Rapid and Efficient Access to Novel Bio-Inspired 3-Dimensional Tricyclic SpiroLactams as Privileged Structures via Meyers’ Lactamization
Abstract
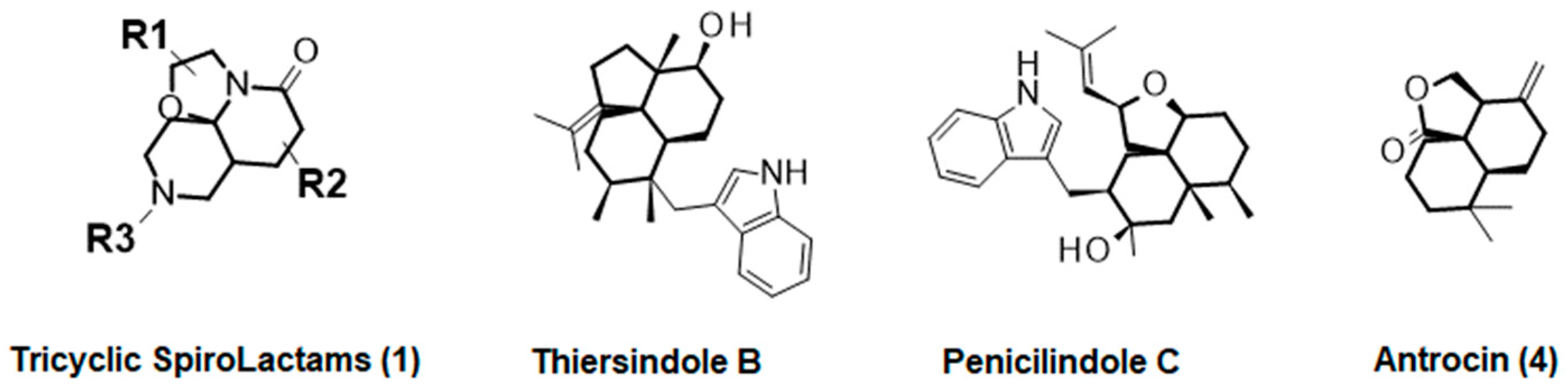
1. Introduction
2. Results and Discussion
2.1. Optimization of Meyers’ Lactamization Reaction
2.2. Scope of Meyers’ Lactamization Reaction
2.2.1. Synthesis of Lactams with Octahydrooxazolo [2,3-j][1,6]naphthyridin-5-ones Core ([5.6.6] Ring System) from Benzyl-Keto-Ester
2.2.2. Synthesis of Lactams with Decahydro-[1,3]oxazino[2,3-j][1,6]naphthyridin-6-one core ([6,6,6] Ring System)
2.2.3. Application of Meyers’ Lactamization Reaction to Other Boc-Keto-Esters and Amino-Alcohols to Produce Tricyclic Spirolactams with Original Scaffolds
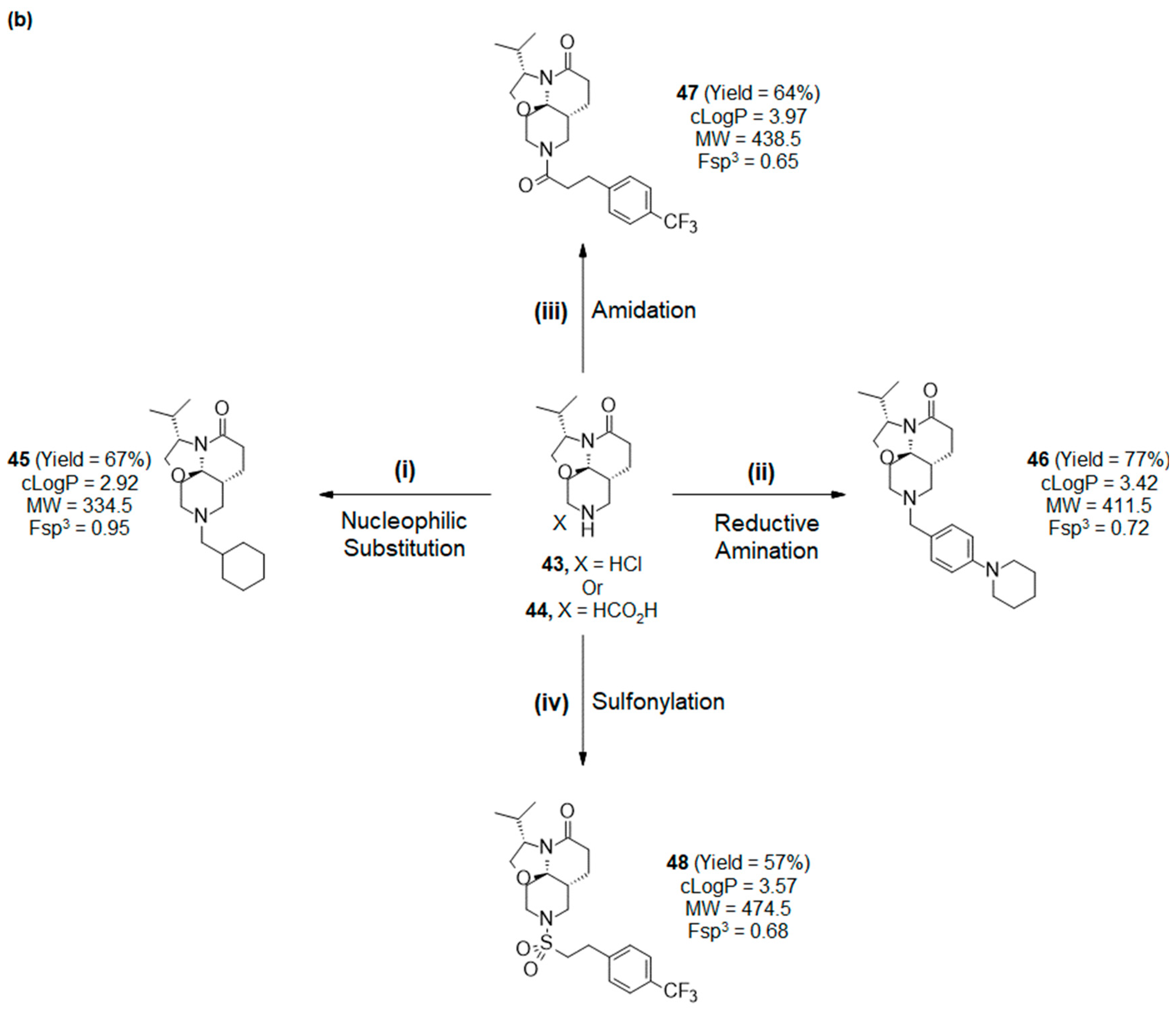
2.3. Functionalization of the Lactam Ring
2.4. Synthesis and Drug-like Properties of Novel Tricyclic Spirolactams Obtained via R3 Position Functionalization
3. Materials and Methods
3.1. General Information
3.2. Chemistry
- 3-(1-benzyl-4-oxo-3-piperidyl)propanoic acid (8):
- Methyl 3-(1-benzyl-4-oxo-3-piperidyl)propanoate (9):
3.2.1. General Protocol 1: Synthesis of Boc-Keto-Esters via Stork Enamine Alkylation
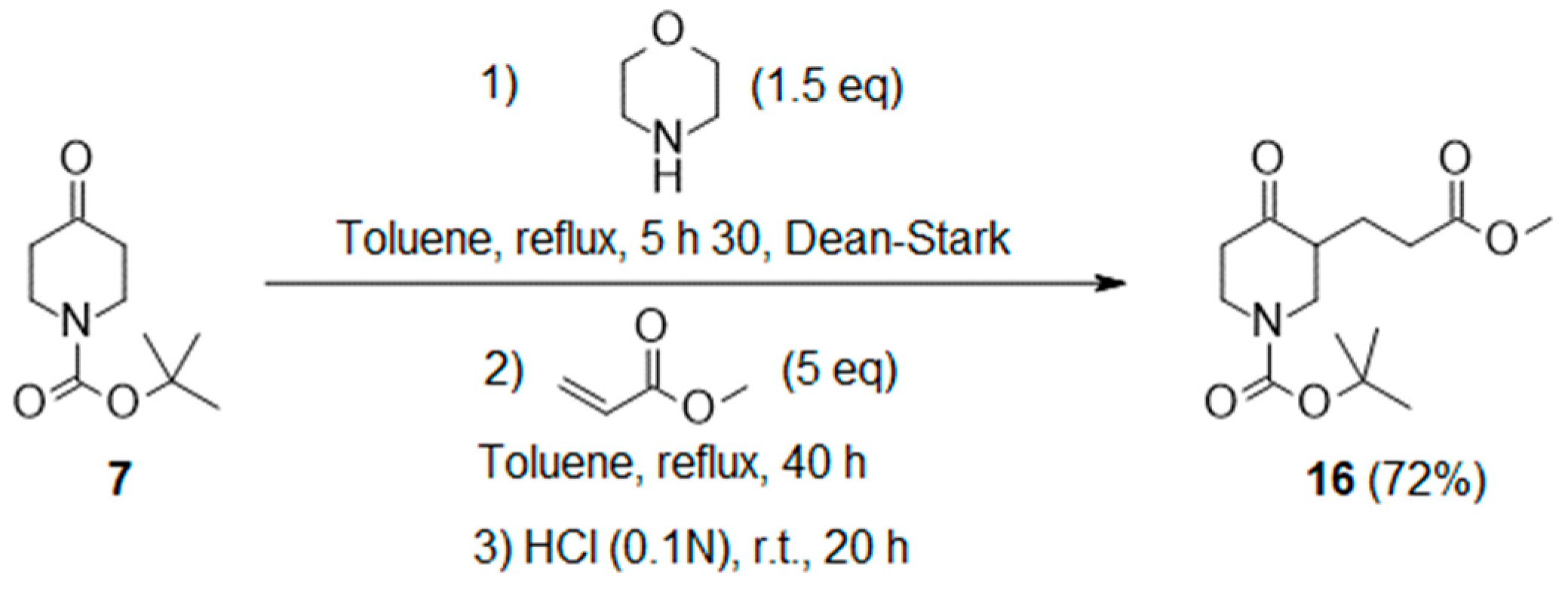
- Tert-butyl 3-(3-methoxy-3-oxo-propyl)-4-oxo-piperidine-1-carboxylate (16):
- Tert-butyl 2-(3-methoxy-3-oxo-propyl)-3-oxo-piperidine-1-carboxylate (30):
- Tert-butyl 3-(3-methoxy-3-oxo-propyl)-4-oxo-pyrrolidine-1-carboxylate (32):
- Tert-butyl 3-(3-methoxy-3-oxo-propyl)-4-oxo-azepane-1-carboxylate (34):
- Tert-butyl 3-(2-methoxy-2-oxo-ethyl)-4-oxo-piperidine-1-carboxylate (36):
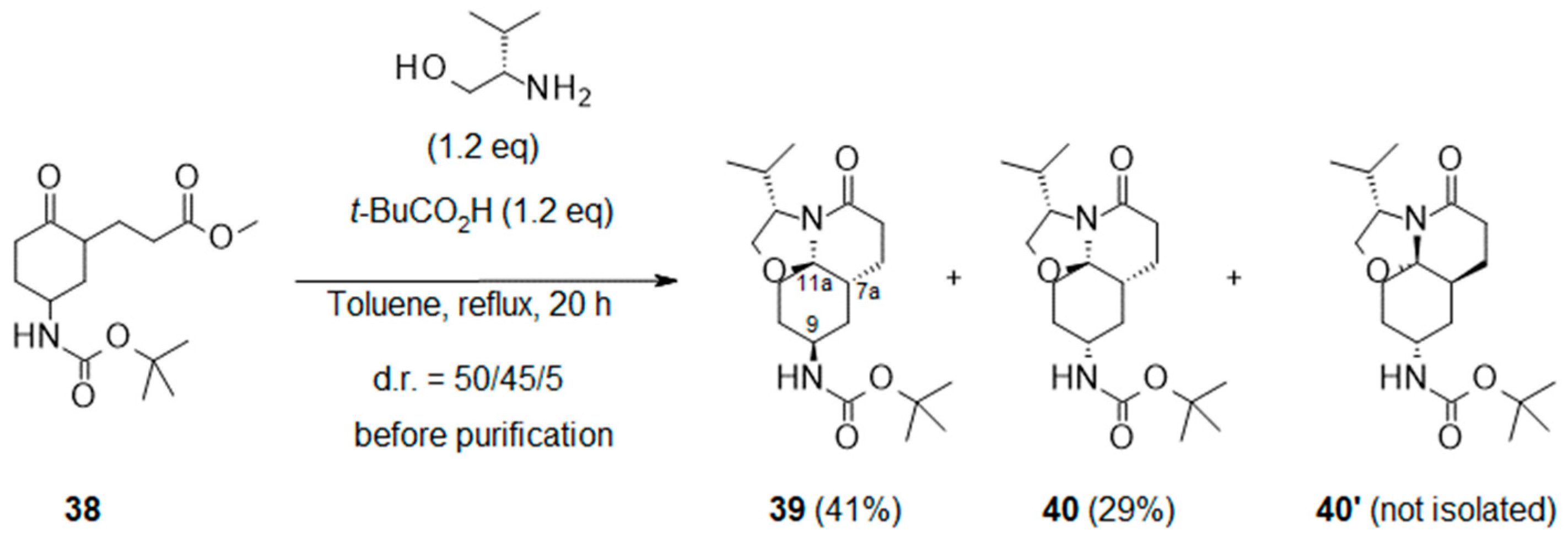
- Methyl 3-[5-(tert-butoxycarbonylamino)-2-oxo-cyclohexyl]propanoate (38):
3.2.2. General Protocol 2: Synthesis of Lactams via Meyers’ Lactamization of Keto-Esters with Amino-Alcohols
- (3S,7aS,11aS)-9-benzyl-3-methyl-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (11) and (3S,7aS,11aS)-9-benzyl-3-methyl-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (11′):
- (3S,7aR,11aR)-9-benzyl-3-isopropyl-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (12):
- (3S,7aR,11aR)-9-benzyl-3-tert-butyl-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (13):
