Honey as a Natural Nutraceutical: Its Combinational Therapeutic Strategies Applicable to Blood Infections—Septicemia, HIV, SARS-CoV-2, Malaria
Abstract
1. Introduction
2. Bioactivities of Honey
3. Blood Infection
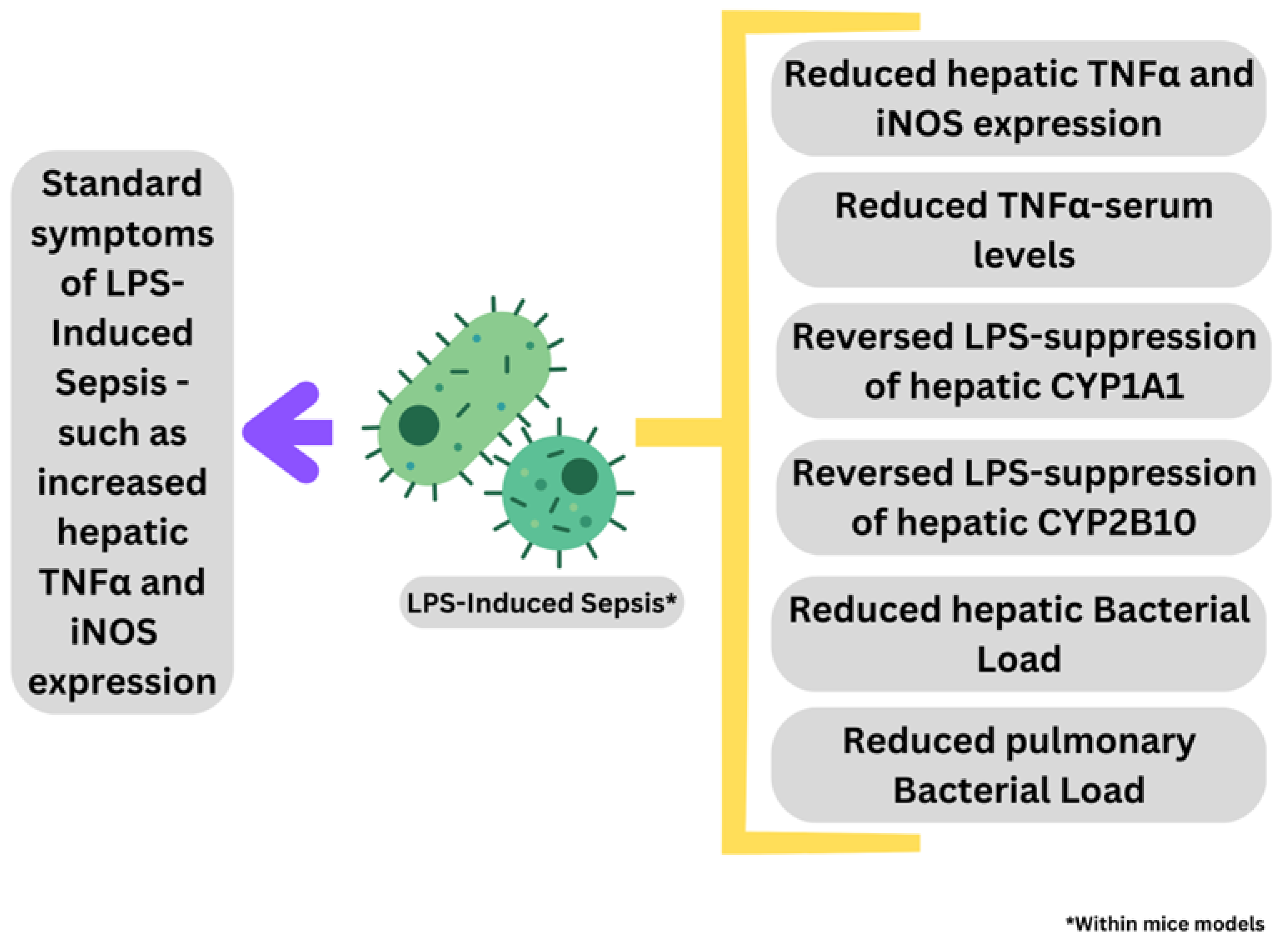
3.1. Septicemia/Sepsis
3.1.1. Combinational Therapy for Sepsis Using Nutraceutical Honey
4. Viral Blood Infections
4.1. Human Immunodeficiency Virus
