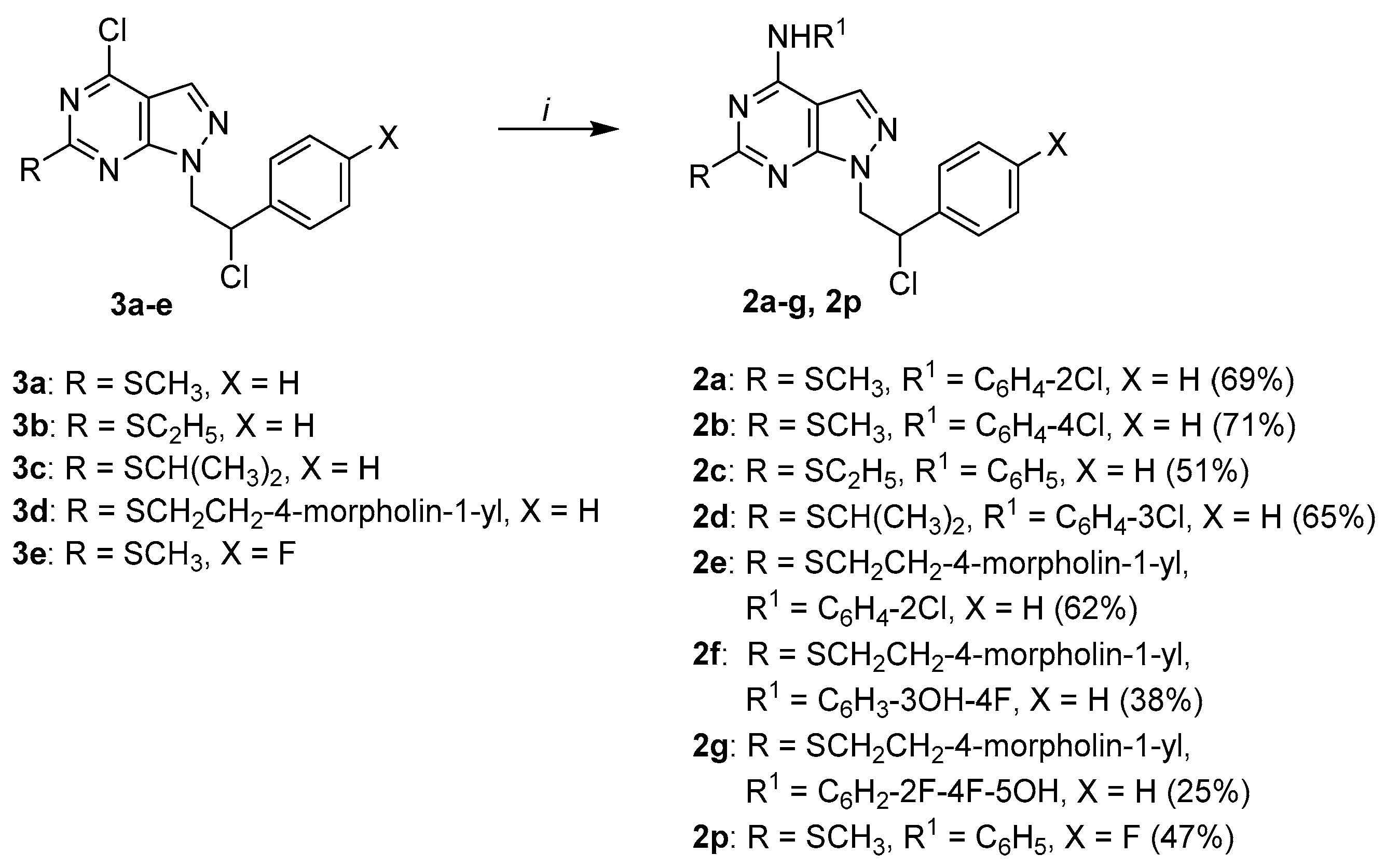
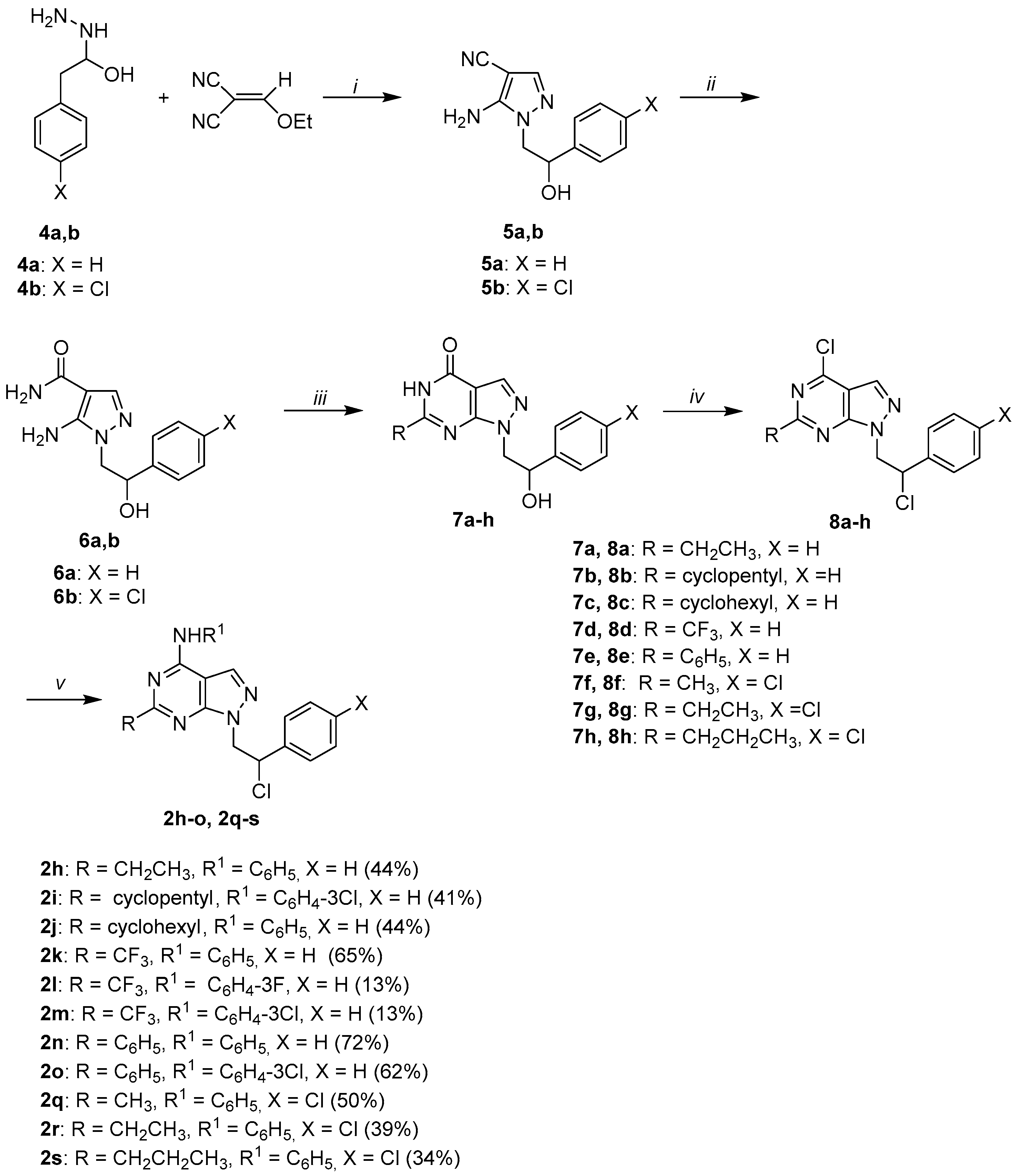
3.1. Chemistry
All commercially available chemicals were used as purchased. DCM was dried over calcium hydride. Anhydrous reactions were run under a positive pressure of dry N2 or argon. TLC was carried out using Merck TLC plates silica gel 60 F254. Chromatographic purifications were performed on columns packed with Merk 60 silica gel, 23–400 mesh, for flash technique. 1H NMR and 13C NMR spectra were recorded on a Brucker Avance DPX400 (at 400 MHz for 1H and 100 MHz for 13C) or using a Varian Gemini 200 (200 MHz for for 1H) in DMSO-d6, CDCl3, or acetone-d6 as solvents as indicated. Chemical shifts (δ) were expressed in parts per million (ppm) relative to tetramethylsilane (TMS), which was used as the internal standard. Data are shown as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, sx = sextet, dd = doublet of doublets, tt = triple of triplets, bs = broad singlet), coupling constant (J) in Hertz (Hz), and integration. Elemental analysis for C, H, N, and S was determined using Thermo Scientific Flash 2000 and results were within ±0.4% of the theoretical value. All target compounds possessed a purity of ≥95%, which was verified by elemental analysis. Microwave irradiation experiments were conducted using a CEM Discover synthesis unit (CEM Corp., Matthews, NC, USA). The machine consists of a continuous focused microwave power delivery system with operator selectable power output from 0 to 300 W. The temperature of the contents of the vessels was monitored using a calibrate infrared temperature control mounted under the reaction vessel. All of the experiments were performed using a stirring option whereby the contents of the vessel are stirred by means of a rotating magnetic plate located below the floor of the microwave cavity and a Teflon-coated magnetic stir bar in the vessel.
Synthesis of the intermediates
3a [
9],
3b [
15],
3c [
14],
3d [
8],
3e and
4a,b [
9] has already been reported by us.
3.1.1. General Procedure for the Synthesis of Final Compounds 2a-e, 2h-j and 2n-s
