. Probing the limits of Q-tag bioconjugation of antibodies. Chem. Commun. 2019, 55, 11342–11345. [Google Scholar] [CrossRef]
- Courtois, F.; Agrawal, N.J.; Lauer, T.M.; Trout, B.L. Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. mAbs 2016, 8, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Zhang, Q.; Zhang, L.; Zhu, X.; Du, C.; Zhang, Z. Development and validation of aggregates analysis method in analytical similarity assessment of HLX04 vs. Avastin®. J. Pharm. Biomed. Anal. 2023, 223, 115121. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timmers, M.; Kipper, A.; Frey, R.; Notermans, S.; Voievudskyi, M.; Wilson, C.; Hentzen, N.; Ringle, M.; Bovino, C.; Stump, B.; et al. Exploring the Chemical Properties and Medicinal Applications of Tetramethylthiocycloheptyne Sulfoximine Used in Strain-Promoted Azide–Alkyne Cycloaddition Reactions. Pharmaceuticals 2023, 16, 1155. https://doi.org/10.3390/ph16081155
Timmers M, Kipper A, Frey R, Notermans S, Voievudskyi M, Wilson C, Hentzen N, Ringle M, Bovino C, Stump B, et al. Exploring the Chemical Properties and Medicinal Applications of Tetramethylthiocycloheptyne Sulfoximine Used in Strain-Promoted Azide–Alkyne Cycloaddition Reactions. Pharmaceuticals. 2023; 16(8):1155. https://doi.org/10.3390/ph16081155
Chicago/Turabian StyleTimmers, Matt, Andi Kipper, Raphael Frey, Stef Notermans, Maksym Voievudskyi, Claire Wilson, Nina Hentzen, Michael Ringle, Clara Bovino, Bernhard Stump, and et al. 2023. "Exploring the Chemical Properties and Medicinal Applications of Tetramethylthiocycloheptyne Sulfoximine Used in Strain-Promoted Azide–Alkyne Cycloaddition Reactions" Pharmaceuticals 16, no. 8: 1155. https://doi.org/10.3390/ph16081155
APA StyleTimmers, M., Kipper, A., Frey, R., Notermans, S., Voievudskyi, M., Wilson, C., Hentzen, N., Ringle, M., Bovino, C., Stump, B., Rijcken, C. J. F., Vermonden, T., Dijkgraaf, I., & Liskamp, R. (2023). Exploring the Chemical Properties and Medicinal Applications of Tetramethylthiocycloheptyne Sulfoximine Used in Strain-Promoted Azide–Alkyne Cycloaddition Reactions. Pharmaceuticals, 16(8), 1155. https://doi.org/10.3390/ph16081155





