Antibacterial Properties of Mesoporous Silica Nanoparticles Modified with Fluoroquinolones and Copper or Silver Species
Abstract
1. Introduction
2. Results
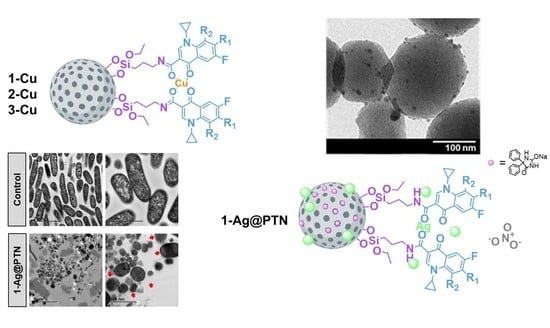
2.1. Synthesis and Physicochemical Characterization of Functionalized NPs

2.1.1. Analysis of Size, Morphology, and Textural Properties
2.1.2. Quantification of the Functionalization Degree by Thermogravimetry and Inductively Coupled Plasma Atomic Emission Spectroscopy
2.1.3. Characterization by Powder X-ray Diffraction Studies
2.2. In Vitro Studies of Antibacterial Activity
2.2.1. Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
2.2.2. Minimal Biofilm Inhibitory Concentration (MBIC) and Minimal Biofilm Eradication Concentration (MBEC)
2.2.3. Effect on Biofilm Development
2.2.4. Inhibition in Wound-like Medium
2.2.5. Bactericidal Mechanism of 1-Ag@PTN
2.2.6. Oxidative Stress Studies and Advanced Oxidation Protein Products (AOPP)
3. Materials and Methods
3.1. General Remarks on Characterization of the Materials
3.2. Synthesis of Mesoporous Silica Nanoparticles (MSNs)
3.3. Functionalization of Silica Materials with Amino Ligand: Synthesis of MSN–AP
3.4. Preparation of MSN–AP with Fluoroquinolone Ligand: Synthesis of Materials 1, 2, and 3
3.5. Preparation of Copper and Silver Materials
3.6. Preparation of 1-Ag@PTN Material
3.7. In Vitro Studies
3.7.1. Bacteria
3.7.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
3.7.3. Minimal Biofilm Inhibitory Concentration and Minimal Biofilm Eradication Concentration
3.7.4. Effect on Biofilm Development
3.7.5. Inhibition in Wound-like Medium
3.7.6. Bactericidal Mechanism of the 1-Ag@PTN Material Using TEM
3.7.7. Oxidative Stress Studies
3.7.8. Advanced Oxidation Protein Products (AOPP)
3.7.9. PTN Release Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015, 10, 354–355. [Google Scholar] [CrossRef]
- McKenna, M. Antibiotic resistance: The last resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Deva, A.K.; Adams, W.P.; Vickery, K. The role of bacterial biofilms in device-associated infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef]
- Castillo, R.R.; Vallet-Regí, M. Recent Advances toward the Use of Mesoporous Silica Nanoparticles for the Treatment of Bacterial Infections. Int. J. Nanomed. 2021, 16, 4409–4430. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.I.; MacGowan, A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Castro, W.; Navarro, M.; Biot, C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013, 5, 81–96. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- de Juan Mora, B.; Filipe, L.; Forte, A.; Santos, M.M.; Alves, C.; Teodoro, F.; Pedrosa, R.; Ribeiro Carrott, M.; Branco, L.C.; Gago, S. Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles. Pharmaceutics 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Alandiyjany, M.N.; Abdelaziz, A.S.; Abdelfattah-Hassan, A.; Hegazy, W.A.H.; Hassan, A.A.; Elazab, S.T.; Mohamed, E.A.A.; El-Shetry, E.S.; Saleh, A.A.; Elsawy, N.A.; et al. Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals 2022, 15, 357. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Ude, Z.; Flothkötter, N.; Sheehan, G.; Brennan, M.; Kavanagh, K.; Marmion, C.J. Multi-targeted metallo-ciprofloxacin derivatives rationally designed and developed to overcome antimicrobial resistance. Int. J. Antimicrob. Agents 2021, 58, 106449. [Google Scholar] [CrossRef]
- Telfer, A.J. Ciprofloxacin Metal Complexes and Linked Dimers as Potential Antimicrobial Agents; University of York: York, UK, 2020. [Google Scholar]