- Tert-butyl 6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3] oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (17):
- Tert-butyl (4R,8aR,12aR)-4-isopropyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (18):
- Tert-butyl (4S,8aR,12aR)-4-methyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (19) and tert-butyl (4S,8aS,12aS)-4-methyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (19′):
- Tert-butyl (3R,8aR,12aR)-3-methyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (20):
- Tert-butyl (3R,8aR,12aR)-3-ethyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (21) and Tert-butyl (3R,8aR,12aR)-3-ethyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (21′):
- Tert-butyl 3-isopropyl-6-oxo-3,4,7,8,8a,9,11,12-octahydro-2H-[1,3]oxazino[2,3-j][1,6]naphthyridine-10-carboxylate (22):
- Tert-butyl (8aR,12aR)-6-oxospiro[2,4,7,8,8a,9,11,12-octahydro-[1,3]oxazino[2,3-j][1,6]naphthyridine-3,1′-cyclopropane]-10-carboxylate (23):
- Tert-butyl (8aR,12aR)-6-oxospiro[2,4,7,8,8a,9,11,12-octahydro-[1,3]oxazino[2,3-j][1,6]naphthyridine-3,1′-cyclobutane]-10-carboxylate (24):
- Tert-butyl (3S,7aR,11aR)-3-isopropyl-5-oxo-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridine-9-carboxylate (25):
- Tert-butyl (3R,7aR,11aR)-3-[(1R)-1-benzyloxyethyl]-5-oxo-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridine-9-carboxylate (26):
- Tert-butyl (3R,7aR,11aR)-5-oxo-3-(trifluoromethyl)-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridine-9-carboxylate (27):
- Tert-butyl 9-oxo-17-oxa-4,10-diazatetracyclo[8.7.0.01,6.011,16]heptadeca-11(16),12,14-triene-4-carboxylate (28):
- Tert-butyl (1R,6R)-9-oxo-15-oxa-4,10-diazatricyclo[8.5.0.01,6]pentadecane-4-carboxylate (29):
- Tert-butyl (3S,7aS,11aR)-3-isopropyl-5-oxo-2,3,6,7,7a,9,10,11-octahydrooxazolo[2,3-e][1,5]naphthyridine-8-carboxylate (31):
- Tert-butyl (1S,4S,9R)-4-isopropyl-6-oxo-2-oxa-5,11-diazatricyclo[7.3.0.01,5]dodecane-11-carboxylate (33):
- Tert-butyl (1R,4S,9R)-4-isopropyl-6-oxo-2-oxa-5,11-diazatricycl [7.5.0.01,5]tetradecane-11-carboxylate (35):
- Tert-butyl (1R,4S,8R)-4-isopropyl-6-oxo-2-oxa-5,10-diazatricyclo[6.4.0.01,5]dodecane-10-carboxylate (37):
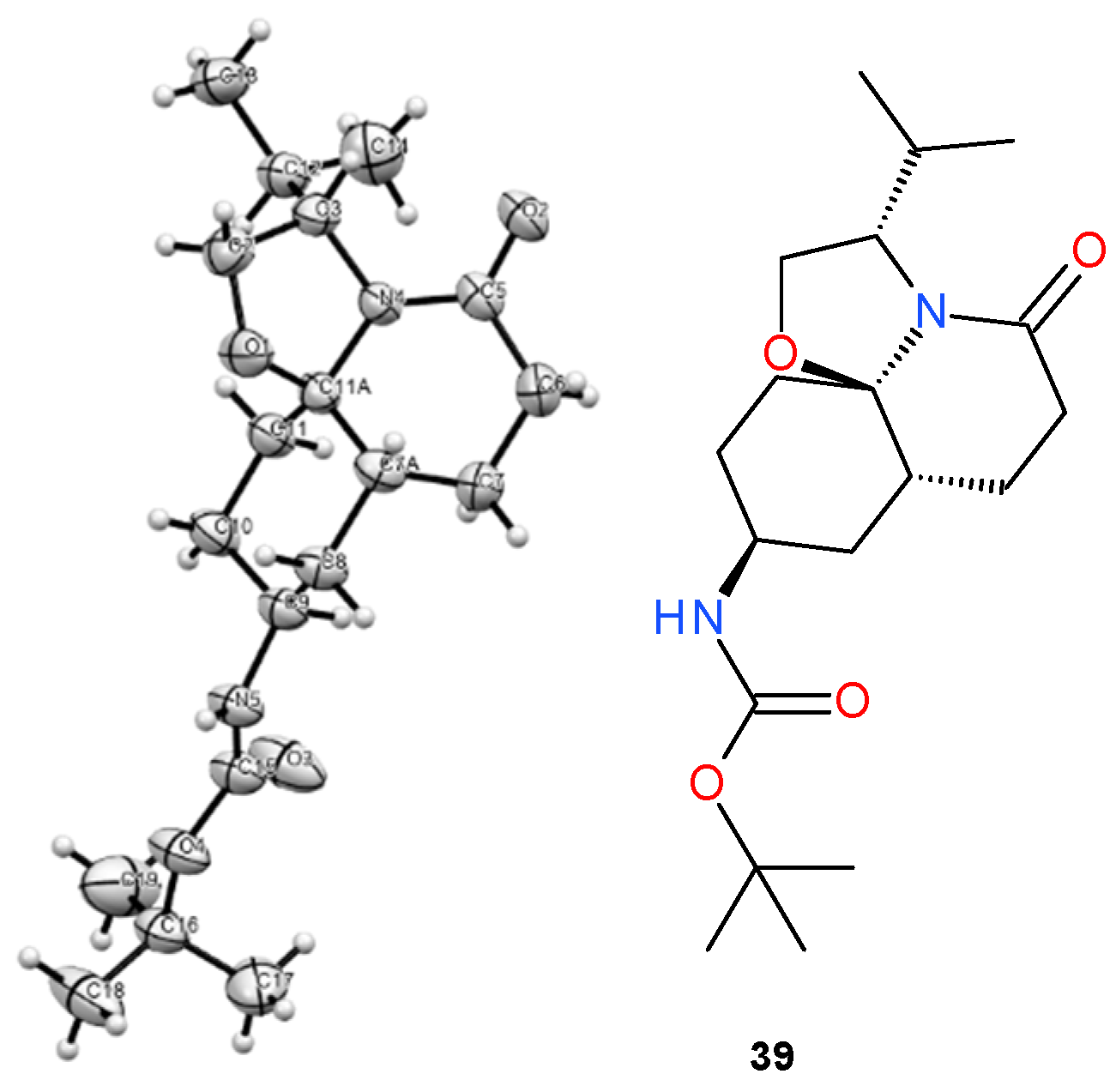
