STA9090 as a Potential Therapeutic Agent for Liver Fibrosis by Modulating the HSP90/TβRII/Proteasome Interplay: Novel Insights from In Vitro and In Vivo Investigations
Abstract
1. Introduction
2. Results
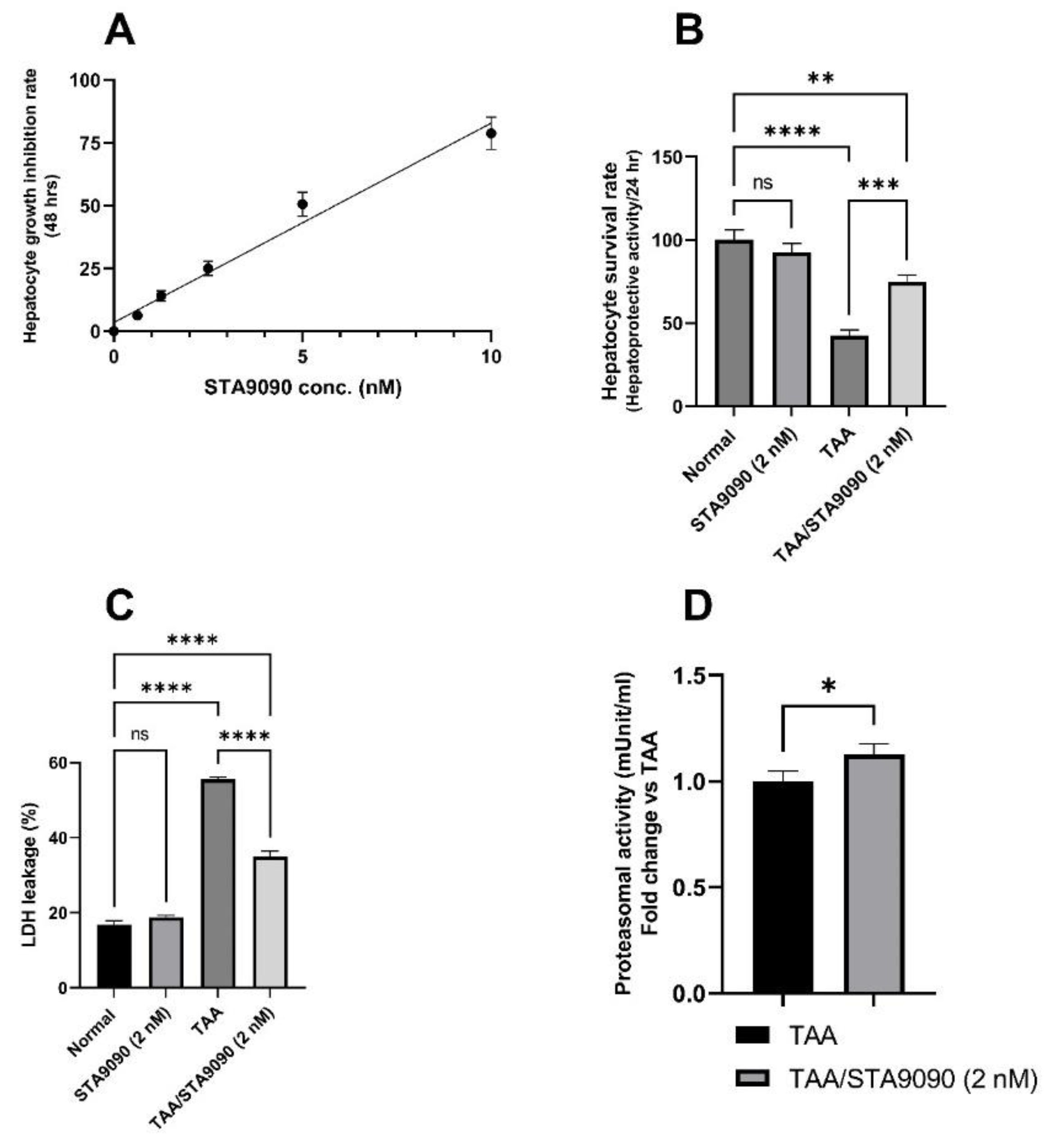
2.1. Effect of STA9090 on Cell Viability
2.2. Effect of STA9090 on Hepatocyte Survival Rate
2.3. Effect of STA9090 on LDH Release
2.4. Effect of STA9090 on 20S Proteasomal Activity (In Vitro)
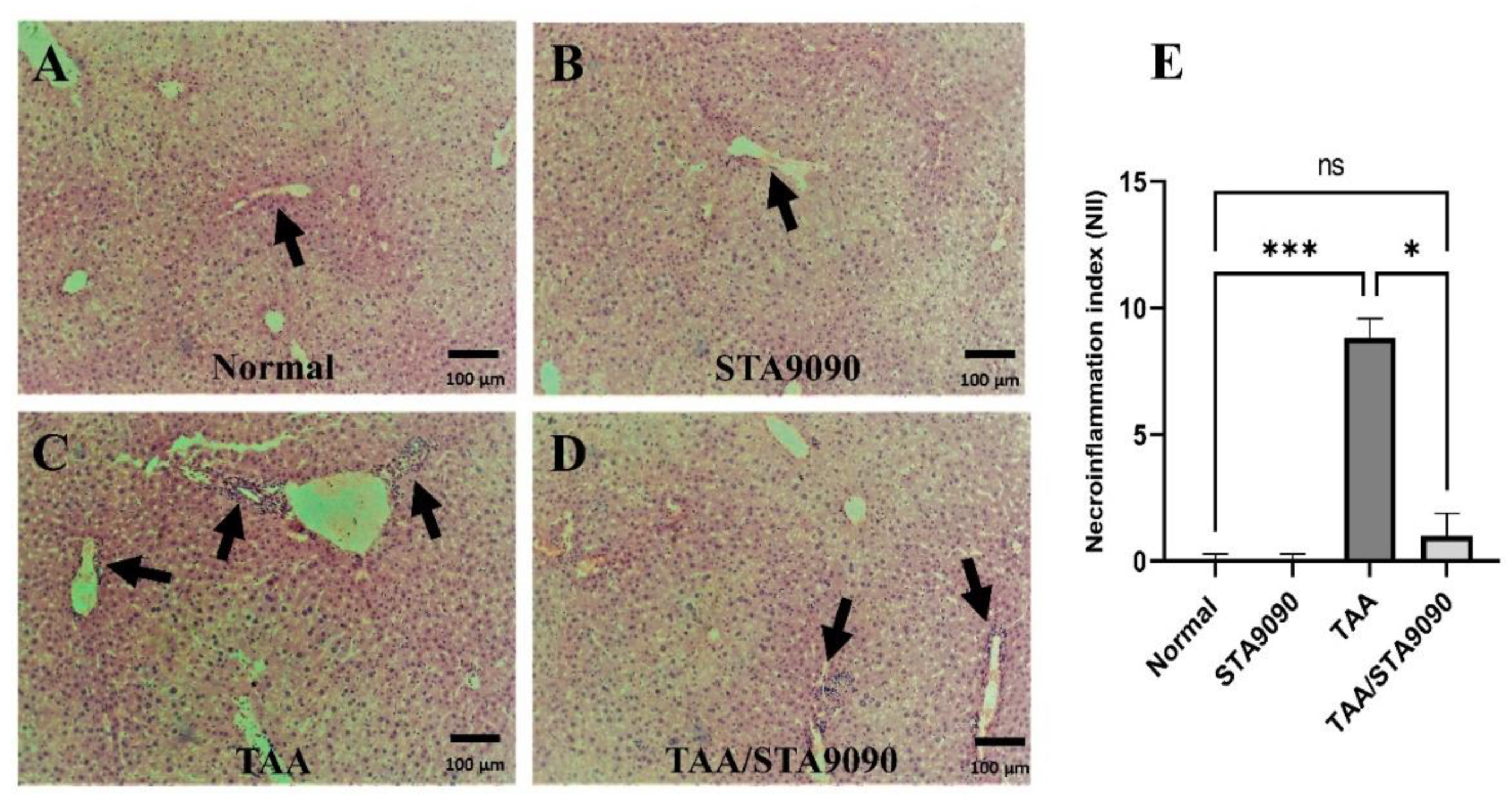
2.5. Effect of STA9090 on Histological Features and the Necro-Inflammation Index
2.6. Effect of STA9090 on the Area of Fibrosis
2.7. Effect of STA9090 on ACTA2 Immunoexpression
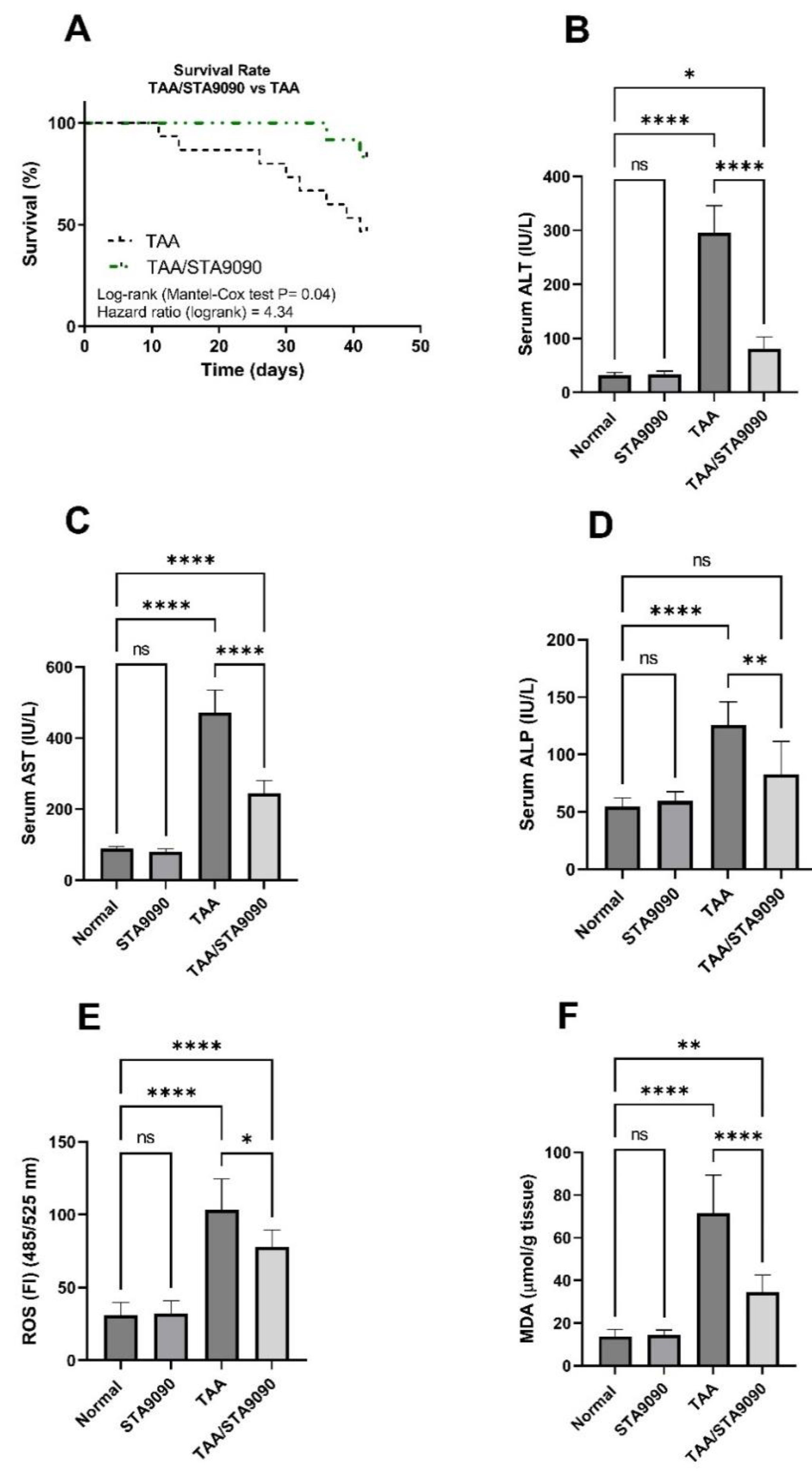
2.8. Effect of STA9090 on Survival Rate
2.9. Effect of STA9090 on Liver Function Enzymes
2.10. Effect of STA9090 on ROS and MDA levels
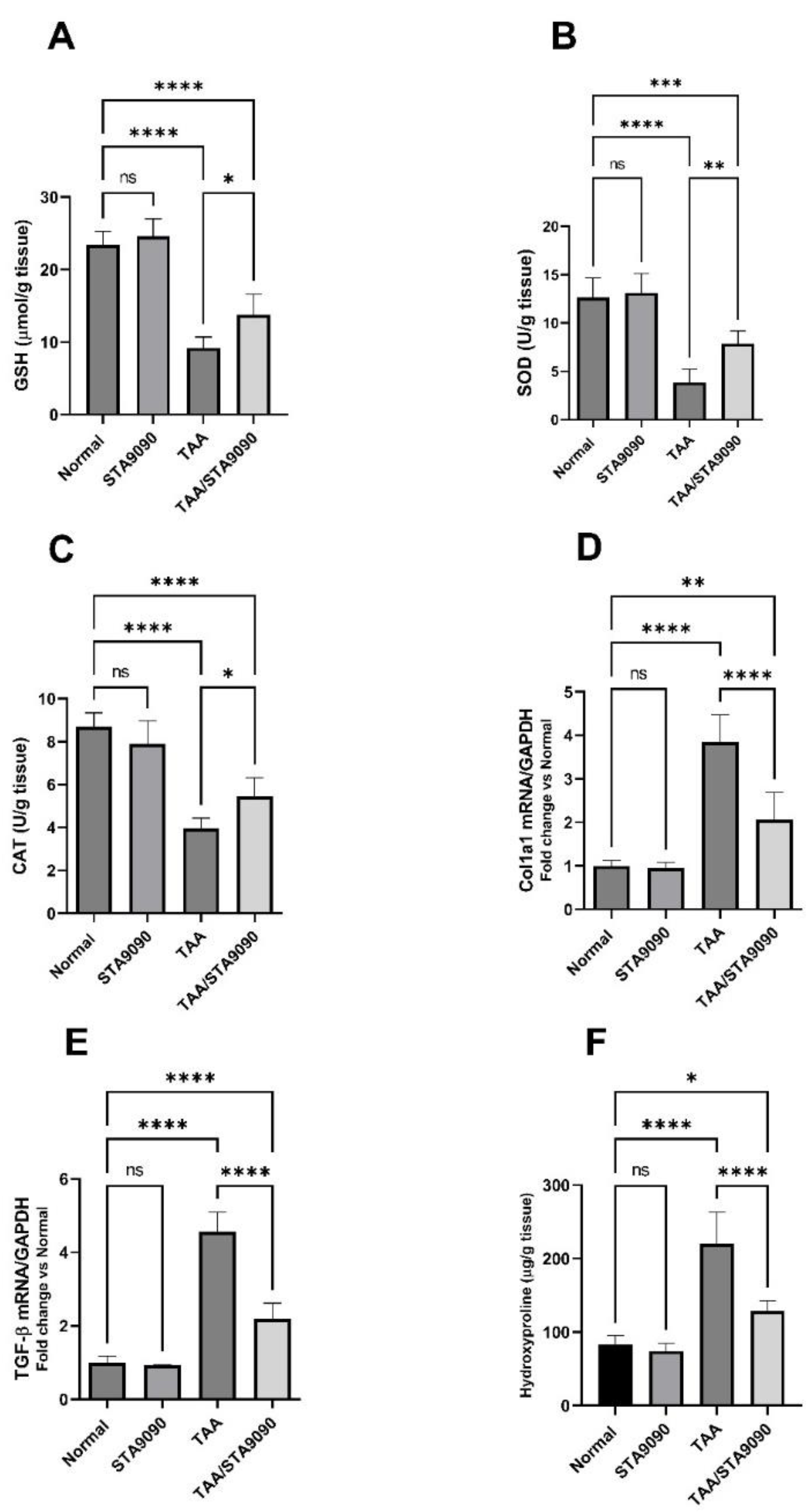
2.11. Effect of STA9090 on GSH, SOD, and CAT
2.12. Effect of STA9090 on Col1a1 and TGF-β mRNA Expression
2.13. Effect of STA9090 on Hydroxyproline Liver Content
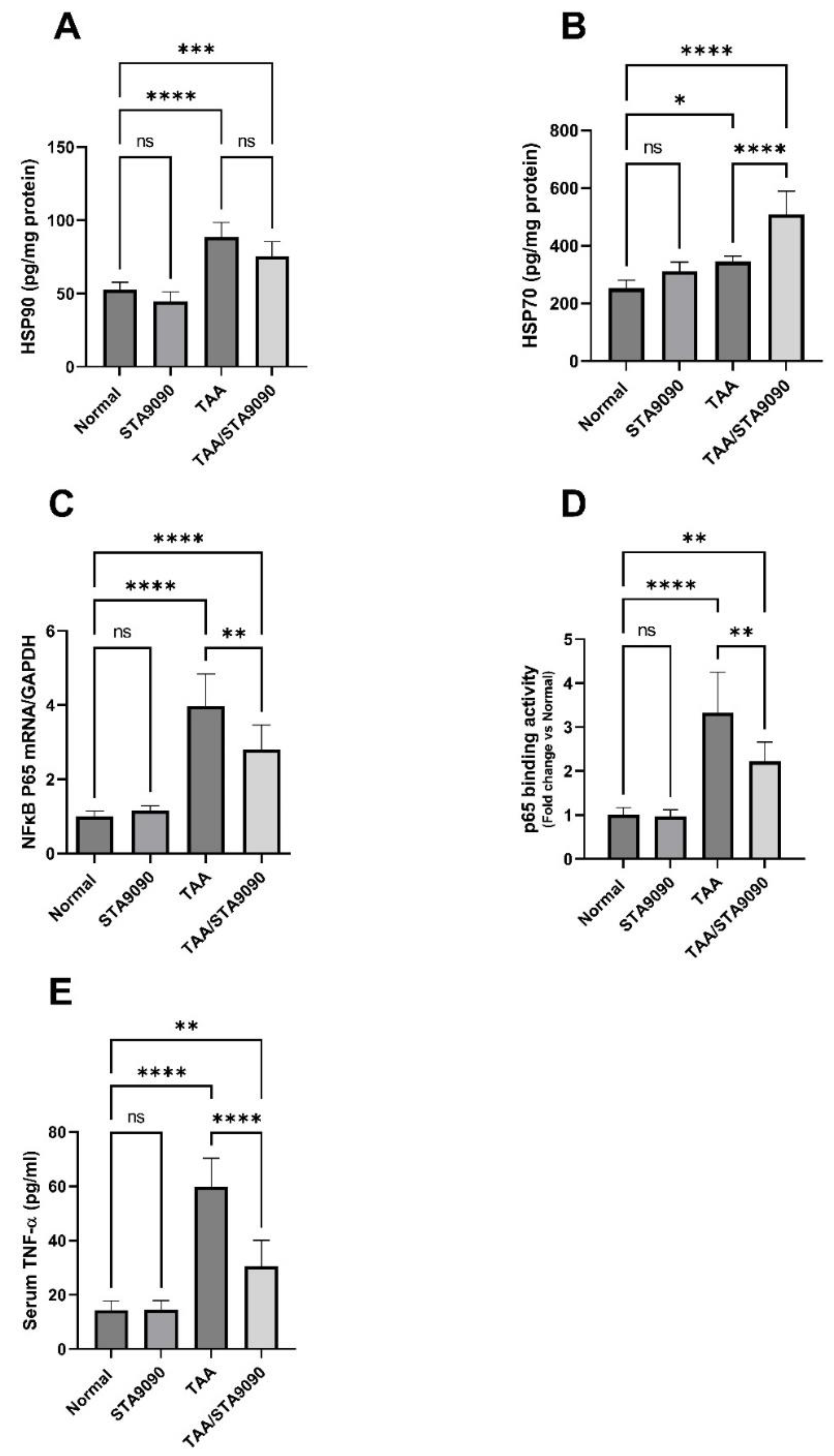
2.14. Effect of STA9090 on HSP90 and HSP70
2.15. Effect of STA9090 on NFκB p65 mRNA Expression, p65 DNA Binding Activity, and the Levels of TNF-α
2.16. Effect of STA9090 on TGF-β and the Ratio of MMP-9/TIMP-1
2.17. Effect of STA9090 on PDGF-BB, HIF-1α, and VEGF
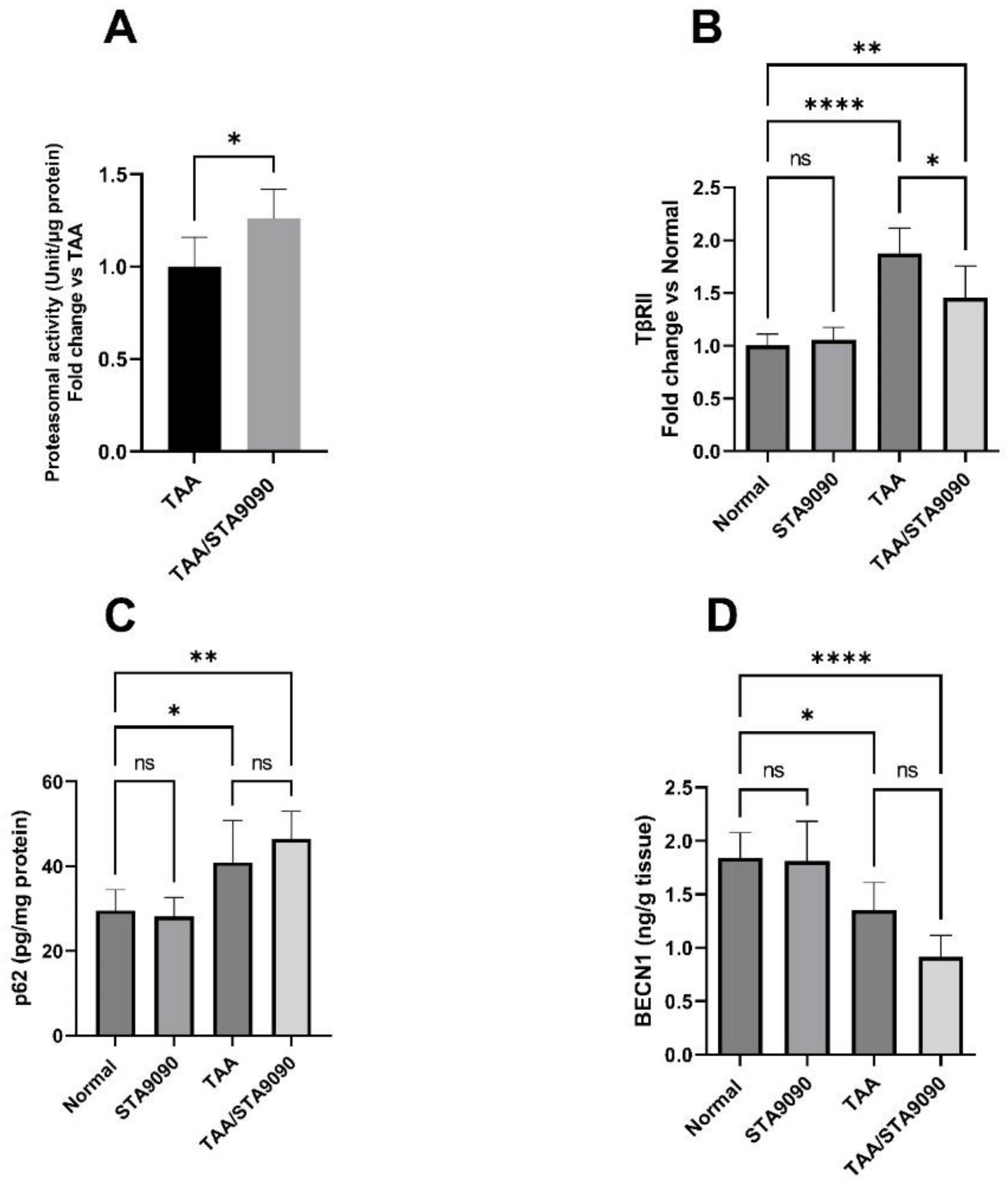
2.18. Effect of STA9090 on 20S Proteasomal Activity in the Liver Tissue
2.19. Effect of STA9090 on TβRII
2.20. Effect of STA9090 on p62 and BECN1
3. Discussion
4. Methods
4.1. Isolation of Primary Rat Hepatocytes
Assessment of Cytotoxicity and Hepatocyte Viability
4.2. Determination of the In Vitro Hepatoprotective Activity of STA9090
4.3. Determination of the Lactate Dehydrogenase (LDH) Leakage in Culture Media
4.4. Measurement of the Proteasomal Activity in Hepatocytes In Vitro
4.5. Animals
4.6. Study Design
4.7. Rational of STA9090 Dosing
4.8. H&E Staining and the Determination of the Necro-Inflammation Index (NII)
4.9. Sirius Red Staining and Determination of the Fibrosis Area %
4.10. ACTA2 Immunostaining
4.11. Survival Analysis
4.12. Determination of Liver Function and Oxidative Stress Markers
4.13. qRT-PCR for the mRNA Expression of Collagen, Type I Alpha 1 (Col1a1), TGF-β1, and NFκB p65