A solution of the appropriate 4-chloro derivatives 3a-e, 8a-c, or 8e-h (1 mmol) and the appropriate aniline (2 mmol) in absolute ethanol was refluxed for 5 h. After cooling, the solvent was evaporated under reduced pressure, and the crude was treated with water (20 mL), and then extracted with CH2Cl2 (20 mL). The organic phase was washed with water (20 mL), dried (MgSO4), and concentrated under reduced pressure. The obtained oil was crystallized by adding a mixture of diethyl ether/petroleum ether (bp 40–60 °C) (1:1) and standing in a refrigerator to afford a white solid, which was recrystallized from absolute ethanol.
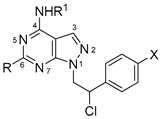
1-(2-Chloro-2-phenylethyl)-N-(2-chlorophenyl)-6-(methylthio)-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2a (SI388). Yield: 69% (white solid). Mp: 169–171 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.02 (s, 1H), 7.67 (br s, 1H), 7.58–7.56 (m, 2H), 7.49–7.47 (m, 2H), 7.41–7.30 (m, 5H), 5.64–5.60 (m, 1H), 4.89–4.83 and 4.76–4.71 (2m, 2H), 2.40 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 169.67, 155.07, 153.98, 137.98, 134.71, 132.21, 131.67, 129.87, 129.09, 128.79, 127.79, 127.44, 126.41, 125.01, 98.57, 60.10, 53.78, 14.37. IR (KBr disc) cm−1: 3156 (NH). Elem. anal. calcd. for C20H17N5Cl2S: C 55.82, H 3.98, N 16.27, S 7.45; found: C 55.78, H 4.11, N 16.45, S 7.07.
1-(2-Chloro-2-phenylethyl)-
N-(4-chlorophenyl)-6-(methylthio)-1
H-pyrazolo[3,4-
d]pyrimidin-4-amine
2b. Yield: 71% (white solid). Analytical and spectroscopic data are in accordance with those previously reported by us [
20].
1-(2-Chloro-2-phenylethyl)-6-(ethylthio)-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2c. Yield: 51% (white solid). Mp: 128–131 °C. 1H NMR (400 MHz, CDCl3): δ 9.37 (br s, 1H), 7.49–7.40, 7.38–7.35 and 7.34–7.30 (3m, 11 H), 6.94 (br s, 1H), 5.43–5.40 (m, 1H), 4.87–4.81 and 4.71–4.44 (2m, 2H), 3.28–3.19 (q, 2H, J = 8.0 Hz), 1.46 (t, 3H, J = 8.0 Hz). 13C NMR (101 MHz, CDCl3): δ 153.98, 137.68, 136.15, 134.31, 130.01, 129.69, 129.37, 129.27, 128.90, 128.86, 127.48, 127.43, 127.31, 60.08, 53.83, 25.65, 14.25. IR (KBr disc) cm−1: 3357 (NH). Elem. anal. calcd. for C21H20N5ClS: C 61.53, H 4.92, N 17.08, S 7.82; found: C 61.31, H 4.66, N 17.23, S 8.02.
