Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology
Abstract
1. Introduction
2. Materials and Methods
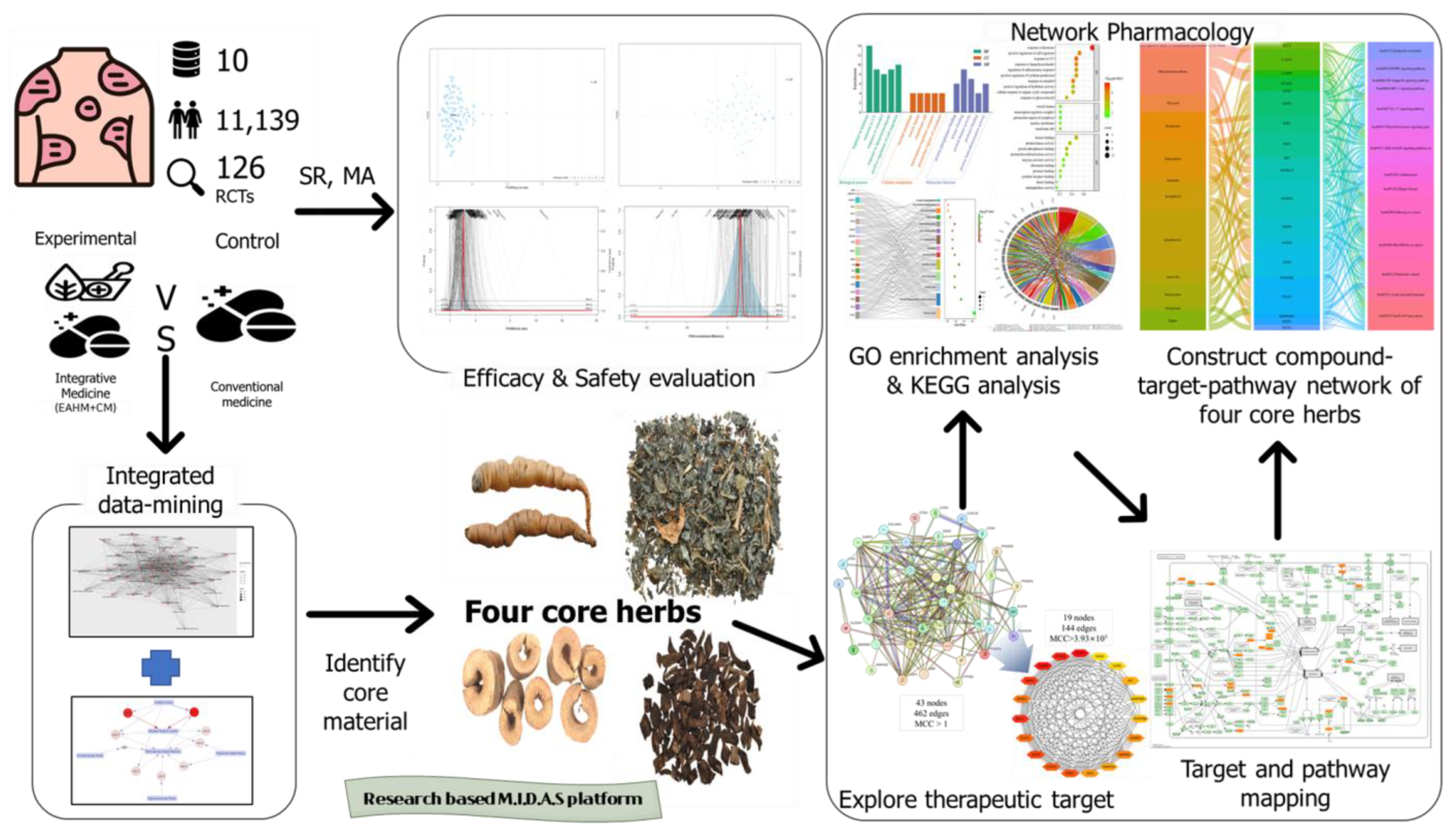
2.1. Research Workflow through Integrated Methodology
2.2. Data Sources and Search Strategy
2.3. Study Selection
2.3.1. Type of Studies
2.3.2. Type of Participants
2.3.3. Type of Interventions
2.3.4. Type of Outcome Measures
2.4. Data Extraction and Management
- Publication information (title, first author, year of publication, and funding source).
- Study characteristics (trial design, randomization method, sample size, treatment duration, and morbidity period).
- Participants (age, sex, diagnostic criteria, and number of participants in each group).
- Intervention (experimental intervention, comparator, ingredients, and detailed information on intervention frequency of medication, dosage, mode of delivery, and course of treatment).
- Outcomes (primary and secondary outcomes, measurement point, blinding of outcome assessment, and AEs).
