Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris
Abstract
:1. Introduction
2. Results
2.1. Effect of A64 and Its Herbal Constituents against the SARS-CoV2 Infected Hamster Model
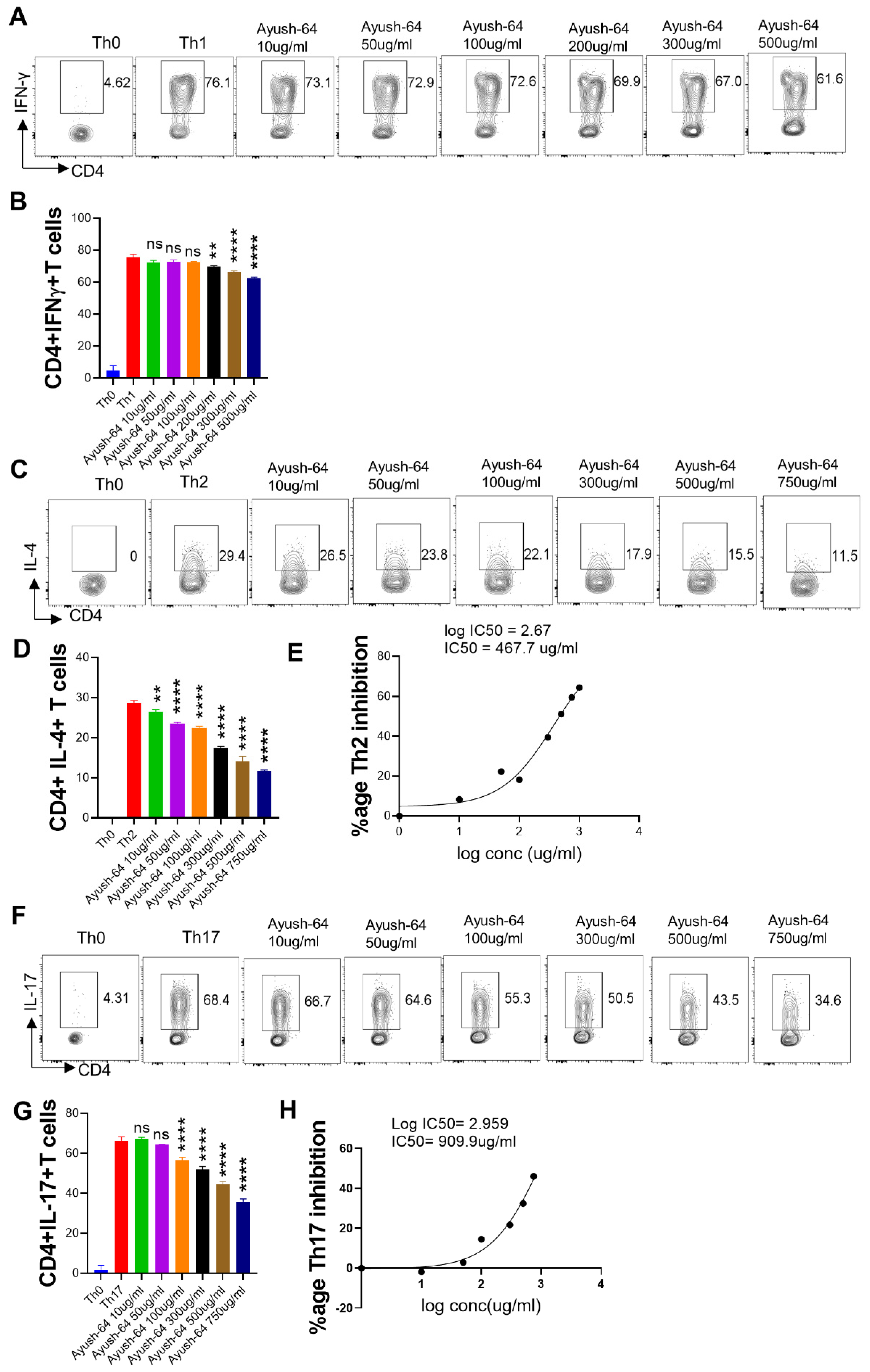
2.2. In Vitro Suppression of T Cell Differentiation by A64
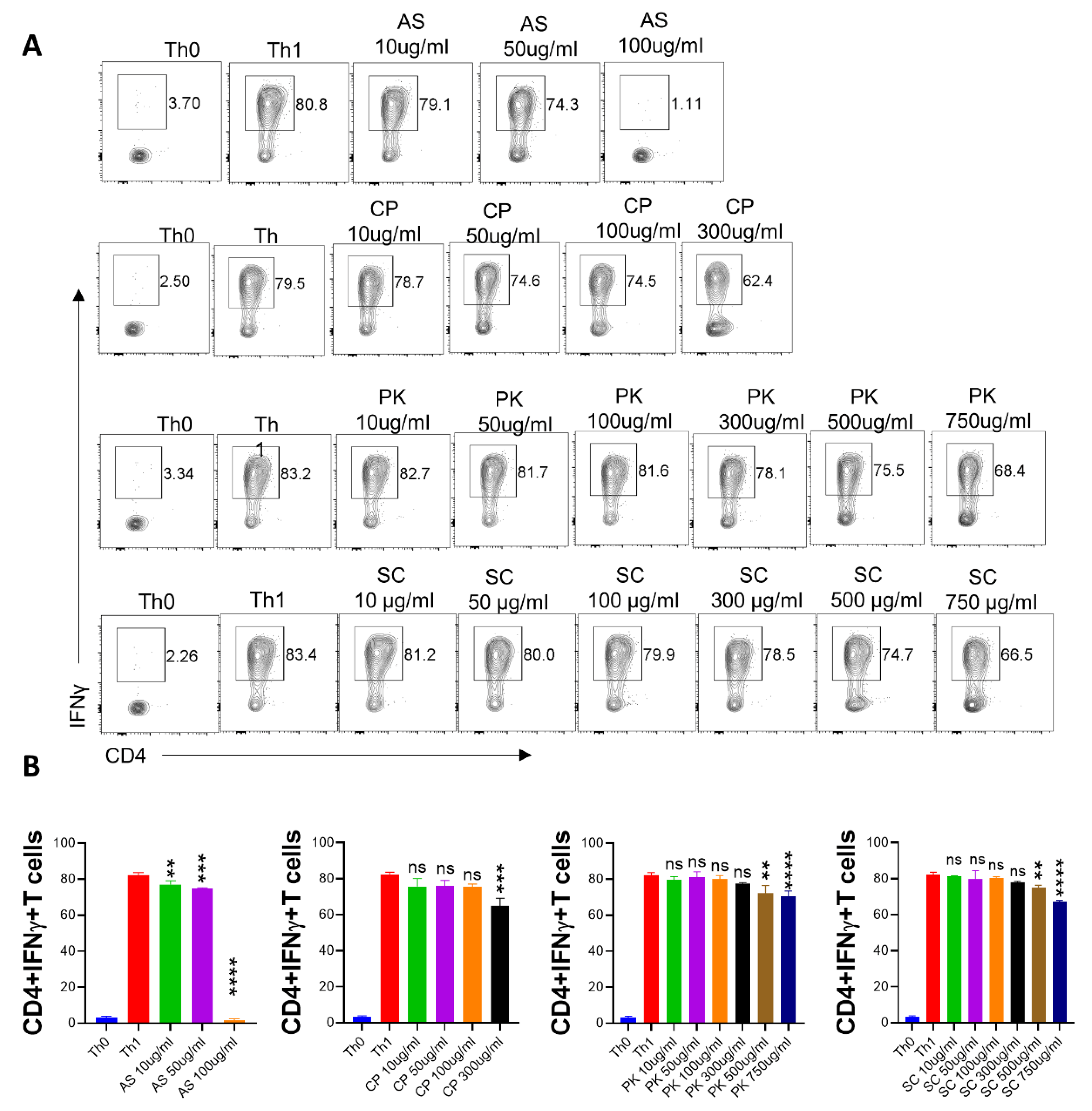
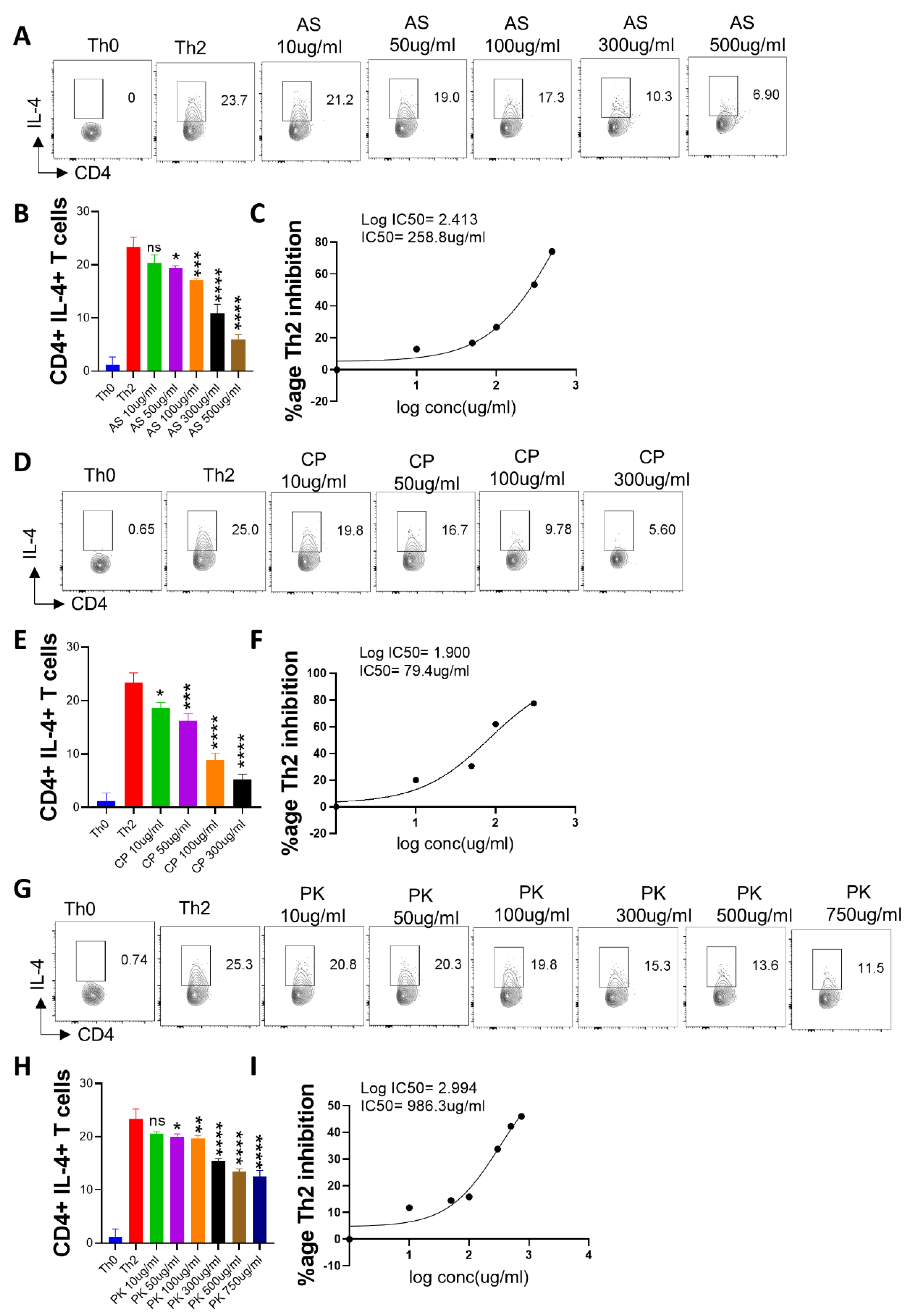
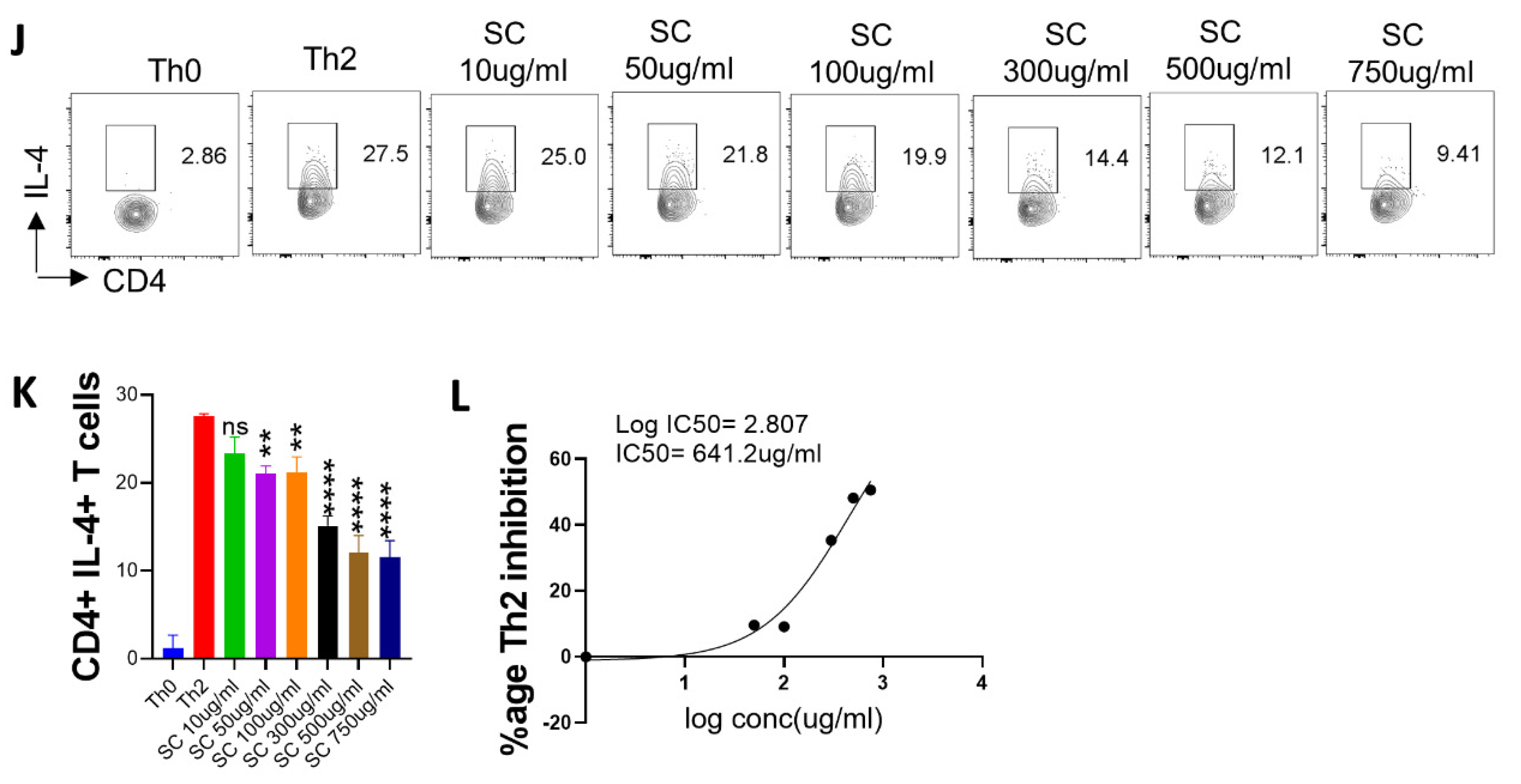
2.3. Anti-Viral and Immunomodulatory Potential of the A64 Ingredients
2.4. In Vitro Effect of A. scholaris, C. crista, P. kurroa, and S. chirata on Th1 Differentiation
2.5. Effect of A. scholaris, C. crista, P. kurroa, and S. chirata on Th2 Differentiation
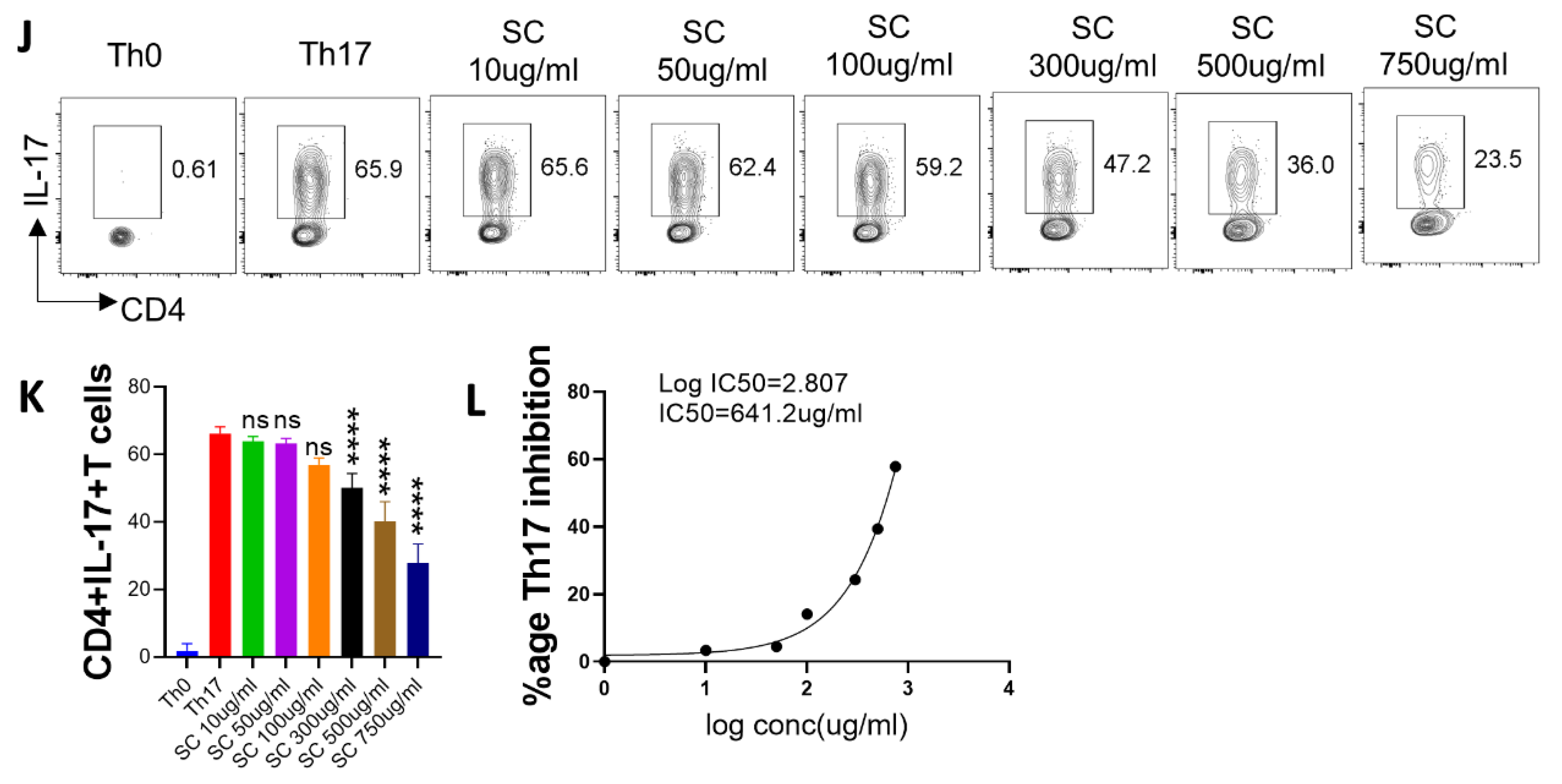
2.6. In Vitro Effect of A. scholaris, C. crista, P. kurroa, and S. chirata Pre-Treatment on Th17 Differentiation
3. Discussion
4. Materials and Methods
4.1. Herbal Extracts
4.2. Preparation and Characterization of Extracts
4.3. SARS-CoV-2-Infected Hamster Model
4.4. Virus Titration
4.5. Gross Parameters of Infected Hamsters
4.6. Lungs Viral Load
| Gene | Forward | Reverse |
| HGPRT | GATAGATCCACTCCCATAACTG | TACCTTCAACAATCAAGACATTC |
| tryptase β2 | TCGCCACTGTATCCCCTGAA | CTAGGCACCCTTGACTTTGC |
| chymase | ATGAACCACCCTCGGACACT | AGAAGGGGGCTTTGCATTCC |
| Muc-1 | CGGAAGAACTATGGGCAGCT | GCCACTACTGGGTTGGTGTAAG |
| Sftp-D | TGAGCATGACAGACGTGGAC | GGCTTAGAACTCGCAGACGA |
| Eotaxin | ATGTGCTCTCAGGTCATCGC | TCCTCAGTTGTCCCCATCCT |
| PAI-1 | CCGTGGAACCAGAACGAGAT | ACCAGAATGAGGCGTGTCAG |
| IFN-γ | TGTTGCTCTGCCTCACTCAGG | AAGACGAGGTCCCCTCCATTC |
| TNF-α | AGAATCCGGGCAGGTCTACT | TATCCCGGCAGCTTGTGTTT |
| IL-13 | AAATGGCGGGTTCTGTGC | AATATCCTCTGGGTCTTGTAGATGG |
| IL-17A | ATGTCCAAACACTGAGGCCAA | GCGAAGTGGATCTGTTGAGGT |
| IL-10 | GGTTGCCAAACCTTATCAGAAATG | TTCACCTGTTCCACAGCCTTG |