- Ferreira, M.; Gameiro, P.; Ruiz, J.; Pons, M.J. Fluoroquinolone-Transition Metal Complexes: A Strategy to Overcome Bacterial Resistance. Microorganisms 2021, 9, 1506. [Google Scholar] [CrossRef]
- Shaikh, A.; Giridhar, R.; Megraud, F.; Yadav, M.R. Metalloantibiotics: Synthesis, characterization and antimicrobial evaluation of bismuth-fluoroquinolone complexes against Helicobacter pylori. Acta Pharm. 2009, 59, 259–271. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Cristina Munteanu, A.; Uivarosi, V. Development perspectives of silver complexes with antibacterial quinolones: Successful or not? J. Organomet. Chem. 2017, 839, 19–30. [Google Scholar] [CrossRef]
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.P.; Carneiro, Z.A.; Ribeiro, C.M.; Lima, M.F.; Paixão, D.A.; Pivatto, M.; De Souza, M.V.N.; Teixeira, L.R.; Lopes, C.D.; De Albuquerque, S.; et al. Three new platinum complexes containing fluoroquinolones and DMSO: Cytotoxicity and evaluation against drug-resistant tuberculosis. J. Inorg. Biochem. 2018, 183, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Bessa, L.J.; Sousa, C.F.; Eaton, P.; Bongiorno, D.; Stefani, S.; Campanile, F.; Gameiro, P. Fluoroquinolone Metalloantibiotics: A Promising Approach against Methicillin-Resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health 2020, 17, 3127. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metal binding and antibacterial activity of ciprofloxacin complexes. J. Enzym. Inhib. Med. Chem. 2005, 20, 303–307. [Google Scholar] [CrossRef]
- El-Gamel, N.E.A. Silver(I) complexes as precursors to produce silver nanowires: Structure characterization, antimicrobial activity and cell viability. Dalt. Trans. 2013, 42, 9884–9892. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. Adv. Microb. Physiol. 2017, 70, 193–260. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Hasamnis, A.A.; Mohanty, B.K.; Muralikrishna; Patil, S. Evaluation of Wound Healing Effect of Topical Phenytoin on Excisional Wound in Albino Rats. J. Young Pharm. 2010, 2, 59–62. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M. Phenytoin repositioned in wound healing: Clinical experience spanning 60 years. Drug Discov. Today 2018, 23, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Liu, H.; Lu, C.; Liang, C.; Sun, Y.; Chen, S.; Sun, B.; Li, Y.; Liu, Y.; Zhang, Q.; et al. Phenytoin silver: A new nanocompound for promoting dermal wound healing via comprehensive pharmacological action. Theranostics 2017, 7, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Páez, P.L.; San Sebastian, E.; Gómez-Ruiz, S. Hybrid Nanosystems Based on Nicotinate-Functionalized Mesoporous Silica and Silver Chloride Nanoparticles Loaded with Phenytoin for Preventing Pseudomonas aeruginosa Biofilm Development. Pharmaceuticals 2022, 15, 884. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Cheng, B.; Lin, J. Synthesis, characterization and photocatalytic activity of mesoporous titania nanorod/titanate nanotube composites. J. Hazard. Mater. 2007, 147, 581–587. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A. A novel preparation of high surface area TiO2 nanoparticles from alkoxide precursor and using active carbon as additive. Catal. Today 2002, 76, 91–101. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Meng, X.; Zhang, Y.; Ren, L.; Nawaz, F.; Liu, J.; Li, Z.; Xiao, F.-S. Ordered hexagonal mesoporous silica materials (SBA-15) with additional disordered large-mesopore networks formed by gaseous expansion. Microporous Mesoporous Mater. 2010, 136, 126–131. [Google Scholar] [CrossRef]
