Neuroprotection in an Experimental Model of Multiple Sclerosis via Opening of Big Conductance, Calcium-Activated Potassium Channels
Abstract
1. Introduction
2. Results
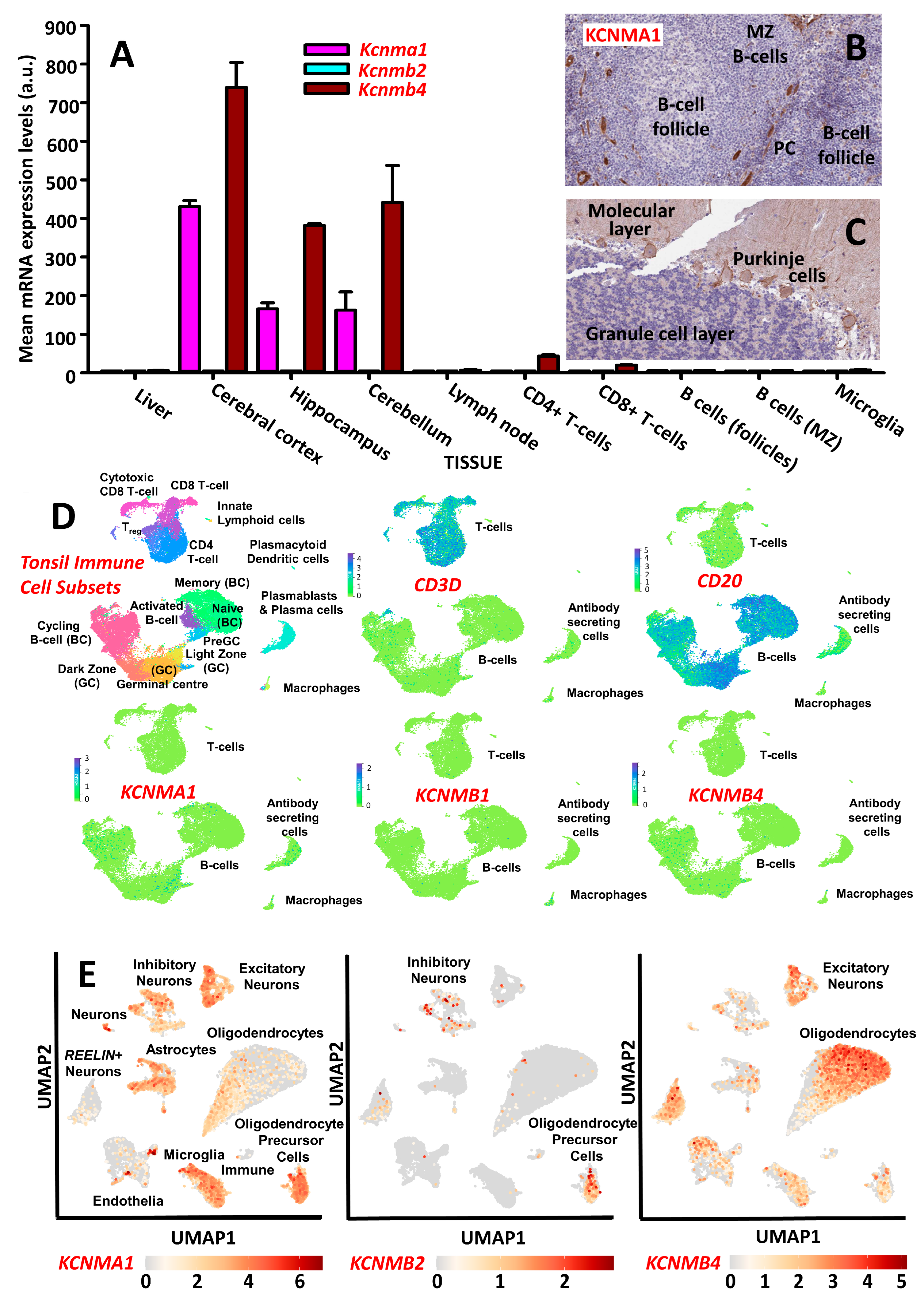
2.1. Tissue Expression of Neural BK Channel
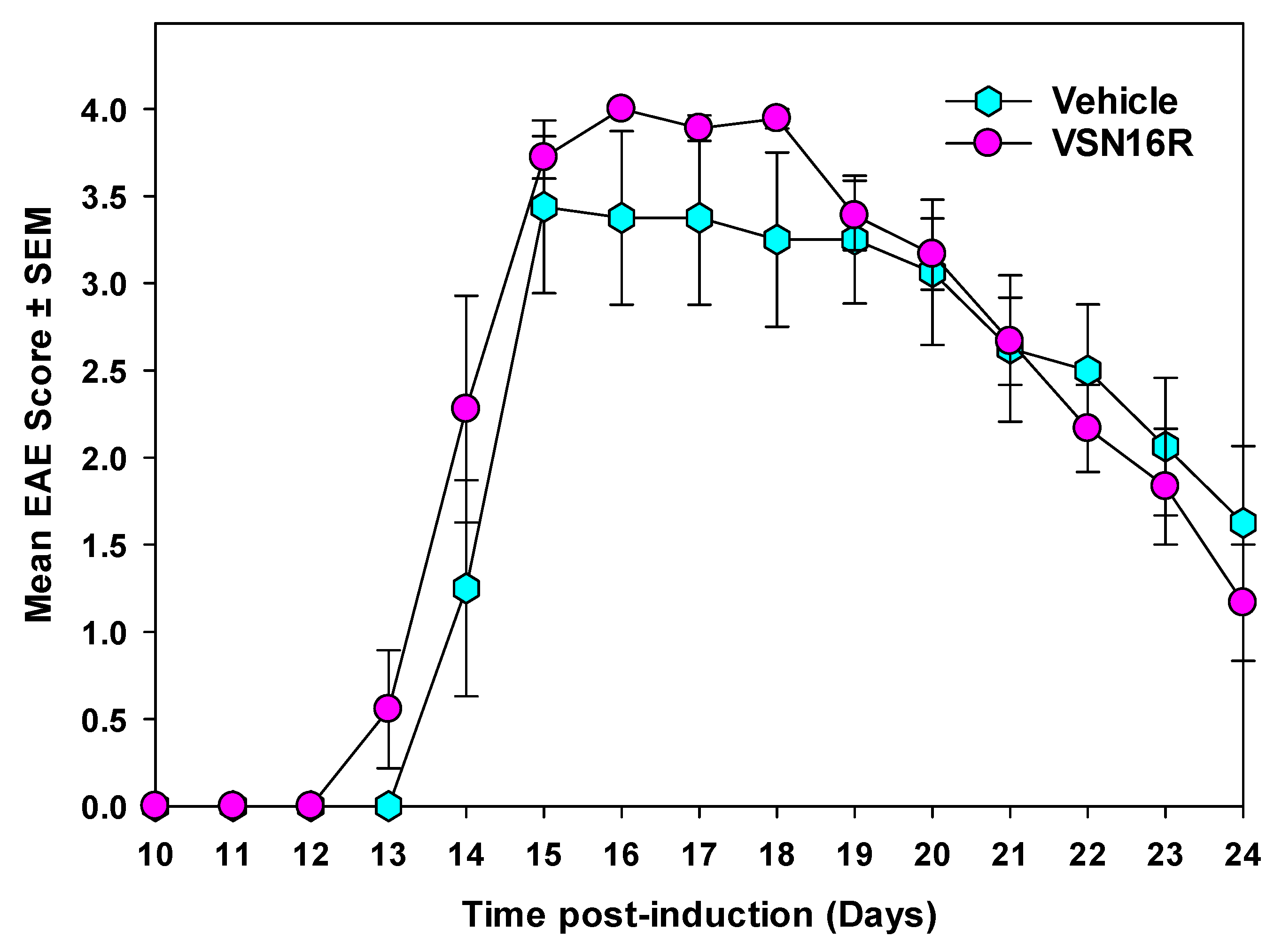
2.2. VSN16R Does Not Induce Immunosuppression
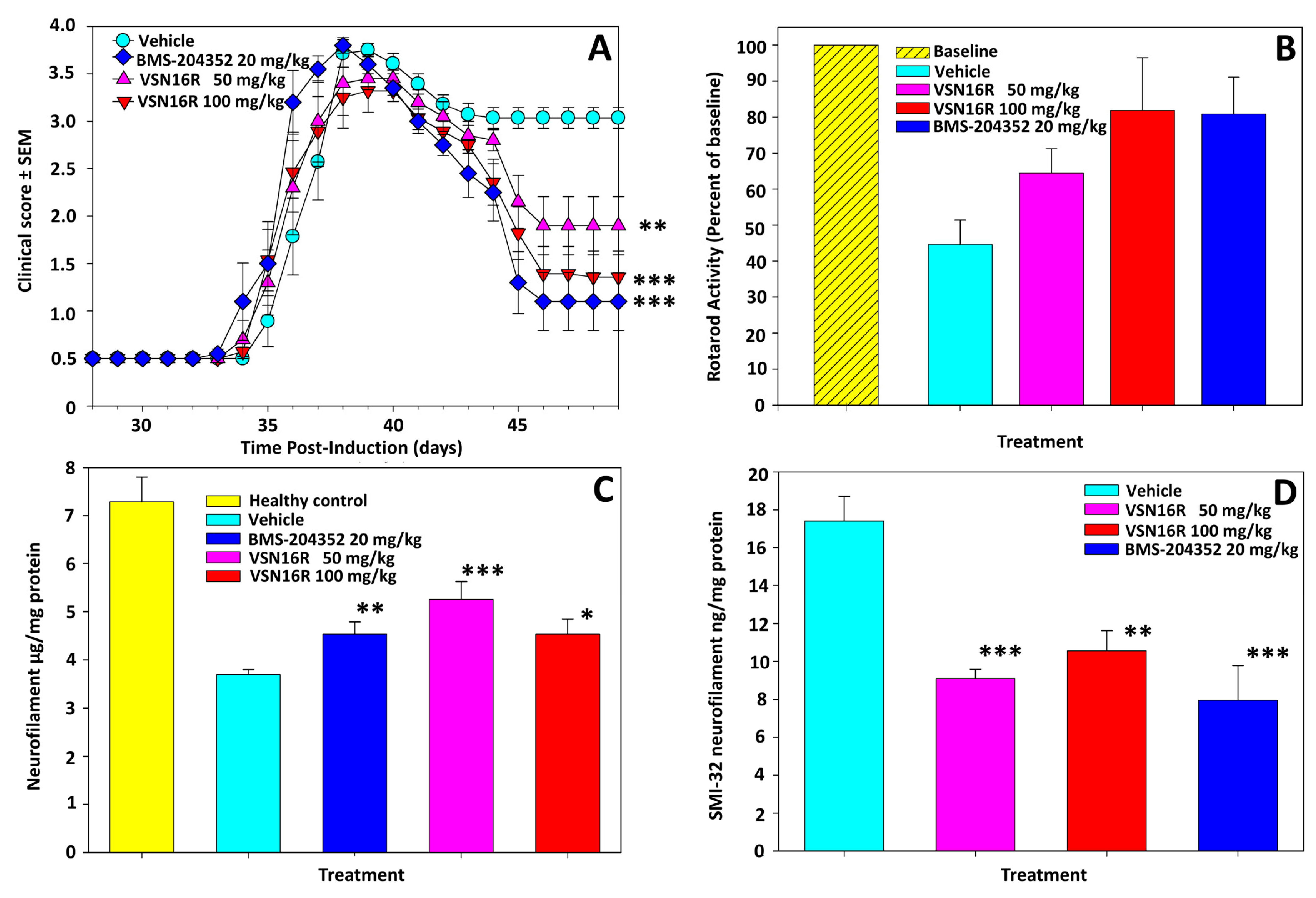
2.3. VSN16R and Neuroprotection
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Humans
4.3. Animals
4.4. Tissue Expression
4.5. Electrophysiology
4.6. Induction of Experimental Autoimmune Encephalomyelitis
4.7. Drug Treatment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hampton, D.W.; Serio, A.; Pryce, G.; Al-Izki, S.; Franklin, R.J.; Giovannoni, G.; Baker, D.; Chandran, S. Neurodegeneration progresses despite complete elimination of clinical relapses in a mouse model of multiple sclerosis. Acta Neuropathol. Commun. 2013, 1, 84. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Davies, M.; Blaker, P.A.; Hall, S.M.; Smith, K.J. Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann. Neurol. 2003, 53, 174–180. [Google Scholar] [CrossRef]
- Morsali, D.; Bechtold, D.; Lee, W.; Chauhdry, S.; Palchaudhuri, U.; Hassoon, P.; Snell, D.M.; Malpass, K.; Piers, T.; Pocock, J.; et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain 2013, 136, 1067–1082. [Google Scholar] [CrossRef]