| IL-6 | GGACAATGACTATGTGTTGTTAGAA | AGGCAAATTTCCCAATTGTATCCAG |
4.7. Histological Assessment of the Hamster Lung Tissues
4.8. In Vitro T cell Differentiation
4.9. Intracellular Cytokine Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Atkinson, B.; Petersen, E. SARS-CoV-2 shedding and infectivity. Lancet 2020, 395, 1339–1340. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L. SARS-CoV-2: Virus dynamics and host response. Lancet Infect. Dis. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e1. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, Z.; Zhu, F.; Cao, Z.; An, Y.; Gao, Y.; Jiang, B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020, 395, e52. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19-Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Ang, L.; Song, E.; Lee, H.W.; Lee, M.S. Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Cheng, T.-J.R.; Juang, Y.-P.; Ma, H.-H.; Wu, Y.-T.; Yang, W.-B.; Cheng, C.-W.; Chen, X.; Chou, T.-H.; Shie, J.-J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Use of herbal drugs to treat COVID-19 should be with caution. Lancet 2020, 395, 1689–1690. [Google Scholar] [CrossRef]
- Panda, A.K.; Kar, S.; Rai, A.K.; Rao, B.C.S.; Srikanth, N. AYUSH-64: A potential therapeutic agent in COVID-19. J. Ayurveda Integr. Med. 2022, 13, 100538. [Google Scholar] [CrossRef] [PubMed]
- Ali, H. “Ayush-64”—A new anti malarial herbal compound. Indian J. Pathol. Microbiol. 1996, 39, 499–500. [Google Scholar]
- Gundeti, M.S.; Bhurke, L.W.; Mundada, P.S.; Murudkar, S.; Surve, A.; Sharma, R.; Mata, S.; Rana, R.; Singhal, R.; Vyas, N.; et al. AYUSH 64, a polyherbal Ayurvedic formulation in Influenza-like illness-Results of a pilot study. J. Ayurveda Integr. Med. 2022, 13, 100325. [Google Scholar] [CrossRef]
