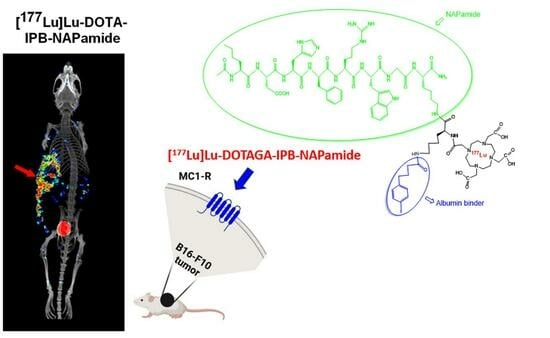
Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals
Abstract
:1. Introduction
2. Results and Discussion
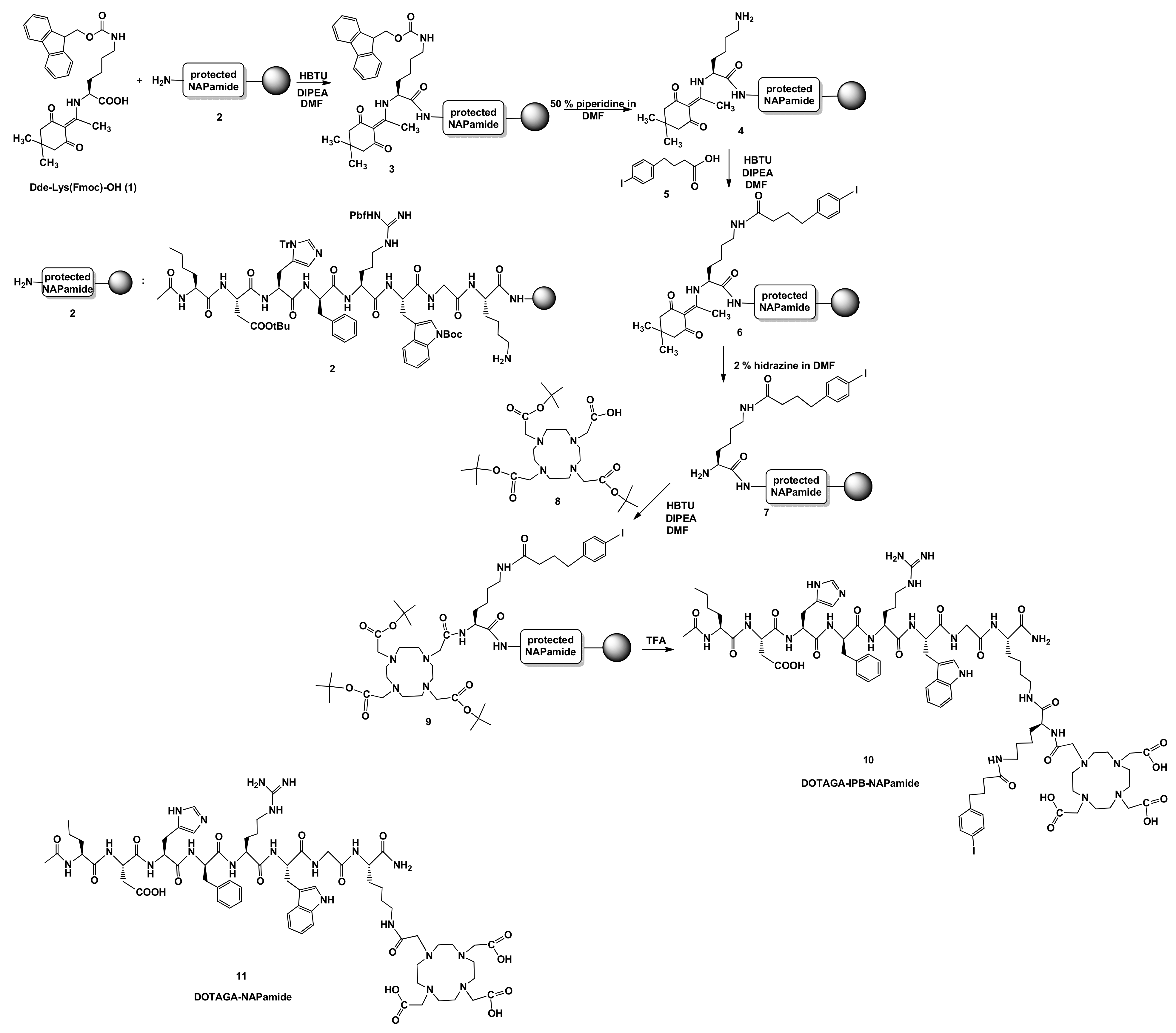
2.1. Synthesis of DOTA-NAPamide Containing 4-(p-iodo-phenyl)butyryl Group (10)
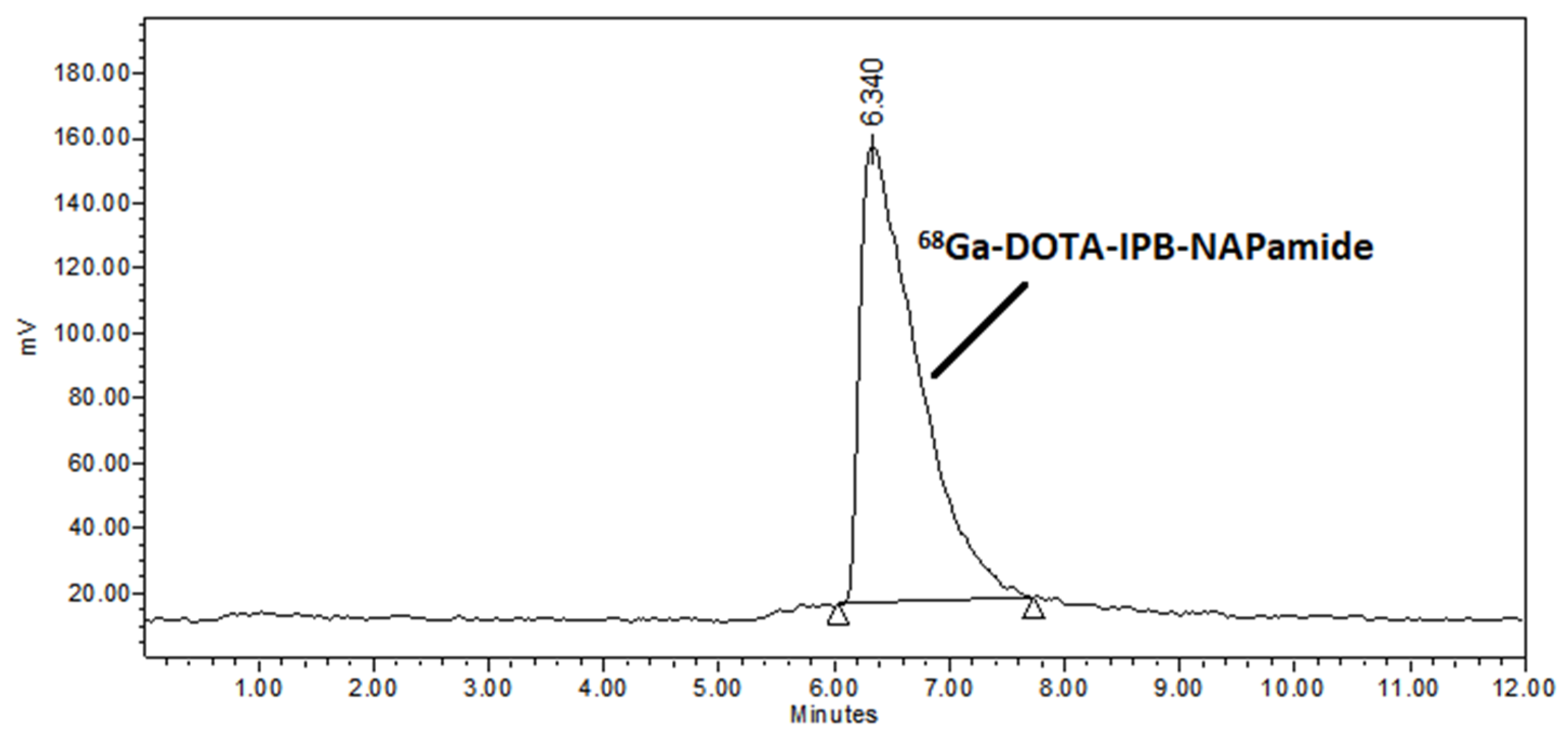
2.2. Radiochemistry
2.3. Biology
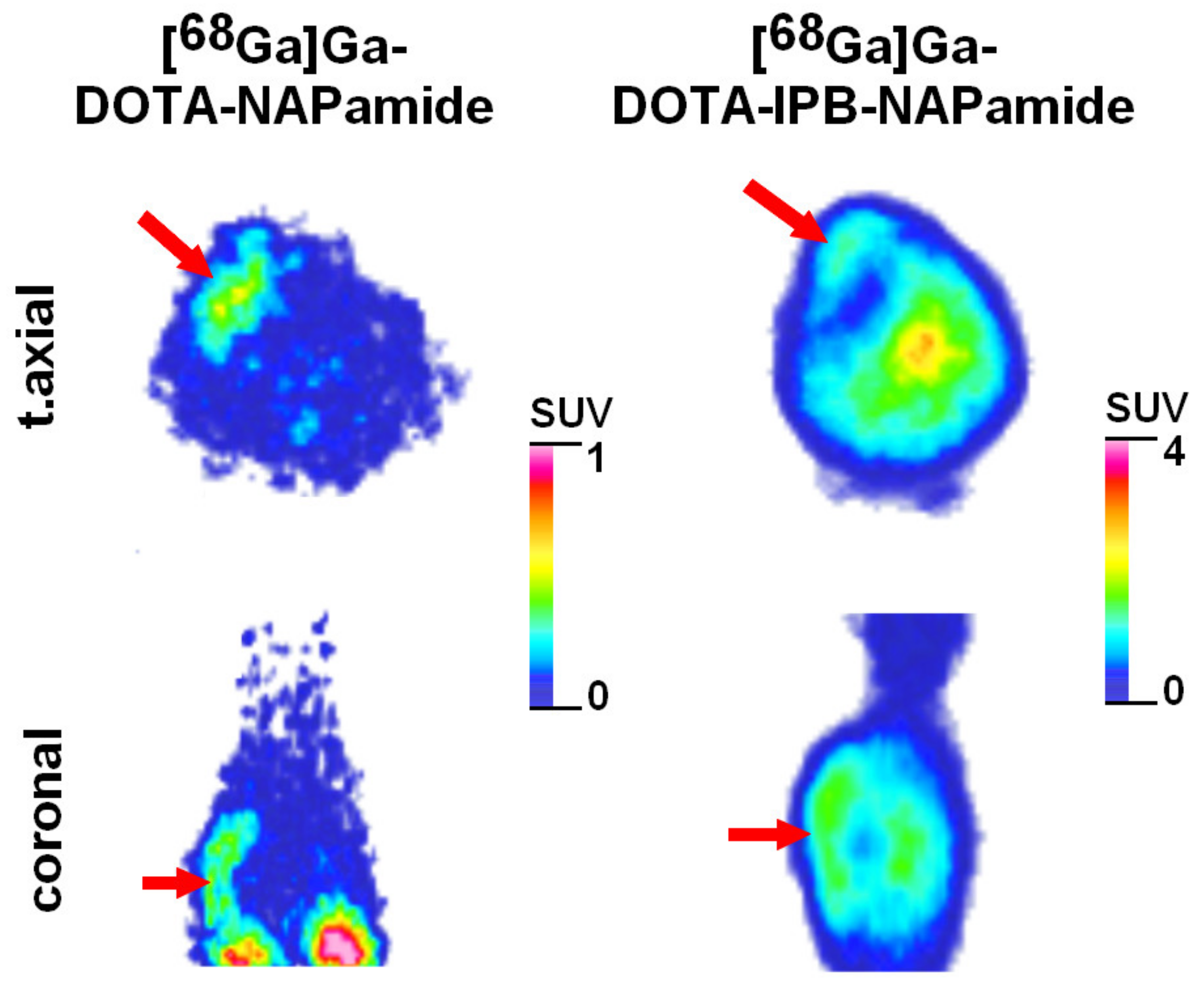
2.3.1. In Vivo and Ex Vivo Biodistribution of 68Ga-Labeled DOTA-NAPamide and DOTA-IPB-NAPamide
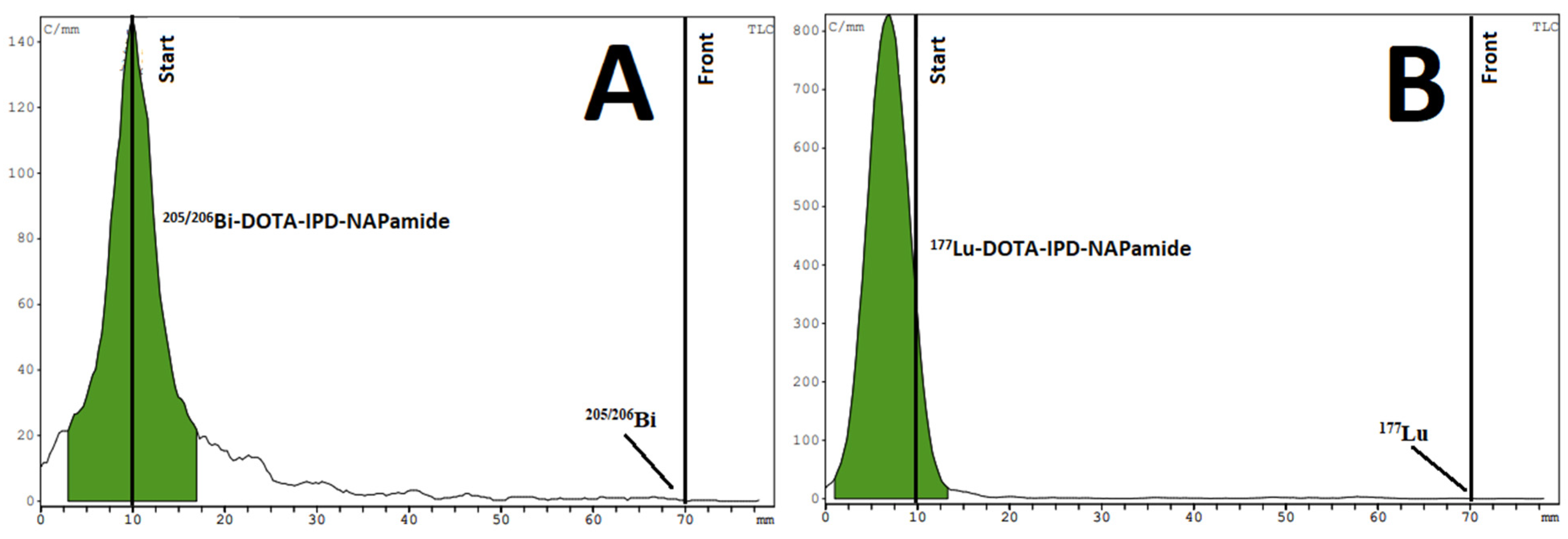
2.3.2. Ex Vivo Biodistribution of [205/206Bi]Bi-DOTA-NAPamide and [205/206Bi]Bi-DOTA-IPB-NAPamide
2.3.3. In Vivo and Ex Vivo Biodistribution Pattern of [177Lu]Lu-DOTA-NAPamide and [177Lu]Lu-DOTA-IPB-NAPamide
3. Materials and Methods
3.1. General
3.2. Chemistry
Synthesis of DOTA-IPB-NAPamide (10)
3.3. Radiochemistry
3.3.1. 68Ga-Labeling of DOTA-NAPamide and DOTA-IPB-NAPamide (10)
3.3.2. 205/206Bi-Labeling of DOTA-NAPamide and DOTA-IPB-NAPamide (10)
3.3.3. 177Lu-Labeling of DOTA-NAPamide and DOTA-IPB-NAPamide (10)
3.3.4. Determination of the logP Values
3.3.5. Stability Test of [68Ga]Ga-DOTA-NAPamide, [68Ga]Ga-DOTA-IPB-NAPamide, [205/206Bi]Bi-DOTA-NAPamide, [205/206Bi]Bi-DOTA-IPB-NAPamide, [177Lu]Lu-DOTA-NAPamide, [177Lu]Lu-DOTA-IPB-NAPamide
3.3.6. EDTA Challenge of [68Ga]Ga-DOTA-NAPamide, [68Ga]Ga-DOTA-IPB-NAPamide, [205/206Bi]Bi-DOTA-NAPamide, [205/206Bi]Bi-DOTA-IPB-NAPamide, [177Lu]Lu-DOTA-NAPamide, [177Lu]Lu-DOTA-IPB-NAPamide
