Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain
Abstract
1. Introduction
2. Results
2.1. Effects of a Single i.p. Mirogabalin and Pregabalin Administration on Pain-Related Behavior Measured 11 Days after CCI in Mice
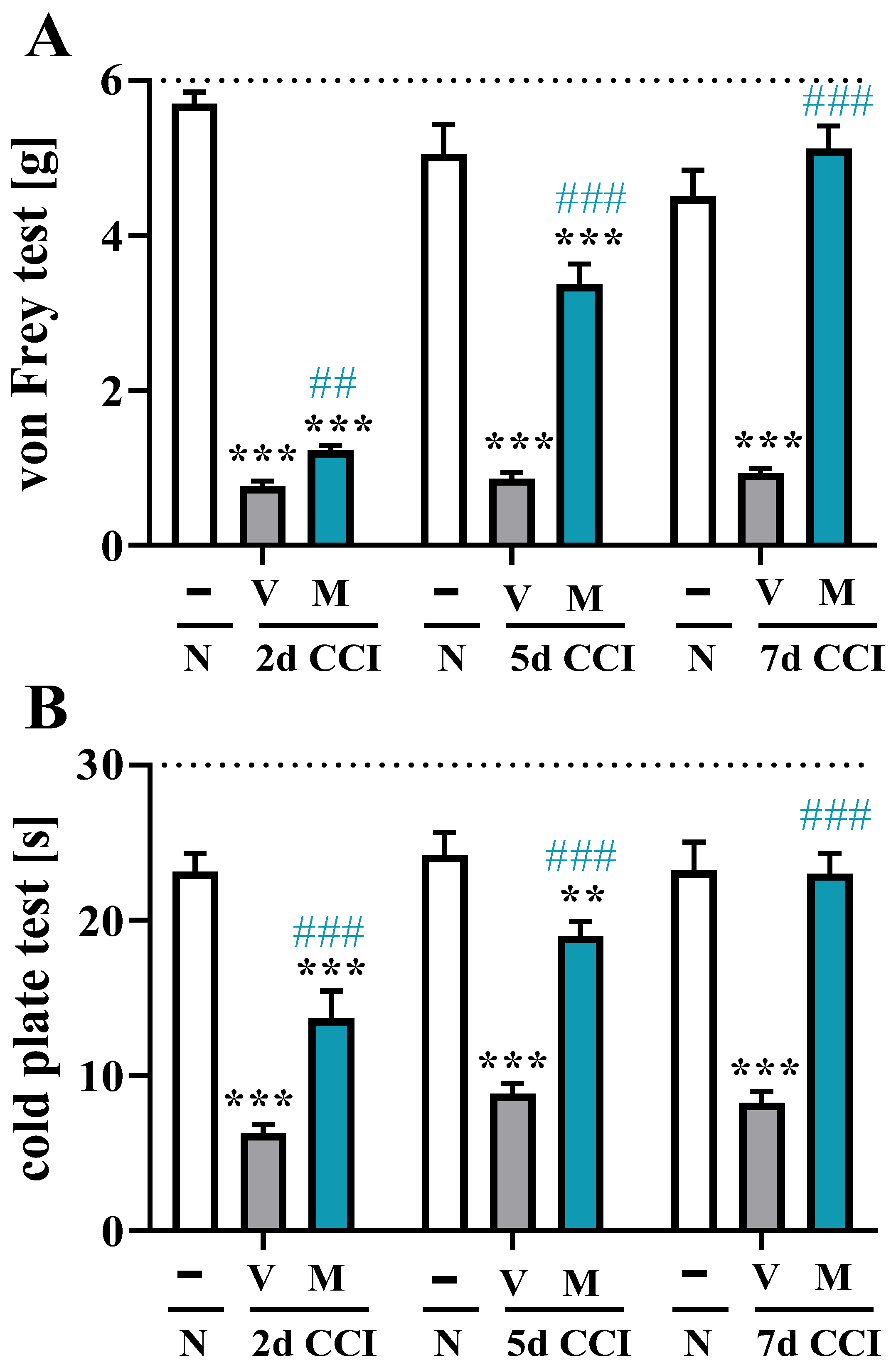
2.2. Effects of Repeated i.p. Mirogabalin Administration on Pain-Related Behavior Measured 2, 5 and 7 Days after CCI in Mice
2.3. Effect of the Repeated Administration of Mirogabalin on IBA-1, GFAP and MPO Protein Levels in the Spinal Cord Measured on Day 7 after CCI in Mice
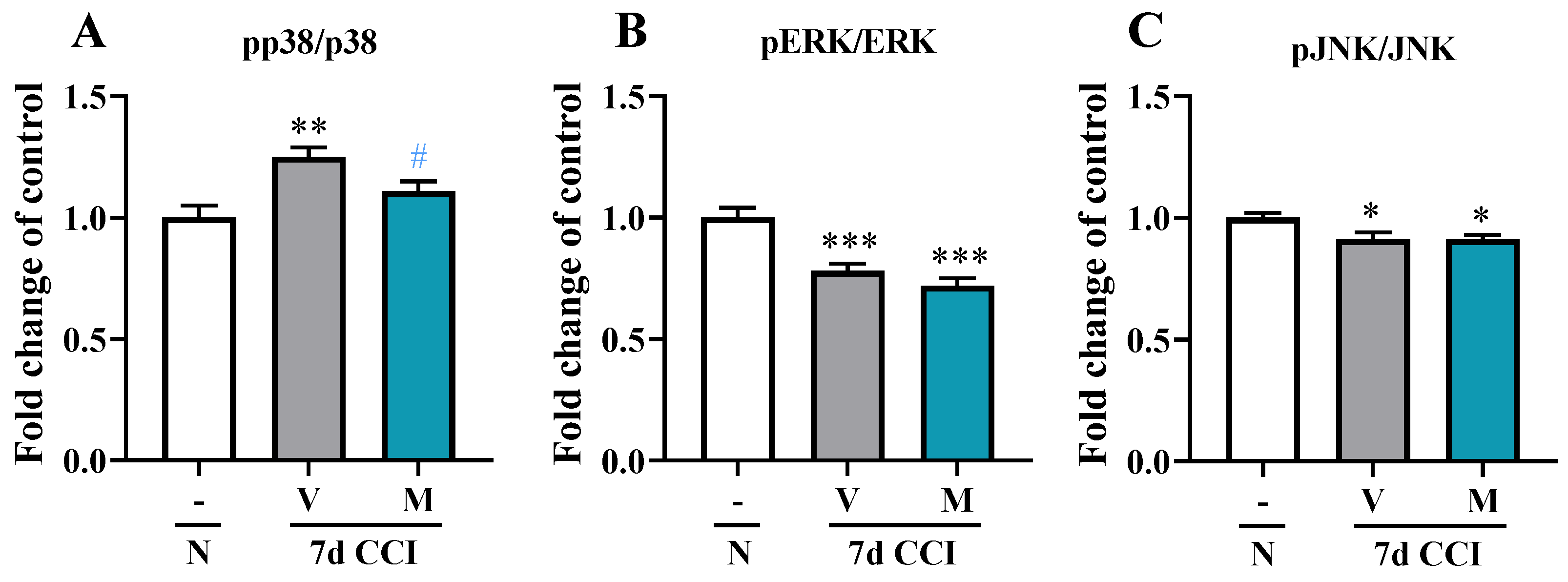
2.4. Effects of Repeated i.p. Administration of Mirogabalin on pp38/p38, pERK/ERK, and pJNK/JNK Protein Levels in the Spinal Cord Measured on Day 7 after CCI in Mice
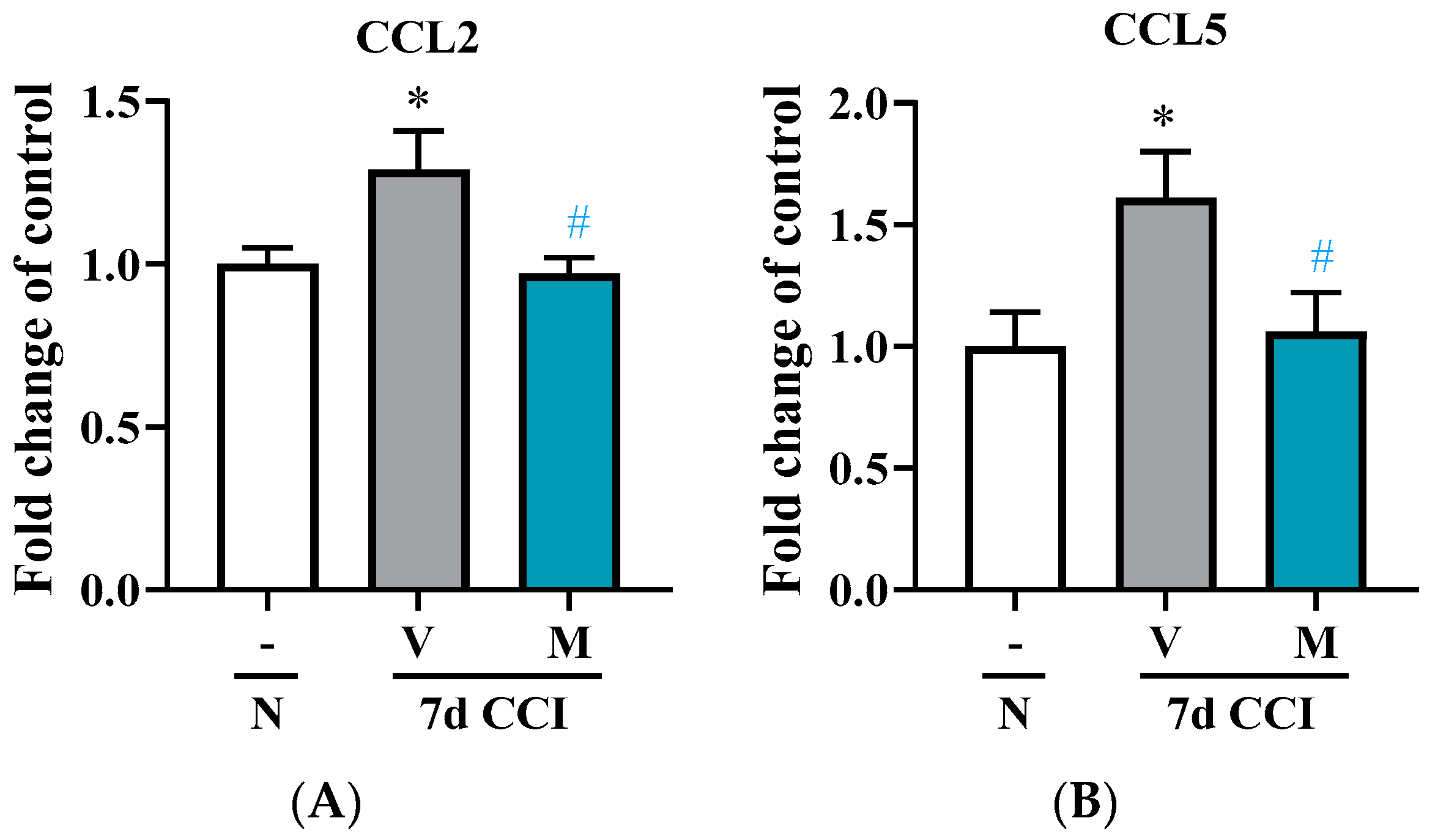
2.5. Effects of Repeated i.p. Administration of Mirogabalin on CCL2 and CCL5 Measured on Day 7 after CCI in the Spinal Cord in Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Sciatic Nerve Surgery
4.3. Drug Administration
4.4. Behavioral Tests
4.4.1. Von Frey Test
4.4.2. Cold Plate Test
4.5. Western Blot
4.6. MILLIPLEX Multiplex Assays Using Luminex®® to Analyze Protein Levels
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A New Definition of Neuropathic Pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The Specific Disease Burden of Neuropathic Pain: Results of a French Nationwide Survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Domon, Y.; Arakawa, N.; Inoue, T.; Matsuda, F.; Takahashi, M.; Yamamura, N.; Kai, K.; Kitano, Y. Binding Characteristics and Analgesic Effects of Mirogabalin, a Novel Ligand for the A2δ Subunit of Voltage-Gated Calcium Channels. J. Pharmacol. Exp. Ther. 2018, 365, 573–582. [Google Scholar] [CrossRef]
- Domon, Y.; Kitano, Y.; Makino, M. Analgesic Effects of the Novel A₂δ Ligand Mirogabalin in a Rat Model of Spinal Cord Injury. Pharmazie 2018, 73, 659–661. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of Glial Activation in Neuropathic Pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Mika, J.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Minocycline and Pentoxifylline Attenuate Allodynia and Hyperalgesia and Potentiate the Effects of Morphine in Rat and Mouse Models of Neuropathic Pain. Eur. J. Pharmacol. 