2.5. Methodological Quality Assessment
2.6. Quality of Evidence according to Outcome Measures
2.7. Statistical Analysis
2.7.1. Data Synthesis of Clinical Outcomes
2.7.2. Deriving Core Herbs Based on Data Mining Approach
2.7.3. Prediction of Anti-Inflammatory Mechanisms Based on Network Pharmacology
3. Results
3.1. Study Identification
3.2. Study Characteristics
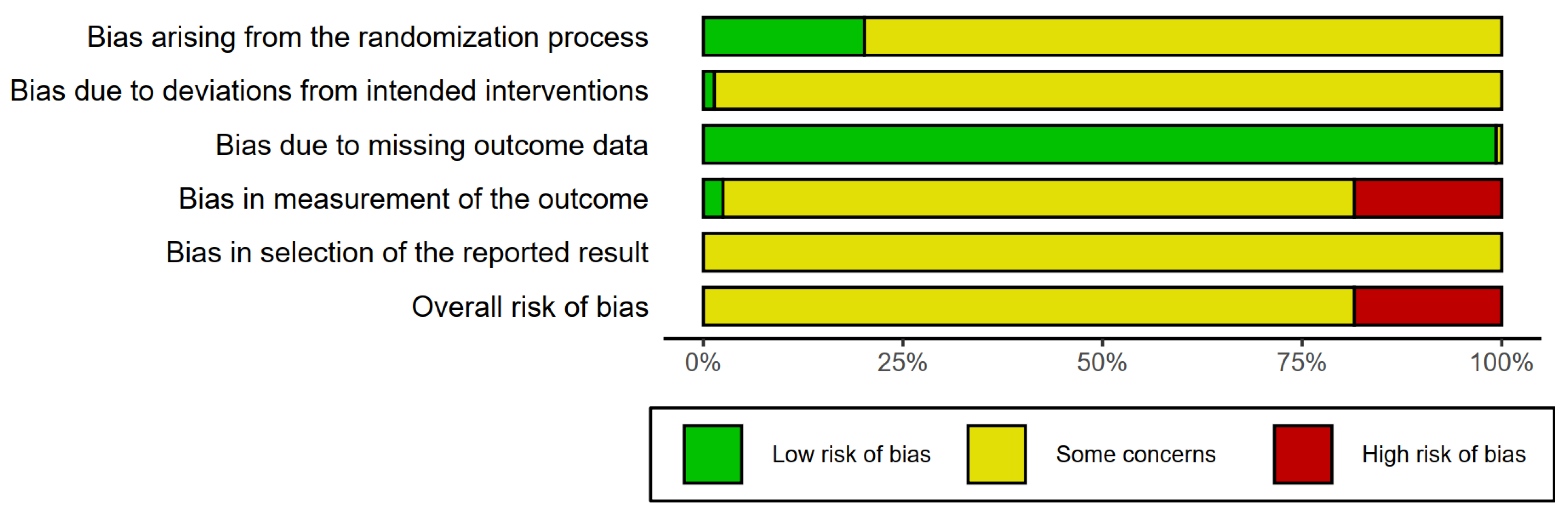
3.3. Risk of Bias
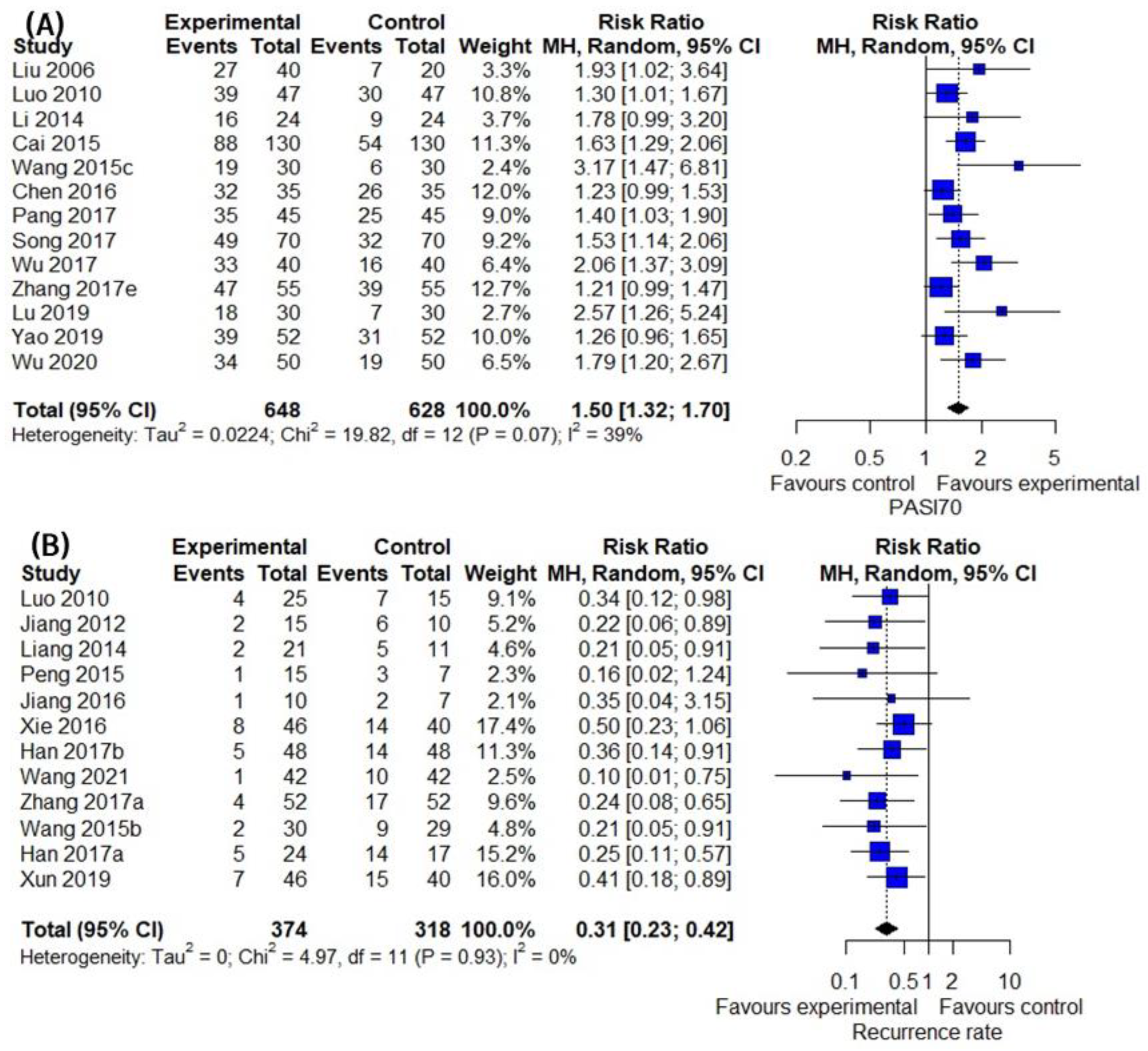
3.4. Primary Outcomes
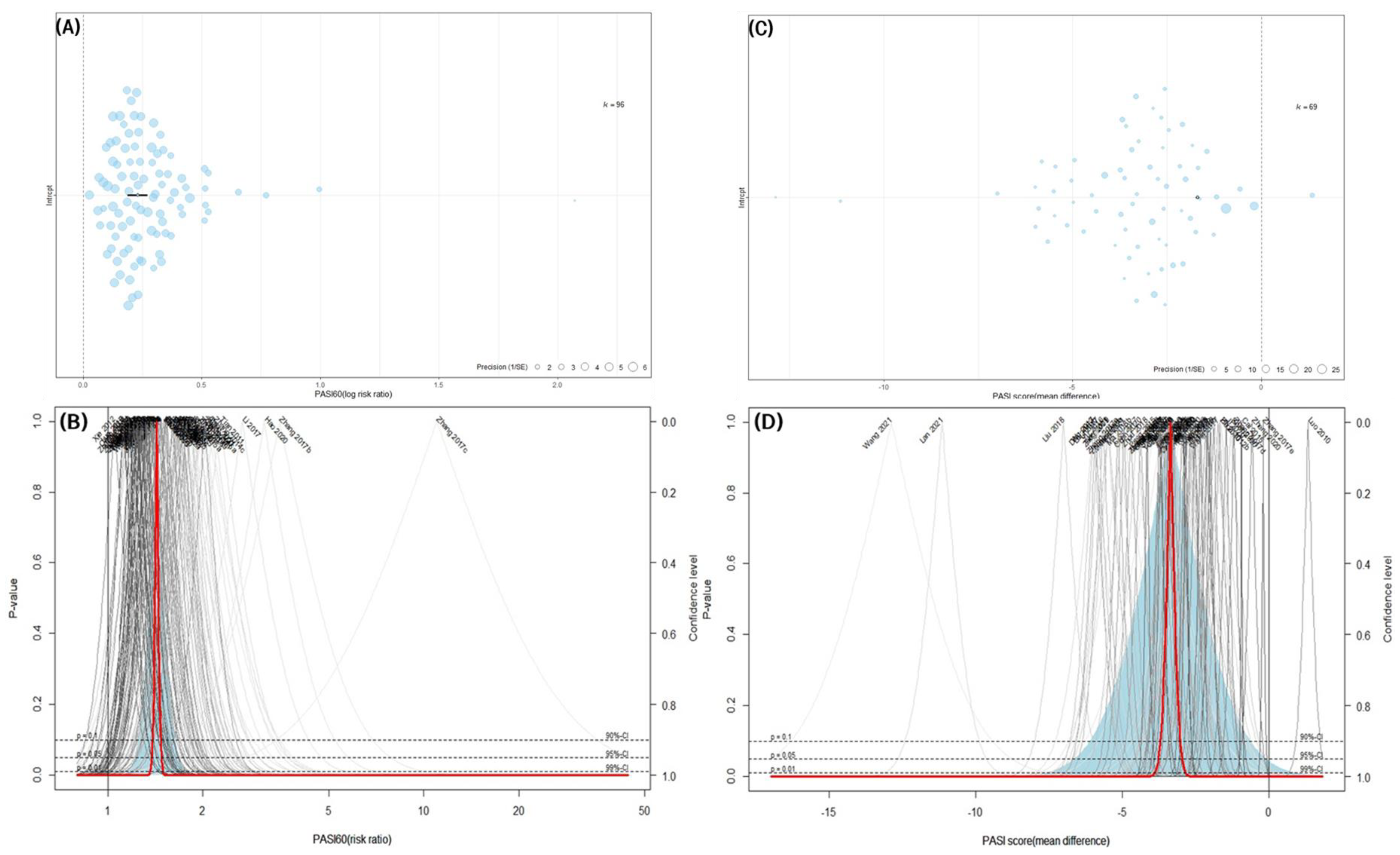
3.4.1. PASI 60
3.4.2. PASI Score
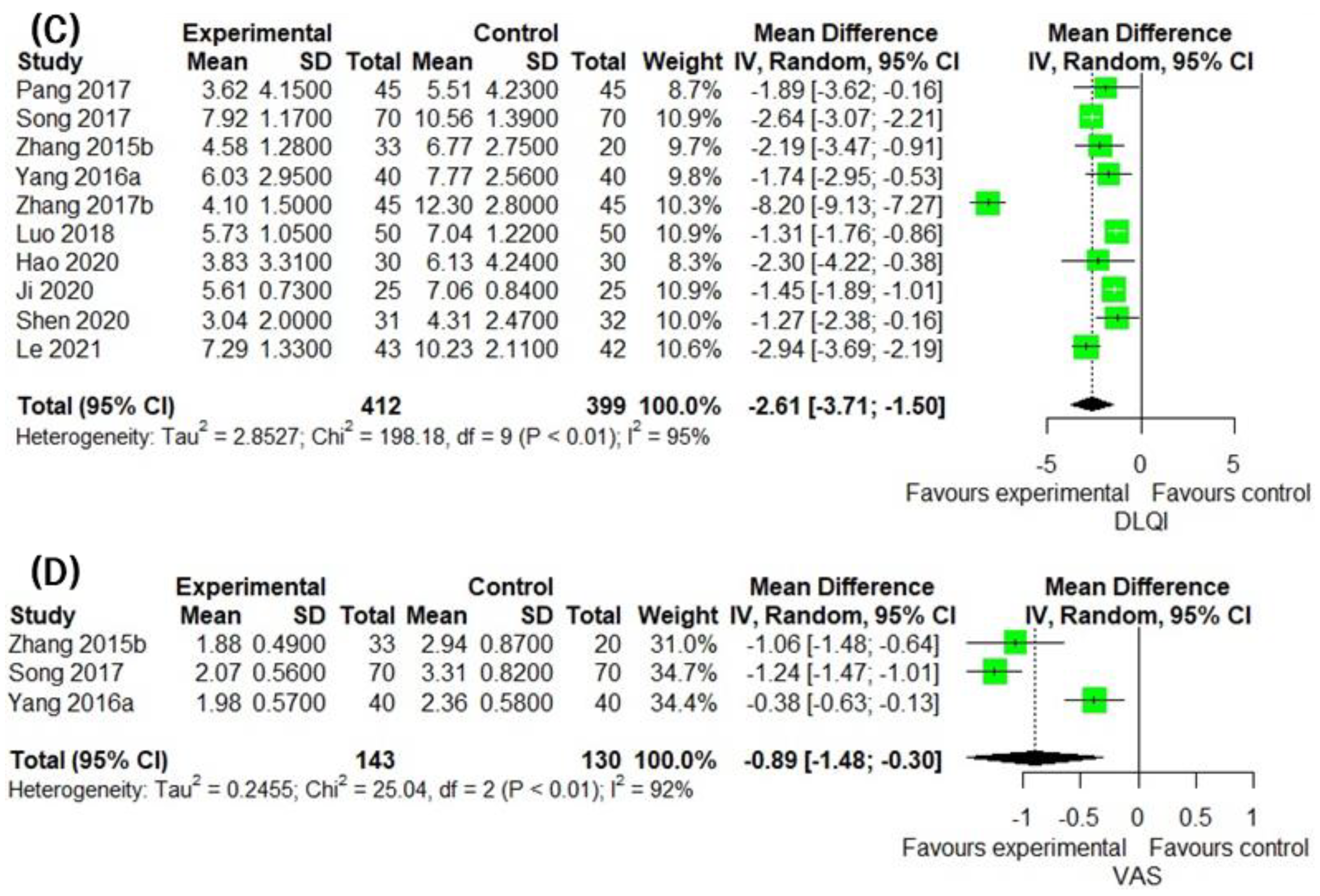
3.5. Secondary Outcomes Group 1: Assessment of Patient Outcome Related to Inflammatory Skin Lesion
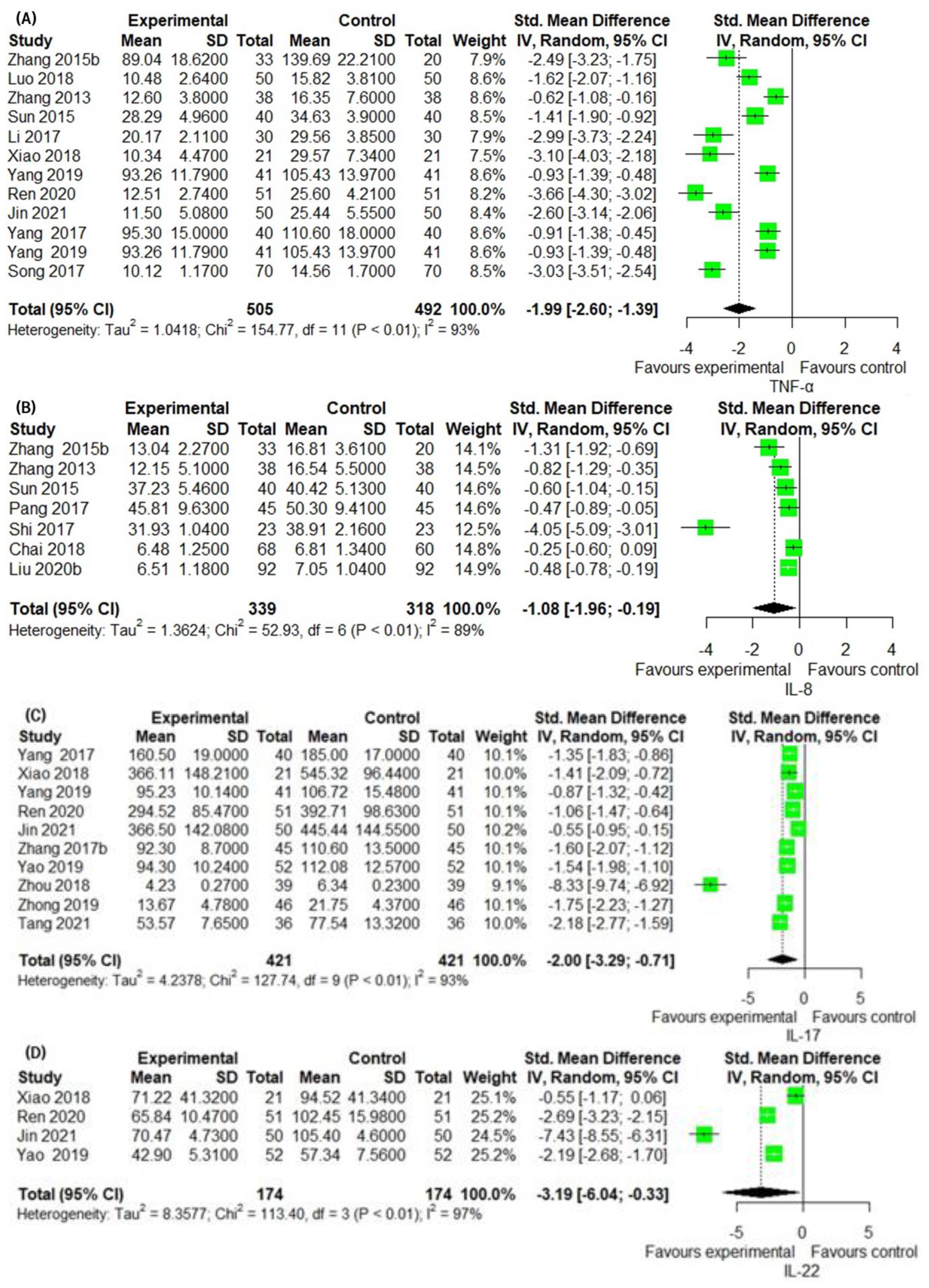
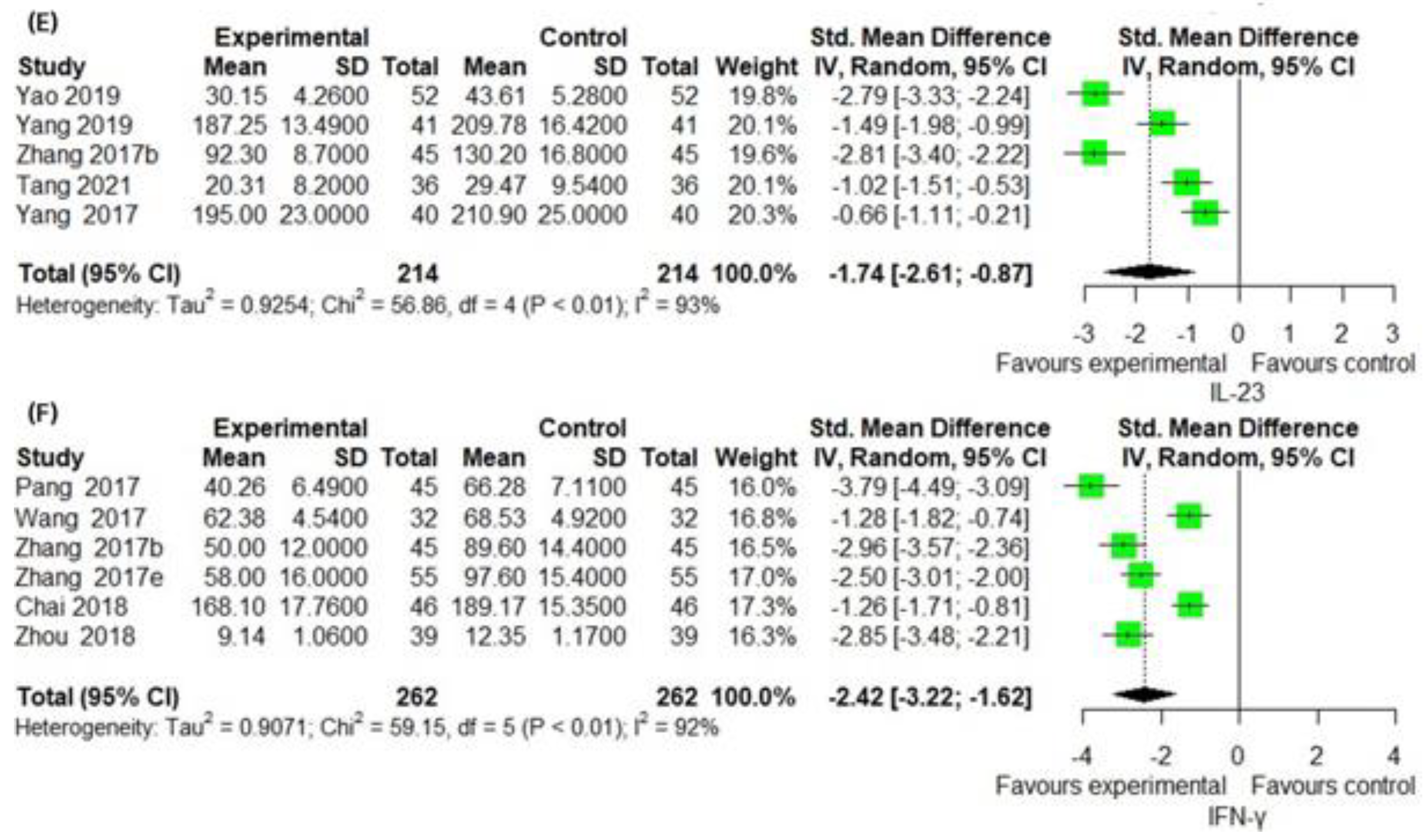
3.6. Secondary Outcomes Group 2: Assessment of Laboratory Biomarkers Related to Inflammatory Skin Lesion
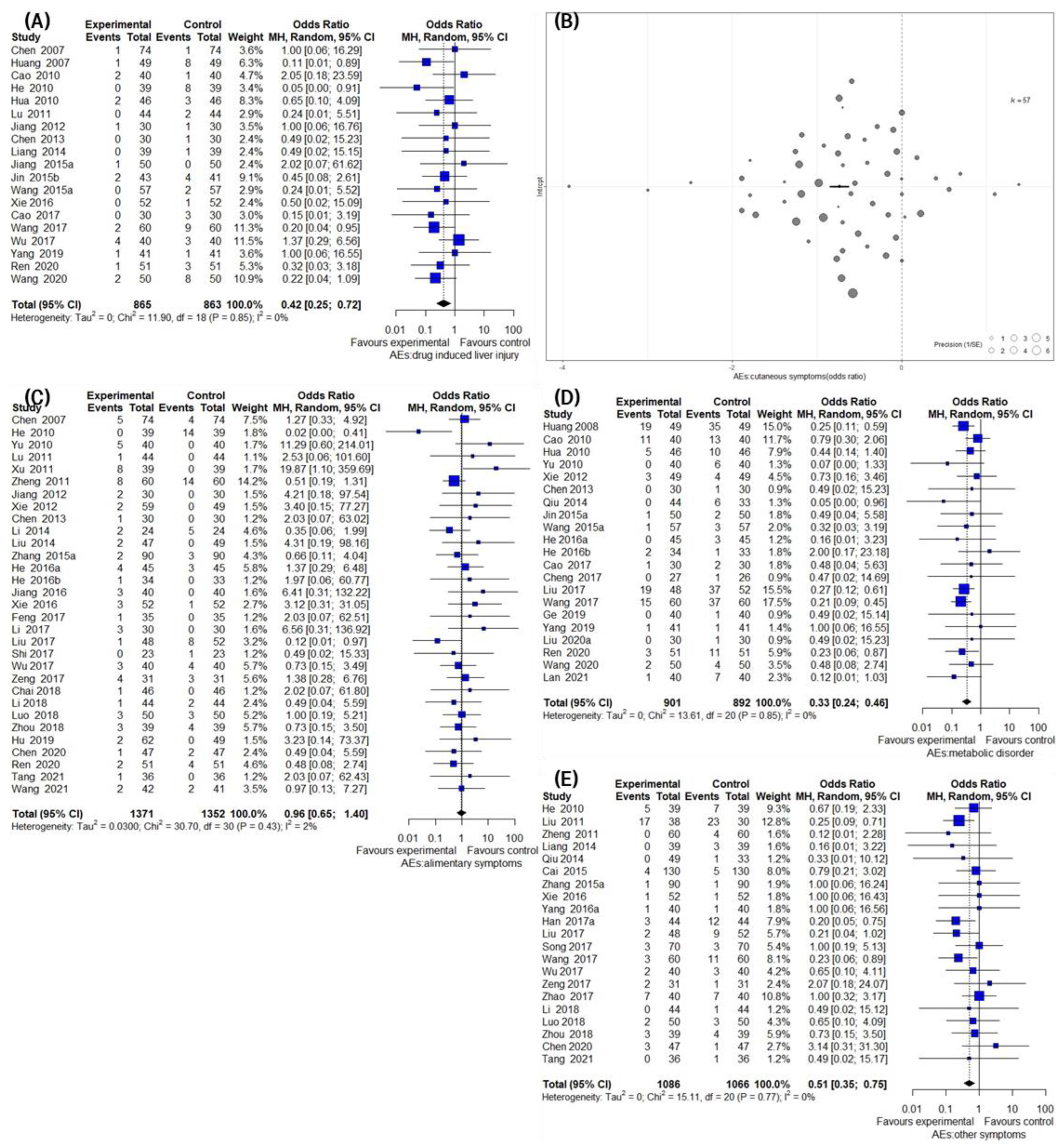
3.7. Secondary Outcomes Group 3: Safety Assessment
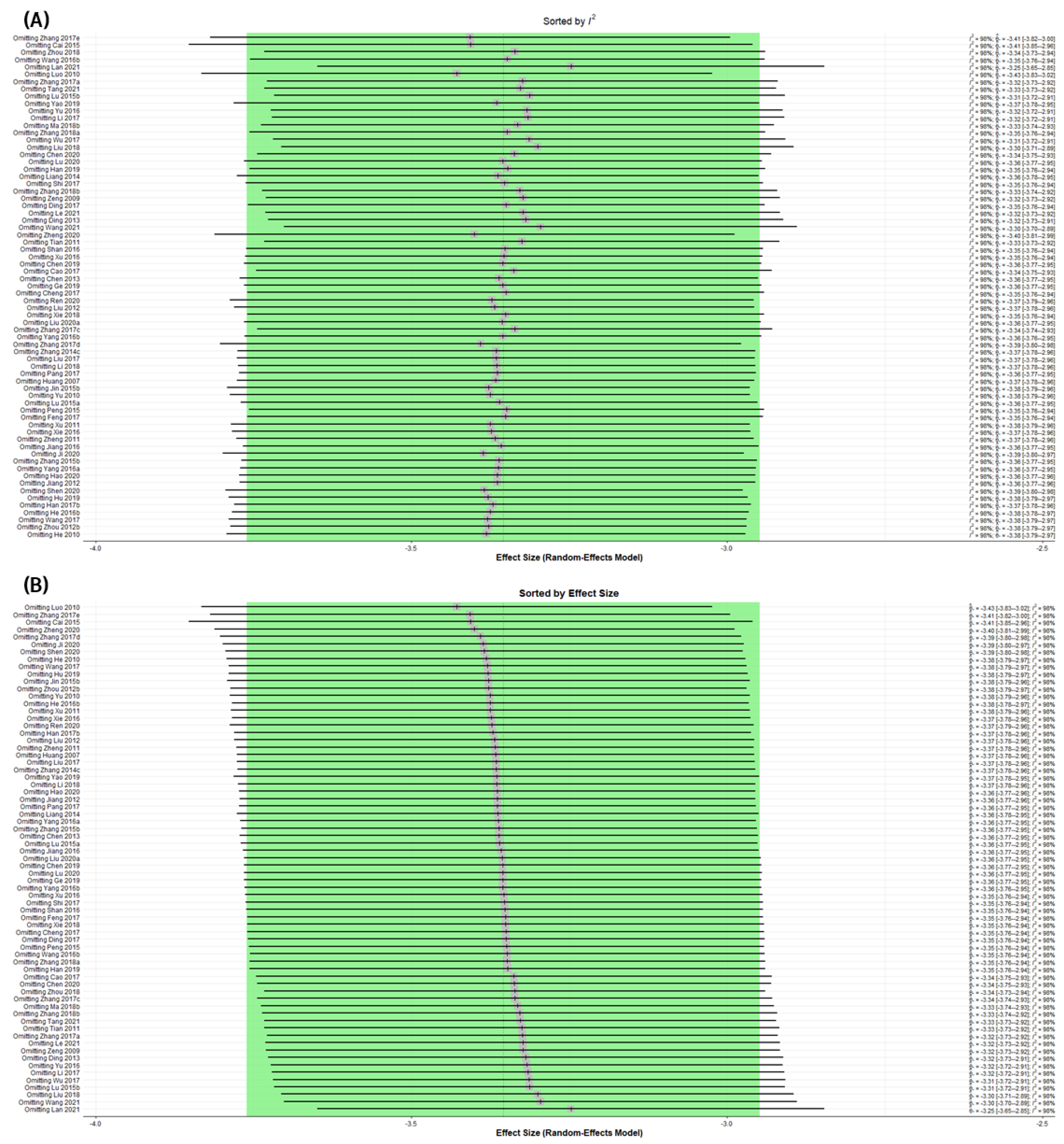
3.8. Assessing Heterogeneity
3.8.1. Sensitivity Analysis
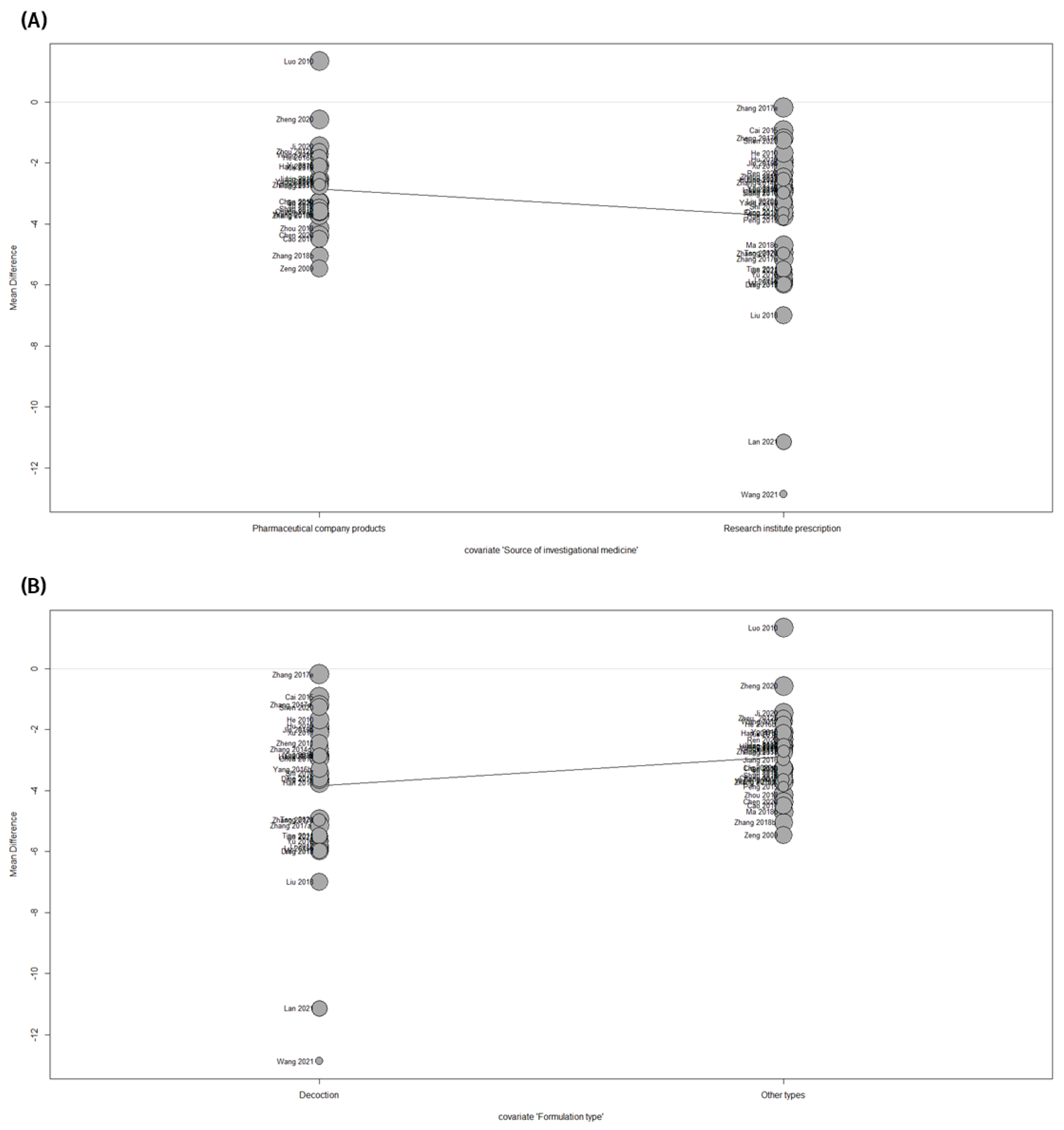
3.8.2. Meta-Regression and Subgroup Analysis
3.9. Assessing Publication Bias
3.10. Summary of Evidence according to Outcome Measures
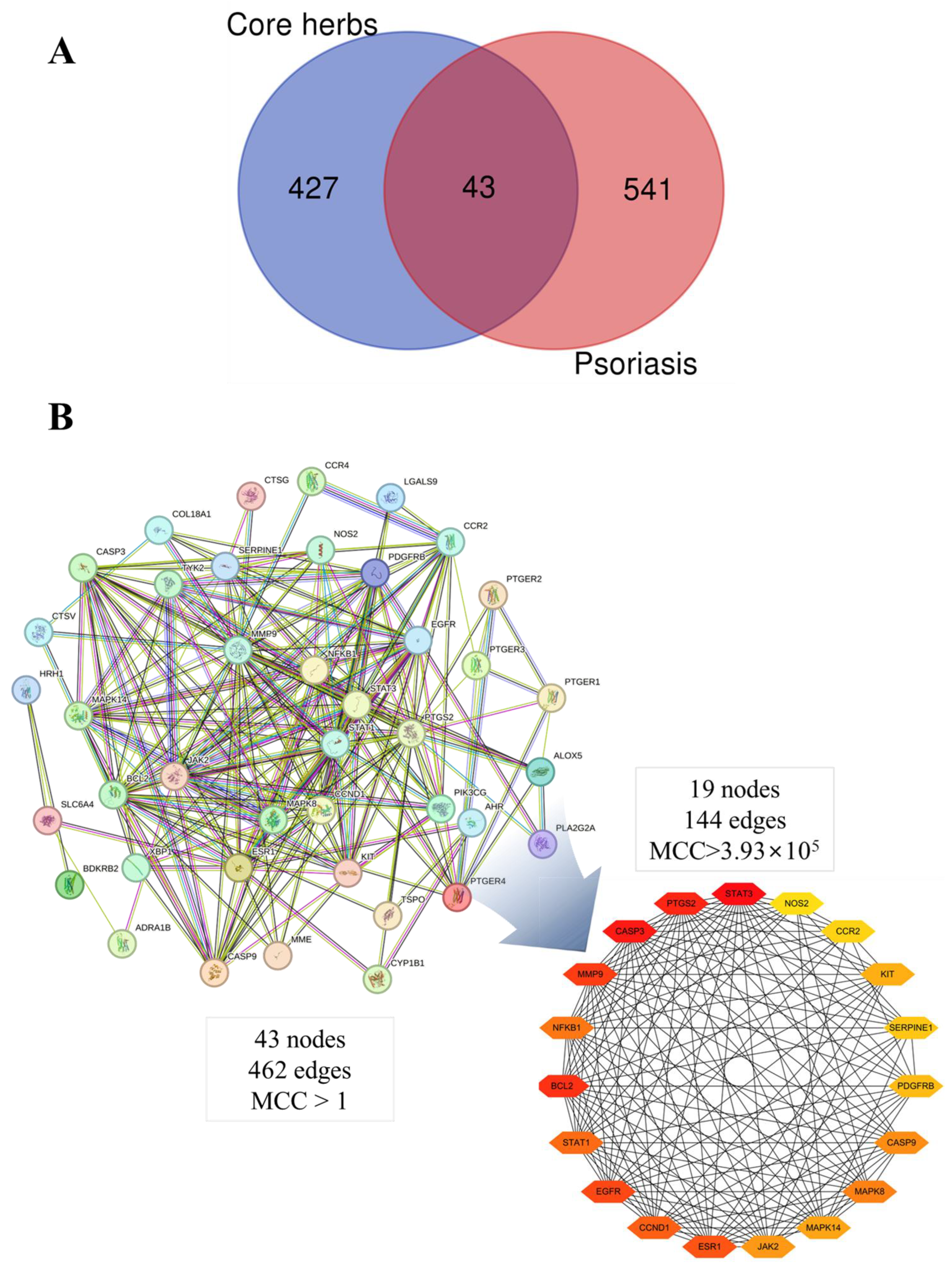
3.11. Core Herbs Discovery Based on Data Mining
3.11.1. Detailed Information on Investigational Medicine Ingredients
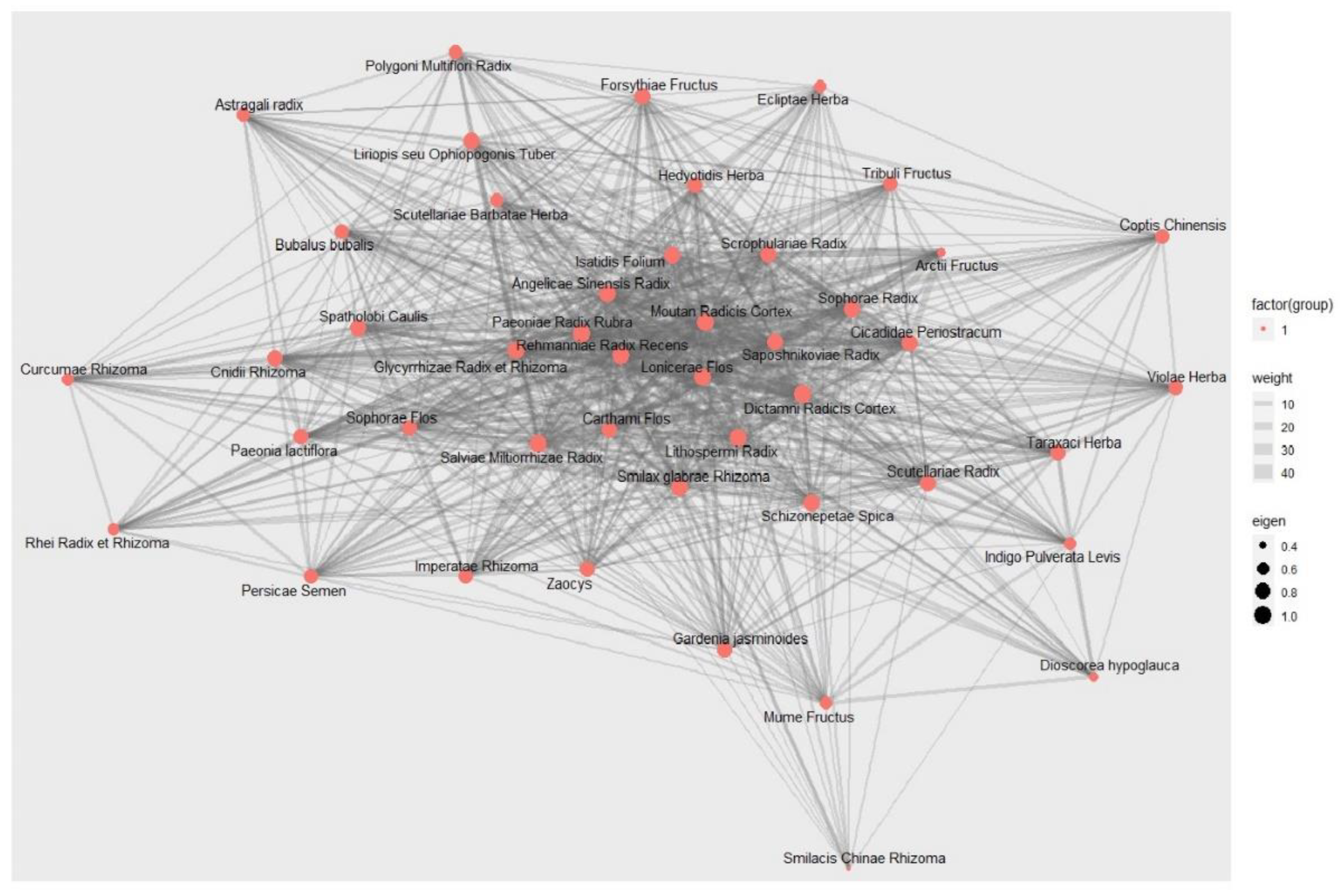
3.11.2. Social Network Analysis
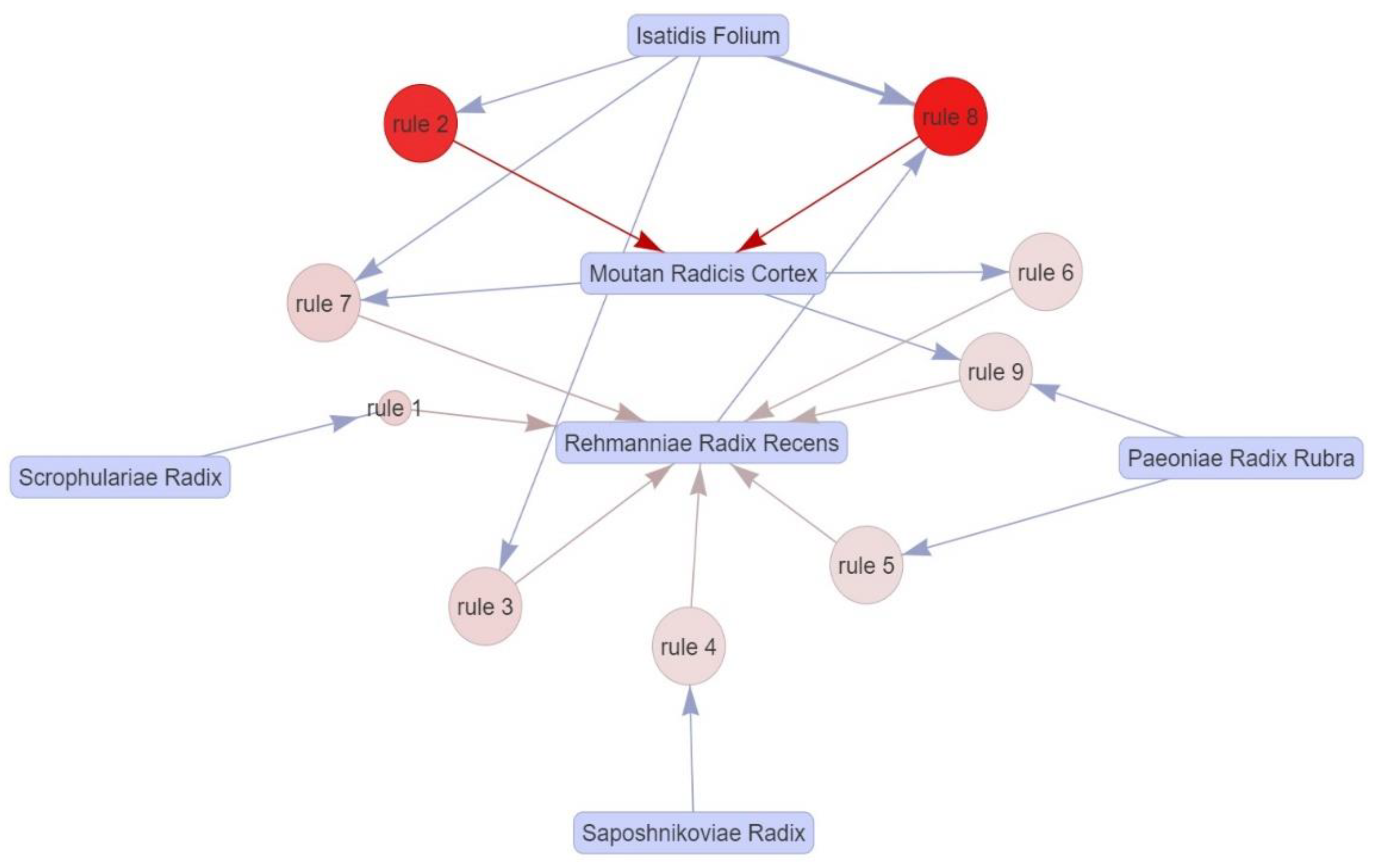
3.11.3. A Priori Algorithm-Based Association Rule Analysis
3.11.4. Derivation of Core Herbs
3.12. Analysis of Four Core Herbs through Network Pharmacology
3.12.1. Active Ingredients and Anti-Psoriasis Targets of Four Core Herbs
3.12.2. PPI Network Construction
3.12.3. GO and KEGG Pathway Enrichment Analyses
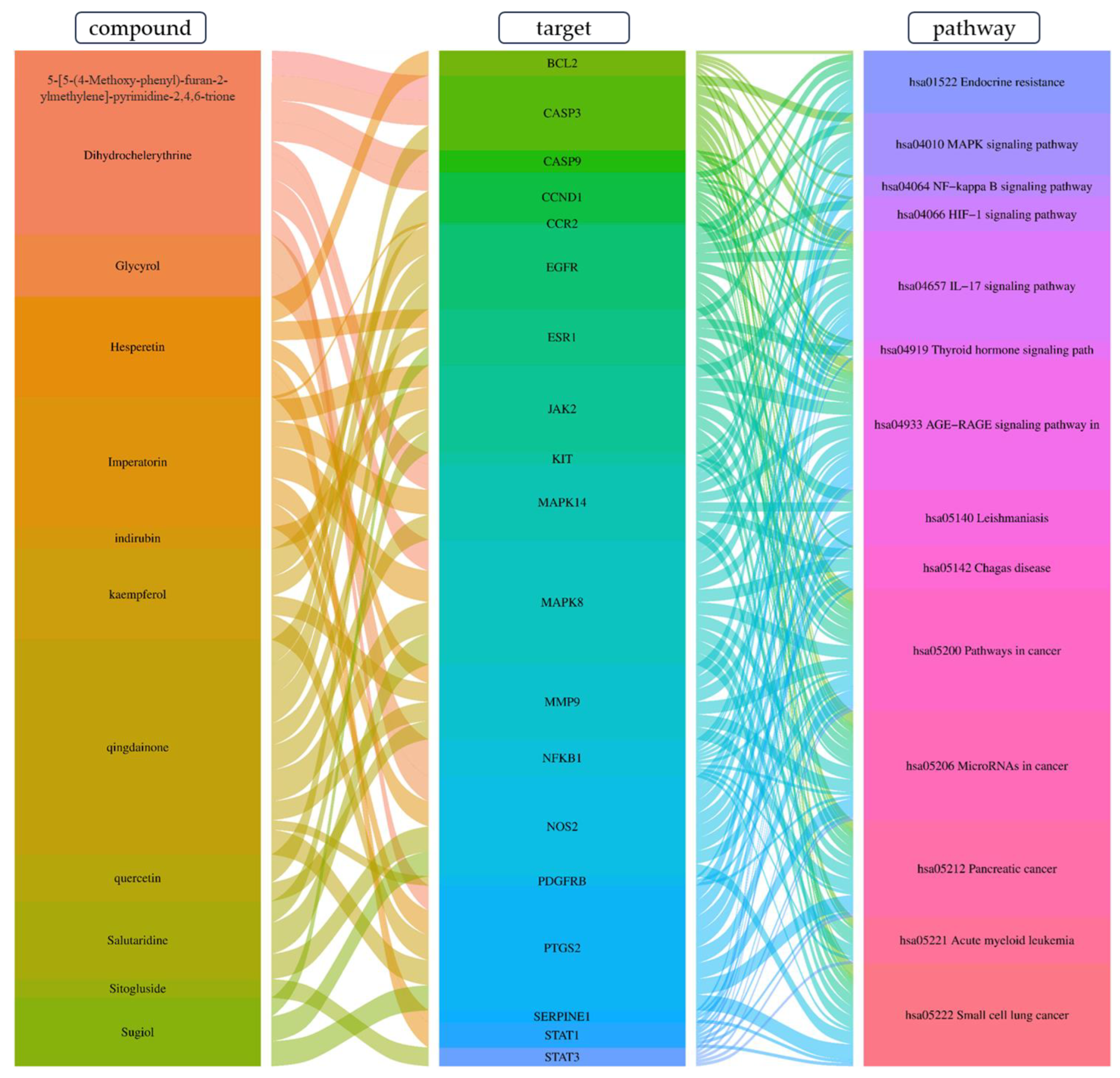
3.12.4. Construction of Compounds–Target-Pathway Network of Four Core Herbs against Psoriasis
4. Discussion
4.1. Summary of the Main Findings and Comparision with Previous Research
4.2. What Are the Limitations and Future Tasks of This Study?
4.3. Implications of the Four Core Herbs That Emerged from This Study
4.4. Possible Pharmacological Mechanisms of Four Core Herbs aginst Psoriasis
4.4.1. Pharmacology of Individual Crude Herbs to Support Efficacy
4.4.2. The Key Therapeutic Targets and Pathways Associated with the Four Core Herbs
4.4.3. Promising Therapeutic Targets in Psoriasis Unexplained by the Pharmacology of Four Core Herbs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Egeberg, A.; Andersen, Y.M.F.; Thyssen, J.P. Prevalence and Characteristics of Psoriasis in Denmark: Findings from the Danish Skin Cohort. BMJ Open 2019, 9, e028116. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound Healing and Keratinocytes. Pharmacol. Res. 2022, 183, 106393. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Greenberg, J.D.; Karki, C.; Mason, M.; Guo, N.; Hur, P.; Zhao, Y.; Herrera, V.; Lin, F.; Lebwohl, M. Impact of Psoriasis Severity on Patient-Reported Clinical Symptoms, Health-Related Quality of Life and Work Productivity among US Patients: Real-World Data from the Corrona Psoriasis Registry. BMJ Open 2019, 9, e027535. [Google Scholar] [CrossRef]
- Mattei, P.L.; Corey, K.C.; Kimball, A.B. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The Correlation between Disease Severity and Psychological Burden in Patients Treated with Biological Therapies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and Suicidality: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440.e2. [Google Scholar] [CrossRef]
- Semenov, Y.R.; Herbosa, C.M.; Rogers, A.T.; Huang, A.; Kwatra, S.G.; Cohen, B.; Anadkat, M.J.; Silverberg, J.I. Psoriasis and Mortality in the United States: Data from the National Health and Nutrition Examination Survey. J. Am. Acad. Dermatol. 2021, 85, 396–403. [Google Scholar] [CrossRef]
- Amin, M.; Lee, E.B.; Tsai, T.-F.; Wu, J.J. Psoriasis and Co-Morbidity. Acta Derm. Venereol. 2020, 100, adv00033. [Google Scholar] [CrossRef]
- Talotta, R.; Atzeni, F.; Sarzi-Puttini, P.; Masala, I.F. Psoriatic Arthritis: From Pathogenesis to Pharmacologic Management. Pharmacol. Res. 2019, 148, 104394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Wu, R.; Li, S.; Su, Y.; Zhang, P. Prevalence of Autoimmune Thyroid Disease in Patients with Psoriasis: A Meta-Analysis. BMJ Open 2022, 12, e055538. [Google Scholar] [CrossRef]
- Korman, N.J. Management of Psoriasis as a Systemic Disease: What Is the Evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Dabholkar, N.; Rapalli, V.K.; Singhvi, G. Potential Herbal Constituents for Psoriasis Treatment as Protective and Effective Therapy. Phytother. Res. 2020, 35, 2429–2444. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris—Part 1: Treatment and Monitoring Recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Hu, S.; Lin, C.; Cai, X.; Zhu, X.; Ji, L. Association between Biologic Therapy and Fracture Incidence in Patients with Selected Rheumatic and Autoimmune Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 2022, 181, 106278. [Google Scholar] [CrossRef]
- Ortiz, N.E.G.; Nijhawan, R.I.; Weinberg, J.M. Acitretin. Dermatol. Ther. 2013, 26, 390–399. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side Effects of Methotrexate Therapy for Rheumatoid Arthritis: A Systematic Review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- An, J.; Zhang, D.; Wu, J.; Li, J.; Teng, X.; Gao, X.; Li, R.; Wang, X.; Xia, L.; Xia, Y. The Acitretin and Methotrexate Combination Therapy for Psoriasis Vulgaris Achieves Higher Effectiveness and Less Liver Fibrosis. Pharmacol. Res. 2017, 121, 158–168. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Kesharwani, P.; Khan, S.; Hussain, F. Phytotherapeutic Potential of Natural Herbal Medicines for the Treatment of Mild-to-Severe Atopic Dermatitis: A Review of Human Clinical Studies. Biomed. Pharmacother. 2017, 93, 596–608. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Ren, J.-L.; Yang, L.; Qiu, S.; Zhang, A.-H.; Wang, X.-J. Efficacy Evaluation, Active Ingredients, and Multitarget Exploration of Herbal Medicine. Trends Endocrinol. Metab. 2023, 34, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.S.; Ko, S.-G. Introduction to the History and Current Status of Evidence-Based Korean Medicine: A Unique Integrated System of Allopathic and Holistic Medicine. Evid. Based Complement. Altern. Med. 2014, 2014, 740515. [Google Scholar] [CrossRef]
- Shim, J.-M.; Kim, J. Cross-National Differences in the Holistic Use of Traditional East Asian Medicine in East Asia. Health Promot. Int. 2018, 33, 536–544. [Google Scholar] [CrossRef]
- Shim, J.-M.; Lee, Y.-S. The Association between the Use of Biomedical Services and the Holistic Use of Traditional East Asian Medicine: A National Survey of Outpatients in South Korea. BMJ Open 2017, 7, e018414. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-M. The Relationship Between the Use of Complementary and Alternative Medicine and the Use of Biomedical Services: Evidence from East Asian Medical Systems. Asia Pac. J. Public Health 2016, 28, 51–60. [Google Scholar] [CrossRef]
- de Seabra Rodrigues Dias, I.R.; Lo, H.H.; Zhang, K.; Law, B.Y.K.; Nasim, A.A.; Chung, S.K.; Wong, V.K.W.; Liu, L. Potential Therapeutic Compounds from Traditional Chinese Medicine Targeting Endoplasmic Reticulum Stress to Alleviate Rheumatoid Arthritis. Pharmacol. Res. 2021, 170, 105696. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Ou, J.; Yu, J.; Lu, C. Effect of Chinese Herbal Medicine Injections for Treatment of Psoriasis Vulgaris: A Systematic Review and Meta-Analysis. Front Pharmacol 2023, 14, 1148445. [Google Scholar] [CrossRef]
- Jo, H.-G.; Baek, E.; Lee, D. Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix–Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology. Pharmaceutics 2023, 15, 1361. [Google Scholar] [CrossRef]
- Kim, H.U.; Ryu, J.Y.; Lee, J.O.; Lee, S.Y. A Systems Approach to Traditional Oriental Medicine. Nat. Biotechnol. 2015, 33, 264–268. [Google Scholar] [CrossRef]
- Cha, W.-S.; Oh, J.-H.; Park, H.-J.; Ahn, S.-W.; Hong, S.-Y.; Kim, N.-I. Historical Difference between Traditional Korean Medicine and Traditional Chinese Medicine. Neurol. Res. 2007, 29 (Suppl. 1), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-W.; Chen, B.-C.; Wang, Y.-C.; Liu, C.-K.; Sun, M.-F.; Chang, C.-M.; Lin, J.-G.; Yen, H.-R. Traditional Chinese Medicine Use among Patients with Psoriasis in Taiwan: A Nationwide Population-Based Study. Evid. Based Complement. Altern. Med. 2016, 2016, 3164105. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Qin, W.; Wu, L.; Yang, B.; Wang, Q.; Kuang, H.; Cheng, G. A Review of Chinese Medicine for the Treatment of Psoriasis: Principles, Methods and Analysis. Chin. Med. 2021, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; El-Shazly, M.; Tsai, Y.-H.; Csupor, D.; Hohmann, J.; Wu, Y.-C.; Tseng, T.-G.; Chang, F.-R.; Wang, H.-C. Uncovering Modern Clinical Applications of Fuzi and Fuzi-Based Formulas: A Nationwide Descriptive Study with Market Basket Analysis. Front. Pharmacol. 2021, 12, 641530. [Google Scholar] [CrossRef] [PubMed]
- Coyle, M.E.; Yu, J.J.; Zhang, A.L.; Jones, L.; Xue, C.C.; Lu, C. Patient Experiences of Using Chinese Herbal Medicine for Psoriasis Vulgaris and Chronic Urticaria: A Qualitative Study. J. Dermatol. Treat. 2020, 31, 352–358. [Google Scholar] [CrossRef]
- Park, M.; Hunter, J.; Kwon, S. Evaluating Integrative Medicine Acute Stroke Inpatient Care in South Korea. Health Policy 2018, 122, 373–379. [Google Scholar] [CrossRef]
- Alschuler, L.; Chiasson, A.M.; Horwitz, R.; Sternberg, E.; Crocker, R.; Weil, A.; Maizes, V. Integrative Medicine Considerations for Convalescence from Mild-to-Moderate COVID-19 Disease. Explore (NY) 2022, 18, 140–148. [Google Scholar] [CrossRef]
- Kim, D.; Shin, J.-S.; Moon, Y.-J.; Ryu, G.; Shin, W.; Lee, J.; Lim, S.; Jeon, H.A.; Seo, J.-Y.; Wang, W.H.; et al. Long-Term Follow-Up of Spinal Stenosis Inpatients Treated with Integrative Korean Medicine Treatment. J. Clin. Med. 2020, 10, 74. [Google Scholar] [CrossRef]
- Yu, H.-H.; Hsieh, C.-J. Integrative Therapy Combining Chinese Herbal Medicines with Conventional Treatment Reduces the Risk of Cardiovascular Disease among Patients with Systemic Lupus Erythematosus: A Retrospective Population-Based Cohort Study. Front. Pharmacol. 2021, 12, 737105. [Google Scholar] [CrossRef]
- Parvizi, M.M.; Salami, M.H.; Moini Jazani, A.; Javaheri, R.; Jaladat, A.M.; Handjani, F. Complementary and Integrative Remedies in the Treatment of Chronic Pruritus: A Review of Clinical Trials. J. Cosmet. Dermatol. 2022, 21, 5360–5369. [Google Scholar] [CrossRef]
- Melby, M.K.; Yoshino, T.; Tonob, D.; Horiba, Y.; Watanabe, K. Differences in Demographics and Complementary and Alternative Medicine Use between Patients Attending Integrative Kampo versus Biomedical Clinics in Japan. Complement. Ther. Med. 2019, 46, 202–209. [Google Scholar] [CrossRef]
- Motoo, Y.; Yukawa, K.; Hisamura, K.; Arai, I. Physician Perspectives on Traditional, Complementary, and Integrative Medicine and the National Evidence-Based Japanese Integrative Medicine Information Website: A Mixed-Method Study. Integr. Med. Res. 2021, 10, 100454. [Google Scholar] [CrossRef]
- Heo, I.; Hwang, M.-S.; Hwang, E.-H.; Cho, J.-H.; Ha, I.-H.; Shin, K.-M.; Lee, J.-H.; Kim, N.-K.; Son, D.-W.; Shin, B.-C. Electroacupuncture as a Complement to Usual Care for Patients with Non-Acute Low Back Pain after Back Surgery: A Pilot Randomised Controlled Trial. BMJ Open 2018, 8, e018464. [Google Scholar] [CrossRef]
- Leung, E.L.-H.; Wu, Q.-B. Concurrent Use of Herbal Products with Prescription Drugs Is a Double-Edged Sword and Evidence-Based Medicine Contributes to Reshaping the Practice. Pharmacol. Res. 2019, 141, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, H.G.; Min, S.Y. East Asian Herbal Medicine Combined with Conventional Therapy for Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Explore 2022, 18, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; May, B.H.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C.; Zhang, H. Integrative Herbal Medicine for Chemotherapy-Induced Peripheral Neuropathy and Hand-Foot Syndrome in Colorectal Cancer: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2019, 18, 1534735418817833. [Google Scholar] [CrossRef]
- Chen, L.; Michalsen, A. Management of Chronic Pain Using Complementary and Integrative Medicine. BMJ 2017, 357, j1284. [Google Scholar] [CrossRef]
- Ang, L.; Song, E.; Lee, M.S. Randomized Controlled Trials of Traditional, Complementary, and Integrative Medicine-Based Interventions for Coronavirus Disease 2019 (COVID-19): A Bibliometric Analysis and Review of Study Designs. Integr. Med. Res. 2021, 10, 100777. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, L.; Ma, R.; Keeler, C.L.; Shen, L.; Bao, Y.; Xu, S. Comprehensive Rehabilitation with Integrative Medicine for Subacute Stroke: A Multicenter Randomized Controlled Trial. Sci. Rep. 2016, 6, 25850. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.Y.-H.; Wu, M.-Y.; Lin, C.-L.; Lin, J.-G.; Chang, C.Y.-Y.; Lin, W.-C.; Yen, H.-R. Adjunctive Chinese Herbal Medicine Treatment Is Associated with an Improved Survival Rate in Patients with Cervical Cancer in Taiwan: A Matched Cohort Study. Integr. Cancer Ther. 2021, 20, 15347354211061752. [Google Scholar] [CrossRef]
- Xiong, Y.; Gao, M.; van Duijn, B.; Choi, H.; van Horssen, F.; Wang, M. International Policies and Challenges on the Legalization of Traditional Medicine/Herbal Medicines in the Fight against COVID-19. Pharmacol. Res. 2021, 166, 105472. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, H.G.; Min, S.Y. East Asian Herbal Medicine for the Treatment of Children with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Explore 2023, 19, 330–355. [Google Scholar] [CrossRef]
- Jo, H.-G.; Seo, J.; Choi, S.; Lee, D. East Asian Herbal Medicine to Reduce Primary Pain and Adverse Events in Cancer Patients: A Systematic Review and Meta-Analysis with Association Rule Mining to Identify Core Herb Combination. Front. Pharmacol. 2021, 12, 800571. [Google Scholar] [CrossRef]
- Jo, H.-G.; Lee, D. Oral Administration of East Asian Herbal Medicine for Peripheral Neuropathy: A Systematic Review and Meta-Analysis with Association Rule Analysis to Identify Core Herb Combinations. Pharmaceuticals 2021, 14, 1202. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Tao, D. Investigation on the Mechanism of Qubi Formula in Treating Psoriasis Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 4683254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Peng, L.; Qin, Y.; Jing, M.; Huang, D.; Guo, J.; Xiao, M.; Chen, M. Benefits and Safety of Chinese Herbal Medicine in Treating Psoriasis: An Overview of Systematic Reviews. Front. Pharmacol. 2021, 12, 680172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Yang, L.; Zhang, A.L.; May, B.H.; Yu, J.J.; Guo, X.; Lu, C.; Xue, C.C. Is Oral Chinese Herbal Medicine Beneficial for Psoriasis Vulgaris? A Meta-Analysis of Comparisons with Acitretin. J. Altern. Complement. Med. 2016, 22, 174–188. [Google Scholar] [CrossRef]
- Deng, S.; May, B.H.; Zhang, A.L.; Lu, C.; Xue, C.C.L. Topical Herbal Formulae in the Management of Psoriasis: Systematic Review with Meta-Analysis of Clinical Studies and Investigation of the Pharmacological Actions of the Main Herbs. Phytother. Res. 2014, 28, 480–497. [Google Scholar] [CrossRef]
- Parker, S.; Zhang, C.S.; Yu, J.J.; Lu, C.; Zhang, A.L.; Xue, C.C. Oral Chinese Herbal Medicine versus Placebo for Psoriasis Vulgaris: A Systematic Review. J. Dermatol. Treat. 2017, 28, 21–31. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Kuai, L.; Zhang, Y.; Ding, X.; Luo, Y.; Ru, Y.; Xing, M.; Li, H.; Sun, X.; et al. Chinese Herbal Medicine for Psoriasis: Evidence from 11 High-Quality Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 599433. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Bae, K.-H.; Lee, Y.; Park, K.-H.; Yoon, Y.; Mun, S.; Lee, S. Perception of Cold and Heat Pattern Identification in Diseases: A Survey of Korean Medicine Doctors. Integr. Med. Res. 2017, 6, 26–32. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.A.; Alraek, T.; Bian, Z.-X.; Birch, S.; Goto, H.; Jung, J.; Kao, S.-T.; Moon, S.-K.; Park, B.; et al. Current Research and Future Directions in Pattern Identification: Results of an International Symposium. Chin. J. Integr. Med. 2016, 22, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Braga, F.C.; Buenz, E.J.; Campana, P.R.V.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kim, H.; Jo, H.-G.; Hwang, J.-H.; Lee, D. Integrative Medicine (East Asian Herbal Medicine Combined with Conventional Medicine) for Psoriasis: A Protocol for Systematic Review and Meta-Analysis. Medicine 2023, 102, e32360. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Lortie, C.J.; Filazzola, A. A Contrast of Meta and Metafor Packages for Meta-Analyses in R. Ecol. Evol. 2020, 10, 10916–10921. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Beyond the Forest Plot: The Drapery Plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef]