- Serra, E.; Mayoral, Á.; Sakamoto, Y.; Blanco, R.M.; Díaz, I. Immobilization of lipase in ordered mesoporous materials: Effect of textural and structural parameters. Microporous Mesoporous Mater. 2008, 114, 201–213. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.R.; Díaz-Sánchez, M.; Mena-Palomo, I.; del Hierro, I.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Copper-functionalized nanostructured silica-based systems: Study of the antimicrobial applications and ROS generation against gram positive and gram negative bacteria. J. Inorg. Biochem. 2020, 203, 110912. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of Synergy between Tetracycline and Quercetin against Antibiotic Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Fang, Y.; Zhu, Z. Boosting antibacterial activity with mesoporous silica nanoparticles supported silver nanoclusters. J. Colloid Interface Sci. 2019, 555, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wei, Q.; Cao, B.; Cheng, X.; Tian, J.; Pu, H.; Yusufu, A.; Cao, L. A multifunctional bioactive material that stimulates osteogenesis and promotes the vascularization bone marrow stem cells and their resistance to bacterial infection. PLoS ONE 2017, 12, e0172499. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Zhu, Y.; Tao, J.; Zhu, F.; Chen, J.; Li, L.; Zhao, J.; Wang, L.; Sun, S.; Yang, Y.; et al. Alginate Lyase Guided Silver Nanocomposites for Eradicating Pseudomonas aeruginosa from Lungs. ACS Appl. Mater. Interfaces 2020, 12, 9050–9061. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Carias-Cálix, R.A.; Jiménez-Jiménez, C.; Esteban, J.; Vallet-Regí, M. Arabic gum plus colistin coated moxifloxacin-loaded nanoparticles for the treatment of bone infection caused by Escherichia coli. Acta Biomater. 2022, 137, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J. Antimicrob. Chemother. 2009, 64, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Skov Pedersen, J.; Arpanaei, A.; Meyer, R.L. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Rangasamy, S.; Purushothaman, B.; Song, J.M. The Application of Bactericidal Silver Nanoparticles in Wound Treatment. Nanomater. Nanotechnol. 2015, 5, 23. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Giachino, A.; Waldron, K.J. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020, 114, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Viviana, C.A.; Onnainty, R.; Mary, V.S.; Theumer, M.G.; Granero, G.E.; Paraje, M.G.; Páez, P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell Biol. 2018, 104, 87–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Wayne, C., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Hernandes, C.; Coppede, J.S.; Bertoni, B.W.; França, S.C.; Pereira, A.M.S. Flash microbiocide: A Rapid and Economic Method for Determination of MBC and MFC. Am. J. Plant Sci. 2013, 4, 850–852. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dowd, S.E.; Smith, E.; Rhoads, D.D.; Wolcott, R.D. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008, 16, 805–813. [Google Scholar] [CrossRef]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic Interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an In Vitro Wound Model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; Lvanov, S.; Van Esse, W.; Voets, G.; Fedorova, E.; Bisseling, T. Medicago N2-Fixing Symbiosomes Acquire the Endocytic Identity Marker Rab7 But Delay the Acquisition of Vacuolar Identity. Plant Cell 2009, 21, 2811–2828. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Wang, J.; Qiu, X.; Wei, W.; Zhao, J. Peptide-Mediated Aqueous Synthesis of NIR-II Emitting Ag2S Quantum Dots for Rapid Photocatalytic Bacteria Disinfection. Angew. Chem. Int. Ed. Eng. 2023, in press. [CrossRef]