- Kasarla, S.S.; Borse, S.P.; Kumar, Y.; Sharma, N.; Dikshit, M. In vitro effect of Withania somnifera, AYUSH-64, and remdesivir on the activity of CYP-450 enzymes: Implications for possible herb-drug interactions in the management of COVID-19. Front. Pharmacol. 2022, 13, 973768. [Google Scholar] [CrossRef]
- Ram, T.S.; Munikumar, M.; Raju, V.N.; Devaraj, P.; Boiroju, N.K.; Hemalatha, R.; Prasad, P.; Gundeti, M.; Sisodia, B.S.; Pawar, S.; et al. In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease. J. Ayurveda Integr. Med. 2022, 13, 100413. [Google Scholar] [CrossRef]
- Thakar, A.; Goyal, M.; Bhinde, S.; Chhotala, Y.; Panara, K.; Chaudhari, S. Impact of AYUSH 64 as an adjunctive to standard of care in mild COVID 19-An open-label randomized controlled pilot study. J. Ayurveda Integr. Med. 2022, 13, 100587. [Google Scholar] [CrossRef]
- Chopra, A.; Tillu, G.; Chuadhary, K.; Reddy, G.; Srivastava, A.; Lakdawala, M.; Gode, D.; Reddy, H.; Tamboli, S.; Saluja, M.; et al. Coadministration of AYUSH 64 as an adjunct to Standard of Care in mild and moderate COVID-19: A randomised, controlled, multicentric clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Zhang, A.J.; Yuan, S.; Poon, V.K.-M.; Chan, C.C.-S.; Lee, A.C.-Y.; Chan, W.-M.; Fan, Z.; Tsoi, H.-W.; Wen, L.; et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020, 71, 2428–2446. [Google Scholar] [CrossRef]
- Shang, J.-H.; Cai, X.-H.; Feng, T.; Zhao, Y.-L.; Wang, J.-K.; Zhang, L.-Y.; Yan, M.; Luo, X.-D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Shang, J.-H.; Pu, S.-B.; Wang, H.-S.; Wang, B.; Liu, L.; Liu, Y.-P.; Hong-Mei, S.; Luo, X.-D. Effect of total alkaloids from Alstonia scholaris on airway inflammation in rats. J. Ethnopharmacol. 2016, 178, 258–265. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; John, J.; Mehta, P.; Vyas, S.P.; Shukla, S. Immunomodulatory activities of the ethanolic extract of Caesalpinia bonducella seeds. J. Ethnopharmacol. 2009, 125, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mehta, A.; Mehta, P.; Vyas, S.P.; Shivaprasad, H.N. In vivo immunomodulatory activities of the aqueous extract of bonduc nut Caesalpinia bonducella seeds. Pharm. Biol. 2010, 48, 227–230. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, Y.K.; Singh, S.; Raj, A. Anti-inflammatory Effect of Picrorhiza kurroa in Experimental Models of Inflammation. Planta Med. 2016, 82, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.; Kroes, B.; Berg, A.v.D.; van der Wal, D.; Worm, E.v.D.; Beukelman, C.; van Dijk, H.; Labadie, R. Immunomodulatory and anti-inflammatory activity of Picrorhiza scrophulariiflora. J. Ethnopharmacol. 2000, 73, 101–109. [Google Scholar] [CrossRef]
- Kumar, V.; Van Staden, J. A Review of Swertia chirayita (Gentianaceae) as a Traditional Medicinal Plant. Front. Pharmacol. 2016, 6, 308. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Ju, J.-M.; Mo, L.-H.; Ma, L.; Hu, W.-H.; You, R.-R.; Chen, X.-Q.; Chen, Y.-Y.; Liu, Z.-Q.; Qiu, S.-Q.; et al. Anti-inflammation action of xanthones from Swertia chirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine 2019, 55, 214–221. [Google Scholar] [CrossRef]
- Singh, H.; Srivastava, S.; Yadav, B.; Rai, A.K.; Jameela, S.; Muralidharan, S.; Mohan, R.; Chaudhary, S.; Singhal, R.; Rana, R.; et al. AYUSH-64 as an adjunct to standard care in mild to moderate COVID-19: An open-label randomized controlled trial in Chandigarh, India. Complement. Ther. Med. 2022, 66, 102814. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.