1-(2-Chloro-2-phenylethyl)-
N-(3-chlorophenyl)-6-(isopropylthio)-1
H-pyrazolo[3,4-
d]pyrimidin-4-amine
2d. Yield: 65% (white solid). Analytical and spectroscopic data are in accordance with those previously reported by us[
20].
1-(2-Chloro-2-phenylethyl)-N-(2-chlorophenyl)-6-((2-morpholinoethyl)thio)-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2e. Yield: 62% (white solid). Mp: 117–118 °C. 1H NMR (400 MHz, CDCl3): δ 8.04 (br s, 1H), 7.52–7.46 (m, 3H), 7.37–7.32 (m, 6 H), 5.50–5.46 (m, 1H), 5.28–5.15 (m, 1H), 4.76–4.71 (m, 2H), 3.87 (br s, 4H), 3.37 (br s, 2H), 2.77 (br s, 6H). 13C NMR (101 MHz, CDCl3): δ 162.52, 154.67, 154.45, 137.71, 137.52, 137.35, 135.78, 134.10, 133.84, 133.37, 129.41, 129.02, 127.43, 126.54, 110.80, 66.20, 60.87, 57.28, 54.24, 53.15, 31.46. IR (KBr disc) cm−1: 3061 (NH). Elem. anal. calcd. for C25H26N6OSCl2: C 56.71, H 4.95, N 15.87, S 6.56; found: C 56.41, H 4.71, N 15.87, S 6.87.
1-(2-Chloro-2-phenylethyl)-6-ethyl-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2h. Yield: 44% (white solid). Mp: 174–176 °C. 1H NMR (400 MHz, DMSO-d6): δ 9.92 (s, 1H), 8.17 (br s, 1H), 7.85 (d, 2H, J = 7.6 Hz), 7.47 (dd, 2H, J = 8.0 Hz, J = 1.6 Hz), 7.35–7.30 (m, 5H), 7.05 (t, 1H, J = 7.2 Hz), 5.67–5.63 (m, 1H), 4.96–4.90 and 4.79–4.72 (2m, 2H), 2.75 (q, 2H, J = 7.6 Hz), 1.27 (t, 3H, J = 7.6 Hz). 13C NMR (400 MHz, DMSO-d6): δ 169.29, 155.14, 154.56, 139.92, 138.51, 132.72, 129.47, 129.23, 129.18, 128.12, 123.66, 121.42, 99.70, 61.04, 52.96, 32.72, 13.13. IR (KBr disc) cm−1: 3155 (NH). Elem. anal. calcd. for C21H20N5Cl: C 66.75, H 5.33, N 18.53; found: C 66.92, H 5.41, N 18.64.
1-(2-Chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-cyclopentyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2i. Yield: 41% (white solid). Mp: 189–190 °C. 1H (400 MHz, DMSO-d6): δ 10.08 (s, 1H,), 8.33 (t, 1H, J = 2.0 Hz), 7.63 (dd, 1H, J = 8.4 Hz, J = 1.2 Hz), 7.46 (dd, 2H, J = 8.0 Hz, J = 1.6 Hz), 7.37–7.28 (m, 4H), 7.07 (dd, 1H, J = 7.2 Hz, J = 1.2 Hz), 5.66–5.63 (m, 1H), 4.95–4.90 and 4.82–4.77 (2m 2H), 3.21 (quint, 1H, J = 8.0 Hz), 2.02–1.96, 1.93–1.82, 1.79–1.78 and 1.65–1.62 (4m, 8H). 13C NMR (101 MHz, DMSO-d6): δ 171.91, 154.96, 154.25, 141.57, 138.45, 133.51, 132.61, 130.77, 129.46, 129.16, 128.10, 122.87, 120.44, 118.92, 99.94, 60.97, 52.96, 48.56, 33.06, 32.91, 26.39. IR (KBr disc) cm−1: 3068 (NH). Elem. anal. calcd. For C24H23N5Cl2: C 63.72, H 5.12, N 15.48; found: C 63.48, H 5.34, N 15.61.
1-(2-Chloro-2-phenylethyl)-6-cyclohexyl-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2j. Yield: 44% (white solid). Mp: 134–136 °C. 1H NMR (400 MHz, CDCl3): δ 7.46–7.45 (m, 4H), 7.40–7.36 (m, 4H), 7.32–7.26 (m, 3H), 7.03 (br s, 1H), 5.48–5.45 (m, 1H), 4.92–4.87 and 4.82–4.77 (2m, 2H), 2.79 (tt, 1H, J = 12.0 Hz, J = 3.6 Hz), 2.04–2.01 (m, 2H), 1.89–1.86 (m, 2H), 1.77–1.65 (m, 3H), 1.47–1.31 (m, 3H). 13C NMR (101 MHz, CDCl3): δ 205.15, 154.56, 137.78, 133.40, 129.88, 129.59, 129.10, 128.77, 128.73, 127.49, 125.28, 98.09, 60.04, 53.66, 47.08, 46.23, 39.33, 31.53, 26.10, 25.97. IR (KBr disc) cm−1: 3361 (NH). Elem. anal. calcd. For C25H26N5Cl: C 69.51, H 16.21, N 6.07; found: C 69.72, H 15.82, N 15.87.
