Natural Products for the Prevention and Treatment of Common Cold and Viral Respiratory Infections
Abstract
1. Introduction
| Plant a | Plant Part, Herbal Drug a | Main Constituents b |
|---|---|---|
| Allium sativum L. | bulb, Allii sativi bulbus | sulfoxide (alliin), steroidal saponins, flavonoids, N-feruloyltyramine, amino acids, adenosine |
| Echinacea angustifolia DC. | root, Echinaceae angustifoliae radix | phenylethanoids, chlorogenic acid, alkamides, polysaccharides, essential oil, glycoproteins, tussilagine, isotussilagine (traces) |
| Echinacea pallida (Nutt.) Nutt. | root, Echinaceae pallidae radix | phenylethanoids, chlorogenic acid, alkamides, polysaccharides, essential oil, glycoproteins |
| Echinacea purpurea (L.) Moench | root, Echinaceae purpureae radix; fresh aerial part, Echinaceae purpureae herba recens | phenylethanoids, chlorogenic acid, alkamides, polysaccharides, essential oil, glycoproteins, tussilagine, isotussilagine |
| Eucalyptus globulus Labill. | essential oil, Eucalypti aetheroleum; leaf, Eucalypti folium | main components: 1,8-cineole, limonene + hydroxycinnamic derivates, flavonoids, triterpenes, euglobals, macrocarpals, essential oil |
| Filipendula ulmaria (L.) Maxim. | flower, Filipendulae ulmariae flos; aerial parts, Filipendulae ulmariae herba | essential oil (main component: salicylaldehyde) flavonoids, tannins, phenolic glycosides, essential oil |
| Foeniculum vulgare Miller subsp. vulgare var. vulgare | essential oil, Foeniculi amari fructus aetheroleum; fruits, Foeniculi amari fructus | main components: trans-anethole, fenchone, limonene, cis-anethole + essential oil, glucosides of hemiterpenoids, monoterpenoids, fatty oil |
| Grindelia robusta Nutt. Grindelia squarrosa (Pursh) Dunal Grindelia humilis Hook. et Arn. Grindelia camporum Greene | aerial part, Grindeliae herba | resin, tannins, triterpenic saponins, flavonoids, phenolic acids, essential oil |
| Glycyrrhiza glabra L. Glycyrrhiza inflata Bat. Glycyrrhiza uralensis Fisch. | root, Liquitiae radix | triterpenic saponins, flavonoids, chalcones, coumarins, polysaccharides, sterols |
| Marrubium vulgare L. | aerial part, Marrubii herba | diterpenes, flavonoids, essential oil, betonicine, choline |
| Matricaria recutita L. | flower, Matricariae flos | flavonoids, essential oil, coumarins, N1,N5,N10,N14-tetra-p-coumaroylspermine, polysaccharides |
| Mentha × piperita L. | essential oil, Menthae piperitae aetheroleum | main components: menthol, menthone, menthylacetate, isomenthone, 1,8-cineole, menthofurane, limonene, pulegone, carvone, isopulegol |
| Origanum dictamnus L. | aerial part, Origani dictamni herba | essential oil, flavonoids, triterpenoids |
| Pelargonium sidoides DC Pelargonium reniforme Curt. | root, Pelargonii radix | coumarins, polyphenols, gallic acid derivatives |
| Pimpinella anisum L. | essential oil, Anisi aetheroleum; fruit, Anisi fructus | main components: trans-anethol, cis-anethol, linalool, α-terpineol + essential oil, flavonoids, fatty oil, coumarins |
| Polygonium aviculare L. | aerial part, Polygoni avicularis herba | flavonoids, coumarins, naphtoquinones, polysaccharides |
| Polypodium vulgare L. | rhizome, Polypodii rhizoma | steroidal saponins, triterpenoids, fatty oil, essential oil |
| Primula veris L. Primula elatior (L.) Hill | flower, Primulae flos; root, Primulae radix | flavonoids, saponins, carotenoids, volemitol, primine |
| Salix purpurea L. Salix daphnoides Vill. Salix fragilis L. | bark, Salicis cortex | phenolic glycosides, flavonoids, tannins (proanthocyanidins) |
| Sambucus nigra L. | flower, Sambuci flos | flavonoids, essential oil, spermidine, tannins, triterpenic acids, mucilage |
| Sideritis scardica Griseb. Sideritis clandestina (Bory and Chaub.) Hayek Sideritis raeseri Boiss. and Heldr. Sideritis syriaca L. | aerial part, Sideritis herba | essential oil, flavonoids, phenylethanoids, diterpenes |