-M.; Chin, A.W.H.; Fung, K.; Choy, K.-T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Dalal, R.; Sadhu, S.; Binayke, A.; Dandotiya, J.; Kumar, Y.; Shrivastava, T.; Gupta, S.K.; Aggarwal, S.; Tripathy, M.R.; et al. Golden Syrian hamster as a model to study cardiovascular complications associated with SARS-CoV-2 infection. eLife 2022, 11, e73522. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Babele, P.; Sadhu, S.; Madan, U.; Tripathy, M.R.; Goswami, S.; Mani, S.; Kumar, S.; Awasthi, A.; Dikshit, M. Prophylactic treatment of Glycyrrhiza glabra mitigates COVID-19 pathology through inhibition of pro-inflammatory cytokines in the hamster model and NETosis. Front. Immunol. 2022, 13, 945583. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.945583 (accessed on 23 February 2022). [CrossRef] [PubMed]
- Rizvi, Z.A.; Sadhu, S.; Dandotiya, J.; Binyka, A.; Sharma, P.; Singh, V.; Das, V.; Khatri, R.; Kumar, R.; Samal, S.; et al. SARS-CoV-2 and its variants, but not Omicron, induces thymic atrophy and impaired T cell development. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Babele, P.; Madan, U.; Sadhu, S.; Tripathy, M.R.; Goswami, S.; Mani, S.; Dikshit, M.; Awasthi, A. Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions. Front. Immunol. 2023, 14, 1138215. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1138215 (accessed on 23 February 2022). [CrossRef]
- Rizvi, Z.A.; Tripathy, M.R.; Sharma, N.; Goswami, S.; Srikanth, N.; Sastry, J.L.N.; Mani, S.; Surjit, M.; Awasthi, A.; Dikshit, M. Effect of Prophylactic Use of Intranasal Oil Formulations in the Hamster Model of COVID-19. Front. Pharmacol. 2021, 12, 746729. Available online: https://www.frontiersin.org/article/10.3389/fphar.2021.746729 (accessed on 23 February 2022). [CrossRef] [PubMed]
