Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
1. Introduction
2. Results
2.1. Screenings and Dropout Characteristics
2.2. Patients
2.3. Primary Outcomes
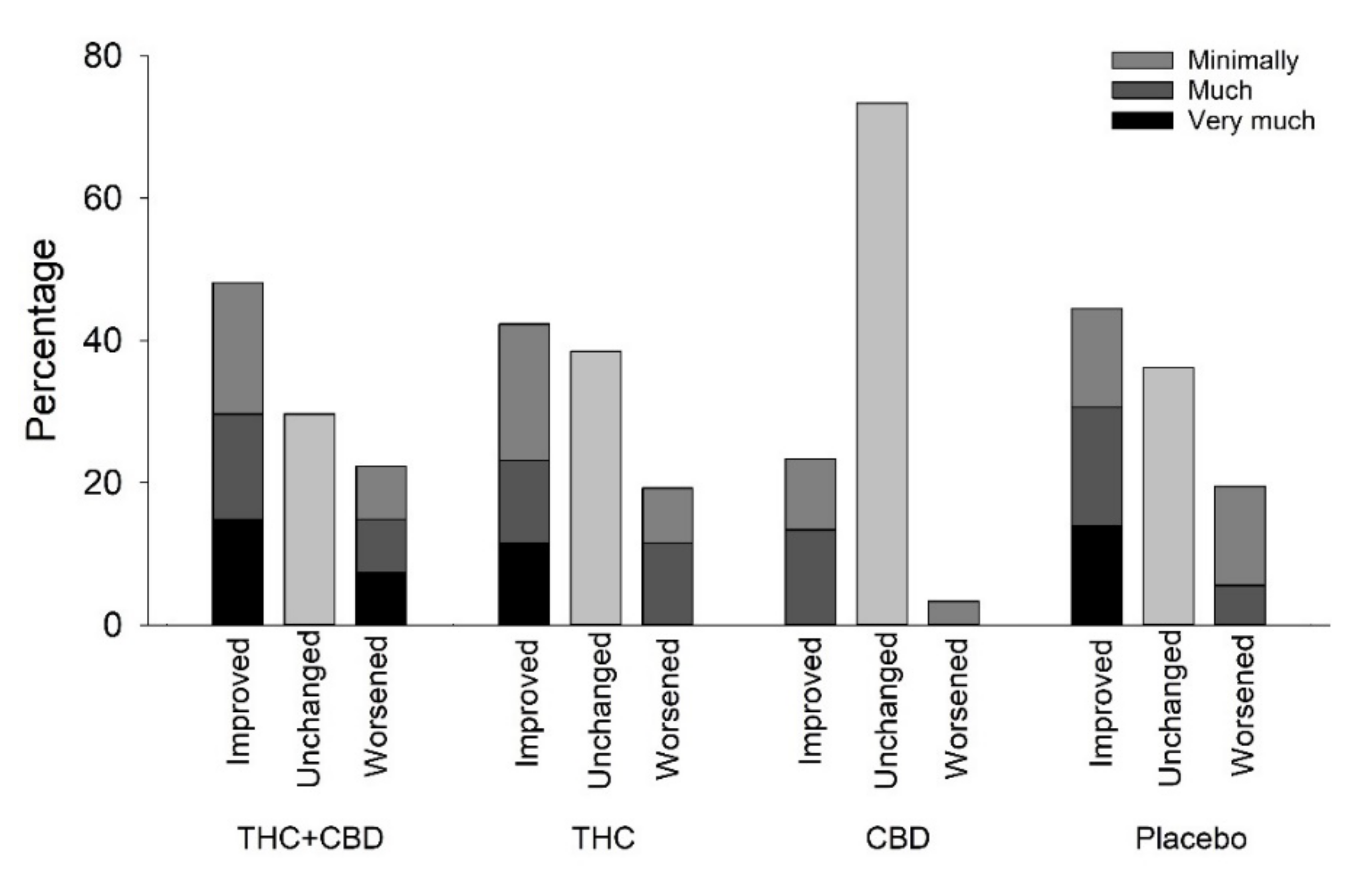
Pain
2.4. Spasticity
2.5. Supplement Analysis and Results
2.6. Secondary Outcomes
2.7. Other Outcomes
2.8. Daily Dose and Blinding Assessment
2.9. Expectation
2.10. Adverse Events and Safety
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Study Patients
4.3. Study Visits
4.4. Randomization and Blinding
4.5. Intervention
4.6. Outcome Measures
4.7. Clinical Laboratory Tests
4.8. Power and Sample Size Considerations
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andresen, S.R.; Hagen, E.M.; Biering-Sørensen, F.; Bach, F.W.; Finnerup, N.B.; Nielsen, J.F. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord 2016, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.B.; Jensen, T.S.; Hansen, H.J.; Bach, F.W. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain 2005, 114, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; van der Stelt, M.; Centonze, D.; Maccarrone, M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Prog. Neurobiol. 2018, 160, 82–100. [Google Scholar] [CrossRef]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Da Rovare, V.P.; Magalhães, G.P.A.; Jardini, G.D.A.; Beraldo, M.L.; Gameiro, M.O.; Agarwal, A.; Luvizutto, G.J.; Paula-Ramos, L.; Camargo, S.E.A.; de Oliveira, L.D.; et al. Cannabinoids for spasticity due to multiple sclerosis or paraplegia: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2017, 34, 170–185. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA-J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Nielsen, S.; Germanos, R.; Weier, M.; Pollard, J.; Degenhardt, L.; Hall, W.; Buckley, N.; Farrell, M. The Use of Cannabis and Cannabinoids in Treating Symptoms of Multiple Sclerosis: A Systematic Review of Reviews. Curr. Neurol. Neurosci. Rep. 2018, 18, 8. [Google Scholar] [CrossRef]