4.1.1. Combinational Therapy for HIV by Nutraceutical Honey
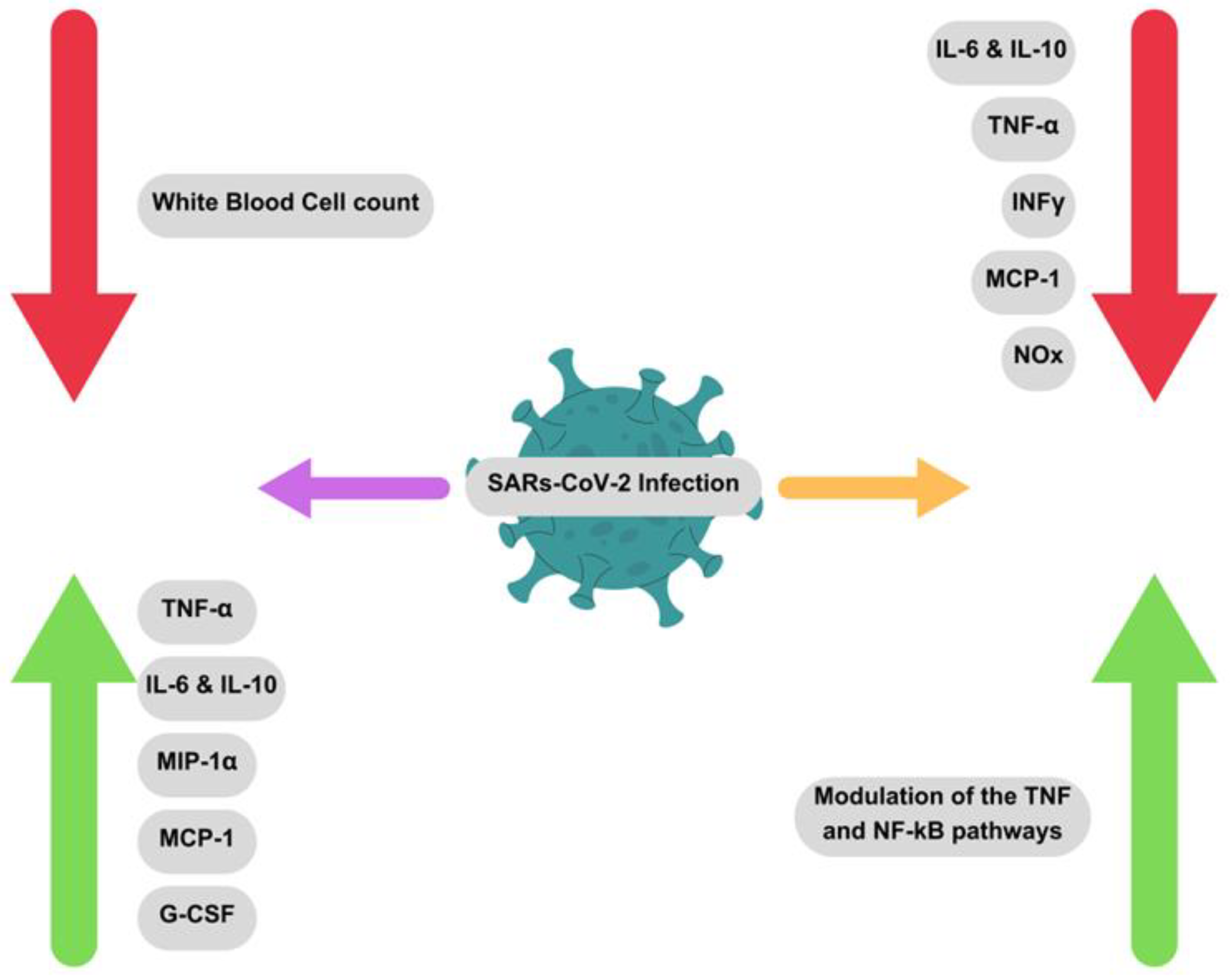
4.2. SARS-CoV-2 (COVID-19 Virus)
4.2.1. Combinational Therapy for SARS-CoV-2 by Nutraceutical Honey
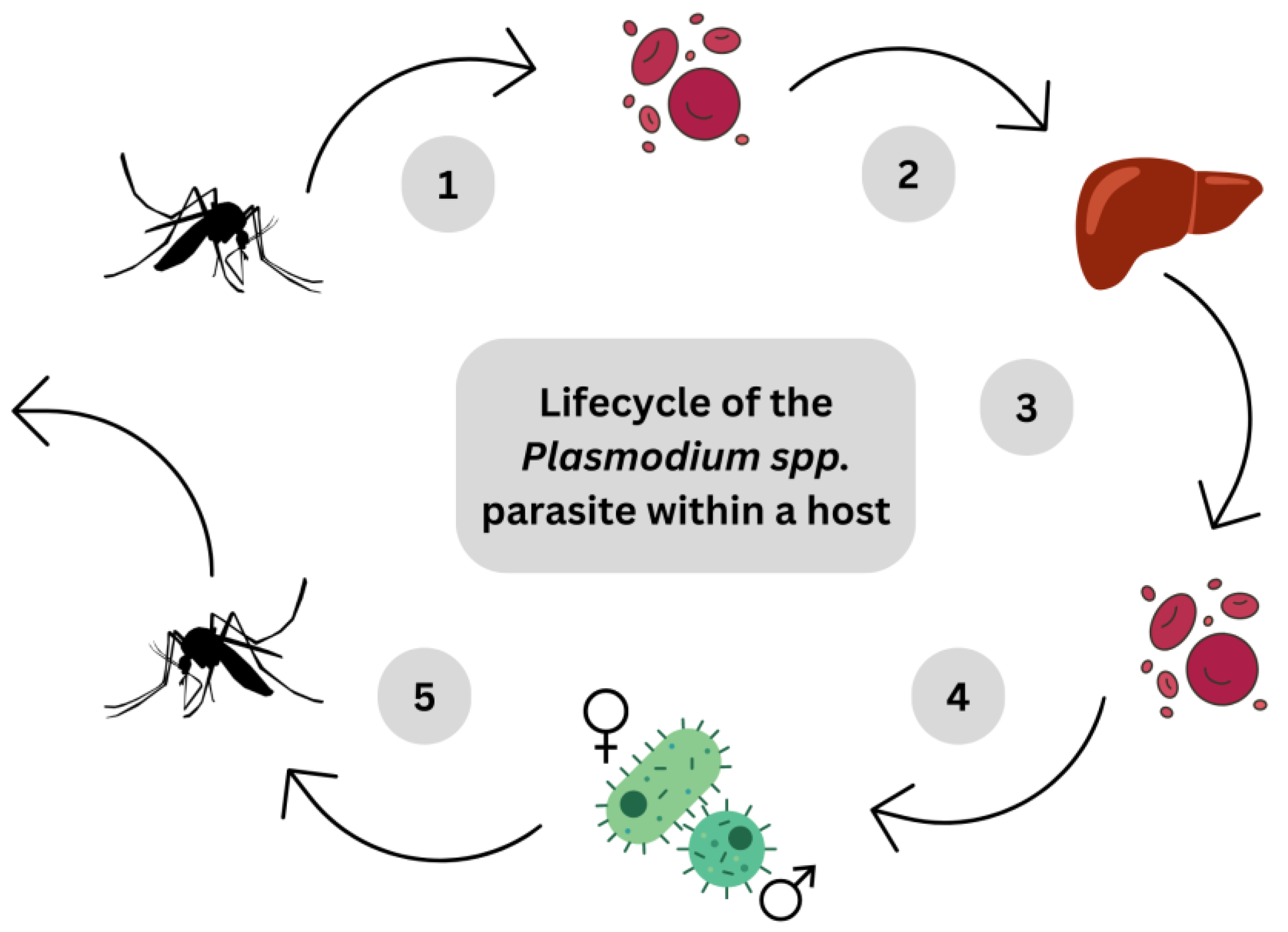
5. Parasitic Infections
5.1. Combinational Therapy for Malaria by Nutraceutical Honey
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murry, C.; Ikuta, K.; Sharara, F.; Swetschiniski, L.; Aguilar, G.; Gray, A. Global burden of bacterial antimicrobial resistance in 2019: A systemic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar] [CrossRef]
- 148 Severe Antibiotic-Resistant Infections a Day in 2021. Available online: https://www.gov.uk/government/news/new-data-shows-148-severe-antibiotic-resistant-infections-a-day-in-2021#full-publication-update-history (accessed on 20 March 2022).