- Rizvi, Z.A.; Puri, N.; Saxena, R.K. Evidence of CD1d pathway of lipid antigen presentation in mouse primary lung epithelial cells and its up-regulation upon Mycobacterium bovis BCG infection. PLoS ONE 2018, 13, e0210116. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Puri, N.; Saxena, R.K. Lipid antigen presentation through CD1d pathway in mouse lung epithelial cells, macrophages and dendritic cells and its suppression by poly-dispersed single-walled carbon nanotubes. Toxicol. Vitr. 2015, 29, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, Z.A.; Dalal, R.; Sadhu, S.; Kumar, Y.; Kumar, S.; Gupta, S.K.; Tripathy, M.R.; Rathore, D.K.; Awasthi, A. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci. Adv. 2021, 7, eabg5016. [Google Scholar] [CrossRef]
- Roy, S.; Rizvi, Z.A.; Clarke, A.J.; Macdonald, F.; Pandey, A.; Zaiss, D.M.W.; Simon, A.K.; Awasthi, A. EGFR-HIF1α signaling positively regulates the differentiation of IL-9 producing T helper cells. Nat. Commun. 2021, 12, 3182. [Google Scholar] [CrossRef]
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell. Infect. Microbiol. 2021, 11, 624483. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat. Rev. Immunol. 2020, 20, 587–588. [Google Scholar] [CrossRef]
- Roncati, L.; Nasillo, V.; Lusenti, B.; Riva, G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020, 99, 1419–1420. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.-S.; Zhang, C.; Tang, G.-Y.; Tan, H.-Y.; Chen, H.-Y.; Wang, N.; Lai, A.Y.-K.; Feng, Y. Edible and Herbal Plants for the Prevention and Management of COVID-19. Front. Pharmacol. 2021, 12, 656103. [Google Scholar] [CrossRef] [PubMed]