3.3.7. Metal Challenge of [68Ga]Ga-DOTA-NAPamide, [68Ga]Ga-DOTA-IPB-NAPamide, [205/206Bi]Bi-DOTA-NAPamide, [205/206Bi]Bi-DOTA-IPB-NAPamide, [177Lu]Lu-DOTA-NAPamide, [177Lu]Lu-DOTA-IPB-NAPamide
3.4. Biology
3.4.1. Experimental Animals
3.4.2. Cell Lines
3.4.3. In Vivo Tumor Models
3.4.4. In Vivo Positron Emission Tomography (PET) Imaging
3.4.5. PET Data Assessment
3.4.6. In Vivo Single-Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) Acquisition
3.4.7. Ex Vivo Organ Distribution Experiments
3.4.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, W.; Solca, F.; Stutz, S.; Giuffrè, L.; Carrel, S.; Girard, J.; Eberle, A.N. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989, 49, 6352–6358. [Google Scholar] [PubMed]
- Eberle, A.N.; Bapst, J.-P.; Calame, M.; Tanner, H.; Froidevaux, S. MSH radiopeptides for melanoma targeting. Adv. Exp. Med. Biol. 2010, 681, 133–142. [Google Scholar]
- Froidevaux, S.; Calame-Christe, M.; Tanner, H.; Sumanovski, L.; Eberle, A.N. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J. Nucl. Med. 2002, 43, 1699–1706. [Google Scholar]
- Bapst, J.-P.; Eberle, A.N. Receptor-Mediated Melanoma Targeting with Radiolabeled α-Melanocyte-Stimulating Hormone: Relevance of the Net Charge of the Ligand. Front. Endocrinol. 2017, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Bapst, J.-P.; Calame, M.; Tanner, H.; Eberle, A.N. Glycosylated DOTA−α-Melanocyte-Stimulating Hormone Analogues for Melanoma Targeting: Influence of the Site of Glycosylation on in Vivo Biodistribution. Bioconj. Chem. 2009, 20, 984. [Google Scholar] [CrossRef]