- Antimicrobial Resistance. Available online: https://www.theguardian.com/society/2022/jan/20/antimicrobial-resistance-antibiotic-resistant-bacterial-infections-deaths-lancet-study (accessed on 20 March 2023).
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731. [Google Scholar] [PubMed]
- Mieles, J.Y.; Vyas, C.; Aslan, E.; Humpheries, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Kapoor, N.; Yadav, R. Manuka honey: A promising wound dressing material for the chronic nonhealing discharging wounds: A retrospective study. J. Maxillofac. Surg. 2021, 12, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Aquil, Z.; Alam, S.S. Historical background of wound care. Hamdan Med. J. 2020, 13, 89–195. [Google Scholar] [CrossRef]
- Hegazi, N.; Elghani, G.; Farag, M. The super-food Manuka honey, a comprehensive review of its analysis and authenticity approaches. J. Food Sci. Technol. 2022, 59, 2527–2534. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Islam, K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant Activity and Phenolic Compound Identification and Quantification in Western Australian Honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef]
- Farkas, Á.; Horváth, G.; Kuzma, M.; Mayer, M.; Kocsis, M. Phenolic compounds in Hungarian acacia, linden, milkweed and goldenrod honeys. Curr. Res. Food Sci. 2023, 6, 100526. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Significance of Nonaromatic Organic Acids in Honey. J. Food Prot. 2003, 66, 2371–2376. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of Organic Acids in Honey by Liquid Chromatography with Tandem Mass Spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Vega, A.; Delgado, N.; Handford, M. Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. Int. J. Mol. Sci. 2022, 23, 5438. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Bifulco, E.; Jerkovic, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl Syringate: A Chemical Marker of Asphodel (Asphodelus microcarpus Salzm. et Viv.) Monofloral Honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Jubri, Z.; Rahim, N.B.A.; Aan, G.J. Manuka honey protects middle-aged rats from oxidative damage. Clinics 2013, 68, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Alamri, A.; Almashjary, M.N.; Qanash, H.; Almishaal, A.A.; Amin, J.; Binsaleh, N.K.; Kraiem, J.; Aldarhami, A.; Alafnan, A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Appl. Sci. 2022, 12, 9928. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef]
- Atrott, J.; Helene, T. Methylglyoxal in manuka honey—Correclation with antibacterial properties. Czech J. Food Sci. 2009, 27, 163–165. [Google Scholar] [CrossRef]
- Hayashi, K.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Effect of methylglyoxal on multidrug-resistant Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Green, K.; Lawag, I.; Locher, C.; Hammer, K. Correlation of the antibacterial activity of commercial manuka and Leptospermum honeys from Australia and New Zealand with methylglyoxal content and other physicochemical characteristics. PLoS ONE 2022, 17, e0272376. [Google Scholar] [CrossRef]
- Yusof, H.I.; Owusu-Apenten, R.; Nigam, P.S. Determination of Iron (III) Reducing Antioxidant Capacity for Manuka Honey and Comparison with ABTS and Other Methods. J. Adv. Biol. Biotechnol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Chau, T.; Owusu-Apenten, R.; Nigam, P. Total Phenols, Antioxidant Capacity and Antibacterial Activity of Manuka Honey Extract. J. Adv. Biol. Biotechnol. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Kirkpatric, G.; Singh, P.; Owusu-Apenten, R.K. Total Phenols, Antioxidant Capacity and Antibacterial Activity of Manuka Honey Chemical Constituents. J. Adv. Biol. Biotechnol. 2017, 15, 1–7. [Google Scholar] [CrossRef][Green Version]
- Henderson, K.; Aldhirgham, T.; Nigam, P.S.; Owusu-Apenten, R.K. Evaluation of Manuka Honey Estrogen Activity Using the MCF-7 Cell Proliferation Assay. J. Adv. Biol. Biotechnol. 2016, 10, 1–11. [Google Scholar] [CrossRef][Green Version]
- Portokalakis, I.; Yusof, H.; Ghanotakis, D.; Nigam, P.; Qwusu-Apenten, R. Manuka Honey-induced Cytotoxicity against MCF7 Breast Cancer Cells is Correlated to Total Phenol Content and Antioxidant Power. J. Adv. Biol. Biotechnol. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. Peer J. 2014, 2, e326. [Google Scholar] [CrossRef]