- Martonik, D.; Parfieniuk-Kowerda, A.; Rogalska, M.; Flisiak, R. The Role of Th17 Response in COVID-19. Cells 2021, 10, 1550. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 Cell’s Function in Immune System. In T Helper Cell Differentiation and Their Function; Sun, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 45–65. [Google Scholar] [CrossRef]
- Akamatsu, M.A.; Castro JT de Takano, C.Y.; Ho, P.L. Off balance: Interferons in COVID-19 lung infections. eBioMedicine 2021, 73, 103642. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Awasthi, A.; Kuchroo, V.K. Th17 cells: From precursors to players in inflammation and infection. Int. Immunol. 2009, 21, 489–498. [Google Scholar] [CrossRef]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; A Hafler, D.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kuchroo, V.K.; Awasthi, A. Emerging new roles of Th17 cells. Eur. J. Immunol. 2012, 42, 2211–2214. [Google Scholar] [CrossRef]
- Parray, H.A.; Narayanan, N.; Garg, S.; Rizvi, Z.A.; Shrivastava, T.; Kushwaha, S.; Singh, J.; Murugavelu, P.; Anantharaj, A.; Mehdi, F.; et al. A broadly neutralizing monoclonal antibody overcomes the mutational landscape of emerging SARS-CoV-2 variants of concern. PLoS Pathog. 2022, 18, e1010994. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Yuan, S.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020, 586, 113–119. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Sasaki, M.; Tabata, K.; Kishimoto, M.; Itakura, Y.; Kobayashi, H.; Ariizumi, T.; Uemura, K.; Toba, S.; Kusakabe, S.; Maruyama, Y.; et al. S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters. Sci. Transl. Med. 2022, 15, eabq4064. [Google Scholar] [CrossRef]