1-(2-Chloro-2-phenylethyl)-N,6-diphenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2n. Yield: 72% (white solid). Mp: 130–140 °C (dec). 1H NMR (400 MHz, CDCl3): δ 8.52–8.50 (m, 2H), 7.54–7.43 (m, 10H), 7.38–7.26 (m, 4H), 5.55–5.52 (m, 1H), 5.04–4.98 and 4.89–4.84 (2m 2H). 13C NMR (101 MHz, CDCl3): δ 170.71, 170.09, 155.20, 145.56, 139.95, 135.55, 129.47, 129.01, 128.87, 128.85, 128.00, 127.53, 126.57, 126.37, 121.84, 117.02, 114.39, 59.58, 53.41. IR (KBr disc) cm−1: 3347 (NH). Elem. anal. calcd. For C25H20N5Cl: C 70.50, H 4.73, N 16.44; found: C 70.63, H 4.89, N 16.43.
1-(2-Chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2o. Yield: 62% (white solid). Mp: 82–84 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.26 (s, 1H), 8.43–8.40 (m, 2H), 8.31 (s, 1H), 8.25 (t, 1H, J = 2.0 Hz), 7.77–7.74 (m, 1H), 7.55–7.50 (m, 5H), 7.43 (t, 1H, J = 8.0 Hz), 7.35–7.25 (m, 3H), 7.16–7.14 (m, 1H), 5.73–5.70 (m, 1H), 5.09–5.06 and 4.9489–4.88 (2m 2H). 13C NMR (101 MHz, DMSO-d6): δ 161.19, 155.27, 154.29, 141.27, 138.51, 138.26, 133.55, 132.94, 131.27, 130.99, 129.51, 129.19, 129.08, 128.69, 128.19, 123.30, 120.88, 119.46, 100.40, 61.09, 53.14. IR (KBr disc) cm−1: 3291 (NH). Elem. anal. calcd. For C25H19N5Cl2: C 65.22, H 4.16, N 15.21; found: C 65.36, H 4.42, N 14.97.
1-(2-Chloro-2-(4-fluorophenyl)ethyl)-6-(methylthio)-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2p. Yield: 47% (white solid). Mp: 227–230 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.27 (s, 1H), 8.26 (br s, 1H), 7.78 (d, 2H, J = 8.0 Hz), 7.54–7.51 (m, 2H), 7.34 (t, 2H, J = 7.6 Hz), 7.17–7.13 (m, 2H), 7.09–7.06 (m, 1H) 5.67–5.63 (m, 1H), 4.90–4.84 and 4.77–4.72 (2m, 2H), 2.50 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ NMR (101 MHz), δ 169.17, 163.88, 161.44, 158.93, 154.61, 139.35, 134.88, 134.85, 133.55, 130.49, 130.41, 129.21, 116.14, 115.92, 99.03, 60.06, 53.06, 14.26. IR (KBr disc) cm−1: 3093 (NH). Elem. anal. calcd. For: C 58.04, H 4.14, N 16.92, S 7.75; found: C 58.09, H 4.07, N 15.75, S 8.33.
1-(2-Chloro-2-(4-chlorophenyl)ethyl)-6-methyl-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2q. Yield: 50% (white solid). Mp: 202–203 °C. 1H NMR (400 MHz, DMSO-d6): δ 9.92 (s, 1H), 8.14 (br s, 1H), 7.82 (d, 2H, J = 8.0 Hz), 7.52–7.49 (m, 2H), 7.41–7.33 (m, 4H), 7.06 (t, 2H, J = 7.6 Hz), 5.67–5.64 (m, 1H), 4.95–4.89 and 4.76–4.71 (2m, 2H), 2.48 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 165.25, 155.19, 154.52, 139.81, 137.61, 134.02, 132.80, 130.15, 129.26, 129.18, 123.87, 121.54, 99.48, 60.02, 52.80, 26.62. IR (KBr disc) cm−1: 3153 (NH). Elem. anal. calcd. For C20H17N5Cl2: C 60.31, H 4.30, N 17.58; found: C 60.51, H 4.60, N 17.40.
1-(2-Chloro-2-(4-chlorophenyl)ethyl)-6-ethyl-N-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2r. Yield: 39% (white solid). Mp: 193–195 °C. 1H NMR (400 MHz, CDCl3): δ 7.68 (s, 1H), 7.54–7.45 (m, 4H), 7.42–7.40 (m, 2H), 7.41–7.38 (m, 2H), 7.35–7.30 (m, 5H), 5.38–5.34 (m, 1H), 4.90–4.84 and 4.76–4.69 (2m, 2H), 2.99 (q, 2H, J = 7.6 Hz), 1.45 (t, 3H, J = 7.6 Hz). 13C NMR (101 MHz, CDCl3): δ 155.56, 155.19, 137.42, 136.51, 134.86, 132.94, 129.76, 128.93, 128.92, 127.16, 125.25, 98.28, 59.10, 53.39, 40.77, 21.76, 14.01. IR (KBr disc) cm−1: 3148 (NH). Elem. anal. calcd. For C21H19N5Cl2: C 61.17, H 4.64, N 16.99; found: C 61.48, H 4.77, N 16.65.
1-(2-Chloro-2-(4-chlorophenyl)ethyl)-N-phenyl-6-propyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2s. Yield: 34% (white solid). Mp: 154–156 °C. 1H NMR (400 MHz, CDCl3): δ 7.68 (br s, 1H), 7.54–7.51 (m, 4H), 7.42–7.40 (m, 2H), 7.34–7.27 (m, 4H), 5.39–5.35 (m, 1H), 4.89–4.81 and 4.74–4.69 (2m, 2H), 2.90 (t, 2H, J = 7.6 Hz), 1.94 (sx, 2H, J = 7.6 Hz), 1.05 (t, 2H, J = 7.6 Hz). 13C NMR (101 MHz, CDCl3): δ 136.35, 135.95, 135.24, 133.55, 130.35, 129.60, 129.13, 128.95, 128.89, 128.82, 126.40, 58.98, 53.67, 40.90, 38.13, 21.47, 21.05, 13.77. IR (KBr disc) cm−1: 3161 (NH). Elem. anal. calcd. For C22H21N5Cl2: C 61.98, H 4.96, N 16.43; found: C 62.14, H 5.04, N 16.49.