- Nakagawa, S.; Lagisz, M.; O’Dea, R.E.; Rutkowska, J.; Yang, Y.; Noble, D.W.A.; Senior, A.M. The Orchard Plot: Cultivating a Forest Plot for Use in Ecology, Evolution, and Beyond. Res. Synth. Methods 2021, 12, 4–12. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-Enhanced Meta-Analysis Funnel Plots Help Distinguish Publication Bias from Other Causes of Asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Natarajan, S.; Jeong, S.Y.; Hong, B.N.; Kang, T.H. Traditional Oriental Medicine for Sensorineural Hearing Loss: Can Ethnopharmacology Contribute to Potential Drug Discovery? J. Ethnopharmacol. 2019, 231, 409–428. [Google Scholar] [CrossRef]
- Agrawal, R.; Imieliński, T.; Swami, A. Mining Association Rules between Sets of Items in Large Databases. In Proceedings of the 1993 ACM SIGMOD International Conference on Management of Data, Washington, DC, USA, 25–28 May 1993; pp. 207–216. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminformatics 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Fang, Z.; Zhang, M.; Yi, Z.; Wen, C.; Shi, T. TCMID: Traditional Chinese Medicine Integrative Database for Herb Molecular Mechanism Analysis. Nucleic Acids Res. 2013, 41, D1089–D1095. [Google Scholar] [CrossRef]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A High-Throughput Experiment- and Reference-Guided Database of Traditional Chinese Medicine. Nucleic Acids Res. 2021, 49, D1197–D1206. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Zhang, Y.-Q.; Liu, Z.-M.; Chen, T.; Lv, C.-Y.; Tang, S.-H.; Zhang, X.-B.; Zhang, W.; Li, Z.-Y.; Zhou, R.-R.; et al. ETCM: An Encyclopaedia of Traditional Chinese Medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG Mapping Tools for Uncovering Hidden Features in Biological Data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Shi, B. 23 Cases of Blood Heat Type Psoriasis Treated with Liangxuexiaofeng Decoction. Guangming J. Chin. Med. 2017, 32, 3122–3124. [Google Scholar] [CrossRef]
- Song, X. 43 Cases of Psoriasis Vulgaris Treated with Xiaoyin Granules. China Pharm. 2013, 22, 82. [Google Scholar]
- Zheng, X. 60 Cases of Psoriasis Vulgaris Treated with Xiaoyinkeji Decoction. Henan Tradit. Chin. Med. 2011, 31, 383–384. [Google Scholar] [CrossRef]
- Chen, B. 74 Cases of Psoriasis Vulgaris Treated by Xiaoyin Granules Combined with Awei A Capsules. Mod. Tradit. Chin. Med. 2007, 27, 29–30. [Google Scholar]
- He, Y.; Yang, Y.; Yong, M.; Li, C.; Xiong, X. A Clinical Study of Compound Qingdai Pill Combined with Acitretin and Calcipotriol in the Treatment of Patients with Moderate to Severe Psoriasis Vulgaris. Chin. J. Dermatovenereology 2016, 30, 769–770. [Google Scholar] [CrossRef]
- Cheng, J. Analysis of Curative Effect and Safety of Compound Glycyrrhizin and Acitretin Capsules in Treatment of Plaque Psoriasis. China Foreign Med. Treat. 2017, 24, 126–128. [Google Scholar] [CrossRef]
- Lu, X. Analysis of Curative Effect of Total Glucosides of Peony Root in the Treatment of Psoriasis. Dermatol. Venereol. 2020, 42, 689–691. [Google Scholar]
- Chen, X. Analysis of the Effect of Combined Use of Calcipotriol Ointment and Traditional Chinese Medicine in the Treatment of Psoriasis. Contemp. Med. Symp. 2016, 14, 17–18. [Google Scholar]
- Han, J. Analysis of the Effect of Qinzhuliangxue Feng Combined with Awei A Capsules in the Treatment of Psoriasis Vulgaris. Guide China Med. 2019, 17, 170. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Shi, X.; Yu, X. Analysis of the Short-Term Effect of Qingreliangxue Decoction in the Treatment of Progressive Psoriasis Vulgaris. Dermatol. Venereol. 2021, 43, 212–213. [Google Scholar]
- Qiu, S.; Lin, A. Avi A Combined Traditional Chinese Medicine Treatment of Skin Diseases, Psoriasis Clinical Experience. China Health Ind. 2014, 12, 5–6. [Google Scholar] [CrossRef]
- Wang, Y. Clinical Analysis of Awei A Capsule Combined with Piminxiao Capsule in the Treatment of Plaque Psoriasis. All Health 2016, 10, 137–138. [Google Scholar]
- Yang, S.; Gao, X. Clinical Analysis of Xiaoyin Granule Combined with Avia Capsule in the Treatment of 78 Cases of Psoriasis. Electron. J. Clin. Med. Lit. 2020, 7, 4–5. [Google Scholar] [CrossRef]
- Lu, Z. Clinical Application Effect of Qingying Decoction in the Treatment of 62 Cases of Psoriasis. Nei Mong. J. Tradit. Chin. Med. 2015, 30, 19. [Google Scholar] [CrossRef]
- Wu, W. Clinical Effect Analysis of Xiaoyin Granules Combined with Awei A Capsules in the Treatment of Psoriasis Vulgaris. World Latest Med. Inf. 2016, 16, 132. [Google Scholar]
- Yang, X.; Sun, D.; Zeng, W.; He, Q.; Ma, L.; Tao, M. Clinical Effect and Regulation of Peripheral-Blood Mir-21 Expression of Qingre Liangxue Decoction on Psoriasis Patients with Blood Heat Syndrome. China J. Tradit. Chin. Med. Pharm. 2016, 31, 2299–2301. [Google Scholar]
- Zhang, X.; Yuan, X.; Liu, C.; Li, H. Clinical Effect Observation of Taohong Ershao Decoction in Treating Psoriasis Vulgaris of Blood Stasis Type. Clin. J. Chin. Med. 2017, 9, 79–80. [Google Scholar]
- Xie, J.; Xi, J.; Li, X.; Xie, W. Clinical Effect of Formulated Granules of Qingying Decoction Combined with Acitretin Capsules in Treatment of Psoriasis Vulgaris with Blood-Heat Syndrome: An Analysis of 60 Cases. Hunan J. Tradit. Chin. Med. 2018, 34, 18–20. [Google Scholar] [CrossRef]
- Wang, H. Clinical Effect of Xiaoyin Granules Combined with Awei A Capsules in the Treatment of Psoriasis Vulgaris. Womens Health Res. 2016, 1, 212. [Google Scholar]
- Song, Y.; Li, F.; Li, Y.; Liu, X.; Yang, F. Clinical Effect of Yangxuetongluo Decoction in the Treatment of Plaque Psoriasis and Its Influence on the Levels of IL-6,TNF-α and VEGF. Mod. J. Integr. Tradit. Chin. West. Med. 2017, 26, 2298–2301. [Google Scholar]
- Li, D.; Zhao, J.; Su, Q. Clinical Effect of Yinxie Capsule Combined with Acitretin in Treatment of Patients with Psoriasis Vulgaris and Its Effect on Immune Function of T Lymphocytes in Peripheral Blood. Clin. Misdiagnosis Mistherapy 2018, 31, 98–101. [Google Scholar] [CrossRef]
- He, G. Clinical Efficacy and Significance of Liangxuerunfu Decoction in the Treatment of Psoriasis Vulgaris. Nei Mong. J. Tradit. Chin. Med. 2016, 31, 23. [Google Scholar] [CrossRef]
- Zhang, J. Clinical Efficacy of Compound Glycyrrhizin Combined with Avi A Capsules in the Treatment of Psoriasis. World Latest Med. Inf. 2018, 18, 107. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Yuan, X.; Li, H. Clinical Efficacy of the Taohong Ershao Decoction on Psoriasis of the Xueyu Type and the Influence on Serum Immune Cells. Clin. J. Chin. Med. 2017, 9, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, X.; Liu, C.; Li, H. Clinical Efficacy of the Taohongershao Decoction on Psoriasis of the Xueyu Type and the Influence on Serum Immune Cells. Clin. J. Chin. Med. 2017, 9, 14–16. [Google Scholar]
- Du, A.; Pan, L.; Li, B.; Zhang, H. Clinical Experience of Calcipotriol Betamethasone Ointment Combined with Paeony Total Glycosides Capsules in the Treatment of Psoriasis Vulgaris. Nei Mong. J. Tradit. Chin. Med. 2017, 33, 79. [Google Scholar] [CrossRef]
- Liu, H.; Li, H. Clinical Observation of 47 Psoriasis Vulgaris Treated by Runzaozhiyang Capsule Combined with Compound Flumetasone Ointment. Chin. J. Dermatovenereology 2014, 28, 654–655. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S. Clinical Observation of Acitretin Capsule Combined with Yin-Xie Capsule in Treating 48 Psoriasis with Blood Deficiency and Wind-Dryness Syndrome. Chin. J. Dermatovenereology 2017, 31, 474–476. [Google Scholar] [CrossRef]
- Lan, X. Clinical Observation of Awei A Capsule Combined with Dangguiyinzi in the Treatment of Psoriasis Vulgaris. Chin. J. Mod. Drug Appl. 2021, 15, 230–232. [Google Scholar] [CrossRef]
- Lu, X. Clinical Observation of Diyin Tablet Combined with Traditional Chinese Medicine in the Treatment of Psoriasis Vulgaris. Intern. Med. China 2015, 10, 197–198. [Google Scholar]
- Hu, R.; Zhou, F.; Zhou, X. Clinical Observation of Liangxuexiaobi Decoction Combined Calcipotriol Betamethasone Ointment in the Treatment of Psoriasis Vulgaris. World Clin. Drugs 2019, 40, 790–794. [Google Scholar] [CrossRef]
- Hao, J.; Xi, J.; Li, L.; Shang, S. Clinical Observation of Liangxuexiaobi Pills Combined with Calcipotriol Ointment in Treating Bloodheat Syndrome of Psoriasis Vulgaris. Clin. J. Tradit. Chin. Med. 2020, 32, 2335–2338. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Nie, Z. Clinical Observation of Liangxuexiaofeng Decoction Combined with Acitretin in the Treatment of 34 Cases of Psoriasis with Blood Heat Type. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2017, 16, 59–60. [Google Scholar]
- Zhong, J. Clinical Observation of Piminxiao Capsule Combined with Awei A Capsule in the Treatment of Psoriasis Vulgaris. World Latest Med. Inf. 2019, 19, 189. [Google Scholar]
- Han, K. Clinical Observation of Xiaoyin Granule Combined with Acitretin Capsule for Treating Psoriasis Vulgaris in 44 Cases. China Pharm. 2017, 26, 83–85. [Google Scholar]
- Zhou, L. Clinical Observation of Xiaoyin Granules Combined with Awei A Capsules in Treating 70 Cases of Psoriasis Vulgaris. J. Aerosp. Med. 2012, 23, 1352–1353. [Google Scholar]
- Du, Q. Clinical Observation of Xiaoyin Granules Combined with Awei A Capsules in Treating 70 Cases of Psoriasis Vulgaris. China Foreign Med. Treat. 2014, 21, 121–122. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, M.; Ban, X. Clinical Observation of Yanghe Decoction Combined with Awei A Capsule in the Treatment of 30 Cases of Pustular Psoriasis. Nei Mong. J. Tradit. Chin. Med. 2014, 30, 1. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J. Clinical Observation of Yinxie Capsule Combined with Acitretin Capsule in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology 2012, 26, 279–280. [Google Scholar]
- Zhao, X. Clinical Observation of Yinxie Capsule Combined with Avi A Capsule in the Treatment of Psoriasis Vulgaris. Med. Forum 2017, 21, 2201–2202. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Y.; Liao, L.; Wang, L. Clinical Observation of Yinxieling Tablet Combinate with Acitretin Capsule in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology 2007, 21, 643–644. [Google Scholar]
- Feng, R.; Xi, J.; Li, X.; Qi, L.; Xie, W. Clinical Observation of Yinxieping Pill Combined with Compound Flumetasone Ointment in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2017, 2017, 57–59. [Google Scholar]
- Jiang, N.; Xi, J.; Li, X.; Qi, L.; Xie, W. Clinical Observation of Yinxieping Pills Combined with Calcipotriol Ointment on Treatment of Psoriasis Vulgaris in Resting Stage. J. Hunan Univ. Chin. Med. 2016, 36, 55–57. [Google Scholar]
- Jin, X. Clinical Observation on 50 Cases of Psoriasis Vulgaris Treated by Awei A Capsule Combined with Matrine Tablets. Chin. J. Ethnomedicine Ethnopharmacy 2015, 22, 72. [Google Scholar]
- Shen, W.; Ma, L.; Fang, Y.; Chen, C.; Ding, H.; Shen, S. Clinical Observation on Liangxuejiedu Decoction Combined with External Application of Western Medicine for Psoriasis Vulgaris with Blood Heat Syndrome. J. New Chin. Med. 2020, 52, 47–49. [Google Scholar] [CrossRef]
- Xun, Y. Clinical Observation on Psoriasis Vulgaris Treated with Psoriasis Granules and Avian Capsules. Electron. J. Clin. Med. Lit. 2019, 6, 6–7. [Google Scholar]
- Yu, X.; Wu, L.; Xiong, W. Clinical Observation on the Treatment of Psoriasis Vulgaris with Acitretin Combined with Runzaozhiyang Capsules. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2010, 9, 111–112. [Google Scholar]
- Mo, Y. Clinical Observation on the Treatment of Psoriasis Vulgaris with Total Glycosides of Paeoniae Alba Combined with Compound Aminopeptide Tablets. Asia Pac. Tradit. Med. 2013, 9, 159–160. [Google Scholar]
- Liu, Z.; Liu, H. Clinical Observation on Treatment of Quiescent Psoriasis Vulgaris with Jianpi Yishen Decoction. Xinjiang J. Tradit. Chin. Med. 2006, 24, 46–47. [Google Scholar]
- Lu, Y. Clinical Research on Psoriasis Treated with Qingrejiedu Decoction Combined with Etretinate, Tigason. China J. Chin. Med. 2011, 26, 1001–1002. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, H. Clinical Study on Keyin Pills Combined with Acitretin Capsules in Treatment of Psoriasis. Drugs Clin. 2018, 33, 101–104. [Google Scholar]
- Wang, Y.; Qi, Y.; Zhang, Y. Clinical Study on the Treatment of Psoriasis Vulgaris with Researcher Prescribed Traditional Chinese Medicine Decoction Combined with Acitretin. Asia Pac. Tradit. Med. 2015, 11, 115–116. [Google Scholar]
- Yao, H.; Xu, K.; Shan, B. Clinical Study on Xiaoyin Granules in Treating Psoriasis Vulgaris. Chin. J. Mod. Drug Appl. 2011, 5, 66–67. [Google Scholar] [CrossRef]
- Xie, C.; Fu, M. Clinical Study on Xiaoying Granules Combined with Acitretin in Treatment of Psoriasis Vulgaris. Drugs Clin. 2016, 31, 1834–1837. [Google Scholar]
- Liu, J.; Liu, Y.; Li, J.; Zhang, Y. Clinical Treatment of Psoriasis Vulgaris with Acitretin and Runzaozhiyang Capsule. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2012, 11, 32–33. [Google Scholar]
- Zhang, N. Comparative Analysis of Combined and Single Use of Avi A Capsules in the Treatment of Psoriasis Vulgaris. Clin. Med. 2014, 34, 43–44. [Google Scholar]
- Ge, M.; Lu, H. Curative Effect of Combination of Compound Glycyrrhizin and Acitretin A Capsule in Treatment of Psoriasis Vulgaris. J. Med. Forum 2019, 40, 29–31. [Google Scholar]
- Ding, Y.; Li, T.; Wang, Z.; Liu, Y.; Chen, Q.; Cheng, X.; Lin, S. Effect of NiupixuanⅡ Decoction on TGF-Β1 of Lymphocytes in Patients with Psoriasis. J. Dermatol. Venereol. 2013, 12, 144–146. [Google Scholar]
- Liu, Y.; Sun, D.; Yan, X. Effect of Banzhilian Decoction on Psoriasis Vulgaris Patients with Blood-Heat Syndrome. Guid. J. Tradit. Chin. Med. Pharm. 2018, 24, 81–83. [Google Scholar] [CrossRef]
- Jin, J. Effect of Xiaoyin Decoction on Immune Function and Serum Levels of IL-17,IL-22 and TNF-α in Patient with Psoriasis Vulgaris. Guangming J. Chin. Med. 2021, 36, 1390–1392. [Google Scholar]
- Yang, C.; Lin, J.; Wang, W.; Wang, Y.; Han, P.; Wang, B. Effects of Compound Qingdai Capsule Combined with Calcipotriol Betamethasone Cream on Serum IL-17, TNF-α and IL-23 Levels in Patients with Psoriasis. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2019, 18, 602–604. [Google Scholar]
- Wang, H.; Huang, P.; Yang, Z. Effects of Danggui-Yinzi Granules on the Skin Barrier Functions of Psoriasis Patients with Blood-Deficiency and Wind-Dryness. J. Hunan Univ. Chin. Med. 2015, 35, 41–43. [Google Scholar]
- Ren, F.; Ni, X.; Liu, J. Effects of Xiaoyin Granules on TNF-α and VEGF Levels in Patients with Blood Heat Type Psoriasis. Mod. Med. Health Res. 2020, 4, 123–124. [Google Scholar]
- Lu, H.; Liu, X. Effects of Yangxuequfeng Granules Combined with Moisturizing Cream on Skin Barrier Function and Quality of Life of Patients with Blood Deficiency and Dryness Psoriasis. Clin. J. Tradit. Chin. Med. 2019, 31, 547–550. [Google Scholar] [CrossRef]
- Chen, S.; Dou, J.; Zhang, Z. Efficacy Analysis of Awei A Combined with Xiaoyin Decoction in the Treatment of Psoriasis Vulgaris. China Pract. Med. 2013, 8, 169–170. [Google Scholar]
- Shan, K.; Li, L. Efficacy Analysis of Compound Glycyrrhizin Combined with Awei A Capsules in the Treatment of Psoriasis. J. North Pharm. 2016, 13, 44–45. [Google Scholar]
- Chen, J. Efficacy Analysis of Compound Glycyrrhizin in Combination with Awei A Capsule in the Treatment of Psoriasis. Sci. Tech. Inf. Gansu 2020, 49, 27–29. [Google Scholar]
- Sun, Y.; Hu, X.; Zhen, Z.; Liu, H.; Chao, D. Efficacy Analysis of Qingrexiaoyin Decoction in the Treatment of Psoriasis Vulgaris with Blood Heat and Wind. Mod. J. Integr. Tradit. Chin. West. Med. 2015, 24, 297–298, 331. [Google Scholar]
- Cao, Y.; Li, B.; Wang, Y. Efficacy Analysis of Researcher Prescribed Yangzhen Decoction Combined with Awei A Capsules in the Treatment of Psoriasis Vulgaris. Chin. J. Misdiagnostics 2010, 10, 1553–1554. [Google Scholar]
- Chai, R. Efficacy Analysis of Xiaoyin Decoction Combined with Calcipotriol in the Treatment of Psoriasis. J. Pract. Tradit. Chin. Med. 2018, 34, 1235–1236. [Google Scholar]
- Wang, J.; Zhang, B.; Gao, L.; Yao, Y. Efficacy and Effect of Serum IL-4, IL-10 and INF-γ of Yinxie Capsule Combined with Acitretin Capsule in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2017, 16, 116–118. [Google Scholar]
- Luo, M. Efficacy and Safety Evaluation of Compound Glycyrrhizin Combined with Avi A Capsules in the Treatment of Psoriasis. Med. Forum 2017, 21, 565–566. [Google Scholar] [CrossRef]
- Cao, Z. Efficacy and Safety of Psoriasis Compound Glycyrrhizin Combined Treatment with Avi A Capsules. China Foreign Med. Treat. 2017, 24, 8–10. [Google Scholar] [CrossRef]
- Han, X.; Yin, D.; Tian, K.; Guo, X.; Yue, Z. Efficacy Observation of Awei A Capsule Combined with Compound Qingdai Capsule in the Treatment of Progressive Psoriasis. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2017, 16, 66–67. [Google Scholar]
- Zeng, W.; Wang, L. Efficacy Observation of Compound Clobetasol Propionate Ointment Combined with Xiaoyin Granules in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2009, 8, 90–91. [Google Scholar]
- Che, X.; Zhang, Z. Efficacy Observation of Diyin Tablet Combined with Erdong Huoxue Decoction in the Treatment of Psoriasis. J. Emerg. Tradit. Chin. Med. 2004, 13, 217. [Google Scholar]