- You, S.; Xiang, Y.; Qi, Y.; Mao, R.; Cai, E.; Lan, Y.; Lu, H.; Shen, J.; Deng, H. Harnessing a biopolymer hydrogel reinforced by copper/tannic acid nanosheets for treating bacteria-infected diabetic wounds. Mater. Today Adv. 2022, 15, 100271. [Google Scholar] [CrossRef]
- Xiong, J.; Cao, Y.; Zhao, H.; Chen, J.; Cai, X.; Li, X.; Liu, Y.; Xiao, H.; Ge, J. Cooperative Antibacterial Enzyme-Ag-Polymer Nanocomposites. ACS Nano 2022, 16, 19013–19024. [Google Scholar] [CrossRef]






| Material | BET Surface (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| MSN | 925 | 0.77 | 2.72 |
| 1-Cu | 45 | 0.09 | <2.00 |
| 1-Ag | 42 | 0.09 | <2.00 |
| 1-Ag@PTN | 27 | 0.07 | <2.00 |
| 2-Cu | 42 | 0.10 | <2.00 |
| 2-Ag | 39 | 0.11 | <2.00 |
| 3-Cu | 144 | 0.12 | <2.00 |
| 3-Ag | 39 | 0.10 | <2.00 |
| Material | AP | FQ | Cu | Ag | PTN |
|---|---|---|---|---|---|
| 1-Cu | 0.70 | 0.28 | 0.20 | - | - |
| 1-Ag | 0.70 | 0.28 | - | 0.32 | - |
| 1-Ag@PTN | 0.70 | 0.28 | - | 0.23 | 1.16 |
| 2-Cu | 0.70 | 0.29 | 0.15 | - | - |
| 2-Ag | 0.70 | 0.29 | - | 0.13 | - |
| 3-Cu | 0.70 | 0.36 | 0.13 | - | - |
| 3-Ag | 0.70 | 0.36 | - | 0.10 | - |
| Material | S. aureus ATCC 29213 | E. faecalis ATCC 29212 | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| MSN | 500 | 1000 | 500 | 1000 |
| 1-Cu | 250 (3.25) | 250 (3.25) | >2000 (26.00) | >2000 (26.00) |
| 2-Cu | 500 (4.50) | 500 (4.50) | >2000 (18.00) | >2000 (18.00) |
| 3-Cu | 250 (2.00) | 500 (4.00) | >2000 (16.00) | >2000 (16.00) |
| 1-Ag | 500 (17.50) | >2000 (70.00) | 1000 (35.00) | >2000 (70.00) |
| 2-Ag | 125 (1.88) | >2000 (30.00) | 500 (7.50) | >2000 (30.00) |
| 3-Ag | 1000 (10.00) | >2000 (20.00) | 1000 (10.00) | >2000 (20.00) |
| Material | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | ||
| MIC | MBC | MIC | MBC | |
| MSN | 250 | 1000 | 250 | 500 |
| 1-Cu | 15.62 (0.20) | 15.62 (0.20) | >2000 (26.00) | >2000 (26.00) |
| 2-Cu | 15.62 (0.14) | 15.62 (0.14) | >2000 (18.00) | >2000 (18.00) |
| 3-Cu | 15.62 (0.12) | 31.25 (0.25) | >2000 (16.00) | >2000 (16.00) |
| 1-Ag | 250 (8.75) | 500 (17.50) | 125 (4.38) | 1000 (35.00) |
| 2-Ag | 250 (3.75) | 250 (3.75) | 125 (1.88) | 250 (3.75) |
| 3-Ag | 250 (2.50) | 500 (5.00) | 250 (2.50) | 2000 (20.00) |
| Material | S. aureus MRSA1 | S. aureus MRSA2 | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| 1-Cu | >2000 (26.00) | >2000 (26.00) | >2000 (26.00) | >2000 (26.00) |
| 2-Cu | >2000 (18.00) | >2000 (18.00) | >2000 (18.00) | >2000 (18.00) |
| 3-Cu | >2000 (16.00) | >2000 (16.00) | >2000 (16.00) | >2000 (16.00) |
| 1-Ag | 1000 (35.00) | 2000 (70.00) | 2000 (70.00) | >2000 (70.00) |
| 2-Ag | 1000 (15.00) | 2000 (30.00) | 1000 (15.00) | >2000 (30.00) |
| 3-Ag | 1000 (10.00) | 2000 (20.00) | 1000 (10.00) | 2000 (20.00) |
| Material | P. aeruginosa PA8 | P. aeruginosa PA13 | ||
| MIC | MBC | MIC | MBC | |
| 1-Ag | 62.5 (2.19) | 250 (8.75) | 31.25 (1.09) | 500 (17.50) |
| 2-Ag | 62.5 (0.94) | 125 (1.88) | 31.25 (0.47) | 500 (7.50) |
| 3-Ag | 62.5 (0.63) | 125 (1.25) | 31.25 (0.31) | 500 (5.00) |
| Material | ATCC 27853 | PA8 | P. aeruginosa PA13 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| 1-Ag@PTN | 15.625 (0.39) | 250 (6.25) | 15.625 (0.39) | 125 (3.13) | 15.625 (0.39) | 250 (6.25) |
| Material | ATCC 27853 | PA8 | PA13 | |||
|---|---|---|---|---|---|---|
| MBIC | MBEC | MBIC | MBEC | MBIC | MBEC | |