- Tert-butyl N-[(3S,7aR,9S,11aR)-3-isopropyl-5-oxo-3,6,7,7a,8,9,10,11-octahydro-2H-oxazolo[2,3-j]quinolin-9-yl]carbamate (39) and Tert-butyl N-[(3S,7aR,9R,11aR)-3-isopropyl-5-oxo-3,6,7,7a,8,9,10,11-octahydro-2H-oxazolo[2,3-j]quinolin-9-yl]carbamate (40):
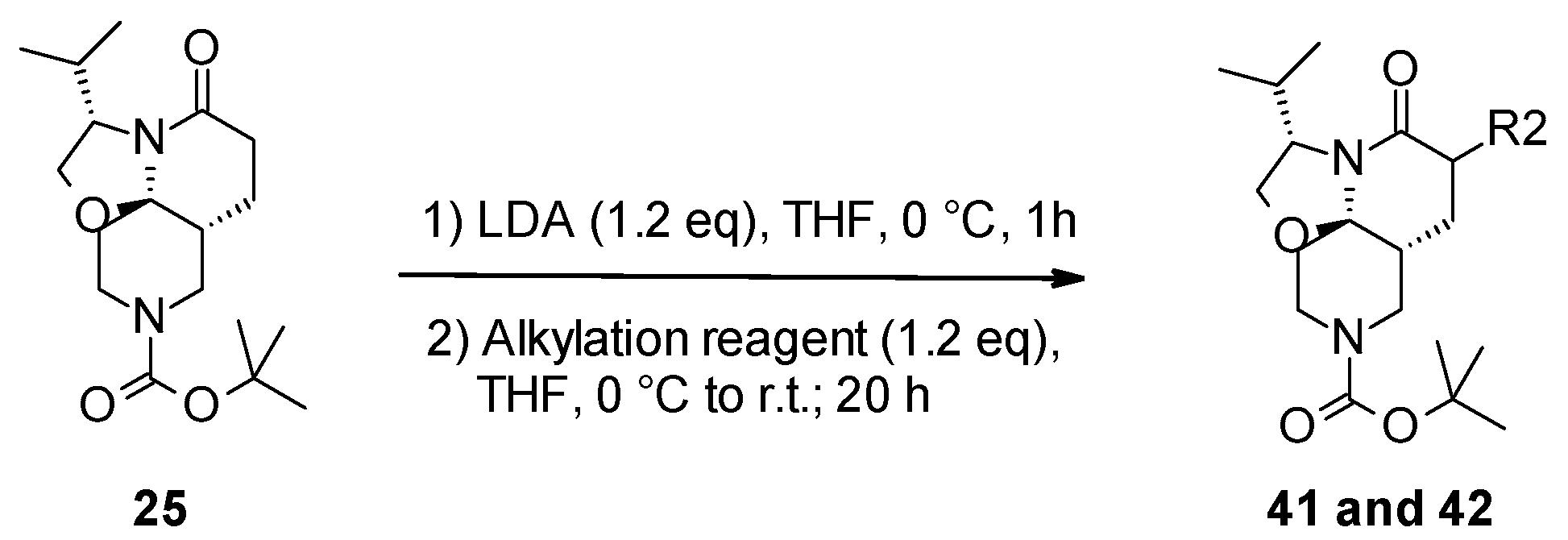
3.2.3. General Protocol 3: Functionalization of Lactam Ring by Alkylation Reaction
- Tert-butyl (3S,7aR,11aR)-3-isopropyl-6-methyl-5-oxo-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridine-9-carboxylate (41):
- Tert-butyl (3S,7aR,11aR)-6-fluoro-3-isopropyl-5-oxo-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridine-9-carboxylate (42):
3.2.4. Deprotection of Lactams
- (3S,7aR,11aR)-3-isopropyl-3,6,7,7a,8,9,10,11-octahydro-2H-oxazolo[2,3-j][1,6]naphthyridin-5-one;hydrochloride (43):
3.2.5. R3 Functionalization of Lactams 43 and 44
- (3S,7aR,11aR)-9-(cyclohexylmethyl)-3-isopropyl-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (45):
- (3S,7aR,11aR)-3-isopropyl-9-[[4-(1-piperidyl)phenyl]methyl]-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (46):
- (3S,7aR,11aR)-3-isopropyl-9-[3-[4-(trifluoromethyl)phenyl]propanoyl]-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (47):
- (3S,7aR,11aR)-3-isopropyl-9-[2-[4-(trifluoromethyl)phenyl]ethylsulfonyl]-2,3,6,7,7a,8,10,11-octahydrooxazolo[2,3-j][1,6]naphthyridin-5-one (48):
3.3. X-ray Structural Determination
3.4. Determination of Compound 44 Solubility
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.W.; Currie, K.S.; Mitchell, S.A.; Darrow, J.W.; Pippin, D.A. Privileged Structures: Applications in Drug Discovery. Comb. Chem. High Throughput Screen. 2004, 7, 473–493. [Google Scholar] [CrossRef]
- Lovering, F.; Biccker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. Med. Chem. Commun. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Grabowski, K.; Baringhaus, K.-H.; Schneider, G. Scaffold diversity of natural products: Inspiration for combinatorial library design. Nat. Prod. Rep. 2008, 25, 892–904. [Google Scholar] [CrossRef]
- Lee, M.-L.; Schneider, G. Scaffold Architecture and Pharmacophoric Properties of Natural Products and Trade Drugs: Application in the Design of Natural Product-Based Combinatorial Libraries. J. Comb. Chem. 2001, 3, 284–289. [Google Scholar] [CrossRef]