- IASP Presidential Task Force on Cannabis and Cannabinoid Analgesia. International Association for the Study of Pain presidential task force on cannabis and cannabinoid analgesia position statement. Pain 2021, 162, S1–S2. [Google Scholar]
- The Danish Sclerosis Association. Cannabis Som Medicin Blandt Mennesker Med Sclerose—Holdninger, Erfaringer Og Barrierer; Scleroseforeningen: København, Danmark, 2019. [Google Scholar]
- Pertwee, R.G. Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014; ISBN 9780199662685 0199662681. [Google Scholar]
- Fisher, E.; Moore, R.A.; Fogarty, A.E.; Finn, D.P.; Finnerup, N.B.; Gilron, I. Cannabinoids, cannabis, and cannabis-based medicine for pain management: A systematic review of randomised controlled trials. Pain 2020, 162, S45–S66. [Google Scholar] [CrossRef]
- Hansen, J.S.; Hansen, R.M.; Petersen, T.; Gustavsen, S.; Oturai, A.B.; Sellebjerg, F.; Sædder, E.A.; Kasch, H.; Rasmussen, P.V.; Finnerup, N.B.; et al. The effect of cannabis-based medicine on neuropathic pain and spasticity in patients with multiple sclerosis and spinal cord injury: Study protocol of a national multicenter double-blinded, placebo-controlled trial. Brain Sci. 2021, 11, 1212. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- The Danish Medicine Agency Medicinal Cannabis Pilot Programme. Available online: https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis-/medicinal-cannabis-pilot-programme/ (accessed on 1 April 2023).