- Rosenke, K.; Hansen, F.; Schwarz, B.; Feldmann, F.; Haddock, E.; Rosenke, R.; Barbian, K.; Meade-White, K.; Okumura, A.; Leventhal, S.; et al. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat. Commun. 2021, 12, 2295. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yin, X.; Meng, X.; Chan, J.F.-W.; Ye, Z.-W.; Riva, L.; Pache, L.; Lai, P.-M.; Chan, C.C.-S.; Poon, V.K.-M.; et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature 2021, 593, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mehta, A.; Mehta, P.; Vyas, S.P.; Shukla, S.; Bajpai, V.K. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem. Toxicol. 2010, 48, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.-E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021, 22, 32–40. [Google Scholar] [CrossRef]
- Liu, B.; Bao, L.; Wang, L.; Li, F.; Wen, M.; Li, H.; Deng, W.; Zhang, X.; Cao, B. Anti-IFN-γ therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice. J. Microbiol. Immunol. Infect. 2021, 54, 396–403. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, Z.A.; Madan, U.; Tripathy, M.R.; Goswami, S.; Mani, S.; Awasthi, A.; Dikshit, M. Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris. Pharmaceuticals 2023, 16, 1333. https://doi.org/10.3390/ph16091333
Rizvi ZA, Madan U, Tripathy MR, Goswami S, Mani S, Awasthi A, Dikshit M. Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris. Pharmaceuticals. 2023; 16(9):1333. https://doi.org/10.3390/ph16091333
Chicago/Turabian StyleRizvi, Zaigham Abbas, Upasna Madan, Manas Ranjan Tripathy, Sandeep Goswami, Shailendra Mani, Amit Awasthi, and Madhu Dikshit. 2023. "Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris" Pharmaceuticals 16, no. 9: 1333. https://doi.org/10.3390/ph16091333
APA StyleRizvi, Z. A., Madan, U., Tripathy, M. R., Goswami, S., Mani, S., Awasthi, A., & Dikshit, M. (2023). Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris. Pharmaceuticals, 16(9), 1333. https://doi.org/10.3390/ph16091333