| Thymus vulgaris L. Thymus zygis L. | essential oil, Thymi typo thymolo aetheroleum aerial part, Thymi herba | main components: thymol, linalool, carvacrol, α-terpinene, 4-terpineol + essential oil, flavonoids, monoterpenoid glucosides, hydroxycinnamic derivatives, polysaccharides |
| Tilia cordata Miller Tilia platyphyllos Scop. Tilia × vulgaris Heyne | flower, Tiliae flos | essential oil, flavonoids, tannins, mucilage, alkaloids (traces) |
| Verbascum thapsum L. Verbascum densiflorum Bertol. Verbascum phlomoides L. | flower, Verbasci flos | mucilage, flavonoids, phenylethanoids, triterpenic saponins, iridoids |
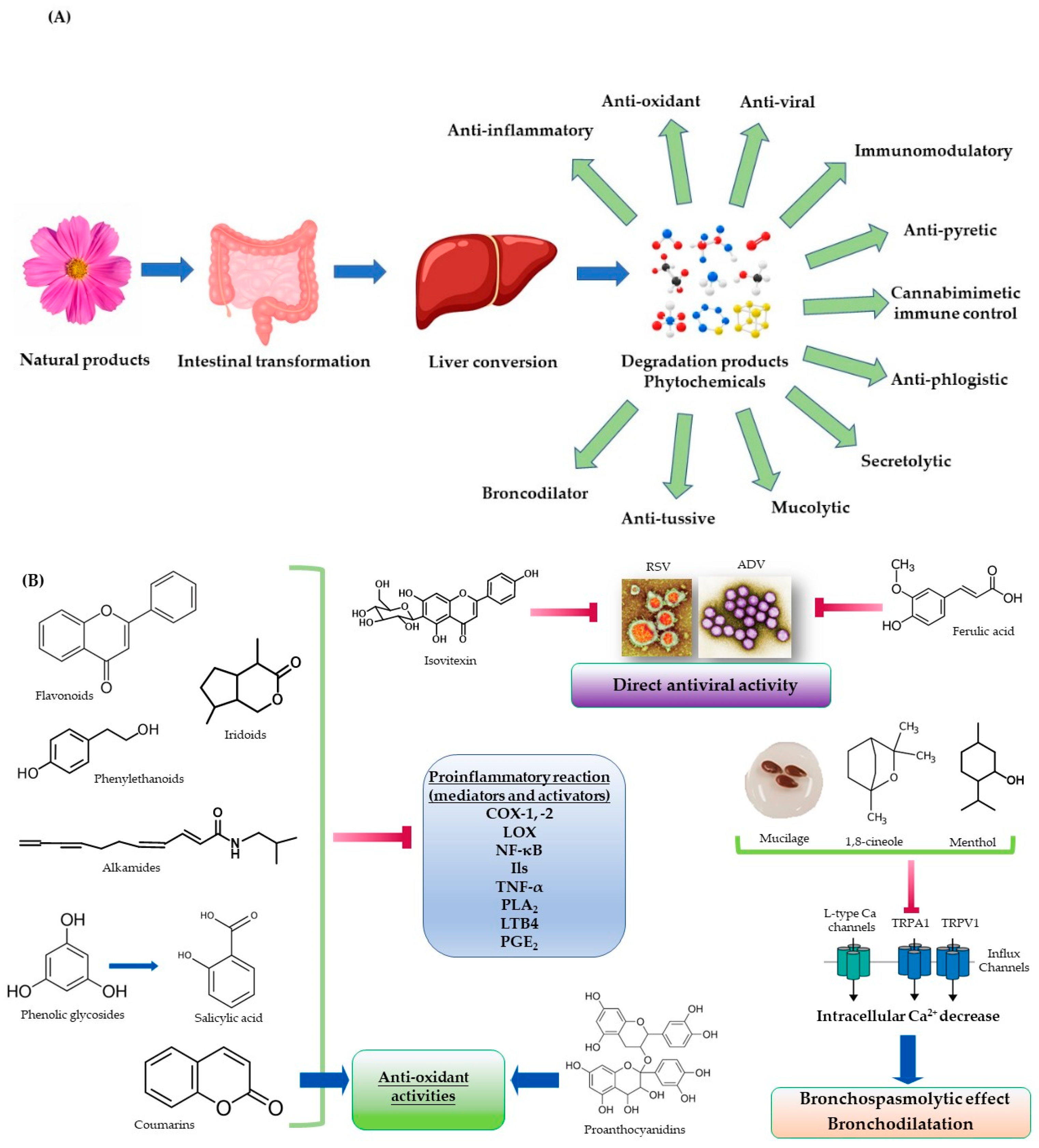
2. Possible Mechanism of Action of Phytochemicals for Colds
3. Most Commonly Used Medicinal Plants for the Treatment of the Common Colds
3.1. Allium sativum L.
3.2. Echinacea angustifolia DC., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench
3.3. Eucalyptus globulus Labill.
3.4. Grindelia robusta Nutt./Grindelia squarrosa (Pursh) Dunal, Grindelia humilis Hook. et Arn., Grindelia camporum Greene
3.5. Glycyrrhiza glabra L., Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch
3.6. Mentha × piperita L.
3.7. Origanum dictamnus L.
3.8. Pelargonium sidoides DC, Pelargonium reniforme Curt
3.9. Pimpinella anisum L.
3.10. Primula elatior (L.) Hill, Primula veris L.
3.11. Sambucus nigra L.
3.12. Sideritis scardica Griseb./Sideritis clandestina (Bory and Chaub.) Hayek./Sideritis raeseri Boiss./and Heldr., Sideritis syriaca L.
3.13. Thymus vulgaris L., Thymus zygis L.
3.14. Tilia cordata Miller, Tilia platyphyllos Scop., Tilia × vulgaris Heyne
3.15. Verbascum thapsum L., Verbascum densiflorum Bertol., Verbascum phlomoides L.
4. Medicinal Plants without EMA Monography with Potential Effects on Common Cold
4.1. Aloe qrborescens Mill.
4.2. Boehmeria jamaicensis Urb.
4.3. Camellia sinensis (L.) Kuntze, Camellia qssamica var. Kucha
4.4. Cistus × incanus. L.
4.5. Cinnamomum verum J. Presl (syn. Cinnamomum cassia J. Presl)
4.6. Larix decidua Mill.
4.7. Paeonia lactiflora Pall.
5. Herbal Combinations Useful for Treating the Common Cold
5.1. Hedera helix/Primula vulgaris/Thymus vulgaris
5.2. Tsumura Bakumondoto
5.3. GeloMyrtol® (Myrtol®)
5.4. Soshiho-Tang
5.5. Kang Jang®
5.6. So-Cheong-Ryong-Tang and Yeon-Gyo-Pae-Dok-San
5.7. Combination of Echinaceae radix, Baptisiae radix, Thujae herba
5.8. Ma-Xing-Shi-Gan-Tang
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worrall, G. Common Cold. Can. Fam. Physician 2011, 57, 1289–1290. [Google Scholar]
- Arroll, B.; Kenealy, T. Antibiotics for the Common Cold. Cochrane Database Syst. Rev. 2002, 3, CD000247. [Google Scholar] [CrossRef]
- Heikkinen, T.; Järvinen, A. The Common Cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Common Cold—Medical Dictionary Online-Medical-Dictionary.Org. Available online: https://www.online-medical-dictionary.org/definitions-c/common-cold.html (accessed on 21 September 2022).
- MedlinePlus. Available online: https://vsearch.nlm.nih.gov/vivisimo/cgi-bin/query-meta?v%3Aproject=medlineplus&v%3Asources=medlineplus-bundle&query=common+cold&_gl=1*125kku4*_ga*MjEyODAzMjEwNi4xNjYzNzYzNjk0*_ga_P1FPTH9PL4*MTY2Mzc2MzY5My4xLjAuMTY2Mzc2MzY5My4wLjAuMA..&_ga=2.234671518.16044576.1663763694-2128032106.1663763694 (accessed on 21 September 2022).
- Common Cold—Symptoms, Diagnosis and Treatment|BMJ Best Practice. Available online: https://bestpractice.bmj.com/topics/en-gb/252 (accessed on 21 September 2022).
- Coerdt, K.M.; Khachemoune, A. Corona Viruses: Reaching Far beyond the Common Cold. Afr. Health Sci. 2021, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Passioti, M.; Maggina, P.; Megremis, S.; Papadopoulos, N.G. The Common Cold: Potential for Future Prevention or Cure. Curr. Allergy Asthma Rep. 2014, 14, 413. [Google Scholar] [CrossRef]
- Common Colds: Overview; Institute for Quality and Efficiency in Health Care (IQWiG): Köln, Germany, 2020.