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; de Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Horsted, T.; Hesthaven, K.L.; Leutscher, P.D.C. Safety and effectiveness of cannabinoids to Danish patients with treatment refractory chronic pain—A retrospective observational real-world study. Eur. J. Pain 2022, 27, 234–247. [Google Scholar] [CrossRef]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, K.; Petersen, M.; Bach, F.W.; Heinesen, A.; Enggaard, T.P.; Almdal, T.P.; Holbech, J.V.; Vase, L.; Jensen, T.S.; Hansen, C.S.; et al. Oral capsules of tetra-hydro-cannabinol (THC), cannabidiol (CBD) and their combination in peripheral neuropathic pain treatment. Eur. J. Pain 2022, 27, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Nurmikko, T.J.; Serpell, M.G.; Hoggart, B.; Toomey, P.J.; Morlion, B.J.; Haines, D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007, 133, 210–220. [Google Scholar] [CrossRef]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef]
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. 2020, 41, 3085–3098. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef]
- Filippini, G.; Minozzi, S.; Borrelli, F.; Cinquini, M.; Dwan, K. Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis (Review). Cochrane Database Syst. Rev. 2022, 5, 1–101. [Google Scholar] [CrossRef]
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296. [Google Scholar] [CrossRef]
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro\, L.; Comi, G.; et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef]
- Ferrè, L.; Nuara, A.; Pavan, G.; Radaelli, M.; Moiola, L.; Rodegher, M.; Colombo, B.; Keller Sarmiento, I.J.; Martinelli, V.; Leocani, L.; et al. Efficacy and safety of nabiximols (Sativex®) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol. Sci. 2016, 37, 235–242. [Google Scholar] [CrossRef]
- Gedin, F.; Blomé, S.; Pontén, M.; Lalouni, M.; Fust, J.; Raquette, A.; Vadenmark Lundquist, V.; Thompson, W.H.; Jensen, K. Placebo Response and Media Attention in Randomized Clinical Trials Assessing Cannabis-Based Therapies for Pain: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2243848. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef]
- Müller-vahl, K.R.; Jakubovski, E.; Fremer, C.; Lenz-ziegenbein, M.; Großhennig, A.; Klages, C.; Koch, A.; Haas, M.; Pisarenko, A. Implications for blinding in clinical trials with THC-containing cannabinoids based on the CANNA-TICS trial. Front. Neurosci. 2022, 16, 793703. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; Neal, L.O.; Mcleod, L.; Delacqua, G.; Delacqua, F.; Kirby, J. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- EuroQol org EuroQol Group 5Q-5D-5L. Available online: https://euroqol.org/ (accessed on 25 September 2022).