- Grigalunas, M.; Burhop, A.; Zinken, S.; Pahl, A.; Gally, J.-M.; Wild, N.; Mantel, Y.; Sievers, S.; Foley, D.J.; Scheel, R.; et al. Natural product fragment combination to performance-diverse pseudo-natural products. Nat. Commun. 2021, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Flitsch, S.L.; Grigalunas, M.; Leeson, P.D.; Quinn, R.J.; Turner, N.J.; Waldmann, H. The Time and Place for Nature in Drug Discovery. JACS Au. 2022, 2, 2400–2416. [Google Scholar] [CrossRef]
- Thomas, G.L.; Johannes, C.W. Natural product-like synthetic libraries. Curr. Opin. Chem. Biol. 2011, 15, 516–522. [Google Scholar] [CrossRef]
- Wildermuth, R.; Speck, K.; Haut, F.-L.; Mayer, P.; Karge, B.; Brönstrup, M.; Magauer, T. A modular synthesis of tetracyclic meroterpenoid antibiotics. Nat. Commun. 2017, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gloer, J.B.; Wicklow, D.T. Thiersindoles A–C: New Indole Diterpenoids from Penicillium thiersii . J. Nat. Prod. 2003, 66, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Bai, M.; Zhou, X.-M.; Huang, G.-L.; Shao, T.-M.; Luo, Y.-P.; Niu, Z.-G.; Niu, Y.-Y.; Chen, G.-Y.; Han, C.-R. Penicilindoles A–C, Cytotoxic Indole Diterpenes from the Mangrove Derived Fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.-M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid.-Based Complement. Altern. Med. 2009, 2011, nep108. [Google Scholar] [CrossRef]
- Venkatachalam, A.; Tai, D.-F. Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction. Molecules 2020, 25, 831. [Google Scholar]
- Rao, Y.K.; Wu, A.T.H.; Geethangili, M.; Huang, M.-T.; Chao, W.-J.; Wu, C.-H.; Deng, W.-P.; Yeh, C.-T.; Tzeng, Y.-M. Identification of Antrocin from Antrodia camphorata as a Selective and Novel Class of Small Molecule Inhibitor of Akt/mTOR Signaling in Metastatic Breast Cancer MDA-MB-231 Cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef]
- Wu, A.T.H.; Lawal, B.; Wei, L.; Wen, Y.-T.; Tzeng, D.T.W.; Lo, W.-C. Multiomics Identification of Potential Targets for Alzheimer Disease and Antrocin as a Therapeutic Candidate. Pharmaceutics 2021, 13, 1555. [Google Scholar] [CrossRef]
- Talele, T.T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem. 2020, 63, 13291–13315. [Google Scholar] [CrossRef]