- Al-Izki, S.; Pryce, G.; Hankey, D.J.; Lidster, K.; von Kutzleben, S.M.; Browne, L.; Clutterbuck, L.; Posada, C.; Edith Chan, A.W.; Amor, S.; et al. Lesional-targeting of neuroprotection to the inflammatory penumbra in experimental multiple sclerosis. Brain 2014, 137, 92–108. [Google Scholar] [CrossRef]
- Raftopoulos, R.; Hickman, S.J.; Toosy, A.; Sharrack, B.; Mallik, S.; Paling, D.; Altmann, D.R.; Yiannakas, M.C.; Malladi, P.; Sheridan, R.; et al. Phenytoin for neuroprotection in patients with acute optic neuritis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 259–269. [Google Scholar] [CrossRef]
- Shakespeare, D.T.; Boggild, M.; Young, C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst. Rev. 2003, 2003, CD001332. [Google Scholar] [CrossRef]
- Zaccara, G.; Giovannelli, F.; Giorgi, F.S.; Franco, V.; Gasparini, S.; Benedetto, U. Tolerability of new antiepileptic drugs: A network meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 811–917. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Furby, J.; Hayton, T.; Smith, K.J.; Altmann, D.R.; Brenner, R.; Chataway, J.; Hughes, R.A.; Miller, D.H. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: A randomized, double blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010, 9, 681–688. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Grant, D.; Morant, S.; Furby, J.; Hayton, T.; Teunissen, C.E.; Leoni, V.; Marta, M.; Brenner, R.; Palace, J.; et al. Biomarker report from the phase II lamotrigine trial in secondary progressive MS-neurofilament as a surrogate of disease progression. PLoS ONE 2013, 8, e70019. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Visintin, C.; Sisay, S.; Bondarenko, A.I.; Ho, W.S.V.; Jackson, S.J.; Williams, T.E.; Al-Izki, S.; Sevastou, I.; et al. Big conductance calcium-activated potassium channel openers control spasticity without sedation. Br. J. Pharmacol. 2017, 174, 2662–2681. [Google Scholar] [CrossRef]
- Nardi, A.; Olesen, S.P. BK channel modulators: A comprehensive overview. Curr. Med. Chem. 2008, 15, 1126–1146. [Google Scholar] [CrossRef]
- N’Gouemo, P. BKca channel dysfunction in neurological diseases. Front. Physiol. 2014, 5, 373. [Google Scholar] [PubMed]
- Hurley, M.J.; Deacon, R.M.J.; Chan, A.W.E.; Baker, D.; Selwood, D.L.; Cogram, P. Reversal of behavioural phenotype by the cannabinoid-like compound VSN16R in fragile X syndrome mice. Brain 2022, 145, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, S.; Song, H.J.; Park, Z.Y.; Park, C.S. Myelin basic protein as a binding partner and calmodulin adaptor for the BKCa channel. Proteomics 2007, 7, 2591–2602. [Google Scholar] [CrossRef]
- Li, B.Y.; Glazebrook, P.; Kunze, D.L.; Schild, J.H. KCa1.1 channel contributes to cell excitability in unmyelinated but not myelinated rat vagal afferents. American journal of physiology. Am. J. Physiol. Cell Physiol. 2011, 300, C1393–C1403. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.; Zivkovic, N.; Stojanovic, I. Multiple sclerosis and glutamate excitotoxicity. Rev. Neurosci. 2013, 24, 71–88. [Google Scholar] [CrossRef]
- Gao, K.; Lin, Z.; Wen, S.; Jiang, Y. Potassium channels and epilepsy. Acta Neurol. Scand. 2022, 146, 699–707. [Google Scholar] [CrossRef]