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Jaume, F.; Valls-Mateus, M.; Mullol, J. Common Cold and Acute Rhinosinusitis: Up-to-Date Management in 2020. Curr. Allergy Asthma Rep. 2020, 20, 28. [Google Scholar] [CrossRef]
- Nakano, Y.; Watari, T.; Adachi, K.; Watanabe, K.; Otsuki, K.; Amano, Y.; Takaki, Y.; Onigata, K. Survey of Potentially Inappropriate Prescriptions for Common Cold Symptoms in Japan: A Cross-Sectional Study. PLoS ONE 2022, 17, e0265874. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Bussey, L.; Eagling-Vose, E.; Rutkowski, K.; Ellis, C.; Argent, C.; Griffin, P.; Kim, J.; Thackwray, S.; Shakib, S.; et al. Efficacy and Safety of a Universal Influenza A Vaccine (MVA-NP+M1) in Adults When given after Seasonal Quadrivalent Influenza Vaccine Immunisation (FLU009): A Phase 2b, Randomised, Double-Blind Trial. Lancet Infect. Dis. 2022, 22, 857–866. [Google Scholar] [CrossRef]
- Mousa, H.A.-L. Prevention and Treatment of Influenza, Influenza-Like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J. Evid. Based Complement. Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A. Non-Pharmacological Remedies for Post-Viral Acute Cough. Monaldi Arch. Chest Dis. 2021, 1, 92. [Google Scholar] [CrossRef] [PubMed]
- Sierocinski, E.; Holzinger, F.; Chenot, J.-F. Ivy Leaf (Hedera helix) for Acute Upper Respiratory Tract Infections: An Updated Systematic Review. Eur. J. Clin. Pharmacol. 2021, 77, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Sambucus nigra (Black Elder) as Alternative Treatment for Cold and Flu. Adv. Tradit. Med. 2021, 21, 405–414. [Google Scholar] [CrossRef]
- Rawangkan, A.; Kengkla, K.; Kanchanasurakit, S.; Duangjai, A.; Saokaew, S. Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 4014. [Google Scholar] [CrossRef]
- Schapowal, A.; Dobos, G.; Cramer, H.; Ong, K.C.; Adler, M.; Zimmermann, A.; Brandes-Schramm, J.; Lehmacher, W. Treatment of Signs and Symptoms of the Common Cold Using EPs 7630—Results of a Meta-Analysis. Heliyon 2019, 5, e02904. [Google Scholar] [CrossRef]
- Kemmerich, B. Evaluation of Efficacy and Tolerability of a Fixed Combination of Dry Extracts of Thyme Herb and Primrose Root in Adults Suffering from Acute Bronchitis with Productive Cough. A Prospective, Double-Blind, Placebo-Controlled Multicentre Clinical Trial. Arzneimittelforschung 2007, 57, 607–615. [Google Scholar] [CrossRef]
- Kemmerich, B.; Eberhardt, R.; Stammer, H. Efficacy and Tolerability of a Fluid Extract Combination of Thyme Herb and Ivy Leaves and Matched Placebo in Adults Suffering from Acute Bronchitis with Productive Cough. A Prospective, Double-Blind, Placebo-Controlled Clinical Trial. Arzneimittelforschung 2006, 56, 652–660. [Google Scholar] [CrossRef]
- Wagner, L.; Cramer, H.; Klose, P.; Lauche, R.; Gass, F.; Dobos, G.; Langhorst, J. Herbal Medicine for Cough: A Systematic Review and Meta-Analysis. Komplementmed 2015, 22, 359–368. [Google Scholar] [CrossRef]
- Henneicke-von Zepelin, H.; Hentschel, C.; Schnitker, J.; Kohnen, R.; Köhler, G.; Wüstenberg, P. Efficacy and Safety of a Fixed Combination Phytomedicine in the Treatment of the Common Cold (Acute Viral Respiratory Tract Infection): Results of a Randomised, Double Blind, Placebo Controlled, Multicentre Study. Curr. Med. Res. Opin. 1999, 15, 214–227. [Google Scholar] [CrossRef]
- EMA Medicines. Available online: https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Herbal (accessed on 21 September 2022).
- Nagy, M.; Mučaji, P.; Grančai, D. Farmakognózia. Biologicky Aktívne Rastlinné Metabolity a Ich Zdroje, 2nd ed.; Herba: Bratislava, Slovakia, 2017; Volume 399. [Google Scholar]
- Jin, M.-Y.; Li, M.-Y.; Huang, R.-M.; Wu, X.-Y.; Sun, Y.-M.; Xu, Z.-L. Structural Features and Anti-Inflammatory Properties of Pectic Polysaccharides: A Review. Trends Food Sci. Technol. 2021, 107, 284–298. [Google Scholar] [CrossRef]
- Catarino, M.D.; Talhi, O.; Rabahi, A.; Silva, A.M.S.; Cardoso, S.M. Chapter 3—The Antiinflammatory Potential of Flavonoids: Mechanistic Aspects. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 65–99. [Google Scholar]
- Pan, J.; Yuan, C.; Lin, C.; Jia, Z.; Zheng, R. Pharmacological Activities and Mechanisms of Natural Phenylpropanoid Glycosides. Pharmazie 2003, 58, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/ Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, B.H.; Chang, I.-M. Inhibitory Potencies of Several Iridoids on Cyclooxygenase-1, Cyclooxygnase-2 Enzymes Activities, Tumor Necrosis Factor-α and Nitric Oxide Production In Vitro. Evid. Based Complement. Altern. Med. 2010, 7, 41–45. [Google Scholar] [CrossRef]
- Müller-Jakic, B.; Breu, W.; Pröbstle, A.; Redl, K.; Greger, H.; Bauer, R. In Vitro Inhibition of Cyclooxygenase and 5-Lipoxygenase by Alkamides from Echinacea and Achillea Species. Planta Med. 1994, 60, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Woelkart, K.; Bauer, R. Alkamides from Echinacea Inhibit Cyclooxygenase-2 Activity in Human Neuroglioma Cells. Biochem. Biophys. Res. Commun. 2007, 360, 441–446. [Google Scholar] [CrossRef]
- Woelkart, K.; Xu, W.; Pei, Y.; Makriyannis, A.; Picone, R.P.; Bauer, R. The Endocannabinoid System as a Target for Alkamides from Echinacea angustifolia Roots. Planta Med. 2005, 71, 701–705. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Okpanyi, S.N.; Kelber, O.; Weiser, D. Mechanisms Involved in the Anti-Inflammatory Effect of a Standardized Willow Bark Extract. Arzneimittelforschung 2005, 55, 677–687. [Google Scholar] [CrossRef]
- Olajide, O.A.; Sarker, S.D. Chapter Five—Anti-Inflammatory Natural Products. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Medicinal Natural Products: A Disease-Focused Approach; Academic Press: Cambridge, MA, USA, 2020; Volume 55, pp. 153–177. [Google Scholar]
- Horváth, G.; Ács, K. Essential Oils in the Treatment of Respiratory Tract Diseases Highlighting Their Role in Bacterial Infections and Their Anti-Inflammatory Action: A Review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- Kim, S.R.; Jung, Y.R.; Kim, D.H.; An, H.J.; Kim, M.K.; Kim, N.D.; Chung, H.Y. Caffeic Acid Regulates LPS-Induced NF-ΚB Activation through NIK/IKK and c-Src/ERK Signaling Pathways in Endothelial Cells. Arch. Pharm. Res. 2014, 37, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic Acid: Pharmacological and Toxicological Aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids-Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Czigle, S.; Bittner Fialová, S.; Tóth, J.; Mučaji, P.; Nagy, M.; on behalf of the OEMONOM. Treatment of Gastrointestinal Disorders—Plants and Potential Mechanisms of Action of Their Constituents. Molecules 2022, 27, 2881. [Google Scholar] [CrossRef] [PubMed]