4.14. Determination of Hydroxyproline Hepatic Content
4.15. Measurements of Hepatic HSP90 and HSP70, p65 Binding Activity, and Serum TNF-α
4.16. Measurements of the Levels of TGF-β, MMP-9, TIMP-1, PDGF-BB, HIF-1α, and VEGF
4.17. Measurement of the Proteasomal Activity in the Liver
4.18. Measurement of TβRII, p62, and BECN1
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Trifan, A.; Muzica, C.M.; Nastasa, R.; Zenovia, S.; Stratina, E.; Stafie, R.; Rotaru, A.; Singeap, A.M.; Cojocariu, C.; Sfarti, C.; et al. High prevalence of liver fibrosis among general population: A Romanian population-based study. Hepatol. Commun. 2023, 7, e0032. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Wells, R.G. Cellular sources of extracellular matrix in hepatic fibrosis. Clin. Liver Dis. 2008, 12, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Arriazu, E.; Ruiz de Galarreta, M.; Cubero, F.J.; Varela-Rey, M.; Pérez de Obanos, M.P.; Leung, T.M.; Lopategi, A.; Benedicto, A.; Abraham-Enachescu, I.; Nieto, N. Extracellular matrix and liver disease. Antioxid. Redox Signal. 2014, 21, 1078–1097. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.E.; Cavalu, S.; Hasan, A.M.; Yahya, G.; Abd-Eldayem, M.A.; Saber, S. Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 5014. [Google Scholar]
- Shaaban, A.A.; Abdelhamid, A.M.; Shaker, M.E.; Cavalu, S.; Maghiar, A.M.; Alsayegh, A.A.; Babalghith, A.O.; El-Ahwany, E.; Amin, N.A.; Mohammed, O.A.; et al. Combining the HSP90 inhibitor TAS-116 with metformin effectively degrades the NLRP3 and attenuates inflammasome activation in rats: A new management paradigm for ulcerative colitis. Biomed. Pharmacother. 2022, 153, 113247. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; Youssef, M.E.; Nasr, M.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; El Adle Khalaf, N.; Ghaffar, D.M.A.; Jamil, L.; Abdel-Nasser, Z.M.; et al. Pimitespib, an HSP90 inhibitor, augments nifuroxazide-induced disruption in the IL-6/STAT3/HIF-1α autocrine loop in rats with bleomycin-challenged lungs: Evolutionary perspective in managing pulmonary fibrosis. Biomed. Pharmacother. 2022, 153, 113487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Huang, W.; Ge, X. The role of heat shock proteins in the regulation of fibrotic diseases. Biomed. Pharmacother. 2021, 135, 111067. [Google Scholar] [CrossRef]