- Jensen, B.S. BMS-204352: A potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2002, 8, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Gribkoff, V.K.; Starrett, J.E., Jr.; Dworetzky, S.I.; Hewawasam, P.; Boissard, C.G.; Cook, D.A.; Frantz, S.W.; Heman, K.; Hibbard, J.R.; Huston, K.; et al. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat. Med. 2001, 7, 471–477. [Google Scholar] [CrossRef]
- Cheney, J.A.; Weisser, J.D.; Bareyre, F.M.; Laurer, H.L.; Saatman, K.E.; Raghupathi, R.; Gribkoff, V.; Starrett, J.E., Jr.; McIntosh, T.K. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J. Cereb. Blood Flow Metab. 2001, 21, 396–403. [Google Scholar] [CrossRef]
- Mancini, M.; Soldovieri, M.V.; Gessner, G.; Wissuwa, B.; Barrese, V.; Boscia, F.; Secondo, A.; Miceli, F.; Franco, C.; Ambrosino, P.; et al. Critical role of large-conductance calcium- and voltage-activated potassium channels in leptin-induced neuroprotection of N-methyl-d-aspartate-exposed cortical neurons. Pharmacol. Res. 2014, 87, 80–86. [Google Scholar] [CrossRef]
- Rupnik, M.; Baker, D.; Selwood, D.L. Oligodendrocytes, BK channels and the preservation of myelin. F1000Research 2021, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.C.; Dixon, A.K.; Campbell, E.A.; Tait, T.M.; Richardson, P.J.; Rice, K.M.; Maslen, G.L.; Metcalfe, A.D.; Streuli, C.H.; Bentley, D.R. Expression mapping of mouse genes. MGI Direct Data Submiss. 1998, 46439. Available online: https://www.informatics.jax.org/image/MGI:1205939 (accessed on 6 July 2023).