- Ambrosini, V.; Fani, M.; Fanti, S.; Forrer, F.; Maecke, H.R. Radiopeptide imaging and therapy in Europe. J. Nucl. Med. 2011, 52, 42s–55s. [Google Scholar] [CrossRef]
- Gharibkandi, N.A.; Conlon, J.N.; Hosseinimehr, S.J. Strategies for improving stability and pharmacokinetic characteristics of radiolabeled peptides for imaging and therapy. Peptides 2020, 133, 170385. [Google Scholar] [CrossRef]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. 177Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Schibli, R.; Müller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties to Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Kolasińska-Ćwikła, A.; Łowczak, A.; Maciejkiewicz, K.M.; Ćwikła, J.B. Peptide Receptor Radionuclide Therapy for Advanced Gastroenteropancreatic Neuroendocrine Tumors—From oncology perspective. Nucl. Med. Rev. 2018, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted alpha therapy with bismuth-213 and actinium-225: Meeting future demand. J. Label. Compd. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, L.; Graves, E.; Xiong, Z.; Dandekar, M.; Chen, X.; Gambhir, S.S. Small-animal PET of melanocortin 1 receptor expression using a 18F-labeled alpha-melanocyte-stimulating hormone analog. J. Nucl. Med. 2007, 48, 987–994. [Google Scholar] [CrossRef]