- Xiong, B.; Li, M.; Xiang, S.; Han, L. A1AR-mediated renal protection against ischemia/reperfusion injury is dependent on HSP27 induction. Int. Urol. Nephrol. 2018, 50, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; Veraldi, K.L.; Thiel, K.J.; Cárdenes, N.; Alvarez, D.; Schneider, F.; Pilewski, J.M.; Rojas, M.; Feghali-Bostwick, C.A. Intracellular Heat Shock Protein 70 Deficiency in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 60, 629–636. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, T.; Liu, X.; Zhang, L.; Yang, Y.; Zhang, J.; Guο, M. Heat shock protein 70 protects rat peritoneal mesothelial cells from advanced glycation end-products-induced epithelial-to-mesenchymal transition through mitogen-activated protein kinases/extracellular signal-regulated kinases and transforming growth factor-β/Smad pathways. Mol. Med. Rep. 2015, 11, 4473–4481. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.-F.; Li, Y.-Y.; Xue, F.-T.; Zhang, S. Decreased HSP70 expression on serum exosomes contributes to cardiac fibrosis during senescence. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3993–4001. [Google Scholar] [PubMed]
- Sun, X.; Zhang, X.-D.; Cheng, G.; Hu, Y.-H.; Wang, H.-Y. Inhibition of hepatic stellate cell proliferation by heat shock protein 90 inhibitors in vitro. Mol. Cell. Biochem. 2009, 330, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Bellaye, P.-S.; Burgy, O.; Causse, S.; Garrido, C.; Bonniaud, P. Heat shock proteins in fibrosis and wound healing: Good or evil? Pharmacol. Ther. 2014, 143, 119–132. [Google Scholar] [CrossRef]
- Taguchi, T.; Nazneen, A.; Al-Shihri, A.A.; Turkistani, K.A.; Razzaque, M.S. Heat Shock Protein 47: A Novel Biomarker of Phenotypically Altered Collagen-Producing Cells. Acta Histochem. Cytochem. 2011, 44, 35–41. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. Hsp90 inhibition and modulation of the proteome: Therapeutical implications for idiopathic pulmonary fibrosis (ipf). Int. J. Mol. Sci. 2020, 21, 5286. [Google Scholar] [PubMed]
- Sontake, V.; Wang, Y.; Kasam, R.K.; Sinner, D.; Reddy, G.B.; Naren, A.P.; McCormack, F.X.; White, E.S.; Jegga, A.G.; Madala, S.K. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2017, 2, e91454. [Google Scholar]