- Sieben, A.; Prenner, L.; Sorkalla, T.; Wolf, A.; Jakobs, D.; Runkel, F.; Häberlein, H. Alpha-Hederin, but Not Hederacoside C and Hederagenin from Hedera Helix, Affects the Binding Behavior, Dynamics, and Regulation of Beta 2-Adrenergic Receptors. Biochemistry 2009, 48, 3477–3482. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, J.; Zhang, X.-L.; Huang, X.-J.; Wu, X.; Zhang, Q.-W.; Li, Y.-L.; Ye, W.-C. Flavone C-Glycosides from the Leaves of Lophatherum Gracile and Their in Vitro Antiviral Activity. Planta Med. 2012, 78, 46–51. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral Activity of Plantago Major Extracts and Related Compounds In Vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chou, T.-W.; Cheng, L.-H.; Ho, C.-W. In Vitro Anti-Adenoviral Activity of Five Allium Plants. J. Taiwan Inst. Chem. Eng. 2011, 2, 228–232. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum Basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Percival, S.S. Aged Garlic Extract Modifies Human Immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef]
- Lissiman, E.; Bhasale, A.L.; Cohen, M. Garlic for the Common Cold. Cochrane Database Syst. Rev. 2014, 11, CD006206. [Google Scholar] [CrossRef]
- Josling, P. Preventing the Common Cold with a Garlic Supplement: A Double-Blind, Placebo-Controlled Survey. Adv. Ther. 2001, 18, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea Species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A Review of Their Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.; Barrett, B.; Bauer, R.; Melchart, D.; Woelkart, K. Echinacea for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2006, 1, CD000530. [Google Scholar] [CrossRef]
- Paulovičová, E.; Paulovičová, L.; Pawlaczyk-Graja, I.; Gancarz, R.; Kopáčová, M.; Capek, P. Effectivity of Polyphenolic Polysaccharide-Proteins Isolated from Medicinal Plants as Potential Cellular Immune Response Modulators. Biologia 2022, 77, 3581–3593. [Google Scholar] [CrossRef]
- Burger, R.A.; Torres, A.R.; Warren, R.P.; Caldwell, V.D.; Hughes, B.G. Echinacea-Induced Cytokine Production by Human Macrophages. Int. J. Immunopharmacol. 1997, 19, 371–379. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In Vitro Virucidal Activity of Echinaforce®, an Echinacea Purpurea Preparation, against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef]
- Kolev, E.; Mircheva, L.; Edwards, M.R.; Johnston, S.L.; Kalinov, K.; Stange, R.; Gancitano, G.; Berghe, W.V.; Kreft, S. Echinacea Purpurea For the Long-Term Prevention of Viral Respiratory Tract Infections During COVID-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. Front. Pharmacol. 2022, 13, 856410. [Google Scholar] [CrossRef]
- Nicolussi, S.; Ardjomand-Woelkart, K.; Stange, R.; Gancitano, G.; Klein, P.; Ogal, M. Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms 2022, 10, 211. [Google Scholar] [CrossRef]
- Weishaupt, R.; Bächler, A.; Feldhaus, S.; Lang, G.; Klein, P.; Schoop, R. Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children 2020, 7, 292. [Google Scholar] [CrossRef]
- Goel, V.; Lovlin, R.; Barton, R.; Lyon, M.R.; Bauer, R.; Lee, T.D.G.; Basu, T.K. Efficacy of a Standardized Echinacea Preparation (Echinilin) for the Treatment of the Common Cold: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Pharm. Ther. 2004, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Schulten, B.; Bulitta, M.; Ballering-Brühl, B.; Köster, U.; Schäfer, M. Efficacy of Echinacea Purpurea in Patients with a Common Cold. A Placebo-Controlled, Randomised, Double-Blind Clinical Trial. Arzneimittelforschung 2001, 51, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Brinkeborn, R.M.; Shah, D.V.; Degenring, F.H. Echinaforce and Other Echinacea Fresh Plant Preparations in the Treatment of the Common Cold. A Randomized, Placebo Controlled, Double-Blind Clinical Trial. Phytomedicine 1999, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Community Herbal Monograph on Eucalyptus Globulus Labill. Eucalyptus polybractea R.T. Baker and/or Eucalyptus smithii R.T. Baker, Aetheroleum. Committee on Herbal Medicinal Products. 2012, 9. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-eucalyptus-globulus-labill-eucalyptus-polybractea-rt-baker/eucalyptus-smithii-rt-baker-aetheroleum_en.pdf (accessed on 27 October 2022).
- Hasegawa, T.; Takano, F.; Takata, T.; Niiyama, M.; Ohta, T. Bioactive Monoterpene Glycosides Conjugated with Gallic Acid from the Leaves of Eucalyptus globulus. Phytochemistry 2008, 69, 747–753. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Value-Added Compound Recovery from Invasive Forest for Biofunctional Applications: Eucalyptus Species as a Case Study. Molecules 2020, 25, 4227. [Google Scholar] [CrossRef]
- Cermelli, C.; Fabio, A.; Fabio, G.; Quaglio, P. Effect of Eucalyptus Essential Oil on Respiratory Bacteria and Viruses. Curr. Microbiol. 2008, 56, 89–92. [Google Scholar] [CrossRef]
- Huang, S.; Constant, S.; De Servi, B.; Meloni, M.; Culig, J.; Bertini, M.; Saaid, A. In Vitro Safety and Performance Evaluation of a Seawater Solution Enriched with Copper, Hyaluronic Acid, and Eucalyptus for Nasal Lavage. Med. Devices 2019, 12, 399–410. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Ring, D.J.; Dalrymple, S.N. Treatment of the Common Cold. Am. Fam. Physician 2019, 100, 281–289. [Google Scholar]
- Smith, A.; Matthews, O. Aromatic Ointments for the Common Cold: What Does the Science Say? Drugs Context 2022, 11, 2022-5-6. [Google Scholar] [CrossRef]
- Her, L.; Kanjanasilp, J.; Chaiyakunapruk, N.; Sawangjit, R. Efficacy and Safety of Eucalyptus for Relieving Cough: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Integr. Complement. Med. 2022, 28, 218–226. [Google Scholar] [CrossRef]
- Gierlikowska, B.; Filipek, A.; Gierlikowski, W.; Kania, D.; Stefańska, J.; Demkow, U.; Kiss, A.K. Grindelia Squarrosa Extract and Grindelic Acid Modulate Pro-Inflammatory Functions of Respiratory Epithelium and Human Macrophages. Front. Pharmacol. 2021, 11, 534111. [Google Scholar] [CrossRef] [PubMed]