3.1.2. General Procedure for the Synthesis of Final Compounds 2f,g
The appropriate 3-aminophenol derivative (5 mmol) was added to a solution of 4-chloro-1-(2-chloro-2-phenylethyl)-6-[(2-morpholinoethyl)thio]-1H-pyrazolo[3,4-d]pyrimidine 3d (1 mmol, 438 mg) in absolute ethanol (10 mL), and the mixture was refluxed for 3−5 h. After cooling to room temperature, the solvent was evaporated under reduced pressure and the crude was solved in ethyl acetate (10 mL), washed with 0.1 N HCl solution (2 × 10 mL), 1 N NaOH solution (10 mL) and brine (2 × 10 mL), dried (MgSO4), filtered, and concentrated under reduced pressure to give a brown oil which crystallized by adding a 1:1 mixture of diethyl ether/petroleum ether (bp 40−60 °C) affording yellow solid.
5-({1-(2-Chloro-2-phenylethyl)-6-[(2-morpholinoethyl)thio]-1H-pyrazolo[3,4-d]pyrimidin-4-yl)amino)-2-fluorophenol hydrochloride 2f. Yield: 38% (yellow solid). Mp: 238–239 °C. 1H NMR (200 MHz, DMSO-d6): δ 10.23 (s, 1H), 10.18 (br s, 1H), 7.63–7.61, 7.41–7.39, and 7.19–7.15 (3m, 9H), 5.68–5.62 (m, 1H), 5.21–5.07 and 4.83–4.78 (2m, 2H), 3.89–3.18 (m, 12H). 13C NMR (50 MHz, DMSO-d6): δ 167.84, 156.93, 152.04, 151.75, 150.39, 138.70, 138.23, 134.50, 129.39, 128.86, 127.54, 117.51, 113.09, 107.78, 106.14, 66.74, 61.80, 54.23, 52.66, 45.49, 31.87. IR (KBr disc) cm−1: 3300–3100 (OH and NH). (NH). Elem. anal. calcd. For C25H27N6O2SCl2F: C 53.10, H 4.81, N 14.26, S 5.67; found: C 53.25, H 4.77, N 14.49, S 5.27.
5-((1-(2-Chloro-2-phenylethyl)-6-((2-morpholinoethyl)thio)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)amino)-2,4-difluorophenol 2g. Yield: 25% (yellow solid). Mp: 146–149 °C. 1H NMR (400 MHz, CDCl3): δ 8.33 (br s, 1H), 7.89 (br s, 1H), 7.47–7.46 (m, 2H), 7.28–7.27 (m, 4H), 6.98–6.96 (m, 1H), 6.98–6.92 (m, 1H), 5.56–5.52 (m, 1H), 4.99–4.92 and 4.753–4.69 (2m, 2H), 3.92–3.91 (br s, 4H), 3.42 (br s, 2H), 3.98 (br s, 2H), 3.73 (br s, 4H). 13C NMR (101 MHz, CDCl3) δ 168.21, 157.49, 151.95, 151.03, 148.20, 146.02, 138.23, 134.50, 132.47, 129.39, 128.86, 127.54, 109.07, 106.92, 105.42, 66.82, 61.80, 54.23, 53.16, 51.43, 31.44. IR (KBr disc) cm−1: 3420–3300 (OH and NH). Elem. anal. calcd. For C25H25N6O2SClF2: C 54.89, H 4.61, N 15.36, S 5.86; found: C 55.00, H 4.70, N 15.47, S 5.67.
3.1.3. General Procedure for the Synthesis of Final Compounds 2k-m
The opportune amine (2.1 mmol) was added to a solution of 4-chloro-1-(2-chloro-2-phenylethyl)-6-(trifluoromethyl)-1H-pyrazolo[3,4-d]pyrimidine 8d (0.25 g, 0.69 mmol) in absolute ethanol (4 mL). The reaction mixture was irradiated in the microwave for 2–10 min at 80 °C in an open vessel. After cooling, the solvent was evaporated under reduced pressure, and the crude was treated with water (5 mL), then extracted with CH2Cl2 (5 mL); the organic phase was washed with water (5 mL), dried (MgSO4), and concentrated under reduced pressure. The obtained oil was crystallized by adding a mixture of CH2Cl2/n-hexane (1:4) and standing in a refrigerator to afford the pure product as a white solid.
1-(2-Chloro-2-phenylethyl)-N-phenyl-6-(trifluoromethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2k. Yield: 65% (white solid). Mp: 139–141 °C. 1H NMR (400 MHz, CDCl3): δ 7.53–7.38 (m, 8H), 7.33–7.29 (m, 3H), 5.46–5.42 (m, 1H), 5.00–4.94 and 4.84–4.79 (2m, 2H). 13C NMR (101 MHz, CDCl3): 155.95, 149.62, 141.42, 137.40, 133.90, 130.18, 129.90, 129.29, 128.87, 127.46, 123.84, 123.18, 110.63, 59.97, 54.15.. IR (KBr disc) cm−1: 3235 (NH). Elem. anal. calcd. For C20H15N5ClF3: C 57.49, H 3.92, N 16.76; found: C 57.44, H 4.15, N 16.43.
1-(2-Chloro-2-phenylethyl)-N-(3-fluorophenyl)-6-(trifluoromethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2l. Yield: 13% (white solid). Mp: 164–166 °C. 1H NMR (400 MHz, CDCl3): δ 8.63 (br s 1H), 7.51 (br s, 1H), 7.42–7.26 (m, 8H), 6.96 (t, 1H, J = 7.6 Hz), 5.51–5.48 (m, 1H), 5.02–4.97 and 4.87–4.82 (2m, 2H). 13C NMR (101 MHz, CDCl3): δ NMR (101 MHz, ) δ 164.34, 161.88, 154.08, 139.30, 138.04, 137.59, 136.35, 135.48, 132.94, 132.69, 130.66, 130.57, 129.18, 128.82, 127.49, 127.48 60.07, 54.06. IR (KBr disc) cm−1: 3281 (NH). Elem. anal. calcd. For C20H14N5ClF4: C 55.12, H 3.24, N 16.07; found: C 55.36, H 3.34, N 15.89.