| 1-Ag | 250 (8.75) | >1000 (35.00) | 500 (17.50) | 1000 (35.00) | 500 (17.50) | >1000 (35.00) |
| 1-Ag@PTN | 250 (6.25) | >1000 (25.00) | 250 (6.25) | 1000 (25.00) | 1000 (25.00) | >1000 (25.00) |
| 2-Ag | 250 (3.75) | >1000 (15.00) | 500 (7.50) | 1000 (15.00) | 500 (7.50) | >1000 (15.00) |
| 3-Ag | 500 (5.00) | >1000 (10.00) | 500 (5.00) | 1000 (10.00) | 500 (5.00) | >1000 (10.00) |
| S. aureus | ||||||
|---|---|---|---|---|---|---|
| Material | ROS | AOPP | ||||
| Concentration | t (h) | % | Concentration | t (h) | % | |
| 1-Cu | MIC/10 | 4 | 195 ± 19 | MIC × 10 | 1 | 705 ± 233 |
| 2-Cu | MIC | 4 | 223 ± 22 | MIC × 10 | 1 | 864 ± 186 |
| 3-Cu | MIC/10 | 4 | 279 ± 29 | MIC × 10 | 1 | 874 ± 198 |
| E. coli | ||||||
| Material | ROS | AOPP | ||||
| Concentration | t (h) | % | Concentration | t (h) | % | |
| 1-Cu | MIC | 4 | 89 ± 6 | MIC × 10 | 1 | 390 ± 4 |
| 2-Cu | MIC/10 | 4 | 209 ± 21 | MIC × 10 | 1 | 647 ± 20 |
| 3-Cu | MIC × 10 | 4 | 139 ± 8 | MIC × 10 | 1 | 764 ± 1 |
| Antibiotic | S. aureus | P. aeruginosa | ||
|---|---|---|---|---|
| MRSA1 | MRSA2 | PA8 | PA13 | |
| Amikacin | - | - | S (2) | S (2) |
| Aztreonam | - | - | S (4) | S (2) |
| Cefepime | - | - | S (1) | R (16) |
| Cefoxitin detection | Pos | Pos | - | - |
| Ceftazidime | - | - | S (2) | S (8) |
| Ceftolozane/Tazobactam | - | - | S (0.5) | S (1) |
| Ciprofloxacin | - | - | S (≤0.25) | R (≥4) |
| Clindamycin | S (≤0.25) | R (≥8) | - | - |
| Colistin | - | - | S (≤0.5) | S (≤0.5) |
| Cotrimoxazole | S (≤10) | S (20) | - | - |
| Daptomycin | S (0.25) | S (0.25) | - | - |
| Erythromycin | R (≥8) | R (≥8) | - | - |
| Fosfomycin | S (≤8) | S (32) | - | - |
| Fusidic Acid | S (≤0.5) | S (≤0.5) | - | - |
| Gentamycin | S (≤0.5) | R (≥16) | S (≤1) | S(≤1) |
| Imipenem | - | - | S (1) | S (1) |
| Inducible clindamycin resistance | Neg (≥8) | Neg | - | - |
| Levofloxacin | R (≥8) | R (≥8) | - | - |
| Linezolid | S (2) | S (2) | - | - |
| Meropenem | - | - | - | - |
| Mupirocin | - | R (≥512) | S (≤0.25) | S (1) |
| Oxacillin | R (≥4) | R (≥4) | - | - |
| Penicillin | R (≥0.5) | R (≥0.5) | - | - |
| Piperacillin-tazobactam | - | - | - | - |
| Teicoplanin | S (≤0.5) | S (≤0.5) | S (≤4) | R (≥128) |
| Tobramycin | R (≥16) | R (≥16) | - | - |
| Vancomycin | S (1) | S (≤0.5) | S (≤1) | S (≤1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; San Sebastian, E.; Páez, P.L.; Nogales, E.; Esteban, J.; Gómez-Ruiz, S. Antibacterial Properties of Mesoporous Silica Nanoparticles Modified with Fluoroquinolones and Copper or Silver Species. Pharmaceuticals 2023, 16, 961. https://doi.org/10.3390/ph16070961
Ugalde-Arbizu M, Aguilera-Correa JJ, San Sebastian E, Páez PL, Nogales E, Esteban J, Gómez-Ruiz S. Antibacterial Properties of Mesoporous Silica Nanoparticles Modified with Fluoroquinolones and Copper or Silver Species. Pharmaceuticals. 2023; 16(7):961. https://doi.org/10.3390/ph16070961
Chicago/Turabian StyleUgalde-Arbizu, Maider, John Jairo Aguilera-Correa, Eider San Sebastian, Paulina L. Páez, Estela Nogales, Jaime Esteban, and Santiago Gómez-Ruiz. 2023. "Antibacterial Properties of Mesoporous Silica Nanoparticles Modified with Fluoroquinolones and Copper or Silver Species" Pharmaceuticals 16, no. 7: 961. https://doi.org/10.3390/ph16070961
APA StyleUgalde-Arbizu, M., Aguilera-Correa, J. J., San Sebastian, E., Páez, P. L., Nogales, E., Esteban, J., & Gómez-Ruiz, S. (2023). Antibacterial Properties of Mesoporous Silica Nanoparticles Modified with Fluoroquinolones and Copper or Silver Species. Pharmaceuticals, 16(7), 961. https://doi.org/10.3390/ph16070961