Protective Mechanism Pathway of Swietenia macrophylla Extract Nanoparticles against Cardiac Cell Damage in Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. Qualitative Phytochemicals Analysis of S. macrophylla Extract Nanoparticles
2.2. Quantitative Phytochemical Analysis of S. macrophylla Extract Nanoparticles
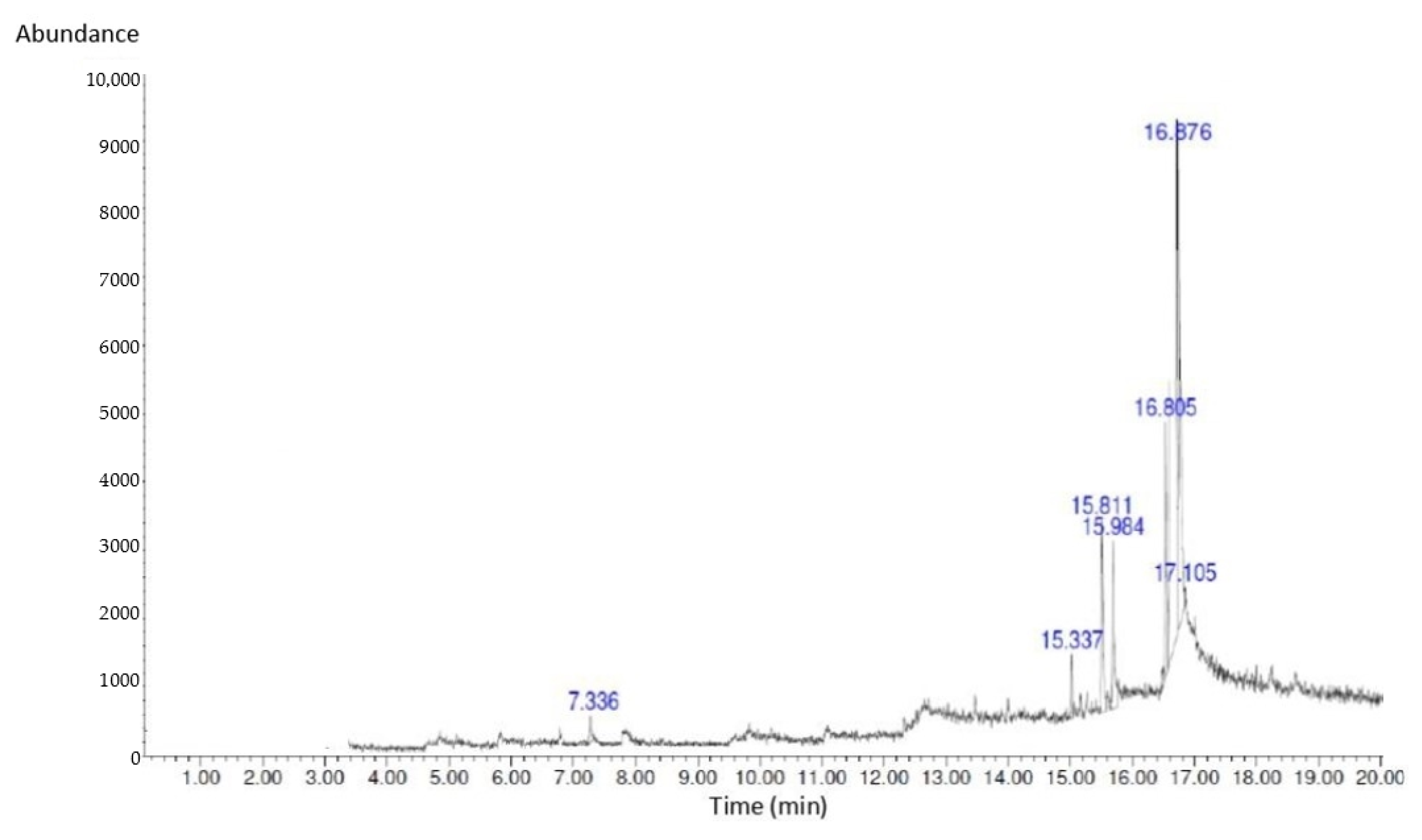
2.3. GC-MS Analysis of Bioactive Compounds in S. macrophylla Extract Nanoparticles
2.4. The Size Distribution of S. macrophylla Extract Nanoparticles
2.5. S. macrophylla Extract Nanoparticles’ Effect on Level of CK-MB and LDH in Serum of Diabetic Rats
2.6. S. macrophylla Extract Nanoparticles’ Effect on Cardiac Tissue MDA Levels in Diabetic Rats
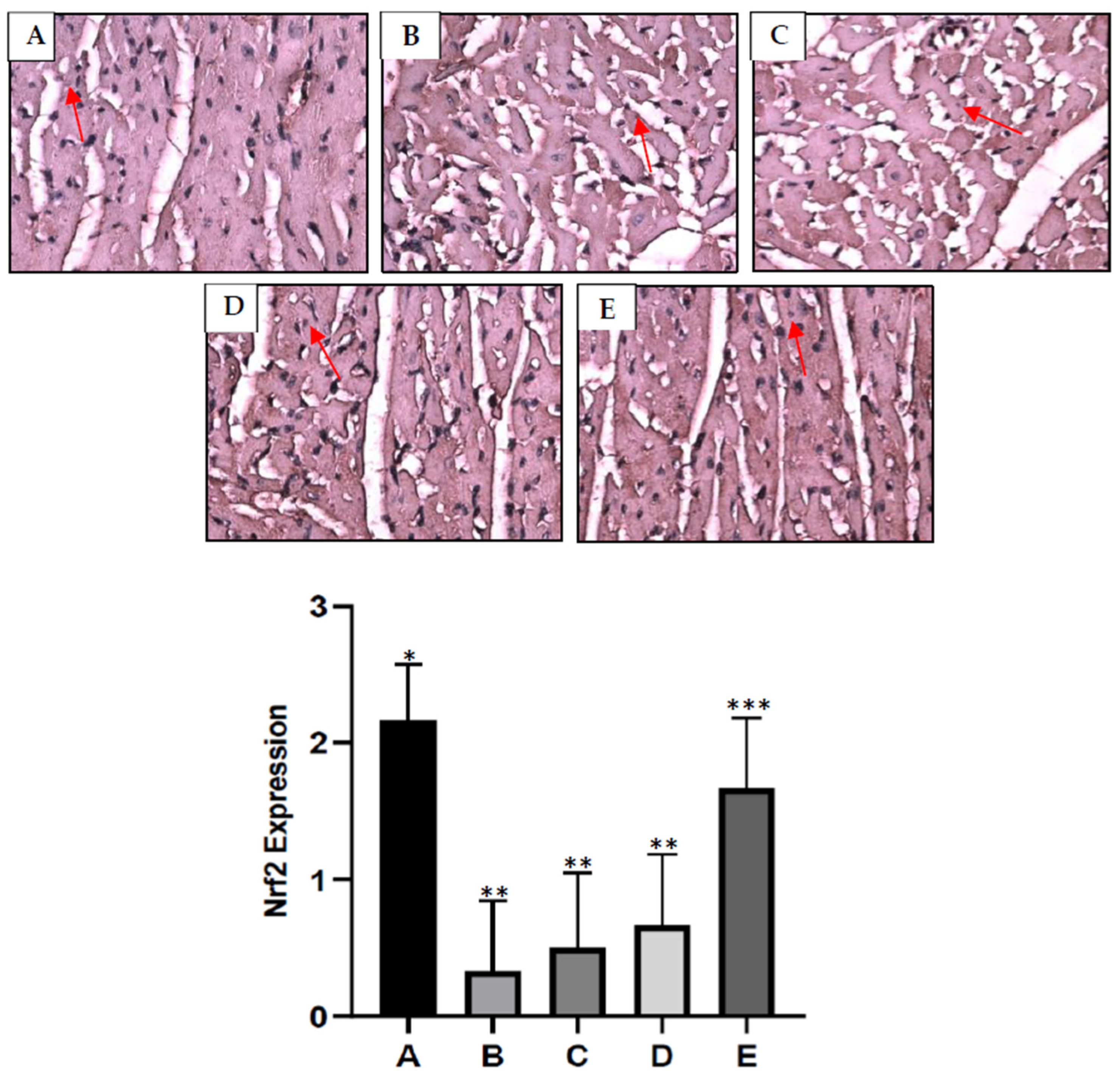
2.7. S. macrophylla Extract Nanoparticles’ Effect on Cardiac Tissue Nrf2 Expression in Diabetic Rats
2.8. S. macrophylla Extract Nanoparticles’ Effect on Cardiac Tissue SOD and GPx Levels in Diabetic Rats
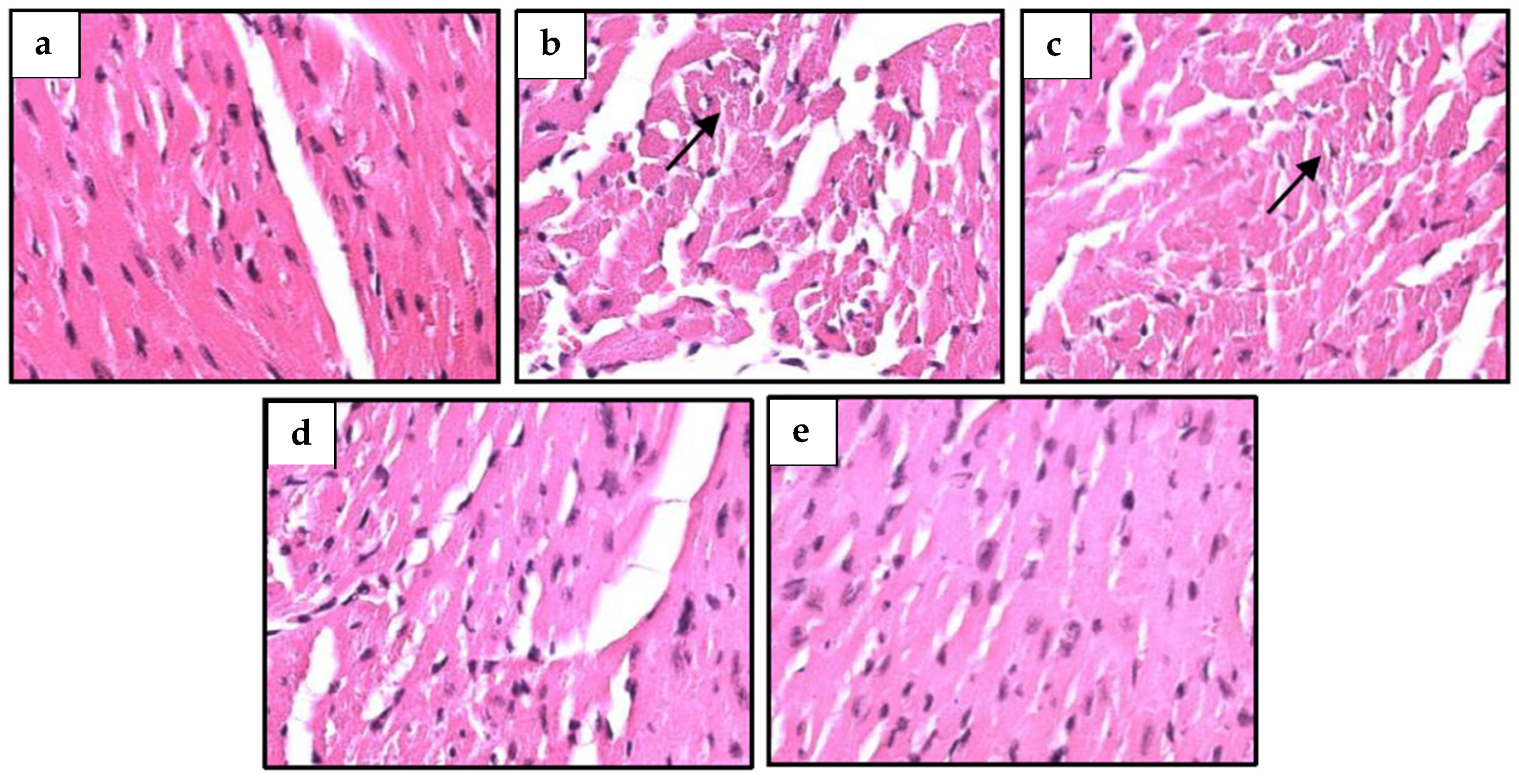
2.9. S. macrophylla Extract Nanoparticles’ Effect on Structural Change in Diabetic Rats’ Cardiac Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of S. macrophylla Extract
4.2. The Manufacturing of S. macrophylla Extract Nanoparticles
4.3. Qualitative Phytochemical Screening of S. macrophylla Extract Nanoparticles
4.3.1. Test for Alkaloids
4.3.2. Test for Flavonoids
4.3.3. Test for Phenols
4.3.4. Test for Saponin
4.3.5. Test for Terpenoids
4.3.6. Test for Tanin
4.4. Quantitative Phytochemical Screening of S. macrophylla Extract Nanoparticles
4.4.1. Total Phenols
4.4.2. Total Flavonoids
4.4.3. Total Alkaloids
4.5. GC-MS Analysis of Bioactive Compounds in S. macrophylla Extract Nanoparticles
4.6. Experimental of Animal
4.7. Model of Diabetic Rat