- Gierlikowska, B.; Gierlikowski, W.; Bekier, K.; Skalicka-Woźniak, K.; Czerwińska, M.E.; Kiss, A.K. Inula Helenium and Grindelia Squarrosa as a Source of Compounds with Anti-Inflammatory Activity in Human Neutrophils and Cultured Human Respiratory Epithelium. J. Ethnopharmacol. 2020, 249, 112311. [Google Scholar] [CrossRef] [PubMed]
- Murgia, V.; Ciprandi, G.; Votto, M.; Filippo, M.D.; Tosca, M.A.; Marseglia, G.L. Natural Remedies for Acute Post-Viral Cough in Children. Allergol. Immunopathol. 2021, 49, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kang, B.; Bok, S.-H.; Cho, S.S.; Park, D.-H. Macmoondongtang Modulates Th1-/Th2-Related Cytokines and Alleviates Asthma in a Murine Model. PLoS ONE 2019, 14, e0224517. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, C.; Wang, Y.; Pan, G.; Jiang, M.; Li, Z.; Zhu, Y. Qualitative and Quantitative Analysis of the Major Constituents in Chinese Medical Preparation Lianhua-Qingwen Capsule by UPLC-DAD-QTOF-MS. Sci. World J. 2015, 2015, 731765. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, J.; Tang, Z.; Liu, H.; Ruan, K.; Song, Z.; Liu, Y.; Yan, K.; Liu, Y.; Tang, Y.; et al. Multi-Evaluating Strategy for Siji-Kangbingdu Mixture: Chemical Profiling, Fingerprint Characterization, and Quantitative Analysis. Molecules 2019, 24, 3545. [Google Scholar] [CrossRef]
- Javid, A.; Motevalli Haghi, N.; Emami, S.A.; Ansari, A.; Zojaji, S.A.; Khoshkhui, M.; Ahanchian, H. Short-Course Administration of a Traditional Herbal Mixture Ameliorates Asthma Symptoms of the Common Cold in Children. Avicenna J. Phytomed. 2019, 9, 126–133. [Google Scholar]
- Barbalho, S. Properties of Mentha Piperita: A Brief Review. World J. Pharm. Med. Res. 2017, 3, 309–313. [Google Scholar]
- Mahendran, G.; Rahman, L.-U. Ethnomedicinal, Phytochemical and Pharmacological Updates on Peppermint (Mentha × piperita L.)—A Review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In Vitro Antiviral, Anti-Inflammatory, and Antioxidant Activities of the Ethanol Extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Brown, J.S.; Marcy, S.A. The Use of Botanicals for Health Purposes by Members of a Prepaid Health Plan. Res. Nurs. Health 1991, 14, 339–350. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Assessment Report on Mentha × piperita L., Folium and Aetheroleum. Committee on Herbal Medicinal Products, 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf (accessed on 27 October 2022).
- Ben-Arye, E.; Dudai, N.; Eini, A.; Torem, M.; Schiff, E.; Rakover, Y. Treatment of Upper Respiratory Tract Infections in Primary Care: A Randomized Study Using Aromatic Herbs. Evid. Based Complement. Alternat. Med. 2011, 2011, 690346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, V.; Rivera, D.; Heinrich, M.; Obón, C.; Ríos, S. An Ethnopharmacological and Historical Analysis of “Dictamnus”, a European Traditional Herbal Medicine. J. Ethnopharmacol. 2015, 175, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.P.; Karampourmpouni, Z.; Chinou, I. Herbal Preparations in Solid Gastro–Resistant Dosage Forms for Oral Use. Committee on Herbal Medicinal Products. 2020, 101. Available online: https://www.ema.europa.eu/en/committees/committee-herbal-medicinal-products-hmpc (accessed on 20 April 2023).
- Careddu, D.; Pettenazzo, A. Pelargonium Sidoides Extract EPs 7630: A Review of Its Clinical Efficacy and Safety for Treating Acute Respiratory Tract Infections in Children. Int. J. Gen. Med. 2018, 11, 91–98. [Google Scholar] [CrossRef]
- Keck, T.; Strobl, A.; Weinhaeusel, A.; Funk, P.; Michaelis, M. Pelargonium Extract EPs 7630 in the Treatment of Human Corona Virus-Associated Acute Respiratory Tract Infections—A Secondary Subgroup-Analysis of an Open-Label, Uncontrolled Clinical Trial. Front. Pharmacol. 2021, 12, 666546. [Google Scholar] [CrossRef]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. Investigation of the Influence of EPs® 7630, a Herbal Drug Preparation from Pelargonium Sidoides, on Replication of a Broad Panel of Respiratory Viruses. Phytomedicine 2011, 18, 384–386. [Google Scholar] [CrossRef]
- Riley, D.S.; Lizogub, V.G.; Zimmermann, A.; Funk, P.; Lehmacher, W. Efficacy and Tolerability of High-Dose Pelargonium Extract in Patients With the Common Cold. Altern. Ther. Health Med. 2018, 24, 16–26. [Google Scholar]
- Lizogub, V.G.; Riley, D.S.; Heger, M. Efficacy of a Pelargonium Sidoides Preparation in Patients with the Common Cold: A Randomized, Double Blind, Placebo-Controlled Clinical Trial. Explore 2007, 3, 573–584. [Google Scholar] [CrossRef]
- Ross, S.M. African Geranium (EPs 7630), Part I: A Proprietary Root Extract of Pelargonium Sidoides (EPs 7630) Is Found to Be Effective in Resolving Symptoms Associated with the Common Cold in Adults. Holist. Nurs. Pract. 2012, 26, 106–109. [Google Scholar] [CrossRef]
- Available online: anise-oil-summary-public_en.pdf (accessed on 27 October 2022).
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) Essential Oil Reduces pro-Inflammatory Cytokines and Stimulates Mucus Secretion in Primary Airway Bronchial and Tracheal Epithelial Cell Lines. Ind. Crops Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Community Herbal Monograph on Primula veris L. and/or Primula elatior (L.) Hill, Radix; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2012.
- European Medicines Agency (EMA). Community Herbal Monograph on Primula veris L. and/or Primula elatior (L.) Hill, Flos; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2012.
- European Medicines Agency (EMA). Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Flos; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2012.
- Bączek, K.; Przybył, J.L.; Mirgos, M.; Kosakowska, O.; Szymborska-Sandhu, I.; Węglarz, Z. Phenolics in Primula veris L. and P. elatior (L.) Hill Raw Materials. Int. J. Anal. Chem. 2017, 2017, 2871579. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Radix; Medicines Agency (EMA): Amsterdam, The Netherlands, 2012.
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Assessment Report on Sambucus nigra L., Flos; Medicines Agency (EMA): Amsterdam, The Netherlands, 2018.
- Gamze Ağalar, H.; Demirci, B.; Demirci, F.; Kırımer, N. The Volatile Compounds of the Elderflowers Extract and the Essential Oil. Nat. Prod. 2017, 11, 491–496. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). European Union Herbal Monograph on Sambucus nigra L., Flos; Medicines Agency (EMA): Amsterdam, The Netherlands, 2018.
- European Medicines Agency (EMA). Assessment Report on Sambucus nigra L., Fructus; Medicines Agency (EMA): Amsterdam, The Netherlands, 2014.