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiong, Z.; Subbarayan, M.; Chen, X.; Gambhir, S.S. 64Cu-labeled alphamelanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjug. Chem. 2007, 18, 765–772. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Huang, X.; Moberg, V.E.; Barkey, N.M.; Sondak, V.K.; Tian, H.; Morse, D.L.; Vagner, J. Synthesis and characterization of a melanoma-targeted fluorescence imaging probe by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. Bioconjug. Chem. 2012, 23, 2451–2459. [Google Scholar] [CrossRef]
- Brandt, F.; Ullrich, M.; Laube, M.; Kopka, K.; Bachmann, M.; Löser, R.; Pietzsch, J.; Pietzsch, H.-J.; van den Hoff, J.; Wodtke, R. “Clickable” Albumin Binders for Modulating the Tumor Uptake of Targeted Radiopharmaceuticals. J. Med. Chem. 2022, 65, 710–733. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, T.; Liu, C.; Guo, X.; Wang, F.; Zhu, H.; Yang, Z. An Albumin-Binding PSMA Ligand with Higher Tumor Accumulation for PET Imaging of Prostate Cancer. Pharmaceuticals 2022, 15, 513. [Google Scholar] [CrossRef]
- Froidevaux, S.; Calame-Christe, M.; Schuhmacher, J.; Tanner, H.; Saffrich, R.; Henze, M.; Eberle, A.N. A gallium-labeled DOTA-alpha-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J. Nucl. Med. 2004, 45, 116–123. [Google Scholar]