- Saldívar-González, F.I.; Lenci, E.; Trabocchi, A.; Medina-Franco, J.L. Exploring the chemical space and the bioactivity profile of lactams: A chemoinformatic study. RSC Adv. 2019, 9, 27105–27116. [Google Scholar] [CrossRef]
- Willand, N.; Beghyn, T.; Nowogrocki, G.; Gesquiere, J.-C.; Deprez, B. Synthesis and structural studies of a novel scaffold for drug discovery: A 4,5-dihydro-3H-spiro[1,5-benzoxazepine-2,40-piperidine]. Tetrahedron Lett. 2004, 45, 1051–1054. [Google Scholar] [CrossRef]
- Beghyn, T.; Deprez-Poulain, R.; Willand, N.; Folleas, B.; Deprez, B. Natural Compounds: Leads or Ideas? Bioinspired Molecules for Drug Discovery. Chem. Biol. Drug Des. 2008, 72, 3–15. [Google Scholar] [PubMed]
- Tran, N.C.; Dhondt, H.; Flipo, M.; Deprez, B.; Willand, N. Synthesis of functionalized 2-isoxazolines as three-dimensional fragments for fragment-based drug discovery. Tetrahedron Lett. 2015, 56, 4119–4123. [Google Scholar] [CrossRef]
- Prevet, H.; Flipo, M.; Roussel, P.; Deprez, B.; Willand, N. Microwave-assisted synthesis of functionalized spirohydantoins as 3-D privileged fragments for scouting the chemical space. Tetrahedron Lett. 2016, 57, 2888–2894. [Google Scholar] [CrossRef]
- Meyers, A.I.; Brengel, G.P. Chiral bicyclic lactams: Useful precursors and templates for asymmetric syntheses. Chem. Commun. 1997, 1, 1–8. [Google Scholar] [CrossRef]
- Groaming, M.D.; Meyers, A.I. Chiral Non-Racemic Bicyclic Lactams. Auxiliary-Based Asymmetric Reactions. Tetrahedron 2000, 56, 9843–9873. [Google Scholar] [CrossRef]
- Amat, M.; Llor, N.; Griera, R.; Pérez, M.; Bosch, J. Enantioselective Synthesis of Alkaloids from Phenylglycinol-Derived Lactams. Nat. Prod. Commun. 2011, 6, 515–526. [Google Scholar] [CrossRef]
- Pinto, A.; Piccichè, M.; Griera, R.; Molins, E.; Bosch, J.; Amat, M. Studies on the Synthesis of Phlegmarine-Type Lycopodium Alkaloids: Enantioselective Synthesis of (−)-Cermizine B, (+)-Serratezomine E, and (+)-Luciduline. J. Org. Chem. 2018, 83, 8364–8375. [Google Scholar] [CrossRef]
- Piccichè, M.; Pinto, A.; Griera, R.; Bosch, J.; Amat, M.J. Total Synthesis of (−)-Cylindricine H. J. Org. Chem. 2022, 24, 5356–5360. [Google Scholar] [CrossRef] [PubMed]