N-(3-Chlorophenyl)-1-(2-chloro-2-phenylethyl)-6-trifluoromethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2m. Yield: 13% (white solid). Mp: 88–90 °C. 1H NMR (400 MHz, CDCl3): δ 8.18 (br s, 1H), 7.58 (br s, 1H), 7.43–7.39 (m, 5H), 7.34–7.28 (m, 4H), 5.49–5.45 (m, 1H), 5.03–4.97 and 4.87–4.82 (2m, 2H). NMR (101 MHz, CDCl3): δ 147.88, 145.37, 137.41, 135.50, 134.72, 132.79, 130.89, 130.67, 129.48, 129.28, 128.90, 128.87, 127.91, 127.47, 127.45, 127.43, 59.98, 54.19. IR (KBr disc) cm−1: 3308 (NH). Elem. anal. calcd. For C20H14N5Cl2F3: C 53.11, H 3.12, N 15.49; found: C 53.12, H 3.15, N 15.46.
3.1.4. General Procedure for the Synthesis of Intermediates 5a,b
Ethoxymethylenemalononitrile (2.44 g, 20 mmol) was added to a solution of the opportune 2-hydrazino-1-phenylethanol 4a,b (20 mmol) in absolute ethanol (40 mL) and the reaction mixture was refluxed for 6 h. The solvent was concentrated to 50% of the initial volume and cooled. The yellow solid obtained was filtered and recrystallized from absolute ethanol.
5-Amino-1-(2-hydroxy-2-phenylethyl)-1
H-pyrazole-4-carbonitrile
5a. Yield: 71% (white solid). Analytical and spectroscopic data are in accordance with those previously reported by us [
20].
5-Amino-1-[2-(4-chlorophenyl)-2-hydroxyethyl]-1H-pyrazole-4-carbonitrile 5b. Yield: 51% (white solid). Mp: 218–220 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.50 (s, 1H), 7.36–7.31 (m, 4H), 6.41 (br s, 2H), 5.72 (d, 1H, J = 4.8 Hz), 4.92–4.88 (m, 1H), 4.05–4.00 and 3.95–3.90 (2m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 152.62, 141.93, 140.63, 132.43, 128.69, 128.54, 115.86, 72.63, 71.01, 54.37. IR (KBr disc) cm−1: 3401–3287 (OH, NH2), 2217 (CN). Elem. anal. calcd. For C12H11N4ClO: C 54.87, H 4.22, N 21.33; found: C55.01, H 4.23, N 21.42.
3.1.5. General Procedure for the Synthesis of Intermediates 6a,b
2M NaOH (10 mL) was added to a solution of the opportune 5-amino-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carboxamide derivative 5a,b (10 mmol) in absolute ethanol (10 mL), and the mixture was refluxed for 2 h. After cooling, the solid obtained was filtered, washed with water and recrystallized from ethanol.
5-Amino-1-(2-hydroxy-2-phenylethyl)-1
H-pyrazole-4-carboxamide
6a. Yield: 66% (white solid). Analytical and spectroscopic data are in accordance with those previously reported by us [
20].
5-Amino-1-[2-(4-chloro-phenyl)-2-hydroxyethyl]-1H-pyrazole-4-carboxamide 6b. Yield: 58% (white solid). Mp: 215–216 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.59 (s, 1H), 7.36–7.31 (m, 4H), 6.05 (br s, 2H), 5.79 (br s, 1H), 4.93–4.90 (m, 1H), 4.00–3.89 (m, 2H). IR (KBr disc) cm−1: 3450–3200 (OH + NH), 1622 (CO). 13C NMR (101 MHz, DMSO-d6): δ 166.67, 150.51, 142.20, 137.67, 132.31, 128.62, 128.53, 97.27, 71.31, 54.16. Elem. anal. calcd. For C12H13N4O2Cl: C 51.34, H 4.67, N 19.96; found: C 51.41, H 4.87, N 19.98.
3.1.6. General Procedure for the Synthesis of 6-Substituted 1-(2-Hydroxy-2-Phenylethyl)-1,5-Dihydro-4H-Pyrazolo[3,4-d]Pyrimidin-4-one Derivatives 7a-h
A solution of sodium ethoxide prepared from sodium (1.38 g, 60 mmol) and absolute ethanol (30 mL) and the appropriate alkyl ester (60 mmol) were added to a solution of the appropriate 5-amino-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carboxamide 6a,b (10 mmol) in absolute ethanol (90 mL). The mixture was refluxed for 6–12 h; after cooling, ice water was added, and the solution was acidified with 3% acetic acid. The obtained precipitate was filtered, washed with water, and recrystallized from absolute ethanol to afford desired compounds 7a-h.
6-Ethyl-1-(2-hydroxy-2-phenylethyl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7a. Alkyl ester used for the synthesis: methyl propionate. Yield: 65% (white solid). Mp: 221–222 °C. 1H NMR (400 MHz, DMSO-d6): δ 11.90 (br s, 1H), 7.94 (s, 1H), 7.26–7.21 (m, 4H), 7.20–7.16 (m, 1H), 5.59 (br s, 1H), 5.05 (br s, 1H), 4.42–4.37 and 4.28–4.23 (2m, 2H), 2.54 (q, 2H, J = 7.6 Hz), 1.14 (t, 3H, J = 7.6 Hz). 13C NMR (101 MHz, DMSO-d6): δ 161.85, 158.59, 153.39, 143.01, 134.53, 128.55, 127.89, 126.54, 104.27, 71.66, 54.48, 28.00, 11.87. IR (KBr disc) cm−1: 3410 (NH), 3350–2990 (OH), 1661 (CO). Elem. anal. calcd. for C15H16N4O2: C 63.37, H 5.67, N 19.71; found: C 63.15, H 5.91, N 19.59.