- De Jong, M.; Breeman, W.A.; Bernard, B.F.; van Gameren, A.; de Bruin, E.; Bakker, W.H.; van der Pluijm, M.E.; Visser, T.J.; Mäcke, H.R.; Krenning, E.P. Tumour uptake of the radiolabelled somatostatin analogue [DOTA0, TYR3]octreotide is dependent on the peptide amount. Eur. J. Nucl. Med. 1999, 26, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, S.; Calame-Christe, M.; Tanner, H.; Eberle, A.N. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: Structural parameters affecting tumor uptake and kidney uptake. J. Nucl. Med. 2005, 46, 887–895. [Google Scholar] [PubMed]
- Cheng, Z.; Chen, J.; Miao, Y.; Owen, N.K.; Quinn, T.P.; Jurisson, S.S. Modification of the structure of a metallopeptide: Synthesis and biological evaluation of (111)In-labeled DOTA-conjugated rhenium-cyclized alpha-MSH analogues. J. Med. Chem. 2002, 45, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Kálmán-Szabó, I.; Képes, Z.; Fekete, A.; Vágner, A.; Nagy, G.; Szücs, D.; Gyuricza, B.; Arató, V.; Varg, J.; Kárpáti, L.; et al. In Vivo evaluation of newly synthesized 213Bi-conjugated alpha-melanocyte stimulating hormone (α-MSH) peptide analogues in melanocortin-1 receptor (MC1-R) positive experimental melanoma model. J. Pharm. Biomed. Anal. 2023, 229, 115374. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg. Med. Chem. Lett. 2013, 23, 2319–2323. [Google Scholar] [CrossRef]

| Organ/Tissue | [68Ga]Ga-DOTA-NAPamide (%ID/g) | [68Ga]Ga-DOTA-IPB-NAPamide (%ID/g) |
|---|---|---|
| blood | 0.35 ± 0.10 | 14.21 ± 1.87 ** |