- Wichtl, M.; Blaschek, W. Wichtl—Teedrogen Und Phytopharmaka: Ein Handbuch Für Die Praxis; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2016; pp. 241–243, 448–449, 666–668. [Google Scholar]
- Assessment Report on Sideritis Scardica Griseb; Sideritis Clandestina (Bory & Chaub.) Hayek; Sideritis Raeseri Boiss. & Heldr.; Sideritis syriaca L., Herba. Repert. Spec. Nov. Regni Veg. Beih. 1939, 30, 257.
- Todorova, M.; Trendafilova, A. Sideritis Scardica Griseb., an Endemic Species of Balkan Peninsula: Traditional Uses, Cultivation, Chemical Composition, Biological Activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, Section Empedoclia in Southeastern Europe and Turkey—Studies in Ethnopharmacology and Recent Progress of Biological Activities. DARU J. Pharm. Sci. 2019, 27, 407. [Google Scholar] [CrossRef]
- EMA Thymi Aetheroleum. Available online: https://www.ema.europa.eu/en/medicines/herbal/thymi-aetheroleum (accessed on 30 May 2022).
- EMA Thymi Herba. Available online: https://www.ema.europa.eu/en/medicines/herbal/thymi-herba (accessed on 30 May 2022).
- Silva, A.S.; Tewari, D.; Sureda, A.; Suntar, I.; Belwal, T.; Battino, M.; Nabavi, S.M.; Nabavi, S.F. The Evidence of Health Benefits and Food Applications of Thymus vulgaris L. Trends Food Sci. Technol. 2021, 117, 218–227. [Google Scholar] [CrossRef]
- Almanea, A.; El-Aziz, G.S.A.; Ahmed, M.M.M. The Potential Gastrointestinal Health Benefits of Thymus Vulgaris Essential Oil: A Review. Biomed. Pharmacol. J. 2019, 12, 1793–1799. [Google Scholar] [CrossRef]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A Systematic Review on Ethnopharmacology, Phytochemistry and Pharmacological Aspects of Thymus Vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial Activity of Essential Oils of Cultivated Oregano (Origanum vulgare), Sage (Salvia officinalis), and Thyme (Thymus vulgaris) against Clinical Isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus Vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Aslani, M.R.; Kiani, S. Relaxant Effect of Thymus Vulgaris on Guinea-Pig Tracheal Chains and Its Possible Mechanism(s). Phytother. Res. 2006, 20, 28–33. [Google Scholar] [CrossRef]
- Vimalanathan, S. Anti-Influenza Virus Activity of Essential Oils and Vapors. Am. J. Essent. Oils Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Lenz, E.; Müller, C.; Mostafa, A.; Dzieciolowski, J.; Kanrai, P.; Dam, S.; Cwientzek, U.; Prenner, L.-N.; Pleschka, S. Authorised Medicinal Product Aspecton® Oral Drops Containing Thyme Extract KMTv24497 Shows Antiviral Activity against Viruses Which Cause Respiratory Infections. J. Herb. Med. 2018, 13, 26–33. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents. Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In-Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2021, 88, 587–603. [Google Scholar] [CrossRef]
- Cohen, A.H. A Randomized, Double-Blind Study to Evaluate the Efficacy and Tolerability of a Cough Syrup Containing Specific Plant Extracts (Poliflav M.A.) and Honey Versus Placebo in Cough Due to Upper Respiratory Tract Infection. 2018. Available online: https://clinicaltrials.gov (accessed on 20 April 2023).
- PharmEvo Pvt Ltd. PEACe: Ivy, Thyme and Cisti Extract (Phytus) Efficacy in Acute Cough. 2018. Available online: https://clinicaltrials.gov (accessed on 20 April 2023).
- Assessment Report on Tilia Cordata Miller, Tilia Platyphyllos Scop., Tilia x Vulgaris Heyne or Their Mixtures, Flos. 20; European Medicines Agency: Amsterdam, The Netherlands, 2012.
- Rathinasabapathy, T.; Sakthivel, L.P.; Komarnytsky, S. Plant-Based Support of Respiratory Health during Viral Outbreaks. J. Agric. Food Chem. 2022, 70, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Salas, J.; Hortigón-Vinagre, M.P.; Morales-Jadán, D.; Ruiz-Téllez, T. Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology 2021, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- EMA Verbasci Flos. Available online: https://www.ema.europa.eu/en/medicines/herbal/verbasci-flos (accessed on 28 October 2022).
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory Effects in THP-1 Cells Treated with Verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Song, X.; He, J.; Xu, H.; Hu, X.-P.; Wu, X.-L.; Wu, H.-Q.; Liu, L.-Z.; Liao, C.-H.; Zeng, Y.; Li, Y.; et al. The Antiviral Effects of Acteoside and the Underlying IFN-γ-Inducing Action. Food Funct. 2016, 7, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Available online: foeniculum-vulgare-miller-subsp-vulgare-var-dulce-miller-thellung-fructus-fennel-fruit-sweet-hmpc_en.pdf (accessed on 20 April 2023).
- Matricaria Recutita—An Overview|ScienceDirect Topics. Available online: https://www-sciencedirect-com.insb.bib.cnrs.fr/topics/immunology-and-microbiology/matricaria-recutita (accessed on 26 October 2022).
- Assessment Report on Polypodium vulgare L., Rhizoma; European Medicines Agency: Amsterdam, The Netherlands, 2008.
- European Union Herbal Monograph on Salix [Various Species Including S. purpurea L., S. daphnoides Vill., S. fragilis L.], Cortex. 9; European Medicines Agency: Amsterdam, The Netherlands, 2017.