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Baunsgaard, C.B.; Nissen, U.V.; Christensen, K.B.; Biering-Sørensen, F. Modified Ashworth scale and spasm frequency score in spinal cord injury: Reliability and correlation. Spinal Cord 2016, 54, 702–708. [Google Scholar] [CrossRef]
- Health Measures, N.Q. PROMIS. Available online: https://www.healthmeasures.net/index.php?option=com_content&view=category&layout=blog&id=148&Itemid=822 (accessed on 2 September 2022).
- Tombaugh, T.N. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch. Clin. Neuropsychol. 2006, 21, 53–76. [Google Scholar] [CrossRef]
- Cleynhens, K.; D’hooghe, M.B.; D’hooge, M.; De Keyser, J.; Nagels, G.; Haelewyck, M.C.; Van Schependom, J. The Symbol Digit Modalities Test as sentinel test for cognitive impairment in multiple sclerosis. Eur. J. Neurol. 2014, 21, 1219–1225. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Goodkin, D.E.; Hertsgaard, D.; Seminary, J. Upper extremity Function in Multiple Sclerosis: Improving Assessment Sensitivity With Box -and-Block and Nine-Hole Peg Test. Arch. Phys. Med. Rehabil. 1988, 69, 850–854. [Google Scholar]

| Placebo | THC | CBD | THC&CBD | |
|---|---|---|---|---|
| Total No. | 40 | 32 | 31 | 31 |
| Diagnosis | ||||
| MS, n (%) | 34 (85.0) | 29 (90.6) | 26 (83.9) | 30 (96.8) |
| SCI, n (%) | 6 (15) | 3 (9.4) | 5 (16.1) | 1 (3.2) |
| Sex | ||||
| Female (n = 99), n (%) | 28 (70.0) | 21 (65.5) | 22 (71.0) | 28 (90.3) |
| Male (n = 35), n (%) | 12 (30.0) | 11 (34.4) | 9 (29.0) | 3 (9.7) |
| Age at randomization | ||||
| Years, mean (SD) range | 52.2 (10.4) 21–70 | 54.1 (10.4) 30–69 | 53.0 (9.8) 30–73 | 51.1 (12.7) 30–84 |
| Time since diagnosis (MS/SCI) | ||||
| Years, mean (SD) range | 13.3 (7.9) 1–32 | 14.8 (11.8) 0–38 | 13.7 (8.2) 1–31 | 11.5 (7.3) 0–30 |
| BMI kg/m2, mean (SD) | 25.5 (5.1) | 26.4 (6.0) | 26.8 (5.1) | 26.0 (5.6) |
| Patients with pain >3, ≤9 at baseline, | ||||
| n (%) (total n = 114) NRS0-10 | 35 (87.5) | 24 (75.0) | 27 (87.1) | 28 (90.3) |
| Pain at baseline mean (SD) | 6.0 (1.3) | 6.6 (1.7) | 6.0 (1.4) | 5.7 (1.4) |
| Patients with spasticity >3 on NRS0-10 at baseline, n (%) (total n = 100) Spasticity at baseline mean (SD) | 29 (72.5) 5.9 (1.4) | 25 (78.1) 5.7 (1.6) | 26 (83.9) 5.9 (1.4) | 20 (64.5) 5.7 (1.5) |
| EDSS (MS) median (IQR) | 4.5 (3.0–6.0) | 6.0 (3.5–6.0) | 6.0 (5.0–6.5) | 4.5 (3.0–6.0) |
| AIS (SCI) | ||||
| A | 1 | - | 2 | - |
| B | 1 | 2 | - | - |
| C | 2 | - | - | - |
| D | 2 | 1 | 3 | 1 |
| Level of lesion | ||||
| Cervical | 3 | 1 | 1 | - |
| Thoracal | 3 | 2 | 3 | 1 |
| Lumbar | - | - | 1 | - |
| SCI type | ||||
| Traumatic | 1 | 1 | 2 | - |
| Non-traumatic | 2 | 2 | 1 | 1 |
| Other/Unknown | 3 | - | 2 | - |
| Other medication | ||||
| Gabapentin/pregabalin | 11 | 10 | 7 | 9 |
| SNRI | 2 | 0 | 4 | 3 |
| TCA | 2 | 4 | 1 | 1 |