- Meera, P.; Wallner, M.; Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 2000, 97, 5562–5567. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Schicht, M.; Stamer, W.D.; Baker, D.; Sherwood, J.M.; Lütjen-Drecoll, E.; Selwood, D.L.; Overby, D.R. The β4-Subunit of the Large-Conductance Potassium Ion Channel KCa1.1 Regulates Outflow Facility in Mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef] [PubMed]
- Al-Izki, S.; Pryce, G.; O’Neill, J.K.; Butter, C.; Giovannoni, G.; Amor, S.; Baker, D. Practical guide to the induction of relapsing progressive experimental autoimmune encephalomyelitis in the Biozzi ABH mouse. Mult. Scler. Relat. Disord. 2012, 1, 29–38. [Google Scholar] [CrossRef]
- Wu, C.; Orozco, C.; Boyer, J.; Leglise, M.; Goodale, J.; Batalov, S.; Hodge, C.L.; Haase, J.; Janes, J.; Huss, J.W., 3rd; et al. BioGPS: An extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009, 10, R130. [Google Scholar] [CrossRef] [PubMed]
- Lattin, J.E.; Schroder, K.; Su, A.I.; Walker, J.R.; Zhang, J.; Wiltshire, T.; Saijo, K.; Glass, C.K.; Hume, D.A.; Kellie, S.; et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008, 4, 5. [Google Scholar] [CrossRef]
- Su, A.I.; Wiltshire, T.; Batalov, S.; Lapp, H.; Ching, K.A.; Block, D.; Zhang, J.; Soden, R.; Hayakawa, M.; Kreiman, G.; et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 2004, 101, 6062–6067. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Baillie, J.K.; Brown, H.; Freeman, T.C.; Hume, D.A. An expression atlas of human primary cells: Inference of gene function from coexpression networks. BMB Genom. 2013, 14, 632. [Google Scholar] [CrossRef]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. MCP 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- King, H.W.; Orban, N.; Riches, J.C.; Clear, A.J.; Warnes, G.; Teichmann, S.A.; James, L.K. Single-cell analysis of human B cell maturation predicts how antibody class switching shapes selection dynamics. Sci. Immunol. 2021, 6, eabe6291. [Google Scholar] [CrossRef] [PubMed]
- Seeker, L.A.; Bestard-Cuche, N.; Jäkel, S.; Kazakou, N.L.; Bøstrand, S.M.K.; Wagstaff, L.J.; Cholewa-Waclaw, J.; Kilpatrick, A.M.; Van Bruggen, D.; Kabbe, M.; et al. Brain matters: Unveiling the distinct contributions of region, age, and sex to glia diversity and CNS function. Acta Neuropathol. Commun. 2023, 11, 84. [Google Scholar] [CrossRef]
- Shi, J.; He, H.Q.; Zhao, R.; Duan, Y.H.; Chen, J.; Chen, Y.; Yang, J.; Zhang, J.W.; Shu, X.Q.; Zheng, P.; et al. Inhibition of martentoxin on neuronal BK channel subtype (alpha+beta4): Implications for a novel interaction model. Biophys. J. 2008, 94, 3706–3713. [Google Scholar] [CrossRef]
- Baker, D.; O’Neill, J.K.; Davison, A.N.; Turk, J.L. Control of immune-mediated disease of the central nervous system requires the use of a neuroactive agent: Elucidation by the action of mitoxantrone. Clin. Exp. Immunol. 1992, 90, 124–128. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.K.; Baker, D.; Davison, A.N.; Allen, S.J.; Butter, C.; Waldmann, H.; Turk, J.L. Control of immune-mediated disease of the central nervous system with monoclonal (CD4-specific) antibodies. J. Neuroimmunol. 1993, 45, 1–14. [Google Scholar] [CrossRef]