- Malaquin, S.; Jida, M.; Courtin, J.; Laconde, G.; Willand, N.; Deprez, B.; Deprez-Poulain, R. Water-based conditions for the microscale parallel synthesis of bicyclic lactams. Tetrahedron Lett. 2013, 54, 562–567. [Google Scholar] [CrossRef]
- Deprez-Poulain, R.; Willand, N.; Boutillon, C.; Nowogrocki, G.; Azaroual, N.; Deprez, B. A simple reaction to produce small structurally complex and diverse molecules. Tetrahedron Lett. 2004, 45, 5287–5290. [Google Scholar] [CrossRef]
- Penhoat, M.; Levacher, v.; Dupas, G. Novel Extension of Meyers’ Methodology: Stereoselective Construction of Axially Chiral 7,5-Fused Bicyclic Lactams. J. Org. Chem. 2003, 68, 9517–9520. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M.D.; Hoffman, R.L.; Ghazal, N.B.; Old, D.W.; Mooney, P.A. Asymmetric Synthesis of Cis-Fused Bicyclic Pyrrolidines and Pyrrolidinones via Chiral Polycyclic Lactams. J. Org. Chem. 1996, 61, 5813–5817. [Google Scholar] [CrossRef]
- Jida, M.; Deprez-Poulain, R.; Malaquin, S.; Roussel, P.; Agbossou-Niedercorn, F.; Deprez, B.; Laconde, G. Solvent-free microwave-assisted Meyers’ lactamization. Green Chem. 2010, 12, 961–964. [Google Scholar] [CrossRef]
- Postikova, S.; Sabbah, M.; Wightman, D.; Nguyen, I.T.; Sanselme, M.; Besson, T.; Brière, J.-F.; Oudeyer, S.; Levacher, V. Developments in Meyers’ Lactamization Methodology: En Route to Bi(hetero)aryl Structures with Defined Axial Chirality. J. Org. Chem. 2013, 78, 8191–8197. [Google Scholar] [CrossRef] [PubMed]
- Membrat, R.; Vasseur, A.; Moraleda, D.; Michaud-Chevallier, S.; Martinez, A.; Giordano, L.; Nuel, D. Platinum–(phosphinito–phosphinous acid) complexes as bi-talented catalysts for oxidative fragmentation of piperidinols: An entry to primary amines. RSC Adv. 2019, 9, 37825–37829. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Baty, J.D.; Jones, G.; Moore, C. Alkylation of 4-Piperidones; Intermediates in the Synthesis of Reduced 2-Pyrindin-6-ones. J. Chem. Soc. C 1969, 11, 1520–1528. [Google Scholar] [CrossRef]
- Schwehm, C.; Li, J.; Song, H.; Hu, X.; Kellam, B.; Stocks, M. Synthesis of New DPP-4 Inhibitors Based on a Novel Tricyclic Scaffold. ACS Med. Chem. Lett. 2015, 6, 324–328. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Idzik, T.J.; Myk, Z.M.; Peruzynska, M.; Maciejewska, G.; Drozdzik, M.; Sosnicki, J.G. Arylation of enelactams using TIPSOTf: Reaction scope and mechanistic insight. Org. Chem. Front. 