- Bastian, P.; Fal, A.M.; Jambor, J.; Michalak, A.; Noster, B.; Sievers, H.; Steuber, A.; Walas-Marcinek, N. Candelabra Aloe (Aloe arborescens) in the Therapy and Prophylaxis of Upper Respiratory Tract Infections: Traditional Use and Recent Research Results. Wien Med. Wochenschr. 2013, 163, 73–79. [Google Scholar] [CrossRef]
- Fal, A.M.; Schönknecht, K.; Jambor, J. Immunomodulatory role of Biostymina® and Bioaron® C in the prevention and treatment of upper respiratory tract infections. Wiad. Lek. 2016, 69, 77–84. [Google Scholar]
- Glatthaar-Saalmüller, B.; Fal, A.M.; Schönknecht, K.; Conrad, F.; Sievers, H.; Saalmüller, A. Antiviral Activity of an Aqueous Extract Derived from Aloe arborescens Mill. against a Broad Panel of Viruses Causing Infections of the Upper Respiratory Tract. Phytomedicine 2015, 22, 911–920. [Google Scholar] [CrossRef]
- Williams, L.a.D.; Igietseme, J.U.; Whittaker, J.A.; Smikle, M.F.; Bailey-Shaw, Y.A.; Barton, E.N. Immunological Evidence Supporting the Use of Extracts from Boehmeria Jamaicensis Urb for Treating the Common Cold and Sinus Infections. West Indian Med. J. 2007, 56, 487–490. [Google Scholar]
- Rowe, C.A.; Nantz, M.P.; Bukowski, J.F.; Percival, S.S. Specific Formulation of Camellia Sinensis Prevents Cold and Flu Symptoms and Enhances Gamma, Delta T Cell Function: A Randomized, Double-Blind, Placebo-Controlled Study. J. Am. Coll. Nutr. 2007, 26, 445–452. [Google Scholar] [CrossRef]
- Lin, P.-R.; Kuo, P.-C.; Li, Y.-C.; Jhuo, C.-F.; Hsu, W.-L.; Tzen, J.T.C. Theacrine and Strictinin, Two Major Ingredients for the Anti-Influenza Activity of Yunnan Kucha Tea. J. Ethnopharmacol. 2020, 262, 113190. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szeremeta, D.; Knaś, M.; Długosz, E.; Ott, P.G.; Kowalska, T.; Sajewicz, M. Antibacterial Potential of the Cistus incanus L. Phenolics as Studied with Use of Thin-Layer Chromatography Combined with Direct Bioautography and In Situ Hydrolysis. J. Chromatogr. A 2018, 1534, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Kalus, U.; Kiesewetter, H.; Radtke, H. Effect of CYSTUS052® and Green Tea on Subjective Symptoms in Patients with Infection of the Upper Respiratory Tract. Phytother. Res. 2010, 24, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kalus, U.; Grigorov, A.; Kadecki, O.; Jansen, J.-P.; Kiesewetter, H.; Radtke, H. Cistus Incanus (CYSTUS052) for Treating Patients with Infection of the Upper Respiratory Tract. A Prospective, Randomised, Placebo-Controlled Clinical Study. Antiviral. Res. 2009, 84, 267–271. [Google Scholar] [CrossRef]
- Droebner, K.; Ehrhardt, C.; Poetter, A.; Ludwig, S.; Planz, O. CYSTUS052, a Polyphenol-Rich Plant Extract, Exerts Anti-Influenza Virus Activity in Mice. Antivir. Res. 2007, 76, 1–10. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Hrincius, E.R.; Korte, V.; Mazur, I.; Droebner, K.; Poetter, A.; Dreschers, S.; Schmolke, M.; Planz, O.; Ludwig, S. A Polyphenol Rich Plant Extract, CYSTUS052, Exerts Anti Influenza Virus Activity in Cell Culture without Toxic Side Effects or the Tendency to Induce Viral Resistance. Antivir. Res. 2007, 76, 38–47. [Google Scholar] [CrossRef]
- Yeh, C.F.; Chang, J.S.; Wang, K.C.; Shieh, D.E.; Chiang, L.C. Water Extract of Cinnamomum Cassia Blume Inhibited Human Respiratory Syncytial Virus by Preventing Viral Attachment, Internalization, and Syncytium Formation. J. Ethnopharmacol. 2013, 147, 321–326. [Google Scholar] [CrossRef]
- Roxas, M.; Jurenka, J. Colds and Influenza: A Review of Diagnosis and Conventional, Botanical, and Nutritional Considerations. Altern. Med. Rev. 2007, 12, 25–48. [Google Scholar]
- Dion, C.; Chappuis, E.; Ripoll, C. Does Larch Arabinogalactan Enhance Immune Function? A Review of Mechanistic and Clinical Trials. Nutr. Metab. 2016, 13, 28. [Google Scholar] [CrossRef]
- Riede, L.; Grube, B.; Gruenwald, J. Larch Arabinogalactan Effects on Reducing Incidence of Upper Respiratory Infections. Curr. Med. Res. Opin. 2013, 29, 251–258. [Google Scholar] [CrossRef]
- Lin, T.-J.; Wang, K.-C.; Lin, C.-C.; Chiang, L.-C.; Chang, J.-S. Anti-Viral Activity of Water Extract of Paeonia Lactiflora Pallas against Human Respiratory Syncytial Virus in Human Respiratory Tract Cell Lines. Am. J. Chin. Med. 2013, 41, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.M.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral Activity and Possible Mechanism of Action of Constituents Identified in Paeonia Lactiflora Root toward Human Rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef] [PubMed]
- Cwientzek, U.; Ottillinger, B.; Arenberger, P. Acute Bronchitis Therapy with Ivy Leaves Extracts in a Two-Arm Study. A Double-Blind, Randomised Study vs. an Other Ivy Leaves Extract. Phytomedicine 2011, 18, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Irifune, K.; Hamada, H.; Ito, R.; Katayama, H.; Watanabe, A.; Kato, A.; Miyoshi, S.; Hamaguchi, N.; Toyozawa, R.; Hamaguchi, S.; et al. Antitussive Effect of Bakumondoto a Fixed Kampo Medicine (Six Herbal Components) for Treatment of Post-Infectious Prolonged Cough: Controlled Clinical Pilot Study with 19 Patients. Phytomedicine 2011, 18, 630–633. [Google Scholar] [CrossRef]
- Gillissen, A.; Wittig, T.; Ehmen, M.; Krezdorn, H.G.; de Mey, C. A Multi-Centre, Randomised, Double-Blind, Placebo-Controlled Clinical Trial on the Efficacy and Tolerability of GeloMyrtol® Forte in Acute Bronchitis. Drug Res. 2013, 63, 19–27. [Google Scholar] [CrossRef]
- Matthys, H.; de Mey, C.; Carls, C.; Ryś, A.; Geib, A.; Wittig, T. Efficacy and Tolerability of Myrtol Standardized in Acute Bronchitis. A Multi-Centre, Randomised, Double-Blind, Placebo-Controlled Parallel Group Clinical Trial vs. Cefuroxime and Ambroxol. Arzneimittelforschung 2000, 50, 700–711. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.; Choi, J.-Y.; Lee, J.A. Soshiho-Tang for Treating Common Cold in Children Younger than 12 Years. Medicine 2018, 97, e13045. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, W.; Jin, C.; Jang, I.; Jung, W.-S.; Moon, S.-K.; Cho, K.-H. Could Herbal Medicine (Soshihotang) Be a New Treatment Option for COVID-19?: A Narrative Review. Integr. Med. Res. 2020, 9, 100480. [Google Scholar] [CrossRef]