4.8. Experimental Design
4.9. Biochemical Estimation of Serum CM-KB and LDH
4.10. Assessment of Cardiac Tissue MDA Levels
4.11. Immunohistochemical Staining of Nrf2 Expression in Cardiac Tissue
4.12. Assessment of Cardiac Tissue SOD and GPx Expression
4.13. Histopathological Observations
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dare, A.; Elrashedy, A.A.; Channa, M.L.; Nadar, A. Cardioprotective effects and in-silico antioxidant mechanism of L-Ergothioneine in experimental type 2 diabetic rats. Cardiovasc. Hematol. Agents Med. Chem. 2022, 20, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Anjalil, S.; Pillai, N.; Soumya, P.; Modal, S.; Mini, S. Cardioprotective effect of Ferulic acid in streptozotocin-induced diabetic rats. Bioact. Compd. Health Dis. 2022, 5, 149–159. [Google Scholar]
- Mandal, M.; Varghese, A.; Gaviraju, V.K.; Talwar, S.N.; Malini, S.S. Impact of hyperglycemia on molecular marker of oxidative stress and antioxidants in type 2 diabetes mellitus. Clin. Diabetol. 2019, 8, 215–222. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Rafieian-Kopaei, M. Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431–438. [Google Scholar]
- Chen, F.; Zhang, H.; He, D.; Rao, C.; Xu, B. Cardioprotective effect of Gynostemma pentaphyllum against streptozotocin induced cardiac toxicity in rats via alteration of AMPK.Nrf2/HO-1 pathway. J. Oleo Sci. 2022, 71, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Wardani, G.; Nugraha, J.; Mustafa, M.R.; Sudjarwo, S.A. Antioxidative stress and ant-inflamatory activity of fucoidan nanoparticles against nephropathy of streptozotocin-induced diabetes in rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, S.; Zhou, Y.; Tang, Z.; Li, F. Mulberry granules protect against diabetic cardiomyopathy through the AMPK.Nrf2 pathway. Int. J. Mol. Med. 2017, 40, 913–921. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Wardani, G.; Nugraha, J.; Kurnijasanti, R.; Mustafa, M.R.; Sudjarwo, S.A. Molecular mechanism of fucoidan nanoparticles as protector on endothelial cell dysfunction in diabetic rats aorta. Nutrients 2023, 15, 568. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species and diabetic complications. Cell Death Dis. 2018, 9, 119–131. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Algrfare, A.I.; Al-Swailmil Mahmoud, A.M. Galangin Attenuate diabetic cardiomyopathy through modulating oaxidative stress, inflammation in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Sitasawad, S.L. Multiple Antioxidants improve cardiac complications and inhibit cardiac cell death in streptozotocin-induced diabetic rats. PLoS ONE 2013, 8, e67009. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, P.; Dong, C.; Zhang, J.; Wang, X.; Pei, H. Oxidative stress signalling mediated pathogenesis of diabetic cardiomyopathy. Oxid. Med. Cell. Longev. 2022, 2022, 5913374. [Google Scholar] [CrossRef]
- Birah, A.; Selvaraj, S.; Holla, S.R.; De, S. Extraction and characterization of total phenolic and flavonoid contents from bark od Swietenia macrophylla and their antimicrobial and antioxidant properties. Arab. J. Chem. 2022, 15, 104370. [Google Scholar] [CrossRef]
- Coello, F.P.; Azuaje, D.R.; Catari, I.P.; Marrero, M.P.; Vargas, C.O. Evaluation of the antioxidant activity of aqueous extracts of leaves and seeds of Swietenia macrophylla King by chemical and biological methods. J. Drug Res.Dev. 2020, 6, 106. [Google Scholar]