- Tonks, A.J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leucoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Ciszkowicz, E.; Sidor, E.; Hęclik, J.; Lecka-Szlachta, K.; Dżugan, M. The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics 2023, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Hikaambo, C.N.A.; Chabalenge, B.; Mfune, R.L.; Mufwambi, W.; Ngazimbi, M.; Matafwali, S.; Daka, V. Antibacterial activities of honey against Escherichia coli and Staphylococcus aureus: A potential treatment for bacterial infections and alternative to antibiotics. J. Pharmacogn. Phytochem. 2023, 12, 6–13. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Kocsis, M.; Farkas, Á. Antibacterial and Antibiofilm Effect of Unifloral Honeys against Bacteria Isolated from Chronic Wound Infections. Microorganisms 2023, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Akaba, D.; Atemkeng, T.F.; Malep-Mayama, B.B.; Moutila, I.L.; Achu, E.; Fokou, E.; Pieme, C.A.; Djam, C.A. Evaluation of Burns Wound Healing Properties of Different Varieties of Honey from Cameroun. J. Appl. Life Sci. Int. 2023, 26, 1–17. [Google Scholar] [CrossRef]
- Onuoha, E.O.; Adekunle, A.A.; Ajike, S.O.; Gbotolorun, O.M.; Adeyemo, W.L. Effect of manuka honey socket dressing on postoperative sequelae and complications following third molar extraction: A randomized controlled study. J. Craniomaxillofac. Surg. 2023, 51, 252–260. [Google Scholar] [CrossRef] [PubMed]
- What is Sepsis? Centres for Disease Control. Available online: https://www.cdc.gov/sepsis/what-is-sepsis (accessed on 24 May 2023).
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Disseminated Intravascular Coagulation (DIC). Available online: https://www.nhlbi.nih.gov/health/disseminated-intravascular-coagulation (accessed on 24 May 2023).
- Septicemia. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/septicemia (accessed on 24 May 2023).
- What’s the Difference between Sepsis and Septicaemia. Available online: https://www.meningitis.org/blogs/difference-sepsis-septicaemia (accessed on 24 May 2023).
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.; Owusu-Apenten, R. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2014, 174, 119–123. [Google Scholar] [CrossRef]
- Wong, L.Y.; Nigam, P.S. Owusu-Apenten RK Effect of Iron and Hydrogen Peroxide Supplementation on the Total Phenols Content and Cytoxicity of Honey for MCF-7 Breast Cancer Cells. J. Adv. Biol. Biotechnol. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Kwok, T.H.; Kirkpatrick, G.; Mohd, Y.H.I.; Portokalakis, I.; Nigam, P. Owusu-Apenten RK Rapid Colorimetric Determination of Methylglyoxal Equivalents for Manuka Honey. J. Adv. Biol. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Koc, F.; Tekeli, M.Y.; Kanbur, M.; Karayigit, M.Ö.; Liman, B.C. The effects of chrysin on lipopolysaccharide-induced sepsis in rats. J. Food Biochem. 2020, 44, e13359. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Ieronymaki, E.; Dimitroulia, E.; Constantinidis, T.C.; Vrioni, G.; Tsatsanis, C.; Tsakris, A. Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms 2022, 10, 2374. [Google Scholar] [CrossRef]
- Gurusamy, U.; Shewade, D. Chapter 46—Pharmacogenomics in India. In Handbook of Pharmacogenomics and Stratified Medicine; Padmanaban, S., Ed.; Academic Press: London, UK, 2014. [Google Scholar] [CrossRef]
- Kwiecińska-Piróg, J.; Przekwas, J.; Majkut, M.; Skowron, K.; Gospodarek-Komkowska, E. Biofilm Formation Reducing Properties of Manuka Honey and Propolis in Proteus mirabilis Rods Isolated from Chronic Wounds. Microorganisms 2020, 8, 1823. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Combarros-Fuertes, L.M.P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Evaluation of Physiological Effects Induced by Manuka Honey upon Staphylococcus aureus and Escherichia coli. Microorganisms 2019, 7, 258. [Google Scholar] [CrossRef]
- Truchado, P.; López-Gálvez, F.; Gil, M.I.; Tomás-Barberán, F.A.; Allende, A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009, 115, 1337–1344. [Google Scholar] [CrossRef]
- Akankwasa, B. Antibacterial Activity of Undiluted Natural Honey against Opportunistic Pathogens Causing Wound Sepsis in Humans. Bachelor’s Thesis, Makerere University, Kampala, Uganda, 2022. Available online: http://hdl.handle.net/20.500.12281/15761 (accessed on 27 May 2023).