| Other pain treatment | 24 | 23 | 18 | 20 |
| Antispastics | 17 | 14 | 12 | 14 |
| Placebo | THC | CBD | THC&CBD | |
|---|---|---|---|---|
| Total n | 40 | 32 | 31 | 31 |
| Impact on sleep (NRS0-10) | ||||
| Baseline, mean (SD) | 4.7 (2.2) | 4.8 (2.4) | 4.9 (2.0) | 3.9 (2.3) |
| NPSI (n = 101 *) | (n = 31) | (n = 20) | (n = 26) | (n = 24) |
| The total sum, median (IQR) | 2.6 (1.6–3.9) | 2.5 (1.7–4.8) | 3.5 (2.2–4.7) | 2.7 (2.0–4.9) |
| Burning pain, median (IQR) | 5.0 (2.0–6.0) | 5.0 (0.5–7.5) | 5.0 (0.0–6.0) | 5.0 (0.0–7.0) |
| Pressing pain, median (IQR) | 3.0 (0.0–5.0) | 3.5 (1.5–7.0) | 4.3 (2.0–7.0) | 3.8 (1.8–5.5) |
| Pins and needles/tingling, median (IQR) | 2.5 (0.0–5.0) | 2.5 (0.0–4.0) | 3.0 (2.0–6.5) | 2.5 (0.0–5.8) |
| Evoked pain, median (IQR) | 1.7 (0.0–3.0) | 1.8 (0.0–3.2) | 3.0 (0.0–5.0) | 2.2 (0.0–4.8) |
| Paresthesia/dysesthesia, median (IQR) | 2.5 (0.0–5.0) | 2.5 (0.0–6.3) | 3.5 (2.0–5.0) | 2.5 (0.5–5.5) |
| The expectation of relief NRS0-10 § | ||||
| Pain * (n = 111), median (IQR) | 8.0 (7.0–10.0) | 8.0 (5.5–9.0) | 8.0 (5.0–10.0) | 8.0 (7.0–10.0) |
| Spasticity ** (n = 99), median (IQR) | 8.0 (6.0–10.0) | 8.0 (6.0–8.0) | 8.0 (6.0–10.0) | 8.0 (7.0–9.0) |
| Quality of life 5Q-5L-5D | ||||
| Index, mean (SD) | 0.6 (0.2) | 0.6 (0.2) | 0.5 (0.2) | 0.6 (0.2) |
| 9-Hole Peg Test (s) | (n = 36/34) | (n = 28/29) | (n = 29/28) | (n = 29/27) |
| Dom hand, median (IQR) | 23.5 (20.0–29.1) | 25.3 (20.8–30.8) | 24.0 (21.0–29.0) | 23.1 (19.0–27.8) |
| Non-dom hand, median (IQR) | 24.6 (20.9–31.0) | 28.6 (23.1–42.0) | 23.9 (22.1–29.7) | 21.9 (18.8–28.8) |
| PASAT (0–60) | (n = 32) | (n = 28) | (n = 28) | (n = 27) |
| Baseline, mean (SD) | 39.3 (9.0) | 38.6 (14.9) | 38.2 (14.8) | 40.4 (15.3) |
| TMT A (s) | (n = 36) | (n = 31) | (n = 31) | (n = 31) |
| Baseline mean (SD) | 45.8 (37.3) | 45.3 (33.3) | 46.4 (26.1) | 42.9 (31.4) |
| TMT B (s) | (n = 36) | (n = 31) | (n = 31) | (n = 29) |
| Baseline, mean (SD) | 116.1 (83.6) | 122.1 (84.8) | 107.8 (62) | 104.4 (62.5) |
| SDMT | (n = 38) | (n = 32) | (n = 31) | (n = 31) |
| mean (SD) | 47.6 (13.1) | 40.4 (14.3) | 39.1 (12.7) | 44.2 (21.5) |
| cMAS (0–48) ** | (n = 19) | (n = 18) | (n = 21) | (n = 11) |
| median, (IQR) | 5.0 (1.0–12.0) | 7.5 (3.0–14.0) | 11 (5.0–18.0) | 12 (3.0–13.0) |
| PROMIS Sleep | (n = 37) | (n = 31) | (n = 29) | (n = 30) |
| mean (SD) | 54.6 (6.0) | 56.3 (6.4) | 55.8 (6.1) | 53.9 (7.2) |
| PROMIS Anxiety | (n = 38) | (n = 31) | (n = 29) | (n = 30) |
| mean (SD) | 50.3 (8.9) | 48.1 (7.3) | 52.0 (8.4) | 50.9 (8.8) |
| PROMIS Depression | (n = 37) | (n = 31) | (n = 26) | (n = 30) |
| mean (SD) | 49.6 (9.2) | 48.9 (9.2) | 53.7 (8.6) | 50.2 (9.6) |
| Primary Outcome (Pain) | Placebo n = 35 | THC n = 24 | CBD n = 27 | THC&CBD n = 28 | p Interaction between THC and CBD | p THC | p CBD |