- Yu, L.Y.; Shi, W.L.; Guo, G.X. Cardioprotective role of gingerol along with prominent ati-diabetic cardiomyopathy action in a streptozotocin-induced diabetic rats model. Cell J. 2017, 19, 469. [Google Scholar]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Pathogenetic role in diabetes mellitus and its complications and therapeutic approach to correction. Bull. Exp. Biol. Med. 2020, 171, 136–149. [Google Scholar] [CrossRef]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complication of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Falah, S.; Safithri, M.; Katayama, T.; Suzuki, T. Hypoglycemic Effect of Mahogany (Swietenia macrophylla King) Bark Extracts in Alloxan-induced Diabetic Rats. Wood Res. J. 2010, 11, 89–95. [Google Scholar]
- Mohammed, S.B.; Azharin, N.H.; Mashitah, Y.M.; Abdurahman, N.H.; Mazza, A.S. In vitro antimicrobial activity and GC-MS analysis of medicinal plant Swietenia macrophylla King. J. Chem. Pharm. Res. 2015, 7, 519–524. [Google Scholar]
- Masendra.; Arisandi, R.; Purba, B.A.V.; Sumantri, F.; Ihda, F.V.; Wati, F.Z.; Lukmandaru, G. Extractives contributing to the color of Swietenia macrophylla Bark. Wood Res. J. 2020, 11, 20–26. [Google Scholar] [CrossRef]
- Hajra, S.; Mehta, A.; Pandey, P. Phenolic compound and antioxidant activity of Swietenia macrophylla seeds. Int. J. Pharm. Pharm. Sci. 2011, 3, 431–434. [Google Scholar]
- Eid, A.M.M.; Elmarzugi, N.A.; EL-enshasy, H.A. A review in the phytopharmacological effect of Swietenia macrophylla. Int. J. Pharm. Pharm. Sci. 2013, 5, 47–53. [Google Scholar]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drugs delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the nano-drug delvery system in treatment of cardiovascular Disease. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical Milling: A superior Nanothecnological tool for fabrication of nanocrystalline and nanocomposite materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, S.; Carillo-Ibarra, S.; Miranda-Dia, A.G. Oaxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Matsumoto, N.; Omagari, D.; Ushikhosi-Nakayama, R.; Yamazaki, T.; Inoue, H.; Saito, I. Hyperglycemia induces generation of reactive oxygen species and accelerates apoptotic cell death in salivary gland cells. Pathobiology 2021, 88, 234–241. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on Chemical Composition, Antioxidant and AntiInflammatory Activities of Hot Water Extract from Picea Mariana Bark and Its Proanthocyanidin-Rich Fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]

| No | Phytochemicals | Presence |
|---|---|---|
| 1 | Phenols | +++ |
| 2 | Flavonoids | +++ |
| 3 | Alkaloids | ++ |
| 4 | Saponins | ++ |
| 5 | Terpenoids | ++ |
| 6 | Tannins | ++ |
| No | Compound Name | RT (min) | Peak (%) | Peak Area |
|---|---|---|---|---|
| 1 | 1-Heptanol,4-methyl | 7.336 | 2.16 | 217,421.25 |
| 2 | Dihexylsulfide | 15.337 | 4.13 | 512,427.28 |
| 3 | Phenol,2,4-bis(1,1-dimethyl) | 15.811 | 7.56 | 839,873.46 |
| 4 | Piperidine | 15.984 | 10.94 | 1,345,839.14 |
| 5 | Imidazole-4,5-d2 | 16.805 | 11.75 | 1,701,547.29 |
| 6 | 7-Hexadecene | 16.876 | 15.64 | 1,936,913.72 |
| 7 | 1-Heptadecanol | 17.105 | 5.13 | 613,201.15 |
| Group | Mean ± SD | |
|---|---|---|
| CK-MB | LDH | |
| Control Rats | 78.4 a ± 2.53 | 108.7 a ± 3.41 |
| Diabetic Rats | 107.6 b ± 2.02 | 158.8 b ± 5.56 |
| S. macrophylla Nano 75 mg/kg BW | 108.7 b ± 3.04 | 154.2 b ± 4.44 |
| S. macrophylla Nano 150 mg/kg BW | 105.2 b ± 6.24 | 151.7 b ± 2.98 |
| S. macrophylla Nano 300 mg/kg BW | 91.7 c ± 2.85 | 133.7 c ± 2.99 |
| Group | Mean ± SD |
|---|---|