- Hussain, M. Role of Honey in Topical and Systemic Bacterial Infections. J. Altern. Complement. Med. 2018, 24, 15–24. [Google Scholar] [CrossRef]
- HIV. Available online: https://www.who.int/health-topics/hiv-aids#tab=tab_1 (accessed on 27 May 2023).
- HIV and Opportunistic Infections, Coinfections, and Conditions. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/what-opportunistic-infection (accessed on 27 May 2023).
- HIV/AIDS. Available online: https://www.mayoclinic.org/diseases-conditions/hiv-aids/symptoms-causes/syc-20373524 (accessed on 27 May 2023).
- Bailey, H.; Zash, R.; Rasi, V.; Thorne, C. HIV treatment in pregnancy. Lancet HIV 2018, 5, e457–e467. [Google Scholar] [CrossRef]
- Moranguinho, I.; Taveira, N.; Bártolo, I. Antiretroviral Treatment of HIV-2 Infection: Available Drugs, Resistance Pathways, and Promising New Compounds. Int. J. Mol. Sci. 2023, 24, 5905. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Bedimo, R.; Hoy-Landovitz, J.F.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; Benson, C.A. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 Recommendations of the International Antiviral Society–USA Panel. JAMA 2023, 329, 63–84. [Google Scholar] [CrossRef]
- Ayoub, W.S.; Zahoor, I.; Dar, A.H.; Farooq, S.; Mir, T.A.; Ganaie, T.A.; Srivastava, S.; Pandey, V.K.; Altaf, A. Exploiting the polyphenolic potential of honey in the prevention of chronic diseases. Food Chem. 2023, 3, 100373. [Google Scholar] [CrossRef]
- Yusuf, W.N.W.; Mohammad, W.M.Z.W.; Gan, S.H.; Mustafa, M.; Aziz, C.B.A.; Sulaiman, S.A. Tualang honey ameliorates viral load, CD4 counts and improves quality of life in asymptomatic human immunodeficiency virus infected patients. J. Altern. Complement. Med. 2019, 9, 249–256. [Google Scholar] [CrossRef]
- Chessum, K.; Chen, T.; Hamid, N.; Kam, R. A comprehensive chemical analysis of New Zealand honeydew honey. Food Res. Int. 2022, 157, 111436. [Google Scholar] [CrossRef]
- Borges, C.V.; Nunes, A.; Costa, V.E.; Orsi, R.O.D.; Basilio, L.S.P.; Monteiro, G.C.; Maraschin, M.; Lima, G.P.P. Tryptophan and biogenic amines in the differentiation and quality of honey. Int. J. Tryptophan Res. 2022, 15, 11786469221102098. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 27 May 2023).
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Ghosh, S.; Huang, J.; Inkman, M.; Zhang, J.; Thotala, S.; Tikhonova, E.; Miheecheva, N.; Frenkel, F.; Ataullakhanov, R.; Wang, X.; et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci. Transl. Med. 2023, 15, eabn6758. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.T.K. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009, 83, 3039–3048. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Shamsuddin, S.H.; Sulaiman, S.A.; Abdullah, J.M. Anti-inflammatory Properties of Stingless Bee Honey May Reduce the Severity of Pulmonary Manifestations in COVID-19 Infections. Malays. J. Med. Sci. MJMS 2020, 27, 165–169. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant, and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Abedi, F.; Ghasemi, S.; Farkhondeh, T.; Azimi-Nezhad, M.; Shakibaei, M.; Samarghandian, S. Possible Potential Effects of Honey and Its Main Components against COVID-19 Infection. Dose-Response 2021, 19, 1559325820982423. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mansora, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef]
- Malaria. Available online: https://www.cdc.gov/malaria/about/faqs (accessed on 13 June 2023).