- Warne, J.; Pryce, G.; Hill, J.M.; Lennerås, F.; Kip, M.; Walker, P.; Chen, A.W.E.; Towers, G.; Duchen, M.; Szabadkai, G.; et al. Selective inhibition of the mitochondrial permeability transition pore protects against neurodegeneration in experimental multiple sclerosis. J. Biol. Chem. 2016, 291, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Hébert, B.; Pietropaolo, S.; Même, S.; Laudier, B.; Laugeray, A.; Doisne, N.; Quartier, A.; Lefeuvre, S.; Got, L.; Cahard, D.; et al. Rescue of fragile X syndrome phenotypes in Fmr1KO mice by a BKCa channel opener molecule. Orphanet. J. Rare Dis. 2014, 9, 124. [Google Scholar] [CrossRef]
- Gocke, A.R.; Lebson, L.A.; Grishkan, I.V.; Hu, L.; Nguyen, H.M.; Whartenby, K.A.; Chandy, K.G.; Calabresi, P.A. Kv1.3 deletion biases T cells toward an immunoregulatory phenotype and renders mice resistant to autoimmune encephalomyelitis. J. Immunol. 2012, 9, 5877–5886. [Google Scholar] [CrossRef]
- Reich, E.P.; Cui, L.; Yang, L.; Pugliese-Sivo, C.; Golovko, A.; Petro, M.; Vassileva, G.; Chu, I.; Nomeir, A.A.; Zhang, L.K.; et al. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur. J. Immunol. 2005, 35, 1027–1036. [Google Scholar] [CrossRef]
- Bittner, S.; Bauer, M.A.; Ehling, P.; Bobak, N.; Breuer, J.; Herrmann, A.M.; Golfels, M.; Wiendl, H.; Budde, T.; Meuth, S.G. The, TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp. Neurol. 2012, 238, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Li, X.; Li, L.; Li, F.; Li, T.; Peng, R.; Wang, C.; Wang, J.; Liu, X.; et al. NS1619 Alleviate brain-derived extracellular vesicle-induced brain injury by regulating BKca channel and Nrf2/HO-1/NF-ĸB Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 2257427. [Google Scholar] [CrossRef]
- Xue, M.; Chen, S.; Xi, J.; Guan, Q.; Chen, W.; Guo, Y.; Chen, Z. Protection against Hypoxia-Reoxygenation Injury of Hippocampal Neurons by H2S via Promoting Phosphorylation of ROCK2 at Tyr722 in Rat Model. Molecules 2022, 27, 4567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Piwońska, M.; Szewczyk, A.; Schröder, U.H.; Reymann, K.G.; Bednarczyk, I. Effectors of large-conductance calcium-activated potassium channel modulate glutamate excitotoxicity in organotypic hippocampal slice cultures. Acta Neurobiol. Exp. 2016, 76, 20–31. [Google Scholar] [CrossRef]
- Gautier, H.O.; Evans, K.A.; Volbracht, K.; James, R.; Sitnikov, S.; Lundgaard, I.; James, F.; Lao-Peregrin, C.; Reynolds, R.; Franklin, R.J.; et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 2015, 6, 8518. [Google Scholar] [CrossRef] [PubMed]
- Bezine, M.; Debbabi, M.; Nury, T.; Ben-Khalifa, R.; Samadi, M.; Cherkaoui-Malki, M.; Vejux, A.; Raas, Q.; de Sèze, J.; Moreau, T.; et al. Evidence of K+ homeostasis disruption in cellular dysfunction triggered by 7-ketocholesterol, 24S-hydroxycholesterol, and tetracosanoic acid (C24:0) in 158N murine oligodendrocytes. Chem. Phys. Lipids 2017, 207, 135–150. [Google Scholar] [CrossRef]
- Hawkins, V.; Butt, A. TASK-1 channels in oligodendrocytes: A role in ischemia mediated disruption. Neurobiol. Dis. 2013, 55, 87–94. [Google Scholar] [CrossRef]