- Liu, B.; Miao, G.; Li, Z. Efficacy Observation of Fangxi Capsule Combined with Fangfengtongsheng Powder in the Treatment of Psoriasis Vulgaris. Shandong Med. J. 2011, 51, 106–107. [Google Scholar]
- Feng, Y. Efficacy Observation of Qingrequshi Decoction Combined with Methotrexate in the Treatment of Psoriatic Arthritis. J. Pract. Tradit. Chin. Med. 2018, 34, 567–568. [Google Scholar]
- Liu, A. Efficacy Observation of Xiaoyin Decoction Combined with Calcipotriol Ointment in the Treatment of Patchy Psoriasis. J. Dermatol. Venereol. 2020, 42, 271–272. [Google Scholar] [CrossRef]
- Peng, D.; Xi, J.; Qi, L.; Li, X. Efficacy Observation of Yinxiping Pill Combined with Awei A Capsule in the Treatment of Psoriasis Vulgaris. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2015, 14, 41–42. [Google Scholar]
- Yuan, C.; Li, L.; Zheng, K.; Ke, J.; Ke, X. Efficacy of Calcipotriol Ointment Combined with Qingrexiaoyin Decoction in Treatment of Progressive Psoriasis Vulgaris. J. Hubei Polytech. Univ. 2015, 31, 51–55. [Google Scholar] [CrossRef]
- Pang, J.; Ye, T.; Shu, Z. Efficacy of Compound Qingdai Capsule in the Treatment of Psoriasis Vulgaris and Its Effect on Serum Inflammatory Factors. World Chin. Med. 2017, 12, 1298–1301. [Google Scholar] [CrossRef]
- Tang, J.; Fei, L. Efficacy of Qingreyangxuejiedu Decoction Combined with Calcipotriol Betamethasone for Psoriasis Vulgaris of Blood-Dryness Type and the Influence on Peripheral Blood IL-17,IL-23. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2021, 20, 146–149. [Google Scholar]
- Liang, Y.; Li, L.; Yang, Y. Efficacy of Qinzhuliangxue Feng Combined with Awei A Capsules in the Treatment of Psoriasis Vulgaris with Blood Heat Syndrome. Guangdong Med. J. 2014, 35, 768–769. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Li, Y. Efficacy of Shentongzhuyu Decoction Combined with Western Medicine in Patients with Blood Stasis of Psoriatic Arthritis. Intern. Med. 2017, 12, 480–483. [Google Scholar] [CrossRef]
- Zhang, N. Efficacy of the Xiaoyin Granules plus Calcipotriene on Psoriasis Vulgaris. Clin. J. Chin. Med. 2018, 10, 116–117. [Google Scholar]
- Le, Q.; Sun, P.; Liu, Y. Efficacy of Tianxian Decoction in the Treatment of Psoriasis Vulgaris and Its Promoting Effect on Skin Healing. J. Sichuan Tradit. Chin. Med. 2021, 39, 169–171. [Google Scholar]
- Xiao, H. Efficacy of Xiaoyin Decoction Combined with Calcipotriol Ointment in the Treatment of Advanced Blood-Heat Type Mild/Moderate (PASI Score < 10) Psoriasis Vulgaris and Its Influence on the Levels of IL-17, IL-22 and TNF-α. J. Clin. Med. Lit. 2018, 5, 158–159. [Google Scholar] [CrossRef]
- Qu, L.; Liu, L.; Lu, X. Efficacy of Yinxie Capsules Combined with Acitretin in the Treatment of Psoriasis Vulgaris and Its Effect on Immune Function of T Lymphocytes. J. Dermatol. Venereol. 2020, 42, 374–375. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, Q.; Wu, J.; Liu, J. Evaluation of Clinical Effect of Qinmei Granules Combined with Compound Xinwei Cream on Psoriasis Vulgaris. Chin. Tradit. Pat. Med. 2016, 38, 1472–1476. [Google Scholar]
- Li, Y.; Zhou, J. Huanglianjiedu Decoction Combined with Methotrexate in the Treatment of 48 Cases of Psoriasis Vulgaris. Henan Tradit. Chin. Med. 2014, 34, 101–102. [Google Scholar] [CrossRef]
- Yao, L.; Wang, X. Liangxuerunzao Decoction Combined with Calcipotriol Ointment in Treatment of Psoriasis Vulgaris with Blood Heat and Wind Dryness Syndrome. Chin. J. Dermatovenereology Integr. Tradit. West. Med. 2019, 18, 309–312. [Google Scholar]
- Liu, G.; Chen, D. Observation of Clinical Efficacy of Compound Glycyrrhizin Combined with Acitretin Capsules in Treatment of Psoriasis. China Mod. Dr. 2020, 58, 105–107. [Google Scholar]
- Zhou, Y.; Yu, R. Observation of Combined Acitretin Capsule and Xiaoyin Granule Therapy for 60 Patients with Psoriasis Vulgaris. Jilin Med. J. 2012, 33, 6504–6505. [Google Scholar]
- Xie, B. Observation of Curative Effect of Acitretin Combined with Runzaozhiyang Capsules in the Treatment of Psoriasis Vulgaris. Guide China Med. 2012, 10, 499. [Google Scholar] [CrossRef]
- Hua, L.; Fan, J. Observation of Curative Effect of Acitretin Combined with Traditional Chinese Medicine Qingyinjiedu Decoction in the Treatment of Psoriasis Vulgaris. J. Chin. Med. Mater. 2010, 33, 1026–1027. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Yuan, S.; Wang, S. Observation of Curative Effect of Compound Glycyrrhizin Combined with Avi A Capsules in the Treatment of Children with Psoriasis. China Pract. Med. 2015, 10, 149–150. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wang, Y. Observation of Curative Effect of Diyin Tablet Combined with Liangxuejiedu Decoction in the Treatment of Psoriasis Vulgaris. J. Ningxia Med. Coll. 2005, 27, 412–413. [Google Scholar]
- Ding, R. Observation of Curative Effect of Traditional Chinese Medicine Ziyinhuoxuerunzao Decoction Combined with Western Medicine in the Treatment of Plaque Psoriasis. Mod. J. Integr. Tradit. Chin. West. Med. 2017, 26, 2030–2032, 2043. [Google Scholar]
- Ma, M. Observation on Curative Effect of Liangxuerunfu Decoction Combined with Western Medicine in Treating Psoriasis Vulgaris. Shanxi J. Tradit. Chin. Med. 2015, 31, 48. [Google Scholar]
- Cai, Y.; Zhang, J.; Yan, Z.; Zhang, L.; Yin, J.; Ren, Y.; Zhang, L.; Yin, Z.; Li, H. Observation on the Clinical Effect of Researcher Prescribed Xiaoyin Feng in the Treatment of Patients with Psoriasis Vulgaris. Chin. J. Clin. Ration. Drug Use 2015, 8, 105–106. [Google Scholar]
- Zeng, M.; Li, Q.; Huang, W.; Chen, J.; Liu, J.; Wang, C.; Dai, H.; Li, J. Observation on the Clinical Efficacy of Compound Glycyrrhizin Combined with Awei A Capsules in the Treatment of Psoriasis. Qingdao Med. J. 2017, 49, 120–122. [Google Scholar]
- He, D. Observation on the Curative Effect of Xiaoranqudan Feng and Awei A Capsules in the Treatment of Psoriasis Vulgaris. Youjiang Med. J. 2010, 38, 434–435. [Google Scholar]
- Ma, L. Observation on the Effect of Xiaoyin Granule in the Adjuvant Treatment of 37 Cases of Psoriasis Vulgaris. J. Dermatol. Venereol. 2018, 40, 840–842. [Google Scholar]
- Wu, S.; Wen, C. Observation on the Efficacy and Adverse Reactions of Compound Glycyrrhizin Combined with Acitretin Capsule in the Treatment of Psoriasis Vulgaris. Chin. Community Dr. 2020, 36, 89–90. [Google Scholar]
- Jin, X. Observation on the Efficacy of Researcher Prescribed Liangxuequfeng Powder Combined with Acitretin in the Treatment of Psoriasis Vulgaris. Gansu Sci. Technol. 2015, 31, 103–104. [Google Scholar]
- Xu, Y. Preliminary Evaluation on the Combined Treatment of Psoriasis with Compound Glycyrrhizin and Avi A Capsules. China Rural Health 2016, 10, 77–78. [Google Scholar]
- Zhang, B. Qingfeiliangxue Decoction and Acitretin in Treating 36 Cases of Psoriasis Vulgaris. West. J. Tradit. Chin. Med. 2014, 27, 106–107. [Google Scholar]
- Tian, Y. Qingfeiliangxue Decoction Clinical Study of Psoriasis Vulgaris. J. Liaoning Univ. Tradit. Chin. Med. 2011, 13, 187–189. [Google Scholar] [CrossRef]
- Chen, J. Study on the Effect of Calcipotriol Ointment, Avitamin A Capsule Combined with Compound Glycyrrhizin Tablets in the Treatment of Psoriasis. Contemp. Med. Symp. 2019, 17, 165–166. [Google Scholar]
- Zhao, C.; Ma, L.; Xia, D. The Clinical Effect Observation of Avermectin A Combined with Traditional Chinese Medicine in the Treatment of Patients with Psoriasis Vulgaris. Chin. J. Clin. Ration. Drug Use 2015, 8, 17–18. [Google Scholar]
- Zhang, X.; Liu, C.; Yuan, X.; Li, H. The Curative Effect of Taohong Ershao Decoction in the Treatment of Blood Stasis Psoriasis Vulgaris and Its Influence on Serum Levels of TH1/TH2. Chin. Med. Mod. Distance Educ. China 2017, 15, 56–57. [Google Scholar]
- Yu, Y.; He, Q.; Liu, Y.; Sun, D.; Cao, Y. The Effect of Peripheral Blood Parathyroid Hormone (PTH) Level of Qingreliangxue Decoction on Psoriasis Patients with Blood-Heat Syndrome. Chin. J. Dermatovenereology 2016, 30, 841–843. [Google Scholar]
- Jiang, Q.; Liu, W.; Chen, H. The Efficacy and Serum TNF-α and IL-8 Content in the Treatment of Blood Heat Psoriasis Vulgaris by Sendi Particles Combined Acitretin Capsules. Asia Pac. Tradit. Med. 2012, 8, 57–58. [Google Scholar]
- Li, J.; Li, H.; Zhang, J. The Efficacy of Qingrejiedu Decoction in the Treatment of Psoriasis Vulgaris with the Pattern of Blood-Heat and the Influence on Level of TNF-α in the Serum. Chin. J. Dermatovenereology 2017, 31, 554–556. [Google Scholar] [CrossRef]
- Ji, T.; Chen, J.; Meng, Y. Therapeutic Effect and Mechanism of Compound Qingdai Capsule Combined with MTX in the Treatment of Psoriasis. Lab. Med. 2020, 35, 120–124. [Google Scholar]
- Zheng, T.; Xiao, J.; Zhou, W.; Feng, D.; Zhu, P.; Niu, F.; Guo, F.; Liu, J.; Wang, S. Therapeutic Effect of Jueyin Granule on Psoriasis Vulgaris with Blood-Heat Syndrome. Gansu Med. J. 2020, 39, 27–28, 31. [Google Scholar]
- Zhang, T.; Jia, Z. To Observe the Effect of Nourishing Yin and Zinyinqingrexiaofengsan Efficacy and Safety in the Treatment of Psoriasis Vulgaris. Clin. J. Chin. Med. 2015, 7, 89–90. [Google Scholar]
- Luo, C.; Ma, L. Topical Calcipotriol Ointment Therapy Combination with Oral Piminxiao Capsule Treatment in Plaque Psoriasis Vulgaris. Mod. Hosp. 2010, 10, 44–46. [Google Scholar]
- Xu, H. Treatment of 39 Cases of Psoriasis Vulgaris with Qingxuanyin Combined with Awei A Capsules. China Pharm. 2011, 20, 72. [Google Scholar]
- Zhang, G. Treatment of 40 Cases of Psoriasis Due to Blood Deficiency and Wind-Dryness with Ziyinyangxuequfeng Decoction. Henan Tradit. Chin. Med. 2014, 34, 1558–1559. [Google Scholar] [CrossRef]
- Zhang, X. Treatment of 76 Cases of Psoriasis Vulgaris with Xiaoyin Capsules. J. Shaanxi Coll. Tradit. Chin. Med. 2013, 36, 69–70. [Google Scholar] [CrossRef]
- Chen, B.; Tan, X. Treatment of Psoriasis Vulgaris with Diyin Tablets Combined with Anti-Psoriasis Formula. Guangdong Med. J. 2004, 25, 727–728. [Google Scholar] [CrossRef]
- Luo, W.; Wang, T.; Wang, C. Treatment of Psoriasis Vulgaris with Zicaohuoxue Decoction Combined with Acitretin. Acta Chin. Med. 2018, 33, 2462–2465. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Ma, J. Xiaoying Granules Combined with A Citretin Capsule in the Treatment of Psoriasis Clinical Analysis. China Med. Cosmetol. 2017, 7, 61–64. [Google Scholar]
- Zhang, J.; Chen, X.; Li, F.; Li, X. Yinxie Capsule Combined with Pyrithione Zinc Aerosol in Treating 65 Cases of Psoriasis Vulgaris. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 169–172. [Google Scholar] [CrossRef]
- Jo, H.-G.; Kim, H.; Lee, D. Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials. Nutrients 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Z.; Gao, T.; Chen, Y.; Yang, Q.; Fu, C.; Zhu, Y.; Wang, F.; Liao, W. Isatidis Radix and Isatidis Folium: A Systematic Review on Ethnopharmacology, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, R.; Caiazzo, G.; Cacciapuoti, S.; Fabbrocini, G.; Scala, E.; Balato, A. Safety Concerns with Current Treatments for Psoriasis in the Elderly. Expert Opin. Drug Saf. 2020, 19, 523–531. [Google Scholar] [CrossRef]
- George, C.J.; Verghese, J.; Izzetoglu, M.; Wang, C.; Holtzer, R. The Effect of Polypharmacy on Prefrontal Cortex Activation during Single and Dual Task Walking in Community Dwelling Older Adults. Pharmacol. Res. 2019, 139, 113–119. [Google Scholar] [CrossRef]
- Panossian, A. Challenges in Phytotherapy Research. Front. Pharmacol. 2023, 14, 1199516. [Google Scholar] [CrossRef]
- Jo, H.-G.; Seo, J.; Lee, D. Clinical Evidence Construction of East Asian Herbal Medicine for Inflammatory Pain in Rheumatoid Arthritis Based on Integrative Data Mining Approach. Pharmacol. Res. 2022, 185, 106460. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Hao, Y.; Du, K.; Du, H.; Ma, C.; Tu, H.; He, Y. A Systematic Review on Botany, Processing, Application, Phytochemistry and Pharmacological Action of Radix Rehmnniae. J. Ethnopharmacol. 2022, 285, 114820. [Google Scholar] [CrossRef]
- Sun, M.; Shen, X.; Ma, Y. Rehmannioside A Attenuates Cognitive Deficits in Rats with Vascular Dementia (VD) through Suppressing Oxidative Stress, Inflammation and Apoptosis. Biomed. Pharmacother. 2019, 120, 109492. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yoon, T.; Jang, J.Y.; Park, S.-J.; Kim, H.K. Topical Application of Rehmannia Glutinosa Extract Inhibits Mite Allergen-Induced Atopic Dermatitis in NC/Nga Mice. J. Ethnopharmacol. 2011, 134, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Cameron, S.; Lee, M.; Cai, S.-Q.; Shoyama, Y. Which East Asian Herbal Medicines Can Decrease Viral Infections? Phytochem. Rev. 2022, 21, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, X.-Y. Research Progress of Chinese Herbal Medicine Radix isatidis (Banlangen). Am. J. Chin. Med. 2013, 41, 743–764. [Google Scholar] [CrossRef]
- Kim, J.H.; Gao, D.; Jeong, W.S.; Kim, C.T.; Cho, C.W.; Kim, H.M.; Kang, J.S. Anti-Wrinkle Effect of Isatis Indigotica Leaf Extract: Evaluation of Antioxidant, Anti-Inflammation, and Clinical Activity. Antioxidants 2021, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.; Xie, X.; Di, T.; Zhao, J.; Lin, Y.; Xu, X.; Li, N.; Zhai, Y.; Wang, Y.; et al. Paeonol Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in BALB/c Mice by Inhibiting the Maturation and Activation of Dendritic Cells. Int. J. Mol. Med. 2017, 39, 1101–1110. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Hwang, E.; Yi, T.-H. Paeonol Extracted from Paeonia suffruticosa Andr. Ameliorated UVB-Induced Skin Photoaging via DLD/Nrf2/ARE and MAPK/AP-1 Pathway. Phytother. Res. 2018, 32, 1741–1749. [Google Scholar] [CrossRef]
- Wu, X.X.; Siu, W.S.; Wat, C.L.; Chan, C.L.; Koon, C.M.; Li, X.; Cheng, W.; Ma, H.; Tsang, M.S.M.; Lam, C.W.-K.; et al. Effects of Topical Application of a Tri-Herb Formula on Inflammatory Dry-Skin Condition in Mice with Oxazolone-Induced Atopic Dermatitis. Phytomedicine 2021, 91, 153691. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Zhao, Z.; Liu, Y.; Wang, Y.; Xiong, H.; Mei, Z. Paeonol Ameliorates Chronic Itch and Spinal Astrocytic Activation via CXCR3 in an Experimental Dry Skin Model in Mice. Front. Pharmacol. 2021, 12, 805222. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, H.-L.; Lee, D.-R.; Choi, B.-K.; Yang, S.-H. Scrophulariae Radix: An Overview of Its Biological Activities and Nutraceutical and Pharmaceutical Applications. Molecules 2021, 26, 5250. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Shen, Z.-Y.; Qin, L.-P.; Zhu, B. Pharmacology, Phytochemistry, and Traditional Uses of Scrophularia Ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, A.Y.; Song, J.-H.; Yang, S.; Park, I.; Lim, J.-O.; Jung, T.-Y.; Ko, J.-W.; Kim, J.-C.; Lim, K.S.; et al. Scrophularia Buergeriana Attenuates Allergic Inflammation by Reducing NF-ΚB Activation. Phytomedicine 2020, 67, 153159. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Ginseng (Part II): The Differential Effects of Red Ginseng and Ginsenoside Rg5 in Cancer and Heart Diseases as Determined by Transcriptomics. Pharmaceuticals 2021, 14, 1010. [Google Scholar] [CrossRef]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Ginseng (Part III): Antitumor Potential of a Fixed Combination of Red Ginseng and Red Sage as Determined by Transcriptomics. Pharmaceuticals 2022, 15, 1345. [Google Scholar] [CrossRef]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Red Ginseng (Part I): Effects of Ginsenoside Rg5 at Physiological and Sub-Physiological Concentrations. Pharmaceuticals 2021, 14, 999. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.-J.; Wikman, G.; Efferth, T. Synergy Assessment of Fixed Combinations of Herba Andrographidis and Radix Eleutherococci Extracts by Transcriptome-Wide Microarray Profiling. Phytomedicine 2015, 22, 981–992. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Ma, W.; Sun, Q. Metformin Inhibits Proliferation and Proinflammatory Cytokines of Human Keratinocytes in Vitro via MTOR-Signaling Pathway. Pharm. Biol. 2016, 54, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, W.; Zhong, H.; Liu, W.; Sun, Q. Metformin Inhibits Proliferation of Human Keratinocytes through a Mechanism Associated with Activation of the MAPK Signaling Pathway. Exp. Ther. Med. 2014, 7, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Q.; Liu, Z.; Cao, Z.; Li, M.; Wei, J.; Du, L.; Han, C.; Zhang, C. Qingre Lishi Decoction Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in SKH-1 Mice by Regulating the Treg-DC-Th17 Axis and Inhibiting MAPK-Mediated DC Maturation. J. Ethnopharmacol. 2023, 318, 116931. [Google Scholar] [CrossRef]
- Xu, Q.; Sheng, L.; Zhu, X.; Liu, Z.; Wei, G.; Zhang, T.; Du, H.; Yang, A.; Yao, J.; Zhang, G.; et al. Jingfang Granules Exert Anti-Psoriasis Effect by Targeting MAPK-Mediated Dendritic Cell Maturation and PPARγ-Mediated Keratinocytes Cell Cycle Progression in Vitro and in Vivo. Phytomedicine 2023, 117, 154925. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, B.; Chen, Z.; Ding, X. The Regulation of Skin Homeostasis, Repair and the Pathogenesis of Skin Diseases by Spatiotemporal Activation of Epidermal MTOR Signaling. Front. Cell Dev. Biol. 2022, 10, 950973. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, L.; Li, X.; Liu, W.; Wang, P.; Lin, Y.; Han, X.; Li, P. Liangxue Jiedu Formula Improves Psoriasis and Dyslipidemia Comorbidity via PI3K/Akt/MTOR Pathway. Front. Pharmacol. 2021, 12, 591608. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Son, M.-J.; Ho, C.-C.; Lee, S.-H.; Kim, Y.; An, J.; Lee, S.-K. Transcriptional Inhibition of STAT1 Functions in the Nucleus Alleviates Th1 and Th17 Cell-Mediated Inflammatory Diseases. Front. Immunol. 2022, 13, 1054472. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Shi, H.-Y.; Lu, Y.-Y.; Lin, J. Centella Asiatica Alleviates Psoriasis through JAK/STAT3-Mediated Inflammation: An in Vitro and in Vivo Study. J. Ethnopharmacol. 2023, 317, 116746. [Google Scholar] [CrossRef] [PubMed]