6-Cyclopentyl-1-(2-hydroxy-2-phenylethyl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7b. Alkyl ester used for the synthesis: methyl cyclopentancarboxylate. Yield: 44% (white solid). Mp: 227–228 °C. 1H NMR (200 MHz, CDCl3): δ 10.95 (br s, 1H), 7.99 (s, 1H), 7.34–7.32, 7.29–7.25 and 7.22–7.17 (3m, 5H), 5.16–5.14 (m, 1H), 4.61–4.56 and 4.50–4.44 (2m, 2H), 4.33 (br s, 1H), 3.04 (t, 1H, J = 7.0 Hz), 2.06–2.04 (m, 2H), 1.88–1.1.79 (m, 4H), 1.71–1.66 (m, 2H). IR (KBr disc) cm−1: 3300–2950 (NH + OH), 1698 (CO). Elem. anal. calcd. for C18H20N4O2: C 66.65, H 6.21, N 17.27; found: C 66.84, H 6.29, N 17.31.
6-Cyclohexyl-1-(2-hydroxy-2-phenylethyl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7c. Alkyl ester used for the synthesis: methyl cyclohexane carboxylate. Yield: 68% (white solid). Mp: 216–218 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.92 (s, 1H), 7.24–7.16 (m, 5H), 5.64 (br s, 1H), 5.07–5.03 (m, 1H), 4.42–4.36 and 4.31–4.26 (2m, 2H), 2.55–2.50 (m, 1H), 1.80–1.62 (m, 4H), 1.66–1.62 (m, 1H), 1.52–1.39 (m, 2H), and 1.29–1.13 (m, 3H). 13C NMR (101 MHz, DMSO-d6): δ 164.31, 158.78, 153.31, 142.97, 134.46, 128.50, 127.86, 126.54, 104.35, 71.68, 54.43, 42.83, 30.73, 30.69, 25.87, 25.81. IR (KBr disc) cm−1: 3330–3000 (NH + OH), 1701 (CO). Elem. anal. calcd. for C19H22N4O2: C 67.44, H 6.55, N 16.56; found C 67.58, H 8.82, N 16.44.
1-(2-Hydroxy-2-phenylethyl)-6-(trifluoromethyl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7d. Alkyl ester used for the synthesis: methyl trifluoroacetate. Yield: 58% (white solid). Mp: 212–214 °C. 1H NMR (200 MHz, DMSO-d6): δ 13.58 (br s, 1H), 8.23 (s, 1H), 7.38–7.19 (m, 5H), 5.71 (d, 1H, J = 6.1 Hz), 5.13–5.06 (m, 1H), 4.58–4.27 (m, 2H). IR (KBr disc) cm−1: 3450–2798 (OH + NH), 1704 (CO). Elem. anal. calcd. for C14H11N4O2F3: C 51.86, H 3.42, N 17.58; found: C 51.78, H 3.77, N 17.34.
1-(2-Hydroxy-2-phenylethyl)-6-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7e. Alkyl ester used for the synthesis: methyl benzoate. Yield: 53% (white solid). Mp: 252–254 °C. 1H NMR (200 MHz, DMSO-d6): δ 12.36 (br s, 1H), 8.12–8.11, 7.60–7.58 and 7.34–7.31 (3m, 11H), 5.61 (d, 1H, J = 4.7 Hz), 5.18–5.06 (m, 1H), 4.61–4.28 (m, 2H). IR (KBr disc) cm−1: 3445–2977 (OH + NH), 1695 (CO). Elem. anal. calcd. for C19H16N4O2: C 68.66, H 4.85, N 16.86; found: C 68.38, H 5.09, N 17.02.
1-[2-(4-Chlorophenyl)-2-hydroxyethyl]-6-methyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7f. Alkyl ester used for the synthesis: ethyl acetate. Yield: 72% (white solid). Mp: 264–266 °C. 1H NMR (200 MHz, DMSO-d6): δ 8.00 (s, 1H), 7.39–7.27 (m, 4H), 5.75 (d, 1H, J = 4.8 Hz), 5.17–5.04 (m, 1H), 4.43–4.39 and 4.30–4.19 (2m, 2H), 2.33 (s, 3H). IR (KBr disc) cm−1: 3230–2950 (NH + OH), 1677 (CO). Elem. anal. calcd. for C14H13N4ClO2: C 55.18, H 4.30, N 18.39; found: C 55.26, H 4.59, N 18.26.
1-[2-(4-Chlorophenyl)-2-hydroxyethyl]-6-ethyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7g. Alkyl ester used for the synthesis: methyl propionate. Yield: 71% (white solid). Mp: 243–245 °C. 1H NMR (200 MHz, DMSO-d6): δ 12.10 (br s, 1H), 8.14 (s, 1H), 7.48–7.35 (m, 4H), 5.39 (br s, 1H), 5.23–5.19 (m, 1H), 4.64–4.41 (m, 2H), 2.71 (q, 2H, J = 7.8 Hz), 1.31 (t, 3H, J = 7.8 Hz). IR (KBr disc) cm−1: 3400–2900 (OH + NH), 1693 (CO). Elem. anal. calcd. for C15H15N4ClO2: C 56.52, H 4.74, N 17.58; found: C 56.78, H 4.94; N 17.60.
1-[2-(4-Chlorophenyl)-2-hydroxyethyl]-6-propyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 7h. Alkyl ester used for the synthesis: methyl butirate. Yield: 80% (white solid). Mp: 257–259 °C. 1H NMR (200 MHz, DMSO-d6): δ 8.00 (s, 1H), 7.34–7.18 (m, 4H), 5.80 (d, 1H, J = 4.6 Hz), 5.17–5.03 (m, 1H), 4.43–4.21 (m, 2H), 1.65 (sx, 2H, J = 7.4 Hz), 0.88 (t, 3H, J = 7.4 Hz). IR (KBr disc) cm−1: 3440–2900 (NH + OH), 1694 (CO). Elem. anal. calcd. for C16H17N4O2Cl: C 57.75, H 5.15, N 16.84; found: C 58.07, H 5.48; N 16.98.
3.1.7. General Procedure for the Synthesis of 6-Alkyl or 6-Phenyl 4-Chloro-1-(2-Chloro-2-Phenylethyl)-1H-Pyrazolo[3,4-d]pyrimidines 8a-h
The Vilsmeier complex, previously prepared from POCl3 (4.60 g, 30 mmol) and anhydrous DMF (2.20 g, 30 mmol), was added to a suspension of the appropriate intermediate 7a-h (1 mmol) in CHCl3 (10 mL). The mixture was refluxed for 6–12 h. The solution was washed with water (2 × 20 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The crude oil was purified by column chromatography (Florisil, 100–200 mesh), using diethyl ether as eluant, to afford compounds 8a-c, f-h as yellow or colorless oils. Compounds 8d,e crystallized standing in a refrigerator by adding a 1:1 mixture of diethyl ether/petroleum ether (bp 40– 60 °C) (1:1) as white solids.