- Sharma, K.; Vabulas, R.M.; Macek, B.; Pinkert, S.; Cox, J.; Mann, M.; Hartl, F.U.J.M. Quantitative proteomics reveals that Hsp90 inhibition preferentially targets kinases and the DNA damage response. Mol. Cell. Proteom. 2012, 11, 3. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Fernandez-Garcia, C.E.; Gomez-Guerrero, C.; Lopez-Franco, O.; Munoz-Garcia, B.; Egido, J.; Blanco-Colio, L.M.; Martin-Ventura, J.L. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc. Res. 2012, 95, 116–123. [Google Scholar] [CrossRef]
- Wyler, E.; Mösbauer, K.; Franke, V.; Diag, A.; Gottula, L.T.; Arsiè, R.; Klironomos, F.; Koppstein, D.; Hönzke, K.; Ayoub, S. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. IScience 2021, 24, 102151. [Google Scholar] [CrossRef]
- Chakraborty, A.; Boel, N.M.; Edkins, A.L. HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation. Cells 2020, 9, 272. [Google Scholar] [CrossRef]
- Coux, O.; Tanaka, K.; Goldberg, A.L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996, 65, 801–847. [Google Scholar] [CrossRef]
- Tanahashi, N.; Tsurumi, C.; Tamura, T.; Tanaka, K. Molecular structure of 20S and 26S proteasomes. Enzym. Protein 1993, 47, 241–251. [Google Scholar] [CrossRef]
- Huang, F.; Chen, Y.-G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, J.; Liang, T.; Feng, X.-H. To Ub or not to Ub: A regulatory question in TGF-β signaling. Trends Biochem. Sci. 2022, 47, 1059–1072. [Google Scholar] [CrossRef]
- Jhaveri, K.; Modi, S. Ganetespib: Research and clinical development. OncoTargets Ther. 2015, 8, 1849–1858. [Google Scholar] [CrossRef]
- Ying, W.; Du, Z.; Sun, L.; Foley, K.P.; Proia, D.A.; Blackman, R.K.; Zhou, D.; Inoue, T.; Tatsuta, N.; Sang, J.; et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012, 11, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Chandarlapaty, S.; Lake, D.; Gilewski, T.; Robson, M.; Goldfarb, S.; Drullinsky, P.; Sugarman, S.; Wasserheit-Leiblich, C.; Fasano, J.; et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin. Breast Cancer 2014, 14, 154–160. [Google Scholar] [CrossRef]
- Goyal, L.; Wadlow, R.C.; Blaszkowsky, L.S.; Wolpin, B.M.; Abrams, T.A.; McCleary, N.J.; Sheehan, S.; Sundaram, E.; Karol, M.D.; Chen, J.; et al. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Investig. New Drugs 2015, 33, 128–137. [Google Scholar] [CrossRef]