- Elango, T.; Thirupathi, A.; Subramanian, S.; Ethiraj, P.; Dayalan, H.; Gnanaraj, P. Methotrexate Treatment Provokes Apoptosis of Proliferating Keratinocyte in Psoriasis Patients. Clin. Exp. Med. 2017, 17, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Lin, C.-F.; Alalaiwe, A.; Yang, S.-C.; Fang, J.-Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Q.; Wang, X.; Zhou, H.; Hu, J.; Gu, L.; Hu, Y.; Zeng, F.; Zhao, F.; Yue, C.; et al. Pathogenesis, Multi-Omics Research, and Clinical Treatment of Psoriasis. J. Autoimmun. 2022, 133, 102916. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-MTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef]
- Gómez-García, F.; Gómez-Arias, P.J.; Montilla-López, A.; Hernández-Parada, J.; Sanz-Cabanillas, J.L.; Ruano, J.; Parra-Peralbo, E. A Scoping Review on Use of Drugs Targeting the JAK/STAT Pathway in Psoriasis. Front. Med. 2022, 9, 754116. [Google Scholar] [CrossRef]
- Kadagothy, H.; Nene, S.; Amulya, E.; Vambhurkar, G.; Rajalakshmi, A.N.; Khatri, D.K.; Singh, S.B.; Srivastava, S. Perspective Insights of Small Molecules, Phytoconstituents and Biologics in the Management of Psoriasis: A Focus on Targeting Major Inflammatory Cytokine Pathways. Eur. J. Pharmacol. 2023, 947, 175668. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Shimizu, T. Therapeutic Target of Leukotriene B4 Receptors, BLT1 and BLT2: Insights from Basic Research. Biochimie 2023, in press. [CrossRef] [PubMed]
- Yan, J.; Zhu, J.; Li, X.; Yang, R.; Xiao, W.; Huang, C.; Zheng, C. Blocking LTB4 Signaling-Mediated TAMs Recruitment by Rhizoma Coptidis Sensitizes Lung Cancer to Immunotherapy. Phytomedicine 2023, 119, 154968. [Google Scholar] [CrossRef]
- Park, G.-S.; Kim, J.-K.; Kim, J.-H. Anti-Inflammatory Action of Ethanolic Extract of Ramulus Mori on the BLT2-Linked Cascade. BMB Rep. 2016, 49, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Joh, B.; Jeon, E.S.; Lim, S.H.; Park, Y.L.; Park, W.; Chae, H. Intercultural Usage of Mori Folium: Comparison Review from a Korean Medical Perspective. Evid. Based Complement. Altern. Med. 2015, 2015, 379268. [Google Scholar] [CrossRef]
- Burger, B.; Sagiorato, R.N.; Cavenaghi, I.; Rodrigues, H.G. Abnormalities of Sphingolipids Metabolic Pathways in the Pathogenesis of Psoriasis. Metabolites 2023, 13, 291. [Google Scholar] [CrossRef]



| Included Study (Reference) | Trial Design | Randomization Method | Number of Participants (Male/Female); Age (Mean ± SD) | Interventions | Morbidity Period (Mean ± SD or Range) | Outcome Index (Intergroup Differencies p-Value) | Course of Treatment | Adverse Events (Case/Symptom) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | Trial | Control | ||||||
| Che 2004 [164] | Randomized; Single center; Parallel | NR | Both group 75 (41/34) 11–44 y Trial: 45 | Both group 75 (41/34) 11–44 y Control: 30 | 1. Erdonghuoxue decoction (t.i.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | Both group 4 m–10 y | Both group 4 m–10 y | 1. PASI 60 (p < 0.05) | 4 w | Trial: 5 AEs Control: 22 AEs Including thirst, hyperhidrosis, pruritus, skin scale, dry lips, cheilitis, dry mouth, dry nose, nausea |
| Chen 2004 [211] | Randomized; Single center; Parallel | NR | 30 (18/12) 32.4 y (16–65 y) | 26 (16/10) 33.2 y (17–63 y) | 1. Anti-psoriasis formula (q.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 6.2 y (3 m-28 y) | 6.1 y (2 m-30 y) | 1. PASI 60 (p < 0.05) | 6 w | Trial: 29 AEs (6 xerostomia, 8 dry lips, 5 xeroderma, 4 pruritus, 6 skin scale) Control: 84 AEs (18 xerostomia, 20 dry lips, 17 xeroderma, 13 pruritus, 16 skin scale) |
| Xu 2005 [186] | Randomized; Single center; Parallel | NR | 45 (27/18) Range 11–63 y | 30 (18/12) Range 15–57 y | 1. Liangxuejiedu decoction (b.i.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 3 w–22 y | 3 w–22 y | 1. PASI 60 (p < 0.05) | 4 w | Trial: 5 AEs Control: 22 AEs Including skin scale, cheilitis, dry nasal cavity, thirst, hyperhidrosis, pruritus, nausea |
| Liu 2006 [137] | Randomized; Single center; Parallel | NR | Both group 60 (31/29) Trial: 40 (NR) 33.63 y (16–53 y) | Both group 60 (31/29) Control: 20 (NR) 32.7 y (21–64 y) | 1. Jianpiyishen decoction (q.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 71.191 m (1–168 m) | 70.95 m (3–120 m) | 1. PASI 70 (p < 0.05) | 8 w | Trial: 41 AEs (8 xerostomia, 14 xeroderma, 8 skin scale, 11 pruritus) Control: 71 AEs (17 xerostomia, 18 xeroderma, 18 skin scale, 18 pruritus) |
| Chen 2007 [92] | Randomized; Single center; Parallel Three arm trial | NR | Both group 222 (139/83) 33 y (19–58 y) Trial (IM): 74 | Both group 222 (139/83) 33 y (19–58 y) Control: 74 | 1. Xiaoyin granule (3.5 g, t.i.d) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | Both group 4.6 y (1–18 y) | Both group 4.6 y (1–18 y) | 1. PASI 60 (p < 0.05) | 16 w | Trial: 36 AEs (26 xeroderma, 3 pruritus, 1 skin poignant itch, 5 gastrointestinal discomfort, 1 hepatic dysfunction) Control: 29 AEs (21 xeroderma, 1 pruritus, 2 skin poignant itch, 4 gastrointestinal discomfort, 1 hepatic dysfunction) |
| Huang 2007 [129] | Randomized; Single center; Parallel; single blind | Simple randomization (envelope concealment method) | 49 (30/19) 37 ± 9.12 y | 49 (32/17) 38 ± 10.27 y | 1. Yinxieling tablet (6t, t.i.d.) 2. Acitretin capsule (0.5 mg/kg/day, q.d.) | 1. Acitretin capsule (0.5 mg/kg/day, q.d.) | 3.36 ± 5.72 y | 3.8 ± 5.44 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.01) | 8 w | Trial: 130 AEs (49 xerostomia, 13 xeroma, 21 xeroderma, 11 pruritus, 2 epistaxis, 14 folliculitis, 1 hepatic dysfunction, 19 hyperlipidemia) Control 238 AEs (49 xerostomia, 40 xeroma, 38 xeroderma, 20 pruritus, 8 epistaxis, 40 folliculitis, 8 hepatic dysfunctions, 35 hyperlipidemia) |
| Zeng 2009 [163] | Randomized; Single center; Parallel; single blind | Simple randomization | 50 (30/20) 38.61 ± 13.12 y | 50 (29/21) 39.41 ± 14.03 y | 1. Xiaoyin granule (3.5 g, t.i.d) 2. Topical corticosteroid (clobetasol propionate ointment, b.i.d.) | 1. Topical corticosteroid (clobetasol propionate ointment, b.i.d.) | 7.68 ± 5.63 y | 6.58 ± 5.92 y | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.01) | 4 w | Trial: 15 AEs Control: 18 AEs Including xeroderma, skin scale, pruritus, erythema, mild stabbing |
| Cao 2010 [157] | Randomized; Single center; Parallel Three arm trial | NR | 40 (24/16) 35.3 y (18–64 y) | 40 (25/15) 33.5 y (19–62 y) | 1. Yangzhen decoction (200 mL, b.i.d.) 2. Acitretin capsule (0.5 mg/kg/day, q.d.) | 1. Acitretin capsule (0.5 mg/kg/day, q.d.) | 43.5 m (6 m–33 y) | 40.5 m (6 m–30 y) | 1. PASI 60 (p < 0.01) | 12 w | Trial: 43 AEs (22 xeroderma and xerostomia, 9 pruritus, 11 hyperlipidemia, 2 hepatic dysfunction) Control: 77AEs (36 xeroderma and xerostomia, 27 pruritus, 13 hyperlipidemia, 1 hepatic dysfunction) |
| He 2010 [191] | Randomized; Single center; Parallel | NR | Both group 78 (36/42) 43.4 ± 6.2 y Trial: 39 | Both group 78 (36/42) 43.4 ± 6.2 y Control: 39 | 1. Xiaoranqudan feng (200 mL, b.i.d) 2. Acitretin capsule (25 mg, q.d.) | 1. Acitretin capsule (25 mg, q.d.) | Both group 3.6 ± 1.1 y | Both group 3.6 ± 1.1 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.01) | 8 w | Trial: 15 AEs (10 xerostomia, 5 headache) Control: 85 AEs (8 hepatic dysfunction, 30 xeroderma, 26 xerostomia, 7 headache, 14 gastrointestinal discomfort) |
| Hua 2010 [184] | Randomized; Single center; Parallel | NR | Both group 90 (50/40) 32.4 y (18–65 y) Trial: 46 | Both group 90 (50/40) 32.4 y (18–65 y) Control: 44 | 1. Qingyinjiedu decoction (30 mg, q.d.) 2. Acitretin capsule (q.d.) | 1. Acitretin capsule (q.d.) | Both group 4.2 y | Both group 4.2 y | 1. PASI 60 (p < 0.01) | 8 w | Trial: 15 AEs (8 skin scale and xerostomia, 5 hyperlipidemia, 2 hepatic dysfunction) Control: 30 AEs (17 skin scale and xerostomia, 10 hyperlipidemia, 3 hepatic dysfunction) |
| Luo 2010 [207] | Randomized; Single center; Parallel | NR | 47 (25/22) 41.5 ± 9.8 y | 47 (28/19) 39.7 ± 7.8 y | 1. Piminxiao capsule (4c, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 9.3 ± 6.8 y | 7.9 ± 5.7 y | 1. PASI 70 (p < 0.05) 2. PASI score (p < 0.01) 3. Recurrence rate (p < 0.05) | 12 w | Trial: No AEs Control: No AEs |
| Yu 2010 [135] | Randomized; Single center; Parallel | NR | 40 (NR) 34.18 y (18–60 y) | 40 (NR) 32.30 y (19–58 y) | 1. Runzaozhiyang capsule (2 g, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 4.2 y (20 d–30 y) | 3.8 y (1 m–29 y) | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.01) | 4 w | Trial: 5 AEs (5 nausea) Control: 6 AEs (6 hyperlipidemia) |
| Liu 2011 [165] | Randomized; Single center; Parallel | NR | Both group 68 (42/26) 35 y (18–65 y) Trial: 38 | Both group 68 (42/26) 35 y (18–65 y) Control: 30 | 1. Fangfengtongsheng powder (6 g, b.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | Both group 5.7 y (2 m–12 y) | Both group 5.7 y (2 m–12 y) | 1. PASI 60 (p < 0.05) | 12 w | Trial: 17 AEs (detailed information NR) Control: 23 AEs (detailed information NR) |
| Lu 2011 [138] | Randomized; Single center; Parallel | NR | 44 (28/16) 34.6 ± 3.82 y | 44 (26/18) 33.96 ± 4.26 y | 1. Qingrejiedu decoction (400 mL, b.i.d.) 2. Etretinate (0.5 mg, q.d.) | 1. Etretinate (0.5 mg, q.d.) | 5.63 ± 1.32 y | 6.02 ± 1.50 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: 1 AE (1 diarrrhea) Control: 2 AEs (2 hepatic dysfunction) |
| Tian 2011 [197] | Randomized; Single center; Parallel | Simple randomization (random number table) | 30 (12/18) 36.2 ± 9.8 y | 30 (15/15) 34.5 ± 10.2 y | 1. Qingfeiliangxue decoction (100 mL, t.i.d.) 2. Vitamin C, Vitamin B6 (NR) | 1. Vitamin C, Vitamin B6 (NR) | 10.4 ± 7.6 y | 10.4 ± 7.6 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 60 d | NR |
| Xu 2011 [208] | Randomized; Single center; Parallel | NR | 39 (23/16) 34.5 y | 39 (20/19) 36.5 y | 1. Qingxuanyin (b.i.d.) 2. Acitretin capsule (20 mg, q.d.) | 1. Acitretin capsule (20 mg, q.d.) | 15 d–29 y | 25 d–31 y | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.01) | 8 w | Trial: 8 AEs (8 diarrhea) Control: 10 AEs (10 xerostomia) |
| Yao 2011 [141] | Randomized; Single center; Parallel | Simple randomization (random number table) | 70 (24/46) 36.5 y (15–62 y) | 62 (34/28) 35.2 y (14–65 y) | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 7 d–45 y | 10 d–47 y | 1. PASI 60 (p < 0.05) | 8 w | Detailed information NR |
| Zheng 2011 [91] | Randomized; Single center; Parallel | NR | 60 (28/32) 42.1 ± 14.6 y | 60 (26/34) 42.3 ± 15.4 y | 1. Xiaoyinkeji decoction (100 mL, b.i.d.) 2. Acitretin capsule (20 mg, q.d.) | 1. Acitretin capsule (20 mg, q.d.) | 7.3 ± 0.9 y | 7.5 ± 1.2 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 8 AEs (6 gastrointestinal discomfort, 2 diarrhea) Control: 14 AEs (10 gastrointestinal discomfort with nausea or vomiting, 4 leukopenia) |
| Jiang 2012 [202] | Randomized; Single center; Parallel; single blind | Simple randomization (random number table) | 30 (16/14) 33.37 ± 4.32 y | 30 (17/13) 34.69 ± 5.01 y | 1. Sendi particles (b.i.d.) 2. Acitretin capsule (10 mg, q.d.) | 1. Acitretin capsule (10 mg, q.d.) | 4.19 ± 2.77 y | 3.98 ± 1.97 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate (p < 0.05) | 12 w | Trial: 3 AEs (2 loose stool, 1 hepatic dysfunction) Control: 1 AE (1 hepatic dysfunction) |
| Liu 2012 [143] | Randomized; Single center; Parallel | NR | 42 (24/18) 40.00 ± 10.26 y | 42 (23/19) 39.00 ± 10.50 y | 1. Runzaozhiyang capsule (4c, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 3.0 ± 4.5 y | 3.0 ± 4.6 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 30 AEs (18 xeroderma, 5 xerostomia, 7 pruritus) Control: 98 AEs (32 xeroderma, 34 xerostomia, 32 pruritus) |
| Xie 2012 [183] | Randomized; Single center; Parallel | NR | Both group 94 (49/45) 35.5 ± 3.2 y Trial: 49 | Both group 94 (49/45) 35.5 ± 3.2 y Control: 45 | 1. Runzaozhiyang capsule (4c, t.i.d.) 2. Acitretin capsule (20 mg, q.d.) 3. Topical corticosteroid (clobetasol propionate ointment, b.i.d.) | 1. Acitretin capsule (20 mg, q.d.) 2. Topical corticosteroid (clobetasol propionate ointment, b.i.d.) | Both group 8.5 y (1 w–18 y) | Both group 8.5 y (1 w–18 y) | 1. PASI 60 (p < 0.05) | 4 w | Trial: 5 AEs (3 hyperlipidemia, 2 gastrointestinal discomfort) Control: 4 AEs (4 hyperlipidemia) |
| Zhang 2012 [127] | Randomized; Single center; Parallel | NR | 40 (24/16) 36.65 ± 9.34 y | 40 (27/13) 35.76 ± 10.26 y | 1. Yinxie capsule (3c, t.i.d.) 2. Acitretin capsule (20 mg, q.d.) | 1. Acitretin capsule (20 mg, q.d.) | 16.43 ± 15.36 m | 15.88 ± 16.48 m | 1. PASI 60 (p < 0.05) | 8 w | Trial: 19 AEs (detailed information NR) Control: 20 AEs (detailed information NR) Including xerostomia, dry lip, xeroma, pruritus, epistaxis |
| Zhou 2012a [124] | Randomized; Single center; Parallel | NR | 70 (46/24) 38.3 y (19–65 y) | 70 (44/26) 33.7 y (16–65 y) | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) 3. 10% zing oxide ointment (b.i.d.) | 1. Acitretin capsule (30 mg, q.d.) 2. 10% zing oxide ointment (b.i.d.) | 5.6 y (3 m–12 y) | 7.6 y (2 m–11 y) | 1. PASI 60 (p < 0.05) | 60 d | NR |
| Zhou 2012b [182] | Randomized; Single center; Parallel | NR | Both group 120 (49/71) 36.85 ± 6.32 y Trial: 49 | Both group 120 (49/71) 36.85 ± 6.32 y Control: 45 | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (10 mg, q.d.) | 1. Acitretin capsule (10 mg, q.d.) | Both group 6.70 ± 0.52 y | Both group 6.70 ± 0.52 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.01) 3. Recurrence rate (p > 0.05) | 12 w | Detailed information NR |
| Chen 2013 [153] | Randomized; Single center; Parallel | NR | 30 (24/7) 31.2 ± 5.3 y | 30 (24/7) 30.8 ± 6.1 y | 1. Xiaoyin decoction (b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 5.1 ± 2.0 y | 5.5 ± 2.3 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 2 AEs (1 xeroderma, 1 gastrointestinal discomfort) Control: 6 AEs (2 xeroderma, 1 xeroma, 1 xerostomia, 1 hyperlipidemia, 1 hepatic dysfunction) |
| Ding 2013 [146] | Randomized; Single center; Parallel | NR | 30 (16/14) 52.32 ± 3.41 y | 30 (12/18) 54.32 ± 2.15 y | 1. NiupixuanⅡ decoction (NR) 2. Acitretin capsule (0.3–1.0 mg/kg/day, NR) | 1. Acitretin capsule (0.3–1.0 mg/kg/day, NR) | 10.3 ± 2.2 y | 11.1 ± 2.5 y | 1. PASI score (p < 0.05) | 4 w | NR |
| Mo 2013 [136] | Randomized; Single center; Parallel | NR | 56 (33/23) 36.2 y (18–58 y) | 54 (33/21) 35.9 y (20–60 y) | 1. Total Glycosides of Paeoniae Alba capsule (2c, b.i.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 5.4 y (0.5 m–32 y) | 5.6 y (1 m–40 y) | 1. PASI 60 (p < 0.05) | 8 w | Trial: No AEs Control No AEs |
| Song 2013 [90] | Randomized; Single center; Parallel | NR | 43 (25/18) 34.5 ± 6.2 y | 43 (26/17) 33.9 ± 6.0 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 8.1 ± 2.3 y | 7.8 ± 2.4 y | 1. PASI 60 (p < 0.05) | 12 w | Trial: No AEs Control No AEs |
| Zhang 2013 [210] | Randomized; Single center; Parallel | NR | 38 (20/18) 37.9 ± 5.9 y | 38 (23/15) 33.9 ± 6.3 y | 1. Xiaoyin capsule (5c, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 8.1 ± 2.7 y | 7.8 ± 2.4 y | 1. TNF-alpha (p < 0.01) 2. IL-8 (p < 0.01) | 12 w | Trial: No AEs Control No AEs |
| Cheng 2014 [126] | Randomized; Single center; Parallel | NR | 30 (17/13) 18–60 y | 30 (16/14) 15–58 y | 1. Yanghe decoction (b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 1 w–5 y | 2 w–6 y | 1. PASI 60 (p < 0.05) | 8 w | Detailed information NR |
| Du 2014 [125] | Randomized; Single center; Parallel | NR | 70 (45/25) 39.6 ± 0.4 y | 70 (45/25) 38.9 ± 0.5 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 6.3 ± 0.4 y | 6.2 ± 0.4 y | 1. PASI 60 (p < 0.05) | 12 w | NR |
| Li 2014 [179] | Randomized; Single center; Parallel | NR | 24 (14/10) 43.6 ± 10.78 y | 24 (11/13) 45.3 ± 11.32 y | 1. Huanglianjiedu decoction (200 mL, t.i.d.) 2. Methotrexate (2.5–5.0 mg, b.i.d.) | 1. Methotrexate (2.5–5.0 mg, b.i.d.) | 7.96 ± 4.41 y | 7.49 ± 4.03 y | 1. PASI 70 (p < 0.05) | 4 w | Trial: 2 AEs Control: 5 AEs Including nausea, anorexia, hepatic dysfunction |
| Liang 2014 [172] | Randomized; Single center; Parallel | NR | 39 (20/19) 38.43 ± 4.12 y | 39 (19/20) 38.48 ± 4.15 y | 1. Qinzhuliangxue feng (200 mL, b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 5.33 ± 1.05 y | 5.38 ± 1.03 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate (p < 0.05) | 60 d | Trial: No AEs Control: 6 AEs (1 hepatic and renal dysfunction, 2 pruritus, 3 tinnitus) |
| Liu 2014 [115] | Randomized; Single center; Parallel | NR | 47 (29/18) 28.9 ± 10.4 y | 49 (26/21) 31.2 ± 12.6 y | 1. Runzaozhiyang capsule (2 g, t.i.d) 2. Topical corticosteroid (compound flumetasone ointment, b.i.d.) | 1. Topical corticosteroid (compound flumetasone ointment, b.i.d.) | 2.3 ± 0.84 y | 2.7 ± 0.91 y | 1. PASI 60 (p < 0.05) | 4 w | Trial: 3 AEs (2 mild abdominal discomfort, 1 mild burning sensation) Control: 1 AE (1 mild stabbing) |