|---|---|---|---|---|---|---|---|
| LOCF Change in pain (0–10 NRS) mean (SD) | −1.8 * (1.8) | −1.4 * (2.0) | −1.4 * (1.6) | −1.6 * (1.8) | |||
|
Effect size for the isolated cannabinoids (95%CI) ^ | THC 0.83 (−0.61–0.75) | CBD 0.71 (−0.55–0.81) | 0.31 | 0.83 | 0.71 | ||
|
Effect size of each treatment group # (95%CI) | 0.42 (−0.54–1.38) | 0.45 (−0.47–1.38) | 0.16 (−0.75–1.08) | One-way p = 0.74 | |||
| BOCF Change in pain (0–10 NRS) mean (SD) | −1.9 * (1.8) | −1.3 * (2.0) | −1.4 * (1.6) | −1.5 * (1.9) | 0.28 | 0.57 | 0.62 |
|
Difference NPSI (no = 101) mean (SD) The total difference Burning pain Pressing pain Paroxysmal pain Evoked pain Paresthesia/dysesthesia | −0.8 * (1.6) −1.7 * (4.0) −0.8 (2.5) −1.3 * (2.1) −0.7 (2.2) −0.4 (3.4) | −1.3 * (1.7) −1.1 (3.5) −1.2 (2.3) −1.5 * (3.0) −0.8 (2.8) −1.5 * (2.7) | −1.5 * (13.4) −1.3 (3.2) −1.1 * (2.3) −2.0 * (2.8) −1.4 * (2.5) −1.4 * (2.7) | −0.8 * (1.3) −1.5 (4.0) −2.1 * (2.1) −1.3 * (2.8) −0.5 (1.7) −0.4 (2.7) | 0.07 0.53 0.54 0.37 0.28 0.06 | 0.74 0.80 0.16 0.61 0.33 0.96 | 0.58 0.96 0.26 0.53 0.51 0.89 |
|
50% pain reduction, n (%) | 8 (22.9) | 5 (20.8) | 4 (14.8) | 7 (25.0) | Fisher 0.81 | ||
|
30% pain reduction, n (%) | 16 (45.7) | 7 (29.2) | 11 (40.7) | 14 (50.0) | Fisher 0.47 | ||
|
Pain relief (1–10) $ Median (IQR) | (n = 27) 2.0 (1.0–5.0) | (n = 15) 5.0 (1.0–7.0) | (n = 25) 1.0 (1.0–3.0) | (n = 20) 3.0 (1.0–6.5) | KW 0.19 | ||
| Primary outcome (spasticity) | Placebo n = 29 | THC n = 25 | CBD n = 26 | THC&CBD n = 20 | p Interaction between THC and CBD | p THC | p CBD |
| LOCF Change in spasticity (0–10 NRS) mean (SD) | −1.7 * (2.3) | −1.5 * (2.0) | −1.3 * (1.9) | −1.6 * (2.7) | |||
|
Effect size for the isolated cannabinoids (95%CI) ^ | THC 0.95 (−0.92–0.86) | CBD 0.61 (−0.70–1.01) | 0.51 | 0.95 | 0.67 | ||
|
Effect size of each treatment group # (95%CI) | 0.24 (−0.67–1.45) | 0.46 (−0.74–1.65) | 0.10 (−1.18–1.39) | One-way p = 0.89 | |||
| BOCF Change in spasticity (0–10 NRS) mean (SD) | 1.8 * (2.6) | 1.5 * (1.9) | 1.3 * (1.9) | 1.5 * (2.8) | 0.65 | 0.88 | 0.49 |
|
cMAS (0–48) Difference, median (IQR) | (n = 15) 0.0 (−5.0–0.0) | (n = 13) −1.0 (−4.0–0.0) | (n = 15) −4.0 * (−8.0–0.0) | (n = 10) −2.0 (−8.0–0.0) | KW 0.53 | ||
| 50% spasticity reduction, n (%) | 7 (24.1) | 7 (28) | 4 (15.4) | 6 (30) | Fisher 0.63 | ||
| 30% spasticity reduction, n (%) | 10 (34.5) | 12 (48.0) | 11 (42.3) | 11 (55.0) | Fisher 0.53 | ||
|
Spasticity relief $ (1–10) Median (IQR) | (n = 21) 2.0 (1.0–4.0) | (n = 12) 3.0 (1.0–6.0) | (n = 20) 1.0 (1.0–4.0) | (n = 12) 2.0 (1.0–4.0) | KW 0.93 |
| Placebo | THC | CBD | THC&CBD | p | p | p | |
|---|---|---|---|---|---|---|---|
| Total | 40 | 32 | 31 | 31 | Interaction THC and CBD | THC | CBD |
| Quality of life 5Q-5L-5D Index difference, mean (SD) | 0.06 (0.1) | 0.03 (0.1) | 0.07 (0.2) | 0.08 (0.2) | 0.47 | 0.66 | 0.44 |