2007, 560, 142–149. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Chen, G.; Ji, R.R. Microglia and Spinal Cord Synaptic Plasticity in Persistent Pain. Neural Plast. 2013, 2013. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Tozaki-Saitoh, H.; Inoue, K. Microglial Regulation of Neuropathic Pain. J. Pharmacol. Sci. 2013, 121, 89–94. [Google Scholar] [CrossRef]
- Mika, J.; Popiolek-Barczyk, K.; Rojewska, E.; Makuch, W.; Starowicz, K.; Przewlocka, B. Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLoS ONE 2014, 9, e104420. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Kwiatkowski, K.; Mika, J. Pharmacological Evidence of the Important Roles of CCR1 and CCR3 and Their Endogenous Ligands CCL2/7/8 in Hypersensitivity Based on a Murine Model of Neuropathic Pain. Cells 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of P38 Mitogen-Activated Protein Kinase in Spinal Hyperactive Microglia Contributes to Pain Hypersensitivity Following Peripheral Nerve Injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef]
- Song, X.S.; Cao, J.L.; Xu, Y.B.; He, J.H.; Zhang, L.C.; Zeng, Y.M. Activation of ERK/CREB Pathway in Spinal Cord Contributes to Chronic Constrictive Injury-Induced Neuropathic Pain in Rats. Acta Pharmacol. Sin. 2005, 26, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Mika, J. The Importance of Chemokines in Neuropathic Pain Development and Opioid Analgesic Potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Murasawa, H.; Kobayashi, H.; Saeki, K.; Kitano, Y. Anxiolytic Effects of the Novel A2δ Ligand Mirogabalin in a Rat Model of Chronic Constriction Injury, an Experimental Model of Neuropathic Pain. Psychopharmacology 2020, 237, 189–197. [Google Scholar] [CrossRef]
- Saeki, K.; Yasuda, S.I.; Kato, M.; Kano, M.; Domon, Y.; Arakawa, N.; Kitano, Y. Analgesic Effects of Mirogabalin, a Novel Ligand for A2δ Subunit of Voltage-Gated Calcium Channels, in Experimental Animal Models of Fibromyalgia. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 723–728. [Google Scholar] [CrossRef]
- Kochi, T.; Nakamura, Y.; Ma, S.; Uemoto, S.; Hisaoka-Nakashima, K.; Irifune, M.; Morioka, N. Mirogabalin Alleviates Nociceptive Hypersensitivity without Causing Sedation in a Mouse Model of Post-Traumatic Trigeminal Neuropathy. Behav. Brain Res. 2022, 425, 113829. [Google Scholar] [CrossRef]