- Bondarenko, A.I.; Panasiuk, O.; Okhai, I.; Montecucco, F.; Brandt, K.J.; Mach, F. Direct activation of Ca2+ and voltage-gated potassium channels of large conductance by anandamide in endothelial cells does not support the presence of endothelial atypical cannabinoid receptor. Eur. J. Pharmacol. 2017, 805, 14–24. [Google Scholar] [CrossRef]
- Moavero, R.; Santarone, M.E.; Galasso, C.; Curatolo, P. Cognitive and behavioral effects of new antiepileptic drugs in pediatric epilepsy. Brain Dev. 2017, 39, 464–469. [Google Scholar] [CrossRef]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Gomez, C.M.; McManus, D.; Mallik, S.; Hobart, J.; et al. CUPID investigator group. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Calabresi, P.A.; Barkhof, F.; Green, A.J.; Kardon, R.; Sastre-Garriga, J.; Schippling, S.; Vermersch, P.; Saidha, S.; Gerendas, B.S.; et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Annal Clin. Translat. Neurol. 2021, 8, 2235–2251. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lu, Z.Y.; Li, N.; Yu, L.H.; Zhao, Y.F.; Ma, B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia 2015, 35, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Hoi, P.M.; Visintin, C.; Okuyama, M.; Gardiner, S.M.; Kaup, S.S.; Bennett, T.; Baker, D.; Selwood, D.L.; Hiley, C.R. Vascular pharmacology of a novel cannabinoid-like compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl) benzamide (VSN16) in the rat. Br. J. Pharmacol. 2007, 152, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.H.; Wang, W.X.; Ye, J.G.; He, L.; Li, Y.J.; Yan, Y.P.; Zhou, Z. Martentoxin, a novel, K+-channel-blocking peptide: Purification, cDNA and genomic cloning, and electrophysiological and pharmacological characterization. J. Neurochem. 2003, 84, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Wang, B. Neurofilament light chain in blood as a diagnostic and predictive biomarker for multiple sclerosis: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0274565. [Google Scholar] [CrossRef]
- Kristensen, L.V.; Sandager-Nielsen, K.; Hansen, H.H. Kv7 (KCNQ) channel openers induce hypothermia in the mouse. Neurosci. Lett. 2011, 488, 178–182. [Google Scholar] [CrossRef]

| Treatment | Sex | n | RBC ×10−12/µL | Cell Numbers × 10−9/µL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils | Platelets | ||||
| A. Rats Treated for 4 Weeks QD | ||||||||||
| Vehicle 100 mg/kg 300 mg/kg 1000 mg/kg | Male Female Male Female Male Female Male Female | 10 9 8 10 9 9 8 10 | 7.86 ± 0.23 7.76 ± 0.20 7.78 ± 0.28 7.77 ± 0.34 7.84 ± 0.34 7.61 ± 0.27 7.97 ± 0.24 7.70 ± 0.16 | 12.19 ± 2.64 8.79 ± 2.67 10.28 ± 1.40 7.47 ± 1.58 13.12 ± 2.41 9.92 ± 4.09 12.63 ± 2.89 9.09 ± 2.55 | 1.52 ± 0.79 0.67 ± 0.18 1.10 ± 0.32 0.91 ± 0.52 2.03 ± 1.71 0.86 ± 0.29 1.70 ± 0.83 0.73 ± 0.31 | 9.99 ± 2.15 7.74 ± 2.54 8.47 ± 1.52 6.21 ± 1.11 10.33 ± 0.98 8.64 ± 3.80 10.24 ± 2.36 8.02 ± 2.30 | 0.24 ± 0.07 0.16 ± 0.04 0.24 ± 0.07 0.12 ± 0.05 0.29 ± 0.17 0.14 ± 0.11 0.27 ± 0.11 0.13 ± 0.08 | 0.12 ± 0.04 0.15 ± 0.05 0.16 ± 0.06 0.17 ± 0.08 0.12 ± 0.06 0.18 ± 0.12 0.12 ± 0.04 0.12 ± 0.0 | 0.16 ± 0.04 0.15 ± 0.05 0.17 ± 0.04 0.17 ± 0.08 0.19 ± 0.06 0.18 ± 0.12 0.18 ± 0.07 0.12 ± 0.06 | 894 ± 148 969 ± 162 1041 ± 184 892 ± 136 956 ± 275 1202 ± 526 1103 ± 181 1112 ± 190 |
| B. Dogs Treated for 4 Weeks QD | ||||||||||