| Qiu 2014 [99] | Randomized; Single center; Parallel | NR | 49 (29/20) 43.7 ± 6.9 y | 33 (22/11) 46.7 ± 7.1 y | 1. EAHM prescription (q.d.) 2. Acitretin capsule (20–50 mg, q.d.) | 1. Acitretin capsule (20–50 mg, q.d.) | 9.5 ± 3.1 y | 9.1 ± 3.9 y | 1. PASI 60 (p < 0.05) | 12 w | Trial: No AEs Control: 26 AEs (1 hypokalemia, 6 hyperlipidemia, 19 xeroderma and xerostomia) |
| Zhang 2014a [144] | Randomized; Single center; Parallel | NR | 38 (26/12) 38.62 ± 6.11 y | 38 (24/14) 36.74 ± 5.23 y | 1. Yinxie capsule (3c, t.i.d.) 2. Acitretin capsule (20 mg, q.d.) | 1. Acitretin capsule (20 mg, q.d.) | 2–78 m | 1–81 m | 1. PASI 60 (p < 0.05) | 8 w | Trial 15 AEs Control 16 AEs Including pruritus, xeroma, epistaxis, xerostomia, dry lib |
| Zhang 2014b [209] | Randomized; Single center; Parallel | NR | 40 (26/14) 35.40 ± 2.83 y | 30 (19/11) 34.69 ± 3.46 y | 1. Ziyinyangxuequfeng decoction (200 mL, b.i.d.) 2. Acitretin capsule (0.75 mg/kg/day, b.i.d.) | 1. Acitretin capsule (0.75 mg/kg/day, b.i.d.) | 6.35 ± 0.74 y | 6.27 ± 0.68 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: No AEs Control: No AEs |
| Zhang 2014c [196] | Randomized; Single center; Parallel | NR | 36 (21/15) 33.5 y | 36 (19/17) 35.3 y | 1. Qingfeiliangxue decoction (200 mL, b.i.d.) 2. Acitretin capsule (0.5 mg/kg/day, b.i.d.) | 1. Acitretin capsule (0.5 mg/kg/day, b.i.d.) | 1–28 y | 1–26 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 2 w | NR |
| Cai 2015 [189] | Randomized; Single center; Parallel | NR | 130 (72/58) 38.5 ± 12.3 y | 130 (67/63) 41.8 ± 11.9 y | 1. Xiaoyin feng (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 4.83 ± 1.39 y | 4.95 ± 1.12 y | 1. PASI 70 (p < 0.05) 2. PASI score (p < 0.05) | 12 w | Trial: 14 AEs (4 hematuria, 10 drug eruption) Control: 16 AEs (5 hematuria, 11 drug eruption) |
| Jin 2015a [132] | Randomized; Single center; Parallel | NR | 50 (28/22) 44.38 ± 2.9 y | 50 (30/20) 43.3 ± 2.5 y | 1. Matrine capsule (2t, t.i.d.) 2. Acitretin capsule (b.i.d.) | 1. Acitretin capsule (b.i.d.) | 22–68 y | 20–65 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: 4 AEs (2 pruritus, 1 hyperlipidemia, 1 hepatic dysfunction) Control: 4 AEs (2 pruritus, 2 hyperlipidemia) |
| Jin 2015b [194] | Randomized; Single center; Parallel | Simple randomization (random number table) | 43 (24/19) 41.33 ± 14.19 y | 41 (26/15) 37.17 ± 11.30 y | 1. Qinzhuliangxue feng (200 mL, b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 5.83 ± 1.60 y | 7.16 ± 0.75 y | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.01) | 8 w | Trial: 21 AEs (18 xeroderma, 1 pruritus, 2 hepatic dysfunction) Control 42 AEs (27 xeroderma, 11 pruritus, 4 hepatic dysfuction) |
| Lu 2015a [102] | Randomized; Single center; Parallel | NR | Both group 62 (36/26) 29.5 ± 3.5 y Trial: 31 | Both group 62 (36/26) 29.5 ± 3.5 y Control: 31 | 1. Qingying decoction (b.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | Both group 4.5 ± 1.2 y | Both group 4.5 ± 1.2 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 2 w | NR |
| Lu 2015b [118] | Randomized; Single center; Parallel | NR | 54 (37/17) 21.2 ± 3.9 y | 54 (33/21) 23.8 ± 2.1 y | 1. Yangxierunfuyin (q.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, b.i.d.) | Both group 3 m–12 y | Both group 3 m–12 y | 1. PASI score (p < 0.05) | 16 w | Trial: 2 AEs (2 burning sensation) Control: 1 AEs (1 burning sensation) |
| Ma 2015 [188] | Randomized; Single center; Parallel | NR | 55 (28/27) 43.7 ± 7.6 y | 55 (29/26) 43.2 ± 7.4 y | 1. Liangxuerunfu decoction (q.d.) 2. Topical boric acid (q.d.) | 1. Topical boric acid (q.d.) | NR | NR | 1. PASI 60 (p < 0.05) | 12 w | NR |
| Peng 2015 [168] | Randomized; Single center; Parallel | NR | 40 (27/13) 36.5 y (18–65 y) | 40 (25/15) 34.3 y (19–65 y) | 1. Yinxiping pill (q.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 3 m–26 y | 1 m–30 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate | 12 w | Detailed information NR |
| Sun 2015 [156] | Randomized; Single center; Parallel | NR | 40 (21/19) 31.4 ± 2.8 y | 40 (20/20) 30.9 ± 2.7 y | 1. Qingrexiaoyin decoction (250 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 4.6 ± 2.7 y | 4.8 ± 2.4 y | 1. PASI 60 (p < 0.05) 2. TNF alpha (p < 0.05) 3. IL-8 (p < 0.05) | 12 w | NR |
| Wang 2015a [185] | Randomized; Single center; Parallel | NR | 57 (30/27) 7.1 ± 2.5 y | 57 (27/30) 7.2 ± 2.4 y | 1. Compound glycyrrhizin (25 mg, t.i.d.) 2. Acitretin capsule (0.5 mg/kg/day, b.i.d.) | 1. Acitretin capsule (0.5 mg/kg/day, b.i.d.) | 4.3 ± 1.4 m | 4.4 ± 1.5 m | 1. PASI 60 (p < 0.05) | 12 w | Trial: 3 AEs (1 hyperlipidemia, 2 hair loss) Control: 10 AEs (3 hyperlipidemia, 5 hair loss, 2 hepatic dysfunction) |
| Wang 2015b [140] | Randomized; Single center; Parallel | Simple randomization (random number table) | Both group 59 (38/21) 37.29 ± 10.24 y Trial: 30 | Both group 59 (38/21) 37.29 ± 10.24 y Control: 29 | 1. Researcher prescription (q.d.) 2. Acitretin capsule (20 mg, q.d.) | 2. Acitretin capsule (20 mg, q.d.) | Both group 7.21 ± 2.13 y | Both group 7.21 ± 2.13 y | 1. PASI 60 (p < 0.05) 2. Recurrence rate (p < 0.05) | 4 w | Trial: No AEs Control: No AEs |
| Wang 2015c [150] | Randomized; Single center; Parallel | NR | 30 (18/12) 22.92 ± 3.08 y | 30 (16/14) 23.08 ± 2.92 y | 1. Danggui-yinzi granule (t.i.d.) 2. Topical urea ointment (t.i.d.) | 1. Topical urea ointment (t.i.d.) | 2.02 ± 0.79 y | 1.98 ± 0.66 y | 1. PASI 70 (p < 0.05) | 4 w | NR |
| Yuan 2015 [169] | Randomized; Single center; Parallel | NR | Both group 80 (35/45) 32.5 ± 4.1 y Trial: 40 | Both group 80 (35/45) 32.5 ± 4.1 y Control: 40 | 1. Qingrexiaoyin decoction (250 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | Both group 2.4 ± 0.7 y | Both group 2.4 ± 0.7 y | 1. PASI 60 (p < 0.05) | 12 w | NR |
| Zhang 2015a [199] | Randomized; Single center; Parallel | Simple randomization (random number table) | 90 (58/32) 44.6 ± 3.8 y | 90 (64/26) 43.8 ± 3.4 y | 1. Liangxuerunfu decoction (200 mL, b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 7.8 ± 0.5 y | 8.7 ± 0.5 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: 12 AEs (6 pruritus, 3 xerostomia, 2 nausea, 1 headache) Control 13 AEs (5 pruritus, 4 xerostomia, 3 nausea, 1 headache) |
| Zhang 2015b [214] | Randomized; Single center; Parallel | Simple randomization (random number table) | 65 (36/29) 35.5 ± 9.7 y | 65 (38/27) 26.76 ± 7.34 y | 1. Yinxie capsule (4c, t.i.d.) 2. Topical Pyrithione Zinc aerosol (t.i.d.) | 1. Topical Pyrithione Zinc aerosol (t.i.d.) | 60.7 ± 21.3 m | 64.6 ± 22.5 m | 1. PASI 60 (p < 0.05) 2. VAS (p < 0.05) 3. DLQI (p < 0.05) 4. TNF alpha (p < 0.05) 5. IL-8 (p < 0.05) | 8 w | NR |
| Zhang 2015c [206] | Randomized; Single center; Parallel | Simple randomization (random number table) | 63 (38/25) 31.29 ± 0.04 y | 65 (38/27) 29.22 y (19–43 y) | 1. Zinyinqingrexiaofengsan (b.i.d.) 2. Acitretin capsule (20 mg, b.i.d.) | 1. Acitretin capsule (20 mg, b.i.d.) | 3 m–10 y | 1–12 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: 8 AEs (5 burning sensation, 2 erythema, 1 pruritus) Control: NR |
| Chen 2016 [96] | Randomized; Single center; Parallel | NR | 35 (18/17) 37.5 ± 6.1 y | 35 (19/16) 36.8 ± 6.0 y | 1. Researcher prescription (b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 9.2 ± 5.7 y | 9.1 ± 5.4 y | 1. PASI 70 (p < 0.05) | 12 w | Trial: 0 AEs Control 0 AEs |
| He 2016a [110] | Randomized; Single center; Parallel | NR | Both group 90 (42/48) 40.1 ± 5.3 y Trial: 45 | Both group 90 (42/48) 40.1 ± 5.3 y Control: 45 | 1. Liangxuerunfu decoction (b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | Both group 11.8 ± 3.3 y | Both group 11.8 ± 3.3 y | 1. PASI 60 (p < 0.05) | 12 w | Trial: 4 AEs (4 gastrointestinal discomfort) Control: 12 AEs (3 gastrointestinal discomfort, 3 hyperlipdemia, 6 xerostomia) |
| He 2016b [93] | Randomized; Single center; Parallel | NR | 34 (18/16) 39.21 ± 18.09 y | 33 (17/16) 38.21 ± 17.68 y | 1. Compound Qingdai pill (25 mg, t.i.d.) 2. Acitretin capsule (0.4 mg/kg/day, b.i.d.) | 1. Acitretin capsule (0.4 mg/kg/day, b.i.d.) | 4.85 ± 3.46 y | 5.02 ± 3.96 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 5 AEs (2 xerostomia, 2 hyperlipidemia, 1 gastrointestinal discomfort) Control: 4 AEs (3 xerostomia, 1 hyperlipidemia) |
| Jiang 2016 [131] | Randomized; Single center; Parallel | NR | 40 (21/19) 34.62 ± 6.56 y | 40 (23/17) 36.12 ± 5.44 y | 1. Yinxiping pill (15 g, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 60.08 ± 41.03 m | 59.45 ± 43.14 m | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate (p < 0.05) | 8 w | Trial: 5 AEs (2 burning sensation with skin rash, 1 gastrointestinal discomfort, 2 loose stool) Control: 3 AEs (burning sensation with skin rash) |
| Shan 2016 [154] | Randomized; Single center; Parallel | Simple randomization (random number table) | Both group 80 (45/35) 54.4 ± 10.4 y Trial: 40 | Both group 80 (45/35) 54.4 ± 10.4 y Control: 40 | 1. Compound glycyrrhizin (50 mg, t.i.d.) 2. Acitretin capsule (50 mg, t.i.d.) | 1. Acitretin capsule (50 mg, t.i.d.) | Both group 5.9 ± 3.2 y | Both group 5.9 ± 3.2 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: No AEs Control: 3 AEs (1 xerostomia, 1 xeroma, 1 xeroderma) |
| Wang 2016a [107] | Randomized; Single center; Parallel | NR | 25 (16/9) 35.8 ± 7.6 y | 25 (15/10) 37.1 ± 8.7 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | NR | NR | 1. PASI 60 (p < 0.05) | 16 w | NR |
| Wang 2016b [100] | Randomized; Single center; Parallel | NR | 60 (35/25) 42.3 ± 6.9 y | 60 (28/32) 39.5 ± 6.2 y | 1. Piminxiao capsule (4c, t.i.d.) 2. Acitretin capsule (25 mg, q.d.) | 1. Acitretin capsule (25 mg, q.d.) | NR | NR | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | NR |
| Wu 2016 [103] | Randomized; Single center; Parallel | NR | 70 (43/27) 38.5 ± 2.6 y | 70 (40/30) 39.1 ± 2.9 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (25 mg, q.d.) | 1. Acitretin capsule (25 mg, q.d.) | 5.5 ± 1.4 y | 5.9 ± 1.7 y | 1. PASI 60 (p < 0.05) | 8 w | Trial: 4 AEs Control: 13 AEs Including xerostomia, xeroderma, conjunctivitis, cheilitis |
| Xie 2016 [142] | Randomized; Single center; Parallel | Simple randomization (random number table) | 52 (22/30) 39.1 ± 2.9 y | 52 (24/28) 40.7 ± 9.5 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (25–30 mg, q.d.) | 1. Acitretin capsule (25–30 mg, q.d.) | 5.32 ± 1.45 y | 5.27 ± 1.42 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate (p < 0.05) 4. IL-17 (p < 0.05) | 4 w | Trial: 6 AEs (2 abdominal pain, 1 anorexia, 1 xerostomia, 1 dizziness, 1 conjunctivitis) Control: 5 AEs (1 pruritus, 1 tinnitus, 1 abdominal pain, 1 xeroma, 1 hepatic dysfunction) |
| Xu 2016 [195] | Randomized; Single center; Parallel | NR | Both group 114 (62/52) 40.5 ± 20.1 y Trial: 57 | Both group 114 (62/52) 40.5 ± 20.1 y Control: 57 | 1. Compound glycyrrhizin (50 mg, t.i.d.) 2. Acitretin capsule (0.4 mg/kg/day, b.i.d.) | 2. Acitretin capsule (0.4 mg/kg/day, b.i.d.) | NR | NR | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 6 w | Trial: 14 AEs Control: 30 AEs Including xeroderma, xeroma, xerostomia |
| Yang 2016a [178] | Randomized; Single center; Parallel | NR | 40 (25/16) 39.33 ± 8.78 y | 40 (30/10) 39.50 ± 9.37 y | 1. Qinmei granule (b.i.d.) 2. Topical urea ointment (t.i.d.) | 1. Topical urea ointment (t.i.d.) | NR | NR | 1. PASI score (p < 0.05) 2. DLQI (p < 0.05) | 12 w | Trial: 1 AE (1 Abnormal findings on urine test) Control: 1 AE (1 Abnormal findings on urine test) |
| Yang 2016b [104] | Randomized; Single center; Parallel | Simple randomization (random number generation) | 23 (15/8) 30.5 y (25–54 y) | 19 (12/7) 34.5 y (29–51 y) | 1. Qingreliangxue decoction (b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 7.62 m (3–26 m) | 6.29 m (2–28 m) | 1. PASI score (p < 0.05) | 4 w | NR |
| Yu 2016 [201] | Randomized; Single center; Parallel | NR | Both group 40 (22/18) 40.3 y (18–74 y) Trial: 20 | Both group 40 (22/18) 40.3 y (18–74 y) Control: 20 | 1. Qingreliangxue decoction (b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 3. Topical corticosteroid (Halometasone cream, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 2. Topical corticosteroid (Halometasone cream, b.i.d.) | Both group 2.5 y (1 m–15 y) | Both group 2.5 y (1 m–15 y) | 1. PASI score (p < 0.05) | 8 w | NR |
| Cao 2017 [161] | Randomized; Single center; Parallel; single blind | Simple randomization (envelope concealment method) | 30 (17/13) 36.02 ± 4.41 y | 30 (18/12) 35.54 ± 4.36 y | 1. Compound glycyrrhizin tablet (2t, t.i.d.) 2. Acitretin capsule (0.4 mg/kg/day, t.i.d.) | 1. Acitretin capsule (0.4 mg/kg/day, t.i.d.) | 4.66 ± 1.21 y | 4.25 ± 1.02 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 6 AEs (2 xeroderma, 3 xeroma, 1 hyperlipidemia) Control: 14 AEs (4 xeroderma, 5 xeroma, 3 hepatic dysfunction, 2 hyperlipidemia) |
| Cheng 2017 [94] | Randomized; Single center; Parallel | NR | 27 (15/112) 38.4 ± 5.8 y | 26 (15/11) 38.2 ± 5.3 y | 1. Compound glycyrrhizin tablet (2–3t, t.i.d.) 2. Acitretin capsule (0.4 mg/kg/day, t.i.d.) | 1. Acitretin capsule (0.4 mg/kg/day, t.i.d.) | 5.6 ± 2.4 y | 5.7 ± 2.5 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 4AEs (4 xeroma, xerostomia, xeroderma) Control: 8 AEs (6 xeroma, xerostomia, xeroderma, 1 hair loss, 1 hyperlipidemia) |
| Ding 2017 [187] | Randomized; Single center; Parallel | Simple randomization (random number table) | 40 (19/11) 36.15 ± 2.11 y | 40 (22/18) 36.20 ± 2.07 y | 1. Ziyinhuoxuerunzao decoction (200 mL, b.i.d.) 2. Acitretin capsule (20–50 mg, q.d.) | 1. Acitretin capsule (20–50 mg, q.d.) | 11.36 ± 1.00 y | 11.41 ± 0.97 | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 1 AEs (1 focal pruritus with rash) Control: 2 AEs (2 focal pruritus with rash) |
| Du 2017 [114] | Randomized; Single center; Parallel | NR | 32 (18/14) 32.2 ± 5.4 y | 32 (21/11) 37.3 ± 5.2 y | 1. Total Glycosides of Paeoniae Alba capsule (0.6 g, b.i.d.) 2. Topical corticosteroid (calcipotriol betamethasone ointment, q.d.) | 1. Topical corticosteroid (calcipotriol betamethasone ointment, q.d.) | NR | NR | 1. PASI 60 (p < 0.05) | 4 w | Trial: 4 AEs (2 skin rash, 1 burning sensation, 1 folliculitis Control: No AE |
| Feng 2017 [130] | Randomized; Single center; Parallel | Simple randomization (random number table) | 35 (21/14) 38.3 ± 4.1 y | 35 (19/16) 35.7 ± 6.4 y | 1. Yinxiping pill (15 g, t.i.d.) 2. Topical corticosteroid (Compound Flumetasone Ointment, b.i.d.) | 1. Topical corticosteroid (Compound Flumetasone Ointment, b.i.d.) | 5.9 ± 3.7 y | 6.2 ± 3.3 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 2 AEs (1 mild diarrhea, 1 mild skin rash) Control: 1 AEs (1 mild skin rash with pruritus) |
| Han 2017a [123] | Randomized; Single center; Parallel | NR | 44 (25/19) 36.04 ± 7.15 y | 44 (26/18) 35.69 ± 6.49 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 5.39 ± 2.48 | 5.21 ± 2.36 | 1. PASI 60 (p < 0.05) 2. Recurrence rate (p < 0.05) 3. IL-17 (p < 0.05) | 12 w | Trial: 3 AEs (detailed information NR) Control 12 AEs (detailed information NR) |
| Han 2017b [162] | Randomized; Single center; Parallel | NR | 48 (26/22) 45.72 ± 5.78 y | 44 (27/21) 43.56 ± 4.43 y | 1. Compound Qingdai capsule (4c, t.i.d.) 2. Acitretin capsule (20 mg, b.i.d.) | 1. Acitretin capsule (20 mg, b.i.d.) | 3.5 y (2 m–40 y) | 4.2 y (3 m–36 y) | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. Recurrence rate (p < 0.05) | 8 w | Detailed information NR |
| Li 2017 [203] | Randomized; Single center; Parallel | NR | 30 (18/12) 36.4 ± 10.0 y | 30 (15/15) 34.2 ± 12.7 y | 1. Qingrejiedu decoction (6 g, b.i.d.) 2. Topical retinoid cream (b.i.d.) | 1. Topical retinoid cream (b.i.d.) | 5.73 ± 3.78 y | NR | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. TNF alpha (p < 0.05) | 4 w | Trial: 3 AEs (3 diarrhea) Control: No AE |
| Liu 2017 [116] | Randomized; Single center; Parallel | NR | 48 (27/21) 33.5 ± 6.5 y | 52 (29/23) 33.8 ± 6.2 y | 1. Yinxie capsule (4c, t.i.d.) 2. Acitretin capsule (20 mg, b.i.d.) | 1. Acitretin capsule (20 mg, b.i.d.) | 5.6 ± 7.2 y | 5.2 ± 6.9 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 65 AEs (13 xeroma, 16 xeroderma, 2 epistaxis, 14 folliculitis, 1 hepatic dysfunction, 19 hyperlipidemia) Control: 178 AEs (42 xeroma, 40 xeroderma, 9 epistaxis, 42 folliculitis, 8 hepatic dysfunction, 37 hyperlipidemia) |
| Luo 2017 [160] | Randomized; Single center; Parallel | NR | 39 (26/13) 31.4 ± 2.3 y | 36 (25/11) 32.7 ± 2.8 y | 1. Compound glycyrrhizin (2–3t, t.i.d.) 2. Acitretin capsule (25–30 mg, t.i.d.) | 1. Acitretin capsule (25–30 mg, t.i.d.) | NR | NR | 1. PASI 60 (p < 0.05) | 8 w | Trial: 5 AEs (2 pruritus, 1 xeroderma, 1 xerostomia, 1 xeroma) Control: 8 AEs (3 pruritus, 2 xeroderma, 2 xerostomia, 1 xeroma) |
| Pang 2017 [170] | Randomized; Single center; Parallel | NR | 45 (22/23) 36.48 ± 14.21 y | 45 (24/21) 37.02 ± 44.47 y | 1. Compound Qingdai capsule (4c, t.i.d.) 2. Immunosuppressant (compound amino peptide tablets 5t, t.i.d.) | 1. Immunosuppressant (compound amino peptide tablets 5t, t.i.d.) | 47.68 ± 18.22 m | 49.13 ± 18.80 m | 1. PASI 70 (p < 0.05) 2. PASI score (p < 0.01) 3. DLQI (p < 0.05) 4. IL-8 (p < 0.05) 5. IFN gamma (p < 0.01) | 8 w | NR |