2021, 8, 708–720. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, J.G.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dam, S.; Tangara, S.; Hamela, C.; Hattabi, T.; Faїon, L.; Carre, P.; Antoine, R.; Herledan, A.; Leroux, F.; Piveteau, C.; et al. Tricyclic SpiroLactams Kill Mycobacteria in vitro and in vivo by Inhibiting Type II NADH Dehydrogenases. J. Med. Chem. 2022, 65, 16651–16664. [Google Scholar] [CrossRef] [PubMed]






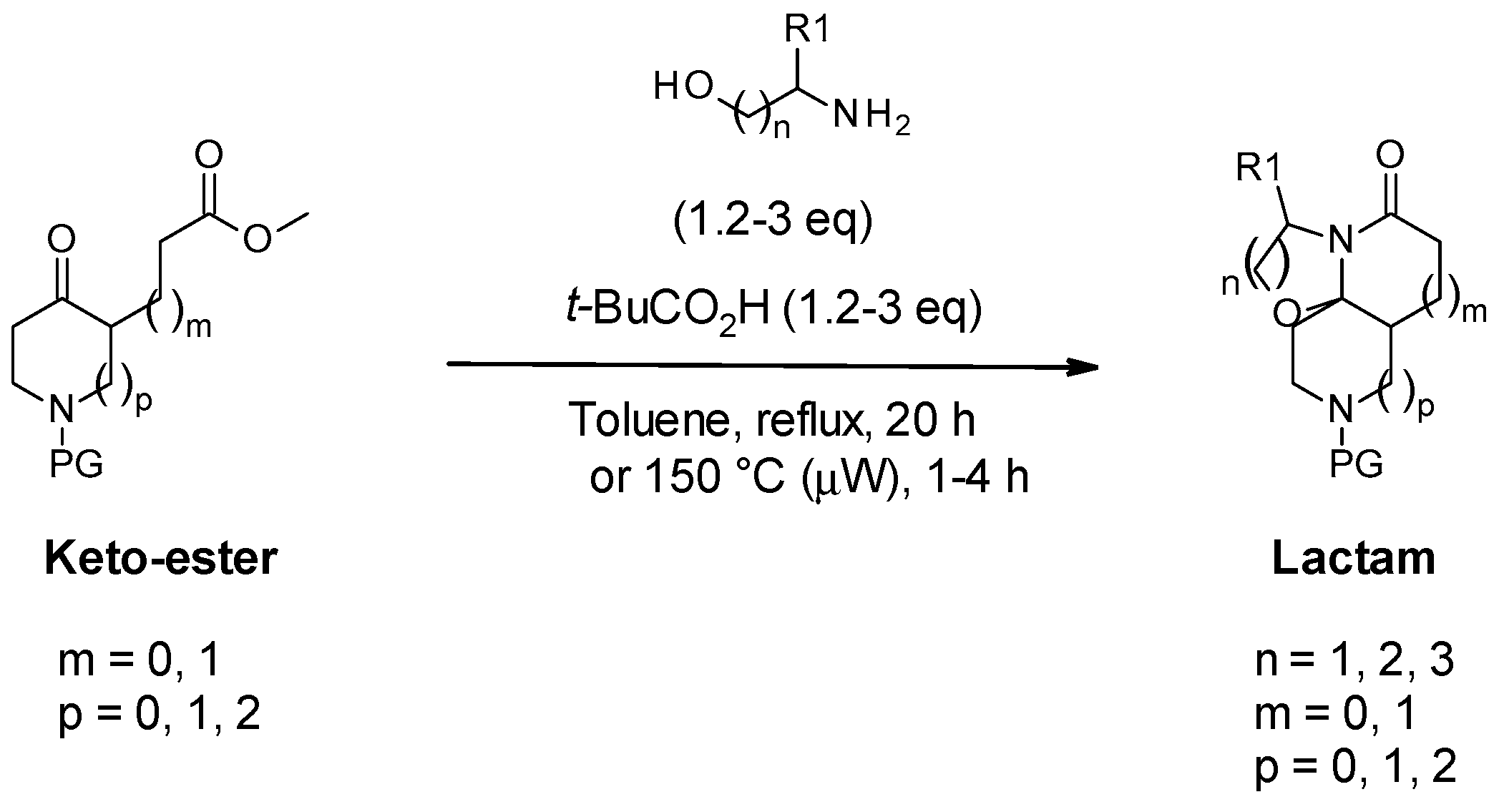
 | |||
|---|---|---|---|
| Entry | Substrate a | Conditions | Conversion b |
| 1 | 8 | Toluene, reflux, 20 h | 66% |
| 2 | 8 | Toluene, 150 °C (µW), 2 h | 76% |
| 3 | 9 | Pivalic acid (1.2 eq), Toluene, 150 °C (µW), 1 h | 100% |
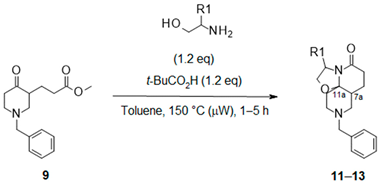 | ||||
|---|---|---|---|---|
| Entry | Amino-Alcohol | Product | Isolated Compound (Yield) a | d.r. b |
| 1 |  |  | 11 + 11′ (46%) | 70:30 |
| 2 |  |  | 12 (75%) | 100:0 |
| 3 |  | 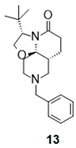 | 13 (40%) | 100:0 |
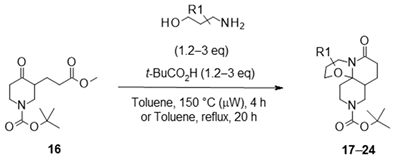 | ||||
|---|---|---|---|---|
| Entry | Amino-Alcohol | Product | Isolated Compound (Yield) d | d.r. e |
| 1 |  | 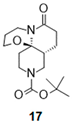 | 17 a (79%) | |
| 2 | 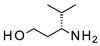 | 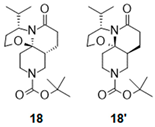 | 18 # (18%) | 90:10 |
| 3 |  | 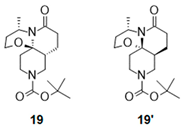 | 19 + 19′ b (86%) | 50:50 |
| 4 | 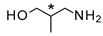 |  | 20 c (63%) | 60:40 |
| 5 | 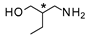 |  | 21 + 21′ b (89%) | 70:30 |
| 6 | 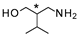 | 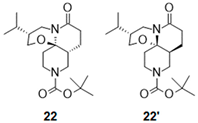 | 22 c (92%) | 96:4 |
| 7 | 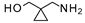 |  | 23 a (88%) | |
| 8 |  | 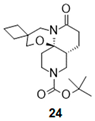 | 24 a (98%) | |
 | |||||
|---|---|---|---|---|---|
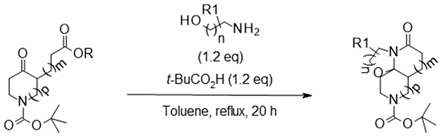
| Entry | Boc-Keto-Ester a | Amino-Alcohol | Product | Isolated Compound (Yield) c | d.r. d |
| 1 | 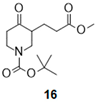 |  |  | 25 (84%) * 25 (88%) | 90:10 |
| 2 | 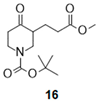 | 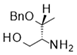 |  | 26 (50%) | 94:6 |