| Vehicle 50 mg/kg 100 mg/kg 200 mg/kg | Male Female Male Female Male Female Male Female | 3 3 3 3 3 3 3 3 | 7.17 ± 0.28 6.75 ± 0.61 7.07 ± 0.45 6.90 ± 0.48 6.89 ± 0.24 7.04 ± 0.20 7.20 ± 0.30 6.47 ± 0.48 | 9.89 ± 1.34 9.15 ± 1.40 9.60 ± 2.58 10.87 ± 1.88 9.78 ± 1.55 9.58 ± 1.85 9.61 ± 0.58 9.34 ± 1.39 | 6.60 ± 1.15 5.37 ± 0.68 6.19 ± 1.97 7.24 ± 1.13 5.89 ± 1.00 6.23 ± 1.31 5.87 ± 1.97 6.00 ± 1.08 | 2.38 ± 0.32 2.89 ± 0.71 2.46 ± 0.43 2.53 ± 0.79 2.76 ± 0.46 2.51 ± 0.45 2.68 ± 0.51 2.47 ± 0.24 | 0.52 ± 0.13 0.45 ± 0.06 0.53 ± 0.18 0.44 ± 0.08 0.61 ± 0.05 0.45 ± 0.13 0.51 ± 0.06 0.49 ± 0.14 | 0.17 ± 0.14 0.21 ± 0.07 0.21 ± 0.09 0.29 ± 0.15 0.26 ± 0.13 0.14 ± 0.08 0.23 ± 0.12 0.19 ± 0.05 | 0.19 ± 0.04 0.20 ± 0.09 0.18 ± 0.04 0.32 ± 0.01 * 0.23 ± 0.06 0.22 ± 0.07 0.27 ± 0.06 0.16 ± 0.04 | 333 ± 37 337 ± 37 369 ± 41 458 ± 87 299 ± 124 397 ± 49 341 ± 20 358 ± 47 |
| C. Humans Treated for 1 Week BID | ||||||||||
| Placebo ~0.8 mg/kg ~3.3 mg/kg ~13 mg/kg | Male Male Male Male | 6 6 6 6 | 4.78 ± 0.32 5.00 ± 0.28 4.76 ± 0.22 4.78 ± 0.32 | 6.70 ± 1.40 5.00 ± 1.00 5.90 ± 1.70 6.70 ± 1.40 | 3.40 ± 1.20 2.10 ± 0.50 3.30 ± 1.40 3.40 ± 1.20 | 2.50 ± 0.70 2.00 ± 0.50 1.90 ± 0.60 2.50 ± 0.70 | 0.60 ± 0.10 0.60 ± 0.10 0.50 ± 0.20 0.60 ± 0.10 | 0.20 ± 0.10 0.20 ± 0.20 0.20 ± 0.20 0.20 ± 0.10 | 0.00 ± 0.10 0.00 ± 0.10 0.00 ± 0.00 0.00 ± 0.10 | 254 ± 47 220 ± 35 204 ± 49 254 ± 47 |
| Treatment | Dose | No. EAE/Total | Group Score | EAE Score | Day of Onset |
|---|---|---|---|---|---|
| Initial Paralytic Episode | |||||
| Untreated Vehicle VSN16R | - 0.1 mL H2O p.o. 40 mg/kg p.o. | 12/12 8/9 10/10 | 3.7 ± 0.2 3.5 ± 0.4 4.0 ± 0.0 | 3.7 ± 0.2 3.9 ± 0.1 4.0 ± 0.0 | 14.6 ± 2.8 15.0 ± 1.7 13.6 ± 1.5 |
| Induced-Relapse | |||||
| Vehicle BMS-204352 | 0.1 mL DCP i.p. 20 mg/kg i.p. | 12/12 10/10 | 4.0 ± 0.0 4.0 ± 0.0 | 4.0 ± 0.0 4.0 ± 0.0 | 36.3 ± 1.2 36.3 ± 0.9 |
| Vehicle VSN16R VSN16R BMS-204352 | 0.1 mL H2O p.o. 50 mg/kg p.o. 100 mg/kg p.o. 20 mg/kg i.p. | 16/16 15/15 14/15 14/15 | 3.9 ± 0.1 3.9 ± 0.2 3.5 ± 0.2 3.7 ± 0.0 | 3.9 ± 0.1 3.9 ± 0.2 3.8 ± 0.1 3.9 ± 0.0 | 36.9 ± 1.3 36.4 ± 1.5 36.1 ± 2.1 35.5 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryce, G.; Sisay, S.; Giovannoni, G.; Selwood, D.L.; Baker, D. Neuroprotection in an Experimental Model of Multiple Sclerosis via Opening of Big Conductance, Calcium-Activated Potassium Channels. Pharmaceuticals 2023, 16, 972. https://doi.org/10.3390/ph16070972
Pryce G, Sisay S, Giovannoni G, Selwood DL, Baker D. Neuroprotection in an Experimental Model of Multiple Sclerosis via Opening of Big Conductance, Calcium-Activated Potassium Channels. Pharmaceuticals. 2023; 16(7):972. https://doi.org/10.3390/ph16070972
Chicago/Turabian StylePryce, Gareth, Sofia Sisay, Gavin Giovannoni, David L. Selwood, and David Baker. 2023. "Neuroprotection in an Experimental Model of Multiple Sclerosis via Opening of Big Conductance, Calcium-Activated Potassium Channels" Pharmaceuticals 16, no. 7: 972. https://doi.org/10.3390/ph16070972
APA StylePryce, G., Sisay, S., Giovannoni, G., Selwood, D. L., & Baker, D. (2023). Neuroprotection in an Experimental Model of Multiple Sclerosis via Opening of Big Conductance, Calcium-Activated Potassium Channels. Pharmaceuticals, 16(7), 972. https://doi.org/10.3390/ph16070972