- Komatsu, S.; Nakamura, S.; Nonaka, T.; Yamada, T.; Yamamoto, T. Analgesic Characteristics of a Newly Developed A2δ Ligand, Mirogabalin, on Inflammatory Pain. Mol. Pain 2021, 17, 7448069211052167. [Google Scholar] [CrossRef]
- Ushida, T.; Katayama, Y.; Hiasa, Y.; Nishihara, M.; Tajima, F.; Katoh, S.; Tanaka, H.; Maeda, T.; Furusawa, K.; Richardson, M.; et al. Mirogabalin for Central Neuropathic Pain After Spinal Cord Injury: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study in Asia. Neurology 2022, 100, e1193–e1206. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Mirogabalin: First Global Approval. Drugs 2019, 79, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yamaguchi, S.; Suzuki, T.; Kato, J.; Chiba, S.; Hirakawa, N.; Yamaguchi, K.; Tanabe, Y.; Takatsuna, H.; Kenyoshi, Y.; et al. Switching From Pregabalin to Mirogabalin in Patients with Peripheral Neuropathic Pain: A Multi-Center, Prospective, Single-Arm, Open-Label Study (MIROP Study). Pain Ther. 2021, 10, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Coraci, D.; Brau, F.; Galluzzo, V.; Loreti, C.; Caliandro, P.; Padua, L.; Maccauro, G.; Biscotti, L.; Bernabei, R. Neuropathic Pain in the Elderly. Diagnostics 2021, 11, 613. [Google Scholar] [CrossRef]
- Giovannini, S.; van der Roest, H.G.; Carfì, A.; Finne-Soveri, H.; Garms-Homolová, V.; Declercq, A.; Jónsson, P.V.; van Hout, H.; Vetrano, D.L.; Gravina, E.M.; et al. Polypharmacy in Home Care in Europe: Cross-Sectional Data from the IBenC Study. Drugs Aging 2018, 35, 145–152. [Google Scholar] [CrossRef]
- Garrison, C.J.; Dougherty, P.M.; Kajander, K.C.; Carlton, S.M. Staining of Glial Fibrillary Acidic Protein (GFAP) in Lumbar Spinal Cord Increases Following a Sciatic Nerve Constriction Injury. Brain Res. 1991, 565, 1–7. [Google Scholar] [CrossRef]
- Eriksson, N.P.; Persson, J.K.E.; Aldskogius, H.; Svensson, M. A Quantitative Analysis of the Glial Cell Reaction in Primary Sensory Termination Areas Following Sciatic Nerve Injury and Treatment with Nerve Growth Factor in the Adult Rat. Exp. Brain Res. 1997, 114, 393–404. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The Neuro-Immune Balance in Neuropathic Pain: Involvement of Inflammatory Immune Cells, Immune-like Glial Cells and Cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Mika, J.; Osikowicz, M.; Rojewska, E.; Korostynski, M.; Wawrzczak-Bargiela, A.; Przewlocki, R.; Przewlocka, B. Differential Activation of Spinal Microglial and Astroglial Cells in a Mouse Model of Peripheral Neuropathic Pain. Eur. J. Pharmacol. 2009, 623, 65–72. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Jurga, A.M.; Makuch, W.; Przewlocka, B.; Mika, J. Involvement of Pro- and Antinociceptive Factors in Minocycline Analgesia in Rat Neuropathic Pain Model. J. Neuroimmunol. 2014, 277, 57–66. [Google Scholar] [CrossRef]
- Kanashiro, A.; Hiroki, C.H.; da Fonseca, D.M.; Birbrair, A.; Ferreira, R.G.; Bassi, G.S.; Fonseca, M.D.; Kusuda, R.; Cebinelli, G.C.M.; da Silva, K.P.; et al. The Role of Neutrophils in Neuro-Immune Modulation. Pharmacol. Res. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Wodarski, R.; Clark, A.K.; Grist, J.; Marchand, F.; Malcangio, M. Gabapentin Reverses Microglial Activation in the Spinal Cord of Streptozotocin-Induced Diabetic Rats. Eur. J. Pain 2009, 13, 807–811. [Google Scholar] [CrossRef]
- Yang, J.L.; Xu, B.; Li, S.S.; Zhang, W.S.; Xu, H.; Deng, X.M.; Zhang, Y.Q. Gabapentin Reduces CX3CL1 Signaling and Blocks Spinal Microglial Activation in Monoarthritic Rats. Mol. Brain 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Rosa, A.S.; Freitas, M.F.; Rocha, I.R.C.; Chacur, M. Gabapentin Decreases Microglial Cells and Reverses Bilateral Hyperalgesia and Allodynia in Rats with Chronic Myositis. Eur. J. Pharmacol. 2017, 799, 111–117. [Google Scholar] [CrossRef]
- Chen, E.Y.; Beutler, S.S.; Kaye, A.D.; Edinoff, A.N.; Khademi, S.H.; Stoltz, A.E.; Rueb, N.R.; Cornett, E.M.; Suh, W.J. Mirogabalin as a Novel Gabapentinoid for the Treatment of Chronic Pain Conditions: An Analysis of Current Evidence. Anesthesiol. Pain Med. 2021, 11, e121402. [Google Scholar] [CrossRef]
- Kitano, Y.; Wakimoto, S.; Tamura, S.; Kubota, K.; Domon, Y.; Arakawa, N.; Saito, M.; Sava, B.; Buisson, B. Effects of Mirogabalin, a Novel Ligand for the A₂δ Subunit of Voltage-Gated Calcium Channels, on N-Type Calcium Channel Currents of Rat Dorsal Root Ganglion Culture Neurons. Pharmazie 2019, 74, 147–149. [Google Scholar] [CrossRef]