| 3 | 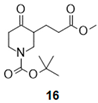 |  | 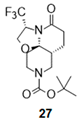 | 27 (59%) | 100:0 |
| 4 | 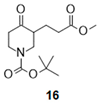 | 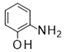 |  | 28 b (98%) | |
| 5 | 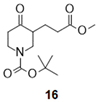 | 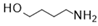 | 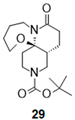 | 29 b (81%) | |
| 6 |  | 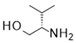 |  | 31 (49%) | 100:0 |
| 7 | 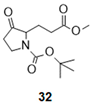 | 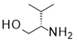 |  | 33 (78%) | 100:0 |
| 8 | 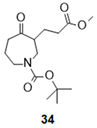 |  | 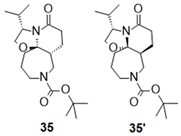 | 35 (89%) | 90:10 |
| 9 | 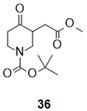 | 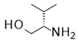 | 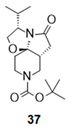 | 37 (90%) | 100:0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangara, S.; Faïon, L.; Piveteau, C.; Capet, F.; Godelier, R.; Michel, M.; Flipo, M.; Deprez, B.; Willand, N.; Villemagne, B. Rapid and Efficient Access to Novel Bio-Inspired 3-Dimensional Tricyclic SpiroLactams as Privileged Structures via Meyers’ Lactamization. Pharmaceuticals 2023, 16, 413. https://doi.org/10.3390/ph16030413
Tangara S, Faïon L, Piveteau C, Capet F, Godelier R, Michel M, Flipo M, Deprez B, Willand N, Villemagne B. Rapid and Efficient Access to Novel Bio-Inspired 3-Dimensional Tricyclic SpiroLactams as Privileged Structures via Meyers’ Lactamization. Pharmaceuticals. 2023; 16(3):413. https://doi.org/10.3390/ph16030413
Chicago/Turabian StyleTangara, Salia, Léo Faïon, Catherine Piveteau, Frédéric Capet, Romain Godelier, Marion Michel, Marion Flipo, Benoit Deprez, Nicolas Willand, and Baptiste Villemagne. 2023. "Rapid and Efficient Access to Novel Bio-Inspired 3-Dimensional Tricyclic SpiroLactams as Privileged Structures via Meyers’ Lactamization" Pharmaceuticals 16, no. 3: 413. https://doi.org/10.3390/ph16030413
APA StyleTangara, S., Faïon, L., Piveteau, C., Capet, F., Godelier, R., Michel, M., Flipo, M., Deprez, B., Willand, N., & Villemagne, B. (2023). Rapid and Efficient Access to Novel Bio-Inspired 3-Dimensional Tricyclic SpiroLactams as Privileged Structures via Meyers’ Lactamization. Pharmaceuticals, 16(3), 413. https://doi.org/10.3390/ph16030413