| Shi 2017 [89] | Randomized; Single center; Parallel | NR | 23 (12/11) 45.74 ± 8.43 y | 23 (13/10) 45.72 ± 8.45 y | 1. Liangxuexiaofeng decoction (200 mL, b.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 7.29 ± 1.25 y | 7.28 ± 1.28 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.01) 3. IL-8 (p < 0.05) | 8 w | Trial: 3 AEs (1 xerostomia, 1 xeroma, 1 xeroderma) Control: 2 AEs (1 xerostomia, 1 nausea) |
| Song 2017 [108] | Randomized; Single center; Parallel | NR | 70 (32/28) 41.02 ± 5.39 y | 70 (39/31) 40.76 ± 5.32 y | 1. Yangxuetongluo decoction (b.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 6.41 ± 1.00 y | 6.49 ± 1.03 y | 1. PASI 70 (p < 0.05) 2. VAS (p < 0.05) 3. DLQI (p < 0.05) 4. TNF alpha (p < 0.05) | 8 w | Trial: 9 AEs (2 conjunctivitis, 4 xerostomia, 3 headache) Control: 8 AEs (3 conjunctivitis, 2 xerostomia, 2 headache, 1 muscular pain) |
| Wang 2017 [159] | Randomized; Single center; Parallel | NR | 60 (32/28) 32.46 ± 6.25 y | 60 (32/28) 33.08 ± 6.32 y | 1. Yinxie capsule (4c, t.i.d.) 2. Acitretin capsule (20 mg, t.i.d.) | 1. Acitretin capsule (20 mg, t.i.d.) | 5.61 ± 7.32 y | 5.29 ± 6.96 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. IL-8 (p < 0.05) | 8 w | Trial: 54 AEs (15 xeroma, 18 xeroderma, 3 epistaxis, 16 folliculitis, 2 hepatic dysfunction, 15 hyperlipidemia) Control: 180 AEs (48 xeroma, 43 xeroderma, 11 epistaxis, 32 folliculitis, 9 hepatic dysfunction, 37 hyperlipidemia) |
| Wu 2017 [173] | Randomized; Single center; Parallel | NR | 40 (26/14) 40.85 ± 15.48 y | 40 (25/15) 41.64 ± 15.86 y | 1. Shentongzhuyu decoction (200 mL, b.i.d.) 2. Methotrexate (10 mg, q.w.) 3. Sulfasalazine tablets (1.0 g, t.i.d.) 4. Diclofenac sodium extended-release tablet (0.1 g, q.d.) 5. Folic acid tablet (10 mg, q.d.) | 1. Methotrexate (10 mg, q.w.) 2. Sulfasalazine tablets (1.0 g, t.i.d.) 3. Diclofenac sodium extended-release tablet (0.1 g, q.d.) 4. Folic acid tablet (10 mg, q.d.) | NR | NR | 1. PASI 70 (p < 0.01) 2. PASI score (p < 0.05) | 12 w | Trial: 9 AEs (2 leukopenia, 3 hepatic dysfunction, 1 hyperbilirubinemia, 3 nausea and vomiting) Control 10 AEs (3 leukopenia, 2 hepatic dysfunction, 1 hyperbilirubinemia, 4 nausea and vomiting) |
| Yang 2017 [213] | Randomized; Single center; Parallel | Simple randomization | 40 (25/15) 30.6 ± 8.21 y | 40 (24/16) 32.5 ± 7.10 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (20 mg, q.d.) | 1. Acitretin capsule (20 mg, q.d.) | 4.51 ± 3.13 y | 4.60 ± 3.02 y | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.05) 3. TNF alpha (p < 0.05) 4. IL-17 (p < 0.05) 5. IL-23 (p < 0.05) | 12 w | Trial: 9AEs (3 pruritus, 5 xerostomia, 1 xeroma) Control: 17 AEs (7 pruritus, 8 xerostomia, 1 dizzines, 1 xeroma) |
| Zeng 2017 [190] | Randomized; Single center; Parallel | NR | 31 (21/11) 35.14 ± 0.15 y | 31 (21/10) 35.29 ± 0.18 y | 1. Compound glycyrrhizin capsule (2-3c, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 32.14 ± 1.25 m | 33.45 ± 1.34 m | 1. PASI 60 (p < 0.05) | 8 w | Trial: 13 AEs (2 xerostomia, 3 xeroma, 2 pruritus, 4 nausea, 2 dizziness) Control: 8 AEs (1 xerostomia, 2 xeroma, 1 pruritus, 3 nausea, 1 dizziness) |
| Zhang 2017a [113] | Randomized; Single center; Parallel | NR | 52 (27/25) 28.5 ± 5.2 y | 52 (26/26) 30.1 ± 4.1 y | 1. Taohongershao decoction (150 mL, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 7.3 ± 4.5 y | 8.5 ± 4.9 y | 1. PASI score (p < 0.05) 2. Recurrence rate (p < 0.05) | 4 w | NR |
| Zhang 2017b [112] | Randomized; Single center; Parallel | NR | 45 (21/24) 27.5 ± 7.4 y | 45 (22/23) 28.0 ± 9.5 y | 1. Taohongershao decoction (150 mL, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 10.5 ± 8.3 y | 10.9 ± 8.0 y | 1. PASI 60 (p < 0.05) 2. DLQI (p < 0.05) 3. IFN gamma (p < 0.05) 4. IL-17 (p < 0.05) 5. IL-23 (p < 0.05) | 4 w | NR |
| Zhang 2017c [105] | Randomized; Single center; Parallel | NR | 50 (23/27) 32.5 ± 5.9 y | 50 (25/25) 30.9 ± 6.1 y | 1. Taohongershao decoction (150 mL, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 12.6 ± 7.5 y | 11.5 ± 6.9 y | 1. PASI 60 (p < 0.01) 2. PASI score (p < 0.05) | 8 w | NR |
| Zhang 2017d [121] | Randomized; Single center; Parallel | NR | 17 (10/7) 46.47 ± 14.06 | 17 (9/8) 46.41 ± 18.45 | 1. Liangxuexiao feng (6 g, b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 16.29 ± 10.49 y | 16.08 ± 12.80 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | NR |
| Zhang 2017e [200] | Randomized; Single center; Parallel | NR | 55 (24/31) 30.1 ± 4.4 y | 55 (22/33) 29.8 ± 7.3 y | 1. Taohongershao decoction (150 mL, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 7.5 ± 6.3 y | 8.1 ± 6.4 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. IFN gamma (p < 0.05) | 4 w | NR |
| Zhao 2017 [128] | Randomized; Single center; Parallel | NR | 40 (23/17) 36.35 ± 2.09 y | 40 (22/18) 36.25 ± 2.13 y | 1. Yinxie capsule (3c, t.i.d.) 2. Acitretin capsule (20 mg, q.d.). | 1. Acitretin capsule (20 mg, q.d.). | NR | NR | 1. PASI 60 (p < 0.05) | 8 w | Trial: 18 AEs (6 pruritus, 7 epistaxis, 5 xerostomia) Control: 19 AEs (7 pruritus, 7 epistaxis, 6 xerostomia) |
| Chai 2018 [158] | Randomized; Single center; Parallel | NR | 46 (26/20) 45.3 ± 3.8 y | 46 (25/21) 45.5 ± 3.6 y | 1. Xiaoyin decoction (b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 2.3 ± 0.5 y | 2.1 ± 0.4 y | 1. PASI 60 (p < 0.05) 2. IFN gamma (p < 0.05) 3. IL-8 (p < 0.05) | 8 w | Trial: 2 AEs (1 skin rash, 1 mild gastrointestinal discomfort) Control: 3 AEs (2 skin rash, 1 pruritus) |
| Li 2018 [109] | Randomized; Single center; Parallel | Simple randomization (random number table) | 44 (23/21) 35.01 ± 7.09 y | 44 (24/20) 35.31 ± 7.29 y | 1. Yinxie capsule (4c, t.i.d.) 2. Acitretin capsule (20 mg, b.i.d.) | 1. Acitretin capsule (20 mg, b.i.d.). | 7.69 ± 3.69 y | 7.71 ± 3.46 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.01) | 8 w | Trial: 1 AEs (1 nausea) Control: 3 AEs (1 headache, 2 nausea) |
| Liu 2018 [147] | Randomized; Single center; Parallel Three arm trial | NR | 25 (11/14) 37.21 ± 9.87 y | 25 (13/12) 39.42 ± 9.23 y | 1. Banzhilian decoction (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.). | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 6.8 ± 5.1 y | 6.2 ± 3.9 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | NR |
| Luo 2018 [212] | Randomized; Single center; Parallel | NR | 50 (31/19) 32.46 ± 10.24 y | 50 (29/21) 33.57 ± 10.82 y | 1. Zicaohuoxue decoction (200 mL, bi.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 2.46 ± 1.24 y | 2.52 ± 1.28 y | 1. PASI 60 (p < 0.05) 2. TNF alpha (p < 0.05) 3. DLQI (p < 0.05) | 8 w | Trial: 8 AEs (1 pruritus, 2 xeroma, 2 headache, 3 nausea) Control: 10 AEs (1 pruritus, 3 xeroma, 3 headache, 3 nausea) |
| Ma 2018a [192] | Randomized; Single center; Parallel | Simple randomization (random number table) | 37 (21/16) 38.1 ± 4.2 y | 42 (23/19) 37.4 ± 4.1 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 3. Acitretin capsule (30 mg, q.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 3.66 ± 1.01 y | 3.7 ± 1.3 y | 1. PASI 60 (p < 0.05) | 4 w | NR |
| Ma 2018b [166] | Randomized; Single center; Parallel | NR | 34 (19/15) 40.2 ± 6.9 y | 34 (21/13) 41.6 ± 7.5 y | 1. Qingrequshi decoction (b.i.d.) 2. Methotrexate (15 mg, q.w.) | 1. Methotrexate (15 mg, q.w.) | 8.7 ± 2.6 y | 8.2 ± 2.4 y | 1. PASI score (p < 0.05) | 12 w | NR |
| Xiao 2018 [176] | Randomized; Single center; Parallel | Simple randomization (random number table) | 21 (11/10) 27.5 ± 2.2 y | 21 (15/6) 27.3 ± 1.2 y | 1. Xiaoyin decoction (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | NR | NR | 1. TNF alpha (p < 0.05) 2. IL-17 (p < 0.05) 3. IL-22 (p < 0.05) | 12 w | NR |
| Xie 2018 [106] | Randomized; Single center; Parallel | NR | 60 (34/26) 36.5 y (19–65 y) | 60 (29/31) 34.3 y (19–64 y) | 1. Qingying decoction (b.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 6.4 y (3 m–26 y) | 6.1 y (1 m–30 y) | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Detailed information NR |
| Zhang 2018a [111] | Randomized; Single center; Parallel | NR | 48 (25/23) 35.43 ± 0.16 | 48 (28/20) 35.28 ± 0.23 | 1. Compound glycyrrhizin (2–3t, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 33.12 ± 1.64 m | 32.65 ± 1.14 m | 1. PASI score (p < 0.05) | 8 w | NR |
| Zhang 2018b [174] | Randomized; Single center; Parallel | Simple randomization | 36 (20/16) 29.15 ± 6.24 y | 36 (19/17) 29.36 ± 6.02 | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 5.2 ± 1.3 y | 5.4 ± 1.2 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | NR |
| Zhou 2018 [139] | Randomized; Single center; Parallel | NR | 39 (17/22) 38.82 ± 1.29 y | 39 (19/20) 38.71 ± 1.22 y | 1. Keyin pills (10 mg, b.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 1. Acitretin capsule (10 mg, t.i.d.) | 7.59 ± 0.78 y | 7.46 ± 0.65 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. IFN gamma (p < 0.05) 4. IL-17 (p < 0.05) | 8 w | Trial: 8 AEs (1 conjunctivitis, 1 skin rash, 2 arthralgia, 1 headache, 3 nausea and vomiting) Control: 11 AEs (1 conjunctivitis, 2 skin rash, 3 arthralgia, 1 headache, 4 nausea and vomiting) |
| Chen 2019 [198] | Randomized; Single center; Parallel | NR | 62 (26/36) 37.62 ± 6.34 y | 62 (28/34) 35.74 ± 5.54 y | 1. Compound glycyrrhizin tablet (2–3t, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 3. Acitretin capsule (25–30 mg, q.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) 2. Acitretin capsule (25–30 mg, q.d.) | 6.92 ± 3.05 y | 6.25 ± 2.47 y | 1. PASI score (p < 0.05) | 8 w | NR |
| Ge 2019 [145] | Randomized; Single center; Parallel | NR | 40 (21/19) 27 ± 2.4 y | 40 (22/18) 26 ± 2.3 y | 1. Compound glycyrrhizin (50 mg, t.i.d.) 2. Acitretin capsule (20 mg, t.i.d.) | 1. Acitretin capsule (20 mg, t.i.d.) | 3 m–31 y | 3 m–31.5 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 5 AEs (including xerostomia, xeroma, xeroderma) Control: 8 AEs (including xerostomia, xeroma, xeroderma, 2 hair loss, 1 hyperlipidemia) |
| Han 2019 [97] | Randomized; Single center; Parallel | NR | Both group 80 (59/21) 37.37 ± 9.48 y | Both group 80 (59/21) 37.37 ± 9.48 y | 1. Qinzhuliangxue feng (b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | Both group 5.29 ± 1.44 y | Both group 5.29 ± 1.44 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 4 w | NR |
| Hu 2019 [119] | Randomized; Single center; Parallel | Simple randomization | 62 (34/28) 36.8 ± 8.1 y | 59 (33/26) 37.8 ± 9.4 y | 1. Liangxuexiaobi decoction (150 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol betamethasone ointment, q.d.) | 1. Topical corticosteroid (Calcipotriol betamethasone ointment, q.d.) | 45.4 ± 12.5 m | 46.1 ± 11.1 m | 1. PASI score (p < 0.05) | 8 w | Trial: 2 AEs (2 loose stool) Control: 1AE (1 skin rash) |
| Lu 2019 [152] | Randomized; Single center; Parallel; single blind | Simple randomization (envelope concealment method) | 30 (15/15) 21–66 y | 30 (16/14) 22–65 y | 1. Yangxuequfeng granule (b.i.d.) 2. Topical urea ointment (t.i.d.) | 1. Topical urea ointment (t.i.d.) | NR | NR | 1. PASI 70 (p < 0.05) 2. DLQI (p < 0.05) | 4 w | NR |
| Xun 2019 [134] | Randomized; Single center; Parallel | NR | 52 (24/28) 40–50 y | 52 (22/30) 40–51 y | 1. Yinxie capsule (15 g, t.i.d.) 2. Acitretin capsule (25–50 mg, t.i.d.) | 1. Acitretin capsule (25–50 mg, t.i.d.) | NR | NR | 1. Recurrence rate (p-value NR) | 4 w | NR |
| Yang 2019 [149] | Randomized; Single center; Parallel | NR | 41 (24/17) 38.24 ± 4.19 y | 41 (25/16) 38.57 ± 4.03 y | 1. Compound Qingdai capsule (4c, t.i.d.) 2. Topical corticosteroid (Calcipotriol betamethasone ointment, q.d.) | 1. Topical corticosteroid (Calcipotriol betamethasone ointment, q.d.) | 14.39 ± 2.78 y | 14.79 ± 1.93 y | 1. PASI 60 (p < 0.05) 2. TNF alpha (p < 0.05) 3. IL-17 (p < 0.05) 4. IL-23 (p < 0.05) | 8 w | Trial: 4 AEs (1 xeroderma, 1 pruritus, 1 hyperlipidemia, 1 hepatic dysfunction) Control: 6 AEs (1 skin scale with edema, 1 xeroderma, 2 pruritus, 1 hyperlipidemia, 1 hepatic dysfunction) |
| Yao 2019 [180] | Randomized; Single center; Parallel | Simple randomization (random number table) | 52 (29/23) 45.37 ± 6.12 y | 52 (27/25) 45.13 ± 6.08 y | 1. Liangxuerunzao decoction (250 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 9.77 ± 1.30 y | 9.86 ± 1.35 y | 1. PASI 70 (p < 0.05) 2. PASI score (p < 0.01) 3.IL-17 (p < 0.01) 4.IL-22 (p < 0.01) 5.IL-23 (p < 0.01) | 8 w | NR |
| Zhong 2019 [122] | Randomized; Single center; Parallel | NR | 46 (22/24) 36.72 ± 6.21 y | 46 (23/23) 37.23 ± 5.78 y | 1. Piminxiao capsule (4c, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 5.10 ± 1.76 y | 5.25 ± 1.28 y | 1. PASI 60 (p < 0.05) 3. IL-17 (p < 0.05) | 12 w | NR |
| Chen 2020 [155] | Randomized; Single center; Parallel | NR | 47 (25/22) 36.9 ± 5.3 y | 47 (24/23) 38.2 ± 5.1 y | 1. Compound glycyrrhizin capsule (3c, t.i.d.) 2. Acitretin capsule (25–30 mg, q.d.) | 2. Acitretin capsule (25–30 mg, q.d.) | NR | NR | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Trial: 7 AEs (1 nausea, 3 hyperesthesia, 2 xeroderma, 1 xerostomia) Control: 5 AEs (2 nausea, 1 hyperesthesia 1 xeroderma, 1 xerostomia) |
| Hao 2020 [120] | Randomized; Single center; Parallel | Simple randomization (random number table) | 30 (22/8) 42 ± 13 y | 30 (21/9) 41 ± 12 y | 1. Liangxuexiaobi pill (6c, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 3.71 ± 3.26 y | 4.28 ± 2.96 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. DLQI (p < 0.05) | 4 w | Trial: 1 AE (1 skin rash) Control: 4 AEs (2 erythema, 1 skin rash with burning sensation, 1 pruritus) |
| Ji 2020 [204] | Randomized; Single center; Parallel | NR | 25 (15/10) 37.8 y (25.3–49.2 y) | 25 (17/8) 37.5 y (27.1–48.2 y) | 1. Compound Qingdai capsule (6 g, t.i.d.) 2. Methotrexate (detailed dosage NR) | 1. Methotrexate (detailed dosage NR) | NR | NR | 1. PASI score (p < 0.05) 2. DLQI (p < 0.05) | 8 w | NR |
| Liu 2020a [181] | Randomized; Single center; Parallel | NR | 30 (18/12) 41.3 ± 5.1 y | 30 (17/13) 42.3 ± 6.2 y | 1. Compound glycyrrhizin tablet (2t, t.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 1. Acitretin capsule (10 mg, b.i.d.) | 5.23 ± 1.13 y | 5.31 ± 1.23 y | 1. PASI score (p < 0.05) 2. DLQI (p < 0.05) | 8 w | Trial: 5 AEs (2 xerostomia, 2 xeroma, 1 xeroderma) Control: 11 AEs (3 xerostomia, 3 xeroma, 2 xeroderma, 2 hair loss, 1 hyperlipidemia) |
| Liu 2020b [167] | Randomized; Single center; Parallel | NR | 92 (46/46) 54.18 ± 4.19 y | 92 (45/47) 54.81 ± 4.33 y | 1. Xiaoyin decoction (6c, t.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 8.43 ± 2.28 y | 8.79 ± 2.30 y | 1. IL-8 (p < 0.05) | 8 w | NR |
| Lu 2020 [95] | Randomized; Single center; Parallel | NR | 56 (31/25) 44.58 ± 7.12 y | 56 (29/27) 45.01 ± 6.93 y | 1. Total Glycosides of Paeoniae Alba capsule (0.6 g, t.i.d.) 2. Acitretin capsule (25–30 mg, q.d.) | 2. Acitretin capsule (25–30 mg, q.d.) | 7.63 ± 1.83 y | 7.79 ± 1.72 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | Detailed information NR |
| Qu 2020 [177] | Randomized; Single center; Parallel | Simple randomization (computer assisted random assignment) | 46 (25/21) 40.44 ± 6.74 y | 46 (26/20) 40.13 ± 6.48 y | 1. Yinxie capsule (4c, t.i.d.) 2. Acitretin capsule (25–30 mg, q.d.) | 2. Acitretin capsule (25–30 mg, q.d.) | 7.75 ± 3.75 y | 7.45 ± 3.46 y | 1. PASI 60 (p < 0.05) | 8 w | NR |
| Ren 2020 [151] | Randomized; Single center; Parallel | NR | 51 (27/24) 38.19 ± 2.14 y | 51 (26/25) 38.62 ± 2.37 y | 1. Xiaoyin granule (10 g, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 2. Acitretin capsule (10 mg, t.i.d.) | 2.25 ± 0.95 y | 2.34 ± 0.83 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 12 w | Trial: 6 AEs (2 xerostomia, 1 pruritus, 2 gastrointestinal discomfort, 1 hepatic dysfunction) Control: 18 AEs (5 xerostomia, 6 pruritus, 4 gastrointestinal discomfort, 3 hepatic dysfunction) |
| Shen 2020 [133] | Randomized; Single center; Parallel | NR | 31 (15/16) 36 ± 14 y | 32 (16/16) 35 ± 11 y | 1. Liangxuejiedu decoction (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, q.d.) 3. Topical corticosteroid (Mometasone furoate cream, q.d.) | 1. Topical corticosteroid (Calcipotriol ointment, q.d.) 2. Topical corticosteroid (Mometasone furoate cream, q.d.) | 6.67 ± 6.30 y | 6.42 ± 6.13 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. DLQI (p < 0.05) | 8 w | NR |
| Wu 2020 [193] | Randomized; Single center; Parallel | NR | 50 (28/22) 35.4 ± 7.9 y | 50 (27/23) 34.4 ± 7.6 y | 1. Compound glycyrrhizin tablet (10 g, t.i.d.) 2. Acitretin capsule (10 mg, t.i.d.) | 2. Acitretin capsule (10 mg, t.i.d.) | 4.6 ± 3.1 y | 4.5 ± 2.9 y | 1. PASI 70 (p < 0.05) | 8 w | Trial: 25 AEs (21 xerostomia, xeroma, xeroderma, 2 hepatic dysfunction, 2 hyperlipidemia) Control: 47 AEs (35 xerostomia, xeroma, xeroderma, 8 hepatic dysfunction, 4 hyperlipidemia) |
| Yang 2020 [101] | Randomized; Single center; Parallel | NR | 78 (44/34) 39.24 ± 6.51 y | 78 (45/33) 39.17 ± 6.48 y | 1. Xiaoyin granule (3.5 g, t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 1. Acitretin capsule (30 mg, q.d.) | 7.71 ± 2.51 y | 7.69 ± 2.53 y | 1. PASI 60 (p < 0.05) | 8 w | NR |
| Zheng 2020 [205] | Randomized; Single center; Parallel | NR | 32 (16/16) 29.8 ± 6.2 y | 32 (14/18) 30.5 ± 5.8 y | 1. Jueyin granule (b.i.d.) 2. Acitretin capsule (20 mg, q.d.) 3. Topical corticosteroid (Compound flumetasone cream, b.i.d.) | 1. Acitretin capsule (20 mg, q.d.) 2. Topical corticosteroid (Compound flumetasone cream, b.i.d.) | NR | NR | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 8 w | NR |