- Barth, A.; Hovhannisyan, A.; Jamalyan, K.; Narimanyan, M. Antitussive Effect of a Fixed Combination of Justicia Adhatoda, Echinacea Purpurea and Eleutherococcus Senticosus Extracts in Patients with Acute Upper Respiratory Tract Infection: A Comparative, Randomized, Double-Blind, Placebo-Controlled Study. Phytomedicine 2015, 22, 1195–1200. [Google Scholar] [CrossRef]
- Byun, J.-S.; Yang, S.-Y.; Jeong, I.-C.; Hong, K.-E.; Kang, W.; Yeo, Y.; Park, Y.-C. Effects of So-Cheong-Ryong-Tang and Yeon-Gyo-Pae-Dok-San on the Common Cold: Randomized, Double Blind, Placebo Controlled Trial. J. Ethnopharmacol. 2011, 133, 642–646. [Google Scholar] [CrossRef]
- Naser, B.; Lund, B.; Henneicke-von Zepelin, H.H.; Köhler, G.; Lehmacher, W.; Scaglione, F. A Randomized, Double-Blind, Placebo-Controlled, Clinical Dose-Response Trial of an Extract of Baptisia, Echinacea and Thuja for the Treatment of Patients with Common Cold. Phytomedicine 2005, 12, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-F.; Lo, C.-W.; Liu, C.-H.; Lin, S.; Yen, H.-R.; Lin, T.-Y.; Horng, J.-T. Mechanism by Which Ma-Xing-Shi-Gan-Tang Inhibits the Entry of Influenza Virus. J. Ethnopharmacol. 2012, 143, 57–67. [Google Scholar] [CrossRef] [PubMed]
| Plant | Family | Infection | References |
|---|---|---|---|
| Allium sativum L. | Amaryllidaceae | Common cold; COVID-19; Rhinoviruses. | [48,49,50] |
| Echinacea purpurea L., Echinacea angustifolia DC. | Asteraceae | Common cold; Coronavirus 229E and SARS-CoV-2; Rhinovirus colds. | [51,52,53,54,55,56,57,58,59,60,61] |
| Eucalyptus globulus Labill. | Myrtaceae | Acute respiratory infection. | [62,63,64,65,66,67,68,69] |
| Grindelia robusta Nutt, Grindelia squarrosa (Pursh) Dunal, Grindelia humilis Hook. et Arn., Grindelia camporum Greene | Asteraceae | Acute respiratory infection. | [15,70,71,72] |
| Glycyrrhiza glabra L., Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch | Fabaceae | Upper respiratory infections; common colds. | [73,74,75,76] |
| Mentha × piperita L. | Lamiaceae | Common colds; respiratory syncytial virus (RSV). | [77,78,79,80,81,82] |
| Origanum dictamnus L. | Lamiaceae | Upper respiratory infections. | [82,83,84] |
| Pelargonium sidoides DC, Pelargonium reniforme Curt. | Geraniaceae | Common cold; Acute respiratory tract infections. | [85,86,87,88,89,90] |
| Pimpinella anisum L. | Apiaceae | Expectorant Common cold. | [91,92] |
| Primula elatior (L.) Hill, Primula veris L. | Primulaceae | Antitussive. | [93,94,95,96,97] |
| Sambucus nigra L. | Adoxaceae | Common cold and influenza (A and B). | [98,99,100,101,102,103] |
| Sideritis scardica Griseb./Sideritis clandestina (Bory and Chaub.) Hayek./Sideritis raeseri Boiss./and Heldr., Sideritis syriaca L. | Lamiaceae | Bronchitis; bronchial asthma;common colds. | [104,105,106] |
| Thymus vulgaris L., Thymus zygis L. | Lamiaceae | Antitussive; common cold; Human rhinovirus. | [17,22,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124] |
| Tilia cordata Miller, Tilia platyphyllos Scop., Tilia × vulgaris Heyne | Tiliaceae | Common cold. | [125] |
| Verbascum thapsum L., Verbascum densiflorum Bertol., Verbascum phlomoides L. | Scrophulariaceae | Common colds; coughs; asthma; bronchit. | [126,127,128,129,130] |
| Foeniculum vulgare Miller subsp. vulgare var. | Apiaceae | Expectorant. | [131] |
| Matricaria recutita L. | Asteraceae | antitussive bronchitis; fever; colds. | [76,132] |
| Polygonium aviculare L./Polypodium vulgare L. | Polygonaceae | Common colds. | [133] |
| Salix purpurea L./Salix daphnoides Vill./Salix fragilis L. | Salicaceae | Common colds. | [134] |
| Aloe arborescens Mill. | Asphodelaceae | Upper respiratory tract infections; Human rhinovirus B (HRV14), influenza A virus (H1N1) and (H3N2), influenza B, respiratory syncytial virus (RSV), parainfluenza type 3 virus (Para 3). | [135,136,137] |
| Boehmeria jamaicensis Urb. | Urticaceae | Common colds. | [138] |
| Camellia sinensis (L.) Kuntze, Camellia assamica var. kucha | Theaceae | Anti-Influenza viral adsorption and suppressed replication Cold viruses Common cold. | [139,140] |
| Cistus × incanus L. | Cistus | Common colds; upper respiratory tract; Anti-Influenza. | [141,142,143,144,145,146] |
| Cinnamomum verum J.S. Presl | Lauraceae | Common cold. Chronic bronchitis Human respiratory syncytial virus. | [147] |
| Larix decidua Mill. | Pinaceae | Common cold. | [148,149,150] |
| Paeonia lactiflora Pall.; Paeonia veitchii Lynch | Fabaceae | Rhinoviruses. | [151,152] |
| Plants | Infection | References |
|---|---|---|
| Ivy (Hedera helix)/primrose (Primula vulgaris)/thyme (Thymus vulgaris) | Common cold Acute bronchitis | [21,22,23,117,118,153] |
| Tsumura bakumondoto | Common cold | [154] |
| Gelo Myrtol® | Common cold | [155,156] |
| Soshiho-tang | Common cold (chills and fever) Pulmonary disease | [157,158] |
| Kan Jang® | Respiratory tract infection | [159] |
| So-cheong-ryong-tang Yeon-gyo-pae-dok-san | Common cold | [160] |
| Echinaceae radix, Baptisiae radix, and Thujae herba. | Acute viral respiratory tract infection | [24,161] |
| Ma-xing-shi-gan-tang: | Common cold, fever, and influenza virus infections | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammari, N.; Albert, Q.; Devocelle, M.; Kenda, M.; Kočevar Glavač, N.; Sollner Dolenc, M.; Mercolini, L.; Tóth, J.; Milan, N.; Czigle, S.; et al. Natural Products for the Prevention and Treatment of Common Cold and Viral Respiratory Infections. Pharmaceuticals 2023, 16, 662. https://doi.org/10.3390/ph16050662
Mammari N, Albert Q, Devocelle M, Kenda M, Kočevar Glavač N, Sollner Dolenc M, Mercolini L, Tóth J, Milan N, Czigle S, et al. Natural Products for the Prevention and Treatment of Common Cold and Viral Respiratory Infections. Pharmaceuticals. 2023; 16(5):662. https://doi.org/10.3390/ph16050662
Chicago/Turabian StyleMammari, Nour, Quentin Albert, Marc Devocelle, Maša Kenda, Nina Kočevar Glavač, Marija Sollner Dolenc, Laura Mercolini, Jaroslav Tóth, Nagy Milan, Szilvia Czigle, and et al. 2023. "Natural Products for the Prevention and Treatment of Common Cold and Viral Respiratory Infections" Pharmaceuticals 16, no. 5: 662. https://doi.org/10.3390/ph16050662
APA StyleMammari, N., Albert, Q., Devocelle, M., Kenda, M., Kočevar Glavač, N., Sollner Dolenc, M., Mercolini, L., Tóth, J., Milan, N., Czigle, S., Varbanov, M., & on behalf of the OEMONOM. (2023). Natural Products for the Prevention and Treatment of Common Cold and Viral Respiratory Infections. Pharmaceuticals, 16(5), 662. https://doi.org/10.3390/ph16050662