- Hundehege, P.; Fernandez-Orth, J.; Römer, P.; Ruck, T.; Müntefering, T.; Eichler, S.; Cerina, M.; Epping, L.; Albrecht, S.; Menke, A.F.; et al. Targeting Voltage-Dependent Calcium Channels with Pregabalin Exerts a Direct Neuroprotective Effect in an Animal Model of Multiple Sclerosis. Neurosignals 2018, 26, 77–93. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Mika, J.; Leppert, W.; Kocot-Kępska, M.; Malec-Milewska, M.; Wordliczek, J. Mirogabalin-A Novel Selective Ligand for the A2δ Calcium Channel Subunit. Pharmaceuticals 2021, 14, 112. [Google Scholar] [CrossRef]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. P38 Mitogen-Activated Protein Kinase Is Activated after a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Zychowska, M.; Makuch, W.; Przewlocka, B.; Mika, J. PD98059 Influences Immune Factors and Enhances Opioid Analgesia in Model of Neuropathy. PLoS ONE 2015, 10, e0138583. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, X.; Huang, H.; Zhu, Y.; Wu, Y. Montelukast Attenuates Neuropathic Pain through Inhibiting P38 Mitogen-Activated Protein Kinase and Nuclear Factor-Kappa B in a Rat Model of Chronic Constriction Injury. Anesth. Analg. 2014, 118, 1090–1096. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. Activation of Extracellular Signal-Regulated Kinase in Sciatic Nerve Contributes to Neuropathic Pain after Partial Sciatic Nerve Ligation in Mice. Anesth. Analg. 2009, 109, 1305–1311. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, C.; Li, Z.H.; Liu, X.Y.; Sun, S.K.; Zhang, T.; Luo, Z.J.; Zhang, H.; Li, W.Y. Inhibition of the Spinal Astrocytic JNK/MCP-1 Pathway Activation Correlates with the Analgesic Effects of Tanshinone IIA Sulfonate in Neuropathic Pain. J. Neuroinflamm. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Li, R.; Dang, S.; Yao, M.; Zhao, C.; Zhang, W.; Cui, J.; Wang, J.; Wen, A. Osthole Alleviates Neuropathic Pain in Mice by Inhibiting the P2Y1-Receptor-Dependent JNK Signaling Pathway. Aging 2020, 12, 7945–7962. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Activated Microglia Contribute to the Maintenance of Chronic Pain after Spinal Cord Injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef]
- Mei, X.P.; Sakuma, Y.; Xie, C.; Wu, D.; Ho, I.; Kotani, J.; Xu, L.X. Depressing Interleukin-1β Contributed to the Synergistic Effects of Tramadol and Minocycline on Spinal Nerve Ligation-Induced Neuropathic Pain. Neurosignals 2014, 22, 30–42. [Google Scholar] [CrossRef]