| MDA (nmol/mg Tissue) | |
| Control Rats | 50.8 a ± 4.02 |
| Diabetic Rats | 76.7 b ± 4.32 |
| S. macrophylla Nano 75 mg/kg BW | 80.0 b ± 2.83 |
| S. macrophylla Nano 150 mg/kg BW | 75.5 b ± 4.18 |
| S.macrophylla Nano 300 mg/kg BW | 60.5 c ± 3.08 |
| Group | Mean ± SD | |
|---|---|---|
| SOD (U/mg Protein) | GPx (U/mg Protein) | |
| Control Rats | 13.83 a ± 1.60 | 2.73 a ± 0.25 |
| Diabetic Rats | 6.67 b ± 0.82 | 0.78 b ± 0.08 |
| S. macrophylla Nano 75 mg/kg BW | 6.33 b ± 0.81 | 0.85 b ± 0.05 |
| S. macrophylla Nano 150 mg/kg BW | 7.17 b ± 0.75 | 0.88 b ± 0.08 |
| S. macrophylla Nano 300 mg/kgBW | 9.17 c ± 0.76 | 1.68 c ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurnijasanti, R.; Wardani, G.; Mustafa, M.R.; Sudjarwo, S.A. Protective Mechanism Pathway of Swietenia macrophylla Extract Nanoparticles against Cardiac Cell Damage in Diabetic Rats. Pharmaceuticals 2023, 16, 973. https://doi.org/10.3390/ph16070973
Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. Protective Mechanism Pathway of Swietenia macrophylla Extract Nanoparticles against Cardiac Cell Damage in Diabetic Rats. Pharmaceuticals. 2023; 16(7):973. https://doi.org/10.3390/ph16070973
Chicago/Turabian StyleKurnijasanti, Rochmah, Giftania Wardani, Mohd. Rais Mustafa, and Sri Agus Sudjarwo. 2023. "Protective Mechanism Pathway of Swietenia macrophylla Extract Nanoparticles against Cardiac Cell Damage in Diabetic Rats" Pharmaceuticals 16, no. 7: 973. https://doi.org/10.3390/ph16070973
APA StyleKurnijasanti, R., Wardani, G., Mustafa, M. R., & Sudjarwo, S. A. (2023). Protective Mechanism Pathway of Swietenia macrophylla Extract Nanoparticles against Cardiac Cell Damage in Diabetic Rats. Pharmaceuticals, 16(7), 973. https://doi.org/10.3390/ph16070973