- Goldman, J.W.; Raju, R.N.; Gordon, G.A.; Vukovic, V.M.; Bradley, R.; Rosen, L.S. A phase I dose-escalation study of the Hsp90 inhibitor STA-9090 administered once weekly in patients with solid tumors. J. Clin. Oncol. 2010, 28, 2529. [Google Scholar] [CrossRef]
- Taldone, T.; Gozman, A.; Maharaj, R.; Chiosis, G. Targeting Hsp90: Small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol. 2008, 8, 370–374. [Google Scholar] [CrossRef]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Lin, W.L.; Lin, T.C.; Liao, H.J.; Lu, Y.W. The Ameliorative Effects of Saikosaponin in Thioacetamide-Induced Liver Injury and Non-Alcoholic Fatty Liver Disease in Mice. Int. J. Mol. Sci. 2021, 22, 11383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Ding, C.; Gu, Y.; Liu, W. Dihydromyricetin Reverses Thioacetamide-Induced Liver Fibrosis Through Inhibiting NF-κB-Mediated Inflammation and TGF-β1-Regulated of PI3K/Akt Signaling Pathway. Front. Pharmacol. 2021, 12, 783886. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y.; Ye, J.; Ying, W.; Ogawa, L.S.; Inoue, T.; Tatsuta, N.; Wada, Y.; Koya, K.; Huang, Q.; et al. A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol. Appl. Pharmacol. 2013, 273, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hasan Khudhair, D.; Al-Gareeb, A.I.; Al-kuraishy, H.M.; El-Kadem, A.H.; Elekhnawy, E.; Negm, W.A.; Saber, S.; Cavalu, S.; Tirla, A.; Alotaibi, S.S.; et al. Combination of Vitamin C and Curcumin Safeguards against Methotrexate-Induced Acute Liver Injury in Mice by Synergistic Antioxidant Effects. Front. Med. 2022, 9, 866343. [Google Scholar] [CrossRef]
- Cavalu, S.; Sharaf, H.; Saber, S.; Youssef, M.E.; Abdelhamid, A.M.; Mourad, A.A.E.; Ibrahim, S.; Allam, S.; Elgharabawy, R.M.; El-Ahwany, E.; et al. Ambroxol, a mucolytic agent, boosts HO-1, suppresses NF-κB, and decreases the susceptibility of the inflamed rat colon to apoptosis: A new treatment option for treating ulcerative colitis. FASEB J. 2022, 36, e22496. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Yahya, G.; Gobba, N.A.; Sharaf, H.; Alshaman, R.; Alattar, A.; Amin, N.A.; El-Shedody, R.; Aboutouk, F.H.; Abd El-Galeel, Y.; et al. The Supportive Role of NSC328382, a P2X7R Antagonist, in Enhancing the Inhibitory Effect of CRID3 on NLRP3 Inflammasome Activation in Rats with Dextran Sodium Sulfate-Induced Colitis. J. Inflamm. Res. 2021, 14, 3443–3463. [Google Scholar] [CrossRef]