- Milligan, E.D.; Twining, C.; Chacur, M.; Biedenkapp, J.; O’Connor, K.; Poole, S.; Tracey, K.; Martin, D.; Maier, S.F.; Watkins, L.R. Spinal Glia and Proinflammatory Cytokines Mediate Mirror-Image Neuropathic Pain in Rats. J. Neurosci. 2003, 23, 1026–1040. [Google Scholar] [CrossRef]
- Obata, K.; Yamanaka, H.; Dai, Y.; Mizushima, T.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. Differential Activation of MAPK in Injured and Uninjured DRG Neurons Following Chronic Constriction Injury of the Sciatic Nerve in Rats. Eur. J. Neurosci. 2004, 20, 2881–2895. [Google Scholar] [CrossRef]
- Wen, Y.R.; Suter, M.R.; Kawasaki, Y.; Huang, J.; Pertin, M.; Kohno, T.; Berde, C.B.; Decosterd, I.; Ji, R.R. Nerve Conduction Blockade in the Sciatic Nerve Prevents but Does Not Reverse the Activation of P38 Mitogen-Activated Protein Kinase in Spinal Microglia in the Rat Spared Nerve Injury Model. Anesthesiology 2007, 107, 312–321. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. P38 MAPK, Microglial Signaling, and Neuropathic Pain. Mol. Pain 2007, 3, 1744–8069. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, S.B.; Gao, Y.J.; Xing, J.L.; Xian, H.; Li, Z.Z.; Shen, S.N.; Wu, S.X.; Luo, C.; Xie, R.G. Spinal CCL2 Promotes Pain Sensitization by Rapid Enhancement of NMDA-Induced Currents Through the ERK-GluN2B Pathway in Mouse Lamina II Neurons. Neurosci. Bull. 2020, 36, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.T.; Mao, C.C.; Ching-Wah Sum, D.; Liu, F.C.; Lai, Y.S.; Li, J.C.; Day, Y.J. Peritoneal Administration of Met-RANTES Attenuates Inflammatory and Nociceptive Responses in a Murine Neuropathic Pain Model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.K. A Peripheral Mononeuropathy in Rat That Produces Disorders of Pain Sensation like Those Seen in Man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Rojewska, E.; Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Kocot-Kępska, M.; Makuch, W.; Wordliczek, J.; Mika, J. Mirogabalin Decreases Pain-like Behaviours and Improves Opioid and Ketamine Antinociception in a Mouse Model of Neuropathic Pain. Pharmaceuticals 2022, 15, 88. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Takasu, K.; Ono, H.; Tanabe, M. Pregabalin, S-(+)-3-Isobutylgaba, Activates the Descending Noradrenergic System to Alleviate Neuropathic Pain in the Mouse Partial Sciatic Nerve Ligation Model. Neuropharmacology 2007, 53, 842–853. [Google Scholar] [CrossRef]