4-Chloro-1-(2-chloro-2-phenylethyl)-6-ethyl-1H-pyrazolo[3,4-d]pyrimidine 8a. Yield: 85% (colorless oil). 1H NMR (400 MHz, CDCl3): δ 11.29 (br s, 1H), 8.05 (s, 1H), 7.40–7.37 (m, 2H), 7.32–7.25 (m, 3H), 5.49–5.46 (m, 1H), 4.92–4.86 and 4.78–4.72 (2m, 2H), 2.74 (q, 2H, J = 7.2 Hz), 1.35 (t, 3H, J = 7.2 Hz). 13C NMR (101 MHz, CDCl3): 161.18, 160.03, 153.79, 137.86, 135.47, 129.04, 128.75, 127.47, 103.99, 60.19, 54.01, 28.56, 11.29. Elem. anal. calcd. for C15H14N4Cl2: C 56.09, H 4.39, N 17.44; found: C 56.18, H 4.51, N 17.63.
4-Chloro-1-(2-chloro-2-phenylethyl)-6-cyclopentyl-1H-pyrazolo[3,4-d]pyrimidine 8b.Yield: 95% (yellow oil). 1H NMR (200 MHz, CDCl3): δ 8.04 (s, 1H), 7.39–7.24 (m, 5H), 5.28–5.21 (m, 1H), 4.67–4.59 and 4.54–4.43 (2m, 2H), 3.12–3.02 (m, 1H), 1.87–1.43 (m, 8H). Elem. anal. calcd. for C18H18N4Cl2: C 58.84, H 5.02, N 15.51; found: C 60.00, H 4.99, N 15.21.
4-Chloro-1-(2-chloro-2-phenylethyl)-6-cyclohexyl-1H-pyrazolo[3,4-]pyrimidine 8c. Yield: 98% (yellow oil). 1H NMR (200 MHz, DMSO-d6): δ 8.45 (s, 1H), 7.48–7.42 and 7.35–7.33 (2m, 5H), 5.78–5.67 (m, 1H), 5.15–4.96 (m, 2H), 2.88 (t, 1H, J = 7.8 Hz), 2.00–1.94 and 1.94–1.33 (2m, 10H). Elem. anal. calcd. for C19H20N4Cl2: C 60.81, H 5.37, N 14.93; found: C 60.96, H 5.44, N 15.03.
4-Chloro-1-(2-chloro-2-phenylethyl)-6-(trifluoromethyl)-1H-pyrazolo[3,4-d]pyrimidine 8d. Yield: 84% (white solid). 1H NMR (200 MHz, DMSO-d6): δ 8.08 (s, 1H), 7.52–7.50 and 7.40–7.35 (2m, 5H), 5.77–5.65 (m, 1H), 5.23–4.98 (m, 2H). Mp: 85–87 °C. Elem. anal. calcd. for C14H9N4Cl2F3: C 46.56, H 2.51, N 15.51; found: 46.78, H 2.80, N 15.20.
4-Chloro-1-(2-chloro-2-phenylethyl)-6-phenyl-1H-pyrazolo[3,4-d]pyrimidine 8e. Yield: 81% (white solid). Mp: 114–117 °C. 1H NMR (200 MHz, CDCl3): δ 8.59–8.56 (m, 2H), 8.16–8.14 (m, 1H), 7.59–7.51 (m, 5H), 7.48–7.31 (m, 3H), 5.63–5.59 (m, 1H), 5.21–4.98 (m, 2H). Elem. anal. calcd. for C19H14N4Cl2: C 61.80, H 3.82, N 15.17; found: C 61.74, H 4.03, N 14.83.
4-Chloro-1-[2-chloro-2-(4-chlorophenyl)ethyl]-6-methyl-1H-pyrazolo[3,4-d]pyrimidine 8f. Yield: 94% (colorless oil). 1H NMR (200 MHz, CDCl3): δ 7.97 (s, 1H), 7.38–7.29 (m, 4H), 5.58–5.50 (m, 1H), 5.18–5.00 and 4.95–4.79 (2m, 2H), 2.70 (s, 3H). Elem. anal. calcd. for C14H11N4Cl3: C 49.22, H 3.25, N 16.40; found: C 49.13, H 2.98, N 16.31.
4-Chloro-1-[2-chloro-2-(4-chloro-phenyl)-ethyl]-6-ethyl-1H-pyrazolo[3,4-d]pyrimidine 8g. Yield: 81% (yellow oil, that slowly crystallized). Mp: 63–65 °C. 1H NMR (200 MHz, DMSO-d6): δ 8.46 (s, 1H), 7.55–7.51 and 7.46–7.39 (2m, 4H), 5.78–5.66 (m, 1H), 5.17–4.92 (m, 2H), 2.96 (q, 2H, J = 7.6 Hz), 1.31 (t, 3H, J = 7.6 Hz). Elem. anal. calcd. for C15H13N4Cl3: C 50.66, H 3.68, N 15.75; found C 50.47, H 3.50, N 15.60.
4-Chloro-1-[2-chloro-2-(4-chlorophenyl)ethyl]-6-propyl-1H-pyrazolo[3,4-d]pyrimidine 8h. Yield: 93% (yellow oil). 1H NMR (200 MHz, DMSO-d6): δ 7.90 (s, 1H), 7.58–7.39 (m, 4H), 5.44–5.38 (m, 1H), 5.09–5.01 (m, 2H), 2.65 (t, J = 7.7 Hz, 2H), 1.80 (sx, 2H, J = 7.7 Hz), 0.89 (t, 3H, J = 7.7 Hz). Elem. anal. calcd. for C16H15N4Cl3: C 51.98, H 4.09, N 15.16; found: C 52.20, H 4.17, N 15.21.