- Dijk, F.; Olinga, P.; Poelstra, K.; Beljaars, L. Targeted therapies in liver fibrosis: Combining the best parts of platelet derived growth factor BB and interferon gamma. Front. Med. 2015, 2, 72. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, Q.; Liu, Z.; Liu, Q.; Deng, X. Hepatic stellate cells produce vascular endothelial growth factor via phospho-p44/42 mitogen-activated protein kinase/cyclooxygenase-2 pathway. Mol. Cell. Biochem. 2012, 359, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- van de Sluis, B.; Groot, A.J.; Vermeulen, J.; van der Wall, E.; van Diest, P.J.; Wijmenga, C.; Klomp, L.W.; Vooijs, M. COMMD1 Promotes pVHL and O2-Independent Proteolysis of HIF-1alpha via HSP90/70. PLoS ONE 2009, 4, e7332. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.M.; Youssef, M.E.; Abd El-Fattah, E.E.; Gobba, N.A.; Gaafar, A.G.A.; Girgis, S.; Shata, A.; Hafez, A.-M.; El-Ahwany, E.; Amin, N.A.; et al. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci. 2021, 286, 120070. [Google Scholar] [CrossRef]
- Marxsen, J.H.; Stengel, P.; Doege, K.; Heikkinen, P.; Jokilehto, T.; Wagner, T.; Jelkmann, W.; Jaakkola, P.; Metzen, E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem. J. 2004, 381, 761–767. [Google Scholar] [CrossRef]
- Saber, S.; Nasr, M.; Saad, A.S.; Mourad, A.A.E.; Gobba, N.A.; Shata, A.; Hafez, A.-M.; Elsergany, R.N.; Elagamy, H.I.; El-Ahwany, E.; et al. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: A new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biomed. Pharmacother. 2021, 142, 112029. [Google Scholar] [CrossRef]
- Saber, S.; Mahmoud, A.; Helal, N.; El-Ahwany, E.; Abdelghany, R. Liver Protective Effects of Renin-Angiotensin System Inhibition Have No Survival Benefits in Hepatocellular Carcinoma Induced by Repetitive Administration of Diethylnitrosamine in Mice. Open Access Maced. J. Med. Sci. 2018, 6, 955–960. [Google Scholar] [CrossRef]