| Impact on sleep (NRS0-10) Mean difference (SD) | −1.8 * (2.3) | −1.7 * (2.2) | −1.3 * (1.9) | −1.6 * (1.9) | 0.60 | 0.81 | 0.33 |
| Dropouts before week 5 n (%) # | 3 (7.5) | 7 (21.9) | 1 (3.2) | 5 (16.1) | Fisher: 0.09 | ||
| Daily dose at endpoint (LOCF) Daily dose, median (IQR) Reaching full dose n (%) | 9.0 (9.0–9.0) 32 (80.0) | 9.0 (5.9–9.0) 16 (50.0) | 9.0 (9.0–9.0) 24 (77.4) | 7.0 (4.9–9.0) 12 (38.7) | KW <0.01 | ||
| PROMIS Sleep, n Difference, mean (SD) | (n = 28) 5.0 * (6.6) | (n = 19) 5.5 * (7.4) | (n = 27) 2.5 * (5.7) | (n = 22) 5.4 * (7.2) | 0.39 | 0.21 | 0.26 |
| PROMIS Anxiety, n Difference, mean (SD) | (n = 28) 4.3 * (7.3) | (n = 20) 1.5 (9.2) | (n = 27) 4.4 * (8.9) | (n = 22) 5.0 * (7.8) | 0.32 | 0.57 | 0.35 |
| PROMIS Depression, n Difference, mean (SD) | (n = 28) 1.8 (6.8) | (n = 20) −0.3 (11.0) | (n = 24) 2.7 (6.5) | (n = 22) 3.2 (7.6) | 0.44 | 0.61 | 0.22 |
| 9-Hole PEG test difference n dom/non-dom diff. dom hand median (IQL) Diff. non-dom hand | (n = 30/28) 1.3 (−0.7–3.0) 0.4 (−1.2–4.0) | (n = 22/21) 1.4 (−0.9–4.0) 1.2 (−1.6–3.6) | (n = 27/27) −0.1 (−1.1–2.4) 0.0 (−4.2–1.5) | (n = 25/23) 0.6 (−1.0–2.0) −0.4 (−2.5–1.5) | KW 0.70 0.21 | ||
| PASAT (No. correct 0–60) n responders Difference, mean (SD) | (n = 27) −3.7 * (6.5) | (n = 20) −0.6 (9.6) | (n = 24) −1.8 (9.8) | (n = 20) 1.6 (6.5) | 0.96 | 0.06 | 0.25 |
| TMT A (s) Difference, mean (SD) | (n = 29) 5.0 (24.4) | (n = 23) 4.2 (10.6) | (n = 28) 0.6 (23.7) | (n = 26) 7.4 (21.5) | 0.88 | 0.09 | 0.77 |
| TMT B (s) Difference, mean (SD) | (n = 30) −5.8 (57.6) | (n = 22) −14.1 (49.0) | (n = 28) −17.9 * (43.5) | (n = 24) 13.2 (48.0) | 0.08 | 0.09 | 0.35 |
| SDMT mean (SD) | (n = 33) −1.1 (5.9) | (n = 25) 1.1 (7.2) | (n = 29) −1.9 (7.3) | (n = 27) −3.1 (11.1) | 0.26 | 0.73 | 0.12 |
| Blinding assessment n Active treatment, n (%) Placebo n (%) Don’t know, n (%) | (n = 36) 16 (44.4) 16 (44.4) 4 (11.1) | (n = 26) 14 (53.8) 9 (34.6) 3 (11.5) | (n = 30) 5 (16.7) 19 (63.3) 6 (20.0) | (n = 28) 18 (64.3) 7 (25.0) 3 (10.7) | Fisher 0.012 | ||
| Reasons ‘Guess of treatment’(>than one option was allowed) Efficacy on pain, n (%) Efficacy on spasticity, n (%) Adverse events, n (%) Others/don’t know, n (%) | 19 (52.7) 19 (52.7) 12 (33.3) 17 (47.2) | 13 (50.0) 11 (42.3) 10 (38.5) 6 (23.1) | 19 (63.3) 15 (50.0) 8 (26.7) 13 (43.3) | 11 (39.3) 5 (17.9) 14 (50.0) 10 (35.7) |
| Placebo n = 40 | THC n = 32 | CBD n = 31 | THC&CBD n = 31 | |
|---|---|---|---|---|
| 1. Mouth dryness, n (%), p | 11 (27.5) | 17 (53.1) 0.03 | 9 (29.0) 0.89 | 21 (67.7) <0.01 |
| 2. Headache n (%), p | 13 (32.5) | 15 (46.9) 0.21 | 10 (32.3) 0.98 | 13 (41.9) 0.41 |
| 3. Depressive thoughts n (%), p | 4 (10.0) | 2 (6.25) 0.57 | 4 (12.9) 0.70 | 2 (6.5) 0.59 |
| 4. Nightmare n (%), p | 1 (2.5) | 1 (3.1) 0.87 | 0 (0.0) 0.38 | 2 (6.5) 0.41 |