| liver | 0.31 ± 0.08 | 2.64 ± 0.11 ** |
| spleen | 0.15 ± 0.02 | 2.37 ± 0.25 ** |
| kidney | 3.07 ± 0.35 | 4.57 ± 0.88 |
| small intestine | 0.35 ± 0.13 | 2.41 ± 0.03 ** |
| large intestine | 0.30 ± 0.01 | 0.92 ± 1.25 |
| stomach | 0.24 ± 0.09 | 2.97 ± 1.38 ** |
| muscle | 0.08 ± 0.02 | 1.01 ± 0.18 ** |
| lung | 0.33 ± 0.06 | 6.32 ± 0.91 ** |
| heart | 0.10 ± 0.06 | 5.14 ± 0.72 ** |
| brain | 0.03 ± 0.01 | 0.28 ± 0.02 ** |
| fat | 0.16 ± 0.03 | 2.02 ± 0.65 ** |
| B16-F10 tumor | 1.18 ± 0.27 | 5.06 ± 1.08 ** |
| Organ/Tissue | [205/206Bi]Bi-DOTA-NAPamide (%ID/g) | [205/206Bi]Bi-DOTA-IPB-NAPamide (%ID/g) |
|---|---|---|
| blood | 0.34 ± 0.08 | 13.75 ± 3.58 ** |
| liver | 1.09 ± 0.17 | 2.54 ± 0.91 * |
| spleen | 0.53 ± 0.12 | 2.07 ± 1.35 ** |
| kidney | 8.78 ± 3.61 | 3.85 ± 0.55 |
| small intestine | 0.31 ± 0.19 | 1.99 ± 0.21 ** |
| large intestine | 0.39 ± 0.11 | 1.02 ± 0.86 |
| stomach | 0.18 ± 0.07 | 1.59 ± 1.11 ** |
| muscle | 0.04 ± 0.02 | 1.20 ± 0.75 ** |
| lung | 0.44 ± 0.08 | 6.14 ± 1.62 ** |
| heart | 0.16 ± 0.03 | 4.52 ± 1.13 ** |
| brain | 0.04 ± 0.03 | 0.25 ± 0.10 ** |
| fat | 0.03 ± 0.02 | 1.50 ± 0.96 ** |
| B16-F10 tumor | 3.14 ± 0.32 | 4.50 ± 0.98 * |
| Organ/Tissue | [177Lu]Lu-DOTA-NAPamide (%ID/g) | [177Lu]Lu-DOTA-IPB-NAPamide (%ID/g) |
|---|---|---|
| blood | 0.02 ± 0.02 | 3.71 ± 0.73 ** |
| liver | 0.24 ± 0.13 | 1.97 ± 0.13 ** |
| spleen | 0.07 ± 0.04 | 1.32 ± 0.20 ** |
| kidney | 1.85 ± 0.79 | 5.58 ± 1.59 |