- Khalil, R.; Shata, A.; Abd El-Kader, E.M.; Sharaf, H.; Abdo, W.S.; Amin, N.A.; Saber, S. Vildagliptin, a DPP-4 inhibitor, attenuates carbon tetrachloride-induced liver fibrosis by targeting ERK1/2, p38α, and NF-κB signaling. Toxicol. Appl. Pharmacol. 2020, 407, 115246. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Z.; Niaz, Q.; Saffari, P.M.; Dehpour, A.R.; Rezayat, S.M.; Jazaeri, F. Evaluation of the effect of heat shock protein 70 targeted drugs on cirrhotic cardiomyopathy in biliary cirrhotic rats. Life Sci. 2021, 273, 119261. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, M.; Cervello, M.; Montalto, G.; Cappello, F.; Gulino, A.; Knape, C.; Specht, H.M.; Multhoff, G. Heat shock protein 70 serum levels differ significantly in patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Front. Immunol. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Hasegawa, H.; Sasaki, D.; Ando, K.; Sawayama, Y.; Imaizumi, Y.; Imanishi, D.; Taguchi, J.; Hata, T.; Tsukasaki, K.; et al. Heat Shock Protein 90 Inhibitor NVP-AUY922 Has Potent Anti-Tumor Activity with Adult T-Cell Leukemia-Lymphoma Cells. Blood 2013, 122, 1829. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Lam, K.-K.; Peng, Y.-J.; Lee, Y.-M.; Yang, C.-Y.; Tsai, Y.-J.; Yen, M.-H.; Cheng, P.-Y. Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J. Cell. Mol. Med. 2016, 20, 1889–1897. [Google Scholar] [CrossRef]
- Saber, S.; Goda, R.; El-Tanbouly, G.S.; Ezzat, D. Lisinopril inhibits nuclear transcription factor kappa B and augments sensitivity to silymarin in experimental liver fibrosis. Int. Immunopharmacol. 2018, 64, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Scutigliani, E.M.; Liang, Y.; IJff, M.; Rodermond, H.; Mei, X.; Korver, M.P.; Orie, V.S.; Hoebe, R.A.; Picavet, D.I.; Oei, A. Evaluation of the Heat Shock Protein 90 Inhibitor Ganetespib as a Sensitizer to Hyperthermia-Based Cancer Treatments. Cancers 2022, 14, 5250. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.M.; Saber, S.; Youssef, M.E.; Gaafar, A.G.A.; Eissa, H.; Abd-Eldayem, M.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Shahien, M.A.; et al. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: Emerging approach for new application. Biomed. Pharmacother. 2022, 145, 112455. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef]



| Exp. Groups | 6 Weeks |
|---|---|
| Normal (n = 8) | - |
| STA9090 (n = 8) | STA9090 (20 mg/kg/every other day, i.p.) |
| TAA (n = 15) | TAA (150 mg/kg twice a week, i.p.) |
| TAA/STA9090 (n = 12) | TAA (150 mg/kg twice a week, i.p.) and STA9090 (20 mg/kg/every other day, i.p.) |