| 5. Euphoria n (%), p | 1 (2.5) | 5 (15.6) 0.05 | 1 (3.2) 0.86 | 4 (12.9) 0.09 |
| 6. Dizziness n (%), p | 12 (30.0) | 19 (59.4) 0.01 | 5 (16.1) 0.17 | 18 (58.1) 0.02 |
| 7. Tinnitus n (%), p | 4 (10.0) | 6 (18.8) 0.29 | 4 (12.9) 0.70 | 4 (12.9) 0.70 |
| 8. Anxiety n (%), p | 1 (2.5) | 3 (9.4) 0.21 | 2 (6.5) 0.41 | 3 (9.7) 0.19 |
| 9. Nervousness n (%), p | 3 (3.5) | 2 (6.3) 0.84 | 3 (9.7) 0.74 | 5 (16.1) 0.25 |
| 10. Hallucinations n (%), p | 1 (2.5) | 3 (9.4) 0.21 | 3 (9.7) 0.41 | 0 (0) 0.38 |
| 11. Fatigue/drowsiness, n (%), p | 14 (35.0) | 17 (53.1) 0.12 | 14 (45.2) 0.39 | 17 (54.8) 0.10 |
| 12. Palpitations n (%), p | 0 (0) | 6 (18.8) 0.004 | 2 (6.5) 0.10 | 3 (9.7) 0.04 |
| 13. Flushing of the face n (%), p | 3 (7.5) | 6 (18.8) 0.15 | 1 (3.2) 0.44 | 2 (6.5) 0.86 |
| 14. Stomach ache n (%), p | 3 (7.5) | 8 (25.0) 0.04 | 6 (19.4) 0.14 | 6 (19.4) 0.14 |
| 15. Nausea n (%), p | 4 (10.0) | 6 (18.8) 0.29 | 5 (16.1) 0.44 | 11 (35.5) 0.01 |
| 16. Diarrhea n (%), p | 2 (5.0) | 3 (9.4) 0.47 | 0 (0) 0.21 | 7 (22.6) 0.03 |
| 17. Muscle pain n (%), p | 10 (25.0) | 6 (18.8) 0.53 | 4 (12.9) 0.20 | 6 (19.4) 0.57 |
| 18. Visual disturbance n (%), p | 3 (7.5) | 5 (16.6) 0.28 | 2 (6.5) 0.86 | 3 (9.7) 0.74 |
| 19. “Other” AEs n (%), p | 3 (7.5) | 7 (21.9) 0.08 | 3 (9.7) 0.74 | 14 (45.2) <0.01 |
| SAR/SUSARS n, (%) | 0 (0) | 2 (6.3) | 1 (3.2) | 0 (0) |
| SAE | 2 (5.0) | 2 (6.5) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, J.S.; Gustavsen, S.; Roshanisefat, H.; Kant, M.; Biering-Sørensen, F.; Andersen, C.; Olsson, A.; Chow, H.H.; Asgari, N.; Hansen, J.R.; et al. Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial. Pharmaceuticals 2023, 16, 1079. https://doi.org/10.3390/ph16081079
Hansen JS, Gustavsen S, Roshanisefat H, Kant M, Biering-Sørensen F, Andersen C, Olsson A, Chow HH, Asgari N, Hansen JR, et al. Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial. Pharmaceuticals. 2023; 16(8):1079. https://doi.org/10.3390/ph16081079
Chicago/Turabian StyleHansen, Julie Schjødtz, Stefan Gustavsen, Homayoun Roshanisefat, Matthias Kant, Fin Biering-Sørensen, Claus Andersen, Anna Olsson, Helene Højsgaard Chow, Nasrin Asgari, Julie Richter Hansen, and et al. 2023. "Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial" Pharmaceuticals 16, no. 8: 1079. https://doi.org/10.3390/ph16081079
APA StyleHansen, J. S., Gustavsen, S., Roshanisefat, H., Kant, M., Biering-Sørensen, F., Andersen, C., Olsson, A., Chow, H. H., Asgari, N., Hansen, J. R., Nielsen, H. H., Hansen, R. M., Petersen, T., Oturai, A. B., Sellebjerg, F., Sædder, E. A., Kasch, H., Rasmussen, P. V., Finnerup, N. B., & Svendsen, K. B. (2023). Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial. Pharmaceuticals, 16(8), 1079. https://doi.org/10.3390/ph16081079