- Coderre, T.J.; Kumar, N.; Laferriere, A.; Yu, J.S.C.; Leavitt, A. Evidence That Pregabalin Reduces Neuropathic Pain by Inhibiting the Spinal Release of Glutamate. J. Neurochem. 2010, 113, 552–561. [Google Scholar] [CrossRef]
- Liu, X.; Fagotto, F. A Method to Separate Nuclear, Cytosolic, and Membrane-Associated Signaling Molecules in Cultured Cells. Sci. Signal. 2011, 4, pl2. [Google Scholar] [CrossRef]
- Baghirova, S.; Hughes, B.G.; Hendzel, M.J.; Schulz, R. Sequential Fractionation and Isolation of Subcellular Proteins from Tissue or Cultured Cells. MethodsX 2015, 2, e440–e445. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New Insights into the Analgesic Properties of the XCL1/XCR1 and XCL1/ITGA9 Axes Modulation under Neuropathic Pain Conditions-Evidence from Animal Studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajączkowska, R.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Ciechanowska, A.; Rojewska, E.; Kocot-Kępska, M.; Makuch, W.; Wordliczek, J.; Mika, J. Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain. Pharmaceuticals 2023, 16, 1023. https://doi.org/10.3390/ph16071023
Zajączkowska R, Pawlik K, Ciapała K, Piotrowska A, Ciechanowska A, Rojewska E, Kocot-Kępska M, Makuch W, Wordliczek J, Mika J. Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain. Pharmaceuticals. 2023; 16(7):1023. https://doi.org/10.3390/ph16071023
Chicago/Turabian StyleZajączkowska, Renata, Katarzyna Pawlik, Katarzyna Ciapała, Anna Piotrowska, Agata Ciechanowska, Ewelina Rojewska, Magdalena Kocot-Kępska, Wioletta Makuch, Jerzy Wordliczek, and Joanna Mika. 2023. "Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain" Pharmaceuticals 16, no. 7: 1023. https://doi.org/10.3390/ph16071023
APA StyleZajączkowska, R., Pawlik, K., Ciapała, K., Piotrowska, A., Ciechanowska, A., Rojewska, E., Kocot-Kępska, M., Makuch, W., Wordliczek, J., & Mika, J. (2023). Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain. Pharmaceuticals, 16(7), 1023. https://doi.org/10.3390/ph16071023