| Gene | GenBank Accession | F | R | Amplicon Size (bp) |
|---|---|---|---|---|
| Col1a1 | NM_053304.1 | 5′-GACATGTTCAGCTTTGTGGACCC-3′ | 5′-AGGGACCCTTAGGCCATTGTGTA-3′ | 120 |
| TGF-β | NM_021578.2 | 5′-CTTCTCCACCAACTACTGCTTC-3′ | 5′-GGGTCCCAGGCAGAAGTT-3′ | 139 |
| NFκB P65 | NM_199267.2 | 5′-TTCCCTGAAGTGGAGCTAGGA-3′ | 5′-CATGTCGAGGAAGACACTGGA-3′ | 185 |
| GAPDH | NM_017008.4 | 5′-TCAAGAAGGTGGTGAAGCAG-3′ | 5′-AGGTGGAAGAATGGGAGTTG-3′ | 111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, O.A.; Abdel-Reheim, M.A.; Alamri, M.M.S.; Alfaifi, J.; Adam, M.I.E.; Saleh, L.A.; Farrag, A.A.; Yahia, A.I.O.; Abdel-Ghany, S.; AlQahtani, A.A.J.; et al. STA9090 as a Potential Therapeutic Agent for Liver Fibrosis by Modulating the HSP90/TβRII/Proteasome Interplay: Novel Insights from In Vitro and In Vivo Investigations. Pharmaceuticals 2023, 16, 1080. https://doi.org/10.3390/ph16081080
Mohammed OA, Abdel-Reheim MA, Alamri MMS, Alfaifi J, Adam MIE, Saleh LA, Farrag AA, Yahia AIO, Abdel-Ghany S, AlQahtani AAJ, et al. STA9090 as a Potential Therapeutic Agent for Liver Fibrosis by Modulating the HSP90/TβRII/Proteasome Interplay: Novel Insights from In Vitro and In Vivo Investigations. Pharmaceuticals. 2023; 16(8):1080. https://doi.org/10.3390/ph16081080
Chicago/Turabian StyleMohammed, Osama A., Mustafa Ahmed Abdel-Reheim, Mohannad Mohammad S. Alamri, Jaber Alfaifi, Masoud I. E. Adam, Lobna A. Saleh, Alshaimaa A. Farrag, Amar Ibrahim Omer Yahia, Sameh Abdel-Ghany, AbdulElah Al Jarallah AlQahtani, and et al. 2023. "STA9090 as a Potential Therapeutic Agent for Liver Fibrosis by Modulating the HSP90/TβRII/Proteasome Interplay: Novel Insights from In Vitro and In Vivo Investigations" Pharmaceuticals 16, no. 8: 1080. https://doi.org/10.3390/ph16081080
APA StyleMohammed, O. A., Abdel-Reheim, M. A., Alamri, M. M. S., Alfaifi, J., Adam, M. I. E., Saleh, L. A., Farrag, A. A., Yahia, A. I. O., Abdel-Ghany, S., AlQahtani, A. A. J., Bahashwan, E., Eltahir, H. B., Mohammed, N. A., El-wakeel, H. S., Hazem, S. H., & Saber, S. (2023). STA9090 as a Potential Therapeutic Agent for Liver Fibrosis by Modulating the HSP90/TβRII/Proteasome Interplay: Novel Insights from In Vitro and In Vivo Investigations. Pharmaceuticals, 16(8), 1080. https://doi.org/10.3390/ph16081080