- Malaria. Available online: https://www.who.int/health-topics/malaria#tab=tab_1 (accessed on 13 June 2023).
- Mawson, A. The pathogenesis of malaria: A new perspective. Pathog. Glob. Health 2013, 107, 122–129. [Google Scholar] [CrossRef]
- Frischknecht, F.; Matuschewski, K. Plasmodium Sporozoite Biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025478. [Google Scholar] [CrossRef]
- Maguire, J.D.; Baird, J.K. Malaria. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: London, UK, 2014; Volume 1, pp. 989–991. [Google Scholar]
- Crutcher, J.M.; Hoffman, S.L. Chapter 83—Malaria. In Medical Microbiology, 4th ed.; Baron, S., Castro, G., Eds.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Laksemi, D.A.A.S.; Tunas, K.; Damayanti, P.A.A.; Sudarmaja, M.; Widyadharma, P.E.; Wiryanthini, I.A.D.; Linawat, N.M. Evaluation of Antimalarial Activity of Combination Extract of Citrus aurantifolia and Honey against Plasmodium berghei–İnfected Mice. Trop. J. Nat. Prod. Res. 2023, 7, 2168–2171. [Google Scholar] [CrossRef]
- Adokoh, C.K.; Asante, D.B.; Acheampong, D.O.; Kotsuchibashi, Y.; Armah, F.A.; Sirikyi, I.H.; Kimura, K.; Gmakame, E.; Abdul-Rauf, S. Chemical profile and in vivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicol. Rep. 2019, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Rinaldi, M.A.; Famodimu, M.T.; Van Veelen, M.; Larson, T.R.; Winzer, T.; Rathbone, D.A.; Harvey, D.; Horrocks, P.; Graham, I.A. Flavonoid versus artemisinin anti-malarial activity in Artemisia annua whole-leaf extracts. Front. Plant Sci. 2019, 10, 984. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. R. Soc. Chem. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients 2023, 15, 2979. [Google Scholar] [CrossRef]

| Activities and Applications of Honey | References |
|---|---|
| Antiproliferative capacity | [19] |
| Capture and reduction of free radicals | [17,18] |
| Ability to invade and evade detection and removal by bacterial efflux mechanisms | [22] |
| Synergistic capacity of MGO with other known antibacterial materials in destruction of Gram+ and Gram− bacteria | [23] |
| Estrogenic activity involving MCF-7 pro- and antiproliferation capacity, and cytotoxicity | [19,27,28] |
| Topical antibiofilm agent relevant to wound healing | [29] |
| Macrophagic stimulation in aid of tissue healing due to bacterial damage and infection | [30] |
| Pronounced antibacterial capacity | [31,32,33] |
| Topical treatment for burn wound healing | [34] |
| Promotion of healing post-craniomaxillofacial surgery, and reduction of complications associated with healing | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackin, C.; Dahiya, D.; Nigam, P.S. Honey as a Natural Nutraceutical: Its Combinational Therapeutic Strategies Applicable to Blood Infections—Septicemia, HIV, SARS-CoV-2, Malaria. Pharmaceuticals 2023, 16, 1154. https://doi.org/10.3390/ph16081154
Mackin C, Dahiya D, Nigam PS. Honey as a Natural Nutraceutical: Its Combinational Therapeutic Strategies Applicable to Blood Infections—Septicemia, HIV, SARS-CoV-2, Malaria. Pharmaceuticals. 2023; 16(8):1154. https://doi.org/10.3390/ph16081154
Chicago/Turabian StyleMackin, Caoimhin, Divakar Dahiya, and Poonam Singh Nigam. 2023. "Honey as a Natural Nutraceutical: Its Combinational Therapeutic Strategies Applicable to Blood Infections—Septicemia, HIV, SARS-CoV-2, Malaria" Pharmaceuticals 16, no. 8: 1154. https://doi.org/10.3390/ph16081154
APA StyleMackin, C., Dahiya, D., & Nigam, P. S. (2023). Honey as a Natural Nutraceutical: Its Combinational Therapeutic Strategies Applicable to Blood Infections—Septicemia, HIV, SARS-CoV-2, Malaria. Pharmaceuticals, 16(8), 1154. https://doi.org/10.3390/ph16081154