| Jin 2021 [148] | Randomized; Single center; Parallel | NR | 50 (24/26) 42.06 ± 4.37 y | 32 (14/18) 41.32 ± 4.93 y | 1. Xiaoyin decoction (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol ointment, b.i.d.) | 3.47 ± 1.40 y | 3.32 ± 1.03 y | 1. TNF alpha (p < 0.05) 2. IL-17 (p < 0.05) 3. IL-22 (p < 0.05) | 4 w | NR |
| Lan 2021 [117] | Randomized; Single center; Parallel | NR | 40 (25/15) 46.21 ± 8.07 y | 40 (23/17) 46.05 ± 8.69 y | 1. Dangguiyinzi (150 mL, b.i.d.) 2. Acitretin capsule (10 mg, b.i.d.) | 2. Acitretin capsule (10 mg, b.i.d.) | 6.25 ± 3.00 y | 6.18 ± 1.95 y | 1. PASI 60 (p < 0.05) 2. PASI score (p < 0.05) | 12 w | Trial: 5 AEs (1 hyperlipidemia, 2 xeroma, 2 xerostomia) Control: 21 AEs (7 hyperlipidemia, 4 xeroma, 8 xerostomia, 2 cheilitis) |
| Le 2021 [175] | Randomized; Single center; Parallel | NR | 43 (24/19) 42.17 ± 2.75 y | 42 (21/21) 42.34 ± 2.66 y | 1. Tianxian decoction (t.i.d.) 2. Acitretin capsule (30 mg, q.d.) | 2. Acitretin capsule (30 mg, q.d.) | 4.68 ± 0.91 y | 4.71 ± 0.88 y | 1. PASI score (p < 0.05) 2. DLQI (p < 0.05) | 12 w | NR |
| Tang 2021 [171] | Randomized; Single center; Parallel | Simple randomization (random number table) | 36 (19/17) 34.17 ± 1.75 y | 36 (20/16) 33.25 ± 1.67 y | 1. Qingreyangxuejiedu decoction (200 mL, b.i.d.) 2. Topical corticosteroid (Calcipotriol betamethasone ointment, b.i.d.) | 1. Topical corticosteroid (Calcipotriol betamethasone ointment, b.i.d.) | 1.96 ± 0.76 y | 1.75 ± 0.49 y | 1.P ASI 60 (p < 0.05) 2. PASI score (p < 0.05) 3. IL-17 (p < 0.05) 4. IL-23 (p < 0.05) | 8 w | Trial: 1 AE (1 gastrointestinal discomfort) Control: 2 AEs (1 telangiectasia, 1 folliculitis) |
| Wang 2021 [98] | Randomized; Single center; Parallel | NR | 42 (22/20) 46.75 ± 21.23 y | 41 (21/20) 45.91 ± 20.89 y | 1. Qingreliangxue decoction (100 mL, b.i.d.) 2. Acitretin capsule (20 mg, q.d.) 3. Topical corticosteroid (Mometasone furoate cream, q.d.) | 1. Acitretin capsule (20 mg, q.d.) 2. Topical corticosteroid (Mometasone furoate cream, q.d.) | 12.74 ± 5.23 y | 11.76 ± 5.48 y | 1. PASI 60 (p < 0.05) 3. Recurrence rate (p < 0.05) | 8 w | Trial: 9AEs (2 xerostomia, 5 xeroderma with pruritus, 2 diarrhea) Control: 10 AEs (2 xerostomia, 6 xeroderma with pruritus, 2 diarrhea) |
| Author | Year | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|
| Che | 2004 | Sc | Sc | L | H | Sc | H |
| Chen | 2004 | Sc | Sc | L | Sc | Sc | Sc |
| Xu | 2005 | Sc | Sc | L | Sc | Sc | Sc |
| Liu | 2006 | Sc | Sc | L | H | Sc | H |
| Chen | 2007 | Sc | Sc | L | H | Sc | H |
| Huang | 2007 | L | L | L | L | Sc | Sc |
| Zeng | 2009 | L | Sc | L | Sc | Sc | Sc |
| Cao | 2010 | Sc | Sc | L | Sc | Sc | Sc |
| He | 2010 | Sc | Sc | L | H | Sc | H |
| Hua | 2010 | Sc | Sc | L | H | Sc | H |
| Luo | 2010 | Sc | Sc | L | Sc | Sc | Sc |
| Yu | 2010 | Sc | Sc | L | H | Sc | H |
| Liu | 2011 | Sc | Sc | L | H | Sc | H |
| Lu | 2011 | Sc | Sc | L | Sc | Sc | Sc |
| Tian | 2011 | L | Sc | L | Sc | Sc | Sc |
| Xu | 2011 | Sc | Sc | L | Sc | Sc | Sc |
| Yao | 2011 | L | Sc | L | Sc | Sc | Sc |
| Zheng | 2011 | Sc | Sc | L | Sc | Sc | Sc |
| Jiang | 2012 | L | L | L | L | Sc | Sc |
| Liu | 2012 | Sc | Sc | L | Sc | Sc | Sc |
| Xie | 2012 | Sc | Sc | L | H | Sc | H |
| Zhang | 2012 | Sc | Sc | L | Sc | Sc | Sc |
| Zhou | 2012a | Sc | Sc | L | Sc | Sc | Sc |
| Zhou | 2012b | Sc | Sc | L | H | Sc | H |
| Chen | 2013 | Sc | Sc | L | Sc | Sc | Sc |
| Ding | 2013 | Sc | Sc | L | Sc | Sc | Sc |
| Mo | 2013 | Sc | Sc | L | Sc | Sc | Sc |
| Song | 2013 | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2013 | Sc | Sc | L | Sc | Sc | Sc |
| Cheng | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Du | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Li | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Liang | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Liu | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Qiu | 2014 | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2014a | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2014b | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2014c | Sc | Sc | L | Sc | Sc | Sc |
| Cai | 2015 | Sc | Sc | L | Sc | Sc | Sc |
| Jin | 2015a | Sc | Sc | L | Sc | Sc | Sc |
| Jin | 2015b | Sc | Sc | L | Sc | Sc | Sc |
| Lu | 2015a | Sc | Sc | L | H | Sc | H |
| Lu | 2015b | Sc | Sc | L | Sc | Sc | Sc |
| Ma | 2015 | Sc | Sc | L | Sc | Sc | Sc |
| Peng | 2015 | Sc | Sc | L | Sc | Sc | Sc |
| Sun | 2015 | Sc | Sc | L | Sc | Sc | Sc |
| Wang | 2015a | Sc | Sc | L | Sc | Sc | Sc |
| Wang | 2015b | L | Sc | L | H | Sc | H |
| Wang | 2015c | Sc | Sc | L | Sc | Sc | Sc |
| Yuan | 2015 | Sc | Sc | L | H | Sc | H |
| Zhang | 2015a | L | Sc | L | Sc | Sc | Sc |
| Zhang | 2015b | L | Sc | L | Sc | Sc | Sc |
| Zhang | 2015c | L | Sc | L | Sc | Sc | Sc |
| Chen | 2016 | Sc | Sc | L | Sc | Sc | Sc |
| He | 2016a | Sc | Sc | L | H | Sc | H |
| He | 2016b | Sc | Sc | L | Sc | Sc | Sc |
| Jiang | 2016 | Sc | Sc | L | Sc | Sc | Sc |
| Shan | 2016 | L | Sc | L | H | Sc | H |
| Wang | 2016a | Sc | Sc | L | H | Sc | H |
| Wang | 2016b | Sc | Sc | L | H | Sc | H |
| Wu | 2016 | Sc | Sc | L | Sc | Sc | Sc |
| Xie | 2016 | L | Sc | L | Sc | Sc | Sc |
| Xu | 2016 | Sc | Sc | L | H | Sc | H |
| Yang | 2016a | Sc | Sc | Sc | H | Sc | H |
| Yang | 2016b | L | Sc | L | Sc | Sc | Sc |
| Yu | 2016 | Sc | Sc | L | H | Sc | H |
| Cao | 2017 | L | Sc | L | L | Sc | Sc |
| Cheng | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Ding | 2017 | L | Sc | L | Sc | Sc | Sc |
| Du | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Feng | 2017 | L | Sc | L | Sc | Sc | Sc |
| Han | 2017a | Sc | Sc | L | Sc | Sc | Sc |
| Han | 2017b | Sc | Sc | L | Sc | Sc | Sc |
| Li | 2017 | Sc | Sc | L | H | Sc | H |
| Liu | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Luo | 2017 | Sc | Sc | L | H | Sc | H |
| Pang | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Shi | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Song | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Wang | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Wu | 2017 | Sc | Sc | L | H | Sc | H |
| Yang | 2017 | L | Sc | L | Sc | Sc | Sc |
| Zeng | 2017 | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2017a | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2017b | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2017c | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2017d | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2017e | Sc | Sc | L | Sc | Sc | Sc |
| Zhao | 2017 | Sc | Sc | L | H | Sc | H |
| Chai | 2018 | Sc | Sc | L | Sc | Sc | Sc |
| Li | 2018 | L | Sc | L | Sc | Sc | Sc |
| Liu | 2018 | Sc | Sc | L | Sc | Sc | Sc |
| Luo | 2018 | Sc | Sc | L | Sc | Sc | Sc |
| Ma | 2018a | L | Sc | L | Sc | Sc | Sc |
| Ma | 2018b | Sc | Sc | L | Sc | Sc | Sc |
| Xiao | 2018 | L | Sc | L | Sc | Sc | Sc |
| Xie | 2018 | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2018a | Sc | Sc | L | Sc | Sc | Sc |
| Zhang | 2018b | L | Sc | L | Sc | Sc | Sc |
| Zhou | 2018 | Sc | Sc | L | Sc | Sc | Sc |
| Chen | 2019 | Sc | Sc | L | Sc | Sc | Sc |
| Ge | 2019 | Sc | Sc | L | Sc | Sc | Sc |
| Han | 2019 | Sc | Sc | L | H | Sc | H |
| Hu | 2019 | L | Sc | L | Sc | Sc | Sc |
| Lu | 2019 | L | Sc | L | L | Sc | Sc |
| Xun | 2019 | Sc | Sc | L | Sc | Sc | Sc |
| Yang | 2019 | Sc | Sc | L | Sc | Sc | Sc |
| Yao | 2019 | L | Sc | L | Sc | Sc | Sc |
| Zhong | 2019 | Sc | Sc | L | Sc | Sc | Sc |
| Chen | 2020 | Sc | Sc | L | H | Sc | H |
| Hao | 2020 | L | Sc | L | Sc | Sc | Sc |
| Ji | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Liu | 2020a | Sc | Sc | L | Sc | Sc | Sc |
| Liu | 2020b | Sc | Sc | L | Sc | Sc | Sc |
| Lu | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Qu | 2020 | L | Sc | L | Sc | Sc | Sc |
| Ren | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Shen | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Wu | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Yang | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Zheng | 2020 | Sc | Sc | L | Sc | Sc | Sc |
| Jin | 2021 | Sc | Sc | L | Sc | Sc | Sc |
| Lan | 2021 | Sc | Sc | L | Sc | Sc | Sc |
| Le | 2021 | Sc | Sc | L | Sc | Sc | Sc |
| Tang | 2021 | L | Sc | L | Sc | Sc | Sc |
| Wang | 2021 | Sc | Sc | L | Sc | Sc | Sc |
| k | MD | 95% CI | Heterogeneity (I2) | Psubgroup | |
|---|---|---|---|---|---|
| Source of investigational medicine | 0.0240 | ||||
| Research institute prescription | 39 | −3.7420 | −4.2580 to −3.2261 | 98.4% | |
| Other conventional medicine | 30 | −2.8494 | −3.4276 to −2.2712 | 95.9% | |
| Formulation type | 0.0116 | ||||
| Decoction | 32 | −2.8951 | −3.3769 to −2.4132 | 95.2% | |
| Other types | 37 | −3.8594 | −4.4331 to −2.2856 | 98.6% |
| Intervention and Comparator Intervention | Outcomes | Number of Participants (Studies) | Anticipated Absolute Effects (95% CI) | Quality of the Evidence (GRADE) |
|---|---|---|---|---|
| IM compared to CM for inflammatory pain of rheumatoid arthritis | PASI60 | 8367 (96) | 224 more per 1000 (from 139 more to 318 more) | ⨁⨁⨁◯ MODERATE a |
| PASI score | 5801 (69) | MD 3.3544 PASI score lower (3.7608 lower to 2.9481 lower) | ⨁⨁⨁◯ MODERATE b | |
| PASI70 | 1276 (13) | 239 more per 1000 (From 154 more to 337 more) | ⨁⨁⨁◯ MODERATE a | |
| Recurrence rate | 692 (12) | 252 fewer per 1000 (From 282 fewer to 210 fewer) | ⨁⨁⨁◯ MODERATE a | |
| DLQI | 811 (10) | MD 2.6072 DLQI lower (3.71 lower to 1.5043 lower) | ⨁⨁⨁◯ MODERATE a | |
| VAS | 273 (3) | MD 0.89 VAS lower (1.4769 lower to 0.3008 lower) | ⨁⨁◯◯ LOW a,b | |
| TNF-α | 997 (12) | SMD 1.9948 SD lower (2.5964 lower to 1.3932 lower) | ⨁⨁◯◯ LOW a,b | |
| IL-8 | 657 (7) | SMD 1.0752 SD lower (1.9647 lower to 0.1856 lower) | ⨁⨁◯◯ LOW a,b | |
| IL-17 | 842 (10) | SMD 2.0009 SD lower (3.2927 lower to 0.7091 lower) | ⨁⨁◯◯ LOW a,b | |
| IL-22 | 348 (4) | SMD 3.1874 SD lower (6.0441 lower to 0.3307 lower) | ⨁⨁◯◯ LOW a,b | |
| IL-23 | 428 (5) | SMD 1.7398 SD lower (2.6139 lower to 0.8657 lower) | ⨁⨁◯◯ LOW a,b | |
| INF-γ | 524 (6) | SMD 2.4211 SD lower (3.2187 lower to 1.6235 lower) | ⨁⨁◯◯ LOW a,b |
| No | Herbal Material (Latin Name) | Frequency of Prescription | Relative Frequency (%) | PageRank Centrality | Eigenvector Centrality |
|---|---|---|---|---|---|
| 1 | Rehmannia glutinosa (Gaertn.) DC. | 80 | 62.5% | 0.0284 | 1 |
| 2 | Glycyrrhiza uralensis Fisch. | 57 | 44.53% | 0.0278 | 0.982 |
| 3 | Dictamnus dasycarpus Turcz. | 54 | 42.19% | 0.0272 | 0.968 |
| 4 | Paeonia × suffruticosa Andrews | 53 | 41.41% | 0.0271 | 0.974 |
| 5 | Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan | 51 | 39.84% | 0.0278 | 0.982 |
| 6 | Smilax glabra Roxb. | 47 | 8.59% | 0.0277 | 0.988 |
| 7 | Angelica sinensis (Oliv.) Diels | 44 | 34.38% | 0.0271 | 0.974 |
| 8 | Lonicera japonica Thunb. | 39 | 30.47% | 0.0266 | 0.953 |
| 9 | Saposhnikovia divaricata (Turcz.) Schischk. | 39 | 30.47% | 0.0266 | 0.945 |
| 10 | Arnebia euchroma (Royle) I.M.Johnst. | 36 | 28.13% | 0.0265 | 0.959 |
| 11 | Sophora flavescens Aiton | 36 | 28.13% | 0.0254 | 0.914 |
| 12 | Isatis tinctoria subsp. athoa (Boiss.) Papan. | 35 | 27.34% | 0.0253 | 0.92 |
| 13 | Scrophularia ningpoensis Hemsl. | 33 | 25.78% | 0.0247 | 0.907 |
| 14 | Salvia miltiorrhiza Bunge | 30 | 23.44% | 0.0277 | 0.988 |
| 15 | Cryptotympana dubia (Haupt) | 28 | 21.88% | 0.0253 | 0.923 |
| 16 | Carthamus tinctorius L. | 27 | 21.09% | 0.0224 | 0.823 |
| 17 | Styphnolobium japonicum (L.) Schott | 21 | 16.41% | 0.0224 | 0.824 |
| 18 | Spatholobus suberectus Dunn | 20 | 15.63% | 0.0253 | 0.92 |
| 19 | Arctium lappa L. | 18 | 14.06% | 0.0128 | 0.442 |
| 20 | Scleromitrion diffusum (Willd.) R.J.Wang | 17 | 13.28% | 0.022 | 0.787 |
| 21 | Paeonia lactiflora Pall. | 17 | 13.28% | 0.023 | 0.843 |
| 22 | Ligusticum striatum DC. | 14 | 10.94% | 0.0219 | 0.793 |
| 23 | Ophiopogon japonicus (Thunb.) Ker Gawl. | 14 | 10.94% | 0.0235 | 0.871 |
| 24 | Scutellaria baicalensis Georgi | 14 | 10.94% | 0.0248 | 0.895 |
| 25 | Scutellaria Nepeta tenuifolia Benth. | 13 | 10.16% | 0.0242 | 0.868 |
| 26 | Taraxacum mongolicum Hand.-Mazz. | 13 | 10.16% | 0.0218 | 0.804 |
| No | Associations Rules | Support | Confidence | Lift |
|---|---|---|---|---|
| 1 | {Scrophularia ningpoensis Hemsl.} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.2500000 | 0.9696970 | 1.551515 |
| 2 | {Isatis tinctoria subsp. athoa (Boiss.) Papan.} => {Paeonia × suffruticosa Andrews} | 0.2578125 | 0.9428571 | 2.277089 |
| 3 | {Isatis tinctoria subsp. athoa (Boiss.) Papan.} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.2578125 | 0.9428571 | 1.508571 |
| 4 | {Saposhnikovia divaricata (Turcz.) Schischk.} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.2812500 | 0.9230769 | 1.476923 |
| 5 | Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.3671875 | 0.9215686 | 1.474510 |
| 6 | {Paeonia × suffruticosa Andrews} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.3828125 | 0.9245283 | 1.479245 |
| 7 | {Isatis tinctoria subsp. athoa (Boiss.) Papan., Paeonia × suffruticosa Andrews} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.2500000 | 0.9696970 | 1.551515 |
| 8 | {Isatis tinctoria subsp. athoa (Boiss.) Papan., Rehmannia glutinosa (Gaertn.) DC.} => {Paeonia × suffruticosa Andrews} | 0.2500000 | 0.9696970 | 2.341910 |
| 9 | {Paeonia × suffruticosa Andrews, Paeonia anomala subsp. veitchii (Lynch) D.Y.Hong and K.Y.Pan} => {Rehmannia glutinosa (Gaertn.) DC.} | 0.2734375 | 0.9210526 | 1.473684 |
| Four Core Herbs | Interquartile Range of Dosage (g/Day) | Interquartile Range of Treatment Duration (Week) |
|---|---|---|
| Rehmannia glutinosa (Gaertn.) DC. | 15–20 g | 4–12 w |
| Isatis tinctoria subsp. athoa (Boiss.) Papan. | 10–30 g | 8–12 w |
| Paeonia × suffruticosa Andrews | 10–15 g | 8–12 w |
| Scrophularia ningpoensis Hemsl. | 10–15 g | 7–12 w |
| Molecule Number | Molecule Name | Chemical Structure | ADME Evaluation | ||||
|---|---|---|---|---|---|---|---|
| Lipsinski | Ghose | Veber | Egan | Muegge | |||
| 1 | Dihydrochelerythrine |  | Yes | Yes | Yes | Yes | Yes |
| 2 | Sitogluside |  | Yes | No | Yes | Yes | No |
| 3 | Glycyrol |  | Yes | Yes | Yes | Yes | Yes |
| 4 | Hesperetin |  | Yes | Yes | Yes | Yes | Yes |
| 5 | Salutaridine |  | Yes | Yes | Yes | Yes | Yes |
| 6 | indirubin |  | Yes | Yes | Yes | Yes | Yes |
| 7 | qingdainone |  | Yes | Yes | Yes | Yes | Yes |
| 8 | indigo |  | Yes | Yes | Yes | Yes | Yes |
| 9 | Quindoline |  | Yes | Yes | Yes | Yes | Yes |
| 10 | Indicaxanthin |  | Yes | No | Yes | Yes | Yes |
| 11 | Paeoniflorgenin |  | Yes | Yes | Yes | Yes | Yes |
| 12 | 6-Deglucosyl-3-O-methylpaeoniflorin |  | Yes | Yes | Yes | Yes | Yes |
| 13 | (2R,3R)-2-(3,5-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one |  | Yes | Yes | Yes | Yes | Yes |
| 14 | Columbianetin acetate |  | Yes | Yes | Yes | Yes | Yes |
| 15 | Cianidanol |  | Yes | Yes | Yes | Yes | Yes |
| 16 | Triptolide |  | Yes | Yes | Yes | Yes | Yes |
| 17 | quercetin |  | Yes | Yes | Yes | Yes | Yes |
| 18 | 5-[5-(4-Methoxy-phenyl)-furan-2-ylmethylene]-pyrimidine-2,4,6-trione |  | Yes | Yes | Yes | Yes | Yes |
| 19 | kaempferol |  | Yes | Yes | Yes | Yes | Yes |
| 20 | Sugiol |  | Yes | Yes | Yes | Yes | No |
| 21 | Imperatorin |  | Yes | Yes | Yes | Yes | Yes |
| 22 | Palmitoleic acid |  | Yes | Yes | No | Yes | No |
| Node Rank | Gene Target | MCC Centrality | Degree Centrality | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|---|
| 1 | STAT3 | 1.01 × 109 | 24 | 75.10296 | 31.58333 |
| 2 | CASP3 | 1.01 × 109 | 19 | 12.27081 | 29.08333 |
| 3 | PTGS2 | 1.01 × 109 | 27 | 284.44838 | 33.08333 |
| 4 | BCL2 | 1.01 × 109 | 24 | 102.82056 | 31.58333 |
| 5 | MMP9 | 1.01 × 109 | 24 | 144.3609 | 31.58333 |
| 6 | EGFR | 1.01 × 109 | 22 | 69.20533 | 30.58333 |
| 7 | ESR1 | 1.01 × 109 | 21 | 305.04296 | 30.33333 |
| 8 | CCND1 | 1.01 × 109 | 18 | 12.97538 | 28.58333 |
| 9 | STAT1 | 1.01 × 109 | 21 | 42.05261 | 30.08333 |
| 10 | NFKB1 | 1.01 × 109 | 22 | 39.82745 | 30.58333 |
| 11 | MAPK8 | 9.59 × 108 | 16 | 8.18678 | 27.58333 |
| 12 | CASP9 | 9.58 × 108 | 15 | 5.20558 | 27.08333 |
| 13 | JAK2 | 5.23 × 108 | 19 | 123.91169 | 29.08333 |
| 14 | MAPK14 | 4.90 × 108 | 16 | 5.94696 | 27.25 |
| 15 | KIT | 4.04 × 107 | 14 | 8.86641 | 26.58333 |
| 16 | PDGFRB | 1.09 × 107 | 17 | 30.99454 | 28.08333 |
| 17 | SERPINE1 | 7,297,946 | 14 | 34.3894 | 26.25 |
| 18 | CCR2 | 453,610 | 15 | 58.75556 | 26.61667 |
| 19 | NOS2 | 413,280 | 12 | 3.37798 | 24.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-G.; Kim, H.; Baek, E.; Lee, D.; Hwang, J.H. Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology. Pharmaceuticals 2023, 16, 1160. https://doi.org/10.3390/ph16081160
Jo H-G, Kim H, Baek E, Lee D, Hwang JH. Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology. Pharmaceuticals. 2023; 16(8):1160. https://doi.org/10.3390/ph16081160
Chicago/Turabian StyleJo, Hee-Geun, Hyehwa Kim, Eunhye Baek, Donghun Lee, and Ji Hye Hwang. 2023. "Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology" Pharmaceuticals 16, no. 8: 1160. https://doi.org/10.3390/ph16081160
APA StyleJo, H.-G., Kim, H., Baek, E., Lee, D., & Hwang, J. H. (2023). Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology. Pharmaceuticals, 16(8), 1160. https://doi.org/10.3390/ph16081160