| small intestine | 0.01 ± 0.01 | 0.60 ± 0.03 ** |
| large intestine | 0.02 ± 0.02 | 0.61 ± 0.11 ** |
| stomach | 0.02 ± 0.01 | 0.69 ± 0.11 ** |
| muscle | 0.00 ± 0.00 | 0.33 ± 0.07 ** |
| lung | 0.04 ± 0.03 | 2.50 ± 0.24 ** |
| heart | 0.01 ± 0.00 | 1.30 ± 0.12 ** |
| brain | 0.00 ± 0.00 | 0.08 ± 0.01 ** |
| fat | 0.01 ± 0.01 | 0.67 ± 0.34 ** |
| B16-F10 tumor | 0.16 ± 0.15 | 4.16 ± 1.86 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szücs, D.; Szabó, J.P.; Arató, V.; Gyuricza, B.; Szikra, D.; Tóth, I.; Képes, Z.; Trencsényi, G.; Fekete, A. Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals. Pharmaceuticals 2023, 16, 1280. https://doi.org/10.3390/ph16091280
Szücs D, Szabó JP, Arató V, Gyuricza B, Szikra D, Tóth I, Képes Z, Trencsényi G, Fekete A. Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals. Pharmaceuticals. 2023; 16(9):1280. https://doi.org/10.3390/ph16091280
Chicago/Turabian StyleSzücs, Dániel, Judit P. Szabó, Viktória Arató, Barbara Gyuricza, Dezső Szikra, Imre Tóth, Zita Képes, György Trencsényi, and Anikó Fekete. 2023. "Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals" Pharmaceuticals 16, no. 9: 1280. https://doi.org/10.3390/ph16091280
APA StyleSzücs, D., Szabó, J. P., Arató, V., Gyuricza, B., Szikra, D., Tóth, I., Képes, Z., Trencsényi, G., & Fekete, A. (2023). Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals. Pharmaceuticals, 16(9), 1280. https://doi.org/10.3390/ph16091280